Abstract
In aiming to decipher the genetic control of shoot architecture in pepper (Capsicum spp.), the allelic late-flowering mutants E-252 and E-2537 were identified. These mutants exhibit multiple pleiotropic effects on the organization of the sympodial shoot. Genetic mapping and sequence analysis indicated that the mutants are disrupted at CaJOINTLESS, the orthologue of the MADS-box genes JOINTLESS and SVP in tomato and Arabidopsis, respectively. Late flowering of the primary and sympodial shoots of Cajointless indicates that the gene functions as a suppressor of vegetative growth in all shoot meristems. While CaJOINTLESS and JOINTLESS have partially conserved functions, the effect on flowering time and on sympodial development in pepper, as well as the epistasis over FASCICULATE, the homologue of the major determinant of sympodial development SELF-PRUNING, is stronger than in tomato. Furthermore, the solitary terminal flower of pepper is converted into a structure composed of flowers and leaves in the mutant lines. This conversion supports the hypothesis that the solitary flowers of pepper have a cryptic inflorescence identity that is suppressed by CaJOINTLESS. Formation of solitary flowers in wild-type pepper is suggested to result from precocious maturation of the inflorescence meristem.
Key words: Flowering time, jointless, pepper, shoot architecture, sympodial growth, tomato.
Introduction
Higher plants exhibit great variation in their shoot architecture, manifested by degree of lateral branching, internode elongation, leaf complexity, determinacy, and inflorescence structure (Alonso-Blanco et al., 2009). Similarly, large variation exists in flowering time and in the mechanisms that control the transition to flowering. Isolation of mutants affected in flowering time or plant architecture has revealed that these two aspects of development are frequently interconnected. The genetic control of plant architecture and flowering time has been studied mostly in a limited number of model species, such as Arabidopsis, rice, maize, tomato, and petunia (Izawa, 2007; Wang and Li, 2008; Michaels, 2009; Yant et al., 2009; Castel et al., 2010). However, while model species are imperative to defining the developmental pathways and genes regulating plant architecture, the extent to which these genes and pathways are conserved in non-model crop species has yet to be determined.
In contrast to Arabidopsis that exhibits a monopodial shoot system in which the shoot apical meristem (SAM) is indeterminate – first producing leaves and later flowers – Solanaceae plants such as pepper, tomato, and petunia exhibit a sympodial growth architecture. Sympodial growth is characterized by the determinacy of the SAM, which converts from vegetative to reproductive meristem comprised of a terminal flower. In pepper, the meristem is arrested at that stage and vegetative sympodial branches are released from basal leaves. In tomato, basal nodes below the flower branch to form another flower, in a reiterative process, leading to the formation of a compound inflorescence made up of several flowers organized in a zigzag pattern (Lifschitz and Eshed, 2006). In both shoots, however, further growth continues from the uppermost axillary meristem located at the node below the inflorescence or flower of the primary stem; this meristem is referred to as sympodial meristem (SYM). Similar to the primary SAM, the SYM terminates after producing a short shoot segment, termed the sympodial unit, consisting of three leaves and an inflorescence in tomato and two leaves and a solitary flower in pepper. Further growth of the sympodial shoot continues from a lateral meristem at the axil of the uppermost leaf of the SYM. These sympodial units are reiterated to form the sympodial shoot structure.
Detailed studies on the regulation of flowering in Arabidopsis have revealed several pathways that control the transition from vegetative to reproductive development (Michaels, 2009; Yant et al., 2009). These include pathways that are environmentally dependent on temperature and day length or on gibberellin, and an autonomous pathway that mediates flowering by monitoring the developmental stage of the plant. To date, more than 100 genes have been identified as regulating this process in Arabidopsis (Srikanth and Schmid, 2011). In cultivated tomato and pepper, which do not respond to photoperiod or vernalization signals (Samach and Lotan, 2007), the autonomous pathway is thought to play a major role in the transition to flowering. However, the molecular mechanisms that underlie the day- neutral flowering response of these plants are just beginning to be revealed (Mizoguchi et al., 2007).
Florigen is a mobile flowering-initiation signal produced in the leaves that induces flowering at the SAM. The identity of florigen was first established as the FLOWERING LOCUS T (FT) homologue SINGLE FLOWER TRUSS (SFT) in tomato, (Lifschitz et al., 2006). Subsequently, the role of FT homologues as florigens in other plant species, such as rice, Arabidopsis, and maize, was established as well (Tamaki et al., 2007; Meng et al., 2011). While FT homologues appear to have a universal function as florigens, their precise action is species specific (Wigge, 2011). Furthermore, the FT/SFT-paralogous genes TERMINAL FLOWER1 (TFL1) and SELF PRUNING (SP) in Arabidopsis and tomato, respectively, have antagonistic functions similar to FT homologues in determining plant shoot determinacy (Conti and Bradley, 2007; Shalit et al., 2009). TFL1/SP homologues were subsequently found to have a species-specific adapted role in controlling plant architecture in diverse plants, such as soybean, poplar, and pepper (Elitzur et al., 2009; Liu et al., 2010; Mohamed et al., 2010; Tian et al., 2010). An emerging conclusion from these and other studies is that, although genes controlling architecture and flowering are common to different plant species, their function may be modified in a species-specific manner.
FT is negatively regulated by a complex of MADS-box genes that includes FLOWERING LOCUS C (FLC) and SHORT VEGETATIVE PHASE (SVP) (Li et al., 2008). SVP is a negative regulator of flowering time (Hartmann et al., 2000) and functions in the floral meristem by activating the floral class B and C homeotic genes that specify floral organ identity (Liu et al., 2009). The tomato orthologue of SVP is JOINTLESS (Mao et al., 2000). The jointless mutant is characterized by reversion of the inflorescence after production of a few flowers into a vegetative shoot (Szymkowiak and Irish, 2006). Analysis of the interactions of jointless with other mutants controlling meristem identity in tomato revealed that the inflorescence and SYMs share common regulatory mechanisms. In addition to its function in inflorescence development, JOINTLESS interacts with the MADS-box gene MACROCALYX to regulate development of the abscission zone in the flower pedicel (Nakano et al., 2012).
Pepper (Capsicum spp.) is an important solanaceous crop, but despite the importance of shoot architecture to crop production, the genetic basis of its variation in growth habit has been poorly explored. Therefore, developmental mechanisms and genes controlling growth habit and transition to flowering in that species remain to be deciphered and the extent to which these genes and pathways are conserved with related model species determined. Because only limited natural variation for these traits exists in pepper, an ethyl methanesulphonate (EMS)-mutagenized population was generated on the background of the blocky-fruited cultivar ‘Maor’ and screened for alterations in plant architecture (Paran et al., 2007). In the present study, the isolation of a late-flowering mutation that is disrupted at CaJOINTLESS, which functions as a floral promoter in primary and sympodial shoots, is reported. Moreover, CaJOINTLESS has a role in suppressing inflorescence development and maintaining the single-flower organization of the pepper truss.
