Abstract
Background
Mild hypothermia is neuroprotective following cerebral ischemia but surgery involving profound hypothermia (PH, temperature <18 °C) is associated with neurological complications. Rewarming (RW) from PH injures hippocampal neurons by glutamate excitotoxicity, N-methyl-D-aspartate receptors and intracellular calcium. Because neurons are protected from hypoxia-ischemia by anesthetics that inhibit N-methyl-D-aspartic acid receptors, we tested whether anesthetics protect neurons from damage caused by PH/RW.
Methods
Organotypic cultures of rat hippocampus were used to model PH/RW injury, with hypothermia at 4°C followed by RW to 37°C and assessment of cell death 1 or 24 h later. Cell death and intracellular Ca2+ were assessed with fluorescent dye imaging and histology. Anesthetics were present in the culture media during PH and RW or only RW.
Results
Injury to hippocampal CA1, CA3, and dentate neurons following PH and RW involved cell swelling, cell rupture, and adenosine triphosphate loss; this injury was similar for 4 through 10 h of PH. Isoflurane (1 and 2%), sevoflurane (3%) and xenon (60%) reduced cell loss but propofol (3 μM) and pentobarbital (100 μM) did not. Isoflurane protection involved reduction in N-methyl-D-aspartate receptor-mediated Ca2+ influx during RW but did not involve γ-amino butyric acid receptors or KATP channels. However, cell death increased over the next day.
Conclusions
Anesthetic protection of neurons rewarmed from 4°C involves suppression of N-methyl-D-aspartate receptor-mediated Ca2+ overload in neurons undergoing adenosine triphosphate loss and excitotoxicity. Unlike during hypoxia/ischemia, anesthetics acting predominately on γ-amino butyric acid receptors do not protect against PH/RW. The durability of anesthetic protection against cold injury may be limited.
Introduction
Controlled mild hypothermia (core temperature 32–34 °C) improves neurologic outcomes following neonatal asphyxia 1,2 and adult cardiac arrest3. However, profound hypothermia (PH), defined here as temperatures less than 18°C, is associated with neurologic injury. Concerns about the deleterious effects of hypothermia date from the early days of cardiac surgery4–6, with deeper levels of profound hypothermia (<18 °C) associated with frequent neurologic complications7,8. The causes of neurologic injuries caused by hypothermia have been little studied compared to hypoxic or ischemic injury.
Experimental studies examining the effects of PH on the central nervous system often have not separated injury caused by hypothermia with that caused by experimental ischemia or the cardiopulmonary bypass techniques. However, a number of laboratory studies suggest that PH/rewarming (RW) injures neurons separately from injury caused by cerebral blood flow insufficiency. For example, in dogs cooled to 12 °C during normal blood flow cardiopulmonary bypass, DeLeon et al. documented extensive neurologic damage in the cerebrocortex9. Similarly, Watanabe et al. 6 found widespread and persistent neural injury, including substantial loss of hippocampal neurons, in dogs following normal flow cardiopulmonary bypass in which the dogs were merely cooled to 20 °C and not subjected to any ischemic stress. Alam et al. 10 found that PH, independent of cerebral ischemia or other factors, causes central nervous system injury in swine. Damage and loss of hippocampal neurons are prominent findings following PH with or without circulatory arrest6,9, similar to hippocampal damage caused by global ischemia 11.
The mechanisms by which PH and/or RW injures neurons remains unclear and little studied. In a recent study12 we found that an increase in intracellular Ca2+ is a key pathological event in hippocampal neurons during PH and/or RW. We found that the increase in Ca2+ was solely due to N-methyl-D-aspartate receptors (NMDARs), with essentially no contribution from voltage gated Ca2+ channels, AMPA-type glutamate receptors, or metabotropic glutamate receptors. This is distinct from excitotoxicity following hypoxia or ischemia, in which multiple Ca2+ entry processes are involved and can be targets for experimental neuroprotection.
Anesthetic agents are neuroprotective in focal or global brain ischemia 13,14. Numerous mechanisms of anesthetic protection have been examined in experimental models of ischemia, including attenuation of glutamate excitotoxicity by block of NMDARs or reduction of glutamate release 15,16, opening of KATP channels 17, augmentation of γ-aminobutyric acid (GABA) receptor currents18, and activation of neuroprotective intracellular signaling pathways and prosurvival gene expression 19–21. Because anesthetics such as isoflurane blunt increases in intracellular Ca2+ mediated by glutamate excitotoxicity during and following ischemic insults and hypothermia involves excitotoxicity, we hypothesized that isoflurane and other anesthetics would also protect against injury caused by PH/RW. Although desflurane protects the brain during deep hypothermic cardiac arrest, the presumption is that desflurane targets the ischemic and not the hypothermic component of the injury 22. There has not been, to our knowledge, any study of how anesthetics affect the survival of neurons during and following PH/RW. The purpose of this study was therefore to determine whether anesthetics improve the cold tolerance of neurons, independent of ischemia-like conditions, and by what mechanisms.
MATERIALS and METHODS
The studies were approved by the University of California San Francisco Committee on Animal Research and conform to relevant National Institutes of Health guidelines for the use of animals in research.
Preparation of hippocampal slice cultures (HSCs)
Organotypic cultures of the hippocampus were prepared by standard methods 23. Eight or 9 day old Sprague Dawley rats (Charles River Laboratories, Hollister, CA) were anesthetized with 3% isoflurane until they did not move in response to a vigorous tail pinch and then decapitated. Seven-day old animals were not anesthetized before decapitation, per University of California San Francisco animal care guidelines. Following decapitation, the hippocampi were quickly removed and placed in 4°C Gey’s Balanced Salt Solution with 20 mM glucose. Further preparation and culture were as described in Bickler et al.24 About 16 slices are harvested from each rat pup, and a total of about 250 pups were used in the entire study.
Study design: cold injury in HSCs and treatment with anesthetics
Slice cultures were to exposed to hypothermia by placing them in a Billups-Rothenberg modular incubator chamber (Del Mar, CA) filled with humidified 95% air/5% carbon dioxide and placed in a 4 ± 1°C cold room for varying amounts of time. In a mock study, a thermocouple probe was placed in the culture media to measure temperature changes and it was found that the media cools to 4°C in about 1 h and that rewarming is complete in a similar period. Survival measurements and histology studies were done 1 h after completion of rewarming to 37°C. In some studies, cell death was measured 24 h after rewarming. To determine if the rate of rewarming is related to damage caused by hypothermia, we also measured cell death following 6 h of PH followed by a 2.5 h rewarming period. For this rewarming profile, slices were first rewarmed to 25 °C over the course of one hour and then warmed to 37°C over the next 1.5 h, achieving an approximately linear rate of warming over the 2.5-h period.
Exposures to anesthetics and study drugs were handled as follows. For the anesthetics isoflurane and sevoflurane, slice culture trays were placed open in Billups-Rothenberg chambers through which the anesthetic was flowed via a calibrated vaporizer with the carrier gas (air/5% CO2) for 5–8 min at 3 liters/min flow to ensure that the desired anesthetic concentration was achieved within the chamber. Isoflurane was studied at 1% and 2% and sevoflurane at 3% and was measured with a calibrated clinical infrared anesthetic analyzer. Isoflurane concentration in slice culture media was also measured in several mock experiments by withdrawing media through a polyethylene tube into a glass syringe. Samples in the syringe were mixed with nitrogen to extract anesthetic vapor and the isoflurane concentration in the nitrogen bubble was measured with a gas chromatograph.
When xenon was studied, we mixed this gas with 5% carbon dioxide/air in 4 liter precision spirometer calibration syringes and flushed through the chamber several times. Xenon concentration was not measured directly. Propofol (3 μM), pentobarbital (100 μM) or other study drugs (e.g., NMDA receptor antagonists and altered concentrations of K+) were present in theculture media 30 min before the start of the hypothermia.
Assessment of cell death in HSCs
Cell death was measured with propidium iodide or Sytox® fluorescence which provide essentially the same assessment of cell death. Each fluorescent dye penetrates damaged plasma membranes and binds to DNA. Confocal microscopy showed that PI and Sytox® both penetrate about 40 microns into the slice from both sides, labeling the vast majority of dead neurons (slice cultures typically thin to about 100 microns). Sytox® (0.5 μM) was added to the wells of the culture trays 1 h after the slices were transferred to the 37° C incubator. After 15 minutes, the Sytox® was washed out and digital images of fluorescence were acquired. The Sytox® excitation light wavelength was 504 nm and the emission was 523 nM. Propidium iodide (2.3 μM) was added to the wells of the culture trays 30 min after the slices were transferred to the 37°C incubator. After 30 min, the HSCs were rinsed in fresh media. The excitation light wavelength was 535 nm and emission was 620 nm for propidium iodide. For both Sytox® and propidium iodide, digital images were taken using a SPOT Jr. Digital Camera (Diagnostic Instruments, Sterling Heights, MI) and an inverted microscope. The fluorescence intensity of these dyes in slice cultures is a linear function of cell death 25,26 and was analyzed in different regions of the cultures (CA1, CA3, and dentate) with Image J software*, by a blinded observed.
