Abstract
Lowland rice roots have a unique physiological response to drought because of their adaptation to flooded soil. Rice root attributes that facilitate growth under flooded conditions may affect rice response to drought, but the relative roles of root structural and functional characteristics for water uptake under drought in rice are not known. Morphological, anatomical, biochemical, and molecular attributes of soil-grown rice roots were measured to investigate the genotypic variability and genotype×environment interactions of water uptake under variable soil water regimes. Drought-resistant genotypes had the lowest night-time bleeding rates of sap from the root system in the field. Diurnal fluctuation predominated as the strongest source of variation for bleeding rates in the field and root hydraulic conductivity (Lp r) in the greenhouse, and was related to expression trends of various PIP and TIP aquaporins. Root anatomy was generally more responsive to drought treatments in drought-resistant genotypes. Suberization and compaction of sclerenchyma layer cells decreased under drought, whereas suberization of the endodermis increased, suggesting differential roles of these two cell layers for the retention of oxygen under flooded conditions (sclerenchyma layer) and retention of water under drought (endodermis). The results of this study point to the genetic variability in responsiveness to drought of rice roots in terms of morphology, anatomy, and function.
Key words: Aquaporin, drought, rice, root anatomy, root hydraulic conductivity, suberin
Introduction
Lowland rice requires ~3000 litres of water per kilogram of grain produced in flooded fields (Bouman et al., 2007)—the greatest water requirement of all cereal crops—yet lowland rice plants often experience drought in rainfed environments when rainfall is not sufficient to maintain flooded paddy conditions. Because rice is adapted to saturated soils, the physiology and drought response of lowland rice are different from those of other crops. Little is known about the exact mechanisms of water uptake in rice. Increased understanding of root attributes affecting water uptake under drought can help rice breeders elucidate genotype×environment interactions and point to important traits that may improve drought resistance (Serraj et al., 2011).
Genetic differences in water uptake in drought-stressed rice have previously been described (Puckridge and O’Toole, 1981; Lilley and Fukai, 1994), which are thought to be due in part to root architecture (root depth and branching, as reviewed by Gowda et al., 2011) and root function [water uptake per length of root and root hydraulic conductivity (Lp r); Kamoshita et al., 2000; Miyamoto et al., 2001]. Because of the strong physiological response of rice roots to rhizosphere conditions (Suralta and Yamauchi, 2008), use of an appropriate root growth medium is critical for studies of root function. Matsuo et al. (2009) have measured Lp r of several rice varieties contrasting for drought resistance in soil, and observed greater Lp r in a drought-resistant variety compared with a drought-sensitive variety. In addition to these initial results, a greater understanding of water uptake dynamics of rice roots under drought, and the contributing root attributes, is needed.
The path of water movement from the root surface to the xylem vessel faces several potential barriers. Unlike in other cereal crops, rice roots in well-watered conditions feature a sclerenchyma layer that is much more suberized than the exodermis and it consists of tightly packed cells of smaller diameter than other cells in the rest of the outer part of the root (OPR). Together, the cell layers of the OPR in rice form a barrier that reduces radial oxygen loss (Colmer et al., 1998) but does not appear to restrict water uptake (Ranathunge et al., 2003, 2004, 2011). However, the role of this barrier in drought response of rice is not known. Aquaporin expression is also probably affecting water uptake by drought-stressed rice roots (Lian et al., 2004, 2006), and has been directly linked to rice root hydraulic conductivity in polyethylene glycol (PEG)-treated solution culture studies (Sakurai et al., 2005).
In this study, variation of water uptake in drought-stressed rice roots grown in soil was investigated in terms of the bleeding rate of sap from the root system, Lp r, and volume of water extracted from the soil. Genetic, drought stress treatment, and time of day effects were examined as sources of variation. It was hypothesized that a combination of root attributes—namely anatomy, suberization, and aquaporin expression—would explain differences in rice water uptake under drought.
Materials and methods
Experiments were conducted in the field and also in more detail with greenhouse and gene expression studies, with the goal of understanding the drought response of different rice genotypes in terms of root morphology, anatomy, gene expression, and hydraulics that are related to water uptake and field performance under drought.
Field experiment
Experiment 1: field measurement of bleeding rate
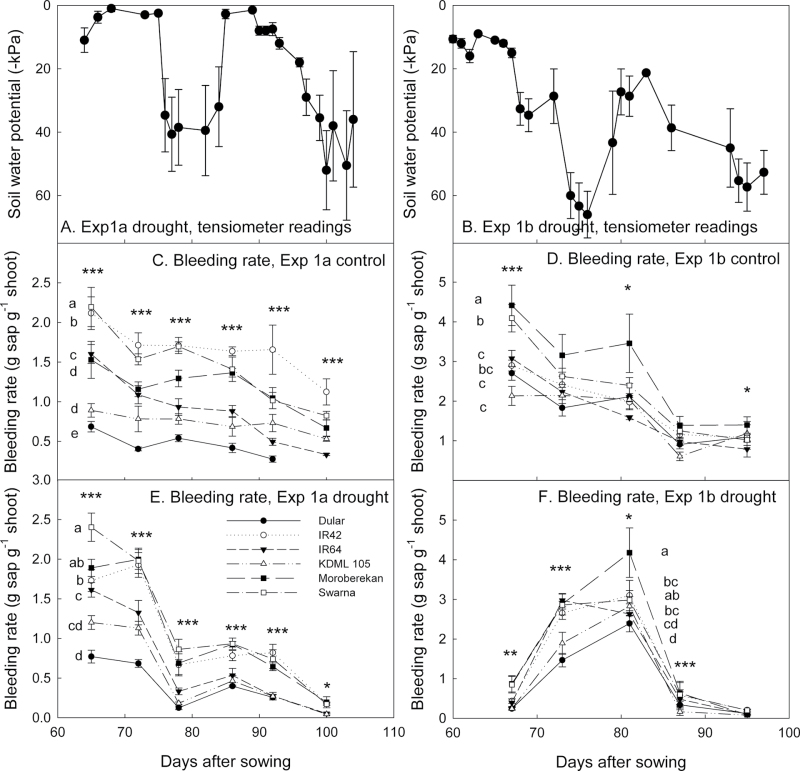
To investigate water translocation from field-grown roots of diverse genotypes, bleeding rate experiments were conducted over two seasons (Exp 1a, 2010 wet season, July–October; and Exp 1b, 2011 dry season, January–April) under transplanted lowland conditions at the IRRI Experiment Station. The soil was classified as an Isohyperthermic Typic Hapludalf, with an average bulk density of 1.1g cm–3 at a depth of 25–30cm. Six genotypes were used based on their diverse previous performance or adaptation: Dular (aus, deep-rooted), IR42 (indica, submergence susceptible), IR64 (indica, popular lowland mega-variety), Khao dawk mali 105 (KDML 105; indica, popular rainfed lowland variety in Thailand), Moroberekan (tropical japonica, upland variety), and Swarna (indica, popular lowland mega-variety in South Asia). Six 3 m rows per plot (15 hills per row) were planted for each genotype, three of which were used for sampling and three for determination of grain yield. Management of the drought and well-watered field plots was carried out as described by Henry et al. (2011). Soil water potential was monitored in the drought stress treatments with three tensiometers (Soilmoisture Equipment Corp., CA, USA) installed at a depth of 30cm. Total seasonal rainfall averaged 1500mm in Exp 1a and 200mm in Exp 1b. Ambient temperatures averaged 28.2 °C in Exp 1a and 26.2 °C in Exp 1b.
