Abstract
Background & Aims
The glucocorticoid-induced tumor necrosis factor receptor family-related protein (GITR; also called TNFRSF18 or CD357) regulates the T-cell mediated immune response and is present on surfaces of T regulatory (Treg) and activated CD4+ T cells. We investigated the roles of GITR in the development of colitis in mice.
Method
Chronic enterocolitis was induced by the transfer of wild-type or GITR−/− CD4+ T cells to GITR−/−xRag−/− or Rag−/− mice. We determined colitis severity using the disease activity index, measured levels of inflammatory cytokines, T cells, and dendritic cells, and performed histologic analysis of colon samples.
Results
Transfer of non-fractionated CD4+ cells from wild-type or GITR−/− donors induced colitis in GITR−/−xRag−/− but not in Rag−/− mice. Among mice with transfer-induced colitis, the percentage of Treg and T-helper (Th)17 cells was reduced but that of Th1 cells increased. Treg cells failed to prevent colitis in GITR−/−xRag−/− recipients; this was not the result of aberrant function of GITR−/− Treg or T effector cells, but resulted from an imbalance between the numbers of tolerogenic CD103+ and PDCA1+ plasmacytoid dendritic cells in GITR−/− mice. This imbalance impaired Treg cell development and expanded the Th1 population in GITR−/−xRag−/− mice following transfer of non-fractionated CD4+ cells.
Conclusions
GITR is not required on the surface of Treg and T effector cells to induce colitis in mice; interactions between GITR and its ligand are not required for colitis induction. GITR instead appears to control dendritic cell and monocyte development; in its absence, mice develop aggravated chronic enterocolitis, via an imbalance of colitogenic Th1 cells and Treg cells.
Keywords: TNF, immune regulation, inflammation, IBD
Introduction
Inflammatory bowel diseases (IBD), which are divided into Crohn's disease and ulcerative colitis, are idiopathic diseases. Some phenotypes of Crohn's diseases can be mimicked by the transfer of CD4 + CD45RBhi naïve T cells into recombination activating gene deficient (Rag−/−) mice, which is preventable by cotransfer of CD4+CD25+ regulatory T cells.
As a TNF receptor superfamily member, GITR may regulate T cell-mediated immune responses due to its constitutive expression on CD4+CD25+ Treg cells and a low but inducible expression on CD4+CD25− Teff cells1. However, it is not conclusive yet whether signaling through GITR will break or enhance immunological tolerance1-4.
GITR is also expressed in antigen presenting cells including monocytes, macrophages, neutrophils, and DCs et al5. The expression levels of TLR-2, TLR-4, CD40 and CD80 in DCs were affected by GITR deficiency when exposed to Candida albicans6. GITR deficiency protects mice from TNBS-induced colitis7, suggesting an involvement of GITR signaling in regulating the function of antigen presenting cells (APCs) and the development of colitis.
GITR-L, the non-promiscuous ligand of GITR, is highly expressed in plasmacytoid dendritic cells (pDCs)8, macrophages and langerhans dendritic cells9, 10. Engagement of GITR ligand (GITR-L or TNFSF18) by GITR in pDCs activates an inhibitory enzyme indoleamine 2,3 dioxygenase (IDO), which protects mice against allergic bronchopulmonary aspergillosis8. Recombinant soluble GITR induces production of inducible nitric oxide synthase, cyclooxygenase-2, and matrix metalloproteinase-9 in the macrophage cell line RAW264.7 and in murine peritoneal macrophages11, 12. GITR-L engagement by a monoclonal antibody blocks the migration of langerhans dendritic cells to the draining lymph node9. These reports indicate that GITR/GITR-L interaction not only induces a costimulatory signal for both Teff and Treg cells but also affects the function of APCs.
Although GITR/GITR-L signaling is potentially implicated in regulating T cell- and APC-mediated immune responses, GITR−/− mice do not have a dramatic phenotype reminiscent to another TNFR family member (HEVM)13. This may reflect the balance between Treg cells, Teff cells and their interaction with APCs, which regulate immune responses oppositely.
To dissect the precise mechanisms how GITR on the surface of Treg cells, effector T cells and antigen presenting cells acts in tolerance induction in vivo, we employed a well-known model of chronic enterocolitis. To this end, we evaluated the pathogenesis of colitis upon the transfer of un-fractionated CD4+ T cells or combinations of GITR-deficient, GITR-L-deficient and –sufficient CD4+ T cell subsets into Rag−/− or GITR−/−xRag−/− recipients. Unexpectedly, the outcomes of these studies demonstrate that the presence of GITR on the surface of dendritic cells and macrophages is requisite for controlling colitis. Upon transfer of non-fractionated CD4 + cells into GITR−/−×Rag−/− mice, disease develops because of an imbalance between Treg and Th1 cell proliferation in the lamina propria and MLN.
Materials and Methods
Mice
B6129SF1, C57BL/6, B6.PL-Thy1a/CyJ, Rag−/− (Recombination Activating Gene 1, Rag-1tm1mom/J) and OTII-Tg transgenic mice [C57BL/6-Tg(TCRαTCRβ)425Cbn/J] were purchased from the Jackson Laboratory. GITR−/− mice were provided by Dr. C. Riccardi and Dr. P.P. Pandolfi14. FoxP3-IRES-EGFP knock-in C57BL/6 mice were generously provided by Dr. V. Kuchroo15. GITR−/− mice were crossed with Rag−/− mice to generate GITR−/−xRag−/− double knockout mice. F2 mice were interbred and used for experiments. GITR-L−/− C57BL/6 mice were generated as described in Supplement Figure S1. All animals were housed in the Center for Life Science animal facility of Beth Israel Deaconess Medical Center. The experiments were performed according to the guidelines of the Institutional Animal Care and Use Committee (IACUC) at BIDMC.
Antibodies
Anti-CD3s-Biotin, CD11b-Pacific Blue, CD4-PE(FITC), CCR7-PE, GITR-PE, IDO and Steptavidin-PerCP were from Biolegend (San Diego, USA). Anti-FoxP3-FITC, IL-17A-PE and GITR-L-PE were products of eBioscience (San Jose, USA). Anti-CCR9-Allophycocyanin was from R&D (Minneapolis, USA). Anti-CD25-PE, CD115-PE, CD11c-PE, CD45RB-FITC, Ly6C-PerCP, CD103-APC, TCRvβ5-Biotin and IFNγ-PE were products from BD Biosciences (San Jose, USA). Anti-PDCA1-FITC was purchased from Miltenyi Biotec (Auburn, USA).
