Abstract
In this paper we describe optimization of SYBR Green I-based real-time PCR parameters and testing of a large number of microbial species with vvh-specific oligonucleotide primers to establish a rapid, specific, and sensitive method for detection of Vibrio vulnificus in oyster tissue homogenate and Gulf of Mexico water (gulf water). Selected oligonucleotide primers for the vvh gene were tested for PCR amplification of a 205-bp DNA fragment with a melting temperature of approximately 87°C for 84 clinical and environmental strains of V. vulnificus. No amplification was observed with other vibrios or nonvibrio strains with these primers. The minimum level of detection by the real-time PCR method was 1 pg of purified genomic DNA or 102 V. vulnificus cells in 1 g of unenriched oyster tissue homogenate or 10 ml of gulf water. It was possible to improve the level of detection to one V. vulnificus cell in samples that were enriched for 5 h. The standard curves prepared from the real-time PCR cycle threshold values revealed that there was a strong correlation between the number of cells in unenriched samples and the number of cells in enriched samples. Detection of a single cell of V. vulnificus in 1 g of enriched oyster tissue homogenate is in compliance with the recent Interstate Shellfish Sanitation Conference guidelines. The entire detection method, including sample processing, enrichment, and real-time PCR amplification, was completed within 8 h, making it a rapid single-day assay. Rapid and sensitive detection of V. vulnificus would ensure a steady supply of postharvest treated oysters to consumers, which should help decrease the number of illnesses or outbreaks caused by this pathogen.
Vibrio vulnificus is a gram-negative halophilic bacterium commonly found in warm coastal waters throughout the world (37, 44). In the United States, this microorganism is indigenous to Gulf of Mexico water (gulf water) and thrives during the warmer months. V. vulnificus is known to cause gastroenteritis and, in some cases, septicemia when it is ingested in raw or poorly cooked oysters (25, 26). Infection by V. vulnificus often results in fatal consequences, and the mortality rate is up to 60%, primarily in individuals who are immunocompromised (10, 11, 17, 18, 28, 31).
Since infection by V. vulnificus is one of the leading causes of seafood-related illnesses in the United States, the Interstate Shellfish Sanitation Conference (ISSC) has proposed that illnesses caused by V. vulnificus due to consumption of shellfish must be reduced by at least 60% by the year 2007 (21). In order to achieve this goal, the ISSC suggests that consumable oysters should not contain more than 3 CFU of V. vulnificus per g of oyster meat (21). Therefore, to meet this objective, a rapid, reliable, and sensitive method for detection of this pathogen is as important as the methods of treatment for reducing the numbers of V. vulnificus in oysters to an acceptable level. Detection of V. vulnificus by conventional biochemical and microbiological culture methods, such as the most-probable-number method or the use of selective agar media, are time-consuming and require several days to obtain confirmatory results (42). A genetically based colorimetric colony hybridization method targeting the V. vulnificus-specific hemolysin gene, vvh, has been described previously and has been recommended as an alternative method for detection of this microorganism in shellfish (24, 33, 52). Although the DNA-DNA colony hybridization method in which the vvh gene segment is used as a probe is reliable and specific for detection of this pathogen, it takes at least 3 days to complete. A conventional PCR method for detection of V. vulnificus and other microbial pathogens in shellfish has also been shown to be effective (8, 22). However, this PCR approach requires analysis of the amplified DNA in an agarose gel or by DNA-DNA hybridization to confirm the results, which again is time-consuming and laborious.
Recently, introduction of a real-time PCR amplification method in which SYBR Green I fluorescent dye is used has made detection of microbial pathogens such as Legionella (46), Escherichia coli (23), Vibrio parahaemolyticus (7), and Campylobacter (29) rapid and cost-effective and the analysis of results simple. The SYBR Green I fluorescent dye binds to the minor grooves of the amplified DNA during the primer annealing and extension steps of each PCR cycle. Accumulation of amplified DNA is measured by determining the increase in fluorescence over time, and this is followed by confirmation of results by melting curve analysis (48). In this paper, we describe development of a method for rapid and sensitive detection of V. vulnificus in shellfish and gulf water by real-time PCR with SYBR Green I fluorescent dye and oligonucleotide primers targeting a segment of the hemolysin (vvh) gene.
MATERIALS AND METHODS
Bacterial strains and growth medium.
All vibrio strains, including V. vulnificus, and nonvibrio strains used in this study are listed in Table 1. V. vulnificus MO6-24(O), isolated from an infected human patient who consumed raw oysters, was used for optimization of real-time PCR with purified DNA, pure cultures, or seeded gulf water and oysters. All V. vulnificus strains were maintained on half-strength (18.7 g/liter) marine agar (Difco, Becton-Dickinson, Franklin Lakes, N.J.); V. parahaemolyticus strains were maintained on nutrient agar (Difco, Becton-Dickinson) supplemented with 3% (wt/vol) NaCl; and Vibrio cholerae was maintained on Luria-Bertani agar (32). All other Vibrio spp. listed in Table 1 were grown on full-strength marine agar (37.4 g/liter). Salmonella, Shigella, Hafnia, Escherichia, Staphylococcus, Pseudomonas, Arthrobacter, Aeromonas, Klebsiella, Enterobacter, Plesiomonas, Streptococcus, Micrococcus, Lactococcus, and Listeria strains were grown on Luria-Bertani agar.
TABLE 1.
Specificity of detection with oligonucleotide primers for vvh tested by using clinical and environmental isolates of V. vulnificus, as well as other Vibrio spp. and nonvibrio isolates
| Strain | Source | Amplification with vvh primersa |
|---|---|---|
| V. vulnificus clinical isolates | ||
| SPRC 10111 | California, 1993 | + |
| SPRC 10113 | California, 1993 | + |
| SPRC 10141 | California, 1993 | + |
| SPRC 10143 | California, 1993 | + |
| SPRC 10145 | California, 1993 | + |
| SPRC 10217 | California, 1993 | + |
| SPRC 10271 | California, 1996 | + |
| SPRC 10273 | California, 1996 | + |
| SPRC 10275 | California, 1996 | + |
| SPRC 10277 | California, 1996 | + |
| MO6-24(O)b | Primary septicemia, California | + |
| BO62316b | Primary septicemia, Maryland | + |
| 6353b | Primary septicemia, Maryland | + |
| NJMSAb | Primary septicemia, New Jersey | + |
