Abstract
Nitric oxide (NO), hydrogen peroxide (H2O2), and calcium (Ca2+)/calmodulin (CaM) are all required for abscisic acid (ABA)-induced antioxidant defence. Ca2+/CaM-dependent protein kinase (CCaMK) is a strong candidate for the decoder of Ca2+ signals. However, whether CCaMK is involved in ABA-induced antioxidant defence is unknown. The results of the present study show that exogenous and endogenous ABA induced increases in the activity of ZmCCaMK and the expression of ZmCCaMK in leaves of maize. Subcellular localization analysis showed that ZmCCaMK is located in the nucleus, the cytoplasm, and the plasma membrane. The transient expression of ZmCCaMK and the RNA interference (RNAi) silencing of ZmCCaMK analysis in maize protoplasts revealed that ZmCCaMK is required for ABA-induced antioxidant defence. Moreover, treatment with the NO donor sodium nitroprusside (SNP) induced the activation of ZmCCaMK and the expression of ZmCCaMK. Pre-treatments with an NO scavenger and inhibitor blocked the ABA-induced increases in the activity and the transcript level of ZmCCaMK. Conversely, RNAi silencing of ZmCCaMK in maize protoplasts did not affect the ABA-induced NO production, which was further confirmed using a mutant of OsCCaMK, the homologous gene of ZmCCaMK in rice. Moreover, H2O2 was also required for the ABA activation of ZmCCaMK, and pre-treatments with an NO scavenger and inhibitor inhibited the H2O2-induced increase in the activity of ZmCCaMK. Taken together, the data clearly suggest that ZmCCaMK is required for ABA-induced antioxidant defence, and H2O2-dependent NO production plays an important role in the ABA-induced activation of ZmCCaMK.
Key words: ABA, antioxidant defence, H2O2, NO, ZmCCaMK.
Introduction
Plants are subjected to various biotic and abiotic stresses during their growth and development and have evolved various mechanisms to adapt to these stresses. The phytohormone abscisic acid (ABA) represents a key signal to regulate plant responses to biotic and abiotic stresses (Zhu, 2002; Cutler et al., 2010). ABA can enhance the antioxidant defence system to protect cells and subcellular systems from the damage caused by excess reactive oxygen species (ROS) (Jiang and Zhang, 2001; Park et al., 2004; Neill et al., 2008). Previous studies have shown that hydrogen peroxide (H2O2), nitric oxide (NO), calcium (Ca2+)/calmodulin (CaM), and mitogen-activated protein kinase (MAPK) are required for ABA-induced up-regulation of the expression and the activities of antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX), and glutathione reductase (GR) in plants (Jiang and Zhang, 2002; Zhang et al., 2006, 2007; Sang et al., 2008; Zhang et al., 2009). However, the detailed mechanisms of ABA-induced antioxidant defence remain unclear.
Ca2+ is a ubiquitous and pivotal second messenger in the signal transduction networks (Pei et al., 2000; Dodd et al., 2010; Kim et al., 2010; Kudla et al., 2010). Various stimuli, such as salinity, drought, cold, heat shock, mechanical disturbances, ABA, H2O2, and pathogen elicitors, trigger changes in the cytosolic Ca2+ concentration, and the transient Ca2+ elevations are recognized by several Ca2+ sensors such as CaM and CaM-like protein (CML), calcium-dependent protein kinase (CDPK), calcineurin B-like protein (CBL), and Ca2+/CaM-dependent protein kinase (CCaMK) (Harmon et al., 2000; Luan et al., 2002; Yang and Poovaiah, 2003; Zhang and Lu, 2003; Harper et al., 2004; Bouché et al., 2005; Harper and Harmon, 2005; Yano et al., 2008; DeFalco et al., 2010). These Ca2+ sensors convert the Ca2+ signals into various physiological responses.
CCaMK is a strong candidate for the decoder of Ca2+ spiking. A CCaMK structure includes a serine/threonine kinase domain, a CaM-binding domain, and three EF-hand motifs, similar to the visinin-like domain (Patil et al., 1995; Takezawa et al., 1996). Many studies have shown that CCaMK is a common symbiosis signalling pathway component and regulates both arbuscular mycorrhiza (AM) and rhizobial symbioses in legumes and non-legumes (Lévy et al., 2004; Godfroy et al., 2006; Tirichine et al., 2006; Chen et al., 2007, 2008; Capoen et al., 2009; Hayashi et al., 2010; Kang et al., 2011). CCaMK was also suggested to play roles in meiosis and mitosis (Patil et al., 1995; Poovaiah et al., 1999; Yang and Poovaiah, 2003). In addition to its development-related roles, CCaMK may also be involved in abiotic stress responses. The wheat CCaMK gene TaCCaMK was down-regulated by ABA, as well as NaCl and polyethylene glycol (PEG) treatments in wheat seedling roots (Yang et al., 2011). Overexpressing TaCCaMK in Arabidopsis reduced their sensitivity to ABA treatment during seed germination and enhanced the seed germination rate under high-salt conditions. These results suggest that TaCCaMK is a negative regulator of ABA signalling involved in abiotic stress responses in wheat.
Previous studies have shown that Ca2+/CaM is required for ABA-induced antioxidant defence, and the cross-talk between Ca2+/CaM and H2O2 and NO plays a pivotal role in the ABA signalling in leaves of maize seedlings (Jiang and Zhang, 2003; Sang et al., 2008). However, it is unknown whether Ca2+/CaM-mediated up-regulation in the antioxidant defence is through the action of CCaMK activated by Ca2+/CaM in ABA signalling and, if so, what the relationship between ZmCCaMK and H2O2 and NO in ABA signalling is.
In this study, the role of CCaMK in ABA-induced up-regulation in the expression of antioxidant genes such as SOD4, encoding a cytosolic isoform of SOD, cAPX, encoding a cytosolic isoform of APX, the total activities of antioxidant enzymes SOD and APX in leaves of maize plants, and the relationship between CCaMK and H2O2 and NO were investigated. By combining pharmacological and biochemical analysis with a genetic approach, evidence is provided to show that ZmCCaMK is required for ABA-induced antioxidant defence, and H2O2-induced NO production plays an important role in the activation of ZmCCaMK in ABA signalling.
Materials and methods
Plant materials and treatments
Maize (Zea mays L. cv. Nongda 108; from Nanjing Agricultural Uni versity, China), rice (Oryza sativa) cultivar Nipponbare, and the rice mutant line NF8513 were used in this study. In the mutant experiments, Nipponbare was used as the wild-type control. Seeds of maize were sown in trays of sand, and rice plants were grown hydroponically with a nutrient solution in a light chamber at a temperature of 22–28 °C, with a photosynthetically active radiation of 200 µmol m–2 s–1 and a photoperiod of 14/10h (light/dark), and watered daily. For protoplast isolation, maize plants were grown at 25 °C under dark conditions. When the second leaves were fully expanded, they were collected and used for investigations.
The plants were excised at the base of the stem and placed in distilled water for 1h to eliminate wound stress. After treatment, the cut ends of the stems were placed in beakers wrapped with aluminium foil containing 100 µM ABA, 10% PEG, 100 µM sodium nitroprusside (SNP), or 10mM H2O2 solution for various times at 25 °C, with a continuous light intensity of 200 µmol m–2 s–1. In order to study the effects of various inhibitors or scavengers, the detached plants were pre-treated with 200 µΜ N-(6-aminohexyl)-5-chloro-1-naphthalene sulphonamide hydrochloride (W7), 100 µΜ trifluoperazine (TFP), 5mM EGTA, 5mM LaCl3, 10mM dimethylthiourea (DMTU), 200U of catalase (CAT), 100 µM diphenyleneiodonium (DPI), 200 µM 2-4-carboxyphenyl-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (cPTIO), and 200 µM N G-nitro-l-Arg methyl ester (l-NAME) for 4h, then exposed to 100 µM ABA or 10mM H2O2 treatment for various times under the same conditions as described above. Detached plants were treated with distilled water under the same conditions for the whole period and served as controls for the above. For fluridone treatment, the seeds soaking in 80 µM fluridone for 14h were sown in trays of sand. After treatments of detached maize plants, the second leaves were sampled and immediately frozen under liquid N2 for further analysis.
Antibody production and immunoprecipitation kinase activity assay
The peptides for ZmCCaMK-C (GDITEPGKLDEVFD) corresponding to the C-terminus of ZmCCaMK were synthesized and conjugated to the keyhole limpet haemocyanin carrier. The ZmCCaMK polyclonal antibody was raised in rabbits and purified by affinity chromatography. Protein was extracted from maize leaves or protoplasts as described previously (Zhang et al., 2006). Protein content was determined according to the method of Bradford (1976) with bovine serum albumin (BSA) as standard. For immunocomplex kinase assay, protein extract (200 µg) was incubated with anti-ZmCCaMK antibody (7.5 µg) in an immunoprecipitation buffer as described previously (Zhang et al., 2006). Kinase activity in the immunocomplex was determined by an in-gel kinase assay using histone S-III as the substrate (Takezawa et al., 1996; Zhang et al., 2002). The immunocomplex was incubated in reaction buffer [25mM Tris, pH 7.5, 5mM MgCl2, 1mM dithiothreitol (DTT), 2.5mM CaCl2, 2 µM CaM, 1mg ml–1 histone S-III] with 200nM ATP plus 1 µCi of [γ-32P]ATP (3000 Ci mM–1) for 30min. An equal volume of SDS sample buffer was added to stop the reaction. The reaction mix was boiled at 100 °C for 5min and resolved by SDS–PAGE. The unincorporated [γ-32P]ATP was removed by washing with 5% trichloroacetic acid (w/v)/1% sodium pyrophosphate (w/v) at least three times. The gel was dried onto Whatman 3 MM paper and exposed to Kodak XAR-5 film. Pre-stained size markers (Bio-Rad) were used to calculate the size of the kinases. Relative activation levels of ZmCCaMK protein were quantitated by Quantity One software (Bio-Rad Laboratories Inc., USA).