Materials and methods
Plant material
The mutants E-252, E-2537, Cablind (Cabl) and Ca-anantha (Ca-an) were isolated from an EMS mutagenesis population using Capsicum annuum cv. ‘Maor’ as a wild-type parent (Paran et al., 2007). Cabl and Ca-an have been described by Jeifetz et al. (2011) and by Lippman et al. (2008), respectively. fasciculate (fa) is a spontaneous mutation described by Elitzur et al. (2009). To map the E-252 mutation, a segregating BC1 population was constructed from a cross of E-252 and Capsicum chinense PI 159234 using E-252 as the recurrent parent.
Double mutants of E-252:Cabl and E-252:fa were derived from F2 populations obtained by crossing the corresponding mutants and screening by cleaved amplified polymorphic sequence markers for double mutations. To generate the double mutant E-252:Ca-an, crosses were performed between E-252 and a heterozygous Ca-an/+ because fertile flowers are not produced in Ca-an, followed by screening an F2 population for double mutants.
Tomato (Solanum lycopersicum) jointless (j) seeds (LA3023) were obtained from the TGRC stock centre at UC Davis, California. Seeds ofcv. M82 SP (wild type), M82 sp, and j:sp double mutants on the background of M82 were obtained from Y. Eshed, The Weizmann Institute, Israel.
Isolation of CaJOINTLESS (CaJ)
To map E-252, selected individuals of mutant and wild-type phenotypes from the BC1 segregating population were used for screening with RFLP markers scattered throughout the genome, according to Ben Chaim et al. (2001). To determine linkage between E-252 and CaJ, tomato JOINTLESS (accession no. AF275345) was amplified with the primers Tom-JF and Tom-JR using tomato genomic DNA as the template (Table 1), and the amplified product was used as an RFLP probe in Southern blot analysis. The same primers were used to amplify a corresponding partial fragment from pepper genomic DNA. A 1502-bp fragment was cloned into pDrive PCR Cloning Vector (Qiagen) and used to screen a bacterial artificial chromosome (BAC) library of Capsicum frutescens BG 2816 obtained from the Arizona Genomics Institute (http://www.genome.arizona.edu/orders). A positive BAC clone (0215J14) was sequenced at the genome centre at Washington University, St. Louis, Missouri. Subsequently, the genomic region containing CaJ identified by BLAST was sequenced in ‘Maor’ and E-252. RNA isolated from leaves was used to create cDNA of the mutant and wild type, which was used as a template for sequencing the open reading frames (ORFs) using primers matching the 5’ and 3’ untranslated regions, CaJ-5UTR and CaJ-3UTR, respectively (Table 1). The ORF of CaJ from ‘Maor’ was deposited in GenBank with accession number JQ698661. For screening the Caj mutation in subsequent experiments, a cleaved amplified polymorphic sequence marker was developed by using PCR primers Tom-JF and CaJ-465R (Table 1) followed by restriction digestion with HphI.
Table 1.
Primers used in this study
| Gene | Primer | Primer sequence (5’–3’) |
| JOINTLESS | Tom-JF | GATCTCTTGAAACTGGATTGAGCC |
| Tom-JR | TTACTTTTTTTTTTCTCCTTCTTCTAATAAC | |
| J-qRTF | TCAATTGATCCTCCTCCTCAA | |
| J-qRTR | AGCCACACCTTGCTTTTGAT | |
| CaJOINTLESS | CaJ-5UTR | GAAAGAAAGACTCGTAATGGC TAGAGAGAAAATT |
| CaJ-3UTR | TTAAGCCAAGTTGAATAAAAAGACTTC | |
| CaJ-465R | GTTGGAGCTGATTAATCTCTCTCATTA | |
| CaJ-qRTF | TTAAGGCAAATGAGGGGTGA | |
| CaJ-qRTR | TCTATAACGCGGCTCAATCC | |
| FASCICULATE | FA-qRTF | CCAGCAGAAGAAAAGGCAAAC |
| FA-qRTR | AGCTACAGGAGAGCCAAGTTC | |
| SELF-PRUNING | SP-qRTF | TATCGAGTGCACCAGTGTCC |
| SP-qRTR | CCTTCTAGCGGCAGTTTCC | |
| CaUBIQUITIN | UBQ-qRTF | GCACAAGCACAAGAAGGTTAAG |
| UBQ-qRTR | GCACCACACTCAGCATTAGGA | |
| UBIQUITIN3 | UBC3-qRTF | GGTCCTGTTGTCCATTTGCT |
| UBC3-qRTR | GTCTCGTATTTGGCCCTGTC |
Analysis of gene expression
Total RNA was extracted from shoot apices using GeneElute Mammalian Total RNA Extraction Miniprep kit (Sigma) according to the manufacturer’s instructions, followed by DNase I treatment (Sigma). Total RNA (400ng) was used for first-strand cDNA synthesis by reverse-transcription PCR (RT-PCR) using a PrimeScript RT Reagent kit (Takara), For quantitative real-time PCR (qRT-PCR), three biological and two technical repeats were used for each sample. For the qRT-PCR experiments, plants were grown in a glasshouse under natural daylight during the winter season in Israel. PCR amplification was performed using the primers CaJ-qRTF and CaJ-qRTR for CaJ and FA-qRTF and FA-qRTR for FA (Table 1). Expression analysis of tomato genes was carried out using the primers J-qRTF and J-qRTR for JOINTLESS and SP-qRTF and SP-qRTR for SP. Amplified products were detected using SYBR Premix Ex Taq II (Takara) in a Rotor-Gene 6000 thermal cycler (Corbett). Results were analysed using Rotor-Gene 6000 Series software 1.7 (Corbett). The relative expression levels of the pepper and tomato genes were normalized against, respectively, pepper CaUBIQUITIN (DQ975458.1) using the primers UBQ-qRTF and UBQ-qRTR, and tomato UBIQUITIN3 (FJ429328.1) using the primers UBC3-qRTF and UBC3-qRTR.
in situ hybridization
Digoxigenin (DIG)-labeled in situ analysis was performed as previously described (Szymkowiak and Irish, 2006; Neta et al., 2011). Meristems were fixed in FAA (formaldehyde/acetic acid/70% ethanol, 10:5:85, v/v), dehydrated and embedded in ParaPlast (McCormick Scientific). The tissue was then cut (10 µm) on a Leica RM2245 microtome (VectaMount, Vector Laboratories) and sections were placed on SuperFrost Plus slides (Menzel-Glaser) for 2 days on a 42 °C hot plate. An antisense DIG-labelled RNA probe was synthesized from the 3’ end of CaJ cDNA, which excludes the conserved domains, using the MEGAscript kit (Ambion) and DIG RNA labelling mix (Roche Applied Science).