| EDL-174b | Primary septicemia, Georgia | + |
| 2809-78b | Primary septicemia, South Carolina | + |
| UM/JSb | Wound, Maryland | + |
| 85A5667b | Primary septicemia, California | + |
| 5C1326b | Primary septicemia, Maryland | + |
| CVD11b | Acapsular mutant of MO6-24(O) | + |
| C1 | Primary septicemia, Atlanta-9579 | + |
| C2 | Primary septicemia, Atlanta-9579 | + |
| P1 | Primary septicemia, Atlanta-9580 | + |
| P2 | Primary septicemia, Atlanta-9580 | + |
| B36-22 | Wound | + |
| B37-22 | Wound | + |
| 276-0576 | Primary septicemia | + |
| ATCC 27562 | Primary septicemia, Florida | + |
| UAB 29 | Primary septicemia, UAB Children's Hospital, Birmingham, Ala. | + |
| FLDHRS 383-C | Primary septicemia, Orlando-1566 | + |
| FLDHRS 360-C | Primary septicemia, Orlando-8073 | + |
| FLDHRS 302-C | Primary septicemia, Orlando-7840 | + |
| FLDHRS 407-C | Primary septicemia, Orlando-7840 | + |
| BPT-H1 190 | Primary septicemia, Atlanta-9580 | + |
| BPT-H1 10 | Primary septicemia, Atlanta-9580 | + |
| Panama city | Primary septicemia, Atlanta-9824 | + |
| Dothan-1 | Primary septicemia, Atlanta-9823 | + |
| Dothan-2 | Primary septicemia, Atlanta-9823 | + |
| G2000-708 | Primary septicemia, Alabama | + |
| NSV 5829 | Primary septicemia, Alabama | + |
| NSV 5830 | Primary septicemia, Alabama | + |
| V. vulnificus environmental isolates | ||
| SEA 10116 | Oyster, Cedar Point, Alabama | + |
| 5-1 | Oyster, Cedar Point, Alabama | + |
| 5-2 | Oyster, Cedar Point, Alabama | + |
| 5-3 | Oyster, Cedar Point, Alabama | + |
| 5-4 | Oyster, Cedar Point, Alabama | + |
| 5-5 | Oyster, Cedar Point, Alabama | + |
| 5-6 | Oyster, Cedar Point, Alabama | + |
| 5-7 | Oyster, Cedar Point, Alabama | + |
| 5-8 | Oyster, Cedar Point, Alabama | + |
| 5-9 | Oyster, Cedar Point, Alabama | + |
| 5-10 | Oyster, Cedar Point, Alabama | + |
| 5-11 | Oyster, Cedar Point, Alabama | + |
| 5-12 | Oyster, Cedar Point, Alabama | + |
| 5-13 | Oyster, Cedar Point, Alabama | + |
| 5-14 | Oyster, Cedar Point, Alabama | + |
| 5-15 | Oyster, Cedar Point, Alabama | + |
| 5-16 | Oyster, Cedar Point, Alabama | + |
| 5-17 | Oyster, Cedar Point, Alabama | + |
| 5-18 | Oyster, Cedar Point, Alabama | + |
| 5-19 | Oyster, Cedar Point, Alabama | + |
| 5-20 | Oyster, Cedar Point, Alabama | + |
| 56511a | Oyster associated with C1 and C2 | + |
| 56516a | Oyster, Galveston Bay, Texas | + |
| 565110a | Oyster, Galveston Bay, Texas | +/PICK> |
| 56512b | Oyster, Galveston Bay, Texas | + |
| 56514b | Oyster, Galveston Bay, Texas | + |
| 56517b | Oyster, Galveston Bay, Texas | + |
| 56518b | Oyster, Galveston Bay, Texas | + |
| 56519b | Oyster, Galveston Bay, Texas | + |
| 56513c | Oyster, Galveston Bay, Texas | + |
| 56515c | Oyster, Galveston Bay, Texas | + |
| 56515c | Oyster, Galveston Bay, Texas | + |
| 565111c | Oyster, Galveston Bay, Texas | + |
| 565112c | Oyster, Galveston Bay, Texas | + |
| FLDHRS A-1 | Oyster associated with Orlando-7840 | + |
| FLDHRS A-2 | Oyster associated with Orlando-7840 | + |
| FLDHRS A-3 | Oyster associated with Orlando-7840 | + |
| WR1 | Water, Willipa Bay, Washington, 1982 | + |
| 27-3A | Oyster, Dauphin Island, Alabama | + |
| 9436 1165-3 | Oyster, Dauphin Island, Alabama, 1994 | + |
| 9436 1165-6 | Oyster, Dauphin Island, Alabama, 1994 | + |
| 1904 653-9 | Malaysian tiger shrimp | + |
| 1904 653-10 | Malaysian tiger shrimp | + |
| Other Vibrio spp. isolates | ||
| V. hollisae 89A1960 | Clinical isolate, California, 1989 | − |
| V. hollisae 89A4206 | Clinical isolate, California, 1989 | − |
| V. campbelli | ATCC 25920 | − |
| V. fluvialis | CDC 1954-82 | − |
| V. fluvialis | CDC 1954-83 | − |
| V. furnissii | CDC 1958-83 | − |
| V. alginolyticus | ATCC 17749 | − |
| V. alginolyticus Z106 | Food and Drug Administration, Washington, D.C. | − |
| V. mimicus | ATCC 33653 | − |
| V. mimicus WMS33 | William Spira, Bangladesh patient, 1986 | − |
| V. cholerae 89A 4555 non-O1, nontoxigenic | Clinical isolate, California, 1989 | − |
| V. cholerae O138 non-O1, nontoxigenic | Clinical isolate, Washington, 1988 | − |
| V. cholerae O145B non-O1 | Clinical isolate, Washington, 1988 | − |
| V. cholerae 154 serovar O:1 Inaba Tox | ATCC 14100 | − |
| V. cholerae C153 non-O1, nontoxigenic | Environmental isolate, California 1984 | − |
| V. cholerae 569B | ATCC 25870 | − |
| V. parahaemolyticus | ATCC 33844 | − |
| V. parahaemolyticus F113A | Oyster, Washington | − |
| V. parahaemolyticus TX 2072, O3:K6 serovar | Clinical isolate, Galveston Bay, Texas, 1998 | − |
| Nonvibrio isolates | ||
| Hafnia alvei | ATCC 29926 | − |
| Enterobacter aerogenes | ATCC 13048 | − |
| Salmonella choleraesuis serotype Typhimurium LT2 | ATCC 19585 | − |
| Salmonella choleraesuis serotype Typhi | ATCC 9993 | − |
| Serratia marcescens | ATCC 13880 | − |
| Staphylococcus aureus | ATCC 29213 | − |
| S. aureus | ATCC 25923 | − |
| Staphylococcus epiderdimis | ATCC 14990 | − |
| Escherichia coli | ATCC 15724 | − |
| E. coli O111 | ATCC 43887 | − |
| E. coli O124: NM | ATCC 43843 | − |
| E. coli O157: H7 | ATCC 43895 | − |
| Escherichia blattae | ATCC 29907 | − |
| Shigella dysenteriae | ATCC 29026 | − |
| Shigella boydii | ATCC 35965 | − |
| Shigella sonnei | ATCC 29930 | − |
| Pseudomonas aeruginosa | ATCC 10145 | − |
| Arthrobacter citreus | ATCC 11624 | − |
| Aeromonas hydrophila | FDA 9271 | − |
| A. hydrophila | C932 | − |
| Aeromonas salmonicida | ATCC 14714 | − |
| Klebsiella pneumoniae | ATCC 13883 | − |
| Listeria monocytogenes | ATCC 43256 | − |
| Plesiomonas shigelloides | ATCC 14029 | − |
| Lactococcus lactis | ATCC 11454 | − |
| Streptococcus pyogenes | ATCC 19615 | − |
| Corneybacterium jeikieum | ATCC 43734 | − |
| Micrococcus cryophilus | ATCC 15174 | − |
+, amplification; −, no amplification.
See reference 14.
DNA purification.
Genomic DNA from a pure culture of V. vulnificus was purified by using the procedure described by Ausubel et al. (5). Briefly, cells were suspended in 567 μl of Tris-EDTA buffer (10 mM Tris-Cl [pH 8.0], 1 mM EDTA) with 30 μl of 10% (wt/vol) sodium dodecyl sulfate and 3 μl of proteinase K (20 mg/ml) (Sigma, St. Louis, Mo.) and were lysed for 1 h at 37°C. Next, 100 μl of 5 M NaCl and 80 μl of cetyltrimethylammonium bromide-NaCl were added and incubated for 10 min at 65°C. DNA was purified by extraction with chloroform-isoamyl alcohol (24:1), followed by extraction with phenol-chloroform-isoamyl alcohol (25:24:1). The DNA was then precipitated with isopropanol, centrifuged for 5 min at 10,000 × g, washed with cold 70% (vol/vol) ethanol, and dried with a DNA SpeedVac (Savant Instrument, Inc., Holbrook, N.Y.). The dried DNA was resuspended in 25 μl of the Tris-EDTA buffer described above, and the DNA concentration measured with a Lambda II spectrophotometer (Perkin-Elmer, Shelton, Conn.) at a wavelength of 260 nm.
Real-time PCR and cycling parameters.
