Abstract
Background
Human parainfluenza virus type 3 (HPIV3) is an important yet underappreciated cause of lower respiratory tract illness in children, and a licensed vaccine is not yet available.
Methods
A live-attenuated investigational HPIV3 vaccine virus designated rcp45 was derived from cDNA using reverse genetics. rcp45 is genetically similar to the biologically-derived cp45 vaccine virus and contains all of the known attenuating mutations of cp45, but has the advantage of a short, well-characterized passage history. We evaluated the tolerability, infectivity, and immunogenicity of two intranasal doses of rcp45 administered 4-10 weeks apart in a placebo-controlled, double-blind trial. A total of 45 infants and children between 6 and 36 months of age participated in this study. Tolerability and antibody responses to vaccine or placebo were assessed in all recipients. Infectivity was assessed by quantitation of vaccine virus shedding in a subset of vaccinated children.
Results
rcp45 was well-tolerated and highly infectious in HPIV3 seronegative children. A second dose of vaccine administered 4-10 weeks after the first dose was restricted in replication and did not boost serum antibody responses. The stability of 9 cp45 mutations, including the 6 major attenuating mutations, was examined and confirmed for viral isolates from 10 children.
Conclusions
The level of attenuation and immunogenicity of cDNA-derived rcp45 is comparable to what was previously observed with the biologically-derived cp45 vaccine, and preliminary data suggest that the attenuating mutations in this vaccine virus are genetically stable. Continued clinical development of rcp45 is warranted.
Keywords: parainfluenza, live-attenuated vaccine
INTRODUCTION
Human parainfluenza virus type 3 (HPIV3) is an important cause of acute lower respiratory illness (ALRI) in infants and young children and accounts for at least 11,000 pediatric hospitalizations annually in the United States. [1-6] Like respiratory syncytial virus (RSV), HPIV3 can cause apnea, bronchiolitis and viral pneumonia in infants and has been associated with wheezing episodes in older children with asthma. [4, 5, 7-9] Although precise estimates do not exist, the number of emergency room and outpatient visits for HPIV3-associated illness are likely to exceed hospitalizations by 10 to 80-fold. [4, 6] Thus, the total clinical burden of HPIV3 illness in children is substantial. Since approximately two-thirds of HPIV3 hospitalizations occur in the first year of life [4, 6], development of a vaccine capable of inducing protective immunity in infancy is urgently needed.
Live-attenuated, intranasally-delivered HPIV3 offers several potential advantages as a vaccine for infants. First, an intranasal vaccine would be easy to administer and should be well-tolerated by infants. Second, intranasal administration reduces the likelihood of interference with the many childhood vaccines that are given parenterally during the first year of life. Third, replication of an intranasally-delivered HPIV3 vaccine in the upper respiratory tract is not restricted by the presence of HPIV3-specific maternal serum antibodies [10, 11] that are present in early infancy, when a first dose of an HPIV3 vaccine would likely be administered. Finally, an intranasally-delivered vaccine is likely to induce local immune responses, including secretory IgA. In challenge studies in adults, secretory antibody correlated with resistance to illness caused by HPIVs. [12, 13] Since the immunogenicity of live-attenuated vaccines administered during the first few months of life is less than that observed in older children, we anticipate that more than one dose of an HPIV3 vaccine will be needed to induce lasting protective immunity in infants. [10, 11, 14, 15]
We have previously described the development and preliminary clinical evaluation of the biologically-derived live attenuated investigational HPIV3 vaccine designated as cp45. [10, 11, 16, 17] The cp45 vaccine was generated by 45 passages of wild-type (wt) HPIV3 at low temperature (cold passage, cp) in primary African green monkey cells. [16] A clonal population of cp45 was used to manufacture clinical trial materials, initially in fetal rhesus lung cells (FRhL-2) [10] and later in Vero cells. [11]. Both the FRhL-2-grown and Vero-grown cp45 were shown to be cold-adapted (ca), temperature-sensitive (ts) and attenuated (att) in rodents and primates [16-18] and were well-tolerated, infectious, immunogenic, and phenotypically stable in HPIV3-seronegative children as young as one month of age. [10, 11] The attenuation phenotype of the biologically-derived cp45 vaccine has been confirmed in phase II studies [19, 20] and has also been evaluated in combination with an experimental RSV vaccine. [21]
Based upon the promising results of these initial studies, further clinical development of the cp45 vaccine appeared to be warranted. To continue development of this investigational vaccine, a cDNA encoding the HPIV3cp45 genome was generated and vaccine seed virus was derived from cDNA using reverse genetics. This procedure provided a seed virus with a short, well-documented passage history, thus minimizing the risks of inadvertent contamination with adventitious agents. rcp45 contains all of the cp45 mutations that appear to be relevant for its biological phenotypes based on studies in hamsters and non-human primates. [22]
The primary purpose of this study was to determine whether the tolerability, infectivity, and antibody response to rcp45 were comparable to what we had previously observed when the biologically-derived cp45 vaccine was administered to HPIV3 naïve children. We also sought to determine whether a 4 to 10 week interval was sufficient to allow reinfection and ‘boosting’ with a second dose of the rcp45 vaccine in this population. Finally, we also assessed the genetic stability of known attenuating mutations following vaccine virus replication.
