Abstract
A pot experiment was conducted to investigate the effect of the non-protein amino acid, β-aminobutyric acid (BABA), on the homeostasis between reactive oxygen species (ROS) and antioxidant defence during progressive soil drying, and its relationship with the accumulation of abscisic acid (ABA), water use, grain yield, and desiccation tolerance in two spring wheat (Triticum aestivum L.) cultivars released in different decades and with different yields under drought. Drenching the soil with 100 µM BABA increased drought-induced ABA production, leading to a decrease in the lethal leaf water potential (Ψ) used to measure desiccation tolerance, decreased water use, and increased water use efficiency for grain (WUEG) under moderate water stress. In addition, at severe water stress levels, drenching the soil with BABA reduced ROS production, increased antioxidant enzyme activity, and reduced the oxidative damage to lipid membranes. The data suggest that the addition of BABA triggers ABA accumulation that acts as a non-hydraulic root signal, thereby closing stomata, and reducing water use at moderate stress levels, and also reduces the production of ROS and increases the antioxidant defence enzymes at severe stress levels, thus increasing the desiccation tolerance. However, BABA treatment had no effect on grain yield of wheat when water availability was limited. The results suggest that there are ways of effectively priming the pre-existing defence pathways, in addition to genetic means, to improve the desiccation tolerance and WUEG of wheat.
Key words: Abscisic acid, β-aminobutyric acid, desiccation resistance, non-hydraulic signals, reactive oxygen species, transpiration efficiency for grain, Triticum aestivum L., water use efficiency for grain
Introduction
Drought is considered to be among the most severe abiotic stresses affecting plant growth and limiting crop yields (Jones and Corlett, 1992; Ozturk et al., 2002). Wheat, as one of the ‘big three’ cereal crops, often suffers water shortage during the growing season with a greater likelihood of water stress in the future because of global climate change and a decline in water resources for agriculture (Turner and Meyer, 2011). Crop plants have evolved a number of biochemical and physiological mechanisms to cope with temporary or terminal water shortage (Turner, 1996; Turner and Asseng, 2005; Wang et al., 2008). There are pre-existing and induced defences in plants to mediate their adaptation to stress conditions (Bray et al., 2000; Pastori and Foyer, 2002; Vallad and Robert, 2004; Jakab et al., 2005).
An early response to soil drying is the closure of stomata without changes of leaf water status through the action of drought-induced abscisic acid (ABA) which originates in the root and is transported to the shoot (Zhang and Davies, 1990; Jakab et al., 2005). This ‘early-warning’ response to soil drying is characterized as a non-hydraulic root signal (nHRS). The early response of nHRS will facilitate the reduced water loss from stomata and serve as the first defence against possible drought (Henson et al., 1989a, b; Davies et al., 1994; Fan et al., 2009). With prolonged soil water depletion, the leaf water status triggers changes in the leaf, hydraulic signals (HS), leading to physiological changes such as osmotic adjustment (Jones and Turner, 1980) and evoking antioxidant defence mechanisms (Wang et al., 2008; Fan et al., 2009). With soil drying, temporary wilting (TW), evident as the drooping of leaves during the day followed by rehydration and recovery during the night, is first observed followed by permanent wilting (PW) when the leaves fail to regain turgor overnight.
Drought inevitably reduces CO2 assimilation and increased electron transfer from photosynthetic electron carriers toward O2, increasing the reactive oxygen species (ROS), namely the superoxide anion radical (O2-), hydrogen peroxide (H2O2) and hydroxyl radical (·OH) (Asada, 1999). In plants, ROS are continuously produced, predominantly in chloroplasts, mitochondria, and peroxisomes (Apel and Hirt, 2004). The homeostasis of production and removal of ROS must be strictly controlled, and this balance can be disturbed by adverse abiotic stresses factors such as high light (Feild et al., 1998), drought (Wang et al., 2008), and extreme temperature (Prasad et al., 1994). Enzymatic constitutents, such as superoxide dismutases (SOD), catalases (CAT), ascorbate peroxidase (APX), and glutathione reductase (GR), and non-enzymatic compounds including ascorbate and glutathione are responsible for ROS scavenging (Noctor and Foyer, 1998; Apel and Hirt, 2004). The activity of antioxidant enzymes and the level of non-enzymatic compounds generally increase during abiotic stress and are correlated with enhanced cellular protection, thereby preventing oxidative damage (Khanna-Chopra and Selote, 2007). During soil drying, endogenous ABA and the water relations of the plant play crucial roles in modulating the equilibrium between ROS and antioxidant defence systems (Jiang and Zhang, 2002a, b; Srivalli et al., 2003; Fan et al., 2009) and this equilibrium between ROS and the antioxidant defence system is also associated with the induction of non-hydraulic and hydraulic signals (Wang et al., 2008; Fan et al., 2009). However, at higher stress levels, the antioxidant defence system collapses and oxidative damage to lipids, proteins, and DNA occurs (Sgherri and Navari-Izzo, 1995; Apel and Hirt, 2004). Drought stress is always associated with an increase in endogenous abscisic acid (ABA), which mediates most plant responses to drought (Leung and Giraudat, 1998; Zhu, 2002). ABA is also a key compound of root-sourced signals that induce stomatal closure (Zhang and Davies, 1990). Jiang and Zhang (2002a, b) proposed that ABA is an essential mediator in triggering a drought-induced antioxidative defence response against oxidative damage in maize seedlings. Therefore, there is a linkage between ABA, root signals, and antioxidant defence in plants exposed to a water deficit.
The non-protein amino acid, β-aminobutyric acid (BABA) is a potent inducer of resistance against infection by various pathogens (Jakab et al., 2001) through the induction of salicylic acid- and/or ABA-dependent defence mechanisms (Zimmerli et al., 2000; Ton and Mauch-Mani, 2004). Recently, BABA was shown to enhance resistance to abiotic stresses, such as drought and salinity, in Arabidopsis by priming the ABA response (Jakab et al., 2005), and heat tolerance through up-regulating heat shock protein 101 expression (Zimmerli et al., 2008). The only research on BABA-induced resistance against abiotic stresses has been in Arabidopsis, not crops. In this study, the effect of BABA on desiccation tolerance and water use efficiency was evaluated in two spring wheat cultivars with different yields under drought. The hypothesis tested in this study is that BABA triggers ABA accumulation, thereby reducing stomatal conductance and water use and increasing the water use efficiency, and improves the desiccation tolerance and grain yield of wheat when water is limited through priming the drought-induced antioxidative defences.
Materials and methods
Plant materials and growth conditions
The two spring wheat (Triticum aestivum L.) cultivars, Gansu 96 (GS) and Longchun 8275 (LC) were selected for their similar phenological development, but different yields under drought in a previous experiment (YL Du, unpublished data). GS was released in the 1950s (referred to as ‘old’) and LC was released in the 1990s (referred to as ‘modern’) for the semiarid regions of the Loess Plateau in northwest China. Seed of both cultivars was obtained from the Institute of Crop Germplasm Resources, Chinese Academy of Agricultural Science, Beijing, China. The experiment was conducted at the Yuzhong Experiment Station of Lanzhou University in Yuzhong County, Gansu Province (35°51' N, 104°07' E, altitude 1620 m) in the growing season (March–July) of 2009. The cultivars were grown under a rainout shelter (50 m long×24 m wide×5.7 m high) that was closed during rain events. Seeds were vernalized at 4 °C for 24h, and germinated in an incubation cabinet. Eighteen seeds were sown in each of 218 plastic pots (220mm diameter×250mm high) containing 4.7kg of sieved peat-based substrate [peat:perlite (v/v)=4:1, dry bulk density of 0.56g cm–3 and a field water capacity (FWC) of 82%] that was sterilized by solar exposure in the rainout shelter for 2 weeks. After emergence, seedlings were thinned to 14 plants per pot in Experiments 1 and 2 and ten plants per pot in Experiment 3. Before sowing, 1.25g N, 0.36g P, and 0.44g K were applied per pot so that nutrition was not limited (Cissé et al., 1996). After emergence, all plants were irrigated daily to maintain the soil at 90% of FWC. To minimize soil evaporation, the pots were covered with 15mm of perlite.
Three days before the treatments started at 30 d after sowing (DAS), the pots of plants used for Experiments 1 and 2 were transferred from the rainout shelter to a growth chamber. The growth chamber was maintained at 22/15 °C (day/night) temperature, 50±5% relative humidity, and 300 µmol m–2 s–1 photosynthetically active radiation (PAR) at the canopy level for a 14h day.
β-Aminobutyric acid (BABA) treatment
β-aminobutyric acid was dissolved in water and applied as a soil drench (100 µM BABA soil concentration) to half of the pots 1 d prior to the imposition of the water treatments. The time of BABA treatment is considered to be day 0.
