Abstract
In contrast to climacteric fruits, where ethylene is known to be pivotal, the regulation of ripening in non-climacteric fruits is not well understood. In the non-climacteric strawberry (Fragaria anannassa), auxin and abscisic acid (ABA) are thought to be important, but the roles of other hormones suggested to be involved in fruit development and ripening are not clear. Here changes in the levels of indole-3-acetic acid (IAA), ABA, GA1, and castasterone from anthesis to fully ripened fruit are reported. The levels of IAA and GA1 rise early in fruit development before dropping to low levels prior to colour accumulation. Castasterone levels are highest at anthesis and drop to very low levels well before ripening commences, suggesting that brassinosteroids do not play an important role in ripening in strawberry. ABA levels are low at anthesis and gradually rise through development and ripening. The synthetic auxin, 1-naphthaleneacetic acid (NAA), can delay ripening, but the application of GA3, the gibberellin biosythesis inhibitor paclobutrazol, and ABA had no significant effect. IAA and ABA levels are higher in the developing achenes than in the receptacle tissue and may be important for receptacle enlargement and ripening, and seed maturation, respectively. Contrary to a recent report, the biologically active GA4 was not detected. The pattern of changes in the levels of the hormones are different from those reported in another well studied non-climateric fruit, grape, suggesting that a single consistent pattern of hormone changes does not occur in this group of fruit during ripening.
Key words: Abscisic acid, auxin, brassinosteroids, gibberellins, ripening, strawberry.
Introduction
Fruit ripening involves dramatic changes in the colour, texture, flavour, and aroma of fleshy fruits. Many characteristics of ripe fruits are highly attractive to humans and, as a consequence, fresh and processed fruits form an integral component of the human diet, providing sugars, fibre, vitamins, minerals, and antioxidants (Barry et al., 2005). Both the palatability and nutritional quality of fruit are highly dependent on its consumption at an optimum stage of ripeness. However, ripe fleshy fruits are also perishable commodities, and this presents problems for fruit production, harvesting, storage, and marketing. Solutions to these problems can only come through a better understanding of the way in which plants regulate and control fruit ripening. This knowledge will enhance our ability to control the ripening process, leading to improved procedures for the production and post-harvest handling of fruits.
The general ripening programmes displayed by most fleshy fruits typically include: (i) modification of colour through the alteration of chlorophyll, carotenoid, and/or anthocyanin accumulation; (ii) modification of texture via alteration of cell turgor and cell wall structure and/or metabolism; (iii) import/accumulation and modification of sugars, acids, and volatiles that affect nutritional quality, flavour, and aroma; and (iv) enhanced susceptibility to pathogens and herbivores (Giovannoni, 2004). These changes in colour, flavour, texture, and aroma make fruit ripening a complex process, which must be very tightly regulated. Key questions relate to how these changes are initiated, controlled, and coordinated (Tucker, 1993; Giovannoni, 2001). Whilst we do not yet have complete answers to these questions, it is clear that plant hormones play important roles in both climacteric and non-climacteric fruits (Tucker, 1993; Giovannoni, 2004).
Fruit species are categorized as either climacteric or non-climacteric, based on physiological differences in their ripening patterns. Climacteric fruits, which include tomato, banana, mango, apple, and avocado, display a well-characterized peak in ethylene production and respiratory activity at the onset of ripening (Seymour et al., 1993; Tucker, 1993; Adams-Phillips et al., 2004; Zaharah et al., 2011). This burst in ethylene production stems from an up-regulation of ethylene biosynthesis genes at the onset of ripening, resulting in autocatalytic ethylene production and an up-regulation of the components of ethylene perception and signalling (Klee and Clark, 2004). These changes act as a key signal for the initiation and coordination of ripening in all climacteric fruits, and have been shown to regulate key genes that control colour change, fruit softening, cell wall breakdown, pathogen defence, and nutrient composition (Hobson and Grierson, 1993; Tucker, 1993; Alexander and Grierson, 2002;Giovannoni, 2004). Blocking ethylene synthesis or action prevents ripening and the associated increase in respiration, and this has been used to regulate ripening in commercially important climacteric species such as tomato and banana (Klee and Clark, 2004). In this case, when ripe fruits are needed, unripe fruits are simply treated with ethylene (Klee and Clark, 2004).
In contrast to climacteric fruits, considerably less is known about the hormonal control of ripening in non-climacteric fruits such as citrus, grape, and strawberry (Seymour et al., 1993; Adams-Phillips et al., 2004). By definition there is no major peak in ethylene levels or respiration during ripening of non-climacteric fruit (Seymour et al., 1993), although more recent evidence of a transient increase in endogenous ethylene levels prior to veraison in grape does not rule out that ethylene may play some role during grape berry development (Chervin et al., 2004). A key unresolved question regarding the non-climacteric fruits is whether this group shares a common ripening mechanism (analogous to the role of ethylene in climacteric fruits) and, if so, what is this mechanism, and which, if any, of the known hormone(s) are involved. There is some evidence implicating auxins, gibberellins (GAs), abscisic acid (ABA), and brassinosteroids (BRs), but much of this evidence was obtained prior to the advent of reliable hormone quantification techniques, or relies solely on exogenous hormone application studies.
Auxin, the first plant hormone identified, may act as an inhibitor of ripening in some non-climacteric fruits (Davies et al., 1997; Trainotti et al., 2005). In strawberry, it appears that auxin from the externally located achenes (seeds) inhibits the ripening of the fleshy receptacle (Given et al., 1988; Manning, 1993). It is thought that as the fruit develops the level of auxin in the achenes, and consequently in the receptacle, falls below a critical level, thus permitting ripening (Manning, 1993). Therefore, removing the achenes promotes ripening, while treating strawberries with synthetic auxins delays ripening (Given et al., 1988; Manning, 1994). Other evidence that auxin inhibits ripening has been obtained from grape. In this case a synthetic auxin-like compound, benzothiazole-2-oxyacetic acid, delays both ripening and the associated increase in endogenous ABA levels (Davies et al., 1997), suggesting that auxin and ABA may interact to control ripening. There is also an increase in endogenous ABA levels associated with the onset of ripening (Davies et al., 1997), and ABA applications can enhance the coloration of grape berries (Ban et al., 2003), while treatments that delay the increase in endogenous ABA levels also delay ripening (Davies et al., 1997). Recent reports using both molecular and applicationtechniques have also provided strong evidence for a role for ABA in strawberry fruit ripening (Chai et al., 2011; Jia et al., 2011).A recent report has also suggested an important role for GAs in the development of the strawberry receptacle, with extremely high levels of GA4 being reported (Csukasi et al., 2011).
