Abstract
Purpose
We examined the role of androgens and estrogens in mammalian sexual differentiation by morphological characterization of adult wt and mutant mouse external genitalia. We tested the hypothesis that external genitalia development depends on androgen and estrogen action.
Materials and Methods
We studied serial sections of the external genitalia of the CD-1 and C57BL6 wt strains of adult mice (Charles River Laboratories, Wilmington, Massachusetts). We recorded linear measurements of key structures in each specimen, including the urethra, erectile tissue, bone and cartilage. We used similar methodology to analyze mice mutant for estrogen receptor α (αERKO) and androgen receptor (XTfm/Y) (Jackson Laboratory, Bar Harbor, Maine).
Results
Morphology in XTfm/Y adult murine external genitalia was remarkably similar to that in wt females. Bone and clitoral length was similar in wt females and XTfm/Y mice. Conversely the αERKO clitoris was 59% longer and bone length in αERKO females was many-fold longer than that in female wt mice or XTfm/Y mutants. The αERKO clitoris contained cartilage, which is typical of the wt penis but never observed in the wt clitoris. Serum testosterone was not increased in female αERKO mice 10 days postnatally when sex differentiation occurs, suggesting that masculinization of the αERKO clitoris is not a function of androgen.
Conclusions
Masculinization of the αERKO clitoris suggests a role for estrogen in the development of female external genitalia. We propose that normal external genital development requires androgen and estrogen action.
Keywords: penis, clitoris, androgens, estrogens, sex differentiation
The conceptual framework of our contemporary understanding of mammalian sexual differentiation was described by Jost and is based on patterns of sex differentiation after fetal castration.1,2 According to Jost ExG masculine development involves androgen action. In the absence of androgens the default female pattern of sex differentiation occurs. In laboratory rodents a unique role for dihydroT3 was noted, especially in regard to the development of skeletal elements.4,5 Since the Jost description of the morphological effects of fetal castration, AR has been cloned and shown to have an essential role in sex differentiation.5–7 While the androgen theory of Jost is correct, is it the whole story?
A possible role of estrogen in normal ExG development is currently debated, stemming from observations of aromatase, and ERα and ERβ in rat developing ExG.8,9 Unfortunately these studies do not identify a single morphogenetic event in normal ExG development that depends on estrogen. Preliminary observations in the spotted hyena on the morphogenetic effects of the aromatase inhibitor letrozole on developing ExG (Cunha and Glickman, unpublished data) stimulated us to examine the role of estrogen in a more conventional animal, the mouse, for which many relevant transgenic strains exist.
The literature on morphology of the penis and clitoris in the adult mouse is inadequate and to some extent inaccurate, especially regarding the exact size, shape and position of certain key internal structures. Adult ExG morphology is by definition the culmination of normal development and, thus, adult morphology was used as the end point of the developmental process. Our goal was to characterize the morphology of the adult wt penis and clitoris by modern morphological technique (3-dimensional reconstruction and morphometrics) to identify specific epithelial and stromal structures, and attribute developmental events to androgen or estrogen action. Previous studies of the sex differentiation of rodent ExG focused on androgenic induction of the os penis and os clitoris in rats and mice.8,9 We propose that the precise morphological organization in adult mouse ExG result from signaling via AR and ERα. By analyzing αERKO and AR mutant (XTfm/Y) mice we suggest a role for estrogen as well as androgens in normal mammalian sexual differentiation.
