Abstract
Many enzymes that produce natriuretic factors such as nitric oxide synthase (NOS), hemeoxygenase-1 (HO-1) and cyclooxygenase-2 (COX-2) are highly expressed in the renal medulla. These enzymes in the renal medulla are up-regulated in response to high salt intake. Inhibition of these enzymes within the renal medulla reduces sodium excretion and increases salt sensitivity of arterial blood pressure, indicating that these enzymes play important roles in kidney salt handling and renal adaptation to high salt challenge. However, it remains a question what mechanisms mediate the activation of these enzymes in response to high salt challenge in the renal medulla. Interestingly, these enzymes are oxygen sensitive genes and regulated by transcription factor hypoxia-inducible factor (HIF)-1α. Our recent serial studies have demonstrated that: 1) High salt intake stimulates HIF-1α-mediated gene expression, such as NOS, HO-1 and COX-2, in the renal medulla, which may augment the production of different antihypertensive factors in the renal medulla, mediating renal adaptation to high salt intake and regulating salt sensitivity of arterial blood pressure. 2) HIF prolyl-hydroxylase 2 (PHD2), an enzyme that promotes the degradation of HIF-1α, is highly expressed in renal medulla. High salt intake suppresses the expression of PHD2 in the renal medulla, which increases HIF-1α-mediated gene expressions in the renal medulla, thereby participates in the control of salt sensitivity of blood pressure. 3) The high salt-induced inhibition in PHD2 and the consequent activation of HIF-1α in the renal medulla is not observed in Dahl salt sensitive hypertensive (Dahl/ss) rats. Correction of these defects in PHD2/HIF-1α-associated molecular adaptation in the renal medulla improves sodium excretion, reduces sodium retention and attenuates saltsensitive hypertension in Dahl/ss rats. In conclusion, PHD2 regulation of HIF-1α-mediated gene activation in the renal medulla is an important molecular adaptation to high salt intake; impaired PHD2 regulation of HIF-1α-mediated gene activation in the renal medulla may be responsible for the salt-sensitive hypertension in Dahl/ss rats; correction of these defects may be used to as therapeutic strategies for the treatment of salt-sensitive hypertension.
Keywords: Salt sensitive hypertension, gene transfection, Dahl S rat, pressure natriuresis, hypoxia inducible factor-1α, transcription factor, sodium excretion, heme oxygenase-1, cyclooxygenase-2, fluid homeostasis
Salt-sensitive hypertension accounts for 50% of hypertensive population [1,2]. Importantly, the salt sensitivity of blood pressure is closely associated with a much greater propensity to develop organ injuries in hypertension [2-4]. Mechanism for salt-sensitive hypertension is not fully understood. It is well known that renal medulla plays critical roles in the regulation of sodium excretion and long-term control of arterial blood pressure [5-7]. Many enzymes that produce natriuretic factors such as nitric oxide synthase (NOS), hemeoxygenase-1 (HO-1) and cyclooxygenase-2 (COX-2) are highly expressed in the renal medulla [8-14]. These enzymes in the renal medulla are up-regulated in response to high salt intake [9,12-15]. Inhibition of these enzymes within the renal medulla reduces sodium excretion and increases salt sensitivity of arterial blood pressure [9-12,14,16-18]. Moreover, in salt sensitive hypertensive animal models, the levels of these enzymes in the renal medulla are much lower [19-21] and the upregulation of these enzymes in response to high salt diet and to angiotensin II are diminished [10,19,22-23] compared with normal controls. Thus, the protective factors produced by these enzymes in the renal medulla play critical roles in the regulation of sodium excretion via actions on blood flow and tubular activity, which is essential in maintaining the homeostasis of body fluid volume and blood pressure. However, it remains a question what mechanisms mediate the activation of these enzymes in the renal medulla in response to high salt challenge.
HIF-1α-mediated gene activation is an important molecular adaptation in the renal medulla in response to high salt challenge
Recent studies have demonstrated that the genes encoding the protective factor-producing enzymes described above are oxygen sensitive genes and regulated by hypoxia-inducible factor (HIF)-1α [24-26], a transcription factor whose level is also very high in the renal medulla [27-29] due to the low oxygen levels in this kidney region [30-33]. HIF-1α is a master regulator of adaptation to hypoxia and activates gene transcription of many oxygen sensitive genes including NOS, COX-2 and HO-1 [24,25,34-36]. Activation of these genes in the renal medulla induces vasodilation and inhibits tubular activity, which promotes sodium excretion and consequently contributes to the control of blood pressure [6-8,26,37]. Therefore, renal medullary functions are in fact associated with genes that are transcriptionally regulated by HIF-1α.
