Abstract
The discovery of the endothelium as a crucial organ for the regulation of the vasculature to physiological needs and the recognition of endothelial dysfunction as a key pathological condition - which is associated with most if not all cardiovascular risk factors - led to a tremendous boost of endothelial research in the past 3 decades. Despite the possibility to measure endothelial function in the individual and its widespread use in research, its use as a clinical tool in daily medicine is not established yet. We review the most common methods to assess vascular function in humans and discuss their advantages and disadvantages. Furthermore we give an overview about clinical settings were endothelial function measurements may be valuable in individual patients. Specifically, we provide information why endothelial function is not only a risk marker for cardiovascular risk but may also provides prognostic information beyond commonly used risk scores in primary prevention, and in patients with already established coronary disease.
We conclude, that non-invasive endothelial function measurements provide valuable additional information, however, to ascertain its use for daily clinical practice, future research should determine whether endothelial function can be used to guide treatment in the individual and if this translates into better outcomes.
Keywords: endothelial function, risk factors, atherosclerosis
Introduction
The discovery of nitric oxide (NO) as a crucial endothelium-derived molecule for vascular relaxation and the recognition of the endothelium as more than a passive interface between blood and the vessel wall, led to substantial progress in the field of vascular research.1 Endothelial dysfunction is a pathological condition, mainly characterized by an imbalance between substances with vasodilating, anti-mitogenic and anti-thrombogenic properties (endothelium-derived relaxing factors)2 and substances with vasoconstricting, pro-thrombotic and proliferative characteristics (endothelium-derived contracting factors).3 Among the most important vasodilator molecule, in muscular arteries in particular, is NO, which also inhibits other key events in the development of atherosclerosis such as platelet adhesion and aggregation, leukocyte adhesion and migration as well as smooth muscle cell proliferation. Particularly in the microcirculation, prostacyclin and endothelial-derived hyperpolarization factors (EDHF; an umbrella term for substances and signals hyperpolarizing vascular myocytes by opening voltage channels4) also play an important role.
Generally, loss of NO bioavailability indicates a broadly dysfunctional phenotype across many properties of the endothelium. Thus, the assessment of its vasodilator properties due to NO and other molecules may provide information on the integrity and function of the endothelium. Interestingly most, if not all, cardiovascular risk factors are associated with endothelial dysfunction,5 and risk factor modification leads to improvement in vascular function. Endothelial dysfunction has been detected in the coronary epicardial and resistance vasculature as well as in peripheral arteries, so that endothelial dysfunction can be regarded as a systemic condition.6 Importantly, the process of atherosclerosis begins early in life, and endothelial dysfunction contributes to atherogenesis and precedes the development of morphological vascular changes.7
Over the past 25 years many methodological approaches have been developed to measure the (patho-)physiological function of the endothelium in humans.8 Although the ability of measuring endothelial function has boosted clinical research in this field, its use as a clinical tool in daily practice is not established, nor has any method been recommended in clinical guidelines for planning primary or secondary prevention of vascular disease.
It is the aim of this review to give a short overview of the most commonly used methods to measure endothelial function in humans, non-invasive techniques in particular (Table 1); to summarize the clinical implications of endothelial dysfunction in the population as well as in individual patients. The possible future role of endothelial function measurement for individualized medicine is also considered.
Table 1.
Advantages and disadvantages of the most commonly used techniques to assess endothelial function
| Technique | Vascular bed | Advantages | Disadvantages | Stimulus (examples) |
|---|---|---|---|---|
| Coronary epicardial vasoreactivity (QCA) | Epicardial macrovascular Conduit arteries |
Assessment directly in the coronary vascular bed Gold standard |
Invasive Expensive Time intensive Limited to those undergoing coronary angiography Challenging for serial measurements |
Ach Exercise Pacing CPT |
| Coronary microvascular function – Doppler wires | Coronary microvascular Resistance arteries |
Assessment directly in the coronary microvasculature | Invasive Expensive Time intensive Limited to those undergoing coronary angiography Challenging for serial measurements |
Ach Adenosine Papaverine |
| Flow- Mediated- Dilation (FMD) | Brachial Artery Conduit artery |
Easy access Correlation with invasive epicardial vascular function Many outcome studies Inexpensive Possibility to assess other important parameters (flow, baseline arterial diameters, FMC) |
Challenging to perform well Disparate protocols for performance and standardizations Need for standardization |
Reactive Hyperemia |
| Venous occlusion plethysmography | Forearm vasculature Microvasculature |
Easy access Vasoactive substances infused to generate a dose-response relationship Contralateral arm as a control |
Invasive (cannulation of the brachial artery) Time consuming |
Ach and other vasoactive substances |
| EndoPAT | Finger Microvasculature |
Easy to access and perform Automated Low inter- and intraobserver variability Correlation with invasive microvascular vascular function |
Expense of disposable finger probes PAT signal influenced by variable non endothelial factors |
Reactive hyperemia |
METHODS TO ASSESS VASCULAR FUNCTION
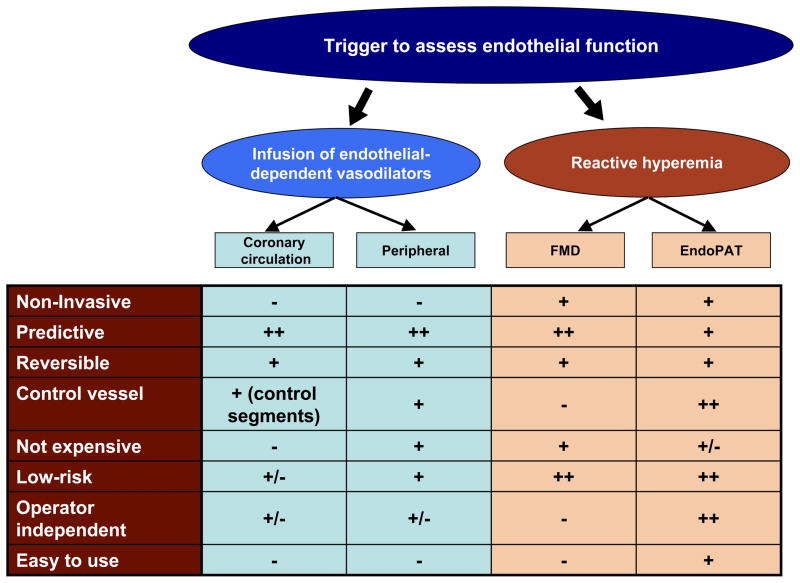
The first demonstration of endothelial dysfunction in atherosclerotic coronary arteries using intracoronary infusion of acetylcholine and quantitative coronary angiography dates back to 1986 by Ganz, Selwyn and colleagues.9 Their seminal studies heralded an important shift in paradigm in understanding of human atherosclerosis, which had previously been regarded as a purely structural disease. Their research drew attention to the functional manifestations of atherosclerosis, such as exaggerated vasoconstriction, as a consequence of poorly functioning endothelium. Later, less invasive techniques were developed mainly using the forearm circulation as a surrogate for coronary arteries.6, 10, 11 All approaches have their advantages and disadvantages and most importantly different vascular beds are examined (Figure 1). The basic principle, however, is similar: healthy arteries such as the coronary or brachial arteries dilate in response to reactive hyperemia (flow-mediated vasodilatation) or after pharmacological stimuli including intra-arterial infusion of endothelium-dependent vasodilators such as acetylcholine (Ach), bradykinin or serotonin, via release of NO and/or other endothelium-derived vasoactive substances.2 In disease states, such endothelium-dependent dilatation is reduced or absent. However, whichever technique is applied, vascular responses are not only determined by the functional status of the vasculature at the place of measurement, but also by the structural condition of the resistance arteries in the microvasculature. Furthermore, to differentiate endothelium-dependent from endothelium-independent responses, exogenous NO donators (e.g. glycerol-trinitrate) or direct non NO donators such as adenosine can be applied. Impaired endothelial-independent function is associated with structural vascular alterations and alterations in smooth muscles cells rather than changes in the endothelium.
Figure 1.
Figure 1 depicts the principles of the most commonly used methods for the assessment of endothelial function.
Coronary epicardial and microvascular function
To assess coronary endothelial function, a “functional” test is performed to measure epicardial as well as resistance vessel endothelial function. Although these methods are limited by the invasive nature, their advantage is to measure endothelial function directly in this clinically important vascular bed.
Epicardial endothelial function
To image vasomotor responses of epicardial coronary arteries, quantitative coronary angiography (QCA) or intravascular ultrasound (IVUS) is used and changes in vessel diameters and cross-sectional areas in response to endothelium-dependent interventions are documented. After acetylcholine infusion, vessels and segments with an intact endothelium vasodilate, whereas vessels and segments with dysfunctional or disrupted endothelium, will respond with vasoconstriction due to a direct activation of muscarinic receptors on vascular smooth muscle cells.9 Similar induced functional changes in vascular reactivity have been demonstrated with salbutamol12 and other substances (Table 2) and with more physiological interventions, for example increased coronary blood-flow. However, dose titration is more difficult. Physical measures of endothelium-dependent responses include exercise,13, 14 or pacing induced tachycardia as a surrogate for exercise,15, 16 and induce an increase in coronary blood flow and thus shear stress on the coronary circulation, which leads to flow mediated endothelium-dependent vasomotion of the epicardial vessels. Similar responses can be seen in response to mental stress.17 The observation of endothelium-dependent flow mediated dilatation in the coronary epicardial vessels and its impairment in atherosclerosis18, 19 provided the rationale to study similar responses in the peripheral vasculature later (see below). Another “physiologic” test to assess epicardial vasoreactivity is the use of the cold pressor test (CPT), where subjects put their hand into ice water. The activation of the sympathetic nervous system leads to release of NO and endothelium-derived hyperpolarizing factors (EDHFs) via stimulation of endothelial α2-adrenergic receptors and consequently vasodilation in healthy.20 However, in dysfunctional endothelium, α1-adrenergic mediated constriction of smooth muscle cells will dominate,21 closely mirroring the responses to Ach. 21, 22
TABLE 2.
Pharmacological triggers for the assessment of coronary vascular function
| Epicardial vessels | Microcirculation | |
|---|---|---|
| Endothelium dependent vascular function |
|
|
| Endothelium independent vascular function |
|
|
Coronary microvascular function
Changes in coronary (or myocardial) blood flow (CBF) can be used as a surrogate parameter for microvascular function.23 Coronary flow reserve (CFR) is the ratio of maximal CBF during maximal coronary hyperemia with provocative stimuli (such as adenosine infusion, pacing or exercise), divided by the resting CBF. This maximal blood flow response (CFR) is both endothelium and non-endothelium dependent and a CFR below 2.0 is considered abnormal.24 To measure endothelium-dependent microvascular function, the percent increase in CBF in response to endothelium-dependent vasodilators (commonly Ach) infused at increasing concentrations is analyzed.
Other methods to estimate microvascular function have been introduced, as for example the measurement of the number of cineangiographic frames that it takes to fill a distal vessel with proximal injection of contrast. The corrected TIMI (Thrombolysis in Myocardial Infarction) frame count (CTFC) provides a semiquantitative assessment of epicardial coronary blood flow.25
Taking the main disadvantage into account – the invasive nature of the above mentioned tests – non-invasive functional tests to assess the coronary microvasculature have been developed, among them positron emission tomography,26 myocardial perfusion imaging,27 blood oxygen level-dependent (BOLD) MRI28 and echocardiography,29 however, in detail discussion would go beyond the scope of this review.
Peripheral techniques to assess endothelial function
The aforementioned techniques to measure both coronary epicardial vascular function and the assessment of the coronary microcirculation are very well suited for patients requiring a coronary angiogram for clinical indications. However, to assess vascular function and health in the asymptomatic patient, performing an invasive functional coronary angiogram is usually not appropriate. Therefore non- or less invasive surrogate techniques to assess macrovascular as well as microvascular endothelial function have been developed. Although these do not measure vascular function in the coronary circulation directly, they have been shown to correlate reasonably with its more invasive counterparts.30–32 Whereas all these techniques assess the generalized function of the vasculature, one has to keep in mind that certain phenomena cannot be explained by systemic endothelial dysfunction; it is likely that local factors (e.g. flow patterns) and local vascular dysregulation observed at branch points related to disturbed shear stresses also contributes to disease.33–37
Plethysmography of the Forearm Circulation
Although still limited by its semi-invasive nature (arterial puncture), with this technique changes in forearm blood flow are measured by venous plethysmography in both arms before and after infusion of vasoactive substances into a cannulated brachial artery.10 The main advantage is that vasoactive molecules, hormones or drugs (for instance Ach or nitroglycerin) can be infused, thus respectively quantifying endothelium-dependent and endothelium-independent vasodilation, in a dose-dependent manner. The dosages required have limited systemic effects, allowing the contralateral limb to serve as an internal control. The results are expressed as the ratio of the changes in flow measured in both arms and are reproducible.38 The response to acetylcholine is significantly reduced by intraarterial infusion of L-NMMA (but not by acetylsalicylic acid),39 demonstrating a key role for NO. However, it has to be taken into account that, especially in patients with multiple risk factors, EDHF also play an essential role for resting microvascular tone,40 as well as for agonist-stimulated vasodilation.41, 42 The technique is well suited to measure differences in blood flow to various stimuli or inhibitors in a single patient, however, due to different initial arterial pressure, forearm blood flow, different sizes of the forearm and other factors, comparisons between groups or serial studies in the same patient are of limited value.43 Although pharmacologically induced vasodilation with this technique gives interesting insights into microvascular pathophysiology, the response not necessarily mimics microvascular vasodilation to transient ischemia or exercise.
Flow-mediated vasodilation of brachial artery
Due to its non-invasive approach, flow-mediated vasodilatation of the arm arteries (FMD) has become the most widely used technique to measure endothelial function. The technique measures the ability of the arteries to respond with endothelial NO release during reactive hyperemia (flow-mediated) after a 5-minute occlusion of the brachial artery with a blood pressure cuff. Celermajer, Deanfield and their colleagues were the first to measure this response in vivo by measuring the respective diameter changes of the brachial or radial artery by ultrasound11 a response later demonstrated to be mainly NO dependent,39, 44, 45 although other vasodilator pathways may contribute as well.46 Importantly, peripheral endothelial function as assessed by FMD correlates with coronary artery endothelial function.30, 32 However, although the principle of this technique seems to be simple, its application is technically challenging and requires extensive training and standardization (Table 3).47–49 Study preparation, image acquisition and site selection, sphygmomanometer probe position, cuff occlusion time, the accurate use of edge-detection software as well as the correct characterization of the FMD response are crucial, as recently outlined in detail in guidelines by Charakida et al.49, Harris et al.50 and Thijssen et al.51 These publications are useful as they draw attention to the need to standardize the different protocols and indeed, if efforts to standardize the technology are followed, reproducibility of FMD can be considerably improved.52
TABLE 3.
