Abstract
The L1 family of cell adhesion molecules (L1CAMs) in vertebrates has long been studied for its roles in nervous system development and function. Members of this family have been associated with distinct neurological disorders that include CRASH, autism, 3p syndrome, and schizophrenia. The conservation of L1CAMs in Drosophila and C. elegans allows the opportunity to take advantage of these simple model organisms and their accessible genetic manipulations to dissect L1CAM functions and mechanisms of action. This review summarizes the discoveries of L1CAMs made in C. elegans, showcasing this simple model organism as a powerful system to uncover L1CAM mechanisms and roles in healthy and diseased states.
INTRODUCTION
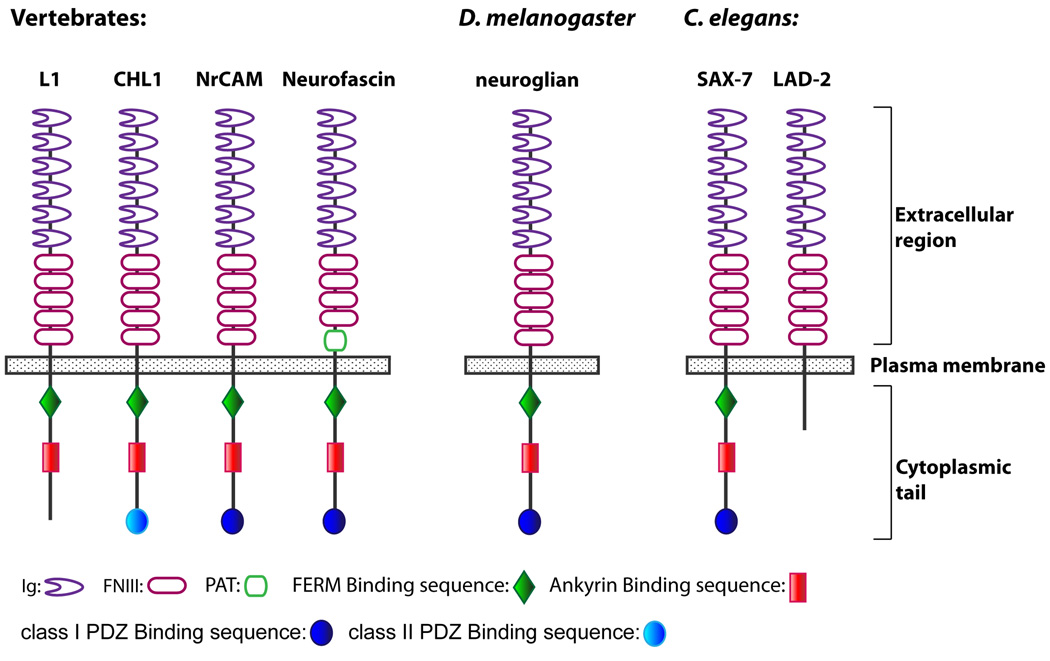
L1CAMs are single-pass transmembrane cell adhesion receptors belonging to the immunoglobulin superfamily (IgSF) that are conserved in both vertebrates and invertebrates, including C. elegans and Drosophila (Moos et al., 1988; Grumet et al., 1991; Volkmer et al., 1992; Holm et al., 1996; Bieber et al., 1989; Chen et al., 2001). Each L1CAM has a conserved protein structure of an extracellular domain consisting of six immunoglobulin-like (Ig) motifs and four or five fibronectin type III (FNIII) repeats, a single transmembrane domain, and a highly conserved cytoplasmic tail (Fig 1).
Fig 1.
The mammalian L1CAM family is composed of four genes – L1, CHL1, NrCAM, and neurofascin – that are highly expressed in the nervous system. The importance of L1CAMs is most apparent in their direct links with human disease. Mutations in L1 can result in the X-linked neurological disorder, CRASH, an acronym that accounts for the clinical symptoms: Corpus callosum hypoplasia, mental Retardation, Adducted thumbs, Spastic paraplegia, and Hydrocephalus (Rosenthal et al., 1992; van Camp et al., 1993; Jouet et al., 1994; reviewed in Fransen et al., 1995). These symptoms are highly variable in their manifestation, ranging from mild mental retardation to pre- and perinatal death resulting from severe hydrocephalus (Jouet et al., 1994). Other L1CAMs are also associated with disease. The link between NrCAM and autism was recently confirmed (Marui et al., 2009) while polymorphisms in CHL1 have been implicated in schizophrenia and non-specific mental retardation associated with the 3p syndrome (Sakurai et al., 2002; Frints et al., 2003). In addition to neurological disorders, L1CAMs have also been associated with cancers where L1 and NrCAM expression correlates with cancer progression, metastasis, and poor prognosis (Conacci-Sorrell et al., 2002; Fogel et al., 2003a; Fogel et al., 2003b).
Cell-based and antibody perturbation studies implicated L1CAMs in multiple neuronal processes, including neuronal migration (Lindner et al., 1983; Asou et al., 1992), myelination (Charles et al., 2002), axon extension and guidance (Fischer et al., 1986; Lagenaur and Lemmon, 1987), and synaptic plasticity (Lüthi et al., 1996). Examination of knockout mice for each mammalian L1CAM, which were subsequently generated, confirmed many of these roles and also revealed each L1CAM as having distinct as well as overlapping roles (Dahme et al., 1997; Fransen et al., 1998; Cohen et al., 1998; Moré et al., 2001; Sakurai et al., 2001; Montag-Sallaz et al., 2002; Sherman et al., 2005). That L1CAMs have redundant functions is evident in double knockout mice of L1 and NrCAM, which exhibit postnatal lethality and severe cerebellar dysgenesis whereas single knockout mice are viable and show subtle brain malformations (Sakurai et al., 2001). L1 and NrCAM knockout mice also exhibit kidney defects (Debiec et al., 2002) and cataracts (Moré et al., 2001), respectively, demonstrating non-neuronal roles for L1CAMs as well. Both neuronal and non-neuronal roles have similarly been identified for neuroglian, the sole Drosophila L1CAM homologue. These roles include axon pathfinding, synapse formation, as well as glial and epithelial septate junction organization (Hall and Bieber, 1997; Genova and Fehon, 2003; Faivre-Sarrailh et al., 2004; Banerjee et al., 2006; Godenschwege et al., 2006).
L1CAMs promote these activities through homophilic and heterophilic interactions via their extracellular domain to mediate cell-cell and cell-extracellular matrix adhesion (reviewed in Haspel and Grumet, 2003). The L1CAM cytoplasmic tail contains conserved consensus binding sites to membrane cytoskeletal linkers such as ankyrin, suggesting the importance of L1CAM association with the cortical cytoskeleton (Davis and Bennett, 1993; Davis and Bennett, 1994). The cytoplasmic tail also harbors phosphorylation sites (Schaefer et al., 1999; Sadoul et al., 1989; Schmid et al., 2000; Jenkins et al., 2001), implying that L1CAMs are subject to regulation by signal transduction pathways.
There have been multiple findings from molecular and cell-based studies conducted on L1CAMs that require further analyses to demonstrate functional relevance. In addition, the redundancies of mammalian L1CAMs can complicate the analysis of L1CAM functions. In contrast to the more complex mammalian system, the simple nervous system, and accessible genetic manipulation in C. elegans make this organism a choice system not only to quickly verify the functional aspects of these L1CAM findings, but also to identify novel mechanistic roles. In this review, we summarize discoveries made from studies performed in C. elegans. These discoveries illustrate the conservation of L1CAM functions and mechanisms of action from C. elegans to mammals thus revealing the potential for C. elegans as a model system to uncover additional L1CAM roles and the mechanistic basis underlying L1CAM-related diseases.
C. elegans L1CAMs
C. elegans has two L1CAM homologues, lad-2 and sax-7/lad-1 (for L1-ADhesion) (Chen et al., 2001; Aurelio et al., 2002; Sasakura et al., 2005; Wang et al., 2005) that do not have overlapping functions (Wang et al., 2008). Each gene has a distinct expression pattern; lad-2 expression is restricted to a subset of neurons while sax-7 is expressed in virtually all cells, as early as the two-cell staged embryo (Chen et al., 2001; Aurelio et al., 2002). Each protein also has a distinct protein structure; the LAD-2 protein has the conserved L1CAM ectodomain but a short and divergent cytoplasmic tail while SAX-7 has all the structural hallmarks of vertebrate L1CAMs, thus revealing SAX-7 as a canonical L1CAM (Fig 1). While it is unclear how the divergent cytoplasmic tail contributes to function, LAD-2 is an L1CAM that participates in processes that also are mediated by mammalian L1CAMs.
lad-2 functions in axon pathfinding
The lad-2 gene produces two alternatively-spliced isoforms – LAD-2L and LAD-2S (Wang et al., 2008). LAD-2L is a full-length membrane-bound isoform (Fig 1) that is localized to the plasma membrane of lad-2-expressing neuronal cell bodies and axons. LAD-2L functions in axon pathfinding along the anterior/posterior and dorsoventral axes. LAD-2S is a secreted isoform that is comprised of the first four Ig motifs and a partial fifth Ig motif. While the role of LAD-2S is not known, it does not appear to be required for axon guidance. The axon defects in lad-2(tm3056) null animals, which lack both isoforms, are not any more severe than the defects exhibited by lad-2(hd31) animals, which lack only the transmembrane isoform but express LAD-2S.