MATERIALS AND METHODS
All animal care and protocols used were approved by the University of California-San Francisco institutional animal care and use committee. ExG of adult wt CD-1 and C57BL6, and mutant αERKO mice and XTfm/y mice were fixed in formalin and serially sectioned at 7 μm for histological staining. We examined the ventral cleft, urethra, clitoris and bone in wt female mice, and the ventral cleft, urethra, bone and cartilage in wt male mice. We examined 5 wt C57BL6 mice of each gender as well as 4 wt male CD-1 mice and 3 wt female CD-1 mice. The C57BL6 strain was chosen because the ERKO mutant colony used in this study also originated from the same strain. The CD-1 strain was used because it is a commonly used, multipurpose outbred strain. Linear measurements of key structures were made by counting sections containing the object of interest. Statistical analysis was done using Student’s t test with p <0.05 considered significant. We created 3-dimensional computer reconstructions from serial sections using Surf-driver® 3.5, as described previously.10 We performed scanning electron microscopy by routine methods.11 Serum T was measured using a solid phase 125I radioimmunoassay kit, as previously described.12
RESULTS
wt Morphology
Male
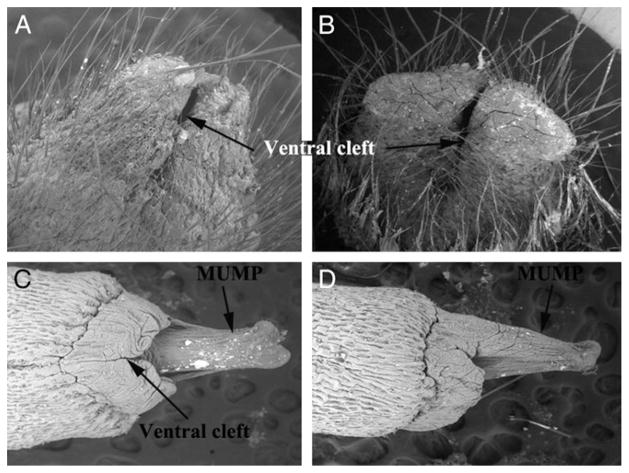
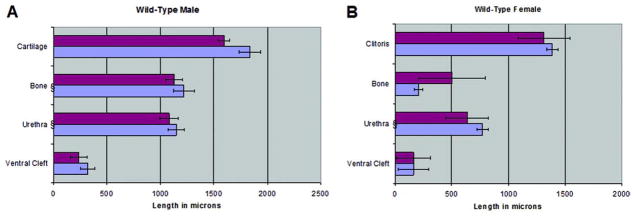
The surface elevation in the perineum of wt males is the prepuce, which is bifid (fig. 1, B). The penis is located internally in the preputial space. The wt penis is adorned by a bifid distal structure which to our knowledge has not been reported previously. It contains a central core of cartilage. This distal bifid projection is called MUMP (figs. 1, C and D, and 2, A). Cartilage begins distal in MUMP and extends proximal to dorsally overlap the os penis. We made linear measurements of penile structures in wt male mice (fig. 3, A). All parameters were remarkably similar in each wt mouse strain. From a qualitative perspective the adult wt mouse penis has 8 diagnostic morphological features (Appendix 1).
Figure 1.
Scanning electron microscopy shows mouse ExG. A, wt female ExG. Reduced from ×50. B, wt male ExG. Reduced from ×50. C, ventral view of wt penis. Reduced from ×100. D, lateral view of wt penis. Reduced from ×100.
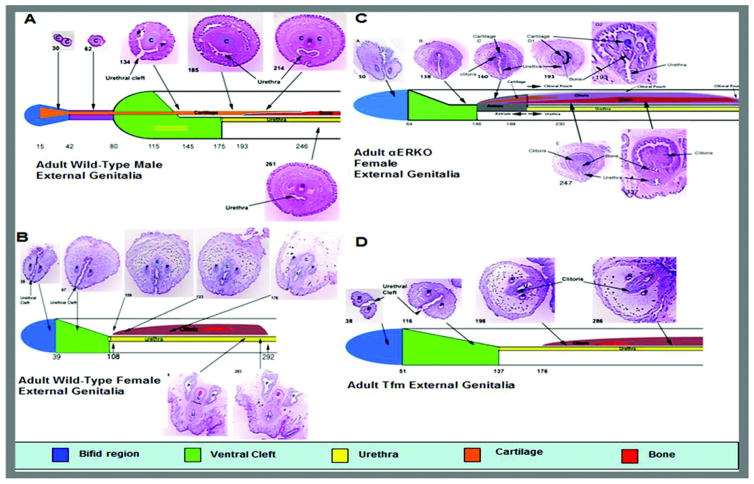
Figure 2.