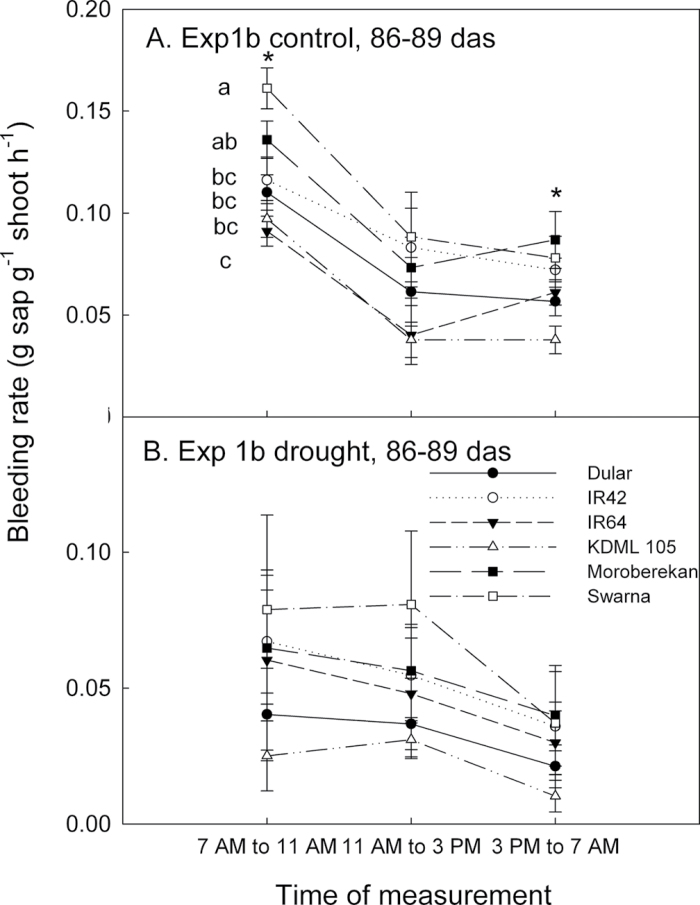
Bleeding rate measurements were carried out according to the method described by Morita and Abe (2002). Starting at 65 days after sowing (DAS) in Exp 1a and 67 DAS in Exp 1b, the bleeding rate from the root zone was measured every 6–8 d in three hills per plot in both control and drought treatments. Starting at ~14:30h, shoots were cut at ~15cm from the soil surface, and cut stems connected to the undisturbed root system were wrapped in a 625cm2 cotton towel, then covered with a polyethylene bag, sealed at the base with a rubber band, and left overnight to absorb xylem sap that flowed from the cut stems. The towel, bag, and rubber band used for each hill were weighed before use in the field. Starting at 07:30h the following day, bags and towels were removed from the stems, sealed, and immediately weighed to quantify the bleeding rate from the intact root system. Shoots were dried and weighed to determine the biomass for each hill for each sampling date in both years. One border row was left between hills for each sampling date. Diurnal changes in bleeding rates (three collections per day) were also monitored in Exp 1b by sampling one replicate per day over 4 d. All bleeding rate values were normalized by the shoot mass of the hill from which sap was collected, in order to account for variation in plant size within and among genotypes.
Greenhouse experiments
Experiment 2: seedling root hydraulic conductivity measurements
The field study was complemented by greenhouse studies with fewer genotypes but more detailed measurements. In this study (Exp 2a), Lp r was measured in soil-grown seedlings in the greenhouse, and the time of day was noted in order to identify any diurnal variation trends in Lp r. Dry, sieved upland soil was put into 50cm long (4cm diameter) mylar tubes to a height of 40cm and a soil bulk density of 1.1g cm–3. Tubes were held in boxes with wire racks to contain 35 tubes per box, which were kept on the cement floor of the greenhouse. Soil for all greenhouse Lp r studies was from the IRRI experiment station, with a pH of 5.76 and 0.26% N (Kjeldahl), 33.6mg kg–1 P (Olsen), and 1.4 meq 100g–1 exchangeable K. No additional fertilizer was added to the soil for the greenhouse Lp r studies. Seeds of IR64 and Dular were sown directly into the tubes at staggered intervals (12 tubes per day) in 10 reps of well-watered (WW) and drydown (DD) conditions (only five reps of the most representative plants were used for measurements). Tubes in the DD treatment started at field capacity and were not watered during the study, and tubes from the WW treatment were watered to maintain standing water above the soil surface. All tubes were weighed daily to monitor water use. Plants were grown in a greenhouse at the IRRI from July to August 2009, with an average temperature of 31.7 °C and relative humidity of 66.7%.
Volumetric soil water content at the time of Lp r measurements, calculated based on the amount of water in the tubes and the density of soil within the tubes, averaged 0.55±0.02 in the WW treatment and 0.10±0.02 in the DD treatment. At 21 d after planting, Lp r was measured at three times during the day [starting at 07:00h (early), 11:00h (mid), and 15:00h (late)] using different plants for each sampling time. Plants from the flooded treatment were drained the night before measurements were made. The protocol for Lp r measurement was adapted from that described by Matsuo et al. (2009). After excising the shoot, each planted tube was placed in a 1.6 l pressure chamber (3000HGBL Plant Water Status Console, Soilmoisture Equipment Corp., CA, USA) with the cut stem of one tiller protruding through the silicone grommet to seal the pressure chamber lid around it. Samples were pressurized with compressed air at 0.2MPa for 10min to equilibrate, and then xylem sap was collected at pressures of 0.2, 0.35, and 0.5MPa for 10min each using a pre-weighed 2ml Eppendorf tube filled with cotton, for a total throughput of 40min per plant sample. The cotton-filled tubes were weighed after xylem sap collection at each pressure. Lp r was calculated as the slope of xylem sap flux at each pressure, and normalized for root surface area. Roots were washed at the end of each day after the Lp r measurements, then stored in 70% ethanol until they were scanned and analysed for total root length, diameter, and surface area using WinRhizo v. 2005 (Regent Instruments, Quebec, Canada). The different root classes were identified by visual inspection of the scanned images, and root diameters of these classes were measured in WinRhizo. The proportion of lateral roots in each sample was calculated by dividing the length of roots <0.2mm in diameter by the total root length.
The Lp r study was repeated (Exp 2b) in July–September 2010 with three genotypes: IR64, Dular, and KDML 105. Temperatures in the greenhouse averaged 31.1 °C and relative humidity averaged 71.7%. Boxes containing 35 tubes each were kept on tables in the greenhouse. In the WW treatment of Exp 2b, soil was maintained at 20% above field capacity (saturated but no standing water). In addition to WW and DD treatments, a third treatment of drydown from 75% of field capacity (DD-75%) was used in order to induce a more severe drought treatment. All tubes were weighed three times per week to monitor water uptake. Seeds were germinated in an incubator (28 °C) for 4 d before planting in the greenhouse. Excess water from tubes in the WW treatment was drained overnight before Lp r measurements. At the time of Lp r measurements, volumetric soil water content averaged 0.44±0.02 in the WW treatment, 0.24±0.025 in the DD treatment, and 0.22±0.05 in the DD-75% treatment. Lp r was measured at three distinct times of day, except for KDML 105, which was measured before 13:00h but not at separate early or mid-day periods due to time constraints with this low-throughput method. Leaf water potential was measured at the time of Lp r measurements on 1–3 leaves in a pressure chamber (3000HGBL Plant Water Status Console, Soilmoisture Equipment Corp., CA, USA) using compressed N2. Maximum root depth was recorded in Exp 2b by unrolling the tube to expose the inner soil and measuring the depth at which the deepest root was located. One nodal root from each plant in the WW and DD treatments of IR64 and Dular was saved for later RNA extraction. The remaining root system was carefully washed and stored in 75% ethanol, and roots were scanned as described above. After scanning, roots from Exp 2b were saved for sectioning and evaluation of anatomy.
Since no recoverable RNA was obtained from the seedlings grown from July to September 2010, WW and DD treatments of IR64 and Dular were grown again (Exp 2c) in October 2010 and harvested at three times during the day [starting at 07:00h (early), 11:00h (mid), and 15:00h (late)], using the same protocols as Exp 2b but with no Lp r measurements preceding harvest. Mean temperature was 31.4 °C and relative humidity averaged 71.5%. At the time of sample collection, volumetric soil water content averaged 0.46±0.02 in the WW treatment and 0.27±0.01 in the DD treatment. Roots were cleaned by gently shaking the soil, then immersed in liquid nitrogen and stored at –80 °C until extraction. This method eliminated the step of immersion in water for root washing, in order to avoid changing the root water status before RNA extraction. Root and leaf samples were ground in liquid nitrogen and RNA was extracted with TRIZOL reagent (Invitrogen). Following phase separation, ethanol was added to the aqueous phase for a final volume of 30% and RNA was purified with silica dioxide (Li et al. 2010). Aquaporin expression was measured with a custom Illumina DASL assay according to the manufacturer’s instructions with 500ng of total RNA per sample (Fan et al., 2004). Probes were selected by Illumina; probe sequences and targets are provided in Supplementary Table S1 available at JXB online. After quantile normalization, expression levels were log2 transformed for further analysis. Three biological replicates were averaged for each factorial combination. Fold change values were calculated as the difference between mean log2-transformed expression values.
