Abstract
The classical model of arrestin-mediated desensitization of cell-surface G protein-coupled receptors (GPCRs) is thought to be universal. However, this paradigm is incompatible with recent reports that the parathyroid hormone (PTH) receptor (PTHR), a crucial GPCR for bone and mineral ion metabolism, sustains GS activity and continues to generate cAMP for prolonged periods after ligand-wash-out; during these periods the receptor is observed mainly in endosomes, associated with the bound ligand, GS and β-arrestins. In this review, we discuss possible molecular mechanisms underlying sustained signaling by the PTHR, including modes of signal generation and attenuation within endosomes, as well as the biological relevance of such non-canonical signaling.
PTHR: a paradoxical and medically critical GPCR
Parathyroid hormone (PTH) and PTH-related protein (PTHrP) play critical and distinct physiological roles by activating a common cell-surface receptor, the PTH type 1 receptor (hereafter noted PTHR), a family 2 GPCR. Circulating and homeostatic PTH regulates blood concentrations of calcium and phosphate ions, as well as vitamin D by acting in bone (osteoblasts, osteocytes) and kidney (proximal and distal tubule cells). PTHrP, a paracrine hormone, controls cell differentiation and proliferation in developing tissues, including the skeleton, the heart, and mammary glands. PTHR, when bound by PTH or PTHrP, stimulates heterotrimeric GS- and Gq/11 proteins, resulting in the activation of signaling pathways involving adenylyl cyclase/cAMP/protein kinase A (PKA) and phospholipase Cβ/inositol trisphosphate (IP3)/Ca2+/protein kinase C (PKC), respectively (1–3). PTHR can also activate other pathways that include G12/13/RhoA/phospholipase D (PLD) and the mitogen-activated protein kinase (MAPK) (extracellular signal-regulated kinase, ERK1/2) signaling cascades (4–6).
Defects in PTHR signaling are directly involved in human diseases of bone and mineral ions metabolism such as those associated with hyper- or hypoparathyroidism (due to a defect in PTH secretion from the parathyroid glands), hypercalcemia of malignancy (due to excessive PTHrP secretion), neonatal lethality in Blomstrand's chondrodysplasia (caused by a defective expression of PTHR) (7), dwarfism and hypercalcemia in Jansen's chondrodysplasia (8) or cartilage tumors of bone in Eiken Syndrome (caused by activating PTHR mutations) (9). PTHR is also a significant therapeutic target, as PTHR agonists can stimulate bone formation. The synthetic N-terminal fragment of PTH, PTH(1–34), and the intact PTH(1–84) polypeptide, are the only bone-anabolic agents currently available that can decrease fracture incidences in severe cases of osteoporosis by the stimulation of trabecular and cortical bone formation. This is accomplished through daily injections of PTH, a regimen known as intermittent PTH treatment (intPTH) (10). However, the therapeutic use for intPTH is limited by the principal side effect of hypercalcemia (elevated Ca2+ in the blood) and a possible risk of osteosarcoma (malignant bone tumor) (11–13). In contrast with the osteoanabolic effect of intPTH, other treatment regimens, such as continuous PTH perfusion, stimulate bone resorption. Understanding molecular and cellular mechanisms by which activation of PTHR by its two native ligands triggers different biological effects and mediates the paradoxical anabolic and catabolic effects that PTH has on bone mass are thus keys for the development of new drugs for diseases of bone and mineral metabolism, such as osteoporosis, hyper- and hypoparathyroidism. Here we review recent findings that not only point to molecular mechanisms that may account for the biological differences between PTH and PTHrP, but also suggest that the internalized PTHR, in complex with GαS and β-arrestin, can sustain cAMP signaling from the early endosomal compartment. The findings thus indicate a paradigm shift in our understanding of GPCR signaling.
Kinetics of PTHR activation
A combination of biochemical, pharmacological and optical techniques, including photo-affinity cross-linking using bisphenol A (BPA)-containing PTH analogs (3, 14–18), coupled with functional assays using mutant receptors and structurally modified ligands (19–22), and Förster resonance energy transfer (FRET)-based approaches (23–26) have revealed important insights about ligand – receptor interaction mechanisms and rate-limiting reactions involved in activation of PTHR and its cognate GS protein (Box 1 and Table 1) (27, 28).
Box 1. Recording activation/deactivation reactions along the PTHR signaling cascade.
PTHR transmits PTH or PTHrP-induced signals via a classical sequence of reactions that takes place initially at the plasma membrane. The first step involves ligand (L) binding to a receptor (R) and its shift from an inactive to active conformation (L–Rrarr; L–R*). The active R* can then bind GS protein (G) in its inactive GDP-bound form (L–R* + GGDPrarr;L–R*–GGDP). This interaction catalyses the GDP–GTP exchange on the Gα subunit, activating the G protein and triggering the dissociation of the GTP-bound Gα (GαGTP) from the receptor and from the Gβγ dimer. The dissociation follows the reaction: L–R* –GGDPrarr;L–R* –GGTPrarr;L–R* + GαGTP+ Gβγ. Next, GαGTP binds and activates adenylyl cyclases that convert ATP into the second messenger cAMP. The intrinsic GTPase activity of Gα hydrolyses GTP into GDP + inorganic phosphate (Pi) resulting in an inactive GαGDP, which then binds Gβγto initiate a new reaction cycle.
PTHR is so far the only receptor for which kinetics of each reaction involved in the signaling cascade from ligand binding to second messenger production has been measured in live cells (25). These kinetics have been measured by FRET-based approaches (28) (Figure I). These techniques are illustrated in the left panels of Figure I (
 GFP,
GFP,
 CFP,
CFP,
 YFP). The right panels represent the time course of individual reactions triggered by PTH or PTHrP. These FRET approaches in live cells, coupled with biochemical-based assays, reveal a series of unexpected findings: i) the PTH–PTHR complex internalizes rapidly into Rab5-positive endosomes (early endosomes) in association GS, β-arrestins, and adenylyl cyclases; ii) the internalization of the PTH–PTHR complex is not associated with desensitization of the GS or cAMP response; iii) blocking PTH–PTHR internalization prevents a sustained cAMP response. In contrast, PTHrP actions are completely reversible and limited to the plasma membrane. The precise mechanisms that mediate the observed prolonged cAMP in response to PTH remain to be determined, but the strong colocalization of PTH with PTHR, Gs and adenylyl cyclase in early ensosomes raises the novel possibility that the internalized PTHR complexes are enzymatically active and can generate cAMP from endosomal membranes, as a means for PTHR-mediated sustained cAMP production (25, 26, 33).
YFP). The right panels represent the time course of individual reactions triggered by PTH or PTHrP. These FRET approaches in live cells, coupled with biochemical-based assays, reveal a series of unexpected findings: i) the PTH–PTHR complex internalizes rapidly into Rab5-positive endosomes (early endosomes) in association GS, β-arrestins, and adenylyl cyclases; ii) the internalization of the PTH–PTHR complex is not associated with desensitization of the GS or cAMP response; iii) blocking PTH–PTHR internalization prevents a sustained cAMP response. In contrast, PTHrP actions are completely reversible and limited to the plasma membrane. The precise mechanisms that mediate the observed prolonged cAMP in response to PTH remain to be determined, but the strong colocalization of PTH with PTHR, Gs and adenylyl cyclase in early ensosomes raises the novel possibility that the internalized PTHR complexes are enzymatically active and can generate cAMP from endosomal membranes, as a means for PTHR-mediated sustained cAMP production (25, 26, 33).
Table 1.