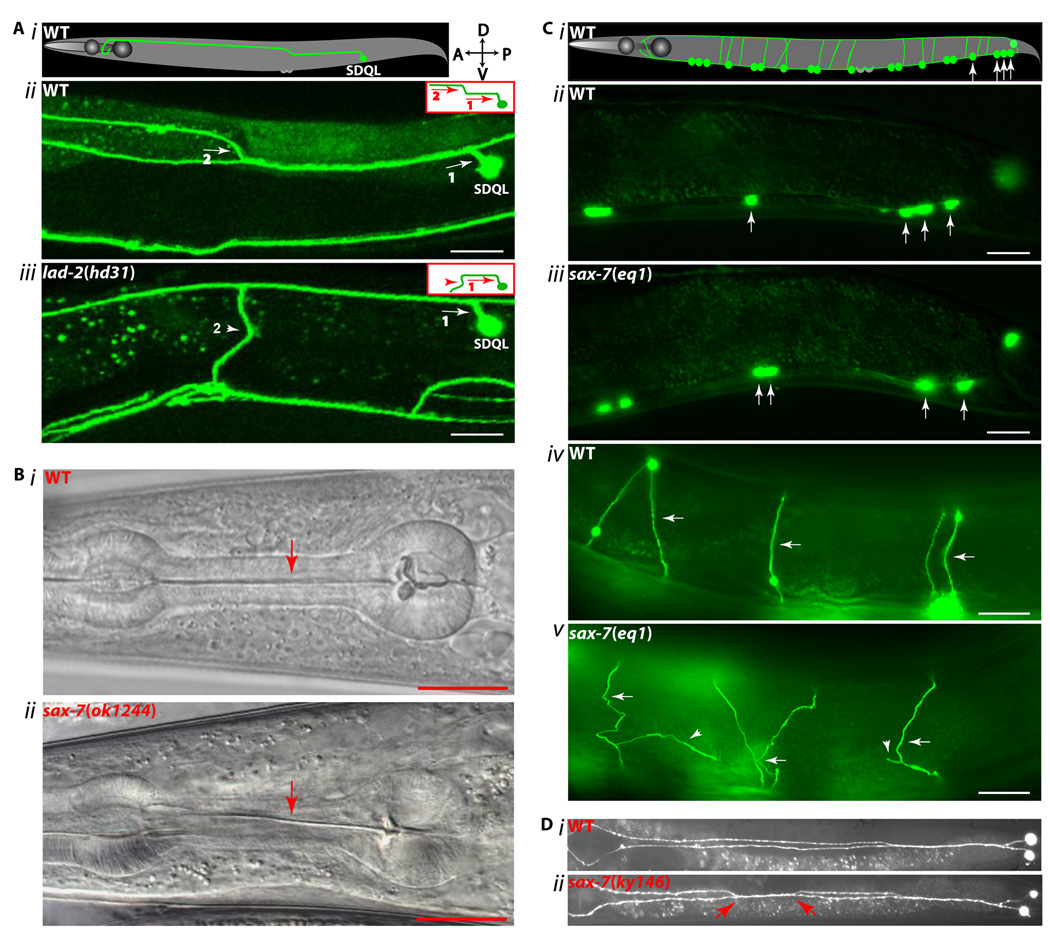
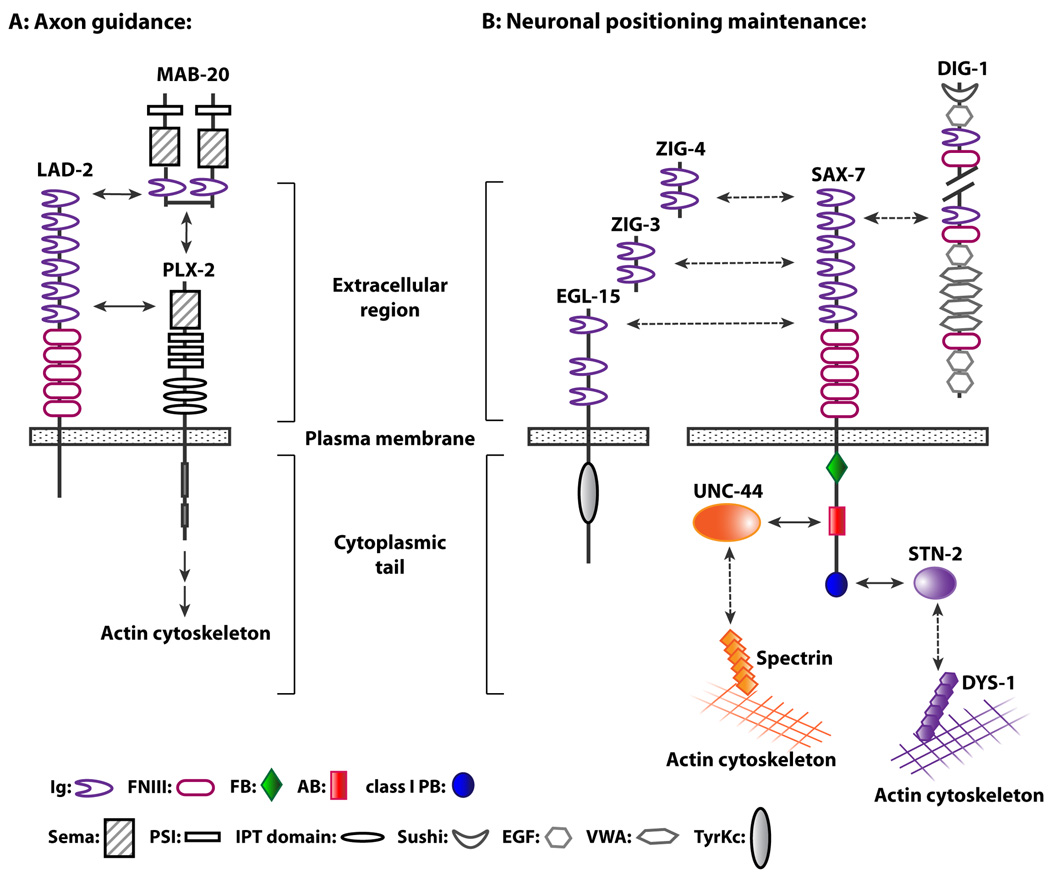
Further analysis revealed that LAD-2L functions cell-autonomously to direct dorsal axon migration of SDQL, a posterior lateral interneuron (Fig 2A), by mediating the repulsive activities of MAB-20/Sema2, a secreted semaphorin, and its PLX-2/plexin receptor (Wang et al., 2008). Consistent with PLX-2/plexin functioning as a semaphorin receptor, MAB-20/Sema2 and PLX-2/plexins can interact, albeit weakly (Nakao et al., 2007; Wang et al., 2008). Additional biochemical studies demonstrated that LAD-2 can form a ternary complex with MAB-20/Sema2 and PLX-2/plexin (Fig 3A). Furthermore, the presence of LAD-2 dramatically enhances the interaction between MAB-20/Sema2 and PLX-2/plexin (Wang et al., 2008). Taken together, these results suggest that LAD-2 guides axon migration by acting as a MAB-20/Sema2 co-receptor and anchoring MAB-20/Sema2 to PLX-2/plexin.
Fig 2.
Fig 3.
Mammalian L1, CHL1, and NrCAM also function as co-receptors for semaphorin-mediated axon pathfinding (Castellani et al., 2000; Falk et al., 2005; Wright et al., 2007). In contrast to LAD-2, each mammalian L1CAM forms a quaternary complex by binding neuropilin, another semaphorin co-receptor that mediates the linkage of the secreted Sema3 to the semaphorin-transducing plexin receptor. Neuropilin is not conserved in C. elegans or Drosophila although it is conserved in the more ancient cniradian, N. vectensis, along with semaphorin and plexin, thus pointing to semaphorin signaling as an ancient process (Putnam et al., 2007). However, L1CAMs do not appear to be present in N. vectensis (C. Magie, pers. comm), suggesting that L1CAMs arose when bilaterians (worms, flies, and humans) emerged. Taken together, we speculate that LAD-2 is an ancestral L1CAM that in its evolution, incorporated the function of neuropilin as a molecule that links semaphorin to plexin due to or resulting in the loss of neuropilin in C. elegans. It is curious that the cytoplasmic tails of LAD-2 and neuropilin, which although do not share significant sequence homology, are both strikingly short, approximately 40 amino acids long (Wang et al., 2008). In mammals, the employment of both L1CAMs and neuropilin in semaphorin signaling may reflect a means by which an additional layer of control was implemented to support a more complex nervous system. Consistent with this hypothesis is a recent finding that activation of the FAK-MAPK cascade during semaphorin-mediated axon guidance in mammals requires the interaction of neuropilin with L1 but not plexin (Bechara et al., 2008).
The axon defects in lad-2 animals are significantly more robust than those seen in mab-20 or plx-2 null animals and also affect neurons that are not perturbed in mab-20 or plx-2 animals (Wang et al., 2008). These data indicate that LAD-2 also mediates axon guidance via MAB-20-independent pathways, either as a direct receptor to a guidance cue or as a regulatory protein in the guidance pathways, and suggests that mammalian L1CAM also mediates axon guidance via additional guidance pathways. Supporting this idea is a recent finding that impaired L1-mediated adhesion can affect pathfinding in axons that rely on the ephrinB/EphB guidance system (Buhusi et al., 2008).
Similar to LAD-2S, soluble forms of mammalian L1CAM, composed of the extracellular portion of the molecule, also exist. They are generated via post-translational proteolytic cleavage at conserved sites, resulting in the release of the L1CAM ectodomain from the cell surface (Nayeem et al., 1999; Kalus et al., 2003; Naus et al., 2004; Maretzky et al., 2005). The soluble L1 ectodomains have been shown to participate in different processes, including promoting cell motility and modulating the response of axons to Sema3 (Mechtersheimer et al., 2001; Castellani et al., 2002; Yang et al., 2009). More studies are required to determine whether these soluble L1 roles are also conserved with LAD-2S.
sax-7 functions to maintain nervous system integrity
In contrast to the developmental roles of LAD-2, SAX-7 functions to maintain the integrity of the nervous system. In sax-7 mutant animals, the nervous system develops normally but neuronal cell bodies and axons eventually become displaced (Fig 2C, D); this phenotype points to defects in maintaining neuronal positioning (Zallen et al., 1999, Sasakura et al., 2005, Wang et al., 2005, Pocock et al., 2008). Displacement of neurons and their axons can be partially suppressed by paralyzing sax-7 animals, revealing SAX-7 as a means to counter the effects of mechanical force (Sasakura et al., 2005; Pocock et al., 2008). Like C. elegans, vertebrates also need to maintain neural integrity against the physical strains exerted by movement, environmental injury, and physical growth. Indeed, zebrafish with impaired or loss of N-cadherin function exhibit defects in maintaining neuronal positioning (Lele et al., 2002; Masai et al., 2003). L1 and CHL1 knockout mice display altered distribution of certain neurons that have thus far been attributed to defective neuronal migration (Demyanenko et al., 2001; Demyanenko et al., 2004). But it is conceivable that these mice as well as NrCAM and neurofascin knockout mice may also display defects in neuronal maintenance. Alternatively, such maintenance defects may require knockouts of multiple L1CAMs due to their functional redundancies.
It is striking that L1CAMs are essential in mammals and Drosophila but not in C. elegans. lad-2 sax-7 double mutant animals are viable unlike neuroglian mutants, which arrest as embryos, and both neurofascin single and NrCAM L1 double knockout mice, which die postnatally (Hall and Bieber, 1997; Sakurai et al., 2001; Sherman et al., 2005; Wang et al., 2005). It is not clear how loss of L1CAMs results in lethality but studies in Drosophila and respective mouse L1CAM mutants suggest that one possible cause is motor defects perhaps resulting from impaired conductance of nerve action potential due to defects in axon ensheathment (Hall and Bieber, 1996; Sakurai et al., 2001; Faivre-Sarrailh et al., 2004; Sherman et al., 2005). By comparison, defective neuronal communication is likely to have more subtle effects in C. elegans, perhaps due to inherent differences in the anatomy and a simpler nervous system. In fact, most C. elegans synaptic transmission defective mutants are viable, unlike in Drosophila or vertebrates (reviewed in J. Richmond, 2005). Because loss of L1CAMs in C. elegans does not result in lethality, it provides a unique opportunity to identify novel functions that might otherwise be difficult to uncover in Drosophila or mice. For example, the neural maintenance defects seen in sax-7 animals may not be present in neuroglian mutants due to their early developmental arrest as embryos and similar defects in mice may require multiple L1CAMs to be knocked out due to compensation among the L1CAMs.
SAX-7 extracellular interactions
Studies focusing on how SAX-7 maintains neuronal positioning have uncovered mechanisms that are conserved in vertebrate L1CAMs. For example, there is evidence to suggest the SAX-7 extracellular domain can mediate cell adhesion via trans homo- and heterophilic interactions, similar to vertebrate L1CAMs. Homophilic adhesion can be induced by expression of SAX-7 in cultured cells as well as in vivo in adjacent neurons that normally do not interact (Sasakura et al., 2005). A particularly good system to study cell adhesion in vivo in a whole animal context consists of two interacting head interneurons, AIY and AVK. In sax-7 mutant animals, both neurons no longer adhere to each other. Consistent with SAX-7 mediating homophilic interactions, adhesion between AIY and AVK can be rescued only when SAX-7 is expressed in both neurons; no rescue is observed when SAX-7 is expressed in only one neuron but not the other (Pocock et al., 2008). In addition to the head neurons, adhesion defects are also observed in neurons located along the ventral nerve cord (VNC) in sax-7 mutant animals (Fig 2C). The positions of both the cell bodies and commissural axons show maintenance defects that can be rescued only when SAX-7 is expressed in the neurons as well as the adjacent hypodermis and body wall muscles (Wang et al., 2005). While this finding is consistent with SAX-7 mediating homophilic adhesion of the VNC neurons to adjacent tissues, it does not rule out heterophilic adhesion.