The BRs have also been implicated in the regulation of grape ripening (Symons et al., 2006). The BRs are a group of steroidal plant hormones that are necessary for normal plant development (Davies, 2004) whose bioactive levels rise dramatically as grape berry ripening begins (Symons et al., 2006). Application of BRs to grape berries also promoted ripening, whereas applying an inhibitor of BR biosynthesis delayed this process. These results provide clear evidence that changes in endogenous BR levels strongly influence ripening in this non-climateric fruit, and raise the question of whether BRs may be an important signal in the ripening of other non-climacteric fruits.
Strawberry has emerged as the most tractable non-climacteric model system (Giovannoni, 2001). Its small size, ease of propagation, short vegetative stage, and rapid fruit development (~30 d from flowering to fruit ripeness) all make strawberry a useful system to study ripening (Perkins-Veazie, 1995). The various stages of strawberry ripening are well characterized (Manning, 1993; Perkins-Veazie, 1995) and numerous ripening-related genes that affect cell wall metabolism, colour, and aroma have been characterized (Giovannoni, 2004). Yet the hormone profiles of the ripening fruits have not been characterized unequivocally. In this study strawberry was therefore used to see how the levels of a number of plant hormones including indole-3-acetic acid (IAA), GA1, ABA, castasterone (CS), and brassinolide (BL) change during ripening and to compare these changes with those reported in the literature for other non-climateric fruit such as grape. Further, application studies were used to see how a range of hormones influence ripening and whether such applications are consistent with the changes in the endogenous levels of these hormones.
Materials and methods
Plant material
For application studies and to monitor hormone levels during fruit development, 1-year-old, bare-rooted strawberry plants (Fragaria×ananassa Duch. cv. Red Gauntlet) were planted in 14cm diameter plastic pots in commercial potting mix (consisting of 8 parts composted fine pine bark and 3 parts coarse river sand with added macro- and micronutrients). Plants were grown in a glasshouse under an 18h photoperiod (consisting of natural daylight extended morning and evening by high pressure sodium lighting, delivering a light intensity of ~100 µmol m–2 s–1 at the pot surface), and were watered daily and fertilized weekly with liquid Aquasol. Glasshouse temperatures ranged from 19–22 °C during the day to 12–14 °C at night during the course of the experiments.
Strawberry fruits for hormone analysis were selected at seven developmental stages: FL, flowering/anthesis; SG, green receptacle; LG, green receptacle with enlarged achenes; SW, small white receptacleand green achenes; LW, large white receptacle with brown achenes; P, colour starting to appear on the receptacle; and R, ripe red receptacle (Fig. 1). The strawberry fruits were harvested, weighed, and placed in a volume of cold (–20 ºC) 80% methanol that was just sufficient to immerse the harvested tissue completely.
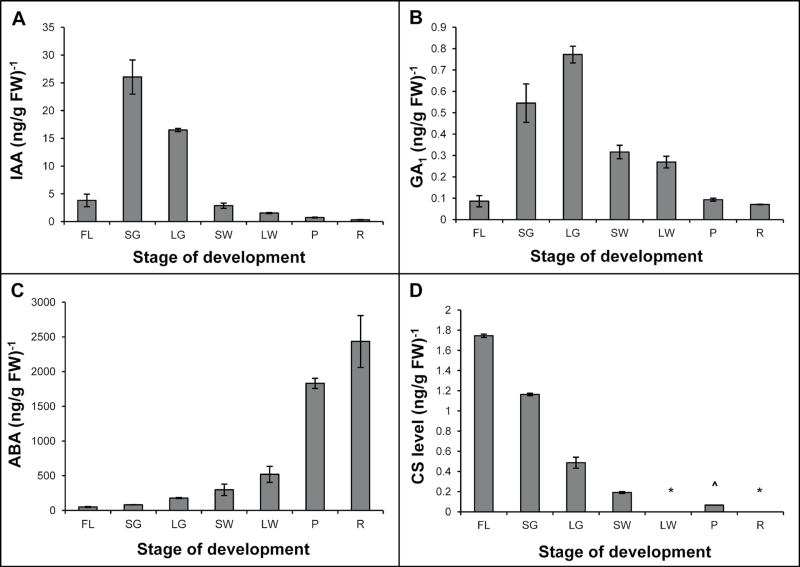
Fig. 1.
(A) The pattern of strawberry fruit development as measured by changes in diameter and fresh weight. (B) The various stages of fruit development: FL, flower; SG, small green; LG, large green; SW, small white; LW, large white; P, pink/turning; R, red. (C) Diagrammatic representation of the changes in hormone levels at the different stages of fruit development based on the results in Fig. 2.
To examine the distribution of hormones between the receptacle and achenes at the white stage of fruit development and to analyse for GA4, strawberries were harvested from the same plants, grown after transfer to a garden in Launceston, Tasmania and harvested from 18 to 23 December 2011.
Changes in ABA, IAA, and GA1 levels during strawberry development
Tissue samples, consisting of both the achenes and receptacles, were homogenized and hormones were extracted with 80% (v/v) methanol at 4 ºC for 12h. The extract was then filtered through a Whatman No. 1 filter paper and an appropriate amount of [13C6]IAA (Cambridge Isotope Laboratories), [2H6]ABA (supplied by Dr S. Abrams, University of Saskatchewan), and [2H2]GA1 and [2H2]GA3 (synthesized by Professor L.N. Mander, Australian National University, Canberra, Australia) internal standards were added. The samples were then concentrated to ~1ml under vacuum at 35 ºC, taken up in 3×3ml of 0.4% (v/v) acetic acid in distilled water, and loaded onto a Sep-Pak C18 cartridge (pre-conditioned first with 15ml of 100% methanol followed by 15ml of 0.4% acetic acid in distilled water). The hormones were eluted from the Sep-Pak with 70% (v/v) methanol in 0.4% (v/v) acetic acid in distilled water. The eluates were then dried under vacuum at 35 ºC, and taken up in 2ml of 20% (v/v) methanol in 0.4% acetic acid in distilled water.