Technical considerations in FMD measurements
Subject preparation
|
Sphygmomanometer probe position and cuff occlusion time
|
Site selection
|
Image acquisition
|
Measurement
|
Recently, some new aspects of this technique have emerged and are under investigation. While FMD measures conduit artery vascular function, the stimulus for FMD itself (reactive hyperemia flow and the induced shear stress on the endothelium) might be an important measure of peripheral microvascular function because reactive hyperemia is highly dependent on maximal forearm resistance.53, 54 Notably, both hyperemia induced shear stress and velocity changes (measured by calculation of the velocity-time integral, adjusted for heart rate) showed even stronger correlations with the presence of cardiovascular risk factors than FMD55 and also predict cardiovascular outcomes.56, 57
Interestingly, some recent multi-center studies demonstrated that simple baseline brachial artery diameter readings correlate with clinical outcomes, nearly as well as the FMD itself.58, 59 One explanation for this intriguing finding might be that the larger the diameter of an artery, the smaller the relative percent changes (FMD) and, additionally, shear stress generated seems to be lower in larger vessels.60, 61 As shear stress is the stimulus for FMD, it seems reasonable to correct for the impact of shear stress on FMD. However, several methodological as well as physiological factors influence shear stress and ratio normalizations of the dilatory response to shear rate have provided conflicting results.51
Recently, Gori et al.62 raised the issue of endothelial function in the resting (not hyperemic) state. While FMD provides crucial information about the ability of the endothelium to respond to a specific stimulus (reactive hyperemia), it is not a measure of the resting production of vasoactive substances. In this respect vasoconstriction of the brachial artery after inflation of a wrist cuff to suprasystolic was first reported years ago,63 and is mainly mediated through vasoconstrictor substances such as endothelin.64 This concept has garnered new attention and the term “low-flow-mediated constriction (L-FMC) was introduced.62, 65, 66 In principle, L-FMC detects the change in brachial artery diameter in response to a decrease in blood flow and shear stress, respectively, after occlusion of the artery by a distally placed cuff. In a recent study an association between traditional risk factors and impaired L-FMC as well as a relationship with the severity of coronary artery disease has been demonstrated.67
Taken together, the information obtained by functional vascular ultrasound is manifold and future protocols should not only measure percent changes in arterial diameter in response to hyperemia but should consider also the above mentioned measurements.
Finger Plethysmography
Recently measuring endothelial function using peripheral arterial tonometry (PAT) has gained increasing attention and a proprietary device has been developed to measure observer independent pulsatile arterial volume changes by finger plethysmography (EndoPAT, Itamar Medical).68, 69 With PAT beat-to-beat plethysmographic recordings of the finger arterial pulse wave amplitude with pneumatic probes are captured. With the device a counterpressure of 70 mmHg on the digit is applied to avoid distal venous distention thus inhibiting venous pooling and venoarteriolar reflex responses.68 In principle, an increase in arterial blood volume in the finger tip causes an increase in pulsatile arterial column changes thus increasing the measured signal. Similar to the assessment of endothelial function with the FMD technique, a pressure cuff is placed on the arm and after obtaining baseline blood volume changes, the blood pressure cuff is inflated above systolic pressure and deflated after 5 minutes to induce reactive hyperemia on one arm. A main advantage of the system is that the contralateral arm serves as its internal control that can be used to correct for any systemic drift in vascular tone during the test and an index between the two arms is calculated to adjust for any such drift. This index is validated marker for endothelial function; however, augmentation of the pulse amplitude after reactive hyperemia is a complex response to ischemia. It reflects changes in flow, as well as in digital microvessel dilatation and is only partly dependent on nitric oxide.70 Validation studies demonstrated that impairment in peripheral finger endothelial function measured with EndoPAT is correlated with coronary microvascular function in patients with early atherosclerosis31 and predicts cardiovascular events.71 Two large cross-sectional studies (in over 1900 patients in the Framingham cohort72, 73 and over 5000 individuals in the Gutenberg Heart Study74) digital vascular dysfunction was associated with traditional and metabolic cardiovascular risk factors but not or only modestly with FMD, thus likely measuring different aspects of vascular biology (see below).
CLINICAL IMPLICATIONS OF ENDOTHELIAL DYSFUNCTION IN POPULATIONS AND IN THE INDIVIDUAL
Is endothelial function a marker for cardiovascular risk?
In the coronary arteries, impairment of endothelial function occurs early in the course of atherosclerosis, in relation to systemic risk factors75 and abnormal hemodynamic shear stresses.37 The more systemic cardiovascular risk factors present, the worse epicardial vascular function.75 There is extensive literature documenting that endothelial dysfunction is associated with almost every condition predisposing to atherosclerosis and cardiovascular disease.76 There are many studies correlating endothelial dysfunction (conduit artery and microvasculature likewise) with cardiovascular risk. For example, endothelial dysfunction has been observed in patients with arterial hypertension,77–79 in normotensive subjects with a family history of hypertension,80 in smokers,81, 82 as well as passive smokers,83, 84 in dyslipidemia,85, 86 in ageing,78 diabetes mellitus,87–91 in obesity,91 in hyperhomocysteinaemia,92, 93 in humans with low intracellular magnesium levels94 and in patients with inflammatory or infectious diseases.95–97 Importantly, the effects of cardiovascular risk on the endothelium can be seen in children as early as 8 years of age.98, 99 Thus, endothelial dysfunction may represent the effect of these risk factors on vascular health.
The fact that endothelial dysfunction is a systemic condition6 may explain why peripheral endothelial function (microvascular and macrovascular) correlates with endothelial function in the coronary arteries.30, 31, 32 The pathophysiology behind the functional changes in impaired endothelial function also leads to structural changes of the vessel over time. In a cross-sectional study in middle aged healthy men, there is no evident correlation between brachial FMD and the carotid intima media thickness (IMT),100 however, in a similar population free of cardiovascular disease, FMD correlated with IMT progression over a 6 years follow up. Interestingly, in this study, in contrast to FMD, Framingham Risk was not correlated with IMT progression.101 Similar, FMD also predicted IMT progression in less healthy patients as demonstrated after 1 year in hypertensive, postmenopausal women.102
Taken together, there is good evidence that endothelial dysfunction is significantly associated with the burden of cardiovascular risk and can be considered as a barometer of the total risk burden (the risk of the risk factors). However, transient endothelial function impairment, for example by intercurrent acute illnesses, 99, 103, 104, after strenuous exercise105, or with certain foods106 have to be taken into account, posing a potential limitation for interpretation. It may thus be that endothelial function measurements should not rely on a single test but rather on the average of several tests.
Does endothelial function provide prognostic information beyond commonly used risk scores, in primary prevention?
As outlined above, endothelial dysfunction is an important mechanism for cardiovascular risk, therefore its association with prognosis is not surprising. In the clinical setting, however, it is relevant to ask whether endothelial dysfunction provides additional information beyond traditional risk score algorithms, defined for example by the Framingham, PROCAM or SCORE projects or if it just reflects cardiovascular risk. Already early, invasive studies demonstrated that endothelial dysfunction measured in the coronary vasculature is an important prognosticator for the incidence of further cardiovascular events even in patients without coronary artery disease.107, 108 However, in the setting of primary prevention, invasive measurements are not feasible and most studies using peripheral endothelial function tests addressing this issue were limited by the small sample size, the evaluation of a particular subset of patients, by the long follow up periods required or by the difficulties to correct for the influence of pre-existing cardiovascular risk factors.
Peripheral flow-mediated vasodilation is predictive of cardiovascular events beyond traditional risk factors in special subsets of patients, such as after elective vascular surgery109 in postmenopausal women110 and in patients with chest pain.111 Several (but not all) large-scale studies recognized the additional value of endothelial function in the primary prevention setting.
In one study of 435 and one of 268 consecutive healthy subjects without heart disease and low clinical risk, brachial artery FMD independently predicted long-term adverse cardiovascular events in addition to traditional risk factor assessment.112, 113 In the Cardiovascular Health Study, the relationship between endothelial function (as measured by FMD) and subsequent cardiovascular events was measured in a cohort of more then 2700 apparently healthy subjects older then 72 years. Over a 5 years follow-up period, event free survival was significantly higher in those patients with normal endothelial function, a relation which still held true after adjusting for traditional risk factors.58 Similarly, in the Multi-Ethnic Study of Atherosclerosis, a study in White, Black, Hispanic and Chinese subjects (>3000 persons), FMD predicted future CV events, again, even after adjusting for the Framingham risk score. Furthermore FMD helped better to classify cardiovascular risk in combination with the Framingham score, compared to FMD or Framingham score alone.59 Similar to the above mentioned study, in another large cohort (n>2000) of asymptomatic postmenopausal women, FMD provided additional prognostic information to the Framingham risk score.114
In these studies the adjustments for traditional risk factors weakened the correlation of endothelial function and outcomes. This is not surprising, as endothelial dysfunction is a key biological mechanism by which cardiovascular risk factors exert their propensity to atherosclerosis and adverse events. However, as seen in a large number of patients in the above mentioned studies, information on endothelial function was able to add additional information, potentially suggesting that we have not yet found all individual risk factors and that in each individual subject, risk factors alone may not be linked predictably to clinical outcome. It also has to be taken into account that predictors found in multivariate analysis may only be of restricted clinical utility and provide only limited information for individual risk assessment.
In discussing whether endothelial dysfunction is able to predict future events beyond traditional risk scores, it also has to be taken into account in which vascular bed and in which patient population the measurements were performed. In the FATE study (Firefighters And Their Endothelium), for example, which included 1574 middle aged apparently healthy men at low cardiovascular risk (Framingham 7.9%), FMD was not associated with future clinical events. One explanation for the neutral findings in terms of FMD might be that these firefighters were very physically active, with the consequence of adaptive vascular remodeling and thus lower FMD.115 An interesting finding in the FATE study was that hyperemic velocity (a measure of microvascular function) in the brachial artery was significantly related to events in a multivariable analysis (also containing Framingham risk score).57 Similar to the above mentioned studies the addition of hyperemic velocity (instead of FMD) to the Framingham Risk score led to a risk-reclassification improvement. In another recent large (1016 persons) community-based cohort study, forearm microvascular endothelium function as assessed with Ach infusion was associated with CV events in elderly patients, whereas FMD was not.116 Again, the addition of microvascular endothelial dysfunction to the Framingham score improved risk discrimination. Similarly, microvascular endothelial dysfunction measured with EndoPAT was useful in predicting non-obstructive coronary atherosclerosis, not well predicted by the Framingham score,117 and in independently predicting adverse cardiac events in 270 outpatients.71 Thus microvascular and macrovascular function may give important extra information, above and beyond traditional risk factors, in certain situations. Altogether if looking at the above mentioned studies it seems that macrovascular endothelial function might be more important in patients with existing atherosclerosis but microvascular function might be more important in the younger subjects; in other words, microvascular function may be an earlier indicator of risk.
Despite data indicating a predictive value for future cardiovascular events, even after adjusting for known risk, endothelial function measurements are not yet recommended by guidelines for prevention, neither by the European (ESC)118 nor the more recent American (AHA/ACC)119, 120 guidelines (Class III indication), and receive lower classification of recommendation than carotid IMT measurements and coronary calcium score. Reasons for this Class III indication were the lack of clear additional prognostic value of endothelial function and the poorly standardized non-invasive methodology (with the exception of EndoPAT). However, most of the above mentioned FMD studies, demonstrating a clear addition of prognostic value, are rather new and are published after the release of the guidelines. Despite the high sensitivity as a prognosticator for future events, specificity still remains a concern. However, with technical modifications and more accurate analysis software the variability of FMD measurements, and thus the specificity, can be shown to be further improved. If endothelial function is appreciated as a dynamic process (perhaps several measurements should be averaged) and if standardized protocols are followed, reproducible measurements can be achieved, especially if performed in high volume experienced centers.49 Furthermore IMT and calcium score, although they can be performed in a more standardized manner and are less impacted on transient abnormalities than endothelial function, these measures give information about the vascular structure (more established disease) rather than function. Additionally these measurements do not change rapidly with interventions, an invaluable advantage of endothelial function measurements.
Is there any role of endothelial function for prognosis in patients with already established coronary artery disease or events?
As endothelial dysfunction plays an important role in the pathogenesis of atherothrombotic disease, it is not surprising that many studies have demonstrated a potential prognostic role of endothelial function in the coronary as well as in the peripheral circulation in secondary prevention. First evidence came from patients with non-obstructive coronary artery disease, where significantly higher incidences in cardiovascular121, 122 and cerebrovascular events in those with impaired coronary vascular function was found.123 Similarly, peripheral endothelial dysfunction assessed with FMD124 and venous occlusion plethysmography predicted CV events in patients with coronary artery disease125 and in patients after acute coronary syndromes.126 In the setting of established coronary artery disease, patients with endothelial dysfunction have higher rates of adverse cardiovascular events, compared to those with normal endothelial function,127 and impaired FMD has been shown to be an independent predictor of in-stent stenosis after single-vessel coronary interventions.128 In patients with advanced ischemic heart failure, endothelial function is a strong and independent predictor of 1 year mortality129 and in patients with graft vasculopathy – atherosclerosis associated with cardiac transplantation – normal endothelial function is associated with lower progression of coronary intimal thickening130 and epicardial endothelial dysfunction independently predicts outcome in these patients.131, 132
In acute myocardial infarction, especially microvascular endothelial dysfunction has been documented to be indicative of a poorer prognosis.133–136 For example, no-reflow on angiography strongly predicts 5-year mortality, independent of infarct size in patients with acute ST-elevation myocardial infarction.137 Interestingly, no-reflow might be reversible in some cases, which is associated with a better prognosis.138
Endothelial function as a contributor to disease progression?