Histologic analysis: Cresyl violet and Fluorojade
HSCs for histologic examinations were grown on membrane “confetti” as described by Lacour.27. Briefly, slices were cultured on discs of permeable membrane (Millipore, FHLC01300) on top of the usual slice culture inserts for 3 days prior to study. After experiments, the cultures et al were fixed in 4% paraformaldehyde in phosphate buffered saline for 1–2 h at 4°C. The cultures were then horizontally “resliced” as follows. Using a Z-axis controlled vibratome (Campden Instruments smz7000, Layfayette, IN), a flat surface was cut on a block of 3% agar. The confetti containing a HSC was removed from the culture insert and glued to the flat agar bed with cyanoacrylate cement, providing an absolutely horizontal tissue for slicing. From the ~100 μm thick HSC, one 30 μm horizontal slice was obtained and mounted on a gelatin slide to dry. The dried and fixed slices were stained with cresyl violet to assess cell morphology or fluorojade to identify degenerating neurons. Confocal microscopy was used to image fluorojade labeled neurons.
Measurement of Intracellular Ca2+
Intracellular Ca2+ in HSCs was measured with the fluorescent indicator calcium green 1-AM (Molecular Probes, Eugene, OR), because this dye loads into neurons in HSCs somewhat better than fura-2, which we have used previously. Cultures were loaded with 5–6 μM of the indicator during the 1-h RW period at 37°C. The cultures were rinsed and the fluorescence was quantified (excitation 488 nm, emission 520 nm) using a inverted microscope with the Spot Jr. camera. The background fluorescent signal from nonslice regions of the images was subtracted from the total fluorescent signal in the slice region. Fluorescence intensity was analyzed with Image J software.
ATP measurements
The ENLITEN® rLuciferase/Luciferin reagent (Promega, FF2021, San Diego, CA) and a luminometer was used to measure adenosine triphosphate (ATP) in hippocampal slice cultures. Slices were removed from culture membranes in cold 5% trichloroacetic acid and frozen in liquid nitrogen to inactivate ATP-degrading enzymes and preserve ATP levels during storage. Before assay, the pH in the samples was neutralized using 100 mM Tris-acetate buffer. ENLITEN® reagent was added to samples and ATP reference standards and the resulting luminescence was measured with a MicroLumat Plus LB96V (EG&G Berthold Technologies, Bad Wildbad, Germany) luminometer. Because individual slice cultures are uniform in size and weight, [ATP] in experimentally treated slices was simply expressed relative to [ATP] in control slices.
Measurement of glutamate release from cultures
Hippocampal slice cultures were grown for 7 days as described above and randomly assigned to these experimental groups: 1) control; 2) 6 h PH/1 h RW; 3) 6 h PH/1h RW with 2% isoflurane present during PH and RW; and 4) control plus 80 mM KCl to cause depolarization and full glutamate release. One ml of slice culture media was placed in each of the wells immediately prior to the experiment. The media was removed at each of the sampling points and snap frozen in ethanol/dry Ice bath before being analyzed for glutamate with a commercially available ELISA kit (BA E-2300; Rocky Mountain Diagnostics, Colorado Springs, CO). Glutamate concentration in the culture media (very low; the media contained no added glutamate) was also measured and subtracted from that in the experiment groups.
Data analysis
Neuron survival/injury experiments were designed to produce a normally distributed pattern of cell death as assayed by propidium iodide or Sytox® fluorescence. Therefore, ANOVA was used to compare the means of these data, and corrections were made for multiple comparisons with the Tukey-Kramer multiple comparison correction procedure; all statistical comparisons involve a two-tailed hypothesis of either an increase or decrease in a measured variable as a result of treatment. Differences were considered significant for P < 0.05. Other statistical comparisons involving multigroup design were also made with ANOVA and the Tukey-Kramer procedure. The GraphPad Prism® software package was used (GraphPad, Inc., La Jolla, CA).
Results
Effects of anesthetics on hypothermia and rewarming injury in hippocampal neurons
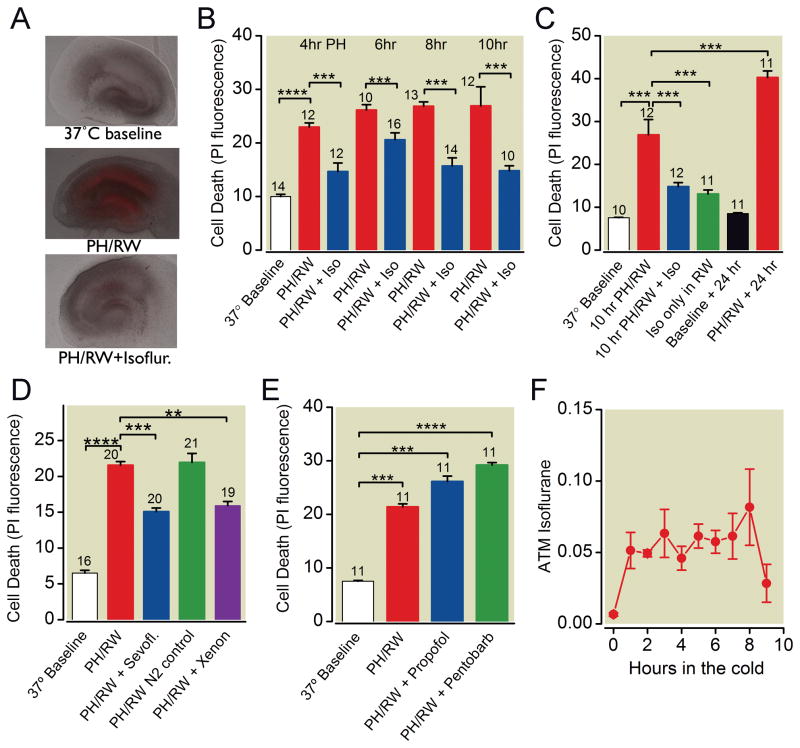
Hippocampal slice cultures exposed to profound hypothermia (4 ± 1 °C) followed by 1 h RW to 37 °C developed cell injury almost exclusively in the neuron cell body regions (fig. 1A). The duration of hypothermia was not significantly related to the amount of cell death (fig. 1B), suggesting that the rewarming period is the critical period in injury. Isoflurane (2%, approx. 1.3 minimal alveolar concentration), when present in the gas phase during hypothermia and rewarming, protected hippocampal neurons from cell death (one-way ANOVA with Tukey’s multiple comparison test, p < 0.001 for 4, 6, 8, and 10-h periods of hypothermia), with roughly similar degree of protection observed after the different durations of profound hypothermia (fig. 1B). We also examined the effects of 1% isoflurane on cell death following PH/RW and found similar protection as with 2% isoflurane (data not shown). Isoflurane reduced PH/RW injury even if it was present only during the rewarming phase of the injury (fig. 1C, one-way ANOVA with Tukey’s, p < 0.001). The anesthetics sevoflurane (3%, approx. 1.3 minimal alveolar concentration28) and xenon (60% of an atmosphere, about 0.4 minimal alveolar concentration for rats29) were also protective against PH/RW injury (fig. 1D, p < 0.001, one-way ANOVA with Tukey’s). Nitrogen substituted for air, as a control in the Xenon experiments, did not increase the PH/RW injury, even though it diluted the carbon dioxide in the chamber to 2%. Experiments examining the effects of reducing the carbon dioxide in the atmosphere in the chamber during hypothermia showed that varying carbon dioxide level between 2 and 10% had no effect on hypothermia and rewarming injury (data not shown). We also examined propofol and pentobarbital and found that these agents, at concentrations commonly used in in vitro neuroprotection studies18, had no effect on cold and rewarming injury (fig. 1E). Measurements of isoflurane concentration in the slice culture media revealed that steady-state concentrations were achieved at 1 h and that less than half of the isoflurane remained in the media 1 h after removal of isoflurane from the gas phase in the study chambers (fig. 1F).
Fig. 1.
