Abstract
This study analysed the contribution of each omega-3 desaturase to the cold response in soybean. Exposure to cold temperatures (5 °C) did not result in great modifications of the linolenic acid content in leaf membrane lipids. However, an increase in the GmFAD3A transcripts was observed both in plant leaves and soybean cells whereas no changes in GmFAD3B or GmFAD3C expression levels were detected. This increase was reversible and accompanied by the accumulation of an mRNA encoding a truncated form of GmFAD3A (GmFAD3A-T), which originated from alternative splicing of GmFAD3A in response to cold. When the expression of plastidial omega-3 desaturases was analysed, a transient accumulation of GmFAD7-2 mRNA was detected upon cold exposure in mature soybean trifoliate leaves while GmFAD7-1 transcripts remained unchanged. No modification of the GmFAD8-1 and GmFAD8-2 transcripts was observed. The functionality of GmFAD3A, GmFAD3B, GmFAD3C and GmFAD3A-T was examined by heterologous expression in yeast. No activity was detected with GmFAD3A-T, consistent with the absence of one of the His boxes necessary for desaturase activity. The linolenic acid content of Sacharomyces cerevisiae cells overexpressing GmFAD3A or GmFAD3B was higher when the cultures were incubated at cooler temperatures, suggesting that reticular desaturases of the GmFAD3 family, and more specifically GmFAD3A, may play a role in the cold response, even in leaves. The data point to a regulatory mechanism of omega-3 fatty acid desaturases in soybean affecting specific isoforms in both the plastid and the endoplasmic reticulum to maintain appropriate levels of linolenic acid under low temperature conditions.
Key words: desaturase, FAD3, FAD7, FAD8, gene expression, cold, soybean
Introduction
Temperature is one of the major environmental factors influencing the distribution of plant species, the range of temperatures experienced by plants being extremely variable both at the spatial and temporal scales (Iba, 2002). The adaptability of plants to their temperature environment will depend directly on their capacity for developing mechanisms of temperature adaptation. These mechanisms are rather complex and include the action of temperature stress factors as well as metabolic changes (Tomashow, 1999; Iba, 2002). Membranes are major targets of the temperature acclimation strategies. Biological membranes are organized structures of lipids and embedded proteins that surround cells and organelles, in which essential processes such as photosynthesis, respiration, or solute transport take place. Thus, membrane lipids provide a dynamic and fluid environment essential for living organisms. Not surprisingly, a strong association has been observed between environmental temperature and the lipid and fatty acid content of plant membranes (Rennie and Tanner, 1989; Nishida and Murata, 1996; Iba, 2002). Certain specific lipids have been directly involved in chilling sensitivity in plants. Thus, a relationship between the levels of palmitic (16:0) and trans-hexadecanoic (t16:1) acids in phosphatidylglycerol (PG) and chilling sensitivity in plants was established (Murata et al., 1982). Furthermore, experiments overexpressing in tobacco the plastidial glycerol-3-phosphate acyl transferase gene (GPAT) from a chilling-sensitive (squash) or resistant (Arabidopsis) plant indicated that increasing levels of saturated PG were correlated to greater sensitivity to cold temperatures (Murata et al., 1992). Apart from the role of specific lipids, cooler temperatures are often associated with an increase in the production of polyunsaturated fatty acids (PUFAs), mainly β-linolenic acid (18:3) (McConn et al., 1994; Heppard et al., 1996; Horiguchi et al., 2000; Martz et al., 2006; Li et al., 2007; Kargiotidou et al., 2008). These PUFAs are thought to maintain membrane fluidity because of their lower melting temperatures (Nishida and Murata, 1996; Iba, 2002; Gushina and Harwood, 2006). The highest increase has been reported in a non-photosynthetic tissue, wheat root tips, which showed a 25% increase in 18:3 levels when exposed to 10 °C (Horiguchi et al., 2000). However, with the exception of Arabidopsis, where significant changes in the 10–15% range have been reported (McConn et al., 1994; Falcone et al., 2004), the extent of the increase in 18:3 levels in leaves has seemed to be rather small in other plant species (Martz et al., 2006; Li et al., 2007; Upchurch and Ramirez, 2011).
Desaturation of fatty acids is performed by a class of enzymes called fatty acid desaturases (FADs). These enzymes are encoded by nuclear genes and differ in their substrate specificity and subcellular localization. The genes encoding plant fatty acid desaturases have been cloned and sequenced from a great variety of plant species. In Arabidopsis, three genes encode the omega-3 desaturases responsible for the synthesis of trienoic fatty acids (TAs): one for the endoplasmic reticulum omega-3 desaturase AtFAD3 and two for the plastidial enzymes AtFAD7 and AtFAD8 (Iba et al., 1993; Yadav et al., 1993; Gibson et al., 1994). AtFAD8 is believed to encode a cold-specific plastidial omega-3 desaturase since its activity has been observed in a fad3/fad7 double mutant when exposed to low temperatures (Gibson et al., 1994). The way that temperature regulates the expression of the genes encoding omega-3 fatty acid desaturases has been the subject of considerable research during recent years. Thus, in maize leaves, a decrease in ZmFAD7 mRNA accompanied by an increase in the ZmFAD8 mRNA was reported in response to low temperatures ( Berberich et al., 1998), suggesting direct transcriptional control. More recently, it was demonstrated that the AtFAD8 protein was destabilized at high temperatures, without changes in mRNA levels, suggesting a post-translational control mechanism regulating AtFAD8 activity in Arabidopsis in which the C-terminus of the mature protein would be involved (Matsuda et al., 2005). Similarly, analysis of wheat root tips subjected to low temperature conditions showed increased enzyme accumulation with higher linolenic acid production without changes in TaFAD3 mRNA levels (Horiguchi et al., 2000). Changes in the protein half-life could also be involved in control of the activity of the soybean seed-specific GmFAD2–2 isoform (Tang et al., 2005) or rape BnFAD3 enzymes (O’Quin et al., 2010) in response to temperature. Unfortunately, many of the studies that have analysed the regulation of plastidial desaturases in response to cold did not study the regulation of endoplasmic reticulum desaturases under the same experimental conditions (Berberich et al., 1998; Matsuda et al., 2005). Similarly, the regulation of endoplasmic reticulum desaturases and their response to cold has been studied in non-photosynthetic tissues such as roots, but not in leaves (Horiguchi et al., 2000; Dyer et al., 2001; Tang et al., 2005; O’Quin et al., 2010). This has resulted in a lack of information about one of the two organelles where 18:3 is synthesized in plants in a concerted manner. As a result, research is far from having an integrated view of how omega-3 desaturase enzymes respond to temperature changes and in which tissue and through which mechanism this role is actually executed.
Increasing evidence indicates that in soybean, the genes encoding omega-3 fatty acid desaturases are grouped in multigene families. Thus, at least three GmFAD3 genes, designated as GmFAD3A, GmFAD3B, and GmFAD3C seem to contribute to 18:3 synthesis in the endoplasmic reticulum membranes of soybean (Bilyeu et al., 2003; Anai et al., 2005). More recently, the presence of two soybean GmFAD7 genes were reported, designated as GmFAD7-1 and GmFAD7-2, which would participate in 18:3 production in plastid membranes (Andreu et al., 2010). Finally, two sequences with homology to known FAD8 genes were detected in the soybean genome (Chi et al., 2011), but their regulation has not been studied yet.
This work studied the effect of low temperatures on the regulation of the expression of omega-3 fatty acid desaturases from soybean in order to analyse the concerted regulation of each gene family in response to temperature. The experiments were performed on mature soybean trifoliate leaves as well as on soybean photosynthetic cell suspension cultures to extend the analysis to a non-tissue differentiating system. The data suggest the existence of regulatory mechanisms of omega-3 fatty acid desaturases affecting specific isoforms in both the plastid and the endoplasmic reticulum to maintain appropriate levels of 18:3 fatty acids under low temperature conditions.