Kinetics of PTHR–ligand association and dissociation at a ligand concentration of 10 μM (reaction 1), PTHR activation and deactivation (reaction 2), PTHR and GS interaction (reaction 3), GS activation and deactivation (reaction 4), cAMP accumulation and degradation (reaction 5). Reactions were recorded from single HEK-293 cells at a saturating concentration of ligand. Values represent the mean ± s.e.m. of the rate constant (τ) and were taken from (24, 25).
| Switch on (s) | Turn off (s) | |||
|---|---|---|---|---|
| PTH(1–34) | PTHrP(1–36) | PTH(1–34) | PTHrP(1–36) | |
| (1) L + R Ä LR | τfast = 0.14 ± 0.01 τslow = 1.15 ± 0.10 |
τfast = 0.17 ± 0.05 τslow = 1.54 ± 0.15 |
τfast = 1.50 ± 0.27 τslow NA |
τfast = 1.38 ± 0.23 τslow = 28.12 ± 0.60 |
| (2) LR Ä LR * | τ = 0.95 ± 0.15 | τ = 1.59 ± 0.11 | NA | τ = 58.54 ± 6.42 |
| (3) LR* + G Ä LR*G | τ = 0.96 ± 0.13 | τ = 1.58 ± 0.19 | NA | τ = 48.14 ± 5.29 |
| (4) G Ä G* | τ = 1.58 ± 0.13 | τ = 2.04 ± 0.14 | NA | τ = 121.50 ± 6.35 |
| (5) cAMP | τ = 10.89 ± 2.26 | τ= 12.66 ± 1.06 | NA | τ = 296.70 ± 17.47 |
We now know that the large (180 amino acid) amino-terminal extracellular domain (N) of PTHR contributes to the initial ligand – receptor (L–R) interaction by docking residues 15–34 of PTH(1–34) to the receptor with kinetics that strictly depend on agonist (A) concentrations as predicted by a simple bimolecular interaction, defined by where kobs is the recorded rate constant (s−1) (24). High-affinity binding between PTH and PTHR depends on the subsequent step, which involves the interaction of the amino-terminal portion of the ligand to the juxtamembrane (J) region of the receptor comprising the seven transmembrane helices and connecting extracellular loops. This interaction stabilizes the active PTHR conformation with a maximal time constant (τ = 1/k) of 1 s (24). This second L–R interaction involving the J region of PTHR, and not the conformational changes to the receptor that take place during activation, is the rate-limiting step for receptor activation (τmax = 950 ms). Once activated, PTHR engages GS at the plasma membrane with time constants that can be as fast as that measured for PTHR activation (τ = 0.96 s for PTH, and τ = 1.6 s for PTHrP). PTHR–GS interaction kinetics are limited by the expression level of G proteins, which supports a diffusion-controlled collision process rather than a receptor-G protein precoupling model (25). The following step, which involves conformational rearrangements and disassembly events between the GαS subunit and the Gβ1γ2 dimer, is rate-limiting for GS activation, and is only moderately faster for PTH (τ = 1.6 s) than for PTHrP (τ = 2.05 s) at saturating ligand concentrations. Cyclic AMP production is detectable a few seconds after GS activation, a delay that presumably reflects the time required for GS activation, its separation from the receptor and activation of adenylyl cyclases.
Conformational selectivity of PTHR deactivation
As described above, the sequence of reactions involved in the activation of PTHR and GS proceed with similar kinetics and mechanisms in response to either PTH or PTHrP. By contrast, the mechanisms of signal termination are quite divergent (Box 1). Recent studies show that a brief pulse of PTH induces a long lasting active state that is characterized by prolonged GS activation and sustained cAMP production even after PTH-bound PTHR internalizes to early endosomes. PTHrP dissociates rapidly from the receptor (τoff = 30 s), prompting rapid GS deactivation and cAMP signal termination at the plasma membrane (25, 26). These studies suggest that PTH and PTHrP stabilize two distinct active conformations of the PTHR. We hypothesized that one of these PTHR conformations, named R0 in reference to prior studies done with the CRF receptor (29, 30), is a high affinity PTHR conformation stabilized by PTH that is not necessarily dependent on G protein coupling, but can nevertheless maintain extended periods of G protein coupling and activation. This R0 PTHR conformation is thus distinct from the classical G protein-dependent high affinity receptor conformation, hereafter noted RG and preferentially stabilized by PTHrP, as predicted by the conventional GPCR signaling paradigm (31).
To study the R0 and RG conformations of PTHR, we utilized membrane-based equilibrium competition binding assays that isolate and quantify binding to each of these two conformations of the PTHR (32–34). Binding to R0 was assessed using 125I-PTH(1–34) in the presence of GTPγS, a non-hydrolyzable GTP analog that antagonizes R–G protein coupling; binding to RG was assessed using a fully functional modified PTH analog that binds weakly when GTPγS is present, 125I-M-PTH(1–15) (where M is Ala/Aib1, Aib3, Gln10, Har11, Ala12, Trp14, Arg19), and membranes prepared from cells expressing PTHR and a dominant negative GαS mutant (GS-ND) that binds the receptor in a nearly irreversible fashion (25, 35). These approaches not only revealed that PTH(1–34) binds with greater selectivity to R0, versus RG, than does PTHrP(1–36) (Figure 1a,1b), but they also led to the identification of PTH analogs, M-PTH(1–28) and M-PTH(1–34), that bind with even higher affinity to R0 than does PTH(1–34) (33, 36). The enhanced selectivity with which these analogs bind to the R0 state is accompanied by markedly prolonged cAMP signaling response in cells, with clear movement of the PTHR to the internalized domain, and, importantly, prolonged hypercalcemic and hypophosphatemic responses when injected in animals (33),(37, 38).
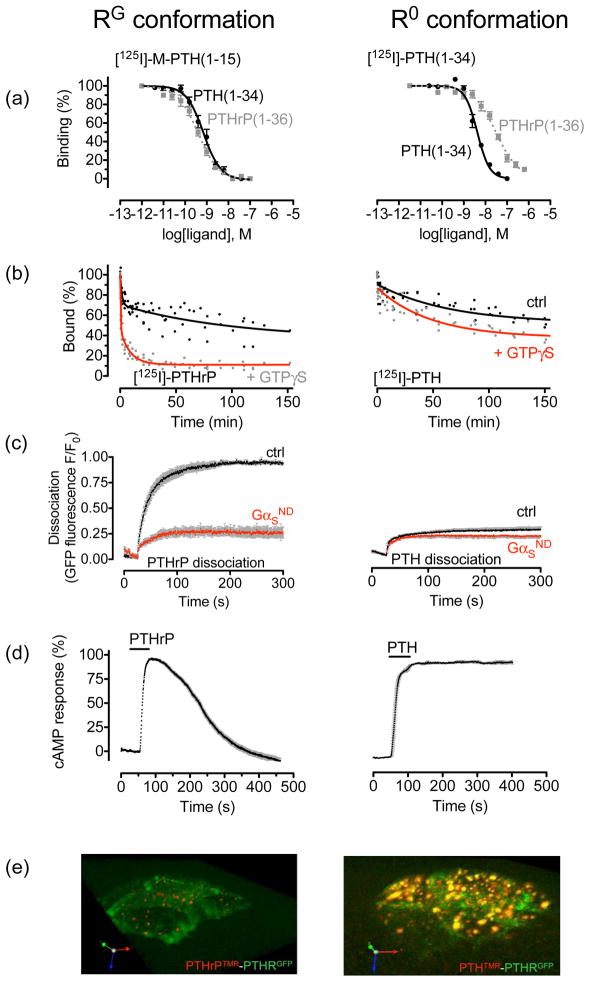
Figure 1.
