Abstract
An invasion-independent pathway has been proposed as a novel mechanism in blood-borne metastasis, where tumour cells enveloped by sinusoidal tumour vessels enter the circulation without vascular invasion. We previously identified the secretory leukocyte protease inhibitor (SLPI) as a candidate gene responsible for this pathway. In this study, the functional role of SLPI in metastatic dissemination was investigated. We transfected the SLPI gene into a poorly metastatic clone of the MCH66 mouse mammary tumour cell line. Over-expression of SLPI promoted in vivo growth and spontaneous metastasis to the lung, whereas it suppressed invasive activity in vitro. The inoculated tumours of SLPI-transfectants exclusively induced a sinusoidal vasculature and subsequently produced endothelial-coated tumour emboli, which are morphological indices of the invasion-independent pathway. In addition, exogenous SLPI inhibited the migration activity through Matrigel of both tumour cells and human umbilical vein endothelial cells (HUVECs). In vivo angiogenesis assays also demonstrated that SLPI suppressed the migration of newly formed blood vessels. These results suggest that an anti-migratory effect of SLPI on tumour-associated endothelial cells may induce vascular remodelling to form a sinusoidal architecture, and consequently promote invasion-independent metastasis. This study provides a new model for metastasis, based on the mechanism regulated by anti-invasive factors, such as SLPI.
Keywords: SLPI, metastasis, invasion, angiogenesis
Introduction
By general consensus, cancer cell invasion is thought to be an essential activity in the metastatic process [1,2]. However, we have described an alternative pathway of blood-borne metastasis that does not require invasion into the vascular wall at either the primary tumour or the target organ [3–5]. The process involves intravasation of tumour nests that are surrounded by blood vessels, transportation of tumour emboli enveloped with endothelial cells and intravascular tumour growth in the lung. Comparative studies between murine tumour cell lines of differential metastatic capability suggested that this pathway requires high angiogenic activity in order to make sinusoidal tumour vasculature [4]. Gene expression analysis of MCH66 clonal cell lines identified several genes whose expression was correlated with metastatic capability [4]. The secretory leukocyte protease inhibitor (SLPI) was one of the candidate genes identified as potentially involved in the invasion-independent metastasis pathway.
SLPI is a serine protease inhibitor serving as an important component for protection of the mucosa and skin [6,7]. In addition, SLPI has reported functions in inhibiting bacterial infection and inflammation [8,9], promoting wound healing [10] and epithelial proliferation [11] independent of anti-protease activity. Increased expression of SLPI has been reported in a wide variety of human cancers, including breast, lung, ovarian and colorectal carcinomas, and glioblastoma [12–14]. Furthermore, several studies have shown a positive correlation of SLPI expression level with tumour aggressiveness and metastatic potential [13,15,16]. To date, it has been unclear how SLPI can enhance the malignant phenotype, which was thought to be based on a degradative function in spite of its tissue-protective roles. In our model of cancer metastasis independent of invasive activity, it does not seem contradictory that SLPI could promote metastasis.
In this study, through transfection methodology and in vivo evaluation, we confirmed that SLPI can confer metastatic potential to cells of low-metastatic capability. Furthermore, we investigated the molecular mechanism of SLPI in cancer metastasis, using assays for metastasis-associated properties, such as growth, invasion and angiogenesis. Our results indicate a new mechanism for cancer metastasis that can be promoted by the suppression of invasive cellular activity.
Materials and methods
Cell lines
The non-metastatic mouse mammary tumour line, MCH66C8 [4], was cultured in DMEM (Sigma-Aldrich, MO, USA) containing 10% fetal bovine serum (FBS). Human umbilical vein endothelial cells (HUVECs) were kindly provided by Kazuhiko Kaji, University of Shizuoka, Japan. HUVECs were cultured in MCDB104 medium (Wako Pure Chemical, Osaka, Japan) containing 10% FBS, 10 mM/l glutamine, 100 μg/ml endothelial cell growth supplement (Sigma-Aldrich).
