Abstract
Purpose
We determined the effect of estrogen on ZEB1 in vitro and tested the hypothesis that ZEB1 is over expressed in the penile skin of subjects with hypospadias.
Materials and Methods
Hs68 cells, a fibroblast cell line derived from human foreskin, were exposed to 0, 1, 10 and 100 nM estrogen, and the expression level of ZEB1 was assessed using reverse transcription real-time polymerase chain reaction, Western blot and immunocytochemical analysis. Next, preputial skin was prospectively collected from case and control subjects at hypospadias repair (37 cases) and circumcision (11). Hypospadias was classified as severe (13 cases) or mild (24) based on the position of the urethral meatus. ZEB1 expression was quantified using reverse transcription real-time polymerase chain reaction, Western blot and immunohistochemical analysis.
Results
Estrogen increased ZEB1 expression at the mRNA and protein levels in Hs68 cells in a concentration dependent fashion (p <0.01). Subjects with severe hypospadias had significantly higher ZEB1 mRNA levels and protein expression compared to controls or subjects with mild hypospadias (both p <0.01). Subjects with severe hypospadias had increased expression of ZEB1 in the basal layers of the preputial epidermis.
Conclusions
Estrogen increases ZEB1 expression in a human foreskin fibroblast cell line in vitro. Furthermore, ZEB1 is significantly over expressed in the penile skin of subjects with severe hypospadias. We propose that ZEB1 overexpression may contribute to development of hypospadias and may mediate the effect of estrogen on developing external male genitalia.
Keywords: estrogens, hypospadias, ZEB1 protein, human
Hypospadias is a common congenital genitourinary anomaly that affects approximately 1 in 125 live male births.1 Although the cause in most cases is unknown, hypospadias has been associated with aberrant androgen signaling during development.2,3 Additionally epidemiological studies have demonstrated an association between fetal estrogen exposure and hypospadias.4,5 The molecular mechanism underlying this association is unknown.
The zinc finger box genes, ZEB1 and ZEB2, have been associated with hypospadias.6,7 ZEB1 is nearly ubiquitously expressed in human tissues and is one of many genes up-regulated in patients with isolated hypospadias. ZEB1 acts primarily as a transcriptional repressor and down-regulates expression of E-cadherin, a cell adhesion molecule expressed in normal epithelial cells.8 Consequently ZEB1 is an important mediator of epithelial to mesenchymal transition, the process by which epithelial cells become migratory mesenchymal cells.9 ZEB1 is hormonally responsive and is up-regulated by estrogen in normal endometrium and uterine cancer.10,11 However, the role of ZEB1 in normal and abnormal penile development is unknown. We sought to determine the effect of estrogen on ZEB1 expression in human foreskin fibroblasts, and to quantify the expression of ZEB1 in boys with normal external genitalia and those with hypospadias.
MATERIALS AND METHODS
Cell Culture
Hs68 cells, a fibroblast cell line derived from human foreskin (ATCC™), were cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum, 1% nonessential amino acid, 10,000 U/ml penicillin, 10,000 μg/ml streptomycin SO4, 0.025 mg/ml amphotericin B and 110 μg/ml sodium pyruvate at 37C in 5% CO2 until 80% confluence. Cells used in the experiments were from passages 5 through 10. At the time of experiment cells were starved of fetal bovine serum for 12 hours and subsequently treated with estrogen (β-estradiol-water soluble, Sigma-Aldrich®) at 0, 1, 10 and 100 nM for 4 hours.
RNA Preparation, Reverse Transcription and Real-Time PCR
The following protocols were used for the Hs68 cells and human foreskin tissue. RNA was isolated with RNeasy® Midi Kit per manufacturer protocol after eliminating contaminating DNA with 1.3 mg proteinase K (Roche Diagnostics GmbH, Mannheim, Germany) and 20 μl RNase-Free DNase I (Qiagen® Inc., Valencia, California). The quantity and purity of RNA were measured by NanoDrop spectrophotometer (Thermo Fisher Scientific Inc., Wilmington, Delaware), and RNA integrity was visualized by the sharpness of the 28s and 18s ribosomal RNA bands in agarose gels.
