Abstract
Reactive nitrogen species (RNS) such as nitrogen dioxide (•NO2), peroxynitrite (ONOO–), and nitrosoperoxycarbonate (ONOOCO2–) are among the most damaging species present in biological systems due to their ability to cause modification of key biomolecular systems through oxidation, nitrosylation and nitration. Nitrone spin traps are known to react with free radicals and non-radicals via electrophilic and nucleophilic addition reactions, and have been employed as reagents to detect radicals using electron paramagnetic resonance (EPR) spectroscopy, and as pharmacological agents against oxidative stress-mediated injury. This study examines the reactivity of cyclic nitrones such as 5,5-dimethylpyrroline N-oxide (DMPO) with, •NO2, ONOO–, ONOOCO2–, SNAP and SIN-1 using EPR. The thermochemistries of nitrone reactivity with RNS, and isotropic hfsc's of the addition products were also calculated at the PCM(water)/B3LYP/6-31+G**//B3LYP/6-31G* level of theory with and without explicit water molecules in order to rationalize the nature of the observed EPR spectra. Spin trapping of other RNS such as azide (•N3), nitrogen trioxide (•NO3), amino (•NH2) radicals, and nitroxyl (HNO) were also theoretically and experimentally investigated by EPR spin trapping and mass spectrometry. This study also shows other spin traps such as AMPO, EMPO and DEPMPO can react with radical and non-radical RNS, thus, making spin traps suitable probes as well as antioxidants against RNS mediated oxidative damage.
Introduction
It has become clear that reactive nitrogen species (RNS) along with reactive oxygen species (ROS) have been implicated in the pathogenesis of various diseases1-6 or as key mediators in cell signaling7 and immune response.8 Nitrosative stress† results from a myriad of biomolecular modifications 9-15 caused by RNS such as the paramagnetic nitric oxide (•NO), nitrogen dioxide (•NO2), as well as the diamagnetic peroxynitrite (ONOO–). Nitric oxide is known to be formed in vivo by nitric oxide synthase (NOS) from L-arginine and O2,16 or can be released directly by synthetic molecules such as S-nitroso-N-acetyl-DL-penicillamine (SNAP).17 The NO-generating function of hemoglobin as an S-nitrosothiol synthase using nitrite as substrate has been proposed.18 Nitric oxide is a free radical that serves as an intracellular messenger and vasodilator, and its toxicity is generally limited to its reaction or oxidation to more highly reactive species such as ONOO– and •NO2 (Scheme 1).19
Scheme 1.
Formation of RNS from •NO.
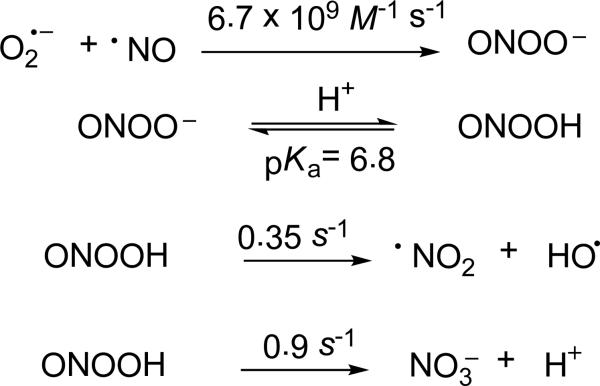
Peroxynitrite is formed from the addition reaction of •NO with superoxide (O2•–) at a diffusion-controlled rate.20,21 Peroxynitrite is known to exist in the relatively stable cis-conformation, or gain a proton to form peroxynitrous acid (ONOOH, pKa 6.8). Like O2•–, ONOO– is capable of reacting with protein active sites containing Cu, Zn, sulfhydryl and Fe-S clusters to cause nitration and protein cleavage resulting in enzyme deactivation.22-25 Currently, indirect methods of ONOO– detection are limited by their sensitivity and specificity, which include fluorescence,26-28 electrochemistry,29 tyrosine, tryptophan and DNA nitration,30,31 and indirect radical detection via electron paramagnetic resonance (EPR) spectroscopy.32 It should be noted however that detection of nitration is not exclusively an indication of peroxynitrite production and that the nitrating agent, NO2, can originate from other routes.33 Attempts to trap •NO and O2•– concurrently using Fe(DTCS)2 and various nitrones, respectively, only yielded individual spectrum of •NO, O2•– and HO• adducts due to the varying rates of reaction of these radicals with the spin traps.34 One emerging method of detection is the selective reaction of ONOO– with boronates coupled with fluorescence spectroscopy.35
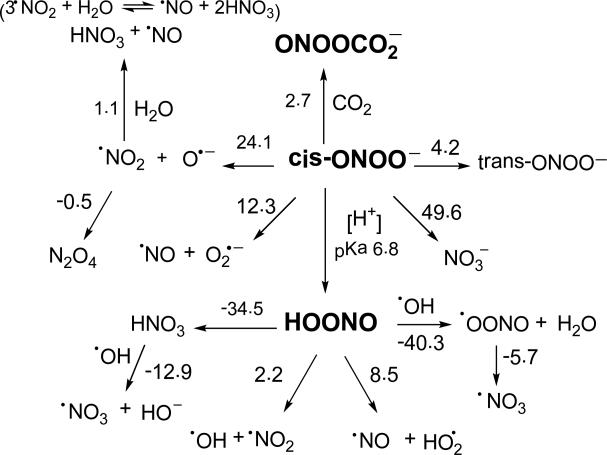
One relevant mechanism for ONOO–/ONOOH decay is its homolytic cleavage through •ONO...O•– and •ONO...•OH intermediates (Scheme 2).36 For ONOOH, the rate of radical cleavage has been reported to be 0.35 ± 0.03 s-1, with about 30% •OH and •NO2 release at pH < 5 via escape from the solvent cage. The rate constant for ONOOH isomerization to nitric acid (HNO3) was found to be 1.1± 0.1 s-1.37 Hydrolysis of •NO2 results in the formation of NO2– and NO3–37,38 . For ONOO–, the rate of radical cleavage has been reported at ≈ 10-6 s-1, with negligible •NO2 and O•– release.39 While the low rate of homolytic cleavage of ONOO– makes the reaction trivial, ONOO– is known to react with dissolved CO2 to form nitrosoperoxycarbonate (ONOOCO2–), an oxidizing species that undergoes homolytic cleavage to form 30% CO3•– and •NO2. 40,41 The decay of ONOOCO2– and ONOOH has been shown to vary depending on the ability of the solvent to hold the intermediate species in the solvent cage, and is therefore, dependent on the viscosity of solvent.42 Peroxynitrite is a strong nucleophile, and has been shown to cause β-scission of carbonyl groups,43,44 where acyl- and H-spin adducts have been observed using EPR spin trapping.45,46 Peroxynitrite has recently been shown to form peroxynitrate (O2NOO–) at neutral pH through combination of ONOO– and ONOOH to form O2NOOH and nitrite (NO2–).47•NO2 is also known to dimerize into N2O4 at moderate concentrations which effectively hides its radical character.48
Scheme 2.
Proposed decomposition and reactions of ONOO–, and their respective ΔG298K,rxn (in kcal/mol) calculated at the PCM/B3LYP/6-31+G**//B3LYP/6-31G* level of theory
Nitrogen trioxide radical (•NO3) is an important oxidant in the troposphere and has been considered as a major oxidant for a variety of unsaturated gas-phase organic species. The reactivity of •NO3 radical with organic compounds occurs via hydrogen atom abstraction or addition to double bond with a rate constant range of 105-107 M-1s-1 and 5 × 107 M-1s-1, respectively.49 Azide anion binds to many heme proteins and metalloenzymes inhibiting their activities. It is also a potent inhibitor of mitochondrial electron transport chain by blocking cytochrome c oxidase and preventing ATP hydrolysis.50 Azidyl radical (•N3) has been generated in solution and their formation was observed using spin trapping technique via oxidation of azide anion by peroxidases in the presence of H2O2,51 succinate-driven respiration in azide-inhibited rat brain submitochondrial particles,52 or via oxidation of azide anion in cadmium sulfide and zinc oxide suspensions.53 Ammonia is one of the most important trace gases in the atmosphere. Activation of NH3 in aqueous solution leads to the formation of a reactive amidogen (•NH2) radical which can later react with a variety of molecular species such as O2, amino acids and melanins.54 Nitroxyl (HNO) (pKa = 11.4) is the one-electron reduction product of •NO and has been shown to regulate cellular function with unique pharmacological properties, specifically to cardiovascular diseases.55
Although EPR has been used to detect RNS in biological systems with hydroxylamine redox probes (e.g., TEMPONE-H),56 the interpretation of the observed signal due to the formation of nitroxide is complicated by the fact that the nitroxide can also be formed from a variety of other reactions.57 In this study we explored the reactivities of various RNS using EPR spin trapping. The spin trapping technique unambiguously identifies free radicals via formation of a persistent radical adduct from the addition reaction of a radical to a nitrone spin trap.58,59 Furthermore, spin adducts have also been shown to be formed via nucleophilic addition reaction to nitrones and subsequent oxidation to the paramagnetic adduct (Forrester-Hepburn mechanism), as demonstrated by others,60,61 making this technique appropriate to study reactive non-radical species as well. Another path is that of inverted spin trapping as proposed by Eberson62 where one-electron oxidation of nitrone yields the nitrone radical cation [nitrone]•+ and its subsequent addition to a nucleophile (Nu–) forms the nitrone-Nu spin adduct. Nitrones have been used for the detection of RNS,63 and have been shown to trap decomposition products and tertiary radicals formed from ONOO–.64 In this study, we also computationally investigated the thermodynamics of RNS reaction to nitrones with the aim to rationalize the nature of the EPR spectral data obtained from the reactions. Studies on the reaction of RNS with nitrones is important, not only for the purpose of RNS detection, but also to partly provide a rationale for the protective properties of nitrones against RNS-mediated cellular toxicity.65
Experimental Procedures
Computational Study
Initial conformational search of the RNS, ROS and spin traps and their respective adducts was carried out using Spartan 04 at the MMFF level. Density functional theory (DFT),66 at the B3LYP/6-31G* level of theory was employed in this study to determine the optimized geometry and each yielded no imaginary vibrational frequency. All calculations were performed with Gaussian 03,67 at the Ohio Supercomputer Center. A scaling factor of 0.9806,68 was used for the zero-point vibrational energy (ZPE) corrections for all the B3LYP/6-31G* geometries. The effects of solvation on the gas-phase calculations were also investigated by using the PCM,69,70 and the spin and charge densities were obtained from natural population analysis (NPA) approach,71 at the PCM/B3LYP/6-31+G** level of theory. Optimization was also performed for adducts with two explicit water molecules for prediction of hyperfine splitting constants at the PCM(water)/B3LYP/6-31+G**//B3LYP/6-31G*. Negligible spin contamination of 0.75 < 〈S2〉 < 0.76 was obtained for all the minima. Using the B3LYP/6-31G* optimized structures, additional calculation was performed for RNS/ROS shown in Table 1 at the G3(MP2) level of theory which is known to perform better than DFT for calculations involving enthalpies of formation, ionization potentials, electron affinities, proton affinities72,73
Table 1.
Free Energies of Reduction, ΔGaq,298K (in kcal/mol), of Selected RNS and ROS at the PCM(water)/B3LYP/6-31+G**//B3LYP/6-31G* and PCM(water)/G3(MP2)//B3LYP/6-31G* (in parentheses) Levels of Theory.
| Entry | Δ G 298K,red |
|---|---|
| ONOOCO2– + 2e– / NO2– + CO32– | -242.5 (-236.5) |
| N2O4 + 2e– / 2NO2– | -240.3 (-232.1) |
| cis-ONOOH + 2e– / HO– + NO2– | -237.1 (230.3) |
| trans-ONOOH + 2e– / HO– + NO2– | -235.6 (-231.8) |
| trans-ONOO– + 2e– / O2– + NO2– | -170.5 (-158.0) |
| cis-ONOO– + 2e– / O2– + NO2– | -166.3 (-153.9) |
| •NO3 + e– / NO3– | -152.3 (-155.0) |
| NO+ + e– / •NO | -146.6 (-140.7) |
| DMPO•+ + e– / DMPO | -139.7 (-147.8) |
| •N3 + e– / N3– | -124.1 (-121.0) |
| •NO2 + e– / NO2– | -120.4 (-116.8) |
| •OH + e– / HO– | -118.9 (-120.0) |
| CO3•– + e– / CO32- | -103.3 (-115.9) |
| N2O4 + e– / N2O4•– | -102.0 (-115.3)a |
| HO2• + e– / HO2– | -97.2 (-97.0) |
| cis-ONOOH + e– / cis-ONOOH•– | -86.5 (-78.6) |
| •NH2 + e– / NH2– | -85.9 (-89.9) |
| trans-ONOOH + e– / trans-ONOOH•– | -83.3 (-80.0)b |
| •NO + e– / 3NO– | -82.6 (-69.0) |
| ONOOCO2– + e– / ONOOCO2•2– | -81.7 (-68.1) |
| trans-ONOO– + e– / trans-ONOO•2– | -62.8 (-50.0) |
| cis-ONOO– + e– / cis-ONOO•2– | -55.7 (-45.9)b |
| O2•– + e– / O22– | -53.7 (-49.6) |
Calculation using NO2– and •NO2 as products for G3(MP2) only and could be an overestimate.
Energies for cis-ONOOH•– and trans-ONOO•2– were used for the G3(MP2) calculation only.