MATERIALS AND METHODS
Vaccine
The investigational rcp45 vaccine used in this study is a recombinant live attenuated HPIV3 virus that is genetically similar to and phenotypically indistinguishable from the biologically-derived HPIV3cp45 vaccine based on studies in cell culture, hamsters and non-human primates. With the exception of two adventitious amino acid substitutions in the hemagglutinin-neuraminidase (HN) glycoprotein, rcp45 is identical at the amino acid level to the biologically-derived HPIV3cp45 vaccine virus. The two substitutions in HN are Pro-370 to Thr and Lys-553 to Thr (mixed population), with the latter substitution being in a region of local sequence divergence for several strains of HPIV3. [23] Importantly, rcp45 contains 15 mutations that include all those responsible for conferring the attenuation, cold-adaptation and temperature-sensitive phenotypes of cp45. [22] The codons of two of the major attenuating mutations in cp45 (F6323 and L13308) were altered in rcp45 to differ by two rather than one nucleotide from the wild-type HPIV3 codons [22]. This was done to reduce the theoretical rate of reversion at these sites to the wild type sequence [24], although loss of attenuation has not been observed in the relatively small studies performed to date. [11, 25]
rcp45 was recovered from cDNA in qualified Vero cells using a set of five plasmids. One plasmid encoded the full-length antigenomic HPIV3cp45 sequence and three additional plasmids encoded the viral proteins that form the viral nucleocapsid and polymerase complex [22]. Expression of these four plasmids was driven by T7 RNA polymerase, which was supplied via a fifth plasmid under the control of a eukaryotic promoter. The seed virus for the production of this investigational vaccine was generated in qualified Vero cells at the Laboratory of Infectious Diseases, NIAID, NIH. The recovered virus was amplified by one passage and a viral clone was selected following three serial terminal dilutions. Virus was passaged twice to generate the seed virus material, which was transferred to Charles River Laboratories (Malvern, PA) for preparation of clinical trial material (CTM). The rcp45 clinical lot (Lot #102A) was tested for sterility, identity, purity, safety, and for the absence of adventitious agents, with satisfactory results in all tests. The final CTM, designated Lot PIV3 102A, had a mean 7.1 infectivity titer of 10 50%-tissue-culture-infectious-doses (TCID50) per mL. The CTM was supplied to the clinical trials site as a frozen concentrate and diluted to dose on-site using qualified Leibovitz L15 medium (Invitrogen, Frederick, MD). L15 medium was also used as the placebo.
Study design
Two cohorts of infants and young children were enrolled in this randomized, double blind, placebo controlled trial at the Johns Hopkins University Center for Immunization Research (clinicaltrials.gov # NCT00308412). In both cohorts, children were randomized in a 2:1 ratio to receive two intranasal doses of the rcp45 vaccine or placebo, administered 4-10 weeks apart. Vaccine or placebo was administered in a total volume of 0.5 mL as nose drops (approximately 0.25 mL in each nostril). Written informed consent was obtained from the parents of study participants prior to enrollment. This study was conducted in accordance with the principles of the Declaration of Helsinki and the Standards of Good Clinical Practice (as defined by the International Conference on Harmonization). The study was performed under a NIAID-held investigational new drug application (BB-IND #12592) reviewed by the US Food and Drug Administration. The clinical protocol, consent form, and Investigators’ Brochure were developed by CIR and NIAID investigators and were reviewed and approved by the NIAID Regulatory Compliance and Human Subjects Protection Branch (RCHSPB) and the Western Institutional Review Board (WIRB). The Data Safety Monitoring Board of the NIAID Division of Clinical Research reviewed all safety data at 6-month intervals.
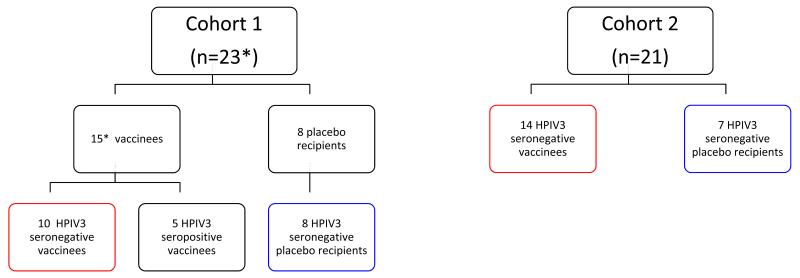
The ‘intensive’ cohort, designated cohort 1 (Figure 1), was designed to assess the infectivity as well as the tolerability and immunogenicity of rcp45. For cohort 1, physical assessments and nasal washes were performed on study days 0; 3 or 4; 6, 7 or 8; 10, 11, or 12; 13 or 14; and 17, 18, or 19 following each dose of vaccine or placebo. Titers of vaccine virus in nasal wash specimens were determined as described below. The ‘less intensive’ cohort, designated cohort 2, was enrolled in order to expand data on the safety, tolerability, and immunogenicity of the rcp45 vaccine. For cohort 2, physical assessments were performed on the same days, but nasal washes were not obtained unless a child was symptomatic. In that case, a single nasal wash was performed and was used solely to identify the possible cause of infection by culture for a number of common pediatric respiratory viruses as well as HPIV3 [11]. For both cohorts, interim history and daily temperatures were obtained from parents via telephone call on all study days between days 0 and 19 on which a study visit did not occur. Fever, upper respiratory illness (URI [rhinorrhea or pharyngitis]), cough, lower respiratory illness (LRI), and otitis media were defined as previously described. [26] A follow-up phone call was completed approximately 6 months after the second dose to determine whether any serious adverse events had occurred after the acute observation period.
