Abstract
Background
Benign MS, traditionally defined as EDSS ≤3 and ≥15 years’ disease duration, is thought to follow a milder course. We determined the extent of visual pathway axonal loss by optical coherence tomography (OCT) retinal nerve fiber layer (RNFL) thickness in a benign MS cohort, and examined the relation to vision and quality of life (QOL).
Methods
In this longitudinal study of vision in MS at three academic centers, a subset of patients with EDSS, visual function, OCT, and QOL assessments was analyzed. Low- and high-contrast letter acuity were performed to assess visual function. RNFL thickness was determined using OCT-3. QOL scales included the 25-Item National Eye Institute Visual Functioning Questionnaire (NEI-VFQ-25) and SF-36.
Results
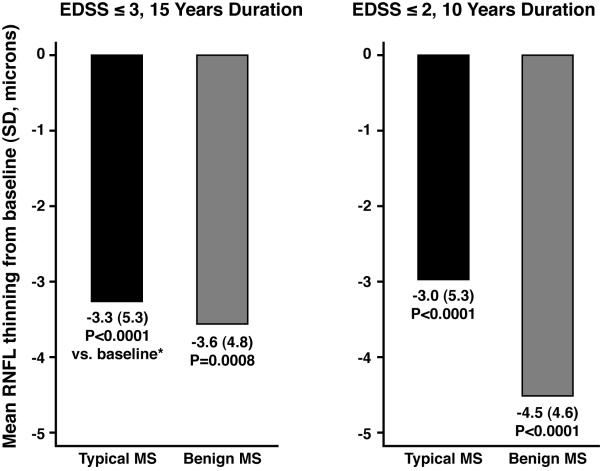
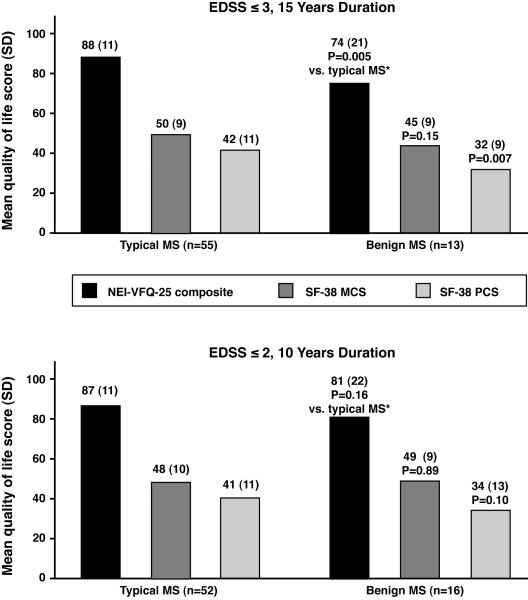
Among 68 patients (135 eyes) studied longitudinally, 13 (26 eyes) had benign MS using criteria of EDSS ≤3 and ≥15 years disease duration. Benign MS eyes had as much RNFL thinning (-3.6 μm, P=0.0008 vs. baseline, paired t-test) as typical MS eyes (-3.3 μm, P<0.0001). Both groups had significant low-contrast acuity loss. Prior history of optic neuritis (ON) was more frequent in benign MS (69% vs. 33% of eyes). History of ON distinguished benign vs. typical MS (P=0.002) and correlated with RNFL thickness at baseline (P=0.002) and disease duration (P=0.03), but not EDSS (P=0.32, logistic regression). NEI-VFQ-25 scores were also worse for benign MS, accounting for age (75±21 vs. 88±11, P=0.005).
Conclusions
Patients with benign MS have RNFL axonal loss that is as marked as that of typical MS, and have reduced vision and QOL. While overall neurologic impairment is mild, visual dysfunction, not well-captured by the EDSS, accounts for a substantial degree of disability in benign MS.
Keywords: multiple sclerosis (MS), visual function, optical coherence tomography (OCT), quality of life
Multiple sclerosis (MS) is characterized by a wide range of clinical presentations (1). Patients with limited neurological signs and symptoms have been described as having a milder or benign form of MS (2-13). Benign MS has been traditionally defined as an Expanded Disability Status Score (EDSS) score ≤3 with ≥15 years disease duration (14). However, a recent consensus definition suggested using an EDSS score ≤2 and disease duration ≥10 years (12). Ultimately, the diagnosis of benign MS can only be made in retrospect (2-14). It is exceedingly difficult to predict which patients will continue to follow a benign clinical trajectory after 10-15 years (6). As few as 20% of patients categorized as having benign MS at 10 years of disease duration, will continue to follow this same course after 20 years of follow-up (2,7).
Despite relatively preserved motor function, patients with benign MS have a similar or even greater MRI T2 lesion burden compared to typical MS (14-27). Possible explanations include sparing of clinically eloquent regions (14-19), paucity of tissue damage in and around MS lesions (19-24), and effective compensatory mechanisms (25-27). EDSS scores do not adequately reflect the frequent and often disabling component of MS associated cognitive impairment, an important feature that correlates with increased MRI lesion burden in benign MS (28-32). Vision is also not well-captured by the EDSS, yet is as important as walking is to patients with early MS (33-38).
Optical coherence tomography (OCT) has brought visual function to the forefront as a model for testing new MS therapies (33-35). We determined the extent of visual pathway axonal loss by OCT retinal nerve fiber layer (RNFL) thickness in a benign MS cohort, and assessed how visual function, history of acute optic neuritis (ON), and QOL differ for benign vs. typical MS. While primary analyses used the traditional definition of EDSS ≤3 and disease duration ≥15 years, the recently revised consensus definition (EDSS ≤2, ≥10 years duration) was also examined.
