Abstract
During Pregnancy, heart develops physiological left ventricular hypertrophy as a result of the natural volume overload. Previously we have characterized the molecular and functional signature of heart hypertrophy during pregnancy. Cardiac hypertrophy during pregnancy is a complex process that involves many changes including in the signalling pathways, composition of extracellular matrix as well as the levels of sex hormones. This review summarises the recent advances and the new frontiers in the context of heart hypertrophy during pregnancy. In particular we focus on structural and extracellular matrix remodelling as well as signalling pathways in pregnancy-induced physiological heart hypertrophy. Emerging evidence shows that various microRNAs modulate key components of hypertrophy, therefore the role of microRNAs in the regulation of gene expression in pregnancy induced hypertrophy is also discussed. We also review the role of ubiquitin proteasome system, the major machinery for the degradation of damaged and misfolded proteins, in heart hypertrophy. The role of sex hormones in particular estrogen in cardiac remodeling during pregnancy is also discussed. We also review pregnancy-induced cardiovascular complications such as peripartum cardiomyopathy and pre-eclampsia and how the knowledge from the animal studies may help us to develop new therapeutic strategies for better treatment of cardiovascular diseases during pregnancy. Special emphasis has to be given to the guidelines on disease management in pregnancy.
Keywords: Pregnancy, physiological heart hypertrophy, signalling pathway, peripartum cardiomyopathy, preeclampsia
Pregnancy-induced physiological heart hypertrophy
Cardiac hypertrophy, defined as an enlargement of the ventricles, is an important compensatory response so the heart can maintain its pumping capacity. Cardiac hypertrophy is often triggered when the heart is subjected to hemodynamic stress from volume or pressure overload [1]. Sustained pressure overload often leads to concentric hypertrophy, which is characterized by increased wall thickness without a concomitant chamber enlargement. Volume overload, on the other hand leads to eccentric hypertrophy characterized by a proportional enlargement of the chamber size and the wall thickness [2]. Heart hypertrophy can be physiological which is beneficial and adaptive or pathological which is maladaptive and detrimental (Figure 1). Pathological heart hypertrophy often leads to heart failure if the stimulus persists for a long time. Heart failure is a clinical syndrome attributable to many factors, which begins as a compensatory response to hypertrophic stimuli, followed by a decompensatory response which eventually results in failure. Physiological hypertrophy which occurs, in response to normal exercise or pregnancy, is not associated with fibrosis, dysfunction, or increased morbidity and mortality. Physiological hypertrophy enables the heart to fulfill its function, and is often reversible without significant long-term detrimental effects on cardiac function [3-5]. In these aspects, pregnancy-and exercise-induced hypertrophies are similar. However, pregnancy is also accompanied by drastic hormonal changes. Both estrogen and testosterone steadily increase and reach their maximum levels at the end of pregnancy. In addition during pregnancy, unlike exercise, the force demand placed on the heart is continuous as opposed to sporadic. Still little is known about the molecular mechanisms that mediate pregnancy-induced hypertrophy, but recent reports have shown that its molecular signature is unique and differs from that induced by exercise [6].
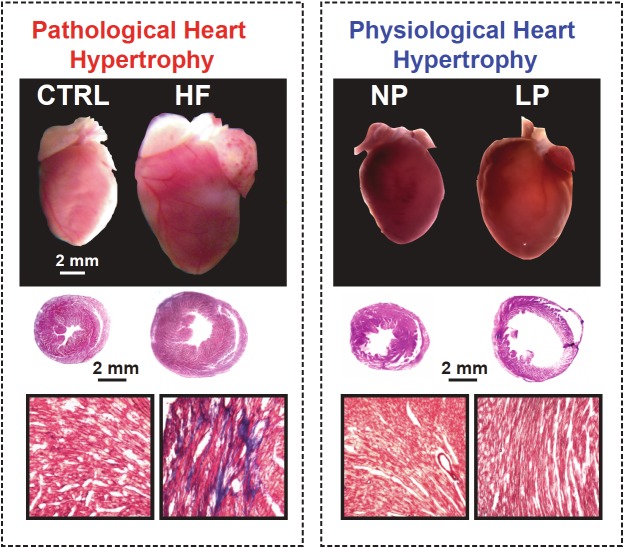
Figure 1.
Pathological hypertrophy vs pregnancy induced physiological hypertrophy. Top panels: Images of the whole heart in pathological hypertrophy and physiological hypertrophy in late pregnancy in mice; Middle panels: Hematoxylin-eosin staining of heart cross-sections; Bottom panels: Masson trichrome staining of heart cross sections, blue colour indicates fibrosis. CTRL: control; HF: heart failure; NP: non-pregnant; LP: late pregnant.
Changes in cardiovascular system during pregnancy
During pregnancy, there is a global increase in the metabolic demand, and changes in the cardiovascular system must occur to meet these needs. Increased blood volume, increased heart rate, together with reduced systemic vascular resistance all lead to increased cardiac output (Table 1). Although pregnancy is associated with hypervolemia partly due to increased retention of water and sodium, the blood pressure is decreased, the renin-angiotensin system is activated and the circulating levels of Angiotensin-II are also increased as the pregnancy progresses [7-9]. The healthy women without heart disease adapt well to these drastic changes that occur during pregnancy. These changes in hemodynamic parameters slowly return to normal values in postpartum period, but complete resolution may take as long as 6 months after delivery.
Table 1.
Hemodynamic and hormonal changes in pregnancy, peripartum and post partum
| Pregnancy | Peripartum | Postpartum | |
|---|---|---|---|
| Human | |||
| Blood volume | ↑[123] | ↑[123] | ↓[123] |
| Systolic blood pressure | ↓[123] | ↑[123] | ↑[123] |
| Diastolic blood pressure | ↓[123] | ↑[123] | ↑[123] |
| Systemic vascular resistance | ↓[123] | ↑[123] | ↑[123] |
| Heart rate | ↑[123] | ↑[123] | ↓[123] |
| Stroke volume | ↑[123] | ↑[123] | ↑[123] |
| Cardiac output | ↑[123] | ↑[123] | ↑[123] |
| Estradiol | |||
| Human | ↑ gradually[124] | ↑ before birth, ↓after birth[124] | ↓[124] |
| Rat | ↑ gradually[125] | ↑ before birth, ↓after birth[125] | ↓[125] |
| Baboon | ↑ gradually[126] | stays high[126] | ↓[126] |
| Progesterone | |||
| Human | ↑ gradually[124] | ↑ before birth, ↓after birth[124] | ↓[124] |
| Rat | ↑ gradually[125] | ↓[125] | ↓[125] |
| Baboon | ↑ gradually[126] | stays high[126] | ↓[126] |
| Testosterone | |||
| Human | ↑gradually (↑↑male fetus, ↑female fetus) [127] | unknown | ↓[128] |
| Rat | ↑ gradually[125] | ↑ up to day 20, ↓ right before birth, ↓ after birth[129] | ↓[129] |
| Baboon | ↑ gradually[126] | stays high[126] | ↓[126] |
| Prolactin | |||
| Human | ↑[130] | ↑ before birth, stays high after birth if lactating ↑[131,132] | Stays high during lactation[131,132] |
| Angiotensin | |||
| Human | ↑[9,133,134] | ↑before birth, ↓sharply 2hr after birth and ↑ afterwards [135] | Unknown |
Changes reflect the hemodynamic and hormonal status of women unless otherwise specified. In women, pregnancy refers to week 1 to 36 of gestation, peripartum refers to the last four weeks pre-partum and the first week post-partum and post-partum reflects the first 3-6 months after delivery. For rat, pregnancy reflects the first 20 days of pregnancy, peripartum refers to the last 4 days of gestation and one day after delivery, and post-partum is one week after parturition. For baboons, pregnancy refers to the first 24 weeks of pregnancy, peripartum refers to one week before delivery up to one week post-delivery and post-partum is 11 weeks after birth.
Structural and extracellular matrix remodeling of the heart during pregnancy
The extracellular matrix (ECM) is an integral part of several tissues and organ systems in the body. In the heart, fibroblasts are the predominant cell type and are primarily responsible for secreting the proteins that compose the cardiac ECM [10]. The cardiac ECM is a network of proteins that provides structural support and facilitates mechanical, electrical and chemical signals during homeostasis and in response to physiological stress or injury. The cardiac ECM maintains a dynamic homeostasis.
Integrins, collagens, fibronectins and other ECM proteins serve to anchor cells to one another and to the ECM. Matrix metalloproteinases (MMPs) and the related A Disintegrin And Metalloproteinase (ADAM) families function to degrade these ECM anchoring proteins. ADAMs and MMPs are in turn inhibited by tissue inhibitors of metalloproteinases (TIMPs). All these players function in equilibrium to maintain the cardiac ECM [10].
However, disruptions in this homeostasis, called ECM remodeling, are emerging as key processes during cardiac hypertrophy, heart failure and recovery, including dilated cardiomyopathy, myocardial infarction, and hypertensive cardiac hypertrophy [11].
Collagen
Collagen is among the most abundant proteins of the cardiac ECM and is a key structural protein responsible for anchoring cells to each other and to the ECM. Excess collagen deposition in the extracellular space, called fibrosis, is a well documented change that occurs during pathological ventricular remodeling [12]. Fibrosis normally accompanies the inflammatory response during cardiac stress and inhibits contractile function and electrical signal conduction in the heart [13]. Fibrosis also occurs in exercise- induced cardiac hypertrophy [14]. Interestingly, there have been some reports that fibrosis is minimal or absent in the overloaded pregnant heart [15,16].
Integrins
Integrins are membrane-spanning structural proteins that mediate interactions between the ECM, cardiomyocytes, and cardiac fibroblasts. Integrins have receptor function, mediate cell-cell communication in the heart, and can have diverse downstream responses [12]. This class of proteins has been found to play a vital role in cardio-protective signaling in the failing myocardium, and also in the prevention of fibrosis leading to heart failure [17,18]. Integrins also inhibit cardiomyocyte apoptosis during failure and contribute to the mechanochemical signaling which leads to hypertrophy [19]. Integrins have been reported to be downregulated in models of heart failure [17,18,20]. Their role in pregnancy has not been characterized.
Matrix Metalloproteinases (MMPs), Disintegrin Metalloproteinases (ADAMs), and Tissue Inhibitors of Metalloproteinases (TIMPs)
Matrix Metalloproteinases are a family of extracellular matrix degrading enzymes that have been implicated in causing adverse remodeling which results in ventricular dysfunction [10,12,21,22]. ADAMs are a closely related family of matrix degrading enzymes that in addition to their metalloproteinase function also have a disintegrin domain. The study of ADAMs in heart failure is a recently emerging field, and ADAMs are proving to be promoters of adverse ventricular remodeling [12,23-25].
