Abstract
Background
Heterozygous deleterious mutations in the gene encoding the tumor necrosis factor receptor superfamily member 13b (TNFRSF13B), or transmembrane activator and CAML interactor (TACI) have been associated with the development of common variable immunodeficiency (CVID). Smith-Magenis syndrome (SMS) is a genetic disorder characterized by developmental delay, behavioral disturbances, craniofacial anomalies, and recurrent respiratory infections. Eighty percent of subjects have a chromosome 17p11.2 microdeletion, which includes TACI. The remaining subjects have mutations sparing this gene. Objective. We examined TACI protein expression and function in SMS patients to define the role of TACI haploinsufficiency in B cell function.
Methods
We studied TACI expression and function in a cohort of 29 SMS subjects.
Results
In SMS subjects with only one TACI allele, we found decreased B cell extra-and intra-cellular expression of TACI, reduced binding of a proliferation inducing ligand (APRIL) and decreased TACI-induced expression of activation-induced cytidine deaminase (AICD) mRNA, but these were normal for SMS cells with two TACI alleles. Impaired upregulation of B cell surface TACI expression by a TLR9 agonist was also observed in cells with one TACI allele. Gene sequence analysis of the remaining TACI allele revealed common polymorphisms with the exception of one patient with an aminoacid change of uncertain significance. SMS subjects with the lowest TACI expression had significantly reduced antibody responses to pneumococcal vaccine serotypes.
Discussion
Our findings suggest that haploinsufficiency of the TACI gene results in humoral immune dysfunction, highlighting the role of genomic copy number variants in complex traits.
Keywords: B cell, Humoral immunity, TACI, CVID, Smith-Magenis Syndrome, Gene haploinsufficiency
Introduction
The tumor necrosis factor receptor superfamily, member 13b (TNFRSF13B), also known as transmembrane activator and CAML interactor (TACI), is a cell membrane receptor for the ligands “a proliferation inducing ligand” or APRIL (TNFSF13) and “B cell-activating factor” or BAFF (TNFSF13B).1 TACI activation of B cells leads to their differentiation and maturation, including antibody isotype switch,2-6 and T cell-independent antibody production.7 Mutations in TACI have been found in 8-10% of patients with common variable immunodeficiency (CVID) 8-12 suggesting a role for these mutations in the development of low serum immunoglobulins and lack of antibody characteristic of this disorder. The mutations found in the immunodeficient subjects appeared to be inherited in a dominant fashion in some cases, suggesting that the mutant protein, even in the heterozygous state usually found in patients, might hinder or abolish the function of the trimeric signaling complex. Alternatively, the mutant protein could lead to insufficient expression of a functional complex with stringent ligand binding requirements.6 Homozygous and heterozygous TACI null mutations have been described in a few CVID cases, 8,11,12 in one of these families, reduced expression of the normal allele in heterozygous relatives was noted,8 suggesting that the pathogenesis of B cell dysfunction might include TACI haploinsufficiency.
Smith-Magenis syndrome (SMS) is a multiple congenital anomalies/mental retardation disorder estimated to occur in 1:15,000 to 1:25,000 persons, most commonly associated with an ∼ 3.7 Mb interstitial deletion within chromosome 17p11.2 (>80 – 90% of patients) or rarely with a point mutation in the retinoic acid induced gene 1, or RAI1, which is viewed as the genetic cause of this syndrome. 17-19 The clinical features include developmental delay, neurobehavioral abnormalities including sleep disturbances, and craniofacial and other skeletal anomalies. Eighty-eight (88%) percent of a cohort of 44 patients with the 17p11.2 deletion were noted to have chronic ear infections while 6 of 10 (60%) SMS patients with point mutations in RAI1 reported these infections. 20,21 TACI maps within the common SMS deletion in chromosome 17p11.2, and therefore most SMS patients are heterozygous null and haploinsufficient for this gene. Although immune defects have not been explored in SMS, we hypothesized that the loss of one TACI allele would lead to deficient expression of the TACI receptor, and would allow us to examine the significance of this protein on the humoral immune function in a cohort of SMS subjects.
Methods
Patients and Controls
Patients with a molecularly established SMS diagnosis who were enrolled in an IRB-approved protocol at Baylor College of Medicine were studied. SMS diagnosis was confirmed by detection of an interstitial deletion of chromosome 17p11.2 that includes the RAI1 gene by fluorescence in-situ hybridization (FISH), or by a mutation in the RAI1 gene identified by direct sequencing. For this study, 25 SMS subjects with the common deletion (including TACI) were compared to 4 others in whom TACI was not deleted. The clinical history was reviewed, and serum IgG, IgA and IgM levels and antibody concentrations to 12 pneumococcal serotypes were determined by ELISA (IBT Technologies, Kansas City). Protective levels of 1 μg/ml were used for each serotype. Demographics of patients are presented in Table I. EBV transformed B cell lines were produced from the subjects' peripheral blood. For comparison, EBV transformed cell lines of normal controls with and without known mutations involving TACI were examined.
Table I. Subject characteristics.
| 17p11.2 common deletion (n=25) | RAI1 mutation and 17p11.2 deletions not including TACI (n=4) | |
|---|---|---|
| Median age (range): | 7.6 years (1.8 – 29 y) | 9.4 years (7.7 – 11.2 y) |
| Gender M/F: | 11/25 | 1/4 |
| Upper respiratory infections | 25/25 | 4/4 |
| IgM Normal for age | 21/21 | 2/2 |
| IgG Normal for age | 16/21 | 2/2 |
| IgA Normal for age | 12/21 | 2/2 |
M, male; F, female
Extracellular and intracellular TACI protein expression
Surface expression of TACI on EBV-transformed B cell lines from SMS subjects and healthy controls was determined by flow cytometry assays using biotinylated polyclonal goat anti TACI (1:400) antibody or a control biotinylated goat IgG and secondary staining with 1:200 streptavidin-phycoerythrin (PE). (BD Pharmingen). Intracellular TACI expression was determined using goat anti-TACI (1:200) antibody after treatment with permeabilization reagents, and followed by streptavidin- PE staining.