The oligonucleotide primers L-vvh (5′-TTCCAACTTCAAACCGAACTATGA-3′) and R-vvh (5′-ATTCCAGTCGATGCGAATA CGTTG-3′), encompassing the 0.205-kbp segment between nucleotides 786 and 990 of the open reading frame of vvh (GenBank accession no. M34670), were used in this study (8). PCR was performed by using 2.5 μl of 10× PCR buffer (10× PCR buffer consisted of 200 mM Tris-Cl [pH 8.4] and 500 mM KCl), 1.25, 2.5, or 3.5 μl of 50 mM MgCl2, each deoxynucleoside triphosphate (Sigma) at a concentration of 200 μM, 2.5 μl of enhancer solution (Invitrogen, Inc., Carlsbad, Calif.), 2.5 or 5 μl of 20× SYBR Green I nucleic acid fluorescent dye (Roche Applied Science, Indianapolis, Ind.), 0.3 μl of a solution containing each of the oligonucleotide primers at a concentration of 20 μM, 0.3 μl of a solution containing 5 U of AmpliTaq DNA polymerase (Promega Corporation, Madison, Wis.) per μl, and enough sterile MilliQ water to bring the total reaction volume to 25 μl. The PCR cycling parameters were as follows: initial denaturation of the template DNA at 94°C for 120 s, followed by 45 cycles of amplification of the template DNA, in which each cycle consisted of denaturation at 94°C for 15 s, primer annealing at 56°C for 15 s, and primer extension on the template DNA at 72°C for 25 s. Following amplification, a melting curve analysis of the amplified DNA was performed at temperatures between 54 and 95°C, with the temperature increasing at a rate of 0.2°C/s. All PCR were performed with a Cepheid smart cycler. During the primer extension step of each amplification cycle, the increase in the fluorescence from the amplified DNA was recorded by using the SYBR Green I optic channel set at a wavelength of 495 nm. The initial threshold value was set at 30 fluorescent units. Following PCR amplification, the amplified DNA was further analyzed in an agarose gel, and the expected molecular weight of the amplicons was confirmed by comparison to a known DNA size marker.
Sensitivity of detection.
The sensitivity of SYBR Green I-based real-time PCR was determined with purified DNA and with an exponentially grown culture of V. vulnificus following cell lysis. Purified genomic DNA of V. vulnificus was serially diluted 10-fold from 0.1 μg to 0.1 pg in Tris-EDTA buffer (10 mM Tris-Cl [pH 7.5], 1 mM Na2EDTA) and subjected to real-time PCR amplification by using the reaction and cycling parameters described above. In addition, a pure culture of V. vulnificus was grown in autoclaved (121°C, 15 lb/in2, 20 min) gulf water (salinity, 16 ppt; 24 ± 1°C; depth, approximately 0.5 m) supplemented with 0.2% (wt/vol) Bacto-Peptone (Difco) (GWP-16) until the optical density at 600 nm (OD600) reached 0.2. Then the culture was 10-fold serially diluted in GWP-16 from 106 CFU/ml to extinction. Aliquots (0.1 ml) of the serially diluted V. vulnificus culture were plated onto modified cellobiose-polymyxin B-colistin (mCPC) agar plates and grown at 37°C overnight (51). The cellobiose-positive yellow colonies were counted to determine the total number of viable V. vulnificus CFU present. Total bacterial cell numbers were determined by the acridine orange direct count procedure described by Hobbie et al. (19) with a Leitz Diaplan epifluorescent microscope. The remaining cultures were centrifuged at 12,000 × g for 10 min; each supernatant was carefully discarded, and the cell pellets were resuspended in 200 μl of Instagene matrix (Bio-Rad Laboratories, Hercules, Calif.). Each sample was then incubated at 56°C for 10 min and then boiled for 20 min. The samples were cooled to room temperature and centrifuged at 3,000 × g for 3 min, and an aliquot (3 μl) of each sample was used for real-time PCR amplification.
Detection in seeded gulf water.
A pure culture of V. vulnificus was grown in sterile GWP-16 until the OD600 reached 0.2. The culture was 10-fold serially diluted in GWP-16, and the total number of viable cells was determined by viable plate and direct microscopic count as described above. Initially, 1-ml aliquots of the 10-fold serially diluted V. vulnificus cultures in GWP-16 were centrifuged at 12,000 × g for 20 min and subjected to DNA purification by using various DNA extraction methods, as shown in Table 2.
TABLE 2.
Effectiveness of V. vulnificus detection in gulf water supplemented with 0.2% (wt/vol) peptone by real-time PCR with various DNA extraction methods
| DNA extraction method | Protocol summary | Sensitivity of detection (gulf water) (CFU/ml)a |
|---|---|---|
| Boiling | Boil at 100°C for 10 min | ≥103 |
| Boiling with Instagene (Bio-Rad) | Incubate at 56°C for 20 min, followed by boiling for 10 min | ≥102 |
| Qiaprep (Qiagen, Valencia, Calif.) | Bacterial pellet resuspended in buffer P1; buffers P2 and N3 added; ethanol precipitation | ≥107 |
| Magnetic Beads (Bugs n' Beads, Genpoint, Oslo, Norway) | Incubate with binding beads; lysis buffer added; ethanol precipitation | ND |
| Nucleotrap DNA purification (Clontech, Palo Alto, Calif.) | Buffer NT2 and Nucleotrap suspension added to DNA extracted by boiling; buffer NT3 added to precipitate DNA; DNA eluted by incubation at 50°C for 5 min | ND |
Results of three consecutive experiments which produced consistent results. ND, no detection.
After optimization and selection of a suitable DNA extraction method, a similarly grown V. vulnificus culture was 10-fold serially diluted to extinction in 10 ml of sterile GWP-16 and enriched for 5 h at 37°C with shaking at 170 rpm in a rotary shaker incubator (Innova 4000; New Brunswick Scientific Inc., Edison, N.J.). The samples were then centrifuged at 12,000 × g for 10 min, and the supernatants were discarded. The cell pellets were treated with 200 μl of Instagene matrix (Bio-Rad) as described above, and the DNA was released. An aliquot (3 μl) of each sample was used for real-time PCR amplification.
Detection of V. vulnificus in cultures exposed to low temperature.
A V. vulnificus strain was grown in sterile GWP-16 (150 ml) until the OD600 reached 0.2, and viable plate and total microscopic count were performed as described above. Equal volumes of the culture were then distributed in three 250-ml sterile flasks and transferred to 4°C. Prior to the low-temperature treatment, 1-ml cultures were centrifuged at 12,000 × g for 10 min, and the cell pellets were treated with Instagene matrix (Bio-Rad) to release the DNA. An aliquot (3 μl) of the DNA was subjected to PCR amplification by using the primers and reaction parameters described above. After 30 days of incubation at 4°C, viable plate counting and direct microscopic counting were performed with each of the cultures. Then, a 1-ml aliquot of each culture was added to 9 ml of fresh GWP-16, enriched for 5 h at 37°C, and amplified by real-time PCR. Similarly, another 1-ml aliquot was PCR amplified without enrichment. Following real-time PCR, an aliquot (10 μl) of each amplified DNA was separated and analyzed by agarose gel electrophoresis to confirm the correct size of the amplicon. This experiment was performed in triplicate, and appropriate PCR-positive and -negative controls were included.
Detection in unseeded gulf water.
Five samples, each consisting of 500 ml of gulf water, were collected in sterile containers from Dauphin Island Bay in Alabama at a location about 50 to 100 ft from the shoreline and at a water column depth of approximately 0.5 m during July and August 2003. The water temperature measured at the time of sample collection was 23 to 25°C, and the salinity ranged from 12 to 18 ppt. Aliquots (10 ml) of these samples were filtered through 0.2-μm-pore-size type HA filters (Millipore, Billerica, Mass.). The filters were placed onto mCPC agar with the cell side up and incubated at 37°C overnight. Individual cellobiose-positive colonies on mCPC agar plates were PCR amplified with the vvh-specific primers to confirm the actual number of viable V. vulnificus cells in the samples. Water samples (10 ml) were enriched with 0.2% (wt/vol) peptone for 5 h at 37°C in a rotary shaker incubator (Innova 4000) set at 170 rpm. After 5 h, a 1-ml aliquot of each sample was centrifuged at 12,000 × g for 10 min, and the cell pellets were treated with 200 μl of Instagene matrix (Bio-Rad) to release the DNA. An aliquot (3 μl) of each sample was used as a template for real-time PCR amplification with SYBR Green I dye. The cycle threshold (Ct) values obtained by real-time PCR were used to calculate the total number of CFU per milliliter present in each of the samples based on the standard curve for enriched gulf water samples. The experiment was performed in triplicate, and appropriate PCR-positive and -negative controls were included.
Detection in seeded oyster tissue homogenate.