Scanning electron microscopy (SEM)
Samples for SEM were fixed directly in 70% ethanol and critically point dried as described by Alvarez et al. (1992). SEM was performed using a Hitachi S-3500N instrument.
Phylogenetic analysis
Multiple sequence alignments were performed with a web-based version of Clustal W (http://www.ebi.ac.uk/Tools/clustalw/) using the default settings. The phylogenetic tree was calculated using the neighbour-joining method and bootstrap analysis with 1000 replicates via MEGA4 software (http://www.megasoftware.net/mega.html). The tree was calculated from alignments of the MADS-box and K-box homeodomains, consisting of the first ~170 amino acids of the proteins.
Results
Pepper shoot architecture and its modifications
Pepper has a sympodial shoot architecture, producing about 10 leaves on the primary stem before termination with a single flower (Fig. 1A). After termination of the primary shoot, further growth occurs from the axils of the two uppermost leaves (Fig. 1B). A SYM differentiates in each axil, which develops into a sympodial unit that consists of two leaves and a single flower (Fig. 1B, 1C). The two leaves grow away from the flower and are positioned above it. The initiation of SYMs in the two leaf axils results in a bifurcated shoot (Fig. 1C). Additional cycles of sympodial development continue from meristems formed at the axils of the two leaves. These cycles of development repeat themselves and can theoretically continue indefinitely.
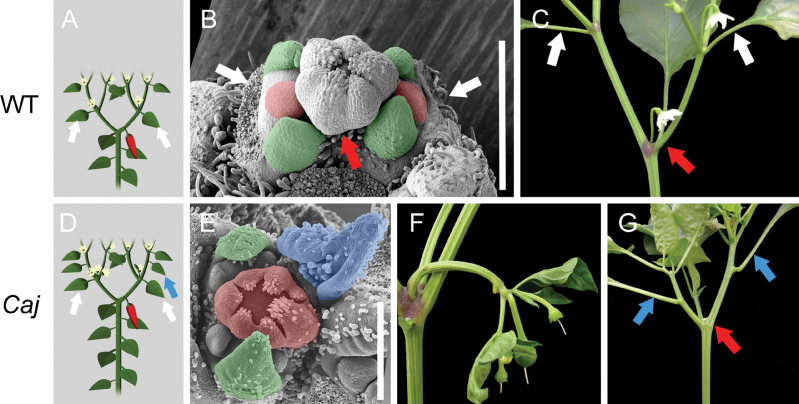
Fig. 1.
Characterization of wild-type pepper and E-252 mutant. (A) Schematic diagram of wild-type parent ‘Maor’. The two leaves that belong to the first sympodial unit are indicated by white arrows. (B) Scanning electron micrograph of wild-type shoot apical meristem, showing a flower (red arrow), locations of flanking leaves (excised, white arrows), and two sympodial meristems, each consisting of two leaf primordia (green) and a flower meristem (red). (C) Wild-type sympodial unit consisting of a flower (red arrow) and two leaves (white arrows). (D) Schematic diagram of E-252 mutant. The extra sympodial leaf is indicated by a blue arrow. (E) Scanning electron micrograph of E-252 sympodial meristem consisting of a flower (red), two leaf primordia (green), and an additional primordial leaf (blue) with no developed meristems in its axil. (F) E-252 flower pedicel carrying multiple flowers and leaves. (G) E-252 sympodial unit consisting of a flower (excised, red arrow) and two extra leaves (blue arrows). Bars, 400 µm.
Phenotypic screening of an EMS-mutagenized population of the blocky cultivar ‘Maor’ for mutants with altered growth habit identified E-252, which exhibits multiple pleiotropic changes in its growth habit that are inherited as a single recessive trait. The E-252 mutant is indistinguishable from the wild type during the vegetative phase and there is no architectural difference between the mutant and wild-type plants before flowering. However, whereas wild-type plants shift from vegetative to reproductive phase after an average of 10 leaves on the primary stem, the mutant plants flower later, after an average 17 leaves. The sympodial units of E-252 often have an extra leaf or two (Fig. 1D, 1E) and the bifurcated shoot architecture is maintained by the two uppermost leaf axils. Later, during sympodial growth, the entire sympodial unit is carried away from the flower and located above it (Fig. 1G). During floral development, two or more flowers may form on the same pedicel with the appearance of vegetative growth along them, resembling the formation of a vegetative inflorescence (Fig. 1F). At flower development, one of the sepals is often converted into a leaf-like organ (not shown).
E-252 is disrupted at CaJ
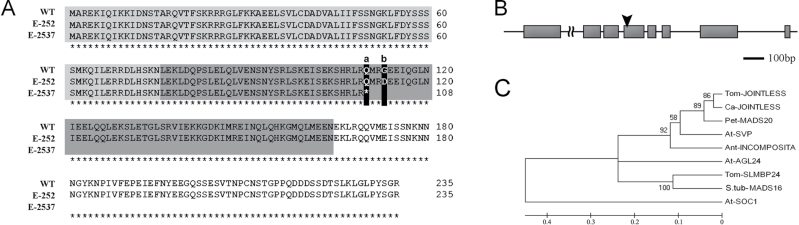
To isolate the gene governing the E-252 mutation, a BC1 segregating population was screened with RFLP markers scattered throughout the pepper genome. One tomato-originated marker, TG194, mapped to chromosome 12 of pepper (Livingstone et al., 1999), was found tightly linked to the mutation (not shown). Searching for genes associated with tomato architecture mapped near this marker enabled detection of the MADS-box transcription factor gene JOINTLESS (J) (Mao et al., 2000; Szymkowiak and Irish, 2006) as a potential candidate for controlling the E-252 mutation. RFLP mapping of J in the BC1 population of 15 mutant and 15 wild-type individuals indicated complete co-segregation with the mutant phenotype. The sequence of the pepper orthologue of JOINTLESS (CaJ) was isolated using cDNA, BAC, and genomic DNA sources and encodes a 234-amino-acid protein (Fig. 2A). Comparison of cDNA and genomic sequences revealed that the gene consists of eight exons (Fig. 2B). Sequencing CaJ in E-252 identified a missense mutation of G to A at position 338 of the ORF that results in a change of glycine to aspartic acid at position 114 of the protein (Fig. 2A). To prove that CaJ controls the E-252 mutation, the EMS population was screened for additional mutants showing a phenotype similar to E-252. This search identified E-2537 which was defined as allelic to E-252 by allelism test (F1 from a cross between the two recessive mutants had a mutant phenotype). Sequencing of E-2537 indicated a single nucleotide change of C to T at position 328 of the ORF, resulting in the formation of a stop codon at position 110 of the protein (Fig. 2A). Both mutations are located in exon 4 in the K-box motif that is involved in a protein–protein interaction of type II MADS-box genes (Egea-Cortines et al., 1999).