Materials and methods
Plant materials and experimental treatments
Soybean plants (Glycine max cv. Volania) were grown hydroponically as described in Andreu et al. (2010), in a bioclimatic chamber under a 16/8 light/darkness photoperiod at 24 °C and a relative humidity of 65%. For cold treatment, mature soybean plants were placed at 5 °C under the same photoperiod and humidity conditions. The plants were kept under these conditions for 3 days and trifoliate leaves (>19 days old) were collected at 24, 48, and 72h of cold exposure. The plants were then placed at normal growth temperature again and samples were collected after 4 days of recovery. Photosynthetic cell suspensions were cultured as described in Collados et al. (2006) and the experiments were performed in the same way as those of soybean plants. When indicated, soybean tissues or cells were collected, frozen in liquid nitrogen, and stored at –80 °C until use.
RNA isolation and cDNA synthesis
Total RNA was isolated from 0.5g of the different soybean tissues using the Trizol Reagent (Invitrogen) and further purified using the RNeasy Plant Mini Kit (Qiagen), following the manufacturer’s instructions. After DNAse I (Roche) treatment to remove contaminating DNA, cDNAs were synthesized from total RNA (4 µg) using M-MLV reverse transcriptase (Promega) and oligo dT primer, according to the manufacturer’s instructions.
Expression analysis of omega-3 fatty acid desaturase genes
The expression patterns of the desaturase genes were examined by semi-quantitative reverse-transcription (RT)-PCR assay. The oligonucleotides used as well as the PCR conditions are shown in Supplementary Table S1 (available in JXB online). ACTIN was used as a housekeeping gene. The amplification reaction was carried out using Platinum Taq DNA polymerase (Invitrogen) according to the manufacturer’s instructions. The amplified products were resolved by electrophoresis on 1% (w/v) agarose gels. As the primers for amplification of GmFAD3 genes recognized and amplified both GmFAD3A and GmFAD3B, a restriction analysis of the amplified fragments was performed using the Van91I enzyme (GE Healthcare), which allowed these different GmFAD3 to be distinguished. The Van91I enzyme generates two fragments of 164 and 755bp from GmFAD3A and three fragments of 161, 164, and 594bp from GmFAD3B. The digestion products were resolved by electrophoresis on 1% (w/v) agarose gel. GmSCOF-1, encoding a transcriptional factor of the C2H2-type zinc finger family that is specifically activated by low temperatures (Kim et al., 2001), was used as an internal control of the cold response under the experimental conditions. Semi-quantification of the relative expression levels was performed through normalization against ACTIN from two independent biological experiments. Densitometric quantification of the PCR bands under non-saturating conditions was performed using an image densitometer (Gel DOC XR, Bio-Rad) and the image analysis software Quantity One (Bio-Rad). The expression value of the control treatment was given the relative value of 1. The rest of the expression values were compared to the control.
Functional expression of GmFAD3 genes in yeast
For the construction of the yeast expression vectors, the corresponding open reading frames of the soybean GmFAD3A (AY204710), GmFAD3B (AY204711), GmFAD3C (AB051215), and GmFAD3A-T (the truncated form of GmFAD3A) were amplified by PCR using Pfu DNA polymerase (Stratagene) and the following primers: 5'-GAGGATCCGCAATGGTTAAAGACACAAAGCCT-3' and 5'-GAACTCGAGACTCAGTCTCGGTGCGAGTG-3' for GmFAD3A, GmFAD3B, and the truncated form of GmFAD3A as well. Clones containing either GmFAD3A or GmFAD3B were differentiated by restriction enzyme digestion and further sequencing. For amplification of GmFAD3C, 5'-GAGGATCCAAATGGTTCAAGCACAG-3' and 5'-GAACTCGAGTTTAGTTGGACTGGGTCC-3' primers were used. All these primers were extended by a BamHI (in the forward primer) and an XhoI (in the reverse primer) restriction site (underlined) for directional ligation behind the inducible GAL1 gene promoter of the yeast expression vector pYES2 (Invitrogen). The resulting PCR product for each specific GmFAD3 isoform was cloned in a pGEM-T-Easy vector, double-digested with BamHI and XhoI, and ligated into the digested destination vector. All constructs were checked by sequencing. Saccharomyces cerevisiae UTL-7A cells were transformed with plasmids pYES2 (negative control), pYES2-GmFAD3A, pYES2-GmFAD3B, pYES2-GmFAD3C, and pYES2-GmFAD3A-T by the lithium acetate protocol (Gietz and Woods, 1994) and selected on minimal agar plates lacking uracil (Ausubel et al., 1995). Strains containing the plasmids of interest were inoculated into complete minimal drop-out uracil (CM-Ura) liquid medium supplemented with 2% (w/v) raffinose as the exclusive carbon source and cultivated at 30 °C. When the cultures reached an OD600nm of 1 absorption unit (exponential phase), they were back-diluted to 0.4 absorption units with fresh medium, and gene expression was induced by adding 2% (w/v) galactose. At the same time, cultures were supplemented with 0.5mM linoleic acid (18:2) and 0.1% (w/v) tergitol (type NP-40) and then grown at 10–35 °C until late log-stationary phase. Yeast cells were harvested by centrifugation at 1500 g for 5min at 4 °C and washed with distilled water. A similar strategy was used to obtain the corresponding constructs in the vector pVT102U (Vernet et al., 1987), which carries the constitutive ADH1 promoter. Strains containing the same constructs in the pVT102U vector were cultivated in a CM-Ura liquid medium supplemented with 2% (w/v) glucose.
Lipid extraction and fatty acid analysis
Total lipids were extracted from mature soybean leaves or photosynthetic cell suspensions (0.5g) with chloroform/methanol (2:1, v/v) as previously described (Bligh and Dyer, 1959). The lipids were trans-esterified with potassium hydroxide in methanol. The resultant fatty acid methyl esters were analysed and quantified using a gas chromatograph (HP model 5890 series 2 plus) equipped with a SE2330 column (30 m length, 0.25mm inner diameter, 0.2 µm film thickness), and flame ionization detector (FID).
Total lipid content and fatty acid composition of whole yeast cells were determined using the one-step method of Garcés and Mancha (1993). Methyl esters were analysed by gas-liquid chromatography (GC), using an HP-7890 (Hewlett-Packard, Palo Alto, CA, USA) fitted with a capillary column (30 m length; 0.32mm inner diameter; 0.2 µm film thickness) of fused silica (Supelco, Bellafonte, PA, USA) and an FID detector. Hydrogen was used as a carrier gas with a linear rate of 1.34ml min–1 and a split ratio of 1/50. The injector and detector temperature was 220 °C, and the oven temperature was 170 °C.
Statistics and data analysis
The results were the mean of three independent experiments, with duplicate determinations of fatty acid composition in each soybean leaf, cell culture, or yeast experiment. Analysis of variance (ANOVA) was applied to compare treatments, and differences between means were tested with Duncan’s multiple range test. Statistical analyses were carried out with the program Statgraphics Plus for Windows 2.1, using a level of significance of 0.05.
Results
Effect of cold temperature exposure on the fatty acid composition and omega-3 fatty acid desaturase gene expression in soybean leaves
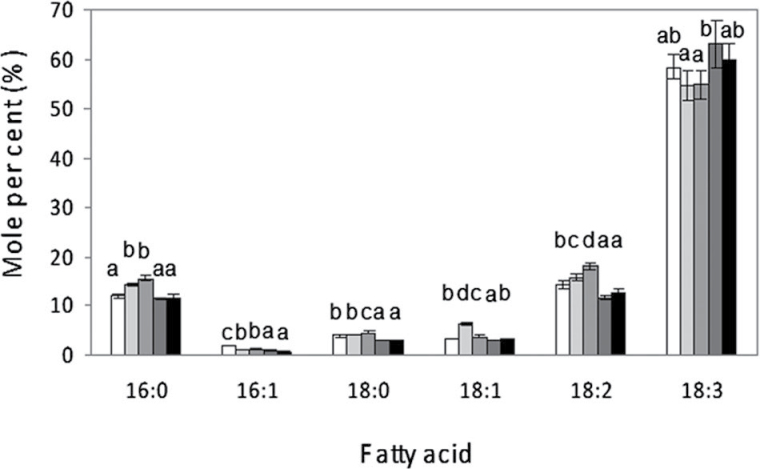
The fatty acid composition of total lipids extracted from mature leaves of soybean plants grown at control temperature (24 °C), subjected to cold (5 °C) exposure for 24, 48, and 72h, and then placed under control temperature conditions again for 4 days is shown in Fig. 1. In mature trifoliate leaves from control plants, the major fatty acid species detected in total lipids corresponded to 18:3, which represented around 60% of total fatty acids. These high 18:3 levels are consistent with those reported previously in soybean plants (Andreu et al., 2010). Upon exposure to 5 °C, slight increases in the mean values for 16:0, 18:1, and 18:2 were detected after 24 and 48h of cold exposure. These increases preceded that for the mean values of 18:3, around 5–8%, which was observed only after 72h of cold exposure. Once the plants were returned to control temperatures (24 °C), levels of 18:3 as well as the rest of the fatty acids returned to normal values after 4 days of recovery. ANOVA analysis of the results indicated that significant changes were obtained when the 18:3 levels detected after 72h of cold exposure were compared with those from 24 or 48h of cold treatment. However, even though the data suggested the existence of a trend in relation to cold exposure, no significant differences between the 18:3 levels after 72h of cold exposure and those obtained from control plants or from plants that were again placed under normal control temperatures for 96h were found.