Reverse transcription PCR was performed according to standard protocol. Briefly 2.5 μg RNA were reverse transcribed in a reaction volume of 20 μl. This product was then diluted with TE buffer (10 mM Tris-HCI, pH 8, 1 mM EDTA). PCR primers were designed according to target gene sequence published on PubMed and were synthesized by Integrated DNA Technologies Inc. (San Diego, California, see table).
Table.
| Gene | Primer | Products Size |
|---|---|---|
| ZEB1 | F: ATGCACAACCAAGTGCAGAAGAGC R: TTGCCTGGTTCAGGAGAAGATGGT |
145 bp |
| GAPDH | F: CATGTTCGTCATGGGTGTGAACCA R: AGTGATGGCATGGACTGTGGTCAT |
160 bp |
Real-time PCR was performed in a 25 μl reaction containing 20 ng template DNA, 1 × Power SYBR® Green PCR master mix and 300 nM primers. Amplification was carried out in 96-well plates in a 7300 Fast Sequence Detection System (Applied Biosystems Inc., Foster City, California) using the default thermal profile. Primer titration and dissociation experiments were performed so that no primer dimers or false amplicons would interfere with the result. Cycle threshold number was extracted for the reference (GAPDH) and target genes with auto baseline and manual threshold. PCR was repeated 3 times for each sample. Expression levels of ZEB1 are reported relative to GAPDH using 2-delta-delta cycle threshold.12 There was no difference in the amplification kinetics of GAPDH and ZEB1.
Protein Isolation and Western Blot
The following protocol was used for the Hs68 cells and in human prepuce samples. Protein from the patient samples was extracted with NE-PER® Nuclear and Cytoplasmic Extraction Reagents kit. Protease inhibitors (Halt Protease Inhibitor Cocktail, Thermo Fisher Scientific Inc.) were added to the extraction reagents. Protein samples from the Hs68 cells were obtained by homogenization of the cells in a lysis buffer containing 1% Igepal® CA-630, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate, aprotinin (10 μl/ml), leupeptin (10 μg/ml) and phosphate buffered saline.
Cell lysates containing 20 μg protein were loaded into a 10% precast sodium dodecyl sulfate-polyacrylamide gel electrophoresis (Bio-Rad Laboratories, Hercules, California), followed by electrophoresis and transfer of the proteins onto a polyvinylidene fluoride membrane (Millipore Corp., Billerica, Massachusetts), which was stained with Ponceau S. Detection of target proteins was performed with an electrochemoluminescence kit (Amersham Life Science Inc., Arlington Heights, Illinois) using primary antibodies for ZEB1 (ab23398, Abcam®, 1:200) and β-actin (Sigma-Aldrich, A5441, 1:3000 dilution). After secondary antibody hybridization the images were analyzed with the ChemiImager 4000 (Cell Biosciences™ Inc., Santa Clara, California). Expression levels of ZEB1 are reported relative to β-actin, another housekeeping gene commonly used as a control in Western blots.
Immunofluorescence Staining
After treatment with estrogen as described the Hs68 cells were fixed with ice-cold methanol, permeabilized with 0.05% Triton® X-100 and blocked with Superblock (Perbio Science GmbH, Heidelberg, Germany). The cells were then incubated with rabbit anti-ZEB1 antibody (H-102, Santa Cruz Biotechnology Inc., Santa Cruz, California) followed by incubation with donkey anti-rabbit Alexa Fluor® 488 fluorescein isothiocyanate conjugated antibody. The cells were stained with 4′, 6-diamidino-2-phenylindole to stain the nuclei and then counted using a Nikon Eclipse E600 fluorescence microscope and a Retiga 1300™ QImaging camera.
Immunohistochemical Analysis
Human tissues were fixed in formalin, paraffin embedded and sectioned (5 μm). Antigens were retrieved using antigen unmasking solution (Vector Laboratories Inc., Burlingame, California). Following blocking, slides were incubated overnight at 4C with rabbit anti-ZEB1 antibody (H-102). Staining of the tissue was performed with the Vectastain Elite ABC Kit (Vector Laboratories Inc.) followed by hematoxylin counterstaining.