IAA and GA samples were then fractionated using a reverse-phase C18 high-performance liquid chromatography (HPLC) system (Waters Associates, Milford, MA, USA). The system consisted of two M-45 Solvent Delivery Systems, a Rheodyne 7725i Manual Injector fitted with a 2ml sample loading loop, a Model 660 solvent programmer, a Z-Model Radial Compression Separation System, and a 10 cm×8mm i.d., 10 µm Radial-Pak C18 cartridge. The solvent program ran from 20% to 75% methanol in 0.4% aqueous acetic acid for 25min with the solvent programmer set on a linear gradient with the flow rate maintained at 2ml min–1. Extracts were passed through a 0.45 µm filter prior to loading. HPLC fractions matching the retention times of IAA and GAs were pooled and dried under vacuum at 35 ºC. The ABA samples were dried without HPLC. The dried samples were then taken up in 200 µl of 100% methanol, methylated with 750 µl of ethereal diazomethane, and dried under a stream of nitrogen. To each sample was added 1ml of distilled water and 400 µl of diethyl ether. The ether fraction was then dried under a stream of nitrogen. ABA was analysed by gas chromatography–mass spectrometry–selected ion monitoring (GC-MS-SIM) as the methyl ester. The IAA and GA1 methyl esters were silylated by adding 10 µl of dry pyridine and 40 µl of N,O-bis(trimethylsilyl)trifluoroacetamide (BSTFA), and heating at 80 ºC for 30min. The samples were then dried and another 15 µl of BSTFA were added before being incubated again at 80 ºC for 30min. The samples were dried and 40 µl of chloroform was added. The analysis of the IAA and ABA samples was performed by GC-MS using tandem MS on a triple quadrupole mass spectrometer (Varian 3800 GC coupled to a Varian 1200 triple quadrupole MS, a Varian 8400 autosampler, and a Varian 1177 injector; Varian Analytical Instruments, CA, USA). The injector was held at 250 ºC in splitless mode and the column used was a Varian Factor Four VF-5ms (30 m×0.25mm i.d., 0.25 µm film thickness). Helium was used as the carrier gas, maintained at a constant flow rate of 1.4ml min–1. The oven temperature was held at 50 ºC for 2min, then ramped to 190 ºC at increments of 30 ºC min–1, then to 270 ºC at increments of 10 ºC min–1, and held at 270 ºC for 5min. MS was operated in selected reaction monitoring mode, with the peak width at 0.9 m/z units, the collision energy at –12eV and –14eV for ABA and IAA, respectively, and with the collision gas argon at 1 mTorr. The ions monitored for endogenous ABA were m/z 134, 162, and 190, and for [2H4]ABA were m/z 138, 166, and 194. The ions monitored for endogenous IAA were m/z 202 and 261, and for [13C6]IAA were m/z 208 and 267.
GAs were analysed by GC-MS using a Hewlett-Packard 5890 GC coupled to a Kratos Concept ISQ mass spectrometer, according to the methods described previously (Hasan et al., 1994). A Hewlett Packard HP1 column (25 m×0.32mm i.d., 0.17 µm film thickness) was used. The carrier gas used was helium maintained at a flow rate of 2ml min–1 with a column head pressure of 15 psi. A volume of 1 µl was injected and the oven temperature was programmed from 60 ºC to 230 ºC at 30 ºC min–1, and then raised to 265 ºC at 3 ºC min–1. To eliminate all impurities, the GC column was taken from 265 ºC to 290 ºC after each run. GA levels were evaluated on the basis of retention times andby monitoring selected pairs of ions characteristic for the hormone.The addition of internal standards resulted in a small contribution of incomplete labelled standards to the endogenous ion peak (Lawrence et al., 1992). Therefore, to compensate for this, pre-determined correction factors were applied when calculating hormone levels.
GA4, ABA, and IAA levels in achenes and receptacles at the white stage of development
Levels of GA4, ABA, and IAA in receptacles and achenes (combined white stages only) were analysed by ultra-performance liquid chromatography–mass spectrometry (UPLC-MS). The two tissue types were separated using a razor blade while the fruits were frozen. The initial extraction and purification procedures were as before, except that hormones were eluted from the Sep-Pak with 50% (v/v) methanol in 0.4% (v/v) acetic acid in distilled water, after a rinse of 10% (v/v) methanol in 0.4% (v/v) acetic acid in distilled water. Eluates were then dried under vacuum at 35 ºC, and the samples for analysis of ABA and IAA were re-suspended in 100 µl of 1% (v/v) acetic acid in distilled water, centrifuged at 13 000rpm for 3min, and analysed directly by UPLC-MS.
Extracts for GA4 analysis were derivatized to improve sensitivity, using a bromocholine-based method adapted from Kojima et al. (2009). Samples were suspended in 10 µl of di-isopropanol ethyl amine and 50 µl of 500mM (2-bromoethyl)triethylammonium bromide in 90% (v/v) acetonitrile in distilled water, and held at 80 ºC for 2h. The samples were then dried under nitrogen gas, resuspended in 100 µl of 1% (v/v) acetic acid, and centrifuged as described above.
Extracts were analysed using a Waters Acquity H-series UPLC coupled to a Waters Xevo triple quadrupole mass spectrometer. A Waters Acquity UPLC BEH C18 column (2.1×100 mm×1.7 µM particles) was used, with mobile phases A = 1% acetic acid (v/v) in water and B = acetonitrile. The flow rate was 0.35ml min–1, and the column was held at 45 °C. For derivatized GA4, the program was 100% A for 0.5min, then a linear gradient to 85% A; 15% B over the next 1.5min, followed by a linear gradient to 30% A; 70% B over the next 5min. For ABA, the program was 80% A; 20% B to 5% A; 95% B over5min, while for IAA, it was 80% A; 20% B to 50% A;50% B over 4.5min. All programs terminated with immediate re-equilibration to starting conditions for 3min.
MS was conducted in the electrospray and multiple reaction monitoring (MRM) modes, monitoring positive ions for IAA and derivatized GA4, and negative ions for ABA. The ion source temperature was 130 °C, the desolvation gas was nitrogen at 950 l h–1, the cone gas was nitrogen at 100 l h–1 (positive ions) or 50 l h–1 (negative ions), the desolvation temperature was 450 °C, and the capillary voltage was 2.8kV (positive ions) or 2.7kV (negative ions).
