Abstract
We reported that the 5-HTTLPR polymorphism in the promoter region of the serotonin transporter gene (SLC6A4) moderates the effect of childhood adversity on posttraumatic stress disorder (PTSD)risk (Xie and others 2009). In the present study, we considered 5178 subjects (a group with generally high substance dependence comorbidity, as for our previous study) using similar methodology to replicate our previous results.
We used logistic regression analyses to explore the interaction effect of 5-HTTLPR genotype and childhood adversity on PTSD risk. We found that, as reported in our previous study, in individuals with childhood adversity, the presence of one or two copies of the S allele of 5-HTTLPR increased the risk to develop PTSD. This gene-environment interaction effect was present in European Americans (EAs), but not in African Americans (AAs) (EAs, OR=1.49, 95% CI=1.07–2.08, P=0.019; AAs, OR=0.90, 95% CI=0.60–1.35, P=0.62). The statistical power to detect this interaction effect was increased when data were combined with those from our previous study (Xie and others 2009).
The findings reported here replicate those from our previous work, adding to a growing body of research demonstrating that the 5-HTTLPR genotype moderates risk for anxiety and depression phenotypes in the context of stress and adverse events.
Keywords: gene-environment interaction, PTSD, childhood adversity, 5-HTTLPR
Introduction
Posttraumatic stress disorder (PTSD) can occur after an individual experiences or witnesses events involving actual or threatened death, serious injury, or sexual violation. However, trauma exposure does not always result in PTSD. Epidemiologic surveys indicate that despite a large proportion of the US population having been exposed to traumatic events during their lifetimes, the prevalence of PTSD is only about 6.8% (Kessler and others 2005). A recent study observed that among the survivors who worked in the World Trade Center towers at the time of the September 11, 2001 terrorist attack, only 15% had developed PTSD two to three years after the event (DiGrande and others 2011). We still have limited knowledge about the biological factors that contribute to inter-individual differences in PTSD vulnerability. As for other complex traits, the identification of risk factors for PTSD in people exposed to trauma is essential both to understand and treat the disorder.
Child abuse frequently produces serious long-term negative effects, and it increases risk for PTSD (Brewin and others 2000; Paolucci and others 2001). Various types of childhood trauma, including sexual abuse (Molnar and others 2001; Widom 1999), physical abuse (Duncan and others 1996; Widom 1999), and other traumatic events (Ahmad and others 2000; Pynoos and others 1993)are linked to a higher risk to develop PTSD later in life. The biological mechanisms that underlie PTSD are far from clear. Animal models and human studies have shown that the long-term effects of childhood trauma may exert some of their actions through epigenetic changes in genes involved in the hypothalamic-pituitary-adrenal (HPA) axis (McGowan and others 2009; Weaver and others 2004). It has also been reported that people with childhood trauma are more likely to develop problem behaviors, which in turn may expose them more to traumatic events (Dodge and others 1990; Widom 1989).
In addition to the environmental factors, genetic components influence PTSD risk. Genetic factors account for one third (or more) of the variance in risk of PTSD (Stein and others 2002; True and others 1993). However, there are no replicable findings of genes associated to PTSD risk using either genetic association or linkage studies (Cornelis and others 2010).
An individual’s genetic background moderates his or her response to environmental risk factors. In a landmark study, Caspi et al (Caspi and others 2003)analyzed a New Zealand birth cohort, and the short allele (S) of a functional polymorphism (5-HTTLPR) in the promoter region of the serotonin transporter gene (SLC6A4) was associated with higher risk for depression in people with stressful life events and childhood maltreatment. 5-HTTLPR is the most studied genetic variant in psychiatry. The serotonin transporter protein is located presynaptically, where it mediates reuptake of serotonin from the synaptic cleft, terminating the action of serotonergic neurotransmission. The L allele of 5-HTTLPR yields about twice the amount of transporter protein as the S allele (Lesch and others 1996). In addition to altering protein expression level, 5-HTTLPR genotype is also associated with variation in brain structure (Pezawas and others 2005).
Since variation in 5-HTTLPR is related to other stress-related behaviors, we tested whether this polymorphism influences PTSD risk for people with early life stress. Previously, we found that in people with a history of childhood adversity, those with one or two copies of the S allele of 5-HTTLPR had a higher risk to develop PTSD than individuals homozygous for the L allele (Xie and others 2009). This gene-environment interaction effect was more prominent in European Americans (EAs) than in African Americans (AAs).
Despite well-known inter-individual variation in trauma response, research on genetic factors moderating childhood adversity effects on PTSD risk is a relatively new area of investigation. So far, in addition to our previous study, only a few studies of the genetic moderation of childhood adversity effects on PTSD risk have been published (Binder and others 2008; Nelson and others 2009; Xie and others 2009; Xie and others 2010). Analyzing a cohort of mostly AA subjects, Binder et al (Binder and others 2008)found that polymorphisms in the FKBP5 gene, which encodes a protein regulating glucocorticoid receptor function, interacted with child abuse to regulate PTSD risk. We replicated this gene-environment interaction effect in our sample, but only in AAs, not in EAs (Xie and others 2010). The third gene in which variation was reported to moderate the effect of child abuse on PTSD risk is GABRA2 (Nelson and others 2009).
As in our previous study, subjects recruited to participate in this study were initially ascertained for genetic studies of cocaine, opioid and alcohol dependence. Because the prevalence of PTSD in people with substance dependence is substantially higher than that in general population (Pierucci-Lagha and others 2005), our ongoing recruitment has made it possible to test the moderating effect of genetic variation on PTSD risk in a new cohort of subjects.
The purposes of this study are, first to replicate our previous finding that 5-HTTLPR genotype moderated the effect of childhood adversity on PTSD risk; and second, to test the same G×E effect again by combining the data from our previous study (1252 subjects) with those from the current study (5178 subjects) to gain greater statistical power to detect a gene-environment interaction effect.