Detailed anatomy of key ExG structures in adult mouse. Numbers indicate section number from distal tip. A, MUMP, cartilage (C), process (P) and bone (B) in adult wt male ExG. B, bifid (blue area), ventrally cleft (green area) distal prepuce, ventrally tethered clitoris (C), bone, preputial duct (P) and urethra (U) in adult wt female ExG. C, ventrally tethered clitoris containing bone and cartilage in adult αERKO female ExG. D, bifid distal portion and clitoris with bone but no cartilage in adult Tfm male ExG.
Figure 3.
Morphometric measurements of key structures were similar in CD-1 (blue bars) and C57BL6 (red bars) adult wt mice. A, 4 CD-1 and 5 C57BL5 wt males. B, 3 CD-1 and 5 C57BL5 wt females. Bars with curley represent distance from ExG tip to beginning of structure. Other bars represent length.
APPENDIX 1.
Qualitative morphological features of adult mouse penis and clitoris
| Penis |
| Circular transverse profile |
| No tethering, freely moving organ |
| Urethra completely within the penis |
| Large os penis |
| Contains cartilage |
| Located in epithelium lined space |
| Surface (penile) spines |
| Large organ size |
| Clitoris |
| U-shaped epithelial lamina |
| Ventral tethering/immobile |
| Urethra never completely within clitoris |
| Miniscule os clitoris |
| No cartilage |
| Never located in epithelium lined space |
| No epithelial spines |
| Small organ size |
Female
The surface elevation in the perineum of the female mouse is not the clitoris but represents the prepuce, which in wt females is also bifid (fig. 1, A). The clitoris is deeply placed in the perineum, mostly defined by a U-shaped solid epithelial lamina and contains a small os clitoris (fig. 2, B). The adult mouse clitoris is tethered to ventral stroma and, thus, is immobile. Figure 2, B shows the anatomy of the distal portion of the adult wt clitoris. Unlike the penis, the adult wt mouse clitoris does not contain cartilage or epithelial spines and is not located in an epithelium lined space. Likewise, the urethra lies completely or partly outside the wt clitoris. Figure 3, B shows linear measurements of structures in the adult wt clitoris. As in the male, all parameters were not significantly different in CD-1 vs C57/6bl wt mice. Qualitatively the adult wt mouse clitoris has 8 characteristic morphological features (Appendix 1).
αERKO Clitoral Morphology
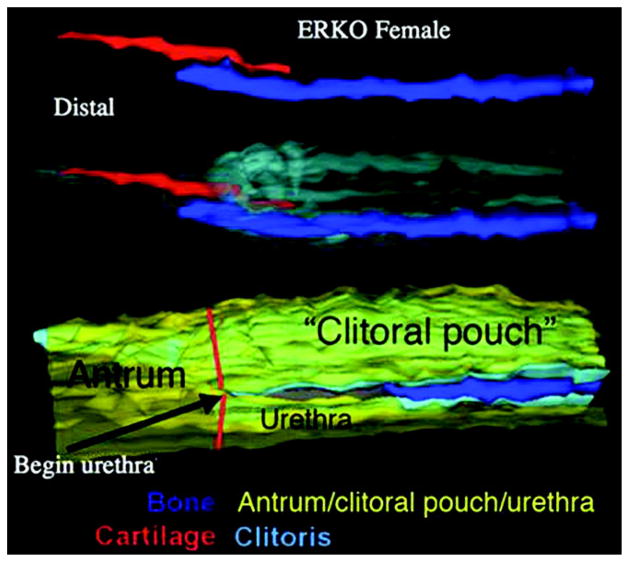
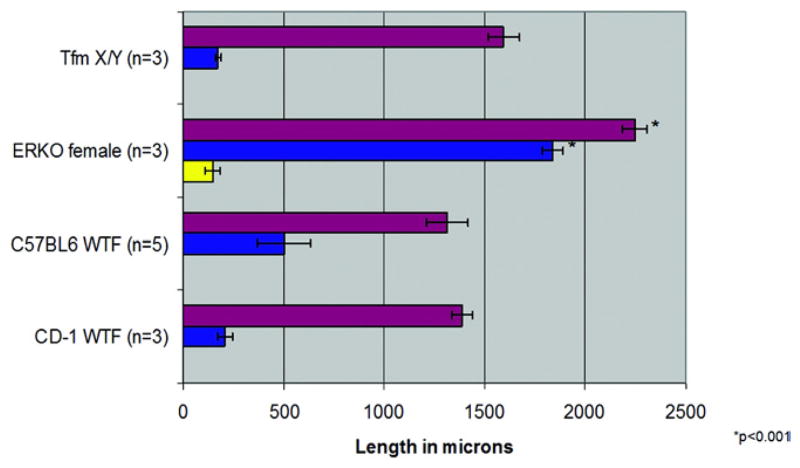