METHODS
Study Participants
Participants were a subset of patients from an ongoing prospective longitudinal study of visual outcome measures in MS at the University of Pennsylvania, University of Texas Southwestern Medical Center at Dallas, and Johns Hopkins University. Subjects represented a subset who had undergone EDSS, visual function testing, OCT, and QOL assessments and were not selected based on clinical features or extent of symptoms. Patients with ≥6 months of follow-up were included in these analyses. The present study represents a unique cohort of patients who fulfilled published definitions for benign MS, and does not represent substantial overlap with previous reports (34,35).
MS was diagnosed by standard criteria (39). All patients had neurologic status graded by the Expanded Disability Status Scale (EDSS). Benign MS has been variably defined in the literature (2-14). We therefore performed analyses using both a more traditional definition of benign MS (EDSS score ≤3 and ≥15 years disease duration) as well as a newer consensus definition (EDSS score ≤2 and ≥10 years disease duration) (12).
Participants with co-morbid ocular conditions, pathologic hyperopia or myopia (spherical refractive error > +8.00 or < −8.00 diopters) were excluded. A past history (months to years prior to enrollment) of acute optic neuritis (ON) was determined by self- and physician report and confirmed by record review. Patients with acute ON that was ongoing, or had occurred within 3 months prior to testing, were not included in this study; optic disc swelling was not noted among any participants. Institutional Review Board (IRB) approval and written informed consent were obtained. The study was conducted in accordance with Health Insurance Portability and Accountability Act (HIPAA) guidelines.
Visual Function Testing
Low-contrast letter acuity was tested for each eye using retroilluminated low-contrast Sloan letter charts (2.5%, 1.25% contrast, 2 meters, Precision Vision, LaSalle, IL) (33,37). High contrast visual acuity was assessed using retroilluminated Early Treatment Diabetic Retinopathy Study (ETDRS) charts at 3.2 meters following detailed refractions. Numbers of letters identified (maximum 70/chart) were recorded for each eye. Testing was performed by trained technicians experienced in research examinations.
Clinically meaningful visual loss over time was also categorized by determining whether changes exceeded the amount that would be expected from repeated testing when there was no real change (35). For high-contrast visual acuity (VA), 2-line, or 10-letter, differences have been used traditionally as criteria for clinically meaningful change, based on studies of test-retest variability (33). However, recent studies have demonstrated that, in patients with relatively good visual acuity, 5-letter or 1-line changes in high-contrast VA are unlikely to be due to testing error (35). Examination of our inter-rater reliability study data has revealed that 5 letters represents two standard deviations of inter-rater difference for VA in patients with MS (35). Correspondingly, two standard deviations of inter-rater difference for low-contrast acuity were equal to 7 letters.
Optical Coherence Tomography (OCT)
Optical coherence tomography (OCT) was performed using OCT-3, version 4.0 software (Carl Zeiss Meditec, Inc., Dublin, CA). While patients in our longitudinal cohort now also undergo spectral-domain OCT, these analyses focused on time-domain OCT since patients have the longest duration of follow-up using this device. Fast retinal nerve fiber layer (RNFL) thickness protocols were used. If the pupils were large enough (≥5 mm) to permit adequate imaging, scanning was completed without use of mydriatic drops. Good quality scans were defined as signal strength ≥7 (maximum 10), centered optic disc, and uniform brightness. Eyes with signal strength <7 were excluded from analyses.
Quality Of Life (QOL) Assessment
QOL instruments were self-administered at study visits; when possible, patients completed all questionnaires prior to vision testing. The NEI-VFQ-25 is the standard vision-specific QOL scale used in ophthalmologic research and clinical trials (36,37). Scores for the NEI-VFQ-25 composite and sub-scales range from 0 to 100 (higher scores=better HRQOL). We included a 10-Item Neuro-Ophthalmic Supplement to the NEI-VFQ-25 designed in a previous study by our group to target additional aspects of vision not captured by the NEI-VFQ-25 in MS (36).
The Short Form-36 Health Survey (SF-36) was used to measure overall HRQOL. Summary scale scores for the SF-36, including the Physical (PCS) and Mental Components Summary (MCS), are calculated based on U.S. population standards, with a standardized score of 50 (standard deviation=10) representing the mean or “norm.”
Statistical Analysis
Statistical analyses were performed using Stata 11.0 software. Changes from baseline in RNFL thickness, VA, low-contrast acuity, and EDSS scores were determined for patients who had received at least one follow-up visit. Changes in RNFL, acuity, and EDSS from baseline to last follow-up were examined for significance using paired t-tests. Proportions of eyes with clinically significant changes in visual function for benign vs. typical MS status were compared using logistic regression, accounting for within-patient, inter-eye correlations. Linear regression was used to examine the association of QOL scores at follow-up to benign vs. typical MS status, accounting for age. Associations of prior history of acute ON, EDSS score, and disease duration with benign vs. typical MS status were examined using logistic regression models. Type I error for significance was P<0.05.
RESULTS
Baseline characteristics for patients with follow-up time ≥6 months (n=68, 135 eyes) are shown in Table 1. Proportions of patients that met criteria for benign MS were similar for the 15-year (13/68, 19%) and 10-year (16/68, 24%) definitions. Age, median EDSS scores, and follow-up in the study were similar among the benign vs. typical MS categories; by definition, the disease duration was substantially greater for the benign MS groups. Most patients in all categories had relapsing-remitting MS (85-93%), with the remainder classified as secondary-progressive. High-contrast visual acuity (VA), low-contrast letter acuity, and retinal nerve fiber layer (RNFL) thickness at baseline were similar for benign vs. typical MS.
TABLE 1.