Subjects and Methods
Subjects
We recruited a total of 5178 subjects for genetic linkage and association studies of cocaine, opioid, and alcohol dependence at three US sites: Yale University School of Medicine (2406 subjects), the University of Connecticut Health Center (2171 subjects), and the University of Pennsylvania School of Medicine (601 subjects). All subjects were interviewed by trained interviewers using the Semi-Structured Assessment for Drug Dependence and Alcoholism (SSADDA), which yields DSM-IV diagnoses for a variety of psychiatric disorders, including PTSD (Pierucci-Lagha and others 2007; Pierucci-Lagha and others 2005). None of the subjects were included in our previous study. The recruitment procedures were nearly identical to the prior study except that the previous sample was ascertained as small nuclear families, and the present sample is composed mostly of unrelated subjects, with only 4.4% of the subjects from small nuclear families. The institutional review board at each of the participating sites approved the study protocol. After receiving a complete description of the study, subjects gave written informed consent to participate and were paid for their participation.
Diagnosis and childhood adversity index
To make a lifetime PTSD diagnosis, participants were interviewed concerning their experience of traumatic events and PTSD symptoms. A diagnosis was made based on DSM-IV criteria. The inter-rater and test-retest reliability [κ] of the PTSD diagnosis using the SSADDA were 0.59 and 0.76, respectively (Pierucci-Lagha and others 2007; Pierucci-Lagha and others 2005). The interview also elicited diagnostic criteria for depression and substance use disorders. Major depressive disorder and substance dependence diagnoses were made based on DSM-IV criteria.
For the assessment of childhood adversity, participants were asked whether, by age 13, they had witnessed or experienced a violent crime, had been sexually abused, or had been physically abused. Participants were asked 3 questions about the relationship with their main caregivers up to age 13 to assess childhood neglect: “whether the person you were closest to was usually available to you when you needed him or her,” “whether you felt you could confide in this person when necessary,” and “whether this person was aware of who your friends were.” Respondents who answered “no” to any of the 3 questions were coded as positive for exposure to childhood neglect.
Genotyping and race information
DNA was extracted from immortalized cell lines or directly from blood or saliva. The short(S) allele and the long (L) allele of 5-HTTLPR system were differentiated using polymerase chain reaction (PCR) amplification followed by size fractionation via agarose gel electrophoresis. The primers used for PCR were: 5′-CCTAACCCCTAATGTCCCTACTG-3′ and 5′-GGACCGCAAGGTGGGCGGGAG-3′. PCR was performed using PC2 buffer and Klentaq DNA polymerase, and cycling conditions of 95/60/72° C for 30 cycles, 30 seconds at each setpoint.
In our previous study, in addition to the 5-HTTLPR VNTR genotype (14 repeats designated traditionally as the S allele and 16 repeats as the L allele), we also genotyped a SNP (rs25531) in the VNTR region, which divides the L variant into LG and LA. However, we observed that including rs25531 genotype did not change the results in our study. Considering that many previous 5-HTTLPR × childhood adversity studies did not include rs25531, we decided to only analyze the L-S bi-allelic classification of the 5-HTTLPR genotype in the present investigation.
Among the 5178 subjects, 3270 subjects were genotyped for 41 ancestry-informative markers (AIMs), including 36 short tandem repeat markers and 5 SNPs. The selection and characteristics of these markers have been described previously (Yang and others 2005). We used STRUCTURE software (Pritchard and Rosenberg 1999)to analyze the AIMs data, and to generate ancestry proportion scores. Subjects with an African ancestry proportion score < 0.500 were classified as EAs; otherwise, they were classified as AAs. Another 704 subjects participated in a genome-wide association study of drug and alcohol dependence, and were genotyped on the Illumina HumanOmni1-Quad 1M array at CIDR or at the Yale Keck center. Population assignments were made by STRUCTURE analysis of all SNPs in the genome array with a genotype call rate > 98% and minor allele frequency ≧ 0.05. We did not have AIMs information for the remaining 1204 subjects, and thus used self-reported race information for these individuals.
Statistical analysis
Logistic regression was used to examine the main effects of 5-HTTLPR genotype (coded as 0 for LL, 1 for LS, and 2 for SS) and childhood adversity (coded as 0 for none, 1 for exposure) on PTSD diagnosis. Sex and age were used as covariates. To explore the interaction effect of the genotype and childhood adversity, an interaction term was entered into the model. Then the interaction effect on PTSD was measured again by adding the diagnosis of major depressive disorder as a covariate in the logistic regression model. Generalized estimating equation (GEE) analyses were applied to fit the logistic regression model to account for the dependence of the data from individuals in the same family. To measure the additive interaction effects from logistic models, we adopted a method proposed by Ai and Norton (Ai and Norton 2003). “Additive interaction effect” is defined as the combined effect of the risk genotype and environmental factor being larger than the sum of their individual effects. We first calculated the marginal effects from logistic regression models. Then the additive interaction effects and standard errors were computed from the marginal effects (Norton and others 2004; Zimmermann and others 2011). EAs and AAs were analyzed separately to prevent population stratification. Because most of the subjects were affected with alcohol, cocaine and/or opioid dependence, additional models including terms for these co-morbidities were examined.
Then, we combined the data from this study (5178 subjects) with those from our previous study (1252 subjects) investigating the interaction effect of 5-HTTLPR and childhood adversity on PTSD risk (Xie and others 2009). The methodology of these two studies, including subject recruitment, PTSD diagnosis and childhood adversity measures were nearly identical (only differing on whether families or unrelated individuals were ascertained). Because in our previous study, association results using the bi-allelic (S vs. L) or the tri-allelic (considering SNP rs25531 in the L allele) genotypes were comparable, only the L-S bi-allelic classification of the 5-HTTLPR genotype was used in the current study. GEE adjusted logistic regression models were applied. We also computed the additive interaction effects from the logistic regression models in the combined sample. All analyses were performed using SAS 9.2 (SAS, Cary, NC)and Stata 12 (Stata, College Station, TX).
Results
Demographics
We observed that 2.3% of the subjects who self-reported as EAs were reclassified as AAs after analyzing the AIMs, and 2.5% of the subjects who self-reported as AAs were reclassified as EAs. We did not have AIMs information for 1204 out of the 5178 subjects, and thus used self-reported race information. Among the 1204 subjects, 860 were self-reported EAs, and 344 were self-reported AAs. Therefore, ~29 subjects were estimated to have been misclassified on race. In total, 2779 EAs and 2399 AAs were included in the study. The mean age of the 5178 subjects (44.0% female)was 40.5 (SD 11.2), ranging from 18 to 85 years. Table 1 lists detailed demographic information for all particpants. There are no statistically significant differences in the mean age, sex proportion, years of education, and overall childhood adversity rate between EAs and AAs. Among EAs, 398 of them had a lifetime diagnosis of PTSD, and 321 of the AAs had a lifetime diagnosis of PTSD. There was no difference in the rate of lifetime PTSD between EAs (14.3%) and AAs (13.4%) ( , P=0.33). In both populations, women had a higher rate of PTSD than men (EA: , P<0.0001; AA: , P=0.0002).