Laser-scanning confocal microscopy
B cell lines were resuspended in Cell Adhesive Solution as instructed by the manufacturer (Crystalgen Inc, Commack NY), then were applied to slides (Gold Seal, Thermo Scientific, Portmouth, NH), fixed with 1.6% (vol/vol) paraformaldehyde and made permeable with 0.2% (vol/vol) Triton X-100 in PBS. Cells were stained with mouse anti-TACI monoclonal antibody ( clone 11H3, eBioscience, San Diego, CA). Negative controls were firstly stained with unconjugated mouse antibody with irrelevant binding activity (Santa Cruz Biotechnology Inc, Santa Cruz, CA). All slides were then stained with secondary antibodies (Donkey anti-mouse IgG AlexaFluor-488, Molecular Probes, Invitrogen Inc, Carlsbad, CA). Nuclei were counterstained with DAPI (Boehrin Mannheim, Germany).Slides were covered with coverslips through the use of FluorSave reagent (Calbiochem) and 22 examined, as previously described.
Ligand APRIL binding
EBV–transformed B cell lines were cultured at 37°C in RPMI 1640 medium (GIBCO, Carlsbad, CA) with L-glutamine and 10% heat-inactivated fetal calf serum (FCS) by triplicate. To assess binding of the ligand APRIL, B cells were incubated with 250 ng/ml FLAG tagged megaAPRIL (Axxora, SanDiego, CA) on ice in the presence of heparin (1000 U/ml); 1 ug/ml biotin-anti FLAG monoclonal M2 antibody (Sigma) was then added. The cells were washed and examined using streptavidin-PE. Flow cytometry (FACS) was performed using a FACSCalibur (Becton Dickinson, Mountain View, CA)
Analysis of TACI isoforms mRNA
Total RNA was extracted from B cell lines cells, and cDNA prepared using a SuperScript™ first-strand synthesis system for RT-PCR (Invitrogen, Carlsbad, CA). First-strand cDNA was synthesized using 100 ng of total RNA in a 20-μl reaction mixture by Invitrogen's protocol. PCR amplification was performed using previously published primers covering exons 1–3 and exons 3–5.19 The exon 3-5 amplicon was used to measure total TACI mRNA expression by quantitative PCR. Briefly, 50-μl reaction mixture was prepared using 5 μl of 10× PCR buffer, 0.5 μl of 25 mM MgCl2, 1.0 μl dNTPs (10 mM), 10 pmol of each primer, and 0.25 unit of Hot-Taq DNA polymerase (Qiagen, Valencia, CA). The PCRs were run under the following conditions: 95°C 15 s, 63°C 30 s, and 72°C 1 min 30 s for 30 cycles. The PCR product was finally visualized on agarose gel electrophoresis containing ethidium bromide. Quantitative RT-PCR was conducted using a LightCycler SYBR Green I Detection System (Roche Diagnostics, Indianapolis, IN). For this, 2 μl samples (cDNA) were run in duplicate for glass capillary reaction tubes in a total volume of 20 μl. The RT-PCR products of gene expression were determined copy number per μg of RNA relative to β-actin. The β-actin primers used were forward, 5′-CCC CCT GAA CCC CAA GGC CAA CCG CGA GAA-3′ and reverse, 5′-TAG CCG CGC TCG GTG AGG ATC TTC ATG AGG-3′.
TACI -induced expression of activation-induced deaminase (AICD) mRNA
50,000 EBV transformed B cells were cultured in 48-well plates with an agonistic monoclonal TACI (clone 174, R&D) or isotype control, with or without 100 ng/ml IL-4 (R&D) to up regulate AICD mRNA (25). To further crosslink anti-TACI, 5 ml of goat-anti-mouse IgG microbeads (Miltenyi, Carlsbad, CA) were added. After 48 hours, mRNA was isolated (RNeasy Mini Kit, Qiagen, Valencia, CA) and reverse transcribed (SuperScript III First-Strand cDNA synthesis kit, Invitrogen, Carlsbad, CA). Quantitative real time PCR was performed using LightCycler Real Time PCR system and FastStart DNA Master SYBR Green I kit (Roche Diagnostics, Indianapolis, IN), using β-actin mRNA as control for cell copy number. The following primers were used: AICD (5′-TGCTCTTCCTCGGCTACATCTC-3′; 5′-AACCTCATACAGGGGCAAAAGG-3′) and β-actin (5′-CTGAACCCCAAGGCCAACAG-3′; 5′-CCAGAGAAGAGGAGGATGCG-3′).
Surface TACI expression by TLR stimulation
Stimulation of peripheral blood B cells by TRL9 ligation enhances the expression of TACI on normal B cells. To determine if this was possible in SMS patients cells, we examined the B cells of two patients with the common deletion, incubating peripheral blood B cells with CpG-DNA, ODN2006 at 0, 0.6, 1.5 and 3.0 μg/ml for 48 hours, and examining the expression of TACI by FACS as described above.
TACI sequencing
Genomic DNA was isolated from EBV cells lines using Puregene DNA Purification Kits (Gentra Systems, Inc. Minneapolis, MN); cDNA was prepared by reverse transcription. Five exons of TACI were PCR amplified using HotStarTaq DNA Polymerase (Qiagen, Valencia, CA) using published primers.12 DNA amplicons were sequenced on an ABI PRISM® 377 DNA Sequencer and aligned to the wild-type TACI sequence using the DNAStar software.
Statistical analysis
Statistical analyses were performed using GraphPad Prism v.4.03 (GraphPad Software Inc, San Diego, CA). The non parametric Mann-Whitney test was used to compare data for SMS patients with normal controls. Data was expressed as mean values and standard deviations, ranges, and as needed, 25th (interquartile) percentiles. P values of <0.05 were considered statistically significant.