MMPs and ADAMs are regulated in normal physiological states by a class of molecules called TIMPs. These endogenous molecules tightly regulate MMPs and ADAMs during physiological states. During pathologic ventricular remodeling, the ratio of metalloproteinases to TIMPs is increased and MMPs and ADAMs are not sufficiently regulated [11,10].
Interestingly, metalloproteinases are also regulated by estrogen [26]. The role of ADAMs and metalloproteinases in the pregnant heart has not yet been explored.
Signaling pathways in pregnancy-induced cardiac hypertrophy
Cardiac hypertrophy is regulated by several receptors and membrane proteins which activate signalling cascades of kinases, phosphatases, and other signalling pathways [27,28]. The signalling pathways involved in cardiac hypertrophy during pregnancy are complex and differ from pathological hypertrophy pathways [29,30]. Gonzalez et al. characterized several of the signalling pathways involved in pregnancy induced cardiac hypertrophy; however, much remains to be elucidated [31].
Key signalling proteins involved in the development of physiological heart hypertrophy are summarized in Table 2 and are discussed below.
Table 2.
Cardiac hypertrophy: physiological vs. pathological hypertrophy
| Physiological Hypertrophy | Pathological Hypertrophy | |||
|---|---|---|---|---|
|
| ||||
| Pregnancy | Exercise | Volume Overload | Pressure Overload | |
| ECM | ||||
| Collagen | No change[15,16] | ↑[14] | ↑[136] | ↑[137,138] |
| MMPs | Unknown | ↑[14] | ↑activity[139-141] | No change[142] |
| TIMPs | Unknown | Unchanged[14] | Unchanged[139-141] | No change[142] |
| ADAMs | Unknown | Unknown | Unknown | Unknown |
| Integrins | Unknown | Unknown | ↓[141] | ↓[17,18,20] |
| Signaling | ||||
| STAT3 | ↑[48] | No change[143] | ↑[143] | |
| ERK1/2 | No change[31] | No change[144] | ↑ activity[143], No change in activity [145] | ↑ activity[145-148], No change in activity[143] |
| JNK | ↓[31] | No change[144] | No change[145] | ↑ activity[147,145] No change[148] |
| P38 | ↓[31] | Upregulated[144] | ↑ activity[145], No change in activity [143] | ↑ activity[145-148], No change in activity[143] |
| AKT | ↓[31] | Required[33] | ↑ activity[149], No change in activity [143] | ↑ protein[150], No change in activity[143] |
| Calcineurin | Unknown | ↑[151] | No change[152] | ↑ activity[153,154], ↑ protein[150,155] |
| NFAT | Unknown | No change[154] | No change[156] | ↑ activity[18], ↑ protein[157] |
| UPS | ||||
| Ubiquitin | Unknown | Unknown | ↑ activity[93] | ↑ protein[92,94] |
| UbB(polyubiquitingene) | Unknown | ↑ mRNA[158] | ||
| 19S proteolytic subunit | Unknown | Unknown | ↑ protein[93] | ↑ RPN1 and RPN2 protein[90] |
| 20S proteolytic subunit | Unknown | Unknown | ↓ caspase and trypsin activity[93] | ↓ mRNA[95,96], ↑ protein[90,159] |
| 26S proteolytic subunit | Unknown | Unknown | ↑ activity[93] | ↓ activity[94], ↑ protein[94], ↑ mRNA of P40[90], ↑ activity[90] |
Akt and GSK3β
Akt signalling plays an important role in mediating survival pathway in cardiac hypertrophy. There are three isoforms of Akt (Akt1, Akt2 and Akt3), Although all three isoforms are broadly expressed, only Akt1 and Akt2 are highly expressed in the heart [32]. Recent studies in Akt knockout mice suggest Akt1 is required for physiological rather than pathological heart growth. Akt1 knockout mice showed a blunted hypertrophic response to swim training but displayed more hypertrophy and cardiac dysfunction in response to pressure overload [33]. It is now generally accepted that Akt1 mediates cardiac cell growth whereas Akt2 is important for cardiac metabolism [33,34]. Akt signalling is known to be upregulated in pathological heart failure as well as exercise-induced cardiac hypertrophy [33-36]. By contrast, Gonazalez et al. observe decreased phospho-Akt/Akt expression during pregnancy [31]. Akt signalling activates GSK3β downstream, which mediates anti-hypertrophic signalling in the heart [37-39]. It has been demonstrated that GSK3β negatively regulates heart growth and that inhibition of GSK3β by hypertrophic stimuli was an important mechanism for stimulating growth [40,41].The role of GSK signalling in the pregnant heart has not been characterized.
ERK 1/2
ERK1/2 mediate anti-hypertrophic signalling in the heart in response to stress. Activated phospho-ERK1/2 is increased in pathological heart hypertrophy induced by trans-aortic constriction [42]. Gonzalez et al. show that there is no change in ERK1/2 expression in the heart during pregnancy, indicating that despite its role in pathological heart hypertrophy ERK1/2 does not play a major role in pregnancy-induced cardiac hypertrophy. However, Gonzalez et al. also note a brief decrease in ERK1/2 expression immediately following pregnancy and returned to normal values by 14 days postpartum [31]. Further studies on the role of ERK in pregnancy are warranted.
P38 MAP kinases
P38 kinases are stress-activated signaling molecules involved in cardioprotection [31,43,44]. P38 is well characterized for its role in inflammation [45]. These kinases are thought to be relatively minor players in the hypertrophic process [46]. Deletion of P38 in the heart leads to increased fibrosis, apoptosis, and reduced cardiac function [47]. However, excess P38 activation also has a detrimental effect on the heart leading to pathological heart hypertrophy [43]. Gonzalez et al. showed that phospho-P38 levels decreased in late pregnancy and were normalized 14 days postpartum indicating that P38 signalling plays a role in pregnancy-induced cardiac hypertrophy [31].
STAT3
STAT3 signalling mediates hypertrophy and angiogenesis in the heart [48,49]. STAT3 is thought to be a critical factor in mediating cardiac hypertrophy under both pathological and physiological conditions [50]. STAT3 promotes anti-apoptotic signalling; its role in this process has been characterized during cancer [51-53].
STAT3 has been characterized for its role in adverse ECM remodeling and inflammation in the heart during pathological heart hypertrophy. STAT3 signalling seems to play an important role in preventing inflammation and cardiac fibrosis. Ablation of STAT3 results in higher sensitivity to inflammation, cardiac fibrosis, and heart failure with advanced age [54-56]. Hilfiker-Kleiner et al. observed increased STAT3 signaling in the heart during pregnancy [48].
Calcineurin and NFAT
Calcineurin is a calcium dependent phosphatase that activates NFAT downstream. Activation of this signaling pathway is sufficient to generate cardiac hypertrophy, and inhibiting this pathway delays the progression of pathological hypertrophy [57-60]. Nothing is known about calcineurin signaling in the heart during pregnancy.
JNK
JNK is another MAPK subfamily that plays a role in heart hypertrophy. JNK is upregulated rapidly following pressure overload, but is not activated during volume overload [61,62]. JNK overexpression leads to hypertrophy [63]. JNK seems to be necessary in maintaining cardiac contractility and preventing failure during mechanical overload [43]. This is emphasized by the fact that JNK is associated with some extracellular matrix remodeling, possibly a response to protect contractile function [64,65]. Gonzalez et al. showed decreased phospho-JNK/JNK during pregnancy, which is restored and possibly elevated post-partum [31].
Role of microRNAs in pregnancy-induced cardiac hypertrophy
MicroRNAs are non-coding RNAs that regulate gene expression by blocking transcription or by repressing translation of their target genes [66,67]. The temporal regulatory role played by Lin-4 and let-7 in controlling the larval development in C. elegans led to the establishment of microRNAs as members of gene regulatory networks [68-71]. Since then, microRNAs have been implicated in almost every aspect of cellular physiology including growth, metabolism, muscle differentiation, stem cell division and apoptosis [72-76]. In the context of cardiac remodeling and hypertrophy, a number of studies have shown the importance of microRNAs in the regulation of gene expression [77,78]. Cardiac hypertrophy is a complex process that involves change in the composition of ECM such as fibrosis, changes in contractility and fetal gene expression, as well as altered angiogenesis. Depending on the type and extent of the stimulus, these changes are manifested in various forms of physiological and pathological hypertrophy [79]. Emerging evidence shows that various microRNAs modulate key components of hypertrophy. Of interest are miR-1 and miR-133 that regulate growth related genes responsible for cardiac hypertrophy [80,81]. MiR-208 is involved in myocardial fibrosis and is a positive regulator of fetal gene expression [82]. MiR-27a regulates cardiac contractility by targeting β myosin heavy chain in cardiac myocytes [83]. Cardiac specific overexpression of miR-195 results in dialated cardiomyopathy in mice [78]. However, majority of these microRNAs are involved in pathological cardiac hypertrophy and very few studies have tried to identify signature microRNAs that are differentially regulated in physiological versus pathological hypertrophy. Of note, miR-29 family has been shown to control physiological cardiac hypertrophy in mice during aerobic training [84]. In another setting of physiological hypertrophy wherein pI3K is constitutively active, miR-222, miR-34a and miR-210 have been demonstrated to be differentially regulated in physiological versus pathological hypertrophy [85]. These studies are an important attempt to delineate the mechanisms involved in physiological versus pathological hypertrophy. However, there are no studies to analyze the role of microRNAs in cardiac adaptations to pregnancy. Further studies in microRNA biology are needed for better understanding and design of clinically relevant therapies for cardiovascular complication during pregnancy.