PTHR conformations. (a–b) Cell membrane binding assays. Binding to the R0 and RG conformations of the PTHR are determined by competition reactions. For R0, [125 I]-PTH(1—34) is used as a tracer radioligand and including GTPγS in the reaction; for RG, [125I]-PTH(1—15) is used as a radioligand in the presence of a high-affinity, negative-dominant GαS subunit (Gαs-ND). (c–e) Life cells FRET-based assays. Averaged dissociation time courses of TMR-labeled ligands, PTH(1–34)TMR (right panel) and PTHrP(1–36)TMR (left panel), from GFP-tagged PTHR, GFPN-PTHR, are shown in the absence or presence of a Gαs-ND. FRET recordings from HEK-293 cells are shown as normalized ratios (c). Average time-courses of cAMP production in response to PTHrP(1–36) (left) and PTH(1–84) (right) in HEK-293 cells stably expressing PTHR and co-transfected with the cAMP biosensor, EpacCFP/YFP. Individual cells were continuously perfused with buffer or with the hormone for the time indicated by the horizontal bar (d). A 3D view of tetramethylrhodamine (TMR)-labeled peptides, and a PTHR N-terminally tagged with GFP (GFPN-PTHR) in live HEK-293 cells by spinning disc confocal microscopy 30 min after ligand wash out. PTH(1–34)TMR (red) and GFPN-PTHR (green) co-localized within endocytic compartments (right). In contrast, PTHrP(1–36)TMR alone is detected as small puntae at internalized sites (left) (e). Adapted from (25, 33).
The capacity of PTH and PTHrP to stabilize distinct PTHR conformations was also confirmed by FRET experiments done in live cells [(25)]. Here, the PTH–PTHR complex was highly stable, whereas that induced by PTHrP(1—36) was reversible after ligand washout (Figure 1c). In agreement with radioligand binding studies performed in vitro, live-cell FRET data showed that dominant negative GS has little or no effect on dissociation of PTH(1—34) from the receptor, but it markedly impedes the dissociation of PTHrP(1—36) (Figure 1b,1c). These results imply that the major component of the dissociation process observed for PTHrP arises from the rapid release of G proteins from the receptor, which does not occur with GS-ND (Figure 1c). Taken together, these studies suggest that with certain ligands, such as PTHrP(1–36), PTHR can form conventional high-affinity complexes that are transient and depend on coupling to G proteins, whereas with other ligands, such as PTH(1–34) and M-PTH(1–34), it can form unusually high-affinity complexes that are not dependent on classical G protein coupling, but yet can sustain activation of GS proteins and cAMP production, even after receptor internalization. Generation of cAMP is abbreviated when the PTH–PTHR complex cannot internalize due to disruption of dynamin activity (25). This, and the apparently complete internalization of PTH–PTHR complexes while a high level of cAMP generation is still recorded, supports the idea that PTHR in fact requires internalization for sustained generation of cAMP.
“Non-canonical” mode of PTHR signaling
Extensive studies of signaling by GPCRs, including but not limited to rhodopsin and the β2-adrenergic receptor (β2AR), have led to what is now considered a classical and general model of GPCR desensitization by arrestins (39, 40). In this “canonical” model, arrestins engage active receptors after ligand binding has stimulated G protein-coupled receptor kinases (GRK) to phosphorylate residues on the C terminus of the receptor. Arrestin binding terminates GPCR signaling by preventing receptor–G-protein coupling (41, 42), and by recruiting diverse enzymes such as phosphodiesterase 4 (PDE4) or diacylglycerol kinase (DGK) to the plasma membrane to degrade the second messengers cAMP and DAG, respectively (43, 44). Arrestin binding also promotes receptor internalization, a process that relies upon the interaction of β-arrestins with the AP-2 subunit of clathrin, a major component of the clathrin-based endocytic machinery (45). However, it is now clear that PTHR does not follow this conventional desensitization paradigm (26). β-arrestins interact rapidly with PTH-bound PTHR (46, 47) without inhibiting the continued generation of cAMP (26, 48). In fact β-arrestins prolong PTH-mediated cAMP in cultured cells that express either recombinant or native PTHR, and fluorescence imaging of live cells shows that PTH induces the internalization of PTHR to early endosomes along with arrestin, GS and adenylyl cyclases (25, 26). Furthermore, the time course of cAMP generation in these cells, which can be measured in real time using FRET-based fluorescent biosensors, correlates temporally with the persistence of arrestin–PTHR–GS complexes on early endosomes. Importantly, analogs of PTH, such as M-PTH(1–28) and M-PTH(1–34), which induce prolonged physiological calcemic and phosphate responses in animals (36) and prolonged cAMP generation in cultured cells, also increase the persistence of receptor-arrestin complexes on endosomes. This, and evidence that an arrestin mutant with increased affinity for active receptor also enhances cAMP generation (26), is consistent only with a model in which arrestin promotes rather than desensitizes cAMP generation by PTHR and further implicates signaling from early endosomes as a key part of the model.
A critical question arises from these findings. How can a long-lived PTH–PTHR–arrestin (L–R–arr) complex mediate prolonged GS/cAMP signaling? Two observations can help to narrow the possibilities: i) there is no evidence that β-arrestins directly bind GαS in any circumstance, suggesting that arrestin plays an indirect rather than a direct role in facilitating PTHR–GαS coupling; and ii) recent data suggest that Gβγ subunits can provide a mechanism for scaffolding β-arrestin (49). If applicable in this case, a long-lived PTH–PTHR–arr ternary complex could contribute to protracted cAMP signaling mediated by PTH by two mechanisms: i) the PTH–PTHR–arr complex could stabilize an interaction with Gβγ that permits multiple rounds of GαS subunit coupling and activation, or ii) each PTH–PTHR–arr–Gβγ complex could mediate sustained coupling and activation of only one, or a few molecules of GαS. However, this model remains entirely hypothetical and the mechanism by which arrestin promotes signaling by PTHR must be determined by future studies.
It seems possible that receptors that bind their ligand with high affinity remain competent to signal while arrestin cycles on and off the cytoplasmic tail of the receptor. If so, then arrestin turnover could leave the G-protein binding site periodically exposed for further rounds of G protein activation. Indeed, FRAP analysis of β-arrestin 1–PTHR complexes on early endosomes revealed a recovery half-life of ~30 seconds, indicating that a significant turnover of arrestin molecules does occur. However, a mutant arrestin that cycles on to and off of the PTHR much more slowly than does native arrestin, mediates prolonged, rather than abbreviated, cAMP generation, as do PTH ligands that cause wild-type β-arrestin 1 to bind the receptor with greater affinity (26). These findings argue against the possibility that sustained cAMP responses involve rapid turnover of arrestin–PTHR complexes. Thus, sustained arrestin interaction, possibly mediated by interaction with Gβγ, is more likely to promote sustained G activation.
Another possibility is that arrestin binds the PTHR independently of G proteins. The PTHR has been shown to dimerize either constitutively or upon activation (50). In this case one protomer could bind arrestin and mediate internalization while the other continues to activate GS. Alternatively, the long PTHR C-terminus contains distinct binding motifs for Gβγ and arrestin that theoretically could allow binding of two accessory proteins at the same time (46, 51). If this is the case, then arrestin mutants with greater affinity for activated receptor, and PTHR ligands that induce more stable arrestin binding, could prolong cAMP generation by blocking access to whichever accessory protein does decouple PTHR from G protein activation.
