Abstract
Much is known about the physiological control of stomatal aperture as a means by which plants adjust to water availability. By contrast, the role played by the modulation of stomatal development to limit water loss has received much less attention. The control of stomatal development in response to water deprivation in the genus Populus is explored here. Drought induced declines in stomatal conductance as well as an alteration in stomatal development in two genotypes of Populus balsamifera. Leaves that developed under water-deficit conditions had lower stomatal indices than leaves that developed under well-watered conditions. Transcript abundance of genes that could hypothetically underpin drought-responsive changes in stomatal development was examined, in two genotypes, across six time points, under two conditions, well-watered and with water deficit. Populus homologues of STOMAGEN, ERECTA (ER), STOMATA DENSITY AND DISTRIBUTION 1 (SDD1), and FAMA had variable transcript abundance patterns congruent with their role in the modulation of stomatal development in response to drought. Conversely, there was no significant variation in transcript abundance between genotypes or treatments for the Populus homologues of YODA (YDA) and TOO MANY MOUTHS (TMM). The findings highlight the role that could be played by stomatal development during leaf expansion as a longer term means by which to limit water loss from leaves. Moreover, the results point to the key roles played by the regulation of the homologues of STOMAGEN, ER, SDD1, and FAMA in the control of this response in poplar.
Key words: Drought, gene regulation, guard cells, Populus balsamifera, stomata, Drought
This paper is available online free of all access charges (see http://jxb.oxfordjournals.org/open_access.html for further details)
Introduction
Water availability is a key determinant of plant growth and survival. In keeping with this, plants have evolved mechanisms to modulate physiological and developmental processes so as to match water use and retention with water availability. Stomata, the pores found on plant surfaces, play a key role in regulating water movement and retention in response to the prevailing environmental conditions. For example, episodic water deficit can invoke a decrease in stomatal aperture with a concomitant decrease in water loss from the plant body (Cowan and Farquhar, 1977; Chaves et al., 2003). Although reduction in stomatal aperture in response to drought limits photosynthesis and affects water-use efficiency, it is a short-term response that enables plants to contend with fluctuating water supply (Chaves et al., 2003). Plants can also mount more lasting stomatal-based responses to persistent water deficit (i.e. drought) by controlling stomatal density during development. Lower stomatal density restricts the number of sites for water loss, with an attendant decrease in water loss. Changes in stomatal density are brought about by modulating stomatal development during leaf formation.
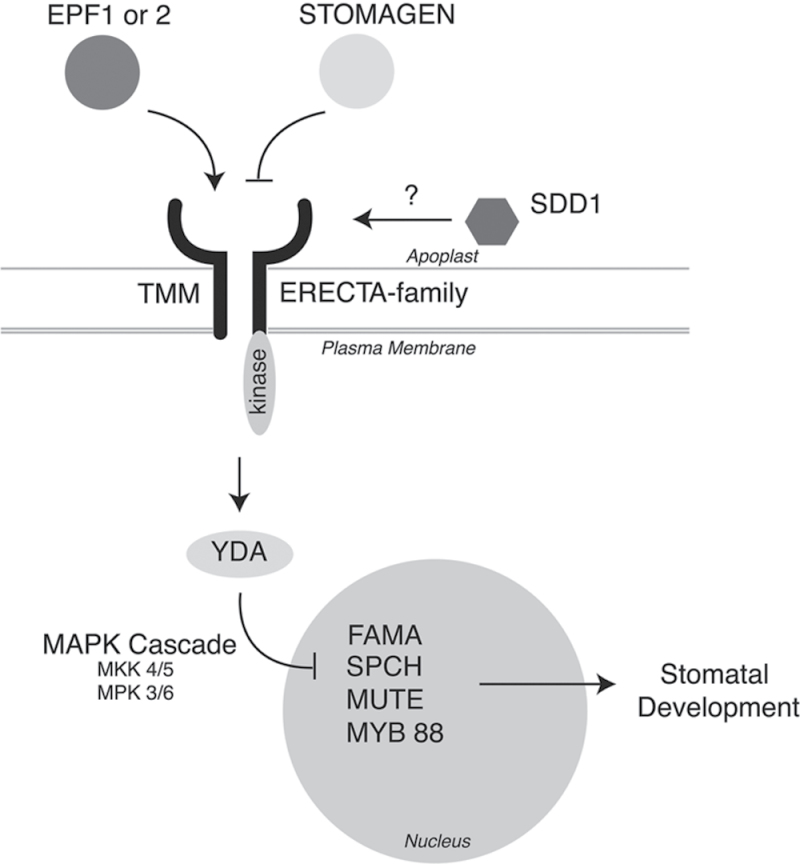
Much is known about stomatal development in Arabidopsis thaliana. Stomatal development proceeds from the asymmetric division of epidermal meristemoid mother cells to the final terminal differentiation of the guard cells that will form the stomate early in leaf development. Many of the components of the regulatory network underlying this terminal differentiation pathway have been characterized (for a review see Bergmann and Sack, 2007; Casson and Hetherington, 2010). Intracellular signalling peptides belonging to the EPIDERMAL PATTERNING FACTOR-LIKE (EPFL) family, such as, EPF-1 and EPF-2, enforce correct stomatal patterning by acting as negative regulators of stomatal development (Hara et al., 2007, 2009). By contrast, STOMAGEN acts as a positive signalling factor in stomatal patterning (Kondo et al., 2010; Sugano et al., 2010). The positive and negative signalling ligands act antagonistically with cell surface receptors, including members of the ERECTA family of leucine rich repeat (LRR) -receptor like kinases (ER, ERL-1, ERL-2) to regulate asymmetric divisions at the onset of stomatal development and spacing divisions (Nadeau and Sack, 2002; Shpak et al., 2005). TOO MANY MOUTHS (TMM) is another LRR-receptor-like protein involved in the modulation of stomatal patterning, which acts synergistically as a signal modulator through interactions with the ER-family of receptors (Lee et al., 2012). In addition, the subtilisin-like protease, STOMATAL DENSITY AND DISTRIBUTION-1 (SDD-1) acts independently of EPF-1 and EPF-2, but also negatively regulates asymmetric cell division (Berger and Altmann, 2000; von Groll et al., 2002). Downstream of the aforementioned receptors, a mitogen activated protein (MAP) kinase signalling cascade is implicated. Activation of the TMM-ER family complex leads to the stimulation of the MAP kinase signalling cascade starting with YODA (YDA), a MAP kinase kinase kinase (Bergmann et al., 2004), which in turn activates MKK4 and MKK5, and finally, MK3 and MK6 (Wang et al., 2007). This signalling cascade negatively regulates stomatal development through three important basic-helix-loop-helix transcription factors, SPEECHLESS (SPCH), MUTE, and FAMA (Fig. 1).
Fig. 1.
The stomatal development signalling network, based on current literature. Arrows represent positive regulation; whereas, blocked lines represent negative regulation. Question marks represent unknown interactions.
The commitment to stomatal development begins with asymmetric cell division of the meristemoid mother cell regulated by a basic-helix-loop-helix transcription factor, SPEECHLESS (MacAlister et al., 2007). Following asymmetric division, cells destined to become guard cells change into a guard mother cell under the control of MUTE (Pillitteri et al., 2007). Each additional amplifying asymmetric division results in the creation of a new meristemoid cell and a larger neighbouring cell. These additional divisions result in the formation of more pavement and stomatal cells. Final differentiation of the stomatal lineage is controlled by another basic-helix-loop-helix transcription factor, FAMA (Pillitteri et al., 2007). Furthermore, an additional class of bHLH transcription factors, SCREAM/ICE1 and SCREAM2 that interact directly with SPCH, MUTE, and FAMA, act to promote the sequential steps in stomatal differentiation (Kanaoka et al., 2008).