Fig. 1.
Effect of exposure to cold temperatures on the fatty acid composition of total lipids from soybean leaves. Total lipids were extracted from plant leaves grown at control temperature (24 °C); after plant exposure for 24, 48, and 72h to 5 °C; and after recovery for 4 days at control temperature. Data are expressed as molar percentages obtained from the quantitative analysis of peak area chromatogram. White bars indicate fatty acids from control leaves; light-grey bars from 24h; medium-grey bars from 48h; dark-grey bars from 72h of cold treatment; and black bars following 4 days at control temperatures after cold exposure. Data are mean ± SD from three experiments. For the same fatty acid, different letters indicate significant differences among treatments (P < 0.05).
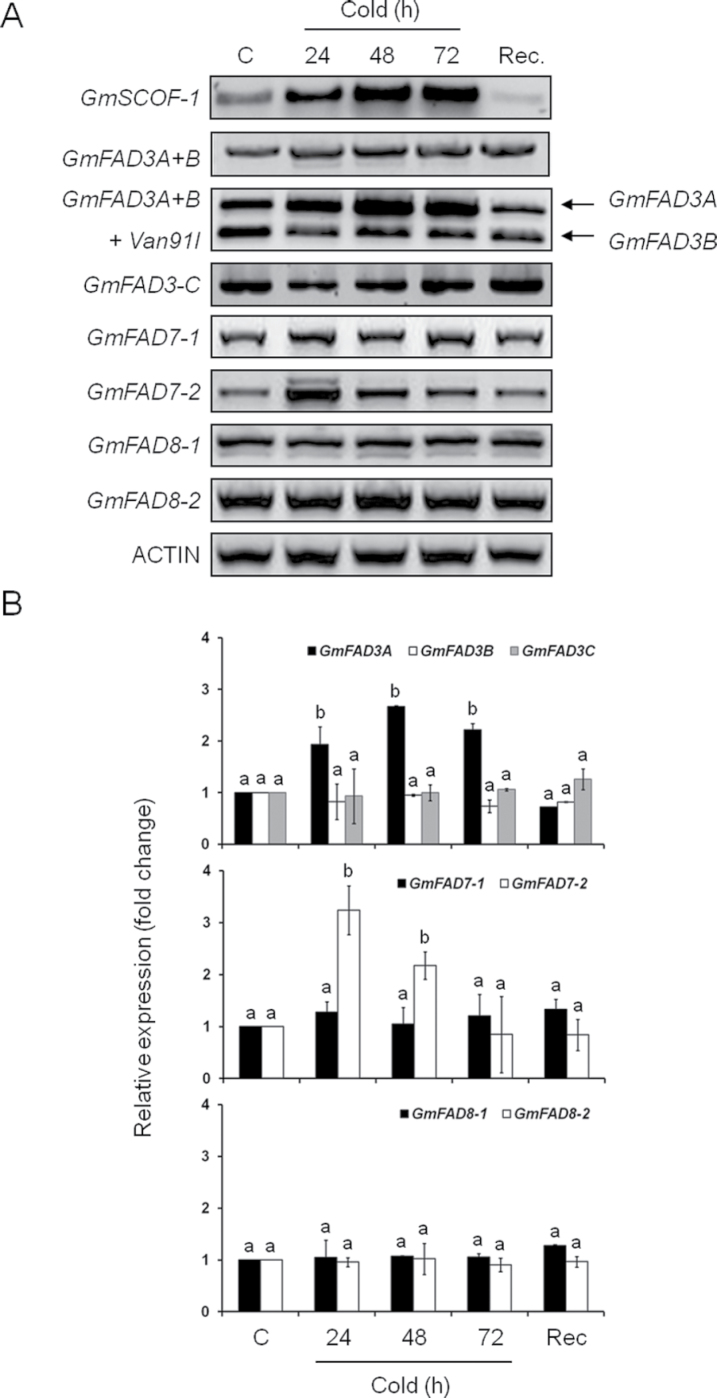
The expression of genes encoding all omega-3 fatty acid desaturases was analysed by semi-quantitative RT-PCR. First, the expression of a cold-sensitive gene was checked as an internal control of the low temperature response under the experimental conditions. To this end, the expression of GmSCOF-1, which encodes a transcription factor of the C2H2 family of zinc finger proteins that is strongly activated in soybean upon low temperature exposure (Kim et al., 2001) was monitored. As shown in Fig. 2A, the expression of GmSCOF-1 was strongly increased upon cold exposure, decreasing once the plants were placed under normal temperatures again. This result indicated that the cold response was induced at the expression level under the experimental conditions. Then the expression of the endoplasmic reticulum omega-3 desaturases was analysed. The PCR primers recognized and amplified both GmFAD3A and GmFAD3B. A slight increase in the amount of GmFAD3A+B transcripts was detected in mature trifoliate leaves upon cold exposure. No significant changes were obtained when the expression of GmFAD3C was analysed (Fig. 2A and B). Interestingly, digestion with Van91I, which allowed for the differentiation of GmFAD3A and GmFAD3B transcripts (Andreu et al., 2010), showed an increase in the amount of GmFAD3A transcripts upon cold exposure (about 2–3 fold according to the normalized analysis). This increase disappeared after the 4-day recovery of cold-treated plants under control temperature conditions, indicating that the increase in GmFAD3A mRNA was a cold-specific response (Fig. 2A and B). It is also worth mentioning that a small band was reproducibly amplified with the GmFAD3A+B specific primers (Fig. 2A). This small band (hereafter designated as GmFAD3A-T) seemed to accumulate specifically in response to cold exposure, since it disappeared once plants returned to the control temperature.
Fig. 2.
(A) Omega-3 fatty acid desaturase gene expression in mature leaves from soybean plants kept at control temperature (24 °C); 5 °C exposure for 24, 48, and 72h; and after recovery for 4 days at control temperature. GmSCOF-1 was used as an internal control for cold-inducible expression. ACTIN was used as a housekeeping gene in all experiments. (B) Normalization of gene expression results against ACTIN. Data are mean ± SD from two experiments. For the same time point, different letters indicate significant differences among treatments (P < 0.05).
With respect to the plastid omega-3 fatty acid desaturases, the expression of GmFAD7-1 was not altered in response to cold exposure (Fig. 2A and B). However, the expression of GmFAD7-2 showed a transient increase upon exposure to cold temperatures for 24h (Fig. 2A and B) and then a progressive decrease with time of cold exposure (48 and 72h), returning to levels similar to control leaves after 4 days of re-exposure to the control temperature. An upper size band was also detected at 24h of cold treatment (Fig. 2A). Sequence analysis revealed that this band was a PCR artefact originating from the unspecific annealing of the reverse primer downstream of GmFAD7-2. Both GmFAD8 transcripts were present in high amounts in soybean leaves at control temperature (Fig. 2A and B). In fact, both GmFAD8 transcripts were detected in total RNA extracted from roots, leaves, stems, flowers, and mature seeds, indicating that both GmFAD8-1 or GmFAD8-2 were expressed in all soybean tissues analysed even at control temperatures (Supplementary Fig. S1). Interestingly, no modification of the transcript levels from GmFAD8-1 or GmFAD8-2 was observed upon cold temperature exposure (Fig. 2A and B). A lower size band was detected in the expression analysis of GmFAD8-1 in mature trifoliate leaves (Fig. 2A). However, this band was detected both under control and cold temperatures, indicating that its accumulation was not temperature specific.
