Abstract
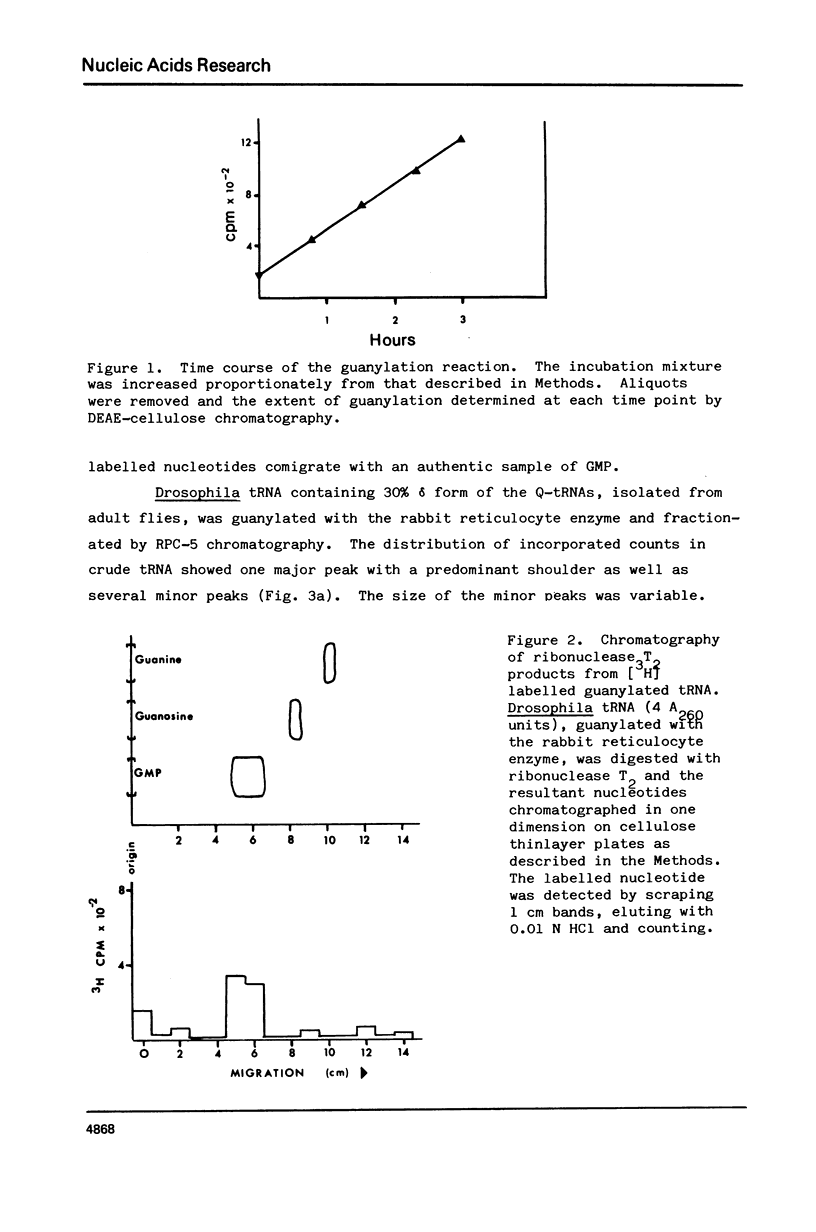
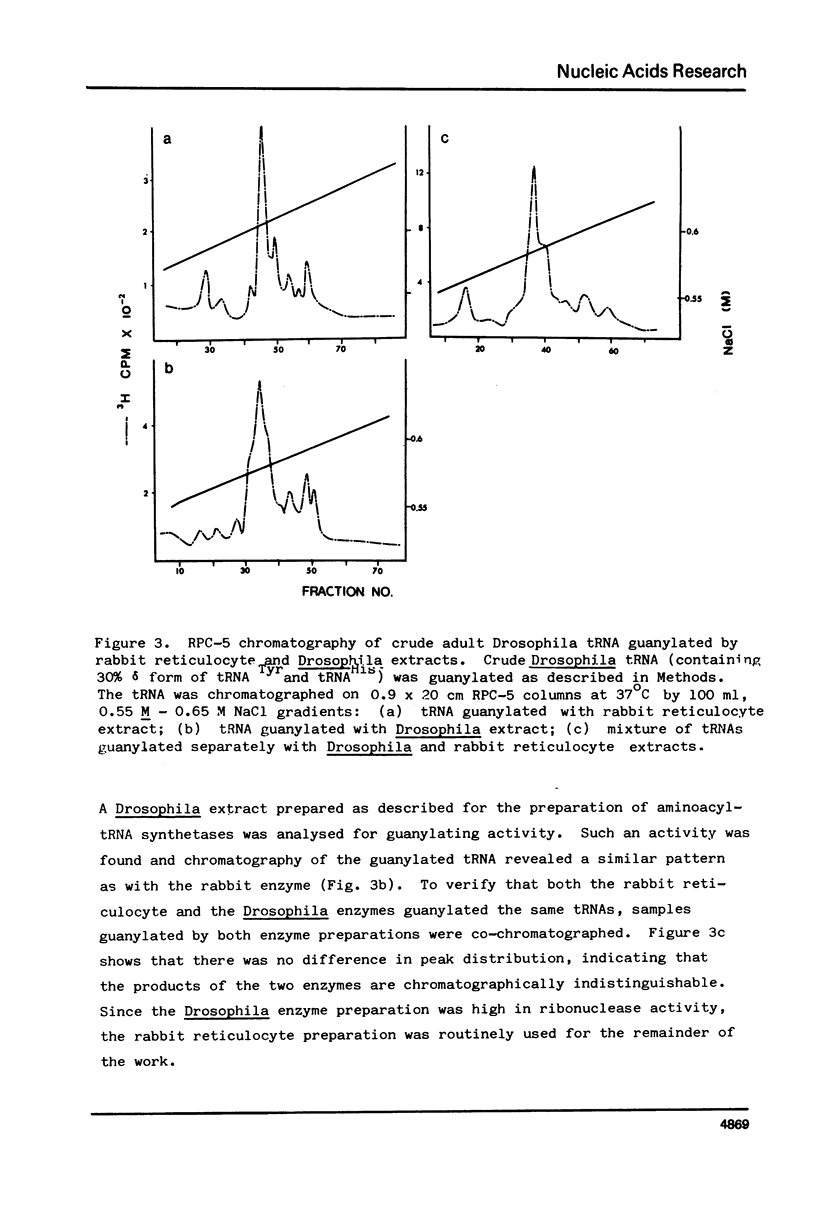
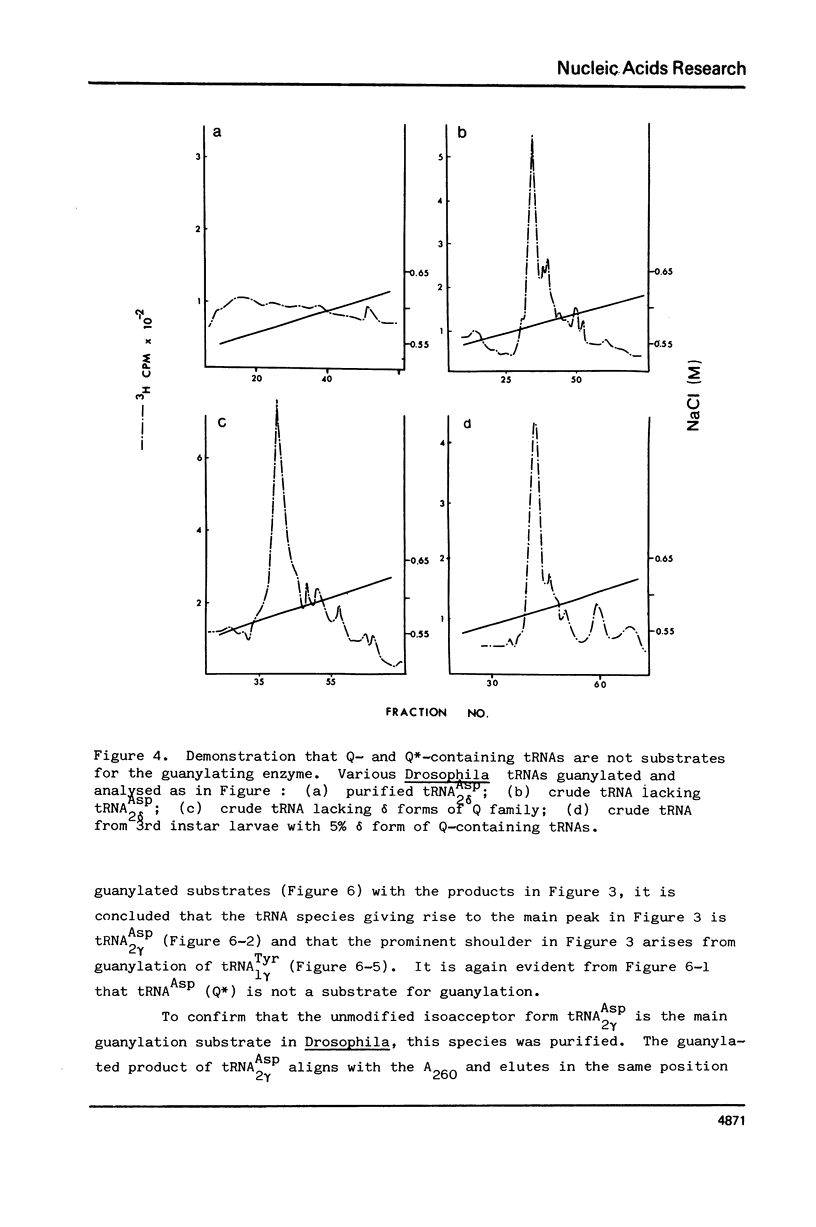
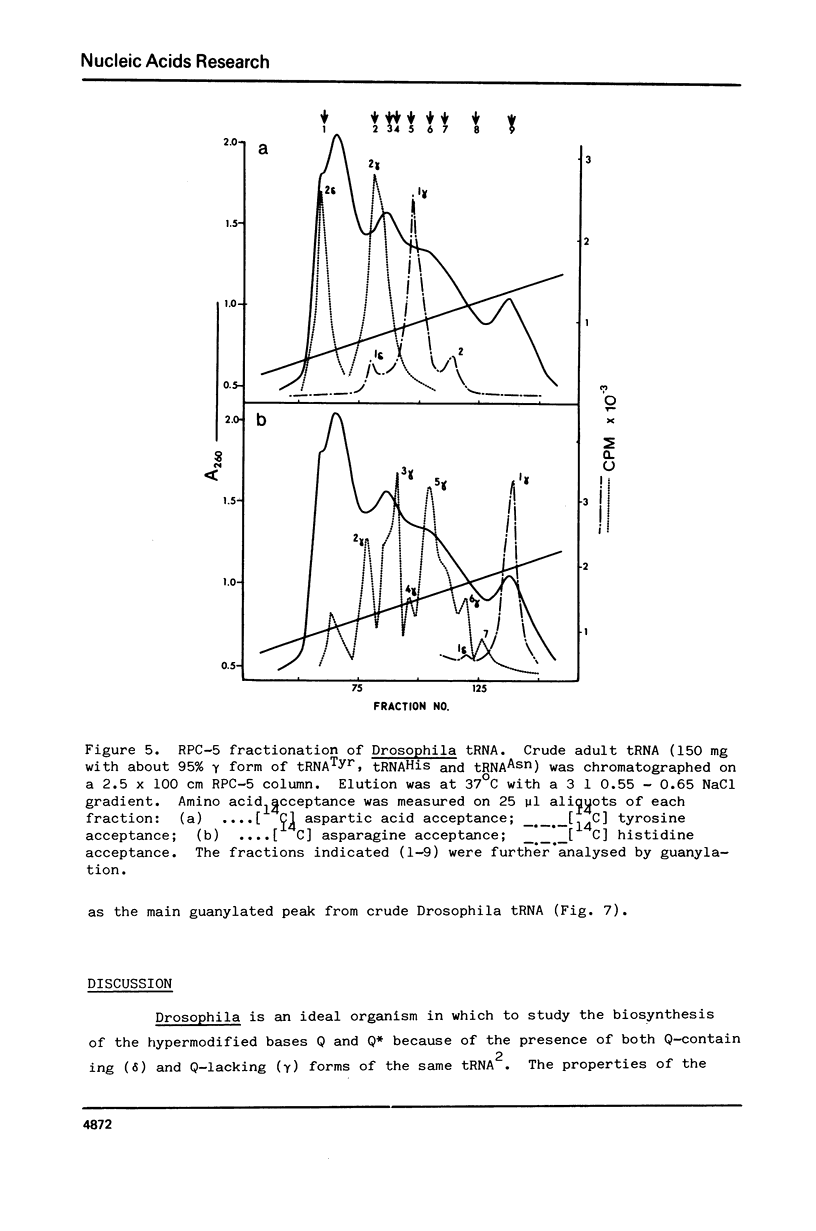
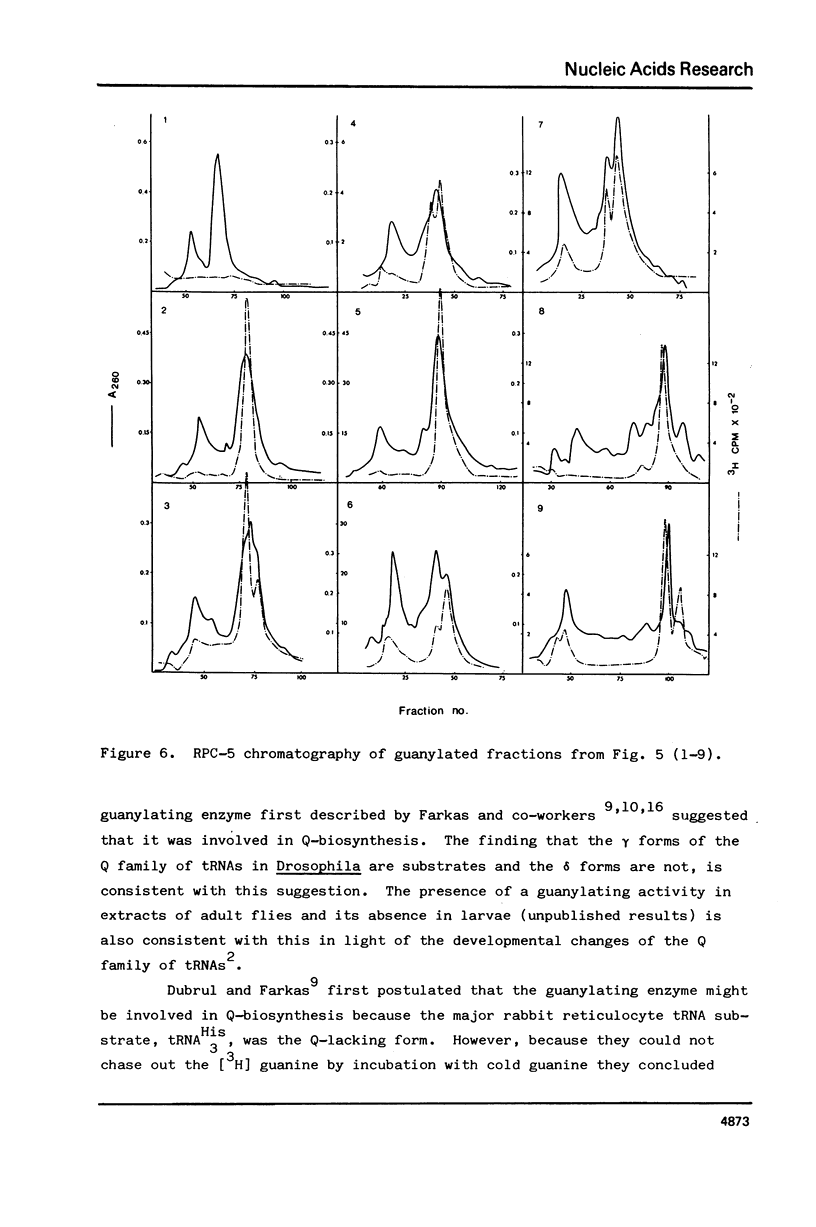
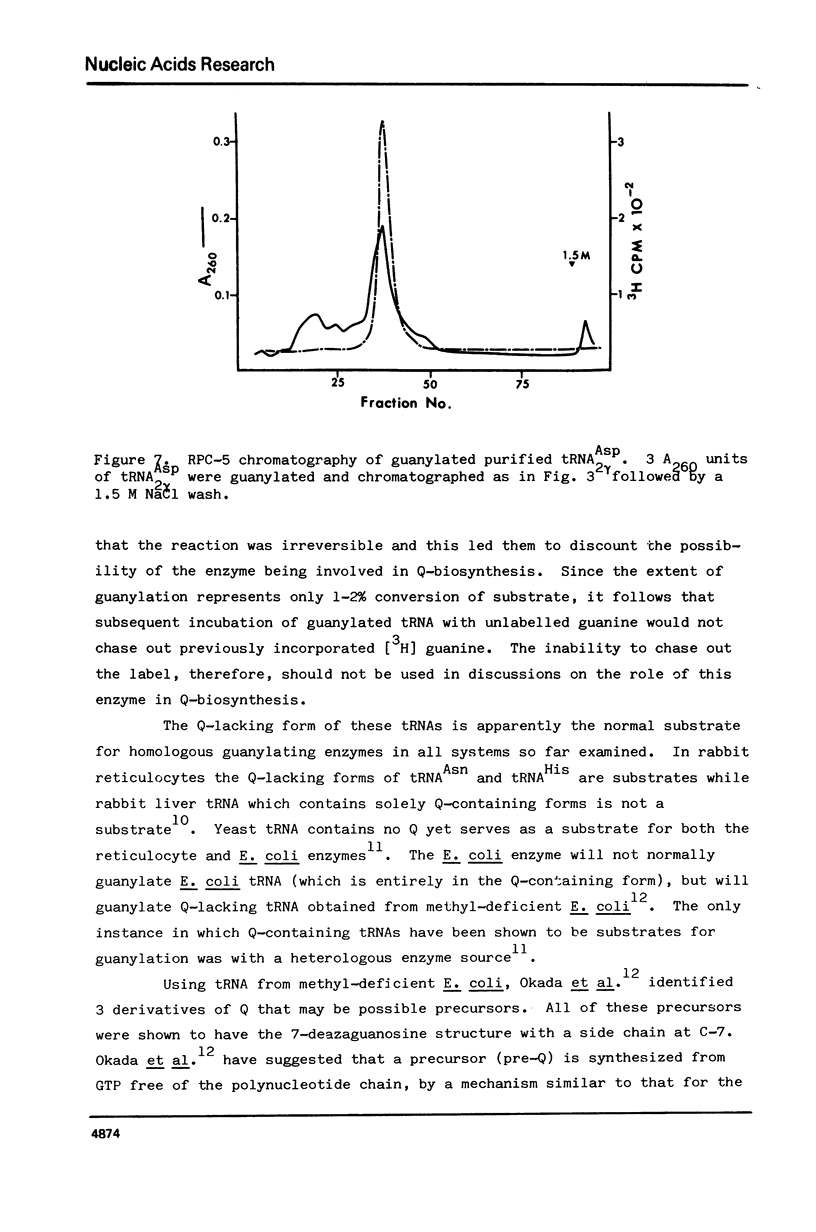
Drosophila tRNA can be guanylated by a crude enzyme from rabbit reticulocytes. Guanylating activity is also present in crude extracts of adult Drosophila. A major product of this reaction as well as several minor ones were resolved by RPC-5 chromatography. The main substrate of both the Drosophila and rabbit reticulocyte enzymes was the non-Q-containing aspartic acid tRNA, tRNA2gammaAsp. The QU-lacking (gamma) forms of asparagine, histidine and tyrosine tRNAs were also substrates and gave rise to the minor products of the reaction. In contrast, the Q- or Q*-containing (delta) forms of these tRNAs appear not to be substrates. The evidence strongly suggests that the guanyating enzyme is involved in Q biosynthesis and would be better termed a guanine replacement or pre-Q insertion enzyme.