Role of ubiquitin proteasome system in pregnancy-induced cardiac hypertrophy
The heart is the only organ in the body that is constantly bearing a heavy workload and a high metabolic rate. Therefore it is essential that cardiac cells maintain a very efficient and tightly controlled system for removal of misfolded or damaged proteins. The ubiquitin-proteasome system (UPS) is the major machinery for the degradation of damaged and misfolded proteins in the heart [86]. Regulation of the proteasome function may occur through the association of the core 20S proteasomal subunit with different regulatory complexes such as 19S or 11S that affect proteasomal assembly and activity [87,88]. Generally however, the covalent binding of ubiquitin molecules to the target protein dictates its degradation by the 26S proteasome. Following attachment of ubiquitin molecules to the target proteins, the 19S regulatory subunits recognize the ubiquitin tags and transfer the protein substrates to be degraded in the inner pore of the 20S catalytic core [89]. A large number of reports have highlighted the functional significance of the UPS in heart hypertrophy. As the cardiac muscle hypertrophies, there is an increase in de novo protein synthesis which could potentially lead to more misfolded or abnormal proteins. As such, an increase in proteasomal activity would be needed to clear these aberrant proteins. Indeed, many studies report an increase in the activity of the proteasome in compensated heart hypertrophy induced by trans-aortic constriction both in mouse and canine models [90,91]. In fact, increased proteasome activity has been suggested to be required for the development of compensated heart hypertrophy [90,91]. Both trypsin-like activity (β2) and chymotrypsin-like activity (β5) were significantly increased in the subendocardium, which is subjected to the highest level of wall stress in a canine model of left-ventricular hypertrophy [90]. In fact, these studies suggest that activation of the proteasome is indeed a requirement for the development of compensated hypertrophy following pressure-overload. The proteasome inhibitor epoxomicin prevented the development of pre-existing hypertrophy and the further reduction in the ejection fraction [90,91]. During the progression of compensated heart hypertrophy to cardiac dysfunction, decreased proteasome activities have been shown [92]. In the isoproteranol-induced volume overload hypertrophy model, Drews et al. [93] also showed increased 26S proteasome activities and noted a significant decrease in the 20S activities, which were attributed to a switch in proteasome subpopulations. The expression of proteasome subunits has also been conflicting in different models of cardiac hypertrophy and failure. Most reports show an increase in 20S and 26S proteasome expression in different models of cardiomyopathy and hypertrophy [90,94], while the transcript levels of representative 20S subunits have been shown to be decreased in failing hearts [95,96]. Since protein ubiquitination is one of the key mechanisms for targeting a peptide to be degraded by the proteasome’s proteolytic pathway, proteasomal activity also depends on the levels of peptide ubiquitination. Immunocytochemical experiments previously revealed markedly increased expression levels of ubiquitin in patients with decompensated cardiomyopathy [94], and other studies show a progressive increase in ubiquitin levels 2-4 weeks post transaortic constriction-induced cardiac hypertrophy in mice [92,90].
During pregnancy as a result of volume-overload, heart develops a physiological heart hypotrophy. However, the precise role of the UPS in physiological heart hypertrophy during pregnancy is not yet known. Unravelling the role of UPS in pregnancy may influence therapeutic strategies for treating pathological heart hypertrophy.
The role of sex hormones in pregnancy-induced heart hypertrophy
Late pregnancy is a high estrogenic state. Estrogen levels in the plasma increase gradually as the pregnancy progresses and peak at the late-pregnant stage (Table 1). The cardioprotective action of estrogen has been well documented in many different experimental models of heart disease. In an experimental model of chronic volume overload induced by aortocaval fistula, female rats were reported to be protected as the mortality following 8 weeks of volume overload was 25% in males, whereas only 2% mortality was observed in females [97]. Furthermore, this apparent cardioprotection, which was lost following ovariectomy, was partially restored by estrogen replacement [98,99]. Estrogen was also effective in male rats in attenuating chronic volume overload induced structural and functional remodeling in the hearts[100]. These findings demonstrate that, in contrast to males, intact, cycling female rats are able to successfully compensate for the increased myocardial stress associated with chronic volume overload and that this cardioprotection is largely due to the action of estrogen. Estrogen is also known to attenuate the development of heart hypotrophy in various animal models. Estrogen also prevents PE-induced cardiomyocyte hypertrophy in vitro[101,102]. Pedram et al. recently showed that E2 therapy can directly inhibit the transition of cardiac fibroblast to myofibroblasts in vitro, preventing the secretion of fibrosis-inducing signal transduction proteins and many of adverse remodeling proteins [103]. Additionally, E2 treatment has been shown to directly mitigate adverse ECM remodelling in left ventricular hypertrophy and failure by attenuating altered collagen, and metalloproteinase expression in vivo[104]. Prevention of cardiac fibrosis and heart hypertrophy by estrogen during pregnancy could be one of the mechanisms protecting the late pregnant heart from failure.
Testosterone levels are also increased in the plasma during pregnancy [105,106]. Estrogen and testosterone have been shown to have opposing effects on chronic cardiac remodeling and function in mice with myocardial infarction (MI). Cavasin et al., suggested that estrogen prevents deterioration of cardiac function and remodeling after MI, but testosterone worsens cardiac dysfunction and remodeling and has a pronounced effect when estrogen levels are reduced [107]. Testosterone has also been implicated in causing cardiac hypertrophy. Clinical data suggest an association of increased serum androgens with cardiovascular mortality in females, but not in males. Detrimental effects of testosterone on post-MI remodelling in female rats have been described [108].
Pregnancy-induced cardiovascular complications
Drastic changes that occur during pregnancy in the cardiovascular system are usually well tolerated in healthy women. However, some healthy women without any cardiovascular complication prior to pregnancy could develop some adverse cardiac events, rarely, which could be fatal. Here, we review some of these complications.
Pregnancy-induced hypertension (preeclampsia) and eclampsia
The blood pressure in healthy pregnant women usually decreases during the second trimester but returns to normal toward the end of pregnancy. Pregnancy-induced hypertension (PIH) or preeclampsia is a medical condition in which the healthy pregnant women start to develop high blood pressure after the 20th week of pregnancy. Severe preeclampsia could lead to dangerous seizure known as eclampsia. Pregnant women with chronic hypertension, multiple gestations, a family history or a previous history of preeclampsia, age under 20 years or over 35 years, obesity or African American ethnicity are at higher risk of developing preeclampsia [109-111]. Although preeclampsia occurs in ~10% of pregnancies and is a life-threatening disease affecting mother and the newborn, the mechanisms underlying the development of preeclampsia are not well known.
During the implantation process, insufficient placental circulation is associated with pregnancy-induced hypertension. This poor circulation produces pro-inflammatory molecules which cause injury to the mother’s endothelial cells and result in increased vascular resistance [112]. A functional imbalance between thromboxane A2 (TXA2) and prostacyclin production has been shown to play a role in the development of preeclampsia. Neurokinin-B with the help of TXA2 may play some role in impaired placental neovascularization and downregulation of vascular endothelial growth factor (VEGF)-mediated signalling [112]. In late pregnancy the placenta secretes VEGF inhibitors like soluble FLT (sFLT), that create an antiangiogenic environment, which is more pronounced in pre-eclampsia and in multiple pregnancies [113]. Suppression of VEGF activity by sFLT in pre-eclampsia impaires diastolic relaxation [113].
Neurokinin-B is found in higher concentration in pregnant women with preeclampsia. The components of renin-angiotensin system (RAS) which have been shown to be increased in healthy pregnancy, are decreased in pregnancy-induced hypertensive mothers [112,114]. Immunologic mechanisms and aberrations of the RAS have been long considered contributors to the disorder. Bridging these two concepts, numerous studies report the presence of the angiotensin II type I receptor agonist autoantibody (AT(1)-AA) in preeclamptic women. This autoantibody induces many key features of the disorder through AT(1) receptor signaling, and has been implicated in the pathogenesis of preeclampsia. Takimoto and colleagues mated female mice expressing human angiotensinogen with male mice expressing human rennin. Pregnant females displayed transient elevations in blood pressure [115]. In another study, IgG from preeclamptic women was injected into pregnant mice, inducing endothelin-1 (ET-1) by AT(1) receptor activation. They showed that tumor necrosis factor alpha (TNFα) and interleukin-6 (IL-6) contribute to a signaling pathway which ultimately leads to increased ET-1 production by AT (1) receptor agonist autoantibody action [116].
Peripartum cardiomyopathy
Healthy women may develop peripartum cardiomyopathy (PPCM), a life-threatening disease of unknown cause, during the last month of pregnancy up to 5 months after delivery. PPCM is characterized by left ventricular systolic dysfunction as a result of dilated cardiomyopathy. PPCM is more common in pregnant women after age 30 and obesity, smoking, alcoholism, African American race, are some of the risk factors [117]. Some possible causes include myocarditis, cardiotrophic viral infection, apoptosis, inflammation and chimerism [118].
Experimental model of PPCM has identified several proteins which play a key role in the development of PPCM. Among them signal transducer and activator of transcription factor-3 (STAT3) has received a lot of attention. STAT3 has been shown to be necessary for protecting mice against PPCM, as female mice with a cardiomyocytes-specific deletion of STAT3 developed postpartum cardiomyopathy with a very high mortality rate [48]. The levels of STAT3 protein have also been shown to be reduced in PPCM patients. STAT3-KO mice also show higher expression of cardiac cathepsin D which cleaves full length prolactin to a subisoform of 16-kDa prolactin [48]. Serum levels of activated cardiac cathepsin D and cleaved prolactin are elevated in PPCM patients. This cleaved form of prolactin is a potent anti-angiogenic, proapoptotic and pro-inflammatory substance. The anti-angiogenic property of prolactin disrupts the cardiac capillary network which is known to play a major role in the transition of compensated hypertrophy to failure. In addition, unbalanced peri/postpartum oxidative stress associated with the proteolytic cleavage of prolactin into 16Kda subform has also been speculated as a potential mechanism for the development of PPCM. The fact that bromocriptine, an inhibitor of prolactin release, prevents the development of PPCM, highlights the role of prolactin as a new therapeutic target in peripartum cardiomyopathy [48,119]. Apoptosis has been shown to play a key role in the development of PPCM. Higher plasma levels of Fas, a cell surface protein that plays essential role in apoptosis, have been reported in some PPCM patients [120]. Inhibition of apoptosis in cardiomyocytes by caspase inhibitor in a mouse model of lethal peripartum cardiomyopathy (Gαq overexpression), abolished the PPCM mortality in these mice [121]. Recent study showed that PPCM is associated with imbalances in angiogenic signalling, and that anti-angiogenic states such as pre-eclampsia or multiple gestation substantially worsen the severity of the disease [113].
Conclusions
Pregnancy-induced heart hypertrophy differs from pathological hypertrophy, as well as physiological hypertrophy during exercise. Further studies are required to identify the role of ubiquitin-proteasome system as well as the key micro-RNAs regulating gene expression in this unique model of physiological heart hypertrophy. The function of the late pregnant heart seems to be preserved under physiological conditions and the drastic hemodynamic changes that occur during pregnancy are usually well tolerated in healthy women. However, some healthy women without any cardiovascular complication prior to pregnancy could develop some adverse cardiac events which, rarely, could prove to be fatal. Therefore these hearts appear to be at a threshold and any additional stress stimuli such as hypertension could drastically deteriorate their function. Management of these life-threatening circumstances and the procurement of women's well-being during pregnancy and postpartum period require a better understanding of the basic molecular mechanisms underlying the remodeling of the heart during this reproductive stage. This review calls for more research in this under explored area that should set the basis for a better treatment of women during pregnancy. Special emphasis has to be given to the guidelines on disease management in pregnancy [122].
Acknowledgments
Supported by NIH grants HL089876 (M.E.) and HL089876S1 (M.E.).