Experiment 3: screening for genetic differences in root anatomy
Another greenhouse study (Exp 3) was conducted to evaluate genetic diversity in root anatomical properties in response to drought. Plants were grown in soil-filled 20 litre pots in a greenhouse at the IRRI under both flooded and drained conditions for 60 d (November 2008–January 2009), with one plant per pot and four replicates per genotype in both treatments. The same six genotypes used in field Exp 1 were used for Exp 3. Dular was replanted after 2 weeks due to germination problems, and therefore was 2 weeks younger than the other genotypes reported here. Pots in the flooded treatment were maintained with standing water above the soil surface, and pots in the drained treatment were watered as needed to maintain well-watered aerobic conditions. All plants were harvested at 62 DAS. Root systems were washed from the soil over a 1mm screen, and root and shoot samples were dried at 70 °C to determine dry mass. Subsamples of nodal roots from each pot were stored in 25% ethanol.
Roots stored in ethanol were hand-sectioned under a dissecting microscope from segments taken half-way between the basal and axial ends. Sections were stained for suberin with Sudan IV (Aldrich, Steinheim, Germany) according to Zeier et al. (1999). Three roots per plant were used for sectioning, and images of 3–5 sections per root were acquired with a Zeiss Axioplan 2 compound microscope at ×50 and ×200 magnification. In each image, anatomical characteristics were determined by measuring the section diameters (whole section and stele), cell layers in the OPR (including the unmodified cortical, sclerenchyma, hypodermal, and exodermal layers), cell size in the sclerenchyma layer with Image J (Abramoff et al. 2004), and the aerenchymatous area of the cortex with Gimp v. 2.4.7 (GNU Image Manipulation Program). Suberization of the sclerenchyma layer and endodermis was rated based on intensity of the stain on a scale of 0–5 by three individuals. The visual suberin intensity scale was determined according to the relative staining intensity observed across all samples. These measurements were repeated on roots from Exp 2b, in which three nodal roots were sectioned at the mid-point along the root axis, which was ~16cm from the root tip in the drought treatments, and ~10cm from the root tip in the well-watered treatment (since roots in the drought treatment were longer than those in the well-watered treatment). Microscopic images were analysed for anatomical parameters as described above.
Statistics
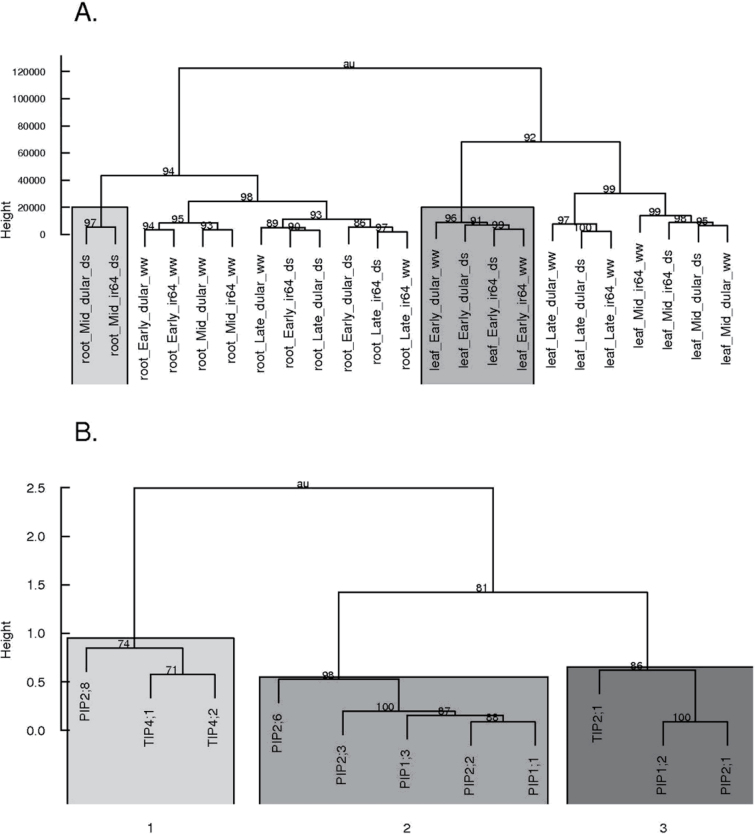
Data were analysed in R v. 2.8.0 (R Development Core Team, 2008) using analysis of variance (ANOVA) and Tukey’s honestly significant difference (HSD) for mean comparison. Aquaporin expression data were quantile normalized in Genome Studio software (Illumina). Hierarchical clustering of expression levels was performed with the hclust function in the R package.
Results
Drought-resistant genotypes showed the lowest bleeding rates in the field
The night-time bleeding rate from the root system in the field (Exps 1a and 1b) was significantly affected by genotype (P < 0.001) and date of measurement (P < 0.001; Table 1). Drought-resistant lines Dular and KDML 105 consistently showed the lowest bleeding rates, in contrast to Swarna and Moroberekan, which showed the greatest bleeding rates across dates and treatments (Fig. 1). Except for the beginning of Exp 2b where bleeding rates were low, trends in the amounts of sap collected per gram of shoot agreed with trends in available soil water, since more sap was collected when soil water potential became less negative, and less sap was collected as stress progressed in the drought treatments (Fig. 1, Table 1). The absolute amount of sap collected and the shoot biomass at each collection date were significantly correlated in the control treatment of Exp 1a (P < 0.001, r 2=0.16) and in the Exp 1b control (P < 0.001, r 2=0.28) and stress (P < 0.001, r 2=0.56) treatments. Diurnal changes in bleeding rates monitored on one date during the season showed that night-time bleeding rates were generally lower than morning and mid-day values in both stress and control treatments (Fig. 2). As in the night-time bleeding rate measurements, Swarna showed the greatest bleeding rate throughout the day in both treatments, but the genotype with the lowest bleeding rate at the early sampling time was IR64 (P < 0.05).
Table 1.
Multivariate effects on rice root functional parameters for water uptake; bleeding rate (field Exps 1a and 1b), root hydraulic conductivity (greenhouse Exps 2a and 2b), and aquaporin expression (greenhouse Exp 2c)
All aquaporin types were analysed together from Exp 2c. Only significant interactions among factors are shown here.
| Bleeding rate | Root hydraulic conductivity | Aquaporin expression | |||
|---|---|---|---|---|---|
| Factor | P-value | Factor | P-value | Factor | P-value |
| Field season | <0.001*** | Experiment | <0.001*** | Tissue | <0.001*** |
| Treatment | <0.001*** | Genotype | 0.698 | Time of day | <0.001*** |
| Genotype | <0.001*** | Treatment | <0.001*** | Treatment | <0.001*** |
| Date | <0.001*** | Time of day | 0.012* | Genotype | 0.014* |
| Field season×treatment | <0.001*** | Soil moisture | 0.906 | Tissue×time | <0.001*** |
| Field season×genotype | <0.001*** | Treatment×soil moisture | 0.037* | Tissue×treatment | <0.001*** |
| Treatment×genotype | 0.085 | Time ×treatment | <0.001*** | ||
| Treatment×date | <0.001*** | From stress treatments | Tissue×genotype | 0.011* | |
| Genotype×date | <0.001*** | Soil moisture | 0.025* | ||
| Field season×treatment×genotype | <0.001*** | Genotype×soil moisture | 0.057 | ||
| From stress treatments | |||||
| Soil water potential | <0.001*** | ||||
| Genotype×soil water potential | 0.043* | ||||
| Time of day | <0.001*** | ||||
| Treatment×time of day | 0.001** | ||||
Fig. 1.
Soil water potential in the drought treatment at a depth of 30cm in the two field studies of this experiment (A and B), and corresponding overnight (17h) bleeding rates for Exp 1a [(C) control and (E) drought] and Exp 1b [(D) control and (F) drought]. Control treatments were maintained continuously flooded. Significance levels among genotypes are ***P < 0.001, **P < 0.01, and *P < 0.05. Letters are indicated next to genotype symbols to signify overall groupings for each treatment.
Fig. 2.