Table 1.
Characteristics of the subjects
| EA (n=2779) | AA (n=2399) | |
|---|---|---|
| Age (SD) | 39.0 (12.5) | 42.2 (9.2) |
| Sex | 43.9% female | 44.0% female |
| Years of education (SD) | 12.9 (2.61) | 12.1 (2.01) |
| PTSD*(%) | 14.3 | 13.4 |
| Childhood adversity(%) | 36.8 | 41.4 |
| Neglect(%) | 22.3 | 19.9 |
| Witnessing or being a victim of a violent crime(%) | 13.4 | 23.1 |
| Sexual abuse(%) | 14.2 | 15.1 |
| Physical abuse(%) | 9.0 | 9.0 |
| Alcohol dependence*(%) | 43.0 | 50.0 |
| Cocaine dependence*(%) | 52.5 | 71.2 |
| Opioid dependence*(%) | 46.7 | 23.9 |
| Major depressive disorder*(%) | 15.6 | 11.8 |
| 5-HTTLPR genotype | 31.0% LL, 48.9% LS and 20.0% SS | 56.2% LL, 36.9% LS, and 6.8% SS |
Diagnoses of lifetime psychiatric disorders were made based on DSM-IV criteria.
Main effects of childhood adversity and 5-HTTLPR genotypes on risk of PTSD
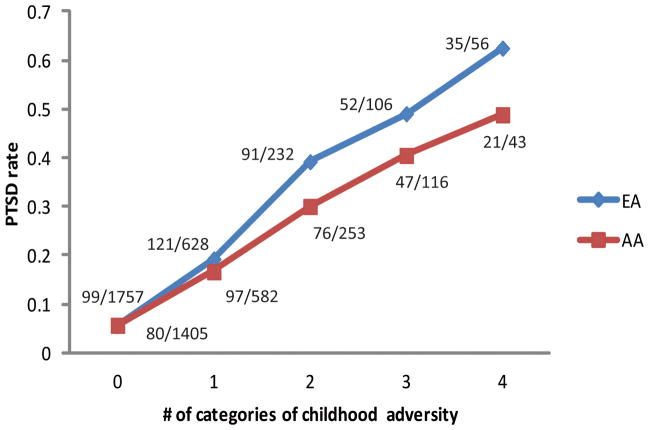
Four categories of adverse childhood experiences before age 13 were assessed in this study: neglect, witnessing or being a victim of a violent crime, sexual abuse, and physical abuse. The correlations among the four categories of childhood adversity were weak to moderate, with Pearson correlation coefficients between 0.15 to 0.31 for AAs, and 0.16 to 0.40 for EAs. The distribution of childhood adversity by race is shown in Table 2. Witnessing or being a victim of a violent crime happened more frequently in AAs than EAs ( , P<0.0001). Other than that, the distribution pattern was similar between the two populations. After adjusting for age and sex, all four categories of childhood adversity were significantly associated with increased risk for a diagnosis of PTSD in EAs. A similar trend was observed in AAs, except for childhood neglect, which was marginally associated with PTSD risk (Table 2). In both populations, having experienced events from a greater number of categories of adverse childhood experiences increased PTSD risk (Figure 1).
Table 2.
Distribution of the four categories of childhood adversity and their effects on lifetime PTSD diagnosis (some individuals experienced more than one type of childhood adversity)
| European American (n=2779) | African American (n=2399) | |||||||
|---|---|---|---|---|---|---|---|---|
| % | PTSD prevalence (%) | OR (95% CI) | P-value | % | PTSD prevalence (%) | OR (95% CI) | P-value | |
| Neglect | 22.3 | 27.8 | 2.04 (1.56–2.67) | <.0001 | 19.9 | 23.6 | 1.35 (0.99–1.84) | 0.058 |
| Witnessing or being a victim of a violent crime | 13.4 | 40.1 | 2.84 (2.06–3.92) | <.0001 | 23.1 | 26.9 | 2.29 (1.71–3.07) | <.0001 |
| Sexual abuse | 14.2 | 41.6 | 2.52 (1.84–3.45) | <.0001 | 15.1 | 37.0 | 3.19 (2.30–4.42) | <.0001 |
| Physical abuse | 9.0 | 45.8 | 2.90 (2.00–4.20) | <.0001 | 9.0 | 36.3 | 2.25 (1.54–3.28) | <.0001 |
Figure 1.
effects of number of categories of childhood adversity experienced on PTSD risk.
The genotype distribution of 5-HTTLPR was 31.0% LL, 48.9% LS and 20.0% SS in EAs; 56.2% LL, 36.9% LS, and 6.8% SS in AAs. The distribution is consistent with previous studies (Caspi and others 2003; Xie and others 2009)and with Hardy-Weinberg equilibrium expectations. After adjusting for age and sex, genotype was not significantly associated with PTSD (EA: OR=0.97, 95% CI=0.83–1.12, P=0.65; AA: OR=1.04, 95% CI=0.87–1.25, P=0.67). These results are similar to our previous observations (Xie and others 2009).