Supporting the notion that SAX-7 can mediate heterophilic adhesion is the ability for SAX-7 to cell-autonomously rescue the positional defect exhibited in sax-7 mutant animals of a single head sensory neuron, AFD, located in the anterior sensory ganglia (Sasakura et al., 2005). Possible heterophilic interactors with SAX-7 include other secreted IgSF proteins that also participate in the same genetic pathway as SAX-7 to maintain ventral nerve cord axon positions (Fig 2D, Benard et al., 2009). These IgSF proteins include the secreted molecules, DIG-1, ZIG-3, and ZIG-4 (Aurelio et al., 2002; Benard et al., 2006; Benard et al., 2009), and EGL-15/Fibroblast Growth Factor Receptor (FGFR), whose role in neuronal maintenance can be mediated non-autonomously by the EGL-15/FGFR ectodomain engineered to be secreted from the hypodermal cell surface (Bülow et al., 2004). In support of a possible interaction between EGL-15/FGFR and SAX-7, a recent study demonstrated that the ectodomains of mammalian FGFR and L1 can biochemically interact with each other (Kulahin et al., 2008). These secreted IgSF molecules can be bound to the extracellular matrix to act as a substrate for SAX-7. Taken together with the fact that DIG-1 functions as a putative component of the basement membrane (Benard et al., 2006), these data are consistent with SAX-7 likely mediating adhesion of neurons to the basal lamina. Vertebrate L1CAMs similarly are known to interact with basement membrane components such as neurocan and laminin (Friedlander et al., 1994; Hall et al., 1997). Alternatively or additionally, these IgSF proteins may function as signaling molecules in their interactions with SAX-7.
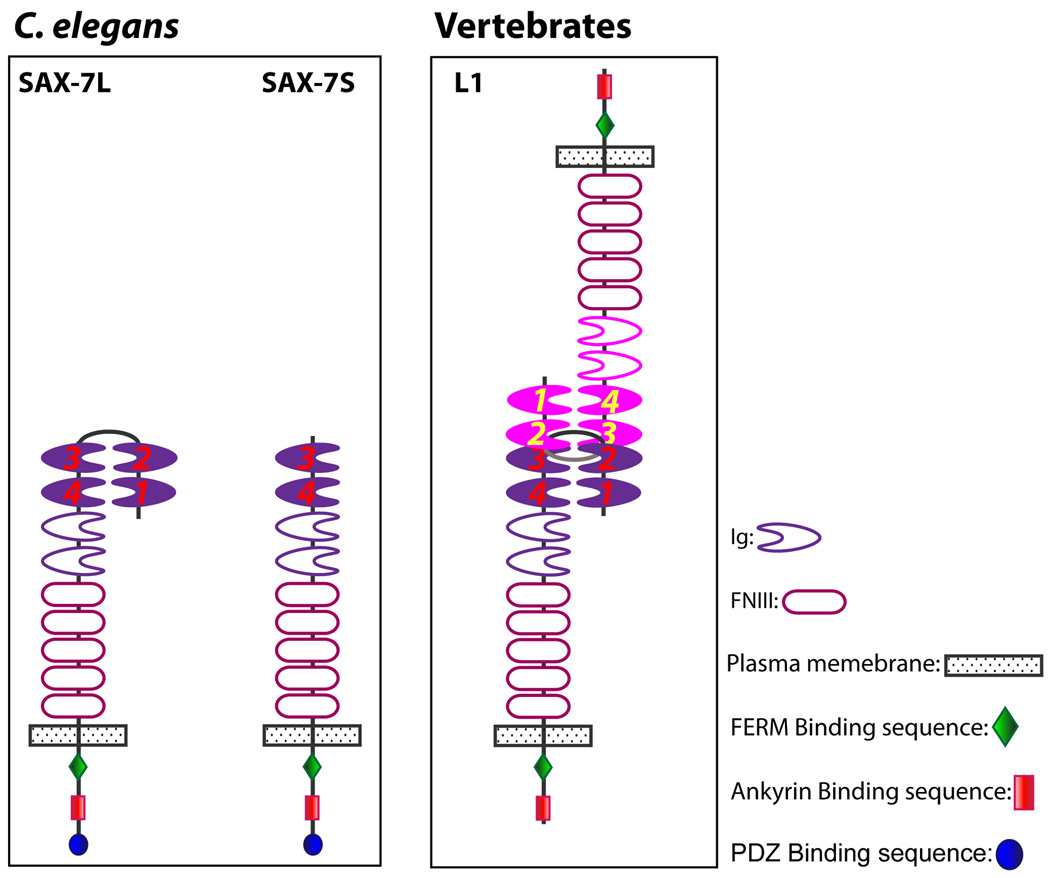
Interestingly, the two alternatively-spliced isoforms of SAX-7 – SAX-7L and SAX-7S, which are distinguished by SAX-7L possessing all six Ig motifs and SAX-7S lacking the first two Ig motifs (Chen et al., 2001) – appear to have different abilities to rescue adhesion of certain neurons in sax-7 animals (Sasakura et al., 2005; Pocock et al., 2008). For example, SAX-7S rescues the AIY-AVK adhesion better than SAX-7L (Pocock et al., 2008). This difference in rescue ability has been proposed to be due to differential adhesive activity; aggregation studies in cultured cells suggest SAX-7S mediates stronger adhesion than SAX-7L (Sasakura et al., 2005). It is not known what accounts for the differences in adhesive activity but genetic rescue experiments with mutated SAX-7 variants suggest that one possible contributing factor may be the distinct protein configurations adopted by SAX-7L and SAX-7S.
Several structural studies revealed that L1 and related Ig molecules assume a “horseshoe” configuration caused by interactions of the first and second Ig motifs folding back onto the fourth and third Ig motifs, respectively (Fig. 4, Su et al., 1998; Freigang et al., 2000; Schurmann et al., 2001; He et al., 2009). The “horseshoe” configuration is present in trans-interacting L1 molecules as revealed by cryoelectron tomographs showing three dimensional views of a homophilic adhesion interface formed between L1 molecules on opposing membranes (Fig 4, He et al., 2009). Based on these structural studies, SAX-7L is predicted to adopt the “horseshoe” configuration, while SAX-7S is anticipated to have an extended “open” configuration due to the lack of the first two Ig motifs (Fig 4). Interestingly, mutations that shorten the linker between the second and third Ig motif in SAX-7L increase the ability for SAX-7L to rescue the AIY/AVK adhesion in sax-7 mutant animals, presumably by preventing the horseshoe configuration (Pocock et al., 2008). This finding suggests that the putative open configuration of SAX-7S allows for increased adhesive activity over that of SAX-7L in an as-yet-unidentified fashion. In fact, this study revealed that of the Ig motifs, only the third and fourth Ig motifs are important for rescue of AIY/AVK adhesion. Unlike SAX-7, mutations in L1 that prevent formation of the horseshoe configuration reduce trans-L1 adhesion (Gouveia et al., 2008). This apparent difference between SAX-7 and L1 reveals a need for additional studies, including structural studies on SAX-7.
Fig 4.
In contrast to the AIY and AVK neurons and sensory neurons that are located in the head, there is no apparent difference in the ability of SAX-7S and SAX-7L to rescue positional defects of VNC neurons and their commissural axons (Zhou et al., 2008). The reason for this dissimilarity between the VNC and head neurons is not known. Perhaps the adjacent tissues and surrounding basement membrane provide additional protein interactions and structural support to the VNC neurons and their commissural axons, unlike the head neurons that mostly require adhesive support of their neuronal neighbors within the ganglia.
SAX-7 intracellular interactions
The SAX-7 cytoplasmic tail contains three distinct consensus binding sites for cytoskeletal linking adaptor proteins (FERM (protein 4.1, Ezrin, Radixin, Moesin) proteins, ankyrin, and PDZ (PSD95, DlgA, ZO-1) proteins) that are also conserved in vertebrate L1CAMs (Fig 1). Mutation and deletion analyses indicate that each of these motifs contributes in an additive fashion to SAX-7 function (Pocock et al., 2008; Zhou et al., 2008), suggesting that proteins are likely to bind to these sites to regulate SAX-7 activity, perhaps by anchoring SAX-7 to the cortical actin cytoskeleton. Indeed, mammalian L1CAMs have been shown to bind ankyrin, an adaptor molecule that can link diverse membrane proteins to the spectrin-actin cytoskeleton (Davis et al, 1994). Moreover, pathologic mutations have been mapped to the cytoplasmic tail of L1, two of which reduce binding to ankyrin (Fransen et al., 1994; Needham et al., 2001).
UNC-44 ankyrin
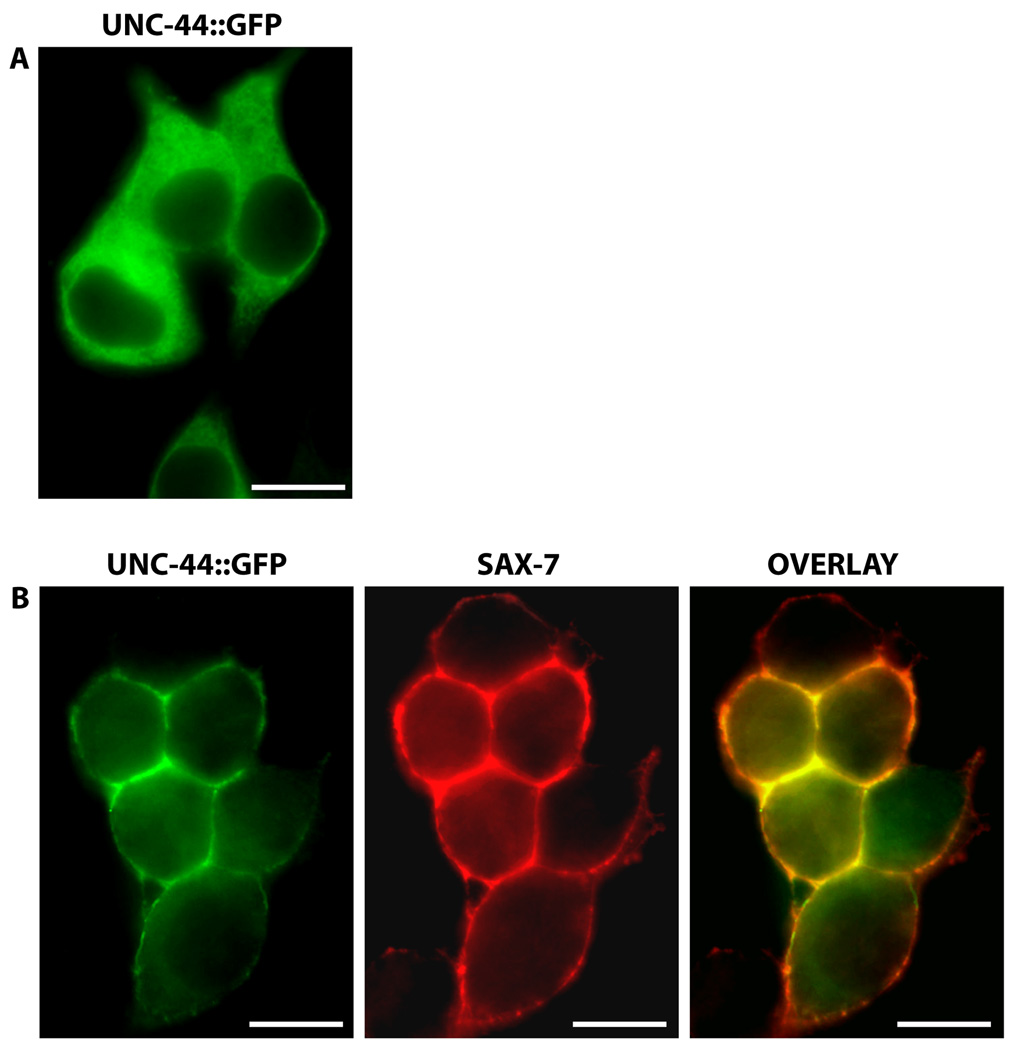
In C. elegans, the interaction of ankyrin to SAX-7 is also conserved, as determined via yeast-two-hybrid assays as well as a protein recruitment assay in human embryonic kidney HEK293 cells (Zhou et al., 2008). In HEK293 cells transfected with UNC-44, the C. elegans ankyrin homologue, UNC-44/ankyrin is primarily localized throughout the cytosol (Fig 5A). But when SAX-7 is co-transfected, UNC-44/ankyrin is dramatically recruited to the cell cortex, overlapping with SAX-7, which is localized at the plasma membrane (Fig 5B). Genetic analysis demonstrates this UNC-44/ankyrin interaction as functionally significant for SAX-7 to maintain neural integrity, presumably by linking SAX-7 to the spectrin-actin cytoskeleton to provide mechanical support (Fig 3).