Clinical Data
Preputial tissue was obtained from 37 males with hypospadias at surgical repair at UCSF between 1995 and 2000. Degree of hypospadias was determined by the position of the urethral meatus and was classified as mild (meatus at or distal to mid shaft of penis, 24 cases) or severe (meatus proximal to mid shaft, 13). Normal preputial skin was obtained from 11 subjects undergoing circumcision during the same period. Fewer subject samples (6 controls, 6 mild cases, 5 severe cases) were used for the Western blot experiments due to insufficient tissue to extract protein in the other samples. For all other experiments the entire sample size was used. All subjects were prospectively enrolled, and written informed consent was obtained from parents or guardians preoperatively. This study was approved by the UCSF Committee on Human Research.
Statistical Analysis
Differences in mean ZEB1 mRNA and protein levels in the Hs68 cells after exposure to increasing estrogen concentrations were determined using ANOVA with Bonferroni correction. The same analysis was performed to determine if mean ZEB1 mRNA and protein levels differed between controls, subjects with mild hypospadias and subjects with severe hypospadias. Statistical analysis was performed with Stata/SE® 11.
RESULTS
Estrogen Increases ZEB1 Expression In Vitro
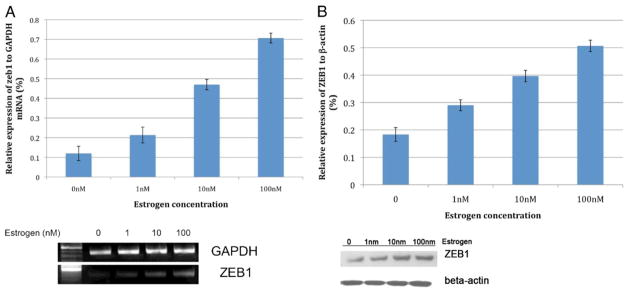
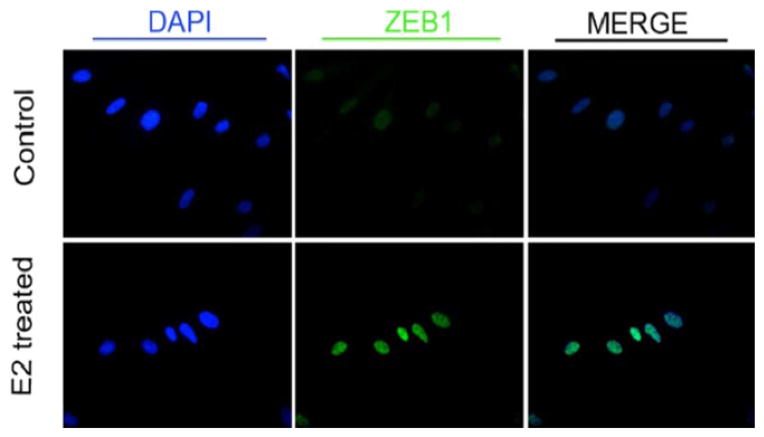
Mean expressions of ZEB1 mRNA in Hs68 cells exposed to 0, 1, 10 and 100 nM estrogen were 0.12, 0.21, 0.47 and 0.71, respectively. Mean levels of ZEB1 protein in Hs68 cells exposed to 0, 1, 10 and 100 nM estrogen were 0.18, 0.29, 0.40 and 0.51, respectively. There was a significant difference in mean expression levels of ZEB1 RNA and protein in cells exposed to increasing concentrations of estrogen (F = 197.03 and F = 122.03, respectively, fig. 1). ZEB1 is expressed in the nucleus and is up-regulated by estrogen (fig. 2).
Figure 1.
Estrogen increases ZEB1 expression in vitro. mRNA (A) and protein (B) levels in Hs68 cells.
Figure 2.
Effect of estrogen (E2) on ZEB1 expression in Hs68 cells. Estrogen increased intensity of ZEB1 immunofluorescence in nucleus of fibroblasts derived from human foreskin.