Interaction effect of childhood adversity and 5-HTTLPR on risk of PTSD
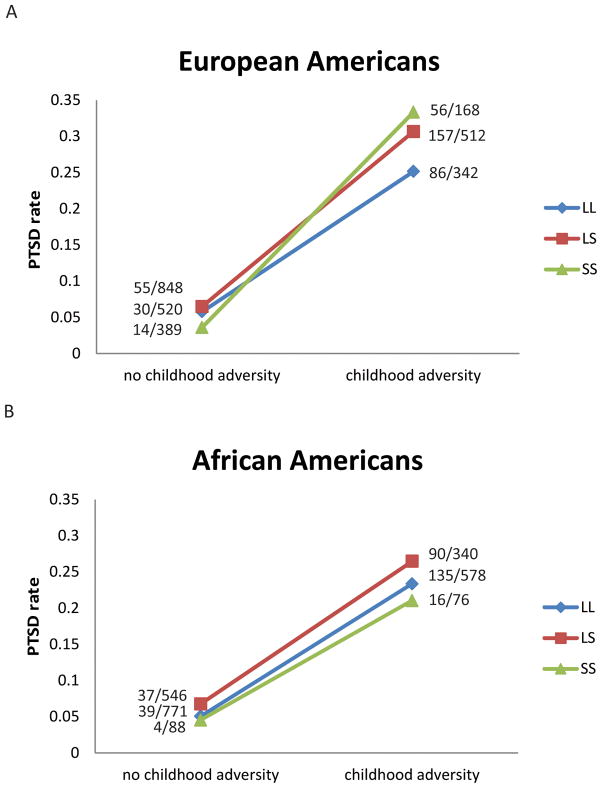
Subjects who reported at least one of the four categories of childhood adversity were considered as having been exposed to early life stress. As in our previous study, the 5-HTTLPR genotype × childhood adversity interaction was significantly associated with lifetime PTSD diagnosis in EAs, but not in AAs (EA, OR=1.49, 95% CI=1.07–2.08, P=0.019; AA, OR=0.90, 95% CI=0.60–1.35, P=0.62. See Table 3 and Figure 2). In EAs without childhood adversity, subjects with different 5-HTTLPR genotypes had similar PTSD rates. However, for subjects with childhood adversity, those carrying the LL genotype had the lowest rate of PTSD; subjects carrying the LS and SS genotypes had significantly higher rates of PTSD ( , P=0.04). By calculating the additive interaction effect, we determined that EA subjects with the LS genotype and childhood adversity had 4.92% higher probability of developing PTSD (95% CI = 0.77%–9.08%, P=0.020), compared with the combined risk for EAs with the LS genotype but without childhood adversity, and EAs with the LL genotype but with childhood adversity. EAs with the SS genotype and with childhood adversity had 9.98% higher probability of developing PTSD (95% CI = 1.26%–18.70%, P=0.025), compared with the combined risk for EAs with the SS genotype but without childhood adversity, and EAs with the LL genotype but with childhood adversity.
Table 3.
Interactive effects of childhood adversity and 5-HTTLPR genotypes on PTSD risk based on logistic regression models.
| European American (n=2779) | African American (n=2339) | |||
|---|---|---|---|---|
| OR (95%CI) | P-value | OR (95%CI) | P-value | |
| Childhood adversity | 4.87 (3.35–7.08) | <.0001 | 5.71 (4.02–8.10) | <.0001 |
| 5-HTTLPR | 0.81 (0.62–1.06) | .13 | 1.14 (0.81–1.60) | .44 |
| 5-HTTLPR × Childhood adversity | 1.49 (1.07–2.08) | .019 | 0.90 (0.60–1.35) | .62 |
| Age | 0.99 (0.98–0.99) | .0015 | 1.00 (0.99–1.02) | .55 |
| Sex | ||||
| Female | 1.0 | |||
| Male | 0.49 (0.39–0.61) | <.0001 | 0.61 (0.48–0.78) | <.0001 |
Figure 2.
5-HTTLPR genotype interacted with childhood adversity to moderate risk for PTSD in European Americans (a), but not in African Americans (b).
In this sample, 30.3% of the subjects with lifetime PTSD also had diagnosis of major depressive disorder. To evaluate the influence of depression on the interaction effect of childhood adversity and 5-HTTLPR genotype on PTSD, the interaction effect was calculated again after adding the diagnosis of major depressive disorder into the logistic regression model as a covariate. Including depression in the model did not change the interaction effect on PTSD (EA, OR=1.49, 95% CI=1.06–2.08, P=0.020; AA, OR=0.85, 95% CI=0.57–1.29, P=0.45). The additive interaction effect also remained largely unchanged after adjusting for depression (Supplementary table 1).
The participants in this study were recruited for studies of the genetics of substance dependence and 68.9% of the EAs and 79.7% of the AAs were dependent on alcohol, cocaine and/or opioids. To explore the effect of these substance dependence phenotypes on PTSD diagnosis, alcohol, cocaine and opioid dependence, and their interactions with childhood adversity and 5-HTTLPR genotype, were added to the original G×E model. For EAs, among the nine newly-added independent variables (three main and six interaction terms), only cocaine dependence and cocaine dependence × childhood adversity had a significant effect on PTSD diagnosis with P < 0.05 (Supplementary table 2). For AAs, the effects of alcohol dependence and cocaine dependence were significant (Supplementary table 2). After adjusting for the effects of substance dependence, the interaction of childhood adversity and 5-HTTLPR genotype on PTSD diagnosis was unchanged.
Analyzing the combined data
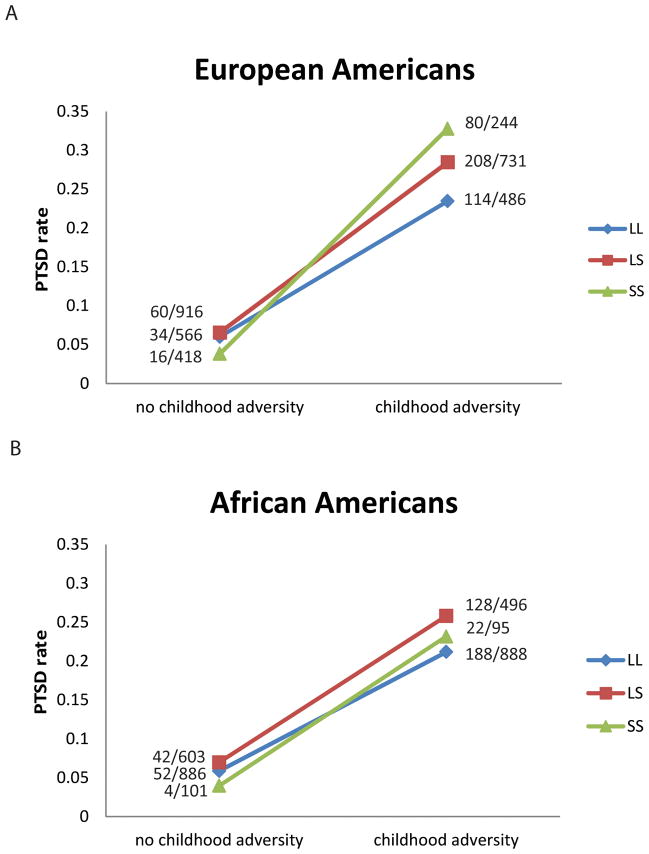
We combined the data from the current study with those from our previous study investigating the interaction effect of 5-HTTLPR and childhood adversity on PTSD risk. A total of 3361EAs and 3069 AAs were included in the analysis. With the increased sample size, the power to detect the interaction effect is increased, but the effect in AAs remained non-significant (EA, OR=1.52, 95% CI=1.12–2.06, P=0.0069; AA, OR=1.14, 95% CI=0.79–1.64, P=0.50. See Table 4 and Figure 3). In the combined sample, EAs with the LS genotype and childhood adversity had 5.40% higher probability of developing PTSD (95% CI = 1.96%–8.84%, P=0.002), compared with the combined risk for EAs with the LS genotype but without childhood adversity, and EAs with the LL genotype but with childhood adversity. EAs with the SS genotype and with childhood adversity had 11.12% higher probability of developing PTSD (95% CI = 3.83%–18.41%, P=0.003), compared with the combined risk for EAs with the SS genotype but without childhood adversity, and EAs with the LL genotype but with childhood adversity. The interaction effect remained almost the same when the model was adjusted by major depressive disorder(EA, OR=1.51, 95% CI=1.11–2.04, P=0.0086; AA, OR=1.10, 95% CI=0.76–1.59, P=0.62; Additive interaction effect see Supplementary table 1).