Figure 2, C shows detailed anatomy of the adult αERKO clitoris. In female αERKO mice the bifid prepuce is followed proximal by a ventrally cleft region and even more proximal by a tubular structure (antrum) formed by fusion of the edges of the ventral cleft. More proximal the antrum divides into the clitoral pouch dorsally and the urethra ventrally (fig. 4). The αERKO clitoris is radically different from the wt clitoris in several respects. The αERKO clitoral body is 1.7-fold longer than the wt clitoris. The αERKO os clitoris is many times longer than that in wt mice (p <0.001, fig. 5). Also, the αERKO clitoris contains cartilage, which is absent in the wt clitoris. Morphometrics of the αERKO male mutant are not significantly different from those of the wt male (data not shown).
Figure 4.
Three-dimensional reconstruction of adult αERKO female ExG reveals cartilage overlapping bone dorsal and antrum divided into clitoral pouch and urethra.
Figure 5.
Clitoral (red bars), bone (blue bars) and cartilage (yellow bars) length in CD-1 and C57BL6 wt females, and αERKO and Tfm mutants. Bone and clitoris were significantly longer in αERKO female vs wt mice and Tfm mutants (p <0.001).
Tfm Morphology
ExG in XTfm/Y mice are remarkably similar to those in wt females (fig. 2, D). Internal structures, including the length of the XTfm/Y clitoris and bone, were not significantly different from those in wt females (fig. 5).
From a purely qualitative perspective ExG in all wt CD-1 and C57/6Bl mice, and in XTfm/Y mice showed an identical female phenotype, in contrast to the wt male phenotype. The phenotype of the αERKO clitoris has several masculine features and, thus, the αERKO clitoris has an intermediate phenotype (Appendix 2).
APPENDIX 2.
External genitalia phenotypes of adult wt and mutant female and male mice
| WT (feature) | XTim/Y (feature) | αERKO (feature) | WT Male (feature) | |
|---|---|---|---|---|
| Shape | Female | Female | Intermediate | Male |
| Ventral tethering | Yes (female) | Yes (female) | Yes (female) | No (male) |
| Urethral site | Female | Female | Female | Male |
| Bone | Small (female) | Small (female) | Large (male) | Large (male) |
| Cartilage | Absent (female) | Absent (female) | Present (male) | Present (male) |
| Epithelial space | Absent (female) | Absent (female) | Present (male) | Present (male) |
| Spines | Absent (female) | Absent (female) | Present (male) | Present (male) |
| Size | Small (female) | Small (female) | Large (male) | Large (male) |
Serum T
Serum T in 10-day-old αERKO female mice was lower than in wt female mice of the same age (fig. 6), although the difference was not statistically significant. Serum T in 10-day-old αERKO and wt female mice was many-fold lower than that reported previously in adult male mice.13
Figure 6.