Effects of inhaled and intravenous anesthetics on total death in neuron cell body regions (average of CA1, CA3 and dentate) in hippocampal slice cultures following profound hypothermia (4°C, PH) and rewarming (RW). A. Images of propidium iodide (PI) fluorescence overlaid on bright field images of cultures at baseline, after 6 h PH/1 h RW and after PH/RW with 2% isoflurane present. Red fluorescence indicates dead cells. B. Cell death assessed with PI fluorescence, in hippocampal slice cultures following 4, 6, 8 or 10 h at 4°C and 1 h of rewarming to 37°C. 2% isoflurane in the gas phase was present in the “Iso” groups during both PH and RW. C. Isoflurane is protective when present throughout hypothermia and rewarming (“10 hr PH/RW+Iso” group) or just during rewarming (“Iso only in RW” group). Data represent means±S.E.M. of PI fluorescence in CA1, CA3 and dentate regions. D. 3% Sevoflurane (“Sevofl”) and 60% xenon protect hippocampal slice cultures from 6 h of PH (4°C) and RW. E. Propofol (3 μM) and sodium pentobarbital (100 μM) present during 6 h of PH (4°C) and RW do not reduce cell death. F. Isoflurane concentrations in slice culture media (in atmospheres, ATM) during a mock hypothermia and rewarming experiment. In this experiment only, 5% isoflurane was used in the gas phase of the Billups-Rothenberg chamber. Samples of media were withdrawn at hourly intervals during hypothermia and after 1 h of rewarming. The chamber was flushed with air at 8 h. The number of cultures in each treatment group are indicated with numbers above the bars. The lines and asterisks above the error bars describe statistical differences between different treatment groups as follows: ** p<0.01, *** p<0.001 and **** p<0.00001 as measured with ANOVA and a Tukey multiple comparison post test.
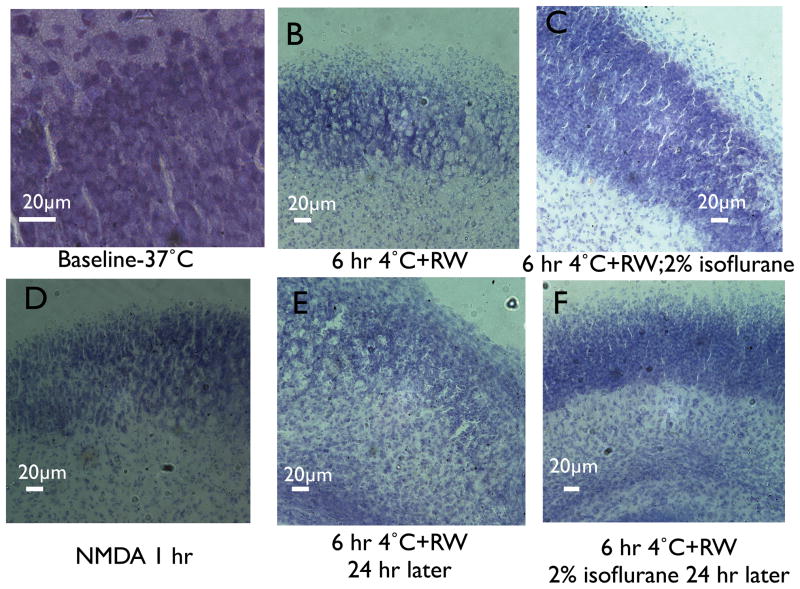
Histologic examination of re-sliced hippocampal cultures with cresyl violet following 6 h of profound hypothermia (4°C) and a 1 h period of rewarming to 37 °C revealed clear effects on the histologic appearance of neurons in the cell body regions. Rupture and loss of neurons was observed in cultures fixed immediately after rewarming (compare fig. 2A and B). Isoflurane prevented apparent cell loss in the cultures (fig 2C). Acute neurodegeneration caused by NMDA application (fig. 2D) caused cell disruption and nuclear condensation similar in histologic appearance to cold and rewarming. In cultures fixed 24 h after rewarming, greater numbers of condensed nuclei were seen, although total cell death, based on histologic appearance, did not appear to be much greater at 24 h after rewarming compared to 1 h after rewarming (fig. 2E, compare to Fig 2B). Isoflurane also improved the histologic appearance of cultures examined 24 h after rewarming (fig. 2F). These findings are consistent with the central role of NMDAR-mediated excitotoxicity in PH/RW injury and observations that NMDAR antagonists uniquely prevent PH/RW injury12.
Fig 2.
Cresyl violet stained re-sliced sections of hippocampal slice cultures, showing the CA1 or CA1-CA3 border region. Horizontal bar is 20 μm. A. Control or baseline conditions. B. Fixation after 1 h of rewarming (RW) to 37°C following 6 h at 4°C (profound hypothermia, PH). C. Same as B but with isoflurane present through the hypothermia and rewarming. D. Fixation 1 h after addition of N-methyl-D-aspartate cocktail (100 μM N-methyl-D-aspartate, Mg2+-free media). E. Fixation 24 h after rewarming. F. Fixation 24 h after rewarming in a culture exposed to isoflurane during profound hypothermia and the 1st h of rewarming.
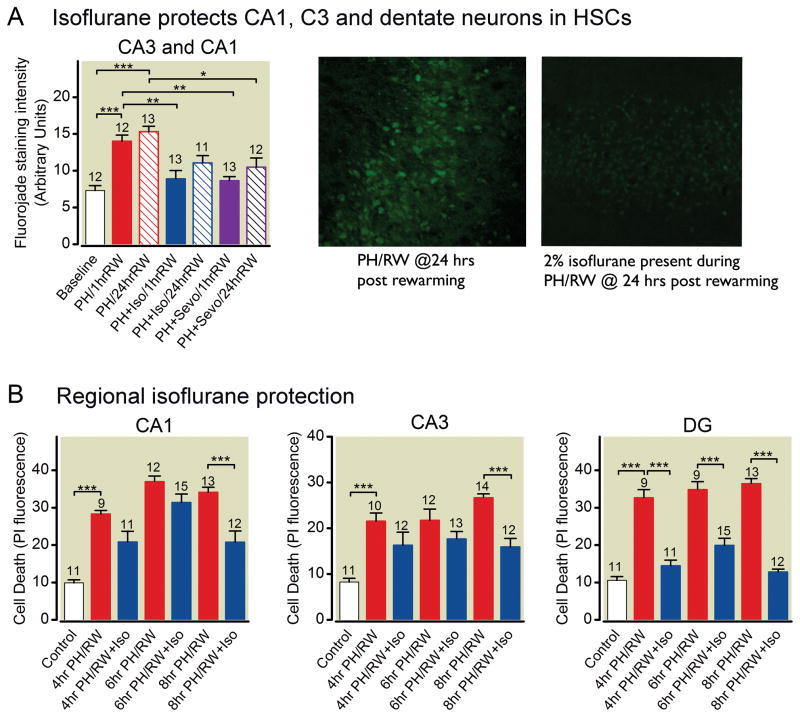
Fluorojade, a neuron-specific dye method for assessing cell death, was also used in the PH/RW studies. We examined the clearest cell body region in each culture, which was typically the CA1 or CA3 region. As with propidium iodide, fluorojade staining revealed that both isoflurane and sevoflurane reduced neuron injury caused by hypothermia and rewarming (fig. 3A). Regional analysis of cell death in the cultures demonstrated isoflurane protection in all the neuronal areas (fig. 3B, one-way ANOVA with Tukey’s multiple comparison test).
Fig 3.
Isoflurane protection from profound hypothermia/rewarming injury is neuron specific. A. Fluorojade staining intensity of neurons in the CA1-CA3 rich regions in hippocampal slice cultures following hypothermia and rewarming with isoflurane (ISO). Images at right were obtained with confocal microscopy of fixed and re-sliced cultures. Bright fluorescence identifies dead cells. B. Region specific cell death (propidium iodide fluorescence) in hippocampal slice cultures in which profound hypothermia (PH) was present for 4,6 or 8 h followed by rewarming to 37°C (RW) for 1 h. DG= dentate gyrus Bars represent means±S.E.M.. The number of cultures in each treatment group are indicated with numbers above the bars. The lines and asterisks above the error bars describe statistical differences between different treatment groups as follows: * p<0.05, ** p<0.01, and *** p<0.001, measured with ANOVA and a Tukey multiple comparison post test.