Effect of cold temperature exposure on the fatty acid composition and omega-3 fatty acid desaturase gene expression in soybean photosynthetic cell cultures
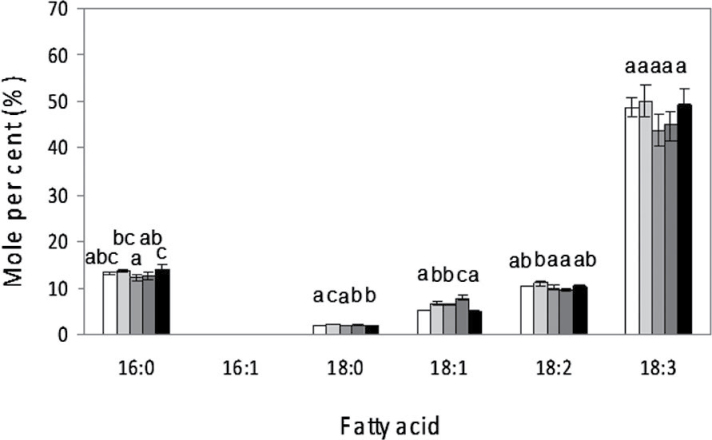
At this point, the effect of cold temperatures on young (1–3 days) developing trifoliate leaves was analysed. However, due to the length of the experiment (7 days) it was extremely difficult to differentiate the changes in 18:3 produced by the cold response from those originating during leaf maturation in all plant species analysed (Horiguchi et al., 1996; data not shown). In an attempt to simplify the current experimental system, the results obtained in plant leaves were compared with similar experiments performed on soybean photosynthetic cell suspension cultures, to separate the effect of developmental and/or tissue differentiation from that of cold response. Such photosynthetic cell suspensions provide a good model system since they behave similarly to young leaf mesophyll cells (Rogers et al., 1987). Furthermore, these cultures have been previously used as a model system to examine fatty acid synthesis and turnover in plant cells, finding similar amounts of phospholipids or galactolipids to those present in leaf cells (MacCarthy and Stumpf, 1980; Martin et al., 1984). The fatty acid composition of photosynthetic cell suspensions after 3 weeks of culture (early-stationary phase) and then exposed to 5 °C is shown in Fig. 3. The fatty acid composition of control cells was similar to that reported previously (Rogers et al., 1987; Collados et al., 2006) and to that obtained in young soybean trifoliate leaves (data not shown). Fatty acid 18:3 constitutes the most abundant fatty acid species, representing around 50% of the total (Fig. 3). Upon exposure to cold temperatures, the fatty acid composition did not change dramatically. A slight but reproducible increase in 18:1 was observed at 72h of cold exposure (Fig. 3). This increase was accompanied by a slight decrease in 18:3 levels (i.e., less than 5%). These results are in agreement with those obtained in similar photosynthetic cell suspensions in which desaturase activity was monitored in a range of temperatures from 15–35 °C using radioactive 14C- labelled fatty acids (MacCarthy and Stumpf, 1980). It is also noteworthy that the increase in 18:1 observed after 72h of cold treatment was similar to that observed in plants during the first 24h of low temperature exposure (Fig. 1). Finally, as occurred with plants cultivated in a growth chamber, re-exposure to the control temperature (24 °C) restored the fatty acid composition to standard values (Fig. 3).
Fig. 3.
Effect of exposure to cold temperatures on fatty acid composition of total lipids from soybean photosynthetic cell suspensions. Total lipids were extracted from cell suspensions kept at control temperatures (24 °C); 5 °C exposure for 24, 48, and 72h; and after recovery for 4 days at control temperature. Fatty acids were determined by gas chromatography (GC). White bars indicate fatty acids from control leaves; light-grey bars from 24h; medium-grey bars from 48h; dark-grey bars from 72h of cold treatment; and black bars after 4 days at control temperatures after cold exposure. Data are mean ± SD from three experiments. For the same fatty acid, different letters indicate significant differences among treatments (P < 0.05).
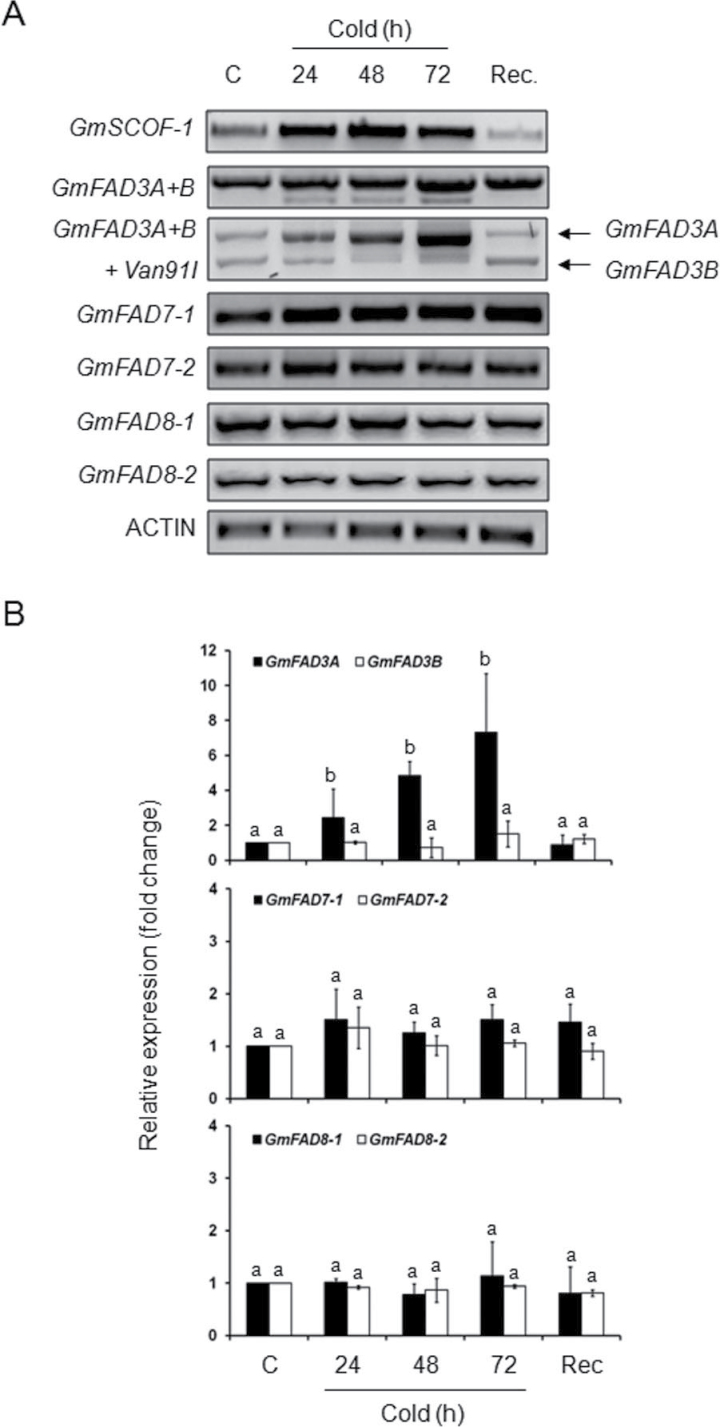
The expression of genes encoding omega-3 desaturases in response to cold in soybean photosynthetic cell suspensions was also monitored. The results are shown in Fig. 4. Expression of GmSCOF-1 was induced upon cold exposure, decreasing after replacement of the cell cultures in normal growth conditions. These results suggested that, as occurred in mature trifoliate leaves, the cold-induced response at the gene expression level was also activated in the photosynthetic suspension cultures. The expression of the GmFAD3 genes was then examined. No expression of GmFAD3C was detected in cell suspensions (data not shown) even at control temperature, so the analysis was focused on GmFAD3A and GmFAD3B. Exposure of photosynthetic cell suspensions to 5 °C produced an increase in the GmFAD3A + GmFAD3B transcripts (Fig. 4A and B). Digestion with Van91I, which allowed the GmFAD3A and GmFAD3B transcripts to be distinguished, showed a similar situation to what happened in mature leaves. A specific increase in the GmFAD3A transcript was detected in cell suspensions upon cold treatment, reverting when the cells were returned to control temperatures (Fig. 4A and B). This increase was accompanied by the accumulation of a smaller transcript that amplified with the GmFAD3A and B specific primers. The smaller transcript was not detected at control temperatures and disappeared upon recovery (Fig. 4A). This behaviour corresponded well to that detected in mature trifoliate leaves under similar temperature conditions (Fig. 2A). It is also worth mentioning that its accumulation seemed to be retarded in photosynthetic cell suspensions when compared with trifoliate leaves (Figs. 2A and 4A).