Results
Patient clinical characteristics
All twenty nine patients had clinical characteristics of SMS, including developmental delay, behavioral disorders and sleep disturbances. (Table 1) Twenty five patients had the common deletion in chromosome 17p11.2, and four patients had genetic lesions sparing the TACI gene. Frequent respiratory tract infections were reported by all patients, most commonly recurrent otitis media requiring pressure-equilibrating tubes. Five patients had recurrent pneumonias. Except for one patient who was diagnosed with psoriasis, there was no history of autoimmune-related disorders or lymphoid hyperplasia or splenomegaly. Sequencing of the remaining TACI allele identified three common polymorphisms that would not result in aminoacid changes (Exon 2: T27T, Exon 3 P92P, Exon 5: S277S). Two patients had the variant P251L, previously reported in normal individuals at similar rates as compared to CVID patients. 10,23 One SMS subject had a novel variant R9Q, in Exon 1, of unknown significance.
Expression of TACI on B cells
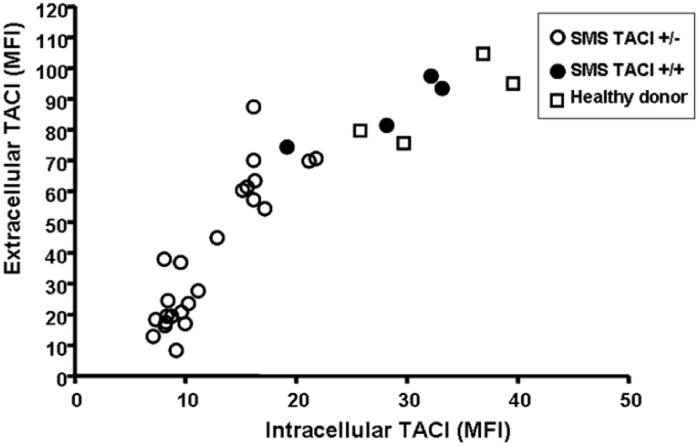
The surface expression of TACI in EBV-transformed B cell lines of SMS subjects with the common 17p11.2 deletion including TACI, was first compared with B cell lines from healthy controls. Although the reduction of protein expression on SMS B cells was variable, the cells from subjects with the common deletion had less surface expression of TACI, with a median reduction of 42% of the mean fluorescent intensity (MFI) of the lowest value of the healthy controls. (Figure 1) Intracellular expression of TACI was also similarly reduced for most SMS subjects, with a median reduction of 39% of the lowest MFI value for the healthy controls. For extracellular expression of TACI, the mean (± 95%IC) MFI value of SMS TACI +/− samples was 44.9 (± 10.8), and of SMS TACI +/+ samples was 88.5 (± 19.8) (p=0.005). Similarly, for intracellular expression of TACI, the mean (95%IC) of SMS TACI +/− samples was 14.3 (± 2.8), and of SMS TACI +/+ was 32.4 (± 10.0) (p=0.001). There was also a direct relationship between surface and intracellular expression of this protein for both SMS subjects and controls (r=0.89, p=0.001). In contrast, B cells from the four SMS patients who had chromosomal deletions not involving TACI, had both intra- and extra-cellular TACI expression at levels comparable to B cells of healthy controls (Figure 1 and 2). Confocal microscopy examination of B cells from SMS subjects using a fluorescent-labeled anti-TACI antibody demonstrated a visible reduction of intra-cytoplasmic TACI expression, only in those subjects with loss of one TACI allele as compared to B cells of subjects with two alleles or healthy controls (Figure 2).
Figure 1. Decreased extracellular and intracellular expression of TACI in SMS.
EBV-transformed B cell lines from SMS subjects (n=29), including 4 SMS subjects with two TACI alleles, and healthy donor controls (n=4) were analyzed. Mean Florescent Intensities (MFI) of both measurements of each individual are shown. Correlation coefficient r= 0.89, p=0.001 Open circles, SMS patients with deletion including TACI; closed circles, SMS patients with two TACI alleles; squares healthy donors.
Figure 2. Decreased TACI protein in cytoplasm of B cells from SMS patients.
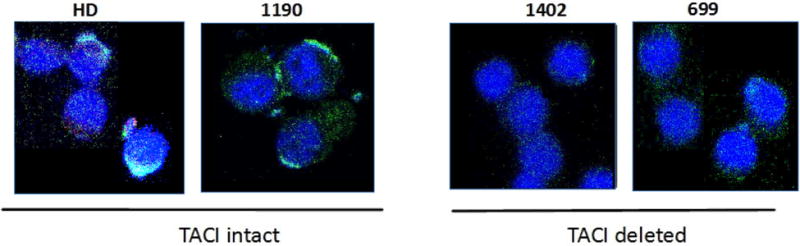
Green fluorescent intensity in B cells stained with FITC-labeled anti-human TACI antibody is decreased in the cytoplasm of SMS patients with one TACI allele deleted. DAPI (4′,6-diamidino-2-phenylindole) was used to stain the cell nucleus. HD, healthy donor control; #1190, SMS patient with two TACI alleles; #1402 and #699, SMS patients with one TACI allele deleted.