Table 4.
Interactive effects of childhood adversity and 5-HTTLPR genotypes on the risk of PTSD based on logistic regression models (analysis of combined data including the present replication study, and our original study).
| European American (n=3361) | African American (n=3069) | |||
|---|---|---|---|---|
| OR (95%CI) | P-value | OR (95%CI) | P-value | |
| Childhood adversity | 4.20 (2.98–5.91) | <.0001 | 4.24 (3.13–5.75) | <.0001 |
| 5-HTTLPR | 0.82 (0.64–1.06) | .13 | 1.02 (0.75–1.40) | .89 |
| 5-HTTLPR × Childhood adversity | 1.52 (1.12–2.06) | .0069 | 1.14 (0.79–1.64) | .50 |
| Age | 0.99 (0.98–1.00) | .02 | 1.01 (1.00–1.02) | .23 |
| Sex | ||||
| Female | 1.0 | |||
| Male | 0.51 (0.42–0.63) | <.0001 | 0.63 (0.51–0.78) | <.0001 |
Figure 3.
5-HTTLPR genotype moderated the effect of childhood adversity on risk for PTSD in European Americans (a), but not in African Americans (b) from the combined sample.
After adjusting for the effects of substance dependence, the interaction of childhood adversity and 5-HTTLPR genotype on PTSD diagnosis remained largely unchanged (See Supplementary table 3).
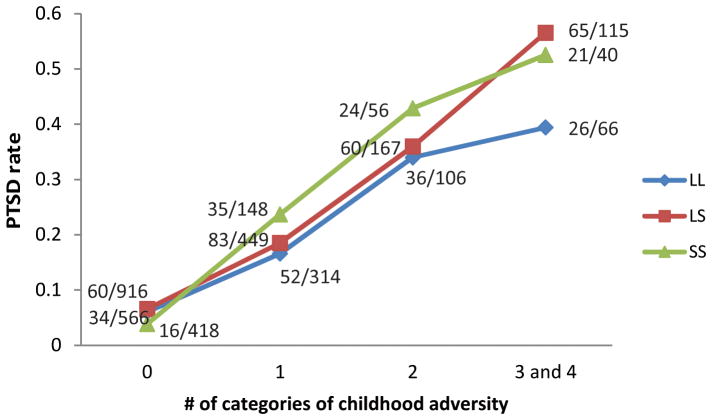
Finally, we examined the interaction of 5-HTTLPR genotype and the number of categories of childhood adversity on risk of PTSD in the combined sample. As shown in Figure 4, the effect of early life stress on the onset of PTSD was evident especially in those EA individuals with an S allele who experienced three or four categories of childhood adversity, compared with those who experienced one or two categories of childhood adversity.
Figure 4.
The interaction of 5-HTTLPR genotype and the number of categories of childhood adversity on risk of PTSD in European Americans from the combined sample.
Discussion
Since the highly influential 2003 study by Caspi and colleagues (Caspi and others 2003), 5-HTTLPR has become one of the most studied genetic variants in psychiatry with respect to G×E interaction. Early life stress has been well validated as a risk factor for PTSD. We first reported that 5-HTTLPR genotypes could moderate the effect of childhood adversity on PTSD onset. By analyzing a mostly substance dependent cohort, which had a rate of PTSD 2–3 times that of the general population, we observed a significant interaction in EAs, but not in AAs. In the current study, we analyzed 719 PTSD cases and 4459 control subjects who were not included in our previous study, and replicated our earlier observation of a significant G×E effect in EAs, with an odds ratio of 1.51 (95% CI=1.07–2.14, P=0.019) based on a logistic regression model. In neither case did we detect an effect in AAs. The study was similarly powered to detect effects in both EAs and AAs. Combining subjects from our previous study with those presented here yielded 948 PTSD cases and 5482 controls. In this larger sample, the odds ratio of the moderating effect of genotype in EAs was 1.52 (95% CI=1.12–2.06, P=0.0069). The G×E effect did not change after adjusting for major depressive disorder and the main effects of substance dependence phenotypes and their interactions with childhood adversity and 5-HTTLPR genotypes.
Interpretation of this study should consider the following limitations. Measurement of early life environment was not systematic and did not employ a validated instrument. This may be of particular concern with retrospective recall of early life experiences, which could be inaccurate or biased, especially for subjects with PTSD or substance dependence. Finally, we did not use a complete list of childhood adverse events; only four categories were investigated in this study.
G×E research in psychiatry is controversial. Two of the three meta-analytic studies of the interaction between 5-HTTLPR, stressful life events, and risk of depression failed to find evidence supporting the original interaction reported by Caspi and colleagues (Karg and others 2011; Munafo and others 2009; Risch and others 2009). However, whether results from these meta-analysis studies reflect the true effect size of the interaction remains unclear. First, because there were insufficient descriptive data from many G×E studies, published studies were excluded in some of these meta-analyses. Second, study designs, environmental measures, and statistical analytic methods differ substantially across G×E studies, which makes it difficult to combine the results. Because statistical power to detect interaction effects is lower than for genetic or environmental main effects, the false positive rate is high (Duncan and Keller 2011). Therefore, a large sample size, examination of genetic and environmental factors with large effect size, and careful measurement of environmental factors are key considerations in G×E studies.
