Summary
Patients with common variable immunodeficiency (CVID) are at high risk of developing immune thrombocytopenia (ITP) and/or autoimmune haemolytic anaemia (AHA). Given their underlying immunodeficiency, immunosuppressive treatment of these manifestations may increase the risk of infection. To assess efficacy and safety of rituximab in patients with CVID-associated ITP/AHA, a multicentre retrospective study was performed. Thirty-three patients, 29 adults and four children, were included. Patients received an average of 2·6 treatments prior to rituximab including steroids, intravenous immunoglobulin and splenectomy (21%). The median ITP/AHA duration at time of first rituximab administration was 12 months [range 1–324] and the indication for using rituximab was ITP (22 cases), AHA (n = 5) or both (n = 7); 1 patient was treated sequentially for ITP and then AHA. The overall initial response rate to rituximab was 85% including 74% complete responses. After a mean follow-up of 39 ± 30 months after rituximab first administration, 10 of the initial responders relapsed and re-treatment with rituximab was successful in 7/9. Severe infections occurred after rituximab in eight adults (24%), four of whom were not on immunoglobulin replacement therapy. In conclusion, rituximab appears to be highly effective and relatively safe for the management of CVID-associated severe immune cytopenias.
Keywords: immune thrombocytopenia, autoimmune haemolytic anaemia, Evans’ syndrome, common variable immunodeficiency, rituximab
Common variable immunodeficiency (CVID) is a heterogeneous syndrome of multiple, often unknown aetiologies characterized by hypogammaglobulinaemia, inability to respond to vaccines, and recurrent bacterial infections (Sicherer & Winkelstein, 1998). A marked decrease in serum immunoglobulin G (IgG) associated with reductions in serum IgM and/or IgA levels is the hallmark of the disease. In addition to recurrent infections, patients with CVID also are at increased risk of developing autoimmune disease (Sneller et al, 1993; Sicherer & Winkelstein, 1998), demonstrating that CVID is characterized by abnormal immunoregulation as well as immunodeficiency (Strober & Chua, 2000). Approximately 20–22% of the patients with CVID develop autoimmune disease, among which immune thrombocytopenia (ITP) and/or autoimmune haemolytic anaemia (AHA) are pre-eminent (Cunningham-Rundles & Bodian, 1999; Cunningham-Rundles, 2002; Chapel et al, 2008; Oksenhendler et al, 2008). The management of CVID-associated cytopenias has been empiric and usually extrapolated from the standard of care of ITP and/or AHA. Corticosteroids are thus commonly used as first-line therapy with good initial response rates (85% for ITP (Michel et al, 2004) and 81% for AHA (Seve et al, 2008)) but their long term use may increase the underlying risk of infections and rates of lasting remission are not well documented. Patients may be on intravenous or subcutaneous immunoglobulin (Ig) for prophylaxis of infections (400 mg/kg every 4 weeks) and may be treated with higher dose (1–2 g/kg) intravenous immunoglobulin (IVIg) upon development of their ITP/AH, although the efficacy of IVIg for ITP and AHA has not been fully demonstrated in this setting. Second-line therapies include vinca-alkaloids, danazol, or intravenous anti-D immunoglobulin (for ITP). For the chronic and most severe forms of ITP/AHA, splenectomy is an option, with reported response rates ranging from 60 to 80% (Michel et al, 2004; Wang & Cunningham-Rundles, 2005; Seve et al, 2008) but with an increased risk of peri-operative complications and over-whelming post-splenectomy infection. Lastly, the risk to benefit ratio of using cytotoxic (cyclophosphamide) or immunosuppressive drugs (azathioprine, cyclosporin) in this setting is uncertain but worrisome in the absence of other than very limited anecdotal data.
Rituximab is a monoclonal chimeric anti-CD20 antibody that has been licenced for the treatment of B-cell lymphoma and rheumatoid arthritis in many countries. Rituximab is also used off-label for treating chronic, severe and/or refractory forms of primary autoimmune cytopenias as clinical studies have shown initial response rates of up to 40–60% in primary ITP (Arnold et al, 2007; Godeau et al, 2008; Zaja et al, 2010) and even higher in warm AHA (Garvey, 2008; Bussone et al, 2009) with an overall good safety profile. However, the fear of increasing the risk of severe infections with rituximab in patients with baseline hypogammaglobulinaemia probably explains why only five cases of rituximab use in CVID have been reported (Wakim et al, 2004; Carbone et al, 2005; El-Shanawany et al, 2007; Kim et al, 2007). To better assess efficacy and safety of rituximab as a treatment of CVID-associated immune cytopenias, a retrospective multicentre collaborative study was performed.
Patients and methods
Study design
This retrospective study was initiated in France throughout the French network of adult primary immune deficiencies [CEREDIH (Centre de Référence Déficits Immunitaires Héréditaires)/DEFI (Deficits Immunitaires)] and the national referral centre for adult immune cytopenias. The study was then extended to the United States in collaboration with the platelet disorders centre of the Weill Cornell Medical College and the referral centre for primary immunodeficiences of the Mount Sinai Hospital both in New York City. Clinical and biological data were collected using the same standardized form for every patient. For all patients, follow-up began at the initiation of rituximab, and ended either at the date of death, or of last visit, or at the latest on April 2011, when the study period ended. Status at the end of follow-up (dead, in remission, in relapse) was systematically recorded.
Criteria of eligibility
To be included, patients had to fulfil the following criteria: (i) Diagnosis of CVID according to the European Society for Immunodeficiencies/Pan-American Group for Immunodeficiency (ESID/PAGID) criteria (Conley et al, 1999), (ii) History of ITP and/or AHA and (iii) Treatment with rituximab given specifically for the management of CVID-associated ITP and/or AHA, regardless of dose. Patients in whom hypogammaglobulinaemia was discovered only after treatment with rituximab were not included.
Secondary (CVID-associated) ITP was defined according to the recently published standardized international criteria (Rodeghiero et al, 2009). AHA was defined as a haemoglobin level ≤100 g/l with a positive direct antiglobulin test (DAT) and evidence of haemolysis. Evans’ syndrome was defined by the simultaneous or sequential occurrence of both AHA and ITP (Michel et al, 2009). Only the main indication(s) of rituximab (i.e. ITP, AHA or both) were considered and analysed in patients with Evans’ syndrome.