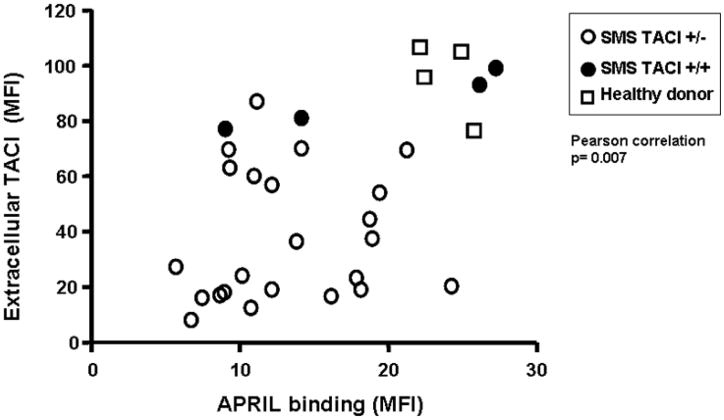
Reduced surface binding of APRIL
Binding of the TACI ligand APRIL was examined in the SMS B cell lines in comparison to normal subjects. Here again binding of APRIL was also reduced when compared to normal B cells. As predicted, the ability of APRIL to bind to these B cells was closely correlated with the extra-cellular expression of TACI protein (Figure 3, p=0.006). The mean MFI of APRIL binding of SMS TACI +/+ samples was 12.99 and the corresponding mean of SMS TACI +/− was 18.98, although this difference did not reach statistical significance (p=0.178).
Figure 3. APRIL binding to TACI receptor is associated with extra-cellular TACI expression.
EBV-transformed B cell lines from SMS subjects and healthy donor controls were incubated with FLAG-tagged megaAPRIL, followed by biotinylated anti-FLAG antibody and subsequent Streptavidin-phycoerythrin, and then analyzed by flow cytometry. Mean Fluorescent Intensities (MFI) is plotted against extracellular TACI MFIs, showing significant association between these two measurements. (Pearson correlation test, p=0.007) Open circles, SMS patients with deletion including TACI; closed circles, SMS patients with two TACI alleles; squares, healthy donor controls.
Analysis of TACI isoforms
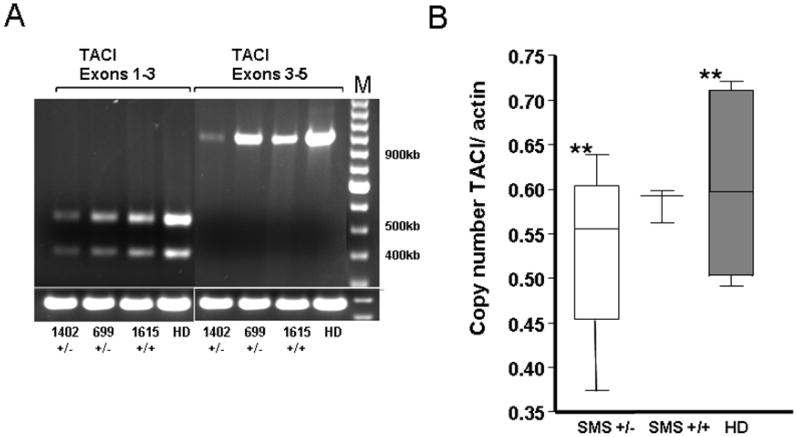
Similar to normal controls, all SMS subjects retained the ability to express two isoforms of TACI, a shorter form (ID: ENST00000343345), in which exon 2 is skipped removing the first cysteine rich domain CDR1, and a second form (ID: ENST00000261652), which has retained this domain. 24 (Figure 4A) We analyzed mRNA expression of TACI relative to beta-actin by obtaining cDNA and using quantitative PCR. B cells from SMS subjects with the common deletion, but not from those with two TACI alleles, had lower mean number of TACI copies as compared to controls; however, this difference did not reach a statistically significant difference. (Figure 4B).
Figure 4. TACI mRNA expression in SMS may be decreased.
A. TACI mRNA expression in B cells from two SMS patients with deletion of one copy of TACI gene (patients #1402, #699) and one subject with no deletion of TACI (patient #1615) were decreased as compared with B cells from a healthy donor. TACI sequences corresponding to exons 1-3 and exons 3- 5 were amplified by RT-PCR and examined by gel electrophoresis. Two bands shown for exon 1-3 correspond to the known 2 isoforms. Beta-actin mRNA was amplified as control for mRNA amount. M, 1Kb Ladder DNA marker; HD, healthy donor control. B. Box plot illustrates mean ± SEMs of TACI exons 3-5 copy number values using beta-actin copies as a reference. HD, healthy donor controls (n=6); SMS +/+, SMS patients with two TACI alleles (n=3); SMS +/−, SMS patients with the common 17p11.2 deletion, one TACI allele deleted (n=4). ** p=0.42.
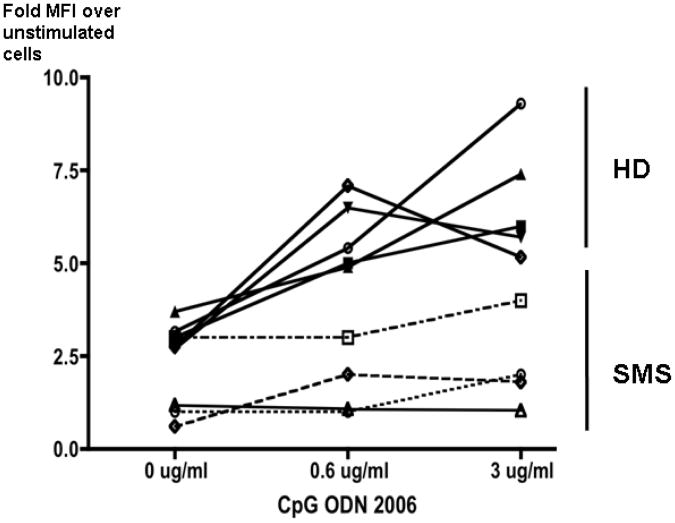
TLR9 stimulation induces TACI up-regulation
Activation of B cells by double stranded DNA TLR ligands is known to increase the TACI expression on the B cell surface. 25 Therefore, we examined this process in peripheral blood B cells from four SMS subjects with the common deletion. TACI expression on peripheral blood B cells of these patients was reduced at baseline, and activation with a TRL9 ligand CpG-DNA oligonucleotide resulted in minimal up-regulation of TACI expression as compared to control B cells examined at the same time. These data support the hypothesis that TACI protein expression is limited by TACI haploinsufficiency (Figure 5).