Figure 1.
Enrollment into the phase 1 clinical trial of the rcp45 vaccine. *, evaluable subjects. As noted in the text, a single vaccinated HPIV3 seronegative child in cohort 1 was withdrawn by his parents on study day 10, so that data from 23 of 24 children in cohort 1 were included. For purposes of reporting, clinical data from all HPIV3 seronegative vaccinees (n=24, groups outlined in red) and all HPIV3 seronegative placebo recipients (n=15, groups outlined in blue) were combined in Table 1.
Sera were obtained to measure antibodies to HPIV3 prior to the first inoculation, approximately 1 to 2 months after the first inoculation and prior to the second inoculation, and approximately 1 month after the second inoculation.
This study was originally designed to enroll infants between 6 and 12 months of age without prior screening and selection based on HPIV3 serostatus. This was done because we assumed that most infants in that age group would likely be HPIV3 seronegative. However, when paired sera from subjects in cohort 1 were tested, we found that 5 of 16 vaccinated children were HPIV3 seropositive prior to inoculation. Because HPIV3 seropositivity would confound the evaluation of the vaccine, the protocol was modified to restrict enrollment to HPIV3 seronegative children, with seronegativity defined as a serum hemagglutination-inhibition (HAI) antibody titer to HPIV3 of <1:8 [26]. In addition, to facilitate enrollment, the protocol was also modified to include children ages 6 to 36 months.
Isolation and quantitation of virus
Nasal washes were performed using a nasal bulb syringe and 15-20 mL of lactated Ringer’s solution. Nasal washes were snap frozen on site and stored at −800C. An aliquot of each nasal wash was rapidly thawed and inoculated onto LLC-MK2 rhesus monkey kidney cells and incubated at 32°C for primary virus isolation.[10, 11] For all specimens that showed cytopathic effect or evidence of infection by hemadsorption, a second aliquot was titered by hemadsorption plaque assay on LLC-MK2 cells at 32°C.[10, 11] Titers of vaccine virus are expressed as the number of plaque-forming units (pfu) per mL of nasal wash fluid.
Immunologic assays
Sera from all subjects were tested for antibodies to HPIV3 (using the HPIV3 Washington/57 strain as the assay virus) by the HAI antibody test [27] starting at a serum dilution of 1:4.
Genotypic analysis of rcp45 isolates
To assess the genetic stability of the mutations conferring attenuation of the rcp45 virus, nucleotide sequencing was performed on isolates obtained on the last day of vaccine virus shedding from the 11 children enrolled in cohort 1 in whom vaccine virus was isolated. Virus isolates were produced by inoculating nasal wash onto LLC-MK2 cells and harvesting the supernatant 6-7 days later. Viral RNA was extracted using Viral RNA Mini Kits (Qiagen, Valencia, CA), and cDNA was generated using Superscript First Strand Kit (Invitrogen, Carlsbad, CA). For each sample, cDNA was amplified with the Clontech Advantage HF2 PCR Kit (Clontech, Mountain View, CA), to generate a PCR product covering most of the rcp45 genome [22], including the temperature-sensitive attenuating mutations L11468, L11618, and L13308; the non-temperature-sensitive attenuating mutations C2076, F6323, and F6419; and several additional mutations that may contribute to the viral phenotypes: N390, N1271, and HN7944. We did not sequence five non-coding nucleotide point mutations near the 3′ end of the genome, nor the M4341 mutation, since these make little or no contribution to the attenuation phenotype [22]. Sequencing was performed using the ABI Big-Dye (Applied Biosystems, Foster City, CA) on an ABI3730 sequencer.
Data analysis
Infection with rcp45 vaccine was defined as either the isolation of vaccine virus or a >4-fold rise in antibody titer as measured by serum HAI. [27] The mean peak titer of vaccine virus shed (log10 pfu/mL) was calculated for infected vaccinees only. The HAI reciprocal titers were transformed to log2 values for calculation of mean log2 titers. The Mann Whitney U test was used to compare ages of children enrolled in cohorts 1 and 2, and Student’s t test was used to compare titers between groups. Rates of illness among vaccines and placebo recipients were compared by the two-tailed Fisher’s exact test.