General EPR Experiments
EPR measurements were taken at room temperature with microwave power 10 mW, modulation amplitude 1 G, receiver gain 1.0 × 105, scan time 21.4 s, time constant 42.0 s, and sweep width 120 G. Solvents used include DMSO or a 10 mM potassium phosphate buffer (pH 7.0) containing 100 μM diethylenetriaminepentaaceticacid (DTPA). All solutions were bubbled with nitrogen gas prior to irradiation. Sample cell used were 50 μL quartz or glass capillary tubes for UV or non-UV irradiation experiments, respectively. For UV irradiation experiments, the sample cell was irradiated with a Spectroline low-pressure mercury vapor lamp with 0.64 cm × 5.4 cm dimensions and a 254-nm wavelength. The spectrum simulation was carried out by an automatic fitting program.74
Peroxynitrite Trapping
Peroxynitrite (≥ 90% in 0.3 M NaOH) was commercially obtained with nitrite/nitrate as impurities and was stored at -86 °C. Peroxynitrite concentration was determined spectrophotometrically at 302 nm. In a typical experiment, solutions contain 20 mM DMPO and 8-12 mM ONOO– in 70% (v/v) DMSO in PBS. For inert atmosphere conditions, solvents (e.g., 70% (v/v) DMSO in PBS) were first purged with argon before making the DMPO and ONOO– solutions. For ONOO– in 100% water, ONOO– was prepared according to the method previously described.75 One milliliter of aqueous solutions of 0.7 M HCl and 0.6 M H2O2 was extruded simultaneously with 1 mL 0.6 M NaNO2 using 1 mL plastic syringes into a stirred ice-cold 0.3 mL solution of 3 M NaOH. The excess H2O2 was removed with MnO2. The concentration of ONOO– solution was calculated to be 15 mM based on an extinction coefficient of 1700 M-1 cm-1 at 302 nm. For ONOOCO2– generation, a solution of DMPO in DMSO was purged with argon and subsequently bubbled with CO2. Stock argon-purged DMSO solution of ONOO– was then added to the DMPO solution to make 70% (v/v) DMSO-30% PBS final solution.
•NO2 Generation
a) A solution containing 20 mM nitrone and 300 mM NaNO2 in PBS was transferred to a quartz capillary tube and was UV-photolyzed in the EPR cavity according to previous procedure.76b) DMSO was bubbled with argon for 15 min, then •NO gas was bubbled through the solvent for 15 min. A 25 μL of •NO-saturated DMSO solution was then added to a 25 μL solution of 50 mM nitrone in DMSO.
Spin Trapping using SNAP
Final aqueous solution contains 100 mM nitrone and 65 mM SNAP. For inert atmosphere conditions, solutions of nitrone and SNAP were argon purged prior to their addition to prevent •NO2 formation. An aliquot of 70 μL was transferred to capillary tube and sample was irradiated in the cavity using visible light while acquiring EPR spectra.
Generation of •N3, •NH2, •NO3 and HNO
PBS (pH 7.4) solutions of NaN3, NH2OH, or HNO3 (100 mM) in the presence of 0.2% H2O2 and 50 mM DMPO were UV irradiated to afford the corresponding radical adducts. Spin adducts from HNO was carried out using Ar purged Angeli's salt solution (120 mM) in distilled water in the presence of DMPO (100 mM) and subsequent acidification with HNO3 to give a final acid concentration of 20 mM.
17O-Isotopic Labeling Studies
O-17 labeled nitrogen dioxide, •N17O2, was prepared according to the procedure shown above by mixing saturated DMSO solutions of •NO and O2 (28% 17O atom) in DMSO.
Mass Spectral Studies
a) GC-MS analysis was carried out using a positive ion electron impact ionization (EI) detection. Ten microliter of the CH3Cl fraction was injected to the column at an initial temperature of 40 oC with ramp of 20 °C min-1 up to a maximum of 250 °C. MS detection was conducted at 200 °C ion source temperature, electron energy of 70 eV and scan speed of 1.6584 scans s-1. In a typical experiment for DMPO-NO2 adduct formation, 1 μL of pure DMPO (~10 M) was added to a 25 μL O2-saturated DMSO solution followed by 25 μL NO-saturated DMSO solution. Seventy microliter of CH3Cl was then added and the resulting mixture was vigorously mixed and 10 μL of the bottom CH3Cl fraction was injected into the column. The procedure was repeated for DMPO alone and O2/NO in the absence of DMPO. For DMPO-OONO, ONOO– was prepared according to the method previously described.75 A 70 μL aliquot solution of ONOO– (15 mM) was added to a 1 μL of pure DMPO followed by 70 μL of CH3Cl. The resulting mixture was vigorously mixed and 10 μL of the bottom CH3Cl fraction was injected into the column. The procedure was repeated for DMPO alone and ONOO– solution in the absence of DMPO. For GC-MS analysis of DMPO-adducts generated from ONOOCO2–, SNAP, •NO3, •NH2, •N3, and HNO, procedures for their generation as mentioned above were followed except that distilled water was used instead of PBS. b) Time-of-flight (TOM) mass spectral analysis was carried out on DMPO-ONOO system only since solution containing DMSO from the generation of DMPO-NO2 is not compatible for this type of analysis. To a 70 μL of freshly prepared aqueous solution of ONOO– (15 mM) was added 1 μL of pure DMPO. The resulting solution (70 μL) was directly infused into the spectrometer. All measurements were done in triplicate.
Results and Discussion
Redox Properties
Free energies (ΔG298K,red) of the various RNS formation and decomposition pathways were computationally studied at the PCM/B3LYP/6-31+G**//B3LYP/6-31G* level of theory. Reduction free energies (ΔG298K,red) for each of the species were calculated and are shown in Table 1. Three oxidizing ROS, O2•–, HO2•, and •OH, were also included for comparison. For one-electron reduction reactions, the ΔG298K,red of •NO3 to NO3– was found to be the most favorable, even more so than the ΔG298K,red of the highly reactive •OH. Reduction of N2O4 resulted in an 18.4 kcal mol-1 decrease in ΔG298K,red compared to its monomeric form •NO2. Cis-peroxynitrite was found to be the least oxidizing of the RNS's studied (ΔG298K,red = -55.7 kcal/mol), and has free energy of reduction close to O2•– (ΔG298K,red = -53.7 kcal/mol). Previous studies77 support our theoretical data showing that trans-ONOO– is more oxidizing than cis-ONOO–, by 7.1 kcal mol-1. In contrast to ONOO–, the protonated form, cis-ONOOH, is more oxidizing by 3.2 kcal mol-1 than its trans analogue but in general, cis- or trans-ONOOH are more oxidizing than the cis- and trans-ONOO–.
The ΔG298K of oxidation of DMPO to DMPO•+ was found to be 139.7 kcal mol-1,78 and therefore, •NO3 is the only RNS in this study that can spontaneously oxidize DMPO with an overall ΔG298K,rxn = - 12.6 kcal mol-1. Thermodynamics of oxidation of DMPO by ONOO– is also unlikely due to the highly endoergic free energy for this process of 84 kcal/mol as had been previously been ruled out by other.64 Due to the high oxidation potential of DMPO (EDMPO•+/DMPO = 1.63 V), and by using [17O] labeling and EPR spin trapping, the formation of DMPO•+ from HO• and SO4•– was discarded.60 Instead, nucleophilic addition to DMPO and its subsequent oxidation to give an EPR detectable species is the more plausible mechanism for radical adduct formation in sulfite/HRP/H2O2 system. RNS reactions with nitrones may not be limited to simple electron transfer reactions and therefore, nucleophilic addition reactions of RNS to nitrones will be explored in the subsequent sections.
We preformed additional calculations at the G3(MP2)//B3LYP/6-31G* level of theory in aqueous phase. G3(MP2) values have been shown to give accurate gas-phase organic thermochemistries for small molecules.79 Using the G3(MP2) approach, more exoergic free energy of reduction was observed for HO• compared to that of •NO2 and is more consistent with the experimental thermochemical data of Eo(HO•g/HO–aq) = 1.98 V80 and Eo(•NO2/NO2–aq) = 1.04 V.81
Nitrogen dioxide radical (•NO2)
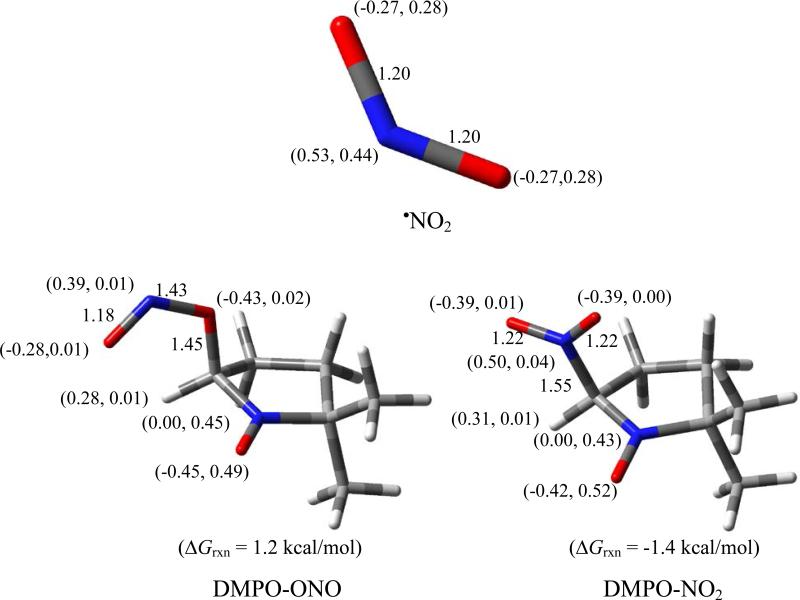
Figure 1 shows the optimized geometries of •NO2, and the O- and N-centered radical adducts, DMPO-NO2. NBO calculation of •NO2 shows negative charges on the two O-atoms and a positive charge on the N-atom with 44% of the spins residing on the N-atom, and 25% on each of the O-atoms. The N-centered radical adduct formation was exoergic with ΔG298K,rxn = -1.4 kcal/mol while the O-centered radical adduct is slightly endoergic with ΔG298K,rxn = 1.2 kcal/mol. Unlike O2•–, the more favored addition of an electropositive center (i.e., the N-atom) to the nitronyl-C indicates that the •NO2 addition to DMPO is electrophilic in nature which may be due to the high spin density distribution on the N-atom. The small energy difference between the N-centered and O-centered addition reaction could also indicate that the latter mechanism can occur as will be discussed below.
Figure 1.
Optimized geometries showing bond lengths (in Å), charge and spin densities (e) (in parentheses), and free energies of •NO2 addition to DMPO at the PCM/B3LYP/6-31+G**//B3LYP/6-31G* level of theory.
Attempts to generate •NO2 by UV irradiation of NaNO2 with DMPO in PBS only yielded an acyclic adduct with aN = 7.3, aH = 4.1, aH = 4.1 (Figure not shown). Spin trapping using •NO/O2 solution in DMSO, however, gave an unidentified O-centered radical adduct which will be called DMPO-OX with aN = 13.8, aH = 11.7 (see Figure 2a). Table 2 and Table S1 list the predicted hfsc's for various •NO2 adducts (in the presence of bulk dielectric effect of water as well as in the presence of two explicit water molecules) and shows that N-centered radical adduct must exhibit a significant nitro-N hfsc, but experimental evidence shows otherwise, indicating that the O-centered radical adduct could be the major product in DMSO solution. Attempt to further prove the formation of O-centered DMPO adduct with NO2, EPR spectrum was obtained from mixing 17O-labeled O2 (28% O-17 atom) and •NO saturated DMSO solutions in the presence of DMPO. Solution only yielded the same spectrum as above with aN = 13.8 G and aH = 11.8 G, and did not yield hfsc originating from 17O due perhaps to the low concentration of N17O2 formed from the complex mechanism of its formation from •NO and O2 as well as the low final yield of DMPO- N17O2. The non-observance of the 17O was exacerbated by its nuclear spin of I = 5/2 that can drastically reduce the amplitude of spectrum by a factor of six. GC-MS analysis, however, showed a distinctive GC peak at ~ 4.4 min with significantly intense base mass peak of 158.98 m/z corresponding to DMPO-NO2 adduct (see Figure S7a of the SI). Considering that the energy difference for the formation of N- and O-centered radical adducts is only 2.6 kcal/mol, the formation of O-centered radical adduct is not improbable. Moreover, addition of the electronegative center (i.e., the O atom) to the nitrone follows that of the nucleophilic addition reaction of O2•– to nitrones. Based on the thermodynamics of O-centered radical adduct formation from NO2 which is only 2.6 kcal/mol difference compared to the formation of the N-centered adduct, the fact that the reaction of NO2 with DMPO yielded an adduct with no additional hfsc due to N, and that the mass spectral analysis is consistent with DMPO-NO2 formula, it is reasonable to assume that the adduct formed has a general structure of DMPO-ONO.
Figure 2.