In this study, interaction effect was observed in EAs, but not in AAs. We reviewed published studies examining the hypothesis that the 5-HTTLPR moderates the effect of stressful life events on PTSD and depression phenotypes. For PTSD, besides the study we published in 2009(Xie and others 2009), five other studies were published (Grabe and others 2009; Kilpatrick and others 2007; Koenen and others 2009; Kolassa and others 2010; Mercer and others 2012). Three of the five studies investigated American populations, but none of them studied AAs. Therefore, so far there are no studies other than ours having analyzed this interaction effect on PTSD risk in AAs. For depression phenotypes, about 18 studies investigating American populations have been published. However, only three studies examined AAs separately (Mitchell and others 2011; Ressler and others 2010; Scheid and others 2011). Ressler et al. (Ressler and others 2010)studied 1392 AAs with high rate of substance abuse, and did not find any interactions between the 5-HTTLPR and child abuse on Beck Depression Inventory (BDI) score. Mitchell et al. (Mitchell and others 2011)studied 1206 women, 760 of whom were AAs. They did not observe any significant interaction effect between education level and the 5-HTTLPR on postpartum depression when AAs were analyzed separately. Scheid et al. (Scheid and others 2011)studied 698 AA women, and found weak interaction effect between the 5-HTTLPR and abuse on midpregnancy depressive symptoms. The interaction effect was not statistically significant after adjustment for multiple comparisons. Therefore, based on the published data, the interaction effect of 5-HTTLPR and stress on depression in AAs is not as clear as it is in EAs, notwithstanding that the results in EAs are also mixed.
There are several possibilities to explain the population-specific G×E effect. First, the minor allele frequency of the 5-HTTLPR in AAs is lower than that in EAs. The genotype distribution of 5-HTTLPR was 31.0% LL, 48.9% LS and 20.0% SS in EAs and 56.2% LL, 36.9% LS, and 6.8% SS in AAs. With the lower genotype frequency of the S allele homozygotes in AAs, the statistical power of calculating G×E effect is lower than for EAs. Studies analyzing larger AA samples may be able to detect an effect. Second, the 5-HTTLPR may not directly interact with early life stress. It may instead reflect the action of variants in linkage disequilibrium with the 5-HTTLPR, which contribute to the interaction effect. Linkage disequilibrium patterns vary by population, providing a possible explanation for failure to detect the signal in EAs but not AAs. Third, multiple differences in gene expression and regulation across racial and ethnic groups and differences in epistatic effects are other possible factors. Gelernter et al. (Gelernter and others 1998)reported that EAs with the 5-HTTLPRS allele had higher scores on neuroticism compared to those with the LL genotype, whereas the opposite pattern of findings was observed for AAs. Williams et al. (Williams and others 2003) reported that in EAs, the 5-HTTLPRSS genotype was associated with lower levels of central nervous system serotonin turnover, compared to the LL and LS genotypes. However, opposite association pattern was observed in AAs.
Serotonin plays numerous roles in the central nervous system. There is substantial evidence for the importance of serotonin in PTSD. First, serotonin-reuptake inhibitors (SSRI) are a first-line medication approved by the Food and Drug Administration to treat PTSD (Brady and others 2000). Second, serotonergic dysregulation is implicated in many symptoms that co-occur with PTSD including depression, impulsivity, and aggression (Southwick and others 1999). The S allele of 5-HTTLPR reduces serotonin transporter gene expression and function (Lesch and others 1996), and thus decreases serotonin reuptake (although the final result depends also on long-term brain homeostatic adaptation to the effects of any particular allele). Animal models and human studies have provided evidence that 5-HTTLPR regulates adaptive capacities to environmental changes (Homberg and Lesch 2011). Therefore, the biological effects of 5-HTTLPR warrant consideration as a candidate for effects in G×E studies of PTSD.
Compared to depression, despite the fact that PTSD is known to have both environmental and genetic risk factors, very little work has been done to examine the interaction of genetic and environmental effects. Further studies are needed to replicate and extend the findings reported here.
Supplementary Material
Acknowledgments
We thank the individuals and families who participated in this study, as well as the interviewers who collected the data. Kathleen Brady, MD, PhD and Raymond F. Anton, MD of the Medical University of South Carolina, David Oslin, MD of the University of Pennsylvania, and Roger Weiss, MD of McLean Hospital and Harvard Medical School oversaw study recruitment at their respective sites. Greg Kay, BS, and Ann Marie Lacobelle, MS, provided technical assistance.
Dr. Kranzler has received consulting fees from Alkermes, GlaxoSmithKline, Gilead, Lundbeck, Roche, and Lilly. He also reports associations with Lilly, Merck, Janssen, Schering Plough, Lundbeck, Alkermes, GlaxoSmithKline, Abbott, and Johnson & Johnson, as these companies provide support to the ACNP Alcohol Clinical Trials Initiative (ACTIVE) and Dr. Kranzler receives support from ACTIVE. Ms. Xie, Dr. Farrerand Dr. Gelernter declare no conflict of interest. This study was supported by NIH grants R01 DA12690, R01 DA12849, R01 AA017535, and R01 AA11330; and by the US VA PTSD Research Center.