Although it was evidenced that the transcriptional expressions of the above enzymes were regulated by HIF-1α, it remained unknown whether this HIF-1α-mediated gene activation was of physiological relevance in the control of renal function, in particular the renal medullary function. We therefore performed a study to test the hypothesis that HIF-1α mediates the activation of the oxygen sensitive genes such as NOS, COX-2 and HO-1 in the renal medulla, and thereby participates in the control of renal medullary functions and consequently regulates blood pressure [38]. In this study, we transfected HIF-1α decoy oligodeoxynucleotides into the renal medulla to inhibit the binding activity of HIF-1α and examined its effect on pressure natriuresis, renal cortical and medullary blood flows in response to the elevations of renal perfusion pressure (RPP) and sodium loading, and then determined its chronic effect on arterial blood pressure. It was demonstrated that blocking the transcriptional activity of HIF-1α to inhibit the expression of its target genes in the renal medulla substantially blunted the increases of renal medullary blood flows and urinary sodium excretion in response to the elevations of RPP, suggesting that HIF-1α, possibly through the actions on its target genes, is importantly involved in the regulation of renal medullary function. Since products of the enzymes encoded by these HIF-1α target genes have been shown to dilate the medullary vasculature and inhibit the tubular activities [7,37,39,40], the effect of HIF-1α-mediated pathway on pressure natriuresis may be through both vascular and tubular actions.
To further evaluate the role of renal medullary HIF-1α on salt handling, we examined the sodium excretion after acute sodium loading and the salt balance after chronic sodium challenge. The results from these experiments demonstrated that inhibition of HIF-1α-mediated gene activation remarkably impaired the capability of the kidneys to remove extra sodium load, which resulted in sodium retention [38]. These data additionally suggest that renal medullary HIF-1α is a regulator in sodium excretion. Since pressure-natriuresis and normal renal medullary function are key determinants to the long-term control of arterial blood pressure [6,7,37,39,41], our data further showed that inhibition of HIF-1α-mediated gene activation led to a considerable increase in arterial blood pressure in response to high salt intake. Interestingly, inhibition of HIF-1α-mediated gene activation did not produce hypertension when rats were not challenged with high salt. Therefore, high saltinduced activation of HIF-1α-regualted pathways is considered as an adaptive mechanism to high salt intake, which results in an induction of various protective factors and consequent promotion of extra sodium excretion. Deficiency of HIF-1α-mediated gene transcription in the renal medulla may decrease the production of various protecting factors, impair renal medullary function, prevent excretion of extra salt intake, consequently disrupt salt adaptation and increase the salt sensitivity of arterial blood pressure. These data suggest that HIF-1α-mediated gene activation may be a common mechanism regulating the expression of various protecting factors in the renal medulla, thereby exerting an antihypertensive action when animals are exposed to high salt challenge.
HIF prolyl-hydoxylase-2 regulates HIF-1α-mediated gene activation in the renal medulla in response to high salt challenge
Our above studies suggest that HIF-1α-mediated gene regulation in the renal medulla represents an important molecular adaptive mechanism in response to high salt intake and plays a crucial role in the maintenance of sodium balance and blood pressure control. However, it remains unclear how high salt intake induces the increases in HIF-1α levels in the renal medulla. It has been recently demonstrated that HIF prolyl-hydroxylases are the major enzymes to promote the degradation of HIF-1α [42-44]. HIF prolyl-hydroxylases catalyze sitespecific proline hydroxylation of HIF-1α using oxygen as a cofactor. After being prolyl-hydroxylated, HIF-1α is recognized and targeted for degradation by the ubiquitin-proteasome pathway. Although HIF prolyl-hydroxylases work as oxygen sensor to regulate the destruction of HIF-1α [42-44], recent evidence has clearly shown that the activities and expressions of HIF prolyl-hydroxylases are also regulated independent of oxygen levels by a variety of factors [45-49].
Three isoforms of HIF prolyl-hydroxylase, including prolyl hydroxylase domain-containing proteins 1, 2, and 3 (PHD1, 2, and 3), have been identified [42,43,50]. Previous studies including ours have demonstrated that PHDs are present in the kidneys with PHD2 as the predominant isoform of PHDs [51-55] and that PHD2 is most abundantly expressed in the renal medulla [51,55]. Our data showed that i.p. injection of L-mimosine, a PHD inhibitor, for 2 weeks substantially upregulated HIF-1α expression in the kidneys, especially in the renal medulla, and remarkably enhanced the natriuretic response to renal perfusion pressure in Sprague-Dawley rats [51]. Inhibition of HIF transcriptional activity by renal medullary transfection of HIF-1α decoy oligodeoxynucleotides attenuated L-mimosine-induced enhancement of pressure natriuresis, which confirmed that HIF-1α mediated the effect of PHD inhibitor. These results indicate that highly expressed PHDs in the renal medulla importantly contribute to the control of renal Na+ excretion through regulation of HIF-1α and its targeted genes [51].