In response to environmental change, plants can modulate stomatal development in new leaves (Casson and Hetherington, 2010). As mature leaves sense environmental conditions, stomatal density is adjusted in developing leaves (Lake et al., 2001; Miyazawa et al., 2006). An increase in light quantity positively influences stomatal numbers through the action of PHYTOCHROME B (PHYB) and the downstream transcription factor phytochrome-interacting FACTOR 4 (PIF4). Elevated concentration of carbon dioxide leads to a decline in stomatal density, a phenomenon that has been observed over geological time (Woodward, 1987). In response to CO2, the gene HIGH CARBON DIOXIDE (HIC) modulates stomatal development in Arabidopsis (Gray et al., 2000). Loss-of-function hic mutants exhibit elevated stomatal numbers when grown under elevated CO2 (Gray et al., 2000).
Modification of stomatal density in response to drought varies between plant species, and is contingent on the severity of water deficit. For example, a drought-induced reduction in stomatal numbers was observed in wheat (Quarrie and Jones, 1977), squash cotyledons (Sakurai et al., 1986), and umbu trees (Silva et al., 2009). By contrast, increased stomatal density was observed in grass with moderate drought stress; although, this increase was reversed under conditions of more severe drought stress (Xu and Zhou, 2008). Variation in stomatal density was observed in response to drought in Mediterranean plants (Galmés et al., 2007). No significant alteration to stomatal density in groundnut was observed under drought (Clifford et al., 1995).
The impact of drought on stomatal density in the ecologically and economically important genus Populus is examined here. Focusing on Populus balsamifera, the aim was to determine the impact of drought on stomatal development during leaf formation by testing the hypothesis that drought-induced modification of the transcription of genes implicated in the stomatal development regulatory network are linked to changes in stomatal density. More specifically, we set out to test the hypothesis that the transcript accumulation of positive regulators of stomatal development will be lower in the developing foliar tissue of water-deficit treated trees and, conversely, that transcript accumulation of negative regulators will be higher in the developing foliar tissue of water-deficit-treated trees. Making use of a transcriptome database for leaf development, and a time-course series during leaf formation in the presence and absence of drought, genes involved in the Populus stomatal development network were identified and a subset shown to show a pattern of transcript abundance in keeping with a role in modifying stomatal numbers in response to drought.
Materials and methods
Plant material
Two Populus balsamifera genotypes (AP-1005 and AP-1006) were propagated from unrooted cuttings (Alberta Pacific, Boyle, Alberta, Canada) in Sunshine mix-1 (Sun Gro Horticulture Inc, Bellevue, WA, USA). The cuttings used in this experiment were obtained from the research stoolbeds at the Alberta-Pacific Mill site (Alberta, Canada); however, genotype AP-1005 historically originates from Slave Lake, Alberta, Canada whereas, genotype AP-1006 originates from Smith, Alberta, Canada. A more detailed description of the two P. balsamifera genotypes can be found in Hamanishi et al. (2010). Trees were grown in a climate-controlled growth chamber at the University of Toronto (Toronto, Ontario, Canada) with conditions described by Hamanishi et al. (2010). After nine weeks of growth under well-watered conditions, half of the trees were placed under water-deficit conditions by withholding water, while temperature and light conditions remained constant. At the onset of the water-deficit experiment, the first fully expanded leaf on day 0 of the experiment was marked with a red thread, and the position of the first expanding leaf relative to the first fully expanded leaf on day 0 was recorded. Fully expanded P. balsamifera leaves were at leaf plastochron index (LPI) 7–8 (Larson and Isebrands, 1971); whereas the developing leaves on day 5 were often at LPI=2 and at LPI=4–5 on day 15 after the onset of the water-deficit experiment.
Plant material was harvested at day 0, and every 5 d thereafter until the completion of the 30 d experiment (days 0, 5, 10, 15, 20, 25, and 30). Using three replicates from each treatment–genotype combination, at the harvesting time-point, the first fully expanded leaf marked on day 0 from two trees was collected, pooled, and flash-frozen using liquid nitrogen. This represented a single sample from a single genotype, treatment, time-point combination. Similarly, the first expanding leaf from day 0 was collected from two trees, pooled, and flash-frozen for future analysis. For each sample collection, only two leaves were removed from each tree: the first fully expanded leaf, and the first expanding leaf from day 0. Once leaves were sampled from a given tree, the tree was no longer included in the experiment.
Physiological measurements and stomatal quantification
For each genotype, physiological responses to drought conditions were monitored every 2 d starting from the onset of the water-withholding experiment. Stomatal conductance (g s) measurements were taken using an infrared gas analyser (LI-6400XT Portable Photosynthesis System, Li-Cor Biosciences Inc., Lincoln, NE, USA). Measurements of g s were taken on the mature, fully expanded leaves at the experimental midday time point (n=3–5 per genotype–treatment group). Temperature and relative humidity were maintained at 21.3±0.6 °C and 62.6±2.17%, respectively, for gas exchange measurements. Productivity and relative water content (RWC) was assessed periodically throughout the 30 d experiment. Height and stem diameter were recorded 5, 10, 20, and 30 d after the onset of the water-withholding experiment. Above-ground biomass was determined at the end of the experiment (day 30); plants (n=10) were harvested and above-ground biomass (fresh weight and dry weight) was measured. Leaf RWC was determined using methods described by Hamanishi et al. (2010) 15 and 30 d after the onset of the water-withholding experiment.
Impressions of the abaxial epidermis were taken 30 d after the onset of the water withholding experiment for two classes of leaves. The two classes of leaves included (a) leaves that were fully developed prior to the onset of the experiment (the first fully expanded leaves at day 0) and (b) leaves that expanded during the water-withholding experiment (leaves that were marked as the first emerging leaf at day 0). Impressions of 10 leaves from each class, genotype, and treatment combination were assessed. Abaxial impressions were taken using methods developed by Ceulemans et al. (1995) at the widest point of the leaf (approximately 3cm wide), and the stomatal index was calculated according to Radoglou and Jarvis (1990).
Gene selection
Putative homologues of genes known to be involved in stomatal development were identified from Populus using P. trichocarpa sequence data available on phytozome v7.0 (http://phytozome.net). The protein sequences from Arabidopsis were used as a query for BLAST (BLASTp/PtPEPv2.0) searches against the databases. FAMA (MacAlister and Bergmann, 2011) and STOMAGEN (Kondo et al., 2010) orthologues in Populus have previously been reported. Poplar GeneChip (Affymetrix) probe sets were identified using the NetAffx resource (http://www.affymetrix.com/analysis/index.affx). Transcript abundance for homologues of genes implicated in stomatal development was assessed through interrogation of Populus balsamifera transcript abundance data available in the PopGenExpress compendium (Wilkins et al., 2009b) of the Bio-Array Resource (BAR, http://bar.utoronto.ca/).
Targeted transcript abundance analysis
Three samples were collected from each genotype (AP-1005 and AP-1006), treatment (well-watered and water-deficit-treated) and developmental stage (first fully expanded leaf from day 0). Flash-frozen plant material collected throughout the experiment (days 5, 10, 15, 20, 25, and 30) was ground to a fine powder under liquid nitrogen. Starting with 1–2g frozen ground leaf tissue per sample, total RNA was isolated according to Chang et al. (1993). RNA quality was assessed electrophoretically and spectrophotometrically. 3 µg total RNA was reverse-transcribed using oligio(dT)18 primers and SuperScript II Reverse Transcriptase (Invitrogen) according to manufacturer’s instructions. cDNA was diluted 4-fold prior to qRT-PCR analysis. Using iQ SYBR Green Supermix (Bio-Rad), qRT-PCR was performed for three biological replicates in triplicate according to published protocols (Raj et al., 2011). Relative transcript abundance was determined using the method described by Pfaffl (2001). Primer sequences can be found in Supplementary Table S1 at JXB online.