ZEB1 Expression Correlates With Hypospadias Severity
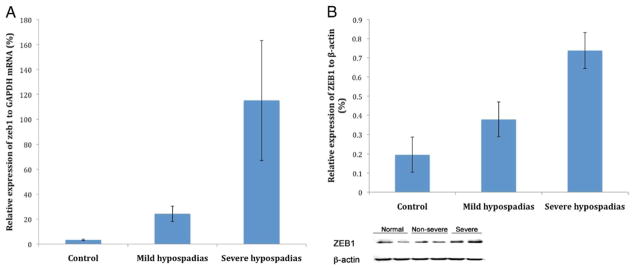
Mean expressions of ZEB1 mRNA in controls and subjects with mild and severe hypospadias were 3.4, 24.33 and 115.17, respectively (fig. 3). There was no difference in the ZEB1 expression levels between controls and subjects with mild hypospadias (p = 0.64). However, there were statistically significant differences in expression of ZEB1 between controls and subjects with severe hypospadias (p <0.001), and between subjects with mild and severe hypospadias (p <0.001).
Figure 3.
Expression of ZEB1 in controls and subjects with hypospadias. Subjects with severe hypospadias had significantly higher mRNA (A) and protein (B) levels of ZEB1 compared to controls and subjects with mild hypospadias.
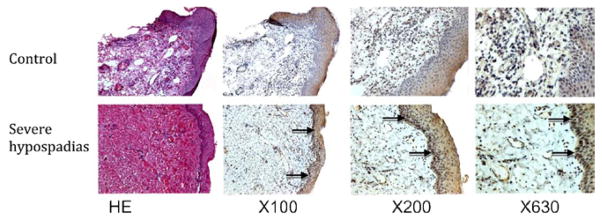
Expression of ZEB1 at the protein level was significantly higher in subjects with mild and severe hypospadias than in controls (p = 0.01 and p <0.01, respectively), and was higher in subjects with severe vs mild hypospadias (p <0.01, fig. 3). Immunohisto-chemical analysis revealed that ZEB1 is highly expressed in the stratum basale of subjects with severe hypospadias but is not seen in controls (fig. 4).
Figure 4.
Expression of ZEB1 in normal subjects and patients with hypospadias. ZEB1 is absent in preputial skin of controls but is highly expressed in stratum basale (arrows) in subjects with severe hypospadias. HE, hematoxylin & eosin.
Subject Characteristics
Of the 24 subjects with mild hypospadias the meatus was glanular in 2 (8%), subcoronal in 4 (17%), at the distal shaft in 10 (42%) and at the mid shaft in 8 (33%). Of the 13 patients with severe hypospadias the meatus was at the proximal penile shaft in 8 (62%), at the penoscrotal junction in 3 (23%) and in the perineum in 2 (15%).
DISCUSSION
We observed that graded estrogen exposure increases ZEB1 mRNA and protein expression in vitro in human foreskin fibroblast cells. Furthermore, we found that ZEB1 is over expressed in preputial tissue of patients with severe hypospadias.
ZEB1 is expressed throughout the developing embryo and is necessary for survival.13 Most research on the function of ZEB1 has focused on the effect of ZEB1 knockouts in development or ZEB1 overexpression in carcinogenesis. To our knowledge, there have not been any reports of human congenital genitourinary anomalies associated with ZEB1 overexpression. Therefore, it is necessary to extrapolate from the known functions of ZEB1 to explain and provide the context for the association we found between ZEB1 expression levels and hypospadias.
The urethral abnormalities seen in hypospadias can be viewed as a failure of epithelial cell adhesion. In hypospadias the urethra terminates proximal to its orthotopic location in the glans penis as a result of failure of the urethral plate and abortive corpus spongiosum to fuse in the midline. We hypothesize that ZEB1 overexpression decreases cellular adhesion in the developing male urethra and ventral penile skin, which results in the abortive penile development seen in hypospadias. It is known that ZEB1 overexpression decreases intercellular adhesion by repressing multiple cellular adhesion molecules such as E-cadherin, which is a transmembrane glycoprotein that mediates cellular adhesion between epithelial cells through interaction with other proteins such as β-catenin.14 –17
To our knowledge, no studies have investigated the expression patterns of E-cadherin in normal and hypospadic urethras. However, poor cellular adhesion is a likely causative factor in hypospadias as mice with mutations of ephrin-B2, a cell surface molecule important in cellular adhesion, manifest severe hypospadias and cloacal anomalies.18 It is plausible that the increased ZEB1 expression in the basal layer of preputial skin in boys with hypospadias reflects decreased expression of cell adhesion molecules in the urethra such that fusion of the urethral folds is aborted prematurely. However, it is possible that the molecular and cellular events occurring in the prepuce, which is of ectodermal origin, do not reflect events occurring in the urethra, which is of endodermal origin.