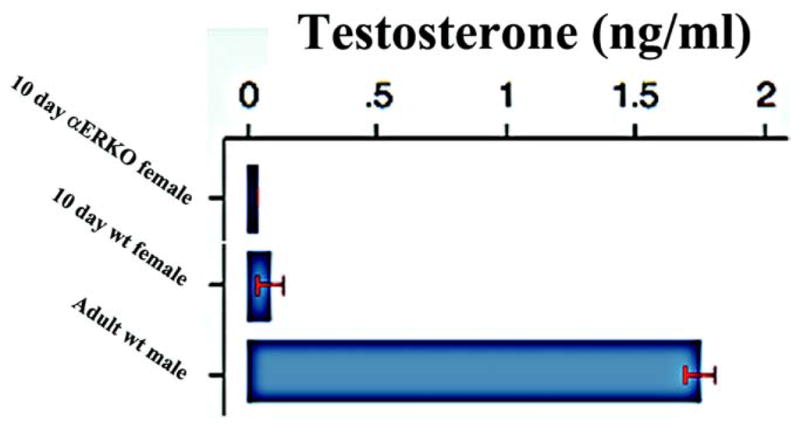
Serum T in WT and αERKO mice with adult wt male value as reported by DePaolo and Masoro.13
DISCUSSION
The current paradigm of sexual differentiation states that in the presence of androgen masculine ExG develop and in the absence of androgen the default female pattern develops. Sparse data exist on internal morphological features of the adult penis, such as the position of the urethral meatus and especially the morphology of the distal penile tip. Morphology of the mouse clitoris is poorly described. Our study shows in detail the external and internal architecture of the wt adult murine male and female ExG, which are used as end points of sexually dimorphic development. Sexually dimorphic structures are remarkably uniform in wt male and female mice. MUMP in males, the amount of bone, and cartilage and erectile tissue appear to be influenced by androgen and estrogen action, based on our data on αERKO and XTfm/Y mutant animals, and those reported previously.5 Initial development of the os penis is androgen independent based on its presence in androgen insensitive of XTfm/Y mice,5 although subsequent bone growth is androgen dependent.14,15 The absolute length of the os clitoris was considerably greater in αERKO vs wt females (p <0.001). These differences may be explained by the effects of estrogen on osteogenesis.16,17 Also, studies of bone metabolism in aromatase KO mice implicate a role for androgen and estrogen in bone metabolism.17 Thus, estrogen insensitivity or androgen acting unopposed by estrogen may contribute to the increased amount of bone in αERKO female ExG.
A substantial cartilaginous element was consistently noted in the αERKO clitoris while cartilage is never observed in the wt clitoris. Neonatal administration of androgen augments bone growth in the mouse clitoris but does not induce cartilage formation.4 Our study suggests that signaling via ER may influence chondrogenesis. In this regard neonatally administered tamoxifen inhibits postnatal differentiation of skeletal elements in the mouse penis, resulting in a decrease in or absence of the os penis and distal cartilaginous segment.18 These changes may be inherent to tamoxifen since treatment with other antiestrogens did not result in cartilage loss. In mice tamoxifen can act as an estrogen agonist or antagonist.
Our findings of a potential role of estrogen in sexual dimorphic development are consistent with previous studies showing ERα, ERβ and aromatase in the developing rat penis,8,9 and ERα in the developing human male fetal ExG.19 Consistent with the ER in the developing ExG is the presence of T and estradiol in the serum of female rodents during the neonatal period, when ExG sex differentiation occurs.20 To our knowledge the biological significance of low T in newborn females is unclear and, thus, especially in αERKO females, must be considered since in this situation androgen can act unopposed by estrogen.
ExG sensitivity was previously established in studies by Goyal et al, in which exogenous estrogen at pharmacological levels was administered during the perinatal period.21 Such treatment elicited penile dysmorphogenesis, including malformations such as hypospadias, abnormal penile muscles and bone formation, and decreased penile length, diameter and weight.21 From these studies Pang et al suggested that ER and AR based mechanisms estrogen induce penile deformity.20 Unfortunately these findings represent nonphysiological conditions and, thus, are not relevant to normal development. Our findings document various developmental effects of the ERα mutant state on normal clitoral development.