Fig. 4.
(A) Omega-3 fatty acid desaturase gene expression in soybean photosynthetic cultured cells kept at control temperature (24 °C); 5 °C exposure for 24, 48, and 72h; and after recovery for 4 days at control temperatures. GmSCOF-1 was used as an internal control for cold-induced expression. ACTIN was used as a housekeeping gene in all experiments. (B) Normalization of gene expression results against ACTIN. Data are mean ± SD from two experiments. For the same time point, different letters indicate significant differences among treatments (P < 0.05).
Transcripts from the plastidial omega-3 fatty acid desaturases (GmFAD7-1, GmFAD7-2, GmFAD8-1, and GmFAD8-2) did not show any noticeable changes upon cold exposure or recovery at control temperatures (Figs. 4A and 4B) in the photosynthetic cell suspension cultures.
Analysis of splice variants originating from the omega-3 desaturase genes in soybean
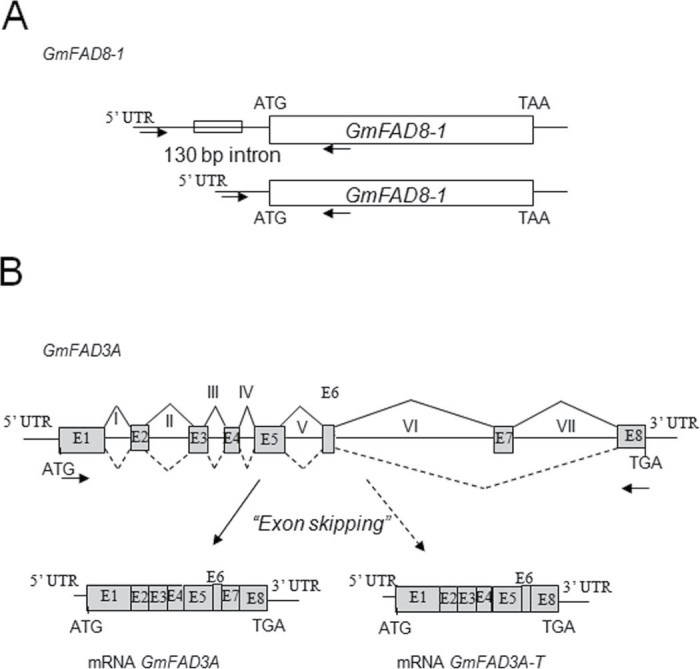
The additional transcript bands detected during the course of the expression analysis of the genes encoding omega-3 desaturases in response to low temperatures were also studied. To analyse their molecular origin in detail, these bands were excised from the agarose gels, purified, cloned in a pGEM-T-Easy vector, and sequenced. In the case of GmFAD8-1, analysis of the lower size band revealed that the only difference between the two bands was the processing of a small intron (130bp) present in the 5'-untranscribed region, 5bp upstream of the ATG of the GmFAD8-1 protein (Fig. 5A). The splicing of this intron might eliminate a canonical Shine-Dalgarno sequence located 7bp upstream of the ATG, suggesting that it could be related with control of the translation of the GmFAD8-1 mRNA. This splicing mechanism seemed to operate independently of temperature since the intron was detected both in control and cold-exposed samples.
Fig. 5.
Schematic diagram showing the proposed alternative splicing mechanisms observed during the expression analysis of GmFAD8-1 (A) and GmFAD3A (B). Boxes represent exons while introns are represented by lines and numbered in Roman numerals. The positions of the ATG and stop codons as well as the primers used for amplification are also shown.
The nature of the small band accumulating during cold exposure upon RT-PCR analysis of the GmFAD3A+B transcripts was also analysed. This small band (GmFAD3A-T) corresponded to a truncated form of the GmFAD3A transcript that presented a deletion of 138 nt eliminating 47 amino acid residues with respect to the mature GmFAD3A protein (Fig. 5B). The study compared the deduced sequences obtained from the analysis of the RT-PCR-amplified bands with the genomic sequence of GmFAD3A obtained from the soybean database. This analysis showed that the GmFAD3A-T transcript originated from an alternative splicing of GmFAD3A that eliminated exon 7, joining exon 6 with exon 8 (Fig. 5B) in a typical exon-skipping mechanism. These results indicate that cold induced an alternative splicing of GmFAD3A, producing a putative truncated form of the GmFAD3A protein. Two features were interesting in this GmFAD3A-T transcript. First, the deletion eliminated one of the three His boxes necessary for the enzymatic desaturase activity (Shanklin et al., 1994). Second, the C-terminus of the GmFAD3A-T form was identical to the mature GmFAD3A protein (Fig. 6), suggesting that all of the sequences necessary for membrane anchoring and insertion were present in GmFAD3A-T.
Fig. 6.
Protein sequence alignment of GmFAD3A and GmFAD3A-T. Black boxes indicate residues that are strictly identical, and dashes show the region that was eliminated in the truncated form after alternative splicing. The His boxes characteristic of the desaturase active site are underlined.
Effect of temperature on the linolenic acid content of S. cerevisiae cells overexpressing the soybean GmFAD3 genes
Yeast has been proven as a suitable heterologous expression system for studying the functionality of endoplasmic reticulum desaturases such as FAD3 (Dyer et al., 2001). In the presence of the appropriate substrates (18:2), the FAD3 enzymes expressed in yeast can obtain reducing power and electrons for the omega-3 desaturase activity (Dyer et al., 2001). Unfortunately, these studies are not suited for the analysis of plastid desaturases as they require electron transport chains from the chloroplast (Shanklin et al., 1994). The current study decided to take advantage of the yeast system to further analyse the functionality of GmFAD3A, GmFAD3B, GmFAD3C, and GmFAD3A-T as a function of temperature. To this end, these four isoforms of GmFAD3 were expressed in S. cerevisiae under the galactose-inducible yeast promoter of the pYES2 vector. The fatty acid analysis of transformed yeast cells revealed a high quantity of linoleic acid (18:2; Table 1 and Supplementary Fig. S2), which was not present in the wild-type yeast (data not shown), showing a correct uptake of the supplemented substrate. Table 1 shows the fatty acid compositions of yeast cells transformed with GmFAD3A, GmFAD3B, GmFAD3C, and GmFAD3A-T using the pYES2 vector. The fatty acid analysis of the GmFAD3A- and GmFAD3B-transformed yeast cells showed the presence of linolenic acid (18:3) that was present neither in wild-type yeast nor in cells transformed with the empty vector. The percentage of 18:3 obtained with the inducible pYES2 vector at normal yeast growth temperature (30 °C) was 3.8, 6.2, and 0.9% for GmFAD3A, GmFAD3B, and GmFAD3C, respectively (Table 1). The percentage of 18:3 obtained was consistent with similar data from the literature (Dyer et al., 2001; O’Quin et al., 2010). These results indicate that the expression of the three genes is functional, as they code for isoforms capable of desaturating exogenous substrate to produce the corresponding 18:3. Under the experimental conditions, the 18:3 content of yeast cells overexpressing GmFAD3B was slightly higher than that obtained for GmFAD3A at yeast growth temperature (Table 1). By contrast, in the case of GmFAD3C, it was always significantly lower than that obtained in yeast transformed with GmFAD3A or GmFAD3B under the same experimental conditions (Table 1). No production of 18:3 was detected in yeast cells transformed with GmFAD3A-T (Table 1), even though the 18:2 levels were similar to those from yeast cells transformed with GmFAD3A, GmFAD3B, or GmFAD3C, indicating that the absence of 18:3 was not due to the low availability of 18:2 as a substrate. Similar results were obtained with the pVT102-U vector, which carries a constitutive promoter (Vernet et al., 1987), except that lower 18:3 percentages were routinely obtained (data not shown).
Table 1.