An alternative, although less likely, explanation is that ZEB1 overexpression decreases urethral collagen. In osteoblasts ZEB1 has been shown to down-regulate type I collagen.19 If ZEB1 also represses collagen I genes in the urethra, it is possible that deficient urethral collagen formation contributes to hypospadias. However, to our knowledge, ZEB1 mediated decreased collagen formation has not been demonstrated in tissues other than cartilage and bone. Additionally there are no differences in the amount or distribution of collagen I or III in hypospadic and normal penises.20
We found a direct relationship between estrogen concentration and ZEB1 foreskin fibroblast expression in vitro. We also found that boys with severe hypospadias have higher levels of ZEB1 mRNA and protein levels than controls. Although the urethral tissue was not directly examined, it is possible that there is also a dose response effect of ZEB1 on urethral development. Chamberlain and Sanders found that estrogen increases ZEB1 transcription through direct interaction of the estrogen receptor complex with the ZEB1 gene.21 Our findings support these results and suggest that the enhancement of ZEB1 transcription by estrogen is concentration dependent. Additionally Graham et al reported that ZEB1 binds to the AR promoter, ZEB1 regulates the response of the AR to androgens, and there is reciprocal suppression between AR and ZEB1.22 These results were observed in an aggressive breast cancer cell line, and it is unknown how ZEB1 and AR interact during normal and abnormal embryonic development. However, when viewed in this context, our findings raise the possibility that ZEB1 mediates some of the effects of sex hormones on penile development.23–25
Alterations in sex hormones during the critical period of external genitalia formation have been hypothesized to increase the risk of hypospadias.26,27 Boys born to women exposed to diethylstilbestrol in utero have higher rates of hypospadias than boys born to women who are not exposed to diethylstilbestrol.4 To our knowledge, no published study has revealed an association between the dose of estrogen exposure and hypospadias severity. However, in a population based cohort boys who were small for gestational age were significantly more likely to have moderate to severe hypospadias but not mild hypospadias compared to normal children.28 It is possible that ZEB1 overexpression links the associated conditions of small for gestational age and severe hypospadias, since ZEB1 represses production of types I and II collagen in osteoblasts and chondrocytes, respectively.19,29
In this study Hs68 cells were used for the in vitro experiments. This cell line has important strengths, including being derived from the foreskin of a newborn male, and previous studies in which Hs68 cells were exposed to estrogen have supported findings in human tissue samples and mouse models.30 However, this cell line has limitations. Hs68 cells might not reflect the gene expression profile or cellular properties of the developing urethral plate, as they are endodermal in origin and derived from neonatal rather than fetal tissue. Given that Hs68 cells are fibroblasts, we cannot definitively state that estrogen increases ZEB1 expression in human foreskin epithelium in vitro. However, since ZEB1 levels vary by tissue type, we believed it was of primary importance to perform the in vitro studies in cells that originated from human neonatal male genital skin. We are not aware of an epithelial cell line that meets these criteria. Despite these limitations, we believe the Hs68 cell line is an acceptable, albeit imperfect, model for investigating hypospadias at a cellular level, given that the foreskin and urethra are affected in hypospadias, and human subject studies support the in vitro experiments.
Given the responsiveness of ZEB1 to estrogen and the association of estrogen exposure and androgen disruption with hypospadias, the mechanism by which ZEB1 mediates the effect of estrogen in the pathogenesis of hypospadias merits further investigation. Future experiments using murine models to investigate the effect of estrogen on ZEB1 expression and the development of hypospadias should help elucidate whether ZEB1 overexpression can cause hypospadias.
CONCLUSIONS
Estrogen up-regulates ZEB1 expression in human foreskin cells in vitro. Additionally boys with severe hypospadias have increased expression of ZEB1 in penile skin. Given the role of ZEB1 in repressing epithelial phenotype and decreasing cell adhesion molecules, and its responsiveness to estrogen, increased ZEB1 expression may be a mechanism that contributes to the development of hypospadias.
Abbreviations and Acronyms
- AR
androgen receptor
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- PCR
polymerase chain reaction
- UCSF
University of California, San Francisco
Footnotes
Study received UCSF Committee on Human Research approval.