All oyster samples were collected from Dauphin Island Bay in Alabama during December 2002 (water temperature, 16 ± 1°C; salinity, 16 ppt). The oysters were kept on ice immediately after harvest, transported to the laboratory, and then cleaned, shucked, and homogenized by using the standard methods recommended by the American Public Health Association (2). At least three aliquots (1 g each) of oyster tissue homogenate from each sample were 10-fold serially diluted in sterile GWP-16 and plated onto mCPC agar to determine the viable plate counts. Individual cellobiose-positive colonies on mCPC agar plates were PCR amplified with the vvh-specific primers to confirm the actual number of viable V. vulnificus cells in the samples. The remainder of the oyster tissue homogenate was stored at −80°C until it was used. A V. vulnificus culture was grown in sterile GWP-16 at 37°C until the OD600 reached 0.2, and viable cell counts were determined as described above. The culture was then 10-fold serially diluted from 105 CFU/ml to extinction in 50 ml of sterile GWP-16 containing 1 g of oyster tissue homogenate and enriched for 5 h at 37°C with shaking at 170 rpm in a rotary shaker incubator (Innova 4000). After 5 h, 5 ml-aliquots were centrifuged at 12,000 × g for 10 min, and the supernatants were discarded. The cell pellets were treated with 200 μl of Instagene matrix (Bio-Rad) as described above, and the DNA was released. An aliquot (3 μl) of each sample was used for real-time PCR amplification with SYBR Green I dye. Unseeded oyster tissue homogenate (1 g) was also diluted in GWP-16, and viable plate counts on mCPC agar were determined as described above.
Statistical analysis.
The sensitivities of detection for 10-fold serially diluted purified genomic DNA samples and V. vulnificus in pure cultures, seeded gulf water, and oyster tissue homogenate were determined in 10 separate experiments. To determine the relationship between the real-time PCR Ct values and the concentrations of DNA or bacterial cells, standard curves were generated by using the Microsoft Excel 2000 statistical software (Microsoft, Seattle, Wash.), followed by regression analysis.
RESULTS
Optimization of real-time PCR.
The real-time PCR was optimized by varying the concentrations of MgCl2 and SYBR Green I dye. It was found that 2.5 μl (5 mM) of MgCl2 and 2.5 μl (2×) of SYBR Green dye were optimal for the reaction. Increasing the SYBR Green I dye concentration in the reaction mixture resulted in an error in reading the fluorescent signals from amplified DNA. Also, the use of an enhancer solution (10%, vol/vol) and equimolar concentrations (0.24 μM) of the oligonucleotide primers reduced the formation of primer artifacts to a nondetectable level.
Specificity of detection.
Real-time PCR amplification of the genomic DNA with the L-vvh and R-vvh oligonucleotide primers and the reaction conditions and temperature cycling parameters described above resulted in the expected 0.205-kbp amplified DNA segment from all 84 strains of V. vulnificus used in this study (Table 1). Amplification of the 0.205-kbp segment of vvh for all V. vulnificus strains was confirmed by melting temperature (87°C) analysis and gel electrophoresis (data not shown). No amplification of vvh was noticed with any of the non-V. vulnificus or other nonvibrio bacterial species tested in this study (Table 1).
Preparation of template DNA.
The results of the various procedures for DNA extraction from V. vulnificus seeded in GWP-16 prior to enrichment are presented in Table 2. Only three of five procedures resulted in PCR amplification of the targeted DNA from V. vulnificus in seeded gulf water. Purification of DNA with a Qiagen DNA purification kit resulted in detection of ≥107 CFU/ml of GWP-16, whereas the detection level for the simple boiling approach was ≥103 CFU/ml. The magnetic bead procedure (GenPoint, Oslo, Norway) and the Nucleotrap suspension method (BD Biosciences Clontech, Palo Alto, Calif.) of DNA purification resulted in no amplification of the targeted gene segment from 108 V. vulnificus cells per ml of GWP-16. The most sensitive detection of V. vulnificus was obtained with the sample treated with Instagene matrix (Bio-Rad), which was less time-consuming and relatively easy to use. Therefore, this method of DNA extraction was used for detection of this pathogen in unenriched and enriched gulf water and oyster tissue homogenates in subsequent experiments.
Sensitivity of detection.
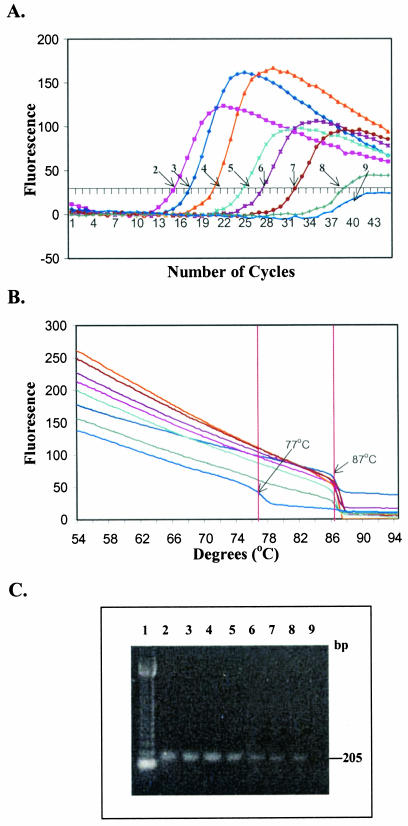
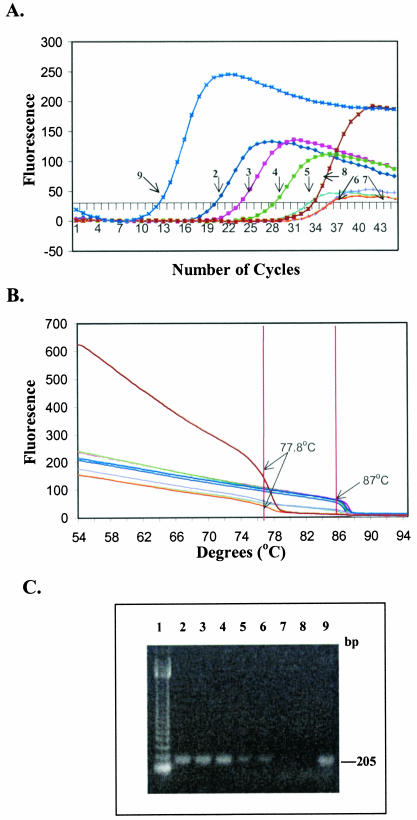
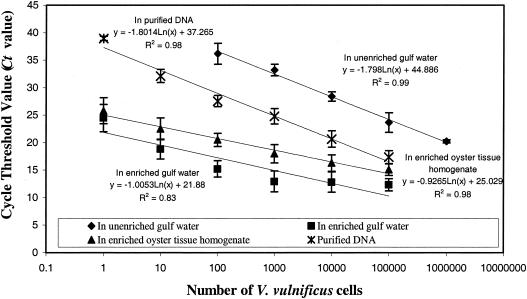
The minimum level of detection of the vvh target from purified genomic DNA was 1 pg with a Ct value of 38.95 ± 0.38 and the expected melting temperature, approximately 87°C (Table 3). This level of detection is equivalent to 100 cells of V. vulnificus, since only one copy of the targeted vvh gene is present in the genome of V. vulnificus (genome accession number, NC 004460) (4). Successive increases in Ct values with the expected melting temperature (87°C) were observed as the concentration of template DNA in the samples decreased (Table 3; Fig. 1). The sensitivity of detection of V. vulnificus in gulf water without enrichment was 102 CFU/ml with a Ct value of 36.15 ± 1.9 (Table 4; Fig. 2). The viable plate count for the exponentially grown V. vulnifcus culture (OD600, 0.2) was 1.5 × 108 CFU/ml (standard deviation, 0.25 × 108 CFU/ml; n = 3), and the total microscopic acridine orange direct count was 2.1 × 108 cells/ml (standard deviation, 0.48 × 108 cells/ml; n = 3). The Ct values increased as the number of CFU per milliliter decreased in the samples. There was a good linear correlation between the Ct values and the concentrations of purified DNA (n = 10; r2 = 0.98) or V. vulnificus cells in pure cultures (n = 10; r2 = 0.99) (Fig. 3). The level of detection of unenriched V. vulnificus in gulf water (102 CFU/ml) correlated with the minimum amount of purified DNA (1 pg) (Fig. 3). However, slightly higher Ct values for one or two cycles for unenriched V. vulnificus in gulf water compared with the values for pure DNA suggests that there may have been inhibition of the real-time PCR.