PTHR signaling stopped by retromer
Depletion of β-arrestins by siRNA reduces the level and the duration of cAMP generation after PTH challenge whereas it increases cAMP induced by β2AR in response to isoproterenol, indicating again that β-arrestins do not desensitize cAMP generation by PTHR (26, 52). If arrestin does not prevents GS coupling from PTHR by streric inhibition, then it is necessary to ask what other protein could do this job. One possibility is that, like certain receptor tyrosine kinases (53), PTHR simply continues to signal until it is sequestered in the multivesicular body prior to degradation in the lysosome. However, this is unlikely, as PTHR does not degrade but rather recycles via an unusually slow pathway (54). Other GPCRs that undergo ligand-dependent internalization such as the β2AR and μ-opioid receptor recycle directly to the plasma membrane, whereas PTHR traffics by retrograde transport to the trans-Golgi network (55) before recycling through the exocytic pathway. It is thus reasonable to suppose that the factor that sorts PTHR from the endosome to the Golgi could also stop cAMP generation by the receptor. The most likely candidate for this activity would be retromer, an endosomal heteropentameric complex that consists of two membrane-bound sorting nexins (Snx1/Snx2) and a soluble heterotrimer of vesicle protein sorting, Vps26, Vps29 and Vps35. Retromer is known to retrieve transmembrane signaling proteins, such as the mannose-6-phosphate receptor and wntless, from endosomes and return them to the Golgi (56). A particularly intriguing observation is that the structures of β-arrestins and the Vps26 subunit of retromer have a striking resemblance, although the functional significance of this similarity remains unknown (57). Regardless, there is strong evidence that retromer influences the signaling and trafficking of PTHR. Fluorescent retromer colocalized and physically interacted with internalized PTHR when co-expressed in HEK293 cells, and over-expression of the soluble Vps26/29/35 trimer both increased PTHR traffic to the Golgi and abbreviated the time course and levels of cAMP generation. It is notable that fluorescent retromer did not colocalize with PTHR immediately upon internalization of active receptors to early endosomes. Rather, three-color live imaging of cells expressing fluorescent PTHR, arrestin and retromer indicated that PTHR and arrestin occupy a distinct endosomal domain from retromer for about 20 min after challenge with PTH, after which time PTHR begins to colocalize with retromer and less strongly with arrestin. This is consistent with a model in which arrestin and retromer occupy exclusive domains of the endosome and act either to sustain (arrestin) or to block (retromer) cAMP generation by PTHR (Figure 2). Depletion of retromer by siRNA resulted in more persistent cAMP generation by PTHR but had no effect on β2AR signaling, also supporting a role for retromer in silencing PTHR. These effects of retromer on PTHR signaling were observed both in HEK293 cells expressing transgenic PTHR as well as in rat osteosarcoma cells that natively express PTHR (26), although it remains unknown how retromer binds PTHR and decouples its signaling. The selectivity of retromer–PTHR binding is shown by the fact that neither PTH, β-arrestins, GS nor adenylyl cyclases colocalize with retromer on the Golgi, and β-arrestins did not colocalize with domains of the early endosome labeled by retromer (Figure 2) (26). A simple and coherent model to explain the unexpected roles played by retromer and arrestin in PTH-mediated cAMP generation would hold that PTHR–arrestin complexes internalize together to the early endosome while cycling between bound and unbound states; any receptor not bound to β-arrestin may instead bind retromer, preventing interactions with arrestin and Gs, or stabilizing the inactive state of the receptor and initiating traffic of PTHR to the trans-Golgi network by way of a distinct domain of the early endosome (Figure 3).
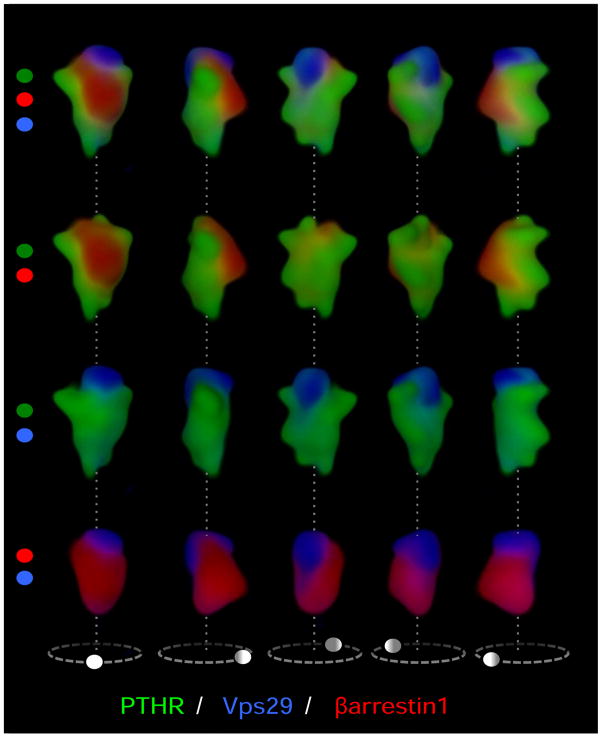
Figure 2.
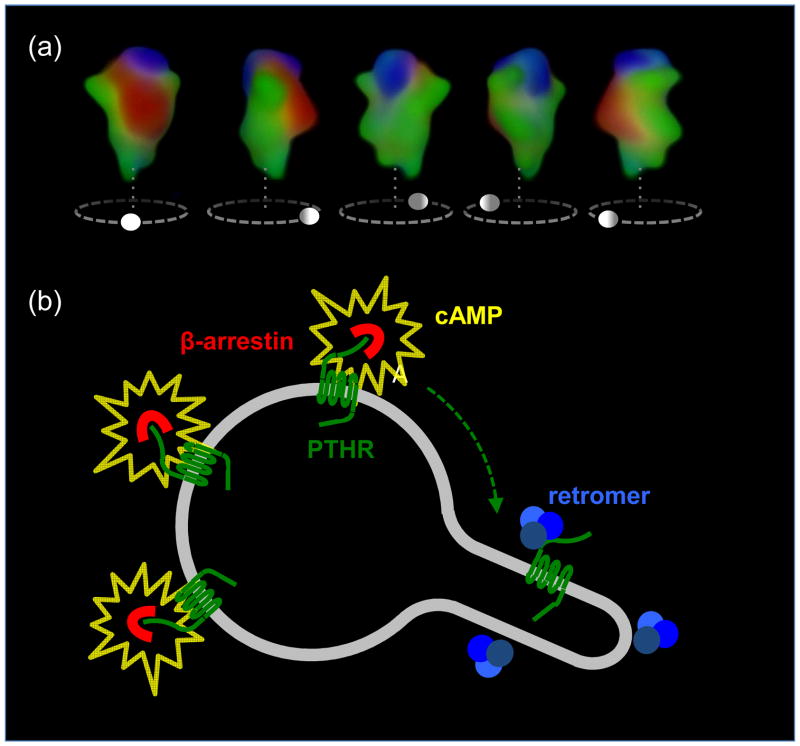
Signaling dynamics of PTHR on the early endosome. (a) We have recently shown that complexes of β-arrestin 1 (red) and PTHR (green) internalize to a compartment of the early endosome that is labeled red-green in a 3D reconstruction of early endosomes visualized with a spectral confocal microscope (top). A second compartment labeled with the sorting complex retromer (blue) is labeled blue-green. Arrestin and retromer do not colocalize, indicating that the two proteins localize in distinct compartments of the endosome, most likely the bulk domain (arrestin) and a domain dedicated to endosome-to-Golgi retrograde traffic (retromer). (b) Persistent complexes of PTHR-arrestin generate cAMP (yellow) from endosomal membranes. However, after arrestin-receptor decoupling, PTHR is free to bind retromer and sort to a compartment that does not support cAMP generation. Retromer-bound inactive PTHR then sorts to the trans-Golgi network before recycling to the plasma membrane. Adapted from (26).
Figure 3.