References
- 1.Paulozzi LJ. International trends in rates of hypospadias and cryptorchidism. Environ Health Perspect. 1999;107:297. doi: 10.1289/ehp.99107297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gray LE, Jr, Wolf C, Lambright C, et al. Administration of potentially antiandrogenic pesticides (procymidone, linuron, iprodione, chlozolinate, p,p′-DDE, and ketoconazole) and toxic substances (dibutyl- and diethylhexyl phthalate, PCB 169, and ethane dimethane sulphonate) during sexual differentiation produces diverse profiles of reproductive malformations in the male rat. Toxicol Ind Health. 1999;15:94. doi: 10.1177/074823379901500109. [DOI] [PubMed] [Google Scholar]
- 3.Aschim EL, Nordenskjöld A, Giwercman A, et al. Linkage between cryptorchidism, hypospadias, and GGN repeat length in the androgen receptor gene. J Clin Endocrinol Metab. 2004;89:5105. doi: 10.1210/jc.2004-0293. [DOI] [PubMed] [Google Scholar]
- 4.Klip H, Verloop J, van Gool JD, et al. Hypospadias in sons of women exposed to diethylstilbestrol in utero: a cohort study. Lancet. 2002;359:1102. doi: 10.1016/S0140-6736(02)08152-7. [DOI] [PubMed] [Google Scholar]
- 5.Brouwers MM, Feitz WF, Roelofs LA, et al. Risk factors for hypospadias. Eur J Pediatr. 2007;166:671. doi: 10.1007/s00431-006-0304-z. [DOI] [PubMed] [Google Scholar]
- 6.Garavelli L, Cerruti-Mainardi P, Virdis R, et al. Genitourinary anomalies in Mowat-Wilson syndrome with deletion/mutation in the zinc finger homeo box 1B gene (ZFHX1B). Report of three Italian cases with hypospadias and review. Horm Res. 2005;63:187. doi: 10.1159/000085894. [DOI] [PubMed] [Google Scholar]
- 7.Wang Z, Liu BC, Lin GT, et al. Up-regulation of estrogen responsive genes in hypospadias: microarray analysis. J Urol. 2007;177:1939. doi: 10.1016/j.juro.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 8.Grooteclaes ML, Frisch SM. Evidence for a function of CtBP in epithelial gene regulation and anoikis. Oncogene. 2000;19:3823. doi: 10.1038/sj.onc.1203721. [DOI] [PubMed] [Google Scholar]
- 9.Takagi T, Moribe H, Kondoh H, et al. DeltaEF1, a zinc finger and homeodomain transcription factor, is required for skeleton patterning in multiple lineages. Development. 1998;125:21. doi: 10.1242/dev.125.1.21. [DOI] [PubMed] [Google Scholar]
- 10.Hurt EM, Saykally JN, Anose BM, et al. Expression of the ZEB1 (deltaEF1) transcription factor in human: additional insights. Mol Cell Biochem. 2008;318:89. doi: 10.1007/s11010-008-9860-z. [DOI] [PubMed] [Google Scholar]
- 11.Spoelstra NS, Manning NG, Higashi Y, et al. The transcription factor ZEB1 is aberrantly expressed in aggressive uterine cancers. Cancer Res. 2006;66:3893. doi: 10.1158/0008-5472.CAN-05-2881. [DOI] [PubMed] [Google Scholar]
- 12.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25:402. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 13.Miyoshi T, Maruhashi M, Van De Putte T, et al. Complementary expression pattern of Zfhx1 genes Sip1 and deltaEF1 in the mouse embryo and their genetic interaction revealed by compound mutants. Dev Dyn. 2006;235:1941. doi: 10.1002/dvdy.20799. [DOI] [PubMed] [Google Scholar]
- 14.Ozaki C, Obata S, Yamanaka H, et al. The extra-cellular domains of E- and N-cadherin determine the scattered punctate localization in epithelial cells and the cytoplasmic domains modulate the localization. J Biochem. 2010;147:415. doi: 10.1093/jb/mvp192. [DOI] [PubMed] [Google Scholar]
- 15.Takeichi M. Morphogenetic roles of classic cadherins. Curr Opin Cell Biol. 1995;7:619. doi: 10.1016/0955-0674(95)80102-2. [DOI] [PubMed] [Google Scholar]