Isolation of total RNA and RT-PCR analysis
Total RNA was isolated from leaves or protoplasts using RNAiso Plus (TaKaRa Bio Inc., China) according to the instructions supplied by the manufacturer. Approximately 2 µg of total RNA were reverse transcribed using oligo(dT)18 primer and M-MLV reverse transcriptase (TaKaRa Bio Inc., China). Transcript levels of several genes were measured by RT-PCR using the following primers: ZmCCaMK (GenBank accession no. DQ403196; size of product, 457bp), forward CGCCGTTCCATGCACCA and reverse AGCCTCATCGCCCTCAGCAC; SOD4 (GenBank accession no.. X17565; size of product, 404bp), forward GGGCACAATCTTCTTCACC and reverse GTCCGATGATCCCACAAG; cAPX (GenBank accession no. EU969033; size of product, 450bp), forward TCACACCCTGGGAAGATG and reverse GCTTCATATCAAACCTTCTCC; GAPDH (glyceraldehyde phosphate dehydrogenase; GenBank accession no. X07156; size of product, 264bp), forward CAACGACCCCTTCATCACC and reverse ACCTTCTTGGCACCACCCT. To standardize the results, the relative abundance of GAPDH was determined and used as the internal standard. The cycle number of the PCRs was adjusted for each gene to obtain barely visible bands in agarose gels. Aliquots of the PCRs were loaded on agarose gels and stained with ethidium bromide.
Real-time quantitative RT-PCR expression analysis
The expression of ZmCCaMK was also measured by qRT-PCR using the DNA Engine Opticon 2 real-time PCR detection system (Bio-Rad Laboratories Inc., USA) with the SYBR® Premix Ex Taq™ (TaKaRa Bio Inc., China) according to the manufacturer’s instructions. The cDNA was amplified by PCR using the following primers: ZmCCaMK (size of product, 172bp), forward CTCAAGCCCGAGAACTGCC and reverse TGGCAGCCGAGACATCC; β-actin (GenBank accession no. J01238; size of product, 152bp), forward GTTTCCTGGGATTGCCGAT and reverse TCTGCTGCTGAAAAGTGCTGAG. To standardize the results, the amplification of β-actin was determined and used as the internal standard. The data were normalized to the amplification of a maize β-actin gene. For each sample, the mean value from three qRT-PCRs was adapted to calculate the expression abundance, and the mean values were then plotted with their SE.
Vector construction and in vitro transcription of the ZmCCaMK gene double-stranded RNA
The full-length cDNA fragment was amplified with the addition of a BsrGI site and then inserted in-frame with yellow fluorescent protein (YFP) into the pXZP008 vector driven by the Cauliflower mosaic virus (CaMV) 35S promoter. The primers used for the PCR amplification were: forward 5′-TGTACAAGATTCCTCGCACAAACCTGCCACA-3′, and reverse 5′-TGTACAGGTGGGAATGAAGTTGAACGAGTTGGAAT-3′ (underlining indicates the BsrGI site).
A template partial-length DNA fragment was amplified by PCR using primers flanked by a T7 promoter, forward (5′-TAATACGACTCACTATAGGGCAAGCCCGAGAACTGCC-3′) and reverse (5′-TAATACGACTCACTATAGGGTGGCAGCCGAGACATCC-3′) (the T7 promoter site is underlined). The PCR amplification consisted of initial denaturation at 94 °C for 3min, then 35 cycles of 94 °C for 20 s, 60 °C for 15 s, and 72 °C for 15 s, and a final extension at 72 °C for 2min. Double-stranded RNA (dsRNA) of ZmCCaMK was synthesized in vitro using the RiboMAX™ Large Scale RNA Production System-T7 (Promega, Madison, WI, USA) according to the manufacturer’s instructions. The purity and concentration of synthesized dsRNA were checked by 2% agarose gel electrophoresis and spectrophotometry.
Protoplast preparation and transfection with constructs or dsRNAs
Protoplast isolation and transfection with constructs or dsRNAs were based on the protocol for maize mesophyll protoplasts provided online by J. Sheen’s laboratory http://genetics.mgh.harvard.edu/sheenweb with minor modifications. For transfection, 1ml of maize protoplasts (usually 5×105 cells ml–1) were transfected with 150 µg of YFP–ZmCCaMK fusion constructs (pXZP008 vector as control) or dsRNAs (H2O as control) using a PEG–calcium-mediated method. Then the transfected protoplasts were incubated in incubation solution overnight in the dark at 25 °C. After that, protoplasts were collected and used for further analysis.
Localization
Expression of YFP–ZmCCaMK fusion constructs and YFP was monitored using a confocal microscope (TCS-SP2, Leica, Bensheim, Germany). The nucleus was stained with the 4′,6-diamidino-2-phenylindole (DAPI) dye, and the plasma membrane was visualized by staining with N-(3-triethylammoniumpropyl]-4-[p-diethyl- aminophenylhexatrienyl) pyridinium dibromide (FM4-64).
Western blot assay
Proteins were extracted from protoplasts tansfected with YFP or YFP-ZmCCaMK, and 20 µg of total protein was subjected to SDS–PAGE. Western blot analysis was performed as described by Sambrook and Russell (2001). Anti-YFP antibody was used to detect the YFP protein or YFP–ZmCCaMK protein.
Antioxidant enzyme assays
Protoplasts were homogenized in 0.5ml of 50mM potassium phosphate buffer (pH 7.0) containing 1mM EDTA and 1% polyvinylpyrrolidone, with the addition of 1mM ascorbate in the case of APX assay. The homogenate was centrifuged at 12 000 g for 15min at 4 °C and the supernatant was immediately used for the susequent antioxidant enzyme assays. The total activities of antioxidant enzymes were determined as previously described (Jiang and Zhang, 2001). Total SOD activity was assayed by monitoring the inhibition of photochemical reduction of nitro blue tetrazolium. One unit of SOD activity was defined as the amount of enzyme that was required to cause 50% inhibition of the reduction of nitro blue tetrazolium, as monitored at 560nm. Total APX activity was measured by monitoring the decrease in absorbance at 290nm as ascorbate was oxidized.
Nitric oxide detection by confocal laser scanning microscopy (CLSM)
Measurement of NO was performed with the specific NO dye 4,5-diaminofluorescein diacetate (DAF-2DA), using the method as described by Corpas et al. (2004) with slight modifications. Leaf segments of ~0.5cm2 or protoplasts were incubated in loading buffer [0.1mM CaCl2, 10mM KCl, 10mM 2-(N-morpholino)ethanesulphonic acid (MES)-TRIS, pH 5.6] and DAF-2DA at a final concentration of 10 µM for 1h in the dark at 25 °C, followed by washing with loading buffer for 1h. All images were visualized using confocal microscopy (excitation 495nm, emission 515nm). To enable the comparison of changes in signal intensity, confocal images were taken under identical exposure conditions for all the samples. The green fluorescence intensity of images acquired was quantified using Leica IMAGE software to integrate the intensity over all pixels within the boundary of each image. The value of each image was normalized to a reference image of the basal state. Data are presented as average fluorescence intensity.