Figure 5. TLR9-induced TACI surface expression is impaired in SMS.
B cells isolated from fresh blood samples of SMS patients with loss of one TACI allele and from healthy normal controls were incubated with or without the oligonucleotide ODN2006 for 48 hrs, and TACI expression was examined by flow cytometry. The ratio of Mean Fluorescent Intensities (MFI) obtained from TACI staining of the TLR9-stimulated cells over un-stimulated cells and measured by flow cytometry were recorded and are presented in the Y-axis with regards to the concentration of the oligonucleotide in the culture media. B cells from SMS patients have impaired up-regulation of B cell surface expression of TACI over baseline. Healthy donor controls (HD, n=5), SMS patients with deletion of one TACI allele (SMS, n=4).
Activation-induced cytidine deaminase (AICD) expression is defective in SMS
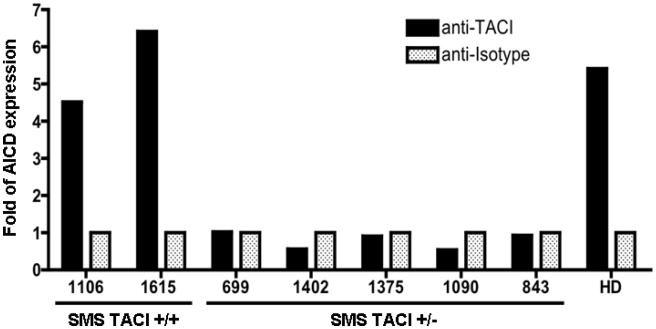
One of the main outcomes of TACI signaling is an increase of AICD mRNA expression which results in isotype switching. We examined this in B cell lines of SMS subjects with and without loss of one copy of TACI, using an agonist anti-TACI antibody. As recently shown for CVID B cells with heterozygous TACI mutations, 22 AICD mRNA was not induced in B cells from SMS subjects with the deletion including TACI. In contrast, SMS subjects 1106 and 1215, who have two TACI alleles, showed an up-regulation of AICD mRNA that was similar to that of B cells of healthy donors. (Figure 6)
Figure 6. Upregulation of AICD expression by TACI activation is compromised in SMS.
AICD mRNA expression was measured by quantitative PCR after incubation of B cells with an agonist anti-TACI antibody or with an isotype as control. Beta-actin mRNA expression was used as a reference to determine fold of AICD expression. B cell lines of SMS patients with one TACI allele deleted (SMS TACI +/−, n=5) were not able to induce AICD mRNA expression after stimulation with an agonist anti-human TACI antibody. A healthy donor controls (HD) and SMS patients with two TACI alleles (SMS TACI +/+, #1106, #1615) showed comparable AICD mRNA induction.
TACI expression and humoral immunity
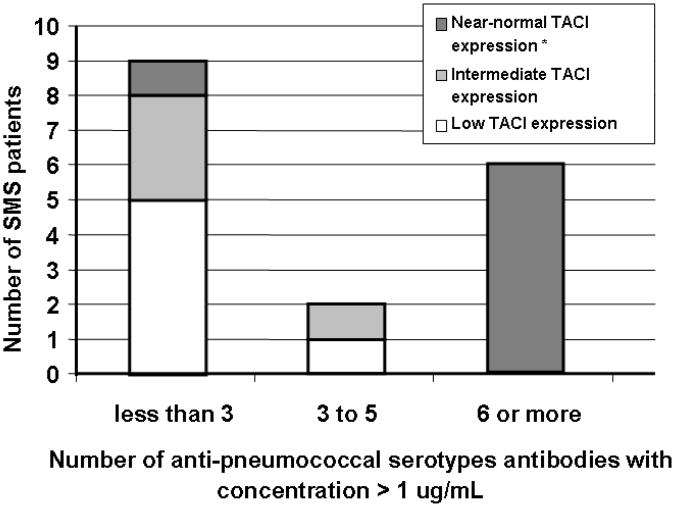
Quantitative immunoglobulins were determined in blood serum samples of 22 SMS subjects, of whom 20 had the common mutation and 2 had two copies of TACI. Five patients had IgG levels below the normal range for age, and 12 patients had abnormally low IgA levels. (Table I) All patients but one had normal serum IgM levels. The lowest IgG level was 361 mg/dL (normal for this patient's age was 592-1723 mg/dL). IgG subclasses were determined for 5 patients; one had a low IgG2. As TACI signaling has been viewed as important in T-independent B cell responses, 7 we then measured the serum antibodies to 12 pneumococcal serotypes for 17 of these SMS patients, all of whom had been immunized. Of the 15 patients with the common chromosomal deletion including TACI, 11 had protective titers to less than 6 of the 12 serotypes; in contrast, the two SMS subjects with chromosomal deletions not involving TACI had protective antibody titers (> 1 μg/mL) to 10 of 12 pneumococcal serotypes (p<0.001). Because the surface expression of TACI on B cells was variable in this SMS cohort, we arbitrarily divided these patients into three groups, depending on the MFI value of B cell TACI extracellular expression: low (MFI < 30), intermediate (MFI > 30, < 70) and near normal (MFI > 70). All 10 SMS subjects with intermediate or low TACI expression had IgG anti-pneumococcal antibodies at protective concentrations for 5 or fewer of the 12 serotypes tested. In contrast, 6 of 7 SMS subjects with “near normal” TACI expression, including those two subjects without TACI deletion, had 6 or more of these specific antibodies at protective levels. (p<0.001, Figure 7).
Figure 7. Protective serum levels of antibody against pneumococcal serotypes were more likely to be present in SMS patients with “near normal” TACI expression.
Blood serum titers of twelve anti-pneumococcal serotypes antibodies were measured in blood serum samples of 17 SMS subjects, including two patients without TACI deletion. The number of these antibodies at protective titers (>1 μg/mL) in each individual is charted with regards to the degree of extracellular TACI expression in B cells. Three categories of TACI expression were established according to the MFI value obtained by flow cytometry: low (MFI < 30), intermediate (MFI > 30, < 70) and near normal (MFI > 70). *Includes two SMS patients without TACI allele deletion.