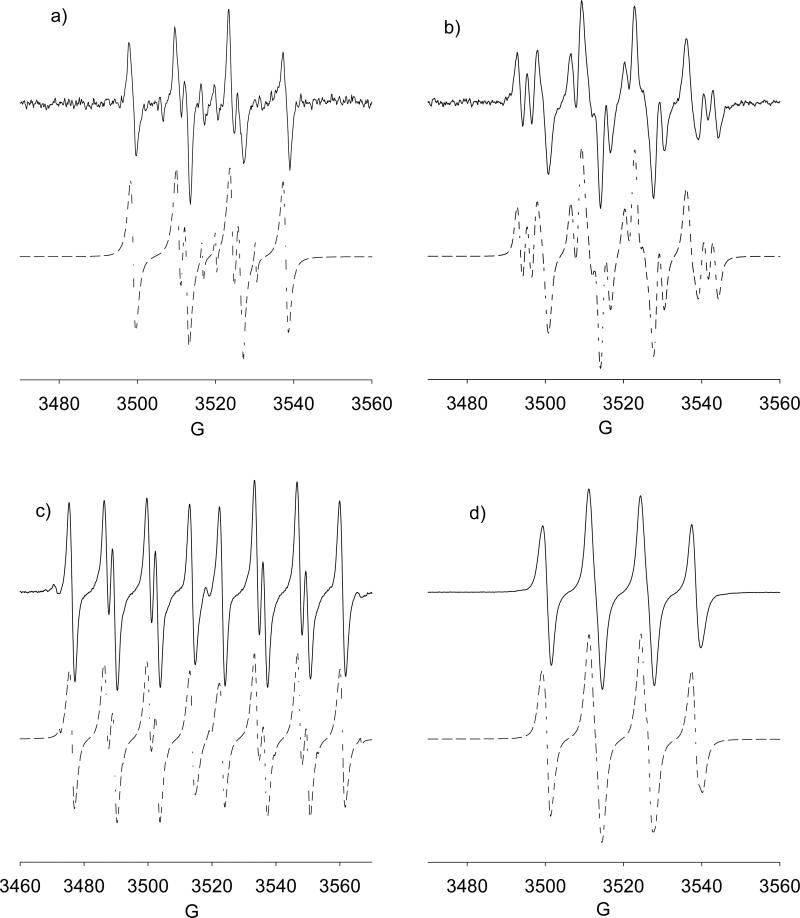
X-Band spectra of adducts generated from •NO/O2 DMSO in the presence of: (a) DMPO (30 mM), [DMPO-ONO, 95%, aN = 13.8, aH = 11.7; non-nitroxide, 5%, aH = 10.5, aH = 10.5, aH = 3.4]; (b) AMPO (10 mM), [AMPO-ONO I, 83.4%, aN = 13.3, aH = 10.8, aN’ = 0.6; AMPO-ONO II, 16.6%, aN = 13.8, aH = 17.7, aN’ = 2.4]; (c) DEPMPO (10 mM), [ DEPMPO-ONO I, 94%, aN = 13.35, aP = 46.97, aH = 10.93; DEPMPO-ONO I aN = 13.51, aP = 45.53, aH = 12.40; DEPMPO-R, 6%, aN = 13.85, aP = 46.94, aH = 20.05]; (d) EMPO (5 mM), [EMPO-ONO I, aN = 13.22, aH = 11.21, aH = 0.3; EMPO-ONO II, aN = 13.37, aH = 12.24, aH = 1.15].
Table 2.
Predicted Hyperfine Splitting Constants (G) of RNS Adducts of DMPO and their Respective Free Energies, ΔG298K,rxn (in kcal/mol), at the PCM(water)/B3LYP/6-31+G**//B3LYP/6-31G* Level of Theory. (Values in Parentheses are in the Presence of Two Explicit Water Molecules Calculated at the Same Level of Theory as Above)a
| predicted hyperfine splitting constants (G) |
||||||
|---|---|---|---|---|---|---|
| Adducts | aN | aβH | aγH | aN (adduct) | aN” for AMPO) aP (for DEPMPO) | ΔGrxn |
| -OONOb | 11.0 (10.4) | 11.8 (14.2) | 0.6 (0.7) | c | c | -26.3 |
| -N(O)O2b | 11.0 | 15.7 | c | 5.8 | -0.5 | |
| -NO | 10.0 (10.3) | 17.2 (19.7) | 0.8 (0.7) | 2.6 (7.1) | c | 14.1 |
| -ONO | 10.7 (10.2) | 7.3 (9.3) | 1.5 (c) | c | c | 1.2 |
| -NO2 | 9.6 (10.2) | 7.8 (7.6) | 1.5 (1.9) | 8.0 (8.4) | c | -1.4 |
| -NH2 | 11.9 (12.5) | 12.1 (11.0) | 0.5 (0.9) | 1.6 (2.0) | -28.3 | |
| -N3 | 11.7 (12.1) | 7.4 (8.1) | 0.8 (0.7) | 3.2 (3.0) | c | -13.4 |
| -SNAP | 11.1 (11.8) | 9.0 (8.8) | 0.9 (1.1) | -1.1 | ||
For complete list of hyperfine splitting constants of various adduct of AMPO, EMPO and DEPMPO, see the supplementary information (Table S1).
Formed from the inverted spin trapping of ONOO– by [nitrone]•+.
Not applicable or not significant.
Spin trapping using •NO/O2 in the presence of AMPO, EMPO, or DEPMPO were also carried out (Figure 2b-2d). Similar to that observed for DMPO, significant amounts of nitrone-ONO adducts were evident. Previously,82 it has been shown that olefins exhibited fast reactions with radicals generated from •NO/O2 systems yielding •NO2 addition product via O-atom attack. Figure 1 shows the thermodynamics of nitrone-NO2 formation which generally shows slightly higher thermodynamic favorability for the formation of the trans-isomers compared to the corresponding cis-isomers, as well as •NO2 addition via the O-atom rather than via the N-atom addition. Figure 2 shows the calculated hfsc's for the more preferred nitrone-ONO adducts, and qualitative trends can be made from the relative magnitudes of aN and aβ-H, as well as the presence of additional aN for the AMPO-ONO cis and trans adducts as experimentally observed (Figure 2b).
The formation of NO2 from NO and O2 is quite complex and may form intermediate species that can react with nitrones. The formation of intermediate species such as N2O4 or NO3 should yield nitrone-ONO and nitrone-ONO2 (to ultimately give DMPO-OH), respectively. Since formation of nitrone-OH adduct was not evident any of the spectra in Figure 2, therefore, one cannot discount the formation of nitrone-ONO from N2O4 since the free energy of N-N homolytic cleavage for N2O4 to give two molecules of NO2 is only endoergic by 0.5 kcal/mol.
Peroxynitrite (ONOO–) and Peroxynitrous Acid (ONOOH)
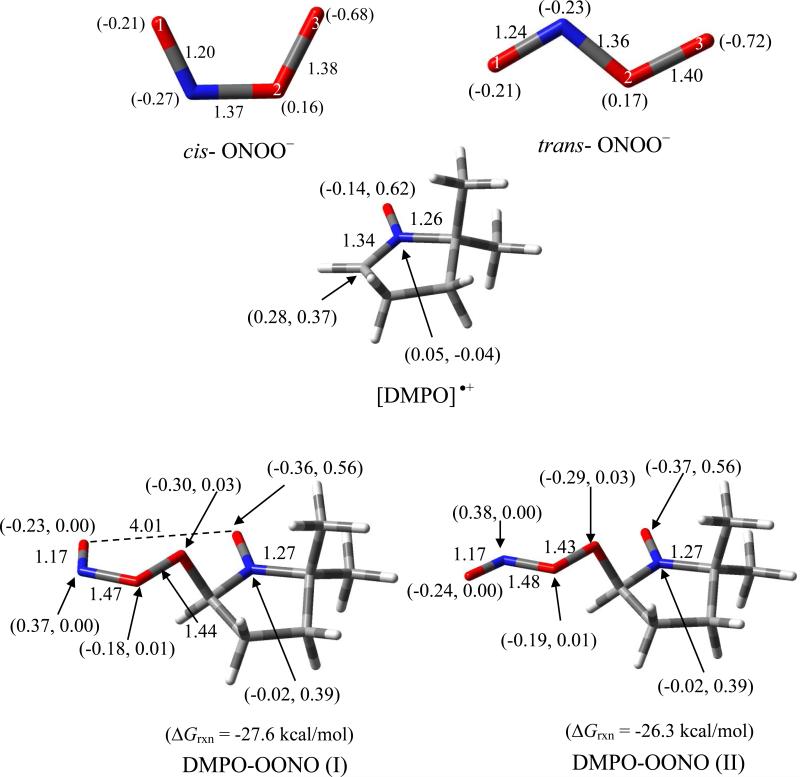
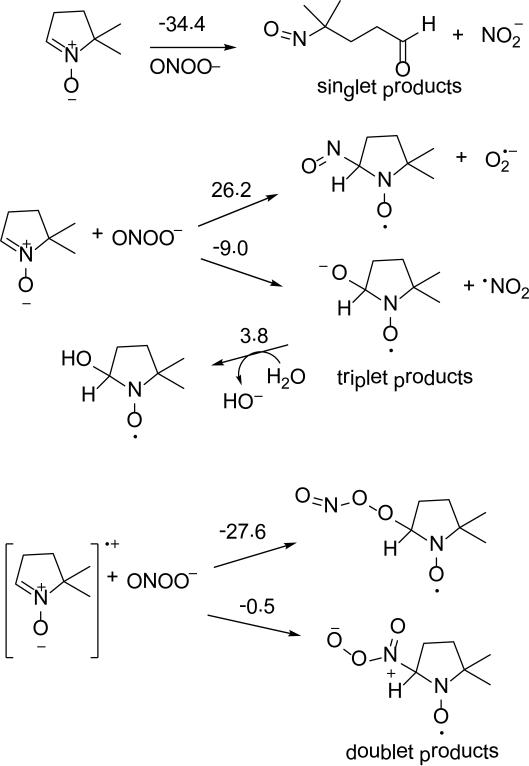
The electronic property of ONOO– as shown in Figure 3, is characterized by high negative charge density on the terminal peroxy-O, O(3), for both cis and trans isomers (-0.68e and -0.72e, respectively). The charge density on the nitroso-O, O(1), is less negative with a charge of -0.21e. The cis-ONOO– conformation is 4.2 kcal/mol more favored than the trans isomer. Scheme 3 shows the various modes of addition reactions of ONOO– to DMPO. Theoretical studies show that nucleophilic addition of ONOO– to DMPO results in ring opening to form the nitroso-aldehyde and nitrite with ΔG298K,rxn of -34.4 kcal/mol. Formation of a triplet adduct from ONOO– and DMPO gave a highly endoergic product for the O(1)-addition (where O(1) corresponds to the doubly-bonded O) with ΔG298K,rxn = 54.1 kcal/mol (Figure not shown). Addition of the terminal peroxyl-O, that is O(3), to DMPO gave exoergic free energy of -9.0 kcal/mol yielding •NO2 and DMPO-O•– radicals, but formation of DMPO-NO and O2•– via addition through the N atom gave endoergic ΔG298K,rxn of 26.2 kcal/mol. It has been previously shown that the reaction of ONOO– with DMPO in 17O-labeled water yielded DMPO-OH spectra under low oxygen tensions, as well as a methyl radical adduct to DBNBS in the presence of ONOO– in DMSO.64 This experimental evidence support that nucleophilic addition of ONOO– to DMPO can result in the formation of DMPO-OH adduct from DMPO-O•– with slightly endoergic ΔG298K,rxn of 3.8 kcal/mol (Scheme 3). The formation of doublet adducts from ONOO– via its addition to [DMPO]•+ is highly exoergic with O(3) addition being the most favorable with ΔG298K,rxn = -26.3 kcal/mol.
Figure 3.
Optimized structures of cis-ONOO–, trans-ONOO–, DMPO•+ and spin adducts formed from DMPO•+ and ONOO–, showing the charge and spin densities (in parentheses), free energies of reaction, and pertinent bond lengths at the PCM/B3LYP/6-31+G**//B3LYP/6-31* level of theory.
Scheme 3.
Proposed reaction mechanisms of ONOO- addition to DMPO and their respective ΔG298K,rxn (in kcal/mol) calculated at the PCM/B3LYP/6-31+G**//B3LYP/6-31G* level of theory.