Baseline characteristics of patients with benign vs. typical MS
| EDSS ≤3, Duration ≥15 Years |
EDSS ≤2, Duration ≥10 Years |
|||
|---|---|---|---|---|
| Benign MS n = 13 (26 eyes) |
Typical MS n = 55 (109 eyes) |
Benign MS n = 16 (30 eyes) |
Typical MS n = 52 (105 eyes) |
|
| Age, mean (SD), y | 52 ± 5 | 50 ± 8 | 51 ± 5 | 50 ± 8 |
| EDSS score, median (range) | 2.0 (1.5 – 3.0) |
2.0 (0 – 7.0) |
2.0 (1.0 – 2.0) |
2.5 (0 – 7.0) |
|
Disease duration, median
(range), y |
19 (15.3 - 29) |
6.0 (>1 - 46) |
15.5 (10.6 - 26) |
5.9 (>1 - 46) |
|
Study follow-up, median (range),
y |
1.5 (1 – 3.5) |
1.4 (0.5 – 5.1) |
1.8 (0.8 – 5.1) |
1.3 (0.5 – 3.7) |
|
Relapsing MS subtype (%
patients) |
85% | 88% | 93% | 86% |
| History of optic neuritis (% eyes) | 69% | 33% | 50% | 37% |
|
High-contrast visual acuity,
median (range), Snellen from ETDRS |
20/20 (20/25 – 20/16) |
20/16 (>20/250 – 20/12.5) |
20/16 (20/25 – 20/12.5) |
20/16 (>20/250 – 20/12.5) |
|
Low-contrast letter acuity (2.5%),
mean (SD), number of letters correct |
28 ± 11 | 31 ± 13 | 30 ± 10 | 30 ± 13 |
|
NEI-VFQ-25 composite score,
mean (SD), best score 100 |
76 ± 19 | 89 ± 15 | 88 ± 20 | 86 ± 15 |
|
10-Item Neuro-Ophthalmic
Supplement to the NEI-VFQ-25, mean (SD) best score 100 |
74 ± 18 | 86 ± 15 | 86 ± 19 | 83 ± 15 |
|
Retinal nerve fiber layer (RNFL)
thickness, TD OCT, mean (SD), μm |
90 ± 14 | 91 ± 16 | 91 ± 14 | 91 ± 16 |
Abbreviations: MS = multiple sclerosis; EDSS = Expanded Disability Status Scale; ETDRS = Early Treatment Diabetic Retinopathy Study; NEI-VFQ-25 = 25-Item National Eye Institute Visual Functioning Questionnaire; TD = time-domain; OCT = optical coherence tomography
The benign MS groups had greater proportions of eyes with a history of acute optic neuritis (ON) prior to study enrollment (69% vs. 33% for the 15-year definition; 50% vs. 37% for the 10-year. The association of benign MS with a prior history of ON was only significant, however, for the 15-year-definition (P=0.002, logistic regression accounting for age and adjusting for within-patient, inter-eye correlations). The level of significance for this association was less when disease duration was accounted for in the models (P=0.01), but did not change when accounting for baseline EDSS score (P=0.002). When only patients with a disease duration of ≥15 years were considered, the occurrence of ON still remained higher in the benign group (69%) compared to those with typical MS (42%). So, even when we equalize the duration of disease, one of the benign groups had a greater proportion of eyes with a history of ON compared to a typical RRMS group. The proportions with history of ON were equal for the 10-year definition (50% of eyes in both groups).
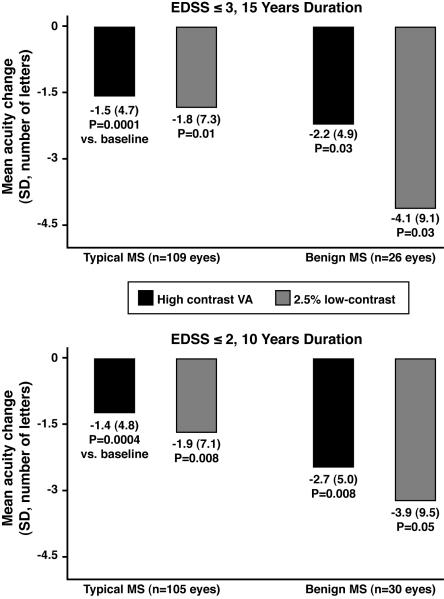
Eyes of patients with benign MS had as much RNFL thinning from baseline as those with typical MS (Fig. 1). Benign MS eyes had similar degrees of thinning, even adjusting for length of follow-up: −2.1 μm/ year for benign MS (15-year definition) vs. −2.1 μm/ year for typical MS; −2.5 μm/ year for benign MS (10-year definition) vs. −2.0 μm for typical MS. RNFL thickness at last follow-up visit was significantly lower compared to baseline values for benign and typical MS (P≤0.0008, paired t-tests, Fig. 1). Greater degrees of RNFL thinning were seen among eyes with a prior history of acute ON (P=0.01, logistic regression). Benign and typical MS eyes demonstrated significant losses of visual function over time (Fig. 2), with patterns similar to those of RNFL thinning. Quality of life (QOL) scores at follow-up were uniformly in the direction of worsening among patients with benign MS compared to typical MS (Fig. 3). Using the 15-year definition, worse scores for vision-specific scales, including the NEI-VFQ-25, were significantly associated with benign MS (P=0.005, linear regression, accounting for age, Fig. 3A). The 10-Item Neuro-Ophthalmic Supplement to the NEI-VFQ-25 showed similar results. Physical aspects of QOL, measured by the SF-36 PCS, were significantly more affected in benign MS, again using the 15-year definition (P=0.007, accounting for age). Using an alternative criterion for benign MS proposed by some authors, EDSS≤2.0 and disease duration ≥20 years (14 eyes of 8 patients in this cohort), results for change in RNFL thickness, visual function, and QOL were similar to those observed for the 15-year definition in Figs. 1-3.
FIG. 1.
Retinal nerve fiber layer (RNFL) thinning from baseline for eyes of patients with benign vs. typical MS, using two definitions for benign MS.
Abbreviations: MS = multiple sclerosis; EDSS = Expanded Disability Status Scale; RNFL = retinal nerve fiber layer thickness
* P values are based on paired t-tests comparing baseline to follow-up values for retinal nerve fiber layer (RNFL) thickness.