Fatty acid composition of S. cerevesiae cells overexpressing soybean GmFAD3 proteins and grown at two different temperatures
| Plasmid | Temperature (ºC) | Fatty acid composition (mol%) | Conversion 18:2 to 18:3 (%) | |||||
| 16:0 | 16:1 | 18:0 | 18:1 | 18:2 | 18:3 | |||
| pYES2 | 30 | 20.6±1.1 | 4.1±0.0 | 6.4±0.3 | 2.3±0.2 | 66.3±1.7 | 0.0±0.0 | 0.0±0.0 |
| 15 | 19.5±1.4 | 5.2±2.8 | 7.3±0.1 | 3.3±2.1 | 64.7±6.3 | 0.0±0.0 | 0.0±0.0 | |
| pYES2-GmFAD3A | 30 | 19.8±1.0 | 5.2±0. | 7.3±0.4 | 3.1±0.2 | 60.7±1.8 | 3.8±0.3 | 5.9±0.5 |
| 15 | 19.5±0.2 | 4.8±0.2 | 8.2±0.3 | 2.9±0.1 | 39.9±1.7 | 24.8±1.8 | 38.8±2.7 | |
| pYES2-GmFAD3A-T | 30 | 18.6±1.1 | 4.2±1.5 | 7.3±1.0 | 2.4±1.1 | 67.2±3.3 | 0.0±0.0 | 0.0±0.0 |
| 15 | 17.4±1.8 | 4.1±0.6 | 7.2±0.2 | 2.4±0.6 | 68.9±3.0 | 0.0±0.0 | 0.0±0.0 | |
| pYES2-GmFAD3B | 30 | 20.2±1.0 | 5.2±1.2 | 7.4±0.2 | 3.0±0.9 | 58.0±1.9 | 6.2±0.9 | 9.7±1.5 |
| 15 | 19.4±1.2 | 5.4±0.4 | 7.7±0.2 | 3.6±0.5 | 34.3±4.9 | 29.6±2.7 | 46.4±5.9 | |
| pYES2-GmFAD3C | 30 | 18.6±0.4 | 6.2±1.1 | 7.7±1.6 | 5.0±1.1 | 61.6±3.9 | 0.9±0.3 | 1.4±0.3 |
| 15 | 16.4±1.2 | 4.7±0.7 | 7.1±0.3 | 3.8±0.5 | 64.0±2.5 | 4.1±0.7 | 6.0±1.2 | |
16:0, Palmitic acid; 16:1, palmitoleic acid; 18:0, stearic acid; 18:1, oleic acid; 18:2, linoleic acid; 18:3, linolenic acid. Data are mean ± SD from three independent experiments.
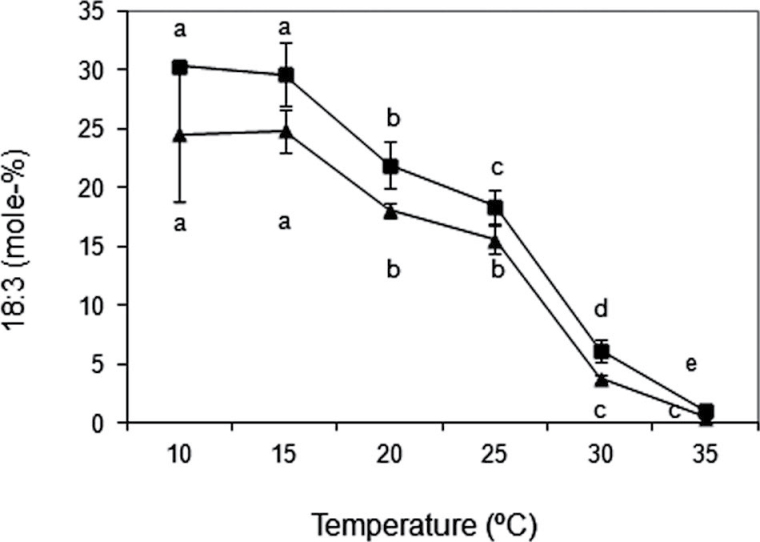
Next was studied the effect of growth temperature on the 18:3 content of S. cerevisiae cells overexpressing GmFAD3A, GmFAD3B, and GmFAD3C isoforms. The results are shown in Fig. 7 and Table 1. The growth temperature modified the 18:3 content in transformed yeast cells. This accumulation of fatty acyl lipid is possibly due to the low levels of β-oxidation displayed by S. cerevisiae cells in the presence of an appropriate carbon source (Veenhuis and Goodman, 1990). Yeast cells transformed with GmFAD3A or GmFAD3B showed the highest amount of linolenic acid at lower temperatures (10–15 °C), with percentages ranging between 25–30%, while at higher temperatures (30–35 °C) the percentage decreased to 4–6% (Table 1 and Fig. 7). Although the percentage of 18:3 production was slightly higher in GmFAD3B- than in GmFAD3A-transformed yeast cells (Fig. 7 and Table 1), the differences were not statistically significant, indicating that overall the ratio of 18:3 conversion in both types of transformed cells was similar independently of the temperature (Table 1). As occurred with GmFAD3A and GmFAD3B, the 18:3 content increased (4.1%) upon exposure of yeast cells transformed with pYES2-GmFAD3C to 15 °C. This result indicated that the 18:3 content of S. cerevisiae cells overexpressing GmFAD3C was also higher at lower temperatures, although this percentage was again lower than that detected for GmFAD3A and GmFAD3B. Finally, it is noteworthy that when similar experiments were performed with the truncated GmFAD3A-T form, no 18:3 was detected irrespective of the temperature, further suggesting that this truncated GmFAD3A-T mRNA, if translated, would give rise to an inactive omega-3 desaturase enzyme (Table 1).
Fig. 7.
Production of linolenic acid in S. cerevisiae cells overexpressing soybean GmFAD3A and GmFAD3B and grown at different temperatures. Yeast cultures harbouring the pYES-GmFAD3A (closed triangles) and pYES-GmFAD3B (closed squares) vectors were grown at the temperatures indicated. After reaching the stationary phase, yeast cells were harvested and the fatty acid composition was determined in whole cells usingGC/FID. Data are mean ± SD from three experiments. For each gene construct, different letters indicate significant differences among treatments (P < 0.05).
Discussion
This work analysed the behaviour of all the endoplasmic reticulum and plastidial omega-3 desaturases in soybean at the level of both fatty acid content and gene expression in order to determine how low temperature exposure affected the concerted contribution of each omega-3 desaturase to the synthesis of 18:3 in response to cold. Exposure of soybean plants to cold did not result in significant modifications of 18:3 in leaf membrane lipids. This result is consistent with previous observations in other plant species. Thus, in birch Martz et al. (2006) reported a 3% increase in 18:3 levels in galactolipids in response to cold. In soybean, Li et al. (2007) reported a 7% increase in 18:3 levels in total lipids from plants exposed to 8 °C for a week. More recently, Upchurch and Ramirez (2011) reported a 4% increase in 18:3 from total leaf lipids isolated from soybean plants exposed to a 20/16 °C day/nighttemperature for 72h. In fact, with the exception of Arabidopsis, where significant changes in the 10–15% range were reported (McConn et al., 1994; Falcone et al., 2004), the extent of 18:3 changes in response to cold seems to be rather small in other plant species, suggesting that the effect of cold temperatures on 18:3 is relatively slight and could be limited to specific plant species, tissues, or growth processes (Iba, 2002). The higher 18:3 content present in soybeans (65–70%) when compared with Arabidopsis (40%) may account for these differences.