Endothelial dysfunction in the periphery and in the coronary arteries not only is a marker for cardiovascular risk, but is also a contributor to the progression of atherosclerosis101 and cardiovascular events. Interestingly, the atherosclerotic epicardial segments which show the most endothelial dysfunction are those with characteristics of vulnerable atherosclerotic plaques.36 These segments are characterized by the loss of NO activity and increase in endothelin-1 activity,139 the same segments progressing more likely to obstructive coronary artery disease.140
Importantly, microvascular dysfunction may contribute to the impaired regulation of myocardial perfusion by reducing the capacity to increase perfusion in response to exercise or mental stress, a circumstance which may lead to myocardial ischemia.141 In the context of myocardial infarction, endothelial microvascular dysfunction is an important mediator of the event, rather than just a consequence.142 This is likely via reduction of coronary blood flow by altering shear stress on the epicardial level and lowering endothelial function and aggravating thrombus formation. Diabetes, and the accumulation of risk factors in the metabolic syndrome, for example, have significant deleterious effects on myocardial perfusion and infarct size in patients with an acute infarction.143, 144, 145, 146 Moreover, patients with pre-procedural impairment of microvascular function are more likely to have post-procedural microvascular impairment as well as procedure-related injury and a worse outcome.147 Thus, pre-existing microvascular endothelial dysfunction leads to a greater vulnerability to myocardial injury, highlighting the potentially clinically relevant role of a dysfunctional microcirculation and damage.
Does endothelial function identify responders and non responders to therapy?
Many medical or lifestyle interventions are able to improve endothelial function and to reduce cardiovascular events. For example statin treatment significantly improves peripheral and coronary vascular function,148 although not all studies were able to prove such an effect within a six months treatment period (ENCORE-1149 and CARATS150). Off note, the impact on risk reduction despite this successful intervention is limited and is in the range of about 20–45% in clinical trials. Even with the combination of all therapies proven to lower risk in secondary prevention (or primary prevention), some patients may still develop later events and therefore are obviously not completely protected by their therapy.
Therefore it is the ultimate goal to identify those patients who will develop future events despite therapy (in order to potentially escalate and intensify current treatment). One concept could be to measure the individual impact of therapy on endothelial function as a parameter of cardiovascular disease and especially target those which no improvement in vascular function. Recently, important studies in this respect have been performed:
In a study in 251 Japanese men with newly diagnosed stable coronary artery disease and concurrent endothelial dysfunction (low FMD), FMD was repeated after 6 month of optimized individualized therapy. Those patients with persistently impaired FMD had significant higher event rates in the follow up period (26% over 31 months) compared to those with normal FMD (10%).151 In a similar study, endothelial function was assessed in 400 post-menopausal hypertensive women without evidence of coronary artery disease at baseline and 6 month after treating blood pressure to normotensive values. In those women whose endothelial function (FMD) has not improved (37.5%) there was a nearly 7-fold increase in CV events over the average follow up of 67 month.152
Both studies convincingly demonstrate that patients who do not respond with improvement of endothelial function with interventions are at a considerable risk of further events. In our view, more studies in this respect need to performed to give definitive answers to one of the most intriguing aspects in using endothelial function measurements.153
Nevertheless, one has to acknowledge in the future that the pathophysiology of vascular dysfunction is extremely complex, probably differs form different vascular beds and between micro- and macrovascular vessels. Thus in the clinical endothelial function evaluation of the future, a more integrative approach should be considered, with the inclusion of peripheral macrovascular endothelial function as well as microvascular function measurements. An ideal test to identify a vulnerable patient should reflect and follow the disease state, thus should be abnormal with disease and possibly reversible with interventions (Table 4).
TABLE 4.
Criteria for an optimal endothelial function test
|
Is improvement in endothelial function an indicator of successful treatment?
It is probably a good sign when endothelial dysfunction is (partly-) reversed with treatments. First proof for this principle came from two controlled studies in 1995 where cholesterol lowering therapy (statins),154, 155 improved endothelial function. Statin treatment now has convincing evidence for its beneficial effect on coronary and peripheral endothelial function,148 likely due to their anti-inflammatory and antioxidant properties, as well as due to the restoration of the vascular NO bioavailability.156 Since the first evidence in humans with statins, numerous interventions in a broad range of patients demonstrated a beneficial effect on endothelial function. Most pharmacological intervention studies with an effect on cardiovascular risk factors also improve endothelial function. For example, antihypertensive therapy in general,157, 158 such as angiotensin-converting enzyme (ACE)-inhibitors,159, 160 angiotensin-receptor blocker,161 calcium channel blockers (CCB)162 and certain β-blockers, in particular the NO-group containing molecule nebivolol,163 might reverse endothelial dysfunction, however, ACE-inhibitors seem to be particularly important.158, 164, 165 CCB reduce calcium entry though L-type voltage-dependent channels of the vascular muscle cells thus dilatating coronary and other arteries. Additionally, some CCB activate endothelial NO synthase or have antioxidative properties thus increasing NO bioavailability.166 The ENCORE-1 and ENCORE-2 trial showed that long acting nifedipine consistently improved coronary endothelial function in patients with stable CAD that persisted even after cessation of the drug.149, 167 ACE-inhibitors and angiotensin receptor blockers are also preferred medication in diabetes mellitus.168 Diabetes modulating drugs like metformin169 or glitazones170, 171 may also improve vascular function in patients with type 2 diabetes, however, the later may have negative effects on cardiovascular risk,172 thus limiting its use.
Not only pharmacological agents but also lifestyle factors and medications that increase the release or prevent the degradation of endothelial derived relaxing factors, NO in particular, and those which decrease production of endothelium-derived constricting factors such as endothelin among others, can improve endothelial function. Many interventions have either demonstrated to be beneficial for microvascular or macrovascular endothelial function by increasing NO bioavailability such as physical exercise,173, 174, 175 weight reduction176–178 (including bariatric surgery),179, 180 or enhanced external counterpulsation181, 182 and dietary interventions with foods rich in polyphenols, especially fruits, tea and cocoa.183–185 An important lifestyle modification with impact on endothelial function is smoking cessation, which clearly demonstrates a favorable effect on epicardial coronary endothelial function,81 which however was not observed in the microvasculature.186 This demonstrates the differences of macrovascular and microvasculature function and its complex not yet fully understood interactions.
While therapies with a proven benefit on morbidity and mortality in cardiovascular patients concordantly improve endothelial function, it is not certain whether the opposite is always true. For example vitamin C or E as well as folic acid supplementation, which were praised for their antioxidative capacity and were associated with significant acute improvement in endothelial function,187 failed to show any benefit in the long-term188 or in cardiovascular disease prevention so far. On the other end, the pathophysiological information derived from studies on endothelial function is not correctly applied in clinical trials. Available evidence demonstrates that vitamin C can improve endothelial function,187 but also that this effect is obtained with concentrations much higher than those reached after oral administration.189 Many studies evaluating pathophysiological aspects were performed in the setting of acute intravenous or intraatrial interventions. Moreover, the response to vitamins may also be dependent on the presence of increased endogenous oxidative stress.190 This kind of information should be carefully evaluated when designing intervention studies. The abundance of current trials with any kind of positive effects on the endothelium are pathophysiologically interesting, but care should be taken to extrapolate the findings to cardiovascular morbidity and mortality. Finally, it is of concern that the drug effects on endothelial function may be different according to which vascular bed is considered.
Additionally, many lifestyle interventions, foods and drugs have been shown to improve endothelial dysfunction in a population as a whole, but this does not give information on the individual patient, a fact which should be addressed in further studies.
Are microvascular and conduit vessel endothelial function comparable?
Although cardiovascular risk factors are associated with endothelial dysfunction in virtually every arterial bed, considering the different physiological role of conduit and resistance arteries, important differences should be considered. Whereas reduced NO release in response to stimuli plays a central role in the pathophysiology of endothelial dysfunction in the conduit arteries, NO in the microcirculation may primarily modulate tissue metabolism.191 Furthermore metabolic and other factors are becoming increasingly important in the regulation of microvascular function. Therefore pharmacologic tests inducing NO release might not reflect the physiologic adaption of endothelial function in the microvasculature in response to exercise or ischemia.
The aforementioned differential effect of smoking on microvasculature and epicardial vasculature as outlined above might be only one example.186 Furthermore, FMD is particularly sensitive to being impaired by traditional risk factors (e.g. age, hypertension), whereas the peripheral arterial tonometry reactive hyperemia index (microvasculature) is more sensitive to metabolic risk factors, especially body mass index and diabetes,72, 73 (and interestingly, shows a paradoxical association with age in the Framingham cohort).72 Micro- and macrovascular dysfunction could also reflect different stages of vascular disease as conduit artery endothelial dysfunction may be more important in patients with existing atherosclerosis and microvascular dysfunction may be an earlier indicator of risk. The fact that micro- and macrovascular endothelial function only show a weak (if any) correlation with each other73 should caution against the extrapolation of findings in one circulation level to the other.
Given that macrovascular and microvascular endothelium are susceptible to different risk factors, whenever possible both should be evaluated.
Is there a role of endothelial function in drug-development programs?
For new drugs the requirement by drug regulation authorities is to prove the principle by “primum non nocere” (first, do no harm); however to test the effect of a certain drug on morbidity and mortality requires large sample sizes. Sometimes, such as for drugs with relatively small effects on the cardiovascular system or in children, such outcome trials are not feasible at all. Clinical endothelial function evaluation is of potential value in reassessing the risk of drug-development programs, especially as it gets more and more challenging to choose novel agents for clinical use.192 With endothelial function as a mechanistic surrogate integrating various types of cardiovascular risks49, 192 sample size can be significantly smaller, compared to clinical endpoint trials.52 Furthermore, endothelial function may respond rapidly to therapies (within hours, days or weeks), long before effects on clinical outcomes are seen. Thus the impact on endothelial function may give important signals of efficacy or, more important, may warn of potential harm. Therefore endothelial function is not only a valuable measure to assess drug efficacy on surrogate endpoints but also may play an important potential role in the evaluation of drug safety as exemplified in the recently completed dal-VESSEL study,193 notably the first multicenter study to use FMD as outcome measure. For certain studies a multimodality approach, which includes peripheral endothelial function measurements, may be of particular value.194
What kind of studies do we need in the future?
As outlined above, endothelial function measurement may differentiate responders versus non-responders to therapy.153 In secondary prevention, studies demonstrate that patients who do not respond with improvement of endothelial function with interventions are at a considerable risk for further events. These early data suggest that individual endothelial function measurements guided therapy might be feasible in these settings but larger studies in this respect are needed to answer the question whether endothelial function guided therapies help to improve outcomes.
In primary prevention, it is still an unanswered question whether endothelial function should be assessed in the apparently healthy persons at low risk from traditional risk factors. To address this issue, endothelial dysfunction, which depicts mechanisms at the core of atherosclerosis and its complications, could be chosen for future similar studies, with different medical and lifestyle interventions to be tested. Designing such a trial would require very careful consideration of which non-invasive test or combination of testes of endothelial function should be included. If such results prove positive, there would be a good rationale to implement endothelial function testing into everyday clinical practice.
Current, clinical guidelines and risk management for prevention are based on the risk factors established in the Framingham study and certain cardiovascular surrogates such as carotid IMT and coronary calcium.119, 120. However, the Framingham score, as well as other scores, when applied to different populations provide inconsistent results195 and adjustment based on different populations might be needed. Additionally, the Framingham Score is limited to the fact that risk factors were collected years ago, where for example no statin therapy was available and most persons smoked and/or were exposed to second-hand smoke. The effect of a changing environment might be better depicted by endothelial function assessments. Assuming that endothelial function provides an integrated functional risk assessment, the question whether endothelial function might be a better predictor for cardiovascular events than the actual scoring systems is intriguing and should be tested with larger scale studies.
When using the Framingham risk score, we are aware how to deal with patients in the high or low risk category. However many patients end up having intermediate risk, where the recommendation are less clear. As demonstrated by the studies discussed above, reclassification of patients with intermediate risk according to their endothelial function seems to be feasible and reasonable, although further studies in this area are required.
CONCLUSION
In the past decade, many studies have suggested that the non invasive assessment of endothelial function may provide important information for individual patient risk, progress and guidance of therapy (in addition to its well established role in clinical research). This is underscored by the low risk of the tests and the valuable information that can be derived from them. Thus further research should be directed to determine whether measurement of endothelial function can be used to effect treatments and change outcomes and ascertain whether detection of endothelial function will be useful in clinical arena.
Acknowledgments
Funding Sources
AJF is supported by the Walter and Gertrud Siegenthaler Foundation, the young academics Support Committee of the University of Zurich, and the Swiss foundation for medical-biological scholarships (SSMBS; SNSF No PASMP3_132551). TJA is funded by as a Senior Scholar of Alberta Innovates-Health Solutions (Edmonton, Canada). JD is supported by grants of the British Heart Foundation. This work was supported by the National Institute of Health (NIH Grant HL-92954 and AG-31750 to AL).
Footnotes
Disclosures
MAC, PG, and AL are on the advisory board of Itamar Medical. TJA received an equipment donation from Itamar Medical. JAV received an equipment donation form Itamar Medical for his laboratory and had a consultative relationship with Angiologix. MS is on the scientific advisory board of Itamar Medical. JD is on the speaker’s bureau or advisory board of Roche, Astra Zeneca, Pfizer, Merck, Danone and Sanofi. TFL has been PI of the Dal-Vessel trial supported by Roche. ST received research grants from Novartis, Servier, Recordati, Menarini and Boehringer and is on the speaker’s bureau for Servier, Recordati, Novartis and Boehringer. The other authors report no actual or potential conflicts of interest in connection with this study.