Response criteria
To assess treatment efficacy, the following criteria were used: for ITP, a complete response (CR) was defined by a platelet count above 100 × 109/l and a response (R) by a platelet count >30 × 109/l with at least a two-fold increase of the pre-treatment count according to the consensus international criteria (Rodeghiero et al, 2009). For AHA, a CR was defined by a haemoglobin (Hb) level ≥120 g/l in the absence of transfusion and without persistent features of haemolysis, and a response (R) by a Hb level ≥100 g/l with an increase of at least 20 g/l from baseline; for the latter, there could be persistent haemolysis if the haemoglobin was stable. Both CR and R could be achieved in response to rituximab only in patients who did not receive any other medication but substitutive IVIg and/or corticosteroids at stable or decreasing doses. A durable response to rituximab was defined as a R or CR lasting more than12 months.
To assess safety, every episode of severe infection occurring after rituximab treatment was recorded, severe infection being defined by an infection requiring in-patient therapy with intravenous antibiotics.
Statistics
Descriptive statistics included means [± standard deviation (SD)] or median (min-max) as appropriate for continuous variables and frequency (percentage) for categorical variables. Rate of initial response to rituximab was compared according to the characteristics of patients using Fisher exact tests for qualitative variables and Mann–Whitney tests for continuous variables. Relapse-free survival rates were estimated according to the Kaplan-Meier method. Relapse-free survival rates were compared according to the characteristics of patients using log rank tests and/or univariate proportional hazard Cox model. Hypothesis of proportional hazard was verified. Due to the small number of events, multivariate analyses were not performed. Categorical variables between groups were compared using the chi-square or Fisher exact test. For all analyses, α risk was set at 5%. All analyses were performed with R software version 2.10.0 (Vienna, Austria).
This retrospective study was performed in accordance with the ethical standards of the Helsinki declaration and was approved by the institutional review boards of the three study hospitals: Henri Mondor, New York Presbyterian, and Mt Sinai.
Results
Patient characteristics (CVID)
Thirty-three patients, 29 adults and four children (48% females), fulfilling the inclusion criteria were included in France (n = 17) and in the United States (n = 16). The mean age at diagnosis of CVID was 35 years ± 15 in adults and 8 ± 5 in children. All patients were of white Caucasian origin, consanguinity was present in only one patient. Eighteen patients (55%) presented with concomitant or sequential ITP and AHA defining Evans’ syndrome, 12 (36%) had solely ITP and 3 (9%) had AHA. The overall rate of recurrent infections (mainly of the upper respiratory tract) was 61%; splenomegaly and/or lymphoid hyperplasia were present in 41% of patients and granulomatous disease in 13%. The baseline characteristics of CVID are described in Table I.
Table I.
Baseline characteristics of the 33 patients.
| Case/sex | Cytopenia treated with rituximab | Age (years) at time of rituximab treatment | IgG/IgA/IgM levels at CVID diagnosis (g/l) ★ | Other autoimmune manifestations | Other CVID-related manifestations |
|---|---|---|---|---|---|
| 1/M | ITP | 57 | 5·6/<0·4/<0·4 | AHA | – |
| 2/M | ITP | 31 | 5·1/0·6/0·22 | AI neutropenia | Recurrent infections, lymphoid hyperplasia |
| 3/F | ITP | 35 | 3·4/0·10/0·60 | AHA | Pulmonary granulomatosis, lymphoid hyperplasia |
| 4/F | ITP | 21 | 1·2/<0·04/<0·01 | AHA | Pulmonary granulomatosis, splenomegaly |
| 5/F | ITP | 32 | 2·9/0·24/0·26 | – | Pneumonia bronchectasis |
| 6/M | ITP | 52 | 5·2/0·8/0·8 | AI thyroiditis, AHA | – |
| 7/F | ITP | 32 | 2·8/0·6/0·06 | AI thyroiditis | – |
| 8/M | ITP | 28 | 0·2/0·9/0·6 | – | – |
| 9/M | ITP then AHA | 40/42 | 3·4/0·05/0·05 | – | Recurrent infections |
| 10/M | ITP | 60 | 5·98/0·07/0·57 | – | – |
| 11/M | ITP | 22 | 2·61/0·8/0·42 | AHA | – |
| 12/M | ITP | 18 | 4·45/0·14/0·06 | – | Recurrent infections |
| 13/F | ITP | 23 | 0·3/0·14/0·15 | – | Recurrent infections |
| 14/M | ITP | 44 | 3·3/0·1/1 | – | – |
| 15/M | ITP | 49 | 4·5/0·6/<0·4 | AHA | Recurrent infections, lymphoid hyperplasia |
| 16/M | ITP | 36 | 3·13/0·39/0·57 | AHA | MALT lymphoma |
| 17/F | ITP | 19 | 4·92/0·4/0·6 | Celiac disease, vitiligo | Systemic granulomatosis |
| 18/M | ITP | 36 | 4·9/2·05/0·3 | – | – |
| 19/M | ITP | 33 | 4·6/0·34/0·21 | – | – |
| 20/F | ITP | 65 | 4·59/0·28/1·46 | – | – |
| 21/F | AHA | 29 | 5/<0·1/1·3 | Celiac disease, ITP | Bronchectasis, systemic granulomatosis, lymphoid hyperplasia |
| 22/F | AHA | 51 | 3·7/0·24//0·38 | – | Splenomegaly |
| 23/M | AHA | 52 | 4·07/<0·5/<0·9 | Pernicious anaemia, celiac disease | Recurrent infections, exsudative enteropathy, lymphoid hyperplasia |
| 24/F | AHA | 60 | 4·5/0·15/NA | AI Hepatitis | Recurrent infections, bronchectasis, cholestatic hepatitis |
| 25/F | Concomitant ITP and AHA | 50 | 2·9/0·36/0·31 | – | Recurrent infections, lymphoid hyperplasia |
| 26/F | Concomitant ITP and AHA | 28 | 0·08/0·28/0·2 | – | Recurrent infections, splenomegaly Pulmonary granulomatosis |
| 27/F | Concomitant ITP and AHA | 29 | 4·9/0·42/1·28 | – | Recurrent infections |
| 28/M | Concomitant ITP and AHA | 76 | 1·8/0·3/<0·1 | Pernicious anaemia | Recurrent infections, bronchectasis, MALT lymphoma |
| 29/F | Concomitant ITP and AHA | 45 | 2·8/0·05/0·24 | – | Recurrent infections, lymphoid hyperplasia |
| 30/F | Concomitant ITP and AHA | 11 | 0·4/0·2/0·3 | – | Recurrent infections, granulomatosis, lymphoid hyperplasia |
| 31/F | Concomitant ITP and AHA | 6 | 5/0·9/0·1 | – | Recurrent infections, splenomegaly |
| 32/M | ITP | 12 | 0·7/0·1/0·06 | AI neutropenia, type I diabete mellitus | Recurrent infections |
| 33/M | ITP | 13 | 3·64/0·07/0·57 | AI neutropenia, AHA | Recurrent infections |
CVID, common variable immune deficiency; AHA, autoimmune haemolytic anaemia; ITP, immune thrombocytopenia; AI, auto-immune; MALT, mucosa-associated lymphoid tissue; NA, not available.