Results
ABA induces increases in the activity and the expression of ZmCCaMK in leaves of maize
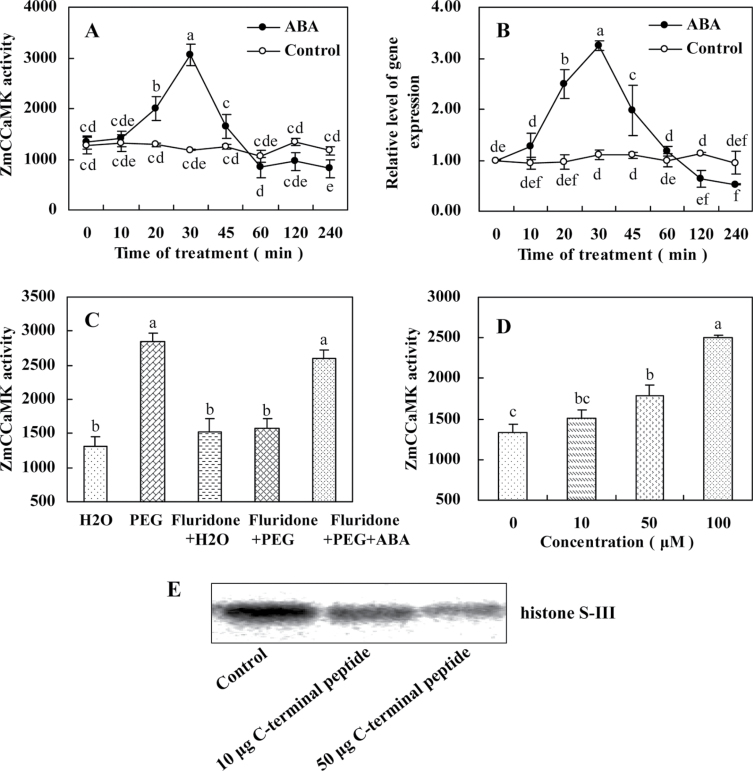
In order to investigate the effect of ABA on the activation of ZmCCaMK in leaves of maize plants, an antibody against the C-terminus of ZmCCaMK was raised and an immunoprecipitation kinase assay was performed on protein extracts from the leaves of maize plants treated or not with ABA, using histone S-III as a substrate (Takezawa et al., 1996; Zhang et al., 2002). Under control conditions, a low background level of ZmCCaMK activity was observed (Fig. 1A). However, when 100 µM ABA was applied, a significant increase in the activation of ZmCCaMK was detected after 20min treatment, was maximizal at 30min, and then decreased (Fig. 1A). Moreover, the ABA-induced activation of ZmCCaMK occurred in a dose-dependent manner in the concentration range of 10–100 µM ABA (Fig. 1D).
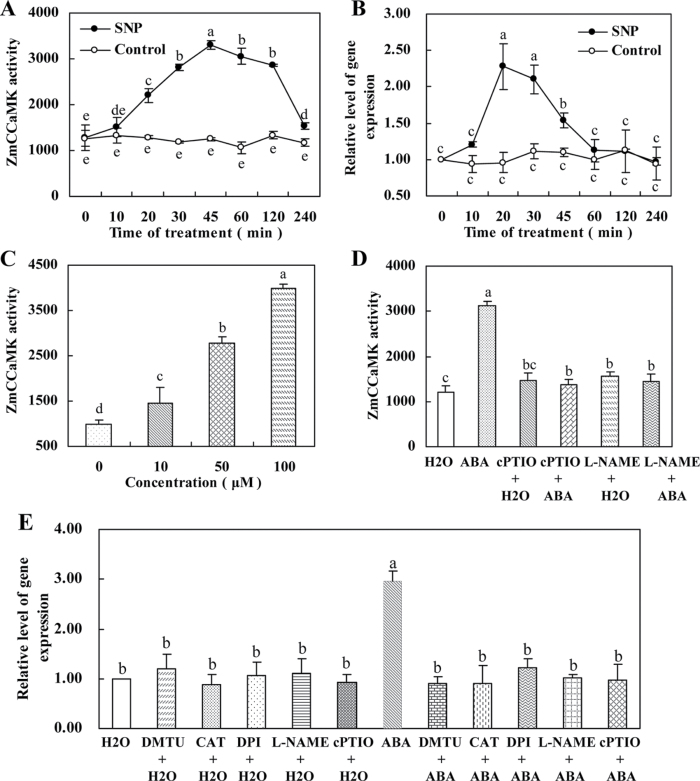
Fig. 1.
ABA induces the up-regulation in the activity of ZmCCaMK and the expression of ZmCCaMK in leaves of maize plants. (A) Time course of ABA-induced ZmCCaMK activation. The detached plants were treated with 100 µM ABA for various times as indicated. Protein extracts from control or ABA-treated leaves were immunoprecipitated with ZmCCaMK antibody and then subjected to an in-gel kinase assay. (B) Time course of ABA-induced gene expression of ZmCCaMK. The detached plants were treated as described in A. Relative expression levels of the ZmCCaMK gene, analysed by qRT-PCR, are normalized to β-actin transcript levels. (C) PEG-induced ABA activates ZmCCaMK. The detached plants were treated as follows: distilled water (control); 10% PEG; 80 µM fluridone+H2O; 80 µM fluridone+10% PEG; 80 µM fluridone+10% PEG+100 µM ABA. Protein extracts were subjected to immunoprecipitation kinase assay. (D) Dose dependence for ABA-induced ZmCCaMK activation. The detached plants were treated with 0, 10, 50, and 100 µM ABA for 30min. Protein extracts were subjected to immunoprecipitation kinase assay. (E) Kinase activity assay of immune complexes. Immunoprecipitation was performed in the absence or presence of 10 µg or 50 µg of competitor peptides corresponding to the C-terminal peptide of ZmCCaMK. Immunocomplex kinase activity was measured using an in-gel kinase assay. Experiments were repeated at least three times with similar results. Values are means ±SE of three different experiments. Means denoted by the same letter did not differ significantly at P < 0.05 according to Duncan’s multiple range test.
To investigate whether the activation of ZmCCaMK can be induced by endogenous ABA, maize seeds were pre-treated by fluridone, an inhibitor of carotenoid biosynthesis, and hence of ABA biosynthesis (Nagamune et al., 2008), and then the pre-treated plants were exposed to PEG treatment. PEG-induced activation of ZmCCaMK was significantly inhibited in 80 µM fluridone-pre-treated plants, but this effect of fluridone was overcome by the application of 100 µM ABA (Fig. 1C), indicating that water stress-induced endogenous ABA accumulation can activate ZmCCaMK.
The effects of ABA on the induction of ZmCCaMK gene expression in leaves of maize plants were further examined, and relative quantitative real-time PCR analysis was performed using total RNA extracted from maize plants treated or not with 100 µM ABA. The experimental results showed that the ZmCCaMK gene was up-regulated by the treatment with 100 µM ABA (Fig. 1B), and the changed pattern of ZmCCaMK gene expression was similar to that of ZmCCaMK activation in leaves of maize exposed to ABA treatment (Fig. 1A, B).
To prove the specificity of the antibody, immunoprecipitations with or without peptide competitors were carried out and immune complexes were assayed for kinase activity. Proteins that could phosphorylate histone S-III were precipitated from extracts of leaves of maize plants. The immune complexes were competed out by the peptide used to raise the antibody against the C-terminal region of ZmCCaMK (Fig. 1E). Further, pre-treatments with the Ca2+ chelator EGTA, the Ca2+ channel blocker LaCl3, and the CaM antagonists TFP and W7 significantly reduced the ABA-induced activation of ZmCCaMK in leaves of maize plants treated with ABA (data not shown).
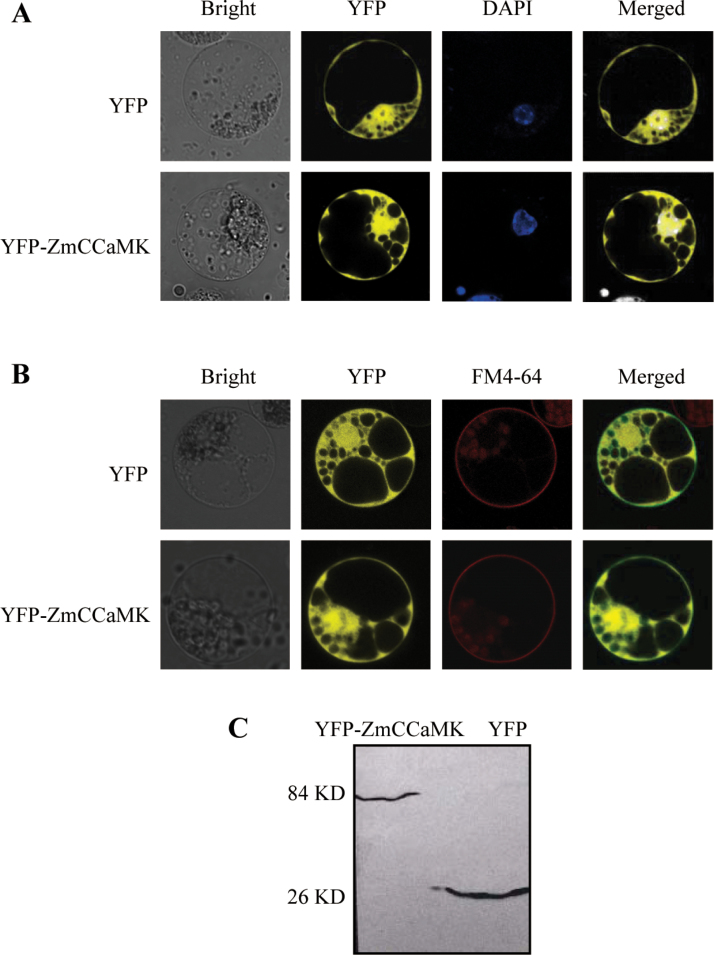
Subcellular localization of ZmCCaMK in maize mesophyll protoplasts
To gain evidence indicative of function, the subcellular localization of ZmCCaMK was investigated in transfected maize protoplasts by using confocal microscopy. YFP fusion constructs for ZmCCaMK were generated under the control of the CaMV 35S promoter. The results showed that YFP–ZmCCaMK was localized in the nucleus, the cytoplasm, and the plasma membrane in maize mesophyll protoplasts (Fig. 2A, B). Nuclear location was confirmed by means of DAPI staining for DNA (Sakamoto et al., 2004), and the plasma membrane location was confirmed by co-localization with the plasma membrane marker, FM4-64 (Levy et al., 2007; Zelazny et al., 2007). In addition, the localization of YFP–ZmCCaMK was not regulated by ABA treatment in maize mesophyll protoplasts (data not shown). Furthermore, the expression of the effector proteins was confirmed by western blot analysis using an anti-YFP antibody in maize mesophyll protoplasts transfected with constructs (Fig. 2C).