FIG. 2.
High-contrast visual acuity (VA, ETDRS charts) and low-contrast letter acuity loss from baseline at 2.5% contrast level for eyes of patients with benign vs. typical MS, using two definitions for benign MS.
Abbreviations: MS = multiple sclerosis; EDSS = Expanded Disability Status Scale; ETDRS = Early Treatment Diabetic Retinopathy Study
* P values are based on paired t-tests comparing baseline to follow-up values for high-contrast visual acuity (VA) and low-contrast letter acuity at 2.5% contrast.
FIG 3.
Follow-up scores for the 25-Item National Eye Institute Visual Functioning Questionnaire (NEI-VFQ-25) and SF-36 Health Survey at follow-up for patients with benign vs. typical MS, using two definitions for benign MS.
Abbreviations: MS = multiple sclerosis; EDSS = Expanded Disability Status Scale; NEI-VFQ-25 = 25-Item National Eye Institute Visual Functioning Questionnaire; SF-36 = SF-36 Health Survey; PCS = Physical Components Summary; MCS = Mental Components Summary
* P values are based on linear regression models, accounting for age, examining the association of quality of life score to benign vs. typical MS.
Among patients who met criteria for benign MS by the 10-year (EDSS ≤2) criteria (n=16), 8 (50%) also had benign MS by the 15-year (EDSS ≤3) definition. Patients with benign MS at baseline by the 15-year definition (n=13) remained benign at follow-up in 62% of cases (Table 2). Using 10-year criteria, 8/16 patients (50%) had benign MS at both baseline and follow-up.
TABLE 2.
Follow-up findings in patients with benign vs. typical MS
| EDSS ≤3, Duration ≥15 Years |
EDSS ≤2, Duration ≥10 Years |
|||
|---|---|---|---|---|
| Benign MS at baseline n = 13 (26 eyes) |
Typical MS at baseline n = 55 (109 eyes) |
Benign MS at baseline n = 16 (30 eyes) |
Typical MS at baseline n = 52 (105 eyes) |
|
| Study follow-up, median (range), y | 1.5 (1 – 3.5) |
1.4 (0.5 – 5.1) |
1.8 (0.8 – 5.1) |
1.3 (0.5 – 3.7) |
| Benign MS at follow-up (%) | 62% | 11% | 50% | 4% |
|
Change in EDSS score, median
(range) |
0.25 (−0.5 – 4.5) |
0.5 (−2.0 – 6.0) |
0.5 (−0.5 – 4.5) |
0.5 (−2.0 – 6.0) |
| Worsening of EDSS by ≥1 point (%) | 39% | 37% | 44% | 35% |
|
Worsening of low-contrast acuity
(2.5%) by ≥7 letters (% of eyes) |
42% | 21% | 33% | 23% |
|
Worsening of EDSS by ≥1 point and
worsening of low-contrast acuity by ≥7 letters (% of eyes) |
12% | 9% | 10% | 10% |
|
Worsening of EDSS by ≥1 point or
worsening of high-contrast VA by ≥5 letters (% of eyes) |
69% | 49% | 63% | 50% |
Abbreviations: MS = multiple sclerosis; EDSS = Expanded Disability Status Scale; VA = high-contrast visual acuity
Twenty-five percent of eyes had visual loss during follow-up based on changes from baseline of ≥5 letters for high-contrast VA or ≥7 letters for low-contrast acuity. While proportions of patients worsening by ≥1 point on the EDSS during the study were similar for benign vs. typical MS groups (38% vs. 37% for 15-year definition, Table 2), clinically significant visual loss, particularly for low-contrast acuity, was predominantly observed among eyes of patients with benign MS (42% vs. 21%). Worsening of EDSS and vision combined was present in both the benign and typical MS groups (12% vs. 9% using 15-year definition); the combined outcome of low-contrast acuity or EDSS worsening captured the most patients and was more frequent among those with benign MS (69% vs. 49%, Table 2).
DISCUSSION
Results of this study demonstrate that, compared to patients with typical MS, those with benign MS have similar and perhaps even greater degrees of visual pathway involvement. Patients in our benign MS cohort had RNFL thinning and low-contrast acuity loss over time, and showed consistent trends of greater worsening for benign MS. Using the traditional definition of benign MS (EDSS ≤3, disease duration ≥15 years), vision-specific and overall QOL at last follow-up were significantly worse for benign MS. These findings suggest that while overall neurologic impairment is mild, visual dysfunction, not well-captured by the EDSS, accounts for a substantial degree of disability in benign MS. Importantly, our results emphasize that the term “benign MS” may not be clinically useful when looking at standard disability measures such as the EDSS.
Our study is novel and important in demonstrating: 1) the occurrence of substantial visual pathway axonal loss even in patients with relatively mild overall neurologic impairment; 2) a greater frequency of a history of acute optic neuritis (ON) among patients with benign MS, with disease duration being more important than EDSS as a factor in the relation between benign MS and ON; and 3) patients with benign MS have worse scores for both vision-specific and overall QOL scales in this longitudinal cohort. Given the importance of vision to QOL, even among patients with low EDSS scores, our results further strengthen the role of the anterior visual pathway as a model for assessing treatment efficacy in MS.
Our findings emphasize the difficulties inherent in defining benign MS by EDSS and disease duration alone (2-14). Patients with benign MS may be categorized based on a definitional bias that overlooks visual pathway disease in the absence of involvement of areas that affect the EDSS. The EDSS incorporates high contrast (black-and-white) visual acuity (VA), a measure that is insensitive to clinical changes over time in MS. VA did not demonstrate treatment effects on sustained visual loss in MS clinical trials of natalizumab, even when tested using standardized research methods (33-35). Similarly in the present study, low-contrast letter acuity, particularly at the 2.5% contrast level, showed greater degrees of change over time compared to high-contrast VA.
