Abstract
Purpose
We have previously demonstrated that prostate tumors that highly express Bcl-2 are not only more tumorigenic, but also more angiogenic than low Bcl-2 expressing tumors. Observed increased rates of angiogenesis are likely due to the secretion of multiple factors from the tumor cells.
Experimental design
Human endothelial cells were subjected to exogenous VEGF or conditioned media from PC-3 cells and assayed by several in vitro systems to better characterize the eVects of tumor microenvironment on endothelial cells.
Results
VEGF stimulation increased Bcl-2 expression in human microvascular endothelial cells (HMVECs), at least partially through stabilization of Bcl-2 mRNA transcripts, and protected these cells from apoptosis. These effects were mimicked by treatment of HMVECs with conditioned media from cultured PC-3 prostate tumor cells manipulated to overexpress Bcl-2. Through the use of kinase inhibitors and molecular profiling, several distinct pathways were implicated in the regulation of Bcl-2 in HMVECs, including those involving PI3K/AKT, PKC, mTOR, STAT-1, and IL-8, factors associated with tumor survival and growth.
Conclusions
This study identifies molecular elements of a link between Bcl-2 expression in distinct cell types within a tumor and reaffirms that strategies designed to target Bcl-2 are desirable as they might enhance treatment response through dual effects.
Keywords: Bcl-2, VEGF, Angiogenesis, Cancer, Gene expression, Prostate
Introduction
Previously, we have demonstrated the concomitant overexpression of Bcl-2 and VEGF in PC-3 human prostate xeno-graft tumors, the latter of which may have been responsible for an associated increased rate of angiogenesis [1]. We also observed that the murine neovasculature in Bcl-2 expressing PC-3 xenografts also expressed high Bcl-2 levels. Given the known anti-apoptotic role of Bcl-2, its expression may render endothelial cells resistant to conventional cancer therapeutics. Although the role of Bcl-2 in endothelial cell survival has been investigated, the induction mechanisms and the molecular changes associated with Bcl-2 expression are poorly understood.
In this study, we show that VEGF can induce Bcl-2 expression in human microvascular endothelial cells (HMVEC), and that this is associated with increased proliferation and resistance to apoptosis. This suggests that VEGF can act as a positive mediator linking Bcl-2 expression in both epithelial and endothelial cells. Better understanding of the molecular mechanisms involved in this link would be beneficial because therapies that can interfere with Bcl-2 expression and/or VEGF function may have synergistic effects on tumor progression by limiting the growth and survival of cancerous tumors expressing Bcl-2.
Materials and methods
Cell lines and culture
HUVEC and HMVEC cells (Cambrex, Wakeville, MD) were grown in supplemented EGM-2-MV media (without the addition of VEGF) (Lonza, Pittsburg, PA). Prostate cancer cell lines, PC-3-Bcl-2 and PC-3-Neo [2], were maintained in supplemented DMEM (Mediatech, Inc. Herndon, VA) and grown in standard conditions.
Reagents
Protein kinase C (PKC) inhibitor, RO31-8220 (2.76 mg/ml) was purchased from Calbiochem (San Diego, CA) and suspended in sterile H2O. Phosphoinistol 3 kinase (PI3K) inhibitor, LY294002 (4 mM); p38 inhibitor, SB203580 (1 mg/ml); ERK inhibitor, PD98059 (5 mg/ml); and actinomycin D (1 mg/ml) were purchased from Calbiochem (San Diego, CA) and dissolved in DMSO before use. mTOR inhibitor, rapamycin (1 mM) was purchased from Alexis Biochemical (San Diego, CA) and dissolved in DMSO. Bevacizamub (25 mg/ml) was purchased from Besse Medical (West Chester, OH) and diluted in sterile PBS.
In vitro proliferation assay
HUVEC and HMVEC cells were seeded in 96-well plates at a density of 5 × 103 cells per well and treated with PBS, VEGF at concentrations ranging from 0 to 50 ng/ml or conditioned media from PC-3-Neo or PC-3- prostate cancer cells. After 24 h, 100 μl of 1 mg/ml MTT (Sigma-Aldrich, St. Louis, MO) solution was added and analyzed as previously described [1].
Quantitative PCR analysis of RNA
HUVEC and HMVEC cells were seeded in 12-well plates at 5 × 104 cells per well and treated with DMEM media without serum, conditioned media from PC-3-Bcl-2 cells, conditioned media from PC-3-Neo cells, DMEM media with VEGF (50 ng/ml) or DMEM media with VEGF (50 ng/ml) and bevacizamub (0.125 mg/ml) for 6 h prior to RNA isolation. Similarly, only HMVEC cells were treated with control, VEGF (50 ng/ml) or VEGF in addition to RO31-8220 (10 μM), rapamycin (30 nM), LY294002 (100 nM), SB203580 (10 μM), or PD98059 (50 μM) for 6 h prior to RNA isolation. At the completion of the experiments, RNA was isolated and analyzed as previously described [3].