Given the important role of PHDs in the regulation of HIF-1α levels and renal function, we hypothesized that PHD2 responds to high salt intake and mediates high salt-induced increase of HIF-1α in the renal medulla. We examined the effect of high salt intake on the expression of PHD2 and determined the role of PHD2 in high salt-induced activation of HIF-1α by transfection of PHD2 expression plasmids into the renal medulla. Our results showed that high salt intake decreased PHD2 levels in the renal medulla, and that over-expression of PHD2 transgene to disrupt high salt-induced PHD2 inhibition in the renal medulla blocked high salt-induced activation in HIF-1α and its target genes, suggesting that high salt increases HIF-1α level and thereby enhances expression of its target genes through inhibition of PHD2 [56]. Most recently, we further demonstrated that over-expression of PHD2 transgene to disrupt the high salt-induced inhibition of PHD2 and subsequently to inhibit the adaptive activation of renal medullary HIF-1α in response to high salt challenge impaired renal medullary function and kidney salt handling, thereafter causing sodium retention and producing a salt sensitive hypertension [57]. These results demonstrated that high salt-induced activation of HIF-1α and its target genes were associated with PHD2 inhibition and that over-expression of PHD2 transgene blocked the activation of HIF-1α and its target genes after high salt challenge, suggesting that PHD2 functions as an upstream signal that regulates HIF-1α-mediated gene activations in the renal medulla in response to high salt. It is concluded that PHD2 regulation of HIF-1α in response to high salt in the renal medulla may represent a novel mechanism involved in renal salt handling and blood pressure regulation.
Impaired PHD2 regulation of HIF-1α-mediated gene activation in the renal medulla in Dahl salt sensitive hypertensive rats
The above information suggests that PHD2/HIF-1α pathway is an important molecular mediator in renal salt adaptation under normal conditions. We wondered whether this PHD2/HIF-1α pathway was involved in the pathogenic mechanism of abnormal renal sodium management in salt-sensitive hypertensive individuals. Dahl salt sensitive hypertensive (Dahl/ss) rat is a widely used genetic model of human salt-sensitive hypertension that exhibits many phenotypic characteristics in common with human hypertension [3,58-61]. Renal medullary dysfunction is one of the major mechanisms for this rat strain to develop hypertension [6,7,10,19]. Most interestingly, the above protective genes regulated by HIF-1α has been shown to be impaired this animal model and deficiencies of these HIF-1α target genes in the renal medulla are considered to be responsible for the development of hypertension in Dahl/ss rats [10,19-23]. For example, it has been reported that there is a defect in renal medullary NOS2, one of HIF-1α target genes [19-21] in Dahl/ss rats, and that the activations of NOS2 in the renal medulla by a high salt diet and by angiotensin II are detected in normal animals but diminished in Dahl/ss rats [10,19,22,23]. These studies indicate that there is possibly an impairment in renal medullary HIF-1α, which produces the deficiencies in the expressions of NOS2 and other genes in the renal medulla in this rat strain.
We therefore determined whether the renal medullary HIF-1α and PHD2 levels were altered in the renal medulla in response to a high salt diet in Dahl/ss rats. Our results showed a decreased expression and reduced response to high salt intake in HIF-1α levels in the renal medulla from Dahl/ss rats compared with that from normal rats, which was accompanied by similar defects in HIF-1α target genes HO-1, NOS2 and COX2 in Dahl/ss rats compared with normal rats [56]. These results indicate that HIF-1α-mediated gene activations in these renal medullary protective factors are impaired in this rat strain. In parallel to these results, a higher level of PHD2 and failed inhibition of PHD2 in response to high salt intake in the renal medulla from Dahl/ss rats were observed [56]. Moreover, reducing PHD2 levels by shRNA restored the upregulatory response to high salt challenge in HIF-1α and its target genes HO-1, NOS2 and COX2 in the renal medulla in Dahl/ss rats [56]. It is suggested that diminished HIF-1α in Dahl/ss rats is caused by abnormal PHD2 response to a high salt diet. These data additionally support the view that inhibitory response of PHD2 facing high salt challenge activates HIF-1α-mediated gene expressions, consequently maintaining a sodium balance. Our results suggest that deficient PHD2/HIF-1α-mediated molecular adaptation in response to high salt intake in the renal medulla may be the pathogenic mechanism responsible for producing salt sensitive hypertension this animal model.
We further investigated whether correction of the defects in PHD/HIF-1α-mediated molecular adaptation in response to high salt intake in the renal medulla would improve the renal sodium excretion and attenuate the salt sensitive hypertension in Dahl/ss rats. We induced the expression of HIF-1α levels in the renal medulla by local over-expression of HIF-1α transgene or infusion of CoCl2, a HIF-1α inducer, into the renal medulla and then determined the improvement of renal sodium handling and saltsensitive hypertension in this animal model. Our results demonstrated that induction of HIF-1α-mediated gene activation in the renal medulla increased the expression of anti-hypertensive genes in the renal medulla, and consequently enhanced the urinary sodium excretion, reduced sodium retention, as a result, attenuated the salt-sensitive hypertension in Dahl/ss rats [62]. It has been shown that high salt-induced activation of HIF-1α-regualted pathways is considered as an adaptive mechanism to high salt intake, which leads to an induction of various protective factors and promotes extra sodium excretion [38]. Therefore, deficiency of HIF-1α-mediated gene transcription in the renal medulla may decrease the production of various protective factors, impair renal medullary function, damage the capability of the kidneys to remove extra sodium load, consequently disrupt salt adaptation and increase the salt sensitivity of arterial blood pressure in Dahl/ss rats. This deficiency in HIF-1α-mediated gene activation may represent an important mechanism for the development of salt sensitive hypertension. Induction of HIF-1α in the renal medulla may restore the molecular adaptation to high salt intake and stimulate the production of different renal medullary protective or antihypertensive factors, thereby, attenuate salt-sensitive hypertension, which may be used to as a therapeutic strategy for salt-sensitive hypertension.