Proposed model sustained PTHR signaling. Left panel, PTH-activated PTHR (green) generating cAMP (grey) by activation of adenyly cyclases internalizes to early endosomes in a process that involves binding of β-arrestin (red). Activated PTHR is then maintained in the early endosome bulk compartment by arrestin binding, where arrestin-mediated activation of ERK1/2 signaling causes inhibition of phosphodiesterases and permits sustained cAMP signaling. Right panel, Binding of PTHR and retromer (blue) causes sorting of the receptor to retrograde trafficking domains. Generation of cAMP is stopped after PTHR–retromer binding in the retrograde domain and retromer-mediated PTHR traffic to the Golgi. Adapted from (26).
Concluding remarks
Studies discussed in this review suggest that PTHR can adopt multiple conformations stabilized by different ligands. This conformational selectivity in turn influences the down-stream signaling responses in target cells. Understanding how these ligand-specific events occur is critical to determine the molecular and cellular mechanisms underlying the anabolic and catabolic effects that PTHR ligands have on bone mass, depending on duration and timing of exposure. Based on available clinical data, Andrew Stewart and colleagues suggested that PTHrP(1–36) has greater efficacy in building bone mass in humans than does PTH(1–34), and thus might be a more effective treatment for osteoporosis (58). These considerations, coupled with our new findings on ligand-based conformational selectivity of PTHR, point to the prediction that R0-selective ligands, due to their prolonged action via endosomal PTHR/GS/cAMP signaling, would favor bone-resorption responses associated with sustained calcium release, and thus be candidate therapies for hypoparathyroidism (59, 60); conversely, RG-selective ligands, due to short and transient action at the receptor, would favor bone anabolism responses, and be candidate therapies for osteoporosis.
Initially revealed for arrestin-dependent ERK and non-receptor tyrosine kinase (src) signaling pathways (61–65), and also receptor tyrosine kinase pathways (66), endosomal signaling via G protein has been documented in yeast (67–69) and is now an emerging topic for GPCR biology in vertebrates. Indeed, sustained cAMP production mediated by endosomal G-protein signaling appears to be a new pathway not only for PTHR function but also for the class 1 GPCRs, the thyroid-stimulating hormone (TSH) receptor (70), and the dopamine D1 receptor (D1R) (67). In a few cases (PTHR, D1R) reported so far, receptor internalization appears to be necessary for sustained generation of cAMP. These recent developments put a finer point on the possibility that exceptions exist to the classic rule of arrestin preventing receptor–G protein coupling and signal termination. For the PTHR, prolonged cAMP signaling is mediated by ligands that bind to a high affinity receptor conformation, R0, and thus form complexes that include GαS and arrestin, and which remain stable within early endosomes. Transit of these complexes to late endosomes results in the exchange of arrestin for retromer, which correlates temporally with signal termination. Future studies will determine the molecular mechanism by which the interaction of arrestin with the PTHR permits a sustained Gs signaling, and reveal its physiological relevance for ions and mineral metabolism.
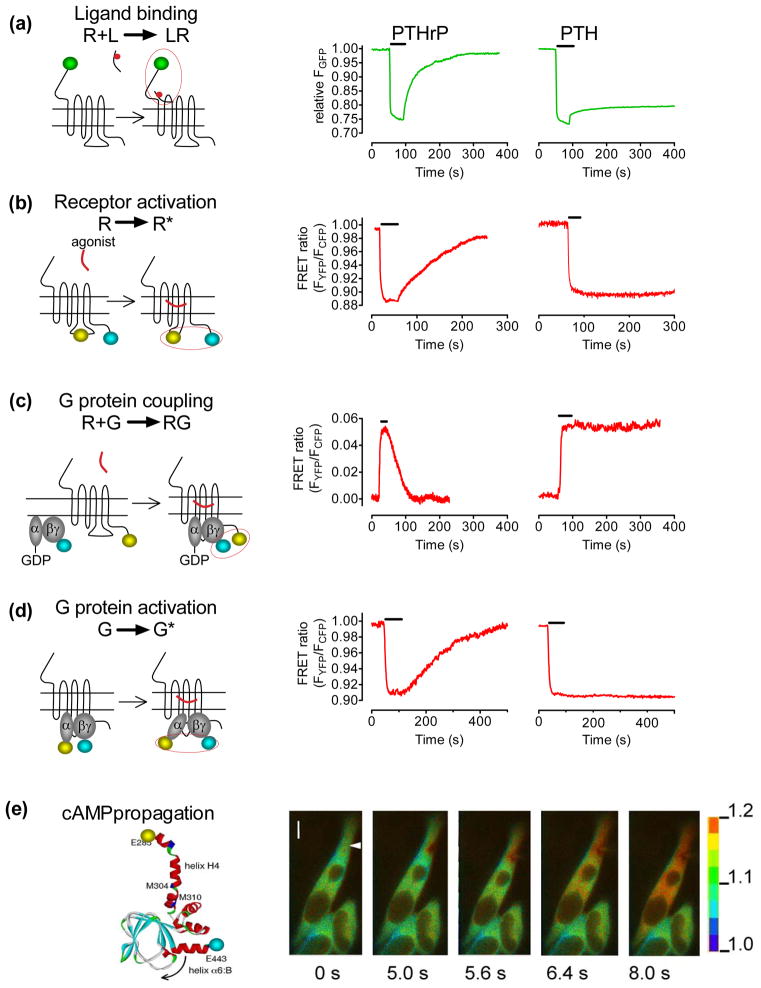
Figure I.
Kinetics of PTHR signaling. (a) Ligand/receptor interaction measured by FRET between GFP-tagged PTHR and tetramethylrhodamine-labeled PTH(1–34) or PTHrP(1–36). Shown are the changes of GFP emission by FRET in response to rapid superfusion of diverse concentrations of ligand-TMR. (b) Following ligand application (horizontal bar), activation of PTHR was monitored in a single HEK-293 cell by a decrease in the FRET signal of PTHRCFP/YFP defined as the ratio of emission intensities of YFP/CFP. (c) The interaction between PTHR and GS proteins in response to ligand binding is measured as an increase in FRET between YFP-labeled PTHR and CFP-labeled Gγ2 in combination with GαS and Gβ1 proteins. (d) Detection of GS activation in cells expressing the wild-type PTHR by recording FRET between YFP-labeled Gαs and CFP-labeled Gγ2-subunits. (e) PTH-mediated cAMP response upon PTHR activation in HEK-293 cells measured as a decrease of FRET in the EpacCFP/YFP sensor. The panels show the propagation of the cAMP response represented as pseudocolored image of the FRET (CFP/YFP emission) ratio before and after stimulation of a single cell with PTH(1–34) via a pipette indicated by an arrow at t = 0 s. The scale bar on the right indicates the pseudocolored scale of the fluorescence ratios. The inner bar represents 5 μm. Adapted from (25).
Acknowledgments
This work was supported by the National Institutes of Health (grant award R01 DK087688 to J.P.V.).