This study also demonstrated that patients with benign MS have substantial degrees of RNFL axonal loss over time. Although patients with benign and typical MS all had baseline mean RNFL thickness values of 91 μm (Table 1), longitudinal RNFL thinning was 3-5 μm for benign MS using both definitions. This is similar to the degree of axonal loss seen in patients with clinically significant visual loss by low-contrast acuity (≥7 letters) over time in a larger, more heterogeneous MS cohort (35). While 12% of eyes in our published longitudinal cohort had clinically significant low-contrast acuity loss over a 1.7 year average follow-up, 42% of eyes with benign MS in the present study had this degree of worsening using the traditional (15-year) definition (compared to 21% with typical MS) in a similar time frame. In contrast, EDSS progression by ≥1 point combined with vision loss occurred in only 12% of benign MS patients in our cohort. Recent pathologic studies of MS eyes also demonstrate that RNFL thinning and ganglion cell loss occur across all disease subtypes (40).
In our study, eyes of patients with benign MS had equal or greater degrees visual and RNFL loss, and were twice as likely to have a prior history of acute ON compared to those with typical MS (69% vs. 33% using the 15-year definition). History of ON correlated with degrees of RNFL thinning and with disease duration, but not with EDSS scores. Stated differently, the association of benign MS with greater frequency of ON was less pronounced when disease duration was accounted for in the regression models, but did not change when accounting for baseline EDSS score. While patients with typical MS and ≥15 years still had lower proportions of eyes with a history of acute ON compared to benign MS (42% vs. 69%), those with typical MS and ≥10 years duration had the same proportions with ON (50%). Furthermore, disease duration was substantially longer in the benign MS cohort even though patient age was similar to those with typical MS (Table 1). Collectively, the findings of greater disease duration and increased frequency of history of ON in the benign cohort may have several potential explanations: 1) patients presenting with ON are likely to be identified earlier in their disease course as visual loss may be recognized as a clinically meaningful and measurable event, therefore making it easier to meet the definition of benign MS; 2) a history of ON at presentation may also increase patients’ risk of further episodes of ON during their course (in the Optic Neuritis Treatment Trial, 35% of patients had recurrent ON over 10 years (41); 3) patients observed over a greater disease duration have a greater probability of ON just by chance alone.
We examined a history of ON at any time prior to enrollment, but other investigations have looked at ON as an initial presentation of MS (42-44). While these studies are often cited as having established an association between ON and a more benign course, they also demonstrated that the course of patients with ON is dependent on the MRI features at presentation (42-44). In one study, baseline MRI was normal in 49% of patients presenting with ON, but was normal in only 18-24% in those with brainstem, spinal cord, or other syndromes (42). When patients with abnormal cranial MRI results at baseline were considered, no differences in the rates of conversion to clinically definite MS were found. Other investigations have demonstrated that T2 lesion load and presence of baseline gadolinium-enhancing lesions on MRI are the greatest predictors of disability and clinical course (43-44). Conversely, while brain atrophy has been found to be less severe among patients with benign MS (16), similar or greater degrees of RNFL loss were seen in our study and are likely reflective of the higher incidence of ON with its inherent visual pathway-specific axonal degeneration. Although patients with benign MS may experience less difficulty with ambulation by definition based on their EDSS, they have reduced QOL scores not only for vision but for overall aspects of physical functioning. This was observed in our study for the SF-36 Physical Components Summary (PCS), as well as for the NEI-VFQ-25 and 10-Item Neuro-Ophthalmic Supplement. These reductions in patient-reported QOL underscore the importance of vision to patients with MS. In a recent survey, patients were asked to rank 13 bodily functions in terms of their importance. Patients ranked vision very highly, second only to lower limb function (38). NEI-VFQ-25 composite scores for vision-specific QOL were lower (worse) among patients with benign MS who met criteria by the 15-year definition compared to typical MS (Fig. 3). Using the 10-year definition, on the other hand, the benign and typical MS groups did not have significantly different scores. These findings are likely due to the fact that the 15-year definition incorporates a longer disease duration; these patients have had a longer time to experience or accumulate disease in the visual pathways that can affect vision-specific QOL.
Given the variable nature of the disease and the location of the lesions, RRMS may encompass a group of patients with varying degrees of disability within several different neurological domains. Our study suggests that vision is one of those domains and that the EDSS inadequately captures dysfunction within the visual system. Patients with visual dysfunction early in the course of their disease may be inappropriately labeled as having benign disease largely because the disability scales we use did not capture dysfunction in this area well. A similar cohort of patients with prominent cognitive symptoms also has been reported (29). Optimizing the treatment of patients with benign MS based on visual and cognitive dysfunction may help this group to better maintain employment and to improve their QOL. New trials for therapies that involve neuroprotection and neurorepair will also benefit from use of the visual pathway as a model for correlating function with structural aspects of axonal and neuronal loss.
One limitation of our study was that this was a convenience sample of patients willing to undergo visual function testing and OCT scanning. Therefore, those with visual disability may have had greater representation than in the general population of MS patients. Visual symptoms and concerns may have motivated this group to pursue study follow-up. Another limitation is the relatively small size of the cohort of patients with benign MS.
Our definitions of benign MS are limited by the tools that we currently use to measure disability, particularly the EDSS. Furthermore, the diagnosis of benign MS can only be made in retrospect. Although current definitions of benign MS are limited, our data would suggest that those using lower EDSS scores (≤2) are probably more reflective of an indolent course. Accordingly, our study further emphasizes that the traditional definition of benign MS, EDSS ≤3 and disease duration ≥15 years, may not truly reflect a benign MS course.
As examination and neuroimaging techniques improve, we will likely recognize that only a small minority of patients ultimately ‘fit’ these definitions. Newer scales such as the Multiple Sclerosis Functional Composite (MSFC) (45), combined with low-contrast letter acuity testing, will refine our capability to more precisely define and prognosticate about whether patients who remain ambulatory, despite longer disease duration, truly have a benign form of MS.