Because high salt-induced PHD2 inhibition and consequent HIF-1α upregulation in the renal medulla was absent in Dahl/ss rats, we also tested whether silencing of PHD2 gene would increase the levels of HIF-1α and its target genes in the renal medulla, consequently enhancing the sodium excretion and attenuating salt-sensitive hypertension in Dahl/ss rats. We transfected PHD2-shRNA plasmids into the renal medulla and then detected the renal sodium excretion and arterial blood pressure after high salt challenge in Dahl/ss rats. It was found that silencing of PHD2 gene to increase HIF-1α levels in the renal medulla in Dahl/ss rats promoted sodium excretion and reduced sodium retention after sodium loading, and consequently attenuated salt sensitive hypertension [63]. It is suggested that the absence of inhibition in PHD2 in the renal medulla after high salt challenge may represent a novel mechanism for the deficient HIF-1α response and the development of hypertension in Dahl/ss rats, and that inhibition of PHD2 in the renal medulla could be used as a therapeutic approach for salt-sensitive hypertension.
Summary
Our serial studies have demonstrated that: 1) High salt intake stimulates HIF-1α-mediated gene activation in the renal medulla. Blockade of this HIF-1α-mediated gene activation in response to high salt challenge reduces sodium excretion, induces sodium retention and produces a salt-sensitive hypertension. It is suggested that HIF-1α-mediated gene activation may increase the production of different renal antihypertensive factors in the renal medulla, mediate renal adaptation to high salt intake and regulate salt sensitivity of arterial blood pressure. 2) PHD2, an enzyme that promotes the degradation of HIF-1α, is the most abundant isoform of PHDs in the kidneys and highly expressed in renal medulla. High salt intake suppresses the expression of PHD2 in the renal medulla (Figure 1). This high salt-induced inhibition of PHD2 is an upstream signal that increases HIF-1α-mediated gene expression in the renal medulla in response to high salt challenge. Disruption of this PHD2-associated adaptive activation of HIF-1α-mediated gene expressions in the renal medulla blunts sodium excretion, induces sodium retention and increases salt sensitivity of blood pressure. Thus, PHD2 regulation of HIF-1α-mediated gene activation in the renal medulla is one of the important adaptive mechanisms to high salt challenge and thereby participates in the control of salt sensitivity of blood pressure. 3) The high salt-induced inhibition in PHD2 and the consequent activation of HIF-1α in the renal medulla is absent in Dahl/ss rats. Induction of HIF-1α levels in the renal medulla in Dahl S rats increases the expression of anti-hypertensive genes, enhances sodium excretion, reduces sodium retention and attenuates the salt-sensitive hypertension. Meanwhile, inhibition of PHD2 in the renal medulla in Dahl S rats corrects the defect in HIF-1α -mediated molecular adaptation, improves sodium excretion, reduces sodium retention and attenuates salt-sensitive hypertension. In conclusion, PHD2 regulation of HIF-1α-mediated gene activation in the renal medulla is an important molecular adaptation to high salt intake; impaired PHD2 regulation of HIF-1α-mediated gene activation in the renal medulla may be responsible for the salt-sensitive hypertension in Dahl S rats; correction of these defects by inhibiting PHD2 or inducing HIF-1α may be used as therapeutic strategies for the treatment of salt-sensitive hypertension. It remains unanswered how high salt inhibits PHD2 expression in the renal medulla. Our interesting finding that the expression of the PHD2 transgene is decreased by high salt intake [56] indicates that high salt-induced PHD2 inhibition may be associated with a reduced mRNA stability. The exact mechanisms for high salt to inhibit PHD2 require further exploration.
Figure 1.
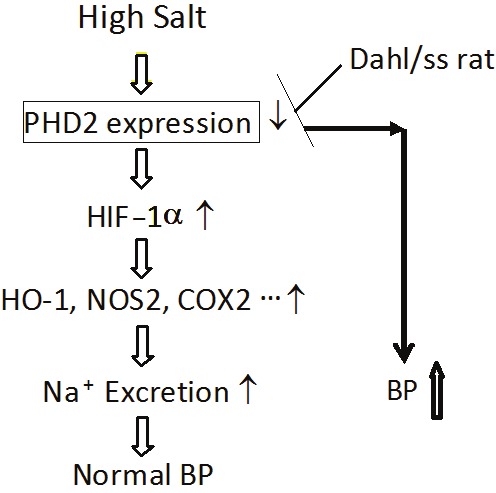
HIF-1α-mediated gene activation in the molecular adaptation to high salt intake in the renal medulla and its role in salt sensitive hypertension. High salt intake inhibits PHD2, an enzyme promoting HIF-1α degradation, leading to the accumulation of HIF-1α in the renal medulla. HIF-1α then activates its target genes such as NOS2, HO-1 and COX-2, which augment the production of anti-hypertensive factors in the renal medulla to remove the extra sodium load, thereby, maintaining a normal blood pressure after high salt challenge. However, in Dahl/ss rats, high salt-induced inhibition of PHD2 is absent. Therefore, there is no HIF-1α accumulation and neither the activation of anti-hypertensive genes in the renal medulla after high salt challenge, which impairs the capability of the kidneys to remove extra sodium load and results in sodium retention, consequently, producing a salt sensitive hypertension in this animal model.