Experiment 1: Progressive soil drying
In Experiment 1, the effect of BABA on the physiological and biochemical responses of the two wheat cultivars was investigated when two watering treatments were imposed from 30 DAS when at the jointing stage: (i) a slow decrease in soil water content, and (ii) the maintenance of a high soil water content (control). For (i), water was withheld from half of the pots one day after the BABA treatment and the pots were not watered for the subsequent 20 d. For (ii), the pots were maintained near 90% FWC by daily watering. Soil water content (SWC) is expressed as a percentage of water available between FWC and dry soil. Thus, there were two cultivars and four treatments, with (+BABA) and without (–BABA) BABA, and with and without water stress. In each cultivar by treatment combination there were 72 pots, 12 pots were used at each of six harvests with each cultivar by treatment combination being replicated three times. Once the drying treatment was imposed, all pots were weighed daily to determine the SWC, and the stomatal conductance (gs) measured on one mature non-senescent leaf in each of the three replicate pots between 08.30h and 10.30h using a LI-6400 portable photosynthesis system (Li-Cor, Lincoln, NE, USA). The stomatal conductance for each leaf was a mean of five readings per leaf. After measuring gs, the leaf relative water content (RWC) was measured on three leaves in each of the three replicate pots at a similar position as the leaf used to measure gs. Two leaf discs (5mm in diameter) were cut with a cork borer from each leaf, and weighed immediately for fresh weight (FW). The discs were floated in freshly-distilled water for 4h under 10 µmol m–2 s–1 PAR, then blotted dry and weighed to obtain the saturated weight (SW). Dry weight (DW) was measured after drying at 80 °C in a forced-draught oven for 24h. RWC was calculated as RWC=[(FW–DW)/(SW–DW)]×100.
The plants were harvested six times during the period that the water treatments were imposed: H1, on the day of initiation of the two water treatments; H2, when the stomata began to close but no reduction of RWC was observed; H3, 8 d after water was withheld; H4, when RWC began to decrease; H5, when temporary wilting was observed; and H6, when permanent wilting (PW) was observed. At the first five harvests after the gs measurements, fully-expanded leaves at the same position were collected and frozen immediately in liquid N2 for biochemical analysis.
Leaf RWC and gs were monitored for 3 d prior to the start of soil drying to ensure that equilibrium with chamber conditions was established (Saab and Sharp, 1989). The data collected for the plants were used to infer relations between leaf RWC or gs and SWC. Non-hydraulic root signals (nHRS) were judged to have been induced when there was a significant reduction in leaf gs (compared with gs in the well-watered plants) without change in leaf RWC, and to end when gs and RWC both decreased simultaneously (the onset of hydraulic signals, HS). These criteria were used to determine the SWC at which nHRS and HS were induced, compared with the well-watered plants. Temporary wilting (TW) was recorded when the leaves began to wilt during the day, but recovered overnight, while PW was recorded when the wilted leaves failed to recover overnight (Fan et al., 2008).
Experiment 2: Desiccation tolerance
The effect of BABA on the desiccation tolerance of the two wheat cultivars was assessed by measuring the lethal leaf water potential (Ψ) (Augé et al., 1998). Experiment 2 used the same cultivars and treatments as Experiment 1, but the soil drying treatments was continued for longer than 20 d. The Ψ of the plants was measured between 08.30h and 10.30h using a pressure chamber (PMS Instrument Company, Albany, OR, USA) and following the precautions recommended by Turner (1988). The lethal Ψ was the value of Ψ when leaves failed to recover the night following their measurement and died. Sampling of Ψ for lethal Ψ commenced when leaves developed extensive necrotic areas as they died. The Ψ of the last surviving leaf was the lethal Ψ. Twenty four pots were used to measure the lethal Ψ, six pots (six replicate samples) of each cultivar and BABA treatment combination (two cultivars and two BABA treatments).
Experiment 3: Yield formation
The effect of BABA treatment on the yield and yield components of the two spring wheat cultivars exposed to three levels of water availability from 30 DAS (jointing stage) was assessed. One day prior to the water treatments, 20 of the 50 pots were pretreated with (+BABA) or without (–BABA) drenching the soil with 100 µM BABA. Water deficits were imposed by withholding water until the SWC reached three predetermined levels of stress: (i) 10 pots were maintained near 90% FWC by daily watering after 18.00h, but were not treated with BABA (control); (ii) 20 pots were allowed to dry until the SWC was about 55% FWC and then maintained at this water content by daily watering after 18.00h (moderate stress, MS); and (iii) 20 pots were allowed to dry until the SWC was about 35% FWC and then watered daily after 18.00h to maintain this water content (severe stress, SS). Each treatment and cultivar combination was replicated five times. Whole plants were harvested at physiological maturity (~110 DAS), as determined by the complete loss of green colour from the glumes (Hanft and Wych, 1982). Wheat roots were washed free of soil using a 0.4mm screen. The plants were divided into grain, shoots (including leaves and husks) and roots, dried for 48h at 80 °C and weighed. Data on water use were obtained by recording the added water from sowing to harvest. The following variables were calculated: Harvest index (HI)=grain dry matter/above-ground dry matter, and water use efficiency for grain (WUEG)=grain yield/water use from sowing to harvest. As soil evaporation was minimized by the covering the soil with perlite, water use is principally a measure of the rate of transpiration and WUEG is in fact transpiration efficiency for grain (TEG ).
Analysis of reactive oxygen species (ROS)
O2- production was measured using nitrite formation from hydroxylamine in the presence of O2- (Elstner and Heupel, 1976). Leaf segments (0.5g) was homogenized with 5ml of 50mM potassium phosphate (pH 7.8) and centrifuged at 5000 g for 600 s at 4 °C. The incubation mixture contained 1ml of 1mM hydroxylamine hydrochloride [using 50mM phosphate buffer (pH 7.8) as solvent] and 1ml of supernatant. After incubation at 25 °C for 1200 s, 17mM sulphanilamide and 7mM α-naphthylamine were added. After reaction at 25 °C for 1200 s, the absorbance was measured in aqueous solution at 530nm. H2O2 was measured by monitoring the absorbance of the titanium-peroxide complex at 415nm (Brennan and Frekel, 1977), calibrated against a standard curve with known H2O2 concentrations.
Enzyme assays
Frozen leaf segments (0.5g) were crushed into fine powder with a mortar and pestle under liquid N2. The soluble proteins were extracted by homogenizing with 10ml of 50mM potassium phosphate buffer(pH 7.0) containing 1mM EDTA and 1% polyvinylpyrrolidone (PVP), with the addition of 1mM ascorbate acid (ASC) for the APX assay. The homogenate was centrifuged at 12 000 g for 1200 s at 4 °C and the supernatant used for the following enzyme assays.
Total superoxide dismutase (SOD, EC 1.15.1.1) activity was assayed by monitoring the inhibition of the photochemical reduction of nitro blue tetrazolium (NBT) according to the method of Giannopolitis and Ries (1977). The 3ml reaction mixture contained 50mM potassium phosphate buffer (pH 7.8), 75 µM NBT, 13mM methionine, 2 µM riboflavin, 0.1mM EDTA, and 100 µl enzyme extract. The reaction mixtures were illuminated for 900 s at a light intensity of 100 µmol m–2 s–1. One unit of SOD activity was defined as the amount of enzyme required to cause 50% inhibition of the reduction of NBT as monitored at 560nm.
Catalase (CAT, EC 1.11.1.6) activity was measured by the disappearance of H2O2 (extinction coefficient 39.4mM–1cm–1) at 240nm for 180 s(Aebi, 1984). The reaction mixture contained 50mM potassium phosphate buffer (pH 7.0), 10mM H2O2 and 200 µl of enzyme extract in a 3ml volume. The reaction was initiated by adding the enzyme extract.
Ascorbate peroxidase (APX, EC 1.11.1.11) activity was measured in a 1ml reaction volume containing 50mM potassium phosphate buffer (pH 7.0), 0.5mM ASC, 0.1mM H2O2, and 200 µl of enzyme extract by following the decrease in A290 (extinction coeffcient 2.8mM–1cm–1) for 60 s. The reaction was started by adding H2O2 (Amako et al., 1994).
Gluthione reductase (GR, EC 1.6.4.2) activity was determined by following the oxidation of NADPH at 340nm (extinction coeffcient 6.2mM–1 cm–1) for 180 s in 1ml of an assay mixture containing 50mM potassium phosphate buffer (pH 7.8), 0.15mM NADPH, 2mM Na2EDTA, 0.5mM oxidized glutathione (GSSG), and 150 µl of enzyme extract. The reaction was initiated by adding NADPH. Corrections were made for the background absorbance at 340nm, without NADPH (Schaedle and Bassham, 1977).
Lipid peroxidation
The level of lipid peroxidation was determined by the content of malondialdehyde (MDA) in the leaf segment homogenates, prepared in 10ml of 10% trichloroacetic acid (TCA) containing 0.5% thiobarbituric acid (TBA) as described by Zhao et al. (1994). The mixture was heated in a water bath at 100 °C for 900 s and then quickly cooled in an ice bath. After centrifugation at 4000 g for 900 s, the absorbance of the supernatant was recorded at 532, 600, and 450nm. The MDA content was calculated by its absorbance (Zhao et al., 1994) and expressed as nmol MDA g–1 dry weight.