Western blot analysis
HMVEC cells were seeded in 12-well plates at 5 × 104 cells per well and treated with control, VEGF (50 ng/ml) or conditioned media from PC-3-Neo or PC-3-Bcl-2 for 24 h. Immunoblotting was performed by first incubating the proteins with primary antibodies against Bcl-2 and γ-tubulin (Santa Cruz Biotechnology, Santa Cruz, CA) and then with secondary antibody (Bio-Rad).
mRNA stability assay
HMVEC cells were cultured in 12-well plates at 5 × 104 cells per well with or without VEGF supplemented media (50 ng/ml) for 6 h, and then with the transcription inhibitor actinomycin D (1 μg/ml). At time points ranging from 0 to 24 h, cells were harvested and total RNA isolated. Bcl-2 transcripts in HMVEC were measured as described above.
Statistical analysis
Differences between experimental groups were analyzed for statistical significance using Student's t tests. A value of P < 0.05 was considered significant.
Gene expression microarray analysis
Duplicate cultures of HMVEC cells were seeded in 6-well plates at a density of 1 × 105 cells per well for 24 h. PC-3-Bcl-2 and PC-3-Neo prostate cancer cells were seeded in 75 cm2 flask at a density of 5 × 105 cells per well for 24 h. In two-thirds of the HMVEC cultures, media was decanted and PC-3-Bcl-2 or PC-3-Neo media was added to the HMVEC cultures for 6 h, whereas the other third of the cultures received naïve DMEM. Total RNA was extracted from each of the samples and prepared for hybridization according to the GeneChip Expression Analysis Technical Manual (Affymetrix, Santa Clara, CA) and as previously described [4]. Signals from significantly regulated genes were z-transformed with a mean of 0 and standard deviation of 1, and hierarchical clustered using Cluster and TreeView program [5]. Biological network relationships among significantly regulated genes were explored using Pathway Studio (Ariadne Genomics, Inc., Rockville, MD) and Res-Net mammalian database [6].
Results and discussion
VEGF induces endothelial cell proliferation in vitro
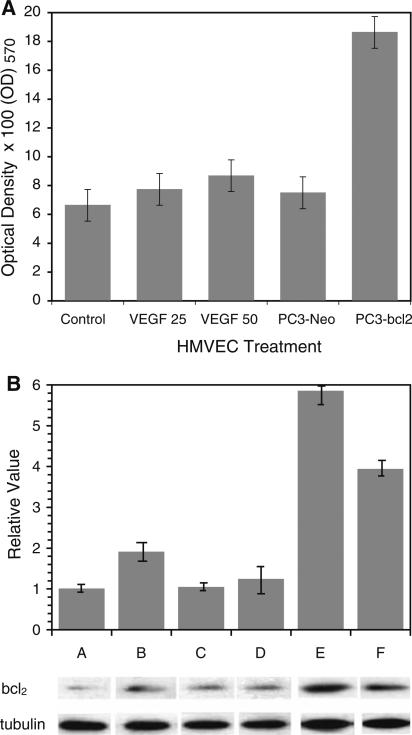
As observed in previous in vitro studies [7, 8], treatment with exogenous VEGF resulted in a significant enhancement of cellular proliferation in HMVEC cells in a dose-dependent manner (Fig. 1a). Previously, our group demonstrated that PC-3-Bcl-2 cells expressed and secreted significantly more VEGF compared to PC-3-Neo cells [9]. With this in mind, next we demonstrated a significant increase in cellular proliferation in HMVEC cells cultured in conditioned media obtained from PC-3-Bcl-2 cells compared to PC-3-Neo cells (P = 0.01) (Fig. 1a). This suggests that VEGF secreted by PC-3-Bcl-2 prostate cancer cells can stimulate endothelial cellular proliferation and promote angiogenesis.
Fig. 1.