References
- 1.Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- 2.Flammer AJ, Luscher TF. Human endothelial dysfunction: EDRFs. Pflugers Arch. 2010;459:1005–1013. doi: 10.1007/s00424-010-0822-4. [DOI] [PubMed] [Google Scholar]
- 3.Virdis A, Ghiadoni L, Taddei S. Human endothelial dysfunction: EDCFs. Pflugers Arch. 2010;459:1015–1023. doi: 10.1007/s00424-009-0783-7. [DOI] [PubMed] [Google Scholar]
- 4.Edwards G, Feletou M, Weston AH. Endothelium-derived hyperpolarising factors and associated pathways: a synopsis. Pflugers Arch. 2010;459:863–879. doi: 10.1007/s00424-010-0817-1. [DOI] [PubMed] [Google Scholar]
- 5.Bonetti PO, Lerman LO, Lerman A. Endothelial dysfunction: a marker of atherosclerotic risk. Arteriosclerosis, thrombosis, and vascular biology. 2003;23:168–175. doi: 10.1161/01.atv.0000051384.43104.fc. [DOI] [PubMed] [Google Scholar]
- 6.Anderson TJ, Gerhard MD, Meredith IT, Charbonneau F, Delagrange D, Creager MA, Selwyn AP, Ganz P. Systemic nature of endothelial dysfunction in atherosclerosis. The American journal of cardiology. 1995;75:71B–74B. doi: 10.1016/0002-9149(95)80017-m. [DOI] [PubMed] [Google Scholar]
- 7.Juonala M, Viikari JS, Laitinen T, Marniemi J, Helenius H, Ronnemaa T, Raitakari OT. Interrelations between brachial endothelial function and carotid intima-media thickness in young adults: the cardiovascular risk in young Finns study. Circulation. 2004;110:2918–2923. doi: 10.1161/01.CIR.0000147540.88559.00. [DOI] [PubMed] [Google Scholar]
- 8.Flammer AJ, Luscher TF. Three decades of endothelium research: from the detection of nitric oxide to the everyday implementation of endothelial function measurements in cardiovascular diseases. Swiss Med Wkly. 2010;140:w13122. doi: 10.4414/smw.2010.13122. [DOI] [PubMed] [Google Scholar]
- 9.Ludmer PL, Selwyn AP, Shook TL, Wayne RR, Mudge GH, Alexander RW, Ganz P. Paradoxical vasoconstriction induced by acetylcholine in atherosclerotic coronary arteries. The New England journal of medicine. 1986;315:1046–1051. doi: 10.1056/NEJM198610233151702. [DOI] [PubMed] [Google Scholar]
- 10.Linder L, Kiowski W, Bühler FR, Lüscher TF. Indirect evidence for release of endothelium-derived relaxing factor in human forearm circulation in vivo. Blunted response in essential hypertension. Circulation. 1990;81:1762–1767. doi: 10.1161/01.cir.81.6.1762. [DOI] [PubMed] [Google Scholar]
- 11.Celermajer DS, Sorensen KE, Gooch VM, Spiegelhalter DJ, Miller OI, Sullivan ID, Lloyd JK, Deanfield JE. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet. 1992;340:1111–1115. doi: 10.1016/0140-6736(92)93147-f. [DOI] [PubMed] [Google Scholar]
- 12.Puri R, Liew GY, Nicholls SJ, Nelson AJ, Leong DP, Carbone A, Copus B, Wong DT, Beltrame JF, Worthley SG, Worthley MI. Coronary {beta}2-adrenoreceptors mediate endothelium-dependent vasoreactivity in humans: novel insights from an in vivo intravascular ultrasound study. European heart journal. doi: 10.1093/eurheartj/ehr359. [DOI] [PubMed] [Google Scholar]
- 13.Gordon JB, Ganz P, Nabel EG, Fish RD, Zebede J, Mudge GH, Alexander RW, Selwyn AP. Atherosclerosis influences the vasomotor response of epicardial coronary arteries to exercise. J Clin Invest. 1989;83:1946–1952. doi: 10.1172/JCI114103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hess OM, Buchi M, Kirkeeide R, Niederer P, Anliker M, Gould KL, Krayenbuhl HP. Potential role of coronary vasoconstriction in ischaemic heart disease: effect of exercise. European heart journal. 1990;11 (Suppl B):58–64. doi: 10.1093/eurheartj/11.suppl_b.58. [DOI] [PubMed] [Google Scholar]
- 15.Nabel EG, Selwyn AP, Ganz P. Paradoxical narrowing of atherosclerotic coronary arteries induced by increases in heart rate. Circulation. 1990;81:850–859. doi: 10.1161/01.cir.81.3.850. [DOI] [PubMed] [Google Scholar]
- 16.Egashira K, Katsuda Y, Mohri M, Kuga T, Tagawa T, Kubota T, Hirakawa Y, Takeshita A. Role of endothelium-derived nitric oxide in coronary vasodilatation induced by pacing tachycardia in humans. Circulation research. 1996;79:331–335. doi: 10.1161/01.res.79.2.331. [DOI] [PubMed] [Google Scholar]
- 17.Yeung AC, Vekshtein VI, Krantz DS, Vita JA, Ryan TJ, Jr, Ganz P, Selwyn AP. The effect of atherosclerosis on the vasomotor response of coronary arteries to mental stress. The New England journal of medicine. 1991;325:1551–1556. doi: 10.1056/NEJM199111283252205. [DOI] [PubMed] [Google Scholar]
- 18.Cox DA, Vita JA, Treasure CB, Fish RD, Alexander RW, Ganz P, Selwyn AP. Atherosclerosis impairs flow-mediated dilation of coronary arteries in humans. Circulation. 1989;80:458–465. doi: 10.1161/01.cir.80.3.458. [DOI] [PubMed] [Google Scholar]
- 19.Nabel EG, Selwyn AP, Ganz P. Large coronary arteries in humans are responsive to changing blood flow: an endothelium-dependent mechanism that fails in patients with atherosclerosis. Journal of the American College of Cardiology. 1990;16:349–356. doi: 10.1016/0735-1097(90)90584-c. [DOI] [PubMed] [Google Scholar]
- 20.Tschudi M, Richard V, Buhler FR, Luscher TF. Importance of endothelium-derived nitric oxide in porcine coronary resistance arteries. The American journal of physiology. 1991;260:H13–20. doi: 10.1152/ajpheart.1991.260.1.H13. [DOI] [PubMed] [Google Scholar]
- 21.Nabel EG, Ganz P, Gordon JB, Alexander RW, Selwyn AP. Dilation of normal and constriction of atherosclerotic coronary arteries caused by the cold pressor test. Circulation. 1988;77:43–52. doi: 10.1161/01.cir.77.1.43. [DOI] [PubMed] [Google Scholar]
- 22.Zeiher AM, Drexler H, Wollschlaeger H, Saurbier B, Just H. Coronary vasomotion in response to sympathetic stimulation in humans: importance of the functional integrity of the endothelium. Journal of the American College of Cardiology. 1989;14:1181–1190. doi: 10.1016/0735-1097(89)90414-2. [DOI] [PubMed] [Google Scholar]
- 23.Beltrame JF, Crea F, Camici P. Advances in coronary microvascular dysfunction. Heart, lung & circulation. 2009;18:19–27. doi: 10.1016/j.hlc.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 24.Camici PG, Crea F. Coronary microvascular dysfunction. The New England journal of medicine. 2007;356:830–840. doi: 10.1056/NEJMra061889. [DOI] [PubMed] [Google Scholar]
- 25.Gibson CM, Cannon CP, Daley WL, Dodge JT, Jr, Alexander B, Jr, Marble SJ, McCabe CH, Raymond L, Fortin T, Poole WK, Braunwald E. TIMI frame count: a quantitative method of assessing coronary artery flow. Circulation. 1996;93:879–888. doi: 10.1161/01.cir.93.5.879. [DOI] [PubMed] [Google Scholar]
- 26.Schindler TH, Schelbert HR, Quercioli A, Dilsizian V. Cardiac PET imaging for the detection and monitoring of coronary artery disease and microvascular health. Jacc. 3:623–640. doi: 10.1016/j.jcmg.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 27.Doyle M, Fuisz A, Kortright E, Biederman RW, Walsh EG, Martin ET, Tauxe L, Rogers WJ, Merz CN, Pepine C, Sharaf B, Pohost GM. The impact of myocardial flow reserve on the detection of coronary artery disease by perfusion imaging methods: an NHLBI WISE study. J Cardiovasc Magn Reson. 2003;5:475–485. doi: 10.1081/jcmr-120022263. [DOI] [PubMed] [Google Scholar]
- 28.Utz W, Jordan J, Niendorf T, Stoffels M, Luft FC, Dietz R, Friedrich MG. Blood oxygen level-dependent MRI of tissue oxygenation: relation to endothelium-dependent and endothelium-independent blood flow changes. Arteriosclerosis, thrombosis, and vascular biology. 2005;25:1408–1413. doi: 10.1161/01.ATV.0000170131.13683.d7. [DOI] [PubMed] [Google Scholar]
- 29.Leung DY, Leung M. Non-invasive/invasive imaging: significance and assessment of coronary microvascular dysfunction. Heart (British Cardiac Society) 2011;97:587–595. doi: 10.1136/hrt.2009.183327. [DOI] [PubMed] [Google Scholar]
- 30.Anderson TJ, Uehata A, Gerhard MD, Meredith IT, Knab S, Delagrage D, Lieberman EH, Ganz P, Creager MA, Yeung AC, Selwyn AP. Close relation of endothelial function in the human coronary and peripheral circulation. Journal of the American College of Cardiology. 1995;26:1235–1241. doi: 10.1016/0735-1097(95)00327-4. [DOI] [PubMed] [Google Scholar]
- 31.Bonetti PO, Pumper GM, Higano ST, Holmes DR, Jr, Kuvin JT, Lerman A. Noninvasive identification of patients with early coronary atherosclerosis by assessment of digital reactive hyperemia. Journal of the American College of Cardiology. 2004;44:2137–2141. doi: 10.1016/j.jacc.2004.08.062. [DOI] [PubMed] [Google Scholar]
- 32.Takase B, Uehata A, Akima T, Nagai T, Nishioka T, Hamabe A, Satomura K, Ohsuzu F, Kurita A. Endothelium-dependent flow-mediated vasodilation in coronary and brachial arteries in suspected coronary artery disease. The American journal of cardiology. 1998;82:1535–1539. A1537–1538. doi: 10.1016/s0002-9149(98)00702-4. [DOI] [PubMed] [Google Scholar]
- 33.el-Tamimi H, Mansour M, Wargovich TJ, Hill JA, Kerensky RA, Conti CR, Pepine CJ. Constrictor and dilator responses to intracoronary acetylcholine in adjacent segments of the same coronary artery in patients with coronary artery disease. Endothelial function revisited. Circulation. 1994;89:45–51. doi: 10.1161/01.cir.89.1.45. [DOI] [PubMed] [Google Scholar]
- 34.Gimbrone MA, Jr, Topper JN, Nagel T, Anderson KR, Garcia-Cardena G. Endothelial dysfunction, hemodynamic forces, and atherogenesis. Ann N Y Acad Sci. 2000;902:230–239. doi: 10.1111/j.1749-6632.2000.tb06318.x. discussion 239-240. [DOI] [PubMed] [Google Scholar]
- 35.Chatzizisis YS, Coskun AU, Jonas M, Edelman ER, Feldman CL, Stone PH. Role of endothelial shear stress in the natural history of coronary atherosclerosis and vascular remodeling: molecular, cellular, and vascular behavior. Journal of the American College of Cardiology. 2007;49:2379–2393. doi: 10.1016/j.jacc.2007.02.059. [DOI] [PubMed] [Google Scholar]
- 36.Lavi S, Bae JH, Rihal CS, Prasad A, Barsness GW, Lennon RJ, Holmes DR, Jr, Lerman A. Segmental coronary endothelial dysfunction in patients with minimal atherosclerosis is associated with necrotic core plaques. Heart (British Cardiac Society) 2009;95:1525–1530. doi: 10.1136/hrt.2009.166017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McLenachan JM, Vita J, Fish DR, Treasure CB, Cox DA, Ganz P, Selwyn AP. Early evidence of endothelial vasodilator dysfunction at coronary branch points. Circulation. 1990;82:1169–1173. doi: 10.1161/01.cir.82.4.1169. [DOI] [PubMed] [Google Scholar]
- 38.Petrie JR, Ueda S, Morris AD, Murray LS, Elliott HL, Connell JM. How reproducible is bilateral forearm plethysmography? Br J Clin Pharmacol. 1998;45:131–139. doi: 10.1046/j.1365-2125.1998.00656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Joannides R, Haefeli WE, Linder L, Richard V, Bakkali EH, Thuillez C, Lüscher TF. Nitric oxide is responsible for flow-dependent dilatation of human peripheral conduit arteries in vivo. Circulation. 1995;91:1314–1319. doi: 10.1161/01.cir.91.5.1314. [DOI] [PubMed] [Google Scholar]
- 40.Ozkor MA, Murrow JR, Rahman AM, Kavtaradze N, Lin J, Manatunga A, Quyyumi AA. Endothelium-derived hyperpolarizing factor determines resting and stimulated forearm vasodilator tone in health and in disease. Circulation. 2011;123:2244–2253. doi: 10.1161/CIRCULATIONAHA.110.990317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Giannarelli C, Virdis A, De Negri F, Magagna A, Duranti E, Salvetti A, Taddei S. Effect of sulfaphenazole on tissue plasminogen activator release in normotensive subjects and hypertensive patients. Circulation. 2009;119:1625–1633. doi: 10.1161/CIRCULATIONAHA.108.782482. [DOI] [PubMed] [Google Scholar]
- 42.Taddei S, Versari D, Cipriano A, Ghiadoni L, Galetta F, Franzoni F, Magagna A, Virdis A, Salvetti A. Identification of a cytochrome P450 2C9-derived endothelium-derived hyperpolarizing factor in essential hypertensive patients. Journal of the American College of Cardiology. 2006;48:508–515. doi: 10.1016/j.jacc.2006.04.074. [DOI] [PubMed] [Google Scholar]
- 43.Benjamin N, Calver A, Collier J, Robinson B, Vallance P, Webb D. Measuring forearm blood flow and interpreting the responses to drugs and mediators. Hypertension. 1995;25:918–923. doi: 10.1161/01.hyp.25.5.918. [DOI] [PubMed] [Google Scholar]
- 44.Lieberman EH, Gerhard MD, Uehata A, Selwyn AP, Ganz P, Yeung AC, Creager MA. Flow-induced vasodilation of the human brachial artery is impaired in patients <40 years of age with coronary artery disease. The American journal of cardiology. 1996;78:1210–1214. doi: 10.1016/s0002-9149(96)00597-8. [DOI] [PubMed] [Google Scholar]