In preliminary experiments with other species, conventional UPLC-MS-MS resulted in interfering peaks in the targeted GA4 channels, as the protonated derivatized molecule at m/z 418.2 yielded only a loss of 59 (trimethylamine) to give a product ion at m/z 359.2. A pseudo MS3 experiment was therefore designed in which ‘in-source’ fragmentation of the protonated derivatized GA4 to yield the m/z 359.2 product ion was optimized by using a relatively high cone voltage of 70V. A full MS-MS scan of this subsequent derivatized GA4 ‘in-source’ product ion at m/z 359.2 from a standard provided a good yield of more diagnostic product ions from which three were selected for monitoring. The transitions monitored for derivatized GA4 were therefore m/z 359.2 to m/z 223.1, m/z 269.15, and m/z 313.2. For derivatized [2H2] GA4, the corresponding transitions were m/z 361.2 to m/z 225.1, m/z 271.15, and m/z 315.2. The collision energy was 30V and the dwell time was 50ms per channel.
The transitions for ABA were m/z 263.2 to 153.1, 204.2, and 219.2, and for [2H6] ABA, m/z 269.2 to 159.1, 207.2 (three deuteriums were lost in this fragmentation), and 225.2. The cone voltage was 32V. The collision energy was 18V for the m/z 263.2 to 153.1 and 204.2 and corresponding deuterium-labelled channels, and 16V for the m/z 263.2 to 219.2 and corresponding deuterium-labelled channel. Dwell time was 50ms per channel. For IAA the transition was m/z 176.1 to 130.1, and for [13C6]IAA, m/z 182.1 to 136.1. The cone voltage was 18V, collision voltage 18V, and dwell time 245ms per channel. Data were analysed using Waters MassLynx and TargetLynx software.
Extraction, purification, and quantification of endogenous brassinolide and castasterone
Tissue samples were homogenized to a fine pulp in 80% methanol and placed in darkness at 4 ºC overnight to facilitate the extraction of hormones. Extracts were filtered through Whatman No. 1 filter paper and rinsed with 100% methanol. Internal standards of [2H6]BL and [2H6]CS (synthesized by Dr Suguru Takatsuto, Joetsu University of Education, Japan) were added and the extracts were concentrated to aqueous phase of ~5ml, under vacuum at 30 ºC. Samples were then resuspended by the addition of 45ml of distilled water and partitioned three times against 50ml of chloroform. The chloroform fraction was then reduced to dryness. The samples were then taken up in 50ml of 80% (v/v) methanol in distilled water, and partitioned three times against 50ml of n-hexane. The methanol-soluble fractions were then retained, dried, and purified using Sep-Pak Vac Silica 20ml cartridges (Waters, Australia) as before (Symons et al., 2006). The samples were then reduced to dryness under vacuum, and 60ml of 30% (v/v) methanol in 0.4% (v/v) acetic acid was added to the residues. The samples were then loaded onto a C18 Sep-Pak cartridge, pre-conditioned earlier with 60ml of methanol followed by 60ml of 30% (v/v) methanol in 0.4% (v/v) acetic acid. The column was rinsed with 60ml of 45% (v/v) methanol in 0.4% (v/v) acetic acid and the samples were eluted with 100ml of 90% (v/v) methanol in 0.4% (v/v) acetic acid. The samples were then dried down in vacuo, and the residues were resuspended in 600 µl of the initial conditions [50% (v/v) methanol in 0.4% (v/v) acetic acid]. The samples were then fractionated using reverse-phase HPLC (10 cm×8mm i.d., 10 µm µBondapak Pak C18 column; Waters, Australia). The solvent program used ran from 50% to 100% (v/v) methanol in 0.4% (v/v) aqueous acetic acid over 40min with the solvent programmer set on a linear gradient with the flow rate maintained at 2ml min–1. The fractions corresponding to the retention times for BL and CS were 22–30min, which were then pooled and dried under vacuum. The residue was then taken up in three times 200 µl of 100% methanol, and derivatized in 30 µl of methane boronic acid in pyridine (2mg ml–1) in the oven at 70 ºC for 30min. A Hewlett Packard 5890 gas chromatograph coupled to the Kratos Concept ISQ mass spectrometer was utilized as described previously (see Symons et al., 2006). The ions monitored for BL were 332.289 (endogenous BL), 338.326 ([2H6]BL internal standard), and 457.294 (common). For CS, the ions monitored were 512.386 (endogenous), 518.423 ([2H6]CS), and 441.299 (common).
Hormone treatment
Strawberry fruits at the small white (SW) stage of development were used for the treatments. The hormones were applied to the outside of the fruit in a small volume of ethanol (controls received ethanol only). For GA3 and the GA biosynthesis inhibitor, paclobutrazol (Butcher et al., 1990), the doses were 5 µg and 20 µg, respectively, in 20 µl of ethanol. The synthetic auxin, 1-naphthaleneacetic acid (NAA), was applied at three doses, 1, 10, and 100 µg, in 20 µl of ethanol, while 100 µg of the auxin action inhibitor, p-chlorophenoxyisobutyric acid (PCIB) (Oono et al., 2003) was applied in 40 µl of ethanol. For ABA the dose was 10 µg in 40 µl of ethanol. This application technique resulted in an even coverage of the majority of the surface of the receptacle.
Stages of fruit ripening
The effects of the various plant hormones on strawberry ripening were assessed by monitoring the development of colour in the fruits using a scale of ripening (Fig. 1) where: 0—small white, green achenes, no sign of colour on receptacle, and treatment applied to fruit of this stage; 1—large white, achenes brown, no sign of colour on receptacle; 2—pink, first sign of pink coloration on receptacle, up to ~90% of the receptacle may be still white; 3—dark pink, receptacle dark pink in colour, covering ~70% of the receptacle surface, some white often still visible on the receptacle; 4— red, light red colour covering the whole of the receptacle, some dark pink patches may remain but no white remaining; 5—commercially ripe, deep red colour, complete and even coverage of the receptacle; 6—over ripe, dark red, obvious softening of the fruit.
Statistical analysis
t-tests were used to test the statistical significance of sample differences, where P < 0.05 was deemed significant.
Results
Fruit ripening
Under the conditions used, it took ~50 d from anthesis for the strawberry fruit to ripen fully. Fruit was harvested at six stages for measurement of fruit weight and diameter. These coincide with the small green, large green, small white, large white, pink, and red stages used by other workers to monitor the development and maturation of strawberry fruit (Aharoni et al., 2002; Fig. 1). The minor differences in the timing and transition between these stages from those observed in other studies are likely simply to reflect the growing conditions and variety used.