Exogenous VEGF and conditioned media from Bcl-2 expressing prostate tumor cells induce endothelial cell proliferation and Bcl-2 expression. a Cell proliferation analysis (MTT assay) of non-treated control HMVEC cells and HMVEC cells treated for 4 days with 25 or 50 ng/ml VEGF and with media conditioned by either PC-3-Neo or PC-3-Bcl-2 cells. Each assay was performed in triplicate, and the mean for all three experiments was calculated. Cellular viability was confirmed by means of the Trypan blue exclusion test. (HUVEC cells subjected to exogenous VEGF or conditioned media from Bcl-2 expressing prostate cancer cells were noted to have an increase in proliferation, data not shown). b Bcl-2 transcript and protein expression in HMVEC cells treated with: A control media; B 50 ng/ml VEGF; C 50 ng/ml VEGF + 125 μg/ml bevacizumab; D PC-3-Neo conditioned media; E PC-3-Bcl-2 conditioned media; F PC-3-Bcl-2 conditioned media + 125 μg/ml bevacizumab. A:B, P < 0.05; A:E P < 0.05; B:C P < 0.05; E:F, P < 0.05. Results represent the mean of triplicates ± SD. The lower panel shows levels of Bcl-2 protein expression compared to that of tubulin by Western analysis (data not shown, HUVEC cells subjected to exogenous VEGF or conditioned media from Bcl-2 expressing prostate cancer cells were not found to over-express Bcl-2 at the RNA or protein level)
In line with the proliferation effect described above, Bcl-2 upregulation was also induced in HMVEC cells subjected to exogenous VEGF (Fig. 1b). This effect has also been demonstrated by other investigators [8]. Similarly, conditioned culture media obtained from PC-3-Bcl-2 prostate cancer cells induced HMVEC cells to over-express Bcl-2 (Fig. 1b). To confirm the contribution of VEGF in the induction of Bcl-2 expression in HMVEC cells subjected to exogenous VEGF or conditioned media from Bcl-2 expressing cells, a monoclonal antibody to VEGF, bevacizumab, was added to the culture media used to treat HMVEC cells. The addition of bevacizumab significantly inhibited the induction of Bcl-2 mRNA by exogenous VEGF and PC-3-Bcl-2 conditioned media (Fig. 1b). Because of this phenomenon, only HMVEC cells were used for subsequent experiments.
VEGF stimulation of HMVEC cells results in the stabilization in Bcl-2 mRNA
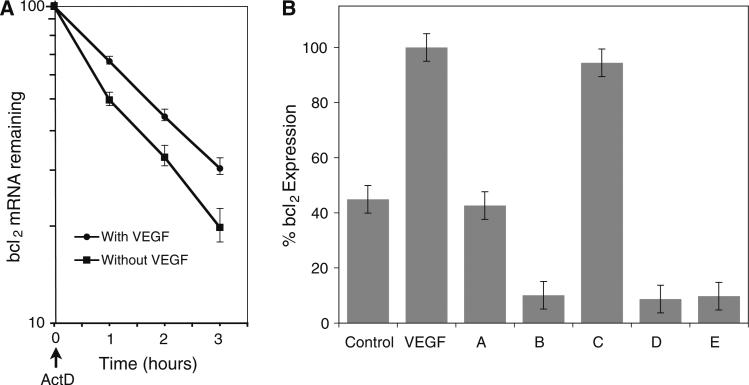
Having shown that VEGF induces Bcl-2 transcript levels, we investigated whether the effect may be mediated through stabilization of Bcl-2 mRNA by performing a mRNA half-life analysis. Analysis of the PCR data shows that HMVEC Bcl-2 mRNA had a half-life of 60 min, and that VEGF treatment extended the half-life to 105 min, *P < 0.05 (Fig. 2a).
Fig. 2.
Exogenous VEGF stabilizes Bcl-2 mRNA and stimulates Bcl-2 expression. a Determination of Bcl-2 mRNA stability in HMVEC cells. HMVECs incubated with or without VEGF (50 ng/ml for 12 h) were treated with actinomycin D (1 μg/ml at 1, 2 and 3 h) prior to RNA harvesting and quantitation of Bcl-2 transcripts by qPCR. The data were used to obtain a best fit linear solution in a semi-log plot and used to determine the relative % remaining mRNA levels for additional samples. Plot shows the percentage of remaining Bcl-2 mRNA relative to the steady-state level detected at time zero. Each variable and time point were performed in triplicate, and the mean for all three experiments was calculated. b Effect of various inhibitors on Bcl-2 mRNA expression in VEGF-stimulated HMVEC cells. A ERK inhibitor PD98059 (50 μM); B mTOR inhibitor, rapamycin (30 nM); C p38 inhibitor, SB203580 (10 μM); D PI3K inhibitor, LY294002 (100 nM); E PKC inhibitor, RO31-8220 (10 μM). Values expressed as a percentage of Bcl-2 levels in 50 ng/ml VEGF-stimulated cells in the absence of inhibitors (VEGF). Control cells were untreated. Results represent the mean of triplicates ± SD. B, D, E versus VEGF, P < 0.05
Inhibition of mTOR, PKC, ERK, and PI3K signaling pathways suppresses VEGF-mediated Bcl-2 expression in endothelial cells
Based on the literature, we hypothesized that upon binding with its receptor, VEGF may activate other signaling molecules in the following pathways to induce Bcl-2 expression: PKC [10], MAPK (ERK1/2) [11], stress-activated protein kinase-2, p38 MAPK [12], or PI3K [11]. To assess whether these specific pathways were involved in the VEGF-mediated induction of Bcl-2 in HMVEC, we analyzed Bcl-2 transcript induction by quantitative RT-PCR in cells stimulated with VEGF treatment and one of five molecular inhibitors to key biologic pathways, namely mTOR, PKC, PI3K, ERK, and p38. As previously illustrated, VEGF stimulation of HMVEC cells resulted in upregulation of Bcl-2 (Fig. 2b). Both LY294002 and RO-31-8220 suppressed VEGF-induced Bcl-2 expression by over 90%. Impressively, rapamycin was able to completely block Bcl-2 upregulation by VEGF in HMVEC cells. This is the first report to show that mTOR is pivotal to VEGF-mediated cellular effects associated with endothelial Bcl-2 expression. PD98059 suppressed Bcl-2 expression by approximately 40%, whereas the one other inhibitor assessed, SB 203580 had no effect on VEGF-mediated Bcl-2 expression. It appears that HMVECs can utilize multiple molecular pathways (mTOR, PKC, PI3K, and ERK, Fig. 2b) to regulate Bcl-2 expression. This redundancy illustrates the importance of Bcl-2 expression in growth and survival of tumor endothelial cells.