Abscisic acid (ABA) extraction, purification, and quantification
The methods for extraction and purification of ABA were modified from those described by Bollmark et al. (1988) and He (1993). The leaf samples were ground in liquid N2 using a mortar and pestle, extracted with ice-cold 80% methanol (v/v) containing 1mM butylated hydroxytoluence to avoid oxidation, and then stored overnight at 4 °C. The extracts were then centrifuged at 10 000 g for 900 s at 4 °C. The residues were suspended in the same ice-cold extraction solution and stored at 4 °C for 1h, and then centrifuged again at 10 000 g for 900 s at 4 °C. The supernatants were combined and passed through Chromosep C18 columns (C18 Sep-Park Cartridge, Waters, Millford, MA, USA), prewashed with 10ml of 100% and 5ml of 80% methanol, respectively. The efflux was collected and dried by evaporation with nitrogen. The residues were dissolved in 1.6ml of phosphate-buffered saline (PBS) containing 0.1% (v/v) Tween 20 and 0.1% (w/v) gelatin (pH 7.5) for analysis by enzyme-linked immunosorbent assay (ELISA).
The mouse monoclonal antigen and antibody against ABA and the immunoglobulin G–horse radish peroxidase (IgG-HRP) used in ELISA were produced at the Phytohormones Research Institute, China Agricultural University, Beijing, China (He, 1993). The method for quantification of ABA by ELISA has been described previously (Yang et al., 2001). In the current study, the percentage recovery of each hormone was calculated by adding known quantities of standard hormone to a split extract. The recovery percentage of ABA in leaves was 85.6±5.2%. The specificity of the monoclonal antibody was confirmed and the possibility of other non-specific inhibitors was excluded in previous studies (Wu et al., 1988; Yang et al., 2001).
Statistical analysis
The values of leaf gs and leaf RWC were expressed relative to the control plants. Other data are presented as means of three replicate samples in Experiment 1, six replicate samples in Experiment 2 and five replicate samples in Experiment 3. All measured variables were examined by analysis of variance (ANOVA). Mean comparisons were made by Fisher’s protected least significant difference (LSD) at P <0.05 significance level. All data analyses were conducted using the Statistical Analysis System (SAS Institute., 2001).
The relative values of gs and RWC equal:
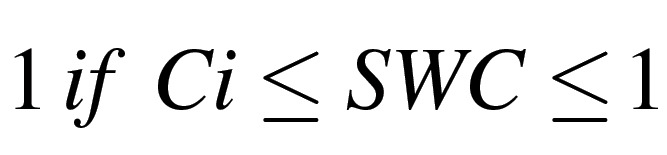 |
(1a) |
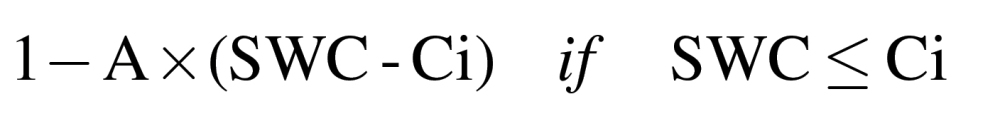 |
(1b) |
where A is the slope of the linear equation (1b) and Ci is the threshold of SWC at which the measured traits started to diverge, i.e. increase or decrease, from 1.
To estimate A and Ci in the linear-plateau model (equation 1), PROC NLIN of PC SAS was employed. The coefficient of determination (r 2) was calculated for each curve as 1–SSE/CSS, where SSE is the residual sum of squares and CSS is the corrected total sum of squares. Statistical separations between different plant physiological processes were from comparisons of coefficients in equation 1b at P <0.05 (Liu et al., 2003).
Results
β-Aminobutyric acid (BABA) enhanced drought-induced ABA accumulation, reduced water use, and increased desiccation tolerance
Table 1 shows the time in days after treatment began and the soil water content at the six harvest times for the two cultivars and two BABA treatments. Harvest 2 (H2) occurred earlier in LC than in GS, while H4 was earlier and at a higher SWC in GS than LC (Table 1). BABA treatment significantly (P <0.05) decreased the rate of soil drying so that while both temporary wilting (TW, harvest 5), and permanent wilting (PW, harvest 6), occurred at the same SWC, this SWC was one day later in the BABA treatment in both cultivars (Table 1).
Table 1.
Time to the six harvests, and soil water content (mean±SD) at harvest in two spring wheat cultivars, Gansu 69 (GS) and Longchun 8275 (LC), with (+) and without (–) treatment with 100 µM β-aminobutyric acid (BABA). The rate of soil drying (% h–1) during progressive soil drying is also given. Means within column with the same letter are statistically similar at P <0.05.
| Cultivars | Chemical treatment | Time (d) | Soil water content (% FWC) | Rate of soildrying | ||||||||||
| H1 | H2 | H3 | H4 | H5 | H6 | H1 | H2 | H3 | H4 | H5 | H6 | (% h–1) | ||
| GS | –BABA | 1 | 6 | 8 | 9 | 15 | 18 | 87.6±0.2 a | 61.9±0.8 c | 50.4±0.8 a | 46.4±0.5 a | 26.7±0.4 a | 22.4±0.2 a | 0.16±0.001 a |
| +BABA | 1 | 5 | 8 | 9 | 16 | 19 | 87.7±0.1 a | 67.8±0.9 b | 52.5±1.1 a | 47.3±0.4 a | 26.7±0.5 a | 21.6±0.5 a | 0.15±0.001 b | |
| LC | –BABA | 1 | 5 | 8 | 12 | 16 | 18 | 87.3±0.2 a | 67.1±1.5 b | 52.4±0.6 a | 36.2±0.5 b | 25.8±0.9 a | 22.4±0.2 a | 0.16±0.001 a |
| +BABA | 1 | 3 | 8 | 12 | 17 | 19 | 87.4±0.3 a | 72.4±0.3 a | 53.1±1.6 a | 37.0±0.3 b | 25.5±0.4 a | 22.1±0.6 a | 0.15±0.002 b | |
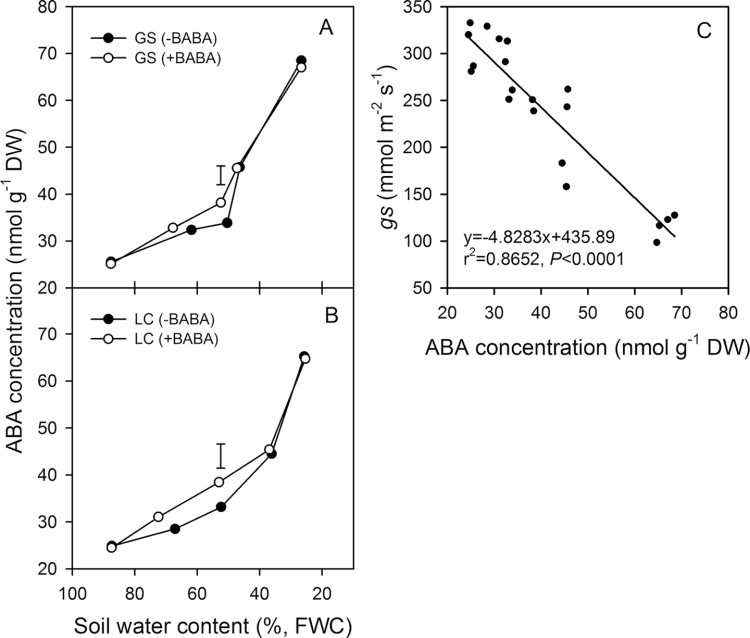
Drenching with BABA significantly (P <0.05) increased ABA accumulation in both cultivars, especially at H2 (P <0.05) when SWC decreased below 62% and 67% in GS and LC, respectively (Table 1), and was higher in GS than in LC (Fig. 1A, 1B), but with the further decrease in SWC there was no difference between BABA treatments after H3. These results indicate that BABA enhances drought-induced ABA accumulation in wheat. The increase in ABA with soil drying was associated with a decrease in stomatal conductance (Fig. 1C). Crucially, ABA increased from 25 nmol g–1 DW to 40 nmol g–1 DW, inducing a decrease in stomatal conductance of 72 mmol m–2 s–1 (Fig. 1C) before RWC decreased. This suggests that ABA was acting as a non-hydraulic root-sourced signal (nHRS). ABA in the leaf continued to increase as the soil dried (Fig. 1A, 1B) and RWC began to decrease, indicating that it may also have played a role in reducing stomatal conductance as RWC decreased (Fig. 1C).
Fig. 1.
Effects of drenching with β-aminobutyric acid (BABA) on the concentration of leaf ABA in two spring wheat cultivars, Gansu 96 (GS) (A) and Longchun 8725 (LC) (B), during progressive soil drying. One day prior to withholding water, plants were pretreated with (+) or without (–) 100 µM BABA. Values are means and the vertical lines show LSD values (n=3, P = 0.05). (C) Relationship between leaf ABA concentration and stomatal conductance (gs) in the two spring wheat cultivars subjected to progressive soil drying. The line is the fitted linear regression.