Endogenous hormone levels
The level of IAA was relatively low at flowering but rose rapidly by the small green stage (12 d post-anthesis) and then declined as fruit growth continued and ripening progressed. The level was low by the fully red stage (Fig. 2A). Similar changes in auxin level have been observed by other workers examining strawberry fruit development (Dreher and Poovaiah, 1982; Given et al., 1988) and are thought to play a key role in regulating ripening (Archbold and Dennis, 1984). The level of the main bioactive GA, GA1, was also low at flowering and then rose during the small green and large green stages before gradually declining as ripening progressed (Fig. 1B). This pattern is similar to that seen for IAA, except that it is slightly displaced in time and would be consistent with the results from other systems (e.g. Ross and Reid, 2010) that show auxin can stimulate GA1 accumulation. The level of other bioactive GAs, GA3 and GA4, which have been reported to affect strawberry fruit development (Montero et al., 1998; Paroussi et al., 2002; Csukasi et al., 2011), were below detection limits in strawberry tissues in these experiments, as a strong internal standard signal was obtained but no dilution with endogenous material was observed. Support for the importance of the early 13-hydroxylation pathway of GA synthesis in strawberries (Blake et al., 2000) came from the dilution of the deuterated internal standard for GA20, the precursor of GA1, in strawberries at the white stage of development.
Fig. 2.
Changes in the levels of IAA (A), GA1 (B), ABA (C), and CS (D) during strawberry fruit development. Values represent the mean ±SE derived from two individual replicates. The asterisk (*) represents values that were below the detection limit, while (^) denotes that the value was obtained from a single replicate only.
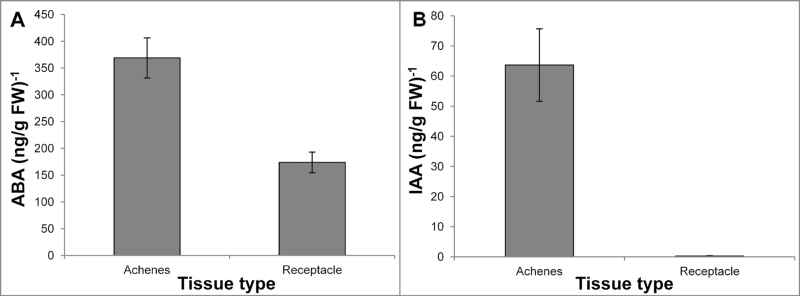
While IAA and GA1 levels exhibited a progressive declineduring strawberry ripening (Fig. 2A, B), the opposite pattern was observed for ABA (Fig. 2C). ABA levels were low at anthesis but increased steadily throughout fruit development. This change was most rapid during the development of colour. ABA levels were highest at the red ripe stage and rose ~500-fold from anthesis, a much larger increase than the results recently reported by Chai et al. (2011). ABA was present in the achenes at about twice the concentration found in the receptacle at the white stage of strawberry development (Fig. 3A; P < 0.01). This concentration in the achenes was even more marked for IAA (Fig. 3B; P < 0.01), consistent with a previous report (Archbold and Dennis, 1984).
Fig. 3.
Levels of ABA (A) and IAA (B) at the combined white stages of strawberry fruit development as analysed by UPLC-MS. The fresh weight of the achenes was 0.93±0.22g and that of the receptacles 10.8±0.97g. Shown are the means ±SE from three replicates.
Fruit development in the strawberry is also accompanied by changes in BR levels (Fig. 2D). The levels of the bioactive BR, CS, peaked in flowers before declining progressively through the successive stages of fruit development. In fact, the CS content had reached a low level prior to the onset of ripening and was below detection limits during the large white and red stages. The most bioactive BR, BL (Wang et al., 2001), was not detected at any stage of strawberry fruit development, although there was a good recovery of the [2H6]BL internal standard during GC-MS analysis (data not shown).
Application experiments
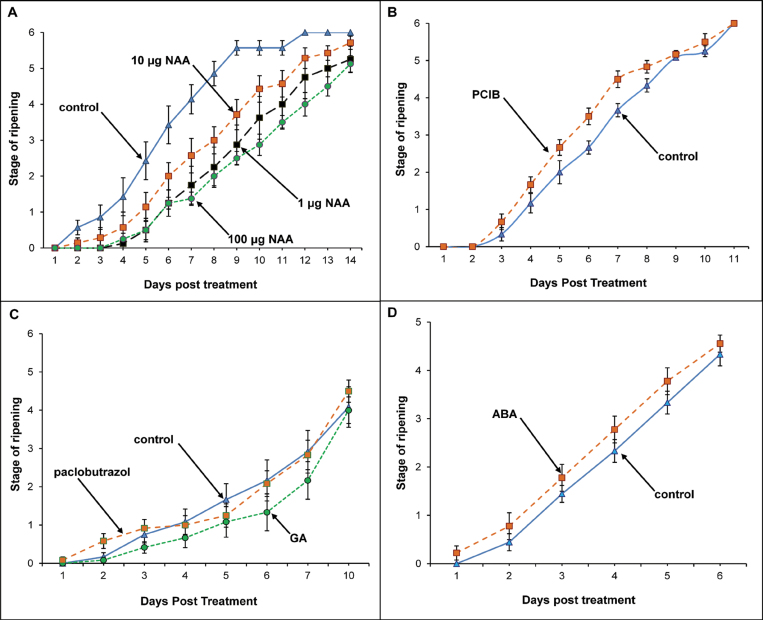
The application of the synthetic auxin, NAA, delayed ripening of strawberry, as judged by the degree of colour development, by up to 6 d (P < 0.001, Fig. 4A). The auxin action inhibitor PCIB had the opposite effect and enhanced fruit coloration compared with the controls (P < 0.05, Fig. 4B). These results are similar to those reported by previous workers (Nitsch, 1950; Lis et al., 1978; Manning, 1994). In contrast, the application of GA3 and the GA biosynthesis inhibitor, paclobutrazol, did not result inany significant changes in ripening (Fig. 4C), and nor did the application of ABA (Fig. 4D). These results do not support the idea that changes in the endogenous levels of GA and ABA are playing a key role in the regulation of the ripening process, although such negative data may only indicate that the applied hormone did not reach a site where it could exert biological activity.
Fig. 4.
Effect of NAA (A), PCIB (B), GA3, and paclobutrazol (C), and ABA (D) on ripening, as measured by colour change. Each point represents the mean for 8–12 replicates; vertical bars indicate standard errors. (This figure is available in colour at JXB online.)