Discussion
TACI signaling on B cells leads to T cell-independent immunoglobulin class switch, B cell proliferation, antibody secretion and prolonged survival of plasma cells. 2-6,26 Although much of the signaling cascade is unclear, TACI stimulation activates the transcription factors nuclear factor–kB (NF-kB), nuclear factor of activated T cells (NF-AT), and activator protein-1 (AP-1) in a pathway that also includes myeloid differentiation primary response gene 88 (MYD88). 77,25-28 Similar to other members of the TNF superfamily, the TACI receptor is a trimeric complex; 1 and the incorporation of one dysfunctional mutant allele into the receptor, might either exert dominant negative effects29 or dilute the overall expression of a sufficient number of functional receptors. As ligand multimerization is needed for TACI receptor function, a sufficient number of intact surface receptors is likely to be required. 5 Haploinsufficiency, defined as a situation in which a single copy of the normal gene is incapable of providing sufficient protein production to ensure normal function, has also been suggested as a molecular mechanism leading to immunodeficiency and immune dysregulation in subjects with TACI mutations. 11,14,30 In fact, selected clinical phenotypes in CVID, especially autoimmunity and lymphoid hyperplasia, are significantly more common in the heterozygous state in CVID as compared to the homozygous states. 11,12 Studies investigating the role of TACI mutations in the pathogenesis of CVID in large patient cohorts have shown that certain common missense mutations, C104R and A181E, are closely associated with the development of impaired humoral immunity. 10 However, family members of patients with heterozygous or the same homozygous missense or even null mutations are not always hypogammaglobulinemic12,16 suggesting that other genes can ameliorate these defects.
In contrast to CVID subjects and their family members with missense mutations, examination of TACI expression and function in the SMS syndrome allows us to examine a unique circumstance, the consequences of haploinsufficiency due to the loss of one TACI allele. B cell lines obtained from subjects who were heterozygous for a splicing mutation that results in absent mRNA expression and protein, showed a reduced level of TACI expression and APRIL-binding capacity, 14 similar to the SMS subjects described here. Immune functions of these heterozygous individuals were not examined. Our data shows that B cells of SMS subjects with loss of one copy of the TACI gene, have significantly decreased intra- and extra-cellular protein expression, while B cell TACI expression for all four SMS subjects in whom the TACI gene copy number was in the normal diploid (n=2) state, was similar to that of control B cells. For subjects with loss of one allele, both TACI mRNA isoforms were decreased in SMS B cells, consistent with decreased protein expression, although this difference did not reach statistical significance.
The expression of TACI depends both on B cell development and the stage of activation. 26,31 While TLR9 ligands in particular up-regulate TACI expression on normal B cells32 we found that TACI up-regulation was blunted in CpG-DNA activated SMS peripheral blood B cells as compared to normal B cells, supporting a haploinsufficiency effect. Our B cell functional studies showed that haploinsufficiency was also associated to loss of up-regulation of AICD upon TACI triggering in B cell lines.
From the clinical point of view, while hypogammaglobulinemia in the range found in CVID was not demonstrated in SMS patients, decreased antibody production to carbohydrate antigens was associated to low TACI expression in B cells. Due to the small sample size, it was not possible to demonstrate this association in each individual; however, these observations are in agreement with the role of TACI in T-independent, anti-glycan antibody production.7,26 Potentially, low B cell expression of TACI in the very young might be associated with the lack of such antibodies leading to a heightened susceptibility to bacterial infections in infancy.33,34
However, the observations in SMS are in contrast to CVID where TACI mutations are closely associated with autoimmunity, lymphadenopathy and splenomegaly, not features of SMS, but somewhat more reminiscent of TNFRSF13B KO mice. The SMS subjects are also different from heterozygous TNFRSF13B +/− mice who were reported to have a B cell phenotype and antibody function similar to litter mate controls; but have findings similar to those recently reported using a mouse model of TACI haploinsufficiency that demonstrated low TACI expression and suboptimal antibody responses to trinitrophenol, a T-independent antigen. The differences of these two experimental mouse models were attributed to environmental variables affecting a mice strain of mixed genetic background.7,35 The SMS subjects with loss of one TACI allele have reduced but variably impaired expression of TACI protein; while as yet unexplained, polymorphisms in the gene promoter or in other regulatory sequences may affect the transcription of the remaining allele.36 Whether haploinsufficiency in SMS will lead to future immune dysfunction is not clear from our studies, as many of the subjects are still in the pediatric age group. It is possible that those with the lowest TACI expression are at higher risk of developing hypogammaglobulinemia over time. In summary, we demonstrate TACI haploinsufficiency in SMS, allowing a further dissection of the role of this complex receptor in human B cell immunity.
Key Messages.
TACI haploinsufficiency leads to decreased protein expression and results in suboptimal B cell activation.
Low TACI protein expression in B cell surface is associated with impaired humoral immunity.
Acknowledgments
We thank patients and their families and also Drs Jordan Orange and Lakshmi Mehta for referral of patients.
Supported by the National Institutes of Health AI-101093, AI-467320, AI-48693 and National Institute of Allergy and Infectious Diseases Contract 03-22 (CCR).