References
- 1.Cantor EJ, Babick AP, Vasanji Z, Dhalla NS, Netticadan T. A comparative serial echocardiographic analysis of cardiac structure and function in rats subjected to pressure or volume overload. J Mol Cell Cardiol. 2005;38:777–786. doi: 10.1016/j.yjmcc.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 2.Dorn GW, Mann DL. Signaling pathways involved in left ventricular remodeling: summation. J Card Fail. 2002;8:S387–S388. doi: 10.1054/jcaf.2002.129266. [DOI] [PubMed] [Google Scholar]
- 3.Carabello BA. Evolution of the study of left ventricular function: everything old is new again. Circulation. 2002;105:2701–2703. doi: 10.1161/01.cir.0000021240.86593.9d. [DOI] [PubMed] [Google Scholar]
- 4.Pluim BM, Swenne CA, Zwinderman AH, Maan AC, van der LA, Doornbos J, Van der Wall EE. Correlation of heart rate variability with cardiac functional and metabolic variables in cyclists with training induced left ventricular hypertrophy. Heart. 1999;81:612–617. doi: 10.1136/hrt.81.6.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schannwell CM, Zimmermann T, Schneppenheim M, Plehn G, Marx R, Strauer BE. Left Ventricular Hypertrophy and Diastolic Dysfunction in Healthy Pregnant Women. Cardiology. 2002;97:73–78. doi: 10.1159/000057675. [DOI] [PubMed] [Google Scholar]
- 6.Chung E, Yeung F, Leinwand LA. Akt and MAPK signaling mediate pregnancy-induced cardiac adaptation. J Appl Physiol. 2012;112:1564–1575. doi: 10.1152/japplphysiol.00027.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weir RJ. Vasopressor substances in normal and abnormal pregnancy. Eur J Obstet Gynecol Reprod Biol. 1975;5:75–85. doi: 10.1016/0028-2243(75)90132-x. [DOI] [PubMed] [Google Scholar]
- 8.Robertson JI, Weir RJ, Dusterdieck GO, Fraser R, Tree M. Renin, angiotensin and aldosterone in human pregnancy and the menstrual cycle. Scott Med J. 1971;16:183–196. doi: 10.1177/003693307101600303. [DOI] [PubMed] [Google Scholar]
- 9.Zheng J, Bird IM, Chen DB, Magness RR. Angiotensin II regulation of ovine fetoplacental artery endothelial functions: interactions with nitric oxide. J Physiol. 2005;565:59–69. doi: 10.1113/jphysiol.2004.082420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bowers SL, Banerjee I, Baudino TA. The extracellular matrix: at the center of it all. J Mol Cell Cardiol. 2010;48:474–482. doi: 10.1016/j.yjmcc.2009.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Swynghedauw B. Molecular mechanisms of myocardial remodeling. Physiol Rev. 1999;79:215–262. doi: 10.1152/physrev.1999.79.1.215. [DOI] [PubMed] [Google Scholar]
- 12.Berk BC, Fujiwara K, Lehoux S. ECM remodeling in hypertensive heart disease. J Clin Invest. 2007;117:568–575. doi: 10.1172/JCI31044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Janicki JS, Brower GL. The role of myocardial fibrillar collagen in ventricular remodeling and function. J Card Fail. 2002;8:S319–S325. doi: 10.1054/jcaf.2002.129260. [DOI] [PubMed] [Google Scholar]
- 14.Benito B, Gay-Jordi G, Serrano-Mollar A, Guasch E, Shi Y, Tardif JC, Brugada J, Nattel S, Mont L. Cardiac arrhythmogenic remodeling in a rat model of long-term intensive exercise training. Circulation. 2011;123:13–22. doi: 10.1161/CIRCULATIONAHA.110.938282. [DOI] [PubMed] [Google Scholar]
- 15.Aljabri MB, Songstad NT, Lund T, Serrano MC, Andreasen TV, Al-Saad S, Lindal S, Sitras V, Acharya G, Ytrehus K. Pregnancy protects against antiangiogenic and fibrogenic effects of angiotensin II in rat hearts. Acta Physiol (Oxf) 2011;201:445–456. doi: 10.1111/j.1748-1716.2010.02234.x. [DOI] [PubMed] [Google Scholar]
- 16.Lemmens K, Doggen K, De Keulenaer GW. Activation of the neuregulin/ErbB system during physiological ventricular remodeling in pregnancy. Am J Physiol Heart Circ Physiol. 2011;300:H931–H942. doi: 10.1152/ajpheart.00385.2010. [DOI] [PubMed] [Google Scholar]
- 17.Shai SY, Harpf AE, Babbitt CJ, Jordan MC, Fishbein MC, Chen J, Omura M, Leil TA, Becker KD, Jiang M, Smith DJ, Cherry SR, Loftus JC, Ross RS. Cardiac myocyte-specific excision of the beta1 integrin gene results in myocardial fibrosis and cardiac failure. Circ Res. 2002;90:458–464. doi: 10.1161/hh0402.105790. [DOI] [PubMed] [Google Scholar]
- 18.Suryakumar G, Kasiganesan H, Balasubramanian S, Kuppuswamy D. Lack of beta3 integrin signaling contributes to calpain-mediated myocardial cell loss in pressure-overloaded myocardium. J Cardiovasc Pharmacol. 2010;55:567–573. doi: 10.1097/FJC.0b013e3181d9f5d4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harston RK, Kuppuswamy D. Integrins are the necessary links to hypertrophic growth in cardiomyocytes. J Signal Transduct. 2011;2011:521742. doi: 10.1155/2011/521742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hessel M, Steendijk P, den AB, Schutte C, van der LA. Pressure overload-induced right ventricular failure is associated with re-expression of myocardial tenascin-C and elevated plasma tenascin-C levels. Cell Physiol Biochem. 2009;24:201–210. doi: 10.1159/000233246. [DOI] [PubMed] [Google Scholar]
- 21.Hutchinson KR, Stewart JA Jr, Lucchesi PA. Extracellular matrix remodeling during the progression of volume overload-induced heart failure. J Mol Cell Cardiol. 2010;48:564–569. doi: 10.1016/j.yjmcc.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Umar S, Hessel M, Steendijk P, Bax W, Schutte C, Schalij M, van der WE, Atsma D, van der LA. Activation of signaling molecules and matrix metalloproteinases in right ventricular myocardium of rats with pulmonary hypertension. Pathol Res Pract. 2007;203:863–872. doi: 10.1016/j.prp.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 23.Wang X, Chow FL, Oka T, Hao L, Lopez-Campistrous A, Kelly S, Cooper S, Odenbach J, Finegan BA, Schulz R, Kassiri Z, Lopaschuk GD, Fernandez-Patron C. Matrix metalloproteinase-7 and ADAM-12 (a disintegrin and metalloproteinase-12) define a signaling axis in agonist-induced hypertension and cardiac hypertrophy. Circulation. 2009;119:2480–2489. doi: 10.1161/CIRCULATIONAHA.108.835488. [DOI] [PubMed] [Google Scholar]
- 24.Gooz M. ADAM-17: the enzyme that does it all. Crit Rev Biochem Mol Biol. 2010;45:146–169. doi: 10.3109/10409231003628015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fedak PW, Moravec CS, McCarthy PM, Altamentova SM, Wong AP, Skrtic M, Verma S, Weisel RD, Li RK. Altered expression of disintegrin metalloproteinases and their inhibitor in human dilated cardiomyopathy. Circulation. 2006;113:238–245. doi: 10.1161/CIRCULATIONAHA.105.571414. [DOI] [PubMed] [Google Scholar]
- 26.Mahmoodzadeh S, Dworatzek E, Fritschka S, Pham TH, Regitz-Zagrosek V. 17beta-Estradiol inhibits matrix metalloproteinase-2 transcription via MAP kinase in fibroblasts. Cardiovasc Res. 2010;85:719–728. doi: 10.1093/cvr/cvp350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dorn GW, Force T. Protein kinase cascades in the regulation of cardiac hypertrophy. J Clin Invest. 2005;115:527–537. doi: 10.1172/JCI24178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heineke J, Molkentin JD. Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat Rev Mol Cell Biol. 2006;7:589–600. doi: 10.1038/nrm1983. [DOI] [PubMed] [Google Scholar]
- 29.Eghbali M, Deva R, Alioua A, Minosyan TY, Ruan H, Wang Y, Toro L, Stefani E. Molecular and Functional Signature of Heart Hypertrophy During Pregnancy. Circ Res. 2005;96:1208–1216. doi: 10.1161/01.RES.0000170652.71414.16. [DOI] [PubMed] [Google Scholar]
- 30.Eghbali M, Wang Y, Toro L, Stefani E. Heart Hypertrophy During Pregnancy: A Better Functioning Heart? Trends in Cardiovascular Medicine. 2006;16:285–291. doi: 10.1016/j.tcm.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 31.Gonzalez AMD, Osorio JC, Manlhiot C, Gruber D, Homma S, Mital S. Hypertrophy signaling during peripartum cardiac remodeling. Am J Physiol Heart Circ Physiol. 2007;293:H3008–H3013. doi: 10.1152/ajpheart.00401.2007. [DOI] [PubMed] [Google Scholar]
- 32.Matsui T, Rosenzweig A. Convergent signal transduction pathways controlling cardiomyocyte survival and function: the role of PI 3-kinase and Akt. J Mol Cell Cardiol. 2005;38:63–71. doi: 10.1016/j.yjmcc.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 33.DeBosch B, Treskov I, Lupu TS, Weinheimer C, Kovacs A, Courtois M, Muslin AJ. Akt1 is required for physiological cardiac growth. Circulation. 2006;113:2097–2104. doi: 10.1161/CIRCULATIONAHA.105.595231. [DOI] [PubMed] [Google Scholar]
- 34.DeBosch B, Sambandam N, Weinheimer C, Courtois M, Muslin AJ. Akt2 regulates cardiac metabolism and cardiomyocyte survival. J Biol Chem. 2006;281:32841–32851. doi: 10.1074/jbc.M513087200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Previlon M, Pezet M, Dachez C, Mercadier JJ, Rouet-Benzineb P. Sequential alterations in Akt, GSK3beta, and calcineurin signalling in the mouse left ventricle after thoracic aortic constriction. Can J Physiol Pharmacol. 2010;88:1093–1101. doi: 10.1139/y10-087. [DOI] [PubMed] [Google Scholar]