RESULTS
Study population
Twenty-four children were enrolled in cohort 1 and 21 children were enrolled in cohort 2. One of the children enrolled in cohort 1 who received the first dose of vaccine was withdrawn by his parents after safety follow-up through study day 15. Nasal washes were obtained only through day 7 for this child, and vaccine virus was not isolated from any nasal wash. None of the data from this vaccinee were included in the study analysis, though we note that this individual was asymptomatic at and before the time of being withdrawn. Forty-four subjects completed the study: 30 were identified by their parents as white, 10 as black, and 4 as other; 2 children were also identified as Hispanic. The median age of subjects in the intensive cohort 1 was 7 months (range, 6-12 months) and the median age in the less intensive cohort 2 was 9 months (range, 7-28 months), P=0.006, Mann-Whitney U test. The median interval between administration of the first and second doses of vaccine or placebo was 4 weeks for cohort 1 and 8 weeks for cohort 2, P=0.0002, Mann-Whitney U test.
As shown in Figure 1, a total of 29 children (15 in cohort 1 and 14 in cohort 2) received 2 doses of 105 TCID50 of vaccine and 15 received 2 doses of placebo (8 in cohort 1 and 7 in cohort 2). As noted above, 5 of the vaccinees in cohort 1 were subsequently determined to be HPIV3 seropositive, and their responses to vaccination are reported separately.
Clinical response to rcp45 vaccine
The rcp45 vaccine appeared to be generally well-tolerated in both study cohorts. Since the clinical response to the vaccine did not differ significantly between cohorts 1 and 2, the clinical data from the 24 seronegative vaccinees (10 from cohort 1 and 14 from cohort 2) and 15 seronegative placebo recipients were combined for reporting purposes (Table 1 and Figure 1). LRI was not observed in any study participant. Rates of fever, upper respiratory illness, cough, and otitis media did not differ significantly between seronegative vaccinees and placebo recipients, nor did rates of illness differ significantly following dose 1 versus dose 2. When rates of febrile illnesses, respiratory illnesses, and otitis media were compared between recipients of rcp45 and recipients of the biologically-derived cp45 vaccine enrolled in a previous study [11], significant differences were not observed (Table 1).
Table 1.
Clinical responses to two 105 pfu doses of rcp45 vaccine, biologically-derived cp45 vaccine,* or placebo in young children
| % with indicated illness |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Subjects’ HPIV3 status |
Vaccine | Dose | No. of subjects |
Fever | URI | LRI | Cough | Otitis Media |
Any respiratory or febrile illness |
| Seropositive | rcp45 | 1 | 5 | 40 | 40 | 0 | 0 | 0 | 60 |
| Seropositive | rcp45 | 2 | 5 | 0 | 20 | 0 | 0 | 0 | 20 |
| Seronegative | rcp45 | 1 | 24 | 13 | 33 | 0 | 4 | 0 | 37 |
| Seronegative | rcp45 | 2 | 24 | 4 | 25 | 0 | 0 | 4 | 25 |
| Seronegative | placebo | 1 | 15 | 13 | 53 | 0 | 7 | 13 | 53 |
| Seronegative | placebo | 2 | 15 | 13 | 47 | 0 | 7 | 0 | 53 |
| Seronegative* | cp45 | 1 | 21 | 24 | 52 | 10 | 19 | 19 | 62 |
| Seronegative* | placebo | 1 | 4 | 25 | 50 | 0 | 25 | 0 | 50 |
URI= upper respiratory illness; LRI= lower respiratory illness. Data from a single seronegative child who withdrew following receipt of rcp45 (and was asymptomatic) are not included.
Data from recipients of the biologically-derived cp45 vaccine are from a previous study (Karron RA et al. Pediatr Infect Dis J, 2003; 22:394-405.)
Infectivity of the first versus second dose of rcp45
Quantitation of vaccine virus shedding in nasal washes was performed for cohort 1 only, and the data from both seropositive and seronegative subjects in cohort 1 are reported here. As shown in Table 2, replication of rcp45 in seropositive children was highly restricted: vaccine virus was detected in nasal wash specimens in only 2 of 5 children. Vaccine virus was undetectable by plaque assay in 1 of these 2 children (i.e., virus was only detected when undiluted nasal wash fluid was inoculated into cell culture, but was not detected in dilutions of nasal wash specimens), and in the other child peak vaccine virus shedding was only 101.1 pfu/mL (Table 2). In contrast, dose 1 of rcp45 infected 100% of seronegative children in cohort 1. Vaccine virus was detected for approximately two weeks in these children, and was shed at a mean peak titer of 103.5 pfu/mL. The infectivity and magnitude of shedding observed for rcp45 are comparable to what was previously observed in seronegative recipients of the biologically-derived cp45 (Table 2). Replication of the second dose of rcp45 was highly restricted: only 1 of the 10 vaccinees in cohort 1 who were seronegative at enrollment shed vaccine virus following dose 2, and the peak titer of virus recovered from that vaccinee was 102.5 pfu/mL (Table 2).
Table 2.