Normal values (ranges): IgG = 6·5–14 g/l; IgA = 0·7–3·2 g/l; IgM = 0·5–3·5 g/l.
Characteristics of cytopenias
Overall, 34 episodes of immune cytopenia were treated with rituximab, one patient with Evans’ syndrome was treated sequentially first for ITP and then (2 years later) for AHA. Rituximab was used to treat ITP in 22 cases, AHA in five and concomitant ITP and AHA in seven cases. The mean age at ITP/AHA diagnosis was 29 years ± 18 among adults, and 7 years ± 4 among children. The median ITP/AHA duration at time of first rituximab administration was 12 months [range 1–324]. The mean lowest platelet count prior to rituximab administration in patients treated for ITP was 13 ± 12 × 109/l; 6/22 (27%) patients had mucosal bleeding. Among patients treated for AHA, the mean lowest haemoglobin level was 65 ± 23 g/l. Two patients were admitted to the intensive care unit because of severe anaemia. (Tables II–IV).
Table II.
Patterns of response and outcome of ITP cases in adults (n = 20 cases).
| Case | ITP duration (months) before rituximab | Previous treatments | Platelet count (×109/l) prior to rituximab | Initial response to rituximab | Severe infection after rituximab (time after 1st infusion) | Substitutive immunoglobulin/Antibiotic prophylaxis | Follow up after rituximab/ITP status at the end of study |
|---|---|---|---|---|---|---|---|
| 1 | 17 | Corticosteroids, IVIg, vincristine, danazol | 9 | CR | No | No/no | 64 months/CR |
| 2 | 216 | Corticosteroids, MTX, CYP, IVIg, ciclosporin, danazol, splenectomy | 74 | CR | No | Yes/no | 34 months/CR |
| 3 | 2 | Corticosteroids, IVIg, platelet transfusion, danazol | 3 | CR | No | Yes/no | 64 months/CR |
| 4 | 108 | Corticosteroids | 5 | CR | Salmonellosis (27 months) | No★/no | 30 months/CR |
| 5 | 72 | Corticosteroids, splenectomy | 69 | CR | No | Yes/no | 22 months/CR |
| 6 | 288 | Corticosteroids, IVIg | 2 | CR | No | Yes/yes | 30 months/CR |
| 7 | 11 | Corticosteroids, IVIg | 12 | CR | No | No/no | 10 months/CR |
| 8 | 24 | Corticosteroids, IVIg, Ig anti D | 51 | CR | No | No/no | 12 months/CR |
| 9 | 6 | Corticosteroids IVIg, vincristin | 20 | CR | No | Yes/no | 52 months/CR |
| 10 | 2 | Corticosteroids, IVIg | 20 | CR | No | No/no | 19 months/CR |
| 11 | 220 | Corticosteroids, IVIg, splenectomy | 12 | CR | No | No/no | 112 months/2 relapses, normal platelet count with maintenance infusions of rituximab |
| 12 | 132 | Corticosteroids, IVIg, Ig anti D | 12 | CR | No | Yes/no | 71 months/normal platelets after re-treatment for 2 relapse |
| 13 | 12 | Corticosteroids, IVIg, Ig anti D | 36 | CR | No | Yes/no | 73 months/normal platelets after re-treatment for a relapse |
| 14 | 84 | Corticosteroids, IVIg, danazol | 3 | CR | No | No/no | 43 months/failure of re-treatment for a relapse, normal platelet count on corticosteroids |
| 15 | 122 | Corticosteroids, IVIg | 6 | CR | Orchiepididimytis | Yes/no | 42 months/normal platelets after a relapse treated with corticosteroids |
| 16 | 288 | Corticosteroids, splenectomy, IVIg | 4 | CR | S. pneumoniae pneumonia (M4) | No†/no | 4 months/Death |
| 17 | 10 | Corticosteroids, IVIg, platelet transfusion, vincristine, vinblastine | 14 | R |
S. maltophila (week 1) and S. paucimobilis (M44) septicemia Salmonellosis (M60) |
Yes‡/no | 78 months/CR ongoing prednisone for granulomatous disease |
| 18 | 12 | Corticosteroids, IVIg | 8 | No response | No | No/no | 11 months/normal platelet count on romiplostim |
| 19 | 324 | Corticosteroids, IVIg, Ig anti D | 6 | No response (after 1 perfusion) | No | No/no | 12 months/normal platelet count after splenectomy |
| 20 | 3 | Corticosteroids, IVIg, Ig anti D | 8 | No response | No | Yes/no | 26 months/Failed eltrombopag and romiplsotim, normal platelet count after splenectomy |
ITP, immune thrombocytopenia; IVIg, intravenous immunoglobulin; MTX, methotrexate; CYP, cyclophosphamide; CR, complete response; R, response.
Gammaglobulin level at time of infection = 0·5 g/l.
Gammaglobulin level at time of infection = 3·3 g/l.
Residual gammaglobulin level at time of infection = 7·6 g/l.
Table IV.