Induction and assessment of colitis
Briefly, CD4+CD45RBhi, CD4+CD25+ or CD4+ T cells were sorted by FACS and i.p. injected into GITR−/−xRag−/− or Rag−/− recipients. Recipient mice were analyzed for disease activity index (DAI) upon the first observance of diarrhea as previously described16. Mice were checked on a daily basis and euthanized if moribund. Histology grades were assigned in a blinded fashion by a pathologist (A.K.B). Lamina propria cells were isolated from colon for analyzing the cellularity. Colon from each mouse was incubated in RPMI medium for 24 hours. Supernatants were collected for cytokine analysis.
Isolation and analysis of lamina propria cells
Lamina propria cells were isolated as previously described17. Briefly, after disruption of epithelial cells from the mucosa in HBSS/EDTA buffer, collagenase D and DNase were used to dissociate lamina propria cells of colon pieces. The cells were then purified using gradient centrifugation.
In vitro CD4+ T cell proliferation and cytokine assays
CD11c+ DCs were isolated from wt or GITR−/− spleens or MLNs using a CD11c+ DC isolation kit (Miltenyi Biotec). DCs primed with 2mg/ml chicken egg ovalbumin were irradiated with an X-ray irradiator (3000 Rad) and used to activate OTII-Tg CD4+ T cells labeled with CFSE (at 5:1 ratio) for 72 hours. TCRvβ5+ cells were compared for the times of proliferation and the percentage of IFN-γ expressing cells. Supernatants were collected to assess the production of cytokines.
IDO Assay
Colon of GITR−/−xRag−/− and Rag−/− mouse were mashed in PBS supplemented with PMSF and protease inhibitors as described before18. IDO protein levels were measured by immunoblotting with an αIDO antibody. IDO activity was quantified by the production of L-kynureinine19.
Cytokine production
Cytokines in cell or colon cultures were analyzed using cytometric bead array Mouse Inflammatory Kits (BD Biosciences, #552364).
Statistical analysis
All data were analyzed with the Prism 4.0c software (GraphPad, San Diego, CA) and presented as the mean values ± Standard Deviation (SD). Statistical comparisons were performed by two-tailed Student's t test. Values P < .05 were considered to be statistically significant.
Results
Transfer of non-fractionated CD4+ T cells into GITR−/−xRag−/− mice induces colitis
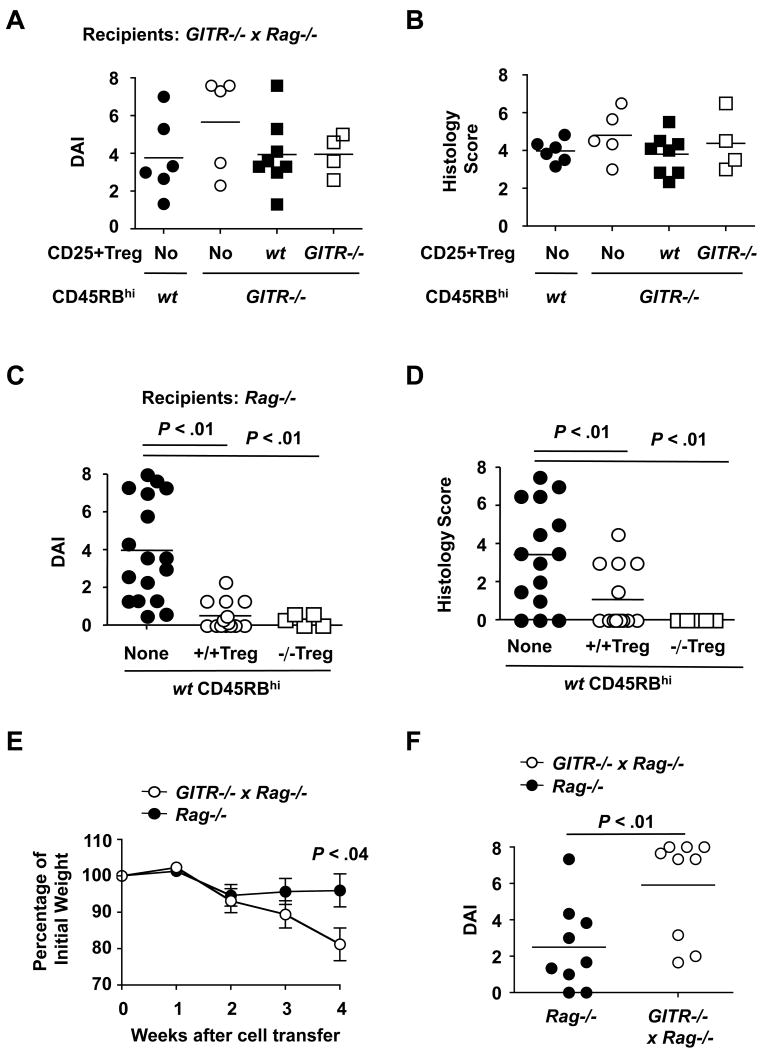
To address the role of GITR/GITR-L interactions in suppression of colitis, we transferred combinations of GITR-deficient or GITR-sufficient CD4+ cell subsets into either GITR−/−xRag−/− or Rag−/− recipients. Surprisingly, when non-fractionated wt CD4+ T cells were transferred into GITR−/−xRag−/− recipients, an aggressive chronic enterocolitis developed. In contrast, weight loss, disease activity indexes (DAI) and histology scores were low in Rag−/− mice kept in the same cage [Figure 1 A-D]. Pro-inflammatory cytokines produced by ex vivo colon cultures (Figure 1E) or in vitro cultures of MLN CD4+ T cells demonstrated a preference for a Th1 type inflammation, while the number of IL-17A producing CD4+ T cells was reduced (Figure 1F).
Fig. 1. CD4 + T cells induce colitis in GITR−/−xRag−/− but not in Rag−/− recipients.