Infectivity and immunogenicity of two 105 pfu doses of rcp45 vaccine, biologically-derived cp45 vaccine* or placebo in young children
| Virus isolation (nasal wash) |
Mean serum HAI antibody response to HPIV3 |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Subjects | Vaccine | Cohort | Dose | No. of subjects |
% infected |
% shedding |
Duration of shedding: mean no. of days |
Mean peak virus titer |
Before vaccination |
After vaccination |
% with 4-fold rise |
| Seropositive | rcp45 | 1 | 1 | 5 | 40 | 40 | 9.0 ±2.8 | 0.9 ±0.4 | 5.4 ±1.3 | 5.4 ±1.5 | 20 |
| Seropositive | rcp45 | 1 | 2 | 5 | 0 | 0 | 0 | ≤0.6 | 5.4 ±1.5 | 5.2 ±1.5 | 0 |
| Seronegative | rcp45 | 1 | 1 | 10 | 100 | 100 | 13.4 ±2.5 | 3.6 ±0.9 | 1.4 ±0.8 | 4.7 ±1.4 | 90 |
| Seronegative | rcp45 | 1 | 2 | 10 | 10 | 10 | 8.0^ | 2.5^ | 4.7 ±1.4 | 4.6 ±1.2 | 0 |
| Seronegative | placebo | 1 | 1 & 2† | 8 | 0 | 0 | 0 | ≤0.6 | 1.0 | 1.0 | 0 |
| Seronegative | rcp45 | 2 | 1 | 14 | 85 | NDX | ND | ND | 1.5 ±0.9 | 4.7 ±1.8 | 86 |
| Seronegative | rcp45 | 2 | 2 | 14 | 14 | ND | ND | ND | 4.7 ±1.8 | 4.9 ±1.4 | 14 |
| Seronegative | placebo | 2 | 1 & 2† | 7 | 0 | ND | ND | ND | 1.0 | 1.0 | 0 |
| Seronegative | cp45* | -- | 1 | 21 | 100 | 100 | 12.3 ±3.0 | 3.3 ±1.3 | 1.8 ±0.9 | 5.9 ±1.5 | 90 |
| Seronegative | placebo | -- | 1 | 4 | 0 | 0 | 0 | ≤0.6 | 2.0 ±0.7 | 2.0 ±0 | 0 |
Infection was defined as isolation of vaccine virus and/or ≥ 4 fold rise in antibody titer. Duration of shedding and mean peak titers of virus shed were calculated for infected subjects only. The lower limit of detection of vaccine virus was 0.7 log10 pfu; titers of 0.6 log10 pfu were assigned to culture-negative samples. Antibody titers are expressed as 1/log2.
Data from recipients of the biologically-derived cp45 vaccine are from a previous study (Karron RA et al. Pediatr Infect Dis J, 2003; 22:394-405.)
Data were identical for those two placebo groups and were pooled for reporting purposes.
Represents data from a single subject and not a mean peak titer.
ND, not done. Nasal washes were not performed for subjects enrolled in cohort 2.
Immune responses following the first and second dose of rcp45
As shown in Table 2, a single 105.0 pfu dose of rcp45 induced a fourfold or greater rise in serum HAI antibody in 86% (cohort 2) to 90% (cohort 1) of seronegative children. Boosting of HAI antibody titers was not observed following a second dose of rcp45 in cohort 1 and in only 14% (2 of 14 vaccinees) in cohort 2 (Table 2). Thus, an increase in the median interval between doses from 4 weeks in cohort 1 to 8 weeks in cohort 2 had little effect on the immune response to the second dose of vaccine.
Genotypic analysis of viral isolates
RT-PCR amplification and sequence analysis was performed to confirm the stability of 9 mutations in rcp45 (2 in N, 1 in C, 2 in F, one in HN, and 3 in the L protein), including the 6 major attenuating mutations (1 in C, 2 in F, and 3 in L). This analysis was performed for isolates obtained on the last day of viral shedding from the 11 children in cohort 1 from whom vaccine virus was recovered. Isolates from 10 of 11 children were confirmed to contain all of the cp45 mutations that were examined (data not shown). The viral isolate from the 11th child was difficult to sequence and confirmation was only obtained for 6 mutations: the three L mutations and the mutations in N and C (no data were available for the mutations in F and HN).
DISCUSSION
The biologically-derived HPIV3cp45 vaccine was previously shown to be highly infectious and well-tolerated in infants as young as 1 month of age and to induce serum HAI antibody responses in children over 6 months of age. [10, 11, 19, 20] While this virus has promising properties as an HPIV3 vaccine candidate, it has a long passage history in primary cells and cell lines. Here, a recombinant rcp45 virus was derived from cDNA to generate a new investigational vaccine with a short, well-defined passage history using qualified reagents and cells. Derivation of a live-attenuated virus from cDNA minimizes the risk of contamination with known or currently unidentified adventitious agents that could potentially be present in the original clinical isolate or could have been introduced during passage, arising either from the primary and continuous cells or from serum, trypsin, or other reagents. Several sequence differences exist between the biologically-derived and the cDNA-derived vaccine virus. While these did not include known attenuating mutations and appeared to be inconsequential for attenuation and immunogenicity in non-human primates, it was essential to confirm comparability of the biologically-derived and cDNA-derived cp45 vaccines in infants and young children.