TABLE 3.
Sensitivity of detection of purified DNA from a pure culture of V. vulnificus MO6-24(O) by using real-time PCR and SYBR Green I fluorescent dyea
| Sampleb | Amt of DNA | Ct value | Melting temp (°C) |
|---|---|---|---|
| 2 (positive control) | 15.19 ± 1.8 | 87.0 ± 0.04 | |
| 3 | 0.1 μg | 17.33 ± 1.2 | 87.68 ± 0.03 |
| 4 | 0.01 μg | 20.65 ± 1.5 | 87.07 ± 0.3 |
| 5 | 1 ng | 24.8 ± 1.4 | 87.93 ± 0.02 |
| 6 | 0.1 ng | 27.53 ± 1.02 | 86.04 ± 1.03 |
| 7 | 0.01 ng | 32.09 ± 1.2 | 87.5 ± 0.06 |
| 8 | 1 pg | 38.95 ± 0.38 | 86.92 ± 0.02 |
| 9 | 0.1 pg | 77.01 ± 1.05 |
The data are means ± standard deviations for 10 independent experiments.
The sample numbers correspond to the lane numbers in Fig. 1C.
FIG. 1.
Real-time PCR sensitivity with primers L-vvh and R-vvh and purified genomic DNA from V. vulnificus MO6-24(O). (A) Representative optic graph for the number of cycles versus the number of fluorescence units for each sample used to calculate the Ct value. (B) Corresponding melting curve analysis, with the results represented by a graph of number of fluorescence units versus temperature. (C) Corresponding agarose gel electrophoresis. Lane 1, 123-bp DNA ladder (Gibco, BRL); lane 2, PCR-positive control; lane 3, 0.1 μg of DNA; lane 4, 0.01 μg of DNA; lane 5, 1 ng of DNA; lane 6, 0.1 ng of DNA; lane 7, 0.01 ng of DNA; lane 8, 1 pg of DNA; lane 9, 0.1 pg of DNA. The lane numbers correspond to the sample numbers shown in Table 3.
TABLE 4.
Sensitivity of detection of an exponentially grown pure culture of V. vulnificus MO6-24(O) with SYBR Green I dye without enrichmenta
| Sampleb | Amt of bacteria (CFU/ml) | Ct value | Melting temp (°C) |
|---|---|---|---|
| 2 | 106 | 20.24 ± 0.03 | 87.05 ± 0.3 |
| 3 | 105 | 23.64 ± 1.8 | 87.41 ± 0.38 |
| 4 | 104 | 28.38 ± 0.85 | 87.12 ± 0.4 |
| 5 | 103 | 33.22 ± 1.05 | 86.5 ± 0.67 |
| 6 | 102 | 36.15 ± 1.9 | 86.71 ± 0.09 |
| 7 | 101 | 36.31 ± 1.6 | 77.8 ± 0.02 |
| 8 (negative control) | 33.76 ± 1 | 77.86 ± 0.8 | |
| 9 (positive control) | 12.45 ± 2.1 | 87.12 ± 0.14 |
The values are means ± standard deviations for 10 independent experiments.
The sample numbers correspond to the lane numbers in Fig. 2C.
FIG. 2.
Real-time PCR sensitivity with primers L-vvh and R-vvh and genomic DNA extracted from 10-fold serially diluted pure cultures of V. vulnificus MO6-24(O) without enrichment. (A) Representative optic graph for the number of cycles versus the number of fluorescence units for each sample used to calculate the Ct value. (B) Corresponding melting curve analysis, with the results represented by a graph of number of fluorescent units versus temperature. (C) Corresponding agarose gel electrophoresis. Lane 1, 123-bp DNA ladder (Gibco, BRL); lane 2, 106 CFU/ml; lane 3, 105 CFU/ml; lane 4, 104 CFU/ml; lane 5, 103 CFU/ml; lane 6, 102 CFU/ml; lane 7, 101 CFU/ml; lane 8, PCR-negative control; lane 9, PCR-positive control. The lane numbers correspond to the sample numbers shown in Table 4.
FIG. 3.
Standard curves for the number of V. vulnificus cells or amount of purified DNA versus Ct values. The error bars indicate the standard deviations obtained in 10 independent experiments.
Detection in seeded and unseeded gulf water following enrichment.
The results of the real-time PCR revealed that there was amplification of the vvh gene target in enriched gulf water samples with an initial cell count of 1 V. vulnificus cell per ml (Table 5). This level of detection was at least 100 times higher than the level of detection for unenriched V. vulnificus cultures and was in compliance with the recent ISSC guidelines (21). An increase in the Ct value from 12.94 ± 1.9 to 24.47 ± 2.5 with the expected melting temperature (approximately 87°C) was observed with samples that were seeded with 103 to 100 V. vulnificus CFU/ml. However, the samples seeded with V. vulnificus cells at concentrations ranging from 105 to 103 CFU/ml exhibited almost the same Ct values. This could have been due to the presence of excess initial template DNA in the reaction mixture, which resulted in accumulation of amplicons to a plateau level (35). A good linear correlation was observed between the Ct values and the concentrations of V. vulnificus cells seeded in 10 ml of gulf water (n = 10; r2 = 0.83) (Fig. 3). Also, V. vulnificus was detected in all five unseeded gulf water samples following 5 h of enrichment with 0.2% (wt/vol) peptone (Table 6). The yellow colonies on mCPC agar that were positive for the vvh gene as determined by PCR were used to determine the viable plate counts. There was a good correlation between the number of CFU per milliliter calculated by real-time PCR and the number of CFU per milliliter calculated by viable plate count (Table 6). The initial concentrations of V. vulnificus cells detected in the samples ranged from 3 to 76 CFU/ml.
TABLE 5.
Detection of V. vulnificus MO6-24(O) from seeded GWP-16 after 5 h of enrichmenta
| Sample | Amt of bacteria (CFU/ml) | Ct value | Melting temp (°C) |
|---|---|---|---|
| 1 | 105 | 12.32 ± 1.1 | 87.08 ± 0.3 |
| 2 | 104 | 12.81 ± 1.85 | 87.93 ± 0.05 |
| 3 | 103 | 12.94 ± 1.9 | 87.05 ± 0.16 |
| 4 | 102 | 15.21 ± 1.5 | 87.0 ± 0.4 |
| 5 | 101 | 18.81 ± 1.8 | 87.31 ± 0.2 |
| 6 | 100 | 24.47 ± 2.5 | 87.05 ± 0.7 |
| 7 | 0 | 29.01 ± 0.3 | 77.2 ± 2.2 |
| 8 (positive control) | 14.50 ± 0.9 | 87.1 ± 0.4 | |
| 9 (negative control)b | 28.71 ± 0.7 | 76.72 ± 2.8 |
The values are means ± standard deviations for 10 independent experiments.
Unseeded gulf water.
TABLE 6.
Detection of V. vulnificus MO6-24(O) from unseeded gulf water following 5 h of enrichmenta
| Sampleb | Ct value | Melting temp (°C) | Calculated concn (CFU/ml) | Viable plate count (CFU/ml) |
|---|---|---|---|---|
| 1 | 20.9 ± 1.5 | 87.4 ± 0.05 | 3 | 4 ± 1 |
| 2 | 18.48 ± 0.85 | 87.6 ± 0.12 | 29 | 32 ± 3.5 |
| 3 | 17.53 ± 1.34 | 87.4 ± 0.2 | 76 | 81 ± 4.72 |
| 4 | 17.95 ± 2.2 | 86.9 ± 0.4 | 52 | 58 ± 4.5 |
| 5 | 20.39 ± 1.66 | 87.2 ± 0.05 | 4 | 3 ± 2 |
| Positive control | 12.37 ± 0.53 | 87.1 ± 0.08 | ||
| Negative control | 27.34 ± 0.6 | 76.89 ± 0.05 |
The values are means ± standard deviations for three independent experiments.