- 45.Joannides R, Richard V, Haefeli WE, Linder L, Lüscher TF, Thuillez C. Role of basal and stimulated release of nitric oxide in the regulation of radial artery caliber in humans. Hypertension. 1995;26:327–331. doi: 10.1161/01.hyp.26.2.327. [DOI] [PubMed] [Google Scholar]
- 46.Parker BA, Tschakovsky ME, Augeri AL, Polk DM, Thompson PD, Kiernan FJ. Heterogenous vasodilator pathways underlie flow-mediated dilation in men and women. American journal of physiology. 2011;301:H1118–1126. doi: 10.1152/ajpheart.00400.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, Deanfield J, Drexler H, Gerhard-Herman M, Herrington D, Vallance P, Vita J, Vogel R. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. Journal of the American College of Cardiology. 2002;39:257–265. doi: 10.1016/s0735-1097(01)01746-6. [DOI] [PubMed] [Google Scholar]
- 48.Deanfield J, Donald A, Ferri C, Giannattasio C, Halcox J, Halligan S, Lerman A, Mancia G, Oliver JJ, Pessina AC, Rizzoni D, Rossi GP, Salvetti A, Schiffrin EL, Taddei S, Webb DJ. Endothelial function and dysfunction. Part I: Methodological issues for assessment in the different vascular beds: a statement by the Working Group on Endothelin and Endothelial Factors of the European Society of Hypertension. J Hypertens. 2005;23:7–17. doi: 10.1097/00004872-200501000-00004. [DOI] [PubMed] [Google Scholar]
- 49.Charakida M, Masi S, Luscher TF, Kastelein JJ, Deanfield JE. Assessment of atherosclerosis: the role of flow-mediated dilatation. European heart journal. 2010;31:2854–2861. doi: 10.1093/eurheartj/ehq340. [DOI] [PubMed] [Google Scholar]
- 50.Harris RA, Nishiyama SK, Wray DW, Richardson RS. Ultrasound assessment of flow-mediated dilation. Hypertension. 2010;55:1075–1085. doi: 10.1161/HYPERTENSIONAHA.110.150821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thijssen DH, Black MA, Pyke KE, Padilla J, Atkinson G, Harris RA, Parker B, Widlansky ME, Tschakovsky ME, Green DJ. Assessment of flow-mediated dilation in humans: a methodological and physiological guideline. American journal of physiology. 2011;300:H2–12. doi: 10.1152/ajpheart.00471.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Donald AE, Halcox JP, Charakida M, Storry C, Wallace SM, Cole TJ, Friberg P, Deanfield JE. Methodological approaches to optimize reproducibility and power in clinical studies of flow-mediated dilation. Journal of the American College of Cardiology. 2008;51:1959–1964. doi: 10.1016/j.jacc.2008.02.044. [DOI] [PubMed] [Google Scholar]
- 53.Mitchell GF, Parise H, Vita JA, Larson MG, Warner E, Keaney JF, Jr, Keyes MJ, Levy D, Vasan RS, Benjamin EJ. Local shear stress and brachial artery flow-mediated dilation: the Framingham Heart Study. Hypertension. 2004;44:134–139. doi: 10.1161/01.HYP.0000137305.77635.68. [DOI] [PubMed] [Google Scholar]
- 54.Mitchell GF, Vita JA, Larson MG, Parise H, Keyes MJ, Warner E, Vasan RS, Levy D, Benjamin EJ. Cross-sectional relations of peripheral microvascular function, cardiovascular disease risk factors, and aortic stiffness: the Framingham Heart Study. Circulation. 2005;112:3722–3728. doi: 10.1161/CIRCULATIONAHA.105.551168. [DOI] [PubMed] [Google Scholar]
- 55.Philpott AC, Lonn E, Title LM, Verma S, Buithieu J, Charbonneau F, Anderson TJ. Comparison of new measures of vascular function to flow mediated dilatation as a measure of cardiovascular risk factors. The American journal of cardiology. 2009;103:1610–1615. doi: 10.1016/j.amjcard.2009.01.376. [DOI] [PubMed] [Google Scholar]
- 56.Huang AL, Silver AE, Shvenke E, Schopfer DW, Jahangir E, Titas MA, Shpilman A, Menzoian JO, Watkins MT, Raffetto JD, Gibbons G, Woodson J, Shaw PM, Dhadly M, Eberhardt RT, Keaney JF, Jr, Gokce N, Vita JA. Predictive value of reactive hyperemia for cardiovascular events in patients with peripheral arterial disease undergoing vascular surgery. Arteriosclerosis, thrombosis, and vascular biology. 2007;27:2113–2119. doi: 10.1161/ATVBAHA.107.147322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Anderson TJ, Charbonneau F, Title LM, Buithieu J, Rose MS, Conradson H, Hildebrand K, Fung M, Verma S, Lonn EM. Microvascular function predicts cardiovascular events in primary prevention: long-term results from the Firefighters and Their Endothelium (FATE) study. Circulation. 2011;123:163–169. doi: 10.1161/CIRCULATIONAHA.110.953653. [DOI] [PubMed] [Google Scholar]
- 58.Yeboah J, Crouse JR, Hsu FC, Burke GL, Herrington DM. Brachial flow-mediated dilation predicts incident cardiovascular events in older adults: the Cardiovascular Health Study. Circulation. 2007;115:2390–2397. doi: 10.1161/CIRCULATIONAHA.106.678276. [DOI] [PubMed] [Google Scholar]
- 59.Yeboah J, Folsom AR, Burke GL, Johnson C, Polak JF, Post W, Lima JA, Crouse JR, Herrington DM. Predictive value of brachial flow-mediated dilation for incident cardiovascular events in a population-based study: the multi-ethnic study of atherosclerosis. Circulation. 2009;120:502–509. doi: 10.1161/CIRCULATIONAHA.109.864801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thijssen DH, Dawson EA, Black MA, Hopman MT, Cable NT, Green DJ. Heterogeneity in conduit artery function in humans: impact of arterial size. American journal of physiology. 2008;295:H1927–1934. doi: 10.1152/ajpheart.00405.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pyke KE, Dwyer EM, Tschakovsky ME. Impact of controlling shear rate on flow-mediated dilation responses in the brachial artery of humans. J Appl Physiol. 2004;97:499–508. doi: 10.1152/japplphysiol.01245.2003. [DOI] [PubMed] [Google Scholar]
- 62.Gori T, Dragoni S, Lisi M, Di Stolfo G, Sonnati S, Fineschi M, Parker JD. Conduit artery constriction mediated by low flow a novel noninvasive method for the assessment of vascular function. Journal of the American College of Cardiology. 2008;51:1953–1958. doi: 10.1016/j.jacc.2008.01.049. [DOI] [PubMed] [Google Scholar]
- 63.Levenson J, Simon A, Pithois-Merli I. Brachial arterial changes in response to wrist occlusion in normotensive and hypertensive men. The American journal of physiology. 1987;253:H217–224. doi: 10.1152/ajpheart.1987.253.2.H217. [DOI] [PubMed] [Google Scholar]
- 64.Spieker LE, Luscher TF, Noll G. ETA receptors mediate vasoconstriction of large conduit arteries during reduced flow in humans. J Cardiovasc Pharmacol. 2003;42:315–318. doi: 10.1097/00005344-200309000-00001. [DOI] [PubMed] [Google Scholar]
- 65.Gori T, Grotti S, Dragoni S, Lisi M, Di Stolfo G, Sonnati S, Fineschi M, Parker JD. Assessment of vascular function: flow-mediated constriction complements the information of flow-mediated dilatation. Heart (British Cardiac Society) 2010;96:141–147. doi: 10.1136/hrt.2009.167213. [DOI] [PubMed] [Google Scholar]
- 66.Gori T, Parker JD, Munzel T. Flow-mediated constriction: further insight into a new measure of vascular function. European heart journal. 2011;32:784–787. doi: 10.1093/eurheartj/ehq412. [DOI] [PubMed] [Google Scholar]
- 67.Gori T, Muxel S, Damaske A, Radmacher MC, Fasola F, Schaefer S, Schulz A, Jabs A, Parker JD, Munzel T. Endothelial function assessment: flow-mediated dilation and constriction provide different and complementary information on the presence of coronary artery disease. European heart journal. 2011 doi: 10.1093/eurheartj/ehr361. [DOI] [PubMed] [Google Scholar]
- 68.Kuvin JT, Patel AR, Sliney KA, Pandian NG, Sheffy J, Schnall RP, Karas RH, Udelson JE. Assessment of peripheral vascular endothelial function with finger arterial pulse wave amplitude. American heart journal. 2003;146:168–174. doi: 10.1016/S0002-8703(03)00094-2. [DOI] [PubMed] [Google Scholar]
- 69.Lavie P, Schnall RP, Sheffy J, Shlitner A. Peripheral vasoconstriction during REM sleep detected by a new plethysmographic method. Nat Med. 2000;6:606. doi: 10.1038/76135. [DOI] [PubMed] [Google Scholar]
- 70.Nohria A, Gerhard-Herman M, Creager MA, Hurley S, Mitra D, Ganz P. Role of nitric oxide in the regulation of digital pulse volume amplitude in humans. J Appl Physiol. 2006;101:545–548. doi: 10.1152/japplphysiol.01285.2005. [DOI] [PubMed] [Google Scholar]
- 71.Rubinshtein R, Kuvin JT, Soffler M, Lennon RJ, Lavi S, Nelson RE, Pumper GM, Lerman LO, Lerman A. Assessment of endothelial function by non-invasive peripheral arterial tonometry predicts late cardiovascular adverse events. European heart journal. 2010;31:1142–1148. doi: 10.1093/eurheartj/ehq010. [DOI] [PubMed] [Google Scholar]
- 72.Hamburg NM, Keyes MJ, Larson MG, Vasan RS, Schnabel R, Pryde MM, Mitchell GF, Sheffy J, Vita JA, Benjamin EJ. Cross-sectional relations of digital vascular function to cardiovascular risk factors in the Framingham Heart Study. Circulation. 2008;117:2467–2474. doi: 10.1161/CIRCULATIONAHA.107.748574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hamburg NM, Palmisano J, Larson MG, Sullivan LM, Lehman BT, Vasan RS, Levy D, Mitchell GF, Vita JA, Benjamin EJ. Relation of brachial and digital measures of vascular function in the community: the Framingham heart study. Hypertension. 2011;57:390–396. doi: 10.1161/HYPERTENSIONAHA.110.160812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schnabel RB, Schulz A, Wild PS, Sinning CR, Wilde S, Eleftheriadis M, Herkenhoff S, Zeller T, Lubos E, Lackner KJ, Warnholtz A, Gori T, Blankenberg S, Munzel T. Noninvasive vascular function measurement in the community: cross-sectional relations and comparison of methods. Circ Cardiovasc Imaging. 2011;4:371–380. doi: 10.1161/CIRCIMAGING.110.961557. [DOI] [PubMed] [Google Scholar]
- 75.Vita JA, Treasure CB, Nabel EG, McLenachan JM, Fish RD, Yeung AC, Vekshtein VI, Selwyn AP, Ganz P. Coronary vasomotor response to acetylcholine relates to risk factors for coronary artery disease. Circulation. 1990;81:491–497. doi: 10.1161/01.cir.81.2.491. [DOI] [PubMed] [Google Scholar]
- 76.Brunner H, Cockcroft JR, Deanfield J, Donald A, Ferrannini E, Halcox J, Kiowski W, Luscher TF, Mancia G, Natali A, Oliver JJ, Pessina AC, Rizzoni D, Rossi GP, Salvetti A, Spieker LE, Taddei S, Webb DJ. Endothelial function and dysfunction. Part II: Association with cardiovascular risk factors and diseases. A statement by the Working Group on Endothelins and Endothelial Factors of the European Society of Hypertension. J Hypertens. 2005;23:233–246. doi: 10.1097/00004872-200502000-00001. [DOI] [PubMed] [Google Scholar]
- 77.Panza JA, Quyyumi AA, Brush JJ, Epstein SE. Abnormal endothelium-dependent vascular relaxation in patients with essential hypertension. The New England journal of medicine. 1990;323:22–27. doi: 10.1056/NEJM199007053230105. [DOI] [PubMed] [Google Scholar]
- 78.Linder L, Kiowski W, Buhler FR, Luscher TF. Indirect evidence for release of endothelium-derived relaxing factor in human forearm circulation in vivo. Blunted response in essential hypertension. Circulation. 1990;81:1762–1767. doi: 10.1161/01.cir.81.6.1762. [DOI] [PubMed] [Google Scholar]
- 79.Treasure CB, Manoukian SV, Klein JL, Vita JA, Nabel EG, Renwick GH, Selwyn AP, Alexander RW, Ganz P. Epicardial coronary artery responses to acetylcholine are impaired in hypertensive patients. Circulation research. 1992;71:776–781. doi: 10.1161/01.res.71.4.776. [DOI] [PubMed] [Google Scholar]
- 80.Taddei S, Virdis A, Mattei P, Ghiadoni L, Sudano I, Salvetti A. Defective L-arginine-nitric oxide pathway in offspring of essential hypertensive patients. Circulation. 1996;94:1298–1303. doi: 10.1161/01.cir.94.6.1298. [DOI] [PubMed] [Google Scholar]
- 81.Celermajer DS, Sorensen KE, Georgakopoulos D, Bull C, Thomas O, Robinson J, Deanfield JE. Cigarette smoking is associated with dose-related and potentially reversible impairment of endothelium-dependent dilation in healthy young adults. Circulation. 1993;88:2149–2155. doi: 10.1161/01.cir.88.5.2149. [DOI] [PubMed] [Google Scholar]
- 82.Zeiher AM, Schachinger V, Minners J. Long-term cigarette smoking impairs endothelium-dependent coronary arterial vasodilator function. Circulation. 1995;92:1094–1100. doi: 10.1161/01.cir.92.5.1094. [DOI] [PubMed] [Google Scholar]