Statistical analysis
Significant variation in relative transcript abundance was analysed using a general linear model. The general linear model for the 2×2×6 factorial experiment (2 genotypes, 2 treatments, and 6 time-points) is represented by:
| yijk=u+Ai+Bj+Ck+(AB)ij+(AC)ik+(BC)jk+(ABC)ijk+ɛijk |
where A corresponds to genotype with i levels, B corresponds to treatment with j levels, and C corresponds to time-point with k levels. Four possible interactions between genotype and treatment are represented by ij, 12 interactions between genotype and time-point are represented by ik, 12 interactions between treatment and time-point are represented by jk, 24 three-way interactions are represented by ijk, and the random error is ɛijk. The α-level was set to 0.05 for all analyses. Analysis of variance (ANOVA) was determined for all relative transcript abundance profiles for each gene. All analyses were performed using R (R Development Core Team, 2011).
Correlation between relative transcript abundance profiles was calculated using Pearson correlation coefficient analysis in R. Pair-wise analysis of transcript profiles across all samples was compared for each given transcript.
Results
Stomatal conductance (g s) and relative water content (RWC) in response to water-deficit stress
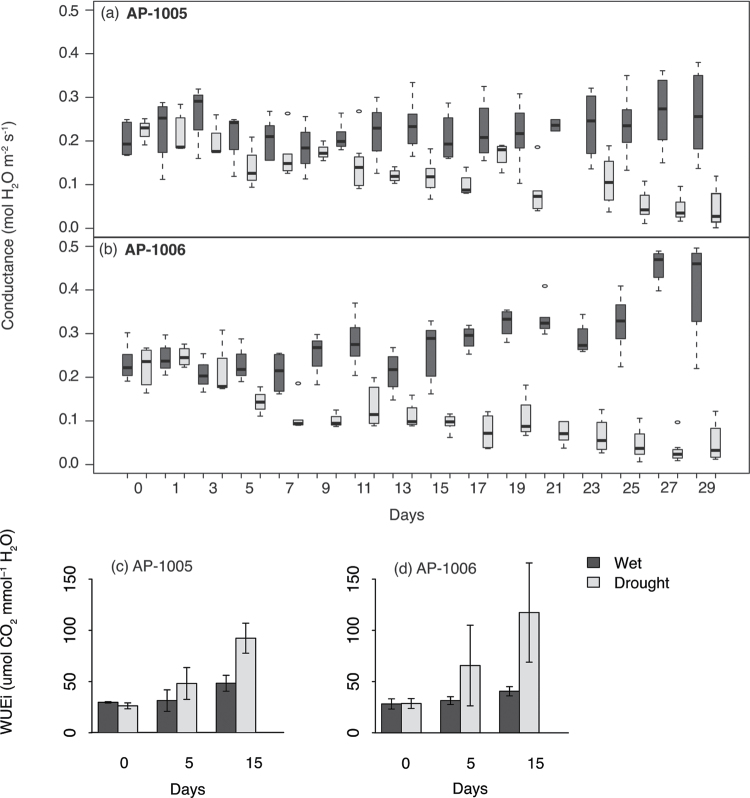
In the two Populus balsamifera genotypes, AP-1005 and AP-1006, g s was significantly lower after 30 d of water withdrawal (t test; P <0.05; Fig. 2). Genotype AP-1006 experienced the largest g s decline in water-deficit-treated plants relative to well-watered samples by day 30 (88% decrease; Fig. 2b). A g s decline in water-deficit-treated samples was observed throughout the drought period for both genotypes; however, AP-1006 exhibited the earliest significant differences between water-deficit and well-watered samples. A significant difference in g s was observed between water-deficit and well-watered samples as early as 7 d after water-withdrawal in AP-1006; whereas, AP-1005 did not show any significant differences in g s until 15 d after the onset of the drought experiment (P <0.05, Fig. 2a, b). In AP-1005 and AP-1006, RWC was significantly lower in water-deficit-treated plants after 30 d of water withdrawal when compared with well-watered trees (see Supplementary Table S2 at JXB online). Similar to the declines observed in g s, genotype AP-1006 had a more severe reduction in RWC when compared with AP-1005. Overall, there was an increase in the intrinsic water use efficiency (WUE=A/g s; Seibt et al., 2008) in drought-treated samples on day 15 (Fig. 2c, d), where a larger increase in intrinsic WUE was observed in genotype AP-1006.
Fig. 2.
Variation in the physiological response to drought stress in genotype AP-1005 and AP-1006. Box plot of the variation in midday stomatal conductance for (a) AP-1005 and (b) AP-1006 for well-watered (shaded boxes) and water-deficit-treated (white boxes) samples. Response of intrinsic water use efficiency (WUEi; A/g s) across well watered and water-deficit-treated samples for (c) AP-1005 and (d) AP-1006 at days 0, 5, and 15.
Stomatal quantification following water-deficit stress
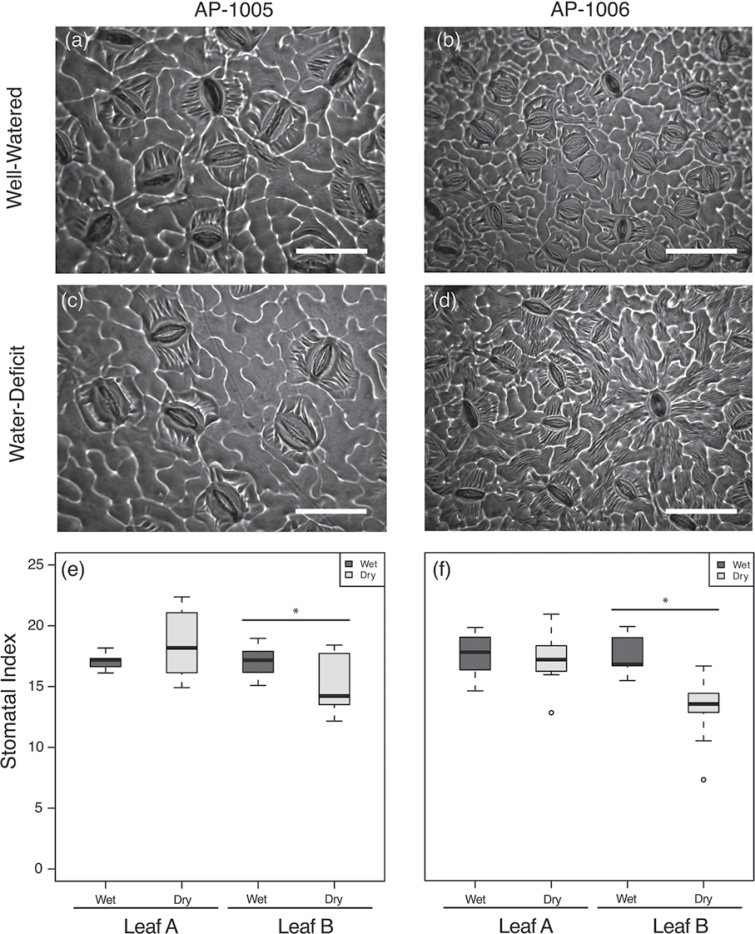
Abaxial leaf stomatal density and index was lower in leaves that developed under water-deficit conditions when compared with leaves that developed under well-watered conditions for both genotypes (Fig. 3). Leaves that were fully developed prior to the onset of the drought experiment had no significant variation between treatments in their stomatal indices. Under well-watered conditions, AP-1006 had the highest stomatal index; however, the leaves of genotype AP-1006 that developed under water-deficit conditions had the lowest stomatal indices. The significant reductions in stomatal index were observed for AP-1005 and AP-1006 at 12% and 25% respectively. Notably, significant variation between treatment and genotype were observed with respect to stomatal index (P <0.05).
Fig. 3.
Variation in leaf epidermis between genotype AP-1005 and AP-1006 under (a, b) well-watered and (c, d) water-deficit conditions, on day 30. White scale bar=50 µm. (e, f) Box plot of variation in stomatal index for well-watered (shaded box) and water-deficit-treated (white box) samples for leaves that were fully developed prior to the onset of the drought experiment (leaf A) and for those that developed during the drought experiment (leaf B). A significant reduction in stomatal index is observed in leaves that developed during the experimental period (leaf B) for each genotype (e) AP-1005 and (f) AP-1006; however, no significant variation in stomatal index is observed for leaves that developed prior to the experiment (leaf A), and the onset of water-deficit conditions. Asterisks represent significant variation between well watered and water-deficit-treated plants. *P <0.05.