The importance of the redundancy that ensures survival of the endothelial cells can have immense therapeutic significance. This redundancy may play a role in radiation resistance or androgen independent prostate cancer. The importance of this redundancy is evident in the recent flourish in the clinical development of over 30 targeted protein kinase inhibitors designed to inhibit angiogenesis, tumor growth and progression [13, 14]. Specifically, Imatinib mesylate [15], Sorafenib [16], Sunitinib malate [17], and Temsirolimus [18] have demonstrated efficiency in solid tumors including renal cell carcinoma.
Bcl-2 expression in HMVEC cells is associated with a unique gene expression profile
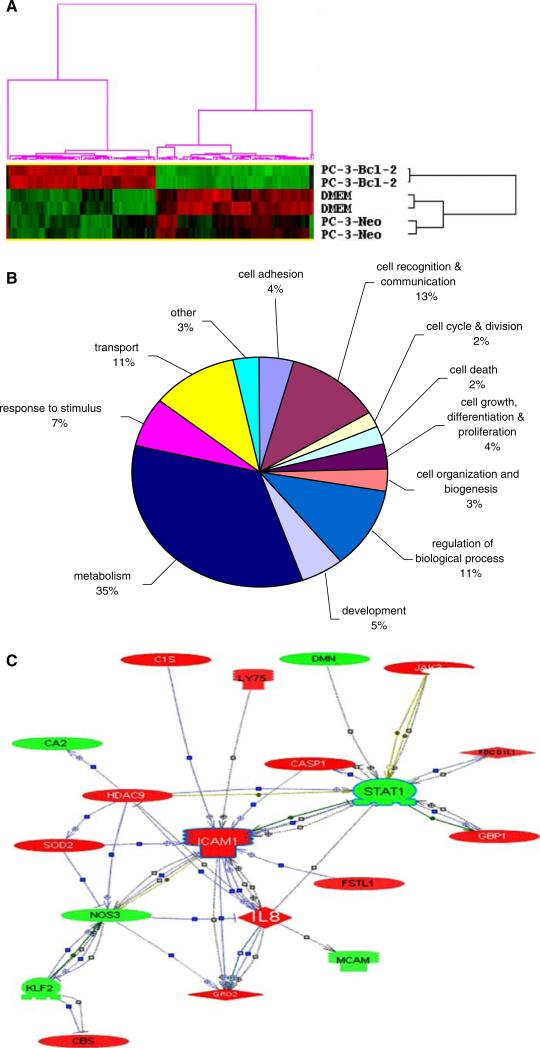
To acquire insight into the broader transcriptome of endothelial cells in the microenvironment of Bcl-2 expressing tumor cells, gene expression profiles of HMVEC (stimulated with PC-3-Bcl-2 conditioned media), HMVEC (stimulated with PC-3-Neo conditioned media), and HMVEC (unconditioned media) cells were evaluated using microarray technology. Using the within-clustering correlation >0.9 as threshold, two distinct clusters were identified for each group and a total of 142 genes were identified as being similarly altered in both groups (Fig. 3a). Of this list of 142 genes, 20 genes of interest are illustrated in Table 1 along with distribution of differentially expressed genes (Fig. 3b). Potential biological links between the regulated genes were revealed by analysis of the 142 gene list using Pathway Studio (Fig. 3c).
Fig. 3.