CD4+ T cells obtained from wt spleens by FACS sorting were i.p. injected into Rag−/− (n=17) or GITR−/−xRag−/− (n=14) hosts [1×106 cells/mouse]. Mice were euthanized when signs of diarrhea, hunching, and wasting disease manifested. Statistical significance was determined by two-tailed Student's t-test.
1A Weight loss as a percentage of the initial weight. Values represent the mean weight ± SD.
1B & 1C DAI and Histology score. Mean and individual values of each group are indicated.
1D Representative Histology. Original magnification 10×.
1E Cytokine production by short-term colon cultures. Colon tissue was isolated from GITR−/−xRag−/− (n=7) and Rag−/− (n=6) mice with wt CD4+ T cells transfer and cultured for 24 hours. Supernatants were collected after centrifugation and analyzed for inflammatory cytokines with the multiplex CBA assay.
1F MLN CD4+ T cells differentiate into Th1 cells upon transfer into GITR−/− x Rag−/− mice. MLN cells isolated from GITR−/−xRag−/− (n=5) and Rag−/− (n=5) mice into which wt CD4+ T cells had been transferred were stimulated with 10 μg/ml of plate bound αCD3ε for 24 hours. Cells were stained with monoclonal antibodies against CD4, IFN-γ and IL-17A. Representative staining of each group is shown.
To assess whether GITR on the surface of donor CD4+ T cells was involved in the CD4+ T cell induced colitis, GITR−/− CD4+ T cells were injected. Again, upon transfer into GITR−/−xRag−/− recipients colitis developed (Figure 2A-B and Supplement Figure S2A), and the same pattern of proinflammatory cytokines was detected in colon samples (Figure 2C). In contrast, upon transfer into Rag−/− mice, no colitis developed. We conclude that although the donor cell population contained both Tregs and colitis inducing CD4+ cells20-22, in the absence of GITR in the recipient mouse suppression appeared to be reduced and a Th1-driven disease prevailed.
Fig. 2. GITR−/− CD4+ T cells induce colitis upon transfer into GITR−/− x Rag−/− recipients.
CD4+ T cells obtained from GITR−/− spleens by FACS sorting were i.p. injected into GITR−/−xRag−/− or Rag−/− hosts [1×106 cells/mouse]. Disease parameters were determined as described in methods and Figure 1.
2A DAI.
2B Histology score.
2C Cytokine production by short-term colon cultures.
Ex vivo colon cultures from GITR−/−xRag−/− (n=8) and Rag−/− (n=8) mice into which GITR−/− CD4+ T cells had been transferred were prepared for determining the inflammatory cytokines as in Figure 1.
The percentage of CD4+FoxP3+ T cells is significantly lower in GITR−/−xRag−/− recipients than that in Rag−/− mice after transfer of non-fractionated CD4+ T cells (Supplement Figure S3), suggesting an imbalance between Teff and Treg cells may be involved in the development of colitis after the transfer of non-fractionated CD4+ T cells into GITR−/−xRag−/− recipients.
Impaired Treg activity upon transfer into GITR−/−x Rag−/− mice
As neither GITR−/− nor GITR−/−xRag−/− mice develop colitis spontaneously (Supplement Figures S4 and S5), the disease may have developed due to either impaired interactions between Treg and CD45RBhi cells or between these cells and a cell in the recipient mouse. We then assessed the influence of GITR-deficiency on the function of colitis-inducing CD45RBhi cells and colitis-preventing Treg cells in GITR−/−x Rag−/− as well as in Rag−/− recipients.
As expected from the previous experiments, GITR-deficient CD45RBhi cells induced colitis as well as GITR-sufficient CD45RBhi cells in GITR−/−xRag−/− recipients. Whilst neither GITR+/+ nor GITR−/− CD25+ Treg cells could suppress colitis (Figure 3A-B and Supplement Figure S2B). In contrast, the absence of GITR did not have an effect on the colitis-inhibiting activity of Treg cells (Figure 3C-D and Supplement Figure S2C), nor did the absence of GITR affect the colitis-inducing activity of Teff cells (Supplement Figure S6) in the Rag−/− recipients.
Fig. 3. Tregs do not suppress colitis after their co-transfer with CD45RBhi cells into GITR−/−xRag−/− recipients.
GITR+/+ or GITR−/− CD4+CD45RBhi cells alone (5×105 cells/mouse) or together with CD4+CD25+ cells (5×104 cells/mouse) were transferred to Rag−/− or GITR−/−xRag−/− recipients. Mice were euthanized at week 4 after cell transfer. DAI and histology score were assigned as described in Figure 1. Each filled or open circle represents one mouse. Statistical significance was determined by the two-tailed Student's t-test. Mean and individual values of each group are indicated.
3A & 3B DAI and histology score upon cell transfer into GITR−/−xRag−/− recipients.
3C & 3D DAI and histology score upon cell transfer into Rag−/− recipients.
3E Weight loss as a percentage of the initial weight after transfer of CD4+CD45RBhi cells into Rag−/− and GITR−/−xRag−/− recipients.
Values represent the mean weight ± SD.
3F DAI upon transfer of CD4+CD45RBhi cells into Rag−/− and GITR−/−xRag−/− recipients.
Furthermore, CD4+CD45RBhi T cells alone induced a faster weight loss (as early as week 4) and a more severe inflammation in the colon of GITR−/−xRag−/− recipients than that of Rag−/− recipients (Figure 3E-F). Conversely, CD4+FoxP3+ Treg cells alone did not induce colitis either in GITR−/−xRag−/− or in Rag−/− recipients (analyzed at week 9). The percentage of donor cells that lost FoxP3 expression was comparable in both donors (Supplement Figure S7). Colitis induced by the transfer of non-fractionated CD4+ T cells in GITR−/−xRag−/− recipients depends on the naïve T cells but not the Treg cells.