Diurnal fluctuations in bleeding rates from field studies, as expressed per unit shoot biomass per hour of collection in (A) Exp 1b control and (B) Exp 1b drought stress. Significant differences among genotypes are *P < 0.05, and letters adjacent to symbols indicate significance levels at the time of measurement.
Root hydraulic conductivity was affected by time of day
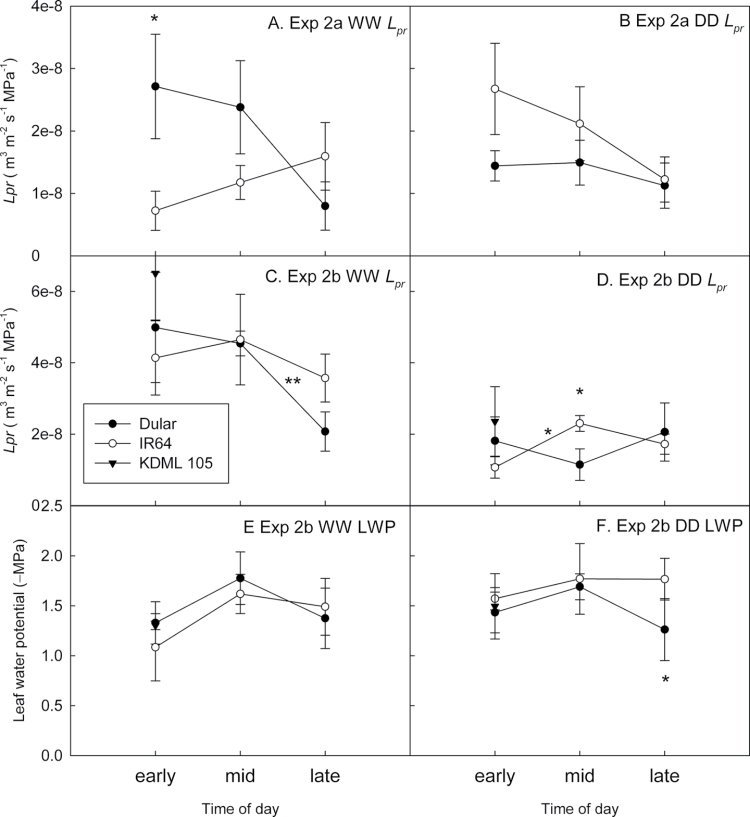
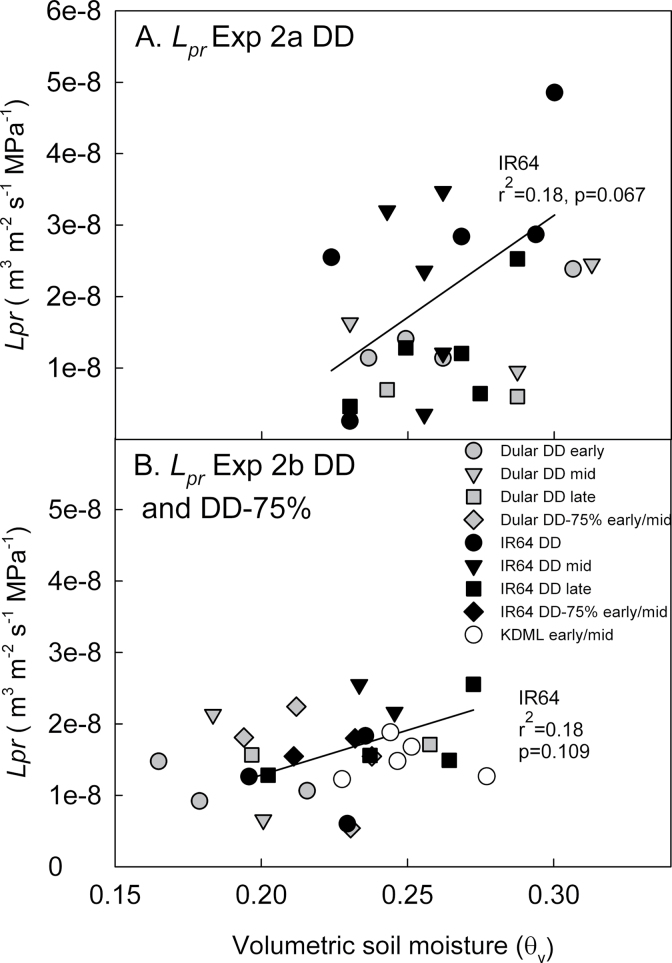
Lp r varied between the two greenhouse studies (P < 0.001), between genotypes (P=0.003), and between treatments (P=0.008; Fig. 3, Table 1), and was lowest at late times of the day in most cases. Significant differences for time of day were observed between early and mid-day Lp r measurements for IR64 (P=0.018) and between mid-day and late measurements for Dular (P=0.008) in Exp 2b. Leaf water potential at the time of Lp r measurement in Exp 2b was affected by treatment when analysed with genotype and time of day as factors (P=0.033) and was lower in the DD treatment in Dular at the late measurement time (P=0.04, Fig. 3). In addition to time of day, the soil moisture level at the time of Lp r measurement significantly affected Lp r readings in the stress treatments (Table 1, Fig. 4). IR64 showed increased Lp r with increasing soil moisture levels at the time of Lp r measurement, but the Lp r of Dular was more stable across varying soil moisture levels (Fig. 4).
Fig. 3.
Root hydraulic conductivity (Lp r) in greenhouse Exp 2a [(A) WW and (B) DD] and Exp 2b [(C) WW and (D) DD] as determined by collected exuded xylem sap at three applied pressures from 0.25MPa to 0.5MPa and normalized for root surface area, and leaf water potential (LWP) in Exp 2b [(E) WW and (F) DD] as determined by pressure chamber measurement in three leaves per plant at the time of Lp r measurement. Measurements of genotype KDML 105 were taken at a range of early to mid times of day. Significant differences are indicated by a * (P < 0.05) or ** above the symbols for differences between genotypes and above the lines for differences between times of day for single genotype.
Fig. 4.
Response of root hydraulic conductivity (Lp r) in the drought stress treatments of greenhouse Exps 2a and 2b to variable soil moisture levels at the time of Lp r measurements.
Plant growth and water uptake
Water loss from tubes in Exps 2a and 2b differed among treatments, but was similar among genotypes, except in Exp 2a when Dular extracted more water than IR64 in the DD treatment (P=0.002; Supplementary Fig. S1 at JXB online). Shoot mass (Supplementary Table S2) and total root length (Table 2) differed by treatment (P < 0.001) and were significantly greater in Dular than IR64 in Exp 2a WW (P=0.04). No consistent differences among genotypes were observed for the root:shoot ratio across the three container studies (Supplementary Table S2). Maximum root depth in Exp 2b was also strongly affected by treatment (P < 0.001; Table 2), and Dular showed greater maximum root depths across treatments than IR64 and KDML 105 (P=0.02). Lateral root formation increased under drought in Exp 2b (P < 0.001), but genotypic differences between Exp 2a and 2b were not consistent (Table 2).
Table 2.
Maximum root depth, total root length, and lateral roots (diameter <0.2mm) as a percentage of total root length in greenhouse Exps 2a and 2b
Values shown are means ±SE. Letters indicate significantly different genotypes within each treatment.
| Treatment | Genotype | Maximum root depth (cm) | Total root length (cm) | Lateral roots (%) | ||
|---|---|---|---|---|---|---|
| Exp 2b | Exp 2a | Exp 2b | Exp 2a | Exp 2b | ||
| WW | Dular | 21.9±1.64 | 3496±296 a | 1382±152 | 79.4±0.8 | 71.0±1.2 |
| IR64 | 17.2±1.25 | 2668±248 b | 1323±128 | 81.2±0.5 | 71.5±0.9 | |
| KDML 105 | 17.8±1.88 | 1057±192 | 66.0±3.7 | |||
| DD | Dular | 34.7±1.65 | 2194±193 | 1460±168 | 81.8±0.8 a | 77.6±1.3 |
| IR64 | 30.7±1.66 | 2211±125 | 1387±157 | 79.1±0.8 b | 80.4±1.3 | |
| KDML 105 | 30.1±3.57 | 1099±181 | 75.1±0.8 | |||
| DD-75% | Dular | 36.0±3.89 | 667±94 | 71.9±1.5 b | ||
| IR64 | 31.6±2.18 | 637±129 | 79.9±1.5 a | |||
| KDML 105 | 29.3±5.52 | 716±147 | 74.1±1.1 b | |||
Aquaporin expression
Multivariate analysis and hierarchical clustering of samples indicate the root/shoot contrast as the predominant factor in aquaporin expression, followed by time of day, treatment, and genotype (Fig. 5A, Table 1). Within leaf samples, the drought treatment did not significantly affect expression. Time of day (P < 0.001) and genotype (P < 0.001) were significant sources of variation in leaf samples, consistent with clustering. The drought treatment did have a significant effect on aquaporin expression in roots; time (P < 0.001), treatment (P < 0.001), and their interaction (P < 0.001) were significant sources of variation in root samples. Mid-day expression in roots was strongly down-regulated by drought in both genotypes, forming a cluster distinct from other samples (Fig. 5A).