- 83.Celermajer DS, Adams MR, Clarkson P, Robinson J, McCredie R, Donald A, Deanfield JE. Passive smoking and impaired endothelium-dependent arterial dilatation in healthy young adults. The New England journal of medicine. 1996;334:150–154. doi: 10.1056/NEJM199601183340303. [DOI] [PubMed] [Google Scholar]
- 84.Heiss C, Amabile N, Lee AC, Real WM, Schick SF, Lao D, Wong ML, Jahn S, Angeli FS, Minasi P, Springer ML, Hammond SK, Glantz SA, Grossman W, Balmes JR, Yeghiazarians Y. Brief secondhand smoke exposure depresses endothelial progenitor cells activity and endothelial function: sustained vascular injury and blunted nitric oxide production. Journal of the American College of Cardiology. 2008;51:1760–1771. doi: 10.1016/j.jacc.2008.01.040. [DOI] [PubMed] [Google Scholar]
- 85.Casino PR, Kilcoyne CM, Quyyumi AA, Hoeg JM, Panza JA. The role of nitric oxide in endothelium-dependent vasodilation of hypercholesterolemic patients. Circulation. 1993;88:2541–2547. doi: 10.1161/01.cir.88.6.2541. [DOI] [PubMed] [Google Scholar]
- 86.Spieker LE, Sudano I, Hurlimann D, Lerch PG, Lang MG, Binggeli C, Corti R, Ruschitzka F, Luscher TF, Noll G. High-density lipoprotein restores endothelial function in hypercholesterolemic men. Circulation. 2002;105:1399–1402. doi: 10.1161/01.cir.0000013424.28206.8f. [DOI] [PubMed] [Google Scholar]
- 87.Makimattila S, Virkamaki A, Groop PH, Cockcroft J, Utriainen T, Fagerudd J, Yki-Jarvinen H. Chronic hyperglycemia impairs endothelial function and insulin sensitivity via different mechanisms in insulin-dependent diabetes mellitus. Circulation. 1996;94:1276–1282. doi: 10.1161/01.cir.94.6.1276. [DOI] [PubMed] [Google Scholar]
- 88.Calver A, Collier J, Vallance P. Inhibition and stimulation of nitric oxide synthesis in the human forearm arterial bed of patients with insulin-dependent diabetes. J Clin Invest. 1992;90:2548–2554. doi: 10.1172/JCI116149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cosentino F, Eto M, De Paolis P, van der Loo B, Bachschmid M, Ullrich V, Kouroedov A, Delli Gatti C, Joch H, Volpe M, Luscher TF. High glucose causes upregulation of cyclooxygenase-2 and alters prostanoid profile in human endothelial cells: role of protein kinase C and reactive oxygen species. Circulation. 2003;107:1017–1023. doi: 10.1161/01.cir.0000051367.92927.07. [DOI] [PubMed] [Google Scholar]
- 90.Smits P, Kapma JA, Jacobs MC, Lutterman J, Thien T. Endothelium-dependent vascular relaxation in patients with type I diabetes. Diabetes. 1993;42:148–153. doi: 10.2337/diab.42.1.148. [DOI] [PubMed] [Google Scholar]
- 91.Steinberg HO, Chaker H, Leaming R, Johnson A, Brechtel G, Baron AD. Obesity/insulin resistance is associated with endothelial dysfunction. Implications for the syndrome of insulin resistance. J Clin Invest. 1996;97:2601–2610. doi: 10.1172/JCI118709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Virdis A, Ghiadoni L, Cardinal H, Favilla S, Duranti P, Birindelli R, Magagna A, Bernini G, Salvetti G, Taddei S, Salvetti A. Mechanisms responsible for endothelial dysfunction induced by fasting hyperhomocystinemia in normotensive subjects and patients with essential hypertension. Journal of the American College of Cardiology. 2001;38:1106–1115. doi: 10.1016/s0735-1097(01)01492-9. [DOI] [PubMed] [Google Scholar]
- 93.Tawakol A, Forgione MA, Stuehlinger M, Alpert NM, Cooke JP, Loscalzo J, Fischman AJ, Creager MA, Gewirtz H. Homocysteine impairs coronary microvascular dilator function in humans. Journal of the American College of Cardiology. 2002;40:1051–1058. doi: 10.1016/s0735-1097(02)02069-7. [DOI] [PubMed] [Google Scholar]
- 94.Shechter M, Sharir M, Labrador MJ, Forrester J, Silver B, Bairey Merz CN. Oral magnesium therapy improves endothelial function in patients with coronary artery disease. Circulation. 2000;102:2353–2358. doi: 10.1161/01.cir.102.19.2353. [DOI] [PubMed] [Google Scholar]
- 95.Hurlimann D, Forster A, Noll G, Enseleit F, Chenevard R, Distler O, Bechir M, Spieker LE, Neidhart M, Michel BA, Gay RE, Luscher TF, Gay S, Ruschitzka F. Anti-tumor necrosis factor-alpha treatment improves endothelial function in patients with rheumatoid arthritis. Circulation. 2002;106:2184–2187. doi: 10.1161/01.cir.0000037521.71373.44. [DOI] [PubMed] [Google Scholar]
- 96.Flammer AJ, Vo NT, Ledergerber B, Hermann F, Gamperli A, Huttner A, Evison J, Baumgartner I, Cavassini M, Hayoz D, Quitzau K, Hersberger M, Sudano I, Ruschitzka F, Luscher TF, Noll G, Weber R. Effect of atazanavir versus other protease inhibitor-containing antiretroviral therapy on endothelial function in HIV-infected persons: randomised controlled trial. Heart (British Cardiac Society) 2009;95:385–390. doi: 10.1136/hrt.2007.137646. [DOI] [PubMed] [Google Scholar]
- 97.Flammer AJ, Sudano I, Hermann F, Gay S, Forster A, Neidhart M, Kunzler P, Enseleit F, Periat D, Hermann M, Nussberger J, Luscher TF, Corti R, Noll G, Ruschitzka F. Angiotensin-converting enzyme inhibition improves vascular function in rheumatoid arthritis. Circulation. 2008;117:2262–2269. doi: 10.1161/CIRCULATIONAHA.107.734384. [DOI] [PubMed] [Google Scholar]
- 98.Sorensen KE, Celermajer DS, Georgakopoulos D, Hatcher G, Betteridge DJ, Deanfield JE. Impairment of endothelium-dependent dilation is an early event in children with familial hypercholesterolemia and is related to the lipoprotein(a) level. J Clin Invest. 1994;93:50–55. doi: 10.1172/JCI116983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Charakida M, Donald AE, Terese M, Leary S, Halcox JP, Ness A, Davey Smith G, Golding J, Friberg P, Klein NJ, Deanfield JE. Endothelial dysfunction in childhood infection. Circulation. 2005;111:1660–1665. doi: 10.1161/01.CIR.0000160365.18879.1C. [DOI] [PubMed] [Google Scholar]
- 100.Yan RT, Anderson TJ, Charbonneau F, Title L, Verma S, Lonn E. Relationship between carotid artery intima-media thickness and brachial artery flow-mediated dilation in middle-aged healthy men. Journal of the American College of Cardiology. 2005;45:1980–1986. doi: 10.1016/j.jacc.2004.12.079. [DOI] [PubMed] [Google Scholar]
- 101.Halcox JP, Donald AE, Ellins E, Witte DR, Shipley MJ, Brunner EJ, Marmot MG, Deanfield JE. Endothelial function predicts progression of carotid intima-media thickness. Circulation. 2009;119:1005–1012. doi: 10.1161/CIRCULATIONAHA.108.765701. [DOI] [PubMed] [Google Scholar]
- 102.Rossi R, Nuzzo A, Olaru AI, Origliani G, Modena MG. Endothelial function affects early carotid atherosclerosis progression in hypertensive postmenopausal women. J Hypertens. 2011;29:1136–1144. doi: 10.1097/HJH.0b013e328345d950. [DOI] [PubMed] [Google Scholar]
- 103.Charakida M, Donald AE, Leary S, Halcox JP, Turner MW, Johnson M, Loukogeorgakis SP, Okorie MI, Davey Smith G, Deanfield JE, Klein NJ. Endothelial response to childhood infection: the role of mannose-binding lectin (MBL) Atherosclerosis. 2010;208:217–221. doi: 10.1016/j.atherosclerosis.2009.07.055. [DOI] [PubMed] [Google Scholar]
- 104.Clapp BR, Hirschfield GM, Storry C, Gallimore JR, Stidwill RP, Singer M, Deanfield JE, MacAllister RJ, Pepys MB, Vallance P, Hingorani AD. Inflammation and endothelial function: direct vascular effects of human C-reactive protein on nitric oxide bioavailability. Circulation. 2005;111:1530–1536. doi: 10.1161/01.CIR.0000159336.31613.31. [DOI] [PubMed] [Google Scholar]
- 105.Dawson EA, Whyte GP, Black MA, Jones H, Hopkins N, Oxborough D, Gaze D, Shave RE, Wilson M, George KP, Green DJ. Changes in vascular and cardiac function after prolonged strenuous exercise in humans. J Appl Physiol. 2008;105:1562–1568. doi: 10.1152/japplphysiol.90837.2008. [DOI] [PubMed] [Google Scholar]
- 106.Plotnick GD, Corretti MC, Vogel RA. Effect of antioxidant vitamins on the transient impairment of endothelium-dependent brachial artery vasoactivity following a single high-fat meal. Jama. 1997;278:1682–1686. [PubMed] [Google Scholar]
- 107.Halcox JP, Schenke WH, Zalos G, Mincemoyer R, Prasad A, Waclawiw MA, Nour KR, Quyyumi AA. Prognostic value of coronary vascular endothelial dysfunction. Circulation. 2002;106:653–658. doi: 10.1161/01.cir.0000025404.78001.d8. [DOI] [PubMed] [Google Scholar]
- 108.Schindler TH, Hornig B, Buser PT, Olschewski M, Magosaki N, Pfisterer M, Nitzsche EU, Solzbach U, Just H. Prognostic value of abnormal vasoreactivity of epicardial coronary arteries to sympathetic stimulation in patients with normal coronary angiograms. Arteriosclerosis, thrombosis, and vascular biology. 2003;23:495–501. doi: 10.1161/01.ATV.0000057571.03012.F4. [DOI] [PubMed] [Google Scholar]
- 109.Gokce N, Keaney JF, Jr, Hunter LM, Watkins MT, Menzoian JO, Vita JA. Risk stratification for postoperative cardiovascular events via noninvasive assessment of endothelial function: a prospective study. Circulation. 2002;105:1567–1572. doi: 10.1161/01.cir.0000012543.55874.47. [DOI] [PubMed] [Google Scholar]
- 110.Rossi R, Chiurlia E, Nuzzo A, Cioni E, Origliani G, Modena MG. Flow-mediated vasodilation and the risk of developing hypertension in healthy postmenopausal women. Journal of the American College of Cardiology. 2004;44:1636–1640. doi: 10.1016/j.jacc.2004.07.027. [DOI] [PubMed] [Google Scholar]
- 111.Neunteufl T, Heher S, Katzenschlager R, Wolfl G, Kostner K, Maurer G, Weidinger F. Late prognostic value of flow-mediated dilation in the brachial artery of patients with chest pain. The American journal of cardiology. 2000;86:207–210. doi: 10.1016/s0002-9149(00)00857-2. [DOI] [PubMed] [Google Scholar]
- 112.Shechter M, Issachar A, Marai I, Koren-Morag N, Freinark D, Shahar Y, Shechter A, Feinberg MS. Long-term association of brachial artery flow-mediated vasodilation and cardiovascular events in middle-aged subjects with no apparent heart disease. Int J Cardiol. 2009;134:52–58. doi: 10.1016/j.ijcard.2008.01.021. [DOI] [PubMed] [Google Scholar]
- 113.Hirsch L, Shechter A, Feinberg MS, Koren-Morag N, Shechter M. The impact of early compared to late morning hours on brachial endothelial function and long-term cardiovascular events in healthy subjects with no apparent coronary heart disease. Int J Cardiol. 2011;151:342–347. doi: 10.1016/j.ijcard.2010.08.069. [DOI] [PubMed] [Google Scholar]
- 114.Rossi R, Nuzzo A, Origliani G, Modena MG. Prognostic role of flow-mediated dilation and cardiac risk factors in post-menopausal women. Journal of the American College of Cardiology. 2008;51:997–1002. doi: 10.1016/j.jacc.2007.11.044. [DOI] [PubMed] [Google Scholar]
- 115.Green DJ. Exercise training as vascular medicine: direct impacts on the vasculature in humans. Exerc Sport Sci Rev. 2009;37:196–202. doi: 10.1097/JES.0b013e3181b7b6e3. [DOI] [PubMed] [Google Scholar]
- 116.Lind L, Berglund L, Larsson A, Sundstrom J. Endothelial function in resistance and conduit arteries and 5-year risk of cardiovascular disease. Circulation. 2011;123:1545–1551. doi: 10.1161/CIRCULATIONAHA.110.984047. [DOI] [PubMed] [Google Scholar]
- 117.Matsuzawa Y, Sugiyama S, Sugamura K, Nozaki T, Ohba K, Konishi M, Matsubara J, Sumida H, Kaikita K, Kojima S, Nagayoshi Y, Yamamuro M, Izumiya Y, Iwashita S, Matsui K, Jinnouchi H, Kimura K, Umemura S, Ogawa H. Digital assessment of endothelial function and ischemic heart disease in women. Journal of the American College of Cardiology. 2010;55:1688–1696. doi: 10.1016/j.jacc.2009.10.073. [DOI] [PubMed] [Google Scholar]
- 118.Graham I, Atar D, Borch-Johnsen K, Boysen G, Burell G, Cifkova R, Dallongeville J, De Backer G, Ebrahim S, Gjelsvik B, Herrmann-Lingen C, Hoes A, Humphries S, Knapton M, Perk J, Priori SG, Pyorala K, Reiner Z, Ruilope L, Sans-Menendez S, Op Reimer WS, Weissberg P, Wood D, Yarnell J, Zamorano JL, Walma E, Fitzgerald T, Cooney MT, Dudina A, Vahanian A, Camm J, De Caterina R, Dean V, Dickstein K, Funck-Brentano C, Filippatos G, Hellemans I, Kristensen SD, McGregor K, Sechtem U, Silber S, Tendera M, Widimsky P, Zamorano JL, Altiner A, Bonora E, Durrington PN, Fagard R, Giampaoli S, Hemingway H, Hakansson J, Kjeldsen SE, Larsen L, Mancia G, Manolis AJ, Orth-Gomer K, Pedersen T, Rayner M, Ryden L, Sammut M, Schneiderman N, Stalenhoef AF, Tokgozoglu L, Wiklund O, Zampelas A. European guidelines on cardiovascular disease prevention in clinical practice: full text. Fourth Joint Task Force of the European Society of Cardiology and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of nine societies and by invited experts) Eur J Cardiovasc Prev Rehabil. 2007;14 (Suppl 2):S1–113. doi: 10.1097/01.hjr.0000277983.23934.c9. [DOI] [PubMed] [Google Scholar]