The samples were collected in the Gulf of Mexico off the coast of Dauphin Island in Alabama.
Detection of V. vulnificus in cultures exposed to low temperature.
PCR performed with the initial culture (day 1), as well as the culture after 30 days of incubation at 4°C, resulted in amplification of a 0.205-kbp segment of the vvh gene. The viable plate counts and the microscopic direct counts on day 1 were approximately 2.2 × 108 CFU/ml (standard deviation, 0.15 × 108 CFU/ml; n = 3) and 2.8 × 108 cells/ml (standard deviation, 0.25 × 108 cells/ml; n = 3), respectively. Real-time PCR amplification with these samples resulted in a Ct value of 18.8 ± 1.2. However, following exposure to 4°C for 30 days, the viable plate counts and microscopic counts decreased to about 1.5 × 101 CFU/ml (standard deviation, 0.21 × 101 CFU/ml; n = 3) and 2.6 × 102 cells/ml (standard deviation, 0.72 × 101 cells/ml; n = 3), respectively. Real-time PCR with DNA extracted from these cold-stressed cultures before enrichment resulted in no amplification. After 5 h of enrichment, the same cultures exhibited Ct values of 19.9 ± 1.3 with the expected melting temperature (approximately 87°C). These results correlate with the Ct values obtained from real-time PCR conducted with seeded gulf water samples that were not exposed to a low temperature, suggesting that amplification resulted only from viable cells present in the samples (Tables 4 and 5). All three replicates in this experiment produced comparable results.
Detection in seeded oyster tissue homogenate following enrichment.
The results of real-time PCR amplification showed that 1 CFU of V. vulnificus was detected in 1 g of seeded oyster tissue homogenate following 5 h of enrichment (Table 7). An increase in the Ct values from 15.1 ± 1.08 to 25.8 ± 2.36 with an increment of one or two cycles was observed for samples that were seeded with 105 to 100 V. vulnificus CFU/ml. This result was unlike the result obtained with the seeded gulf water samples, in which successive increases in Ct values were observed only for samples with concentrations ranging from 103 to 100 CFU/ml. All positive samples had a melting temperature of approximately 87°C. A good linear correlation was observed between the Ct values and the concentrations of V. vulnificus cells seeded in 1 g of oyster tissue homogenate (n = 10; r2 = 0.98) (Fig. 3). Based on the optimum doubling time for this pathogen (18 to 20 min), the Ct values obtained by real-time PCR for the unenriched gulf water seeded with 10-fold serially diluted V. vulnificus cells exhibited a good correlation with the values for seeded gulf water or oyster samples enriched for 5 h (37). The Ct values for the seeded oyster tissue homogenate were two to three cycles higher than the values for the seeded gulf water, indicating that the oyster tissue matrix had a greater inhibitory effect on the real-time PCR than the gulf water samples had (Fig. 3).
TABLE 7.
Detection of V. vulnificus MO6-24 from seeded oyster issue homogenate following 5 h of enrichmenta
| Sample | Concn (CFU/g) | Ct value | Melting temp (°C) |
|---|---|---|---|
| 1 | 105 | 15.10 ± 1.08 | 87.7 ± 0.03 |
| 2 | 104 | 16.28 ± 1.5 | 87.6 ± 0.05 |
| 3 | 103 | 17.99 ± 1.66 | 87.4 ± 0.02 |
| 4 | 102 | 20.5 ± 1.2 | 87.6 ± 0.08 |
| 5 | 101 | 22.5 ± 1.98 | 87.7 ± 0.02 |
| 6 | 100 | 25.8 ± 2.36 | 87.3 ± 0.09 |
| 7 | 0 | 28.17 ± 2.1 | 77.4 ± 1.4 |
| 8 (positive control) | 12.35 ± 1.8 | 87.2 ± 0.03 | |
| 9 (negative control)b | 28.52 ± 1.9 | 77.11 ± 1.1 |
The values are means ± standard deviations for 10 independent experiments.
Unseeded oyster tissue homogenate.
PCR amplification with vvh-specific oligonucleotide primers and the cellobiose-positive colonies on mCPC agar plates, which were indigenous to the unseeded enriched or unenriched oyster tissue homogenates, was negative, suggesting that there were no viable V. vulnificus cells in the oysters used. The absence of viable V. vulnificus in oysters was not unusual, as the samples were collected from Dauphin Island Bay in Alabama during December, when this microorganism has been reported to remain in the undetectable state (38, 39). The level of detection, 1 CFU of V. vulnificus in 1 g of oyster tissue homogenate following enrichment, correlated with the results of the gulf water study (Fig. 3) and is well within the detection limit required by the ISSC.
DISCUSSION
V. vulnificus is the leading cause of seafood-related deaths in the United States (6, 24). V. vulnificus-related sporadic illnesses, such as diarrhea and septicemia, during the summer months have been reported every year primarily in the United States Gulf of Mexico coast region. As a result, the oyster industry in this region continues to suffer from problems related to elevated V. vulnificus levels in raw oysters and associated fatal illnesses (12, 25). For example, during the past several years, the fishery industry in Texas lost potential revenue of 3.4 million dollars from oysters and crabs because of closure due to microbiologically unsafe products (50). Recently, California released emergency restrictions on the sale of all oysters harvested between April and October from the Gulf of Mexico, unless they are treated with a scientifically validated process to reduce the concentration of V. vulnificus to a nondetectable level (<3 CFU/g) (California Department of Health Services, April 2003 [http://www.dhs.ca.gov]). In the last 2 years alone, California has experienced 16 illnesses resulting in 10 deaths, despite educational efforts directed at high-risk populations to warn them of the potential hazards of eating raw oysters. The emergency restrictions implemented in California, which are the first of their kind in the United States, would significantly affect the seafood industries located in the states along the Gulf of Mexico. In order to ensure a steady supply of consumable postharvest treated oysters that would reduce the risk of V. vulnificus-related illnesses to 60% within the next 3 to 4 years, it is necessary to develop a rapid method that can detect less than 3 V. vulnificus cells per g of oyster tissue homogenate (21). In this study, we developed an optimal and rapid method for detection of this pathogen in gulf water and shellfish tissue homogenate by real-time PCR. As a first step in this effort, we evaluated and established a simple and effective Instagene matrix-based DNA purification method that can be used for detection of V. vulnificus in enriched gulf water or oyster tissue homogenate without compromising the sensitivity of detection. For unenriched gulf water samples, the sensitivity of detection by this method was 100 CFU/ml. Collection of relatively low numbers of V. vulnificus cells by centrifugation was possible as the particulates in gulf water could have helped with coprecipitation of bacterial cells, thus facilitating recovery of template DNA. The relatively less sensitive detection of this pathogen with samples purified by other methods (Table 2) could have been due to the loss of template DNA during multiple processing steps or inadequate inactivation of PCR-inhibiting substances that potentially interfered with the amplification process.
Although vvh has been used for conventional PCR detection of V. vulnificus by other investigators, an insufficient number of V. vulnificus isolates were tested in these studies (3, 8, 9, 16, 27). Therefore, it was necessary to verify the specificity of the oligonucleotide primers with a real-time PCR platform. The L-vvh and R-vvh primers used in this study provided specific detection of a large number of V. vulnificus strains that were isolated from infected human patients, oysters, or other environmental sources. Other Vibrio species and nonvibrios did not show any amplification, suggesting that these primers and the real-time PCR parameters were optimum for specific and reliable detection of V. vulnificus.