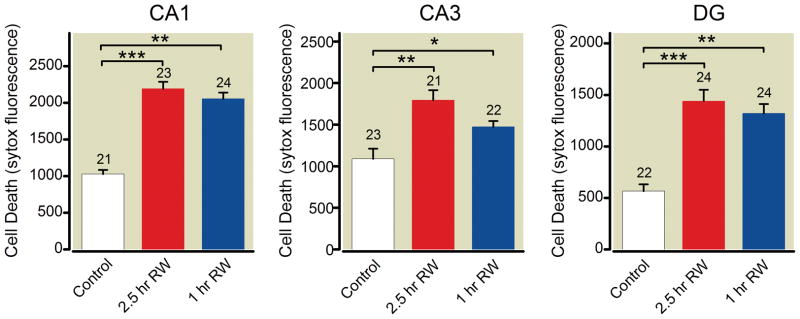
In experimental models of neural injury following profound hypothermia and cardiopulmonary bypass, the rate of rewarming may influence the degree of neurologic damage30. Therefore, we compared our standard rewarming protocol (rewarming from 4°C to 37°C in ~1 h) with a slower rewarming protocol (4°C to 37°C over 2.5 h). We found that slower rewarming had no effect on neuronal death (fig. 4).
Figure 4.
Rate of rewarming (RW) does not affect cell death in CA1, CA3 or dentate regions following profound hypothermia (PH, 4°C for 6 h). Controls are cultures remaining at 37°C; slow RW is rewarming to 25°C over 1 h, then to 37°C over the following 1.5 h; and 1h RW is our “standard rewarming” protocol involving RW from 4°C to 37°C over 1 h. DG= dentate gyrus. The number of cultures in each treatment group are indicated with numbers above the bars. The lines and asterisks above the error bars describe statistical differences between different treatment groups as follows: * p<0.05, ** p<0.01, and *** p<0.001, measured with ANOVA and a Tukey multiple comparison post test.
Isoflurane reduces ATP loss during profound hypothermia
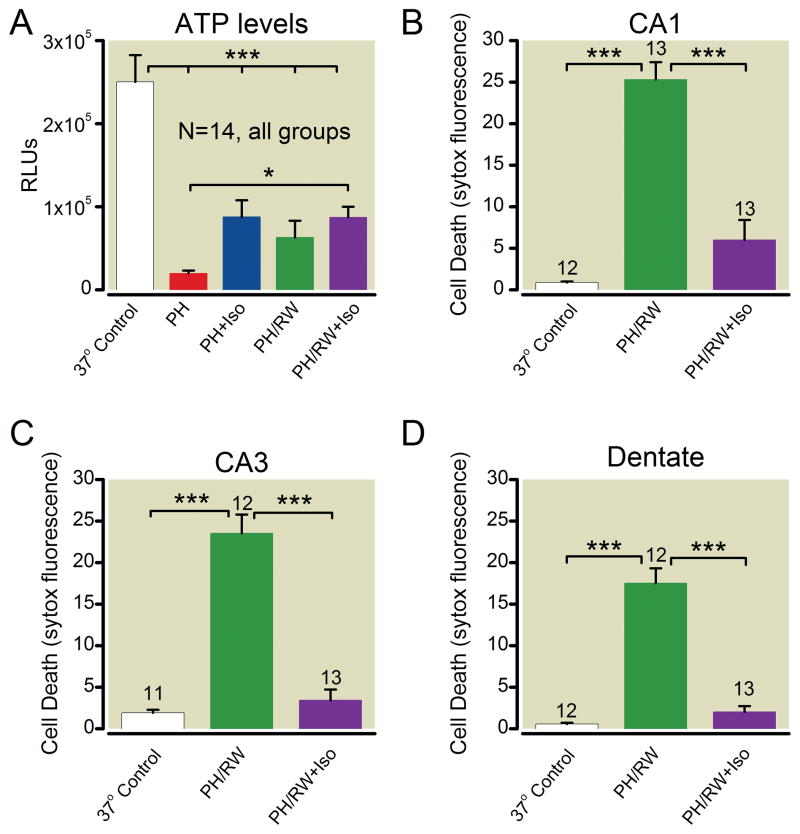
ATP levels and cell death in hippocampal slice cultures after 6 h at 4°C followed by rewarming to 37°C are shown in figure 5. ATP measured in cultures at the end of 8 h at 4 °C (frozen for ATP analysis before rewarming) was reduced by more than 80% compared to control. ATP measured immediately after rewarming rebounded moderately from this nadir (one way ANOVA with Tukey’s, P < 0.01), but remained low. Isoflurane present during hypothermia reduced ATP loss significantly during the period of hypothermia (p < 0.05) but isoflurane did not preserve ATP after rewarming. The moderately higher levels of ATP during hypothermia were apparently critical to cell survival because cell death in “sister” cultures from the same experiments in which the ATP measurements were made demonstrated that isoflurane substantially reduced cell death. This protection was found in CA1, CA3, and dentate regions of the slices (fig. 5B–D, one-way ANOVA p < 0.001 in all regions).
Fig. 5.
Adenosine tri-phosphate (ATP) levels and cell death in sister cultures of hippocampal slices. A. ATP levels. B–C. Cell death in CA1, CA3 and dentate regions. Profound hypothermia (PH) indicates cultures cooled to 4°C for 6 h, PH/RW indicates 1 h rewarming after PH, and Iso indicates groups with 2% isoflurane present during PH or PH/RW. The number of cultures in each treatment group are indicated with numbers above the bars. The lines and asterisks above the error bars describe statistical differences between different treatment groups as follows: * p<0.05 and *** p<0.001, measured with ANOVA and a Tukey multiple comparison post test.
Is isoflurane protection durable?
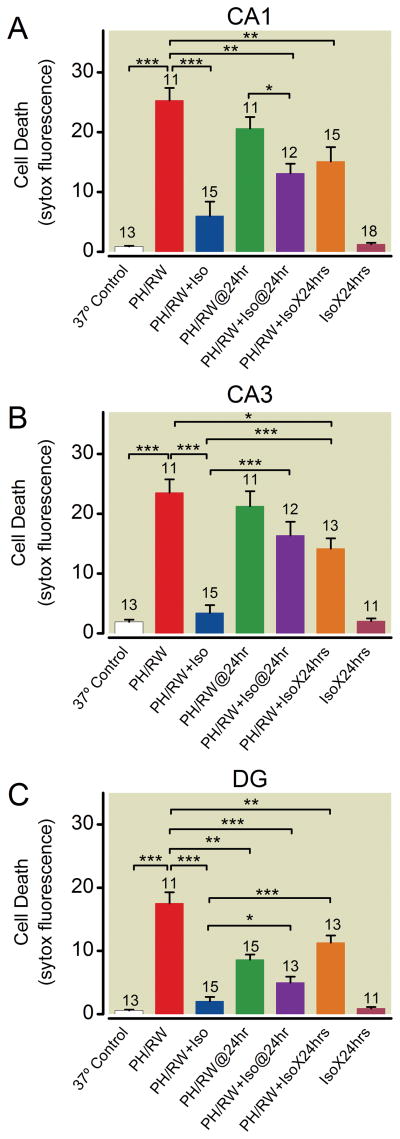
The durability of volatile anesthetic neuroprotection was a major concern in previous studies of neuroprotection following global or regional hypoxia or ischemia13. Indeed, from figure 3A, it appears that both isoflurane and sevoflurane improve survival measured within 1 h after rewarming, but not 24 h later. We thought that this might be due to continuing excitotoxicity during the 24 h following rewarming. To test this, we exposed cultures to 1% isoflurane during PH/RW and for 24 h after rewarming. Figure 6 confirms that isoflurane protection from PH/RW injury fades significantly when assessed at 24 h after rewarming (protection significantly less in CA3 and dentate at 24 h, ANOVA with Tukey’s, p < 0.001 and p < 0.05, respectively), and additionally shows that the continuous presence of 1% isoflurane during the 24-h period after rewarming was of no benefit in protecting neurons (p > 0.05 for all comparisons, one-way ANOVA with Tukey’s multiple comparison test). This pattern was seen in CA1, CA3, and to a lesser degree in dentate neurons.
Fig. 6.
Isoflurane protection of neurons in CA1, CA3 and dentate regions of slice cultures following profound hypothermia/rewarming (PH/RW) is time dependent. In panels A–C, cell death was nominally measured 1 h after completion of rewarming from 6 h of PH. The PH/RW@24hr and PH/RW+Iso@24hr groups had cell death assessed 24 h after the rewarming. The PH/RW+IsoX24 hrs group had 1% isoflurane present during PH, RW and during the 24 h period after rewarming. The IsoX24 hr group was in 1% isoflurane for 24 h only. Note that isoflurane protection seen at 1 h after rewarming fades by 24 h, whether isoflurane (Iso) is present for the subsequent 24 h or not. The number of cultures in each treatment group are indicated with numbers above the bars. The lines and asterisks above the error bars describe statistical differences between different treatment groups as follows: * p<0.05, ** p<0.01 and *** p<0.001, measured with ANOVA and a Tukey multiple comparison post test.