EPR spin trapping of ONOO– in the presence of DMPO was initially performed in DMSO/H2O. Figure 4a shows that the spectrum obtained under normal atmospheric conditions was markedly different than those obtained when the solution was purged with argon (Figure 4b). EPR simulation of the resulting spectra under normal atmospheric conditions revealed hfsc's corresponding to an unknown O-centered radical adduct, DMPO-OX (80%; aN = 14.0, aH = 9.8, aHγ = 1.8) and a C-centered radical adduct, DMPO-R (20%; aN = 15.5, aH = 21.5). The nature of the DMPO-OX will be further discussed in the succeeding section. Using the same spin trapping conditions with an argon purge yielded a different mixture of radical adducts; a C-centered radical adduct DMPO-R (80%; aN = 15.3, aH = 21.5), DMPOOH (16%; aN = 15.4, aH = 15.0) and an adduct assigned to DMPO-ONX (4%; aN = 9.4, aH = 19.9). The DMPO-R adduct can be assigned to the formation of DMPO-CH3, perhaps due to the formation of HO• from the O-O homolytic cleavage of ONOOH. The O-O homolytic cleavage of ONOOH to form HO• and •NO2 was calculated to be slightly endoergic (ΔG298K,rxn = 2.2 kcal/mol) (Scheme 2), and while this process is likely to occur in solution, the formation of DMPO-OH, where the HO• originates from ONOOH, was previously confirmed using 17O-labeled water.64 The hfsc's observed for the formation of small amounts of DMPO-ONX (aN = 9.4, aH = 19.9) follow a qualitative trend predicted for the formation of DMPO-OONO (Table 2) in which the aH is more improved (i.e., 14.2 G) in the presence of explicit interaction of water molecules compared to the just considering the bulk dielectric effect of water. However, DMPO-OONO can only be formed via inverted spin trapping through ONOO– addition to [DMPO]•+. The question on how [DMPO]•+ could be formed in solution can be accounted for by the •NO3 oxidation of DMPO to DMPO•+. However, one could argue that HO• can further react with nitrate impurity (as HNO3) to yield •NO3 (ΔG298K,rxn = -12.9 kcal/mol), or via H-atom abstraction from HOONO and isomerization of the •OONO formed to •NO3 with a total ΔG298K,rxn = -46.0 kcal/mol (see Scheme 2). Hence, •NO3 can oxidize DMPO to DMPO•+ to form DMPO-OONO via inverted spin trapping with ONOO–. This oxidation process is exoergic with ΔG298K,rxn of -12.6 kcal/mol, subsequent addition of ONOO– to DMPO•+ gave highly favorable ΔG298K,rxn of -27.6 kcal/mol (Figure 3) giving a net ΔG298K,rxn of -40.2 kcal/mol for the formation of an EPR-detectable DMPO-OONO. The initial endoergic process for the •NO3 oxidation of DMPO to DMPO•+ could explain the low yield (4%) of DMPO-OONO formed in solution. Also, the formation of DMPO-N(O)O2 can be ruled out due to the lack of additional aN from the EPR spectrum, and because the formation of DMPO-OONO is more exoergic with ΔG298K,rxn = -40.2 kcal/mol than the formation of DMPO-N(O)O2 with ΔG298K,rxn = -0.5 kcal/mol (Scheme 3).
Figure 4.
X-Band spectra of adducts of DMPO generated from (a) ONOO– in 70% DMSO [CO3•– adduct, 80%, aN = 14.0, aH = 9.8, aHβ = 1.8; alkyl adduct, 20%, aN = 15.5, aH 21.5], (b) ONOO– in 70% DMSO under inert atmosphere [alkyl adduct, 80%, aN = 15.3, aH = 21.5; •OH adduct, 16%, aN = 15.4, aH = 15.0; DMPO-ONX, 4%, aN = 9.4, aH = 19.9], (c) ONOO– in 70% DMSO under CO2 atmosphere [CO3•– adduct, 93%, aN = 14.0, aH = 9.8, aHβ = 1.8; alkyl adduct, 7.4%, aN = 15.6, aH 21.2], (d) ONOO– in 100% PBS under inert atmosphere [alkyl adduct, 27%, aN = 16.0, aH = 22.6; DMPO-NX, 57%, aN = 14.5, aN’ = 3.55, aH = 0.93; DMPO-ONO, 6%, aN = 14.4, aH = 11.3].
Under inert atmosphere in aqueous solution (Figure 4d), ONOO– yielded an N-centered radical adduct, DMPO-NX, as the major product (~57%) with aN = 14.5, aN’ = 3.55, aH = 0.93. Formation of a minor product, DMPO-ONO (6%) was also observed with aN = 14.4, aH = 11.3. The formation of the O- and N-centered adducts was further confirmed by GC-MS and showed distinctive peak at ~ 6.2 min (not observed with DMPO or ONOO– alone) with significantly intense base peaks at 175.05 m/z and 159.02 m/z corresponding to DMPO/ONOO– and DMPO-ONO adducts (Figure S8 of SI). Time-of-flight mass spectrum also confirmed the formation of a base peak at 175.14 m/z which was not observed with ONOO– or DMPO alone (Figure S9 of SI). In addition to the formation of O- and N-centered adducts, the same DMPO-R adduct as also observed as in DMSO suggesting that the origin of the DMPO-R is independent of the solvent used. The only C-centered radical source would be from the nitrone itself via trapping of another nitrone. As proposed above, •NO3 at high [ONOO–] may oxidize DMPO to DMPO•+ leading to the addition of excess DMPO to DMPO•+ to form [DMPO-DMPO] •+ with ΔG298K,rxn = 0.5 kcal/mol. The predicted hfsc values for the nitronyl-N and peroxynitrite-N (i.e., 11.0 and 5.8, respectively) of DMPO-N(O)OO gave reasonable agreement with the experimental, while significant discrepancy was observed between the predicted and experimental hfsc for aH with 15.7 and 0.9, respectively (Table 2). Optimization of DMPO-N(O)OO with 2 explicit water molecules was problematic but we predict that the hfsc will not change significantly within ~2 G as also observed for the other adducts with explicit water interaction. Therefore, the nature of DMPO-NX warrants further investigation.
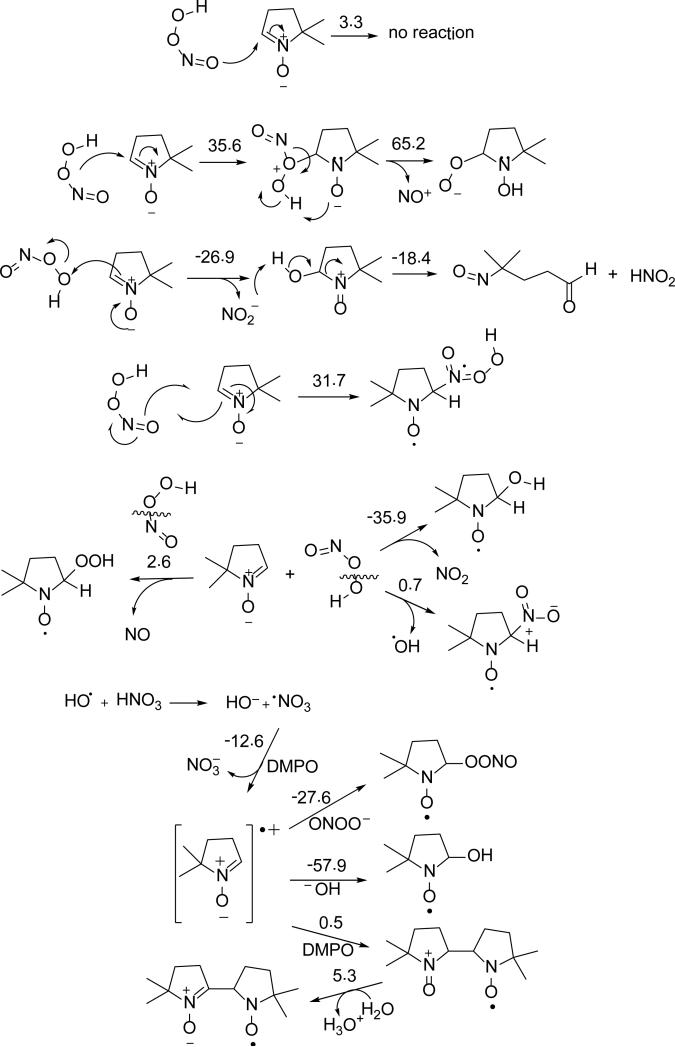
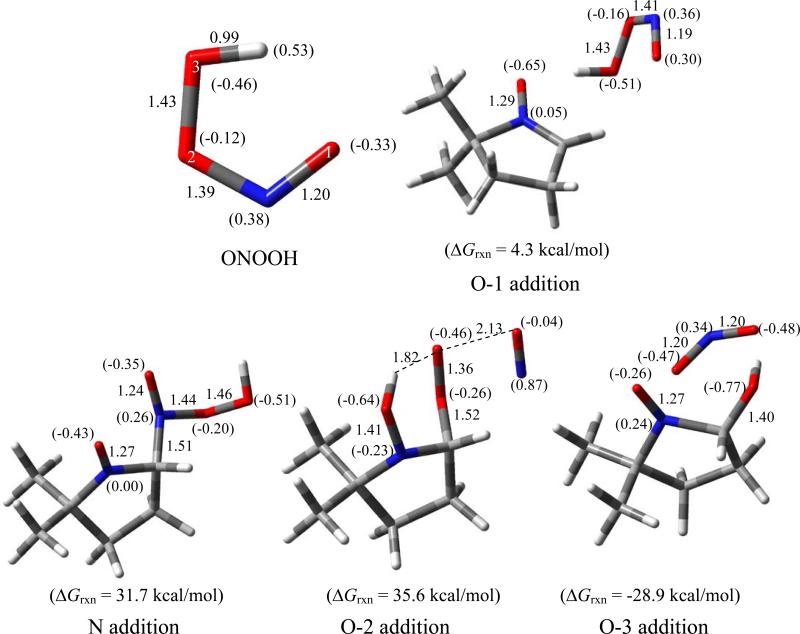
Scheme 4 shows the various modes of ONOOH addition to DMPO where two favorable pathways can be seen: 1) the formation of singlet species, nitroso aldehyde and nitrous acid, via electrophilic addition of O(3) with highly favorable overall ΔG298K's of -45.3 kcal/mol; and 2) the formation of doublet species, nitrogen dioxide and DMPO-OH via O-O homolytic cleavage of ONOOH with also highly favorable ΔG298K,rxn of -35.9 kcal/mol. Figure 5 shows the various modes of ONOOH addition to DMPO with only the O(3) addition to form DMPO-OH and •NO2 being the most favorable pathway.
Scheme 4.
Proposed reaction mechanisms of ONOO– with DMPO with the respective ΔG298K,rxn calculated at the PCM/B3LYP/6-31+G**//B3LYP/6-31G* level of theory.
Figure 5.
Optimized structures of ONOOH and energetics of various modes of ONOOH addition to DMPO, showing the charge densities (in parentheses), and pertinent bond lengths at the PCM/B3LYP/6-31+G**//B3LYP/6-31* level.
Using the same spin trapping conditions employed for DMPO (Figure 4b), the reactivity of ONOO- with EMPO, DEPMPO and AMPO were also explored in an argon-purged 70% DMSO solution. Shown in Figure S1, similar to DMPO, significant amounts of nitrone-R were formed from all nitrones, perhaps via formation of •CH3. While not observed for DEPMPO, nitrone-OX adducts were observed for EMPO and AMPO which can be assigned to the formation of DMPO-OONO most likely via inverted spin trapping. Figure 3 shows the thermodynamics of nitrone-OONO formation from [nitrone]•+ and its addition to ONOO– giving exoergic free energies of ~28 kcal/mol, with the formation of the cis-isomer only slightly more exoergic by < 1 kcal/mol compared to the trans-isomer. The approximated calculated hfsc's for the ONOO– adducts of DMPO, EMPO, DEPMPO, and AMPO are shown in Table 2 (for DMPO) and S1 (for the rest of the nitrones), and generally shows higher aβH than aN by as much as ~4 G. This qualitative trend in the relative magnitudes of aβH and aN follows the experimentally observed hfsc's with the exception of EMPO where the observed aβH is lower than aN.
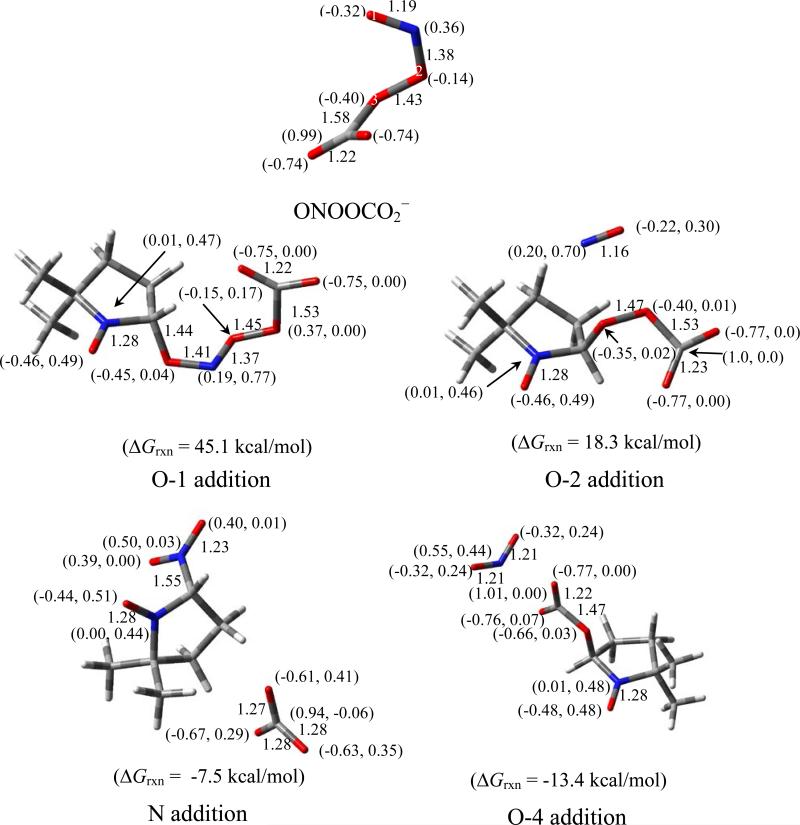
Nitrosoperoxycarbonate (ONOOCO2–)
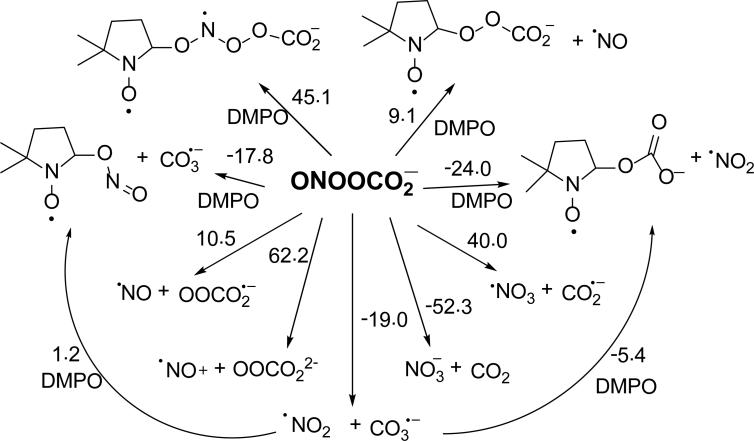
The optimized structure of ONOOCO2– was consistent with that of previous theoretical studies,83 which show a 1.58 Å bond distance between the carboxylate-C and peroxyl-O, and a C-O-O-N dihedral angle of 82o (Figure 6). The ΔG298K,rxn of formation of ONOOCO2– from trans-ONOO– is 0.5 kcal mol-1,83 and is more favorable than its formation from cis-ONOO– with ΔG298K,rxn = 2.8 kcal mol-1. However, the energy required for the conformational change from cis-ONOO– to trans-ONOO– was lowered upon complexation with CO2 (the free energies of conformation change for free and CO2-complexed ONOO– were determined to be 4.2 and 1.9 kcal mol-1, respectively). During its short half-life, the ONOOCO2– complex can act as a strong oxidant and, as previously discussed, can undergo homolytic cleavage to form CO3•– and •NO2 species (ΔG298K,rxn = -19.0 kcal mol-1), or rearrange to form NO3– and CO2 (ΔG298K,rxn = -52.3kcal mol-1) (Scheme 5). These calculations are consistent with previous models in which the yield of radical products was 30%. Thus, in neutral pH to moderately basic solution, ONOO– is energetically able to exist in equilibrium with ONOOCO2– where it can act as a strong oxidizing agent and spontaneously form radical species. At neutral pH, ONOO– can undergo competing mechanisms for radical formation such as protonation to form ONOOH, or ONOOCO2– formation. As the pH is lowered, the protonation of ONOO– can dominate, forming ONOOH.