Patterns of response and outcome of ITP/AHA in children (n = 4 cases).
| Case | Duration (months) of ITP/AHA before rituximab | Previous treatments | Platelet count/Hb level prior to rituximab | Initial response to rituximab | Severe infection after rituximab | Substitutive Immunoglobulin/antibiotic prophylaxis | Follow up after rituximab/disease status at the end of study |
|---|---|---|---|---|---|---|---|
| 30 | 96 | Corticosteroids, IVIg, PRBC transfusion | 5 × 109/l 108 g/l | CR | No | Yes/no | 12 months/CR |
| 31 | 6 | Corticosteroids, IVIg | 22 × 109/l 93 g/l | CR | No | Yes/no | 60 months/CR after re-treatment with rituximab for 2 relapses |
| 32 | 60 | IVIg | 23 × 109/l | CR | No | Yes/no | 3 months/CR |
| 33 | 12 | Corticosteroids, IVIg, Ig anti D, splenectomy | 10 × 109/l | CR | No | No/no | 96 months/CR after successful re-treatment with rituximab (4 courses) for several relapses |
AHA, autoimmune haemolytic anaemia; ITP, immune thrombocytopenia; IVIg, intravenous immunoglobulin; PRBC, packed red blood cell; CR, complete response; R, response.
Previous treatments for immune cytopenias
On average, patients received 2·6 treatments for the management of ITP or AHA prior to rituximab. Of 34 episodes of cytopenia, 31 (91%) were treated with at least one course of corticosteroids as first-line therapy; continuous or intermittent treatment with corticosteroids was required to maintain a response in 19 of these 34 cases (56%). IVIg was given at a dose of 1 g/kg or higher in 28/34 (82%) cases. Seven patients (21%) including four patients with Evans’s syndrome had previously undergone splenectomy, which lead to a transient initial response in 6 cases. Relapse of cytopenias occurred in all cases after splenectomy, after a mean interval of 49 months. Fifteen patients were treated with immunomodulatory drugs (vinca alkaloids, danazol, anti D immunoglobulin), three with immunosuppressives (cyclophosphamide, cyclosporin, azathioprine), and one with romiplostim for chronic severe ITP. (Tables II–IV).
Rituximab dosing
Rituximab was usually given at standard dose, namely 375 mg/m2 once a week for 4 weeks in 31 cases, at 1 g on days 1 and 15 in two cases, and one patient received only a single infusion of rituximab at 375 mg/m2 as severe (transient) pancytopenia occurred within days following the infusion. All patients received premedication with i.v dexchlorpheniramine, acetaminophen and intravenous methylprednisolone or oral prednisone.
Concurrent medications (corticosteroids, IVIg, prophylactic antibiotic)
At the time of the first rituximab infusion, concurrent treatment was ongoing in 27 cases including prednisone at a mean daily dose of 34 ± 21 mg in 13 (52%) cases, monthly courses of dexamethasone in 6 (24%) cases, high dose IVIg alone (7 cases) and romiplostim in one case. Seventeen of the 33 (52%) patients were on Ig replacement therapy (300–500 mg/kg every 3–4 weeks) at the time of the initial rituximab administration, and four patients were receiving antibiotic phophylaxis.
Rates and patterns of initial and lasting response to rituximab in adults
After a median delay of 4 weeks [range 2–8 weeks] following the first infusion of rituximab, a CR was observed in 21 of the 30 cases (70%) of cytopenias including 16 of 20 ITP cases (80%), four of five AHAs (80%) and one among the five patients treated for concomitant cytopenias. (Tables II–IV, Fig 1). Four patients (one with ITP and three with concomitant cytopenias) achieved a response (R) (Table IV). In total, an overall response (R + CR) was observed in 25 of 30 cases (83%) in adults. Five patients did not achieve any response to rituximab, three of these patients eventually underwent splenectomy, which was successful in all cases (3 CR).
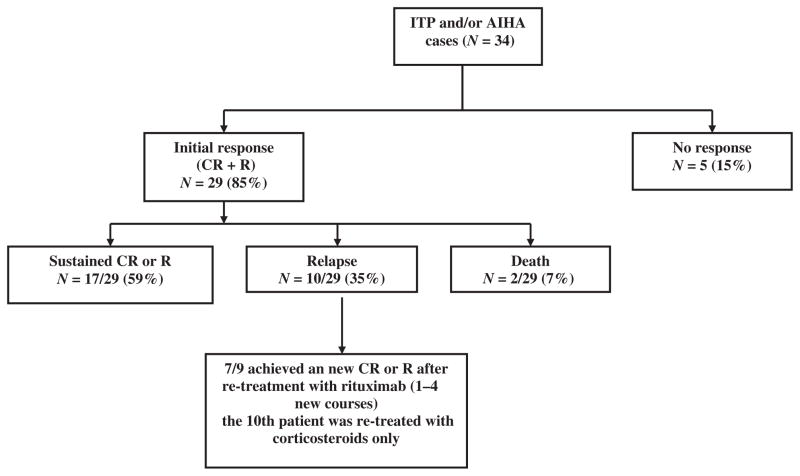
Fig. 1.
Overall response after rituximab (mean follow-up after rituximab 1st administration = 39 ± 30 months). AHA, autoimmune haemolytic anaemia; ITP, immune thrombocytopenia; CR, complete response; R, response.
After a mean follow up of 39 ± 30 months following the first administration of rituximab, the overall rate of durable response was 50% (60% among the initial responders). Two patients had died within 12 months after receiving rituximab (see below) and eight among initial responders relapsed after a mean of 31 ± 24 months from the first rituximab infusion. Among the five patients with ITP who relapsed, four were successfully re-treated with rituximab (Table II).
Initial and long term response among children
After a mean delay of 4 weeks, all the four children treated with rituximab (two with ITP and two with concomitant ITP and AHA) achieved a CR. The two treated for ITP relapsed (at 36 and 48 months, respectively, after the first rituximab infusion) and were successfully re-treated with rituximab. One child who experienced four relapses was successfully retreated with rituximab each time. He is currently treated with a single infusion of rituximab at 375 mg/m2 every 4 months as a maintenance therapy. (Table IV).
In total by pooling the data of adults and children, an initial response (CR + R) was achieved in 29/34 cases (85%), including 86% of ITP cases (19/22), 80% of AHAs (4/5) and 86% of concomitant cytopenias (6/7). The overall rate of durable response was 50% (17/34), and reached 59% among initial responders (see Fig 1). Among responders, corticosteroids could be stopped in 13 of the 19 (72%) patients who were receiving concomitant treatment with either continuous (prednisone) or intermittent (dexamethasone) corticosteroids at time of first rituximab administration.