- 36.Ivanova M, Janega P, Matejikova J, Simoncikova P, Pancza D, Ravingerova T, Barancik M. Activation of Akt kinase accompanies increased cardiac resistance to ischemia/reperfusion in rats after short-term feeding with lard-based high-fat diet and increased sucrose intake. Nutr Res. 2011;31:631–643. doi: 10.1016/j.nutres.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 37.Hardt SE, Sadoshima J. Glycogen synthase kinase-3beta: a novel regulator of cardiac hypertrophy and development. Circ Res. 2002;90:1055–1063. doi: 10.1161/01.res.0000018952.70505.f1. [DOI] [PubMed] [Google Scholar]
- 38.Sugden PH, Fuller SJ, Weiss SC, Clerk A. Glycogen synthase kinase 3 (GSK3) in the heart: a point of integration in hypertrophic signalling and a therapeutic target? A critical analysis. Br J Pharmacol. 2008;153(Suppl 1):S137–S153. doi: 10.1038/sj.bjp.0707659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Markou T, Cullingford TE, Giraldo A, Weiss SC, Alsafi A, Fuller SJ, Clerk A, Sugden PH. Glycogen synthase kinases 3alpha and 3beta in cardiac myocytes: regulation and consequences of their inhibition. Cell Signal. 2008;20:206–218. doi: 10.1016/j.cellsig.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 40.Antos CL, McKinsey TA, Frey N, Kutschke W, McAnally J, Shelton JM, Richardson JA, Hill JA, Olson EN. Activated glycogen synthase-3 beta suppresses cardiac hypertrophy in vivo. Proc Natl Acad Sci USA. 2002;99:907–912. doi: 10.1073/pnas.231619298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haq S, Choukroun G, Kang ZB, Ranu H, Matsui T, Rosenzweig A, Molkentin JD, Alessandrini A, Woodgett J, Hajjar R, Michael A, Force T. Glycogen synthase kinase-3beta is a negative regulator of cardiomyocyte hypertrophy. J Cell Biol. 2000;151:117–130. doi: 10.1083/jcb.151.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meng R, Pei Z, Zhang A, Zhou Y, Cai X, Chen B, Liu G, Mai W, Wei J, Dong Y. AMPK activation enhances PPARalpha activity to inhibit cardiac hypertrophy via ERK1/2 MAPK signaling pathway. Arch Biochem Biophys. 2011;511:1–7. doi: 10.1016/j.abb.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 43.Rose BA, Force T, Wang Y. Mitogen-activated protein kinase signaling in the heart: angels versus demons in a heart-breaking tale. Physiol Rev. 2010;90:1507–1546. doi: 10.1152/physrev.00054.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bogoyevitch MA, Sugden PH. The role of protein kinases in adaptational growth of the heart. Int J Biochem Cell Biol. 1996;28:1–12. doi: 10.1016/1357-2725(95)00142-5. [DOI] [PubMed] [Google Scholar]
- 45.Kyriakis JM, Avruch J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol Rev. 2001;81:807–869. doi: 10.1152/physrev.2001.81.2.807. [DOI] [PubMed] [Google Scholar]
- 46.Zhang S, Weinheimer C, Courtois M, Kovacs A, Zhang CE, Cheng AM, Wang Y, Muslin AJ. The role of the Grb2-p38 MAPK signaling pathway in cardiac hypertrophy and fibrosis. J Clin Invest. 2003;111:833–841. doi: 10.1172/JCI16290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nishida K, Yamaguchi O, Hirotani S, Hikoso S, Higuchi Y, Watanabe T, Takeda T, Osuka S, Morita T, Kondoh G, Uno Y, Kashiwase K, Taniike M, Nakai A, Matsumura Y, Miyazaki J, Sudo T, Hongo K, Kusakari Y, Kurihara S, Chien KR, Takeda J, Hori M, Otsu K. p38alpha mitogen-activated protein kinase plays a critical role in cardiomyocyte survival but not in cardiac hypertrophic growth in response to pressure overload. Mol Cell Biol. 2004;24:10611–10620. doi: 10.1128/MCB.24.24.10611-10620.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hilfiker-Kleiner D, Kaminski K, Podewski E, Bonda T, Schaefer A, Sliwa K, Forster O, Quint A, Landmesser U, Doerries C, Luchtefeld M, Poli V, Schneider MD, Balligand JL, Desjardins F, Ansari A, Struman I, Nguyen NQ, Zschemisch NH, Klein G, Heusch G, Schulz R, Hilfiker A, Drexler H. A cathepsin D-cleaved 16 kDa form of prolactin mediates postpartum cardiomyopathy. Cell. 2007;128:589–600. doi: 10.1016/j.cell.2006.12.036. [DOI] [PubMed] [Google Scholar]
- 49.Kurdi M, Booz GW. JAK redux: a second look at the regulation and role of JAKs in the heart. Am J Physiol Heart Circ Physiol. 2009;297:H1545–H1556. doi: 10.1152/ajpheart.00032.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kunisada K, Negoro S, Tone E, Funamoto M, Osugi T, Yamada S, Okabe M, Kishimoto T, Yamauchi-Takihara K. Signal transducer and activator of transcription 3 in the heart transduces not only a hypertrophic signal but a protective signal against doxorubicin-induced cardiomyopathy. Proc Natl Acad Sci USA. 2000;97:315–319. doi: 10.1073/pnas.97.1.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alas S, Bonavida B. Rituximab inactivates signal transducer and activation of transcription 3 (STAT3) activity in B-non-Hodgkin's lymphoma through inhibition of the interleukin 10 autocrine/paracrine loop and results in down-regulation of Bcl-2 and sensitization to cytotoxic drugs. Cancer Res. 2001;61:5137–5144. [PubMed] [Google Scholar]
- 52.Alas S, Bonavida B. Inhibition of constitutive STAT3 activity sensitizes resistant non-Hodgkin's lymphoma and multiple myeloma to chemotherapeutic drug-mediated apoptosis. Clin Cancer Res. 2003;9:316–326. [PubMed] [Google Scholar]
- 53.Catlett-Falcone R, Landowski TH, Oshiro MM, Turkson J, Levitzki A, Savino R, Ciliberto G, Moscinski L, Fernandez-Luna JL, Nunez G, Dalton WS, Jove R. Constitutive activation of Stat3 signaling confers resistance to apoptosis in human U266 myeloma cells. Immunity. 1999;10:105–115. doi: 10.1016/s1074-7613(00)80011-4. [DOI] [PubMed] [Google Scholar]
- 54.Hilfiker-Kleiner D, Hilfiker A, Fuchs M, Kaminski K, Schaefer A, Schieffer B, Hillmer A, Schmiedl A, Ding Z, Podewski E, Podewski E, Poli V, Schneider MD, Schulz R, Park JK, Wollert KC, Drexler H. Signal transducer and activator of transcription 3 is required for myocardial capillary growth, control of interstitial matrix deposition, and heart protection from ischemic injury. Circ Res. 2004;95:187–195. doi: 10.1161/01.RES.0000134921.50377.61. [DOI] [PubMed] [Google Scholar]
- 55.Kodama H, Fukuda K, Pan J, Makino S, Sano M, Takahashi T, Hori S, Ogawa S. Biphasic activation of the JAK/STAT pathway by angiotensin II in rat cardiomyocytes. Circ Res. 1998;82:244–250. doi: 10.1161/01.res.82.2.244. [DOI] [PubMed] [Google Scholar]
- 56.Jacoby JJ, Kalinowski A, Liu MG, Zhang SS, Gao Q, Chai GX, Ji L, Iwamoto Y, Li E, Schneider M, Russell KS, Fu XY. Cardiomyocyte-restricted knockout of STAT3 results in higher sensitivity to inflammation, cardiac fibrosis, and heart failure with advanced age. Proc Natl Acad Sci USA. 2003;100:12929–12934. doi: 10.1073/pnas.2134694100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Molkentin JD, Lu JR, Antos CL, Markham B, Richardson J, Robbins J, Grant SR, Olson EN. A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell. 1998;93:215–228. doi: 10.1016/s0092-8674(00)81573-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Molkentin JD. Calcineurin and beyond: cardiac hypertrophic signaling. Circ Res. 2000;87:731–738. doi: 10.1161/01.res.87.9.731. [DOI] [PubMed] [Google Scholar]
- 59.Sussman MA, Lim HW, Gude N, Taigen T, Olson EN, Robbins J, Colbert MC, Gualberto A, Wieczorek DF, Molkentin JD. Prevention of cardiac hypertrophy in mice by calcineurin inhibition. Science. 1998;281:1690–1693. doi: 10.1126/science.281.5383.1690. [DOI] [PubMed] [Google Scholar]
- 60.Wilkins BJ, De Windt LJ, Bueno OF, Braz JC, Glascock BJ, Kimball TF, Molkentin JD. Targeted disruption of NFATc3, but not NFATc4, reveals an intrinsic defect in calcineurin-mediated cardiac hypertrophic growth. Mol Cell Biol. 2002;22:7603–7613. doi: 10.1128/MCB.22.21.7603-7613.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fischer TA, Ludwig S, Flory E, Gambaryan S, Singh K, Finn P, Pfeffer MA, Kelly RA, Pfeffer JM. Activation of cardiac c-Jun NH(2)-terminal kinases and p38-mitogen-activated protein kinases with abrupt changes in hemodynamic load. Hypertension. 2001;37:1222–1228. doi: 10.1161/01.hyp.37.5.1222. [DOI] [PubMed] [Google Scholar]
- 62.Sopontammarak S, Aliharoob A, Ocampo C, Arcilla RA, Gupta MP, Gupta M. Mitogenactivated protein kinases (p38 and c-Jun NH2- terminal kinase) are differentially regulated during cardiac volume and pressure overload hypertrophy. Cell Biochem Biophys. 2005;43:61–76. doi: 10.1385/CBB:43:1:061. [DOI] [PubMed] [Google Scholar]
- 63.Wang Y, Su B, Sah VP, Brown JH, Han J, Chien KR. Cardiac hypertrophy induced by mitogen-activated protein kinase kinase 7, a specific activator for c-Jun NH2-terminal kinase in ventricular muscle cells. J Biol Chem. 1998;273:5423–5426. doi: 10.1074/jbc.273.10.5423. [DOI] [PubMed] [Google Scholar]