Functional association of gene clusters and network of genes related to exposure of HMVEC cells to conditioned media from Bcl-2 expressing tumors. RNA extracted from duplicate cultures of treated HMVEC cells was hybridized to Affymetrix U133 Plus 2.0 arrays. Genes absent in all samples were removed from the data set. Due to the small replicate number, local-pooled-error (LPE) test was performed to identify differentially expressed genes between conditions PC-3-Bcl-2 media versus DMEM media and PC-3-Bcl-2 media versus PC-3-Neo media. Genes with bonferroni corrected P value < 0.001 and at least twofold change were considered significantly regulated. Of the 54,613 targets on the array, the expression of 629 genes were significantly differentially expressed (greater than a twofold change and P < 0.001) between PC-3-Bcl-2 stimulated cells and unstimulated cells and 313 genes were significantly differentially expressed between PC-3-Bcl-2 stimulated cells and PC-3-Neo stimulated cells. Two-way hierarchical clustering was performed on the 629 (PC-3-Bcl-2 vs. control) genes and the 313 (PC-3-Bcl-2 vs. PC-3-Neo) genes in order to group together genes with expression patterns that were similar in response to treatment. a K-means clustering of normalized signal values for genes with a significant (P < 0.001) association with treatment of HMVEC with conditioned media obtained from Bcl-2 expressing human prostate cancer PC-3 cells. Each row represents a sample and each column a gene. Red represents a higher level of gene expression and green a lower level, relative to the mean across all samples for each gene. b Functional associations of genes regulated by HMVEC stimulation. Pie chart shows the distribution of 142 differentially expressed genes identified by exposure of human endothelial cells to conditioned media from Bcl-2 expressing PC-3 cells. Gene annotations were derived from the GO Consortium to place genes into functional classes. The percentage of genes in each class is shown. Genes encoding hypothetical proteins or without current annotation are included in ‘others’. The most common cellular functions to be changed in Bcl-2 expressing cells were metabolism (32%), cell communication and recognition (17%) and transport (15%): all functions that are linked to tumor growth. c Interactive network of genes regulated by Bcl-2 in PC-3 prostate tumor cells. A map of protein-binding interactivity between genes revealed to be significantly regulated in stimulated HMVEC endothelial cells was created by Pathway Studio (Ariadne Genomics, Inc., Rockville, MD). Red icons depict genes upregulated by Bcl-2, green icons depict down-regulated genes. Major hubs include ICAM1 (intercellular adhesion molecule 1), STAT1 (signal transducers and activators of transcription 1) and CXCL8 (interleukin-8). All microarray data obtained within this project is available at http://genomics.biotech.ufl.edu/people/rosser/
Table 1.
Genes regulated by Bcl-2 tumoral environment on the HMVEC endothelial cell line (P < 0.001)
| Genes | Affymetrix ID | Cellular localization | Function(s) | Org 6 media vs. PC-3 media | Org 6 media vs. DMEM |
|---|---|---|---|---|---|
| Direction of regulation (fold change) | Direction of regulation (fold change) | ||||
| Caspase 1 | 211366_x_at | Cytoplasma | Proteolysis, apoptosis | ⇑ (41) | ⇑ (3) |
| Janus kinase 3 | 227677_at | Cell membrane | Amino acid phosphorylation, male gonad development | ⇑ (4) | ⇑ (3) |
| Superoxide dismutase 2, mitochondrial | 216841_s_at | Mitochondrion | Regulation of transcription, response to oxidative stress | ⇑ (9) | ⇑ (2) |
| Stanniocalcin 2 | 203438_at | Secreted | Receptor linked signal transduction, cell–cell signaling | ⇑ (6) | ⇑ (7) |
| Arresting domain containing 4 | 225283_at | – | – | ⇑ (5) | ⇑ (4) |
| Thrombospondin, type I, domain containing 4 | 226506_at | Secreted | Metalloendopeptidase activity | ⇑ (4) | ⇑ (2) |
| Proliferation-inducing protein 38 | 219501_at | Cell membrane, secreted | – | ⇑ (4) | ⇑ (2) |
| Muskelin 1 | 204423_at | Cytoplasma | Cell motility, cell–matrix adhesion | ⇓ (0.5) | ⇓ (0.5) |
| Cingulin-like 1 | 225817_at | – | – | ⇓ (0.3) | ⇓ (0.4) |
| Periostin, osteoblast specific factor | 210809_s_at | Secreted | Skeletal development, cell adhesion | ⇓ (0.3) | ⇓ (0.5) |
| Carbonic anhydrase II | 209301_at | Cytoplasma | Carbon dioxide transport, secretion | ⇓ (0.3) | ⇓ (0.3) |
| Growth differentiation factor 3 | 220053_at | Secreted | Cytokine activity, growth factor activity | ⇓ (0.3) | ⇓ (0.5) |
| P8 protein (candidate of metastasis 1) | 209230_s_at | Nucleus | Apoptosis, cell growth | ⇑ (2) | ⇑ (3) |
| Intercellular adhesion molecule 1 | 20263 8_s_at | Cell membrane | Cell–matrix adhesion, receptor linked signal transduction, cell–cell signaling | ⇑ (6) | ⇑ (3) |