Fig 5.
Previously, ankyrin-binding to mammalian L1 and neurofascin was shown to be abolished by the phosphorylation of the tyrosine residue in the ankyrin-binding motif, SFIGQY (Garver et al., 1997), revealing possible regulation of ankyrin-binding to L1CAMs in vivo. The importance of the SFIGQY tyrosine residue was confirmed by the identification of a SFIGQY-to-H disease-causing mutation in L1 (Fransen et al, 1995). L1 containing this pathological SFIGQH mutation cannot bind ankyrin and affects axon migration in murine retinal ganglion cells, underscoring the importance of this ankyrin-binding sequence (Needham et al., 2001; Buhusi et al., 2008). However, it is not known whether it is the loss of ankyrin binding and/or the loss of tyrosine phosphorylation that is the cause of the axon defects.
SAX-7 is similarly phosphorylated (Chen et al., 2001). To test the functional significance of this phosphorylation in C. elegans, SAX-7 containing the SFIGQY-to-F mutation was assayed for activity; this mutation in mammalian L1CAMs prevents phosphorylation but does not affect ankyrin-binding (Zhang et al., 1998). This engineered form of SAX-7 cannot completely rescue the neuronal positioning defect in sax-7 mutant animals (Zhou et al., 2008), thus revealing the functional significance of this phosphotyrosine in SAX-7 in neuronal position maintenance. Consistent with this finding, phosphorylation of SAX-7 is dependent on EGL-15/FGFR (Chen et al., 2001), which also functions in neuronal maintenance. Interestingly, EGL-15/FGFR maintains axonal positioning in a kinase-independent manner (Bülow et al., 2004), thus raising the possibility that SAX-7 is phosphorylated by an as-yet-unidentified tyrosine kinase that is activated upon interaction of EGL-15/FGFR with SAX-7.
The functional importance of SFIGQY phosphorylation was also confirmed in Drosophila neuroglian. Neuroglian mutant animals exhibit defects in central synapse formation. These defects can be rescued with pre- and post-synaptic expression of wild-type neuroglian but not with neuroglian containing the SFIGQF mutation (Godenschwege et al., 2006). The functional conservation of this phosphotyrosine in both SAX-7 and neuroglian suggests that phosphorylation of the SFIGQY-tyrosine likely contributes to mammalian L1CAM function via additional mechanisms other than ankyrin-binding. For example, the phosphotyrosine may provide a de novo binding site for phosphotyrosine-binding proteins. Indeed, the microtubule-binding protein doublecortin was identified in a peptide-binding screen as binding to phosphorylated L1CAMs (Kizhatil et al., 2002).
STN-2 γ-syntrophin
The last four amino acids of SAX-7 form a type I PDZ-binding motif. A yeast-two-hybrid screen for interacting type I PDZ proteins identified γ-syntrophin encoded by the stn-2 gene (Zhou et al., 2008). The specificity of this interaction is underscored by the finding that the α/β syntrophin encoded by stn-1, also a type I PDZ protein, does not interact with SAX-7. Genetic analysis confirmed that the STN-2 γ-syntrophin interaction with SAX-7 is functionally significant (Zhou et al., 2008). Indeed, one mutant copy of stn-2 (stn-2/+) can enhance the neuronal defects of sax-7 animals homozygous for a hypomorphic sax-7 allele whereas stn-2/+ animals by themselves show no neuronal defects; in contrast, no enhancement was seen with one mutant copy of stn-1. Taken together, these molecular and genetic results indicate STN-2/γ-syntrophin as a novel regulator of L1CAM activity (Fig 3).
The mammalian syntrophin family is composed of five members – α, β1, β2, γ1, and γ2 (Ahn et al., 1996; Adams et al., 1995; Piluso et al., 2000). While less well characterized, there is evidence that γ1- and γ2-syntrophins can function similarly to the better studied α-, β1-, and β2-syntrophins; i.e. syntrophins act as adaptor molecules to link signaling and membrane proteins to dystrophin and dystrobrevin, the integral components of the dystrophin-glycoprotein complex that are required to maintain muscle integrity (Mokri and Engel, 1975; Petrof et al., 1993; Ahn et al., 1996; Piluso et al., 2000; Ou et al, 2003;). Thus, in a similar fashion, STN-2/γ-syntrophin may provide SAX-7 linkage to dystrophin and the associated protein complex.
Of the mammalian L1CAMs, only NrCAM and neurofascin contain a type I PDZ-binding motif (Fig 1), thus raising the possibility that γ-syntrophin may also interact with these L1CAMs. Loss of dystrophin in humans causes Duchenne muscular dystrophy, a progressive muscle degeneration disease (Koenig et al., 1987; Medori et al., 1989). Interestingly, about a third of Duchenne muscular dystrophy patients exhibit mental retardation, impaired cognitive function, and increased incidence of neuropsychiatric disorders, including autism (Lenk et al., 1993; Wibawa et al., 2000; Cotton et al., 2005; Wu et al., 2005). Despite these neurological symptoms, dystrophin studies have largely focused on dystrophin function in muscles. It is intriguing to speculate on the possible interplay between the L1CAM and dystrophin pathways, particularly in mental retardation and autism, which are manifested in L1CAM-associated disorders and Duchenne muscular dystrophy.
PERSPECTIVES
Functional relevance
The described findings underscore the conservation of L1CAM functions and mechanisms from C. elegans to human as well as illustrate the power of using C. elegans to uncover novel functional interactions with L1CAMs. The finding that γ-syntrophin binds and regulates SAX-7 reveals a novel mechanism that may be conserved in mammalian L1CAMs. Multiple molecular interactions with mammalian L1CAMs also have been identified in yeast-two-hybrid and peptide-binding screens, but the functional relevance for many of them have yet to be demonstrated. For example, SAP (synapse-associated protein) 97 and SAP102 are PDZ proteins that were identified as interactors of NrCAM (Davey et al., 2005; Dirks et al., 2006), but it is presently not clear how these interactions regulate NrCAM activities. The accessible genetics of C. elegans provides a more convenient medium to test the role of these NrCAM interactions. The SAP family is represented in C. elegans by a single gene, dlg-1. Interestingly, dlg-1 has no apparent neuronal role but functions in epithelial junction formation (Bossinger et al., 2001; Firestein and Rongo, 2001). Of the mammalian SAP family members, only SAP97 is expressed in epithelia like C. elegans DLG-1 (reviewed in Fujita and Kurachi, 2000). A potential interaction between SAX-7 and DLG-1 suggests a role for SAX-7 in epithelial junctions; such a role would not be completely unexpected, as phosphorylated SAX-7 is localized to epithelial junctions (Chen et al., 2001). Moreover, a role in the epithelial septate junctions was previously defined for Drosophila neuroglian (Genova and Fehon, 2003).
In C. elegans, the DLG-1-containing epithelial junction is distinct from the more apical adherens junction mediated by the cadherin-catenin complex. This distinct DLG-1-containing junction also contains proteins typically found in Drosophila septate junctions (Knust and Bossinger, 2002; Lynch and Hardin, 2009). Synergistic regulation of cell adhesion by DLG-1 and the cadherin complex (McMahon et al., 2001) suggests the presence of an as-yet-unidentified adhesion molecule that likely binds DLG-1 in this epithelial junction. SAX-7 fits the profile for such a molecule, based on the SAP97 interaction with NrCAM and the role for Drosophila neuroglian in the septate junctions. Additional studies are required to verify this possibility.
The contribution of the FERM-binding motif to SAX-7 function indicates regulation of SAX-7 by a FERM protein (Zhou et al., 2008). In mammals, ezrin interacts with both L1 and neurofascin. Moreover, this interaction appears to regulate axon branching (Dickson et al., 2002; Cheng et al., 2005; Gunn-Moore et al., 2006). Interestingly, sax-7 mutant animals show ectopic axon branching (Fig 2Cv, Wang et al., 2005), raising the possibility that interaction with the ezrin homolog, ERM-1, or another of the 16 FERM proteins predicted in the C. elegans genome (Göbel et al., 2004; van Fürden et al., 2004) may similarly regulate SAX-7 function in axon branching.
Coordination of L1CAM interactions
An important aspect of understanding how L1CAMs function is to determine how the identified interactions are coordinated. For example, do these proteins bind to a specific L1CAM in the same cell and if so, do they bind simultaneously? If the proteins do not bind concurrently, what mechanisms orchestrate the interactions? In C. elegans, SAX-7 is required in neurons and the adjacent hypodermis and body-wall muscles for VNC neuronal position maintenance (Wang et al, 2005). STN-2/γ-syntrophin is expressed in both neurons and body-wall muscles (Zhou et al., 2008) while UNC-44/ankyrin is widely expressed, similar to SAX-7 (Chen et al., 2001). The co-expression of both STN-2/γ-syntrophin and UNC-44/ankyrin in neurons and muscle raises the question of whether both proteins concurrently bind SAX-7 or whether a mechanism exists to coordinate binding of each protein. The fact that both proteins are known to associate with distinct cytoskeletons – UNC-44 ankyrin with spectrin and STN-2 γ-syntrophin with dystrophin (fig 3) – suggests a mechanism to confer distinct roles and activity to SAX-7.