Fig. 5.
Hierarchical clustering of samples based on aquaporin expression in greenhouse Exp 2c (A). The primary division was based on tissue. Mid-day drought-stressed root samples clustered away from other root samples (left box); morning leaf samples clustered together, regardless of drought treatment (right box). Hierarchical clustering of aquaporin gene expression in roots (B) divided into three main clusters.
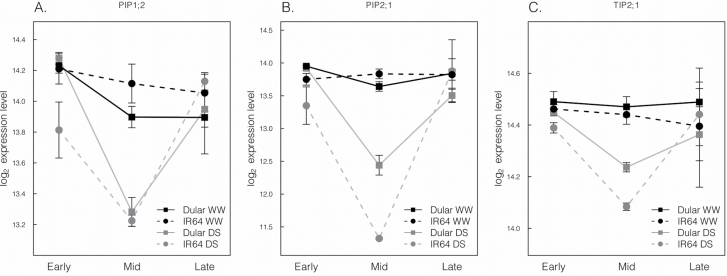
Clustering of aquaporins by tissue identified a root expression cluster with significant genotype×treatment×time interactions (P=0.038, Fig. 5B). This cluster consisted of aquaporins PIP1;2, PIP2;1, and TIP2;1. Morning expression of these three genes was higher in Dular than in IR64 under drought stress, and IR64 in the DD treatment had higher expression of these genes in the evening. Treatment was the only significant factor in mid-day expression, with expression down-regulated in both genotypes under drought stress (Fig. 6). Overall, Dular generally exhibited elevated expression in the root relative to IR64 in the morning, particularly under drought stress (Supplementary Fig. S2 at JXB online).
Fig. 6.
Root expression patterns of cluster 3 aquaporins from greenhouse Exp 2c with significant treatment×time×genotype interaction. Bars indicate the standard error.
The endodermis and sclerenchyma layersexhibited opposing responses to drought
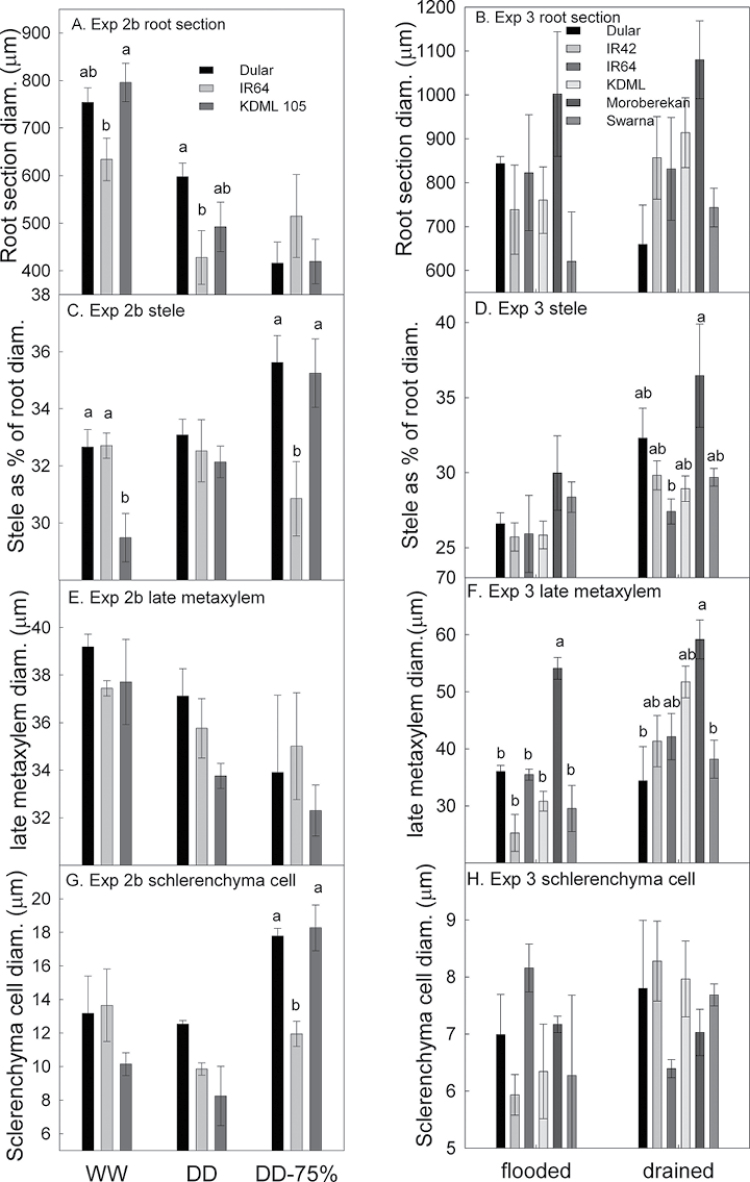
Anatomical parameters were more strongly affected by treatments than by genotypes (Figs. 7 and 8; Supplementary Table S3 at JXB online). In Exp 3, the drained pots showed greater stele diameter as a percentage of total root diameter (P < 0.001) and greater suberization of the endodermis (P=0.34). In Exp 2b, drought caused increases in the stele diameter as a percentage of total root diameter (P=0.02), diameter of cells in the sclerenchyma layer (P < 0.001), and suberization of the endodermis (P < 0.001). Drought caused decreases in the root cross-sectional diameter (P < 0.001), number of cells in the OPR (P < 0.001), width of the OPR (P < 0.001), percentage of the root cortex as aerenchyma (P < 0.001), and suberization of the sclerenchyma layer (P < 0.001). Suberization of the central stele was also variable, but was not rated in this experiment. Xylem vessel diameter and number showed significant genotypic variation in Exps 2a and 3 (P < 0.01), and significant treatment effects were observed in Exp 2b, where xylem vessel diameter and number became smaller with increasing drought stress severity (Fig. 8; Supplementary Table S3).
Fig. 8.
Diameters of root sections (A and B), stele (as a percentage of root diameter; C and D), late metaxylem vessels (E and F), and sclerenchyma cells (G and H) in nodal roots sectioned at the mid-point in greenhouse Exps 2b (seedling stage, severe drought stress) and 3 (mature plants, mild drought stress), respectively.
Discussion
This study allowed a comprehensive comparison of a number of attributes affecting rice water uptake and their roles in drought response (Table 3). Diurnal variation stood out as a predominant source of variation among genotypes in terms of bleeding rates of sap from the root system, Lp r, and aquaporin expression. These trends were evident in both drought and well-watered treatments. Although some genetic variation was observed, morphological and anatomical differences as well as suberization of different cell layers were most apparent among treatments rather than genotypes.
Table 3.