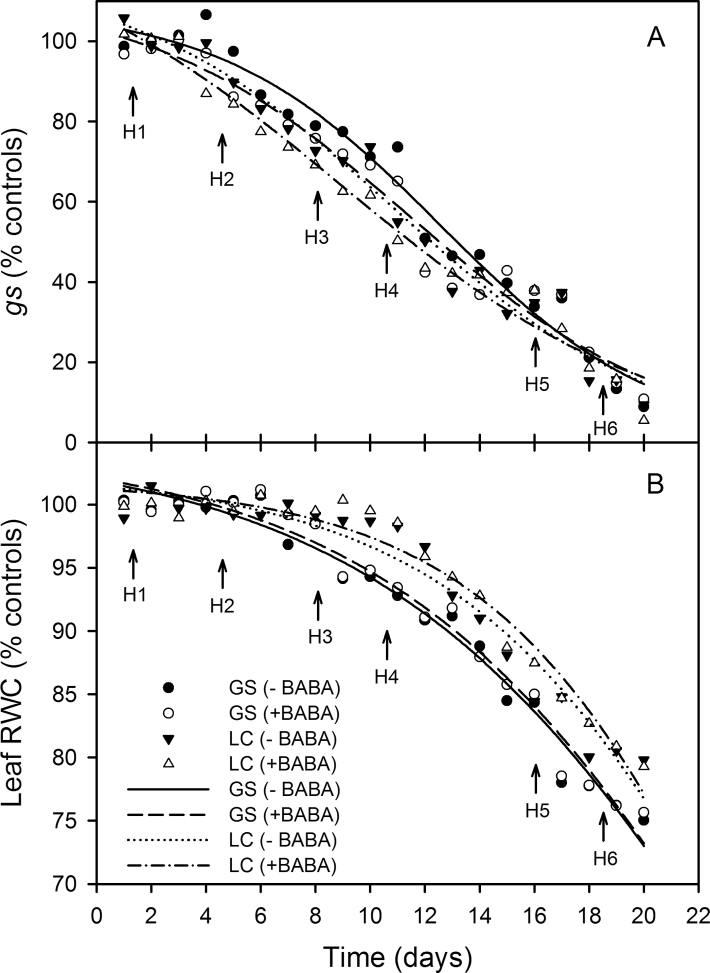
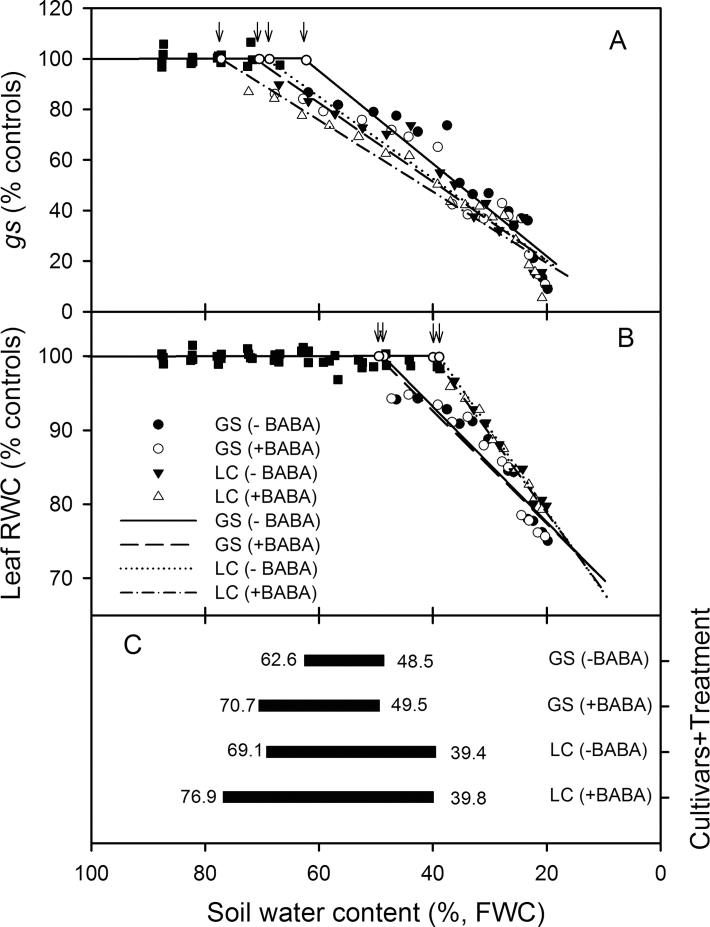
The relative leaf gs and RWC in drought-affected plants with or without BABA treatment were plotted as function of time (Fig. 2A, 2B). Leaf RWC decreased earlier in GS than LC and the addition of BABA did not influence this in both cultivars. However, the leaf gs decreased earlier in LC than GS and the addition of BABA decreased the stomatal conductance at any one time (Fig. 2A). The SWC thresholds of leaf gs and RWC, relative to the well-watered controls, were determined by linear-plateau functions (equation 1; Fig. 3A, 3B). When SWC dropped below a threshold value, gs and RWC decreased linearly. The SWC thresholds for gs were 62.6, 70.7, 69.1, and 76.9% (Fig. 3A) and for leaf RWC were 48.5, 49.5, 39.4, and 39.8% (Fig. 3B), in GS –BABA, GS +BABA, LC –BABA, and LC +BABA (Fig. 3C), respectively. The SWC threshold range over which gs decreased but RWC was unchanged, the indication of nHRS activity, was 29.7% for LC (69.1–39.4%), much greater than the 14.1% for GS (62.6–48.5%) (Fig. 3C). Drenching the soil with BABA significantly broadened the SWC threshold range in both cultivars to 21.2% (70.7–49.5%) in GS and to 37.1% (76.9–39.8%) in LC (Fig. 3C).
Fig. 2.
Change of stomatal conductance (gs) as a percentage of the controls (A) and leaf relative water content (RWC) as a percentage of the controls (B) with time after water was withheld in two spring wheat cultivars, Gansu 96 (GS) and Longchun 8725 (LC), with (+) and without (–) treatment with 100 µM β-aminobutyric acid (BABA). Sigmoid curves (y=100/[1+exp{(–x–x0)}]/b) were fitted. Arrows indicate the six harvest times (H1–H6).
Fig. 3.
Relationships between (A) stomatal conductance (gs, % controls) and soil water content (SWC, % field water capacity, FWC); (B) leaf relative water content (RWC, % controls) and SWC (% FWC), and (C) the change in SWC threshold between the threshold for a decrease in gs and the threshold for a decrease in RWC for two spring wheat cultivars, Gansu 96 (GS) and Longchun 8725 (LC), with (+) and without (–) 100 µM β-aminobutyric acid (BABA). Lines were fitted by a linear-plateau function (equation 1). Arrows indicate the threshold values which are summarized in (C). In (C) values to the left of the black bars are the threshold values for a decrease in gs from (A) and those to the right of the black bars are the threshold values for a decrease in RWC from (B).
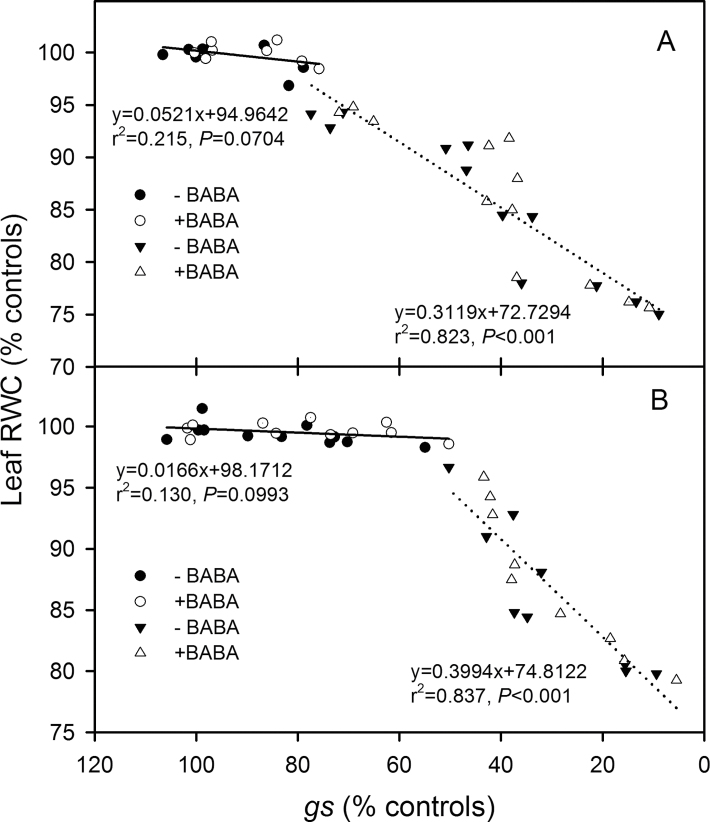
When the relationship between leaf gs and RWC was plotted, it was clear that gs decreased by about 20% in GS and by about 50% in LC without any change in RWC whether the soil was drenched with BABA or not (Fig. 4); this result indicates that gs is governed by ABA rather than leaf RWC during the nHRS range. When the leaf RWC significantly decreased, gs was positively correlated with leaf RWC (P <0.001), but there was no difference in slope when drenched or not with BABA (Fig. 4). This suggests that BABA does not influence the sensitivity of the stomata to leaf water status.
Fig. 4.
Relationship between leaf relative water content (RWC, % controls) and stomatal conductance (gs, % controls) in two spring wheat cultivars, Gansu 96 (A) and Longchun 8275 (B) with (+, open symbols) and without (–, closed symbols) 100 µM β-aminobutyric acid (BABA). The liner regressions (y=ax+b) were fitted separately to data in which leaf RWC was unchanged (circles, solid line) and data in which RWC decreased (triangles, dotted line).