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dubrul E. F., Farkas W. R. Partial purification and properties of the reticulocyte guanylating enzyme. Biochim Biophys Acta. 1976 Sep 6;442(3):379–390. doi: 10.1016/0005-2787(76)90312-9. [DOI] [PubMed] [Google Scholar]
- Farkas W. R., Chernoff D. Identification of the minor guanylated tRNA of rabbit reticulocytes. Nucleic Acids Res. 1976 Oct;3(10):2521–2528. doi: 10.1093/nar/3.10.2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankins W. D., Farkas W. R. Guanylation of transfer RNA by rabbit reticulocytes. Biochim Biophys Acta. 1970 Jul 16;213(1):77–89. doi: 10.1016/0005-2787(70)90009-2. [DOI] [PubMed] [Google Scholar]
- Harada F., Nishimura S. Possible anticodon sequences of tRNA His , tRNA Asm , and tRNA Asp from Escherichia coli B. Universal presence of nucleoside Q in the first postion of the anticondons of these transfer ribonucleic acids. Biochemistry. 1972 Jan 18;11(2):301–308. doi: 10.1021/bi00752a024. [DOI] [PubMed] [Google Scholar]
- KIRBY K. S. A new method for the isolation of ribonucleic acids from mammalian tissues. Biochem J. 1956 Nov;64(3):405–408. doi: 10.1042/bj0640405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai H., Kuchino Y., Nihei K., Nishimura S. Distribution of the modified nucleoside Q and its derivatives in animal and plant transfer RNA's. Nucleic Acids Res. 1975 Oct;2(10):1931–1939. doi: 10.1093/nar/2.10.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai H., Nakanishi K., Macfarlane R. D., Torgerson D. F., Ohashi Z., McCloskey J. A., Gross H. J., Nishimura S. Letter: The structure of Q* nucleoside isolated from rabbit liver transfer ribonucleic acid. J Am Chem Soc. 1976 Aug 4;98(16):5044–5046. doi: 10.1021/ja00432a071. [DOI] [PubMed] [Google Scholar]