Populus homologues of genes implicated in stomatal development
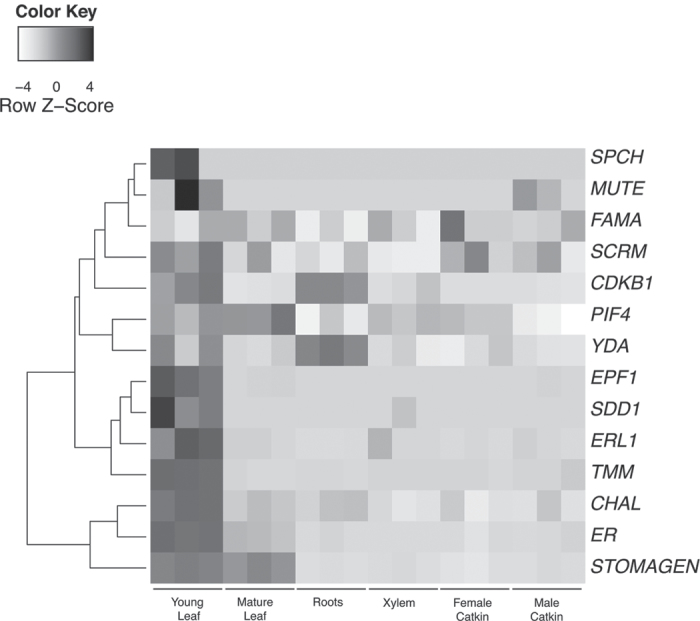
Stomatal development is a function of the integration of many different endogenous and exogenous signals. Many of the genes involved in the underlying pathways regulating stomatal development in Arabidopsis have been identified (for a review see Bergmann and Sack, 2007). Homologues of genes underlying stomatal development in Arabidopsis thaliana are found in Populus. The PopGenExpress transcript abundance compendium (Wilkins et al., 2009a) shows that many of these homologues have relatively greater transcript abundance in young leaves compared with other plant organs, consistent with their role in modulating stomatal development (Fig. 4).
Fig. 4.
Heat map of transcript abundance across a range of tissues for Populus homologues of genes implicated in stomatal differentiation and patterning. Transcript accumulation for the 14 Populus homologues that had differential transcript abundance across the dataset, was derived from the PopGenExpress microarray compendium made available via http://bar.utoronto.ca (Wilkins et al., 2009a). As per the scale provided, elevated transcript abundance is represented by dark grey and diminished transcript abundance is represented by light grey. The highest levels of transcript abundance for this group of genes are in young leaves in contrast to other tissue types. Each column represents a discrete biological sample, and data are represented as biological triplicate replicates for each tissue type. Data are row normalized.
Developmental variation in transcript abundance of stomatal development genes after water deficit
The transcript abundance profiles of six genes with putative roles modulating stomatal development in Populus were examined through development under well-watered and water-deficit conditions using qPCR. Based on previous studies (Bergmann and Sack, 2007; Casson and Hetherington, 2010), the genes selected are believed to play roles in the development pathway, ranging from receptors in the signalling cascade to transcription factors regulating the final differentiation step in the stomatal lineage. The fold change variation in transcript abundance between well-watered and water-deficit-treated samples revealed variation between time-points, treatments or genotypes (Fig. 5). Total cumulative transcript abundance after 30 d of water-withdrawal varied considerably among genotypes for all genes analysed in this experiment (Table 1). A factorial ANOVA analysis revealed significant variation among days for all genes analyzed, with peaks in transcript abundance observed early in the experiment, corresponding to earlier in leaf development (see Supplementary Table S3 at JXB online).
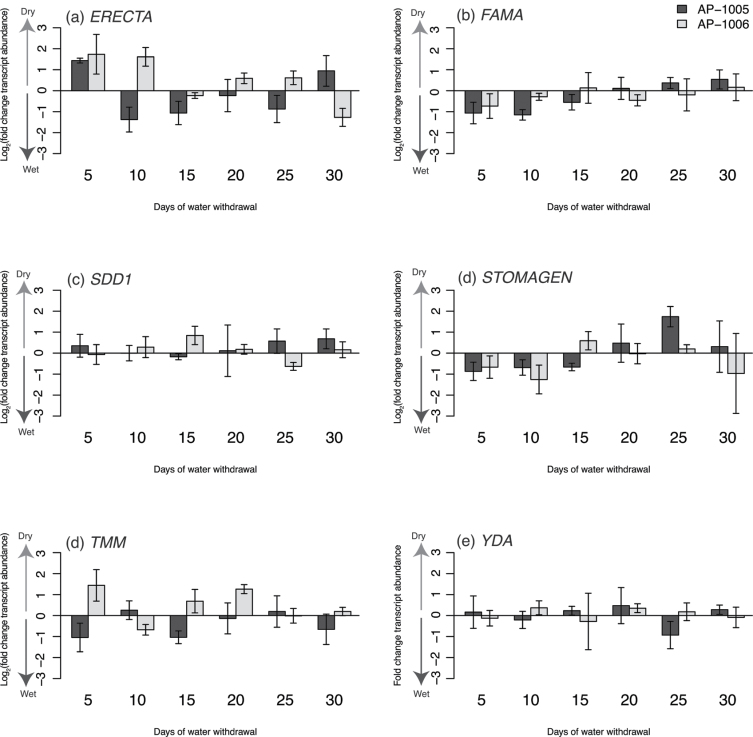
Fig. 5.
Variation in transcript abundance between well-watered and water-deficit-treated trees at six time points (days 5, 10, 15, 20, 25, and 30) for genotype AP-1005 (dark grey) and AP-1006 (light grey) represented by the log2 (fold change transcript abundance) for genes involved in stomatal development. A positive log2 (fold change transcript abundance) value is an indicator of higher transcript abundance in water-deficit-treated samples, whereas a negative log2 (fold change transcript abundance) value is an indicator of lower transcript abundance in water-deficit-treated samples.
Table 1. Mean cumulative transcript abundance across all time-points for genotype AP-1005 and AP-1006 in well-watered and water-deficit-treated samples
| AP-1005 | AP-1006 | |||
| Wet | Dry | Wet | Dry | |
| ER | 13.48±1.66 | 10.21±1.07 | 6.46±1.18 | 8.90±1.64 |
| FAMA | 8.04±0.81 | 5.81±0.27 | 10.82±1.67 | 6.55±0.43 |
| SDD1 | 7.67±0.29 | 8.34±0.19 | 8.39±1.61 | 6.57±0.65 |
| STOMAGEN | 11.84±1.94 | 9.55±1.03 | 14.78±2.25 | 9.03±1.57 |
| TMM | 7.67±0.58 | 5.22±0.34 | 8.21±0.67 | 8.91±0.31 |
| YDA | 7.47±0.38 | 7.12±0.34 | 10.39±1.99 | 7.04±0.53 |
TMM (see Supplementary Fig. S2c at JXB online) and YDA (see Supplementary Fig. S3c at JXB online) only had significant differential transcript abundance among days (ANOVA; P <0.05). The highest transcript levels of TMM were observed on day 10 in the experimental period; whereas, YDA had peak transcript accumulation on day 5, with no significant difference in transcript accumulation among the first three time points (see Supplementary Fig. S3c at JXB online). Both TMM and YDA exhibited gradual declines in transcript abundance after the peak in transcript abundance was observed, with mean transcript levels declining below a 1-fold induction by the end of the experimental timeframe (see Supplementary Figs. S1 and S3 at JXB online).