Production of stable SLPI transfectants
The full-length murine SLPI coding region was amplified from cDNA of MCH66 cells, using forward primer 5′-ATAAGAATGCGGCCGCTAAACTATAT-GAAGTCCTGCGGCCT-3′ and a reverse primer, 5′-CCATCGATGGTCACAGATCCTCTTCAGAGATG-AGTTTCTGCTCCATCGGGGGCAGGCA-3′, fused with a sequence encoding the Myc-tag. The construct pLNCX2–SLPI was made by subcloning this cDNA into the pLNCX2 retroviral vector (Clon-tech, CA, USA). Stable lines were generated using two distinct methods: lipofection-mediated transfection and viral infection. PT67 packaging cells (Clon-tech) were transfected with SLPI/pLNCX2 retroviral vector using Lipofectamine 2000 (Invitrogen, CA, USA). Stable transfectants were selected with G418 (1 mg/ml; Promega, WI, USA) for 3 weeks; 30% confluent MCH66C8 cells were infected by incubation with the supernatants of PT67 cells containing polybrene as vehicle (8 μg/ml). Stable transfectants were selected with 1 mg/ml G418 for 2 weeks. As negative controls, target cells were infected with a retrovirus bearing the pLNCX2 empty vector.
Western blot analysis
Protein lysates (10 μg) were separated on 15% SDS–PAGE gels and transferred to PVDF membrane (Millipore, MA, USA). After blocking with 5% skimmed milk for 1 h, the membrane was incubated with goat polyclonal antibody against mouse SLPI (v-17; Santa Cruz Biotechnology, CA, USA) or mouse monoclonal antibody against β-actin (Sigma-Aldrich), then anti-goat or mouse IgG conjugated with horseradish peroxidase (Sigma-Aldrich). The signals were visualized by enhanced chemiluminescence (ECL Advance; Amer-sham Biosciences, NJ, USA). The autoradiograms were analysed on a densitometer with a computerized image analyser (Image-Pro Plus; Media Cybernetics, MD, USA).
Determination of in vitro growth
For growth rate determination in vitro, 1 × 104 cells were plated in 12-well tissue-culture plates and incubated in DMEM containing 10% FBS. After trypsinization, the cells were counted every day. Growth assays were performed in triplicate.
In vitro invasion/migration assay
Cell migration assays were performed in culture inserts with a polystyrene membrane (8 μm pore; Falcon) in a 24-well tissue culture plate. Culture inserts were coated with Matrigel (BD Bioscience) at 12.5 μg/filter. The upper chamber was filled with 1 × 105 cells in culture media with 0.1% BSA. The lower chamber was filled with culture media with 0.1% BSA and chemoattractants, mouse fibronectin (10 μg/ml; Chemicon, CA, USA) for tumour cells or bFGF (0 or 100 ng/ml; R&D Systems, MN, USA) for HUVECs. For invasion or migration assays to examine the effect of exogenous SLPI, recombinant SLPI (R&D Systems) was added to the upper chamber. After incubation at 37 °C for 6 h, cells on the upper surface of the filters were removed with a cotton swab, and the filters were fixed with 100% methanol, stained with haematoxylin. The cells that invaded to the lower side of the filters were counted in five adjacent diagonal fields (×200).
Spontaneous metastasis assay
Cells (1 × 107) suspended in 200 μl PBS were inoculated into the mammary fat pads of 6 week-old female C3H/He mice. At 10–13 weeks after inoculation, the animals were sacrificed and the number of lung colonies was counted [4]. The numbers of animals used in this assay were 28, 8, 8 and 9 for C8-SLPI2, C8-SLPI4, C8-SLPI5 and C8-control, respectively. The metastatic ability of C8-SLPI2 cells was also evaluated by size-matched comparison, using nine C8-SLPI2 tumours under 5 g in weight, of which the average weight was equivalent to that of the control group. The animal experiments were approved by the Institutional Animal Care and Use Committee in Fukushima Medical University and all procedures were performed in accordance with the Animal experimentation guidelines.
Morphological and immunohistochemical examinations
Tumours and lungs were fixed with 10% formalin for paraffin-embedded sections. Sections were stained with haematoxylin and eosin (H&E) and Elastica Masson stain to detect the elastic layers of blood vessels. Immunohistochemistry was performed using antibodies to SLPI (v-17), Myc-tag (MBL, Nagoya, Japan), mouse CD31 (Abcam, MA, USA), mouse LYVE-1 (MBL), Prox1 (Angiobio, CA, USA), Ki67 (LabVision) and a streptavidin–biotin kit (Nichirei, Tokyo, Japan), according to the manufacturer’s protocol. Double immunohistochemical staining with anti-CD31 and anti-Ki67 antibodies was performed using the diaminobenzidine–TMB technique [17].