Traits observed in this study and their suggested roles in rice root water uptake under drought
| Trait | Trends observed | Suggested function for water uptake under drought |
|---|---|---|
| Morphological | ||
| Lateral root formation | Increased lateral root formation withdrought stress | Improved contact with shrinking water columns in the soil, differential conductivity due to differential anatomy/biochemistry compared with coarse roots |
| Nodal root diameter | Decreased under drought | Finer root formation to conserve resources |
| Anatomical | ||
| Proportion of root cross-sectional diameter represented by stele | Increased under drought | Prioritization of retaining water in vascular tissue rather than reducing radial oxygen loss as drought occurs |
| Diameter/number of xylem vessels | Decreased under severe drought | Reduced risk of xylem vessel cavitation |
| Width of/number of cells in the outer partof the root | Decreased under drought | Reduced impedance to water uptake from the soil, and/or senescence of outer cells due to stress |
| Sclerenchyma cell diameter | Increased under drought | Tightly packed cells not needed for retention of oxygen as drought occurs |
| Suberization of sclerenchyma layer | Decreased under drought stress | Effect on water uptake not apparent: probably most important for reducing radial oxygen loss under flooded conditions |
| Suberization of endodermis | Increased under drought stress | Important for water transport through retention of water in vascular cells during drought, rather than for water uptake |
| Aerenchyma formation | Decreased under drought | Effect on water uptake not apparent: probably most important for supplying oxygen under flooded conditions |
| Functional | ||
| Aquaporin expression | Mid- and late-day decrease under drought | Response to lowered transpirational demand, conservation of soil water |
| Diurnal fluctuations in root hydraulicconductivity and bleeding rate | All genotypes showed reduced levels at night, differential levels early and mid-day | Genotypes that time water uptake and transport to the shoots with periods of the day when transpiration is most efficient (i.e. morning) may have more efficient water use |
| Synchronization of diurnal changes in leaf water potential and root hydraulic conductivity | Differential trends between genotypes: Dularwas better synchronized than IR64 | Synchronization of root and leaf function may allow for more efficient water use |
In terms of bleeding rate, Dular and KDML 105, which are both considered moderately drought tolerant (De Datta et al., 1975; Jearakongman et al., 1995), consistently showed the lowest overnight values in the field in both well-watered and drought conditions (Fig. 1). Swarna consistently showed the greatest bleeding rates in all treatments in the field, and is known to show large yield losses under drought (Venuprasad et al., 2009). Low Lp r has previously been hypothesized as an important drought trait for improved yield in wheat (Richards and Passioura 1989), possibly to conserve soil water for later uptake during grain filling. Conservative water use in drought-resistant lines has also been reported based on transpiration patterns in soybean (Sadok and Sinclair, 2009) and pearl millet (Kholová et al., 2010). The results of the present study also suggest conservative water uptake in drought-resistant lines, but indicate that rice hydraulic conductivity may fluctuate to different degrees in different genotypes over the course of the day (Table 3).
Reduced bleeding rates late in the day or at night have been reported previously in rice (Morita and Abe, 2002). Diurnal variation in water uptake/Lp r under drought may allow for synchronization of water uptake from the soil with stomatal aperture and transpiration demand, in order for the plant to use the limited amounts of water in the soil efficiently. Greater fluctuations in aquaporin expression with the time of day were observed in IR64 than in Dular, which may contribute to the ability of Dular to maintain more stability in terms of bleeding rates across different times of day (Fig. 2) and Lp r values across different soil moisture levels (Fig. 4). Although genotypic differences were observed for fluctuations in Lp r in Exp 2b (Fig. 3), these genotypes did not differ in daily transpiration rates (Supplementary Fig. S1 at JXB online), indicating that more frequent weighing intervals would have been necessary to detect genetic differences in water uptake patterns.
Whether diurnal aquaporin expression patterns are driven by circadian, environmental, or physiological cues is still a matter of debate (Sakurai-Ishikawa et al., 2011; Takase et al., 2011). In this study, leaf aquaporin expression mainly varied by time of day, with little impact of stress treatment, but root expression was significantly affected by the interaction of drought treatment and time of day, suggesting that leaf functional parameters such as transpiration rates may have an effect on root function. Ambient humidity in the field and greenhouse typically drops at mid-day, along with an increase in temperature, and these resulting fluctuations in vapour pressure deficit (VPD) may be the key signal for changes in Lp r, as opposed to time of day or photoperiod. Morning hours may be the most effective time for root water uptake to occur in order to be used for transpiration by leaves and reduce evaporative loss. This is supported by observed diurnal changes in leaf rolling (O’Toole and Cruz, 1980) and xylem vessel cavitation (Stiller et al., 2003). Greater bleeding rates in the morning may also be driven by an increase in mineral concentration of xylem sap that occurs during the night, as described by Pomper and Grusak (2004).
Trends in aquaporin expression were related to trends in Lp r. Aquaporin expression in well-watered leaves was higher in IR64 than in Dular, possibly to help support greater transpiration and water transport in the improved variety (IR64). In roots of the WW treatment, aquaporin expression of Dular was modestly higher in the morning. This pattern was amplified by drought, and is in agreement with the general genotypic trend in morning Lp r. IR64 had greater root aquaporin expression in the evening, consistent with Lp r measurements and overnight field measurements in which Dular generally ranked lowest among genotypes for bleeding rate. It was found that one root cluster of aquaporins had a significant genotype×treatment×time of day interaction. Notably, this group includes PIP1;2 and PIP2;1, which were previously identified as the two most abundant PIP transcripts, accounting for >50% of all root PIP expression (Sakurai-Ishikawa et al., 2011).
At mid-day, there was no clear genotypic pattern for aquaporin expression in roots in the drought treatment, though expression in both genotypes was suppressed relative to the well-watered controls. In drought-stressed leaves at mid-day, expression of OsPIP1;1 (RWC1), OsPIP1;3 (RWC3), OsPIP2;3, and OsPIP2;7 was >2-fold higher in IR64 than in Dular. Although aquaporin expression often mirrored Lp r, this relationship may have been complicated by other factors, such as regulation of aquaporin trafficking within the endomembrane system (Vera-Estrella et al., 2004), chemical modification of aquaporin proteins (Santoni et al., 2006), or cellular acidosis (Tournaire-Roux et al., 2003). Furthermore, although stress-induced changes in aquaporin expression have been shown to correlate with protein level expression (Boursiac et al., 2005), water transport activity varies greatly among aquaporin family members (Sakurai et al., 2005). Previous studies of diurnal variation in aquaporin expression reported morning peaks for all roots (Sakurai-Ishikawa et al., 2011), and up-regulation was observed during the evening for several aquaporins, including TIP4;1 in both root and leaf, and OsPIP2;7 in the leaf. These genes may be involved in controlling water dynamics related to changing source/sink status during the night (Patrick et al., 2001).
Aquaporin expression, root anatomy, and root morphology have all been previously reported to be of importance for explaining differences in hydraulics between wheat and lupin (Bramley et al., 2009), and a number of anatomical parameters have also been described as related to genotypic differences in Lp r of soybean (Rincon et al., 2003). Large differences in root morphology or anatomy were not detected in the present study among the three rice genotypes investigated, except for xylem vessel diameter and number. Interestingly, Dular and KDML 105 showed smaller xylem vessel diameters and number than drought-susceptible IR64 in the severe drought stress treatment (DD-75%; Fig. 8; Supplementary Table S3 at JXB online). The trend of narrowing xylem vessel diameter with increasing drought severity was opposite to the trends in stele size, and is in agreement with Uga et al. (2008) whose QTL mapping work suggests that stele and xylem vessel size in rice are under separate genetic control. Smaller root xylem vessel diameters have been observed previously in rice roots with reduced water supply (Yambao et al., 2003; Mostajeran and Rahimi-Eichi, 2008), and may be beneficial under drought stress by reducing the risk of xylem vessel cavitation.