A potential explanation for the partial masculinization of the αERKO clitoris may be serum T, which is increased 40-fold in adult αERKO mice.22 However, serum T fails to explain the phenotype of the αERKO clitoris for several reasons. Adult serum T does not necessarily have any relation to sex differentiation in the neonatal period. Serum T is not increased in female αERKO mice 21 days postnatally23 or at day 10, when sex differentiation occurs. Given that masculinization in the αERKO clitoris appears to be independent of systemic androgen, the masculinized phenotype of the αERKO clitoris may be due to absent ERα signaling in developing ExG tissue.
CONCLUSIONS
To our knowledge we describe precise morphometrics of the adult male and female ExG for the first time. Overall key structures are uniform in wt male and female mice while XTfm/Y mutant ExG are almost identical to those of wt females. In contrast, the αERKO clitoris contains significantly more bone as well as cartilage, which is typical of the wt male penis. We suggest that the masculinized phenotype of αERKO mice is due to absent estrogen action since serum T is not increased during ExG sex differentiation. We propose that disruption of the balance between androgen and estrogen action alters normal development and leads to abnormal ExG morphology.
Acknowledgments
Supported by National Science Foundation Grant IOS-0920793.
Jennifer Bogener and Dennis Lubahn, University of Missouri, provided αERKO mice.
Abbreviations and Acronyms
- αERKO
ERα knockout
- AR
androgen receptor
- ER
estrogen receptor
- ERKO
ER knockout
- ExG
external genitalia
- MUMP
male urogenital mating protuberance
- T
testosterone
- XTfm/Y
androgen receptor knockout
Footnotes
Study received University of California-San Francisco institutional animal care and use committee approval.
References
- 1.Jost A. Problems of fetal endocrinology: the gonadal and hypophyseal hormones. Rec Prog Horm Res. 1953;8:379. [Google Scholar]
- 2.Jost A. Gonadal hormones in the sex differentiation of the mammalian fetus. In: Urpsrung RL, DeHaan H, editors. Organogenesis. New York: Holt, Rinehart and Winston; 1965. pp. 611–628. [Google Scholar]
- 3.Wilson JD, George FW, Renfree MB. The endocrine role in mammalian sexual differentiation. Recent Prog Horm Res. 1995;50:349. doi: 10.1016/b978-0-12-571150-0.50021-4. [DOI] [PubMed] [Google Scholar]
- 4.Glucksmann A, Ooka-Souda S, Miura-Yasugi E, et al. The effect of neonatal treatment of male mice with antiandrogens and of females with androgens on the development of the os penis and os clitoridis. J Anat. 1976;121:363. [PMC free article] [PubMed] [Google Scholar]
- 5.Murakami R. A histological study of the development of the penis of wild-type and androgen-insensitive mice. J Anat. 1987;153:223. [PMC free article] [PubMed] [Google Scholar]
- 6.Glucksmann A, Ooka-Souda S, Miura-Yasugi E, et al. The effect of neonatal treatment of male mice with antiandrogens and of females with androgens on the development of the os penis and os clitoridis. J Anat. 1976;121:363. [PMC free article] [PubMed] [Google Scholar]
- 7.Glucksmann A, Cherry CP. The hormonal induction of an os clitoridis in the neonatal and adult rat. J Anat. 1972;112:223. [PMC free article] [PubMed] [Google Scholar]
- 8.Jesmin S, Mowa CN, Matsuda N, et al. Evidence for a potential role of estrogen in the penis: detection of estrogen receptor-alpha and -beta messenger ribonucleic acid and protein. Endocrinology. 2002;143:4764. doi: 10.1210/en.2002-220628. [DOI] [PubMed] [Google Scholar]