NMDA receptors and Ca2+ are involved in isoflurane protection against cold and rewarming injury, but not GABA receptors
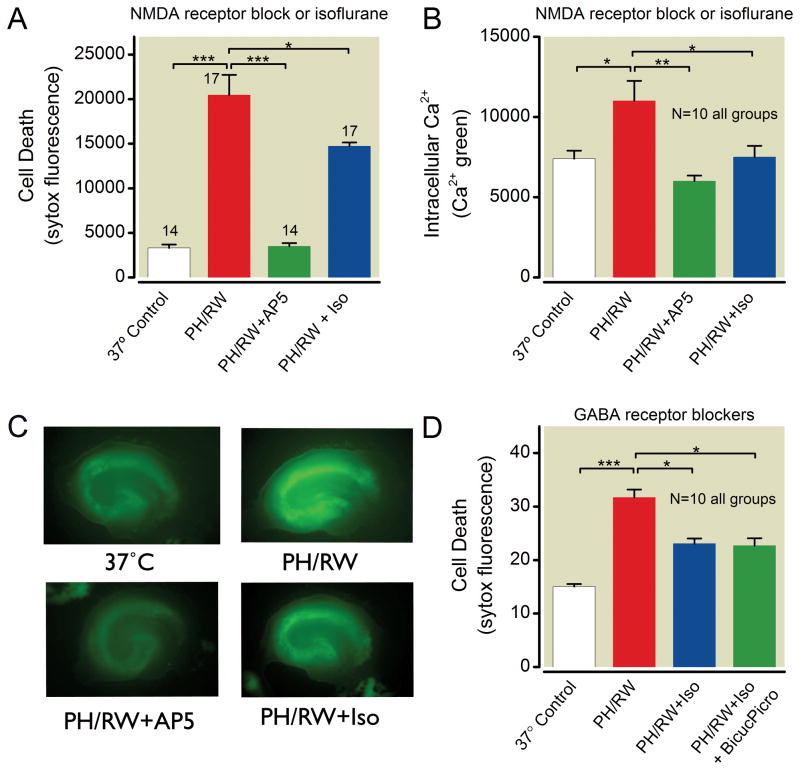
PH/RW injury is caused by glutamate excitotoxicity involving NMDA receptors12. Because isoflurane attenuates NMDAR currents31 and NMDA -based excitotoxicity both in the context of hypoxia/ischemia and exogenously applied neurotoxic concentrations of glutamate or NMDA 32, we reasoned that isoflurane protection of neurons exposed to PH/RW would also involve this mechanism. We found that the selective NMDA receptor antagonist AP5 reduces PH/RW injury and prevents Ca2+ entry (fig. 7A–C). Isoflurane follows this pattern as well, suggesting that isoflurane is acting to antagonize NMDAR-mediated neuronal death in PH/RW injury.
Fig. 7.
Isoflurane protection of hippocampal neurons after profound hypothermia/rewarming (PH/RW) involves N-methyl-D-aspartate (NMDA) receptors but not gamma-amino butyric acid (GABA) receptors. A. Isoflurane (Iso) and the competitive NMDA receptor antagonist AP5 both reduce PH/RW injury in slice cultures. B. Isoflurane and AP5 prevent increases in intracellular Ca2+ in cultures exposed to 4 h PH and 1 h RW. C. Typical images of calcium green fluorescence in slice cultures at baseline (37°C), after PH/RW, after PH/RW with the competitive NMDAR antagonist AP5 and after PH/RW when isoflurane was present. D. Lack of involvement of GABAA receptors in isoflurane protection against profound hypothermia and rewarming injury. The GABAA antagonists bicuculline (50 μM) and picrotoxin (100 μM) were present during PH and RW. Bar graphs show means±S.E.M. The number of cultures in each treatment group are indicated with numbers above the bars. The lines and asterisks above the error bars describe statistical differences between different treatment groups as follows: * p<0.05, ** p<0.01 and *** p<0.001, measured with ANOVA and a Tukey multiple comparison post test.
Isoflurane’s protective effect in hypoxic-ischemic neuronal injury is partly mediated by GABAA receptor activation or augmentation11. GABAA receptors are probably not related to protection against PH/RW injury because isoflurane remained protective when the GABAA antagonists bicuculline and picrotoxin were present in concentrations sufficient to block all GABAA receptor activity (fig. 7D, one-way ANOVA with Tukey’s, p < 0.05 compared to PH/RW group).
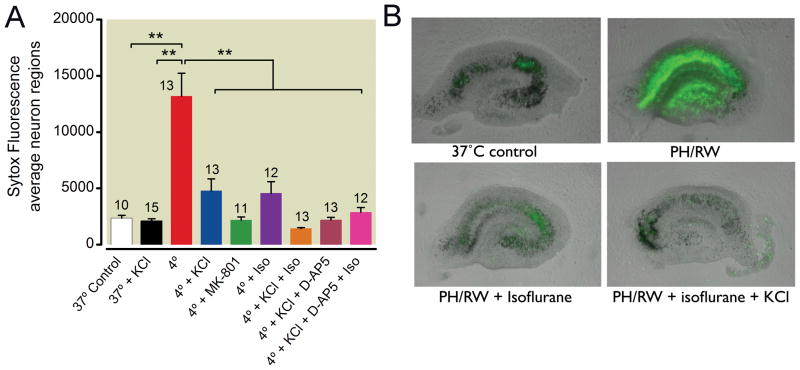
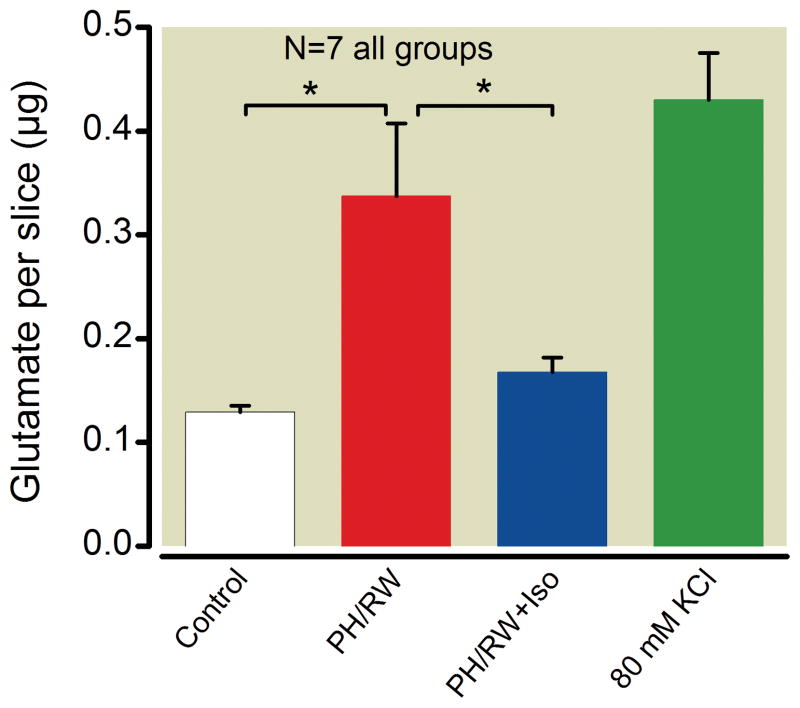
To further define the role of isoflurane in modulating glutamate excitotoxicity in PH/RW injury, we used media with 30 mM K+ during hypothermia to block glutamate release during the subsequent period of rewarming. This approach was based on the work of Hogins et al.33 who showed that 30 mM K+ conferred a preconditioning effect against a stress of oxygen/glucose deprivation that was clearly related to pre-synaptic silencing of glutamate release. 30 mM KCl was markedly protective against PH/RW injury, consistent with the importance of glutamate excitotoxicity in PH/RW injury (fig. 8, p < 0.01 one-way ANOVA with Tukey’s multiple comparison test). Isoflurane combined with 30 mM KCl produced additional protection, as did the NMDAR antagonist AP5. Because 30 mM K+ depolarizes and clamps hippocampal neurons to about −13 mV 12, membrane potential per se must not be the parameter that is responsible for isoflurane protection in PH/RW injury. We also investigated whether isoflurane decreases the release of glutamate during PH/RW injury. We found that isoflurane significantly reduced the release of glutamate into the culture media during the period of hypothermia (fig. 9), from a mean of about 0.35 μg of glutamate per slice to to 0.16 μg per slice (p < 0.05, one-way ANOVA with Tukey’s multiple comparison test).
Fig. 8.