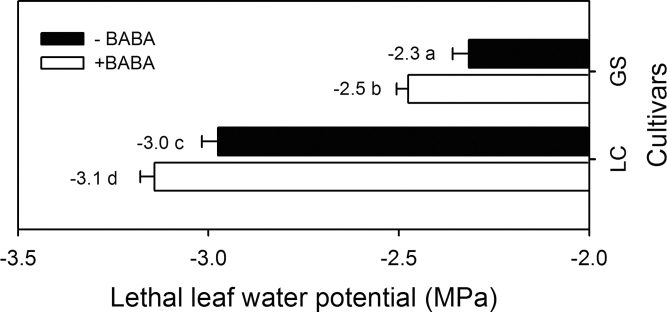
The lethal Ψ of GS (–2.3MPa) was higher than that of LC (–3.0MPa), indicating that GS was more sensitive to foliar dehydration (Fig. 5). Treatment with BABA significantly reduced the lethal Ψ, compared with no BABA treatment; the lethal Ψ of GS and LC with BABA treatment were –2.5 and –3.1MPa, respectively (Fig. 5). As lethal Ψ was used as an indicator of desiccation tolerance, these results show that BABA enhanced the desiccation tolerance in response to soil drying.
Fig. 5.
The effect of treatment with β-aminobutyric acid (BABA) on the lethal leaf water potential of two spring wheat cultivars, Gansu 96 (GS) and Longchun 8725 (LC) treated with (open bars) and without (solid bars) 100 µM BABA. Values are means +1 standard error of the mean (n=6).
β-Aminobutyric acid (BABA) reduced the reactive oxygen species (ROS) and lipid membrane peroxide levels
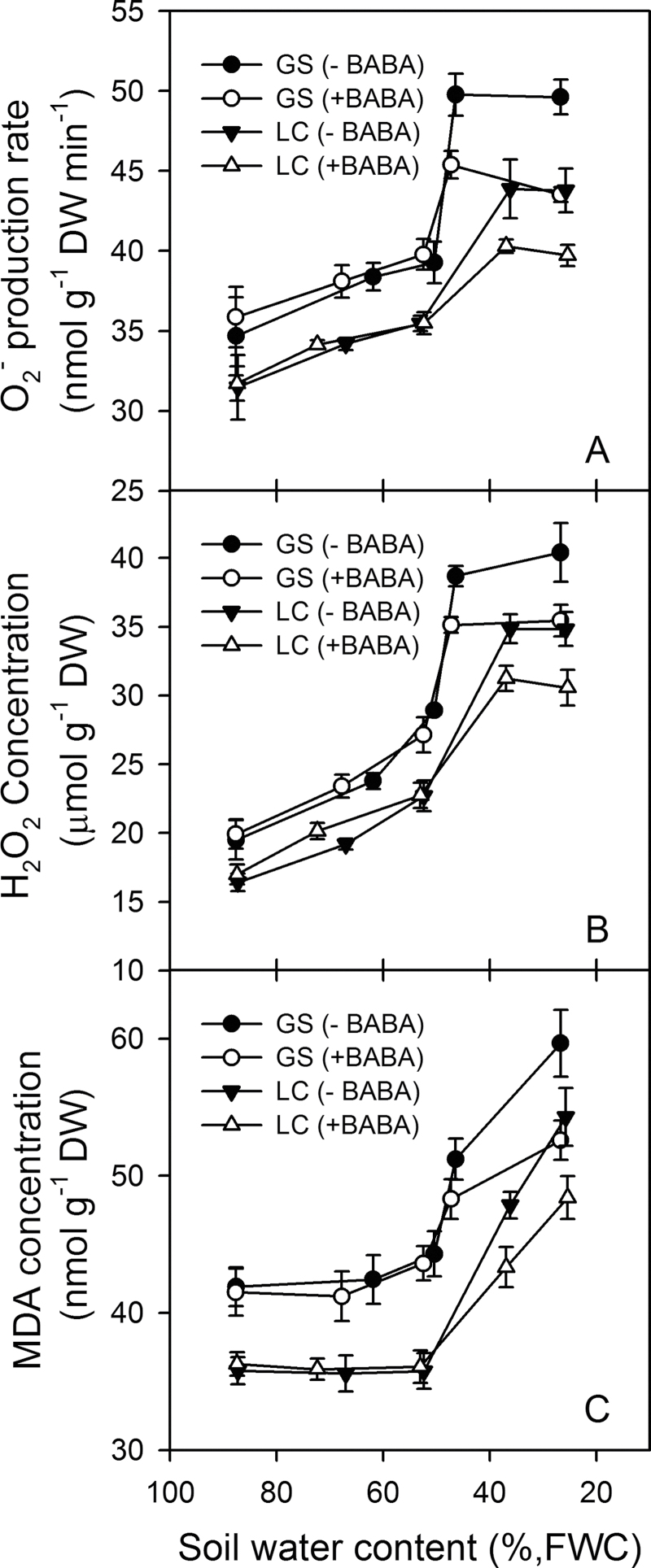
O2-
and H2O2 contents of the leaves were higher in GS than LC and increased linearly as the soil dried to about 50% SWC and then increased significantly at SWC values below this (Fig. 6A, 6B). Lipid membrane peroxide, as measured by MDA content (Fig. 6C), was always higher in GS than in LC during SWC depletion, but unlike
Fig. 6.
Changes with soil water content (% field water capacity, FWC) in (A), production rate of reactive oxygen species (O2-); (B) hydrogen peroxide concentration (H2O2), and (C) malondialdehyde (MDA) concentration in leaves of two spring wheat cultivars, Gansu 96 (GS) and Longchun 8725 (LC), treated with (+) and without (–) 100 µM β-aminobutyric acid (BABA). Values are means ±1 standard error of the mean (n=3).
O2-
and H2O2 it did not increase until the SWC was below 50%. Drenching the soil with BABA decreased the drought-induced
O2-
, H2O2, and MDA accumulation when SWC fell below 50% in both cultivars (Fig. 6). The plants in the BABA treatment had significantly reduced MDA content at severe stress levels, thereby reducing the oxidative damage to membranes.
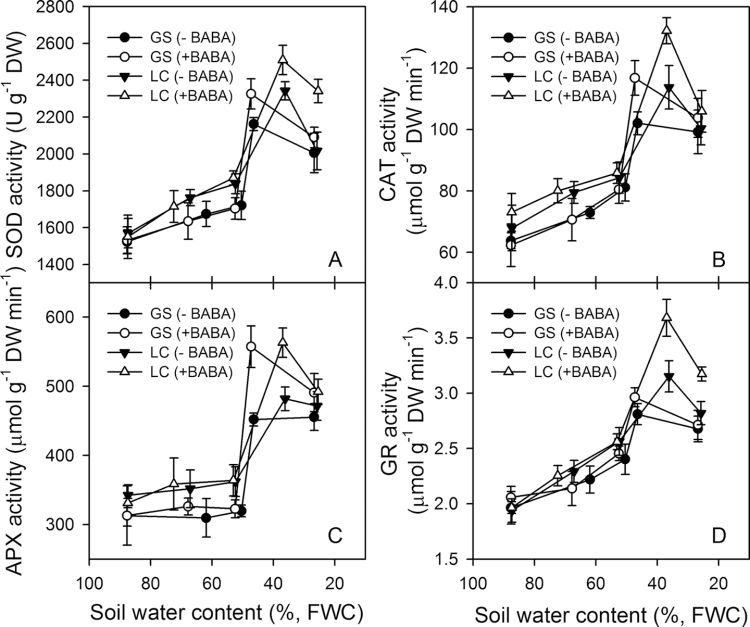
β-Aminobutyric acid (BABA) increased antioxidant enzyme activities
The accumulation of drought-induced antioxidant enzymes are shown in Fig. 7. At SWC above about 50%, there were no significant differences in SOD (Fig. 7A), CAT (Fig. 7B), and GR (Fig. 7D) activities between the two cultivars, regardless of whether drenched with BABA or not. The activity of SOD, CAT, and GR increased linearly as the soil dried to about 50% SWC and then increased markedly at lower SWCs in both cultivars (Fig. 7A, 7B, 7D). The APX activity (Fig. 7C) was unchanged until the SWC decreased below 50% and then increased markedly. At severe water deficits below 50% SWC, the BABA-treated plants had higher levels of all four enzymes and tended to be higher in LC than GS (Fig. 7).
Fig. 7.
Changes with soil water content (% field water capacity, FWC) on the activity of the antioxidant enzymes: (A) superoxidae dismutase (SOD), (B) catalase (CAT), (C) ascorbate peroxidae (APX), and (D) glutathione reductase (GR) in leaves of two spring wheat cultivars, Gansu 96 (GS) and Longchun 8725 (LC), treated with (+) and without (–) 100 µM β-aminobutyric acid (BABA). Values are means ±1 standard error of the mean (n=3).