- 119.Greenland P, Alpert JS, Beller GA, Benjamin EJ, Budoff MJ, Fayad ZA, Foster E, Hlatky MA, Hodgson JM, Kushner FG, Lauer MS, Shaw LJ, Smith SC, Jr, Taylor AJ, Weintraub WS, Wenger NK, Jacobs AK. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2010;122:e584–636. doi: 10.1161/CIR.0b013e3182051b4c. [DOI] [PubMed] [Google Scholar]
- 120.Greenland P, Alpert JS, Beller GA, Benjamin EJ, Budoff MJ, Fayad ZA, Foster E, Hlatky MA, Hodgson JM, Kushner FG, Lauer MS, Shaw LJ, Smith SC, Jr, Taylor AJ, Weintraub WS, Wenger NK, Jacobs AK, Smith SC, Jr, Anderson JL, Albert N, Buller CE, Creager MA, Ettinger SM, Guyton RA, Halperin JL, Hochman JS, Kushner FG, Nishimura R, Ohman EM, Page RL, Stevenson WG, Tarkington LG, Yancy CW. ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Journal of the American College of Cardiology. 2010;56:e50–103. doi: 10.1016/j.jacc.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 121.Schachinger V, Britten MB, Zeiher AM. Prognostic impact of coronary vasodilator dysfunction on adverse long-term outcome of coronary heart disease. Circulation. 2000;101:1899–1906. doi: 10.1161/01.cir.101.16.1899. [DOI] [PubMed] [Google Scholar]
- 122.Suwaidi JA, Hamasaki S, Higano ST, Nishimura RA, Holmes DR, Jr, Lerman A. Long-term follow-up of patients with mild coronary artery disease and endothelial dysfunction. Circulation. 2000;101:948–954. doi: 10.1161/01.cir.101.9.948. [DOI] [PubMed] [Google Scholar]
- 123.Targonski PV, Bonetti PO, Pumper GM, Higano ST, Holmes DR, Jr, Lerman A. Coronary endothelial dysfunction is associated with an increased risk of cerebrovascular events. Circulation. 2003;107:2805–2809. doi: 10.1161/01.CIR.0000072765.93106.EE. [DOI] [PubMed] [Google Scholar]
- 124.Chan SY, Mancini GB, Kuramoto L, Schulzer M, Frohlich J, Ignaszewski A. The prognostic importance of endothelial dysfunction and carotid atheroma burden in patients with coronary artery disease. Journal of the American College of Cardiology. 2003;42:1037–1043. doi: 10.1016/s0735-1097(03)00927-6. [DOI] [PubMed] [Google Scholar]
- 125.Heitzer T, Schlinzig T, Krohn K, Meinertz T, Munzel T. Endothelial dysfunction, oxidative stress, and risk of cardiovascular events in patients with coronary artery disease. Circulation. 2001;104:2673–2678. doi: 10.1161/hc4601.099485. [DOI] [PubMed] [Google Scholar]
- 126.Fichtlscherer S, Breuer S, Zeiher AM. Prognostic value of systemic endothelial dysfunction in patients with acute coronary syndromes: further evidence for the existence of the “vulnerable” patient. Circulation. 2004;110:1926–1932. doi: 10.1161/01.CIR.0000143378.58099.8C. [DOI] [PubMed] [Google Scholar]
- 127.Lerman A, Zeiher AM. Endothelial function: cardiac events. Circulation. 2005;111:363–368. doi: 10.1161/01.CIR.0000153339.27064.14. [DOI] [PubMed] [Google Scholar]
- 128.Patti G, Pasceri V, Melfi R, Goffredo C, Chello M, D’Ambrosio A, Montesanti R, Di Sciascio G. Impaired flow-mediated dilation and risk of restenosis in patients undergoing coronary stent implantation. Circulation. 2005;111:70–75. doi: 10.1161/01.CIR.0000151308.06673.D2. [DOI] [PubMed] [Google Scholar]
- 129.Shechter M, Matetzky S, Arad M, Feinberg MS, Freimark D. Vascular endothelial function predicts mortality risk in patients with advanced ischaemic chronic heart failure. Eur J Heart Fail. 2009;11:588–593. doi: 10.1093/eurjhf/hfp053. [DOI] [PubMed] [Google Scholar]
- 130.Davis SF, Yeung AC, Meredith IT, Charbonneau F, Ganz P, Selwyn AP, Anderson TJ. Early endothelial dysfunction predicts the development of transplant coronary artery disease at 1 year posttransplant. Circulation. 1996;93:457–462. doi: 10.1161/01.cir.93.3.457. [DOI] [PubMed] [Google Scholar]
- 131.Kubrich M, Petrakopoulou P, Kofler S, Nickel T, Kaczmarek I, Meiser BM, Reichart B, von Scheidt W, Weis M. Impact of coronary endothelial dysfunction on adverse long-term outcome after heart transplantation. Transplantation. 2008;85:1580–1587. doi: 10.1097/TP.0b013e318170b4cd. [DOI] [PubMed] [Google Scholar]
- 132.Hollenberg SM, Klein LW, Parrillo JE, Scherer M, Burns D, Tamburro P, Oberoi M, Johnson MR, Costanzo MR. Coronary endothelial dysfunction after heart transplantation predicts allograft vasculopathy and cardiac death. Circulation. 2001;104:3091–3096. doi: 10.1161/hc5001.100796. [DOI] [PubMed] [Google Scholar]
- 133.Morishima I, Sone T, Okumura K, Tsuboi H, Kondo J, Mukawa H, Matsui H, Toki Y, Ito T, Hayakawa T. Angiographic no-reflow phenomenon as a predictor of adverse long-term outcome in patients treated with percutaneous transluminal coronary angioplasty for first acute myocardial infarction. Journal of the American College of Cardiology. 2000;36:1202–1209. doi: 10.1016/s0735-1097(00)00865-2. [DOI] [PubMed] [Google Scholar]
- 134.Sorajja P, Gersh BJ, Costantini C, McLaughlin MG, Zimetbaum P, Cox DA, Garcia E, Tcheng JE, Mehran R, Lansky AJ, Kandzari DE, Grines CL, Stone GW. Combined prognostic utility of ST-segment recovery and myocardial blush after primary percutaneous coronary intervention in acute myocardial infarction. European heart journal. 2005;26:667–674. doi: 10.1093/eurheartj/ehi167. [DOI] [PubMed] [Google Scholar]
- 135.Wu KC, Zerhouni EA, Judd RM, Lugo-Olivieri CH, Barouch LA, Schulman SP, Blumenthal RS, Lima JA. Prognostic significance of microvascular obstruction by magnetic resonance imaging in patients with acute myocardial infarction. Circulation. 1998;97:765–772. doi: 10.1161/01.cir.97.8.765. [DOI] [PubMed] [Google Scholar]
- 136.Prasad A, Stone GW, Aymong E, Zimetbaum PJ, McLaughlin M, Mehran R, Garcia E, Tcheng JE, Cox DA, Grines CL, Gersh BJ. Impact of ST-segment resolution after primary angioplasty on outcomes after myocardial infarction in elderly patients: an analysis from the CADILLAC trial. American heart journal. 2004;147:669–675. doi: 10.1016/j.ahj.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 137.Ndrepepa G, Tiroch K, Fusaro M, Keta D, Seyfarth M, Byrne RA, Pache J, Alger P, Mehilli J, Schomig A, Kastrati A. 5-year prognostic value of no-reflow phenomenon after percutaneous coronary intervention in patients with acute myocardial infarction. Journal of the American College of Cardiology. 2010;55:2383–2389. doi: 10.1016/j.jacc.2009.12.054. [DOI] [PubMed] [Google Scholar]
- 138.Galiuto L, Lombardo A, Maseri A, Santoro L, Porto I, Cianflone D, Rebuzzi AG, Crea F. Temporal evolution and functional outcome of no reflow: sustained and spontaneously reversible patterns following successful coronary recanalisation. Heart (British Cardiac Society) 2003;89:731–737. doi: 10.1136/heart.89.7.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lerman A, Edwards BS, Hallett JW, Heublein DM, Sandberg SM, Burnett JC., Jr Circulating and tissue endothelin immunoreactivity in advanced atherosclerosis. The New England journal of medicine. 1991;325:997–1001. doi: 10.1056/NEJM199110033251404. [DOI] [PubMed] [Google Scholar]
- 140.Stone GW, Maehara A, Lansky AJ, de Bruyne B, Cristea E, Mintz GS, Mehran R, McPherson J, Farhat N, Marso SP, Parise H, Templin B, White R, Zhang Z, Serruys PW. A prospective natural-history study of coronary atherosclerosis. The New England journal of medicine. 2011;364:226–235. doi: 10.1056/NEJMoa1002358. [DOI] [PubMed] [Google Scholar]
- 141.Hasdai D, Gibbons RJ, Holmes DR, Jr, Higano ST, Lerman A. Coronary endothelial dysfunction in humans is associated with myocardial perfusion defects. Circulation. 1997;96:3390–3395. doi: 10.1161/01.cir.96.10.3390. [DOI] [PubMed] [Google Scholar]
- 142.Lerman A, Holmes DR, Herrmann J, Gersh BJ. Microcirculatory dysfunction in ST-elevation myocardial infarction: cause, consequence, or both? European heart journal. 2007;28:788–797. doi: 10.1093/eurheartj/ehl501. [DOI] [PubMed] [Google Scholar]
- 143.Angeja BG, de Lemos J, Murphy SA, Marble SJ, Antman EM, Cannon CP, Braunwald E, Gibson CM. Impact of diabetes mellitus on epicardial and microvascular flow after fibrinolytic therapy. American heart journal. 2002;144:649–656. doi: 10.1067/mhj.2002.124869. [DOI] [PubMed] [Google Scholar]
- 144.Kurisu S, Inoue I, Kawagoe T, Ishihara M, Shimatani Y, Nishioka K, Umemura T, Nakamura S, Yoshida M. Diabetes mellitus is associated with insufficient microvascular reperfusion following revascularization for anterior acute myocardial infarction. Intern Med. 2003;42:554–559. doi: 10.2169/internalmedicine.42.554. [DOI] [PubMed] [Google Scholar]
- 145.Celik T, Turhan H, Kursaklioglu H, Iyisoy A, Yuksel UC, Ozmen N, Isik E. Impact of metabolic syndrome on myocardial perfusion grade after primary percutaneous coronary intervention in patients with acute ST elevation myocardial infarction. Coronary artery disease. 2006;17:339–343. doi: 10.1097/00019501-200606000-00003. [DOI] [PubMed] [Google Scholar]
- 146.Clavijo LC, Pinto TL, Kuchulakanti PK, Torguson R, Chu WW, Satler LF, Kent KM, Suddath WO, Pichard AD, Waksman R. Metabolic syndrome in patients with acute myocardial infarction is associated with increased infarct size and inhospital complications. Cardiovasc Revasc Med. 2006;7:7–11. doi: 10.1016/j.carrev.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 147.Albertal M, Voskuil M, Piek JJ, de Bruyne B, Van Langenhove G, Kay PI, Costa MA, Boersma E, Beijsterveldt T, Sousa JE, Belardi JA, Serruys PW. Coronary flow velocity reserve after percutaneous interventions is predictive of periprocedural outcome. Circulation. 2002;105:1573–1578. doi: 10.1161/01.cir.0000012514.15806.dd. [DOI] [PubMed] [Google Scholar]
- 148.Reriani MK, Dunlay SM, Gupta B, West CP, Rihal CS, Lerman LO, Lerman A. Effects of statins on coronary and peripheral endothelial function in humans: a systematic review and meta-analysis of randomized controlled trials. Eur J Cardiovasc Prev Rehabil. 2011 doi: 10.1177/1741826711398430. [DOI] [PubMed] [Google Scholar]
- 149.Effect of nifedipine and cerivastatin on coronary endothelial function in patients with coronary artery disease: the ENCORE I Study (Evaluation of Nifedipine and Cerivastatin On Recovery of coronary Endothelial function) Circulation. 2003;107:422–428. doi: 10.1161/01.cir.0000046488.52939.bf. [DOI] [PubMed] [Google Scholar]
- 150.Vita JA, Yeung AC, Winniford M, Hodgson JM, Treasure CB, Klein JL, Werns S, Kern M, Plotkin D, Shih WJ, Mitchel Y, Ganz P. Effect of cholesterol-lowering therapy on coronary endothelial vasomotor function in patients with coronary artery disease. Circulation. 2000;102:846–851. doi: 10.1161/01.cir.102.8.846. [DOI] [PubMed] [Google Scholar]
- 151.Kitta Y, Obata JE, Nakamura T, Hirano M, Kodama Y, Fujioka D, Saito Y, Kawabata K, Sano K, Kobayashi T, Yano T, Nakamura K, Kugiyama K. Persistent impairment of endothelial vasomotor function has a negative impact on outcome in patients with coronary artery disease. Journal of the American College of Cardiology. 2009;53:323–330. doi: 10.1016/j.jacc.2008.08.074. [DOI] [PubMed] [Google Scholar]
- 152.Modena MG, Bonetti L, Coppi F, Bursi F, Rossi R. Prognostic role of reversible endothelial dysfunction in hypertensive postmenopausal women. Journal of the American College of Cardiology. 2002;40:505–510. doi: 10.1016/s0735-1097(02)01976-9. [DOI] [PubMed] [Google Scholar]
- 153.Ganz P, Hsue PY. Individualized approach to the management of coronary heart disease: identifying the nonresponders before it is too late. Journal of the American College of Cardiology. 2009;53:331–333. doi: 10.1016/j.jacc.2008.10.021. [DOI] [PubMed] [Google Scholar]
- 154.Anderson TJ, Meredith IT, Yeung AC, Frei B, Selwyn AP, Ganz P. The effect of cholesterol-lowering and antioxidant therapy on endothelium-dependent coronary vasomotion. The New England journal of medicine. 1995;332:488–493. doi: 10.1056/NEJM199502233320802. [DOI] [PubMed] [Google Scholar]
- 155.Treasure CB, Klein JL, Weintraub WS, Talley JD, Stillabower ME, Kosinski AS, Zhang J, Boccuzzi SJ, Cedarholm JC, Alexander RW. Beneficial effects of cholesterol-lowering therapy on the coronary endothelium in patients with coronary artery disease. The New England journal of medicine. 1995;332:481–487. doi: 10.1056/NEJM199502233320801. [DOI] [PubMed] [Google Scholar]