In this study, we sought to determine whether rcp45 was as well tolerated, immunogenic and infectious as the biologically-derived vaccine virus. We initially evaluated the vaccine in an unscreened population of 6-to-12 month old children. However, serologic testing that was performed subsequent to inoculation showed that 5 of 23 children in this cohort were HPIV3 seropositive (HAI antibody titers >1:8), and all of these children received vaccine. This rate of seropositivity is consistent with epidemiologic studies that highlight the importance of HPIV3 as a respiratory pathogen in the first year of life. [4, 6, 7] Replication of rcp45 was highly restricted in these seropositive infants, as we previously observed with the cp45 vaccine. [28] This study confirms that in children over 6 months of age, serum HPIV3 HAI antibody is an appropriate marker for resistance to infection with a live-attenuated HPIV3 vaccine.
In contrast to the limited infectivity observed in HPIV3 seropositive children, rcp45 was highly infectious in HPIV3 seronegative children. Following the first dose of vaccine, 22 of 24 seronegative children (92%) were infected, and the titer and duration of vaccine virus shedding were comparable to what had been previously observed with the biological cp45 vaccine. [10, 11, 19] Seroconversion following the first dose was observed in 83% of the seronegative vaccinees. The HAI titers achieved in vaccinees were lower than the prevaccination HAI titers in the seropositive subjects in this study and lower than has been observed following natural infection with wt HPIV3 [11], indicating that the restricted replication of the vaccine virus induces a less robust antibody response than does infection with wt HPIV3. This finding suggests that two or more doses of vaccine will be necessary to achieve an immune response comparable to that induced by a single infection with wt HPIV3. However, we found that the first dose of rcp45 induced an immune response that was sufficient to prevent vaccine virus shedding in most children who received a second dose of vaccine 4 to 8 weeks after the first dose. This restriction of replication of the second dose of vaccine also prevented boosting of serum antibody titers, though we cannot exclude the possibility that subclinical infection may have occurred and that other components of immunity, such as as secretory or cellular immunity, were augmented by this second dose. This protection against replication of a second dose of rcp45 vaccine virus would likely translate to restriction of replication of a wt HPIV3 during this interval.
Of note, this high level of protection against ‘challenge’ with a second dose of vaccine in children 6 months of age and older is different from what we previously observed when biologically-derived cp45 vaccine was administered to 1 and 2 month old infants. [11] In that study, replication of the second dose of vaccine virus was observed in 4 of 17 (24%) of infants who received a second dose at approximately 4 weeks and 8 of 13 (62%) of infants who received a second dose at 12 weeks after the first dose of vaccine. While the numbers of vaccinees are small and the intervals between doses are not identical in these two studies, these data suggest that rcp45 HPIV3 vaccine may induce a more potent and durable local immune response in older HPIV3-naïve infants and children than in the very youngest infants, which would be consistent with what has been previously observed following natural infection with respiratory syncytial virus (RSV) in young children. [29-32] Additional studies would be needed to confirm this finding.
Studies are also underway to determine whether a longer interval between doses might be sufficient to allow reinfection and boosting of the antibody response. Currently, we are conducting studies to determine the infectivity and immunogenicity of a second dose of rcp45 vaccine administered 6 months after the first dose. This interval was chosen based upon previous studies that suggest that local immunity to other respiratory viruses or live-attenuated respiratory virus vaccines wanes within 3 to 6 months following the initial infection. [30, 31] Data provided from this ongoing study could be helpful in selecting dosing intervals for subsequent phase II studies of the rcp45 vaccine.
The cp45 vaccine virus has been shown to be biologically stable in vitro, through extensive phenotypic analysis of viral isolates, [10, 11] and in vivo, by administration of viral isolates to susceptible non-human primates. [18] In this study, we extended this biological analysis by assessing the genetic stability of 9 attenuating mutations in rcp45 isolates obtained from the 11 children in cohort 1 who shed vaccine virus. We chose to evaluate specimens obtained from the last day of vaccine virus recovery to determine whether revertant viruses might have emerged that had a selective advantage over viruses containing the attenuating mutations. Because of the inherently high rate of nucleotide substitution in RNA viruses, we had designed rcp45 so that two of the major attenuating mutations (one in the F gene and one in the L gene) would each require two (rather than one) nucleotide substitutions to revert to the wild type codon. The presence of 9 rcp45 mutations, including all 6 amino acid substitutions that contribute to the attenuation phenotype of rcp45 in non-human primates, was confirmed for isolates from 10 children. For the 11th child, the presence of four attenuating mutations was confirmed in the L, N, and C genes, but sequence for the remaining mutations could not be derived. While additional data from larger studies will be needed, the preliminary data generated from this clinical trial support previous findings regarding the biologic stability of the cp45 virus and suggest that the attenuating mutations present in the rcp45 vaccine virus are stable following replication in seronegative children.
In summary, rcp45 appears to be an appropriately attenuated vaccine that is infectious, immunogenic, and genetically stable in HPIV3 seronegative infants and children. The infectivity, tolerability, and immune response to rcp45 are comparable to what was previously observed following administration of the biologically-derived cp45 vaccine. However, rcp45 is preferred for future evaluation in phase 2 and 3 clinical trials because of its derivation from cDNA using qualified reagents and cells. Further clinical development of this promising investigational recombinant respiratory virus vaccine is warranted and in progress.