Figure 6.
Optimized structures of ONOOCO2– and the various spin adducts formed with DMPO showing charge and spin densities (in parentheses), and pertinent bond lengths at the PCM/B3LYP/6-31+G**//B3LYP/6-31* level of theory.
Scheme 5.
Proposed reaction mechanisms for the formation of nitrone spin adducts with ONOOCO2–, and their respective ΔG298K,rxn (in kcal/mol) calculated at the PCM/B3LYP/6-31+G**//B3LYP/6-31G* level of theory.
We further investigated the nature of the DMPO-OX EPR signal in Figure 4a. Under normal atmospheric condition, the observed formation of DMPO-OX adduct could originate from DMPOOCO2–. This hypothesis was confirmed by bubbling pure CO2 through argon-purged DMSO before ONOO– spin trapping (Figure 4c). The spectra obtained from the CO2 bubbled solution yielded one radical adduct at low ONOO– concentration, which is identified as DMPO-OCO2– based on our previous reported hfsc's values of aN = 14.32, aH = 10.68, aβ-H = 1.37 for DMPO-OCO2–.84 Figure S2 shows that only when [ONOO–] >> [CO2] the DMPO-R adduct is formed, while at [ONOO–] << [CO2], the DMPO-OCO2 adduct is more predominant due to the formation of •CH3 from HO• via O-O homolytic cleavage of ONOOH as discussed above. Figure 6 shows the optimized geometries of DMPO-ONOOCO2– arising from the various modes of ONOOCO2– addition reaction to DMPO. Results suggest that N- and carboxylate-O(4) addition to DMPO gave the most exoergic ΔG298K,rxn with -7.5 and -13.4 kcal/mol, respectively. Due to the highly negative charge density of the carboxylate-O(4)'s ONOOCO2– (-0.74e), its addition to DMPO is therefore nucleophilic in nature and is the most favorable to give DMPO-OCO2– and •NO2, further confirming the EPR results observed in the presence of CO2 and ONOO–. GC-MS analysis of the sample showed evidence of DMPO-OCO2 adduct formation where the chromatogram showed a unique peak at RT 3.1 min with a base mass peak 174.91 m/z corresponding to [DMPO-OCO2H + H]+ (Figure S10 of SI).
In DMSO, the possibility of forming the decomposition products, CH3S=O(NO2) + •CH3 and CH3S=O(CO3)– + •CH3, due to oxidation by •NO2 and CO3•–, respectively, was also considered. However, results show endoergic free energies of 40.5 kcal mol-1 and 22.3 kcal mol-1, for reactions of •NO2 or CO3•– with DMSO, respectively, compared to the exoergic •OH reaction to DMSO to form CH3S=O(OH) + •CH3 with ΔG298K,rxn = -2.3 kcal/mol indicate that •CH3 formation is less likely to occur from •NO2 and CO3•– oxidation of DMSO. Furthermore, formation of N2O4 from •NO2 with ΔG298K,rxn = -0.5 kcal/mol can compete with •NO2 oxidation of DMSO.
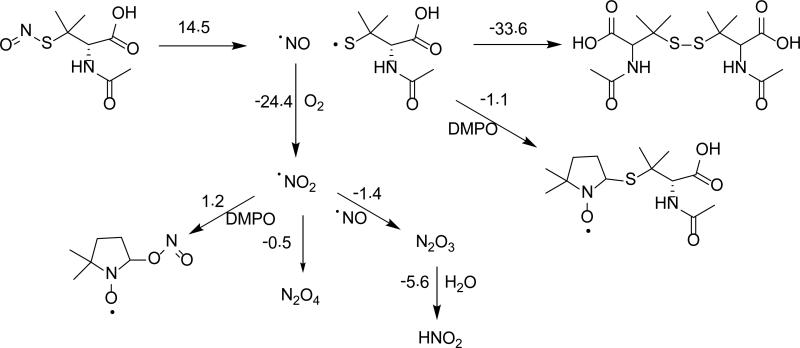
Spin Trapping Studies using S-Nitroso-N-Acetyl-D,L-Penicillamine (SNAP) and 3-Morpholinosydnonimine (SIN-1)
Scheme 6 shows the reaction of SNAP with DMPO. Homolytic cleavage of the S-N bond of SNAP into •NO and thiol radical (RS•) was determined to be endoergic, while the ionic cleavage of SNAP into NO+ and thiyl anion (RS–) was calculated to be highly unfavorable with ΔG298K,rxn = 14.5 kcal/mol and ΔG298K,rxn = 50.5 kcal/mol, respectively, suggesting that the only relevant mechanism of SNAP decomposition is the release of •NO and RS•. While it is energetically favorable for the free RS• to form an adduct with DMPO (ΔG298K,rxn = -1.1 kcal/mol), it was calculated that the formation of a disulfide from two RS• molecules was significantly more favorable with ΔG298K,rxn = -33.6 kcal/mol.
Scheme 6.
Proposed reaction mechanisms for the decomposition of SNAP in the presence of DMPO and their respective ΔG298K,rxn (in kcal/mol) calculated at the PCM/B3LYP/6-31+G**//B3LYP/6-31G* level of theory.
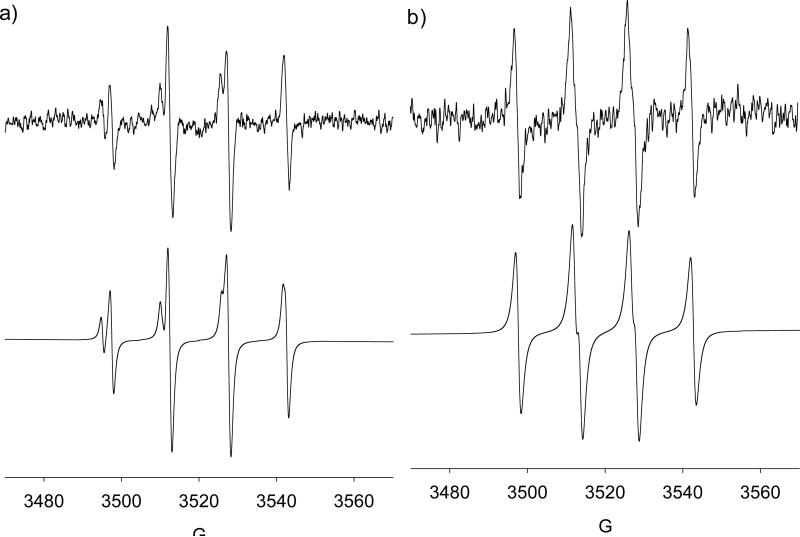
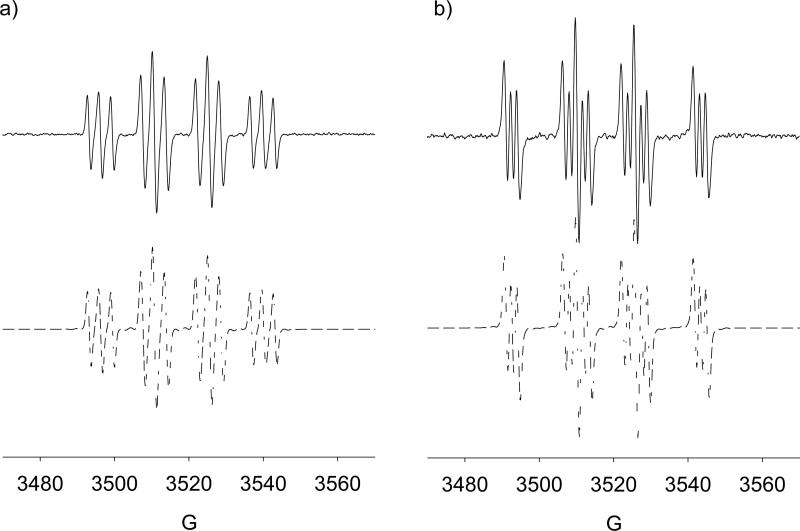
EPR spin trapping from SNAP decomposition was performed. Solutions of irradiated SNAP in water alone under inert atmosphere and air did not give any quartet EPR signal. The EPR spectra of DMPO in the presence of SNAP under normal atmosphere was determined to be HO• adducts (Figure 7a) with slightly different g values (i.e., major species: aN = 15.2, aH = 14.8; and minor; aN = 15.8, aH = 15.2) indicating that the presence O2 can lead to the formation of DMPO-OH via perhaps the initial reaction of O2 with the NO to form an oxidizing species. However, under Ar atmosphere, formation of an RS• adduct was observed (Figure 7b) with aN = 14.6, aH = 16.0 close to the reported hfsc values for DMPO-SNAP of aN = 15.3, aH = 17.7 and DMPO-SG of aN = 15.2, aH = 16.3.85 GC-MS analysis of the irradiated DMPO/SNAP solution gave a significantly intense GC peak at 6.45 min compared to DMPO and SNAP alone with base mass peak of 302.94 m/z (see Figure S11 of SI).
Figure 7.
X-Band spectra generated from visible light irradiation of DMPO (100 mM) and SNAP (65 mM) in aqueous solution: (a) under ambient atmosphere, HO• adducts: (major) aN = 15.2, aH = 14.8; (minor) aN = 15.8, aH = 15.2; (b) under Ar atmosphere, RS• adduct: aN = 14.6, aH = 16.0 (lit. value for DMPO-SNAP: aN = 15.3, aH = 17.7).85
The formation of S-centered radical adducts from SNAP were further confirmed by spin trapping using AMPO, EMPO and DEPMPO, showing hfsc's consistent to that of the formation of S-centered radical (see Figure S3). The signal intensity increased significantly when the experiment was repeated under inert atmosphere (Figure 7b) which suggests •NO2 formation under atmospheric conditions can annihilate the signal, probably via its direct reaction to the DMPO-SR adduct. It has been previously shown that as •NO concentration increases in the presence of O2, the rate of •NO2 formation increases, such that at 1.0 mM •NO, the half-life of •NO due to •NO2 formation is 0.56 s.19 Calculations suggest that the formation of a DMPO-NO adduct is thermodynamically unfavorable, with ΔG298K,rxn = 14.0 kcal/mol,86 while formation of a DMPO-NO2 adduct is favorable, with ΔG298K,rxn = -1.4-1.2 kcal/ mol. While DMPO may display thermodynamically favorable trapping reactions with •NO2, the radical characteristic of •NO2 can be ‘hidden’ via its complexation to form N2O4 which can compete with its addition to the nitrone, hence, no •NO2 adduct was observed (see Scheme 6).
As expected, the unfavorable reactivity of •NO to DMPO only yielded O2•– adduct using SIN-1 as shown in Figure S4. While the SIN-1 decomposition mechanism was proposed87 to include the formation of a C-centered radical, only DMPO-OOH was observed in this study.