Assessment of neovascularization
The development of sinusoidal vessels was assessed by the area of blood vessels within a tumour [4]. The H&E-stained sections were used to assess neovascular development. Blood vessel morphometry was evaluated in three low-power fields (×10) in the periphery and the centre of every three tumours of transfectants. The areas of blood vessels and tumour parenchyma exclusive of necrosis were measured in each field, using a computerized image analyser. The percentage of total vascular area in each tumour was used as an index of vascularity.
Dorsal air sac assay
The dorsal air sac assay was applied to mice to examine the angiogenesis activities of C8-SLPI2 and C8-control [4]. Cell suspensions (1 × 106 in 100 μl PBS) were injected into a diffusion chamber consisting of a ring (Millipore Corp., MA, USA) covered with Millipore filters (0.45 μm pore size) on each side. The chamber was implanted into an air sac produced by injecting 10 ml air on the dorsal subcutaneous tissue of the mouse. On day 4, vertical histological sections of the subcutaneous tissues were made. Angiogenesis activities were assessed by counting the number of blood vessels and measuring the distance of vascular sprouts extending toward the chamber from the subcutaneous muscular layer, using a computerized image analyser. All experiments were performed using three animals.
Matrigel plug assay
The effect of rSLPI on in vivo angiogenesis was assayed by the Matrigel plug assay, as described previously [18]. Briefly, Matrigel was mixed with bFGF (0 and 0.2 μg/ml), and rSLPI (0, 10, 100 ng/ml). The Matrigel mixture (0.5 ml) was subcutaneously injected into mice. After 7 days, the Matrigel plugs were removed and fixed in formalin for paraffin-embedded sections. Sections stained with H&E were examined by light microscopy, and the vascular density was calculated using a computerized image analyser. All experiments were made using three animals.
Statistics
The results were analysed using Statview Software (SAS Institute, NC, USA). Differences between samples were determined using an unpaired Student’s t -test or one-way ANOVA followed by Fisher’s PLSD procedure. Statistical significance was defined as p <0.05.
Results
Generation of stable clones over-expressing SLPI
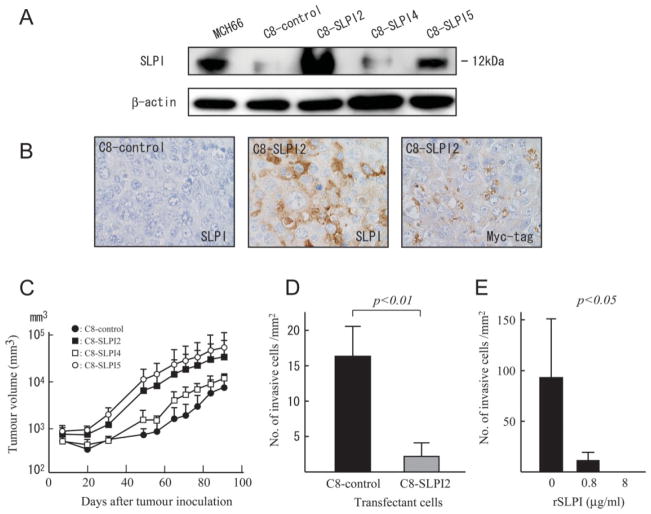
Transfection of SLPI using the retroviral vector system and G418 selection generated stable bulk transfectant clonal lines. Western blot analysis using an anti-SLPI antibody identified clonal lines of high SLPI expressors (C8-SLPI2 and -5), low SLPI expressors (C8-SLPI4) and C8-control, a stable transfectant with empty vector (Figure 1A). C8-SLPI2, in which the expression level was 10.6-fold higher than the C8-control, was mainly used for further analysis. Immuno-histochemistry using antibodies to SLPI and a Myc-tag demonstrated that higher expression levels of SLPI derived from the transfected cDNA of C8-SLPI2 were persistent in vivo (Figure 1B).
Figure 1.