Acknowledgments
Funding Source: NIH R01 EY 014993, K24 EY 018136; National MS Society RG 4212-A-4, TR 3760-A-3; DADs Foundation; McNeill Foundation.
Footnotes
Conflict of Interest: Dr. Frohman has received speaking and consulting honoraria from Biogen-Idec, Novartis, TEVA, and Acorda. Dr. Calabresi has received personal compensation for consulting and serving on scientific advisory boards from; Biogen-Idec, Teva, and Novartis; and has received research funding from companies Biogen-Idec, Teva, EMD Serono, Vertex, Genentech, Abbott, and Bayer. Dr. S. Galetta has received speaking and consulting honoraria from Biogen-Idec, Novartis, and Teva. Dr. Balcer has received speaking and consulting honoraria from Biogen-Idec, Bayer, and Novartis.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Compston A, Coles A. Multiple sclerosis. Lancet. 2008;372:1502–1517. doi: 10.1016/S0140-6736(08)61620-7. [DOI] [PubMed] [Google Scholar]
- 2.Hawkins SA, McDonnell G. Benign multiple sclerosis? Clinical course, long term follow up, and assessment of prognostic factors. J Neurol Neurosurg Psychiatry. 1999;67:148–152. doi: 10.1136/jnnp.67.2.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramsaransing GSM, De Keyser J. Benign course in multiple sclerosis: a review. Acta Neurol Scand. 2006;113:359–369. doi: 10.1111/j.1600-0404.2006.00637.x. [DOI] [PubMed] [Google Scholar]
- 4.Glad S, Nyland H, Myhr KM. Benign multiple sclerosis. Acta Neurol Scand Suppl. 2006;183:55–57. doi: 10.1111/j.1600-0404.2006.00617.x. [DOI] [PubMed] [Google Scholar]
- 5.Ramsaransing GSM, De Keyser J. Predictive value of clinical characteristics of ‘benign’ multiple sclerosis. Eur J Neurol. 2007;14:885–889. doi: 10.1111/j.1468-1331.2007.01810.x. [DOI] [PubMed] [Google Scholar]
- 6.Sayao AL, Devonshire V, Tremlett H. Longitudinal follow-up of “benign” multiple sclerosis at 20 years. Neurology. 2007;68:496–500. doi: 10.1212/01.wnl.0000253185.03943.66. [DOI] [PubMed] [Google Scholar]
- 7.Hirst C, Ingram G, Swingler R, Compston DA, Pickersgill T, Robertson NP. Change in disability in patients with multiple sclerosis: a 20-year prospective population-based analysis. J Neurol Neurosurg Psychiatry. 2008;79:1137–1143. doi: 10.1136/jnnp.2007.133785. [DOI] [PubMed] [Google Scholar]
- 8.Pittock SJ, Rodriguez M. Benign multiple sclerosis: a distinct clinical entity with therapeutic implications. Curr Top Microbiol Immunol. 2008;318:1–17. doi: 10.1007/978-3-540-73677-6_1. [DOI] [PubMed] [Google Scholar]
- 9.Costelloe L, Thompson A, Walsh C, Tubridy N, Hutchinson M. Long-term clinical relevance of criteria for designating multiple sclerosis as benign after 10 years of disease. J Neurol Neurosurg Psychiatry. 2008;79:1245–1248. doi: 10.1136/jnnp.2008.143586. [DOI] [PubMed] [Google Scholar]
- 10.Glad SB, Nyland HI, Aarseth JH, Riise T, Myhr KM. Long-term follow-up of benign multiple sclerosis in Hordaland County, Western Norway. Mult Scler. 2009;15:942–950. doi: 10.1177/1352458509106511. [DOI] [PubMed] [Google Scholar]
- 11.Masterodemos V, Nikolakaki, Tzagournissakis M, Kotzamani D, Panou T, Spanaki C, Klados G, Maris T, Kontolaimaki E, Psaroudaki K, Chlouverakis G, Georgakakis G, Plaitakis A. Benign multiple sclerosis in Crete. Mult Scler. 2010;16:701–706. doi: 10.1177/1352458510364631. [DOI] [PubMed] [Google Scholar]
- 12.Glad SB, Nyland H, Aarseth JH, Riise T, Kjell-Morten M. Benign multiple sclerosis: a need for a consensus. Acta Neurol Scand Suppl. 2010;122:44–50. doi: 10.1111/j.1600-0404.2010.01375.x. [DOI] [PubMed] [Google Scholar]
- 13.Glad SB, Nyland H, Arseth JH, Riise T, Myhr KM. How long can you keep working with benign multiple sclerosis? J Neurol Neurosurg Psychiatry. 2010;82:78–82. doi: 10.1136/jnnp.2010.210732. [DOI] [PubMed] [Google Scholar]
- 14.Rovaris M, Barkhof F, Calabrese M, De Stefano N, Fazekas F, Miller DH, Montalban X, Polman C, Rocca MA, Thompson AJ, Yousry TA, Filippi M. MRI features of benign multiple sclerosis: Toward a new definition of this disease phenotype. Neurology. 2009;72:1693–1701. doi: 10.1212/WNL.0b013e3181a55feb. [DOI] [PubMed] [Google Scholar]
- 15.Strasser-Fuchs S, Enzinger C, Ropele S, Wallner M, Fazekas F. Clinically benign multiple sclerosis despite large T2 lesion load: Can we explain this paradox? Mult Scler. 2008;14:205–211. doi: 10.1177/1352458507082354. [DOI] [PubMed] [Google Scholar]
- 16.Gauthier SA, Berger AM, Liptak Z, Duan Y, Egorova S, Buckle GJ, Glanz BI, Khoury SJ, Bakshi R, Weiner HL, Guttmann CR. Rate of brain atrophy in benign vs. early multiple sclerosis. Arch Neurol. 2009;66:234–237. doi: 10.1001/archneurol.2008.567. [DOI] [PubMed] [Google Scholar]