- 64.Petrich BG, Wang Y. Stress-activated MAP kinases in cardiac remodeling and heart failure; new insights from transgenic studies. Trends Cardiovasc Med. 2004;14:50–55. doi: 10.1016/j.tcm.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 65.Petrich BG, Liao P, Wang Y. Using a gene-switch transgenic approach to dissect distinct roles of MAP kinases in heart failure. Cold Spring Harb Symp Quant Biol. 2002;67:429–437. doi: 10.1101/sqb.2002.67.429. [DOI] [PubMed] [Google Scholar]
- 66.Bartel DP, Chen CZ. Micromanagers of gene expression: the potentially widespread influence of metazoan microRNAs. Nat Rev Genet. 2004;5:396–400. doi: 10.1038/nrg1328. [DOI] [PubMed] [Google Scholar]
- 67.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ambros V. A hierarchy of regulatory genes controls a larva-to-adult developmental switch in C. elegans. Cell. 1989;57:49–57. doi: 10.1016/0092-8674(89)90171-2. [DOI] [PubMed] [Google Scholar]
- 69.Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75:843–854. doi: 10.1016/0092-8674(93)90529-y. [DOI] [PubMed] [Google Scholar]
- 70.Olsen PH, Ambros V. The lin-4 regulatory RNA controls developmental timing in Caenor-habditis elegans by blocking LIN-14 protein synthesis after the initiation of translation. Dev Biol. 1999;216:671–680. doi: 10.1006/dbio.1999.9523. [DOI] [PubMed] [Google Scholar]
- 71.Reinhart BJ, Slack FJ, Basson M, Pasquinelli AE, Bettinger JC, Rougvie AE, Horvitz HR, Ruvkun G. The 21-nucleotide let-7 RNA regulates developmental timing in Caenorhabditis elegans. Nature. 2000;403:901–906. doi: 10.1038/35002607. [DOI] [PubMed] [Google Scholar]
- 72.Xu P, Vernooy SY, Guo M, Hay BA. The Drosophila microRNA Mir-14 suppresses cell death and is required for normal fat metabolism. Curr Biol. 2003;13:790–795. doi: 10.1016/s0960-9822(03)00250-1. [DOI] [PubMed] [Google Scholar]
- 73.Hatfield SD, Shcherbata HR, Fischer KA, Nakahara K, Carthew RW, Ruohola-Baker H. Stem cell division is regulated by the microRNA pathway. Nature. 2005;435:974–978. doi: 10.1038/nature03816. [DOI] [PubMed] [Google Scholar]
- 74.Nguyen HT, Frasch M. MicroRNAs in muscle differentiation: lessons from Drosophila and beyond. Curr Opin Genet Dev. 2006;16:533–539. doi: 10.1016/j.gde.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 75.Lynn FC. Meta-regulation: microRNA regulation of glucose and lipid metabolism. Trends Endocrinol Metab. 2009;20:452–459. doi: 10.1016/j.tem.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 76.Magill ST, Cambronne XA, Luikart BW, Lioy DT, Leighton BH, Westbrook GL, Mandel G, Goodman RH. microRNA-132 regulates dendritic growth and arborization of newborn neurons in the adult hippocampus. Proc Natl Acad Sci USA. 2010;107:20382–20387. doi: 10.1073/pnas.1015691107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Callis TE, Pandya K, Seok HY, Tang RH, Tatsuguchi M, Huang ZP, Chen JF, Deng Z, Gunn B, Shumate J, Willis MS, Selzman CH, Wang DZ. MicroRNA-208a is a regulator of cardiac hypertrophy and conduction in mice. J Clin Invest. 2009;119:2772–2786. doi: 10.1172/JCI36154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.van RE, Sutherland LB, Liu N, Williams AH, McAnally J, Gerard RD, Richardson JA, Olson EN. A signature pattern of stress-responsive microRNAs that can evoke cardiac hypertrophy and heart failure. Proc Natl Acad Sci USA. 2006;103:18255–18260. doi: 10.1073/pnas.0608791103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mann DL, Bristow MR. Mechanisms and models in heart failure: the biomechanical model and beyond. Circulation. 2005;111:2837–2849. doi: 10.1161/CIRCULATIONAHA.104.500546. [DOI] [PubMed] [Google Scholar]
- 80.Sayed D, Hong C, Chen IY, Lypowy J, Abdellatif M. MicroRNAs play an essential role in the development of cardiac hypertrophy. Circ Res. 2007;100:416–424. doi: 10.1161/01.RES.0000257913.42552.23. [DOI] [PubMed] [Google Scholar]
- 81.Care A, Catalucci D, Felicetti F, Bonci D, Addario A, Gallo P, Bang ML, Segnalini P, Gu Y, Dalton ND, Elia L, Latronico MV, Hoydal M, Autore C, Russo MA, Dorn GW, Ellingsen O, Ruiz-Lozano P, Peterson KL, Croce CM, Peschle C, Condorelli G. MicroRNA-133 controls cardiac hypertrophy. Nat Med. 2007;13:613–618. doi: 10.1038/nm1582. [DOI] [PubMed] [Google Scholar]
- 82.van RE, Sutherland LB, Qi X, Richardson JA, Hill J, Olson EN. Control of stress-dependent cardiac growth and gene expression by a microRNA. Science. 2007;316:575–579. doi: 10.1126/science.1139089. [DOI] [PubMed] [Google Scholar]
- 83.Nishi H, Ono K, Horie T, Nagao K, Kinoshita M, Kuwabara Y, Watanabe S, Takaya T, Tamaki Y, Takanabe-Mori R, Wada H, Hasegawa K, Iwanaga Y, Kawamura T, Kita T, Kimura T. MicroRNA-27a regulates beta cardiac myosin heavy chain gene expression by targeting thyroid hormone receptor beta1 in neonatal rat ventricular myocytes. Mol Cell Biol. 2011;31:744–755. doi: 10.1128/MCB.00581-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Soci UP, Fernandes T, Hashimoto NY, Mota GF, Amadeu MA, Rosa KT, Irigoyen MC, Phillips MI, Oliveira EM. MicroRNAs 29 are involved in the improvement of ventricular compliance promoted by aerobic exercise training in rats. Physiol Genomics. 2011;43:665–673. doi: 10.1152/physiolgenomics.00145.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lin RC, Weeks KL, Gao XM, Williams RB, Bernardo BC, Kiriazis H, Matthews VB, Woodcock EA, Bouwman RD, Mollica JP, Speirs HJ, Dawes IW, Daly RJ, Shioi T, Izumo S, Febbraio MA, Du XJ, McMullen JR. PI3K(p110 alpha) protects against myocardial infarction-induced heart failure: identification of PI3K-regulated miRNA and mRNA. Arterioscler Thromb Vasc Biol. 2010;30:724–732. doi: 10.1161/ATVBAHA.109.201988. [DOI] [PubMed] [Google Scholar]
- 86.Predmore JM, Wang P, Davis F, Bartolone S, Westfall MV, Dyke DB, Pagani F, Powell SR, Day SM. Ubiquitin proteasome dysfunction in human hypertrophic and dilated cardiomyopathies. Circulation. 2010;121:997–1004. doi: 10.1161/CIRCULATIONAHA.109.904557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Glickman MH, Ciechanover A. The ubiquitinproteasome proteolytic pathway: destruction for the sake of construction. Physiol Rev. 2002;82:373–428. doi: 10.1152/physrev.00027.2001. [DOI] [PubMed] [Google Scholar]
- 88.Glickman MH, Raveh D. Proteasome plasticity. FEBS Lett. 2005;579:3214–3223. doi: 10.1016/j.febslet.2005.04.048. [DOI] [PubMed] [Google Scholar]
- 89.Hochstrasser M. Ubiquitin-dependent protein degradation. Annu Rev Genet. 1996;30:405–439. doi: 10.1146/annurev.genet.30.1.405. [DOI] [PubMed] [Google Scholar]
- 90.Depre C, Wang Q, Yan L, Hedhli N, Peter P, Chen L, Hong C, Hittinger L, Ghaleh B, Sadoshima J, Vatner DE, Vatner SF, Madura K. Activation of the cardiac proteasome during pressure overload promotes ventricular hypertrophy. Circulation. 2006;114:1821–1828. doi: 10.1161/CIRCULATIONAHA.106.637827. [DOI] [PubMed] [Google Scholar]
- 91.Hedhli N, Lizano P, Hong C, Fritzky LF, Dhar SK, Liu H, Tian Y, Gao S, Madura K, Vatner SF, Depre C. Proteasome inhibition decreases cardiac remodeling after initiation of pressure overload. Am J Physiol Heart Circ Physiol. 2008;295:H1385–H1393. doi: 10.1152/ajpheart.00532.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tsukamoto O, Minamino T, Okada K, Shintani Y, Takashima S, Kato H, Liao Y, Okazaki H, Asai M, Hirata A, Fujita M, Asano Y, Yamazaki S, Asanuma H, Hori M, Kitakaze M. Depression of proteasome activities during the progression of cardiac dysfunction in pressure-overloaded heart of mice. Biochem Biophys Res Commun. 2006;340:1125–1133. doi: 10.1016/j.bbrc.2005.12.120. [DOI] [PubMed] [Google Scholar]
- 93.Drews O, Tsukamoto O, Liem D, Streicher J, Wang Y, Ping P. Differential regulation of proteasome function in isoproterenol-induced cardiac hypertrophy. Circ Res. 2010;107:1094–1101. doi: 10.1161/CIRCRESAHA.110.222364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Otsuka K, Terasaki F, Shimomura H, Tsukada B, Horii T, Isomura T, Suma H, Shibayama Y, Kitaura Y. Enhanced expression of the ubiquitin-proteasome system in the myocardium from patients with dilated cardiomyopathy referred for left ventriculoplasty: an immunohistochemical study with special reference to oxidative stress. Heart Vessels. 2010;25:474–484. doi: 10.1007/s00380-010-0006-3. [DOI] [PubMed] [Google Scholar]
- 95.Kaab S, Barth AS, Margerie D, Dugas M, Gebauer M, Zwermann L, Merk S, Pfeufer A, Steinmeyer K, Bleich M, Kreuzer E, Steinbeck G, Nabauer M. Global gene expression in human myocardium-oligonucleotide microarray analysis of regional diversity and transcriptional regulation in heart failure. J Mol Med (Berl) 2004;82:308–316. doi: 10.1007/s00109-004-0527-2. [DOI] [PubMed] [Google Scholar]
- 96.Zolk O, Schenke C, Sarikas A. The ubiquitin-proteasome system: focus on the heart. Cardiovasc Res. 2006;70:410–421. doi: 10.1016/j.cardiores.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 97.Gardner JD, Brower GL, Janicki JS. Gender differences in cardiac remodeling secondary to chronic volume overload. J Card Fail. 2002;8:101–107. doi: 10.1054/jcaf.2002.32195. [DOI] [PubMed] [Google Scholar]