With soil drying β-aminobutyric acid (BABA) decreased water use without affecting grain yield
Drought stress significantly decreased root dry weight, above-ground dry weight, and grain yield in both cultivars (Table 2), but drenching with BABA had no effect on these parameters in either cultivar. Consistent with its reportedly higher drought resistance, the grain yield of the modern cultivar LC was significantly higher than that of the older cultivar GS under moderate drought stress (55% FWC), but there were no significant difference in both well-watered (90% FWC) and severe drought stress (35% FWC). The harvest index (HI) of the modern cultivar, LC, was significant higher than that of the older cultivar, GS, in all three water treatments. The 1000-kernel weight (TKW) of LC was markedly higher than that of GS, but water shortage has no effect on kernel size. Water shortage reduced the grain number per pot in both cultivars, but to compensate for the smaller kernels in GS, there were more kernels produced in GS than LC at all three levels of water stress. Treatment with BABA had no effect on any of the components of grain yield (Table 2).
Table 2.
Root dry weight (g pot–1), above-ground dry weight (g pot–1), grain number (pot–1), 1000-kernel weight (TKW, g), grain yield (g pot–1), harvest index (HI), water use (kg pot–1) and water use efficiency for grain (WUEG, grain yield g kg–1 water use) of two spring wheat cultivars, Gansu 96 (GS) and Longchun 8275 (LC) given three different water treatments [maintained about 90% field water capacity (FWC); maintained about 55% FWC, and maintained at 35% FWC] and treated with (+) or without (–) 100 µM β-aminobutyric acid (BABA)
| Cultivar | Watertreatment | Chemical treatment | Root dry weight | Above-grounddry weight | Grain number | TKW | Grain yield | HI | Water use | WUEG |
| GS | 90% | Control | 4.34 | 113.78 | 1617.4 | 25.03 | 40.48 | 0.36 | 38.0 | 1.07 |
| 55% | –BABA | 3.61 | 78.73 | 1088.0 | 25.97 | 28.25 | 0.36 | 24.2 | 1.17 | |
| +BABA | 3.62 | 79.12 | 1089.2 | 26.26 | 28.57 | 0.36 | 22.9 | 1.25 | ||
| 35% | –BABA | 3.13 | 49.52 | 703.0 | 26.03 | 18.29 | 0.37 | 20.4 | 0.90 | |
| +BABA | 3.14 | 51.06 | 708.0 | 25.40 | 17.98 | 0.35 | 20.5 | 0.88 | ||
| LC | 90% | Control | 3.74 | 102.03 | 1267.6 | 32.14 | 40.73 | 0.40 | 32.8 | 1.24 |
| 55% | –BABA | 3.35 | 76.99 | 921.4 | 34.39 | 31.68 | 0.41 | 22.6 | 1.40 | |
| +BABA | 3.39 | 76.24 | 926.8 | 34.10 | 31.56 | 0.41 | 21.5 | 1.47 | ||
| 35% | –BABA | 2.87 | 46.47 | 557.8 | 33.39 | 18.60 | 0.40 | 18.7 | 1.00 | |
| +BABA | 2.90 | 47.85 | 553.0 | 33.10 | 18.30 | 0.38 | 19.1 | 0.96 | ||
| LSD0.05 | 0.21 | 4.18 | 53.5 | 1.14 | 1.40 | 0.03 | 0.96 | 0.05 |
The two soil drying treatments decreased water use significantly in both cultivars, while BABA application reduced water use significantly at the moderate stress level (55% FWC) (Table 2). The WUEG or TEG was greater in LC than GS at all levels of water availability and increased with moderate stress (55% FWC), but not severe stress (35% FWC), in both cultivars (Table 2). The decrease in water use in the BABA treatment at moderate stress with no effect on grain yield led to a significant increase in WUEG and TEG.
Discussion
In this study, BABA was shown to enhance drought-induced ABA accumulation in the leaves, resulting in partial stomatal closure and reduced water use at moderate stress. As the grain yield was not affected by BABA, WUEG (as soil evaporation was minimized, it was really TEG) was higher at the moderate stress level after the soil was drenched with BABA than in the control treatment in both cultivars of wheat. Previous research has shown that BABA induced abiotic stress tolerance in Arabidopsis through priming ABA- and/or SA-dependent responses (Jakab et al., 2005). ABA plays an important role in ‘root-to-shoot’ communication (Zhang and Davies, 1990) and water stress signal transduction (Jiang and Zhang, 2002a, b) in plants. The earlier and faster ABA accumulation in response to soil drying (Fig. 1A, 1B) after drenching the soil with BABA, that is, BABA-primed ABA accumulation, triggered stomatal closure (Fig. 1C), thus leading to partial stomatal closure at a higher SWC in both wheat cultivars (Fig. 3A, 3C; Table 1). One of the interesting findings is that such a small drought-induced increase of endogenous ABA in the leaves of wheat triggered stomata closure. Considering the small increases of ABA concentration induced by BABA treatment (Fig. 1), the results suggest that the BABA increased the sensitivity of stomata to ABA; the high sensitivity of stomata to small changes in ABA concentration were also found in Phaseolus vulgaris L. (Trejo and Davies, 1991), but not previously reported in wheat. However, addition of BABA to the soil did not alter the SWC at which the leaf RWC declined (Fig. 3B), thereby broadening the soil water threshold range of nHRS from 14.1% and 29.7% FWC in GS and LC without BABA to 21.2% and 37.1% FWC, respectively, after the drenching with BABA (Fig. 3C). Previous research showed that the threshold range of nHRS at the different times of release of spring wheat cultivars (Xiong et al., 2006a; Fan et al., 2008), winter wheat cultivars (Wang et al., 2008), and the different ploidy levels of wheat (Xiong et al., 2006b) were significantly correlated with increased desiccation tolerance (lower lethal Ψ), a higher WUEG, and a smaller percentage reduction in biomass. This led Wang et al. (2008) to propose that the threshold range of nHRS was associated with increased drought resistance. In the present study, the enhancement of desiccation tolerance and WUEG was associated with a wider threshold range of nHRS with BABA treatment. However, BABA did not affect the grain yield in both the moderate and severe stress treatments. As grain yield with limited water is the true measure of drought resistance in grain crops, by this measure BABA, does not appear to increase drought resistance.
Drought, salt, and cold stress all induce the production of reactive oxygen species (ROS) such as superoxide, hydrogen peroxide, and hydroxyl radicals (Apel and Hirt, 2004), leading to oxidative stress in plant tissues. ROS, especially hydrogen peroxide, acts as a signal in guard cells (McAinsh et al., 1996). Jiang and Zhang, (2002a, b) found that ABA could trigger ROS accumulation in maize seedlings. In the present study, the levels of ROS significantly increased with ABA accumulation in two wheat cultivars, both with or without BABA treatment; and the modern cultivar, LC, had lower ROS levels than that of old cultivar, GS (Fig. 6). This result is similar to that observed previously in three winter wheat cultivars (Wang et al., 2008) and three spring wheat cultivars (Fan et al., 2009). In addition, when water shortage became more severe, the ROS accumulation of plants with BABA treatment was lower than that without BABA treatment (Fig. 6A, 6B). However, the oxidative damage to lipid membranes only occurred when the soil water was almost depleted, not when the ROS began to increase at moderate levels of water depletion (Fig. 6C). The data suggest that the antioxidant enzymes SOD, CAT, and GR, that all increased in concert with ROS (Fig. 7), may have mitigated the effects of the ROS. SOD catalyses the dismutation of O2-
to H2O2, while CAT, APX, and GR are responsible for the removal of H2O2 (Apel and Hirt, 2004). The MDA content in the modern cultivar was lower than that of the old cultivar, and drenching with BABA reduced the MDA content when the soil was very dry and the plants under severe stress (Fig. 6C). While there was no significant differences between the cultivars in the levels of SOD, CAT, APX, and GR at moderate stress levels, the antioxidant enzymes may have played a role in mitigating the effects of ROS that were higher in the old than in the modern cultivar, and may be a part explanation for the reduced level of membrane damage at severe water shortage when the soil was drenched with BABA (Fig. 6C). Alternatively, BABA reduced the level of ROS in the leaves which may also be a reason for the reduced lipid damage, as expressed by MDA concentration, at severe water shortage (Fig. 6C). These results are similar to those observed in winter wheat and spring wheat subjected to soil drying (Wang et al., 2008; Fan et al., 2009). Recently, BABA has been shown to alter the stress-related gene expression of Arabidopsis under salt, drought (Jakab et al., 2005), and heat stress (Zimmerli et al., 2008). These results in wheat suggested that the enhancement the activities of antioxidant enzymes may have been associated with the up-regulation of these antioxidant-enzyme genes.
Previous research has shown that tree seedlings (Tyree et al., 2003) and wheat (Xiong et al., 2006a) with a lower lethal Ψ had higher desiccation tolerance. In the present study, the addition of BABA significantly decreased (more negative) the lethal Ψ of both cultivars of wheat (Fig. 5). In addition, drenching with BABA decreased water use and improved the WUEG, but did not increase the grain yield under moderate water stress conditions (Table 2). Thus, the BABA treatment increased the desiccation tolerance of the wheat, but this did not influence its drought resistance, defined as yield under water-limited conditions.