- 16.Drake JM, Strohbehn G, Bair TB, et al. ZEB1 enhances transendothelial migration and represses the epithelial phenotype of prostate cancer cells. Mol Biol Cell. 2009;20:2207. doi: 10.1091/mbc.E08-10-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kakihana M, Ohira T, Chan D, et al. Induction of E-cadherin in lung cancer and interaction with growth suppression by histone deacetylase inhibition. J Thorac Oncol. 2009;4:1455. doi: 10.1097/JTO.0b013e3181bc9419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dravis C, Yokoyama N, Chumley MJ, et al. Bidirectional signaling mediated by ephrin-B2 and EphB2 controls urorectal development. Dev Biol. 2004;271:272. doi: 10.1016/j.ydbio.2004.03.027. [DOI] [PubMed] [Google Scholar]
- 19.Terraz C, Toman D, Delauche M, et al. Delta Ef1 binds to a far upstream sequence of the mouse pro-alpha 1(I) collagen gene and represses its expression in osteoblasts. J Biol Chem. 2001;276:37011. doi: 10.1074/jbc.M104185200. [DOI] [PubMed] [Google Scholar]
- 20.Erol A, Baskin LS, Li YW, et al. Anatomical studies of the urethral plate: why preservation of the urethral plate is important in hypospadias repair. BJU Int. 2000;85:728. doi: 10.1046/j.1464-410x.2000.00486.x. [DOI] [PubMed] [Google Scholar]
- 21.Chamberlain EM, Sanders MM. Identification of the novel player deltaEF1 in estrogen transcriptional cascades. Mol Cell Biol. 1999;19:3600. doi: 10.1128/mcb.19.5.3600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Graham TR, Yacoub R, Taliaferro-Smith L, et al. Reciprocal regulation of ZEB1 and AR in triple negative breast cancer cells. Breast Cancer Res Treat. 2010;123:139. doi: 10.1007/s10549-009-0623-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goyal HO, Braden TD, Cooke PS, et al. Estrogen receptor alpha mediates estrogen-inducible abnormalities in the developing penis. Reproduction. 2007;133:1057. doi: 10.1530/REP-06-0326. [DOI] [PubMed] [Google Scholar]
- 24.Goyal HO, Braden TD, Williams CS, et al. Permanent induction of morphological abnormalities in the penis and penile skeletal muscles in adult rats treated neonatally with diethylstilbestrol or estradiol valerate: a dose-response study. J Androl. 2005;26:32. [PubMed] [Google Scholar]
- 25.Agras K, Willingham E, Liu B, et al. Ontogeny of androgen receptor and disruption of its mRNA expression by exogenous estrogens during morphogenesis of the genital tubercle. J Urol. 2006;176:1883. doi: 10.1016/S0022-5347(06)00613-6. [DOI] [PubMed] [Google Scholar]
- 26.Silver RI, Russell DW. 5Alpha-reductase type 2 mutations are present in some boys with isolated hypospadias. J Urol. 1999;162:1142. doi: 10.1016/S0022-5347(01)68102-3. [DOI] [PubMed] [Google Scholar]
- 27.Silver RI, Rodriguez R, Chang TS, et al. In vitro fertilization is associated with an increased risk of hypospadias. J Urol. 1999;161:1954. [PubMed] [Google Scholar]
- 28.Ghirri P, Scaramuzzo RT, Bertelloni S, et al. Prevalence of hypospadias in Italy according to severity, gestational age and birthweight: an epidemiological study. Ital J Pediatr. 2009;35:18. doi: 10.1186/1824-7288-35-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murray D, Precht P, Balakir R, et al. The transcription factor deltaEF1 is inversely expressed with type II collagen mRNA and can repress Col2a1 promoter activity in transfected chondrocytes. J Biol Chem. 2000;275:3610. doi: 10.1074/jbc.275.5.3610. [DOI] [PubMed] [Google Scholar]
- 30.Liu B, Lin G, Willingham E, et al. Estradiol upregulates activating transcription factor 3, a candidate gene in the etiology of hypospadias. Pediatr Dev Pathol. 2007;10:446. doi: 10.2350/06-04-0079.1. [DOI] [PubMed] [Google Scholar]