The transcript abundance profiles for ERECTA (ER), FAMA, STOMAGEN, and STOMATAL DENSITY AND DISTRIBUTION 1 (SDD1) exhibited significant genotype×day interactions (ANOVA; P <0.05). Transcript abundance profiles of ER for both genotypes had many differences (see Supplementary Fig. S4 at JXB online). The highest levels of ER transcript abundance for well-watered samples in AP-1005 were observed later in the experimental period (day 15 and day 20) when compared with water-deficit-treated samples; however, the shift in transcript abundance between treatments was not as evident in AP-1006. For ER (see Supplementary Fig. S4c at JXB online) and SDD1 (see Supplementary Fig. S5c at JXB online), a more rapid and severe decline in transcript abundance was observed in AP-1006; however, the rapid decline in transcript abundance was not as evident in AP-1005.
Significant variation in ER and SDD1 transcript abundance was observed between genotypes (ANOVA; P <0.05). An overall reduction in transcript abundance for ER and SDD1 was observed in AP-1006 (see Supplementary Figs. S4d and S5d at JXB online). ER had a 55% reduction in total transcript abundance in AP-1006 relative to AP-1005. The reduction observed in genotype AP-1006 for SDD1 was not as severe.
FAMA and STOMAGEN also had significant treatment×day interactions (ANOVA; P <0.05). Genotypic influences on transcript abundance variation were less evident for FAMA and STOMAGEN. Significantly higher transcript abundance was found on days 5 and 10 for FAMA in well-watered samples (see Supplementary Fig. S6 at JXB online). Although there was reduced variation in FAMA transcript abundance in the later part of the experiment (days 15 through 30), FAMA had a significant treatment main effect. Like FAMA, STOMAGEN had significantly higher transcript abundance in well-watered samples on day 10 compared with the low variation observed between treatments for all other days examined (see Supplementary Fig. S7c at JXB online). Peak transcript abundance of STOMAGEN on day 10 is observed for both genotypes; however, the highest STOMAGEN transcript abundance for water-deficit-treated samples was day 10 for AP-1005 and day 15 for AP-1006 (see Supplementary Fig. S7 at JXB online). Significant variation between treatments was also observed for STOMAGEN (ANOVA; P <0.1; see Supplementary Fig. S4 at JXB online).
Genes acting as positive regulators in stomatal development have correlated transcript profiles
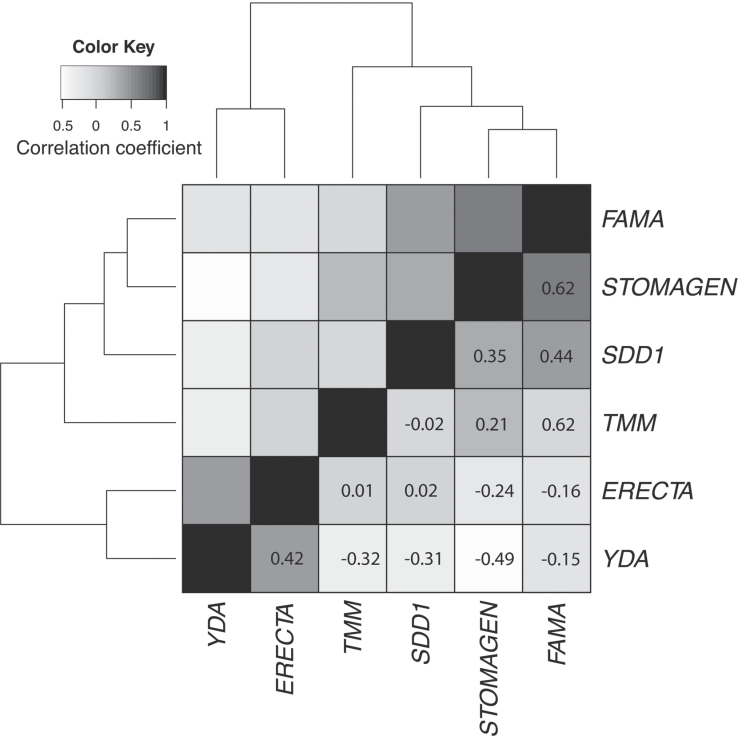
The transcript abundance profiles of FAMA and STOMAGEN are more highly correlated (r=0.62; Fig. 6) than the negative regulators of stomatal development. The homologuous FAMA and STOMAGEN show inverse correlation with other negative regulators of stomatal development analysed in this experiment.
Fig. 6.
Pearson correlation coefficient (PCC) heat map representing the transcript abundance profiles across AP-1005 and AP-1006 and six time-points. The PCC was determined for each pair-wise comparison (gene–gene), and is represented by the colour in the corresponding cell. All genes are represented in the same order on the x- and y-axes.
Discussion
Optimization of carbon uptake and water loss, regulated through the modulation of stomatal function and development, is critical for plant survival against a background of fluctuating environmental conditions. Environmental cues such as light and CO2 concentrations have been shown to modulate stomatal development in Arabidopsis thaliana (Casson and Hetherington, 2010). There is relatively scant information about the role that water availability plays in the control of stomatal development in terrestrial plants (Casson and Hetherington, 2010). The modulation of stomatal development in response to water deficit in Populus was explored here and the role of candidate genes in the regulation of this process was examined.
Drought response varied between Populus balsamifera genotypes over time
An increase in intrinsic WUE was observed in water-deficit-treated trees, probably attributable to the decline in stomatal conductance observed in the water-deficit-treated plants (Fig. 2). After 30 d of water-deficit, AP-1006 had the most severe reduction in stomatal conductance, yet it also had the largest reduction in RWC. Variation between poplar genotypes in their physiological response to drought stress is often observed, and hypothesized to be a result of various strategies to contend with drought-stress (Marron et al., 2002; Zhang et al., 2004). The changes in physiological status of the trees in response to water-deficit stress, including an increase in intrinsic WUE, and the decline in RWC and stomatal conductance in both genotypes after the imposition of water-deficit conditions may have been responsible for drought-induced modifications to leaf development.
In response to changes in water availability, leaf morphology can vary considerably (Pena-Rojas et al., 2005). Specifically, with respect to stomatal numbers, modification to environmental factors that influence mature leaf g s will have lasting effects on the stomatal differentiation of new leaves (Miyazawa et al., 2006). Two P. balsamifera genotypes, genotype AP-1005 and AP-1006, had a reduction in stomatal index in response to drought stress, indicating a potential impact on leaf development by a reduction in water availability. A greater reduction in stomatal index was observed in AP-1006 (Fig. 3). The anatomical variation observed between these two genotypes with respect to stomatal numbers supports the greater reduction in g s observed in AP-1006 at the end of the experiment (Fig. 2). The greater reduction in stomatal index in AP-1006 may be a result of the reduction in water availability and its influence on whole plant water status. A reduction in stomatal index would result in a long-term strategy to regulate water loss during prolonged periods of drought stress, which would ultimately be reflected in larger declines in g s between well-watered and water-deficit-treated samples. As integral regulators of carbon uptake and plant water relations, modulation of stomatal differentiation will influence long-term plant WUE and productivity (Casson and Hetherington, 2010).
Transcript abundance of the Populus homologues of key stomatal development regulatory genes varied through leaf development in a manner consistent with their proposed molecular functions
Transcript abundance for the Populus TMM orthologue was highly variable between days (Fig. 5d; see Supplementary Fig. S2 at JXB online). TMM, a LRR receptor like protein, functions primarily in the modulation of stomatal number and regulation of stomatal patterning in A. thaliana (Nadeau and Sack, 2002). In A. thaliana, a single loss of function mutation in TMM results in an excess of stomata arranged in clusters (Nadeau and Sack, 2002). Although limited variation in TMM transcript abundance was observed in P. balsamifera, peak transcript abundance was observed on day 10 of the experimental period, early in leaf development (see Supplementary Fig. S2c at JXB online). In A. thaliana, the transcript abundance of TMM is highest in the early stages of the stomatal lineage, in the young meristemoid mother cell; whereas transcripts were absent in mature guard cells (Nadeau and Sack, 2002). Transcript abundance for the P. balsamifera TMM homologue peaked at the early stages of leaf development, and may be congruent with its role previously described in A. thaliana.