Despite the small changes in 18:3 levels, the data showed specific changes at the level of the expression of genes encoding omega-3 desaturases in response to cold in soybean. The data suggest the existence of changes in the regulatory mechanism of omega-3 fatty acid desaturases affecting specific isoforms in both cell compartments to maintain appropriate levels of 18:3 under low temperature conditions. Thus, with respect to the plastidial desaturases, this study detected a rapid transient activation of GmFAD7-2 in response to cold that was only present in mature trifoliate leaves but not in photosynthetic cell suspensions. This increase preceded the small changes observed in the 18:3 content. Interestingly, it has been previously reported that the GmFAD7-1 isoform seemed to be more sensitive to the wound response than GmFAD7-2 (Andreu et al., 2010). These results together might suggest a certain degree of specialization among GmFAD7 isoforms, with a specific role for the GmFAD7-2 isoform in the cold response. It has generally been inferred from the results obtained in Arabidopsis that the increase in 18:3 observed as a response to low temperatures was due to FAD8 induction. Thus, Gibson et al. (1994) identified the FAD8 locus in a fad3/fad7 double mutant from Arabidopsis that was capable of producing TAs only at cold temperatures. Low temperatures seem to induce FAD8 mRNA in Arabidopsis (Gibson et al., 1994), maize (Berberich et al., 1998), rice (Wang et al., 2006), and birch (Martz et al., 2006). The current data in soybean showed high levels of both GmFAD8 transcripts even at control temperatures (i.e., unlike Arabidopsis, maize, rice, or birch) with no apparent changes upon cold exposure. This result suggests that, if there is a specific effect of cold temperatures on the GmFAD8 genes, it is not at the transcriptional level. In this sense, a post-translational regulatory mechanism acting on the stability of the AtFAD8 protein in response to temperature has been described in Arabidopsis (Matsuda et al., 2005). Given the results presented in this paper, and in the absence of data on specific protein or enzyme activity, the existence of additional control points controlling the amount and activity of GmFAD8 proteins in response to cold remains to be elucidated.
The current data show that in soybean leaves, the expression of endoplasmic reticulum omega-3 desaturases is also tightly regulated in response to cold temperatures. An increase in GmFAD3A transcripts was detected both in mature leaves and cell suspensions. These results were consistent with previous observations for BnFAD3 (Tasseva et al., 2004). The higher activity levels (Fig. 7 and Table 1) observed for GmFAD3A and GmFAD3B compared to GmFAD3C in transformed yeast indicate that these two genes/isoforms might contribute to the 18:3 content to a greater extent than GmFAD3C. As occurred with the plastidial GmFAD7-2, the selection towards the GmFAD3A isoform in response to cold might suggest a more specific role for this GmFAD3 isoform in these conditions. However, the yeast expression experiments showed no significant differences in activity at a low temperature between the GmFAD3A and B isoforms, suggesting that this exchange was not related with higher activity of GmFAD3A at a low temperature. It cannot be ruled out that in its natural environment this could be the case. However, other factors differentially regulating the expression of GmFAD3A in response to cold might account for the different behaviour of these two GmFAD3 isoforms. Another important point that can be inferred from the data on transformed yeast expression is the high GmFAD3 omega-3 desaturase activity detected in yeast at low temperatures (Fig. 7 and Table 1). These values are consistent with those previously reported in other plant species (Dyer et al., 2001; O’Quin et al., 2010), and are also consistent with the highest percentage of 18:3 reported in wheat root tips exposed to cold (Horiguchi et al., 2000). Endoplasmic reticulum enzymes have been shown to be the major contributors to root linolenic acid levels (Yadav et al., 1993). All these results suggest that the role of FAD3 and the endoplasmic reticulum membranes in the cold response cannot be precluded, even in leaves.
Finally, another interesting question emerging from these data is the involvement of alternative splicing mechanisms in the regulation of the expression of omega-3 desaturases in soybean. One of the spliced variants seemed to be cold specific (GmFAD3A), while that derived from GmFAD8-1 was not related with temperature. The existence of an intron in the 5'-untranscribed region strongly suggests a role in the translation of the GmFAD8-1 protein that could be more closely related with the relative abundance of GmFAD8 isoforms. By contrast, this study detected an alternative spliced form of GmFAD3A that gave rise to a putative truncated form of the GmFAD3A protein that was specifically accumulated upon cold exposure of soybean plants. It is worth noting that a similar truncated form of AtFAD3 was also found in the databases (accession NM179808), suggesting that this alternative splicing is not a unique feature of soybean. Although the specific role of these spliced variants is still far from being understood, the list of the alternatively spliced genes associated with abiotic stress responses is rapidly expanding (Reddy, 2007). The putative truncated form of GmFAD3A proved to be inactive in yeast, consistent with the loss of one of the three His boxes that have been reported to be essential for the desaturation activity (Shanklin et al., 1994). It is tempting to speculate on other functions more closely related with gene regulation, as for example acting as regulatory RNAs in order to control gene expression by different means.
In conclusion, these data show that in soybean there is a cold-specific response by omega-3 desaturases at least at the transcriptional level, involving both endoplasmic reticulum (GmFAD3A) and plastidial (GmFAD7-2) omega-3 desaturases, in order to maintain appropriate 18:3 levels in membrane lipids. Given this coordinated expression of these omega-3 desaturase genes and their different subcellular localization, the data highlight the relevance of the mechanisms of lipid exchange between membranes in these acclimation responses in plants.
Supplementary Material
Supplementary material
Supplementary data are available at JXB online.
Supplementary Table S1. Gene specific primers used in this study
Supplementary Fig. S1. Tissue-specific expression of GmFAD8-1 and GmFAD8-2 genes in soybean plants
Supplementary Fig. S2. GC-FID chromatograms showing the fatty acid profile obtained in yeast transformants containing the different constructions corresponding to each of the GmFAD3 isoforms
Acknowledgements
The authors wish to thank Soledad Gracia, of the Laboratorio Medioambiental (Aragón Government) for fatty acid analysis and Victoria López (EEAD-CSIC) for her invaluable help with the statistical analysis. This work was supported by the Aragón Government (PIP 140/2008), the Andalusian Government (P09-AGR-4516), and the Spanish Ministry of Science and Innovation (AGL2008-00377 and AGL2008-00258). AR and BL are recipients of a fellowship from the JAE-Predoctoral CSIC Program. VA was the recipient of a predoctoral fellowship from the Aragón Government. LH was the recipient of a contract from the JAE-Postdoctoral CSIC Program.
References
- Anai T , Yamada T , Kinoshita T , Rahman SM , Takagi Y . 2005. Identification of corresponding genes for three low-α-linolenic acid mutants and elucidation of their contribution to fatty acid biosynthesis in soybean seed Plant Science 168 1615–1623 [Google Scholar]
- Andreu V , Lagunas B , Collados R , Picorel R , Alfonso M . 2010. The GmFAD7 gene family from soybean: identification of novel genes and tissue-specific conformations of the FAD7 enzyme envolved in desaturase activity Journal of Experimental Botany 61 3371–3384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ausubel FM , Brent R , Kingston RE , Moore DD , Seidman JG , Smith JA , Struhl K , Albright LM , Coen DM , Varki A . Current protocols in molecular biology. John Wiley and Sons; New York, USA: 1995. [Google Scholar]
- Berberich T , Harada M , Sugawara K , Kodama H , Iba K , Kusano T . 1998. Two maize genes encoding ω-3 fatty-acid desaturase and their differential expression to temperature Plant Molecular Biology 36 297–306 [DOI] [PubMed] [Google Scholar]
- Bilyeu KD , Palavalli L , Sleper DA , Beuselinck PR . 2003. Three microsomal desaturase genes contribute to soybean linolenic acid levels Crop Science 43 1833–1838 [Google Scholar]