Acknowledgements
This study was supported by the Intramural Research Program of the National Institutes of Allergy and Infectious Diseases (NIAID), National Institutes of Health. Clinical trials were conducted as part of contracts between NIAID and the Johns Hopkins Bloomberg School of Public Health. (N01-A1-15444 and HHSN272200900010C).
We thank Barbara Burns, Elizabeth Schappell, Karen Loehr, Racine Harris, Amy Hoffman, Susan DiLorenzo and Nicole Yoder for expert clinical and technical assistance and Romeo Paredes and Jason Morsell for data management and administrative assistance. We would also like to thank the Regulatory Compliance and Human Subjects Protection Program within the NIAID Division of Clinical Research and the NIAID Data Safety Monitoring Board for their support. We are grateful to the physicians and staff of Primary Care Pediatrics, Dundalk Pediatrics, Bright Oaks Pediatrics, The Pediatric Group, and the Harriet Lane Clinic and to the families whose children participated in this study. We are grateful to Mario Skiadopoulos for his contributions to the preclinical development of this investigational vaccine.
Supported by NIH contracts N01-AI-15444 and HHSN272200900010C
REFERENCES
- 1.Chanock RM, Parrott RH. Acute respiratory disease in infancy and childhood: Present understanding and prospects for prevention. Pediatrics. 1965;36:21–39. [PubMed] [Google Scholar]
- 2.Glezen WP, Denny FW. Epidemiology of acute lower respiratory disease in children. N Engl J Med. 1973;288:498–505. doi: 10.1056/NEJM197303082881005. [DOI] [PubMed] [Google Scholar]
- 3.Counihan ME, et al. Human parainfluenza virus-associated hospitalizations among children less than five years of age in the United States. Pediatr Infect Dis J. 2001;20(7):646–53. doi: 10.1097/00006454-200107000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Weinberg GA, et al. Parainfluenza Virus Infection of Young Children: Burden of Hospitalization and Seasonal Patterns of Infection. Society for Pediatric Research; San Francisco, CA: 2004. p. 1824. [Google Scholar]
- 5.Weinberg GA. Parainfluenza viruses: an underappreciated cause of pediatric respiratory morbidity. Pediatr Infect Dis J. 2006;25(5):447–8. doi: 10.1097/01.inf.0000218037.83110.c4. [DOI] [PubMed] [Google Scholar]
- 6.Weinberg GA, et al. Parainfluenza virus infection of young children: estimates of the population-based burden of hospitalization. J Pediatr. 2009;154(5):694–9. doi: 10.1016/j.jpeds.2008.11.034. [DOI] [PubMed] [Google Scholar]
- 7.Glezen WP, et al. Parainfluenza virus type 3: seasonality and risk of infection and reinfection in young children. J Infect Dis. 1984;150(6):851–7. doi: 10.1093/infdis/150.6.851. [DOI] [PubMed] [Google Scholar]
- 8.Karron RA, et al. Molecular epidemiology of a parainfluenza type 3 virus outbreak on a pediatric ward. J Infect Dis. 1993;167(6):1441–5. doi: 10.1093/infdis/167.6.1441. [DOI] [PubMed] [Google Scholar]
- 9.Reed G, et al. Epidemiology and clinical impact of parainfluenza virus infections in otherwise healthy infants and young children < 5 years old. J Infect Dis. 1997;175(4):807–13. doi: 10.1086/513975. [DOI] [PubMed] [Google Scholar]
- 10.Karron RA, et al. Evaluation of a live attenuated bovine parainfluenza type 3 vaccine in 2-to-6-month-old infants. Pediatr Infect Dis J. 1996;15:650–654. doi: 10.1097/00006454-199608000-00003. [DOI] [PubMed] [Google Scholar]
- 11.Karron RA, et al. A live human parainfluenza type 3 virus vaccine is attenuated and immunogenic in young infants. Pediatr Infect Dis J. 2003;22(5):394–405. doi: 10.1097/01.inf.0000066244.31769.83. [DOI] [PubMed] [Google Scholar]
- 12.Smith CB, et al. Protective effect of antibody to parainfluenza type 1 virus. N Engl J Med. 1966;275(21):1145–52. doi: 10.1056/NEJM196611242752101. [DOI] [PubMed] [Google Scholar]
- 13.Tremonti LP, Lin JL, Jackson GG. Neutralizing activity in nasal secretions and serum in resistance of volunteers to parainfluenza virus type 2 1. J Immunol. 1968;101:572–577. [PubMed] [Google Scholar]
- 14.Clements ML, et al. Effective immunization with live attenuated influenza A virus can be achieved in early infancy. J Infect Dis. 1996;173:44–51. doi: 10.1093/infdis/173.1.44. [DOI] [PubMed] [Google Scholar]
- 15.Wright PF, et al. Evaluation of a live, cold-passaged, temperature-sensitive, respiratory syncytial virus vaccine candidate in infancy. J Infect Dis. 2000;182(5):1331–1342. doi: 10.1086/315859. [DOI] [PubMed] [Google Scholar]