Fig. 2.
Subcellular localization of ZmCCaMK in maize protoplasts. (A, B) Protoplasts were isolated from the leaves of maize, and then were transfected with constructs carrying 35S:YFP-ZmCCaMK or 35S:YFP by PEG–calcium-mediated transformation. Fluorescence micrographs were taken after 16h of incubation by a laser confocal microscope. The nucleus was stained with DAPI dye (blue, A). The plasma membrane was labelled with FM4-64 steryl dye (red, B). Experiments were repeated at least five times with similar results. (C) Western blot analysis for YFP–ZmCCaMK fusion proteins with an anti-YFP antibody. Experiments were repeated at least five times with similar results. (This figure is available in colour at JXB online.)
ZmCCaMK modulates the ABA-induced antioxidant defence
To investigate whether ZmCCaMK mediates the ABA-induced antioxidant defence in maize, a transient gene expression analysis and a transient RNA interference (RNAi) analysis in maize mesophyll protoplasts, which have been proven to be efficient for functional analysis of plant genes (Sheen, 2001; Chen et al., 2006; Zhai et al., 2009; Kim and Somers, 2010; Gao et al., 2011), were used for the functional analysis of ZmCCaMK in ABA-induced antioxidant defence.
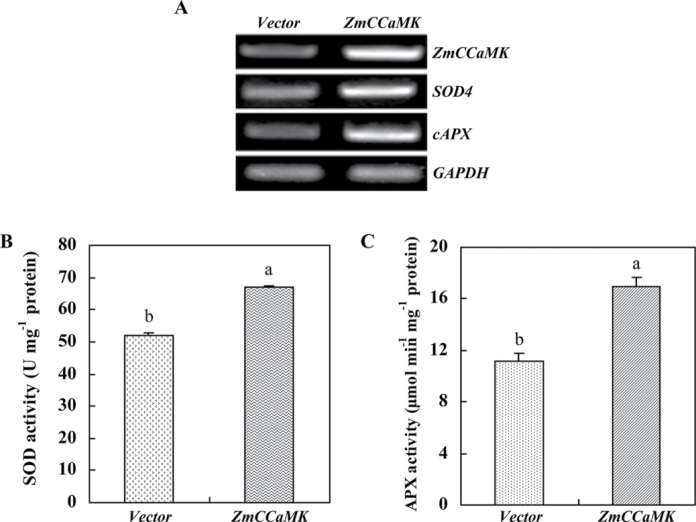
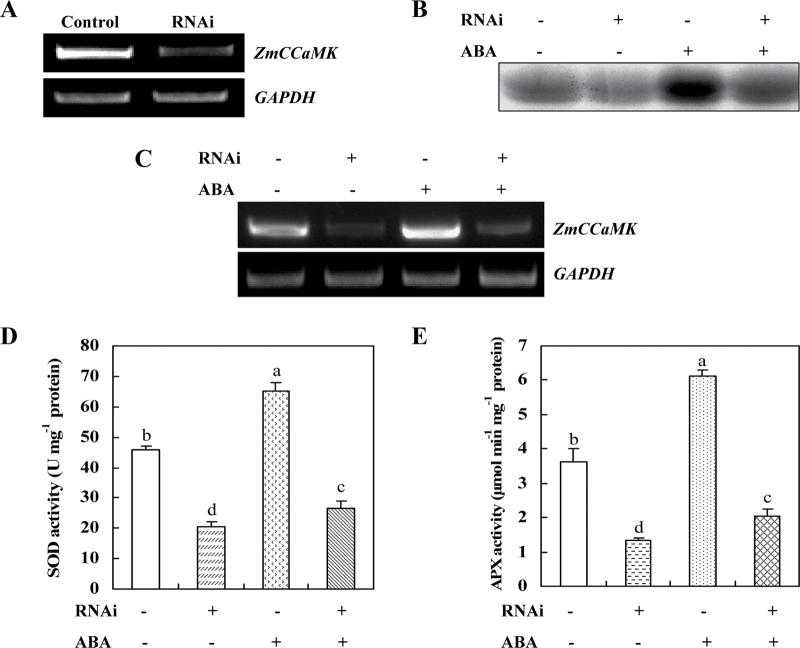
As anticipated, transient expression of ZmCCaMK in protoplasts resulted in a significant enhancement in the transcript levels of ZmCCaMK, and the antioxidant genes, SOD4 and cAPX, when compared with those in the protoplasts transfected with the empty vector (Fig. 3A). Similar to the expression of antioxidant genes, the total activities of the antioxidant enzymes SOD and APX were also obviously increased in protoplasts showing transient expression of ZmCCaMK (Fig. 3B, C). On the other hand, RNAi-mediated silencing of ZmCCaMK, which resulted in an obvious reduction in the expression of ZmCCaMK (Fig. 4A) and the activity of ZmCCaMK (Fig. 4B), significantly decreased the activities of SOD and APX compared with control (Fig. 4D, E). Further, treatment with 10 µM ABA only slightly increased the expression of ZmCCaMK and the activities of ZmCCaMK, SOD, and APX in protoplasts subjected to RNAi silencing of ZmCCaMK (Fig. 4B–E). However, treatment with 10 µM ABA induced significant increases in the expression of ZmCCaMK and the activities of ZmCCaMK, SOD, and APX in the control protoplasts (Fig. 4B–E). Taken together, these data demonstrate unequivocally that ZmCCaMK is required for ABA-induced antioxidant defence in maize protoplasts.
Fig. 3.
The gene expression and the total activities of antioxidant enzymes in maize mesophyll protoplasts transiently expressing ZmCCaMK. (A) The expression of ZmCCaMK, SOD4, and cAPX. Protoplasts isolated from the leaves of maize were transfected with constructs carrying YFP-ZmCCaMK (ZmCCaMK), and control protoplasts were transfected with empty vector (Vector). Relative expression of antioxidant enzymes was analysed by RT-PCR, and was normalized to GAPDH transcript levels. Experiments were repeated at least three times with similar results. (B and C) The activities of SOD and APX. Protoplasts were isolated as described in A, and the activities of SOD and APX were measured as described in the Materials and methods. Values are means ±SE of three different experiments. Means denoted by the same letter did not differ significantly at P < 0.05 according to Duncan’s multiple range test.
Fig. 4.
The expression of ZmCCaMK and the activities of ZmCCaMK and antioxidant enzymes in the protoplasts with transiently silenced ZmCCaMK. (A) The expression of ZmCCaMK. Protoplasts were transfected with dsRNA against ZmCCaMK (RNAi) or with water (Control) and incubated for 24h. Silencing of ZmCCaMK was analysed by RT-PCR, and was normalized to GAPDH transcript levels. Experiments were repeated at least three times with similar results. (B) ABA-induced activation of ZmCCaMK. The protoplasts were treated with 10 µM ABA for 5min. Protein extracts from ABA-treated or untreated protoplasts were immunoprecipitated with ZmCCaMK antibody and then subjected to an in-gel kinase assay. Experiments were repeated at least three times with similar results. (C) ABA-induced gene expression of ZmCCaMK. Protoplasts were treated with 10 µM ABA for 15min, and the expression of ZmCCaMK was analysed by RT-PCR. Experiments were repeated three times with similar results. (D, E) ABA increased the activities of SOD (D) and APX (E). Protoplasts were treated with 10 µM ABA for 15min, and the activities of SOD and APX were measured as described in the Materials and methods. Values are means ±SE of three different experiments. Means denoted by the same letter did not differ significantly at P < 0.05 according to Duncan’s multiple range test.
NO induces the activation and gene expression of ZmCCaMK in leaves of maize plants
It has been reported that NO is involved in the ABA-induced antioxidant defence system and NO functions both upstream and downstream of Ca2+/CaM in plants (Zhang et al., 2007; Sang et al., 2008; Aboul-Soud et al., 2009). To establish a link between NO and ZmCCaMK in ABA signalling, the NO donor SNP was used. Treatment with SNP led to a rapid activation of ZmCCaMK (Fig. 5A). A rapid increase in the activity of ZmCCaMK was detected within 10min and maximized at 45min after SNP treatment which has been shown to result in NO effects itself by using sodium ferricyanide as control (Zhang et al., 2007). The activity of ZmCCaMK decreased after 60min of SNP treatment. SNP treatment also induced a significant increase in the expression of ZmCCaMK in a similar manner as the activity of ZmCCaMK in leaves of maize plants (Fig. 5B). In addition, the activation of ZmCCaMK occurred at SNP concentrations as low as 10 µM and the activity of ZmCCaMK appeared to reach a maximum at 100 µM SNP (Fig. 5C).
Fig. 5.