Fig. 2.
Molecular characterization of CaJ. (A) Amino acid sequence of the CaJ protein. The MADS-box domain is shaded in light grey and the K-box motif is shaded in dark grey. The mutations in E-2537 and E-252 are indicated by a and b, respectively. (B) Schematic description of CaJ. Introns and exons are indicated by solid lines and grey boxes, respectively. The region containing the EMS mutations in exon 4 is indicated by an arrowhead (note that the size of the first intron is larger than 2kb and is not to scale). (C) Phylogenetic tree of the conserved domains of JOINTLESS-related proteins from the subclade family StMADS11 and SOC1 from Arabidopsis. Accession numbers: AGL24, NP_194185.1; INCOMPOSITA, CAG27846.1; JOINTLESS, AAG09811.1; MADS16, AAV65504.1; MADS20, ACY82403.1; SLMBP24, TC161634; SOC1, NP_182090.1; SVP, NP_001154528.1.
CaJ contains two conserved domains at its N-terminal end (Fig. 2A) and belongs to the StMADS11 subclade (García-Maroto et al., 2000; Becker and Theissen, 2003). Phylogenetic analysis showed that CaJ is closely related to tomato J, and together they are clustered in the same clade with Arabidopsis SVP, Petunia MADS20 and Antirrhinum INCOMPOSITA (Fig. 2C).
CaJ is expressed in all shoot meristems
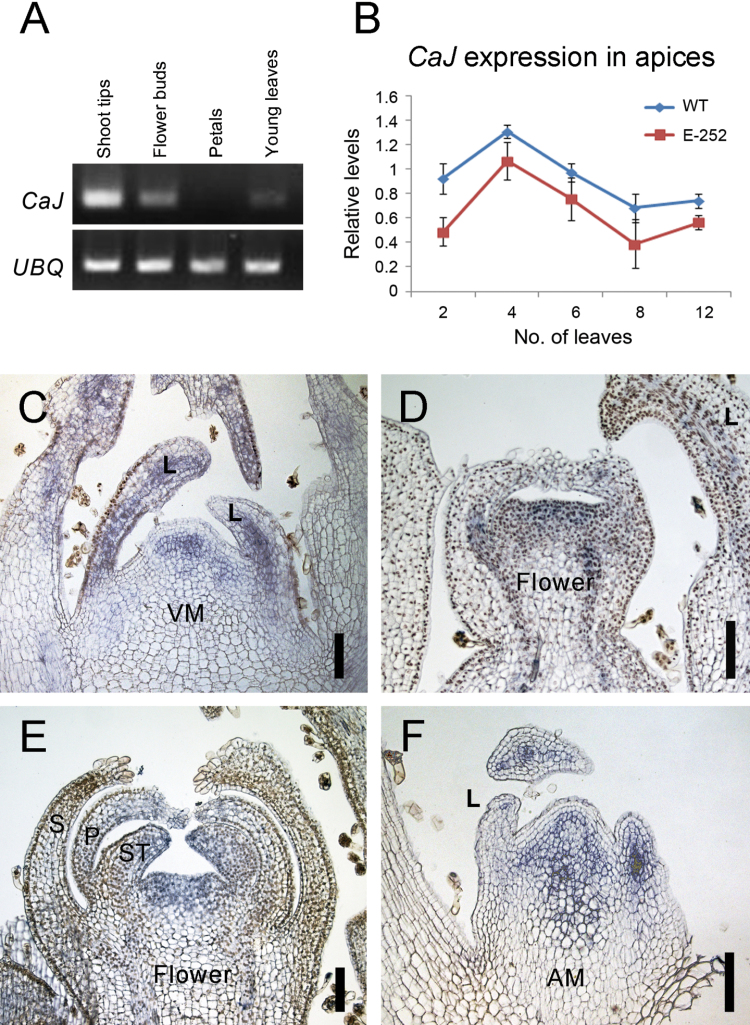
The expression level of CaJ was analysed in different plant tissues. The strongest expression level was observed in sympodial apices, followed by flower buds and young leaves, whereas no expression was observed in mature flower petals (Fig. 3A). The spatial and temporal expression of CaJ was further explored using qRT-PCR and in situ hybridization. Sampling mRNA from shoot apices at different stages of development in ‘Maor’ and E-252 indicated that CaJ is already expressed before the transition to flowering (2-expanded leaf stage; Fig. 3B). Transcript level was highest just before transition to flowering (4-leaf stage) and declined later on (8- to 12-leaf stages). The expression pattern in E-252 resembled that of ‘Maor’ although at a lower level, indicating possible auto-regulation of CaJ. In situ hybridization analysis revealed CaJ transcripts in the central zone of the SAM at the vegetative stage, as well as in the leaf primordia (Fig. 3C). CaJ transcripts were also detected in floral meristems at different stages. In the early flowering meristem, expression was observed in the central meristematic zone but not in the sepal primordia (Fig. 3D). At a more advanced stage of flower development, expression was observed in the central zone and in the petal and stamen primordia but not in the sepal primordia (Fig. 3E). In addition, expression was detected in the axillary meristems (Fig. 3F).
Fig. 3.
Expression pattern of CaJ. (A) Reverse-transcription PCR of CaJ transcripts in different tissues of ‘Maor’. (B) Expression of CaJ transcripts in wild-type and mutant apices at different leaf stages on the primary stem. (C–F) In situ hybridization of CaJ transcripts in vegetative apex at 2-leaf stage (C), young flower at 6-leaf stage, (D), flower at 8-leaf stage (E), and axillary meristem (F). Bars, 100 µm. AM, axillary meristem; L, leaf; P, petal; S, sepal; ST, stamen; VM, vegetative meristem.
CaJ and Ca-AN independently control floral meristem identity
Because Caj mutants are altered in their transition to flowering, floral meristem identity, and sympodial development, the existence of a regulatory hierarchy between CaJ and other genes affecting these processes in pepper was explored. Double mutants of Caj with other available mutants affected in transition to flowering, floral meristem identity, or sympodial development were created. These included Ca-anantha (Ca-an), fasciculate (fa), and Cablind (Cabl), controlled by the orthologues of tomato ANANTHA, SELF PRUNING and BLIND, respectively (Lippman et al., 2008; Elitzur et al., 2009; Jeifetz et al., 2011).