Abbreviations
- TNFRSF13B
tumor necrosis factor receptor superfamily member 13b
- TACI
transmembrane activator and CAML interactor
- CVID
common variable immunodeficiency
- SMS
Smith-Magenis syndrome
- RAI1
retinoic acid induced gene 1
- FISH
fluorescence in-situ hybridization
- APRIL
a proliferation inducing ligand
- AICD
activation induced cytosine deaminase
- MFI
mean fluorescence intensity
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wu Y, Bressette D, Carrell JA, Kaufman T, Feng P, Taylor K, et al. Tumor necrosis factor (TNF) receptor superfamily member TACI is a high affinity receptor for TNF family members APRIL and BLyS. J Biol Chem. 2000;275:35478–85. doi: 10.1074/jbc.M005224200. [DOI] [PubMed] [Google Scholar]
- 2.von Bülow GU, Russell H, Copeland NG, Gilbert DJ, Jenkins NA, Bram RJ. Molecular cloning and functional characterization of murine transmembrane activator and CAML interactor (TACI) with chromosomal localization in human and mouse. Mamm Genome. 2000;11:628–32. doi: 10.1007/s003350010125. [DOI] [PubMed] [Google Scholar]
- 3.Castigli E, Scott S, Dedeoglu F, Bryce P, Jabara H, Bhan AK, et al. Impaired IgA class switching in APRIL-deficient mice. Proc Natl Acad Sci U S A. 2004;101:3903–8. doi: 10.1073/pnas.0307348101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Castigli E, Wilson SA, Elkhal A, Ozcan E, Garibyan L, Geha RS. Transmembrane activator and calcium modulator and cyclophilin ligand interactor enhances CD40-driven plasma cell differentiation. J Allergy Clin Immunol. 2007;120:885–91. doi: 10.1016/j.jaci.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Castigli E, Wilson SA, Scott S, Dedeoglu F, Xu S, Lam KP, et al. TACI and BAFF-R mediate isotype switching in B cells. J Exp Med. 2005;201:35–9. doi: 10.1084/jem.20032000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bossen C, Cachero TG, Tardivel A, Ingold K, Willen L, Dobles M, et al. TACI, unlike BAFF-R, is solely activated by oligomeric BAFF and APRIL to support survival of activated B cells and plasmablasts. Blood. 2008;111:1004–12. doi: 10.1182/blood-2007-09-110874. [DOI] [PubMed] [Google Scholar]
- 7.von Bülow GU, van Deursen JM, Bram RJ. Regulation of the T-independent humoral response by TACI. Immunity. 2001;14:573–82. doi: 10.1016/s1074-7613(01)00130-3. [DOI] [PubMed] [Google Scholar]
- 8.Salzer U, Chapel HM, Webster AD, Pan-Hammarström Q, Schmitt-Graeff A, Schlesier M, et al. Mutations in TNFRSF13B encoding TACI are associated with common variable immunodeficiency in humans. Nat Genet. 2005;37:820–8. doi: 10.1038/ng1600. [DOI] [PubMed] [Google Scholar]
- 9.Castigli E, Wilson SA, Garibyan L, Rachid R, Bonilla F, Schneider L, Geha RS. TACI is mutant in common variable immunodeficiency and IgA deficiency. Nat Genet. 2005;37:829–34. doi: 10.1038/ng1601. [DOI] [PubMed] [Google Scholar]
- 10.Pan-Hammarström Q, Salzer U, Du L, Björkander J, Cunningham-Rundles C, Nelson DL, et al. Reexamining the role of TACI coding variants in common variable immunodeficiency and selective IgA deficiency. Nat Genet. 2007;39:429–30. doi: 10.1038/ng0407-429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salzer U, Bacchelli C, Buckridge S, Pan-Hammarström Q, Jennings S, Lougaris V, et al. Relevance of biallelic versus monoallelic TNFRSF13B mutations in distinguishing disease-causing from risk-increasing TNFRSF13B variants in antibody deficiency syndromes. Blood. 2009;113:1967–76. doi: 10.1182/blood-2008-02-141937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang L, Radigan L, Salzer U, Behrens TW, Grimbacher B, Diaz G, et al. Transmembrane activator and calcium-modulating cyclophilin ligand interactor mutations in common variable immunodeficiency: clinical and immunologic outcomes in heterozygotes. J Allergy Clin Immunol. 2007;120:1178–85. doi: 10.1016/j.jaci.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee JJ, Rauter I, Garibyan L, Ozcan E, Sannikova T, Dillon SR, et al. The murine equivalent of the A181E TACI mutation associated with common variable immunodeficiency severely impairs B-cell function. Blood. 2009;114:2254–62. doi: 10.1182/blood-2008-11-189720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mohammadi J, Liu C, Aghamohammadi A, Bergbreiter A, Du L, Lu J, et al. Novel mutations in TACI (TNFRSF13B) causing common variable immunodeficiency. J Clin Immunol. 2009;29:777–785. doi: 10.1007/s10875-009-9317-5. [DOI] [PubMed] [Google Scholar]
- 15.Dong X, Hoesltzle MV, Hagn JB, Park MA, Li JT, Abraham RS. Phenotypic and clinical heterogeneity associated with monoallelic TNFRSF13B-A181E mutations in common variable immunodeficiency. Hum Immunol. 2010;71:5505–11. doi: 10.1016/j.humimm.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 16.Martinez-Pomar N, Detkova D, Arostegui JI, Alvarez A, Soler-Palacin P, Vidalier A, et al. Role of TNFRSF13B variants in patients with common variable immunodeficiency. Blood. 2009;114:2846–2848. doi: 10.1182/blood-2009-05-213025. [DOI] [PubMed] [Google Scholar]