To elucidate the fate of naïve T cells and the Treg cells after their transfer into GITR−/−xRag−/− recipients, naïve CD4+CD45RBhi cells from Thy1.1+ C57BL/6 congenic mice were mixed with CD4+FoxP3+ Treg cells FACS sorted from Thy1.2+ FoxP3-IRES-GFP reporter mice at 9:1 ratio and adoptively transferred to GITR−/−xRag−/− or in Rag−/− recipients. As shown in Figure 4A-B, a mix of Thy1.1+ naïve T cells and Thy1.2+ Treg cells induced an accelerated weight loss and severe colitis in GITR−/−xRag−/− but not in Rag−/− recipients. The percentage of Thy1.1+ naïve T cells was significantly increased, whilst the percentage of Thy1.2+FoxP3(GFP)+ Treg cells was reduced particularly in the mesenteric lymph node and in the lamina propria of GITR−/−xRag−/− recipients as compared to that of Rag−/− recipients (Figure 4C-D). Thy1.1+FoxP3+ cells, which represent the inducible Treg cells converted from naïve T cells in the peripheral lymphoid organ, was also lower in GITR−/−xRag−/− recipients than in Rag−/− recipients (Figure 4E). Surprisingly, Treg-mediated suppression is not ameliorated in vitro by the presence of GITR−/−xRag−/− splenocytes as APCs (Supplement Figure S8), suggesting an in vitro suppression may not mimic the condition in vivo.
Fig. 4. GITR deficiency in Rag−/− recipients caused an over expansion of naïve T cells as well as a loss of FoxP3 expression on Treg cells.

CD4+CD45RBhi naïve T cells sorted from Thy1.1+ B6 congenic mice were mixed with CD4+FoxP3+ Treg cells from Thy1.2+ FoxP3-IRES-GFP reporter mice at 9:1 ratio and adoptively transferred to GITR−/−xRag−/− or Rag−/− hosts (5 mice for each group). Mice were weighed weekly and euthanized for colitis activity as described in Figure 1. Spleen, MLN and lamina propria cells from GITR−/−xRag−/− or Rag−/− recipients were isolated and evaluated for FoxP3+, Thy1.1+ and Thy1.2+ cells.
4A Weight loss as a percentage of the initial weight. Values represent the mean weight ± SD.
4B DAI. Mean and individual values of each group are indicated.
4C Ratio of Thy1.1+ and Thy1.2+ cells after cell transfer. Thy1.1+ cells represent CD4+CD45RBhi naïve T cells. Thy1.2+ cells represent FoxP3+(GFP+) cells.
4D Percentage of FoxP3(GFP)+/Thy1.2+ cells after cell transfer.
4E Percentage of FoxP3+ cells expressing Thy1.1+ and Thy1.2+. Splenocytes from GITR−/−xRag−/− or Rag−/− recipients were stained with CD4, Thy1.1 and FoxP3. Thy1.1 and FoxP3 expressing cells were compared among gated CD4+ T cells.
We conclude that the expression of GITR is neither required for the colitis-inducing activity of CD45RBhi T cells nor for the suppressor function of Treg cells in Rag−/− recipients. However, CD4+ CD45RBhi T cells are refractory to the inhibition either by GITR-deficient or GITR-sufficient Treg cells and induce colitis in GITR−/−xRag−/− recipients.
Treg cells isolated from GITR-L-deficient mice do not ameliorate disease upon co-transfer with CD45RBhi T cells into GITR−/−xRag−/− mice
The loss of their ability to ameliorate colitis upon the transfer of Treg into GITR−/−x Rag−/− mice could also be due to an inability of GITR-L on the surface of Treg cells to appropriately interact with a GITR+ cell in the recipient mouse. To evaluate this possibility, we first analyzed by monoclonal antibody staining and PCR whether T cells express GITR-L23. Although none of these analyses detected GITR-L in CD4 + T cells (Supplement Figure S9), it was conceivable that this cell surface ligand could be expressed at some stage during activation in vivo. We therefore transferred combinations of GITR-L−/− CD45RBlo and CD45RBhi cells into Rag−/− recipients. As shown in Figure 5 and Supplement Figure S2D, GITR-L−/− Tregs ameliorate CD45RBhi induced colitis in Rag−/− mice. Thus, if GITR-L were to be fleetingly expressed on the surface of CD4+ T cells, its absence did not impair their ability to induce or suppress chronic enterocolitis in the Rag−/− mouse. By contrast, upon co-transferring GITR-L−/− CD45RBlo cells together with CD45RBhi cells into GITR−/−x Rag−/− recipients disease developed.
Fig. 5. GITR expression by non-T hematapoietic cells, but not GITR-L by CD4+ T cells, abrogated the Treg-mediated suppression.
5A DAI.
5B Histology score.
GITR-L−/− CD4+CD45RBhi (5×105 cells/mouse) and CD4+CD45RBlo (5×105 cells/mouse) T cells were co-injected into GITR−/−xRag−/− or Rag−/− hosts. DAI and Histology scores were determined as in Figure 1. Mean and individual values of each group are indicated.
We conclude that the impaired Treg function observed after transfer into GITR−/− x Rag−/− mice was not caused by the absence of GITR-L on CD4+ T cells. More importantly, the experiments convincingly demonstrate that the absence of GITR in the recipient Rag−/− mouse affects the suppressive effect by Tregs and this effect is not due to a direct interaction between two T cell subsets or between a GITR+ APC and a GITR-L+ T cell. It is therefore more likely that the absence of GITR affects an APC–intrinsic functions.
Expression of GITR on the surface of dendritic cells and macrophages
Development of colitis in Rag−/− mice requires an interplay in the lamina propria and mesenteric lymph nodes between CD4+ cells and bacterial antigens presented by specialized dendritic cells and macrophages. Migration of aggressor macrophages or tolerogenic dendritic cells determines the outcome of the inflammatory responses, which are completed by neutrophil infiltration. Because expression of GITR on the surface of these cells types is poorly understood5, 7, we first employed anti-GITR to detect expression on dendritic cells and macrophages in colitic Rag−/− recipients upon transfer of CD45RBhi CD4+ T cells. As shown in Figure 6A, GITR is expressed on the surface of dendritic cells and macrophages in the lamina propria of colitic mice. In contrast, without the transfer of CD45RBhi CD4+ T cells, lamina propria cells isolated from the colon of Rag−/− mice do not express GITR (Supplement Figure S10). Because LPS-activation also induces the expression of GITR on the surface of splenic DCs and macrophages (Figure 6B-C), we conclude that GITR is an activation marker for monocyte-derived cells.
Fig. 6. GITR expression on the surface of lamina propria DCs and macrophages of colitic mice and on splenic monocyte-derived cells upon activation.