Effects of 30 mM K+, isoflurane (Iso) or N-methyl-D-aspartate (NMDA) antagonists on neuron death following profound hypothermia/rewarming (PH/RW). 30 mM K+ was added to the media prior to hypothermia to prevent glutamate release at later times (i.e. during rewarming). 30 mM K+ will clamp the membrane potential to ~−13 mV12. Sytox® fluorescence values are means±SEM. At right are typical images of Sytox® fluorescence overlaid on the corresponding bright field images of cultures from several treatment groups. The number of cultures in each treatment group are indicated with numbers above the bars. The lines and asterisks above the error bars describe statistical differences between different treatment groups as follows: ** p<0.01, measured with ANOVA and a Tukey multiple comparison post test.
Fig. 9.
Isoflurane (Iso) decreases glutamate concentration in the media during profound hypothermia (PH) and rewarming (RW). Glutamate was assayed in control cultures and after 6 h at 4°C followed by 1h of rewarming (PH/RW), and with 2% isoflurane in the gas phase during the entire PH/RW period. The KCl group was treated with 80 mM KCl at 37°C to cause maximal glutamate release. In all panels, the number of cultures in each treatment group are indicated with numbers above the bars. The lines and asterisks above the error bars describe statistical differences between different treatment groups (p<0.05), measured with ANOVA and a Tukey multiple comparison post test.
KATP channels are not involved in isoflurane protection against cold and rewarming injury
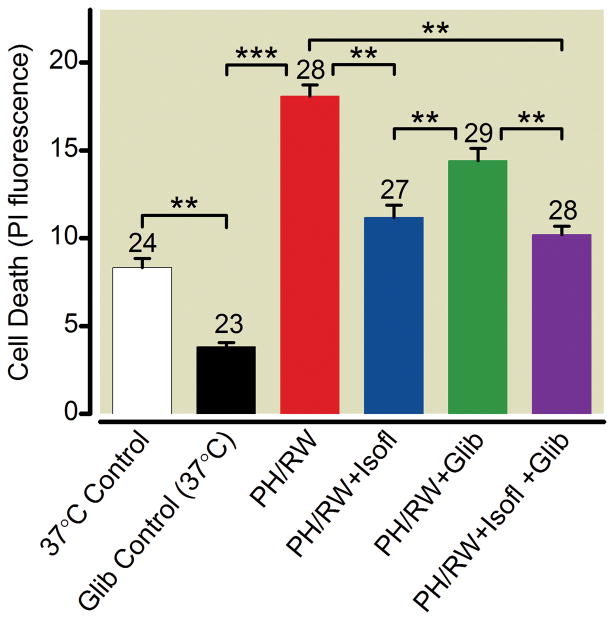
ATP-sensitive K channels are proposed to mediate part of isoflurane’s protection of hypoxic or ischemic myocardium and brain tissue17,34. To determine if this mechanism accounts for isoflurane protection following PH/RW, slice cultures were preincubated in the KATP channel blocker glibenclamide for 30 min. before cooling them to 4 °C for 6 h. Gliblenclamide did not prevent isoflurane from protecting neurons when they were rewarmed from this period of hypothermia (fig. 10, ANOVA with Tukey’s multiple comparison test, p > 0.05).
Fig. 10.
KATP channels are not involved in isoflurane protection against profound hypothermia and rewarming injury in hippocampal slice cultures. Glibenclamide (100 μM, Glib) or isoflurane (2%, Isofl) was present during the entire profound hypothermia (PH) and rewarming (RW) period in the PH/RW+Isofl, PH/RW+glib and PH/RW+Glib+Iso groups. Data represent means±SEM. The number of cultures in each treatment group are indicated with numbers above the bars. The lines and asterisks above the error bars describe statistical differences between different treatment groups as follows: ** p<0.01 and *** p<0.001, measured with ANOVA and a Tukey multiple comparison post test.
Discussion
Deep hypothermia is currently used to facilitate complex surgical procedures including those that involve cardiac arrest 35,36. Such procedures are frequently complicated by adverse neurological outcomes, but it is unclear whether ischemia, hypothermia or both cause the injury. The results of this study, and another from our laboratory12 show that hypothermia (4 °C) and rewarming damages rat hippocampal neurons. The experimental conditions for both these studies are such that we can exclude ischemia-like damage from the injury to the neurons. Studies in dogs suggest that profound hypothermia, independent of other factors, causes neuronal injury 6. The rate of rewarming from hypothermia may influence the severity of injury caused by hypothermia or ischemia during the period of hypothermia, which is an important issue that is debated in the clinical use of hypothermia for cardiopulmonary bypass37. However, when we slowed the rewarming period after hypothermia from 1 h to 2.5 h, no difference in neuron death was seen (fig. 4). Whether rewarming rate matters for less severe hypothermia and rewarming injury is currently under investigation in our laboratory.
We found that the volatile anesthetics isoflurane, sevoflurane and xenon, but not the intravenous anesthetics propofol and pentobarbital, protect neurons from the injury caused by profound hypothermia (4°C) and rewarming. The mechanism of isoflurane protection appears to involve limiting NMDA receptor-dependent Ca2+ overload that may be related to suppressing the release of glutamate caused by the stress of hypothermia and rewarming or by antagonizing NMDA receptors. Isoflurane also reduced the loss of ATP during hypothermia itself, but not when the entire hypothermia and rewarming period. We excluded several other targets that were thought to be involved in isoflurane protection; including ATP-sensitive K+ channels and GABA receptors. Because protection from cold injury was achieved when isoflurane was present only during rewarming, it appears that the predominant target for isoflurane is NMDA receptors during the rewarming phase when energy depletion is severe and the potential for excitotoxicity may be greatest.
A major finding was that isoflurane protection of neurons injured by PH/RW was not completely durable; that is, we observed protection after 1 h of rewarming, but 24 h later, the injury increased. This decrease in protection was also seen when the isoflurane was continued during the entire 24-h post-rewarming period. There are several possibilities for these observations. The first is that PH/RW injury continues to evolve long after completion of rewarming, even though injury measured by propidium iodide or Sytox® does not suggest this12. The second is that isoflurane is relatively weak in preventing PH/RW injury and that excitotoxicity still kills many neurons if excitotoxicity persists for a long enough period of time. This possibility could be tested in experiments in which NMDA receptor antagonists remain in the culture media after rewarming, although NMDA antagonist toxicity was a problem when this was attempted.
Blocking NMDA receptors was effective in preventing PH/RW injury in hippocampal neurons (figs. 6 and 7), as was reducing extracellular [Ca2+] with the chelating agent EGTA12. Similar to Ca2+-related neuron injury following hypoxia or ischemia, hypothermia-rewarming injury involves glutamatergic excitotoxicity, where uncontrolled Ca2+ influx through NMDA receptors is caused by release of glutamate from depolarizing neurons. In contrast to hypoxic-ischemic injury, T-type Ca2+ channels and L-type Ca2+ channels and the reverse mode Na+/Ca2+ exchanger are not involved in PH/RW injury12. Volatile anesthetics are known to modulate excitotoxicity by several processes, including augmentation of glutamate re-uptake transporters, inhibition of voltage-gated Ca2+ channels and activation of KATP channels, and suppression of glutamate release. Although we only examined the mechanisms of protection in the case of isoflurane, it is reasonable to suggest that similar processes apply in the case of sevoflurane and xenon. Neither propofol nor pentobarbital have significant effects on NMDA receptors and this is probably why they were ineffective in preventing PH/RW injury.
We observed that increasing extracellular K+ to 30 mM protects neurons from cold injury (fig. 7). This effect is observed in a variety of preparations, including brain slices and dissociated neurons 38,39 and is most likely due to suppression of presynaptic glutamate release33. These findings support our belief that glutamate neurotransmission is important to the excitotoxicity involved in PH/RW injury. As seen in figure 9, PH/RW involves glutamate release from the cultures. At much higher K+ concentrations (80–120 mM), neurons are killed by Ca2+ accumulation from activation of voltage-dependent Ca2+ channels39. The data in figure 8 also make it unlikely that maintenance of a range of membrane potential is the key to surviving PH/RW; based on previous patch-clamp studies of isolated hippocampal neurons we showed that 30 mM K+ drives the membrane potential to about −13 mV. 30 mM K+ was markedly neuroprotective, making it unlikely that maintenance of normal or hyperpolarized membrane potential is uniquely necessary for cold survival. Furthermore, because isoflurane added to the neuroprotection afforded by 30 mM K+, it is unlikely that K channels (i.e., background or tandem-pore K channels) exclusively confer isoflurane’s protection against PH/RW injury because the membrane potential of neurons is effectively clamped to depolarized potentials by the high extracellular K+ concentration.