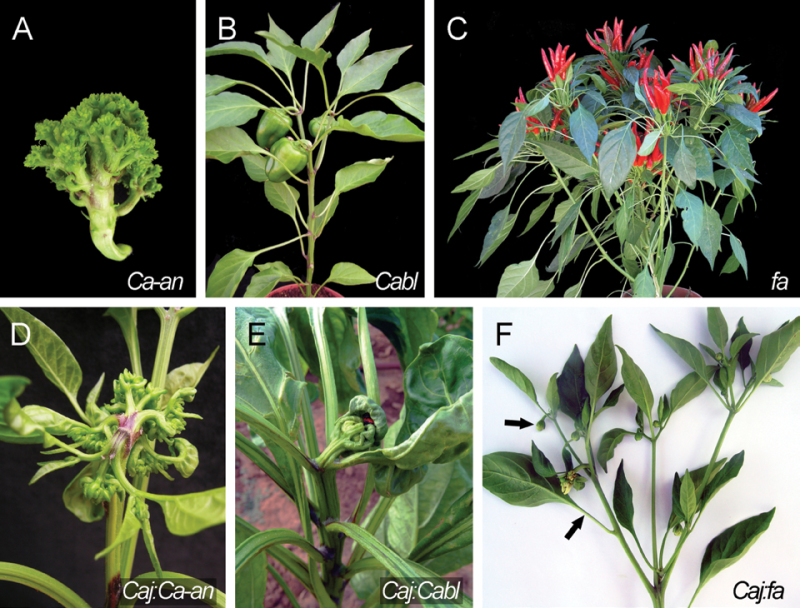
Ca-AN is required for normal differentiation of flower organs. The Ca-an mutant produces an indeterminate shoot-like structure instead of a single flower, resulting in a cauliflower-like inflorescence (Fig. 4A). No change in flowering time is observed in the mutant compared to ‘Maor’ (Lippman et al., 2008). The double mutant Caj:Ca-an had a similar late-flowering phenotype and an extra leaf in the sympodial unit as in Caj. The floral meristem produced an indeterminate shoot consisting of leaves and anantha-like structures (Fig. 4D), combining the floral characteristics of both mutants. Therefore, these phenotypes suggest that Caj and Ca-an have additive effects and that their corresponding genes operate in independent pathways to promote floral meristem identity.
Fig. 4.
Pepper mutants affected in growth habit and their interaction with Caj. (A) Ca-an indeterminate shoot-like inflorescence. (B) Cabl mutant exhibits no axillary growth and early termination of sympodial growth. (C) fa mutant exhibits fruit clusters and determinate growth. (D) Caj:Ca-an exhibits vegetative indeterminate inflorescence structure. (E) Caj:Cabl exhibits late flowering, lack of axillary meristems, and early termination of the sympodial shoot by a single flower. (F) Caj:fa sympodium resembles Caj single mutant characteristics. Arrows indicate extra sympodial leaf and single flower.
CaJ and CaBL interact with each other in controlling flowering time and sympodial development
CaBL is required for lateral meristem differentiation and normal sympodial development. Cabl is characterized by a lack of axillary meristems in the primary shoot and precocious termination of the sympodial shoot after development of a few sympodial units. In addition, transition to flowering is delayed by 5.5 leaves compared to ‘Maor’, one of the sepals is converted into a leaf-like organ, and the sympodial unit consists of an extra leaf compared to that of ‘Maor’ (Fig. 4B; Jeifetz et al., 2011). The double mutant Caj:Cabl lacked axillary meristems and flowered after 30 leaves on the primary stem, producing a single terminal flower with a leaf-like sepal that completely terminated shoot growth as all axillary shoots were blocked (Fig. 4E). The synergistic late-flowering phenotype of the double mutant indicated that the two genes interact with each other in controlling the transition to flowering. Furthermore, the complete elimination of lateral shoot growth in Caj:Cabl indicated that the two genes redundantly regulate lateral shoot outgrowth.
CaJ is epistatic to FA in determining shoot architecture
FA is a flowering repressor in the primary and sympodial shoots and its action is antagonistic to that of CaJ. Pepper fa is characterized by early flowering time and a ‘determinate’ growth habit, resulting in the formation of floral clusters separated by short internodes and leaves (Fig. 4C). However, in fa plants, the basic structure of the sympodial unit remains unchanged (Elitzur et al., 2009). The phenotype of the Caj:fa double mutant was similar to that of Caj, i.e. it flowered after 17 leaves, no floral clusters were observed, the sympodial unit had an additional leaf, and occasionally, one of the sepals developed into a leaf-like organ (Fig. 4F). These phenotypes suggest that Caj is epistatic to fa with respect to development of apical, sympodial, and floral meristems.
Because of the antagonistic interaction of CaJ and FA, their interaction in controlling pepper growth habit was further examined by measuring their expression level by qRT-PCR in four different genotypes: wild type (‘Maor’) and the Caj, fa, and Caj:fa mutants. Expression levels were determined in the meristems of the second sympodial unit, after floral transition. Expression of CaJ in the Caj mutant was lower than in the wild type, indicating auto-regulation of CaJ, which is common for SVP-related genes (de Folter et al., 2005). Expression of CaJ was upregulated in the fa mutant compared to the wild type (Fig. 5A). In contrast, expression of CaJ as well as FA was downregulated in the Caj:fa mutant compared to fa and to the wild type (Fig. 5A, 5B). These results are in accordance with the epistatic action of CaJ over FA in the double mutant and indicate that FA inhibits CaJ.
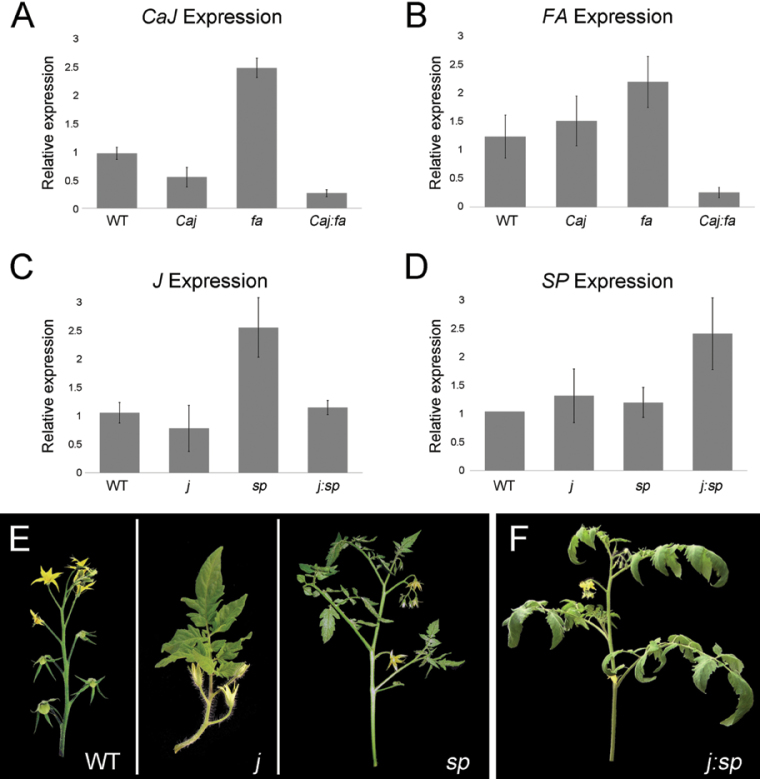
Fig. 5.