The results in the present study show that BABA had no effect on dry matter accumulation and partitioning, and therefore grain yield, in both wheat cultivars when the soil was dried (Table 2) despite the increase in ABA production, stomatal closure, reduced water use, increased desiccation tolerance, and anti-oxidative defence at the leaf level. This is surprising in light of the observations of Travaglia et al. (2010) who found that, in a water-limited environment, spraying 300 µg g–1 ABA directly on to the leaves of wheat increased the grain yield in the field. One possible explanation for the lack of an effect on grain yield is that BABA did not increase the level of ABA in the leaf to a high enough concentration to affect the yield. The ABA concentration in the leaf was only increased by about 20% by drenching the soil with BABA, possibly because of the metabolism of BABA by soil microbes after drenching, even though the soil in the present study was sterilized by solarization. As far as is known there are no reports of the fate of BABA in the soil and it would be interesting to see whether increased grain yields in water-limited environments could be generated by more frequent or higher concentrations of drenching with BABA, or by exogenous application directly to the leaves.
Abiotic stress, especially by salinity and drought, are the primary causes of crop losses worldwide, causing average yield losses of more than 50% for major crops (Bray et al., 2000). The yield loss is exacerbated by the predicted reduction in available arable land and available water resources in the future. Recently, the integration of new technologies, including traditional trait-based breeding with molecular breeding, have been used to breed crop cultivars with a higher yield potential and greater stress tolerance, thereby accelerating the rates of genetic gain (Reynolds et al., 2009). Jakab et al. (2005) proposed that priming the existing defence mechanisms, rather than manipulation of the genome, is another way of increasing the stress tolerance of crops. BABA-induced resistance through priming stress-tolerance mechanisms has been proposed as a way of generating crop varieties with an enhanced defensive capacity against biotic and abiotic stresses (Ton et al., 2005).
Beckers and Conrath (2007) showed that BABA can be used to prime crops against biotic stresses. Foliar application of BABA has already successfully primed grapevines (Vitis vinifera L.) against Plasmopara viticola (downy mildew) (Reuveni et al., 2001), while soil drenching has been used to prime melons (Cucumis melo L.) against Monosporascus cannonballus (Cohen, 2002). In the present study, BABA effectively primed pre-existing defence pathways that reduced water use and increased the desiccation tolerance and WUEG of wheat. There are several application methods for priming plants with BABA, such as soil drenching, foliar spraying, and stem injection (Cohen, 2002). Future work should determine which combination of application methods and intervals between treatments is the most effective and cost-effective in increasing yields under water-limited conditions. In addition, the present results in this study open the possibility of the future use of BABA in agriculture, as long as the cost of BABA is not too high. Its use will not be harmful to the environment because BABA, as an organic compound, can be degraded by microorganisms.
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/2.0/uk/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Acknowledgements
This research was supported by National Nature Science Foundation of China (30625025), the ‘111’ programme (B07051), and the Program for Changjiang Scholars and Innovative Research Team in University of Ministry of Education of China, the UWA Institute of Agriculture and Centre for Legumes in Mediterranean Agriculture at the University of Western Australia. We also thank two anonymous reviewers for their helpful comments on an earlier version of this paper.
© 2012 The Author(s).
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0), which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Glossary
Abbreviations:
- ABA
abscisic acid
- APX
ascorbate peroxidase
- ASC
ascorbic acid
- BABA
β-aminobutyric acid
- CAT
catalase
- DAS
day after sowing
- FWC
field water capacity
- GR
glutathione reductase
- GSSG
oxidized glutathione
- gs
stomatal conductance
- HS
hydraulic signals
- MDA
malondialdehyde
- NADPH
nicotinamide adenine dinucleotide phosphate
- NBT
nitro blue tetrazolium
- nHRS
non-hydraulic root-sourced signals
- PVP
polyvinylpyrrolidone
- PW
permanent wilting
- ROS
reactive oxygen species
- RWC
relative water content
- SA
salicylic acid
- SOD
superoxide dismutase
- SWC
soil water content
- TCA
trichloroacetic acid
- TEG
transpiration efficiency for grain
- TW
temporary wilting
- WUEG
water use efficiency for grain
- Ψ
leaf water potential
Footnotes
This paper is available online free of all access charges (see http://jxb.oxfordjournals.org/open_access.html for further details)
References
- Aebi H. 1984. Catalase in vitro In: Packer L, ed. Methods in enzymology Vol. 105 London: Academic Press; 121 126 [DOI] [PubMed] [Google Scholar]
- Amako K, Chen GX, Asada K. Separate assays specific for ascorbate peroxidase and guaiacol peroxidase and for the chloroplastic and cytosolic isozymes of ascorbate peroxidase in plants. Plant and Cell Physiology. 1994;35:497–504. [Google Scholar]
- Apel K, Hirt H. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annual Review of Plant Biology. 2004;55:373–399.. doi: 10.1146/annurev.arplant.55.031903.141701. [DOI] [PubMed] [Google Scholar]
- Asada K. The water–water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photons. Annual Review of Plant Physiology and Plant Molecular Biology. 1999.;50:601–639. doi: 10.1146/annurev.arplant.50.1.601. [DOI] [PubMed] [Google Scholar]
- Augé RM, Duan X, Croker JL, Witte WT, Green CD. Foliar dehydration tolerance of 12 deciduous tree species. Journal of Experimantal Botany. 1998;49:753–759. [Google Scholar]
- Beckers GJM, Conrath U. Priming for stress resistance: from the lab to the field. Current Opinion in Plant Biology. 2007;10:425–431. doi: 10.1016/j.pbi.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Bollmark M, Kubat B, Eliasson L. Variation in endogenous cytokinin content during adventitious root formation in pea cuttings. Journal of Plant Physiology. 1988;132:262–265. [Google Scholar]
- Bray EA, Bailey-Serres J, Weretilnyk E. 2000. Responses to abiotic stresses. In: Gruissem W, Buchannan B, Jones R, eds. Biochemistry and molecular biology of plants Rockville, MD:American Society of Plant Physiologists; 1158 1249 [Google Scholar]
- Brennan T, Frekel C. Involvement of hydrogen peroxide in the regulation of senescence in pear. Plant Physiology. 1977;59:411–416. doi: 10.1104/pp.59.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cissé N, Thiaw S, Ndiaye M, Hall A. Guide de production de niébé. Fiches Techniques ISRA. 1996;6:1–12. [Google Scholar]
- Cohen YR. β-aminobutyric acid-induced resistance against plant pathogens. Plant Disease. 2002;86:448–457. doi: 10.1094/PDIS.2002.86.5.448. [DOI] [PubMed] [Google Scholar]
- Davies WJ, Tardieu F, Trejo CL. 1994. How do chemicalsignals work in plants that grow in drying soil? Plant Physiology 104 309 314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elstner EF, Heupel A. Inhibition of nitrite formation from hydroxylammoniumchloride: a simple assay for superoxide dismutase. Analytical Biochemistry. 1976;70:616–620. doi: 10.1016/0003-2697(76)90488-7. [DOI] [PubMed] [Google Scholar]
- Fan XW, Li FM, Song L, Xiong YC, An LZ, Jia Y, Fang XW. Defense strategy of old and modern spring wheat varieties during soil drying. Physiologia Plantarum. 2009;136:310–323. doi: 10.1111/j.1399-3054.2009.01225.x. [DOI] [PubMed] [Google Scholar]
- Fan XW, Li FM, Xiong YC, An LZ, Long RJ. The cooperative relation between non-hydraulic root signals and osmotic adjustment under water stress improves grain formation for spring wheat varieties. Physiologia Plantarum. 2008;132:283–292. doi: 10.1111/j.1399-3054.2007.01007.x. [DOI] [PubMed] [Google Scholar]
- Feild TS, Nedbal L, Ort DR. Nonphotochemical reduction of the plastoquinone pool in sunflower leaves originates from chlororespiration. Plant Physiology. 1998;116:1209–1218. doi: 10.1104/pp.116.4.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannopolitis CN, Ries SK. Superoxide dismutases. I. Occurrence in higher plants. Plant Physiology. 1977.; 59:309–314. doi: 10.1104/pp.59.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanft JM, Wych RD. Visual indicators of physiological maturity of hard red spring wheat. Crop Science. 1982;22:584–588. [Google Scholar]
- He Z. 1993. Enzyme linked immunosorbent assay for endogenous plant hormones. In: He Z, ed. Guidance to experiment on chemical control in crop plants Beijing: Beijing Agricultural University Publishers; 60 68 [Google Scholar]
- Henson I, Jensen C, Turner N. Leaf gas exchange and water relations of lupins and wheat. I. Shoot responses to soil water deficits. Australian Journal of Plant Physiology. 1989a;16:401–413. [Google Scholar]