- 17.Calabrese M, Filippi M, Rovaris M, Bernardi V, Atzori M, Mattisi I, Favaretto A, Grossi P, Barachino L, Rinaldi L, Romualdi C, Perini P, Gallo P. Evidence for relative cortical sparing in benign multiple sclerosis: a longitudinal magnetic resonance imaging study. Mult Scler. 2009;15:36–41. doi: 10.1177/1352458508096686. [DOI] [PubMed] [Google Scholar]
- 18.Portaccio E, Zipoli V, Goretti B, De Stefano N, Amato MP. Evidence for relative cortical sparing in benign multiple sclerosis: a longitudinal magnetic resonance imaging study [letter] Mult Scler. 2009;15:403. doi: 10.1177/1352458508100237. [DOI] [PubMed] [Google Scholar]
- 19.Benedetti B, Rovaris M, Rocca MA, Caputo D, Zaffaroni M, Capra R, Bertolotto A, Martinelli V, Comi G, Filippi M. In-vivo evidence for stable neuroaxonal damage in brain of patients with benign multiple sclerosis. Mult Scler. 2009;15:789–794. doi: 10.1177/1352458509103714. [DOI] [PubMed] [Google Scholar]
- 20.Rigotti DJ, Gonen O, Grossman RI, Babb JS, Falini A, Benedetti B, Filippi M. Global N-acetylaspartate declines even in benign multiple sclerosis. AJNR Am J Neuroradiol. 2010;32:204–209. doi: 10.3174/ajnr.A2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DeStefano N, Battaglini M, Stromillo ML, Zipoli V, Bartolozzi ML, Guidi L, Siracusa G, Portaccio E, Giorgio A, Sorbi S, Federico A, Amato MP. Brain damage as detected by magnetization transfer imaging is less pronounced in benign than in early relapsing multiple sclerosis. Brain. 2006;129:2008–2016. doi: 10.1093/brain/awl152. [DOI] [PubMed] [Google Scholar]
- 22.Barkhof F. “Benign” and “normal appearing”: it’s in the eye of the beholder. J Neurol Neurosurg Psychiatry. 2010;81:4. doi: 10.1136/jnnp.2009.180638. [DOI] [PubMed] [Google Scholar]
- 23.Benedetti B, Rocca MA, Rovaris M, Caputo D, Zaffaroni M, Capra R, Bertolotto A, Martinelli V, Comi G, Filippi M. A diffusion tensor MRI study of cervical cord damage in benign and secondary progressive multiple sclerosis patients. J Neurol Neurosurg Psychiatry. 2010;81:26–30. doi: 10.1136/jnnp.2009.173120. [DOI] [PubMed] [Google Scholar]
- 24.Spano B, Cercignani M, Basile B, Romano S, Mannu R, Centonze D, Caltagirone C, Bramanti P, Nocentini U, Bozzali M. Multiparametric MR investigation of the motor pyramidal system in patients with “truly benign” multiple sclerosis. Mult Scler. 2010;16:178–188. doi: 10.1177/1352458509356010. [DOI] [PubMed] [Google Scholar]
- 25.Giorgio A, Portaccio E, Stromillo ML, Marino S, Zipoli V, Battaglini M, Blandino A, Bartolozzi ML, Siracusa G, Amato MP, De Stefano N. Cortical functional reorganization and its relationship with brain structual damage in patients with benign multiple sclerosis. Mult Scler. 2010;16:1326–1334. doi: 10.1177/1352458510377333. [DOI] [PubMed] [Google Scholar]
- 26.Rocca MA, Ceccarelli A, Rodegher M, Misci P, Riccitelli G, Falini A, Comi G, Filippi M. Preserved brain adaptive properties in patients with benign multiple sclerosis. Neurology. 2010;74:142–149. doi: 10.1212/WNL.0b013e3181c91a00. [DOI] [PubMed] [Google Scholar]
- 27.Vazquez-Marrufo M, Gonzalez-Rosa JJ, Vaquero E, Duque P, Borges M, Gomez C, Izquierdo G. Quantitative electroencephalography reveals different physiological profiles between benign and remitting-relapsing multiple sclerosis patients. BMC Neurology. 2008;8:44. doi: 10.1186/1471-2377-8-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Amato MP, Zipoli V, Goretti B, Portaccio E, De Caro MF, Ricchiuti L, Siracusa G, Masini M, Sorbi S, Trojano M. Benign multiple sclerosis: Cognitive, psychological and social aspects in a clinical cohort. J Neurol. 2006;253:1054–1059. doi: 10.1007/s00415-006-0161-8. [DOI] [PubMed] [Google Scholar]
- 29.Rovaris M, Riccitelli G, Judica E, Possa F, Caputo D, Ghezzi A, Bertolotto A, Capra R, Falautano M, Mattioli F, Martinelli V, Comi G, Filippi M. Cognitive impairment and structural brain damage in benign multiple sclerosis. Neurology. 2008;71:1521–1525. doi: 10.1212/01.wnl.0000319694.14251.95. [DOI] [PubMed] [Google Scholar]
- 30.Benedict RHB, Fazekas F. Benign or not benign MS: a role for routine neuropsychological assessment? [editorial] Neurology. 2009;73:494–495. doi: 10.1212/WNL.0b013e3181b35225. [DOI] [PubMed] [Google Scholar]
- 31.Portaccio E, Stromillo ML, Goretti B, Zipoli V, Siracusa G, Battaglini M, Giorgio A, Bartolozzi ML, Guidi L, Sorbi S, Federico A, Amato MP, De Stefano N. Neuropsychological and MRI measures predict short-term evolution in benign multiple sclerosis. Neurology. 2009;73:498–503. doi: 10.1212/WNL.0b013e3181b351fd. [DOI] [PubMed] [Google Scholar]