Genetic modifiers in the CRASH syndrome
The clinical symptoms of the neurological disorder CRASH are highly variable among interfamilial as well as intrafamilial members (Fransen et al., 1995). Within a family, hydrocephalus can be presented with varying severity in some affected male members but not at all in others (Jouet et al., 1994). Different degrees of hydrocephalus are also seen in L1 knockout and L1-6D knock-in mice that are bred in the C57BL/6J background but not the 129/Sv background (Dahme et al., 1997; Cohen et al., 1998; Fransen et al., 1998; Rolf et al., 2001; Itoh et al., 2004), thus suggesting the presence of modifier genes in the C57BL/6J strain that genetically interact with L1. An extensive genetic screen for L1 modifiers was recently performed; L1-6D knock-in mice were used in this screen because they are fertile, unlike L1 knockout mice (Tapanes-Castillo et al., 2009). While the identity of the L1 modifier(s) requires finer mapping, single nucleotide polymorphism analysis narrowed the genomic region harboring the genetic L1 modifier(s). Candidate L1 modifiers includes a polycomb-like transcription factor, Mtf2, which when knocked out in mice, can result in the development of hydrocephalus (Wang et al., 2007). Because the L1-6D protein still retains some activity (Itoh et al., 2004), candidate modifiers for L1-6D mice can include genes that regulate L1 function as well as those that have overlapping L1 functions that can compensate for the loss of L1.
Genetic modifiers for NrCAM also exist. In a mutagenesis screen for genes underlying peripheral neuropathy, a mutant strain isolated for its dramatic adult-onset hindlimb paralysis phenotype was determined to be a double mutant for NrCAM and Lpin1, a phosphatidate phosphatase that functions in lipid metabolism and adipogenesis. Both genes interact synergistically to cause muscle wasting in the hind limbs, a phenotype that is not seen in either single mutant (Douglas et al., 2009). While the mechanism underlying this synthetic phenotype is not clear, the interaction of two seemingly unrelated genes points to the importance of identifying L1CAM genetic modifiers to better define L1CAM roles as well as the context within which L1CAMs act.
The broad expression of SAX-7 as early as the two-cell staged embryo suggests additional SAX-7 roles other than maintaining neuronal positioning. Indeed, sax-7 mutant animals can also exhibit ectopic axon branching (Fig 2Cv) and a twisted pharynx phenotype (Fig 2B), indicating a role for SAX-7 in regulating axon branching and pharyngeal morphogenesis (Wang et al., 2005; Axäng et al., 2007). Additional SAX-7 functions may not be readily apparent because of genes with overlapping functions. Mutations in such genes would cause a synthetic phenotype only in conjunction with the loss of sax-7 function. The previously reported aldicarb resistance exhibited by sax-7(eq1) animals (Wang et al., 2005) is in fact caused by a genetic interaction between sax-7 and a closely linked second-site mutation (Yochem and Chen, unpublished), thus revealing a sax-7 modifier gene as well as a novel role for sax-7. Although aldicarb resistance is a phenotype commonly associated with defective synaptic transmission in C. elegans (Jorgensen et al., 1995; Miller et al., 1996; Rand and Russell, 1985), the process underlying this aldicarb resistance in animals that are mutant for sax-7 and the interacting locus has yet to be determined. It is interesting to note that a role in synapse formation has been defined for Drosophila neuroglian and mammalian L1 (Godenschwege et al., 2006).
Synthetic screens to identify genetic redundancies have been successfully performed in C. elegans using classical forward genetic screens (e.g. Ferguson and Horvitz, 1989; Mani and Fay, 2009). The ability to perform RNAi on a whole organismal level, together with availability of RNAi libraries encompassing 94% of ∼19,000 genes encoded in the C. elegans genome, has made it possible to conduct high throughput genome-wide RNAi screens in C. elegans (Kamath et al., 2003; Rual et al., 2004), thus dramatically facilitating the identification of genetic interactions and modifiers on a comprehensive scale (Lehner et al., 2006a; Lehner et al., 2006b; Suzuki and Han, 2006). An extensive screen for additional sax-7 interacting genes can similarly be performed using RNAi, which provides an obvious advantage of easily identifying a sax-7-interacting gene based on the sequence of the RNAi clone, thus eliminating the time-consuming process of mapping and cloning that is typical of a classical genetic screen.
It is clear that the CRASH disorder joins a growing number of monogenic diseases for which the phenotypes cannot all be accounted for by mutations at a single locus. For example, phenotypic variation in the monogenic disorder, cystic fibrosis, can be attributed to genetic modifiers that play considerable roles in determining the severity of the disease (Collaco et al., 2008). As there is evidence to suggest that modifier genes identified in one organism are likely to similarly function in the same context in another organism (reviewed in Lehner, 2007), together with the conservation of LAD-2 and SAX-7 mechanistic roles as vertebrate L1CAMs, C. elegans presents a powerful in vivo discovery system for dissecting the underpinnings of the CRASH disorder as well as other L1CAM-associated diseases.
Acknowledgements
We thank Claire Benard and Marc Pilon for contributing the respective micrographs of the PVQ axon flipping and twisted pharynx phenotypes exhibited by sax-7 mutant animals, Craig Magie for providing unpublished findings on L1CAMs in N. vectensis, and Ann Rougvie for constructive editorial suggestions. L. Chen is supported by grant NS045873 from the National Institute of Neurological Disorders and Stroke.
References
- Adams ME, Dwyer TM, Dowler LL, White RA, Froehner SC. Mouse alpha 1- and beta 2-syntrophin gene structure, chromosome localization, and homology with a discs large domain. J.Biol.Chem. 1995;270:25859–25865. doi: 10.1074/jbc.270.43.25859. [DOI] [PubMed] [Google Scholar]
- Ahn AH, Freener CA, Gussoni E, Yoshida M, Ozawa E, Kunkel LM. The three human syntrophin genes are expressed in diverse tissues, have distinct chromosomal locations, and each bind to dystrophin and its relatives. J.Biol.Chem. 1996;271:2724–2730. doi: 10.1074/jbc.271.5.2724. [DOI] [PubMed] [Google Scholar]
- Asou H, Miura M, Kobayashi M, Uyemura K. The cell adhesion molecule L1 has a specific role in neural cell migration. Neuroreport. 1992;3:481–484. doi: 10.1097/00001756-199206000-00006. [DOI] [PubMed] [Google Scholar]
- Aurelio O, Hall DH, Hobert O. Immunoglobulin-domain proteins required for maintenance of ventral nerve cord organization. Science. 2002;295:686–690. doi: 10.1126/science.1066642. [DOI] [PubMed] [Google Scholar]
- Axäng C, Rauthan M, Hall DH, Pilon M. The twisted pharynx phenotype in C. elegans. BMC Dev.Biol. 2007;7:61. doi: 10.1186/1471-213X-7-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee S, Pillai AM, Paik R, Li J, Bhat MA. Axonal ensheathment and septate junction formation in the peripheral nervous system of Drosophila. J Neurosci. 2006;26:3319–3329. doi: 10.1523/JNEUROSCI.5383-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Nawabi H, Moret F, Yaron A, Weaver E, Bozon M, Abouzid K, Guan JL, Tessier-Lavigne M, Lemmon V, Castellani V. FAK-MAPK-dependent adhesion disassembly downstream of L1 contributes to semaphorin3A-induced collapse. EMBO J. 2008;27:1549–1562. doi: 10.1038/emboj.2008.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benard C, Tjoe N, Boulin T, Recio J, Hobert O. The Small, Secreted Immunoglobulin Protein ZIG-3 Maintains Axon Position in Caenorhabditis elegans. Genetics. 2009;183:917–927. doi: 10.1534/genetics.109.107441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benard CY, Boyanov A, Hall DH, Hobert O. DIG-1, a novel giant protein, non-autonomously mediates maintenance of nervous system architecture. Development. 2006;133:3329–3340. doi: 10.1242/dev.02507. [DOI] [PubMed] [Google Scholar]
- Bieber AJ, Snow PM, Hortsch M, Patel NH, Jacobs JR, Traquina ZR, Schilling J, Goodman CS. Drosophila neuroglian: a member of the immunoglobulin superfamily with extensive homology to the vertebrate neural adhesion molecule L1. Cell. 1989;59:447–460. doi: 10.1016/0092-8674(89)90029-9. [DOI] [PubMed] [Google Scholar]
- Bossinger O, Klebes A, Segbert C, Theres C, Knust E. Zonula adherens formation in Caenorhabditis elegans requires dlg-1, the homologue of the Drosophila gene discs large. Dev.Biol. 2001;230:29–42. doi: 10.1006/dbio.2000.0113. [DOI] [PubMed] [Google Scholar]
- Buhusi M, Schlatter MC, Demyanenko GP, Thresher R, Maness PF. L1 interaction with ankyrin regulates mediolateral topography in the retinocollicular projection. J.Neurosci. 2008;28:177–188. doi: 10.1523/JNEUROSCI.3573-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bülow HE, Boulin T, Hobert O. Differential functions of the C. elegans FGF receptor in axon outgrowth and maintenance of axon position. Neuron. 2004;42:367–374. doi: 10.1016/s0896-6273(04)00246-6. [DOI] [PubMed] [Google Scholar]
- Castellani V, Chedotal A, Schachner M, Faivre-Sarrailh C, Rougon G. Analysis of the L1-deficient mouse phenotype reveals cross-talk between Sema3A and L1 signaling pathways in axonal guidance. Neuron. 2000;27:237–249. doi: 10.1016/s0896-6273(00)00033-7. [DOI] [PubMed] [Google Scholar]
- Castellani V, DeAngelis E, Kenwrick S, Rougon G. Cis and trans interactions of L1 with neuropilin-1 control of axon responses to semaphorin 3A. EMBO. 2002;21:6348–6357. doi: 10.1093/emboj/cdf645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles P, Reynolds R, Seilhean D, Rougon G, Aigrot MS, Niezgoda A, Zalc B, Lubetzki C. Re-expression of PSA-NCAM by demyelinated axons: an inhibitor of remyelination in multiple sclerosis? . Brain. 2002;125:1972–1979. doi: 10.1093/brain/awf216. [DOI] [PubMed] [Google Scholar]