Discussion
In this study it has been shown that the levels of the four plant hormones, IAA, GA1, ABA, and CS, change dramatically during fruit development and fruit ripening in strawberry (Fig. 2). This provides the first simultaneous monitoring for this range of hormones, and novel results for the BRs, and is essential given the numerous reports suggesting that one or more of these hormones are involved in regulating development and ripening of strawberries. The results for IAA are consistent with previous evidence that the seeds (achenes) on the surface of the false fruit (receptacle) produce IAA that then promotes receptacle enlargement and ripening of the fruit (Nitsch, 1950; Dreher and Poovaiah, 1982; Archbold and Dennis, 1984). Results from application experiments with the synthetic auxin, NAA, also support this conclusion (Fig. 3; Given et al., 1988), and are consistent with molecular studies that show that auxin inhibits the expression of ripening-related genes (Manning, 1994; Aharoni et al., 2002; Liu et al., 2011).
While the changes in GA1 levels follow those seen for IAA (Fig. 2), the evidence from the application experiments does not suggest that GAs are important in regulating fruit development and ripening (Fig. 4). This is contrary to the view put forward by others (Martinez et al., 1994, 1996), but the difference might be explained by a lack of penetration of the applied GA3 and paclobutrazol in the present case. Until recently, GA levels have only been monitored during the development and ripening of strawberry fruit by bioassay (Lis et al., 1978), although potentially bioactive GA1 and GA3 have been identified previouslyby physiochemical methods (Blake et al., 2000). However,a recent report by Csukasi et al. (2011) reported that the three biologically active GAs, GA1, GA3, and GA4, were all present in strawberry receptacles, with GA4 levels reaching 150ng g–1 FW. This was reported to be the highest level recorded for a bioactive form of GA in any plant tissue and led to the claim that GA4 plays a major role in strawberry development (Csukasi et al., 2011). However, the present results are consistent with the view that the early 13-hydroxylation pathway leading to GA1 is the key pathway in strawberry fruit as suggested by Blake et al. (2000), in addition to vegetative tissues (Taylor et al., 1994; Wiseman and Turnbull, 1999). The results provide clarification not only of the level of the bioactive molecule in developing fruit but also of the metabolic pathway present since GA20 was identified from strawberries at the white stage of development. The timing of changes in GA1 levels is also consistent with the view that they may be regulated by the level of IAA, as has been shown in numerous other species and tissues (Ross and Reid, 2010). However, this requires direct experimental verification. No evidence was found for GA3 or GA 4 during the experiments. The detection limit for GA4 was 0.15ng g–1 FW. This means that GA4, if present at all, was at extremely low levels, no more than 1/1000 of the level reported by Csukasi et al. (2011). The results therefore call into question the validity of the findings of Csukasi et al., which were based on a technique that did not use stable isotope-labelled internal standards.
The increase in ABA levels observed during fruit development and ripening is similar to previous results (e.g. Archbold and Dennis, 1984; Chai et al., 2011), although high levels at anthesis were not found. The most rapid rise coincided with the onset of colour development. The ABA concentration was higher in the achenes and is probably involved in seed maturation(Finkelstein, 2010). However, application experiments did not show a promotion of ripening by ABA (Fig. 4), even when dimethylsulphoxide was included in the treatment solution to improve the response (data not shown). The start of the increase in ABA levels coincides with the drop in both IAA and GA1 levels (Fig. 1). Exogenous application of ABA has been reported to increase anthocyanin levels and ripening in strawberry (Jiang and Joyce, 2003; Jia et al., 2011) and to stimulate the accumulation of sucrose and other assimilates in the fruit tissue of strawberry in vitro(Archbold, 1988; Ofosu-Anim et al., 1996). The reason for the apparent difference is not known, but different growth conditions and ABA application techniques were used.
The level of the bioactive BR, CS, dropped dramatically from the onset of flowering through to the small white stage of fruit development. In the later stages of fruit development, including ripening, its level was very low or below the detection limits (Fig. 2D). The most biologically active BR, BL, was not detected during the present experiments. Work on other species shows that some produce BL, while in others CS is the main biologically active molecule present (Hong et al., 2002; Mori et al., 2002; Kim et al., 2005; Montoya et al., 2005; Jager et al., 2007). This difference can also occur within the one species, with for example the vegetative tissues of tomato only containing CS, whilst the developing fruit contains BL (Montoya et al., 2005). This difference is dependent upon the nature and function of their BR C-6 oxidase enzymes which are members of the CYP85A family of P450 monooxygenases (Jager et al., 2007). The present results do not suggest that the BRs play an important role during the ripening phases of strawberry development, although a role during the early stages is possible.
One of the aims of the present work was to compare the patterns of hormonal changes during fruit development in non- climateric fruit in order to see if there was a consistent pattern as broadly observed amongst climateric fruits. The most studied non-climateric fruits are grape, strawberry, citrus, and pepper. Previous work has examined the same group of hormones studiedhere in grape (Davies et al., 1997; Symons et al., 2006). These two model species exhibit different fruit structures; grape berries are simple fruits that have a fleshy inner layer of the fruit wall and have many small seeds contained within a gel, while strawberries are false fruits, formed from the swollen base of the flower (receptacle) with the many seeded fruits (achenes) located on its outer surface. These structural differences might reflect underlying differences in their ripening mechanisms.A comprehensive investigation of the hormone levels in these two species during fruit development allows key hormone signals that may be important in all non-climacteric fruits to be distinguished from those that are specific to a certain species or fruit type. The results indicate substantial differences in the patterns observed. For example, there is a strong rise in CS levels at the onset of fruit ripening (veraison) in grape that does not appear to occur at the onset of ripening in strawberry (Fig. 2D). Work in grape suggests that this is an important signal in ripening in this non-climateric fruit, but this does not appear to be the case in strawberry. The pattern of change of GA1 levels is also different, with the level being consistently low in grape except at flowering, but showing a pronounced peak during the early stages of fruit development in strawberry. The changes in ABA levels are also different, with a steady rise through development in strawberry (Fig. 2C) but an initial decline in grape before a rise at veraison (Davies et al., 1997). While these changes may, in part, reflect the different tissues sampled in the various studies, they do not support the view that there are similar hormone signals controlling ripening in grape and strawberry. They suggest that non-climateric fruit may not form a consistent group which would be in agreement with their different fruit structures and phylogenetic relationships.
Acknowledgements
We wish to thank Ian Cummings, Tracy Winterbottom and Matthew Reid for technical support, and the Australian Research Council for financial support.