- 17.Greenberg F, Lewis RA, Potocki L, Glaze D, Parke J, Killian J, et al. Multi-disciplinary clinical study of Smith-Magenis syndrome (deletion 17p11.2) Am J Med Genet. 1996;62:247–54. doi: 10.1002/(SICI)1096-8628(19960329)62:3<247::AID-AJMG9>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 18.Potocki L, Shaw CJ, Stankiewicz P, Lupski JR. Variability in clinical phenotype despite common chromosomal deletion in Smith-Magenis syndrome [del(17)(p11.2p11.2)] Genet Med. 2003;5:430–4. doi: 10.1097/01.gim.0000095625.14160.ab. [DOI] [PubMed] [Google Scholar]
- 19.Edelman EA, Girirajan S, Finucane B, Patel PI, Lupski JR, Smith AC, Elsea SH. Gender, genotype, and phenotype differences in Smith-Magenis syndrome: a meta-analysis of 105 cases. Clin Genet. 2007;71:540–50. doi: 10.1111/j.1399-0004.2007.00815.x. [DOI] [PubMed] [Google Scholar]
- 20.Bi W, Saifi GM, Shaw CJ, Walz K, Fonseca P, Wilson M, et al. Mutations of RAI1, a PHD-containing protein, in nondeletion patients with Smith-Magenis syndrome. Hum Genet. 2004;115:515–24. doi: 10.1007/s00439-004-1187-6. [DOI] [PubMed] [Google Scholar]
- 21.Bi W, Lupski JR. RAI1, The Smith-Magenis, and Potocki-Lupski Syndromes. In: Epstein CJ, Erickson RP, Wynshaw-Boris A, editors. Inborn Errors of Development. 2nd. Oxford University Press; New York, N.Y.: 2008. pp. 1078–1088. [Google Scholar]
- 22.He B, Santamaria R, Xu W, Cols M, Chen K, Puga I, et al. The transmembrane activator TACI triggers immunoglobulin class switching by activating B cells through the adaptor MyD88. Nat Immunol. 2012 Aug 1; doi: 10.1038/ni.1914. PMID: 20676093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bethesda (MD): National Center for Biotechnology Information, National Library of Medicine; Database of Single Nucleotide Polymorphisms (dbSNP) dbSNP accession: rs34262254, (dbSNP Build ID:130). Available from: http://www.ncbi.nlm.nih.gov/SNP/ [Google Scholar]
- 24.Hymowitz SG, Patel DR, Wallweber JA, Runyon S, Yan M, Yin J, Shriver SK, et al. Structures of APRIL-receptor complexes: like BCMA, TACI employs only a single cysteine-rich domain for high affinity ligand binding. J Biol Chem. 2005;280:7218–7227. doi: 10.1074/jbc.M411714200. [DOI] [PubMed] [Google Scholar]
- 25.Katsenelson N, Kanswal S, Puig M, Mostowski H, Verthelyi D, Akkoyunlu M. Synthetic CpG oligodeoxynucleotides augment BAFF- and APRIL-mediated immunoglobulin secretion. Eur J Immunol. 2007;37:1785–95. doi: 10.1002/eji.200636800. [DOI] [PubMed] [Google Scholar]
- 26.Mackay F, Schneider P. TACI, an enigmatic BAFF/APRIL receptor, with new unappreciated biochemical and biological properties. Cytokine Growth Factor Rev. 2008;19:263–76. doi: 10.1016/j.cytogfr.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 27.Xia XZ, Treanor J, Senaldi G, Khare SD, Boone T, Kelley M, et al. TACI is a TRAF-interacting receptor for TALL-1, a tumor necrosis factor family member involved in B cell regulation. J Exp Med. 2000;192:137–43. doi: 10.1084/jem.192.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schneider P. The role of APRIL and BAFF in lymphocyte activation. Curr Opin Immunol. 2005;17:282–9. doi: 10.1016/j.coi.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 29.Garibyan L, Lobito AA, Siegel RM, Call ME, Wucherpfennig KW, Geha RS. Dominant-negative effect of the heterozygous C104R TACI mutation in common variable immunodeficiency (CVID) J Clin Invest. 2007;117:1550–7. doi: 10.1172/JCI31023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee JJ, Ozcan E, Rauter I, Geha RS. Transmembrane activator and calcium-modulator and cyclophilin ligand interactor mutations in common variable immunodeficiency. Curr Opin Allergy Clin Immunol. 2008;8:520–6. doi: 10.1097/ACI.0b013e3283141200. [DOI] [PubMed] [Google Scholar]
- 31.Darce JR, Arendt BK, Wu X, Jelinek DF. Regulated expression of BAFF-binding receptors during human B cell differentiation. J Immunol. 2007;179:7276–86. doi: 10.4049/jimmunol.179.11.7276. [DOI] [PubMed] [Google Scholar]
- 32.Ozcan E, Garibyan L, Lee JJ, Bram RJ, Lam KP, Geha RS. Transmembrane activator, calcium modulator, and cyclophilin ligand interactor drives plasma cell differentiation in LPS-activated B cells. J Allergy Clin Immunol. 2009;123:1277–86. doi: 10.1016/j.jaci.2009.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaur K, Chowdhury S, Greenspan NS, Schreiber JR. Decreased expression of tumor necrosis factor family receptors involved in humoral immune responses in preterm neonates. Blood. 2007;110:2948–54. doi: 10.1182/blood-2007-01-069245. [DOI] [PubMed] [Google Scholar]
- 34.Kanswal S, Katsenelson N, Selvapandiyan A, Bram RJ, Akkoyunlu M. Deficient TACI expression on B lymphocytes of newborn mice leads to defective Ig secretion in response to BAFF or APRIL. J Immunol. 2008;181:976–90. doi: 10.4049/jimmunol.181.2.976. [DOI] [PubMed] [Google Scholar]
- 35.Lee JJ, Jabara HH, Garibyan L, Rauter I, Sannikova T, Dillon SR, et al. The C104R mutant impairs the function of transmembrane activator and cyclophillin ligand interactor (TACI) through haploinsufficiency. J Allergy Clin Immunol. 2010;126:1234–41. doi: 10.1016/j.jaci.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kurotaki N, Shen JJ, Touyama M, Kondoh T, Visser R, Ozaki T, et al. Phenotypic consequences of genetic variation at hemizygous alleles: Sotos syndrome is a contiguous gene syndrome incorporating coagulation factor twelve (FXII) deficiency. Genet Med. 2005;7:479–83. doi: 10.1097/01.gim.0000177419.43309.37. [DOI] [PubMed] [Google Scholar]