Like TMM, there was significant variation in Populus YDA transcript abundance throughout leaf development; however, peak transcript abundance for P. balsamifera YDA occurred on day 5, presaging the TMM peak (see Supplementary Fig. S3 at JXB online). YDA encodes a mitogen-activated protein (MAP) kinase signalling cascade that is involved in the regulation of stomatal differentiation downstream of the TMM–ERF receptors. In A. thaliana, loss-of-function mutations in the YDA-encoded MAP kinase kinase kinase result in excess stomatal proliferation (Bergmann et al., 2004). Although transcript abundance for the Populus YDA homologue did not have a significant treatment or genotype effect in the experiments described here, it had the highest transcript abundance early in the experiment, and development, congruent with its early role in stomatal development.
Elevated Populus ERECTA (ER) transcript abundance early in development corresponded with decreased stomatal indices
Transcript abundance analysis revealed significant genotype, day, treatment×day and genotype×day effects for a Populus ER homologue (see Supplementary Fig. S4 at JXB online) over the course of the experiment. In A. thaliana, ER appears to regulate the initial decision of cells to enter the stomatal lineage and is important for correct stomatal differentiation. Consistent with this, a single loss-of-function mutation, er, in A. thaliana results in a higher number of stomatal-lineage ground cells as well as guard cells (Shpak et al., 2005). In the experiment described here, higher transcript abundance in water-deficit-treated samples, relative to well-watered samples, was observed 5 d after the onset of the drought experiment for both poplar genotypes (Fig. 5a). In both poplar genotypes, a negative relationship between the stomatal indices of leaves that developed under water-deficit stress and ER transcript abundance (see Supplementary Fig. S8a at JXB online). Declines in stomatal numbers have been observed in response to increased ER transcript abundance in A. thaliana (Masle et al., 2005). Similarly, over-expression in Arabidopsis of a Populus ER orthologue (PdERECTA) conferred decreased stomatal numbers. Thus, transcript abundance patterns early in the experiments are consistent with ER playing a role in the suppression of stomatal numbers in Populus under water withdrawal conditions.
Although declines in stomatal index were observed in samples with high ER transcript abundance on day 5, this pattern was not consistent throughout the developmental period. Increased variability in transcript abundance patterns between well-watered and water-deficit samples were observed after day 10 for both genotypes. The variation observed for the day×genotype interaction (see Supplementary Fig. S4c at JXB online) highlights the variation observed between AP-1005 and AP-1006. A gradual decline in ER transcript abundance was observed in AP-1006, earlier in development; whereas a decline in transcript abundance was not observed until after day 15 in AP-1005. This could be a result of genotypic plasticity or the influence of the redundancy of ER and functional paralogues, ERECTA-LIKE 1 (ERL1) and ERECTA-LIKE 2 (ERL2), that are known to act together in the negative regulation of stomatal development (Shpak et al., 2005). However, the significant decline in overall ER transcript abundance as determined by the genotypic variation (see Supplementary Fig. S4c, d at JXB online) may indicate a more fundamental role of ER in plant development. ER plays an important role in regulating leaf and whole plant development, and is not restricted to its involvement in stomatal development (Tisne et al., 2011). Elevated ER transcript abundance was observed in AP-1005 that demonstrated significantly more height growth than AP-1006 (Table 2).
Table 2. Mean plant height (in cm) on day 30 ±standard error of the mean, n ≥6
| AP-1005 | AP-1006 | P-value | |
| Well-watered | 78.81±2.73 | 67.70±3.03 | 0.0083* |
| Water deficit | 69.00±3.81 | 63.06±2.98 | 0.1811 |
STOMATAL DENSITY AND DISTRIBUTION 1 (SDD1) and genotype-specific control of stomatal development
Loss of SDD1 function in A. thaliana leads to significant increases in stomatal density. SDD1 is a subtilisin-like Ser protease that is predominantly expressed in stomatal precursor cells, and activates ER and TMM to repress stomatal development (von Groll et al., 2002). Despite its prominent role in A. thaliana stomatal development, no significant variation with respect to treatment was observed in the transcript abundance pattern of the Populus SDD1 homologue over the course of the experiment in either genotype (see Supplementary Fig. S5 at JXB online); however, significant variation between genotypes was observed. SDD1 transcript abundance in AP-1005 was significantly higher than in AP-1006 (see Supplementary Fig. S5c at JXB online). The reduction in transcript abundance in AP-1006 may reflect an alteration in signalling mechanisms to the underlying signalling cascade regulating stomatal development in this genotype.
Stomatal development and the regulation of Populus STOMAGEN and FAMA transcript abundance in response to water deficit
STOMAGEN encodes a peptide that positively regulates stomatal density. The STOMAGEN peptide is thought to act through antagonistic competition with other peptide signalling molecules, EPIDERMAL PATTERNING FACTOR 1 and 2 (EPF1 and EPF2), through the LRR-receptor like protein, TMM (Kondo et al., 2010; Sugano et al., 2010). In A. thaliana, STOMAGEN, is derived from the mesophyll-tissue, unlike EPF1 and EPF2 that are primarily expressed in the leaf epidermis, specifically the early meristemoid cells, guard mother cells and guard cells (Kondo et al., 2010; Sugano et al., 2010). In A. thaliana, over-expression of STOMAGEN increased stomatal density; whereas loss of STOMAGEN function decreased stomatal density (Kondo et al., 2010; Sugano et al., 2010).
In the P. balsamifera drought experiment described here, there was significant variation in STOMAGEN transcript abundance among days and between treatments (see Supplementary Fig. S7 and Table S3 at JXB online). ANOVA revealed significant day×treatment and day×genotype interactions for STOMAGEN transcript abundance, suggesting a role for changes in STOMAGEN transcript abundance in genotype- and treatment-dependent differences in the regulation of stomatal development. The most severe log2(fold-change) reduction in STOMAGEN transcript abundance between well-watered and water-deficit-treated samples were observed in AP-1006 on day 10 (Fig. 5d). Notably, the genotype with the lowest stomatal index in the water-deficit treated samples was AP-1006. A positive relationship between STOMAGEN transcript abundance on day 10 and stomatal index was observed (see Supplementary Fig. S8b at JXB online), consistent with the Populus homologue of STOMAGEN playing a role in the control of stomatal density.
In Populus hybrids, the stomatal index of new leaves is highly correlated with stomatal conductance and the physiological status of fully developed leaves suggesting that a long-distance signalling mechanism is used to regulate stomatal development (Miyazawa et al., 2006). STOMAGEN is expressed in the mesophyll tissue of immature Arabidopsis leaves (Sugano et al., 2010); however, stomata are derived from cells on the epidermal layer of leaves. STOMAGEN may play a role in this long-distance signalling mechanism by acting as a signalling molecule between the mesophyll and the epidermal layer in leaves. In the Populus drought experiment, reduced stomatal conductance was observed in response to water-deficit conditions and, similarly, plants exposed to water-deficit conditions had reduced transcript accumulation of the STOMAGEN homologue together with reduced stomatal indices. It may be that, in response to water-deficit stress in Populus, STOMAGEN optimizes long-term carbon uptake and water loss through its role as a mesophyll-derived signalling factor modulating stomatal development.
Similar to STOMAGEN, FAMA transcript accumulation was highest in the early stages of development, with peak transcript abundance at day 5 and 10 (see Supplementary Fig. S6 at JXB online). In A. thaliana, FAMA is required for the final stages of stomatal differentiation, exhibiting the greatest transcript abundance in differentiating guard cells, with declining transcript accumulation as guard cells mature (Ohashi-Ito and Bergmann, 2006). In the Populus drought experiment, significant variation in FAMA transcript accumulation was observed between days and treatment (see Supplementary Fig. S6 at JXB online). The lower levels of transcript accumulation in water-deficit-treated samples on days 5 and 10 for both genotypes (Fig. 5b) and the lower stomatal numbers observed in water-deficit-treated P. balsamifera samples (see Supplementary Fig. S8a, b at JXB online) are consistent with the role described for FAMA in A. thaliana. Variation among water-deficit-treated samples throughout the experimental period is considerably less than in the well-watered samples. As a transcription factor that is both required and sufficient for the final stages of differentiation, a minimum accumulation of FAMA transcript may be required for correct stomatal development. Regardless, the elevated FAMA transcript abundance suggests a role for modulation of this gene to control stomatal development in Populus under drought conditions.