References
- Adams-Phillips L, Barry C, Giovannoni J. Signal transduction systems regulating fruit ripening. Trends in Plant Science. 2004;9:331–338. doi: 10.1016/j.tplants.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Aharoni A, Keizer LCP, Van Den Broeck HC, Blanco-Portales R, Muñoz-Blanco J, Bois G, Smit P, De Vos RCH, O’Connell AP. Novel insight into vascular, stress, and auxin-dependent and -independent gene expression programs in strawberry, a non-climacteric fruit. Plant Physiology. 2002;129:1019–1031. doi: 10.1104/pp.003558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander L, Grierson D. Ethylene biosynthesis and action in tomato: a model for climacteric fruit ripening. Journal of Experimental Botany. 2002;53:2039–2055. doi: 10.1093/jxb/erf072. [DOI] [PubMed] [Google Scholar]
- Archbold DD. Abscisic acid facilitates sucrose import by strawberry fruit explants and cortex disks in vitro . HortScience. 1988;23:880–881. [Google Scholar]
- Archbold DD, Dennis FGJ. Quantification of free ABA and free and conjugated IAA in strawberry achene and receptacle tissue during fruit development. Journal of the American Society for Horticultural Science. 1984;109:330–335. [Google Scholar]
- Ban T, Ishimaru M, Kobayashi S, Shiozaki S, Goto-Yamamoto N, Horiuchi S. Abscisic acid and 2,4-dichlorophenoxyacetic acid affect the expression of anthocyanin biosynthetic pathway genes in ‘Kyoho’ grape berries. Journal of Horticultural Science and Biotechnology. 2003;78:586–589. [Google Scholar]
- Barry CS, McQuinn RP, Thompson AJ, Seymour GB, Grierson D, Giovannoni JJ. Ethylene insensitivity conferred by the Green-ripe and Never-ripe 2 ripening mutants of tomato. Plant Physiology. 2005;138:267–275. doi: 10.1104/pp.104.057745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake PS, Taylor DR, Crisp CM, Mander LN, Owen DJ. . Identification of endogenous gibberellins in strawberry, including thenovel gibberellins GA123, GA124and GA125. Phytochemistry. 2000;55:887–890. doi: 10.1016/s0031-9422(00)00237-5. [DOI] [PubMed] [Google Scholar]
- Butcher DN, Clark JA, Lenton JR. Gibberellins and the growth of excised tomato roots: comparison of gib-1 mutant and wild type and responses to applied GA3 and 2S,3S paclobutrazol. Journal of Experimental Botany. 1990;41:715–722. [Google Scholar]
- Chai YM, Jia HF, Li CL, Dong QH, Shen YY. FaPYR1 is involved in strawberry fruit ripening. Journal of Experimental Botany. 2011;62:5079–5089. doi: 10.1093/jxb/err207. [DOI] [PubMed] [Google Scholar]
- Chervin C, El-Kereamy A, Roustan JP, Latche A, Lamon J, Bouzayen M. Ethylene seems required for the berry development and ripening in grape, a non-climacteric fruit. Plant Science. 2004;167:1301–1305. [Google Scholar]
- Csukasi F, Osorio S, Gutierrez JR, et al. Gibberellin biosynthesis and signalling during development of the strawberry receptacle. New Phytologist. 2011;191:376–390. doi: 10.1111/j.1469-8137.2011.03700.x. [DOI] [PubMed] [Google Scholar]
- Davies C, Boss PK, Robinson SP. Treatment of grape berries, a nonclimacteric fruit with a synthetic auxin, retards ripening and alters the expression of developmentally regulated genes. Plant Physiology. 1997;115:1155–1161. doi: 10.1104/pp.115.3.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies PJ. 2004. Introduction. The plant hormones: their nature, occurrence, and functions. In: Davies PJ, ed. Plant hormones biosynthesis, signal transduction, action! Dordrecht, The Netherlands: Kluwer Academic Publishers; 1 15 [Google Scholar]
- Dreher TW, Poovaiah BW. Changes in auxin content during development in strawberry fruits. Journal of Plant Growth Regulation. 1982;1:267–276. [Google Scholar]
- Finkelstein RR. 2010. The role of hormones during seed development and germination. In: Davies PJ, ed. Plant hormones biosynthesis, signal transduction, action!, 3rd edn. revised, Dordrecht, The Netherlands: Springer; 549 573 [Google Scholar]
- Giovannoni JJ. Molecular biology of fruit maturation and ripening. Annual Review of Plant Physiology and Plant Molecular Biology. 2001;52:725–749. doi: 10.1146/annurev.arplant.52.1.725. [DOI] [PubMed] [Google Scholar]
- Giovannoni JJ. Genetic regulation of fruit development and ripening. The Plant Cell. 2004;16:S170–S180. doi: 10.1105/tpc.019158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Given NK, Venis MA, Gierson D. Hormonal regulation ofripening in the strawberry, a non-climacteric fruit. Planta. 1988;174:402–406. doi: 10.1007/BF00959527. [DOI] [PubMed] [Google Scholar]
- Hasan O, Ridoutt BG, Ross JJ, Davies NW, Reid JB. Identification and quantification of endogenous gibberellins in apical buds and the cambial region of Eucalyptus. Physiologia Plantarum. 1994;90:475–480. [Google Scholar]
- Hobson G, Grierson D. 1993. Tomato. In: Seymour GB, Taylor JE, Tucker GA, eds. Biochemistry of fruit ripening. London: Chapman and Hall; 405 442 [Google Scholar]
- Hong S-H, Kim I-J, Yang DC, Chung W-I. Characterization of an abscisic acid responsive gene homologue from Cucumis melo. Journal of Experimental Botany. 2002;53:2271–2272. doi: 10.1093/jxb/erf075. [DOI] [PubMed] [Google Scholar]
- Jager CE, Symons GM, Nomura T, Yamada Y, Smith JJ, Yamaguchi S, Kamiya Y, Weller JL, Yokota T, Reid JB. Characterization of two brassinosteroid C-6 oxidase genes in pea. Plant Physiology. 2007;143:1894–1904. doi: 10.1104/pp.106.093088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia HF, Chai YM, Li CL, Lu D, Luo JJ, Qin L, Shen YY. Abscisic acid plays an important role in the regulation of strawberry fruit ripening. Plant Physiology. 2011;157:188–199. doi: 10.1104/pp.111.177311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Joyce DC. ABA effects on ethylene production, PAL activity, anthocyanin and phenolic contents of strawberry fruit. Plant Growth Regulation. 2003;39:171–174. [Google Scholar]