Use of SYBR Green I dye and selected oligonucleotide primers in real-time PCR resulted in an expected 205-bp amplicon with a consistent melting temperature of approximately 87°C, confirming the reliability of this approach. The detection of microbial pathogens in various biological samples by real-time PCR with SYBR Green I dye has been shown to be rapid, reliable, and cost-effective (1, 23, 30, 34, 40, 46). Also, a recent study in which the SYBR Green I-based PCR was compared with the Taqman assay for detection of fecal pathogens showed that the two assays had the same level of sensitivity of detection (30). It has been suggested that in the SYBR Green I-based real-time PCR method a relatively more expensive hot-start thermostable DNA polymerase should be used to avoid the formation of PCR primer artifacts or nonspecific amplicons (15). However, in our study, we accomplished this by using a less expensive enhancer solution (Invitrogen) and regular recombinant Taq DNA polymerase (Promega). By using the optimum PCR temperature cycling parameters, reaction parameters, and enrichment of the pathogen, it was possible to detect V. vulnificus at the single-cell level. Besides the improvement in the sensitivity of detection from unenriched 102 CFU/ml to the single-cell level, the enrichment step helps in recovery and multiplication of injured cells following application of the postharvest treatment methods, thereby avoiding false-negative detection (24, 42). This conclusion is supported by the results obtained with V. vulnificus cultures exposed to low temperature, in which detection was possible with stressed but viable cells. The level of detection of V. vulnificus cells in gulf water and oyster samples following enrichment achieved in this study is well within the recent ISSC guidelines. Currently, there is no specific guideline that describes a minimum level of V. vulnificus in gulf water that could potentially be hazardous to humans. However, a number of studies have suggested that V. vulnificus can cause wound infections and septicemia, often with fatal consequences, during recreational activities in coastal waters (13, 20, 36, 41, 43, 45, 47, 49). Therefore, we felt that it was necessary to demonstrate that the real-time PCR detection method described in this study could potentially be used for detection of this pathogen in gulf water.
It was possible to complete the entire method, including sample processing, enrichment, and real-time PCR amplification, in less than 8 h, making it an expeditious single-day assay for detection of V. vulnificus in oysters or in gulf water samples. This is in contrast to the conventional methods, like the most-probable-number method followed by biochemical testing and colony blot hybridization, that take about 3 to 4 days to complete (24, 42). In addition, the standard curves from the real-time PCR Ct values showed that there was a strong correlation between the enriched and unenriched cells, which can potentially be used to provide quantitative estimates of the levels of V. vulnificus in oysters or gulf water samples, as shown by the calculated numbers of CFU per milliliter for unseeded gulf water samples. Slightly higher (one to two cycles) Ct values for the seeded oyster tissue homogenate than for the seeded gulf water could have been due to the complexity of the oyster tissue, which resulted in inhibition of the PCR. However, this did not affect detection of V. vulnificus at the single-cell level following an enrichment step. The data presented here also showed that a 5-h enrichment was sufficient to enable detection of 1 CFU/ml in seeded, unseeded, and stressed cultures of V. vulnificus, since the optimum doubling time for this pathogen (18 to 20 min) provided enough cells at the end of the enrichment period for accurate and sensitive detection (37). Rapid detection of this pathogen in consumable oysters and in coastal water, especially in and around approved oyster-harvesting sites, at a level that meets the recent ISSC guidelines would help reduce the incidence of illness and fatality that result from ingestion of raw shellfish or from exposure to coastal water.
Acknowledgments
This work was supported in part by funds from the Mississippi-Alabama Sea Grant Consortium, the National Oceanic and Atmospheric Administration, the Department of Commerce, and the University of Alabama at Birmingham (research grant NA86RG0039-4, project R/SP-1).
We thank Angelo DePaola, Jr., and Charles A. Kaysner for providing the V. vulnificus strains and some of the other vibrio strains and for their suggestions. Also, we thank Anita C. Wright and Cepheid for their helpful suggestions.
REFERENCES
- 1.Aarts, H. J., R. G. Joosten, M. H. Henkens, H. Stegeman, and A. H. van Hoek. 2001. Rapid duplex PCR assay for the detection of pathogenic Yersinia enterocolitica strains. J. Microbiol. Methods 47:209-217. [DOI] [PubMed] [Google Scholar]
- 2. American Public Health Association. 1970. Recommended procedures for the examination of seawater and shellfish, 4th ed. American Public Health Association, Washington, D.C.
- 3.Arias, C. R., E. Garay, and R. Aznar. 1995. Nested PCR method for rapid and sensitive detection of Vibrio vulnificus in fish, sediments, and water. Appl. Environ. Microbiol. 61:3476-3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Atlas, R. M., and A. K. Bej. 1991. Detecting bacterial pathogens in environmental water samples by using PCR and gene probes, p. 399-406. In M. A. Innis, D. H. Gelfand, J. J. Sninsky, and T. J. White (ed.), PCR protocols: a guide to methods and applications. Academic Press, Inc., San Diego, Calif.
- 5.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Smith, J. G. Sideman, and K. Struhl (ed.). 1987. Current protocols in molecular biology, p. 2.10-2.11. John Wiley & Sons, Inc., New York, N.Y.
- 6.Birkenhauer, J. M., and J. D. Oliver. 2003. Use of diacetyl to reduce the load of Vibrio vulnificus in the eastern oyster, Crassostrea virginica. J. Food Prot. 66:38-43. [DOI] [PubMed] [Google Scholar]
- 7.Blackstone, G. M., J. L. Nordstrom, M. C. L. Vickery, M. D. Bowen, R. F. Meyer, and A. DePaola, Jr. 2003. Detection of pathogenic Vibrio parahaemolyticus in oyster enrichments by real time PCR. J. Microbiol. Methods 53:149-155. [DOI] [PubMed] [Google Scholar]
- 8.Brasher, C. W., A. DePaola, D. D. Jones, and A. K. Bej. 1998. Detection of microbial pathogens in shellfish with multiplex PCR. Curr. Microbiol. 37:101-107. [DOI] [PubMed] [Google Scholar]
- 9.Brauns, L. A., M. C. Hudson, and J. D. Oliver. 1991. Use of the polymerase chain reaction in detection of culturable and nonculturable Vibrio vulnificus cells. Appl. Environ. Microbiol. 57:2651-2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention. 1996. Vibrio vulnificus infections associated with eating raw oysters—Los Angeles, 1996. Morb. Mortal. Wkly. Rep. 45:621-624. [PubMed] [Google Scholar]
- 11.Cohen, M. L. 2000. Changing patterns of infectious disease. Nature 406:762-767. [DOI] [PubMed] [Google Scholar]
- 12.Glatzer, M. 2001. Vibrio vulnificus shellfish case file from 1989-2000. U.S. Food and Drug Administration, Washington, D.C.
- 13.Halow, K. D., R. C. Harner, and L. J. Fontenelle. 1996. Primary skin infections secondary to Vibrio vulnificus: the role of operative intervention. J. Am. Coll. Surg. 183:329-334. [PubMed] [Google Scholar]
- 14.Hayat, U., G. P. Reddy, C. A. Bush, J. A. Johnson, A. C. Wright, and J. G. J. Morris. 1993. Capsular types of Vibrio vulnificus: an analysis of strains from clinical and environmental sources. J. Infect. Dis. 168:758-762. [DOI] [PubMed] [Google Scholar]
- 15.Hein, I., A. Lehner, P. Rieck, K. Klein, E. Brandl, and M. Wagner. 2001. Comparison of different approaches to quantify Staphylococcus aureus cells by real-time quantitative PCR and application of this technique for examination of cheese. Appl. Environ. Microbiol. 67:3122-3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hill, W. E., S. P. Keasler, M. W. Truckess, P. Feng, C. A. Kaysner, and K. A. Lampel. 1991. Polymerase chain reaction identification of Vibrio vulnificus in artificially contaminated oysters. Appl. Environ. Microbiol. 57:707-711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hlady, W. G. 1997. Vibrio infections associated with raw oyster consumption in Florida, 1991-1994. J. Food Prot. 60:353-357. [DOI] [PubMed] [Google Scholar]
- 18.Hlady, W. G., and K. C. Klontz. 1996. The epidemiology of Vibrio infections in Florida, 1981-1993. J. Infect. Dis. 173:1176-1183. [DOI] [PubMed] [Google Scholar]
- 19.Hobbie, J. E., R. J. Daley, and S. Jasper. 1977. Use of Nuclepore filters for counting bacteria by fluorescence microscopy. Appl. Environ. Microbiol. 33:1225-1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoyer, J., E. Engelmann, R. M. Liehr, A. Distler, H. Hahn, and T. Shimada. 1995. Septic shock due to Vibrio vulnificus serogroup 04 wound infection acquired from the Baltic Sea. Eur. J. Clin. Microbiol. Infect. Dis. 14:1016-1018. [DOI] [PubMed] [Google Scholar]
- 21. Interstate Shellfish Sanitation Conference. 2000. Issue relating to a Vibrio vulnificus risk management plan for oysters. Interstate Shellfish Sanitation Conference, Columbia, S.C.