| Growth differentiation factor 15 | 221577_x_at | Secreted | Regulation of transcription, TGFbeta signaling, chromatin modification | ⇑ (3) | ⇑ (3) |
| Interleukin 8 | 202859_x_at | Secreted | Angiogenesis, cell motility, chemotaxis | ⇑ (7) | ⇑ (7) |
| Chemokine ligand 2 | 209774_x_at | Secreted | Chemotaxis, inflammatory response | ⇑ (22) | ⇑ (2) |
| Growth arrest-specific 1 | 204457_s_at | Cell membrane | Apoptosis, cell cycle | ⇓ (0.4) | ⇓ (0.4) |
| Desmuslin | 212730_at | Cytoplasma | – | ⇓ (0.2) | ⇓ (0.3) |
| p21 (CDKN1A)-activated kinase 2 | 208875_s_at | Nucleus, cytoplasm | Translation, signal transduction | ⇓ (0.4) | ⇓ (0.5) |
| Oxytocin receptor | 206825_at | Cell membrane | Muscle contraction, signal transduction | ⇓ (0.1) | ⇓ (0.2) |
Functional groups of genes were assigned using gene-association files from the GO Consortium and by GenMapp and Kegg pathway analysis. Full gene list is available at http://genomics.biotech.ufl.edu/people/rosser/
Adhesion of tumor cells to endothelium via cell-adhesion molecules is a key step in tumor growth and metastasis. Intercellular adhesion molecule 1 (ICAM-1) is an endothelium protein known to bind tumor cells. In addition, cells binding to ICAM-1 can auto-upregulate endothelial ICAM-1 expression at the leading edge of the tumor, promoting release of other chemoattractants that can sustain the growth of tumors [19]. One such chemoattractant is chemokine ligand 2 (CCL2), recently found to be a prominent regulator of prostate cancer growth and metastasis [20]. CCL2 was also upregulated in our model. Researchers have shown that intratumoral injection of CCL2 induces effective interaction between monocytes and endothelial cells in the peritumoral area, accompanied by the upregulation of ICAM-1 [21].
CXCL8 belongs to the superfamily of chemokines that possess an assortment of proinflammatory effects. CXCL8 is produced by a wide range of cells, including tumor cells [22]. CXCL8 expression can modulate angiogenesis and invasiveness and/or extracellular matrix remodeling in the tumor microenvironment and cellular CXCL8 levels have been correlated with VEGF levels [23]. CXCL8 interacts directly with many of the same genes as ICAM-I (Fig. 3c) illustrating considerable biological overlap between CXCL8 and ICAM-I. Recent work by Zeitlin and others demonstrated in an in vivo model that targeting endothelial Bcl-2 with small molecule inhibitors resulted in inhibition of the production of potent pro-angiogenic (e.g., CXCL1 and CXCL8) molecules with a marked reduction in angiogenesis [24].
The other key hub revolves around the gene STAT1 (signal transducers and activators of transcription 1) which was downregulated in stimulated HMVEC cells. STAT1 has been previously shown to inhibit angiogenesis when induced by interferon gamma [23], and serves as a potent inhibitor of cell growth and as a promoter of apoptosis. STAT1 is negatively regulated by Janus kinase 3 [25], which we found to be upregulated in the Bcl-2 stimulated endothelial cells. Limited data exist on a role for STAT-1 in angiogenesis; however, it has been demonstrated that siRNA targeting of STAT-1 is associated with reduced IFN-gamma and increased VEGF secretion, leading to retardation of angiogenesis [26].
While it is intriguing to suggest that the increase in VEGF secreted by PC-3-Bcl-2 stimulates endothelial cellular proliferation and that bevacizumab possess the ability to block this stimulation, we cannot excluded that multiple other factors may be involved (e.g., bFGF, CXCL8). Thus more research is needed to better elucidate other factors that may be involved in this critical event.
Further analysis into the molecular pathways mediating VEGF-induced Bcl-2 expression in endothelial cells will identify critical mechanisms that might be valuable targets for anti-angiogenic therapies, but more importantly, strategies designed to overcome the survival advantage afforded by Bcl-2 and VEGF could provide doubly effective treatments that target both tumor cells and the supporting endothelial cells essential to tumor progression.
Abbreviations
- VEGF
Vascular endothelial growth factor
- Bcl-2
B-cell CLL/lymphoma 2
Footnotes
Y. Sakai and S. Goodison are co-first authors.
Conflict of interest statement None.
Contributor Information
Yoshihisa Sakai, Department of Urology, University of Florida College of Medicine, PO Box 100247, Suite N215, Gainesville, FL 32610, USA.
Steve Goodison, Department of Surgery, University of Florida, Jacksonville, FL, USA.
Wengang Cao, Department of Urology, University of Florida College of Medicine, PO Box 100247, Suite N215, Gainesville, FL 32610, USA.
Virginia Urquidi, Department of Medicine, University of Florida, Jacksonville, FL, USA.
Kazunori Namiki, Department of Urology, University of Florida College of Medicine, PO Box 100247, Suite N215, Gainesville, FL 32610, USA.