Nitrogen trioxide (•NO3), Amino (•NH2), Azidyl (•N3), and Nitroxyl (HNO)
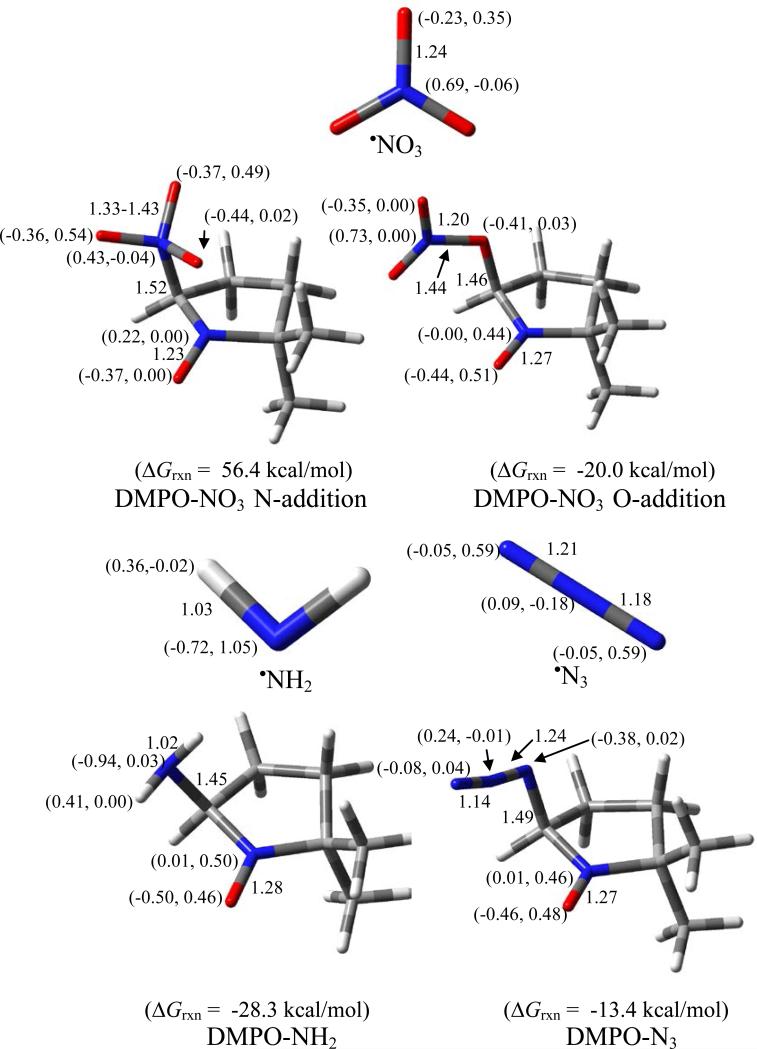
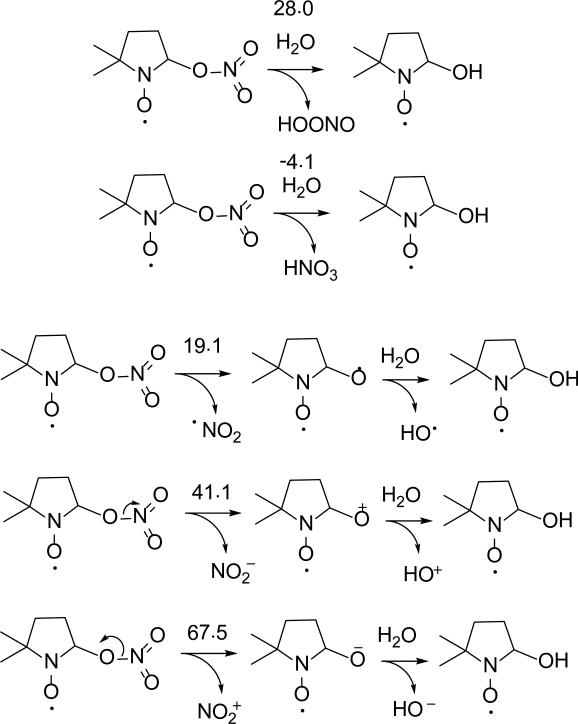
Figure 8 shows the various radical adducts formed from •NO3, •NH2 and •N3–. The formation of O-centered radical adduct, DMPO-ONO2, is more favorable than the N-centered radical adduct, DMPO-NO3, with ΔG298K,rxn of - 20.0 kcal/mol and 56.4 kcal/mol, respectively. Due to the higher reduction potential of NO3 Eo(•NO3/ NO3–) = 1.9-2.6 V88 compared to the oxidation potential of DMPO Eo(DMPO/DMPO•+) of Eo = 1.6 V89 (consistent with the free energies of reduction shown in Table 1), it is expected that the mechanism of DMPO-NO3 formation should proceed via inverted-spin trapping where DMPO is oxidized to DMPO•+ •NO3 and subsequent addition of NO3– to DMPO•+. The high negative charge on the O atoms of NO3– favored the nucleophilic addition of the radical to the nitrone. Spin density distribution on the nitro-N in DMPO-ONO2 is negligible. EPR spin trapping of •NO3 only gave spectrum corresponding to DMPOOH. Although the formation of DMPO-ONO2 was already exoergic, its hydrolysis to DMPO-OH and HNO3 is also favorable with ΔG298K,rxn = -4.1 kcal/mol (Scheme 7)—significantly more favorable than the highly endoergic formation of DMPO-OH and HOONO with ΔG298K,rxn = 28.0 kcal/mol. Scheme 7 further shows that DMPO-OH and HNO3 are more likely to be formed via O-N homolytic cleavage of DMPO-ONO2 to form DMPO-O• and •NO2 with ΔG298K,rxn = 19.1 kcal/mol compared to the O-N heterolytic cleavages resulting in the formation of DMPO-O+ and NO2–, or DMPO-O– and NO2+ with ΔG298K,rxn's of 41.1 and 67.5 kcal/mol, respectively.
Figure 8.
Optimized structures of •NO3, •NH2, •N3 and their respective adducts with DMPO showing the free energies of reaction, charge and spin densities (in parentheses), and pertinent bond lengths at the PCM/B3LYP/6-31+G**//B3LYP/6-31* level of theory.
Scheme 7.
Proposed decomposition mechanisms for DMPO-NO3 and their respective ΔG298K,rxn (in kcal/mol) calculated at the PCM/B3LYP/6-31+G**//B3LYP/6-31G* level of theory.
Since only DMPO-OH adduct was observed during irradiation of DMPO in the presence of HNO3 and H2O2, the effect of HNO3 concentration on DMPO-OH formation was further investigated. It is expected that the DMPO-OH concentration will be dependent on HNO3 concentration at constant H2O2 concentration. Results show that lower DMPO-OH is formed at higher HNO3 concentration which indicates that HNO3 can be competing with DMPO for reaction with HO• (see Figure S5 of SI) Moreover, DMPO-OH formation was evident even at large excess of HNO3 over H2O2 (i.e., 800 mM HNO3: 140 mM H2O2) which may also indicate •NO3 addition to DMPO, however, the use of HN17O3 would have been more straightforward for the elucidation of the mechanism of DMPO-OH formation. Nevertheless, the calculated thermochemistries and formation of DMPO-OH adduct at large excess of HNO3 suggest origination of DMPO-OH from DMPO-NO3.
Formations of DMPO-NH2 and DMPO-N3– gave exoergic ΔG298K,rxn's of -28.3 and -13.4 kcal/mol, respectively, with the most electronegative N-atoms adding to the nitrone. Spin densities of the N-atom directly bound to the nitrone range from 2%-3%, which is enough to impart additional aN due to the N-atom as shown in Figure 9. The formation of DMPO-NH2 was previously reported by Kirino, et al.90 (aN = 15.9, aH = 19.0 and aN-amino = 1.7) as well as by Chignell, et al.91 (aN = 15.9, aH = 19.3 and aN-amino = 1.60). These values are consistent with the current data of aN = 15.8, aH = 19.3 and aN-amino = 1.6. GC- GC analysis and showed evidence of DMPO-NH2 adduct formation where chromatogram showed a unique peak at 3.18 min with a base mass peak at 128.97 m/z (Figure S12 of SI). Generation of DMPON3 and its detection by EPR spin trapping was previously reported51 giving a quartet of triplets with hfcs values of aN = 14.8, aH = 14.2 and aN-azide = 3.1. These values are consistent with the current data of aN = 14.8, aH = 14.2 and aN-amino = 3.4. GC-MS analysis showed evidence of DMPO-N3 adduct formation in which the chromatogram gave a unique peak at 6.21 min with a base mass peak 155.03 m/z. (Figure S13 of SI).
Figure 9.
X-Band spectra in PBS of DMPO adducts of (a) •N3: aN = 14.75, aβ-H = 14.18, aN’ = 3.4 (lit. value for DMPO-N3: aN = 14.8, aH = 14.2 and aN-azide = 3.1)51; and (b) •NH2: aN = 15.75, aβ-H = 19.30, aN’ = 1.62; (lit. value for DMPO-NH2: aN = 15.9, aH = 19.3 and aN-amino = 1.60).93
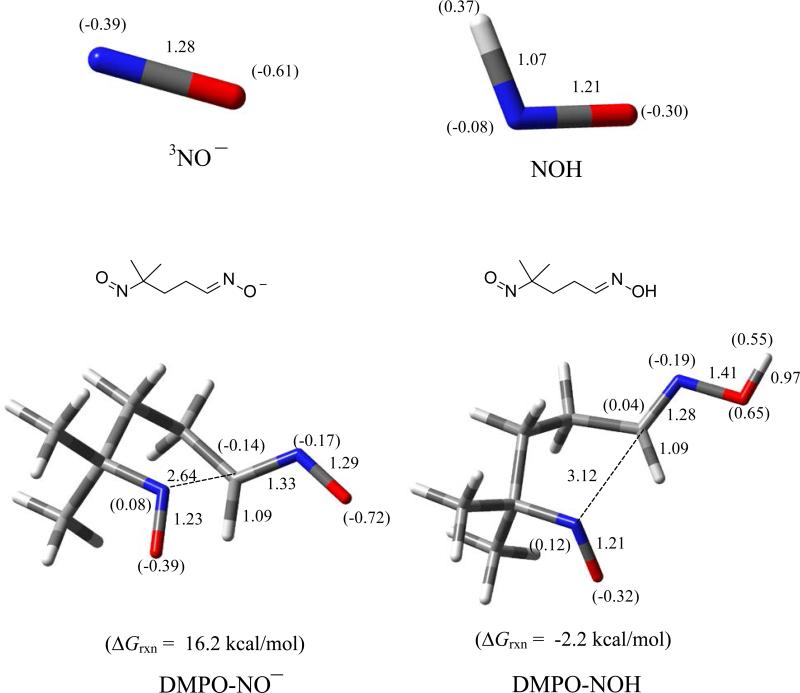
Addition of 3NO– and HNO to DMPO (Figure 10) via the N-atom each resulted in ring opening to form the nitroso-oximate and nitroso-oxime, with the latter being exoergic having ΔG298K,rxn of -2.2 kcal/mol. Previously,92 spin trapping studies using DMPO and Angeli's salt only showed formation of DMPO-OH and C-centered adducts as also confirmed in this work (see Figure S6 of SI). GC-MS analysis revealed a unique GC peak at 3.21 min with base mass peak 113.08 m/z (Figure S14 of SI) corresponding to an ene-aldoxime which can be formed from the elimination of the NO from the C-nitroso moiety of the originally formed nitroso-oxime.
Figure 10.
Optimized structures of 3NO–, HNO and their respective addition products (i.e., nitroso-oximate and nitroso-oxime, respectively) with DMPO showing the free energies of reaction, charge densities (in parentheses), and pertinent bond lengths at the PCM/B3LYP/6-31+G**//B3LYP/6-31* level of theory.
Conclusions
EPR and theoretical studies of the reaction between DMPO and •NO2 suggest formation of an O-centered radical adduct, DMPO-ONO. The formation of an O-centered radical adduct from •NO2 was also demonstrated using AMPO, EMPO and DEPMPO. The addition reactions of DMPO with ONOO–/ONOOH primarily result in ring opening to form the nitroso-aldehyde and nitrite/nitrous acid. The formation of DMPO-OH, as detected by EPR, from the O-O homolytic cleavage of ONOOH was also thermodynamically favorable. Decomposition of ONOOCO2– in the presence of DMPO yielded CO3•– adduct at high CO2 concentration. However, alkyl adduct formation in an all PBS system suggests inverted spin trapping of DMPO by DMPO•+ which is likely generated from the highly oxidizing •NO3.The EPR-detectable nitronyl adduct as DMPO-OONO, whose formation can also be rationalized by inverted spin trapping, was shown to be also formed using AMPO and EMPO as well. EPR studies of the NO-donor SNAP in the presence of DMPO show S-centered radical adduct formation under inert atmosphere. Reaction of SIN-1 with DMPO only yielded O2•–, while •NO3 addition to DMPO initially formed DMPO-ONO2, its subsequent hydrolysis yielded DMPO-OH and HNO3 as the former detected by EPR, consistent with the calculated exoergic free energy. Additions of •N3 and •NH2 to DMPO yielded N-centered radicals with significant hyperfine contributions from the N-atoms of the radical moieties. Reaction of HNO to DMPO only yielded a ring opening product to give nitroso-oxime and DMPO-OH. This study shows a myriad of RNS reaction with nitrones, and through the use of spin traps, can provide insights into the mechanisms of RNS-mediated reactions in chemical and biological systems. Moreover, this study can lead to the development of better probe designs for RNS detection, and to the understanding the antioxidative properties of nitrones.
Supplementary Material
Acknowledgments
Funding
This publication was made possible by Grant RO1 HL81248 from the NIH National Heart, Lung, and Blood Institute. This work was supported by an allocation of computing time from the Ohio Supercomputer Center.