- 98.Brower GL, Gardner JD, Janicki JS. Gender mediated cardiac protection from adverse ventricular remodeling is abolished by ovariectomy. Mol Cell Biochem. 2003;251:89–95. [PubMed] [Google Scholar]
- 99.Gardner JD, Brower GL, Voloshenyuk TG, Janicki JS. Cardioprotection in female rats subjected to chronic volume overload: synergistic interaction of estrogen and phytoestrogens. Am J Physiol Heart Circ Physiol. 2008;294:H198–H204. doi: 10.1152/ajpheart.00281.2007. [DOI] [PubMed] [Google Scholar]
- 100.Gardner JD, Murray DB, Voloshenyuk TG, Brower GL, Bradley JM, Janicki JS. Estrogen attenuates chronic volume overload induced structural and functional remodeling in male rat hearts. Am J Physiol Heart Circ Physiol. 2010;298:H497–H504. doi: 10.1152/ajpheart.00336.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pedram A, Razandi M, Aitkenhead M, Levin ER. Estrogen inhibits cardiomyocyte hypertrophy in vitro. Antagonism of calcineurin-related hypertrophy through induction of MCIP1. J Biol Chem. 2005;280:26339–26348. doi: 10.1074/jbc.M414409200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Babiker FA, De Windt LJ, van EM, Thijssen V, Bronsaer RJ, Grohe C, van BM, Doevendans PA. 17beta-estradiol antagonizes cardiomyocyte hypertrophy by autocrine/paracrine stimulation of a guanylyl cyclase A receptor-cyclic guanosine monophosphate-dependent protein kinase pathway. Circulation. 2004;109:269–276. doi: 10.1161/01.CIR.0000105682.85732.BD. [DOI] [PubMed] [Google Scholar]
- 103.Pedram A, Razandi M, O'Mahony F, Lubahn D, Levin ER. Estrogen receptor-beta prevents cardiac fibrosis. Mol Endocrinol. 2010;24:2152–2165. doi: 10.1210/me.2010-0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Voloshenyuk TG, Gardner JD. Estrogen improves TIMP-MMP balance and collagen distribution in volume-overloaded hearts of ovariectomized females. Am J Physiol Regul Integr Comp Physiol. 2010;299:R683–R693. doi: 10.1152/ajpregu.00162.2010. [DOI] [PubMed] [Google Scholar]
- 105.Bammann BL, Coulam CB, Jiang NS. Total and free testosterone during pregnancy. Am J Obstet Gynecol. 1980;137:293–298. doi: 10.1016/0002-9378(80)90912-6. [DOI] [PubMed] [Google Scholar]
- 106.Harrison RF, Mansfield MD. Maternal plasma androgens in early human pregnancy. Br J Obstet Gynaecol. 1980;87:695–704. doi: 10.1111/j.1471-0528.1980.tb04603.x. [DOI] [PubMed] [Google Scholar]
- 107.Cavasin MA, Sankey SS, Yu AL, Menon S, Yang XP. Estrogen and testosterone have opposing effects on chronic cardiac remodeling and function in mice with myocardial infarction. Am J Physiol Heart Circ Physiol. 2003;284:H1560–H1569. doi: 10.1152/ajpheart.01087.2002. [DOI] [PubMed] [Google Scholar]
- 108.Frantz S, Hu K, Widder J, Weckler B, Scheuermann H, Bauersachs J, Ertl G, Callies F, Allolio B. Detrimental effects of testosterone on post-myocardial infarction remodelling in female rats. J Physiol Pharmacol. 2007;58:717–727. [PubMed] [Google Scholar]
- 109.Cunningham FG GNLKGLHJWK. Williams Obstetrics. 21st Edition. New York, NY: McGraw-Hill; 2001. pp. 567–618. [Google Scholar]
- 110.Walker JJ. Pre-eclampsia. Lancet. 2000;356:1260–1265. doi: 10.1016/S0140-6736(00)02800-2. [DOI] [PubMed] [Google Scholar]
- 111.Sibai BM, Ewell M, Levine RJ, Klebanoff MA, Esterlitz J, Catalano PM, Goldenberg RL, Joffe G. Risk factors associated with preeclampsia in healthy nulliparous women. The Calcium for Preeclampsia Prevention (CPEP) Study Group. Am J Obstet Gynecol. 1997;177:1003–1010. doi: 10.1016/s0002-9378(97)70004-8. [DOI] [PubMed] [Google Scholar]
- 112.Furuya M, Ishida J, Aoki I, Fukamizu A. Pathophysiology of placentation abnormalities in pregnancy-induced hypertension. Vasc Health Risk Manag. 2008;4:1301–1313. doi: 10.2147/vhrm.s4009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Patten IS, Rana S, Shahul S, Rowe GC, Jang C, Liu L, Hacker MR, Rhee JS, Mitchell J, Mahmood F, Hess P, Farrell C, Koulisis N, Khankin EV, Burke SD, Tudorache I, Bauersachs J, del MF, Hilfiker-Kleiner D, Karumanchi SA, Arany Z. Cardiac angiogenic imbalance leads to peripartum cardiomyopathy. Nature. 2012;485:333–338. doi: 10.1038/nature11040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hanssens M, Keirse MJ, Spitz B, Van Assche FA. Measurement of individual plasma angiotensins in normal pregnancy and pregnancy-induced hypertension. J Clin Endocrinol Metab. 1991;73:489–494. doi: 10.1210/jcem-73-3-489. [DOI] [PubMed] [Google Scholar]
- 115.Takimoto E, Ishida J, Sugiyama F, Horiguchi H, Murakami K, Fukamizu A. Hypertension induced in pregnant mice by placental renin and maternal angiotensinogen. Science. 1996;274:995–998. doi: 10.1126/science.274.5289.995. [DOI] [PubMed] [Google Scholar]
- 116.Zhou CC, Irani RA, Dai Y, Blackwell SC, Hicks MJ, Ramin SM, Kellems RE, Xia Y. Autoantibody-mediated IL-6-dependent endothelin-1 elevation underlies pathogenesis in a mouse model of preeclampsia. J Immunol. 2011;186:6024–6034. doi: 10.4049/jimmunol.1004026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ramaraj R, Sorrell VL. Peripartum cardiomyopathy: Causes, diagnosis, and treatment. Cleve Clin J Med. 2009;76:289–296. doi: 10.3949/ccjm.76a.08004. [DOI] [PubMed] [Google Scholar]
- 118.Bultmann BD, Klingel K, Nabauer M, Wallwiener D, Kandolf R. High prevalence of viral genomes and inflammation in peripartum cardiomyopathy. Am J Obstet Gynecol. 2005;193:363–365. doi: 10.1016/j.ajog.2005.01.022. [DOI] [PubMed] [Google Scholar]
- 119.Yamac H, Bultmann I, Sliwa K, Hilfiker-Kleiner D. Prolactin: a new therapeutic target in peripartum cardiomyopathy. Heart. 2010;96:1352–1357. doi: 10.1136/hrt.2009.179218. [DOI] [PubMed] [Google Scholar]
- 120.Sliwa K, Forster O, Libhaber E, Fett JD, Sundstrom JB, Hilfiker-Kleiner D, Ansari AA. Peripartum cardiomyopathy: inflammatory markers as predictors of outcome in 100 prospectively studied patients. Eur Heart J. 2006;27:441–446. doi: 10.1093/eurheartj/ehi481. [DOI] [PubMed] [Google Scholar]
- 121.Hayakawa Y, Chandra M, Miao W, Shirani J, Brown JH, Dorn GW, Armstrong RC, Kitsis RN. Inhibition of cardiac myocyte apoptosis improves cardiac function and abolishes mortality in the peripartum cardiomyopathy of G-alpha(q) transgenic mice. Circulation. 2003;108:3036–3041. doi: 10.1161/01.CIR.0000101920.72665.58. [DOI] [PubMed] [Google Scholar]
- 122.Regitz-Zagrosek V, Blomstrom LC, Borghi C, Cifkova R, Ferreira R, Foidart JM, Gibbs JS, Gohlke-Baerwolf C, Gorenek B, Iung B, Kirby M, Maas AH, Morais J, Nihoyannopoulos P, Pieper PG, Presbitero P, Roos-Hesselink JW, Schaufelberger M, Seeland U, Torracca L, Bax J, Auricchio A, Baumgartner H, Ceconi C, Dean V, Deaton C, Fagard R, Funck-Brentano C, Hasdai D, Hoes A, Knuuti J, Kolh P, McDonagh T, Moulin C, Poldermans D, Popescu BA, Reiner Z, Sechtem U, Sirnes PA, Torbicki A, Vahanian A, Windecker S, Baumgartner H, Deaton C, Aguiar C, Al-Attar N, Garcia AA, Antoniou A, Coman I, Elkayam U, Gomez-Sanchez MA, Gotcheva N, Hilfiker-Kleiner D, Kiss RG, Kitsiou A, Konings KT, Lip GY, Manolis A, Mebaaza A, Mintale I, Morice MC, Mulder BJ, Pasquet A, Price S, Priori SG, Salvador MJ, Shotan A, Silversides CK, Skouby SO, Stein JI, Tornos P, Vejlstrup N, Walker F, Warnes C. ESC Guidelines on the management of cardiovascular diseases during pregnancy: the Task Force on the Management of Cardiovascular Diseases during Pregnancy of the European Society of Cardiology (ESC) Eur Heart J. 2011;32:3147–3197. doi: 10.1093/eurheartj/ehr218. [DOI] [PubMed] [Google Scholar]
- 123.Candice K, Silversides JMC. Heart disease in pregnancy, chapter 2. second edition 2007. [Google Scholar]
- 124.Martin VT, Behbehani M. Ovarian hormones and migraine headache: understanding mechanisms and pathogenesis--part 2. Headache. 2006;46:365–386. doi: 10.1111/j.1526-4610.2006.00370.x. [DOI] [PubMed] [Google Scholar]
- 125.Lapolt PS, Matt DW, Judd HL, Lu JK. The relation of ovarian steroid levels in young female rats to subsequent estrous cyclicity and reproductive function during aging. Biol Reprod. 1986;35:1131–1139. doi: 10.1095/biolreprod35.5.1131. [DOI] [PubMed] [Google Scholar]
- 126.Altmann J, Lynch JW, Nguyen N, Alberts SC, Gesquiere LR. Life-history correlates of steroid concentrations in wild peripartum baboons. Am J Primatol. 2004;64:95–106. doi: 10.1002/ajp.20064. [DOI] [PubMed] [Google Scholar]
- 127.Klinga K, Bek E, Runnebaum B. Maternal peripheral testosterone levels during the first half of pregnancy. Am J Obstet Gynecol. 1978;131:60–62. doi: 10.1016/0002-9378(78)90474-x. [DOI] [PubMed] [Google Scholar]
- 128.Bammann BL, Coulam CB, Jiang NS. Total and free testosterone during pregnancy. Am J Obstet Gynecol. 1980;137:293–298. doi: 10.1016/0002-9378(80)90912-6. [DOI] [PubMed] [Google Scholar]
- 129.Bridges RS, Todd RB, Logue CM. Serum concentrations of testosterone throughout pregnancy in rats. J Endocrinol. 1982;94:21–27. doi: 10.1677/joe.0.0940021. [DOI] [PubMed] [Google Scholar]