Despite our knowledge of other genes that influence stomatal development, it is not yet known how their expression is influenced by water-deficit stress or how they may influence the stomatal index in Populus. Further exploration of these known players in the stomatal development pathway may provide an increased insight into the long-term modulation of stomatal development, including genotypic variation, in response to water-deficit stress. Although it is evident that some players in the basal stomatal development pathway show altered transcript abundance under water-deficit conditions, an important question to consider is how water-deficit cues are perceived and integrated into the stomatal developmental pathway. Such foci will undoubtedly provide fertile grounds for future research.
Conclusion
In response to water-deficit stress, P. balsamifera demonstrated significant declines in stomatal conductance and RWC. Intraspecific variation in physiological responses between two P. balsamifera genotypes was observed. The largest declines in physiological status were observed in AP-1006 in response to the imposition of water-deficit conditions. Reductions in stomatal indices were also observed in both genotypes; however, declines in stomatal index in AP-1006 were markedly larger than AP-1005. Quantification of transcript abundance profiles of a subset of genes involved in stomatal development under well-watered and water-deficit-treated conditions revealed variation between genotypes, as well as between treatments. Notably, STOMAGEN, a mesophyll-derived signalling peptide, had significantly higher transcript abundance in well-watered samples on days 5 and 10 for both genotypes that corresponded with higher stomatal indices, congruent with its role as a positive regulator in stomatal development. ERECTA transcript abundance was reduced in well-watered samples on day 5 for both genotypes; however, variation in transcript abundance later in development may be a result of the other roles of ERECTA in plant development. Variation in transcript accumulation of ER and SDD1 between genotypes may indicate variation in drought-response strategies, specifically with respect to the modulation of development in response to water-deficit stress. TMM and YDA may not have notable roles in the regulation of stomatal differentiation in response to drought.
Supplementary Material
Supplementary data
Supplementary data can be found at JXB online.
Supplementary Table S1. Primers used for qRT-PCR analysis.
Supplementary Table S2. Relative water content (RWC) on day 30.
Supplementary Table S3. ANOVA results: tanscript abundance.
Supplementary Fig. S1. Absolute fold change transcript abundance between well-watered and water-deficit-treated samples for genotype AP-1005 (dark grey) and AP-1006 (light grey) at the six time-points through the water-withdrawal experiment for (a) ER, (b) FAMA, (c) SDD1, (d) STOMAGEN, (e) TMM, and (f) YDA.
Supplementary Fig. S2. Variation in the relative transcript accumulation of a Populus TMM homologue as determined by qRT-PCR.
Supplementary Fig. S3. Variation in the relative transcript accumulation of a Populus YODA homologue as determined by qRT-PCR.
Supplementary Fig. S4. Variation in the relative transcript accumulation of a Populus ERECTA homologue as determined by qRT-PCR.
Supplementary Fig. S5. Variation in the relative transcript accumulation of a Populus STOMATAL DENSITY AND DISTRIBUTION-1 homologue as determined by qRT-PCR.
Supplementary Fig. S6. Variation in the relative transcript accumulation of a Populus FAMA homologue as determined by qRT-PCR.
Supplementary Fig. S7. Variation in the relative transcript accumulation of a Populus STOMAGEN homologue as determined by qRT-PCR.
Supplementary Fig. S8. Pearson correlation coefficient (PCC) heat map representing the transcript accumulation profiles and stomatal indices in P. balsamifera at (a) 5 d, (b) 10 d, and (c) 15 d after the imposition of water-deficit stress.
© 2012 The Authors.This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/2.0/uk/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Acknowledgements
We are most grateful to Bruce Hall and Andrew Petrie for excellent greenhouse assistance, John McCarron for the experimental set-up, Joan Ouellette for technical assistance, and Dave Kamelchuk (Al-Pac) for collecting all the plant materials. We would also like to extend our gratitude for helpful comments and feedback received by two anonymous reviewers. Research infrastructure and technical support was generously provided by the Centre for Analysis of Genome Evolution and Function at the University of Toronto. ETH was supported by an Ontario Graduate Scholarship in Science and Technology. This work was supported by generous funding from the Natural Sciences and Engineering Research Council of Canada (NSERC) and the University of Toronto to MMC.
References
- Berger D, Altmann T. A subtilisin-like serine protease involved in the regulation of stomatal density and distribution in Arabidopsis thaliana. Genes and Development. 2000;14:1119–1131. [PMC free article] [PubMed] [Google Scholar]
- Bergmann DC, Lukowitz W, Somerville CR. Stomatal development and pattern controlled by a MAPKK kinase. Science. 2004;304:1494–1497. doi: 10.1126/science.1096014. [DOI] [PubMed] [Google Scholar]
- Bergmann DC, Sack FD. Stomatal development. Annual Review of Plant Biology. 2007;58:163–181. doi: 10.1146/annurev.arplant.58.032806.104023. [DOI] [PubMed] [Google Scholar]
- Casson SA, Hetherington AM. Environmental regulation of stomatal development. Current Opinion in Plant Biology. 2010;13:90–95. doi: 10.1016/j.pbi.2009.08.005. [DOI] [PubMed] [Google Scholar]
- Ceulemans R, van Praet L, Jiang XN. Effects of CO2 enrichment, leaf position and clone on stomatal index and epidermal cell density in poplar (Populus) New Phytologist. 1995;131:99–107. doi: 10.1111/j.1469-8137.1995.tb03059.x. [DOI] [PubMed] [Google Scholar]
- Chang S, Puryear J, Cairney J. A simple and efficient method for isolating RNA from pine trees. Plant Molecular Biology Reporter. 1993;11:113–116. [Google Scholar]
- Chaves MM, Maroco JP, Pereira JS. Understanding plant responses to drought: from genes to the whole plant. Functional Plant Biology. 2003;30:239–264. doi: 10.1071/FP02076. [DOI] [PubMed] [Google Scholar]
- Clifford SC, Black CR, Roberts JA, Stronach IM, Singleton-Jones PR, Mohamed AD, Azam-Ali SN. The effect of elevated atmospheric CO2 and drought on stomatal frequency in groundnut (Arachis hypogaea L.) Journal of Experimental Botany. 1995;46:847–852. [Google Scholar]
- Cowan L, Farquhar G. 1977. Stomatal function in relation to leaf metabolism and environment . In: Jennings DH , ed. Integration of activity in the higher plant Cambridge University Press: 471 505 [PubMed] [Google Scholar]
- Galmés J, Flexas J, Savé R, Medrano H. Water relations and stomatal characteristics of Mediterranean plants with different growth forms and leaf habits: responses to water stress and recovery. Plant and Soil. 2007;290:139–155. [Google Scholar]
- Gray JE, Holroyd GH, van der Lee FM, Bahrami AR, Sijmons PC, Woodward FI, Schuch W, Hetherington AM. The HIC signalling pathway links CO2 perception to stomatal development. Nature. 2000;408:713–716. doi: 10.1038/35047071. [DOI] [PubMed] [Google Scholar]
- Hamanishi ET, Raj S, Wilkins O, Thomas BR, Mansfield SD, Plant AL, Campbell MM. Intraspecific variation in the Populus balsamifera drought transcriptome. Plant, Cell and Environment. 2010;33:1742–1755. doi: 10.1111/j.1365-3040.2010.02179.x. [DOI] [PubMed] [Google Scholar]