Abbreviations
- EPR
electron paramagnetic resonance
- ROS
reactive oxygen species
- RNS
reactive nitrogen species
- SNAP
S-nitroso-N-acetyl-DL-penicillamine
- TEMPONE-H
1-hydroxy-2,2,6,6-tetramethyl-4-oxo-piperidine
- HRP
horseradish peroxidase
- DMPO
5,5-dimethyl-pyrroline N-oxide
- AMPO
5-carbamoyl-5-methyl-pyrroline N-oxide
- EMPO
5-ethoxycarbonyl-5-methyl-pyrroline N-oxide
- DEPMPO
5-(diethoxyphosphoryl)-5-methyl-1-pyrroline N-oxide
Footnotes
The term “nitrosative stress” is a misnomer as commonly used in the literature because most manifestations of which are not due to nitrosation reaction but will be referred to in general as stress caused by RNS.
Supporting Information
Additional EPR spectra, tables, thermodynamic data of all RNS, ROS, nitrones and their respective spin adducts, and complete reference 67. This material is available free of charge via internet www.pubs.acs.org.
References
- 1.Bossy-Wetzel E, Schwarzenbacher R, Lipton SA. Molecular pathways to neurodegeneration. Nat. Med. 2004;10(Suppl):S2–9. doi: 10.1038/nm1067. [DOI] [PubMed] [Google Scholar]
- 2.Escobales N, Crespo MJ. Oxidative-nitrosative stress in hypertension. Curr. Vasc. Pharmacol. 2005;3:231–246. doi: 10.2174/1570161054368643. [DOI] [PubMed] [Google Scholar]
- 3.Kaneki M, Shimizu N, Yamada D, Chang K. Nitrosative stress and pathogenesis of insulin resistance. Antioxid. Redox Signal. 2007;9:319–329. doi: 10.1089/ars.2006.1464. [DOI] [PubMed] [Google Scholar]
- 4.Llorens S, Nava E. Cardiovascular diseases and the nitric oxide pathway. Curr. Vasc. Pharmacol. 2003;1:335–346. doi: 10.2174/1570161033476637. [DOI] [PubMed] [Google Scholar]
- 5.Sugiura H, Ichinose M. Nitrative stress in inflammatory lung diseases. Nitric Oxide. 2011;25:138–144. doi: 10.1016/j.niox.2011.03.079. [DOI] [PubMed] [Google Scholar]
- 6.Szabo C. Role of nitrosative stress in the pathogenesis of diabetic vascular dysfunction. Br. J. Pharmacol. 2009;156:713–727. doi: 10.1111/j.1476-5381.2008.00086.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thomas DD, Ridnour LA, Isenberg JS, Flores-Santana W, Switzer CH, Donzelli S, Hussain P, Vecoli C, Paolocci N, Ambs S, Colton CA, Harris CC, Roberts DD, Wink DA. The chemical biology of nitric oxide: implications in cellular signaling. Free Radical Biol. Med. 2008;45:18–31. doi: 10.1016/j.freeradbiomed.2008.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferguson LR. Chronic inflammation and mutagenesis. Mutat. Res. 2010;690:3–11. doi: 10.1016/j.mrfmmm.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 9.Bartesaghi S, Valez V, Trujillo M, Peluffo G, Romero N, Zhang H, Kalyanaraman B, Radi R. Mechanistic studies of peroxynitrite-mediated tyrosine nitration in membranes using the hydrophobic probe N-tert-BOC-L-tyrosine tert-butyl ester. Biochemistry. 2006;45:6813–6825. doi: 10.1021/bi060363x. [DOI] [PubMed] [Google Scholar]
- 10.Kalyanaraman B. Nitrated lipids: a class of cell-signaling molecules. Proc. Natl. Acad. Sci. U S A. 2004;101:11527–11528. doi: 10.1073/pnas.0404309101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lancaster JR., Jr. Protein cysteine thiol nitrosation: Maker or marker of reactive nitrogen species-induced non-erythroid cellular signaling? Nitric Oxide. 2008;19:68–72. doi: 10.1016/j.niox.2008.04.028. [DOI] [PubMed] [Google Scholar]
- 12.Nuriel T, Hansler A, Gross SS. Protein nitrotryptophan: Formation, significance and identification. J. Proteomics. 2011 doi: 10.1016/j.jprot.2011.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ridnour LA, Thomas DD, Mancardi D, Espey MG, Miranda KM, Paolocci N, Feelisch M, Fukuto J, Wink DA. The chemistry of nitrosative stress induced by nitric oxide and reactive nitrogen oxide species. Putting perspective on stressful biological situations. Biol. Chem. 2004;385:1–10. doi: 10.1515/BC.2004.001. [DOI] [PubMed] [Google Scholar]
- 14.Shahani N, Sawa A. Nitric oxide signaling and nitrosative stress in neurons: role for S-nitrosylation. Antioxid. Redox Signal. 2011;14:1493–1504. doi: 10.1089/ars.2010.3580. [DOI] [PubMed] [Google Scholar]
- 15.Tiago T, Palma PS, Gutierrez-Merino C, Aureliano M. Peroxynitrite-mediated oxidative modifications of myosin and implications on structure and function. Free Radical Res. 2010;44:1317–1327. doi: 10.3109/10715762.2010.502170. [DOI] [PubMed] [Google Scholar]
- 16.Rosen GM, Tsai P, Pou S. Mechanism of free-radical generation by nitric oxide synthase. Chem. Rev. 2002;102:1191–1200. doi: 10.1021/cr010187s. [DOI] [PubMed] [Google Scholar]
- 17.McAninly J, Williams DLH, Askew SC, Butler AR, Russell C. Metal ion catalysis in nitrosothiol (RSNO) decomposition. Chem. Commun. 1993;23:1758–1759. [Google Scholar]
- 18.Angelo M, Singel DJ, Stamler JS. An S-nitrosothiol (SNO) synthase function of hemoglobin that utilizes nitrite as a substrate. Proc. Natl. Acad. Sci. U S A. 2006;103:8366–8371. doi: 10.1073/pnas.0600942103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and the ugly. Am. J. Physiol. 1996;271:C1424–C1437. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- 20.Beckman JS, Beckman TW, Chen J, Marshall PA, Freeman BA. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc. Natl. Acad. Sci. U S A. 1990;87:1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Czapski G, Goldstein S. The role of the reactions of NO with superoxide and oxygen in biological systems: a kinetic approach. Free Radical Biol. Med. 1995;19:785–794. doi: 10.1016/0891-5849(95)00081-8. [DOI] [PubMed] [Google Scholar]
- 22.Alvarez B, Demicheli V, Duran R, Trujillo M, Cervenansky C, Freeman BA, Radi R. Inactivation of human Cu,Zn superoxide dismutase by peroxynitrite and formation of histidinyl radical. Free Radical Biol. Med. 2004;37:813–822. doi: 10.1016/j.freeradbiomed.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 23.Ischiropoulos H, Zhu L, Chen J, Tsai M, Martin JC, Smith CD, Beckman JS. Peroxynitrite-mediated tyrosine nitration catalyzed by superoxide dismutase. Arch. Biochem. Biophys. 1992;298:431–437. doi: 10.1016/0003-9861(92)90431-u. [DOI] [PubMed] [Google Scholar]
- 24.Pearce LL, Martinez-Bosch S, Manzano EL, Winnica DE, Epperly MW, Peterson J. The resistance of electron-transport chain Fe-S clusters to oxidative damage during the reaction of peroxynitrite with mitochondrial complex II and rat-heart pericardium. Nitric Oxide. 2009;20:135–142. doi: 10.1016/j.niox.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rubbo H, Denicola A, Radi R. Peroxynitrite inactivates thiol-containing enzymes of Trypanosoma cruzi energetic metabolism and inhibits cell respiration. Arch. Biochem. Biophys. 1994;308:96–102. doi: 10.1006/abbi.1994.1014. [DOI] [PubMed] [Google Scholar]
- 26.Yang D, Wang H-L, Sun Z-N, Chung N-W, Shen J-G. A highly selective fluorescent probe for the detection and imaging of peroxynitrite in living cells. J. Am. Chem. Soc. 2006;128:6004–6005. doi: 10.1021/ja0603756. [DOI] [PubMed] [Google Scholar]
- 27.Sun Z-N, Wang H-L, Liu F-Q, Chen Y, Tam PKH, Yang D. BODIPY-based fluorescent probe for peroxynitrite detection and imaging in living cells. Org. Lett. 2009;11:1887–1890. doi: 10.1021/ol900279z. [DOI] [PubMed] [Google Scholar]
- 28.Lancaster JR., Jr. The use of diaminofluorescein for nitric oxide detection: Conceptual and methodological distinction between NO and nitrosation. Free Radical Biol. Med. 2010;49:1145. doi: 10.1016/j.freeradbiomed.2010.06.015. [DOI] [PubMed] [Google Scholar]
- 29.Cortes JS, Granados SG, Ordaz AA, Jimenez JAL, Griveau S, Bedioui F. Electropolymerized manganese tetraaminophthalocyanine thin films onto platinum ultramicroelectrode for the electrochemical detection of peroxynitrite in solution. Electroanalysis. 2007;19:61–64. [Google Scholar]
- 30.Lorch S, Lightfoot R, Ohshima H, Virag L, Chen Q, Hertkorn C, Weiss M, Souza J, Ischiropoulos H, Yermilov V, Pignatelli B, Masuda M, Szabo C. Detection of peroxynitrite-induced protein and DNA modifications. Methods Mol. Biol. 2002;196:247–275. doi: 10.1385/1-59259-274-0:247. [DOI] [PubMed] [Google Scholar]
- 31.Ikeda K, Yukihiro Hiraoka B, Iwai H, Matsumoto T, Mineki R, Taka H, Takamori K, Ogawa H, Yamakura F. Detection of 6-nitrotryptophan in proteins by Western blot analysis and its application for peroxynitrite-treated PC12 cells. Nitric Oxide. 2006;16:18–28. doi: 10.1016/j.niox.2006.04.263. [DOI] [PubMed] [Google Scholar]
- 32.Kalyanaraman B, Karoui H, Singh RJ, Felix CC. Detection of thiyl radical adducts formed during hydroxyl radical- and peroxynitrite-mediated oxidation of thiols-a high resolution ESR spin-trapping study at Q-band (35 GHz). Anal. Biochem. 1996;241:75–81. doi: 10.1006/abio.1996.0380. [DOI] [PubMed] [Google Scholar]
- 33.Goldstein S, Czapski G, Lind J, Merényi G. Tyrosine nitration by simultaneous generation of NO and O2 under physiological conditions. J. Biol. Chem. 2000;275:3031–3036. doi: 10.1074/jbc.275.5.3031. [DOI] [PubMed] [Google Scholar]
- 34.Tsai P, Porasuphatana S, Pou S, Rosen GM. Investigations into the spin trapping of nitric oxide and superoxide: models to explore free radical generation by nitric oxide synthase. J. Chem. Soc., Perkin Trans. 2000;2:983–988. [Google Scholar]
- 35.Sikora A, Zielonka J, Lopez M, Joseph J, Kalyanaraman B. Direct oxidation of boronates by peroxynitrite: Mechanism and implications in fluorescence imaging of peroxynitrite. Free Radical Biol. Med. 2009;47:1401–1407. doi: 10.1016/j.freeradbiomed.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goldstein S, Lind J, Merenyi G. Chemistry of peroxynitrites as compared to peroxynitrates. Chem. Rev. 2005;105:2457–2470. doi: 10.1021/cr0307087. [DOI] [PubMed] [Google Scholar]
- 37.Mere'nyi G. b., Lind J, Czapski G, Goldstein S. Direct determination of the Gibbs’ energy of formation of peroxynitrous acid. Inorg. Chem. 2003;42:3796–3800. doi: 10.1021/ic025698r. [DOI] [PubMed] [Google Scholar]
- 38.Goldstein S, Rabani J. Mechanism of nitrite formation by nitrate photolysis in aqueous solutions: the role of peroxynitrite, nitrogen dioxide, and hydroxyl radical. J. Am. Chem. Soc. 2007;129:10597–10601. doi: 10.1021/ja073609+. [DOI] [PubMed] [Google Scholar]
- 39.Merenyi G, Lind J, Goldstein S, Czapski G. Mechanism and thermochemistry of peroxynitrite decomposition in water. J. Phys. Chem. 1999;103:5685–5691. [Google Scholar]
- 40.Bonini MG, Radi R, Ferrer-Sueta G, Ferreira AMDC, Augusto O. Direct EPR detection of the carbonate radical anion produced from peroxynitrite and carbon dioxide. J. Biol. Chem. 1999;274:10802–10806. doi: 10.1074/jbc.274.16.10802. [DOI] [PubMed] [Google Scholar]
- 41.Meli R, Nauser T, Latal P, Koppenol WH. Reaction of peroxynitrite with carbon dioxide: intermediates and determination of the yield of CO3 and NO2. J. Biol. Inorg. Chem. 2002;7:31–36. doi: 10.1007/s007750100262. [DOI] [PubMed] [Google Scholar]
- 42.Goldstein S, Czapski G. Viscosity effects on the reaction of peroxynitrite with CO2: Evidence for radical formation in a solvent cage. J. Am. Chem. Soc. 1999;121:2444–2447. [Google Scholar]
- 43.Massari J, Tokikawa R, Zanolli L, Tavares MFM, Assuncao NA, Bechara EJH. Acetyl radical production by the methylglyoxal-peroxynitrite system: A possible route for L-lysine acetylation. Chem. Res. Toxicol. 2010;23:1762–1770. doi: 10.1021/tx1002244. [DOI] [PubMed] [Google Scholar]