NO induces the activation of ZmCCaMK and the expression of ZmCCaMK in leaves of maize plants. (A) Time course of SNP-induced ZmCCaMK activation. The detached plants were treated with 100 µM SNP for various times as indicated. Protein extracts from control or SNP-treated leaves were immunoprecipitated with ZmCCaMK antibody and then subjected to an in-gel kinase assay. (B) Time course of SNP-induced gene expression of ZmCCaMK. The detached plants were treated as described in A. Relative expression levels of the ZmCCaMK gene, analysed by qRT-PCR, are normalized to β-actin transcript levels. (C) Dose dependence for SNP-induced ZmCCaMK activation. The detached plants were treated with 0, 10, 50, and 100 µM SNP for 45min and then subjected to immunoprecipitation kinase assay. (D) Effects of pre-treatments with cPTIO and l-NAME on ABA-induced ZmCCaMK activation. The detached plants were treated as follows: distilled water (control); 100 µM ABA; 200 µM cPTIO+H2O; 200 µM cPTIO+100 µM ABA; 200 µM l-NAME+H2O; 200 µM l-NAME+100 µM ABA. The detached plants were pre-treated with the scavenger or inhibitor for 4h then exposed to 100 µM ABA treatment for 30min. (E) Effects of pre-treatments with the H2O2 scavengers or inhibitor, DMTU, CAT, and DPI, and the NO scavenger or inhibitor, cPTIO and l-NAME, on the expression of ZmCCaMK in leaves of maize plants exposed to ABA treatment. The detached plants were treated as follows: distilled water (control); 10mM DMTU+H2O; 200U of CAT+H2O; 100 µM DPI+H2O; 200 µM l-NAME+H2O; 200 µM cPTIO+H2O; 100 µM ABA; 10mM DMTU+100 µM ABA; 200U of CAT+100 µM ABA; 100 µM DPI+100 µM ABA; 200 µM l-NAME+100 µM ABA; 200 µM cPTIO+100 µM ABA. The detached plants were pre-treated with scavengers or inhibitors for 4h then exposed to 100 µM ABA treatment for 30min. Values are means ±SE of three different experiments. Means denoted by the same letter did not differ significantly at P < 0.05 according to Duncan’s multiple range test.
In order to determine that the induction of ZmCCaMK by ABA is related to ABA-induced NO generation, the effects of the NO scavenger cPTIO and the NOS inhibitor l-NAME on ABA-induced activation of ZmCCaMK were assessed. The detached plants were pre-treated with cPTIO and l-NAME, and then exposed to ABA treatment. ABA-induced increases in the activity of ZmCCaMK and the gene expression of ZmCCaMK were greatly inhibited in the presence of cPTIO and l-NAME, as shown in Fig. 5D and E. Alone, cPTIO and l-NAME has little effect on the activation of ZmCCaMK and the gene expression of ZmCCaMK. Together these data suggest that ABA-induced NO production is required for ABA-induced activation of ZmCCaMK.
ABA-activated ZmCCaMK does not mediate the ABA-induced NO production
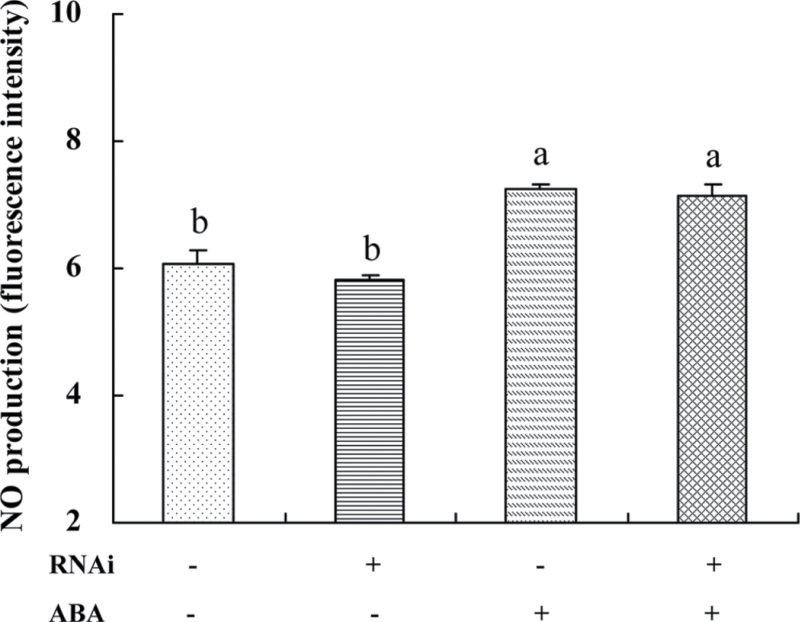
The role of ZmCCaMK in ABA-induced NO production was also examined by monitoring NO synthesis in response to ABA treatment, using protoplasts transfected with dsRNA against ZmCCaMK. Protoplasts were loaded with the NO-specific fluorescent dye DAF-2DA, and CLSM was used to monitor changes in NO-induced fluorescence in mesophyll protoplasts in maize leaves. Treatment with 10 µM ABA led to a rapid increase in NO-induced fluorescence in mesophyll protoplasts compared with control, and a similar induction was also observed in protoplasts transfected with dsRNA against ZmCCaMK (Fig. 6), suggesting that ABA-induced ZmCCaMK activation is not required for ABA-induced NO production.
Fig. 6.
NO production in transiently silenced ZmCCaMK protoplasts. The protoplasts were treated with 10 µM ABA for 30min, and then loaded with 4,5-diaminofluorescein diacetate (DAF-2DA) and detected by confocal laser scanning microscopy. Values are the mean ±SE of three different experiments. Means denoted by the same letter did not differ significantly at P < 0.05 according to Duncan’s multiple range test.
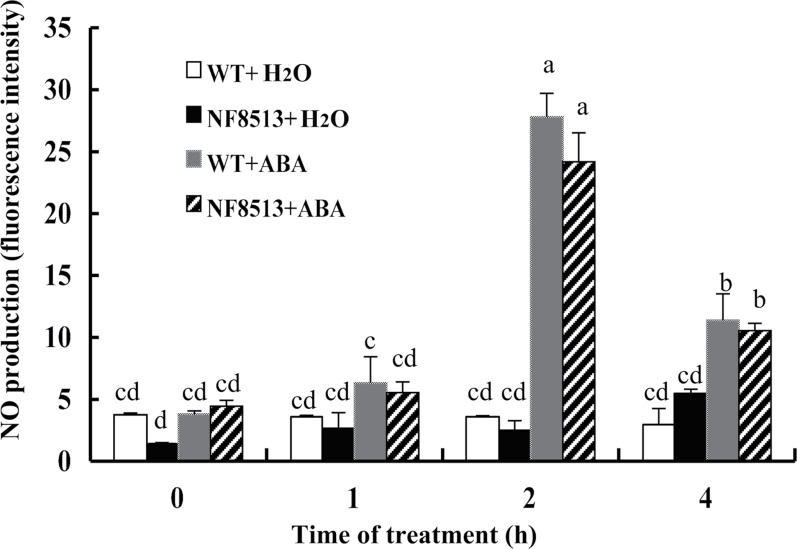
To gain further evidence that ZmCCaMK is not essential for ABA-induced NO production, the rice mutant line NF8513 (‘Nipponbare’) containing the Tos17 insertion in OsDMI3, a homologous gene of ZmCCaMK in rice, was screened and the homozygous mutant in NF8513 was isolated. As shown in Fig. 7, a significant increase in NO-induced fluorescence was observed in ABA-treated leaves of the wild type compared with the control leaves. NO production was observed as early as 1h after the addition of 100 µM ABA, was maximizal after 2h of ABA treatment, and then decreased after 4h treatment. Importantly, leaves of the rice mutant line NF8513 also exhibited a significant increase in the level of NO in response to exogenous ABA in the same manner as did those of the wild type, and ABA-induced NO production in the wild type and mutant was not significantly different (Fig. 7). These data further confirm that ZmCCaMK is not involved in ABA-induced NO accumulation in ABA signalling.
Fig. 7.
Time course analysis of NO production in mesophyll cells of the rice mutant of OsDMI3 and the wild type exposed to ABA treatment. The detached plants were treated with 100 µM ABA or distilled water for various times as indicated, and then the leaf segments were loaded with 4,5-diaminofluorescein diacetate (DAF-2DA) and detected by confocal laser scanning microscopy. Values are means ±SE of three different experiments. Means denoted by the same letter did not differ significantly at P < 0.05 according to Duncan’s multiple range test.