References
- Ahmad A, Sofi MA, Sundelin-Wahlsten V, von Knorring AL. Posttraumatic stress disorder in children after the military operation “Anfal” in Iraqi Kurdistan. Eur Child Adolesc Psychiatry. 2000;9(4):235–43. doi: 10.1007/s007870070026. [DOI] [PubMed] [Google Scholar]
- Ai C, Norton EC. Interaction terms in logit and probit models. Economics Letters. 2003;80:123–9. [Google Scholar]
- Binder EB, Bradley RG, Liu W, Epstein MP, Deveau TC, Mercer KB, Tang Y, Gillespie CF, Heim CM, Nemeroff CB, et al. Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. JAMA. 2008;299(11):1291–305. doi: 10.1001/jama.299.11.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady K, Pearlstein T, Asnis GM, Baker D, Rothbaum B, Sikes CR, Farfel GM. Efficacy and safety of sertraline treatment of posttraumatic stress disorder: a randomized controlled trial. JAMA. 2000;283(14):1837–44. doi: 10.1001/jama.283.14.1837. [DOI] [PubMed] [Google Scholar]
- Brewin CR, Andrews B, Valentine JD. Meta-analysis of risk factors for posttraumatic stress disorder in trauma-exposed adults. J Consult Clin Psychol. 2000;68(5):748–66. doi: 10.1037//0022-006x.68.5.748. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301(5631):386–9. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Cornelis MC, Nugent NR, Amstadter AB, Koenen KC. Genetics of post-traumatic stress disorder: review and recommendations for genome-wide association studies. Curr Psychiatry Rep. 2010;12(4):313–26. doi: 10.1007/s11920-010-0126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiGrande L, Neria Y, Brackbill RM, Pulliam P, Galea S. Long-term posttraumatic stress symptoms among 3,271 civilian survivors of the September 11, 2001, terrorist attacks on the World Trade Center. Am J Epidemiol. 2011;173(3):271–81. doi: 10.1093/aje/kwq372. [DOI] [PubMed] [Google Scholar]
- Dodge KA, Bates JE, Pettit GS. Mechanisms in the cycle of violence. Science. 1990;250(4988):1678–83. doi: 10.1126/science.2270481. [DOI] [PubMed] [Google Scholar]
- Duncan LE, Keller MC. A critical review of the first 10 years of candidate gene-by-environment interaction research in psychiatry. Am J Psychiatry. 2011;168(10):1041–9. doi: 10.1176/appi.ajp.2011.11020191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan RD, Saunders BE, Kilpatrick DG, Hanson RF, Resnick HS. Childhood physical assault as a risk factor for PTSD, depression, and substance abuse: findings from a national survey. Am J Orthopsychiatry. 1996;66(3):437–48. doi: 10.1037/h0080194. [DOI] [PubMed] [Google Scholar]
- Gelernter J, Kranzler H, Coccaro EF, Siever LJ, New AS. Serotonin transporter protein gene polymorphism and personality measures in African American and European American subjects. Am J Psychiatry. 1998;155(10):1332–8. doi: 10.1176/ajp.155.10.1332. [DOI] [PubMed] [Google Scholar]
- Grabe HJ, Spitzer C, Schwahn C, Marcinek A, Frahnow A, Barnow S, Lucht M, Freyberger HJ, John U, Wallaschofski H, et al. Serotonin transporter gene (SLC6A4) promoter polymorphisms and the susceptibility to posttraumatic stress disorder in the general population. Am J Psychiatry. 2009;166(8):926–33. doi: 10.1176/appi.ajp.2009.08101542. [DOI] [PubMed] [Google Scholar]
- Homberg JR, Lesch KP. Looking on the bright side of serotonin transporter gene variation. Biol Psychiatry. 2011;69(6):513–9. doi: 10.1016/j.biopsych.2010.09.024. [DOI] [PubMed] [Google Scholar]
- Karg K, Burmeister M, Shedden K, Sen S. The serotonin transporter promoter variant (5-HTTLPR), stress, and depression meta-analysis revisited: evidence of genetic moderation. Arch Gen Psychiatry. 2011;68(5):444–54. doi: 10.1001/archgenpsychiatry.2010.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Kilpatrick DG, Koenen KC, Ruggiero KJ, Acierno R, Galea S, Resnick HS, Roitzsch J, Boyle J, Gelernter J. The serotonin transporter genotype and social support and moderation of posttraumatic stress disorder and depression in hurricane-exposed adults. Am J Psychiatry. 2007;164(11):1693–9. doi: 10.1176/appi.ajp.2007.06122007. [DOI] [PubMed] [Google Scholar]
- Koenen KC, Aiello AE, Bakshis E, Amstadter AB, Ruggiero KJ, Acierno R, Kilpatrick DG, Gelernter J, Galea S. Modification of the association between serotonin transporter genotype and risk of posttraumatic stress disorder in adults by county-level social environment. Am J Epidemiol. 2009;169(6):704–11. doi: 10.1093/aje/kwn397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolassa IT, Ertl V, Eckart C, Glockner F, Kolassa S, Papassotiropoulos A, de Quervain DJ, Elbert T. Association study of trauma load and SLC6A4 promoter polymorphism in posttraumatic stress disorder: evidence from survivors of the Rwandan genocide. J Clin Psychiatry. 2010;71(5):543–7. doi: 10.4088/JCP.08m04787blu. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, Benjamin J, Muller CR, Hamer DH, Murphy DL. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274(5292):1527–31. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- McGowan PO, Sasaki A, D’Alessio AC, Dymov S, Labonte B, Szyf M, Turecki G, Meaney MJ. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat Neurosci. 2009;12(3):342–8. doi: 10.1038/nn.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer KB, Orcutt HK, Quinn JF, Fitzgerald CA, Conneely KN, Barfield RT, Gillespie CF, Ressler KJ. Acute and posttraumatic stress symptoms in a prospective gene × environment study of a university campus shooting. Arch Gen Psychiatry. 2012;69(1):89–97. doi: 10.1001/archgenpsychiatry.2011.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell C, Notterman D, Brooks-Gunn J, Hobcraft J, Garfinkel I, Jaeger K, Kotenko I, McLanahan S. Role of mother’s genes and environment in postpartum depression. Proc Natl Acad Sci U S A. 2011;108(20):8189–93. doi: 10.1073/pnas.1014129108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnar BE, Buka SL, Kessler RC. Child sexual abuse and subsequent psychopathology: results from the National Comorbidity Survey. Am J Public Health. 2001;91(5):753–60. doi: 10.2105/ajph.91.5.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munafo MR, Durrant C, Lewis G, Flint J. Gene X environment interactions at the serotonin transporter locus. Biol Psychiatry. 2009;65(3):211–9. doi: 10.1016/j.biopsych.2008.06.009. [DOI] [PubMed] [Google Scholar]