Stacy Porvasnik, Department of Urology, University of Florida College of Medicine, PO Box 100247, Suite N215, Gainesville, FL 32610, USA.
Cydney Urbanek, Department of Urology, University of Florida College of Medicine, PO Box 100247, Suite N215, Gainesville, FL 32610, USA.
Charles Joel Rosser, Department of Urology, University of Florida College of Medicine, PO Box 100247, Suite N215, Gainesville, FL 32610, USA; Department of Pharmacology and Therapeutics, University of Florida, Gainesville, FL, USA.
References
- 1.Anai S, Goodison S, Shiverick K, Hirao Y, Brown BD, Rosser CJ. Knock-down of Bcl-2 by antisense oligodeoxynucleotides induces radiosensitization and inhibition of angiogenesis in human PC-3 prostate tumor xenografts. Mol Cancer Ther. 2007;6:101–111. doi: 10.1158/1535-7163.MCT-06-0367. doi:10.1158/1535-7163.MCT-06-0367. [DOI] [PubMed] [Google Scholar]
- 2.Schumacher G, Bruckheimer EM, Beham AW, Honda T, Brisbay S, Roth JA, et al. Molecular determinants of cell death induction following adenovirus-mediated gene transfer of wild-type p53 in prostate cancer cells. Int J Cancer. 2007;91:159–600. doi: 10.1002/1097-0215(200002)9999:9999<::aid-ijc1026>3.3.co;2-e. doi:10.1002/1097-0215(200002)9999:9999<::AID-IJC1026>3.3.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 3.Urquidi V, Sloan D, Kawai K, Agarwal D, Woodman AC, Tarin D, Goodison S. Contrasting expression of thrombospondin-1 and osteopontin correlates with absence or presence of metastatic phenotype in an isogenic model of spontaneous human breast cancer metastasis. Clin Cancer Res. 2002;8:61–64. [PubMed] [Google Scholar]
- 4.Eisen MB, Spellman PT, Brown PO, Botstein D. Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci USA. 1998;95:14863–14868. doi: 10.1073/pnas.95.25.14863. doi:10.1073/pnas.95.25.14863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nikitin A, Egorov S, Daraselia N, Mazo I. Pathway studio—the analysis and navigation of molecular networks. Bioinformatics. 2003;19:2155–2157. doi: 10.1093/bioinformatics/btg290. doi:10.1093/bioinformatics/btg290. [DOI] [PubMed] [Google Scholar]
- 6.Addison CL, Nör JE, Zhao H, Linn SA, Polverini PJ, Delaney CE. The response of VEGF-stimulated endothelial cells to angiostatic molecules is substrate-dependent. BMC Cell Biol. 2005;6:38. doi: 10.1186/1471-2121-6-38. doi:10.1186/1471-2121-6-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grosjean J, Kiriakidis S, Reilly K, Feldmann M, Paleolog E. Vascular endothelial growth factor signaling in endothelial cell survival: a role for NFkappaB. Biochem Biophys Res Commun. 2006;340:984–994. doi: 10.1016/j.bbrc.2005.12.095. doi:10.1016/j.bbrc.2005.12.095. [DOI] [PubMed] [Google Scholar]
- 8.Nör JE, Christensen J, Mooney DJ, Polverini PJ. Vascular endothelial growth factor (VEGF)-mediated angiogenesis is associated with enhanced endothelial cell survival and induction of Bcl-2 expression. Am J Pathol. 1999;154:375–384. doi: 10.1016/S0002-9440(10)65284-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sakai Y, Goodison S, Kusmartsev S, Fletcher B, Eruslanov E, Cao W, Porvasnik S, Namiki K, Anai S, Rosser CJ. Bcl-2 mediated modulation of vascularization in prostate cancer xenografts. Prostate. 2009;69(5):459–470. doi: 10.1002/pros.20888. [DOI] [PubMed] [Google Scholar]
- 10.Harhaj NS, Felinski EA, Wolpert EB, Sundstrom JM, Gardner TW, Antonetti DA. VEGF activation of protein kinase C stimulates occludin phosphorylation and contributes to endothelial permeability. Invest Ophthalmol Vis Sci. 2006;47:5106–5115. doi: 10.1167/iovs.06-0322. doi:10.1167/iovs.06-0322. [DOI] [PubMed] [Google Scholar]
- 11.Trisciuoglio D, Iervolino A, Zupi G, Del Bufalo D. Involvement of PI3K and MAPK signaling in Bcl-2-induced vascular endothelial growth factor expression in melanoma cells. Mol Biol Cell. 2005;16:4153–4162. doi: 10.1091/mbc.E04-12-1087. doi:10.1091/mbc.E04-12-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu WL, Guo X, Chen QQ, Guo ZG. VEGF protects bovine aortic endothelial cells from TNF-alpha- and H2O2-induced apoptosis via co-modulatory effects on p38-and p42/p44-CCDPK signaling. Acta Pharmacol Sin. 2002;23:45–49. [PubMed] [Google Scholar]