H2O2 is required for ABA-induced activation of ZmCCaMK and the activation is regulated by NO
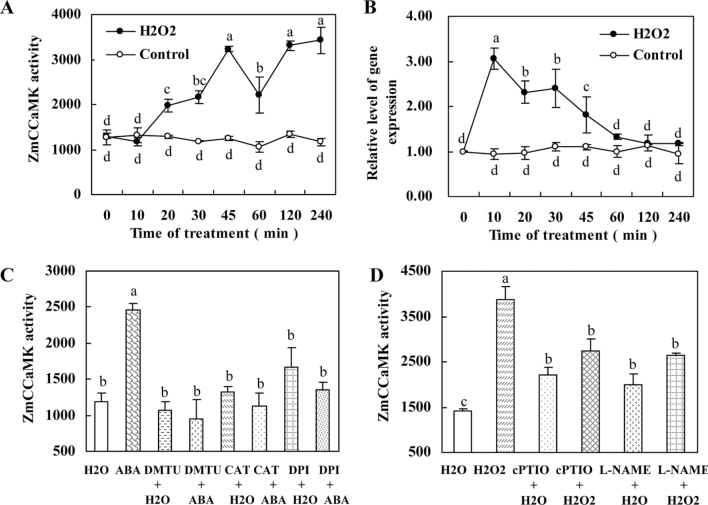
Previous work showed that ABA-induced NO generation was mediated by H2O2 in ABA signalling in leaves of maize (Zhang et al., 2007). To investigate whether H2O2 also plays a role in NO-induced activation of ZmCCaMK in ABA signalling, the activation and gene expression of ZmCCaMK induced by H2O2 were examined in leaves of maize plants. Exogenous 10mM H2O2 treatment rapidly induced an increase in the activity of ZmCCaMK in leaves of maize plants (Fig. 8A). Several ROS manipulators, such as DPI, an inhibitor of NADPH oxidase, and DMTU and CAT, H2O2 scavengers, were used to assess the effect of endogenous H2O2 on the induction of ZmCCaMK. Pre-treatments with DPI, DMTU, and CAT clearly blocked the ABA-induced activation of ZmCCaMK in maize plants (Fig. 8C), suggesting that H2O2 is required for the activation of ZmCCaMK in ABA signalling. Similarly, the gene expression of ZmCCaMK was also significantly up-regulated by both exogenous and endogenous H2O2 (Figs 5E, 8B). Furthermore, pre-treatment with cPTIO and l-NAME significantly blocked the H2O2-induced activation of ZmCCaMK in leaves of maize (Fig. 8D). Moreover, a previous study showed that exogenous H2O2 and ABA-induced H2O2 could induce the production of NO in leaves maize (Zhang et al., 2007). Together with these data, the present results suggest that NO is required for H2O2-induced activation of ZmCCaMK in ABA signalling in leaves of maize plants.
Fig. 8.
H2O2 is involved in the ABA-induced activation of ZmCCaMK and the activation is mediated by NO. (A) Time course of H2O2-induced ZmCCaMK activation. The detached plants were treated with 10mM H2O2 for various times as indicated. Protein extracts from control or H2O2-treated leaves were immunoprecipitated with ZmCCaMK antibody and then subjected to an in-gel kinase assay. (B) Time course of H2O2-induced gene expression of ZmCCaMK. The detached plants were treated as described in A. Relative expression levels of the ZmCCaMK gene, analysed by qRT-PCR, are normalized to β-actin transcript levels. (C) Effects of H2O2 scavengers or an inhibitor on ABA-activated ZmCCaMK. The detached plants were treated as follows: distilled water (control); 100 µM ABA; 10mM DMTU+H2O; 10mM DMTU+100 µM ABA; 200U of CAT+H2O; 200U of CAT+100 µM ABA; 100 µM DPI+H2O; 100 µM DPI+100 µM ABA. The detached plants were pre-treated with scavengers or inhibitor for 4h then exposed to 100 µM ABA treatment for 30min. (D) Effects of an NO scavenger or inhibitor on H2O2-activated ZmCCaMK. The detached plants were treated as follows: distilled water (control); 10mM H2O2; 200 µM cPTIO+H2O; 200 µM cPTIO+10mM H2O2; 200 µM l-NAME+H2O; 200 µM l-NAME+10mM H2O2. The detached plants were pre-treated with scavengers or inhibitors for 4h then exposed to 10mM H2O2 treatment for 45min. Values are means ±SE of three different experiments. Means denoted by the same letter did not differ significantly at P < 0.05 according to Duncan’s multiple range test.
Discussion
In animal cells, CaMKs have been shown to be involved in H2O2 signal transduction that results in the regulation of various cellular processes (Nguyen et al., 2004; Bouallegue et al., 2009; Palomeque et al., 2009). In plants, CCaMKs have high homology to mammalian CaMKs in both the kinase and CaM-binding domains (Yang et al., 2007) and are thought to function in a manner analogous to CaMKII (Mitra et al., 2004; Yang et al., 2007). It has been well documented that CCaMKs play important roles in mediating symbiotic relationships with bacteria and fungi (Lévy et al., 2004; Godfroy et al., 2006; Tirichine et al., 2006; Chen et al., 2007, 2008; Capoen et al., 2009; Hayashi et al., 2010; Kang et al., 2011). CCaMKs were also suggested to play roles in meiosis and mitosis (Yang and Poovaiah, 2003) and in abiotic stress responses (Yang et al., 2011). CCaMKs were obviously regulated at the transcriptional level by ABA, NaCl, or PEG treatment (Yang et al., 2011). However, it is not clear whether CCaMKs are involved in oxidative stress responses in plants as CaMKs are in animals. In the present study, the results showed that ABA treatment induced the expression of ZmCCaMK and the activity of ZmCCaMK in leaves of maize plants (Fig. 1A, B, D) and in maize mesophyll protoplasts (Fig. 4B, C). Water stress-induced endogenous ABA also increased the activity of ZmCCaMK (Fig. 1C). These results suggest that ZmCCaMK is very likely to participate in ABA signalling as reported by Yang et al. (2011). To investigate further the involvement of ZmCCaMK in ABA signalling, the role of ZmCCaMK in ABA-induced antioxidant defence was investigated by means of transient overexpression or transient silencing of ZmCCaMK in maize mesophyll protoplasts. Transiently expressing ZmCCaMK in maize mesophyll protoplasts significantly enhanced the activities of SOD and APX (Fig. 3). In contrast, the activities of SOD and APX were reduced in the protoplasts transfected with dsRNA against ZmCCaMK (Fig. 4). More importantly, ABA treatment failed to induce an increase in the activities of SOD and APX in the protoplasts transfected with dsRNA against ZmCCaMK, although ABA treatment significantly increased the activities of SOD and APX in the control protoplasts (Fig. 4). These results provide conclusive evidence that ZmCCaMK is required for the enhancement of ABA-induced antioxidant defence. Furthermore, in a rice mutant of OsCCaMK (OsDMI3), a gene homologous to ZmCCaMK in rice, ABA treatment was also not able to induce the increases in the activities of these antioxidant enzymes in the leaves of rice (unpublished data). These data clearly indicate that ZmCCaMK and its orthologue in rice play an important role in the ABA-induced antioxidant defence.
However, a recent study showed that in wheat, the expression of TaCCaMK, which is closely related to maize ZmCCaMK and rice OsCCaMK, was down-regulated by ABA, as well as NaCl and PEG treatments in wheat seedlings roots (Yang et al., 2011). Overexpression of TaCCaMK in Arabidopsis reduced ABA sensitivity in seed germination and enhanced the seed germination rate under high-salt conditions. These results suggest that TaCCaMK is, as a negative regulator for ABA signalling, involved in abiotic stress responses in wheat. The conclusion seems to be in contrast to the conclusion of this study. There exist several explanations for the discrepancy. In the study by Yang et al. (2011), the expression of TaCCaMK in wheat roots exposed to ABA, NaCl, and PEG treatments was analysed over a 3h period, and a transient change in the expression of TaCCaMK at times >3h was not analysed. In the present study, ABA treatment induced a significant increase in the expression of ZmCCaMK within 20min (Fig. 1B). The expression of ZmCCaMK reached the maximum after 30min of ABA treatment, and then decreased to the control level within 60min of ABA treatment. After 2h of ABA treatment, the expression of ZmCCaMK induced by ABA was significantly lower than that in the control. Moreover, CCaMK does not exist in the Arabidopsis genome (Harper et al., 2004; DeFalco et al., 2010). Overexpression of TaCCaMK in Arabidopsis could interfere with the function of Ca2+ sensors in Arabidopsis. The phenotypes of transgenic plants that reduced ABA sensitivity in seed germination and enhanced the seed germination rate under high-salt conditions might be not from the direct role of TaCCaMK. Another possibility is that the difference between the study by Yang et al. (2011) and the present study may be related to the different physiological process. It is also possible that different plant species or organs have different responses to ABA.
In Medicago trunculata, CCaMK (DMI3) was localized in the nucleus in epidermal root cells and root hairy cells (Kaló et al., 2005; Smit et al., 2005). However, in Triticum aestivum, TaCCaMK with a 3′ end green fluorescent protein (GFP) fusion has been shown to be located both on the plasma membrane and in the nucleus (Yang et al., 2011). In this study, transient expression of YFP-ZmCCaMK revealed that the fluorescence of this construct was detected in the nucleus, the cytoplasm, and the plasma membrane in maize protoplasts (Fig. 2). The fluorescence of OsDMI3–YFP was also detected in the nucleus, the cytoplasm, and the plasma membrane in rice protoplasts that were transformed with an OsDMI3-YFP fusion construct under the control of the 35S promoter or the OsDMI3 native promoter (unpublished data). These results suggest that ZmCCaMK is located not only in the nucleus, but also in the cytosol and the plasma membrane. Different subcellular localizations of CCaMKs may be related to their distinct functions in plants.