- 156.Bonetti PO, Lerman LO, Napoli C, Lerman A. Statin effects beyond lipid lowering--are they clinically relevant? European heart journal. 2003;24:225–248. doi: 10.1016/s0195-668x(02)00419-0. [DOI] [PubMed] [Google Scholar]
- 157.Lüscher TF, Vanhoutte PM, Raij L. Antihypertensive treatment normalizes decreased endothelium-dependent relaxations in rats with salt-induced hypertension. Hypertension. 1987;9 (Suppl III):193–197. doi: 10.1161/01.hyp.9.6_pt_2.iii193. [DOI] [PubMed] [Google Scholar]
- 158.Taddei S, Virdis A, Ghiadoni L, Sudano I, Salvetti A. Effects of antihypertensive drugs on endothelial dysfunction: clinical implications. Drugs. 2002;62:265–284. doi: 10.2165/00003495-200262020-00003. [DOI] [PubMed] [Google Scholar]
- 159.Taddei S, Virdis A, Ghiadoni L, Mattei P, Salvetti A. Effects of angiotensin converting enzyme inhibition on endothelium- dependent vasodilatation in essential hypertensive patients. J Hypertens. 1998;16:447–456. doi: 10.1097/00004872-199816040-00006. [DOI] [PubMed] [Google Scholar]
- 160.Creager MA, Roddy M-A, Coleman SM, Dzau VJ. The effect of ACE inhibition on endothelium-dependent vasodilation in hypertension. J Vasc Res. 1992;29:97. [Google Scholar]
- 161.Flammer AJ, Hermann F, Wiesli P, Schwegler B, Chenevard R, Hurlimann D, Sudano I, Gay S, Neidhart M, Riesen W, Ruschitzka F, Luscher TF, Noll G, Lehmann R. Effect of losartan, compared with atenolol, on endothelial function and oxidative stress in patients with type 2 diabetes and hypertension. J Hypertens. 2007;25:785–791. doi: 10.1097/HJH.0b013e3280287a72. [DOI] [PubMed] [Google Scholar]
- 162.Taddei S, Virdis A, Ghiadoni L, Uleri S, Magagna A, Salvetti A. Lacidipine restores endothelium-dependent vasodilation in essential hypertensive patients. Hypertension. 1997;30:1606–1612. doi: 10.1161/01.hyp.30.6.1606. [DOI] [PubMed] [Google Scholar]
- 163.Prisant LM. Nebivolol: pharmacologic profile of an ultraselective, vasodilatory beta1-blocker. J Clin Pharmacol. 2008;48:225–239. doi: 10.1177/0091270007310378. [DOI] [PubMed] [Google Scholar]
- 164.Mancini GB, Henry GC, Macaya C, O’Neill BJ, Pucillo AL, Carere RG, Wargovich TJ, Mudra H, Luscher TF, Klibaner MI, Haber HE, Uprichard AC, Pepine CJ, Pitt B. Angiotensin-converting enzyme inhibition with quinapril improves endothelial vasomotor dysfunction in patients with coronary artery disease. The TREND (Trial on Reversing ENdothelial Dysfunction) Study. Circulation. 1996;94:258–265. doi: 10.1161/01.cir.94.3.258. [DOI] [PubMed] [Google Scholar]
- 165.Chen JW, Hsu NW, Wu TC, Lin SJ, Chang MS. Long-term angiotensin-converting enzyme inhibition reduces plasma asymmetric dimethylarginine and improves endothelial nitric oxide bioavailability and coronary microvascular function in patients with syndrome X. The American journal of cardiology. 2002;90:974–982. doi: 10.1016/s0002-9149(02)02664-4. [DOI] [PubMed] [Google Scholar]
- 166.Tang EH, Vanhoutte PM. Endothelial dysfunction: a strategic target in the treatment of hypertension? Pflugers Arch. 459:995–1004. doi: 10.1007/s00424-010-0786-4. [DOI] [PubMed] [Google Scholar]
- 167.Luscher TF, Pieper M, Tendera M, Vrolix M, Rutsch W, van den Branden F, Gil R, Bischoff KO, Haude M, Fischer D, Meinertz T, Munzel T. A randomized placebo-controlled study on the effect of nifedipine on coronary endothelial function and plaque formation in patients with coronary artery disease: the ENCORE II study. European heart journal. 2009;30:1590–1597. doi: 10.1093/eurheartj/ehp151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Tabit CE, Chung WB, Hamburg NM, Vita JA. Endothelial dysfunction in diabetes mellitus: molecular mechanisms and clinical implications. Rev Endocr Metab Disord. 2010;11:61–74. doi: 10.1007/s11154-010-9134-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Meaney E, Vela A, Samaniego V, Meaney A, Asbun J, Zempoalteca JC, Elisa ZN, Emma MN, Guzman M, Hicks J, Ceballos G. Metformin, arterial function, intima-media thickness and nitroxidation in metabolic syndrome: the mefisto study. Clin Exp Pharmacol Physiol. 2008;35:895–903. doi: 10.1111/j.1440-1681.2008.04920.x. [DOI] [PubMed] [Google Scholar]
- 170.Hanefeld M, Marx N, Pfutzner A, Baurecht W, Lubben G, Karagiannis E, Stier U, Forst T. Anti-inflammatory effects of pioglitazone and/or simvastatin in high cardiovascular risk patients with elevated high sensitivity C-reactive protein: the PIOSTAT Study. Journal of the American College of Cardiology. 2007;49:290–297. doi: 10.1016/j.jacc.2006.08.054. [DOI] [PubMed] [Google Scholar]
- 171.Esposito K, Ciotola M, Carleo D, Schisano B, Saccomanno F, Sasso FC, Cozzolino D, Assaloni R, Merante D, Ceriello A, Giugliano D. Effect of rosiglitazone on endothelial function and inflammatory markers in patients with the metabolic syndrome. Diabetes care. 2006;29:1071–1076. doi: 10.2337/diacare.2951071. [DOI] [PubMed] [Google Scholar]
- 172.Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. The New England journal of medicine. 2007;356:2457–2471. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- 173.Hambrecht R, Wolf A, Gielen S, Linke A, Hofer J, Erbs S, Schoene N, Schuler G. Effect of exercise on coronary endothelial function in patients with coronary artery disease. The New England journal of medicine. 2000;342:454–460. doi: 10.1056/NEJM200002173420702. [DOI] [PubMed] [Google Scholar]
- 174.Clarkson P, Montgomery HE, Mullen MJ, Donald AE, Powe AJ, Bull T, Jubb M, World M, Deanfield JE. Exercise training enhances endothelial function in young men. Journal of the American College of Cardiology. 1999;33:1379–1385. doi: 10.1016/s0735-1097(99)00036-4. [DOI] [PubMed] [Google Scholar]
- 175.Hambrecht R, Fiehn E, Weigl C, Gielen S, Hamann C, Kaiser R, Yu J, Adams V, Niebauer J, Schuler G. Regular physical exercise corrects endothelial dysfunction and improves exercise capacity in patients with chronic heart failure. Circulation. 1998;98:2709–2715. doi: 10.1161/01.cir.98.24.2709. [DOI] [PubMed] [Google Scholar]
- 176.Dod HS, Bhardwaj R, Sajja V, Weidner G, Hobbs GR, Konat GW, Manivannan S, Gharib W, Warden BE, Nanda NC, Beto RJ, Ornish D, Jain AC. Effect of intensive lifestyle changes on endothelial function and on inflammatory markers of atherosclerosis. The American journal of cardiology. 2010;105:362–367. doi: 10.1016/j.amjcard.2009.09.038. [DOI] [PubMed] [Google Scholar]
- 177.Meyer AA, Kundt G, Lenschow U, Schuff-Werner P, Kienast W. Improvement of early vascular changes and cardiovascular risk factors in obese children after a six-month exercise program. Journal of the American College of Cardiology. 2006;48:1865–1870. doi: 10.1016/j.jacc.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 178.Shechter M, Beigel R, Freimark D, Matetzky S, Feinberg MS. Short-term sibutramine therapy is associated with weight loss and improved endothelial function in obese patients with coronary artery disease. The American journal of cardiology. 2006;97:1650–1653. doi: 10.1016/j.amjcard.2005.12.059. [DOI] [PubMed] [Google Scholar]
- 179.Sturm W, Tschoner A, Engl J, Kaser S, Laimer M, Ciardi C, Klaus A, Weiss H, Sandhofer A, Patsch JR, Ebenbichler CF. Effect of bariatric surgery on both functional and structural measures of premature atherosclerosis. European heart journal. 2009;30:2038–2043. doi: 10.1093/eurheartj/ehp211. [DOI] [PubMed] [Google Scholar]
- 180.Gokce N, Vita JA, McDonnell M, Forse AR, Istfan N, Stoeckl M, Lipinska I, Keaney JF, Jr, Apovian CM. Effect of medical and surgical weight loss on endothelial vasomotor function in obese patients. The American journal of cardiology. 2005;95:266–268. doi: 10.1016/j.amjcard.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 181.Bonetti PO, Barsness GW, Keelan PC, Schnell TI, Pumper GM, Kuvin JT, Schnall RP, Holmes DR, Higano ST, Lerman A. Enhanced external counterpulsation improves endothelial function in patients with symptomatic coronary artery disease. Journal of the American College of Cardiology. 2003;41:1761–1768. doi: 10.1016/s0735-1097(03)00329-2. [DOI] [PubMed] [Google Scholar]
- 182.Shechter M, Matetzky S, Feinberg MS, Chouraqui P, Rotstein Z, Hod H. External counterpulsation therapy improves endothelial function in patients with refractory angina pectoris. Journal of the American College of Cardiology. 2003;42:2090–2095. doi: 10.1016/j.jacc.2003.05.013. [DOI] [PubMed] [Google Scholar]
- 183.Corti R, Flammer AJ, Hollenberg NK, Luscher TF. Cocoa and cardiovascular health. Circulation. 2009;119:1433–1441. doi: 10.1161/CIRCULATIONAHA.108.827022. [DOI] [PubMed] [Google Scholar]
- 184.Flammer AJ, Hermann F, Sudano I, Spieker L, Hermann M, Cooper KA, Serafini M, Luscher TF, Ruschitzka F, Noll G, Corti R. Dark chocolate improves coronary vasomotion and reduces platelet reactivity. Circulation. 2007;116:2376–2382. doi: 10.1161/CIRCULATIONAHA.107.713867. [DOI] [PubMed] [Google Scholar]
- 185.Sudano I, Spieker LE, Hermann F, Flammer A, Corti R, Noll G, Luscher TF. Protection of endothelial function: targets for nutritional and pharmacological interventions. J Cardiovasc Pharmacol. 2006;47(Suppl 2):S136–150. doi: 10.1097/00005344-200606001-00008. discussion S172-136. [DOI] [PubMed] [Google Scholar]
- 186.Lavi S, Prasad A, Yang EH, Mathew V, Simari RD, Rihal CS, Lerman LO, Lerman A. Smoking is associated with epicardial coronary endothelial dysfunction and elevated white blood cell count in patients with chest pain and early coronary artery disease. Circulation. 2007;115:2621–2627. doi: 10.1161/CIRCULATIONAHA.106.641654. [DOI] [PubMed] [Google Scholar]
- 187.Taddei S, Virdis A, Ghiadoni L, Magagna A, Salvetti A. Vitamin C improves endothelium-dependent vasodilation by restoring nitric oxide activity in essential hypertension. Circulation. 1998;97:2222–2229. doi: 10.1161/01.cir.97.22.2222. [DOI] [PubMed] [Google Scholar]
- 188.Kinlay S, Behrendt D, Fang JC, Delagrange D, Morrow J, Witztum JL, Rifai N, Selwyn AP, Creager MA, Ganz P. Long-term effect of combined vitamins E and C on coronary and peripheral endothelial function. Journal of the American College of Cardiology. 2004;43:629–634. doi: 10.1016/j.jacc.2003.08.051. [DOI] [PubMed] [Google Scholar]
- 189.Sherman DL, Keaney JF, Jr, Biegelsen ES, Duffy SJ, Coffman JD, Vita JA. Pharmacological concentrations of ascorbic acid are required for the beneficial effect on endothelial vasomotor function in hypertension. Hypertension. 2000;35:936–941. doi: 10.1161/01.hyp.35.4.936. [DOI] [PubMed] [Google Scholar]
- 190.Versari D, Daghini E, Rodriguez-Porcel M, Sattler K, Galili O, Pilarczyk K, Napoli C, Lerman LO, Lerman A. Chronic antioxidant supplementation impairs coronary endothelial function and myocardial perfusion in normal pigs. Hypertension. 2006;47:475–481. doi: 10.1161/01.HYP.0000201445.77125.26. [DOI] [PubMed] [Google Scholar]
- 191.Trochu JN, Bouhour JB, Kaley G, Hintze TH. Role of endothelium-derived nitric oxide in the regulation of cardiac oxygen metabolism: implications in health and disease. Circulation research. 2000;87:1108–1117. doi: 10.1161/01.res.87.12.1108. [DOI] [PubMed] [Google Scholar]
- 192.Charakida M, Masi S, Loukogeorgakis SP, Deanfield JE. The role of flow-mediated dilatation in the evaluation and development of antiatherosclerotic drugs. Curr Opin Lipidol. 2009;20:460–466. doi: 10.1097/MOL.0b013e3283330518. [DOI] [PubMed] [Google Scholar]
- 193.Luscher TF, Taddei S, Kaski JC, Jukema JW, Kallend D, Munzel T, Kastelein JJ, Deanfield JE. Vascular effects and safety of dalcetrapib in patients with or at risk of coronary heart disease: the dal-VESSEL randomized clinical trial. European heart journal. 2012 doi: 10.1093/eurheartj/ehs019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Fayad ZA, Mani V, Woodward M, Kallend D, Abt M, Burgess T, Fuster V, Ballantyne CM, Stein EA, Tardif JC, Rudd JH, Farkouh ME, Tawakol A. Safety and efficacy of dalcetrapib on atherosclerotic disease using novel non-invasive multimodality imaging (dal-PLAQUE): a randomised clinical trial. Lancet. 2011 doi: 10.1016/S0140-6736(11)61383-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Brindle P, Emberson J, Lampe F, Walker M, Whincup P, Fahey T, Ebrahim S. Predictive accuracy of the Framingham coronary risk score in British men: prospective cohort study. Bmj. 2003;327:1267. doi: 10.1136/bmj.327.7426.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]