- 13.Dancey J, Sausville EA. Issues and progress with protein kinase inhibitors for cancer treatment. Nat Rev Drug Discov. 2003;2:296–313. doi: 10.1038/nrd1066. doi:10.1038/nrd1066. [DOI] [PubMed] [Google Scholar]
- 14.Manning G, Whyte DB, Martinez R, Hunter T, Sudarsanam S. The protein kinase complement of the human genome. Science. 2002;298:1912–1934. doi: 10.1126/science.1075762. doi:10.1126/science.1075762. [DOI] [PubMed] [Google Scholar]
- 15.Wilhelm SM, Adnane L, Newell P, Villanueva A, Llovet JM, Lynch M. Preclinical overview of sorafenib, a multikinase inhibitor that targets both Raf and VEGF and PDGF receptor tyrosine kinase signaling. Mol Cancer Ther. 2008;7(10):3129–3140. doi: 10.1158/1535-7163.MCT-08-0013. doi:10.1158/1535-7163.MCT-08-0013 Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ryan C, Bukowski R, Figlin R, et al. The Advanced Renal Cell Carcinoma Sorafenib (ARCCS) expanded access trial: long-term outcomes in first-line patients (pts). J Clin Oncol. 2007;25:5096. [Google Scholar]
- 17.Motzer RJ, Rini BI, Bukowski RM, et al. Sunitinib in patients with metastatic renal cell carcinoma. JAMA. 2006;295:2516–2524. doi: 10.1001/jama.295.21.2516. doi:10.1001/jama.295.21.2516. [DOI] [PubMed] [Google Scholar]
- 18.Hudes G, Carducci M, Tomczak P, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356:2271–2281. doi: 10.1056/NEJMoa066838. doi:10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 19.Finzel AH, Reininger AJ, Bode PA, Wurzinger LJ. ICAM-1 supports adhesion of human small-cell lung carcinoma to endothelial cells. Clin Exp Metastasis. 2004;21:185–189. doi: 10.1023/b:clin.0000037696.36108.27. doi:10.1023/B:CLIN.0000037696.36108.27. [DOI] [PubMed] [Google Scholar]
- 20.Loberg RD, Ying C, Craig M, Day LL, Sargent E, Neeley C, et al. Targeting CCL2 with systemic delivery of neutralizing antibodies induces prostate cancer tumor regression in vivo. Cancer Res. 2007;67:9417–9424. doi: 10.1158/0008-5472.CAN-07-1286. doi:10.1158/0008-5472.CAN-07-1286. [DOI] [PubMed] [Google Scholar]
- 21.Okudaira K, Tsuzuki Y, Hokari R, Miyazaki J, Mataki N, Komoto S, et al. Effects of intratumoral injection of CCL2 on monocyte–endothelial cell interactions in mouse pancreatic cancer. Microcirculation. 2007;14:241–251. doi: 10.1080/10739680601139393. doi:10.1080/10739680601139393. [DOI] [PubMed] [Google Scholar]
- 22.Kitadai Y, Haruma K, Sumii K, Yamamoto S, Ue T, Yokozaki H, et al. Expression of interleukin-8 correlates with vascularity in human gastric carcinomas. Am J Pathol. 1998;152:93–100. [PMC free article] [PubMed] [Google Scholar]
- 23.Kitaya K, Yasuo T, Yamaguchi T, Fushiki S, Honjo H. Genes regulated by interferon-gamma in human uterine microvascular endothelial cells. Int J Mol Med. 2007;20:689–697. [PubMed] [Google Scholar]
- 24.Zeitlin BD, Joo E, Dong Z, Warner K, Wang G, Nikolovska-Coleska Z, et al. Antiangiogenic effect of TW37, a small-molecule inhibitor of Bcl-2. Cancer Res. 2006;66:8698–8706. doi: 10.1158/0008-5472.CAN-05-3691. doi:10.1158/0008-5472.CAN-05-3691. [DOI] [PubMed] [Google Scholar]
- 25.Takemoto S, Mulloy JC, Cereseto A, Migone TS, Patel BK, Matsuoka M, et al. Proliferation of adult T cell leukemia/lymphoma cells is associated with the constitutive activation of JAK/STAT proteins. Proc Natl Acad Sci USA. 1997;94:13897–13902. doi: 10.1073/pnas.94.25.13897. doi:10.1073/pnas.94.25.13897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Battle TE, Lynch RA, Frank DA. Signal transducer and activator of transcription 1 activation in endothelial cells is a negative regulator of angiogenesis. Cancer Res. 2006;66:3649–3657. doi: 10.1158/0008-5472.CAN-05-3612. doi:10.1158/0008-5472.CAN-05-3612. [DOI] [PubMed] [Google Scholar]