- 32.Amato MP, Portaccio E, Stromillo ML, Goretti B, Zipoli V, Siracusa G, Battaglini M, Giorgio A, Bartolozzi ML, Guidi L, Sorbi S, Federico A, De Stefano N. Cognitive assessment and quantitative magnetic resonance metrics can help to identify benign multiple sclerosis. Neurology. 2008;71:632–638. doi: 10.1212/01.wnl.0000324621.58447.00. [DOI] [PubMed] [Google Scholar]
- 33.Balcer LJ, Galetta SL, Calabresi PA, Confavreux C, Giovannoni G, Havrdova E, Hutchinson M, Kappos L, Lublin FD, Miller DH, O’Connor PW, Phillips JT, Polman CH, Radue EW, Rudick RA, Stuart WH, Wajgt A, Weinstock-Guttman B, Wynn DR, Lynn F, Panzara MA. Natalizumab reduces visual loss in patients with relapsing multiple sclerosis. Neurology. 2007;68:1299–1304. doi: 10.1212/01.wnl.0000259521.14704.a8. [DOI] [PubMed] [Google Scholar]
- 34.Fisher JB, Jacobs DA, Markowitz CE, Galetta SL, Volpe NJ, Nano-Schiavi ML, Baier ML, Frohman EM, Winslow H, Frohman TC, Calabresi PA, Maguire MG, Cutter GR, Balcer LJ. Relation of visual function to retinal nerve fiber layer thickness in multiple sclerosis. Ophthalmology. 2006;113:324–332. doi: 10.1016/j.ophtha.2005.10.040. [DOI] [PubMed] [Google Scholar]
- 35.Talman LS, Bisker ER, Sackel DJ, Long DA, Jr, Galetta KM, Ratchford JN, Lile DJ, Farrell SK, Loguidice MJ, Remington G, Conger A, Frohman TC, Jacobs DA, Markowitz CE, Cutter GR, Ying GS, Dai Y, Maguire MG, Galetta SL, Frohman EM, Calabresi PA, Balcer LJ. Longitudinal study of vision and retinal nerve fiber layer thickness in multiple sclerosis. Ann Neurol. 2010;67:749–760. doi: 10.1002/ana.22005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Raphael BA, Galetta KM, Jacobs DA, Markowitz CE, Liu GT, Nano-Schiavi ML, Galetta SL, Maguire MG, Mangione CM, Globe DR, Balcer LJ. Validation and test characteristics of 10-Item Neuro-Ophthalmic Supplement to the NEI-VFQ-25. Am J Ophthalmol. 2006;142:1026–1035. doi: 10.1016/j.ajo.2006.06.060. [DOI] [PubMed] [Google Scholar]
- 37.Mowry EM, Loguidice MJ, Daniels AB, Jacobs DA, Markowitz CE, Galetta SL, Nano-Schiavi ML, Cutter GR, Maguire MG, Balcer LJ. Vision related quality of life in multiple sclerosis: correlation with new measures of low and high contrast letter acuity. J Neurol Neurosurg Psychiatry. 2009;80:767–772. doi: 10.1136/jnnp.2008.165449. [DOI] [PubMed] [Google Scholar]
- 38.Heesen C, Böhm J, Reich C, Kasper J, Goebel M, Gold SM. Patient perception of bodily functions in multiple sclerosis: gait and visual function are the most valuable. Mult Scler. 2008;14:988–991. doi: 10.1177/1352458508088916. [DOI] [PubMed] [Google Scholar]
- 39.Polman CH, Reingold SC, Edan G, Filippi M, Hartung HP, Kappos L, Lublin FD, Metz LM, McFarland HF, O’Connor PW, Sandberg-Wollheim M, Thompson AJ, Weinshenker BG, Wolinsky JS. Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria.”. Ann Neurol. 2005;58:840–846. doi: 10.1002/ana.20703. [DOI] [PubMed] [Google Scholar]
- 40.Green AJ, McQuaid S, Hauser SL, Allen IV, Lyness R. Ocular pathology in multiple sclerosis: retinal atrophy and inflammation irrespective of disease duration. Brain. 2010;133:1591–1601. doi: 10.1093/brain/awq080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Opitc Neuritis Study Group Visual function more than 10 years after optic neuritis. Am J Ophthalmol. 2004;137:77–83. doi: 10.1016/s0002-9394(03)00862-6. [DOI] [PubMed] [Google Scholar]
- 42.Tintoré M, Rovira A, Rio J, Nos C, Grivé E, Téllez N, Pelayo R, Comabella M, Montalban X. Is optic neuritis more benign than other first attacks in multiple sclerosis? Ann Neurol. 2005;57:210–215. doi: 10.1002/ana.20363. [DOI] [PubMed] [Google Scholar]
- 43.Di Filippo M, Anderson VM, Altmann DR, Swanton JK, Plant GT, Thompson AJ, Miller DH. Brain atrophy and lesion load measures over 1 year relate to clinical status after 6 years in patients with clinically isolated syndromes. J Neurol Neurosurg Psychiatry. 2010;81:204–208. doi: 10.1136/jnnp.2009.171769. [DOI] [PubMed] [Google Scholar]
- 44.Finisku LK, Brex PA, Altmann DR, Miszkiel KA, Benton CE, Lanyon R, Thompson AJ, Miller DH. Disabilty and T2 MRI lesions: a 20-year follow-up of patients with relapse onset of multiple sclerosis. Brain. 2008;121:808–817. doi: 10.1093/brain/awm329. [DOI] [PubMed] [Google Scholar]
- 45.Polman CH, Rudick RA. The multiple sclerosis functional composite: a clinically meaningful measure of disability. Neurology. 2010;74:S8–15. doi: 10.1212/WNL.0b013e3181dbb571. [DOI] [PubMed] [Google Scholar]