Acknowledgements
Support: National Institutes of Health Grant HL89563 and HL106042.
References
- 1.Weinberger MH, Miller JZ, Luft FC, Grim CE, Fineberg NS. Definitions and characteristics of sodium sensitivity and blood pressure resistance. Hypertension. 1986;8:II127–134. doi: 10.1161/01.hyp.8.6_pt_2.ii127. [DOI] [PubMed] [Google Scholar]
- 2.Chrysant GS, Bakir S, Oparil S. Dietary salt reduction in hypertension--what is the evidence and why is it still controversial? Prog Cardiovasc Dis. 1999;42:23–38. doi: 10.1016/s0033-0620(99)70007-1. [DOI] [PubMed] [Google Scholar]
- 3.Campese V. Salt sensitivity in hypertension. Renal and cardiovascular implications [clinical conference] . Hypertension. 1994;23:531–550. doi: 10.1161/01.hyp.23.4.531. [DOI] [PubMed] [Google Scholar]
- 4.Weinberger MH, Fineberg NS, Fineberg SE, Weinberger M. Salt Sensitivity, Pulse Pressure, and Death in Normal and Hypertensive Humans. Hypertension. 2001;37:429–432. doi: 10.1161/01.hyp.37.2.429. [DOI] [PubMed] [Google Scholar]
- 5.Cowley AW Jr MD, Lu S, Roman RJ. The renal medulla and hypertension. Hypertension. 1995;25:663–673. doi: 10.1161/01.hyp.25.4.663. [DOI] [PubMed] [Google Scholar]
- 6.Bergstrom G, Evans RG. Mechanisms underlying the antihypertensive functions of the renal medulla. Acta Physiologica Scandinavica. 2004;181:475–486. doi: 10.1111/j.1365-201X.2004.01321.x. [DOI] [PubMed] [Google Scholar]
- 7.Mattson DL. Importance of the renal medullary circulation in the control of sodium excretion and blood pressure. Am J Physiol Regul Integr Comp Physiol. 2003;284:R13–27. doi: 10.1152/ajpregu.00321.2002. [DOI] [PubMed] [Google Scholar]
- 8.Zou A-P, Billington H, Su N, Cowley AW Jr. Expression and Actions of Heme Oxygenase in the Renal Medulla of Rats. Hypertension. 2000;35:342–347. doi: 10.1161/01.hyp.35.1.342. [DOI] [PubMed] [Google Scholar]
- 9.Mattson DL, Higgins DJ. Influence of Dietary Sodium Intake on Renal Medullary Nitric Oxide Synthase. Hypertension. 1996;27:688–692. doi: 10.1161/01.hyp.27.3.688. [DOI] [PubMed] [Google Scholar]
- 10.Tan DY, Meng S, Cason GW, Manning RD Jr. Mechanisms of salt-sensitive hypertension: role of inducible nitric oxide synthase. Am J Physiol Regul Integr Comp Physiol. 2000;279:R2297–2303. doi: 10.1152/ajpregu.2000.279.6.R2297. [DOI] [PubMed] [Google Scholar]
- 11.Mattson DL, Maeda CY, Bachman TD, Cowley AW Jr. Inducible Nitric Oxide Synthase and Blood Pressure. Hypertension. 1998;31:15–20. doi: 10.1161/01.hyp.31.1.15. [DOI] [PubMed] [Google Scholar]
- 12.Zewde T, Mattson DL. Inhibition of Cyclooxygenase-2 in the Rat Renal Medulla Leads to Sodium-Sensitive Hypertension. Hypertension. 2004;44:424–428. doi: 10.1161/01.HYP.0000140924.91479.03. [DOI] [PubMed] [Google Scholar]
- 13.Yang T, Singh I, Pham H, Sun D, Smart A, Schnermann JB, Briggs JP. Regulation of cyclooxygenase expression in the kidney by dietary salt intake. Am J Physiol Renal Physiol. 1998;274:F481–489. doi: 10.1152/ajprenal.1998.274.3.F481. [DOI] [PubMed] [Google Scholar]
- 14.Li N, Yi F, dos Santos EA, Donley DK, Li P-L. Role of Renal Medullary Heme Oxygenase in the Regulation of Pressure Natriuresis and Arterial Blood Pressure. Hypertension. 2007;49:148–154. doi: 10.1161/01.HYP.0000250086.06137.fb. [DOI] [PubMed] [Google Scholar]
- 15.Harris RC, Breyer MD. Physiological regulation of cyclooxygenase-2 in the kidney. Am J Physiol Renal Physiol. 2001;281:F1–11. doi: 10.1152/ajprenal.2001.281.1.F1. [DOI] [PubMed] [Google Scholar]
- 16.Zou AP CAJ. Role of nitric oxide in the control of renal function and salt sensitivity. Current Hypertension Reports. 1999;1:178–186. doi: 10.1007/s11906-999-0016-7. [DOI] [PubMed] [Google Scholar]
- 17.Yao B, Harris RC, Zhang M-Z. Interactions between 11{beta}-hydroxysteroid dehydrogenase and COX-2 in kidney. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1767–1773. doi: 10.1152/ajpregu.00786.2004. [DOI] [PubMed] [Google Scholar]