- Kasai H., Oashi Z., Harada F., Nishimura S., Oppenheimer N. J., Crain P. F., Liehr J. G., von Minden D. L., McCloskey J. A. Structure of the modified nucleoside Q isolated from Escherichia coli transfer ribonucleic acid. 7-(4,5-cis-Dihydroxy-1-cyclopenten-3-ylaminomethyl)-7-deazaguanosine. Biochemistry. 1975 Sep 23;14(19):4198–4208. doi: 10.1021/bi00690a008. [DOI] [PubMed] [Google Scholar]
- Kelmers A. D., Novelli G. D., Stulberg M. P. Separation of transfer ribonucleic acids by reverse phase chromatography. J Biol Chem. 1965 Oct;240(10):3979–3983. [PubMed] [Google Scholar]
- Nishimura S. Minor components in transfer RNA: their characterization, location, and function. Prog Nucleic Acid Res Mol Biol. 1972;12:49–85. [PubMed] [Google Scholar]
- Okada N., Harada F., Nishimura S. Specific replacement of Q base in the anticodon of tRNA by guanine catalyzed by a cell-free extract of rabbit reticulocytes. Nucleic Acids Res. 1976 Oct;3(10):2593–2603. doi: 10.1093/nar/3.10.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada N., Yasuda T., Nishimura S. Detection of nucleoside Q precursor in methyl-deficient E.coli tRNA. Nucleic Acids Res. 1977 Dec;4(12):4063–4075. doi: 10.1093/nar/4.12.4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson R. L., Weiss J. F., Kelmers A. D. Improved separation of transfer RNA's on polychlorotrifuoroethylene-supported reversed-phase chromatography columns. Biochim Biophys Acta. 1971 Feb 11;228(3):770–774. doi: 10.1016/0005-2787(71)90748-9. [DOI] [PubMed] [Google Scholar]
- Suhadolnik R. J., Uematsu T. Biosynthesis of the pyrrolopyrimidine nucleoside antibiotic, toyocamycin. VII. Origin of the pyrrole carbons and the cyano carbon. J Biol Chem. 1970 Sep 10;245(17):4365–4371. [PubMed] [Google Scholar]
- Twardzik D. R., Grell E. H., Jacobson K. B. Mechanism of suppression in Drosophila: a change in tyrosine transfer RNA. J Mol Biol. 1971 Apr 28;57(2):231–245. doi: 10.1016/0022-2836(71)90343-3. [DOI] [PubMed] [Google Scholar]
- White B. N. Chromatographic changes in specific tRNAs after reaction with cyanogen bromide and sodium periodate. Biochim Biophys Acta. 1974 Jul 11;353(3):283–291. doi: 10.1016/0005-2787(74)90021-5. [DOI] [PubMed] [Google Scholar]
- White B. N., Tener G. M. Activity of a transfer RNA modifying enzyme during the development of Drosophila and its relationship to the su(s) locus. J Mol Biol. 1973 Mar 15;74(4):635–651. doi: 10.1016/0022-2836(73)90054-5. [DOI] [PubMed] [Google Scholar]
- Wosnick M. A., White B. N. Purification and nucleoside composition of a Q* - containing aspartic acid tRNA from Drosophila. Biochem Biophys Res Commun. 1978 Apr 28;81(4):1131–1138. doi: 10.1016/0006-291x(78)91254-8. [DOI] [PubMed] [Google Scholar]