6A Dendritic cells and macrophages isolated from the lamina propria of colitic Rag−/− mice express GITR. CD4+CD45RBhi wt T cells were transferred to Rag−/− recipients (5×105 cells/mouse). Once diarrhea started, monocyte derived cells were isolated from the colonic lamina propria and stained with αCD11c, αCD11b, αLy6C and αGITR. Different cell subsets were gated to compare the GITR expression levels. Bold black line represents αGITR; gray shaded area represents IgG isotype control.
6B & 6C LPS induces GITR expression on splenic DCs and macrophages. Freshly isolated wt or GITR−/− splenocytes (upper panel) or those stimulated with LPS (lower panel) for 24 hours were stained with monoclonal antibodies directed against CD11c, Ly6C and GITR The expression levels of GITR on different cell subpopulations (gated as in 6B) were compared. Bold black line represents GITR+/+ cells; gray shaded area represents GITR−/− cells.
6D & 6E IDO protein levelsiand enzymatic activity in the colon of GITR−/− x Rag−/− mice are identical to that of Rag −/− mice. IDO protein levels and enzymatic activity in the colon extracts of GITR−/−xRag−/− and Rag−/− mouse were measured by immunoblot with an αIDO antibody and by the production of L-kynureinine respectively.
To examine whether the absence of GITR in Rag−/− recipients altered the induction of colitis, we used an agonistic anti-CD40 monoclonal antibody, which causes colitis in Rag−/− mice24. Because the response in GITR−/−xRag−/− mice was similar to that in Rag−/− mice, the effect of GITR deficiency on colitis was not simply due to a gross abnormality of DC or macrophage functions (Supplement Figure S11).
Furthermore, soluble GITR is thought to initiate a signaling pathway in GITR-L expressing pDCs, which results in activation of the enzyme IDO. We found that the levels of IDO activity in the colon of GITR−/−xRag−/− mice in which colitis had been induced by the transfer of non-fractionated CD4+ cells was comparable to that of Rag−/− mice (Figure 6D-E). Thus, this DC-intrinsic pathway was unlikely to be involved in the disease of GITR−/−xRag−/− recipients.
Collectively, our data demonstrate that the absence of GITR on the surface of dendritic cells and macrophages does not cause colitis by itself, but rather indicates that the inflammation is caused by the aberrant interaction of APCs with the donor CD4+ T cells.
DCs from MLN of GITR−/− mice preferentially drive Th1 responses instead of Treg expansion
Due to the influence of GITR on the nonlymphocyte immune system and the inducible expression of GITR on DCs, we assessed whether GITR−/− DCs differ functionally from their wt counterparts in vitro. Interestingly, OVA-loaded CD11c+ DCs from GITR−/− MLN aggressively drove a Th1 response of OTII CD4+ cells (Figure 7A and Supplement Figure S12A-B), which was consistent with the Th1-colitis in GITR−/−xRag−/− recipients after transfer of CD4+ T cells (Figure 1). In contrast, OTII CD4+ cells secreted equal amounts of IFNγ when activated by wt or GITR−/− splenic DCs (Figure 7B)
Fig. 7. DCs from MLN of colitic GITR−/− mice preferentially drive Th1 responses instead of Treg expansion.

7A GITR−/− DCs from MLN preferentially stimulate Th1 cells. CD11c+ DCs from MLN of wt and GITR−/− mice were primed with 2 mg/ml of chicken ovalbumin for 18 hours. OVA-loaded DCs were irradiated [3000 Rad] and cocultured with OTII CD4+ T cells at 1 : 5 ratio for 3 days. Supernatants were used for assessing the cytokines by CBA. Data represent mean ± SD of triplicate. Results are representative of 3 individual experiments.
7B Cytokines secreted by CD4+ T cells in the presence of splenic DCs. CD11c+ DCs from spleen of wt and GITR−/− mice were loaded with OVA protein and used to stimulate OTII CD4+ T cells as described in 7A. Supernatants were used for assessing the cytokines by CBA. Data represent mean ± SD of triplicate.
7C-D Percentage of MLN CD103+ DCs and splenic PDCA1+CCR9+ pDCs of GITR−/− mice was reduced under steady state. MLN (7C) and spleen (7D) cells from wt and GITR−/− were stained for comparing the percentage of different DC subsets. Each filled or open circle represents one mouse. Mean and individual values of each group are indicated.
7E Reduced number of pDCs in spleen of GITR−/−xRag−/− mice without transfer of CD4+ T cells. Splenocytes from Rag−/− and GITR−/−xRag−/− mice (5 for each group) were stained for MHCII, CD11c and PDCA1.
7F Reduced number of pDCs in GITR−/−xRag−/− mice during inflammation induced by CD4+ T cells. CD4+ T cells from wt mice were adoptively transferred into GITR−/−xRag−/− and Rag−/− recipients (5 for each group). Mice were euthanized at week 4. Spleen, MLN or colon LP cells were pooled for CD11c, PDCA1 staining. Result represents two separate experiments.
To address why the absence of GITR on the surface of DCs causes an enhanced induction of Th1 cells, the frequency of tolerogenic PDCA1+ CD11c+ pDCs and CD103+ DCs was assessed. As shown in Figure 7C-D and Supplement Figure S12C-D, the numbers of both CD103+ DCs in the MLN and PDCA1+CCR9+ pDCs in the spleen were reduced in GITR−/− mice compared to that of wt mice under steady state. The number of PDCA1+ pDCs in GITR−/−xRag−/− mice, with or without inflammation induced by the transfer of CD4+ T cells, was significantly lower than in their wt counterparts (Figure 7E-F).
We conclude that the composition of tolerogenic DC subsets is altered by GITR deficiency, which causes a Th1-colitis after transfer of CD4+ T cells into the GITR−/−xRag−/−recipients.
Discussion
GITR is highly expressed on the surface of naturally occurring CD4+CD25+ Treg cells and on the surface of effector CD4+ T cells upon activation of naïve cells1. However, naïve GITR−/− CD4+ CD45RBhi T cells are as equally efficient as their wt counterparts in the induction of colitis in Rag−/− recipients. CD45RBhi CD4+ T cell-induced colitis is preventable by the co-transfer of GITR−/− Treg cells. We conclude that GITR on the surface of CD4+ T cells is dispensable for the induction and suppression of colitis. The results suggest that in general Treg / Teff interactions that are known to operate in vivo are not dependent on the presence of GITR.