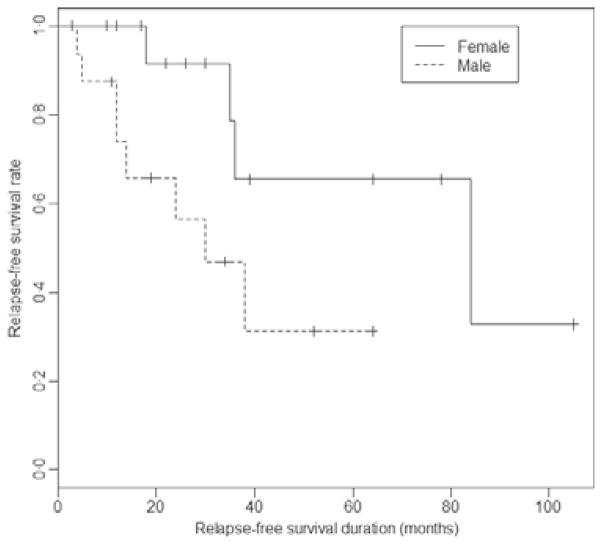
None of the characteristics (age, sex, time from diagnosis) was statistically different when initial responders and non-responders were compared (Table V), whereas among responders the rate of relapse over time was significantly higher in males (Fig 2). Except for gender, none of the other parameters (age, characteristics of CVID, time elapsed between ITP/AHA onset and rituximab) was significantly associated with the duration of response (data not shown).
Table V.
Comparison of main characteristics between responders and non-responders.
| Characteristics | Initial response to rituximab N = 28 | No response to rituximab N = 5 | P value |
|---|---|---|---|
| Age, n (%) | |||
| Child | 4 (14) | 0 (0) | 1 |
| Adult | 24 (86) | 5 (100) | |
| Sex, n (%) | |||
| Female | 13 (46) | 3 (60) | 0·6 |
| Male | 15 (54) | 2 (40) | |
| Type of cytopenia, n (%) | |||
| ITP | 19 (68) | 3 (60) | 0·8 |
| AHA | 3 (11) | 1 (20) | |
| Evans syndrome | 6 (21) | 1 (20) | |
| Other auto-immune manifestations, n (%) | |||
| Yes | 15 (54) | 1 (20) | 0·3 |
| No | 13 (46) | 4 (80) | |
| Other CVID-associated manifestations, n (%) | |||
| Granuloma | 5 (18) | 0 | 0·6 |
| Splenomegaly | 4 (14) | 0 | 1 |
| Infections | 16 (57) | 2 (40) | 0·6 |
| Lymphoid hyperplasia | 9 (32) | 1 (20) | 1 |
| Ig replacement therapy at the time of rituximab, n (%) | |||
| Yes | 15 (54%) | 2 (40%) | 0·66 |
| No | 13 (46%) | 3 (60%) | |
| Time between diagnosis and Rituximab, median (Q1–Q3) | 12 (5–63) | 24 (7–120) | 0·4 |
CVID, common variable immune deficiency; AHA, autoimmune haemolytic anaemia; ITP, immune thrombocytopenia; Ig, immunoglobulin.
Fig. 2.
Relapse-free survival according to gender. (Log rank test, P = 0·04, univariate HR (men vs women) = 3·8 (95% CI: 1·0–14·3), P = 0·05).
Short and long-term safety
No immediate or post-infusion severe adverse reactions were observed. Eleven episodes of severe infections occurred in eight patients (24%) after rituximab infusions (see Tables II–III for details). Four of these eight patients were on Ig replacement therapy at the time of infection. In the four receiving Ig, the residual level of gammaglobulin at the time of infection was respectively 5·9 g/l, 7·2 g/l, 6·3 g/l and 7·6 g/l but all of them had previously received high dose corticosteroids as well as immunosuppressive agents. Of the four patients not on Ig who developed severe infections, two had previously undergone splenectomy only one of whom received prophylactic antibiotics. In three of the four patients in whom IgG data was available, a significant decrease in gammaglobulin level compared to the pre-rituximab level was seen (mean decrease = 1·7g/l, mean residual IgG level = 3·1 g/l). Overall, the rate of severe infections observed was not statistically different when patients on (n = 20) or off (n = 13) Ig replacement therapy after rituximab were compared (20% vs. 31%, P = 0·68).
Table III.
Patterns of response and outcome of AHA (n = 5) and concomitant cytopenias (n = 5) cases in adults.
| Case/cytopenia | Cytopenia duration (months) before rituximab | Previous treatments | Hb level and/or platelet count before rituximab | Initial response to rituximab | Severe infection after rituximab (time elapsed after the 1st infusion) | Substitutive immunoglobulin/Antibiotic prophylaxis | Follow up after rituximab /Disease status at the end of study |
|---|---|---|---|---|---|---|---|
| 10/AHA | 6 | Corticosteroids IVIg, vincristine | 63 g/l | CR | No | Yes/no | 28 months/CR |
| 21/AHA | 1 | Corticosteroids, CYP, PRBC transfusion | 30 g/l | CR | Pyosalpinx (67 months) | No★/yes | 105 months/CR |
| 22/AHA | 60 | Corticosteroids, IVIg, PRBC transfusion, splenectomy | 70 g/l | CR | No | Yes/no | 44 months/normal Hb after 1 relapse treated with rituximab, ongoing prednisone 7 mg/d |
| 23/AHA | 16 | Corticosteroids, PRBC transfusion | 64 g/l | CR | No | Yes/yes | 24 months/failure of re-treatment with rituximab after relapse – normal Hb after splenectomy |
| 24/AHA | 120 | Corticosteroids, CYP, AZA, danazol | 71 g/l | No response | Pneumonia (1 month) | Yes_/no | 10 months/normal Hb after splenectomy |
| 25/concomitant ITP and AHA | 1 | Corticosteroids, PRBC transfusion | 28 g/l, 2 × 109/l | CR | Meningitis (1 week) | No‡/no | 22 months/CR |
| 26/concomitant ITP and AHA | 2 | Corticosteroids, IVIg, romiplostim | 88 g/l, 16 × 109/l | R | No | Yes/no | 17 months/R |
| 27/concomitant ITP and AHA | 33 | Corticosteroids | 55 g/l, 10 × 109/l | R | No | Yes/yes | 50 months/ongoing rituximab every 3 months after a relapse |
| 28/concomitant ITP and AHA | 7 | Corticosteroids, PRBC transfusion, splenectomy | 65 g/l, 19 × 109/l | CR ITP R AHA | Aspergilloma (11 months) | Yes§/no | 12 months/Death |
| 29/concomitant ITP and AHA | 24 | IVIg, vincristine | 70 g/l, 2 × 109/l | No response | No | Yes/no | 39 months/normal platelet on IVIg repeated infusions |
AHA, autoimmune haemolytic anaemia; ITP, immune thrombocytopenia; IVIg, intravenous immunoglobulin, PRBC, packed red blood cells; CYP, cyclophosphamide; AZA, azathioprine; CR, complete response; R, response; Hb, haemoglobin.