- Bligh EG , Dyer WS . 1959. A rapid method of total lipid extraction and purification Canadian Journal of Biochemistry and Physiology 37 911–917 [DOI] [PubMed] [Google Scholar]
- Chi X , Yang Q , Lu Y , Wang J , Zhang Q , Pan L , Chen M , He Y , Yu S . 2011. Genome-wide analysis of fatty acid desaturases in soybean (Glycine max) Plant Molecular Biology Reports 29 769–783 [Google Scholar]
- Collados R , Andreu V , Picorel R , Alfonso M . 2006. A light-sensitive mechanism differently regulates transcription and transcript stability of ω3 fatty-acid desaturases (FAD3, FAD7 and FAD8) in soybean photosynthetic cell suspensions FEBS Letters 580 4934–4940 [DOI] [PubMed] [Google Scholar]
- Dyer JM , Chapital DC , Cary JW , Pepperman AB . 2001. Chilling-sensitive, post-transcriptional regulation of a plant fatty acid desaturase expression in yeast Biochemical and Biophysical Research Communications 282 1019–1025 [DOI] [PubMed] [Google Scholar]
- Falcone DL , Ogas JP , Somerville C . 2004. Regulation of membrane fatty acid composition by temperature in mutants of Arabidopsis with alterations in membrane lipid composition BMC Plant Biology 4 17–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcés R , Mancha M . 1993. One-step lipid extraction and fatty acid methyl esters preparation from fresh plant tissues Analytical Biochemistry 211 139–143 [DOI] [PubMed] [Google Scholar]
- Gietz RD , Woods RA . 1994. High-efficiency transformation in yeast. In: Johnson JA, ed. Molecular genetics of yeast: practical approaches Oxford University Press, New York: pp 121–134 [Google Scholar]
- Gibson S , Arondel V , Iba K , Somerville C . 1994. Cloning of a temperature-regulated gene encoding a chloroplast ω-3 desaturase from Arabidopsis thaliana Plant Physiology 106 1615–1621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gushina IA , Harwood JL . 2006. Mechanisms of temperature adaptation in poikilotherms FEBS Letters 580 5477–5483 [DOI] [PubMed] [Google Scholar]
- Heppard EP , Kinney AJ , Stecca KL , Miao G . 1996. Developmental and growth temperature regulation of different microsomal ω-6 desaturase genes in soybeans Plant Physiology 110 311–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiguchi G , Fuse T , Kawakami N , Kodama H , Iba K . 2000. Temperature-dependent translational regulation of the ER omega-3 fatty acid desaturase gene in wheat root tips The Plant Journal 24 805–813 [DOI] [PubMed] [Google Scholar]
- Horiguchi G , Kodama H , Nishimura M , Iba K . 1996. Role of ω-3 fatty-acid desaturases in the regulation of the level of trienoic fatty acids during leaf cell maturation Planta 199 439–442 [Google Scholar]
- Iba K . 2002. Acclimative response to temperature stress in higher plants: approaches of gene engineering for temperature tolerance Annual Review of Plant Biology 53 225–245 [DOI] [PubMed] [Google Scholar]
- Iba K , Gibson S , Nishiuchi T , Fuse T , Nishimura M , Arondel V , Hugly S , Somerville C . 1993. A gene encoding a chloroplast omega-3 fatty acid desaturase complements alterations in fatty acid desaturation and chloroplast copy number of the fad7 mutant of Arabidopsis thaliana Journal of Biological Chemistry 268 24099–24105 [PubMed] [Google Scholar]
- Kargiotidou A , Deli D , Galanopolou D , Tsaftaris A , Farmaki T . 2008. Low temperature and light regulate delta 12 fatty acid desaturases (FAD2) at a transcriptional level in cotton (Gossypium hirsutum) Journal of Experimental Botany 59 2043–2056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JC , Lee SH , Chong YM , et al. 2001. A novel cold-inducible zinc finger protein from soybean, SCOF-1, enhances cold tolerance in transgenic plants The Plant Journal 25 247–259 [DOI] [PubMed] [Google Scholar]
- Li L , Wang X , Gai J , Yu D . 2007. Molecular cloning and characterization of a novel microsomal oleate desaturase gene from soybean Journal of Plant Physiology 164 1516–1526 [DOI] [PubMed] [Google Scholar]
- MacCarthy JJ , Stumpf PK . 1980. The effect of different temperatures on fatty-acid synthesis and polyunsaturation in cell suspension cultures Planta 147 389–395 [DOI] [PubMed] [Google Scholar]
- Martin BA , Horn MF , Widholm JM , Rinne R.W . 1984. Synthesis, composition and location of glycerolipids in photoautotrophic soybean cell cultures Biochimica et biophysica Acta 796 146–154 [Google Scholar]
- Martz F , Kiviniemi S , Plava TE , Sutinen M.L . 2006. Contribution of omega-3 fatty acid desaturase and 3-ketoacyl-ACP synthase II (KASII) genes in the modulation of glycerolipid fatty acid composition during cold acclimation in birch leaves Journal of Experimental Botany 57 897–909 [DOI] [PubMed] [Google Scholar]
- Matsuda O , Sakamoto H , Hashimoto T , Iba K . 2005. A temperature-sensitive mechanism that regulates post-translational stability of a plastidial ω-3 fatty-acid desaturase (FAD8) in Arabidopsis leaf tissues Journal of Biological Chemistry 280 3597–3604 [DOI] [PubMed] [Google Scholar]
- McConn M , Hugly S , Browse J , Somerville C . 1994. A mutation at the fad8 locus of Arabidopsis identifies a second chloroplast ω-3 desaturase Plant Physiology 106 1609–1614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata N , Ishizaki-Nishizawa O , Higashi S , Hayashi H , Tasaka Y , Nishida I . 1992. Genetically engineered alteration in the chilling sensitivity of plants Nature 356 710–713 [Google Scholar]
- Murata N , Sato N , Takahashi N , Hamazaki Y . 1982. Compositions and positional distributions of fatty acids in phospholipids from leaves of chilling-sensitive and chilling-resistant plants Plant Cell Physiology 23: 1071–1079 [Google Scholar]
- Nishida I , Murata N . 1996. Chilling sensitivity in plants and cyanobacteria: the crucial contribution of membrane lipids Annual Review of Plant Physiology and Plant Molecular Biology 47 541–568 [DOI] [PubMed] [Google Scholar]
- O’Quin JB , Bourassa L , Zhang D , Shockey JM , Gidda SK , Fosnot S , Chapman KD , Mullen RT , Dyer JM . 2010. Temperature-sensitive post-translational regulation of plant omega-3 fatty acid desaturases is mediated by the endoplasmic reticulum-associated degradation pathway Journal of Biological Chemistry 285 21781–21796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy ASN . 2007. Alternative splicing of pre-messenger RNAs in plants in the genomic era Annual Review of Plant Biology 58 267–294 [DOI] [PubMed] [Google Scholar]
- Rennie BD , Tanner JW . 1989. Fatty acid composition of oil from soybean seeds grown at extreme temperatures Journal of American Oil Chemistry Society 66 1622–1624 [Google Scholar]
- Rogers SMD , Ogren WL , Widholm JM . 1987. Photosynthetic characteristics of a photoautotrophic cell suspension culture of soybean Plant Physiology 84 1451–1456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanklin J , Whittle E , Fox BG . 1994. Eight histidine residues are catalytically essential in a membrane-associated iron enzyme, stearoyl-CoA desaturase, and are conserved in alkane hydroxylase and xylene monooxygenase Biochemistry 33 12787–12794 [DOI] [PubMed] [Google Scholar]
- Tang GQ , Novitzky WP , Griffin HC , Huber SC , Dewey RE . 2005. Oleate desaturase enzymes of soybean: evidence of regulation through differential stability and phosphorylation The Plant Journal 44 433–446 [DOI] [PubMed] [Google Scholar]
- Tasseva G , de Virville JD , Cantrel C , Moreau F , Zachowski A . 2004. Changes in endoplasmic reticulum lipid properties in response to low temperature in Brassica napus Biochimica et Biophysica Acta 42 811–822 [DOI] [PubMed] [Google Scholar]
- Tomashow MF . 1999. Plant cold acclimation: freezing tolerance genes and regulatory mechanisms Annual Review of Plant Physiology and Plant Molecular Biology 50 571–599 [DOI] [PubMed] [Google Scholar]
- Upchurch RG , Ramirez ME . 2011. Soybean plastidial omega-3 fatty acid desaturase genes GmFAD7 and GmFAD8: structure and expression Crop Science 51 1673–1682 [Google Scholar]
- Veenhuis M , Goodman J . 1990. Peroxisomal assembly: membrane proliferation precedes the induction of abundant matrix proteins in the methylotrophic yeast Candida boidinii Journal of Cell Science 96 583–590 [DOI] [PubMed] [Google Scholar]
- Vernet T , Dignard D , Thomas DY . 1987. A family of yeast expression vector genes containing the phage f1 intergenic region Gene 52 225–233 [DOI] [PubMed] [Google Scholar]
- Wang J , Ming F , Pittman J , Han Y , Hu J , Guo B , Shen D . 2006. Characterization of rice (Oryza sativa L.) gene encoding a temperature-dependent chloroplast ω-3 fatty acid desaturase Biochemical and Biophysical Research Communications 340 1209–1216 [DOI] [PubMed] [Google Scholar]
- Yadav NS , Wierzbicki A , Aegerter M , et al. 1993. Cloning of higher plant ω3 fatty-acid desaturases Plant Physiology 103 467–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.