The observation that isoflurane was additive in protection to that conferred by 30 mM K+ (fig. 7) suggests that isoflurane’s effects on glutamate excitotoxicity is mediated by inhibition post-synaptic NMDARs rather than only by suppression of glutamate release. This is because 30 mM K+ acts to mute synaptic release of glutamate33; the fact that isoflurane produces additional protection in the presence of the high K+ means that it must have a separate protective effect. However, isoflurane did reduce the release of glutamate during hypothermia and rewarming. Taken together, we suggest that these data mean that isoflurane probably has both a pre and postsynaptic effect on limiting glutamate excitotoxicity during PH/RW injury.
Study Limitations
The inference that isoflurane’s main target in protecting neurons from PH/RW injury is the NMDA receptor is limited by the fact that we did not directly measure inhibition of NMDARs in hypothermic or rewarming neurons. This was attempted, but was defeated by the difficulty of patch-clamping neurons in rewarming cultures (swelling makes the neurons rupture easily). The conclusion that isoflurane inhibits the NMDARs and that NMDARs are related to PH/RW injury and calcium overload is based on solid evidence, however. Isoflurane also decreases the release of glutamate, an effect that also would act to limit NMDAR-mediated Ca2+ overload during PH/RW injury.
With the exception of isoflurane, we studied only single concentrations of each anesthetic and although the concentrations were similar to those used clinically, we cannot exclude the possibility that the protective effects of these compounds is dose-dependent. Another issue relates to anesthetic potency at different temperatures. What is the relevance of minimal alveolar concentration or anesthetic concentration at temperatures below 28°C where low temperature itself produces immobility? It is important to consider that, when volatile anesthetics are used in hypothermic patients, no specific temperature correction is used to adjust the delivered concentration. Further, we believe that the protective effect of isoflurane or the other anesthetics is exerted during the rewarming period when excitotoxicity must peak. This does not explain why isoflurane reduces ATP loss during the period of hypothermia beyond suggesting that ATPase activity must be suppressed by isoflurane in hypothermic neurons.
Innate differences in the hypothermia tolerances of different species of animals means that extrapolation of our results to other species of animals is difficult. Rats are probably more hypothermia tolerant than humans and 7-day old rats are extremely tolerant of hypothermia compared to adult rats40. It is therefore likely that the neuronal damage following exposure of hippocampal slice cultures to 4°C would occur at higher temperatures or with shorter durations of hypothermia in less hypothermia tolerant species, including humans.
Conclusions
We conclude that the most important neuroprotective target for volatile anesthetics in neurons undergoing PH/RW is NMDA receptor-mediated Ca2+ influx caused by glutamate excitotoxicity, which may be mediated both by reduced glutamate release and reduced NMDAR activity. Excitotoxicity is probably most significant during rewarming, when energy depleted neurons experience dissipated ion gradients and peak risk of excitotoxic Ca2+ accumulation. Despite these protective actions, neuroprotection following profound hypothermia and rewarming decreased with time, with protection fading by 24 h even when isoflurane was present during the entire post-rewarming period.
Summary Statement.
Isoflurane, sevoflurane and xenon reduce damage to hippocampal neurons exposed to cold and rewarming injury. With isoflurane, the mechanism involves limiting glutamate excitotoxicity/N-methyl-D-aspartate receptor-mediated Ca2+ overload, but the protection may be transient.
What we already know about this topic
While moderate hypothermia can be neuroprotective, profound hypothermia is associated with neurological injury by poorly understood mechanisms
Profound hypothermia and rewarming are associated with detrimental increases in intracellular calcium via N-methyl-D-aspartic acid (NMDA) type glutamate receptors
What this article tells us that is new
In a rat hippocampal slice model, inhaled, but not intravenous, anesthetics provided early neuroprotection following profound hypothermia and rewarming
The transient protective effect of isoflurane involved reduced calcium entry by NMDA receptor blockade, in contrast to ischemic protection that involves potentiation of type A γ-aminobuyric acid receptors
Acknowledgments
Supported by a grant (RO1 GM 52212) from the US National Institutes of Health (Washington, D.C.) to P. Bickler. We thank Ted Eger, M.D., Professor of Anesthesia, University of California, San Francisco, San Francisco, California) for help measuring isoflurane concentration in media samples.
Footnotes
(http://rsb.info.nih.gov/ij), last accessed March, 12, 2012.
Presented in part at the annual meeting of the Society for Neuroanesthesia and Critical Care, San Diego, California, October 15, 2010.
References
- 1.Gluckman PD, Wyatt JS, Azzopardi D, Ballard R, Edwards AD, Ferriero DM, Polin RA, Robertson CM, Thoresen M, Whitelaw A, Gunn AJ. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: Multicentre randomised trial. Lancet. 2005;365:663–70. doi: 10.1016/S0140-6736(05)17946-X. [DOI] [PubMed] [Google Scholar]
- 2.Shankaran S, Laptook AR, Ehrenkranz RA, Tyson JE, McDonald SA, Donovan EF, Fanaroff AA, Poole WK, Wright LL, Higgins RD, Finer NN, Carlo WA, Duara S, Oh W, Cotten CM, Stevenson DK, Stoll BJ, Lemons JA, Guillet R, Jobe AH. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005;353:1574–84. doi: 10.1056/NEJMcps050929. [DOI] [PubMed] [Google Scholar]
- 3.Hypothermia after Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346:549–56. doi: 10.1056/NEJMoa012689. [DOI] [PubMed] [Google Scholar]
- 4.Egerton N, Egerton WS, Kay JH. Neurologic changes following profound hypothermia. Ann Surg. 1963;157:366–74. doi: 10.1097/00000658-196303000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khaladj N, Shrestha M, Meck S, Peterss S, Kamiya H, Kallenbach K, Winterhalter M, Hoy L, Haverich A, Hagl C. Hypothermic circulatory arrest with selective antegrade cerebral perfusion in ascending aortic and aortic arch surgery: A risk factor analysis for adverse outcome in 501 patients. J Thorac Cardiovasc Surg. 2008;135:908–14. doi: 10.1016/j.jtcvs.2007.07.067. [DOI] [PubMed] [Google Scholar]
- 6.Watanabe Y, Kawaura Y, Iwa T. Brain damage after deep hypothermia in dogs. Jap J Surgery. 1982;12:61–70. doi: 10.1007/BF02469017. [DOI] [PubMed] [Google Scholar]
- 7.Levin DA, Seay AR, Fullerton DA, Simoes EA, Sondheimer HM. Profound hypothermia with alpha-stat pH management during open-heart surgery is associated with choreoathetosis. Pediatr Cardiol. 2005;26:34–8. doi: 10.1007/s00246-004-0669-6. [DOI] [PubMed] [Google Scholar]
- 8.Visconti KJ, Rimmer D, Gauvreau K, del Nido P, Mayer JE, Jr, Hagino I, Pigula FA. Regional low-flow perfusion versus circulatory arrest in neonates: One-year neurodevelopmental outcome. Ann Thorac Surg. 2006;82:2207–11. doi: 10.1016/j.athoracsur.2006.06.069. [DOI] [PubMed] [Google Scholar]
- 9.DeLeon SY, Thomas C, Roughneen PT, King N, Lehne R, DeLeon AM, Walenga J, Pifarre R. Experimental evidence of cerebral injury from profound hypothermia during cardiopulmonary bypass. Pediatr Cardiol. 1998;19:398–403. doi: 10.1007/s002469900335. [DOI] [PubMed] [Google Scholar]
- 10.Alam HB, Chen Z, Li Y, Velmahos G, DeMoya M, Keller CE, Toruno K, Mehrani T, Rhee P, Spaniolas K. Profound hypothermia is superior to ultraprofound hypothermia in improving survival in a swine model of lethal injuries. Surgery. 2006;140:307–14. doi: 10.1016/j.surg.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 11.Lipton P. Ischemic cell death in brain neurons. Physiol Rev. 1999;79:1431–567. doi: 10.1152/physrev.1999.79.4.1431. [DOI] [PubMed] [Google Scholar]