Unexpectedly, upon transfer of non-fractionated CD4+ T cells or naïve CD4+ T cells alone into GITR−/−xRag−/− recipients an accelerated Th1 type inflammation develops in the colon as compared to Rag−/− recipients, which manifests as early as 3-4 weeks. Non-fractionated CD4+ T cell-induced colitis in GITR−/−xRag−/− recipients does not depend on Treg cells, while naïve CD4+ T cells somehow are refractory to Treg-mediated suppression in vivo and induce inflammation in the colon of GITR−/−xRag−/− mice. Abrogation of Treg-mediated suppression in vivo is not caused by an aberrant interaction between GITR on the surface of a cell in the recipient Rag−/− mouse (and not in the GITR−/−xRag−/− animal) could interact with GITR-L on the surface of either a Treg or a Teff cells. Because CD4+ T cells do not express GITR-L in contrast with a previous report23 as judged by FACS analysis with αGITR-L monoclonal antibodies and by RT-PCR both ex vivo and in vitro, nor does a GITR-L deficiency affect the colitis-inducing function of naïve T cells as well as the colitis-preventing function of regulatory T cells.
We do detect Helicobacter bilis in our GITR−/−x Rag−/− and Rag−/− mice by a PCR-based method. However, H. bilis may not play a key role in the development of colitis in GITR−/−xRag−/− recipients after transfer of non-fractionated CD4+ T cells. Because the Rag−/− recipients, which are kept in the same cage with GITR−/−xRag−/− recipients and are positive for H. bilis, develop milder colitis after the transfer of CD4+CD45RBhi naïve T cells as well as the transfer of non-fractionated CD4+ T cells (Figure 3E-F and Figure 4). However, we cannot rule out the possibility that GITR-deficient Rag−/− mice are more prone to the infection by other pathogenic bacteria. GITR−/− APCs exposed to the pathogenic bacterial antigens drive a strong Th1 type inflammation.
GITR−/−xRag−/− mice develop an accelerated colitis by the transfer of naïve CD4+ T cells in recipients reminiscent that of the HVEM−/−xRag−/− mice13, in which a radio-resistant cell but not DCs is proposed to cause the accelerated disease. In contrast to HVEM−/− mice, in which the function of DCs is not greatly affected, GITR-deficiency causes a significant reduction of tolerogenic CD103+ DCs and PDCA1+ pDCs. GITR−/− MLN DCs induced CD4+ T cells to secrete significantly higher IFNγ than their wt counterparts in vitro, consistent with the previous reports that CD103− DCs are more efficient at driving Th1 immune responses than CD103+ DCs25-28. IFNγ alone may not induce the devastating disease in GITR−/−xRag−/− mice, it may initiate the escalating Th1 inflammation.
Treg-mediated suppression is abrogated in GITR−/−xRag−/− mice, which cannot be mimicked by a traditional in vitro suppression assay, suggesting a migratory factor may be involved in the abrogation of Treg-mediated suppression in vivo. As the precursor of the pro-inflammatory macrophages and DCs29, 30, monocytes are the strong candidates with migratory ability. Increased Th1-cytokines produced by an abnormal DC-CD4+ T cell interaction inevitably affect the migration of monocytes to the inflamed sites and the subsequent differentiation into aggressor macrophages or dendritic cells, which determines the outcome of the inflammatory responses5, 7. The effect of GITR deficiency on the function of monocytes is still under active investigation.
There is also significantly increased IL-6 in ex vivo colon cultures of GITR−/−xRag−/− recipients after transfer of CD4+ T cells. T cells secrete trace amount of IL-6; therefore, it must be produced by an APC (macrophages and/or DCs) accumulated in the colon of GITR−/−xRag−/− mice during the inflammation. IL-6 trans-signaling is known to inhibit the generation of inducible Treg cells from naive T cells and to render CD4+CD25− Teff cells resistant to Treg-mediated suppression31, 32. Increased IL-6 in inflamed GITR−/−xRag−/− mice by the transfer of CD4+ cells may explain the reduction of FoxP3+ Treg cells, which is caused both by the loss of FoxP3 expression in Treg cells and by the de novo generation of inducible FoxP3+ Treg cells from naïve T cells. The percentage of Treg cells lose FoxP3 expression in GITR−/−xRag−/− recipients, in which no colitis is induced, is comparable to that in Rag−/− recipients after the transfer of CD4+FoxP3+ Treg cells alone. This suggests the reduction of CD4+FoxP3+ Treg cells in GITR−/−xRag−/−recipients after transfer of non-fractionated CD4+ T cells is secondary to the inflammation.
Taken together, by using a CD4+ T cell transfer model of chronic enterocolitis we establish that the expression of GITR on the surface of regulatory and effector CD4+ T cells is dispensable for development of disease. In contrast, the abnormal development and function of DC subsets in recipient GITR−/−xRag−/− mice results in a Th1-inflammatory phenotype and reduced activity of Treg cells when unfractionated CD4+ T cells are transferred.
Supplementary Material
Acknowledgments
We thank Dr C. Riccardi and Dr P. P. Pandolfi for generously sharing the GITR−/− mice, Dr V. Kuchroo for providing the C57BL/6 FoxP3-IRES-GFP reporter mice, Dr X. Romero for helpful discussion, M. O'Keeffe for proofreading the manuscript, the BIDMC histology core for processing colon tissue slides with H&E staining.
Grant support: National Institutes of Health (P01 HL078810 and R01 DK-52510 to C.T. and PHS 5T32AI7512-24 to G.L.)