- Nelson EC, Agrawal A, Pergadia ML, Lynskey MT, Todorov AA, Wang JC, Todd RD, Martin NG, Heath AC, Goate AM, et al. Association of childhood trauma exposure and GABRA2 polymorphisms with risk of posttraumatic stress disorder in adults. Mol Psychiatry. 2009;14(3):234–5. doi: 10.1038/mp.2008.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton EC, Wang H, Ai C. Computing interaction effects and standard errors in logit and probit models. The Stata Journal. 2004;4(1):154–67. [Google Scholar]
- Paolucci EO, Genuis ML, Violato C. A meta-analysis of the published research on the effects of child sexual abuse. J Psychol. 2001;135(1):17–36. doi: 10.1080/00223980109603677. [DOI] [PubMed] [Google Scholar]
- Pezawas L, Meyer-Lindenberg A, Drabant EM, Verchinski BA, Munoz KE, Kolachana BS, Egan MF, Mattay VS, Hariri AR, Weinberger DR. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nat Neurosci. 2005;8(6):828–34. doi: 10.1038/nn1463. [DOI] [PubMed] [Google Scholar]
- Pierucci-Lagha A, Gelernter J, Chan G, Arias A, Cubells JF, Farrer L, Kranzler HR. Reliability of DSM-IV diagnostic criteria using the semi-structured assessment for drug dependence and alcoholism (SSADDA) Drug Alcohol Depend. 2007;91(1):85–90. doi: 10.1016/j.drugalcdep.2007.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierucci-Lagha A, Gelernter J, Feinn R, Cubells JF, Pearson D, Pollastri A, Farrer L, Kranzler HR. Diagnostic reliability of the Semi-structured Assessment for Drug Dependence and Alcoholism (SSADDA) Drug Alcohol Depend. 2005;80(3):303–12. doi: 10.1016/j.drugalcdep.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Pritchard JK, Rosenberg NA. Use of unlinked genetic markers to detect population stratification in association studies. Am J Hum Genet. 1999;65(1):220–8. doi: 10.1086/302449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pynoos RS, Goenjian A, Tashjian M, Karakashian M, Manjikian R, Manoukian G, Steinberg AM, Fairbanks LA. Post-traumatic stress reactions in children after the 1988 Armenian earthquake. Br J Psychiatry. 1993;163:239–47. doi: 10.1192/bjp.163.2.239. [DOI] [PubMed] [Google Scholar]
- Ressler KJ, Bradley B, Mercer KB, Deveau TC, Smith AK, Gillespie CF, Nemeroff CB, Cubells JF, Binder EB. Polymorphisms in CRHR1 and the serotonin transporter loci: gene × gene × environment interactions on depressive symptoms. Am J Med Genet B Neuropsychiatr Genet. 2010;153B(3):812–24. doi: 10.1002/ajmg.b.31052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risch N, Herrell R, Lehner T, Liang KY, Eaves L, Hoh J, Griem A, Kovacs M, Ott J, Merikangas KR. Interaction between the serotonin transporter gene (5-HTTLPR), stressful life events, and risk of depression: a meta-analysis. JAMA. 2009;301(23):2462–71. doi: 10.1001/jama.2009.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheid JM, Holzman CB, Jones N, Friderici KH, Jernigan KA, Symonds LL, Sikorskii A, Fisher R. Life stressors and 5-HTTLPR interaction in relation to midpregnancy depressive symptoms among African-American women. Psychiatr Genet. 2011;21(6):271–80. doi: 10.1097/YPG.0b013e32834603e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southwick SM, Paige S, Morgan CA, 3rd, Bremner JD, Krystal JH, Charney DS. Neurotransmitter alterations in PTSD: catecholamines and serotonin. Semin Clin Neuropsychiatry. 1999;4(4):242–8. doi: 10.153/SCNP00400242. [DOI] [PubMed] [Google Scholar]
- Stein MB, Jang KL, Taylor S, Vernon PA, Livesley WJ. Genetic and environmental influences on trauma exposure and posttraumatic stress disorder symptoms: a twin study. Am J Psychiatry. 2002;159(10):1675–81. doi: 10.1176/appi.ajp.159.10.1675. [DOI] [PubMed] [Google Scholar]
- True WR, Rice J, Eisen SA, Heath AC, Goldberg J, Lyons MJ, Nowak J. A twin study of genetic and environmental contributions to liability for posttraumatic stress symptoms. Arch Gen Psychiatry. 1993;50(4):257–64. doi: 10.1001/archpsyc.1993.01820160019002. [DOI] [PubMed] [Google Scholar]
- Weaver IC, Cervoni N, Champagne FA, D’Alessio AC, Sharma S, Seckl JR, Dymov S, Szyf M, Meaney MJ. Epigenetic programming by maternal behavior. Nat Neurosci. 2004;7(8):847–54. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- Widom CS. The cycle of violence. Science. 1989;244(4901):160–6. doi: 10.1126/science.2704995. [DOI] [PubMed] [Google Scholar]
- Widom CS. Posttraumatic stress disorder in abused and neglected children grown up. Am J Psychiatry. 1999;156(8):1223–9. doi: 10.1176/ajp.156.8.1223. [DOI] [PubMed] [Google Scholar]
- Williams RB, Marchuk DA, Gadde KM, Barefoot JC, Grichnik K, Helms MJ, Kuhn CM, Lewis JG, Schanberg SM, Stafford-Smith M, et al. Serotonin-related gene polymorphisms and central nervous system serotonin function. Neuropsychopharmacology. 2003;28(3):533–41. doi: 10.1038/sj.npp.1300054. [DOI] [PubMed] [Google Scholar]
- Xie P, Kranzler HR, Poling J, Stein MB, Anton RF, Brady K, Weiss RD, Farrer L, Gelernter J. Interactive effect of stressful life events and the serotonin transporter 5-HTTLPR genotype on posttraumatic stress disorder diagnosis in 2 independent populations. Arch Gen Psychiatry. 2009;66(11):1201–9. doi: 10.1001/archgenpsychiatry.2009.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie P, Kranzler HR, Poling J, Stein MB, Anton RF, Farrer LA, Gelernter J. Interaction of FKBP5 with childhood adversity on risk for post-traumatic stress disorder. Neuropsychopharmacology. 2010;35(8):1684–92. doi: 10.1038/npp.2010.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang BZ, Zhao H, Kranzler HR, Gelernter J. Practical population group assignment with selected informative markers: characteristics and properties of Bayesian clustering via STRUCTURE. Genet Epidemiol. 2005;28(4):302–12. doi: 10.1002/gepi.20070. [DOI] [PubMed] [Google Scholar]
- Zimmermann P, Bruckl T, Nocon A, Pfister H, Binder EB, Uhr M, Lieb R, Moffitt TE, Caspi A, Holsboer F, et al. Interaction of FKBP5 gene variants and adverse life events in predicting depression onset: results from a 10-year prospective community study. Am J Psychiatry. 2011;168(10):1107–16. doi: 10.1176/appi.ajp.2011.10111577. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.