- Chen L, Ong B, Bennett V. LAD-1, the Caenorhabditis elegans L1CAM homologue, participates in embryonic and gonadal morphogenesis and is a substrate for fibroblast growth factor receptor pathway-dependent phosphotyrosine-based signaling. J.Cell Biol. 2001;154:841–855. doi: 10.1083/jcb.200009004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng L, Itoh K, Lemmon V. L1-mediated branching is regulated by two ezrin-radixin-moesin (ERM)-binding sites, the RSLE region and a novel juxtamembrane ERM-binding region. J Neurosci. 2005;25:395–403. doi: 10.1523/JNEUROSCI.4097-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen NR, Taylor JS, Scott LB, Guillery RW, Soriano P, Furley AJ. Errors in corticospinal axon guidance in mice lacking the neural cell adhesion molecule L1. Curr.Biol. 1998;8:26–33. doi: 10.1016/s0960-9822(98)70017-x. [DOI] [PubMed] [Google Scholar]
- Collaco JM, Vanscoy L, Bremer L, McDougal K, Blackman SM, Bowers A, Naughton K, Jennings J, Ellen J, Cutting GR. Interactions between secondhand smoke and genes that affect cystic fibrosis lung disease. JAMA. 2008;299:417–424. doi: 10.1001/jama.299.4.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conacci-Sorrell ME, Ben-Yedidia T, Shtutman M, Feinstein E, Einat P, Ben-Ze'ev A. Nr-CAM is a target gene of the beta-catenin/LEF-1 pathway in melanoma and colon cancer and its expression enhances motility and confers tumorigenesis. Genes Dev. 2002;16:2058–2072. doi: 10.1101/gad.227502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotton SM, Voudouris NJ, Greenwood KM. Association between intellectual functioning and age in children and young adults with Duchenne muscular dystrophy: further results from a meta-analysis. Dev.Med.Child Neurol. 2005;47:257–265. doi: 10.1017/s0012162205000496. [DOI] [PubMed] [Google Scholar]
- Dahme M, Bartsch U, Martini R, Anliker B, Schachner M, Mantei N. Disruption of the mouse L1 gene leads to malformations of the nervous system. Nat.Genet. 1997;17:346–349. doi: 10.1038/ng1197-346. [DOI] [PubMed] [Google Scholar]
- Davey F, Hill M, Falk J, Sans N, Gunn-Moore FJ. Synapse associated protein 102 is a novel binding partner to the cytoplasmic terminus of neurone-glial related cell adhesion molecule. J.Neurochem. 2005;94:1243–1253. doi: 10.1111/j.1471-4159.2005.03271.x. [DOI] [PubMed] [Google Scholar]
- Davis JQ, Bennett V. Ankyrin-binding activity of nervous system cell adhesion molecules expressed in adult brain. J.Cell Sci.Suppl. 1993;17:109–117. doi: 10.1242/jcs.1993.supplement_17.16. [DOI] [PubMed] [Google Scholar]
- Davis JQ, Bennett V. Ankyrin binding activity shared by the neurofascin/L1/NrCAM family of nervous system cell adhesion molecules. J.Biol.Chem. 1994;269:27163–27166. [PubMed] [Google Scholar]
- Debiec H, Kutsche M, Schachner M, Ronco P. Abnormal renal phenotype in L1 knockout mice: a novel cause of CAKUT. Nephrol.Dial.Transplant. 2002;(17 Suppl 9):42–44. doi: 10.1093/ndt/17.suppl_9.42. [DOI] [PubMed] [Google Scholar]
- Demyanenko GP, Shibata Y, Maness PF. Altered distribution of dopaminergic neurons in the brain of L1 null mice. Brain Res.Dev.Brain Res. 2001;126:21–30. doi: 10.1016/s0165-3806(00)00129-2. [DOI] [PubMed] [Google Scholar]
- Demyanenko GP, Schachner M, Anton E, Schmid R, Feng G, Sanes J, Maness PF. Close homolog of L1 modulates area-specific neuronal positioning and dendrite orientation in the cerebral cortex. Neuron. 2004;44:423–437. doi: 10.1016/j.neuron.2004.10.016. [DOI] [PubMed] [Google Scholar]
- Dickson TC, Mintz CD, Benson DL, Salton SR. Functional binding interaction identified between the axonal CAM L1 and members of the ERM family. J Cell Biol. 2002;157:1105–1112. doi: 10.1083/jcb.200111076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirks P, Thomas U, Montag D. The cytoplasmic domain of NrCAM binds to PDZ domains of synapse-associated proteins SAP90/PSD95 and SAP97. Eur.J.Neurosci. 2006;24:25–31. doi: 10.1111/j.1460-9568.2006.04899.x. [DOI] [PubMed] [Google Scholar]
- Douglas DS, Moran JL, Bermingham JR, Jr, Chen XJ, Brindley DN, Soliven B, Beier DR, Popko B. Concurrent Lpin1 and Nrcam mouse mutations result in severe peripheral neuropathy with transitory hindlimb paralysis. J.Neurosci. 2009;29:12089–12100. doi: 10.1523/JNEUROSCI.2029-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faivre-Sarrailh C, Banerjee S, Li J, Hortsch M, Laval M, Bhat MA. Drosophila contactin, a homolog of vertebrate contactin, is required for septate junction organization and paracellular barrier function. Development. 2004;131:4931–4942. doi: 10.1242/dev.01372. [DOI] [PubMed] [Google Scholar]
- Falk JA, Bechara A, Fiore R, Nawabi H, Zhou H, Hoyo-Becerra C, Bozon M, Rougon G, Grumet M, Püschel AW, Sanes JR, Castellani V. Dual functional activity of semaphorin 3B is required for positioning the anterior commissure. Neuron. 2005;48:63–75. doi: 10.1016/j.neuron.2005.08.033. [DOI] [PubMed] [Google Scholar]
- Ferguson EL, Horvitz HR. The multivulva phenotype of certain Caenorhabditis elegans mutants results from defects in two functionally redundant pathways. Genetics. 1989;123:109–121. doi: 10.1093/genetics/123.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firestein BL, Rongo C. DLG-1 is a MAGUK similar to SAP97 and is required for adherens junction formation. Mol.Biol.Cell. 2001;12:3465–3475. doi: 10.1091/mbc.12.11.3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer G, Kunemund V, Schachner M. Neurite outgrowth patterns in cerebellar microexplant cultures are affected by antibodies to the cell surface glycoprotein L1. J.Neurosci. 1986;6:605–612. doi: 10.1523/JNEUROSCI.06-02-00605.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogel M, Gutwein P, Mechtersheimer S, Riedle S, Stoeck A, Smirnov A, Edler L, Ben-Arie A, Huszar M, Altevogt P. L1 expression as a predictor of progression and survival in patients with uterine and ovarian carcinomas. Lancet. 2003;362:869–875. doi: 10.1016/S0140-6736(03)14342-5. [DOI] [PubMed] [Google Scholar]
- Fogel M, Mechtersheimer S, Huszar M, Smirnov A, Abu-Dahi A, Tilgen W, Reichrath J, Georg T, Altevogt P, Gutwein P. L1 adhesion molecule (CD 171) in development and progression of human malignant melanoma. Cancer Lett. 2003;189:237–247. doi: 10.1016/s0304-3835(02)00513-x. [DOI] [PubMed] [Google Scholar]
- Fransen E, Schrander-Stumpel C, Vits L, Coucke P, Van Camp G, Willems PJ. X-linked hydrocephalus and MASA syndrome present in one family are due to a single missense mutation in exon 28 of the L1CAM gene. Hum.Mol.Genet. 1994;3:2255–2256. doi: 10.1093/hmg/3.12.2255. [DOI] [PubMed] [Google Scholar]
- Fransen E, Lemmon V, Van Camp G, Vits L, Coucke P, Willems PJ. CRASH syndrome: clinical spectrum of corpus callosum hypoplasia, retardation, adducted thumbs, spastic paraparesis and hydrocephalus due to mutations in one single gene, L1. Eur.J.Hum.Genet. 1995;3:273–284. doi: 10.1159/000472311. [DOI] [PubMed] [Google Scholar]
- Fransen E, D'Hooge R, Van Camp G, Verhoye M, Sijbers J, Reyniers E, Soriano P, Kamiguchi H, Willemsen R, Koekkoek SK, De Zeeuw CI, De Deyn PP, Van der Linden A, Lemmon V, Kooy RF, Willems PJ. L1 knockout mice show dilated ventricles, vermis hypoplasia and impaired exploration patterns. Hum.Mol.Genet. 1998;7:999–1009. doi: 10.1093/hmg/7.6.999. [DOI] [PubMed] [Google Scholar]
- Freigang J, Proba K, Leder L, Diederichs K, Sonderegger P, Welte W. The crystal structure of the ligand binding module of axonin-1/TAG-1 suggests a zipper mechanism for neural cell adhesion. Cell. 2000;101:425–433. doi: 10.1016/s0092-8674(00)80852-1. [DOI] [PubMed] [Google Scholar]
- Friedlander DR, Milev P, Karthikeyan L, Margolis RK, Margolis RU, Grumet M. The neuronal chondroitin sulfate proteoglycan neurocan binds to the neural cell adhesion molecules Ng- CAM/L1/NILE and N-CAM, and inhibits neuronal adhesion and neurite outgrowth. J.Cell Biol. 1994;125:669–680. doi: 10.1083/jcb.125.3.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frints SG, Marynen P, Hartmann D, Fryns JP, Steyaert J, Schachner M, Rolf B, Craessaerts K, Snellinx A, Hollanders K, D'Hooge R, De Deyn PP, Froyen G. CALL interrupted in a patient with non-specific mental retardation: gene dosage-dependent alteration of murine brain development and behavior. Hum.Mol.Genet. 2003;12:1463–1474. doi: 10.1093/hmg/ddg165. [DOI] [PubMed] [Google Scholar]
- Fujita A, Kurachi Y. SAP family proteins. Biochem Biophys Res Commun. 2000;269(1):1–6. doi: 10.1006/bbrc.1999.1893. 5. [DOI] [PubMed] [Google Scholar]
- Garver TD, Ren Q, Tuvia S, Bennett V. Tyrosine phosphorylation at a site highly conserved in the L1 family of cell adhesion molecules abolishes ankyrin binding and increases lateral mobility of neurofascin. J.Cell Biol. 1997;137:703–714. doi: 10.1083/jcb.137.3.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genova JL, Fehon RG. Neuroglian, Gliotactin, and the Na+/K+ ATPase are essential for septate junction function in Drosophila. J. Cell Biol. 2003;161:979–989. doi: 10.1083/jcb.200212054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobel V, Barrett PL, Hall DH, Fleming JT. Lumen morphogenesis in C. elegans requires the membrane-cytoskeleton linker erm-1. Dev.Cell. 2004;6:865–873. doi: 10.1016/j.devcel.2004.05.018. [DOI] [PubMed] [Google Scholar]
- Godenschwege TA, Kristiansen LV, Uthaman SB, Hortsch M, Murphey RK. A conserved role for Drosophila Neuroglian and human L1-CAM in central-synapse formation. Curr Biol. 2006;16:12–23. doi: 10.1016/j.cub.2005.11.062. [DOI] [PubMed] [Google Scholar]
- Gouveia RM, Gomes CM, Sousa M, Alves PM, Costa J. Kinetic analysis of L1 homophilic interaction: role of the first four immunoglobulin domains and implications on binding mechanism. J.Biol.Chem. 2008;283:28038–28047. doi: 10.1074/jbc.M804991200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grumet M, Mauro V, Burgoon MP, Edelman GM, Cunningham BA. Structure of a new nervous system glycoprotein, Nr-CAM, and its relationship to subgroups of neural cell adhesion molecules. J.Cell Biol. 1991;113:1399–1412. doi: 10.1083/jcb.113.6.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunn-Moore FJ, Hill M, Davey F, Herron LR, Tait S, Sherman D, Brophy PJ. A functional FERM domain binding motif in neurofascin. Mol Cell Neurosci. 2006;33:441–446. doi: 10.1016/j.mcn.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Hall H, Carbonetto S, Schachner M. L1/HNK-1 carbohydrate- and beta 1 integrin-dependent neural cell adhesion to laminin-1. J.Neurochem. 1997;68:544–553. doi: 10.1046/j.1471-4159.1997.68020544.x. [DOI] [PubMed] [Google Scholar]