- 18.Nakanishi K, Hara N, Nagai Y. Salt-sensitive hypertension in conscious rats induced by chronic nitric oxide blockade. American Journal of Hypertension. 2002;15:150–156. doi: 10.1016/s0895-7061(01)02267-1. [DOI] [PubMed] [Google Scholar]
- 19.Szentivanyi M Jr, Zou A-P, Mattson DL, Soares P, Moreno C, Roman RJ, Cowley AW Jr. Renal medullary nitric oxide deficit of Dahl S rats enhances hypertensive actions of angiotensin II. Am J Physiol Regul Integr Comp Physiol. 2002;283:R266–272. doi: 10.1152/ajpregu.00461.2001. [DOI] [PubMed] [Google Scholar]
- 20.Ikeda Y, Saito K, Kim J-I, Yokoyama M. Nitric Oxide Synthase Isoform Activities in Kidney of Dahl Salt-Sensitive Rats. Hypertension. 1995;26:1030–1034. doi: 10.1161/01.hyp.26.6.1030. [DOI] [PubMed] [Google Scholar]
- 21.Cowley AW Jr, Mori T, Mattson D, Zou A-P. Role of renal NO production in the regulation of medullary blood flow. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1355–1369. doi: 10.1152/ajpregu.00701.2002. [DOI] [PubMed] [Google Scholar]
- 22.Yoshihara F, Suga S, Yasui N, Horio T, Tokudome T, Nishikimi T, Kawano Y, Kangawa K. Chronic administration of adrenomedullin attenuates the hypertension and increases renal nitric oxide synthase in Dahl salt-sensitive rats. Regul Pept. 2005;128:7–13. doi: 10.1016/j.regpep.2004.12.028. [DOI] [PubMed] [Google Scholar]
- 23.Tojo A, Onozato ML, Kobayashi N, Goto A, Matsuoka H, Fujita T. Antioxidative effect of p38 mitogen-activated protein kinase inhibitor in the kidney of hypertensive rat. J Hypertens. 2005;23:165–174. doi: 10.1097/00004872-200501000-00027. [DOI] [PubMed] [Google Scholar]
- 24.Jung F, Palmer LA, Zhou N, Johns RA. Hypoxic Regulation of Inducible Nitric Oxide Synthase via Hypoxia Inducible Factor-1 in Cardiac Myocytes. Circ Res. 2000;86:319–325. doi: 10.1161/01.res.86.3.319. [DOI] [PubMed] [Google Scholar]
- 25.Komatsu DE, Hadjiargyrou M. Activation of the transcription factor HIF-1 and its target genes, VEGF, HO-1, iNOS, during fracture repair. Bone. 2004;34:680–688. doi: 10.1016/j.bone.2003.12.024. [DOI] [PubMed] [Google Scholar]
- 26.Yang Z-Z, Zou A-P. Transcriptional regulation of heme oxygenases by HIF-1alpha in renal medullary interstitial cells. Am J Physiol Renal Physiol. 2001;281:F900–908. doi: 10.1152/ajprenal.2001.281.5.F900. [DOI] [PubMed] [Google Scholar]
- 27.Manotham K, Tanaka T, Ohse T, Kojima I, Miyata T, Inagi R, Tanaka H, Sassa R, Fujita T, Nangaku M. A biologic role of HIF-1 in the renal medulla. Kidney Int. 2005;67:1428–1439. doi: 10.1111/j.1523-1755.2005.00220.x. [DOI] [PubMed] [Google Scholar]
- 28.Bianciardi P, Fantacci M, Caretti A, Ronchi R, Milano G, Morel S, von Segesser L, Corno A, Samaja M. Chronic in vivo hypoxia in various organs: hypoxia-inducible factor-1alpha and apoptosis. Biochem Biophys Res Commun. 2006;342:875–880. doi: 10.1016/j.bbrc.2006.02.042. [DOI] [PubMed] [Google Scholar]
- 29.Zou A-P, Yang Z-Z, Li P-L, Cowley AW Jr. Oxygen-dependent expression of hypoxia-inducible factor-1{alpha} in renal medullary cells of rats. Physiol. Genomics. 2001;6:159–168. doi: 10.1152/physiolgenomics.2001.6.3.159. [DOI] [PubMed] [Google Scholar]
- 30.Cowley AW Jr. Long-term control of arterial blood pressure. Physiol. Rev. 1992;72:231–300. doi: 10.1152/physrev.1992.72.1.231. [DOI] [PubMed] [Google Scholar]
- 31.Brezis M, Heyman SN, Dinour D, Epstein FH, Rosen S. Role of nitric oxide in renal medullary oxygenation. Studies in isolated and intact rat kidneys. J Clin Invest. 1991;88:390–395. doi: 10.1172/JCI115316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang W, Edwards A. Oxygen transport across vasa recta in the renal medulla. Am J Physiol Heart Circ Physiol. 2002;283:H1042–1055. doi: 10.1152/ajpheart.00074.2002. [DOI] [PubMed] [Google Scholar]
- 33.Epstein FH. Oxygen and renal metabolism. Kidney Inter. 1997;51:381–385. doi: 10.1038/ki.1997.50. [DOI] [PubMed] [Google Scholar]