- 44.Royer LO, Knudsen FS, De Oliveira MA, Tavares MFM, Bechara EJH. Peroxynitrite-initiated oxidation of acetoacetate and 2-methylacetoacetate esters by oxygen: Potential sources of reactive intermediates in keto acidoses. Chem. Res. Toxicol. 2004;17:1725–1732. doi: 10.1021/tx049821y. [DOI] [PubMed] [Google Scholar]
- 45.Imaram W, Gersch C, Kim KM, Johnson RJ, Henderson GN, Angerhofer A. Radicals in the reaction between peroxynitrite and uric acid identified by electron spin resonance spectroscopy and liquid chromatography mass spectrometry. Free Radical Biol. Med. 2010;49:275–281. doi: 10.1016/j.freeradbiomed.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Imaram W, Johnson RJ, Angerhofer A. ESR spin trapping of the reaction between urate and peroxynitrite: the hydrogen adduct. Appl. Magn. Reson. 2009;37:463–472. [Google Scholar]
- 47.Olson LP, Bartberger MD, Houk KN. Peroxynitrate and peroxynitrite: A complete basis set investigation of similarities and differences between these NOx species. J. Am. Chem. Soc. 2003;125:3999–4006. doi: 10.1021/ja029619m. [DOI] [PubMed] [Google Scholar]
- 48.Shiri M, Zolfigol MA, Kruger HG, Tanbakouchian Z. Advances in the application of N2O4/NO2 in organic reactions. Tetrahedron. 2010;66:9077–9106. [Google Scholar]
- 49.Batinic-Haberle I, Spasojevic I, Stevens RD, Hambright P, Neta P, Okado-Matsumoto A, Fridovich I. New class of potent catalysts of O2.bul.-dismutation. Mn(III) ortho-methoxyethylpyridyl- and di-ortho-methoxyethylimidazolylporphyrins. Dalton Trans. 2004:1696–1702. doi: 10.1039/b400818a. [DOI] [PubMed] [Google Scholar]
- 50.Bowler MW, Montgomery MG, Leslie AGW, Walker JE. How azide inhibits ATP hydrolysis by the F-ATPases Proc. Natl. Acad. Sci. USA. 2006;103:8646–8649. doi: 10.1073/pnas.0602915103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kalyanaraman B, Janzen EG, Mason RP. Spin trapping of the azidyl radical in azide/catalase/hydrogen peroxide and various azide/peroxidase/hydrogen peroxide peroxidizing systems. J. Biol. Chem. 1985;260:4003–4006. [PubMed] [Google Scholar]
- 52.Partridge RS, Monroe SM, Parks JK, Johnson K, Parker WD, Jr., Eaton GR, Eaton SS. Spin trapping of azidyl and hydroxyl radicals in azide-inhibited rat brain submitochondrial particles Arch. Biochem. Biophys. 1994;310:210–217. doi: 10.1006/abbi.1994.1159. [DOI] [PubMed] [Google Scholar]
- 53.Amadelli R, Maldotti A, Bartocci C, Carassiti V. ESR spin-trapping investigation of azide oxidation on cadmium sulfide and zinc oxide suspension. J. Phys. Chem. 1989;93:6448–6453. [Google Scholar]
- 54.Clarke K, Edge R, Johnson V, Land EJ, Navaratnam S, Truscott TG. Direct observation of NH2 reactions with oxygen, amino acids, and melanins. J. Phys. Chem. A. 2008;112:1234–1237. doi: 10.1021/jp076395r. [DOI] [PubMed] [Google Scholar]
- 55.Kemp-Harper BK. Nitroxyl (HNO): a novel redox signaling molecule. Antioxid. Redox Signal. 2011;14:1609–1613. doi: 10.1089/ars.2011.3937. [DOI] [PubMed] [Google Scholar]
- 56.Dikalov S, Skatchkov M, Fink B, Bassenger E. Quantification of superoxide radicals and peroxynitrite in vascular cells using oxidation of sterically hindered hydroxylamines and electron spin resonance. Nitric Oxide. 1997;1:423–431. doi: 10.1006/niox.1997.0139. [DOI] [PubMed] [Google Scholar]
- 57.Rosen GM, Rauckman l. J. Formation and reduction of a nitroxide radical by liver microsomes. Biochem. Pharmacol. 1977;26:675–678. doi: 10.1016/0006-2952(77)90049-1. [DOI] [PubMed] [Google Scholar]
- 58.Finkelstein E, Rosen GM, Rauckman EJ. Spin trapping. Kinetics of the reaction of superoxide and hydroxyl radicals with nitrones. J. Am. Chem. Soc. 1980;102:4994–4999. [Google Scholar]
- 59.Rosen GM, Britigan BE, Halpern HJ, Pou S. Free radicals: Biology and detection by spin trapping. Oxford University Press; New York, New York: 1999. [Google Scholar]
- 60.Ranguelova K, Mason RP. The fidelity of spin trapping with DMPO in biological systems. Magn. Reson. Chem. 2011;49:152–158. doi: 10.1002/mrc.2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Makino K, Hagiwara T, Hagi A, Nishi M, Murakami A. Cautionary note for DMPO spin trapping in the presence of iron ion. Biochem. Biophys. Res. Commun. 1990;172:1073–1080. doi: 10.1016/0006-291x(90)91556-8. [DOI] [PubMed] [Google Scholar]
- 62.Eberson L. Inverted spin trapping. Part III. Further studies on the chemical and photochemical oxidation of spin traps in the presence of nucleophiles. J. Chem. Soc., Perkin Trans. 1994;2:171–176. [Google Scholar]
- 63.Astolfi P, Greci L, Panagiotaki M. Spin trapping of nitrogen dioxide and of radicals generated from nitrous acid. Free Radical Res. 2005;39:137–144. doi: 10.1080/10715760500045582. [DOI] [PubMed] [Google Scholar]
- 64.Gatti RM, Alvarez B, Vasquez-Vivar J, Radi R, Augusto O. Formation of spin trap adducts during the decomposition of peroxynitrite. Arch. Biochem. Biophys. 1998;349:36–46. doi: 10.1006/abbi.1997.0451. [DOI] [PubMed] [Google Scholar]
- 65.Durand G, Prosak RA, Han Y, Ortial S, Rockenbauer A, Pucci B, Villamena FA. Spin trapping and cytoprotective properties of fluorinated amphiphilic carrier conjugates of cyclic versus linear nitrones. Chem. Res. Toxicol. 2009;22:1570–1581. doi: 10.1021/tx900114v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Labanowski JW, Andzelm J. Density Functional Methods in Chemistry. Springer; New York, N. Y.: 1991. [Google Scholar]
- 67.Firsch MJ, et al. Gaussian 03. Gaussian, Inc.; Pittsburgh, PA: 2004. [Google Scholar]
- 68.Scott AP, Radom L. Harmonic vibrational frequencies: An evaluation of Hartree-Fock, Moeller-Plesset, quadratic configuration interaction, density functional theory, and semiempirical scale factors. J. Phys. Chem. 1996;100:16502–16513. [Google Scholar]
- 69.Cossi M, Barone V. Analytical second derivatives of the free energy in solution by polarizable continuum models. J. Chem. Phys. 1998;109:6246–6254. [Google Scholar]
- 70.Cossi M, Barone V, Cammi R, Tomasi J. Ab initio study of solvated molecules: a new implementation of the polarizable continuum model. Chem. Phys. Lett. 1996;255:327–335. [Google Scholar]
- 71.Reed AE, Curtiss LA, Weinhold F. Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint. Chem. Rev. 1988;88:899–926. [Google Scholar]
- 72.Baboul AG, Curtiss LA, Redfern PC. Gaussian-3 theory using density functional geometries and zero-point energies. J. Chem. Phys. 1999;110:7650–7657. [Google Scholar]
- 73.Izgorodina EI, Brittain DR, Hodgson JL, Krenske EH, Lin CY, Namazian M, Coote ML. Should contemporary density functional theory methods be used to study the thermodynamics of radical reactions? J. Phys. Chem. A. 2007;111:10754–10768. doi: 10.1021/jp075837w. [DOI] [PubMed] [Google Scholar]
- 74.Rockenbauer A, Korecz L. Automatic computer simulations of ESR spectra. Appl. Magn. Reson. 1996;10:29–43. [Google Scholar]
- 75.Robinson KM, Beckman JS. Synthesis of peroxynitrite from nitrite and hydrogen peroxide. Methods in Enzymol. 2005;396:207–214. doi: 10.1016/S0076-6879(05)96019-9. [DOI] [PubMed] [Google Scholar]
- 76.Pace MD, Carmichael AJ. Quantitative EPR spin trapping. 1. Nitrogen dioxide radicals and nitrite ions from energetic materials in alkaline aqueous solution. J. Phys. Chem. A. 1997;101:1848–1853. [Google Scholar]
- 77.Crow JP, Spruell C, Chen J, Gunn C, Ischiropoulos H, Tsai M, Smith CD, Radi R, Koppenol WH, Beckman JS. On the pH-dependent yield of hydroxyl radical products from peroxynitrite. Free Radical Biol. Med. 1994;16:331–338. doi: 10.1016/0891-5849(94)90034-5. [DOI] [PubMed] [Google Scholar]
- 78.Villamena FA. Superoxide radical anion adduct of 5,5-dimethyl-1-pyrroline N-oxide. 6: Redox properties. J. Phys. Chem. A. 2010;114:1153–1160. doi: 10.1021/jp909614u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Henry DJ, Parkinson CJ, Radom L. An assessment of the performance of high-level theoretical procedures in the computation of the heats of formation of small open-shell molecules. J. Phys. Chem. A. 2002;106:7927–7936. [Google Scholar]
- 80.Koppenol WH, Liebman JF. The oxidizing nature of the hydroxyl radical. A comparison with the ferryl ion (FeO2+). J. Phys. Chem. A. 1984;88:99–101. [Google Scholar]
- 81.Stanbury DM. Reduction potentials involving inorganic free radicals in aqueous solution. Adv. Inorg. Chem. 1989;33:69–138. [Google Scholar]
- 82.Rockenbauer A, Györ M, Tüdös F. Spin trapping reactions with nitric oxides IV. Reactions with olefins. Tetrahedron Lett. 1986;27:3425–3428. [Google Scholar]
- 83.Houk KN, Condroski KR, Pryor WA. Radical and concerted mechanisms in oxidations of amines, sulfides, and alkenes by peroxynitrite, peroxynitrous acid, and the peroxynitrite-CO2 adduct: Density functional theory transition structures and energetics. J. Am. Chem. Soc. 1996;118:13002–13006. [Google Scholar]
- 84.Villamena FA, Locigno EJ, Rockenbauer A, Hadad CM, Zweier JL. Theoretical and experimental studies of the spin trapping of inorganic radicals by 5,5- dimethyl-1-pyrroline N-oxide (DMPO). 2. Carbonate radical anion. J. Phys. Chem. 2007;111:384–391. doi: 10.1021/jp065692d. [DOI] [PubMed] [Google Scholar]
- 85.Singh RJ, Hogg N, Joseph J, Kalyanaraman B. Mechanism of nitric oxide release from S-nitrosothiols. J. Biol. Chem. 1996;271:18596–18603. doi: 10.1074/jbc.271.31.18596. [DOI] [PubMed] [Google Scholar]
- 86.Villamena FA, Hadad CM, Zweier JL. Comparative DFT study of the spin trapping of methyl, mercapto, hydroperoxy, superoxide, and nitric oxide radicals by various substituted cyclic nitrones. J. Phys. Chem. A. 2005;109:1662–1674. doi: 10.1021/jp0451492. [DOI] [PubMed] [Google Scholar]
- 87.Rojas Wahl RU. Decomposition mechanism of 3-N-morpholinosydnonimine (SIN-1)-a density functional study on intrinsic structures and reactivities. J. Mol. Model. 2004;10:121–129. doi: 10.1007/s00894-004-0178-9. [DOI] [PubMed] [Google Scholar]
- 88.Dutton AS, Fukuto JM, Houk KN. Theoretical reduction potentials for nitrogen oxides from CBS-QB3 energetics and (C)PCM solvation calculations. Inorg. Chem. 2005;44:4024–4028. doi: 10.1021/ic048734q. [DOI] [PubMed] [Google Scholar]
- 89.McIntire GL, Blount HN, Stronks HJ, Shetty RV, Janren EG. Spin trapping in electrochemistry. 2. Aqueous and nonaqueous electrochemical characterizations of spin traps. J. Phys. Chem. A. 1980;84:916–921. [Google Scholar]
- 90.Kirino Y, Ohkuma T. a., Kwan T. Spin trapping with 5,5-dimethylpyrroline-N-oxide in aqueous solution. Chem.Pharm. Bull. 1981;29:29–34. [Google Scholar]
- 91.Chignell CF, Kalyanaraman B, Sik RH, Mason RP. Spectroscopic studies of cutaneous photosensitizing agents. II. Spin trapping of photolysis products from sulfanilamide and 4-aminobenzoic acid using 5,5-dimethyl-1-pyrroline-1-oxide. Photochem. Photobiol. 1981;34:147–156. [Google Scholar]
- 92.Stoyanovsky DA, Clancy R, Cederbaum AI. Decomposition of sodium trioxodinitrate (Angeli's Salt) to hydroxyl radical: An ESR spin-trapping study. J. Am. Chem. Soc. 1999;121:5093–5094. [Google Scholar]
- 93.Chignell CF, Kalyanaraman B, Mason RP, Sik RH. Spectroscopic studies of cutaneous photosensitizing agents. I. Spin trapping of photolysis products from sulfanilamide, 4-aminobenzoic acid, and related compounds. Photochem. Photobiol. 1980;32:563–571. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.