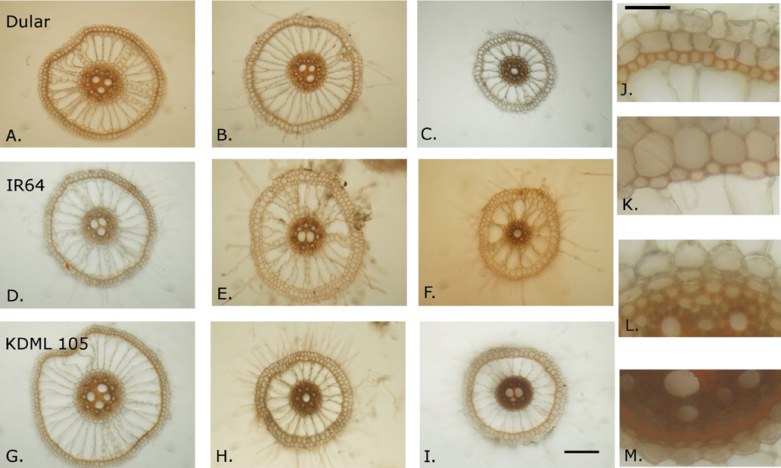
Results from this study showed interesting trends in root anatomy and suberization between drought and well-watered treatments that suggest differential roles for the OPR and the stele for drought response (Table 3). Suberization of the OPR of rice in solution culture studies has been reported either to reduce water uptake (Miyamoto et al., 2001) or to have no effect on water uptake (Ranathunge et al., 2011), and could possibly play a role in water retention by the root under drought. An increased size and decreased suberization of sclerenchyma cells was observed, as well as decreased width of the OPR under drought (Figs 7, 78; Supplementary Table S3 at JXB online). The decreased prevalence of tight packing of cells in the suberized sclerenchyma layer would reduce its effectiveness as a barrier, suggesting a decreased importance of the OPR as drought occurs. In contrast, suberization of the endodermis and in the proportion of root diameter represented by the stele increased under drought. The endodermis has been previously described as impeding water flow in rice roots (Miyamoto et al., 2001; Ranathunge et al., 2011). These trends suggest an increasingly important role for the endodermis and stele, perhaps in water transport by helping to retain root water under drought, rather than in water uptake under drought. Suberin staining intensity and root anatomy in general are likely to vary according to the spatio-temporal development of root zones in response to drought. Observation of additional root zones in addition to at the longitudinal mid-point (as observed this study) may reveal more apparent genetic differences in root anatomical response to drought. The decreased responsiveness in terms of anatomical changes between treatments in Exp 3 compared with Exp 2b is probably due to the advanced maturity of the plants and the mild stress applied in Exp 3. In Exp 2b, the root anatomy of Dular and KDML 105 appeared to be more responsive to treatment than that of IR64 (Fig. 8).
Fig. 7.
Nodal root anatomy at the longitudinal mid-point in greenhouse Exp 2b differed among treatments for most parameters measured, and among genotypes in terms of sclerenchyma cell diameter and stele as a percentage of root diameter. Suberization of the sclerenchyma layer decreased with drought, and suberization of the endodermis increased with drought. Genotypes shown on the left are Dular [(A) WW (well-watered control); (B) DD (drydown from field capacity); (C) DD-75% (drydown from 75% of field capacity)], IR64 [(D) WW, (E) DD, and (F) DD-75%], and KDML 105 [(G) WW, (H), DD, and (I) DD-75%]. Images A–I are shown at the same scale, which was a magnification of ×100, and the bar in I.represents 150 µm. Images on the right show differences in staining intensity of the suberin lamellae of the sclerenchyma layer [(J) WW and (K) DD-75%] and endodermis [(L) WW and (M) DD-75%] in KDML 105. Images J–M are shown on the same scale, and the bar in J represents 30 µm.
Variability in soil moisture had a strong effect on Lp r and bleeding rate measurements, as indicated by the differences between drought and well-watered treatments and with fluctuating soil water potential in the field. Differences in Lp r, plant growth, and water uptake between Exps 2a and 2b were likely to be due to differences in growth location within the greenhouse (floor versus table) that probably affected plant temperatures. In addition to effects on soil moisture and soil conductivity, greater plant water uptake could have increased the resistance of the root–soil interface (Passioura, 1988), thereby also reducing Lp r. Differences in phenology were apparent in the field, which could affect water uptake dynamics, and these genotypes have been previously characterized to differ in root growth at depth (Lafitte et al., 2001; Henry et al., 2011). Although soil moisture varied within the drought stress treatments, trends among genotypes in the field for bleeding rate across different growth stages and soil water potentials were consistent, and time of day showed a strong effect on bleeding rate, Lp r, and aquaporin expression.
In summary, genotypes classified as drought resistant showed consistently lower bleeding rates in the field, more stable Lp r with variation in soil moisture, more responsiveness of root anatomy to drought, and greater levels of aquaporin expression early in the day. This range of traits may confer the ability to regulate water uptake and root hydraulic conductivity, to allow efficient control of plant water status under drought. More research is necessary to understand the signals that initiate these responses to drought in rice roots.
Supplementary data
Supplementary data are available at JXB online.
Fig. S1. Transpiration rate per plant determined by weighing of pots in (A) Exp 2a and (B) Exp 2b.
Fig. S2. Genotypic differences in aquaporin expression in leaves and roots in greenhouse Exp 2c.
Table S1. Shoot mass and root:shoot ratio from the three greenhouse studies in this experiment.
Table S2. Probe sequences and targets from the Illumina DASL assay for aquaporin expression, in reference to the Michigan State University (MSU) and Rice Annotation Project (RAP) genome assemblies.
Table S3. Root anatomical parameters examined in Exps 2b and 3 from nodal roots sectioned at the longitudinal mid-point.
Supplementary Material
Acknowledgements
This work was funded by the Generation Challenge Program project ‘Targeting Drought-Avoidance Root Traits to Enhance Rice Productivity under Water-Limited Environments’, and the Bill and Melinda Gates Foundation project ‘Stress-Tolerant Rice for Africa and South Asia.’ Many thanks go to L. Holongbayan, N. Turingan, A. Los Añes, L. Satioquia, E. Mico, E. Arcillas B. Punzalan, A. Reyes, N. Driz, A. Perjes, N. Sanchez, and D. Morales for technical support, R. Oane for help with microscopy, and M. Thomson for assistance with the Bead Express. We thank J. Lynch, M. Wissuwa, and L. Wade for helpful discussions, as well as S. Heuer and A. Kohli for reviewing a draft of this manuscript.
References
- Abramoff MD, Magalhaes PJ, Ram SJ. Image processing with ImageJ. Biophotonics International. 2004;11:36–42. [Google Scholar]
- Bouman BAM, Humphreys E, Tuong TP, Barker R. Rice and water. Advances in Agronomy. 2007;92:187–237. [Google Scholar]
- Boursiac Y, Chen S, Luu D-T, Sorieul M, van den Dries N, Maurel C. Early effects of salinity on water transport in Arabidopsis roots. Molecular and cellular features of aquaporin expression. Plant Physiology. 2005;139:790–805. doi: 10.1104/pp.105.065029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramley H, Turner NC, Turner DW, Tyerman SD. Roles of morphology, anatomy, and aquaporins in determining contrasting hydraulic behavior of roots. Plant Physiology. 2009;150:348–364. doi: 10.1104/pp.108.134098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colmer TD, Gibberd MR, Wiengweera A, Tinh TK. The barrier to radial oxygen loss from roots of rice (Oryza sativa L.) is induced by growth in stagnant solution. Journal of Experimental Botany. 1998;49:1431–1436. [Google Scholar]
- De Datta SK, Chang TT, Yoshida S. 1975. Drought tolerance in upland rice. In: Upland rice IRRI; Los Baños, Philippines: 101 106 [Google Scholar]
- Fan JB, Yeakley JM, Bibikova M, et al. A versatile assay for high-throughput gene expression profiling on universal array matrices. Genome Research. 2004;14:878–885. doi: 10.1101/gr.2167504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowda VRP, Henry A, Yamauchi A, Shashidhar HE, Serraj R. Root biology and genetic improvement for drought avoidance in rice. Field Crops Research. 2011;122:1–13. [Google Scholar]
- Henry A, Gowda VRP, Torres RO, McNally KL, Serraj R. Variation in root system architecture and drought response in rice (Oryza sativa): phenotyping of the OryzaSNP panel in rainfed lowland fields. Field Crops Research. 2011;120:205–214. [Google Scholar]
- Jearakongman S, Rajatasereekul S, Naklang K, Romyen P, Fukai S, Skulkhu E, Jumpaket B, Nathabutr K. Growth and grain yield of contrasting rice cultivars grown under different conditions of water availability. Field Crops Research. 1995;44:139–150. [Google Scholar]