Expression patterns of CaJ, FA and their tomato homologues in sympodial apices. (A, B) Expression levels of CaJ and FA in sympodial apices of wild-type, Caj (E-252), fa, and Caj:fa pepper. (C, D) Expression levels of JOINTLESS (J) and SP in sympodial apices of wild-type, j, sp, and j:sp tomato. (E) Inflorescences and sympodiums of tomato genotypes: left, wild type; centre, j forming a few flowers followed by leaves; right, sp showing reduction of leaf number between inflorescences. (F) Inflorescence and sympodium of tomato double mutant j:sp. The sympodial shoot and inflorescence are similar to sp.
To further study the relationship between CaJ and FA in pepper in comparison to the homologous genes in tomato, a tomato j:sp double mutant was obtained and the expression of the corresponding genes in the individual and double mutants was measured. The sympodial unit of wild-type tomato consists of three leaves and an inflorescence composed of multiple flowers (Fig. 5E). This structure is maintained in the sympodial unit of j, but the inflorescence produces a few flowers and then converts to vegetative growth (Fig. 5E). In sp mutants, the number of leaves within the sympodial unit is progressively reduced until growth is terminated by two sequential inflorescences (Pnueli et al., 1998). The phenotype of j:sp resembled that of sp, i.e. the number of leaves between the inflorescences was progressively reduced and no vegetative growth was observed in the inflorescence (Fig. 5F), indicating that unlike in pepper, sp is epistatic to j with respect to sympodial growth and inflorescence development.
Expression of J in SYMs of tomato was upregulated in sp and downregulated in j:sp (Fig. 5C), in a pattern similar to that of CaJ in pepper-homologous genotypes. In contrast, the expression pattern of SP and its pepper homologue FA differed between tomato and pepper. SP was upregulated in j:sp (Fig. 5D), whereas FA was downregulated in the homologous Caj:fa pepper double mutant. These findings show that CaJ and its homologue from tomato have the same expression patterns in both the wild-type and homologous mutants. In contrast, the expression pattern of SP and FA is not conserved in the two species, which may contribute to the different epistatic relationship between the two genes in pepper and tomato.
Discussion
Role of CaJ in controlling transition to flowering
The Caj mutant was initially discovered because of its late-flowering phenotype, exhibiting a delay of seven leaves to first flower on the primary stem compared to ‘Maor’. A late-flowering phenotype was also reported for a tomato j mutant, but the delay in flowering was weaker than in pepper (1–3-leaf delay, depending on the genetic background, Emery and Munger, 1970; Thouet et al., 2012). Therefore, CaJ and J function to promote termination of the SAM and accelerate flowering. Interestingly, SVP, the closest homologue of J in Arabidopsis, is a flowering repressor operating via repression of the flowering integrator SOC1 in the SAM and FT in leaves (Hartmann et al., 2000; Li et al., 2008). In contrast, Arabidopsis AGL24, the closest homologue of SVP, is a flowering promoter that activates SOC1 (Gregis et al., 2006; Liu et al., 2008). Homologues of SVP in other plant species, such as INCOMPOSITA in Antirrhinum (Masiero et al., 2004), TaVRT-2 in wheat (Kane et al., 2005), and OsMADS55 in rice, function as flowering repressors when expressed in Arabidopsis (Lee et al., 2012). Other homologues from the StMADS11 subclade, such as MPF1 from Physalis, function as flowering promoters (He et al., 2010). Therefore, while members of the StMADS11 subclade MADS-box genes have a conserved role in regulating flowering time in diverse plant species, their role as promoters or repressors of flowering is case specific.
The pronounced late-flowering phenotypes of Caj and Caj:Cabl indicate that the function of CaJ in controlling flowering time in pepper is stronger than that of its tomato homologue J. In addition to late flowering, Caj and Cabl share other common characteristics, including addition of leaves to the sympodial units and conversion of one sepal in the flower into a leaf-like organ. These characteristics are also common to other late-flowering pepper mutants such as E-172-1 (data not shown). The increase in vegetativeness in different parts of the shoot in these mutants indicates that all shoot meristems, including the apical, sympodial, and floral meristems, can independently delay their termination. The enhanced precocious sympodial termination in Caj:Cabl compared to Cabl is similar to the homologous tomato double mutant that produces a single terminal flower (Szymkowiak and Irish, 2006). The synergistic action of CaJ and CaBL in controlling flowering time and sympodial development as inferred from the double mutant indicate that the two genes interact with each other in controlling pepper architecture.
The stage-specific effects of mutations in genes such as SP and TERMINATING FLOWER (TMF) on termination of the sympodial shoot and primary shoot of tomato, respectively, led to the conclusion that two flowering systems exist in tomato: one for the primary shoot and a second for the sympodial shoot (Lifschitz and Eshed, 2006). The late-flowering mutants of pepper (Cabl, Caj, and E-172–1) do not show such stage-specific phenotypes of the two shoot systems, as both the primary and sympodial shoots are late flowering. Similarly, the early termination phenotype of fa is observed in both shoot systems. Although this coordination may be lost in other mutants, it is possible that in pepper, flowering regulation of the two shoot systems is more tightly coordinated than in tomato.
Role of CaJ in controlling sympodial development
The main effect of Caj on sympodial development is the sporadic addition of leaves within the sympodial units. In contrast, tomato j has been mostly associated with lack of abscission zone on the flower pedicel and to a lesser extent with reversion of the inflorescence into vegetative growth (Szymkowiak and Irish, 2006). According to Emery and Munger (1970), an increased number of leaves between inflorescences was also observed in tomato j; however, other studies indicate no change in the sympodial growth pattern between the wild type and j (Thouet et al., 2012). Since wild-type pepper lacks an abscission zone on the flower pedicel, the effect of CaJ on abscission zone development in the pedicel cannot be assessed.
Increased expression of CaJ in the fa mutant and strong epistasis of Caj over fa in the Caj:fa double mutant indicated that FA, the pepper orthologue of tomato SP, is upstream of CaJ in the pathway controlling sympodial development and inhibits it (Fig. 4F, 5A). In tomato, inactivation of SP results in early termination of the sympodial shoot but with no change in flowering time of the primary shoot (Pnueli et al., 1988). In pepper, fa is characterized by early flowering of the primary shoot and compact sympodial units (Elitzur et al., 2009). Therefore, in both tomato and pepper, SP and FA have functions opposite to those of J and CaJ, respectively, by repressing the termination of shoot apices. However, while in pepper, Caj is epistatic to fa in all aspects of primary and sympodial shoot development, the relationship between the two homologous mutations in tomato is more complex and depends on the genetic background. sp has been reported to be epistatic to j with respect to inflorescence development (Rick and Butler, 1956; Szymkowiak and Irish, 2006), while late flowering of sp:j was similar to j.