- 34.Palmer LA, Semenza GL, Stoler MH, Johns RA. Hypoxia induces type II NOS gene expression in pulmonary artery endothelial cells via HIF-1. Am J Physiol Lung Cell Mol Physiol. 1998;274:L212–219. doi: 10.1152/ajplung.1998.274.2.L212. [DOI] [PubMed] [Google Scholar]
- 35.Lukiw WJ, Ottlecz A, Lambrou G, Grueninger M, Finley J, Thompson HW, Bazan NG. Coordinate Activation of HIF-1 and NF-{kappa}B DNA Binding and COX-2 and VEGF Expression in Retinal Cells by Hypoxia. Invest Ophthalmol Vis Sci. 2003;44:4163–4170. doi: 10.1167/iovs.02-0655. [DOI] [PubMed] [Google Scholar]
- 36.Hierholzer C, Harbrecht BG, Billiar TR, Tweardy DJ. Hypoxia-inducible factor-1 activation and cyclo-oxygenase-2 induction are early reperfusion-independent inflammatory events in hemorrhagic shock. Arch Orthop Trauma Surg. 2001;121:219–222. doi: 10.1007/s004020000211. [DOI] [PubMed] [Google Scholar]
- 37.Evans RG, Majid DS, Eppel GA. Mechanisms mediating pressure natriuresis: what we know and what we need to find out. Clin Exp Pharmacol Physiol. 2005;32:400–409. doi: 10.1111/j.1440-1681.2005.04202.x. [DOI] [PubMed] [Google Scholar]
- 38.Li N, Chen L, Yi F, Xia M, Li P-L. Salt-Sensitive Hypertension Induced by Decoy of Transcription Factor Hypoxia-Inducible Factor-1 {alpha} in the Renal Medulla. Circ Res. 2008;102:1101–1108. doi: 10.1161/CIRCRESAHA.107.169201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Granger JP, Alexander BT, Llinas M. Mechanisms of pressure natriuresis. Curr Hypertens Rep. 2002;4:152–159. doi: 10.1007/s11906-002-0040-3. [DOI] [PubMed] [Google Scholar]
- 40.Wang T, Sterling H, Shao WA, Yan Q, Bailey MA, Giebisch G, Wang W-H. Inhibition of heme oxygenase decreases sodium and fluid absorption in the loop of Henle. Am J Physiol Renal Physiol. 2003;285:F484–490. doi: 10.1152/ajprenal.00135.2003. [DOI] [PubMed] [Google Scholar]
- 41.Hall JE. The Kidney, Hypertension, and Obesity. Hypertension. 2003;41:625–633. doi: 10.1161/01.HYP.0000052314.95497.78. [DOI] [PubMed] [Google Scholar]
- 42.Bruick RK, McKnight SL. A Conserved Family of Prolyl-4-Hydroxylases That Modify HIF. Science. 2001;294:1337–1340. doi: 10.1126/science.1066373. [DOI] [PubMed] [Google Scholar]
- 43.Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, Salic A, Asara JM, Lane WS, Kaelin WG Jr. HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science. 2001;292:464–468. doi: 10.1126/science.1059817. [DOI] [PubMed] [Google Scholar]
- 44.Jaakkola P, Mole DR, Tian Y-M, Wilson MI, Gielbert J, Gaskell SJ, Kriegsheim Av, Hebestreit HF, Mukherji M, Schofield CJ, Maxwell PH, Pugh CW, Ratcliffe PJ. Targeting of HIF-alpha to the von Hippel-Lindau Ubiquitylation Complex by O2-Regulated Prolyl Hydroxylation. Science. 2001;292:468–472. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- 45.Callapina M, Zhou J, Schnitzer S, Metzen E, Lohr C, Deitmer JW, Brune B. Nitric oxide reverses desferrioxamine- and hypoxia-evoked HIF-1[alpha] accumulation--Implications for prolyl hydroxylase activity and iron. Experimental Cell Research. 2005;306:274–284. doi: 10.1016/j.yexcr.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 46.Baek JH, Mahon PC, Oh J, Kelly B, Krishnamachary B, Pearson M, Chan DA, Giaccia AJ, Semenza GL. OS-9 Interacts with Hypoxia-Inducible Factor 1[alpha] and Prolyl Hydroxylases to Promote Oxygen-Dependent Degradation of HIF-1[alpha] . Molecular Cell. 2005;17:503–512. doi: 10.1016/j.molcel.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 47.Page EL, Chan DA, Giaccia AJ, Levine M, Richard DE. Hypoxia-inducible Factor-1{alpha} Stabilization in Nonhypoxic Conditions: Role of Oxidation and Intracellular Ascorbate Depletion. Mol Biol Cell. 2008;19:86–94. doi: 10.1091/mbc.E07-06-0612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McMahon S, Charbonneau M, Grandmont S, Richard DE, Dubois CM. Transforming Growth Factor beta1 Induces Hypoxia-inducible Factor-1 Stabilization through Selective Inhibition of PHD2 Expression. J Biol Chem. 2006;281:24171–24181. doi: 10.1074/jbc.M604507200. [DOI] [PubMed] [Google Scholar]