Gammaglobulin level at time of infection = 5·5 g/l.
Residual gammaglobulin level at time of infection = 6·3 g/l.
Gammaglobulin level at time of infection = 4·4 g/l.
Residual gammaglobulin level = 7·2 g/l.
A severe pancytopenia occurred after the first infusion of rituximab in one patient treated for ITP, who eventually achieved a CR after splenectomy. One case of neutropenia of mild severity occurred 3 months after rituximab. The nadir neutrophil count was 1·07 × 109/l and the patient spontaneously recovered within a month without any infection.
Deaths (n = 2)
Two patients died after they had received rituximab treatment. The first patient had been previously heavily treated by corticosteroids and chemotherapy for a diffuse large B-cell - lymphoma (which occurred more than 2 years after the diagnosis of CVID). Ten months after receiving the last rituximab infusion for concomitant ITP and AHA, he developed a pulmonary aspergilloma as a complication of pre-existing bronchiectasis. He was on IVIg when the infection was diagnosed and his IgG level was 7·2 g/l. A lobectomy was performed, and the patient died from post-operative pulmonary haemorrhage despite having a normal platelet count. The second patient had undergone splenectomy 12 years earlier for chronic ITP and was neither on IVIG nor antibiotic prophylaxis. He died from Streptococcus pneumoniae pneumonia with sepsis 4 months after receiving rituximab for a late relapse of ITP. His last IgG level was 3·3 g/l.
Discussion
Treating patients with pre-existing hypogammaglobulinaemia with rituximab could be seen at least as paradoxical, if not contraindicated. Considering the estimated prevalence of CVID (Stray-Pedersen et al, 2000) and the expected rate of associated ITP and/or AHA (Cunningham-Rundles & Bodian, 1999; Chapel et al, 2008; Oksenhendler et al, 2008), this series of 33 patients with CVID treated for 34 episodes of immune cytopenias thus provides substantial new data that may be helpful to the consulting haematologists and immunologists caring for patients with CVID.
Based on this retrospective study, and despite possible bias and confounding factors, the overall safety of rituximab was demonstrated, particularly for subjects on prophylactic Ig. After a mean follow-up of 39 months after the first rituximab infusion, eight of the 33 patients (24%) had experienced a severe infection, a rate of infection comparable to that observed in previously reported series of CVID-associated immune cytopenias (Michel et al, 2004; Seve et al, 2008). In four of these eight patients, the severe infection occurred while the patients were not receiving Ig replacement therapy, two of whom had been previously splenectomized. A substantial decrease in gammaglobulin level from baseline to a level approximately half of the lower limit of normal was observed in these patients after rituximab, which might have increased the risk of infection. It is therefore quite possible that these infections could have been avoided by systematic Ig replacement therapy. If so, the rate of serious infection over 3 years might have been reduced to 12% (4/33).
However, four severe infections occurred after rituximab despite ongoing Ig (mean residual IgG level >5 g/l) and the rate of severe infections in this subgroup of patients on Ig replacement therapy was not significantly lower than the one observed in patients not receiving Ig. The impact of rituximab in these four patients is difficult to assess as they had previously received long term corticosteroids and immunosuppressive therapy, and one of them had experienced severe viral and fungal infections prior to rituximab administration. On the other hand, no severe infections were observed after rituximab in any of the four children who were on prophylactic IVIg. Taken together, even if there are no statistics to support this recommendation, these observations in a group of patients with a low baseline IgG level prompt us to re-enforce the need for systematic use of Ig replacement therapy (400 mg/kg every 3–4 weeks) in every patient with CVID treated with rituximab, even if a CR has been achieved and in the absence of previous recurrent and/or severe infections.
The data in this series show that the overall rate of lasting response observed in CVID-associated ITP was substantially higher than the rates observed in primary ITP (Arnold et al, 2007; Godeau et al, 2008) and, in CVID-associated AHA, was in keeping with the published data for primary or other secondary cases of AHAs (Garvey, 2008; Bussone et al, 2009). In all cases of CR but two, the response to rituximab was achieved within few weeks following the first infusion. Of the eight adults who had an initial CR and then relapsed, re-treatment of rituximab was successful in 6/7. In addition, 13 of 19 responders to rituximab who were on concomitant steroids tolerated complete taper of the steroids.
Given that the mechanisms of action of rituximab in primary autoimmune diseases are far from being well established, in the setting of CVID they can only be speculative. A profound, transient B lymphocyte depletion in the peripheral blood lasting for at least 6 months is commonly observed after rituximab but it is not predictive of either a good or lasting clinical response in most of immune diseases (Cambridge et al, 2003; Edwards & Cambridge, 2006; Smith et al, 2006). In the present study, the impact of rituximab on circulating B CD19+ cells could not be assessed because sequential data on B-cell counts in the peripheral blood after rituximab were lacking in many patients. However, the higher than usual response rate in ITP (Cooper et al, 2004) seems to confirm an important role of abnormal B cells and/or T cells subsets (Boileau et al, 2011). A possible effect on the T cells subsets, including restoration of the Th1/Th2 and cytokine balance (Stasi et al, 2007) and/or the number and/or function of regulatory T cells, have been suggested in patients with primary ITP (Bussel et al, 1991; Stasi et al, 2007; Taylor & Lindorfer, 2008) In CVID, one could also hypothesize that rituximab could reset the deficient effector and regulatory B and T cell networks (Lund & Randall, 2010). In futures studies, a prospective and careful analysis of the impact of rituximab on B and T cells subsets in the peripheral blood over time in patients with underlying CVID would be useful in achieving a better understanding of the mechanisms promoting autoimmunity in patients with CVID.