Footnotes
Conflicts of interest. J.-P.V. holds a patent on the FRET technology of measuring GPCR activation/deactivation by FRET (EP 1581811B1; US8084575).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Juppner H, Abou-Samra AB, Freeman M, Kong XF, Schipani E, Richards J, Kolakowski LF, Jr, Hock J, Potts JT, Jr, Kronenberg HM, et al. A G protein-linked receptor for parathyroid hormone and parathyroid hormone-related peptide. Science. 1991;254:1024–1026. doi: 10.1126/science.1658941. [DOI] [PubMed] [Google Scholar]
- 2.Schwindinger WF, Fredericks J, Watkins L, Robinson H, Bathon JM, Pines M, Suva LJ, Levine MA. Coupling of the PTH/PTHrP receptor to multiple G-proteins. Direct demonstration of receptor activation of Gs, Gq/11, and Gi(1) by [alpha-32P]GTP-gamma-azidoanilide photoaffinity labeling. Endocrine. 1998;8:201–209. doi: 10.1385/ENDO:8:2:201. [DOI] [PubMed] [Google Scholar]
- 3.Gensure RC, Gardella TJ, Juppner H. Parathyroid hormone and parathyroid hormone-related peptide, and their receptors. Biochem Biophys Res Commun. 2005;328:666–678. doi: 10.1016/j.bbrc.2004.11.069. [DOI] [PubMed] [Google Scholar]
- 4.Singh AT, Gilchrist A, Voyno-Yasenetskaya T, Radeff-Huang JM, Stern PA. G alpha12/G alpha13 subunits of heterotrimeric G proteins mediate parathyroid hormone activation of phospholipase D in UMR-106 osteoblastic cells. Endocrinology. 2005;146:2171–2175. doi: 10.1210/en.2004-1283. [DOI] [PubMed] [Google Scholar]
- 5.Gesty-Palmer D, Chen M, Reiter E, Ahn S, Nelson CD, Wang S, Eckhardt AE, Cowan CL, Spurney RF, Luttrell LM, Lefkowitz RJ. Distinct beta-arrestin- and G protein-dependent pathways for parathyroid hormone receptor-stimulated ERK1/2 activation. J Biol Chem. 2006;281:10856–10864. doi: 10.1074/jbc.M513380200. [DOI] [PubMed] [Google Scholar]
- 6.Sneddon WB, Yang Y, Ba J, Harinstein LM, Friedman PA. Extracellular signal-regulated kinase activation by parathyroid hormone in distal tubule cells. Am J Physiol Renal Physiol. 2007;292:F1028–1034. doi: 10.1152/ajprenal.00288.2006. [DOI] [PubMed] [Google Scholar]
- 7.Zhang P, Jobert AS, Couvineau A, Silve C. A homozygous inactivating mutationin the parathyroid hormone/parathyroid hormone-related peptide receptor causing Blomstrand chondrodysplasia. J Clin Endocrinol Metab. 1998;83:3365–3368. doi: 10.1210/jcem.83.9.5245. [DOI] [PubMed] [Google Scholar]
- 8.Bastepe M, Raas-Rothschild A, Silver J, Weissman I, Wientroub S, Juppner H, Gillis D. A form of Jansen's metaphyseal chondrodysplasia with limited metabolic and skeletal abnormalities is caused by a novel activating parathyroid hormone (PTH)/PTH-related peptide receptor mutation. J Clin Endocrinol Metab. 2004;89:3595–3600. doi: 10.1210/jc.2004-0036. [DOI] [PubMed] [Google Scholar]
- 9.Duchatelet S, Ostergaard E, Cortes D, Lemainque A, Julier C. Recessive mutations in PTHR1 cause contrasting skeletal dysplasias in Eiken and Blomstrand syndromes. Hum Mol Genet. 2005;14:1–5. doi: 10.1093/hmg/ddi001. [DOI] [PubMed] [Google Scholar]
- 10.Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY, Hodsman AB, Eriksen EF, Ish-Shalom S, Genant HK, Wang O, Mitlak BH. Effect of parathyroid hormone (1–34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001;344:1434–1441. doi: 10.1056/NEJM200105103441904. [DOI] [PubMed] [Google Scholar]
- 11.Miller PD, Bilezikian JP, Diaz-Curiel M, Chen P, Marin F, Krege JH, Wong M, Marcus R. Occurrence of hypercalciuria in patients with osteoporosis treated with teriparatide. J Clin Endocrinol Metab. 2007;92:3535–3541. doi: 10.1210/jc.2006-2439. [DOI] [PubMed] [Google Scholar]
- 12.Miller PD, Schwartz EN, Chen P, Misurski DA, Krege JH. Teriparatide in postmenopausal women with osteoporosis and mild or moderate renal impairment. Osteoporos Int. 2007;18:59–68. doi: 10.1007/s00198-006-0189-8. [DOI] [PubMed] [Google Scholar]
- 13.Antoniucci DM, Sellmeyer DE, Bilezikian JP, Palermo L, Ensrud KE, Greenspan SL, Black DM. Elevations in serum and urinary calcium with parathyroid hormone (1–84) with and without alendronate for osteoporosis. J Clin Endocrinol Metab. 2007;92:942–947. doi: 10.1210/jc.2006-1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vilardaga JP, Lin I, Nissenson RA. Analysis of parathyroid hormone (PTH)/secretin receptor chimeras differentiates the role of functional domains in the pth/ pth-related peptide (PTHrP) receptor on hormone binding and receptor activation. Mol Endocrinol. 2001;15:1186–1199. doi: 10.1210/mend.15.7.0665. [DOI] [PubMed] [Google Scholar]
- 15.Juppner H, Schipani E, Bringhurst FR, McClure I, Keutmann HT, Potts JT, Jr, Kronenberg HM, Abou-Samra AB, Segre GV, Gardella TJ. The extracellular amino-terminal region of the parathyroid hormone (PTH)/PTH-related peptide receptor determines the binding affinity for carboxyl-terminal fragments of PTH-(1–34) Endocrinology. 1994;134:879–884. doi: 10.1210/endo.134.2.8299582. [DOI] [PubMed] [Google Scholar]
- 16.Gardella TJ, Luck MD, Wilson AK, Keutmann HT, Nussbaum SR, Potts JT, Jr, Kronenberg HM. Parathyroid hormone (PTH)-PTH-related peptide hybrid peptides reveal functional interactions between the 1–14 and 15–34 domains of the ligand. J Biol Chem. 1995;270:6584–6588. doi: 10.1074/jbc.270.12.6584. [DOI] [PubMed] [Google Scholar]
- 17.Gardella TJ, Wilson AK, Keutmann HT, Oberstein R, Potts JT, Jr, Kronenberg M, Nussbaum SR. Analysis of parathyroid hormone's principal receptor-binding region by site-directed mutagenesis and analog design. Endocrinology. 1993;132:2024–2030. doi: 10.1210/endo.132.5.8386605. [DOI] [PubMed] [Google Scholar]
- 18.Gardella TJ, Juppner H. Molecular properties of the PTH/PTHrP receptor. Trends Endocrinol Metab. 2001;12:210–217. doi: 10.1016/s1043-2760(01)00409-x. [DOI] [PubMed] [Google Scholar]
- 19.Hoare SR, Gardella TJ, Usdin TB. Evaluating the signal transduction mechanism of the parathyroid hormone 1 receptor. Effect of receptor-G-protein interaction on the ligand binding mechanism and receptor conformation. J Biol Chem. 2001;276:7741–7753. doi: 10.1074/jbc.M009395200. [DOI] [PubMed] [Google Scholar]
- 20.Shimizu M, Carter PH, Khatri A, Potts JT, Jr, Gardella TJ. Enhanced activity in parathyroid hormone-(1–14) and -(1–11): novel peptides for probing ligand-receptor interactions. Endocrinology. 2001;142:3068–3074. doi: 10.1210/endo.142.7.8253. [DOI] [PubMed] [Google Scholar]
- 21.Shimizu M, Potts JT, Jr, Gardella TJ. Minimization of parathyroid hormone. Novel amino-terminal parathyroid hormone fragments with enhanced potency in activating the type-1 parathyroid hormone receptor. J Biol Chem. 2000;275:21836–21843. doi: 10.1074/jbc.M909861199. [DOI] [PubMed] [Google Scholar]