Abbreviations used in this paper
- APC
antigen-presenting cell
- CBA
cytometric bead array
- CFSE
carboxyfluorescein diacetate, succinimidyl ester
- DAI
Disease Activity Index
- DC
dendritic cell
- GITR
Glucocorticoid-Induced TNF Receptor-related gene
- GITR-L
GITR ligand
- IFN
interferon
- IL
interleukin
- LPS
lipopolysaccharide
- MLN
mesenteric lymph node
- OTII-Tg
C57BL/6-Tg(TCRaTCRb)425Cbn/J transgenic
- SD
Standard Deviation
- Treg
CD4+CD25+ regulatory T cells
- Teff
effector T cells
- WT
wild type
Footnotes
Author Contributions: GL performed all IBD inducing experiments, DC and monocyte subsets fractionation, in vitro DC functional assays; CD helped processing samples and editing the manuscript; SBB performed the CBA cytokine assays and analysis; PE developed several anti-GITR-L monoclonal antibodies for FACS analysis; RM generated GITR-L deficient mice; RWH helped discuss and write manuscript; AKB helped assessing histology score; CT is the major organizer of this work and designed the experiments with GL. All authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Gongxian Liao, Email: gliao@bidmc.harvard.edu, Division of Immunology, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston MA 02115. USA. Tel: 1-617-735-4141, Fax: (617) 735-4135.
Cynthia Detre, Email: cdetre@bidmc.harvard.edu, Division of Immunology, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston MA 02115. USA.
Scott B. Berger, Email: sberger@bidmc.harvard.edu, Division of Immunology, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston MA 02115. USA.
Pablo Engel, Email: pengel@ub.edu, Immunology Unit, Department of Cell Biology, Immunology and Neurosciences, Medical School, University of Barcelona, C/Casanova 143, Barcelona E-08036, Spain.
Rene de Waal Malefyt, Email: rene.de.waal.malefyt@merck.com, Biologics Discovery, Merck Research Laboratories, Palo Alto, 901 California Avenue, Palo Alto, CA 94304-1104, USA.
Roland W. Herzog, Email: rherzog@ufl.edu, University of Florida, Cancer and Genetics Research Center, 1376 Mowry Road, Room 203, Gainesville, FL 32610, USA.
Atul K. Bhan, Email: abhan@partners.org, Department of Pathology, Massachusetts General Hospital, Harvard Medical School, Boston, MA 02114, USA.
Cox Terhorst, Email: cterhors@bidmc.harvard.edu, Division of Immunology, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston MA 02115. USA. Phone: (617) 735-4131; Fax: (617) 735-4135.
References
- 1.Shimizu J, Yamazaki S, Takahashi T, Ishida Y, Sakaguchi S. Stimulation of CD25(+)CD4(+) regulatory T cells through GITR breaks immunological self-tolerance. Nat Immunol. 2002;3:135–42. doi: 10.1038/ni759. [DOI] [PubMed] [Google Scholar]
- 2.Ko K, Yamazaki S, Nakamura K, Nishioka T, Hirota K, Yamaguchi T, Shimizu J, Nomura T, Chiba T, Sakaguchi S. Treatment of advanced tumors with agonistic anti-GITR mAb and its effects on tumor-infiltrating Foxp3+CD25+CD4+ regulatory T cells. J Exp Med. 2005;202:885–91. doi: 10.1084/jem.20050940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Olffen RW, Koning N, van Gisbergen KP, Wensveen FM, Hoek RM, Boon L, Hamann J, van Lier RA, Nolte MA. GITR triggering induces expansion of both effector and regulatory CD4+ T cells in vivo. J Immunol. 2009;182:7490–500. doi: 10.4049/jimmunol.0802751. [DOI] [PubMed] [Google Scholar]
- 4.Liao G, Nayak S, Regueiro JR, Berger SB, Detre C, Romero X, de Waal Malefyt R, Chatila TA, Herzog RW, Terhorst C. GITR engagement preferentially enhances proliferation of functionally competent CD4+CD25+FoxP3+ regulatory T cells. Int Immunol. 22:259–70. doi: 10.1093/intimm/dxq001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Azuma M. Role of the glucocorticoid-induced TNFR-related protein (GITR)-GITR ligand pathway in innate and adaptive immunity. Crit Rev Immunol. 30:547–57. doi: 10.1615/critrevimmunol.v30.i6.40. [DOI] [PubMed] [Google Scholar]
- 6.Vecchiarelli A, Pericolini E, Gabrielli E, Agostini M, Bistoni F, Nocentini G, Cenci E, Riccardi C. The GITRL-GITR system alters TLR-4 expression on DC during fungal infection. Cell Immunol. 2009;257:13–22. doi: 10.1016/j.cellimm.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 7.Santucci L, Agostini M, Bruscoli S, Mencarelli A, Ronchetti S, Ayroldi E, Morelli A, Baldoni M, Riccardi C. GITR modulates innate and adaptive mucosal immunity during the development of experimental colitis in mice. Gut. 2007;56:52–60. doi: 10.1136/gut.2006.091181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grohmann U, Volpi C, Fallarino F, Bozza S, Bianchi R, Vacca C, Orabona C, Belladonna ML, Ayroldi E, Nocentini G, Boon L, Bistoni F, Fioretti MC, Romani L, Riccardi C, Puccetti P. Reverse signaling through GITR ligand enables dexamethasone to activate IDO in allergy. Nat Med. 2007;13:579–86. doi: 10.1038/nm1563. [DOI] [PubMed] [Google Scholar]
- 9.Kamimura Y, Iwai H, Piao J, Hashiguchi M, Azuma M. The glucocorticoid-induced TNF receptor-related protein (GITR)-GITR ligand pathway acts as a mediator of cutaneous dendritic cell migration and promotes T cell-mediated acquired immunity. J Immunol. 2009;182:2708–16. doi: 10.4049/jimmunol.0803704. [DOI] [PubMed] [Google Scholar]
- 10.Heng TS, Painter MW. The Immunological Genome Project: networks of gene expression in immune cells. Nat Immunol. 2008;9:1091–4. doi: 10.1038/ni1008-1091. [DOI] [PubMed] [Google Scholar]
- 11.Shin HH, Kwon BS, Choi HS. Recombinant glucocorticoid induced tumour necrosis factor receptor (rGITR) induced COX-2 activity in murine macrophage Raw 264.7 cells. Cytokine. 2002;19:187–92. doi: 10.1006/cyto.2002.1962. [DOI] [PubMed] [Google Scholar]