- 9.Jesmin S, Mowa CN, Sakuma I, et al. Aromatase is abundantly expressed by neonatal rat penis but downregulated in adulthood. J Mol Endocrinol. 2004;33:343. doi: 10.1677/jme.1.01548. [DOI] [PubMed] [Google Scholar]
- 10.Yucel S, Cavalcanti AG, Desouza A, et al. The effect of oestrogen and testosterone on the urethral seam of the developing male mouse genital tubercle. BJU Int. 2003;92:1016. doi: 10.1111/j.1464-410x.2003.04511.x. [DOI] [PubMed] [Google Scholar]
- 11.Nakanishi Y, Sugiura F, Kishi J, et al. Scanning electron microscopic observation of mouse embryonic submandibular glands during initial branching: preferential localization of fibrillar structures at the mesenchymal ridges participating in cleft formation. J Embryol Exp Morphol. 1986;96:65. [PubMed] [Google Scholar]
- 12.Cota CD, Liu RR, Sumberac TM, et al. Genetic and phenotypic studies of the dark-like mutant mouse. Genesis. 2008;46:562. doi: 10.1002/dvg.20432. [DOI] [PubMed] [Google Scholar]
- 13.DePaolo L, Masoro E. Endocrine hormones in laboratory animals. In: Loeb W, Quimby F, editors. Clinical Chemistry of Laboratory Animals. Elmsford, New York: Pergamon Press; 1989. [Google Scholar]
- 14.Glucksmann A, Cherry CP. The hormonal induction of an os clitoridis in the neonatal and adult rat. J Anat. 1972;112:223. [PMC free article] [PubMed] [Google Scholar]
- 15.Yoshida H, Huggins CB. Induction of ossification in the clitoris of neonatal female rats by administration of androgens. Endocrinology. 1980;106:1956. doi: 10.1210/endo-106-6-1956. [DOI] [PubMed] [Google Scholar]
- 16.Rochira V, Balestrieri A, Faustini-Fustini M, et al. Role of estrogen on bone in the human male: insights from the natural models of congenital estrogen deficiency. Mol Cell Endocrinol. 2001;178:215. doi: 10.1016/s0303-7207(01)00446-4. [DOI] [PubMed] [Google Scholar]
- 17.Matsumoto C, Inada M, Toda K, et al. Estrogen and androgen play distinct roles in bone turnover in male mice before and after reaching sexual maturity. Bone. 2006;38:220. doi: 10.1016/j.bone.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 18.Iguchi T, Irisawa S, Uesugi Y, et al. Abnormal development of the os penis in male mice treated neonatally with tamoxifen. Acta Anat. 1990;139:201. doi: 10.1159/000146998. [DOI] [PubMed] [Google Scholar]
- 19.Crescioli C, Maggi M, Vannelli GB, et al. Expression of functional estrogen receptors in human fetal male external genitalia. J Clin Endocrinol Metab. 2003;88:1815. doi: 10.1210/jc.2002-021085. [DOI] [PubMed] [Google Scholar]
- 20.Pang SF, Caggiula AR, Gay VL, et al. Serum concentrations of testosterone, oestrogens, luteinizing hormone and follicle-stimulating hormone in male and female rats during the critical period of neural sexual differentiation. J Endocrinol. 1979;80:103. doi: 10.1677/joe.0.0800103. [DOI] [PubMed] [Google Scholar]
- 21.Goyal HO, Braden TD, Williams CS, et al. Estrogen-induced developmental disorders of the rat penis involve both estrogen receptor (ESR)- and androgen receptor (AR)-mediated pathways. Biol Reprod. 2009;81:507. doi: 10.1095/biolreprod.108.071951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Couse JF, Yates MM, Walker VR, et al. Characterization of the hypothalamic-pituitary-gonadal axis in estrogen receptor (ER) Null mice reveals hypergonadism and endocrine sex reversal in females lacking ERalpha but not ERbeta. Mol Endocrinol. 2003;17:1039. doi: 10.1210/me.2002-0398. [DOI] [PubMed] [Google Scholar]
- 23.Rosenfeld CS, Cooke PS, Welsh TH, Jr, et al. The differential fate of mesonephric tubular-derived efferent ductules in estrogen receptor-alpha knockout versus wild-type female mice. Endocrinology. 2000;141:3792. doi: 10.1210/endo.141.10.7694. [DOI] [PubMed] [Google Scholar]