In conclusion, based on this series of 34 episodes of cytopenias in 33 CVID patients, rituximab was a highly effective and relatively safe option for the management of ITP and AHA in patients with CVID. We therefore believe that this treatment should be considered as the primary second-line treatment, prior to splenectomy and/or immunosuppressors, in this group of patients at high risk of infection and especially in those with AHA or Evans’ syndrome. To minimize the risk of infection, initiating or continuing Ig replacement therapy, is strongly recommended. Whether other measures, such as antibiotic prophylaxis, would be routinely helpful in this setting still needs to be addressed, but they certainly need to be utilized in patients who have undergone splenectomy.
Footnotes
Authorship
DG performed research, collected and analysed the data and wrote the manuscript; MM and OH designed the research, MM also contributed to the analysis and interpretation of the data and the writing of the manuscript as did JB and CCR; JB, CCR, and all others co-authors contributed to patient inclusion and data collection, AD performed statistical analysis.
Conflict of interest
The authors declare no competing financial conflict of interest.
References
- Arnold DM, Dentali F, Crowther MA, Meyer RM, Cook RJ, Sigouin C, Fraser GA, Lim W, Kelton JG. Systematic review: efficacy and safety of rituximab for adults with idiopathic thrombocytopenic purpura. Annals of Internal Medicine. 2007;146:25–33. doi: 10.7326/0003-4819-146-1-200701020-00006. [DOI] [PubMed] [Google Scholar]
- Boileau J, Mouillot G, Gérard L, Carmagnat M, Rabian C, Oksenhendler E, pasquali JL, Korganow AS DEFI study group. Autoimmunity in common variable immunodeficiency: correlation with lymphocyte phenotype in the French DEFI study. Journal of Autoimmunity. 2011;36:25–32. doi: 10.1016/j.jaut.2010.10.002. [DOI] [PubMed] [Google Scholar]
- Bussel JB, Graziano JN, Kimberly RP, Pahwa S, Aledort LM. Intravenous anti-D treatment of immune thrombocytopenic purpura: analysis of efficacy, toxicity, and mechanism of effect. Blood. 1991;77:1884–1893. [PubMed] [Google Scholar]
- Bussone G, Ribeiro E, Dechartres A, Viallard JF, Bonnotte B, Fain O, Godeau B, Michel M. Efficacy and safety of rituximab in adults’ warm antibody autoimmune haemolytic anemia: retrospective analysis of 27 cases. American Journal of Hematology. 2009;84:153–157. doi: 10.1002/ajh.21341. [DOI] [PubMed] [Google Scholar]
- Cambridge G, Leandro MJ, Edwards JC, Ehrenstein MR, Salden M, Bodman-Smith M, Webster AD. Serologic changes following B lymphocyte depletion therapy for rheumatoid arthritis. Arthritis & Rheumatism. 2003;48:2146–2154. doi: 10.1002/art.11181. [DOI] [PubMed] [Google Scholar]
- Carbone J, Escudero A, Mayayo M, Ballesteros M, Perez-Corral A, Sanchez-Ramon S, Sarmiento E, Micheloud D, Fernandez-Cruz E. Partial response to anti-CD20 monoclonal antibody treatment of severe immune thrombocytopenic purpura in a patient with common variable immunodeficiency. Annals New York Academy of Sciences. 2005;1051:666–671. doi: 10.1196/annals.1361.111. [DOI] [PubMed] [Google Scholar]
- Chapel H, Lucas M, Lee M, Bjorkander J, Webster D, Grimbacher B, Fieschi C, Thon V, Abedi MR, Hammarstrom L. Common variable immunodeficiency disorders: division into distinct clinical phenotypes. Blood. 2008;112:277–286. doi: 10.1182/blood-2007-11-124545. [DOI] [PubMed] [Google Scholar]
- Conley ME, Notarangelo LD, Etzioni A. Diagnostic criteria for primary immunodeficiencies. Representing PAGID (Pan-American Group for Immunodeficiency) and ESID (European Society for Immunodeficiencies) Clinical Immunology. 1999;93:190–197. doi: 10.1006/clim.1999.4799. [DOI] [PubMed] [Google Scholar]
- Cooper N, Stasi R, Cunningham-Rundles S, Feuerstein MA, Leonard JP, Amadori S, Bussel JB. The efficacy and safety of B-cell depletion with anti-CD20 monoclonal antibody in adults with chronic immune thrombocytopenic purpura. British Journal of Haematology. 2004;125:232–239. doi: 10.1111/j.1365-2141.2004.04889.x. [DOI] [PubMed] [Google Scholar]
- Cunningham-Rundles C. Hematologic complications of primary immune deficiencies. Blood Reviews. 2002;16:61–64. doi: 10.1054/blre.2001.0185. [DOI] [PubMed] [Google Scholar]
- Cunningham-Rundles C, Bodian C. Common variable immunodeficiency: clinical and immunological features of 248 patients. Clinical Immunology. 1999;92:34–48. doi: 10.1006/clim.1999.4725. [DOI] [PubMed] [Google Scholar]
- Edwards JC, Cambridge G. B-cell targeting in rheumatoid arthritis and other autoimmune diseases. Nature Reviews Immunology. 2006;6:394–403. doi: 10.1038/nri1838. [DOI] [PubMed] [Google Scholar]
- El-Shanawany TM, Williams PE, Jolles S. Response of refractory immune thrombocytopenic purpura in a patient with common variable immunodeficiency to treatment with rituximab. Journal of Clinical Pathology. 2007;60:715– 716. doi: 10.1136/jcp.2006.041426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garvey B. Rituximab in the treatment of autoimmune haematological disorders. British Journal of Haematology. 2008;141:149–169. doi: 10.1111/j.1365-2141.2008.07054.x. [DOI] [PubMed] [Google Scholar]