- 12.Lee HS, Shin HH, Kwon BS, Choi HS. Soluble glucocorticoid-induced tumor necrosis factor receptor (sGITR) increased MMP-9 activity in murine macrophage. J Cell Biochem. 2003;88:1048–56. doi: 10.1002/jcb.10456. [DOI] [PubMed] [Google Scholar]
- 13.Steinberg MW, Turovskaya O, Shaikh RB, Kim G, McCole DF, Pfeffer K, Murphy KM, Ware CF, Kronenberg M. A crucial role for HVEM and BTLA in preventing intestinal inflammation. J Exp Med. 2008;205:1463–76. doi: 10.1084/jem.20071160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ronchetti S, Nocentini G, Riccardi C, Pandolfi PP. Role of GITR in activation response of T lymphocytes. Blood. 2002;100:350–2. doi: 10.1182/blood-2001-12-0276. [DOI] [PubMed] [Google Scholar]
- 15.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–8. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 16.de Jong YP, Abadia-Molina AC, Satoskar AR, Clarke K, Rietdijk ST, Faubion WA, Mizoguchi E, Metz CN, Alsahli M, ten Hove T, Keates AC, Lubetsky JB, Farrell RJ, Michetti P, van Deventer SJ, Lolis E, David JR, Bhan AK, Terhorst C. Development of chronic colitis is dependent on the cytokine MIF. Nat Immunol. 2001;2:1061–6. doi: 10.1038/ni720. [DOI] [PubMed] [Google Scholar]
- 17.Simpson SJ, Hollander GA, Mizoguchi E, Allen D, Bhan AK, Wang B, Terhorst C. Expression of pro-inflammatory cytokines by TCR alpha beta+ and TCR gamma delta+ T cells in an experimental model of colitis. Eur J Immunol. 1997;27:17–25. doi: 10.1002/eji.1830270104. [DOI] [PubMed] [Google Scholar]
- 18.Liao G, Zhang M, Harhaj EW, Sun SC. Regulation of the NF-kappaB-inducing kinase by tumor necrosis factor receptor-associated factor 3-induced degradation. J Biol Chem. 2004;279:26243–50. doi: 10.1074/jbc.M403286200. [DOI] [PubMed] [Google Scholar]
- 19.Mellor AL, Keskin DB, Johnson T, Chandler P, Munn DH. Cells expressing indoleamine 2,3-dioxygenase inhibit T cell responses. J Immunol. 2002;168:3771–6. doi: 10.4049/jimmunol.168.8.3771. [DOI] [PubMed] [Google Scholar]
- 20.Powrie F, Correa-Oliveira R, Mauze S, Coffman RL. Regulatory interactions between CD45RBhigh and CD45RBlow CD4+ T cells are important for the balance between protective and pathogenic cell-mediated immunity. J Exp Med. 1994;179:589–600. doi: 10.1084/jem.179.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Powrie F, Leach MW, Mauze S, Caddle LB, Coffman RL. Phenotypically distinct subsets of CD4+ T cells induce or protect from chronic intestinal inflammation in C. B-17 scid mice. Int Immunol. 1993;5:1461–71. doi: 10.1093/intimm/5.11.1461. [DOI] [PubMed] [Google Scholar]
- 22.Mottet C, Uhlig HH, Powrie F. Cutting edge: cure of colitis by CD4+CD25+ regulatory T cells. J Immunol. 2003;170:3939–43. doi: 10.4049/jimmunol.170.8.3939. [DOI] [PubMed] [Google Scholar]
- 23.Shevach EM, Stephens GL. The GITR-GITRL interaction: co-stimulation or contrasuppression of regulatory activity? Nat Rev Immunol. 2006;6:613–8. doi: 10.1038/nri1867. [DOI] [PubMed] [Google Scholar]
- 24.Uhlig HH, McKenzie BS, Hue S, Thompson C, Joyce-Shaikh B, Stepankova R, Robinson N, Buonocore S, Tlaskalova-Hogenova H, Cua DJ, Powrie F. Differential activity of IL-12 and IL-23 in mucosal and systemic innate immune pathology. Immunity. 2006;25:309–18. doi: 10.1016/j.immuni.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 25.Hadeiba H, Sato T, Habtezion A, Oderup C, Pan J, Butcher EC. CCR9 expression defines tolerogenic plasmacytoid dendritic cells able to suppress acute graft-versus-host disease. Nat Immunol. 2008;9:1253–60. doi: 10.1038/ni.1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, Hall J, Sun CM, Belkaid Y, Powrie F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757–64. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Annacker O, Coombes JL, Malmstrom V, Uhlig HH, Bourne T, Johansson-Lindbom B, Agace WW, Parker CM, Powrie F. Essential role for CD103 in the T cell-mediated regulation of experimental colitis. J Exp Med. 2005;202:1051–61. doi: 10.1084/jem.20040662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jaensson E, Uronen-Hansson H, Pabst O, Eksteen B, Tian J, Coombes JL, Berg PL, Davidsson T, Powrie F, Johansson-Lindbom B, Agace WW. Small intestinal CD103+ dendritic cells display unique functional properties that are conserved between mice and humans. J Exp Med. 2008;205:2139–49. doi: 10.1084/jem.20080414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu K, Nussenzweig MC. Origin and development of dendritic cells. Immunol Rev. 234:45–54. doi: 10.1111/j.0105-2896.2009.00879.x. [DOI] [PubMed] [Google Scholar]
- 30.Siddiqui KR, Laffont S, Powrie F. E-cadherin marks a subset of inflammatory dendritic cells that promote T cell-mediated colitis. Immunity. 32:557–67. doi: 10.1016/j.immuni.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fujimoto M, Nakano M, Terabe F, Kawahata H, Ohkawara T, Han Y, Ripley B, Serada S, Nishikawa T, Kimura A, Nomura S, Kishimoto T, Naka T. The influence of excessive IL-6 production in vivo on the development and function of Foxp3+ regulatory T cells. J Immunol. 186:32–40. doi: 10.4049/jimmunol.0903314. [DOI] [PubMed] [Google Scholar]
- 32.Dominitzki S, Fantini MC, Neufert C, Nikolaev A, Galle PR, Scheller J, Monteleone G, Rose-John S, Neurath MF, Becker C. Cutting edge: trans-signaling via the soluble IL-6R abrogates the induction of FoxP3 in naive CD4+CD25 T cells. J Immunol. 2007;179:2041–5. doi: 10.4049/jimmunol.179.4.2041. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.