Role of CaJ in flower development
Instead of the single flower that develops in wild-type pepper, pedicels developing from the Caj mutant often carry multiple (two or three) flowers and vegetative growth is evident on the flower pedicel (Fig. 1F). The appearance of multiple flowers and leaves on the flower pedicel indicates that the pepper’s solitary flower has a cryptic inflorescence identity and that this inflorescence has a sympodial nature, similar to that of tomato. Support for this conclusion comes from the indeterminate flower phenotype of pepper Ca-an mutant (Lippman et al., 2008) and the double mutant Caj:Ca-an (Fig. 4D). A model suggesting the rate of meristem maturation as a key factor in the regulation of inflorescence architecture was proposed by Park et al. (2012). According to that model, delayed meristem maturation promotes branching inflorescences, such as the compound inflorescence (s) mutant in tomato (Lippman et al., 2008), while rapid maturation of the inflorescence meristem leads to formation of a single flower in species such as pepper. Deceleration of inflorescence meristem maturation in the Caj background leads to the formation of a sympodial shoot-like structure composed of leaves and flowers. Therefore, similar to the function of J in repressing sympodial identity of the inflorescence in tomato, CaJ has evolved to suppress sympodial inflorescence growth in pepper and to promote early maturation of the inflorescence meristem to produce a single flower.
In conclusion, CaJ functions as a repressor of vegetative growth in all shoot meristems. In agreement with this function, its expression is observed in all shoot meristems. While the pepper gene has functions similar to its tomato orthologue, the effect in pepper on controlling flowering time and sympodial development is more pronounced than in tomato. Moreover, both genes also evolved to have specific functions. While J is involved in abscission-zone formation in the flower pedicel of tomato, CaJ maintains single-flower formation in pepper. This study demonstrates that induced mutagenesis and identification of multiple mutant alleles is an effective method to generate variation in shoot architecture and study the function of the major genes controlling this trait. Such an approach is particularly useful in species such as pepper for which transformation is inefficient as a tool to study gene function.
Acknowledgements
The authors thank Saadia Nahon for technical support, Arnon Brand for graphic design, and Hanita Zemach for assistance with microscopic analyses. They also thank Prof. Yuval Eshed (Weizmann Institute) for fruitful discussions, critical reading of the manuscript, and help with the mutant screening, and Prof. Dani Zamir (Hebrew University) for PhD guidance of Oded Cohen. This research was supported by the Israel Science Foundation (grant no. 1349/10).
References
- Alonso-Blanco C, Aarts MGM, Bentsink L, Keurentjes JJB, Reymond M, Vreugdenhil D, Koornneef M. 2009. What has natural variation taught us about plant development, physiology, and adaptation The Plant Cell 21 1877– 1896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez J, Guli CL, Yu XH, Smyth DR. 1992. Terminal Flower. A gene affecting inflorescence development in Arabidopsis thaliana The Plant Journal 2 103– 116 [Google Scholar]
- Becker A, Theissen G. 2003. The major clades of MADS-box genes and their role in the development and evolution of flowering plants Molecular Phylogenetics and Evolution 29 464– 489 [DOI] [PubMed] [Google Scholar]
- Ben Chaim A, Paran I, Grube RC, Jahn M, van Wijk R, Peleman J. 2001. QTL mapping of fruit-related traits in pepper (Capsicum annuum) Theoretical and Applied Genetics 102 1016– 1028 [Google Scholar]
- Castel R, Kusters E, Koes R. 2010. Inflorescence development in petunia: through the maze of botanical terminology Journal of Experimental Botany 61 2235– 2246 [DOI] [PubMed] [Google Scholar]
- Conti L, Bradley D. 2007. TERMINAL FLOWER1 is a mobile signal controlling Arabidopsis architecture The Plant Cell 19 767– 778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Folter S, Immink RGH, Kieffer M, et al. 2005. Comprehensive interaction map of the Arabidopsis MADS box transcription factors The Plant Cell 17 1424– 1433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egea-Cortines M, Saedler H, Sommer H. 1999. Ternary complex formation between the MADS-box proteins SQUAMOSA, DEFICIENS and GLOBOSA is involved in the control of floral architecture in Antirrhinum majus EMBO Journal 18 5370– 5379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elitzur T, Nahum H, Borovsky Y, Pekker I, Eshed Y, Paran I. 2009. Co-ordinated regulation of flowering time, plant architecture and growth by FASCICULATE: the pepper orthologue of SELF PRUNING Journal of Experimental Botany 60 869– 880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery GC, Munger HM. 1970. Alteration of growth and flowering in tomatoes by the jointless genotype The Journal of Heredity 61 51– 52 [Google Scholar]
- García-Maroto F, Ortega N, Lozano R, Carmona MJ. 2000. Characterization of the potato MADS-box gene StMADS16 and expression analysis in tobacco transgenic plants Plant Molecular Biology 42 499– 513 [DOI] [PubMed] [Google Scholar]
- Gregis V, Sessa A, Colombo L, Kater MM. 2006. AGL24, SHORT VEGETATIVE PHASE, and APETALA1 redundantly control AGAMOUS during early stages of flower development in Arabidopsis. The Plant Cell 18 1373– 1382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann U, Höhmann S, Nettesheim K, Wisman E, Saedler H, Huijser P. 2000. Molecular cloning of SVP: a negative regulator of the floral transition in Arabidopsis The Plant Journal 21 351– 360 [DOI] [PubMed] [Google Scholar]
- He C, Tian Y, Saedler R, Efremova N, Riss S, Khan MR, Yephremov A, Saedler H. 2010. The MADS-domain protein MPF1 of Physalis floridana controls plant architecture, seed development and flowering time Planta 231 767– 777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izawa T. 2007. Adaptation of flowering-time by natural and artificial selection in Arabidopsis and rice Journal of Experimental Botany 58 3091– 3097 [DOI] [PubMed] [Google Scholar]
- Jeifetz D, David-Schwartz R, Borovsky Y, Paran I. 2011. CaBLIND regulates axillary meristem initiation and transition to flowering in pepper Planta 234 1227– 1236 [DOI] [PubMed] [Google Scholar]