- Henson I, Jensen C, Turner N. Leaf gas exchange and water relations of lupins and wheat. III. Abscisic acid and drought-induced stomatal closure. Australian Journal of Plant Physiology. 1989b;16:429–442. [Google Scholar]
- Jakab G, Cottier V, Toquin V, Rigoli G, Zimmerli L, Métraux JP, Mauch-Mani B. β-Aminobutyric acid-induced resistance in plants. European Journal of Plant Pathology. 2001;107:29–37. [Google Scholar]
- Jakab G, Ton J, Flors V, Zimmerli L, Métraux JP, Mauch-Mani B. Enhancing Arabidopsis salt and drought stress tolerance by chemical priming for its abscisic acid responses. Plant Physiology. 2005; 139:267–274. doi: 10.1104/pp.105.065698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang M, Zhang J. Role of abscisic acid in water stress-induced antioxidant defense in leaves of maize seedlings. Free Radical Research. 2002a;36:1001–1015. doi: 10.1080/1071576021000006563. [DOI] [PubMed] [Google Scholar]
- Jiang M, Zhang J. Water stress-induced abscisic acid accumulation triggers the increased generation of reactive oxygen species and up-regulates the activities of antioxidant enzymes in maize leaves. Journal of Experimantal Botany. 2002b;53:2401–2410. doi: 10.1093/jxb/erf090. [DOI] [PubMed] [Google Scholar]
- Jones HG, Corlett JE. Current topics in drought physiology. Journal of Agricultural Science. 1992;119:291–296. [Google Scholar]
- Jones MM, Turner NC. Osmotic adjustment in expanding and fully expanded leaves of sunflower in response to water deficits. Australian Journal of Plant Physiology. 1980;7:181–192. [Google Scholar]
- Khanna-Chopra R, Selote DS. Acclimation to drought stress generates oxidative stress tolerance in drought-resistant than -susceptible wheat cultivars under field conditions. Environmental and Experimental Botany. 2007;60:276–283. [Google Scholar]
- Leung J, Giraudat J. Abscisic acid signal transduction. Annual Review of Plant Physiology and Plant Molecular Biology. 1998;49:199–222. doi: 10.1146/annurev.arplant.49.1.199. [DOI] [PubMed] [Google Scholar]
- Liu F, Jensen CR, Andersen MN. Hydraulic and chemical signals in the control of leaf expansion and stomatal conductance in soybean exposed to drought stress. Functional PlantBiology. 2003;30:67–73. doi: 10.1071/FP02170. [DOI] [PubMed] [Google Scholar]
- McAinsh MR, Clayton H, Mansfield TA, Hetherington AM. Changes in stomatal behavior and guard cell cytosolic free calcium in response to oxidative stress. Plant Physiology. 1996;111:1031–1042. doi: 10.1104/pp.111.4.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noctor G, Foyer CH. Ascorbate and glutathione: keeping active oxygen under control. Annual Review of Plant Physiology and Plant Molecular Biology. 1998;49:249–279. doi: 10.1146/annurev.arplant.49.1.249. [DOI] [PubMed] [Google Scholar]
- Ozturk ZN, Talamé V, Deyholos M, Michalowski CB, Galbraith DW, Gozukirmizi N, Tuberosa R, Bohnert HJ. Monitoring large-scale changes in transcript abundance in drought-and salt-stressed barley. Plant Molecular Biology. 2002;48:551–573. doi: 10.1023/a:1014875215580. [DOI] [PubMed] [Google Scholar]
- Pastori GM, Foyer CH. Common components, networks, and pathways of cross-tolerance to stress. The central role of ‘redox’ and abscisic acid-mediated controls. Plant Physiology. 2002;129:460–468. doi: 10.1104/pp.011021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad TK, Anderson MD, Martin BA, Stewart CR. Evidence for chilling-induced oxidative stress in maize seedlings and a regulatory role for hydrogen peroxide. The Plant Cell. 1994;6:65–74.. doi: 10.1105/tpc.6.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuveni M, Zahavi T, Cohen Y. 2001. Controlling downy mildew (Plasmopara viticola) in field-grown grapevine with β-aminobutyric acid (BABA) Phytoparasitica 29 125 133 [Google Scholar]
- Reynolds M, Foulkes MJ, Slafer GA, Berry P, Parry MA, Snape JW, Angus WJ. Raising yield potential in wheat. Journal of Experimantal Botany. 2009;60:1899–1918. doi: 10.1093/jxb/erp016. [DOI] [PubMed] [Google Scholar]
- Saab IN, Sharp RE. Non-hydraulic signals from maize roots in drying soil: inhibition of leaf elongation but not stomatal conductance. Planta. 1989;179:466–474. doi: 10.1007/BF00397586. [DOI] [PubMed] [Google Scholar]
- SAS Institute 2001. The SAS system for windows, Version 8.02 Cary, NC: SAS Institute; [Google Scholar]
- Schaedle M, Bassham JA. Chloroplast glutathione reductase. Plant Physiology. 1977;59:1011–1012. doi: 10.1104/pp.59.5.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sgherri CLM, Navari-Izzo F. Sunflower seedlings subjected to increasing water deficit stress: oxidative stress and defence mechanisms. Physiologia Plantarum. 1995;93:25–30. [Google Scholar]
- Srivalli B, Sharma G, Khanna-Chopra R. Antioxidative defense system in an upland rice cultivar subjected to increasing intensity of water stress followed by recovery. Physiologia Plantarum. 2003;119:503–12. [Google Scholar]
- Ton J, Jakab G, Toquin V, Flors V, Iavicoli A, Maeder MN, Metraux JP, Mauch-Mani B. Dissecting the beta-aminobutyric acid-induced priming phenomenon in Arabidopsis. The Plant Cell. 2005;17:987–999. doi: 10.1105/tpc.104.029728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ton J, Mauch-Mani B. 2004. β-amino-butyric acid-induced resistance against necrotrophic pathogens is based on ABA-dependent priming for callose The Plant Journal 38 119 130 [DOI] [PubMed] [Google Scholar]
- Travaglia C, Reinoso H, Cohen A, Luna C, Tommasino E, Castillo C, Bottini R. Exogenous ABA increases yield in field-grown wheat with moderate water restriction. Journal of Plant Growth Regulation. 2010;29:366–374. [Google Scholar]
- Trejo C. Davies WJ. Drought-induced closure of Phaseolus vulgaris L. stomata precedes leaf water deficit and any increase in xylem ABA concentration. Journal of Experimental Botany. 1991;42:1507–1516. [Google Scholar]
- Turner NC. Measurement of plant water status by the pressure chamber technique. Irrigation Science. 1988;9:289–308. [Google Scholar]
- Turner NC. Further progress in crop water relations. Advances in Agronomy. 1996;58:293–338. [Google Scholar]
- Turner NC, Asseng S. Productivity, sustainability,and rainfall-use efficiency in Australian rainfed Mediterranean agricultural systems. Australian Journal of AgriculturalResearch. 2005;56:1123–1136. [Google Scholar]
- Turner NC, Meyer R. 2011.. Synthesis of regional impacts and global agricultural adjustments. In: Yadav SS, Redden RJ, Hatfield JL, Lotze-Campen H, Hall AE, eds. Crop adaptation to climate change Chichester, UK: Wiley-Blackwell; 156 165 [Google Scholar]
- Tyree MT, Engelbrecht BMJ, Vargas G, Kursar TA. Desiccation tolerance of five tropical seedlings in Panama. Relationship to a field assessment of drought performance. Plant Physiology. 2003;132:1439–1447. doi: 10.1104/pp.102.018937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallad GE, Goodman RM. Systemic acquired resistance and induced systemic resistance in conventional agriculture. Crop Science. 2004;44:1920–1934. [Google Scholar]
- Wang ZY, Li FM, Xiong YC, Xu BC. Soil-water threshold range of chemical signals and drought tolerance was mediated by ROS homeostasis in winter wheat during progressive soil drying. Journal of Plant Growth Regulation. 2008;27:309–319. [Google Scholar]
- Wu SR, Chen WF, Zhou X. Enzyme linked immunosorbent assay for endogenous plant hormones. Plant Physiology Communications. 1988;5:53–57. [Google Scholar]
- Xiong YC, Li FM, Xu BC, Hodgkinson KC. Hydraulic and non-hydraulic root-sourced signals in old and modern spring wheat cultivars in a semiarid area. Journal of Plant Growth Regulation. 2006a;25:120–136. [Google Scholar]
- Xiong YC, Li FM, Zhang T. Performance of wheatcrops with different chromosome ploidy: root-sourced signals,drought tolerance, and yield performance. Planta. 2006b;224: 710–718. doi: 10.1007/s00425-006-0252-x. [DOI] [PubMed] [Google Scholar]
- Yang J, Zhang J, Wang Z, Zhu Q, Wang W. Hormonal changes in the grains of rice subjected to water stress during grain filling. Plant Physiology. 2001;127:315–323. doi: 10.1104/pp.127.1.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Davies W. Changes in the concentration of ABA in xylem sap as a function of changing soil water status can account for changes in leaf conductance and growth. Plant, Cell and Environment. 1990;13:277–285. [Google Scholar]
- Zhao SJ, Xu C, Zou Q, Meng Q. Improvements of method for measurement of malondialdehyde in plant tissues. Plant Physiology Communications. 1994;30:207–210. [Google Scholar]
- Zhu JK. Salt and drought stress signal transduction in plants. Annual Review of Plant Biology. 2002;53:247–273. doi: 10.1146/annurev.arplant.53.091401.143329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerli L, Hou BH, Tsai CH, Jakab G, Mauch B-Mani, Somerville S. 2008. The xenobioticβ-aminobutyric acid enhances Arabidopsis thermotolerance. The Plant Journal 53 144 156 [DOI] [PubMed] [Google Scholar]
- Zimmerli L, Jakab G, Metraux JP, Mauch-Mani B. Potentiation of pathogen-specific defence mechanisms in Arabidopsis by beta-aminobutyric acid. Proceedings of the National Academy of Sciences, USA. 2000;97:12920–12925. doi: 10.1073/pnas.230416897. [DOI] [PMC free article] [PubMed] [Google Scholar]