- Kim TW, Hwang JY, Kim YS, Joo SH, Chang SC, Lee JS, Takatsuto S, Kim SK. Arabidopsis CYP85A2, a cytochrome P450, mediates the Baeyer–Villiger oxidation of castasterone to brassinolide in brassinosteroid biosynthesis. The Plant Cell. 2005;17:2397–2412. doi: 10.1105/tpc.105.033738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klee HJ, Clark DG. 2004. Ethylene signal transduction in fruits and flowers. In: Davies PJ, ed. Plant hormones biosynthesis, signal transduction, action! Dordrecht, The Netherlands: Kluwer Academic Publishers; 369 390 [Google Scholar]
- Kojima M, Kamada-Nobusada T, Komatsu H, et al. Highly sensitive and high-throughput analysis of plant hormones using MS-probe modification and liquid chromatography-tandem mass spectrometry: an application for hormone profiling in Oryza sativa . Plant and Cell Physiology. 2009;50:1201–1214. doi: 10.1093/pcp/pcp057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence N, Ross JJ, Mander LN, Reid JB. Internode length in Pisum: mutants lk, lka, and lkb do not accumulate gibberellins. Journal of Plant Growth Regulation. 1992;11:35–37. [Google Scholar]
- Lis EK, Borkowska B, Antoszewski R. Growth regulators in the strawberry fruit. Fruit Science Report. 1978;V:17–29. [Google Scholar]
- Liu DJ, Chen JY, Lu WJ. Expression and regulation of the early auxin-responsive Aux/IAA genes during strawberry fruit development. Molecular Biology Reports. 2011;38:1187–1193. doi: 10.1007/s11033-010-0216-x. [DOI] [PubMed] [Google Scholar]
- Manning K. 1993. Soft fruit. In: Seymour GB, Taylor JE, Tucker GA,eds. Biochemistry of fruit ripening London: Chapman & Hall; 347 378 [Google Scholar]
- Manning K. Changes in gene expression during strawberry fruit ripening and their regulation by auxin. Planta. 1994;194:62–68. [Google Scholar]
- Martínez GA, Chaves AR, Añón MC. Effect of gibberellic acid on ripening of strawberry fruits (Fragaria-ananassa Duch.) Journal of Plant Growth Regulation. 1994;13:87–91. [Google Scholar]
- Martínez GA, Chaves AR, Añón MC. Effect of exogenous application of gibberellic acid on color change and phenylalanine ammonia-lyase, chlorophyllase, and peroxidase activities during ripening of strawberry fruit (Fragaria x ananassa Duch) Journal of Plant Growth Regulation. 1996;15:139–146. [Google Scholar]
- Montero T, Mollá E, Martín-Cabrejas MA, López-Andréu FJ. Effects of gibberellic acid (GA3) on strawberry PAL (phenylalanine ammonia-lyase) and TAL (tyrosine ammonia-lyase) enzyme activities. Journal of the Science of Food and Agriculture. 1998;77:230–234. [Google Scholar]
- Montoya T, Nomura T, Yokota Y, Farrar K, Harrison K, Jones JDG, Kaneta T, Kamiya Y, Szekeres M, Bishop GJ. Patterns of Dwarf expression and brassinosteroid accumulation in tomato reveal the importance of brassinosteroid synthesis during fruit development. The Plant Journal. 2005;42:262–269. doi: 10.1111/j.1365-313X.2005.02376.x. [DOI] [PubMed] [Google Scholar]
- Mori M, Nomura T, Ooka H, et al. Isolation and characterization of a rice dwarf mutant with a defect in brassinosteroid biosynthesis. Plant Physiology. 2002;130:1152–1161. doi: 10.1104/pp.007179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsch JP. Growth and morphogenesis of the strawberry as related to auxin. American Journal of Botany. 1950;37:211–215. [Google Scholar]
- Ofosu-Anim J, Kanayama Y, Yamaki S. Sugar uptake into strawberry fruit is stimulated by abscisic acid and indoleacetic acid. Physiologia Plantarum. 1996;97:169–174. [Google Scholar]
- Oono Y, Ooura C, Rahman A, Aspuria ET, Hayashi K-i, Tanaka A, Uchimiya H. p-Chlorophenoxyisobutyric acid impairs auxin response in Arabidopsis root. Plant Physiology. 2003;133:1135–1147. doi: 10.1104/pp.103.027847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paroussi G, Voyiatzis DG, Paroussis E, Drogoudi PD. Growth, flowering and yield responses to GA3 of strawberry grown under different environmental conditions. Scientia Horticulturae. 2002;96:103–113. [Google Scholar]
- Perkins-Veazie PM. Growth and ripening of strawberry fruit. Horticultural Reviews. 1995;17:267–297. [Google Scholar]
- Ross JJ, Reid JB. Evolution of growth-promoting plant hormones. Functional Plant Biology. 2010;37:795–805. [Google Scholar]
- Seymour GB Taylor JE Tucker GAeds. 1993. Biochemistry of fruit ripening London: Chapman & Hall; [Google Scholar]
- Symons GM, Davies C, Shavrukov Y, Dry IB, Reid JB, Thomas MR. Grapes on steroids. Brassinosteroids are involved in grape berry ripening. Plant Physiology. 2006;140:150–158. doi: 10.1104/pp.105.070706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor DR, Blake PS, Browning G. Identification of gibberellins in leaf tissues of strawberry (Fragaria x ananassa Duch.) grown under different photoperiods. Plant Growth Regulation. 1994;15:235–240. [Google Scholar]
- Trainotti L, Pavanello A, Casadoro G. Different ethylene receptors show an increased expression during the ripening of strawberries: does such an increment imply a role for ethylene in the ripening of these non-climacteric fruits? Journal of Experimental Botany. 2005;56:2037–2046. doi: 10.1093/jxb/eri202. [DOI] [PubMed] [Google Scholar]
- Tucker GA. 1993. Introduction. In: Seymour GB, Taylor JE, Tucker GA, eds. Biochemistry of fruit ripening. London: Chapman and Hall; 1 52 [Google Scholar]
- Wang Z-Y, Seto H, Fujioka S, Yoshida S, Chory J. BRI1 is a critical component of a plasma-membrane receptor for plant steroids. Nature. 2001;410:380–383. doi: 10.1038/35066597. [DOI] [PubMed] [Google Scholar]
- Wiseman NJ, Turnbull CGN. Endogenous gibberellin content does not correlate with photoperiod-induced growth changes in strawberry petioles. Functional Plant Biology. 1999;26:359–366. [Google Scholar]
- Zaharah SS, Singh Z, Symons GM, Reid JB. Role of brassinosteroids, ethylene, abscisic acid, and indole-3-acetic acid in mango fruit ripening. Journal of Plant Growth Regulation 2011 [Google Scholar]