- Hara K, Kajita R, Torii KU, Bergmann DC, Kakimoto T. The secretory peptide gene EPF1 enforces the stomatal one-cell-spacing rule. Genes and Development. 2007;21:1720–1725. doi: 10.1101/gad.1550707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara K, Yokoo T, Kajita R, Onishi T, Yahata S, Peterson KM, Torii KU, Kakimoto T. Epidermal cell density is autoregulated via a secretory peptide, EPIDERMAL PATTERNING FACTOR 2 in Arabidopsis leaves. Plant and Cell Physiology. 2009;50:1019–1031. doi: 10.1093/pcp/pcp068. [DOI] [PubMed] [Google Scholar]
- Kanaoka MM, Pillitteri LJ, Fujii H, Yoshida Y, Bogenschutz NL, Takabayashi J, Zhu J-K, Torii KU. SCREAM/ICE1 and SCREAM2 specify three cell-state transitional steps leading to Arabidopsis stomatal differentiation. The Plant Cell. 2008;20:1775–1785. doi: 10.1105/tpc.108.060848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo T, Kajita R, Miyazaki A, et al.2010Stomatal density is controlled by a mesophyll-derived signaling molecule Plant and Cell Physiology 511–8. [DOI] [PubMed] [Google Scholar]
- Lake JA, Quick WP, Beerling DJ, Woodward FI. Plant development: signals from mature to new leaves. Nature. 2001;411:154–154. doi: 10.1038/35075660. [DOI] [PubMed] [Google Scholar]
- Larson PR, Isebrands JG. The Plastochron Index as applied to developmental studies of cottonwood. Canadian Journal of Forest Research. 1971;1:1–11. [Google Scholar]
- Lee JS, Kuroha T, Hnilova M, Khatayevich D, Kanaoka MM, McAbee JM, Sarikaya M, Tamerler C, Torii KU. Direct interaction of ligand–receptor pairs specifying stomatal patterning. Genes and Development. 2012;26:126–136. doi: 10.1101/gad.179895.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacAlister CA, Bergmann DC. Sequence and function of basic helix–loop–helix proteins required for stomatal development in Arabidopsis are deeply conserved in land plants. Evolution and Development. 2011;13:182–192. doi: 10.1111/j.1525-142X.2011.00468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacAlister CA, Ohashi-Ito K, Bergmann DC. Transcription factor control of asymmetric cell divisions that establish the stomatal lineage. Nature. 2007;445:537–540. doi: 10.1038/nature05491. [DOI] [PubMed] [Google Scholar]
- Marron N, Delay D, Petit J-M, Dreyer E, Kahlem G, Delmotte FM, Brignolas F. Physiological traits of two Populus×euramericana clones, Luisa Avanzo and Dorskamp, during a water stress and re-watering cycle. Tree Physiology. 2002;22:849–858. doi: 10.1093/treephys/22.12.849. [DOI] [PubMed] [Google Scholar]
- Masle J, Gilmore SR, Farquhar GD. The ERECTA gene regulates plant transpiration efficiency in Arabidopsis. Nature. 2005;436:866. doi: 10.1038/nature03835. [DOI] [PubMed] [Google Scholar]
- Miyazawa S-I, Livingston NJ, Turpin DH. Stomatal development in new leaves is related to the stomatal conductance of mature leaves in poplar (Populus trichocarpa×P. deltoides) Journal of Experimental Botany. 2006;57:373–380. doi: 10.1093/jxb/eri278. [DOI] [PubMed] [Google Scholar]
- Nadeau JA, Sack FD. Control of stomatal distribution on the Arabidopsis leaf surface. Science. 2002;296:1697–1700. doi: 10.1126/science.1069596. [DOI] [PubMed] [Google Scholar]
- Ohashi-Ito K, Bergmann DC. Arabidopsis FAMA controls the final proliferation/differentiation switch during stomatal development. The Plant Cell Online. 2006;18:2493–2505. doi: 10.1105/tpc.106.046136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pena-Rojas K, Aranda X, Joffre R, Fleck I. Leaf morphology, photochemistry and water status changes in resprouting Quercus ilex during drought. Functional Plant Biology. 2005;32:117–130. doi: 10.1071/FP04137. [DOI] [PubMed] [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Research. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillitteri LJ, Sloan DB, Bogenschutz NL, Torii KU. Termination of asymmetric cell division and differentiation of stomata. Nature. 2007;445:501–505. doi: 10.1038/nature05467. [DOI] [PubMed] [Google Scholar]
- Quarrie SA, Jones HG. Effects of abscisic acid and water stress on development and morphology of wheat. Journal of Experimental Botany. 1977;28:192–203. [Google Scholar]
- R Development Core Team 2011. R: a language and environment for statistical computing Vienna, Austria: : R Foundation for Statistical Computing; [Google Scholar]
- Radoglou KM, Jarvis PG.1990Effects of CO 2 enrichment on four poplar clones. I. Growth and leaf anatomy Annals of Botany 65617–626. [Google Scholar]
- Raj S, Brautigam K, Hamanishi ET, Wilkins O, Thomas BR, Schroeder W, Mansfield SD, Plant AL, Campbell MM. Clone history shapes Populus drought responses. Proceedings of the National Academy of Sciences, USA. 2011;108:12521–12526. doi: 10.1073/pnas.1103341108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai N, Akiyama M, Kuraishi S. Irreversible effects of water stress on growth and stomatal development in cotyledons of etiolated squash seedlings. Plant and Cell Physiology. 1986;27:1177–1185. [Google Scholar]
- Seibt U, Rajabi A, Griffiths H, Berry J. Carbon isotopes and water use efficiency: sense and sensitivity. Oecologia. 2008;155:441–454. doi: 10.1007/s00442-007-0932-7. [DOI] [PubMed] [Google Scholar]
- Shpak ED, McAbee JM, Pillitteri LJ, Torii KU. Stomatal patterning and differentiation by synergistic interactions of receptor kinases. Science. 2005;309:290–293. doi: 10.1126/science.1109710. [DOI] [PubMed] [Google Scholar]
- Silva EC, NogueiraI RJMC, Vale FHA, Araújo FPd, Pimenta MA. Stomatal changes induced by intermittent drought in four umbu tree genotypes. Brazilian Journal of Plant Physiology. 2009;121:33–42. [Google Scholar]
- Sugano SS, Shimada T, Imai Y, Okawa K, Tamai A, Mori M, Hara-Nishimura I. Stomagen positively regulates stomatal density in Arabidopsis. Nature. 2010;463:241–244. doi: 10.1038/nature08682. [DOI] [PubMed] [Google Scholar]
- Tisne S, Barbier F, Granier C. The ERECTA gene controls spatial and temporal patterns of epidermal cell number and size in successive developing leaves of Arabidopsis thaliana . Annals of Botany. 2011;108:159–168. doi: 10.1093/aob/mcr091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Groll U, Berger D, Altmann T. The subtilisin-like serine protease SDD1 mediates cell-to-cell signaling during Arabidopsis stomatal development. The Plant Cell. 2002;14: 1527–1539. doi: 10.1105/tpc.001016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Ngwenyama N, Liu Y, Walker JC, Zhang S. Stomatal development and patterning are regulated by environmentally responsive mitogen-activated protein kinases in Arabidopsis. The Plant Cell. 2007;19:63–73. doi: 10.1105/tpc.106.048298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins O, Nahal H, Foong J, Provart NJ, Campbell MM.2009aExpansion and diversification of the Populus R2R3-MYB family of transcription factors Plant Physiology 149981–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins O, Waldron L, Nahal H, Provart NJ, Campbell MM.2009bGenotype and time of day shape the Populus drought response The Plant Journal 60703–715. [DOI] [PubMed] [Google Scholar]
- Woodward FI. Stomatal numbers are sensitive to increases in CO2 from pre-industrial levels. Nature. 1987;327:617–618. [Google Scholar]
- Xu Z, Zhou G. Responses of leaf stomatal density to water status and its relationship with photosynthesis in a grass. Journal of Experimental Botany. 2008;59:3317–3325. doi: 10.1093/jxb/ern185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Zang R, Li C. Population differences in physiological and morphological adaptations of Populus davidiana seedlings in response to progressive drought stress. Plant Science. 2004;166:791–797. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.