- 130.Biswas S, Rodeck CH. Plasma prolactin levels during pregnancy. Br J Obstet Gynaecol. 1976;83:683–687. doi: 10.1111/j.1471-0528.1976.tb00913.x. [DOI] [PubMed] [Google Scholar]
- 131.Riordan J. Breastfeeding and Human Lactation. 3rd ed. Goston and London: Jones and Bartlett; 2005. pp. 75–77. [Google Scholar]
- 132.Walker M. Breastfeeding Management for the Clinician: Using the Evidence. Boston: Jones and Bartlett; 2006. pp. 63–66. [Google Scholar]
- 133.Hanssens M, Keirse MJ, Spitz B, Van Assche FA. Angiotensin II levels in hypertensive and normotensive pregnancies. Br J Obstet Gynaecol. 1991;98:155–161. doi: 10.1111/j.1471-0528.1991.tb13361.x. [DOI] [PubMed] [Google Scholar]
- 134.Magness RR, Cox K, Rosenfeld CR, Gant NF. Angiotensin II metabolic clearance rate and pressor responses in nonpregnant and pregnant women. Am J Obstet Gynecol. 1994;171:668–679. doi: 10.1016/0002-9378(94)90080-9. [DOI] [PubMed] [Google Scholar]
- 135.Broughton PF, Oats JJ, Symonds EM. Sequential changes in the human renin-angiotensin system following delivery. Br J Obstet Gynaecol. 1978;85:821–827. doi: 10.1111/j.1471-0528.1978.tb15836.x. [DOI] [PubMed] [Google Scholar]
- 136.Lu H, Melendez GC, Levick SP, Janicki JS. Prevention of adverse cardiac remodeling to volume overload in female rats is the result of an estrogen-altered mast cell phenotype. Am J Physiol Heart Circ Physiol. 2012;302:H811–H817. doi: 10.1152/ajpheart.00980.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bradshaw AD, Baicu CF, Rentz TJ, Van Laer AO, Boggs J, Lacy JM, Zile MR. Pressure overload-induced alterations in fibrillar collagen content and myocardial diastolic function: role of secreted protein acidic and rich in cysteine (SPARC) in post-synthetic procollagen processing. Circulation. 2009;119:269–280. doi: 10.1161/CIRCULATIONAHA.108.773424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Weber KT, Janicki JS, Shroff SG, Pick R, Chen RM, Bashey RI. Collagen remodeling of the pressure-overloaded, hypertrophied nonhuman primate myocardium. Circ Res. 1988;62:757–765. doi: 10.1161/01.res.62.4.757. [DOI] [PubMed] [Google Scholar]
- 139.Melendez GC, Li J, Law BA, Janicki JS, Supowit SC, Levick SP. Substance P induces adverse myocardial remodelling via a mechanism involving cardiac mast cells. Cardiovasc Res. 2011;92:420–429. doi: 10.1093/cvr/cvr244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Givvimani S, Qipshidze N, Tyagi N, Mishra PK, Sen U, Tyagi SC. Synergism between arrhythmia and hyperhomo-cysteinemia in structural heart disease. Int J Physiol Pathophysiol Pharmacol. 2011;3:107–119. [PMC free article] [PubMed] [Google Scholar]
- 141.Ulasova E, Gladden JD, Chen Y, Zheng J, Pat B, Bradley W, Powell P, Zmijewski JW, Zelickson BR, Ballinger SW, rley-Usmar V, Dell'italia LJ. Loss of interstitial collagen causes structural and functional alterations of cardiomyocyte subsarcolemmal mitochondria in acute volume overload. J Mol Cell Cardiol. 2011;50:147–156. doi: 10.1016/j.yjmcc.2010.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Nagatomo Y, Carabello BA, Coker ML, McDermott PJ, Nemoto S, Hamawaki M, Spinale FG. Differential effects of pressure or volume overload on myocardial MMP levels and inhibitory control. Am J Physiol Heart Circ Physiol. 2000;278:H151–H161. doi: 10.1152/ajpheart.2000.278.1.H151. [DOI] [PubMed] [Google Scholar]
- 143.Miyamoto T, Takeishi Y, Takahashi H, Shishido T, Arimoto T, Tomoike H, Kubota I. Activation of distinct signal transduction pathways in hypertrophied hearts by pressure and volume overload. Basic Res Cardiol. 2004;99:328–337. doi: 10.1007/s00395-004-0482-7. [DOI] [PubMed] [Google Scholar]
- 144.Iemitsu M, Maeda S, Jesmin S, Otsuki T, Kasuya Y, Miyauchi T. Activation pattern of MAPK signaling in the hearts of trained and untrained rats following a single bout of exercise. J Appl Physiol. 2006;101:151–163. doi: 10.1152/japplphysiol.00392.2005. [DOI] [PubMed] [Google Scholar]
- 145.Sopontammarak S, Aliharoob A, Ocampo C, Arcilla RA, Gupta MP, Gupta M. Mitogen-activated protein kinases (p38 and c-Jun NH2-terminal kinase) are differentially regulated during cardiac volume and pressure overload hypertrophy. Cell Biochem Biophys. 2005;43:61–76. doi: 10.1385/CBB:43:1:061. [DOI] [PubMed] [Google Scholar]
- 146.Esposito G, Prasad SV, Rapacciuolo A, Mao L, Koch WJ, Rockman HA. Cardiac overexpression of a G(q) inhibitor blocks induction of extracellular signal-regulated kinase and c-Jun NH(2)-terminal kinase activity in in vivo pressure overload. Circulation. 2001;103:1453–1458. doi: 10.1161/01.cir.103.10.1453. [DOI] [PubMed] [Google Scholar]
- 147.Harris IS, Zhang S, Treskov I, Kovacs A, Weinheimer C, Muslin AJ. Raf-1 kinase is required for cardiac hypertrophy and cardiomyocyte survival in response to pressure overload. Circulation. 2004;110:718–723. doi: 10.1161/01.CIR.0000138190.50127.6A. [DOI] [PubMed] [Google Scholar]
- 148.Takeishi Y, Huang Q, Abe J, Glassman M, Che W, Lee JD, Kawakatsu H, Lawrence EG, Hoit BD, Berk BC, Walsh RA. Src and multiple MAP kinase activation in cardiac hypertrophy and congestive heart failure under chronic pressure-overload: comparison with acute mechanical stretch. J Mol Cell Cardiol. 2001;33:1637–1648. doi: 10.1006/jmcc.2001.1427. [DOI] [PubMed] [Google Scholar]
- 149.Alvin ZV, Laurence GG, Coleman BR, Zhao A, Hajj-Moussa M, Haddad GE. Regulation of the instantaneous inward rectifier and the delayed outward rectifier potassium channels by Captopril and Angiotensin II via the Phosphoinositide-3 kinase pathway in volume-overload-induced hypertrophied cardiac myocytes. Med Sci Monit. 2011;17:BR165–BR172. doi: 10.12659/MSM.881843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wu CH, Liu JY, Wu JP, Hsieh YH, Liu CJ, Hwang JM, Lee SD, Chen LM, Chang MH, Kuo WW, Shyu JC, Tsai JH, Huang CY. 17[beta] -Estradiol reduces cardiac hypertrophy mediated through the up-regulation of PI3K/Akt and the suppression of calcineurin/NF-AT3 signaling pathways in rats. Life Sciences. 2005;78:347–356. doi: 10.1016/j.lfs.2005.04.077. [DOI] [PubMed] [Google Scholar]
- 151.Eto Y, Yonekura K, Sonoda M, Arai N, Sata M, Sugiura S, Takenaka K, Gualberto A, Hixon ML, Wagner MW, Aoyagi T. Calcineurin is activated in rat hearts with physiological left ventricular hypertrophy induced by voluntary exercise training. Circulation. 2000;101:2134–2137. doi: 10.1161/01.cir.101.18.2134. [DOI] [PubMed] [Google Scholar]
- 152.Bartelds B, Borgdorff MA, Smit-van OA, Takens J, Boersma B, Nederhoff MG, Elzenga NJ, van Gilst WH, De Windt LJ, Berger RM. Differential responses of the right ventricle to abnormal loading conditions in mice: pressure vs. volume load. Eur J Heart Fail. 2011;13:1275–1282. doi: 10.1093/eurjhf/hfr134. [DOI] [PubMed] [Google Scholar]
- 153.Bartelds B, Borgdorff MA, Smit-van OA, Takens J, Boersma B, Nederhoff MG, Elzenga NJ, van Gilst WH, De Windt LJ, Berger RM. Differential responses of the right ventricle to abnormal loading conditions in mice: pressure vs. volume load. Eur J Heart Fail. 2011;13:1275–1282. doi: 10.1093/eurjhf/hfr134. [DOI] [PubMed] [Google Scholar]
- 154.Wilkins BJ, Dai YS, Bueno OF, Parsons SA, Xu J, Plank DM, Jones F, Kimball TR, Molkentin JD. Calcineurin/NFAT Coupling Participates in Pathological, but not Physiological, Cardiac Hypertrophy. Circ Res. 2004;94:110–118. doi: 10.1161/01.RES.0000109415.17511.18. [DOI] [PubMed] [Google Scholar]
- 155.Lebeche D, Kaprielian R, del MF, Tomaselli G, Gwathmey JK, Schwartz A, Hajjar RJ. In vivo cardiac gene transfer of Kv4.3 abrogates the hypertrophic response in rats after aortic stenosis. Circulation. 2004;110:3435–3443. doi: 10.1161/01.CIR.0000148176.33730.3F. [DOI] [PubMed] [Google Scholar]
- 156.Bartelds B, Borgdorff MA, Smit-van OA, Takens J, Boersma B, Nederhoff MG, Elzenga NJ, van Gilst WH, De Windt LJ, Berger RM. Differential responses of the right ventricle to abnormal loading conditions in mice: pressure vs. volume load. Eur J Heart Fail. 2011;13:1275–1282. doi: 10.1093/eurjhf/hfr134. [DOI] [PubMed] [Google Scholar]
- 157.Lebeche D, Kaprielian R, del MF, Tomaselli G, Gwathmey JK, Schwartz A, Hajjar RJ. In vivo cardiac gene transfer of Kv4.3 abrogates the hypertrophic response in rats after aortic stenosis. Circulation. 2004;110:3435–3443. doi: 10.1161/01.CIR.0000148176.33730.3F. [DOI] [PubMed] [Google Scholar]
- 158.Razeghi P, Baskin KK, Sharma S, Young ME, Stepkowski S, Essop MF, Taegtmeyer H. Atrophy, hypertrophy, and hypoxemia induce transcriptional regulators of the ubiquitin proteasome system in the rat heart. Biochem Biophys Res Commun. 2006;342:361–364. doi: 10.1016/j.bbrc.2006.01.163. [DOI] [PubMed] [Google Scholar]
- 159.Hedhli N, Lizano P, Hong C, Fritzky LF, Dhar SK, Liu H, Tian Y, Gao S, Madura K, Vatner SF, Depre C. Proteasome inhibition decreases cardiac remodeling after initiation of pressure overload. Am J Physiol Heart Circ Physiol. 2008;295:H1385–H1393. doi: 10.1152/ajpheart.00532.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]