- 12.Warren DE, Bickler PE, Clark JP, Gregersen M, Brosnan H, McKleroy W, Gabatto P. Hypothermia and rewarming injury in hippocampal neurons involves intracellular Ca2+ and glutamate excitotoxicity. Neuroscience. 2012 doi: 10.1016/j.neuroscience.2011.12.034. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 13.Sakai H, Sheng H, Yates RB, Ishida K, Pearlstein RD, Warner DS. Isoflurane provides long-term protection against focal cerebral ischemia in the rat. Anesthesiology. 2007;106:92–9. doi: 10.1097/00000542-200701000-00017. [DOI] [PubMed] [Google Scholar]
- 14.Weigl M, Tenze G, Steinlechner B, Skhirtladze K, Reining G, Bernardo M, Pedicelli E, Dworschak M. A systematic review of currently available pharmacological neuroprotective agents as a sole intervention before anticipated or induced cardiac arrest. Resuscitation. 2005;65:21–39. doi: 10.1016/j.resuscitation.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 15.Eilers H, Bickler PE. Hypothermia and isoflurane similarly inhibit glutamate release evoked by chemical anoxia in rat cortical brain slices. Anesthesiology. 1996;85:600–7. doi: 10.1097/00000542-199609000-00020. [DOI] [PubMed] [Google Scholar]
- 16.Kudo M, Aono M, Lee Y, Massey G, Pearlstein RD, Warner DS. Effects of volatile anesthetics on N-methyl-D-aspartate excitotoxicity in primary rat neuronal-glial cultures. Anesthesiology. 2001;95:756–65. doi: 10.1097/00000542-200109000-00031. [DOI] [PubMed] [Google Scholar]
- 17.Wang J, Cottrell JE, Kass IS. Effects of desflurane and propofol on electrophysiological parameters during and recovery after hypoxia in rat hippocampal slice CA1 pyramidal cells. Neuroscience. 2009;160:140–8. doi: 10.1016/j.neuroscience.2009.02.027. [DOI] [PubMed] [Google Scholar]
- 18.Bickler PE, Warner DS, Stratmann G, Schuyler JA. γ-Aminobutyric acid-A receptors contribute to isoflurane neuroprotection in organotypic hippocampal cultures. Anesth Analg. 2003;97:564–71. doi: 10.1213/01.ANE.0000068880.82739.7B. [DOI] [PubMed] [Google Scholar]
- 19.Bickler PE, Fahlman CS. Enhanced hypoxic preconditioning by isoflurane: Signaling gene expression and requirement of intracellular Ca2+ and inositol triphosphate receptors. Brain Res. 2010;1340:86–95. doi: 10.1016/j.brainres.2010.04.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bickler PE, Zhan X, Fahlman CS. Isoflurane preconditions hippocampal neurons against oxygen-glucose deprivation: Role of intracellular Ca2+ and mitogen-activated protein kinase signaling. Anesthesiology. 2005;103:532–9. doi: 10.1097/00000542-200509000-00016. [DOI] [PubMed] [Google Scholar]
- 21.Gray JJ, Bickler PE, Fahlman CS, Zhan X, Schuyler JA. Isoflurane neuroprotection in hypoxic hippocampal slice cultures involves increases in intracellular Ca2+ and mitogen-activated protein kinases. Anesthesiology. 2005;102:606–15. doi: 10.1097/00000542-200503000-00020. [DOI] [PubMed] [Google Scholar]
- 22.Kurth CD, Priestley M, Watzman HM, McCann J, Golden J. Desflurane confers neurologic protection for deep hypothermic circulatory arrest in newborn pigs. Anesthesiology. 2001;95:959–64. doi: 10.1097/00000542-200110000-00027. [DOI] [PubMed] [Google Scholar]
- 23.Stoppini L, Buchs PA, Muller D. A simple method for organotypic cultures of nervous tissue. J Neurosci Methods. 1991;37:173–82. doi: 10.1016/0165-0270(91)90128-m. [DOI] [PubMed] [Google Scholar]
- 24.Bickler PE, Fahlman CS, Gray JJ. Hypoxic preconditioning failure in aging hippocampal neurons: Impaired gene expression and rescue with intracellular calcium chelation. J Neurosci Res. 2010;88:3520–9. doi: 10.1002/jnr.22508. [DOI] [PubMed] [Google Scholar]
- 25.Laake JH, Haug F-M, Weiloch T, Ottersen OP. A simple in vitro model of ischemia based on hippocampal slice cultures and propidium iodide fluorescence. Brain Res Brain Res Protoc. 1999;4:173–84. doi: 10.1016/s1385-299x(99)00021-5. [DOI] [PubMed] [Google Scholar]
- 26.Newell DW, Barth A, Papermaster V, Malouf AT. Glutamate and non-glutamate receptor mediated toxicity caused by oxygen and glucose deprivation in organotypic hippocampal cultures. J Neuroscience. 1995;15:7702–11. doi: 10.1523/JNEUROSCI.15-11-07702.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lacour P, Heimrich B, Prols F. Induction of cellular stress and chaperone activation in organotypic slice cultures of hippocampus. J Neurosci Methods. 2007;166:24–31. doi: 10.1016/j.jneumeth.2007.06.019. [DOI] [PubMed] [Google Scholar]
- 28.Orliaguet G, Vivien B, Langeron O, Bouhemad B, Coriat P, Riou B. Minimum alveolar concentration of volatile anesthetics in rats during postnatal maturation. Anesthesiology. 2001;95:734–9. doi: 10.1097/00000542-200109000-00028. [DOI] [PubMed] [Google Scholar]
- 29.Koblin DD, Fang Z, Eger EI, 2nd, Laster MJ, Gong D, Ionescu P, Halsey MJ, Trudell JR. Minimum alveolar concentrations of noble gases, nitrogen, and sulfur hexafluoride in rats: Helium and neon as nonimmobilizers (nonanesthetics) Anesth Analg. 1998;87:419–24. doi: 10.1097/00000539-199808000-00035. [DOI] [PubMed] [Google Scholar]
- 30.Gordan ML, Kellermann K, Blobner M, Nollert G, Kochs EF, Jungwirth B. Fast rewarming after deep hypothermic circulatory arrest in rats impairs histologic outcome and increases NFkappaB expression in the brain. Perfusion. 2010;25:349–54. doi: 10.1177/0267659110377946. [DOI] [PubMed] [Google Scholar]
- 31.Ogata J, Shiraishi M, Namba T, Smothers CT, Woodward JJ, Harris RA. Effects of anesthetics on mutant N-methyl-D-aspartate receptors expressed in Xenopus oocytes. J Pharmacol Exp Ther. 2006;318:434–43. doi: 10.1124/jpet.106.101691. [DOI] [PubMed] [Google Scholar]
- 32.Bickler PE, Buck LT, Hansen BM. Effects of isoflurane and hypothermia on glutamate receptor-mediated calcium influx in brain slices. Anesthesiology. 1994;81:1461–9. doi: 10.1097/00000542-199412000-00022. [DOI] [PubMed] [Google Scholar]
- 33.Hogins J, Crawford DC, Jiang X, Mennerick S. Presynaptic silencing is an endogenous neuroprotectant during excitotoxic insults. Neurobiol Dis. 2011;43:516–25. doi: 10.1016/j.nbd.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nakayama M, Fujita S, Kanaya N, Tsuchida H, Namiki A. Blockade of ATP-sensitive K+ channel abolishes the anti-ischemic effects of isoflurane in dog hearts. Acta Anaesthesiol Scand. 1997;41:531–5. doi: 10.1111/j.1399-6576.1997.tb04737.x. [DOI] [PubMed] [Google Scholar]
- 35.Elmistekawy EM, Rubens FD. Deep hypothermic circulatory arrest: Alternative strategies for cerebral perfusion. A review article Perfusion. 2011;26(Suppl 1):27–34. doi: 10.1177/0267659111407235. [DOI] [PubMed] [Google Scholar]
- 36.Sundt TM, Flemming MD, Oderich GS, Torres NE, Li Z, Lenoch J, Kalra M. Spinal cord protection during open repair of thoracic and thoracoabdominal aortic aneurysms using profound hypothermia and circulatory arrest. J Am Coll Surg. 2011;212:678–83. doi: 10.1016/j.jamcollsurg.2010.12.022. [DOI] [PubMed] [Google Scholar]
- 37.Grigore AM, Murray CF, Ramakrishna H, Djaiani G. A core review of temperature regimens and neuroprotection during cardiopulmonary bypass: Does rewarming rate matter? Anesth Analg. 2009;109:1741–51. doi: 10.1213/ANE.0b013e3181c04fea. [DOI] [PubMed] [Google Scholar]
- 38.Scott BS, Fisher KC. Potassium concentration and number of neurons in cultures of dissociated ganglia. Exp Neurol. 1970;27:16–22. doi: 10.1016/0014-4886(70)90197-4. [DOI] [PubMed] [Google Scholar]
- 39.Gallo V, Kingsbury A, Balazs R, Jorgensen OS. The role of depolarization in the survival and differentiation of cerebellar granule cells in culture. J Neurosci. 1987;7:2203–13. doi: 10.1523/JNEUROSCI.07-07-02203.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Adolph E. Tolerance to cold and anoxia in infant rats. Am J Physiol. 1948;155:366–77. doi: 10.1152/ajplegacy.1948.155.3.366. [DOI] [PubMed] [Google Scholar]