- 16.Belshe RB, Hisson RK. Cold adaptation of parainfluenza virus type 3: induction of three phenotypic markers. J Med Virol. 1982;10:235–242. doi: 10.1002/jmv.1890100403. [DOI] [PubMed] [Google Scholar]
- 17.Crookshanks FK, Belshe RB. Protection of weanling hamsters from experimental infection with wild-type parainfluenza virus type 3 (para 3) by cold-adapted mutants of para 3. J Med Virol. 1986;18:131–137. doi: 10.1002/jmv.1890180205. [DOI] [PubMed] [Google Scholar]
- 18.Hall SL, et al. A cold-adapted mutant of parainfluenza virus type 3 in attenuated and protective in chimpanzees. J Infect Dis. 1993;167:958–962. doi: 10.1093/infdis/167.4.958. [DOI] [PubMed] [Google Scholar]
- 19.Belshe RB, et al. Phase 2 evaluation of parainfluenza type 3 cold passage mutant 45 live attenuated vaccine in healthy children 6-18 months old. J Infect Dis. 2004;189(3):462–470. doi: 10.1086/381184. [DOI] [PubMed] [Google Scholar]
- 20.Madhi SA, et al. Transmissibility, infectivity and immunogenicity of a live human parainfluenza type 3 virus vaccine (HPIV3cp45) among susceptible infants and toddlers. Vaccine. 2006;24(13):2432–2439. doi: 10.1016/j.vaccine.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 21.Belshe RB, et al. Evaluation of live attenuated bivalent RSV-parainfluenza 3 virus vaccine in infants and young children. J Infect Dis. 2004;190(12):2096–103. doi: 10.1086/425981. [DOI] [PubMed] [Google Scholar]
- 22.Skiadopoulos MH, et al. Identification of mutations contributing to the temperature-sensitive, cold-adapted, and attenuation phenotypes of the live attenuated cold-passage 45 (cp 45) human parainfluenza virus 3 candidate vaccine. J Virol. 1999;73(2):1374–1381. doi: 10.1128/jvi.73.2.1374-1381.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Wyke Coelingh KL, Winter CC, Murphy BR. Nucleotide and deduced amino acid sequence of hemagglutinin-neuraminidase genes of human type 3 parainfluenza viruses isolated from 1957 to 1983. Virology. 1988;162(1):137–43. doi: 10.1016/0042-6822(88)90402-3. [DOI] [PubMed] [Google Scholar]
- 24.McAuliffe JM, et al. Codon substitution mutations at two positions in the L polymerase protein of human parainfluenza virus type 1 yield viruses with a spectrum of attenuation in vivo and increased phenotypic stability in vitro. J Virol. 2004;78(4):2029–2036. doi: 10.1128/JVI.78.4.2029-2036.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hall SL, et al. Cold-passaged human parainfluenza type 3 viruses contain ts and non-ts mutations leading to attenuation in rhesus monkeys. Virus Res. 1992;22(3):173–84. doi: 10.1016/0168-1702(92)90049-f. [DOI] [PubMed] [Google Scholar]
- 26.Karron RA, et al. A live attenuated bovine parainfluenza type 3 virus vaccine is safe, infectious, immunogenic, and phenotypically stable in infants and children. J Infect Dis. 1995;171:1107–1114. doi: 10.1093/infdis/171.5.1107. [DOI] [PubMed] [Google Scholar]
- 27.Clements ML, et al. Evaluation of bovine, cold-adapted human, and wild-type human parainfluenza type 3 viruses in adult volunteers and in chimpanzees. J Clin Microbiol. 1991;29:1175–1182. doi: 10.1128/jcm.29.6.1175-1182.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karron RA, et al. A live human parainfluenza type 3 virus vaccine is attenuated and immunogenic in healthy infants and children. J Infect Dis. 1995;172:1445–1450. doi: 10.1093/infdis/172.6.1445. [DOI] [PubMed] [Google Scholar]
- 29.McIntosh K, et al. The immunologic response to infection with respiratory syncytial virus in infants. J Infect Dis. 1978;138:24–32. doi: 10.1093/infdis/138.1.24. [DOI] [PubMed] [Google Scholar]
- 30.Welliver RC, et al. The antibody response to primary and secondary infection with respiratory syncytial virus: Kinetics of class-specific responses. J Pediatr. 1980;96(5):808–813. doi: 10.1016/s0022-3476(80)80547-6. [DOI] [PubMed] [Google Scholar]
- 31.Kaul TN, et al. Secretory antibody response to respiratory syncytial virus infection. Am J Dis Child. 1981;135:1013–1016. doi: 10.1001/archpedi.1981.02130350017007. [DOI] [PubMed] [Google Scholar]
- 32.Murphy BR, et al. Effect of age and preexisting antibody on serum antibody response of infants and children to the F and G glycoproteins during respiratory syncytial virus infection. J Clin Microbiol. 1986;24:894–898. doi: 10.1128/jcm.24.5.894-898.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]