- 22.Shimizu N, Dean T, Tsang JC, Khatri A, Potts JT, Jr, Gardella TJ. Novel parathyroid hormone (PTH) antagonists that bind to the juxtamembrane portion of the PTH/PTH-related protein receptor. J Biol Chem. 2005;280:1797–1807. doi: 10.1074/jbc.M408270200. [DOI] [PubMed] [Google Scholar]
- 23.Vilardaga JP, Bunemann M, Krasel C, Castro M, Lohse MJ. Measurement of the millisecond activation switch of G protein-coupled receptors in living cells. Nat Biotechnol. 2003;21:807–812. doi: 10.1038/nbt838. [DOI] [PubMed] [Google Scholar]
- 24.Castro M, Nikolaev VO, Palm D, Lohse MJ, Vilardaga JP. Turn-on switch in parathyroid hormone receptor by a two-step parathyroid hormone binding mechanism. Proc Natl Acad Sci U S A. 2005;102:16084–16089. doi: 10.1073/pnas.0503942102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferrandon S, Feinstein TN, Castro M, Wang B, Bouley R, Potts JT, Gardella TJ, Vilardaga JP. Sustained cyclic AMP production by parathyroid hormone receptor endocytosis. Nat Chem Biol. 2009;5:734–742. doi: 10.1038/nchembio.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feinstein TN, Wehbi VL, Ardura JA, Wheeler DS, Ferrandon S, Gardella TJ, Vilardaga JP. Retromer terminates the generation of cAMP by internalized PTH receptors. Nat Chem Biol. 2011;7:278–284. doi: 10.1038/nchembio.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vilardaga JP, Romero G, Friedman PA, Gardella TJ. Molecular basis of parathyroid hormone receptor signaling and trafficking: a family B GPCR paradigm. Cell Mol Life Sci. 2011 doi: 10.1007/s00018-010-0465-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vilardaga JP, Bunemann M, Feinstein TN, Lambert N, Nikolaev VO, Engelhardt S, Lohse MJ, Hoffmann C. GPCR and G proteins: drug efficacy and activation in live cells. Mol Endocrinol. 2009;23:590–599. doi: 10.1210/me.2008-0204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoare SR, Sullivan SK, Pahuja A, Ling N, Crowe PD, Grigoriadis DE. Conformational states of the corticotropin releasing factor 1 (CRF1) receptor: detection, and pharmacological evaluation by peptide ligands. Peptides. 2003;24:1881–1897. doi: 10.1016/j.peptides.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 30.Hoare SR, Sullivan SK, Schwarz DA, Ling N, Vale WW, Crowe PD, Grigoriadis DE. Ligand affinity for amino-terminal and juxtamembrane domains of the corticotropin releasing factor type I receptor: regulation by G-protein and nonpeptide antagonists. Biochemistry. 2004;43:3996–4011. doi: 10.1021/bi036110a. [DOI] [PubMed] [Google Scholar]
- 31.De Lean A, Stadel J, Lefkowitz R. A Ternary complex model explains the agonist-specific binding properties of the adenylate cyclase-coupled β-adrenergic receptor. J Biol Chem. 1980;255:7108–7117. [PubMed] [Google Scholar]
- 32.Dean T, Linglart A, Mahon MJ, Bastepe M, Juppner H, Potts JT, Jr, Gardella TJ. Mechanisms Of Ligand Binding To The PTH/PTHrp Receptor: Selectivity Of A Modified PTH(1–15) Radioligand For G{Alpha}S-Coupled Receptor Conformations. Mol Endocrinol. 2006;20:931–942. doi: 10.1210/me.2005-0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dean T, Vilardaga JP, Potts JT, Jr, Gardella TJ. Altered Selectivity of Parathyroid Hormone (PTH) and PTH-Related Protein (PTHrP) for Distinct Conformations of the PTH/PTHrP Receptor. Mol Endocrinol. 2008;22:156–166. doi: 10.1210/me.2007-0274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hoare SR, de Vries G, Usdin TB. Measurement of agonist and antagonist ligand-binding parameters at the human parathyroid hormone type 1 receptor: evaluation of receptor states and modulation by guanine nucleotide. J Pharmacol Exp Ther. 1999;289:1323–1333. [PubMed] [Google Scholar]
- 35.Berlot CH. A highly effective dominant negative alpha s construct containing mutations that affect distinct functions inhibits multiple Gs-coupled receptor signaling pathways. J Biol Chem. 2002;277:21080–21085. doi: 10.1074/jbc.M201330200. [DOI] [PubMed] [Google Scholar]
- 36.Okazaki M, Ferrandon S, Vilardaga JP, Bouxsein ML, Potts JT, Jr, Gardella TJ. Prolonged signaling at the parathyroid hormone receptor by peptide ligands targeted to a specific receptor conformation. Proc Natl Acad Sci U S A. 2008;105:16525–16530. doi: 10.1073/pnas.0808750105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Okazaki M, Nagai S, Dean T, Potts JJ, Gardella T. Analysis of PTH-PTH Receptor Interaction Mechanisms Using a New, Long-Acting PTH(1–28) Analog Reveals Selective Binding to Distinct PTH Receptor Conformations and Biological Consequences In Vivo. J Bone Min Res. 2007;22(Suppl):Abstract 1190. [Google Scholar]
- 38.Nagai S, Okazaki M, Potts JJ, Juppner H, Gardella T. Dissection of the Mechanisms of PTH-mediated Inhibition of Sodium-dependent Phosphate Transport Using a Long-acting PTH(1–28) Analog. J Bone Min Res. 2007;22(Suppl):Abstract 1189. [Google Scholar]
- 39.Lohse MJ, Benovic JL, Codina J, Caron MG, Lefkowitz RJ. beta-Arrestin: a protein that regulates beta-adrenergic receptor function. Science. 1990;248:1547–1550. doi: 10.1126/science.2163110. [DOI] [PubMed] [Google Scholar]
- 40.Lohse MJ, Andexinger S, Pitcher J, Trukawinski S, Codina J, Faure JP, Caron MG, Lefkowitz RJ. Receptor-specific desensitization with purified proteins. Kinase dependence and receptor specificity of beta-arrestin and arrestin in the beta 2-adrenergic receptor and rhodopsin systems. J Biol Chem. 1992;267:8558–8564. [PubMed] [Google Scholar]
- 41.Pitcher J, Lohse MJ, Codina J, Caron MG, Lefkowitz RJ. Desensitization of the isolated beta 2-adrenergic receptor by beta-adrenergic receptor kinase, cAMP-dependent protein kinase, and protein kinase C occurs via distinct molecular mechanisms. Biochemistry. 1992;31:3193–3197. doi: 10.1021/bi00127a021. [DOI] [PubMed] [Google Scholar]
- 42.Pippig S, Andexinger S, Daniel K, Puzicha M, Caron MG, Lefkowitz RJ, Lohse MJ. Overexpression of beta-arrestin and beta-adrenergic receptor kinase augment desensitization of beta 2-adrenergic receptors. J Biol Chem. 1993;268:3201–3208. [PubMed] [Google Scholar]
- 43.Nelson CD, Perry SJ, Regier DS, Prescott SM, Topham MK, Lefkowitz RJ. Targeting of diacylglycerol degradation to M1 muscarinic receptors by beta-arrestins. Science. 2007;315:663–666. doi: 10.1126/science.1134562. [DOI] [PubMed] [Google Scholar]
- 44.Perry SJ, Baillie GS, Kohout TA, McPhee I, Magiera MM, Ang KL, Miller WE, McLean AJ, Conti M, Houslay MD, Lefkowitz RJ. Targeting of cyclic AMP degradation to beta 2-adrenergic receptors by beta-arrestins. Science. 2002;298:834–836. doi: 10.1126/science.1074683. [DOI] [PubMed] [Google Scholar]