- Hall SG, Bieber AJ. Mutations in the Drosophila neuroglian cell adhesion molecule affect motor neuron pathfinding and peripheral nervous system patterning. J Neurobiol. 1997;32:325–340. [PubMed] [Google Scholar]
- Haspel J, Grumet M. The L1CAM extracellular region: a multi-domain protein with modular and cooperative binding modes. Front.Biosci. 2003;8:s1210–s1225. doi: 10.2741/1108. [DOI] [PubMed] [Google Scholar]
- He Y, Jensen GJ, Bjorkman PJ. Cryo-electron tomography of homophilic adhesion mediated by the neural cell adhesion molecule L1. Structure. 2009;17:460–471. doi: 10.1016/j.str.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm J, Hillenbrand R, Steuber V, Bartsch U, Moos M, Lubbert H, Montag D, Schachner M. Structural features of a close homologue of L1 (CHL1) in the mouse: a new member of the L1 family of neural recognition molecules. Eur.J.Neurosci. 1996;8:1613–1629. doi: 10.1111/j.1460-9568.1996.tb01306.x. [DOI] [PubMed] [Google Scholar]
- Itoh K, Cheng L, Kamei Y, Fushiki S, Kamiguchi H, Gutwein P, Stoeck A, Arnold B, Altevogt P, Lemmon V. Brain development in mice lacking L1-L1 homophilic adhesion. J.Cell Biol. 2004;165:145–154. doi: 10.1083/jcb.200312107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins SM, Kizhatil K, Kramarcy NR, Sen A, Sealock R, Bennett V. FIGQY phosphorylation defines discrete populations of L1 cell adhesion molecules at sites of cell-cell contact and in migrating neurons. J.Cell.Sci. 2001;114:3823–3835. doi: 10.1242/jcs.114.21.3823. [DOI] [PubMed] [Google Scholar]
- Jorgensen EM, Hartwieg E, Schuske K, Nonet ML, Jin Y, Horvitz HR. Defective recycling of synaptic vesicles in synaptotagmin mutants of Caenorhabditis elegans. Nature. 1995;378:196–199. doi: 10.1038/378196a0. [DOI] [PubMed] [Google Scholar]
- Jouet M, Rosenthal A, Armstrong G, MacFarlane J, Stevenson R, Paterson J, Metzenberg A, Ionasescu V, Temple K, Kenwrick S. X-linked spastic paraplegia (SPG1), MASA syndrome and X-linked hydrocephalus result from mutations in the L1 gene. Nat.Genet. 1994;7:402–407. doi: 10.1038/ng0794-402. [DOI] [PubMed] [Google Scholar]
- Kalus I, Schnegelsberg B, Seidah NG, Kleene R, Schachner M. The proprotein convertase PC5A and a metalloprotease are involved in the proteolytic processing of the neural adhesion molecule L1. J Biol Chem. 2003;278:10381–10388. doi: 10.1074/jbc.M208351200. [DOI] [PubMed] [Google Scholar]
- Kamath RS, Ahringer J. Genome-wide RNAi screening in Caenorhabditis elegans. Methods. 2003;30:313–321. doi: 10.1016/s1046-2023(03)00050-1. [DOI] [PubMed] [Google Scholar]
- Kizhatil K, Wu YX, Sen A, Bennett V. A new activity of doublecortin in recognition of the phospho-FIGQY tyrosine in the cytoplasmic domain of neurofascin. J.Neurosci. 2002;22:7948–7958. doi: 10.1523/JNEUROSCI.22-18-07948.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knust E, Bossinger O. Composition and formation of intercellular junctions in epithelial cells. Science. 2002;298:1955–1959. doi: 10.1126/science.1072161. [DOI] [PubMed] [Google Scholar]
- Koenig M, Hoffman EP, Bertelson CJ, Monaco AP, Feener C, Kunkel LM. Complete cloning of the Duchenne muscular dystrophy (DMD) cDNA and preliminary genomic organization of the DMD gene in normal and affected individuals. Cell. 1987;50:509–517. doi: 10.1016/0092-8674(87)90504-6. [DOI] [PubMed] [Google Scholar]
- Kulahin N, Li S, Hinsby A, Kiselyov V, Berezin V, Bock E. Fibronectin type III (FN3) modules of the neuronal cell adhesion molecule L1 interact directly with the fibroblast growth factor (FGF) receptor. Mol.Cell.Neurosci. 2008;37:528–536. doi: 10.1016/j.mcn.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Lagenaur C, Lemmon V. An L1-like molecule, the 8D9 antigen, is a potent substrate for neurite extension. Proc.Natl.Acad.Sci.U.S.A. 1987;84:7753–7757. doi: 10.1073/pnas.84.21.7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehner B. Modelling genotype-phenotype relationships and human disease with genetic interaction networks. J.Exp.Biol. 2007;210:1559–1566. doi: 10.1242/jeb.002311. [DOI] [PubMed] [Google Scholar]
- Lehner B, Crombie C, Tischler J, Fortunato A, Fraser AG. Systematic mapping of genetic interactions in Caenorhabditis elegans identifies common modifiers of diverse signaling pathways. Nat.Genet. 2006;38:896–903. doi: 10.1038/ng1844. [DOI] [PubMed] [Google Scholar]
- Lehner B, Tischler J, Fraser AG. RNAi screens in Caenorhabditis elegans in a 96-well liquid format and their application to the systematic identification of genetic interactions. Nat.Protoc. 2006;1:1617–1620. doi: 10.1038/nprot.2006.245. [DOI] [PubMed] [Google Scholar]
- Lele Z, Folchert A, Concha M, Rauch GJ, Geisler R, Rosa F, Wilson SW, Hammerschmidt M, Bally-Cuif L. parachute/n-cadherin is required for morphogenesis and maintainted integrity of the zebrafish neural tube. Development. 2002;129:3281–3294. doi: 10.1242/dev.129.14.3281. [DOI] [PubMed] [Google Scholar]
- Lenk U, Hanke R, Kraft U, Grade K, Grunewald I, Speer A. Non-isotopic analysis of single strand conformation polymorphism (SSCP) in the exon 13 region of the human dystrophin gene. J.Med.Genet. 1993;30:951–954. doi: 10.1136/jmg.30.11.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindner J, Rathjen FG, Schachner M. L1 mono- and polyclonal antibodies modify cell migration in early postnatal mouse cerebellum. Nature. 1983;305:427–430. doi: 10.1038/305427a0. [DOI] [PubMed] [Google Scholar]
- Luthi A, Mohajeri H, Schachner M, Laurent JP. Reduction of hippocampal long-term potentiation in transgenic mice ectopically expressing the neural cell adhesion molecule L1 in astrocytes. J.Neurosci.Res. 1996;46:1–6. doi: 10.1002/(SICI)1097-4547(19961001)46:1<1::AID-JNR1>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Lynch AM, Hardin J. The assembly and maintenance of epithelial junctions in C. elegans. Front Biosci. 2009;14:1414–1432. doi: 10.2741/3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani K, Fay DS. A mechanistic basis for the coordinated regulation of pharyngeal morphogenesis in Caenorhabditis elegans by LIN-35/Rb and UBC-18-ARI-1. PLoS Genet. 2009;5:e1000510. doi: 10.1371/journal.pgen.1000510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maretzky T, Schulte M, Ludwig A, Rose-John S, Blobel C, Hartmann D, Altevogt P, Saftig P, Reiss K. L1 is sequentially processed by two differently activated metalloproteases and presenilin/gamma-secretase and regulates neural cell adhesion, cell migration, and neurite outgrowth. Mol Cell Biol. 2005;25:9040–9053. doi: 10.1128/MCB.25.20.9040-9053.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marui T, Funatogawa I, Koishi S, Yamamoto K, Matsumoto H, Hashimoto O, Nanba E, Nishida H, Sugiyama T, Kasai K, Watanabe K, Kano Y, Sasaki T, Kato N. Association of the neuronal cell adhesion molecule (NRCAM) gene variants with autism. Int.J.Neuropsychopharmacol. 2009;12:1–10. doi: 10.1017/S1461145708009127. [DOI] [PubMed] [Google Scholar]
- Masai I, Lele Z, Yamaguchi M, Komori A, Nakata A, Nishiwaki Y, Wada H, Tanaka H, Nojima Y, Hammerschmidt M, Wilson SW, Okamoto H. N-cadherin mediates retinal lamination, maintenance of forebrain compartments and patterning of retinal neurites. Development. 2003;130:2479–2494. doi: 10.1242/dev.00465. [DOI] [PubMed] [Google Scholar]
- McMahon L, Legouis R, Vonesch JL, Labouesse M. Assembly of C. elegans apical junctions involves positioning and compaction by LET-413 and protein aggregation by the MAGUK protein DLG-1. J Cell Sci. 2001;114:2265–2277. doi: 10.1242/jcs.114.12.2265. [DOI] [PubMed] [Google Scholar]
- Mechtersheimer S, Gutwein P, Agmon-Levin N, Stoeck A, Oleszewski M, Riedle S, Postina R, Fahrenholz R, Fogel M, Lemmon V, Altevogt P. Ectodomain shedding of L1 adhesion molecule promotes cell migration by autocrine binding to integrins. J Cell Biol. 2001;155:661–673. doi: 10.1083/jcb.200101099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medori R, Brooke MH, Waterston RH. Genetic abnormalities in Duchenne and Becker dystrophies: clinical correlations. Neurology. 1989;39:461–465. doi: 10.1212/wnl.39.4.461. [DOI] [PubMed] [Google Scholar]
- Miller KG, Alfonso A, Nguyen M, Crowell JA, Johnson CD, Rand JB. A genetic selection for Caenorhabditis elegans synaptic transmission mutants. Proc.Natl.Acad.Sci.U.S.A. 1996;93:12593–12598. doi: 10.1073/pnas.93.22.12593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokri B, Engel AG. Duchenne dystrophy: electron microscopic findings pointing to a basic or early abnormality in the plasma membrane of the muscle fiber. Neurology. 1975;25:1111–1120. doi: 10.1212/wnl.25.12.1111. [DOI] [PubMed] [Google Scholar]
- Montag-Sallaz M, Schachner M, Montag D. Misguided axonal projections, neural cell adhesion molecule 180 mRNA upregulation, and altered behavior in mice deficient for the close homolog of L1. Mol.Cell.Biol. 2002;22:7967–7981. doi: 10.1128/MCB.22.22.7967-7981.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moos M, Tacke R, Scherer H, Teplow D, Fruh K, Schachner M. Neural adhesion molecule L1 as a member of the immunoglobulin superfamily with binding domains similar to fibronectin. Nature. 1988;334:701–703. doi: 10.1038/334701a0. [DOI] [PubMed] [Google Scholar]