Previous studies showed that ABA-induced NO production up-regulated the expression and the activities of antioxidant enzymes in ABA signalling, and there exists a cross-talk mechanism between Ca2+/CaM and NO in ABA-induced antioxidant defence in maize leaves (Jiang and Zhang, 2003; Zhang et al., 2007; Sang et al., 2008). To investigate further the mechanisms of ZmCCaMK in ABA-induced antioxidant defence, the relationship between NO and ZmCCaMK was studied. The results showed that treatment with the NO donor SNP induced an increase in the activity of ZmCCaMK and the expression of ZmCCaMK in maize leaves (Fig. 5A–C), and pre-treatment with an NO scavenger or inhibitor substantially reduced the ABA-induced increases in the activity and the transcription level of ZmCCaMK (Fig. 5D, E). These results suggest that NO is involved in the activation of ZmCCaMK in ABA signalling. Conversely, the expression of ZmCCaMK was down-regulated through RNAi to investigate the effects of ZmCCaMK on the production of NO. The results showed that the RNAi silencing of ZmCCaMK in maize protoplasts did not block the ABA-induced increase in the production of NO (Fig. 6). Similarly, the mutant of OsDMI3, a homologous gene of ZmCCaMK in rice, also did not affect the ABA-induced increase in the production of NO within 4h of ABA treatment (Fig. 7). These results provide conclusive evidence that NO is required for the ABA-induced up-regulation in the expression and the activity of ZmCCaMK, and ZmCCaMK does not mediate the ABA-induced NO production in maize leaves.
ROS have also been demonstrated to be important signal transduction molecules (Miller et al., 2008, 2010; Mittler et al., 2011). In ABA signalling, ROS play an important role in the regulation of stomatal closure, stress survival, and growth processes (Neill et al., 2008; Mittler et al., 2011). It has been well established that H2O2 induces NO synthesis and accumulation in ABA signalling, and the ABA–H2O2–NO cascade is involved in ABA-induced stomatal closure (Bright et al., 2006; Neill et al., 2008) and antioxidant defence (Zhang et al., 2007). However, it is not clear whether the ABA–H2O2–NO cascade is involved in the ABA-induced activation of ZmCCaMK in ABA signalling. In this study, H2O2 treatment obviously induced increases in the activity of ZmCCaMK and gene expression of ZmCCaMK (Fig. 8A, B). Pre-treatments with H2O2 scavengers or inhibitor significantly blocked the ABA-induced activation of ZmCCaMK and the gene expression of ZmCCaMK (Figs 5E, 8C). These results suggest that ABA-induced H2O2 production is required for the ABA-induced activation of ZmCCaMK. A previous study showed that exogenous H2O2 and ABA-induced H2O2 could increase the accumulation of NO in leaves of maize plants (Zhang et al., 2007). Furthermore, in the present study, pre-treatments with an NO scavenger and inhibitor inhibited the H2O2-induced increase in the activity of ZmCCaMK (Fig. 8D). All these data suggest that the H2O2-NO pathway is involved in ABA-induced activation of ZmCCaMK.
In conclusion, the present data indicate that ZmCCaMK is required for ABA-induced antioxidant defence in maize leaves. ABA-induced NO production mediated by H2O2 activates ZmCCaMK, thus resulting in the up-regulation of the expression and the activities of antioxidant enzymes in ABA signalling.
Acknowledgements
This work was supported by the National Basic Research Program of China (grant no. 2012CB114300 to MJ), the National Natural Science Foundation of China (30700491, 31071344, to AZ, and 90717108, 30970238, and 31070254 to MJ), the Natural Science Foundation of Jiangsu Province (BK2010455 to AZ), the Fundamental Research Funds for the Central Universities (KYZ201157 to AZ, and KYZ200905 and KYT201001 to MJ), the Program for New Century Excellent Talents in University (NCET-10-0498 to AZ), and the Priority Academic Program Development of Jiangsu Higher Education Institutions.
References
- Aboul-Soud MA, Aboul-Enein AM, Loake GJ. 2009. Nitric oxide triggers specific and dose-dependent cytosolic calcium transients in Arabidopsis Plant Signaling and Behavior 4 191–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouallegue A, Pandey NR, Srivastava AK. 2009. CaMKII knockdown attenuates H2O2-induced phosphorylation of ERK1/2, PKB/Akt and IGF-1R in vascular smooth muscle cells Free Radical Biology and Medicine 47 858–866 [DOI] [PubMed] [Google Scholar]
- Bouché N, Yellin A, Snedden WA, Fromm H. 2005. Plant-specific calmodulin binding proteins Annual Review of Plant Biology 56 435–466 [DOI] [PubMed] [Google Scholar]
- Bradford MM. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding Analytical Biochemistry 72 248–254 [DOI] [PubMed] [Google Scholar]
- Bright J, Desikan R, Hancock JT, Weir IS, Neill SJ. 2006. ABA-induced NO generation and stomatal closure in Arabidopsis are dependent on H2O2 synthesis The Plant Journal 45 113–122 [DOI] [PubMed] [Google Scholar]
- Capoen W, Herder JD, Sun J, Verplancke C, Keyser AD, Rycke RD, Goormachtig S, Oldroyd G, Holsters M. 2009. Calcium spiking patters and the role of the calcium/calmodulin-dependent kinase CCaMK in lateral root base nodulation of Sesbania rostrata The Plant Cell 21 1526–1540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Ané JM, Zhu H. 2008. OsIPD3, an ortholog of the Medicago truncatula DMI3 interacting protein IPD3, is required for mycorrhizal symbiosis in rice New Phytologist 180 311–315 [DOI] [PubMed] [Google Scholar]
- Chen C, Gao M, Liu J, Zhu H. 2007. Fungal symbiosis in rice requires an ortholog of a legume common symbiosis gene encoding a Ca2 +/calmodulin-dependent protein kinase Plant Physiology 145 1619–1628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Tao L, Zeng L, Vega-Sanchez ME, Umemura K, Wang GL. 2006. A highly efficient transient protoplast system for analyzing defence gene expression and protein–protein interactions in rice Molecular Plant Pathology 7 417–427 [DOI] [PubMed] [Google Scholar]
- Corpas FJ, Barroso JB, Carreras A, et al. 2004. Cellular and subcellular localization of endogenous nitric oxide in young and senescent pea plants Plant Physiology 136 2722–2733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler SR, Rodriguez PL, Finkelstein RR, Abrams SR. 2010. Abscisic acid: emergence of a core signalling network Annual Review of Plant Biology 61 651–679 [DOI] [PubMed] [Google Scholar]
- DeFalco TA, Bender KW, Snedden WA. 2010. Breaking the code: Ca2+ sensors in plant signaling Biochemical Journal 425 27–40 [DOI] [PubMed] [Google Scholar]
- Dodd AN, Kudla J, Sanders D. 2010. The language of calcium signaling Annual Review of Plant Biology 61 593–620 [DOI] [PubMed] [Google Scholar]
- Gao X, Wheeler T, Li Z, Kenerley CM, He P, Shan L. 2011. . Silencing GhNDR1 and GhMKK2 compromises cotton resistance to Verticillium wilt The Plant Journal 66, 293–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfroy O, Debellé F, Timmers T, Rosenberg C. 2006. A rice calcium- and calmodulin-dependent protein kinase restores nodulation to a legume mutant Molecular Plant-Microbe Interactions 19 495–501 [DOI] [PubMed] [Google Scholar]
- Harmon AC, Gribskov M, Harper J. 2000. CDPKs—a kinase for every Ca2+ signal? Trends in Plant Science 5 154–159 [DOI] [PubMed] [Google Scholar]
- Harper JF, Breton G, Harmon A. 2004. Decoding Ca2+ signals through plant protein kinases Annual Review of Plant Biology 55 263–288 [DOI] [PubMed] [Google Scholar]
- Harper JF Harmon A 2005. Plants, symbiosis and parasites: a calcium signalling connection Nature Reviews Molecular Cell Biology 6,555–566 [DOI] [PubMed] [Google Scholar]
- Hayashi T, Banba M, Shimoda Y, Kouchi H, Hayashi M, Imaizumi-Anraku H. 2010. A dominant function of CCaMK in intracellular accommodation of bacterial and fungal endosymbionts The Plant Journal 63 141–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang MY, Zhang JH. 2001. Effect of abscisic acid on active oxygen species, antioxidative defence system and oxidative damage in leaves of maize seedlings Plant and Cell Physiology 42 1265–1273 [DOI] [PubMed] [Google Scholar]
- Jiang MY, Zhang JH. 2002. Water stress-induced abscisic acid accumulation triggers the increased generation of reactive oxygen species and up-regulates the activities of antioxidant enzymes in maize leaves Journal of Experimental Botany 53 2401–2410 [DOI] [PubMed] [Google Scholar]
- Jiang MY, Zhang JH. 2003. Cross-talk between calcium and reactive oxygen species originated from NADPH oxidase in abscisic acid-induced antioxidant defense in leaves of maize seedlings Plant, Cell and Environment 26 929–939 [DOI] [PubMed] [Google Scholar]
- Kaló P, Gleason C, Edwards A, et al. 2005. Nodulation signaling in legumes requires NSP2, a member of the GRAS family of transcriptional regulators Science 308 1786–1789 [DOI] [PubMed] [Google Scholar]