- 49.Tug S, Reyes BD, Fandrey J, Berchner-Pfannschmidt U. Non-hypoxic activation of the negative regulatory feedback loop of prolylhydroxylase oxygen sensors. Biochemical and Biophysical Research Communications. 2009;384:519–523. doi: 10.1016/j.bbrc.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 50.Epstein AC, Gleadle JM, McNeill LA, Hewitson KS, O'Rourke J, Mole DR, Mukherji M, Metzen E, Wilson MI, Dhanda A, Tian YM, Masson N, Hamilton DL, Jaakkola P, Barstead R, Hodgkin J, Maxwell PH, Pugh CW, Schofield CJ, Ratcliffe PJ. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell. 2001;107:43–54. doi: 10.1016/s0092-8674(01)00507-4. [DOI] [PubMed] [Google Scholar]
- 51.Li N, Yi F, Sundy CM, Chen L, Hilliker ML, Donley DK, Muldoon DB, Li P-L. Expression and actions of HIF prolyl-4-hydroxylase in the rat kidneys. Am J Physiol Renal Physiol. 2007;292:F207–216. doi: 10.1152/ajprenal.00457.2005. [DOI] [PubMed] [Google Scholar]
- 52.Takeda K, Cowan A, Fong G-H. Essential Role for Prolyl Hydroxylase Domain Protein 2 in Oxygen Homeostasis of the Adult Vascular System. Circulation. 2007;116:774–781. doi: 10.1161/CIRCULATIONAHA.107.701516. [DOI] [PubMed] [Google Scholar]
- 53.Rosenberger C, Rosen S, Shina A, Frei U, Eckardt K-U, Flippin LA, Arend M, Klaus SJ, Heyman SN. Activation of hypoxia-inducible factors ameliorates hypoxic distal tubular injury in the isolated perfused rat kidney. Nephrol Dial Transplant. 2008;23:3472–3478. doi: 10.1093/ndt/gfn276. [DOI] [PubMed] [Google Scholar]
- 54.Takeda K, Aguila HL, Parikh NS, Li X, Lamothe K, Duan L-J, Takeda H, Lee FS, Fong G-H. Regulation of adult erythropoiesis by prolyl hydroxylase domain proteins. Blood. 2008;111:3229–3235. doi: 10.1182/blood-2007-09-114561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schodel J, Klanke B, Weidemann A, Buchholz B, Bernhardt W, Bertog M, Amann K, Korbmacher C, Wiesener M, Warnecke C, Kurtz A, Eckardt K-U, Willam C. HIF-Prolyl Hydroxylases in the Rat Kidney: Physiologic Expression Patterns and Regulation in Acute Kidney Injury. Am J Pathol. 2009;174:1663–1674. doi: 10.2353/ajpath.2009.080687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang Z, Zhu Q, Xia M, Li PL, Hinton SJ, Li N. Hypoxia-inducible factor prolyl-hydroxylase 2 senses high-salt intake to increase hypoxia inducible factor 1alpha levels in the renal medulla. Hypertension. 2010;55:1129–1136. doi: 10.1161/HYPERTENSIONAHA.109.145896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhu Q, Liu M, Han W, Li P-L, Wang Z, Li N. Overexpression of HIF Prolyl-Hydoxylase-2 transgene in the renal medulla induced a salt-sensitive hypertension. J Cell Mol Med. 2012 doi: 10.1111/j.1582-4934.2012.01590.x. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rapp JP. Dahl salt-susceptible and salt-resistant rats. A review. Hypertension. 1982;4:753–763. doi: 10.1161/01.hyp.4.6.753. [DOI] [PubMed] [Google Scholar]
- 59.Tobian L. Salt and hypertension. Lessons from animal models that relate to human hypertension. Hypertension. 1991;17:I52–58. doi: 10.1161/01.hyp.17.1_suppl.i52. [DOI] [PubMed] [Google Scholar]
- 60.Rapp JP. Genetic Analysis of Inherited Hypertension in the Rat. Physiol Rev. 2000;80:135–172. doi: 10.1152/physrev.2000.80.1.135. [DOI] [PubMed] [Google Scholar]
- 61.Cowley AW Jr. The genetic dissection of essential hypertension. Nat Rev Genet. 2006;7:829–840. doi: 10.1038/nrg1967. [DOI] [PubMed] [Google Scholar]
- 62.Zhu Q, Wang Z, Xia M, Li P-L, Zhang F, Li N. Overexpression of HIF-1α transgene in the renal medulla attenuated salt sensitive hypertension in Dahl S rats. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease. 2012;1822:936–941. doi: 10.1016/j.bbadis.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhu Q, Liu M, Han W, Li P-L, Li N. Gene silencing of HIF prolyl-hydroxylase 2 in the renal medulla attenuated sodium retention in Dahl S Rats. FASEB J. 2012;26:684.614. (EB2012 Meeting Abstract) [Google Scholar]