- 22.Jones D. D., and A. K. Bej. 1994. Applications of polymerase chain reaction (PCR) in food microbiology, p. 341-365. In H. Griffin and A. Griffin (ed.), PCR technology: current innovations. CRC Press, Boca Raton, Fla.
- 23.Jothikumar, N., and M. W. Griffiths. 2002. Rapid detection of Escherichia coli O157:H7 with multiplex real-time PCR assays. Appl. Environ. Microbiol. 68:3169-3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaysner, C. A., and A. DePaola, Jr. 2001. Vibrio, p. 405-420. In F. P. Downes and K. Ito (ed.), Compendium of methods for the microbiological examination of food. American Public Health Association, Washington, D.C.
- 25.Koenig, K. L., J. Mueller, and T. Rose. 1991. Vibrio vulnificus. Hazard on the half shell. West. J. Med. 155:421-422. [PMC free article] [PubMed] [Google Scholar]
- 26.Kumamoto, K. S., and D. J. Vukich. 1998. Clinical infections of Vibrio vulnificus: a case report and review of the literature. J. Emerg. Med. 16:61-66. [DOI] [PubMed] [Google Scholar]
- 27.Lee, S. E., S. Y. Kim, S. J. Kim, H. S. Kim, J. H. Shin, S. H. Choi, S. S. Chung, and J. H. Rhee. 1998. Direct identification of Vibrio vulnificus in clinical specimens by nested PCR. J. Clin. Microbiol. 36:2887-2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Linkous, D. A., and J. D. Oliver. 1999. Pathogenesis of Vibrio vulnificus. FEMS Microbiol. Lett. 174:207-214. [DOI] [PubMed] [Google Scholar]
- 29.Logan, J. M., K. J. Edwards, N. A. Saunders, and J. Stanley. 2001. Rapid identification of Campylobacter spp. by melting peak analysis of biprobes in real-time PCR. J. Clin. Microbiol. 39:2227-2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Malinen, E., A. Kassinen, T. Rinttila, and A. Palva. 2003. Comparison of real-time PCR with SYBR Green I or 5′-nuclease assays and dot-blot hybridization with rDNA-targeted oligonucleotide probes in quantification of selected fecal bacteria. Microbiology 149:269-277. [DOI] [PubMed] [Google Scholar]
- 31.Mead, P. S., L. Slutsker, P. M. Griffin, and R. V. Tauxe. 1999. Food-related illness and death in the United States. Reply to Dr. Hedberg. Emerg. Infect. Dis. 5:841-842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 33.Morris, J. G. J., A. C. Wright, D. M. Roberts, P. K. Wood, L. M. Simpson, and J. D. Oliver. 1987. Identification of environmental Vibrio vulnificus isolates with a DNA probe for the cytotoxin-hemolysin gene. Appl. Environ. Microbiol. 53:193-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morrison, T. B., Y. Ma, J. H. Weis, and J. J. Weis. 1999. Rapid and sensitive quantification of Borrelia burgdorferi-infected mouse tissues by continuous fluorescent monitoring of PCR. J. Clin. Microbiol. 37:987-992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mullis, K. B., and F. A. Faloona. 1987. Specific synthesis of DNA in vitro via a polymerase-catalyzed chain reaction. Methods Enzymol. 155:335-350. [DOI] [PubMed] [Google Scholar]
- 36.Olga Perez-Moreno, M. M., G. Romera, G. Pous, A. M. Jardi, J. Zaragoza, J. I. Buj, and F. J. Perez. 1996. Bacteremia caused by Vibrio vulnificus in a patient with a skin ulcer exposed to sea water. Enferm. Infecc. Microbiol. Clin. 14:512-513. [PubMed] [Google Scholar]
- 37.Oliver, J. D. 1989. Vibrio vulnificus, p. 552-554. In M. P. Doyle (ed.), Foodborne bacterial pathogens. Marcel Dekker Inc., New York, N.Y.
- 38.Oliver, J. D., R. A. Warner, and D. R. Cleland. 1982. Distribution and ecology of Vibrio vulnificus and other lactose-fermenting marine vibrios in coastal waters of the southeastern United States. Appl. Environ. Microbiol. 44:1404-1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oliver, J. D., R. A. Warner, and D. R. Cleland. 1983. Distribution of Vibrio vulnificus and other lactose-fermenting vibrios in the marine environment. Appl. Environ. Microbiol. 45:985-998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.O'Mahony, J., and C. Hill. 2002. A real time PCR assay for the detection and quantitation of Mycobacterium avium subsp. paratuberculosis using SYBR Green and the Light Cycler. J. Microbiol. Methods 51:283-293. [DOI] [PubMed] [Google Scholar]
- 41.Patel, V. J., E. Gardner, and C. S. Burton. 2002. Vibrio vulnificus septicemia and leg ulcer. J. Am. Acad. Dermatol. 46:S144-145. [DOI] [PubMed] [Google Scholar]
- 42.Peeler, J. T., G. A. Houghtby, and A. P. Rainosek. 1992. The most probable number technique, p. 105-120. In F. P. Downes and K. Ito (ed.), Compendium of methods for the microbiological examination of food. American Public Health Association, Washington, D.C.
- 43.Penman, A. D., D. C. J. Lanier, W. T. R. Avara, K. E. Canant, J. W. DeGroote, B. T. Brackin, M. M. Currier, and R. L. Hotchkiss. 1993. Vibrio vulnificus wound infections from the Mississippi Gulf coastal waters: June to August 1993. South. Med. J. 88:531-533. [DOI] [PubMed] [Google Scholar]
- 44.Potasman, I., A. Paz, and M. Odeh. 2002. Infectious outbreaks associated with bivalve shellfish consumption: a worldwide perspective. Clin. Infect. Dis. 35:921-928. [DOI] [PubMed] [Google Scholar]
- 45.Pressly, K. B., and L. S. Quattlebaum. 2000. Vibrio vulnificus sepsis. Bay Medical Center, Panama City, Fla., USA. Crit. Care Nurse 20:78-83. [PubMed] [Google Scholar]
- 46.Rantakokko-Jalava, K., and J. Jalava. 2001. Development of conventional and real-time PCR assays for detection of Legionella DNA in respiratory specimens. J. Clin. Microbiol. 39:2904-2910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reed, K. C., M. C. Crowell, M. D. Castro, and M. L. Sloan. 1999. Skin and soft-tissue infections after injury in the ocean: culture methods and antibiotic therapy for marine bacteria. Mil. Med. 164:198-201. [PubMed] [Google Scholar]
- 48.Simpson, D. A. C., S. Feeney, C. Boyle, and A. W. Stitt. 2000. Retinal VEGF mRNA measured by SYBR Green I fluorescence: a versatile approach to quantitative PCR. Mol. Vision. 6:178-183. [PubMed] [Google Scholar]
- 49.Stabellini, N., A. Camerani, D. Lambertini, M. R. Rossi, V. Bettoli, I. A. Virgil, and P. Gilli. 1998. Fatal sepsis from Vibrio vulnificus in a hemodialyzed patient. Nephron 78:221-224. [DOI] [PubMed] [Google Scholar]
- 50.Sustainable Coastal Margins and Program. 2003. Coastal resources of the state of Texas: economic impact. Texas A&M University, College Station, Tex.
- 51.Tamplin, M. L., A. L. Martin, A. D. Ruple, D. W. Cook, and C. W. Kasper. 1991. Enzyme immunoassay for identification of Vibrio vulnificus in seawater, sediment, and oysters. Appl. Environ. Microbiol. 57:1235-1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wright, A. C., J. G. J. Morris, D. R. J. Maneval, K. Richardson, and J. B. Kaper. 1985. Cloning of the cytotoxin-hemolysin gene of Vibrio vulnificus. Infect. Immun. 50:922-924. [DOI] [PMC free article] [PubMed] [Google Scholar]