- More MI, Kirsch FP, Rathjen FG. Targeted ablation of NrCAM or ankyrin-B results in disorganized lens fibers leading to cataract formation. J.Cell Biol. 2001;154:187–196. doi: 10.1083/jcb.200104038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao F, Hudson ML, Suzuki M, Peckler Z, Kurokawa R, Liu Z, Gengyo-Ando K, Nukazuka A, Fujii T, Suto F, Shibata Y, Shioi G, Fujisawa H, Mitani S, Chisholm AD, Takagi S. The PLEXIN PLX-2 and the ephrin EFN-4 have distinct roles in MAB-20/Semaphorin 2A signaling in Caenorhabditis elegans morphogenesis. Genetics. 2007;176:1591–1607. doi: 10.1534/genetics.106.067116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayeem N, Silletti S, Yang X, Lemmon VP, Reisfeld RA, Stallcup WB, Montgomery AM. A potential role for the plasmin(ogen) system in the posttranslational cleavage of the neural cell adhesion molecule L1. J Cell Sci. 1999;112:4739–4749. doi: 10.1242/jcs.112.24.4739. [DOI] [PubMed] [Google Scholar]
- Naus S, Richter M, Wildeboer D, Moss M, Schachner M, Bartsch JW. Ectodomain shedding of the neural recognition molecule CHL1 by the metalloprotease-disintegrin ADAM8 promotes neurite outgrowth and suppresses neuronal cell death. J Biol Chem. 2004;279:16083–16090. doi: 10.1074/jbc.M400560200. [DOI] [PubMed] [Google Scholar]
- Needham LK, Thelen K, Maness PF. Cytoplasmic domain mutations of the L1 cell adhesion molecule reduce L1-ankyrin interactions. J.Neurosci. 2001;21:1490–1500. doi: 10.1523/JNEUROSCI.21-05-01490.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou Y, Strege P, Miller SM, Makielski J, Ackerman M, Gibbons SJ, Farrugia G. Syntrophin gamma 2 regulates SCN5A gating by a PDZ domain-mediated interaction. J.Biol.Chem. 2003;278:1915–1923. doi: 10.1074/jbc.M209938200. [DOI] [PubMed] [Google Scholar]
- Petrof BJ, Shrager JB, Stedman HH, Kelly AM, Sweeney HL. Dystrophin protects the sarcolemma from stresses developed during muscle contraction. Proc.Natl.Acad.Sci.U.S.A. 1993;90:3710–3714. doi: 10.1073/pnas.90.8.3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piluso G, Mirabella M, Ricci E, Belsito A, Abbondanza C, Servidei S, Puca AA, Tonali P, Puca GA, Nigro V. Gamma1- and gamma2-syntrophins, two novel dystrophin-binding proteins localized in neuronal cells. J.Biol.Chem. 2000;275:15851–15860. doi: 10.1074/jbc.M000439200. [DOI] [PubMed] [Google Scholar]
- Pocock R, Benard CY, Shapiro L, Hobert O. Functional dissection of the C. elegans cell adhesion molecule SAX-7, a homologue of human L1. Mol.Cell.Neurosci. 2008;37:56–68. doi: 10.1016/j.mcn.2007.08.014. [DOI] [PubMed] [Google Scholar]
- Putnam NH, Srivastava M, Hellsten U, Dirks B, Chapman J, Salamov A, Terry A, Shapiro H, Lindquist E, Kapitonov VV, Jurka J, Genikhovich G, Grigoriev IV, Lucas SM, Steele RE, Finnerty JR, Technau U, Martindale MQ, Rokhsar DS. Sea anemone genome reveals ancestral eumetazoan gene repertoire and genomic organization. Science. 2007;317:86–94. doi: 10.1126/science.1139158. [DOI] [PubMed] [Google Scholar]
- Rand JB, Russell RL. Molecular basis of drug-resistance mutations in C. elegans. Psychopharmacol.Bull. 1985;21:623–630. [PubMed] [Google Scholar]
- Richmond J. Synaptic function. WormBook. 2005;30:1–14. doi: 10.1895/wormbook.1.69.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolf B, Kutsche M, Bartsch U. Severe hydrocephalus in L1-deficient mice. Brain Res. 2001;891:247–252. doi: 10.1016/s0006-8993(00)03219-4. [DOI] [PubMed] [Google Scholar]
- Rosenthal A, Jouet M, Kenwrick S. Aberrant splicing of neural cell adhesion molecule L1 mRNA in a family with X-linked hydrocephalus. Nat.Genet. 1992;2:107–112. doi: 10.1038/ng1092-107. [DOI] [PubMed] [Google Scholar]
- Rual JF, Ceron J, Koreth J, Hao T, Nicot AS, Hirozane-Kishikawa T, Vandenhaute J, Orkin SH, Hill DE, van den Heuvel S, Vidal M. Toward improving Caenorhabditis elegans phenome mapping with an ORFeome-based RNAi library. Genome Res. 2004;14:2162–2168. doi: 10.1101/gr.2505604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadoul R, Kirchhoff F, Schachner M. A protein kinase activity is associated with and specifically phosphorylates the neural cell adhesion molecule L1. J.Neurochem. 1989;53:1471–1478. doi: 10.1111/j.1471-4159.1989.tb08540.x. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Lustig M, Babiarz J, Furley AJ, Tait S, Brophy PJ, Brown SA, Brown LY, Mason CA, Grumet M. Overlapping functions of the cell adhesion molecules Nr-CAM and L1 in cerebellar granule cell development. J.Cell Biol. 2001;154:1259–1273. doi: 10.1083/jcb.200104122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai K, Migita O, Toru M, Arinami T. An association between a missense polymorphism in the close homologue of L1 (CHL1, CALL) gene and schizophrenia. Mol.Psychiatry. 2002;7:412–415. doi: 10.1038/sj.mp.4000973. [DOI] [PubMed] [Google Scholar]
- Sasakura H, Inada H, Kuhara A, Fusaoka E, Takemoto D, Takeuchi K, Mori I. Maintenance of neuronal positions in organized ganglia by SAX-7, a Caenorhabditis elegans homologue of L1. EMBO J. 2005;24:1477–1488. doi: 10.1038/sj.emboj.7600621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer AW, Kamiguchi H, Wong EV, Beach CM, Landreth G, Lemmon V. Activation of the MAPK signal cascade by the neural cell adhesion molecule L1 requires L1 internalization. J.Biol.Chem. 1999;274:37965–37973. doi: 10.1074/jbc.274.53.37965. [DOI] [PubMed] [Google Scholar]
- Schmid RS, Pruitt WM, Maness PF. A MAP kinase-signaling pathway mediates neurite outgrowth on L1 and requires Src-dependent endocytosis. J.Neurosci. 2000;20:4177–4188. doi: 10.1523/JNEUROSCI.20-11-04177.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schurmann G, Haspel J, Grumet M, Erickson HP. Cell adhesion molecule L1 in folded (horseshoe) and extended conformations. Mol.Biol.Cell. 2001;12:1765–1773. doi: 10.1091/mbc.12.6.1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman DL, Tait S, Melrose S, Johnson R, Zonta B, Court FA, Macklin WB, Meek S, Smith AJ, Cottrell DF, Brophy PJ. Neurofascins are required to establish axonal domains for saltatory conduction. Neuron. 2005;48:737–742. doi: 10.1016/j.neuron.2005.10.019. [DOI] [PubMed] [Google Scholar]
- Su XD, Gastinel LN, Vaughn DE, Faye I, Poon P, Bjorkman PJ. Crystal structure of hemolin: a horseshoe shape with implications for homophilic adhesion. Science. 1998;281:991–995. doi: 10.1126/science.281.5379.991. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Han M. Genetic redundancy masks diverse functions of the tumor suppressor gene PTEN during C. elegans development. Genes Dev. 2006;20:423–428. doi: 10.1101/gad.1378906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapanes-Castillo A, Weaver EJ, Smith RP, Kamei Y, Caspary T, Hamilton-Nelson KL, Slifer SH, Martin ER, Bixby JL, Lemmon VP. A modifier locus on chromosome 5 contributes to L1 cell adhesion molecule X-linked hydrocephalus in mice. Neurogenetics. 2010;11:53–71. doi: 10.1007/s10048-009-0203-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Camp G, Vits L, Coucke P, Lyonnet S, Schrander-Stumpel C, Darby J, Holden J, Munnich A, Willems PJ. A duplication in the L1CAM gene associated with X-linked hydrocephalus. Nat.Genet. 1993;4:421–425. doi: 10.1038/ng0893-421. [DOI] [PubMed] [Google Scholar]
- Van Furden D, Johnson K, Segbert C, Bossinger O. The C. elegans ezrin-radixin-moesin protein ERM-1 is necessary for apical junction remodelling and tubulogenesis in the intestine. Dev.Biol. 2004;272:262–276. doi: 10.1016/j.ydbio.2004.05.012. [DOI] [PubMed] [Google Scholar]
- Volkmer H, Hassel B, Wolff JM, Frank R, Rathjen FG. Structure of the axonal surface recognition molecule neurofascin and its relationship to a neural subgroup of the immunoglobulin superfamily. J.Cell Biol. 1992;118:149–161. doi: 10.1083/jcb.118.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, He F, Xiong W, Gu S, Liu H, Zhang T, Yu X, Chen Y. Polycomb-like-2-deficient mice exhibit abnormal left-right asymmetry. Dev. Dyn. 2007;236:853–861. doi: 10.1002/dvdy.21070. [DOI] [PubMed] [Google Scholar]
- Wang X, Kweon J, Larson S, Chen L. A role for the C. elegans L1CAM homologue lad-1/sax-7 in maintaining tissue attachment. Dev.Biol. 2005;284:273–291. doi: 10.1016/j.ydbio.2005.05.020. [DOI] [PubMed] [Google Scholar]
- Wang X, Zhang W, Cheever T, Schwarz V, Opperman K, Hutter H, Koepp D, Chen L. The C. elegans L1CAM homologue LAD-2 functions as a coreceptor in MAB-20/Sema2 mediated axon guidance. J.Cell Biol. 2008;180:233–246. doi: 10.1083/jcb.200704178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wibawa T, Takeshima Y, Mitsuyoshi I, Wada H, Surono A, Nakamura H, Matsuo M. Complete skipping of exon 66 due to novel mutations of the dystrophin gene was identified in two Japanese families of Duchenne muscular dystrophy with severe mental retardation. Brain Dev. 2000;22:107–112. doi: 10.1016/s0387-7604(99)00126-6. [DOI] [PubMed] [Google Scholar]
- Wright AG, Demyanenko GP, Powell A, Schachner M, Enriquez-Barreto L, Tran TS, Polleux F, Maness PF. Close homolog of L1 and neuropilin 1 mediate guidance of thalamocortical axons at the ventral telencephalon. J.Neurosci. 2007;27:13667–13679. doi: 10.1523/JNEUROSCI.2888-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JY, Kuban KC, Allred E, Shapiro F, Darras BT. Association of Duchenne muscular dystrophy with autism spectrum disorder. J.Child Neurol. 2005;20:790–795. doi: 10.1177/08830738050200100201. [DOI] [PubMed] [Google Scholar]
- Yang M, Adla S, Temburni MK, Patel VP, Lagow EL, Brady OA, Tian J, Boulos MI, Galileo DS. Stimulation of glioma cell motility by expression, proteolysis, and release of the L1 neural cell recognition molecule. Cancer Cell Int. 2009;9:27. doi: 10.1186/1475-2867-9-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zallen JA, Kirch SA, Bargmann CI. Genes required for axon pathfinding and extension in the C. elegans nerve ring. Development. 1999;126:3679–3692. doi: 10.1242/dev.126.16.3679. [DOI] [PubMed] [Google Scholar]
- Zhang X, Davis JQ, Carpenter S, Bennett V. Structural requirements for association of neurofascin with ankyrin. J.Biol.Chem. 1998;273:30785–30794. doi: 10.1074/jbc.273.46.30785. [DOI] [PubMed] [Google Scholar]
- Zhou S, Opperman K, Wang X, Chen L. unc-44 Ankyrin and stn-2 gamma-syntrophin regulate sax-7 L1CAM function in maintaining neuronal positioning in Caenorhabditis elegans. Genetics. 2008;180:1429–1443. doi: 10.1534/genetics.108.091272. [DOI] [PMC free article] [PubMed] [Google Scholar]