- Kane NA, Danyluk J, Tardif G, Ouellet F, Laliberté JF, Limin AE, Fowler DB, Sarhan F. 2005. TaVRT-2, a member of the StMADS-11 clade of flowering repressors, is regulated by vernalization and photoperiod in wheat Plant Physiology 138 2354– 2363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Park SH, Ahn JH. 2012. Functional conservation and diversification between rice OsMADS22/OsMADS55 and Arabidopsis SVP proteins Plant Science 185–186 97– 104 [DOI] [PubMed] [Google Scholar]
- Li D, Liu C, Shen L, Wu Y, Chen H, Robertson M, Helliwell CA, Ito T, Meyerowitz E, Yu H. 2008. A repressor complex governs the integration of flowering signals in Arabidopsis Developmental Cell 15 110– 120 [DOI] [PubMed] [Google Scholar]
- Lifschitz E, Eshed Y. 2006. Universal florigenic signals triggered by FT homologues regulate growth and flowering cycles in perennial day-neutral tomato Journal of Experimental Botany 57 3405– 3414 [DOI] [PubMed] [Google Scholar]
- Lifschitz E, Eviatar T, Rozman A, Shalit A, Goldshmidt A, Amsellem Z, Alvarez JP, Eshed Y. 2006. The tomato FT ortholog triggers systemic signals that regulate growth and flowering and substitute for diverse environmental stimuli Proceedings of the National Academy of Sciences, USA 103 6398– 6403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippman ZB, Cohen O, Alvarez JP, Abu-Abied M, Pekker I, Paran I, Eshed Y, Zamir D. The making of a compound inflorescence in tomato and related nightshades. PLoS Biology. 2008;6:e288. doi: 10.1371/journal.pbio.0060288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Watanabe S, Uchiyama T, et al. 2010. The soybean stem growth habit gene DT1 is an ortholog of Arabidopsis TERMINAL FLOWER1 Plant Physiology 153 198– 210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Chen H, Er HL, Soo HM, Kumar PP, Han JH, Liou YC, Yu H. 2008. Direct interaction of AGL24 and SOC1 integrates flowering signals in Arabidopsis Development 135 1481– 1491 [DOI] [PubMed] [Google Scholar]
- Liu C, Xi W, Shen L, Tan C, Yu H. 2009. . Regulation of floral patterning by flowering time genes Developmental Cell 16 711– 722 [DOI] [PubMed] [Google Scholar]
- Livingstone KD, Lackney VK, Blauth J, Wijk VR, Jahn MK. 1999. Genome mapping in Capsicum and the evolution of genome structure in the Solanaceae Genetics 152 1183– 1202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao L, Begum D, Chuang HW, Budiman MA, Szymkowiak EJ, Irish EE, Wing RA. 2000. JOINTLESS is a MADS-box gene controlling tomato flower abscission zone development Nature 406 910– 913 [DOI] [PubMed] [Google Scholar]
- Masiero S, Li MA, Will I, Hartmann U, Saedler H, Huijser P, Schwarz-Sommer Z, Sommer H. 2004. INCOMPOSITA: a MADS-box gene controlling prophyll development and floral meristem identity in Antirrhinum Development 131 5981– 5990 [DOI] [PubMed] [Google Scholar]
- Meng X, Muszynski MG, Danilevskaya ON. 2011. The FT-Like ZCN8 gene functions as a floral activator and is involved in photoperiod sensitivity in maize The Plant Cell 23 942– 960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaels SD. 2009. Flowering time regulation produces much fruit Current Opinion in Plant Biology 12 75– 80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizoguchi T, Niinuma K, Yoshida R. 2007. . Day-neutral response of photoperiodic flowering in tomatoes: possible implications based on recent molecular genetics of Arabidopsis and rice Plant Biotechnology 24 83– 86 [Google Scholar]
- Mohamed R, Wang C, Ma C, et al. 2010. Populus CEN/TFL1 regulates first onset of flowering, axillary meristem identityand dormancy release in Populus The Plant Journal 62 674– 688 [DOI] [PubMed] [Google Scholar]
- Nakano T, Kimbara J, Fujisawa M, Kitagawa M, Ihashi N, Maeda H, Kasumi T, Ito Y. 2012. MACROCALYX and JOINTLESS interact in the transcriptional regulation of tomato fruit abscission zone development Plant Physiology 158 439– 450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neta R, David-Schwartz R, Peretz Y, Sela I, Rabinowitch HD, Flaishman M, Kamenetsky R. 2011. Flower development ingarlic: the ups and downs of gaLFY expression Planta 233 1063– 1072 [DOI] [PubMed] [Google Scholar]
- Paran I, Borovsky Y, Nahon S, Cohen O. 2007. . The use of induced mutations to study shoot architecture in Capsicum Israel Journal of Plant Sciences 55 125– 131 [Google Scholar]
- Park SJ, Jiang K, Schatz MC, Lippman ZB. 2012. Rate of meristem maturation determines inflorescence architecture in tomato Proceedings of the National Academy of Sciences, USA 109 639– 644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pnueli L, Carmel-Goren L, Hareven D, Gutfinger T, Alvarez J, Ganal M, Zamir D, Lifschitz E. 1998. The SELF-PRUNING gene of tomato regulates vegetative to reproductive switching of sympodial meristems and is the ortholog of CEN and TFL1 Development 125 1979– 1989 [DOI] [PubMed] [Google Scholar]
- Rick C, Butler L. 1956. Cytogenetics of tomato Advances in Genetics 8 267– 382 [Google Scholar]
- Samach A, Lotan H. 2007. The transition to flowering in tomato Plant Biotechnology 24 71– 82 [Google Scholar]
- Shalit A, Rozman A, Goldshmidt A, Alvarez John P, Bowman JL, Eshed Y, Lifschitz E. 2009. The flowering hormone florigen functions as a general systemic regulator of growth and termination Proceedings of the National Academy of Sciences, USA 106 8392– 8397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srikanth A, Schmid M. 2011. Regulation of flowering time: all roads lead to Rome Cellular and Molecular Life Sciences 68 2013– 2037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szymkowiak EJ, Irish EE. 2006. JOINTLESS suppresses sympodial identity in inflorescence meristems of tomato Planta 223 646– 658 [DOI] [PubMed] [Google Scholar]
- Tamaki S, Matsuo S, Wong HL, Yokoi S, Shimamoto K. 2007. Hd3a protein is a mobile flowering signal in rice Science 316 1033– 1036 [DOI] [PubMed] [Google Scholar]
- Thouet J, Quinet M, Lutts S, Kinet JM, Perilleux C. Repression of floral meristem fate is crucial in shaping tomato inflorescence. PLoS ONE. 2012;7:e31096. doi: 10.1371/journal.pone.0031096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Z, Wang X, Lee R, Li Y, Specht JE, Nelson RL, McClean PE, Qiu L, Ma J. 2010. Artificial selection for determinate growth habit in soybean Proceedings of the National Academy of Sciences, USA 107 8563– 8568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Li J. 2008. Molecular basis of plant architecture Annual Review of Plant Biology 59 253– 279 [DOI] [PubMed] [Google Scholar]
- Wigge PA. 2011. FT, a mobile developmental signal in plants Current Biology 21 R374– R378 [DOI] [PubMed] [Google Scholar]
- Yant L, Mathieu J, Schmid M. 2009. Just say no: floral repressors help Arabidopsis bide the time Current Opinion in Plant Biology 12 580– 586 [DOI] [PubMed] [Google Scholar]