- 45.Ferguson SS, Downey WE, 3rd, Colapietro AM, Barak LS, Menard L, Caron MG. Role of beta-arrestin in mediating agonist-promoted G protein-coupled receptor internalization. Science. 1996;271:363–366. doi: 10.1126/science.271.5247.363. [DOI] [PubMed] [Google Scholar]
- 46.Vilardaga JP, Krasel C, Chauvin S, Bambino T, Lohse MJ, Nissenson RA. Internalization determinants of the parathyroid hormone receptor differentially regulate beta-arrestin/receptor association. J Biol Chem. 2002;277:8121–8129. doi: 10.1074/jbc.M110433200. [DOI] [PubMed] [Google Scholar]
- 47.Ferrari SL, Behar V, Chorev M, Rosenblatt M, Bisello A. Endocytosis of ligand-human parathyroid hormone receptor 1 complexes is protein kinase C-dependent and involves beta-arrestin2. Real-time monitoring by fluorescence microscopy. J Biol Chem. 1999;274:29968–29975. doi: 10.1074/jbc.274.42.29968. [DOI] [PubMed] [Google Scholar]
- 48.Castro M, Dicker F, Vilardaga JP, Krasel C, Bernhardt M, Lohse MJ. Dual regulation of the parathyroid hormone (PTH)/PTH-related peptide receptor signaling by protein kinase C and beta-arrestins. Endocrinology. 2002;143:3854–3865. doi: 10.1210/en.2002-220232. [DOI] [PubMed] [Google Scholar]
- 49.Yang M, He RL, Benovic JL, Ye RD. beta-Arrestin1 interacts with the G-protein subunits beta1gamma2 and promotes beta1gamma2-dependent Akt signalling for NF-kappaB activation. Biochem J. 2009;417:287–296. doi: 10.1042/BJ20081561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pioszak AA, Harikumar KG, Parker NR, Miller LJ, Xu HE. Dimeric arrangement of the parathyroid hormone receptor and a structural mechanism for ligand-induced dissociation. J Biol Chem. 2010;285:12435–12444. doi: 10.1074/jbc.M109.093138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mahon MJ, Bonacci TM, Divieti P, Smrcka AV. A docking site for G protein βγ subunits on the parathyroid hormone 1 receptor supports signaling through multiple pathways. Mol Endocrinol. 2006;20:136–146. doi: 10.1210/me.2005-0169. [DOI] [PubMed] [Google Scholar]
- 52.Violin JD, DiPilato LM, Yildirim N, Elston TC, Zhang J, Lefkowitz RJ. beta2-adrenergic receptor signaling and desensitization elucidated by quantitative modeling of real time cAMP dynamics. J Biol Chem. 2008;283:2949–2961. doi: 10.1074/jbc.M707009200. [DOI] [PubMed] [Google Scholar]
- 53.Platta HW, Stenmark H. Endocytosis and signaling. Curr Opin Cell Biol. 2011;23:393–403. doi: 10.1016/j.ceb.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 54.Wang B, Bisello A, Yang Y, Romero GG, Friedman PA. NHERF1 regulates parathyroid hormone receptor membrane retention without affecting recycling. J Biol Chem. 2007;282:36214–36222. doi: 10.1074/jbc.M707263200. [DOI] [PubMed] [Google Scholar]
- 55.Garrido JL, Wheeler D, Vega LL, Friedman PA, Romero G. Role of phospholipase D in parathyroid hormone type 1 receptor signaling and trafficking. Mol Endocrinol. 2009;23:2048–2059. doi: 10.1210/me.2008-0436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bonifacino JS, Rojas R. Retrograde transport from endosomes to the trans-Golgi network. Nat Rev Mol Cell Biol. 2006;7:568–579. doi: 10.1038/nrm1985. [DOI] [PubMed] [Google Scholar]
- 57.Aubry L, Guetta D, Klein G. The arrestin fold: variations on a theme. Curr Genomics. 2009;10:133–142. doi: 10.2174/138920209787847014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bisello A, Horwitz MJ, Stewart AF. Parathyroid hormone-related protein: an essential physiological regulator of adult bone mass. Endocrinology. 2004;145:3551–3553. doi: 10.1210/en.2004-0509. [DOI] [PubMed] [Google Scholar]
- 59.Winer KK, Ko CW, Reynolds JC, Dowdy K, Keil M, Peterson D, Gerber LH, McGarvey C, Cutler GB., Jr Long-term treatment of hypoparathyroidism: a randomized controlled study comparing parathyroid hormone-(1–34) versus calcitriol and calcium. J Clin Endocrinol Metab. 2003;88:4214–4220. doi: 10.1210/jc.2002-021736. [DOI] [PubMed] [Google Scholar]
- 60.Mittelman SD, Hendy GN, Fefferman RA, Canaff L, Mosesova I, Cole DE, Burkett L, Geffner ME. A hypocalcemic child with a novel activating mutation of the calcium-sensing receptor gene: successful treatment with recombinant human parathyroid hormone. J Clin Endocrinol Metab. 2006;91:2474–2479. doi: 10.1210/jc.2005-2605. [DOI] [PubMed] [Google Scholar]
- 61.Reiter E, Ahn S, Shukla AK, Lefkowitz RJ. Molecular mechanism of beta-arrestin-biased agonism at seven-transmembrane receptors. Annu Rev Pharmacol Toxicol. 52:179–197. doi: 10.1146/annurev.pharmtox.010909.105800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shenoy SK, Barak LS, Xiao K, Ahn S, Berthouze M, Shukla AK, Luttrell LM, Lefkowitz RJ. Ubiquitination of beta-arrestin links seven-transmembrane receptor endocytosis and ERK activation. J Biol Chem. 2007;282:29549–29562. doi: 10.1074/jbc.M700852200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Drake MT, Shenoy SK, Lefkowitz RJ. Trafficking of G protein-coupled receptors. Circ Res. 2006;99:570–582. doi: 10.1161/01.RES.0000242563.47507.ce. [DOI] [PubMed] [Google Scholar]
- 64.Hanyaloglu AC, von Zastrow M. Regulation of GPCRs by endocytic membrane trafficking and its potential implications. Annu Rev Pharmacol Toxicol. 2008;48:537–568. doi: 10.1146/annurev.pharmtox.48.113006.094830. [DOI] [PubMed] [Google Scholar]
- 65.Shukla AK, Xiao K, Lefkowitz RJ. Emerging paradigms of beta-arrestin-dependent seven transmembrane receptor signaling. Trends Biochem Sci. 2011;36:457–469. doi: 10.1016/j.tibs.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pyne NJ, Pyne S. Receptor tyrosine kinase-G-protein-coupled receptor signalling platforms: out of the shadow? Trends Pharmacol Sci. 32:443–450. doi: 10.1016/j.tips.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 67.Kotowski SJ, Hopf FW, Seif T, Bonci A, von Zastrow M. Endocytosis promotes rapid dopaminergic signaling. Neuron. 2011;71:278–290. doi: 10.1016/j.neuron.2011.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Slessareva JE, Routt SM, Temple B, Bankaitis VA, Dohlman HG. Activation of the phosphatidylinositol 3-kinase Vps34 by a G protein alpha subunit at the endosome. Cell. 2006;126:191–203. doi: 10.1016/j.cell.2006.04.045. [DOI] [PubMed] [Google Scholar]
- 69.Slessareva JE, Dohlman HG. G protein signaling in yeast: new components, new connections, new compartments. Science. 2006;314:1412–1413. doi: 10.1126/science.1134041. [DOI] [PubMed] [Google Scholar]
- 70.Calebiro D, Nikolaev VO, Gagliani MC, de Filippis T, Dees C, Tacchetti C, Persani L, Lohse MJ. Persistent cAMP-signals triggered by internalized G-protein-coupled receptors. PLoS Biol. 2009;7:e1000172. doi: 10.1371/journal.pbio.1000172. [DOI] [PMC free article] [PubMed] [Google Scholar]