- Godeau B, Porcher R, Fain O, Lefrere F, Fenaux P, Cheze S, Vekhoff A, Chauveheid MP, Stirnemann J, Galicier L, Bourgeois E, Haiat S, Varet B, Leporrier M, Papo T, Khellaf M, Michel M, Bierling P. Rituximab efficacy and safety in adult splenectomy candidates with chronic immune thrombocytopenic purpura: results of a prospective multicenter phase 2 study. Blood. 2008;112:999–1004. doi: 10.1182/blood-2008-01-131029. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Thrasher AJ, Jones AM, Davies EG, Cale CM. Rituximab for the treatment of autoimmune cytopenias in children with immune deficiency. British Journal of Haematology. 2007;138:94–96. doi: 10.1111/j.1365-2141.2007.06616.x. [DOI] [PubMed] [Google Scholar]
- Lund F, Randall TE. effector and regulatory B cells: modulators of CD4+ T cell immunity. Nature Reviews Immunology. 2010;4:236–247. doi: 10.1038/nri2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel M, Chanet V, Galicier L, Ruivard M, Levy Y, Hermine O, Oksenhendler E, Schaeffer A, Bierling P, Godeau B. Autoimmune thrombocytopenic purpura and common variable immunodeficiency: analysis of 21 cases and review of the literature. Medicine (Baltimore) 2004;83:254–263. doi: 10.1097/01.md.0000133624.65946.40. [DOI] [PubMed] [Google Scholar]
- Michel M, Chanet V, Dechartres A, Morin AS, Piette JC, Cirasino L, Emilia G, Zaja F, Ruggeri M, Andres E, Bierling P, Godeau B, Rodeghiero F. The spectrum of Evans syndrome in adults: new insight into the disease based on the analysis of 68 cases. Blood. 2009;114:3167–3172. doi: 10.1182/blood-2009-04-215368. [DOI] [PubMed] [Google Scholar]
- Oksenhendler E, Gerard L, Fieschi C, Malphettes M, Mouillot G, Jaussaud R, Viallard JF, Gardembas M, Galicier L, Schleinitz N, Suarez F, Soulas-Sprauel P, Hachulla E, Jaccard A, Gardeur A, Theodorou I, Rabian C, Debre P. Infections in 252 patients with common variable immunodeficiency. Clinical Infectious Diseases. 2008;46:1547–1554. doi: 10.1086/587669. [DOI] [PubMed] [Google Scholar]
- Rodeghiero F, Stasi R, Gernsheimer T, Michel M, Provan D, Arnold DM, Bussel JB, Cines DB, Chong BH, Cooper N, Godeau B, Lechner K, Mazzucconi MG, McMillan R, Sanz MA, Imbach P, Blanchette V, Kuhne T, Ruggeri M, George JN. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group. Blood. 2009;113:2386–2393. doi: 10.1182/blood-2008-07-162503. [DOI] [PubMed] [Google Scholar]
- Seve P, Bourdillon L, Sarrot-Reynauld F, Ruivard M, Jaussaud R, Bouhour D, Bonotte B, Gardembas M, Poindron V, Thiercelin MF, Broussolle C, Oksenhendler E. Autoimmune hemolytic anemia and common variable immunodeficiency: a case-control study of 18 patients. Medicine (Baltimore) 2008;87:177–184. doi: 10.1097/MD.0b013e31817a90ba. [DOI] [PubMed] [Google Scholar]
- Sicherer SH, Winkelstein JA. Primary immunodeficiency diseases in adults. Journal of the American Medical Association. 1998;279:58–61. doi: 10.1001/jama.279.1.58. [DOI] [PubMed] [Google Scholar]
- Smith KG, Jones RB, Burns SM, Jayne DR. Long-term comparison of rituximab treatment for refractory systemic lupus erythematosus and vasculitis: remission, relapse, and re-treatment. Arthritis & Rheumatism. 2006;54:2970– 2982. doi: 10.1002/art.22046. [DOI] [PubMed] [Google Scholar]
- Sneller MC, Strober W, Eisenstein E, Jaffe JS, Cunningham-Rundles C. NIH conference. New insights into common variable immunodeficiency. Annals of Internal Medicine. 1993;118:720–730. doi: 10.7326/0003-4819-118-9-199305010-00011. [DOI] [PubMed] [Google Scholar]
- Stasi R, Del Poeta G, Stipa E, Evangelista ML, Trawinska MM, Cooper N, Amadori S. Response to B-cell depleting therapy with rituximab reverts the abnormalities of T-cell subsets in patients with idiopathic thrombocytopenic purpura. Blood. 2007;110:2924–2930. doi: 10.1182/blood-2007-02-068999. [DOI] [PubMed] [Google Scholar]
- Stray-Pedersen A, Abrahamsen TG, Froland SS. Primary immunodeficiency diseases in Norway. Journal of Clinical Immunology. 2000;20:477–485. doi: 10.1023/a:1026416017763. [DOI] [PubMed] [Google Scholar]
- Strober W, Chua K. Common variable immunodeficiency. Clinical Reviews in Allergy & Immunology. 2000;19:157–181. doi: 10.1385/CRIAI:19:2:157. [DOI] [PubMed] [Google Scholar]
- Taylor RP, Lindorfer MA. Immunotherapeutic mechanisms of anti-CD20 monoclonal antibodies. Current Opinion in Immunology. 2008;20:444–449. doi: 10.1016/j.coi.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakim M, Shah A, Arndt PA, Garratty G, Weinberg K, Hofstra T, Church J. Successful anti-CD20 monoclonal antibody treatment of severe autoimmune hemolytic anemia due to warm reactive IgM autoantibody in a child with common variable immunodeficiency. American Journal of Hematology. 2004;76:152–155. doi: 10.1002/ajh.20072. [DOI] [PubMed] [Google Scholar]
- Wang J, Cunningham-Rundles C. Treatment and outcome of autoimmune hematologic disease in common variable immunodeficiency (CVID) Journal of Autoimmunity. 2005;25:57–62. doi: 10.1016/j.jaut.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Zaja F, Baccarani M, Mazza P, Bocchia M, Gugliotta L, Zaccaria A, Vianelli N, Defina M, Tieghi A, Amadori S, Campagna S, Ferrara F, Angelucci E, Usala E, Cantoni S, Visani G, Fornaro A, Rizzi R, De Stefano V, Casulli F, Battista ML, Isola M, Soldano F, Gamba E, Fanin R. Dexamethasone plus rituximab yields higher sustained response rates than dexamethasone monotherapy in adults with primary immune thrombocytopenia. Blood. 2010;115:2755–2762. doi: 10.1182/blood-2009-07-229815. [DOI] [PubMed] [Google Scholar]