- Kamoshita A, Wade LJ, Yamauchi A. Genotypic variation in response of rainfed lowland rice to drought and rewatering. III. Water extraction during the drought period. Plant Production Science. 2000;3:189–196. [Google Scholar]
- Kholová J, Hash CT, Kumar L, Yadav RS, Kocová M, Vadez V. Terminal drought-tolerant pearl millet [Pennisetum glaucum (L.) R. Br.] have high leaf ABA and limit transpiration at high vapour pressure deficit. Journal of Experimental Botany. 2010;61:1431–1440. doi: 10.1093/jxb/erq013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafitte HR, Champoux MC, McLaren G, O’Toole JC. Rice root morphological traits are related to isozyme group and adaptation. Field Crops Research. 2001;71:57–70. [Google Scholar]
- Li J-F, Li L, Sheen J. Protocol: a rapid and economical procedure for purification of plasmid or plant DNA with diverse applications in plant biology. Plant Methods. 2010;6:1. doi: 10.1186/1746-4811-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian H-L, Yu X, Lane D, Sun W-N, Tang Z-C, Su W-A. Upland rice and lowland rice exhibited different PIP expression under water deficit and ABA treatment. Cell Research. 2006;16:651–660. doi: 10.1038/sj.cr.7310068. [DOI] [PubMed] [Google Scholar]
- Lian H-L, Yu X, Ye Q, Ding X-S, Kitagawa Y, Kwak S-S, Su W-Am, Tang Z-C. The role of aquaporin RWC3 in drought avoidance in rice. Plant and Cell Physiology. 2004;45:481–489. doi: 10.1093/pcp/pch058. [DOI] [PubMed] [Google Scholar]
- Lilley JM, Fukai S. Effect of timing and severity of water deficit on four diverse rice cultivars. I. Rooting pattern and soil water extraction. Field Crops Research. 1994;37:205–213. [Google Scholar]
- Matsuo N, Ozawa K, Mochizuki T. Genotypic differences in root hydraulic conductance of rice (Oryza sativa L.) in response to water regimes. Plant and Soil. 2009;316:25–34. [Google Scholar]
- Miyamoto N, Steudle E, Hirasawa T, Lafitte R. Hydraulic conductivity of rice roots. Journal of Experimental Botany. 2001;52:1835–1846. doi: 10.1093/jexbot/52.362.1835. [DOI] [PubMed] [Google Scholar]
- Morita S, Abe J. Diurnal and phenological changes of bleeding rate in lowland rice plants. Japanese Journal of Crop Science. 2002;71:383–388. [Google Scholar]
- Mostajeran A, Rahimi-Eichi V. Drought stress effects on root anatomical characteristics of rice cultivars (Oryza sativa L.) Pakistan Journal of Biological Sciences. 2008;11:2173–2183. doi: 10.3923/pjbs.2008.2173.2183. [DOI] [PubMed] [Google Scholar]
- O’Toole JC, Cruz RT. Response of leaf water potential, stomatal resistance, and leaf rolling to water stress. Plant Physiology. 1980;65:428–432. doi: 10.1104/pp.65.3.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passioura JB. Water transport in and to roots. Annual Review of Plant Physiology and Plant Molecular Biology. 1988;39:245–265. [Google Scholar]
- Patrick JW, Zhang W, Tyerman SD, Offler CE, Walker A. Role of membrane transport in phloem translocation of assimilates and water. Australian Journal of Plant Physiology. 2001;28:697–709. [Google Scholar]
- Pomper KW, Grusak MA. Calcium uptake and whole-plant water use influence pod calcium concentration in snap bean plants. Journal of the American Society for Horticultural Science. 2004;129:890–895. [Google Scholar]
- Puckridge DW, O’Toole JC. Dry matter and grain production of rice, using a line source sprinkler in drought studies. Field Crops Research. 1981;3:303–319. [Google Scholar]
- R Development Core Team 2008. A language and environment for statistical computing Vienna, Austria: R Foundation for Statistical Computing; [Google Scholar]
- Ranathunge K, Kotula L, Steudle E, Lafitte R. Water permeability and reflection coefficient of the outer part of young rice roots are differently affected by closure of water channels (aquaporins) or blockage of apoplastic pores. Journal of Experimental Botany. 2004;55:433–447. doi: 10.1093/jxb/erh041. [DOI] [PubMed] [Google Scholar]
- Ranathunge K, Lin J, Steudle E, Schreiber L. Stagnant deoxygenated growth enhances root suberization and lignifications, but differentially affects water and NaCL permeabilities in rice (Oryza sativa L.) roots. Plant, Cell and Environment. 2011;34:1223–1240. doi: 10.1111/j.1365-3040.2011.02318.x. [DOI] [PubMed] [Google Scholar]
- Ranathunge K, Steudle E, Lafitte R. Control of water uptake by rice (Oryza sativa L.): role of the outer part of the root. Planta. 2003;217:193–205. doi: 10.1007/s00425-003-0984-9. [DOI] [PubMed] [Google Scholar]
- Richards RA, Passioura JB. A breeding program to reduce the diameter of the major xylem vessel in the seminal roots of wheat and its effect on grain yield in rain-fed environments. Australian Journal of Agricultural Research. 1989;40:943–950. [Google Scholar]
- Rincon CA, Raper CD, Patterson RP., Jr Genotypic differences in root anatomy affecting water movement through roots of soybean. International Journal of Plant Science. 2003;164:543–551. [Google Scholar]
- Sadok W, Sinclair T. Genetic variability of transpiration response to vapor pressure deficit among soybean cultivars. Crop Science. 2009;49:955–960. [Google Scholar]
- Sakurai J, Ishikawa F, Yamaguchi T, Uemura M, Maeshima M. Identification of 33 rice aquaporin genes and analysis oftheir expression and function. Plant and Cell Physiology. 2005;46:1568–1577. doi: 10.1093/pcp/pci172. [DOI] [PubMed] [Google Scholar]
- Sakurai-Ishikawa J, Murai-Hatano M, Hayashi H, Ahamed A, Fukushi K, Matsumoto T, Kitagawa Y. Transpiration from shoots triggers diurnal changes in root aquaporin expression. Plant, Cell and Environment. 2011;34:1150–1163. doi: 10.1111/j.1365-3040.2011.02313.x. [DOI] [PubMed] [Google Scholar]
- Santoni V, Verdoucq L, Sommerer N, Vinh J, Pflieger D, Maurel C. Methylation of aquaporins in plant plasma membrane. Biochemical Journal. 2006;400:189–197. doi: 10.1042/BJ20060569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serraj R, McNally KL, Slamet-Loedin I, Kohli A, Haefele SM, Atlin G, Kumar A. Drought resistance improvement in rice: an integrated genetic and resource management strategy. Plant Production Science. 2011;14:1–14. [Google Scholar]
- Stiller V, Lafitte HR, Sperry JS. Hydraulic properties of rice and the response of gas exchange to water stress. Plant Physiology. 2003;132:1698–1706. doi: 10.1104/pp.102.019851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suralta RR, Yamauchi A. Root growth, aerenchyma development, and oxygen transport in rice genotypes subjected to drought and waterlogging. Environmental and Experimental Botany. 2008;64:75–82. [Google Scholar]
- Takase T, Ishikawa H, Murakami H, Kikuchi J, Sato-Nara K, Suzuki H. The circadian clock modulates water dynamics and aquaporin expression in Arabidopsis roots. Plant and Cell Physiology. 2011;52:373–383. doi: 10.1093/pcp/pcq198. [DOI] [PubMed] [Google Scholar]
- Tournaire-Roux C Sutka M Javot H Gout E Gerbeau P Luu DT Bligny R Maurel C 2003Cytosolic pH regulates root water transport during anoxic stress through gating of aquaporins.Nature 425,393 397 [DOI] [PubMed] [Google Scholar]
- Uga Y, Okuno K, Yano M. QTLs underlying natural variation in stele and xylem structures of rice root. Breeding Science. 2008;58:7–14. [Google Scholar]
- Venuprasad R, Dalid CO, Del Valle M, Zhao D, Espiritu M, Sta Cruz MT, Amante M, Kumar A, Atlin GN. Identification and characterization of large-effect quantitative trait loci for grain yield under lowland drought stress in rice using bulk-segregant analysis. Theoretical and Applied Genetics. 2009;120:177–190. doi: 10.1007/s00122-009-1168-1. [DOI] [PubMed] [Google Scholar]
- Vera-Estrella R, Barkla B, Bohnert H, Pantoja O. Novel regulation of aquaporins during osmotic stress. Plant Physiology. 2004;35:2318–2329. doi: 10.1104/pp.104.044891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yambao EB, Ingram KT, Real JG. Root xylem influence on the water relations and drought resistance of rice. Journal of Experimental Botany. 1992;43:925–932. [Google Scholar]
- Zeier J, Ruel K, Ryser U, Schreiber L. Chemical analysis and immunolocalisation of lignin and suberin in endodermal and hypodermal/rhizodermal cell walls of developing maize (Zea mays L.) primary roots. Planta. 1999;209:1–12. doi: 10.1007/s004250050601. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.