- Kang H, Zhu H, Chu X, Yang Z, Yuan S, Yu D, Wang C, Hong Z, Zhang Z. 2011. A novel interaction between CCaMK and a protein containing the Scythe_N ubiquitin-like domain in Lotus japonicus Plant Physiology 155 1312–1324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Somers DE. 2010. Rapid assessment of gene function in the circadian clock using artificial microRNA in Arabidopsis thaliana mesophyll protoplasts Plant Physiology 154, 611–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TH, Böhmer M, Hu H, Nishimura N, Schroeder JI. 2010. Guard cell signal transduction network: advances in understanding abscisic acid, CO2, and Ca2+ signaling Annual Review of Plant Biology 61 561–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudla J, Batistič O, Hashimoto K. 2010. Calcium signals: the lead currency of plant information processing The Plant Cell 22 541–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy A, Erlanger M, Rosenthal M, Epel BL. 2007. A plasmodesmata-associated β-1,3-glucanase in Arabidopsis The Plant Journal 49 669–682 [DOI] [PubMed] [Google Scholar]
- Lévy J, Bres C, Geurts R, et al. 2004. A putative Ca2+ and calmodulin-dependent protein kinase required for bacterial and fungal symbioses Science 303 1361–1364 [DOI] [PubMed] [Google Scholar]
- Luan S, Kudla J, Rodriguez-Concepcion M, Yalovsky S, Gruissem W. 2002. Calmodulins and calcineurin B-like proteins: calcium sensors for specific signal response coupling in plants The Plant Cell 14 S389–S400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G, Shulaev V, Mittler R. 2008. Reactive oxygen signaling and abiotic stress Physiologia Plantarum 133 481–489 [DOI] [PubMed] [Google Scholar]
- Miller G Suzuki N Ciftci-Yilmaz S Mittler R 2010. Reactive oxygen species homeostasis and signaling during drought and salinity stresses Plant, Cell and Environent 33 453–467 [DOI] [PubMed] [Google Scholar]
- Mitra RM, Gleason CA, Edwards A, Hadfield J, Downie JA, Oldroyd GED, Long SR. 2004. A Ca2+/calmodulin-dependent protein kinase required for symbiotic nodule development: gene identification by transcript-based cloning Proceedings of the National Academy of Sciences, USA 101 4701–4705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittler R, Vanderauwera S, Suzuki N, Miller G, Tognetti VB, Vandepoele K, Gollery M, Shulaev V, Van Breusegem F. 2011. ROS signaling: the new wave? Trends in Plant Science 16 300–309 [DOI] [PubMed] [Google Scholar]
- Nagamune K, Hicks LM, Fux B, Brossier F, Chini EN, Sibley LD. 2008. Abscisic acid controls calcium-dependent egress and development in Toxoplasma gondii Nature 451 207–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neill S, Barros R, Bright J, Desikan R, Hancock J, Harrison J, Morris P, Ribeiro D, Wilson I. 2008. Nitric oxide, stomatal closure, and abiotic stress Journal of Experimental Botany 59,165–176 [DOI] [PubMed] [Google Scholar]
- Nguyen A, Chen P, Cai H. 2004. Role of CaMKII in hydrogen peroxide activation of ERK1/2, p38MAPK, HSP27 and actin reorganization in endothelial cells FEBS Letters 572 307–313 [DOI] [PubMed] [Google Scholar]
- Palomeque J, Rueda OV, Sapia L, Valverde CA, Salas M, Petroff MV, Mattiazzi A. 2009. Angiotensin II-induced oxidative stress resets the Ca2+ dependence of Ca2+–calmodulin protein kinase II and promotes a death pathway conserved across different species Circulation Research 105 1204–1212 [DOI] [PubMed] [Google Scholar]
- Park SY, Ryu SH, Jang IC, Kwon SY, Kim JG, Kwak SS. 2004. Molecular cloning of a cytosolic ascorbate peroxidase cDNA from cell cultures of sweetpotato and its expression in response to stress Molecular Genetics and Genomics 271 339–346 [DOI] [PubMed] [Google Scholar]
- Patil S, Takezawa D, Poovaiah BW. 1995. Chimeric plant calcium/calmodulin-dependent protein kinase gene with a neural visinin-like calcium-binding domain Proceedings of the National Academy of Sciences, USA 92 4897–4901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei ZM, Murata Y, Benning G, Thomine S, Klüsener B, Allen GJ, Grill E, Schroeder JI. 2000. Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells Nature 406, 731–734 [DOI] [PubMed] [Google Scholar]
- Poovaiah BW, Xia M, Liu Z, Wang W, Yang T, Sathyanarayanan PV, Franceschi VR. 1999. Developmental regulation of the gene for chimeric calcium/calmodulin-dependent protein kinase in anthers Planta 209 161–171 [DOI] [PubMed] [Google Scholar]
- Sakamoto H, Maruyama K, Sakuma Y, Meshi T, Iwabuchi M, Shinozaki K, Yamaguchi-Shinozaki K. 2004. Arabidopsis Cys2/His2-type zinc-finger proteins function as transcription repressors under drought, cold, and high-salinity stress conditions Plant Physiology 136 2734–2746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Russell DW. 2001. Molecular cloning: a laboratory manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; [Google Scholar]
- Sang JR, Zhang AY, Lin F, Tan MP, Jiang MY. 2008. Cross-talk between calcium–calmodulin and nitric oxide in abscisic acid signaling in leaves of maize plants Cell Research 18 577–588 [DOI] [PubMed] [Google Scholar]
- Sheen J. 2001. Signal transduction in maize and Arabidopsis mesophyll protoplasts Plant Physiology 127 1466–1475 [PMC free article] [PubMed] [Google Scholar]
- Smit P, Raedts J, Portyanko V, Debellé F, Gough C, Bisseling T, Geurts R. 2005. NSP1 of the GRAS protein family is essential for rhizobial Nod factor-induced transcription Science 308 1789–1791 [DOI] [PubMed] [Google Scholar]
- Takezawa D, Ramachandiran S, Paranjape V, Poovaiah BW. 1996. Dual regulation of a chimeric plant serine/threonine kinase by calcium and calcium/calmodulin Journal of Biological Chemistry 271 8126–8132 [DOI] [PubMed] [Google Scholar]
- Tirichine L, Imaizumi-Anraku H, Yoshida S, et al. 2006. Deregulation of a Ca2+/calmodulin-dependent kinase leads to spontaneous nodule development Nature 441 1153–1156 [DOI] [PubMed] [Google Scholar]
- Yang C, Li A, Zhao Y, et al. 2011. Overexpression of a wheat CCaMK gene reduces ABA sensitivity of Arabidopsis thaliana during seed germination and seedling growth Plant Molecular Biology Reporter 29 681–692 [Google Scholar]
- Yang T, Du L, Poovaiah BW. 2007. Concept of redesigning proteins by manipulating calcium/calmodulin-binding domains to engineer plants with altered traits Functional Plant Biology 34 343–352 [DOI] [PubMed] [Google Scholar]
- Yang T, Poovaiah BW. 2003. Calcium/calmodulin-mediated signal network in plants Trends in Plant Science 8 505–512 [DOI] [PubMed] [Google Scholar]
- Yano K, Yoshida S, Müller J, et al. 2008. CYCLOPS, a mediator of symbiotic intracellular accommodation Proceedings of the National Academy of Sciences, USA 105 20540–20545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelazny E, Borst JW, Muylaert M, Batoko H, Hemminga MA, Chaumont F. 2007. FRET imaging in living maize cells reveals that plasma membrane aquaporins interact to regulate their subcellular localization Proceedings of the National Academy of Sciences, USA 104 12359–12364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai Z, Sooksa-nguan T, Vatamaniuk OK. 2009. Establishing RNA interference as a reverse-genetic approach for functional analysis in protoplasts Plant Physiology 149 642–652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang AY, Jiang MY, Zhang JH, Ding HD, Xu SC, Hu XL, Tan MP. 2007. Nitric oxide induced by hydrogen peroxide mediates abscisic acid-induced activation of the mitogen-activated protein kinase cascade involved in antioxidant defense in maize leaves New Phytologist 175 36–50 [DOI] [PubMed] [Google Scholar]
- Zhang AY, Jiang MY, Zhang JH, Tan MP, Hu XL. 2006. Mitogen-activated protein kinase is involved in abscisic acid-induced antioxidant defense and acts downstream of reactive oxygen species production in leaves of maize plants Plant Physiology 141 475–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Liu BF, Liang S, Jones RL, Lu YT. 2002. Molecular and biochemical characterization of a calcium/calmodulin-binding protein kinase from rice Biochemical Journal 368 145–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Lu YT. 2003. Calmodulin-binding protein kinases in plants Trends in Plant Science 8 123–127 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Tan J, Guo Z, Lu S, He S, Shu W, Zhou B. 2009. Increased abscisic acid levels in transgenic tobacco over-expressing 9 cis-epoxycarotenoid dioxygenase influence H2O2 and NO production and antioxidant defences Plant, Cell and Environment 32 509–519 [DOI] [PubMed] [Google Scholar]
- Zhu JK. 2002. Salt and drought stress signal transduction in plants Annual Review of Plant Biology 53 247–273 [DOI] [PMC free article] [PubMed] [Google Scholar]