Over-expression of SLPI promoted tumour growth and suppressed in vitro invasion. (A) Western blotting. SLPI protein expression was determined in cell lysates. Lane 1, MCH66 (parent cell line of MCH66C8); lane 2, C8-control; lane 3, C8-SLPI2; lane 4, C8-SLPI4; lane 5, C8-SLPI5. Relative protein expression levels of SLPI to β-actin in C8-SLPI2, -4, and -5 were 10.6-, 1.2-and 3.0-fold, respectively, higher than that in the C8-control. (B) Immunohistochemistry of C8-SLPI2 and C8-control tumours. Myc-tagged SLPI expression was analysed in the transfectants, using antibodies to SLPI and Myc-tag. (C) Tumour growth in vivo. Tumour volumes of tumours were monitored at weekly intervals. Tumour growth of C8-SLPI2 and -5 (high SLPI expressers) were more rapid than C8-control and C8-SLPI4 (low expressers). (D, E) Histograms of in vitro invasion assays through Matrigel. (D) The number of C8-SLPI2 cells migrating through the filter were significantly fewer than that of C8-control cells (p < 0.01). (E) Recombinant SLPI treatment demonstrates a dose-dependent effect on invasion-suppressive activity on MCH66C8 cells (p <.0.05 for dose effect by one-way ANOVA, followed by Fisher’s PLSD procedure). Each value in (C–E) represents mean ± SD of triplicate measurements
Growth properties in vitro and in vivo
To investigate the effect of SLPI on cell growth, we compared C8-SLPI2, -4 and -5 with the C8-control. There was no significant difference in growth rate in vitro (doubling time: C8-control, 14.4 h; C8-SLPI2, 13.2 h; C8-SLPI4, 17.0 h; C8-SLPI5, 14.5 h). In contrast, tumour growth in vivo monitored as tumour volumes was more rapid in mice inoculated with C8-SLPI2 and -5 (high SLPI expressers) than in C8-control and C8-SLPI4 (low SLPI expressers) (Figure 1C). Tumour weights of C8-SLPI2 and -5 at autopsy were also significantly higher than that of the C8-control (p < 0.01 and p < 0.05, respectively; Table 1).
Table 1.
Incidence of primary tumours and spontaneous metastases produced by transfectants in vivo
| Tumour type | Tumourigenicity [n (%)] | Tumour weight (g) | Lung metastases [n (%)]
|
Lymph node metastasis [n (%)] | |
|---|---|---|---|---|---|
| Incidence | Colonies | ||||
| C8-control | 9/9 (100) | 2.3 ± 1.3 | 1/12 (8.3) | 0.1 (0–1) | 0/12 (0) |
| C8-SLPI21 | 28/28 (100) | 5.5 ± 2.2* | 16/28 (57) | 4.2 (0–40)** | 3/28 (11) |
| Small (1–5 g) | 9/9 (100) | 3.0 ± 1.2 | 5/9 (56)a | 2.7 (0–8)** | 1/9 (11) |
| Large (>5 g) | 19/19 (100) | 6.8 ± 1.2* | 11/19 (58)a | 4.9 (0–40)** | 2/19 (11) |
| C8-SLPI4 | 8/8 (100) | 2.3 ± 2.0 | 1/8 (13) | 0.3 (0–2) | 0/8 (0) |
| C8-SLPI5 | 8/8 (100) | 3.53 ± 0.8** | 3/8 (38) | 1.3 (0–4) | 0/8 (0) |
C8-SLPI2 tumours were divided into two groups; small (1–5 g) and large (>5 g). Values are mean ± SD.
p < 0.01 compared with control groups.
p < 0.05 compared with control groups.
SLPI suppresses the invasive potential of cancer cells in vitro
We examined the effect of SLPI over-expression on invasive property using an in vitro Matrigel invasion assay. C8-SLPI2 over-expressing SLPI exhibited significantly lower invasive activity than the C8-control (p < 0.01; Figure 1D). To investigate the effect of exogenous SLPI on cancer cell invasion, we added recombinant SLPI (rSLPI) protein to MCH66C8 cells in the same assay system. This experiment showed that invasion was significantly suppressed in cells treated with increasing doses of rSLPI (p < 0.05 for dose effect by one-way ANOVA, followed by Fisher’s PLSD procedure; Figure 1E), an observation which predicts that SLPI over-expressing tumours would be less metastatic. To evaluate this hypothesis, the effect of SLPI over-expression on metastasis was evaluated in a xenograft model.
SLPI promotes spontaneous metastases to the lungs and lymph nodes
We examined the spontaneous metastasis capability of transfectants with SLPI and empty vector. Contrary to the hypothesis, tumours of C8-SLPI2 and C8-SLPI5 (high SLPI expressors) exhibited more metastatic potential to the lung (51.7% and 37.5%; colony number, 0–40; p < 0.05 compared with C8-control and 0–4, respectively) than C8-control and C8-SLPI4 (low SLPI expressors: 8.3% and 12.5%; colony number, 0–1 and 0–2, respectively; Table 1). Axillary lymph node metastasis was generated exclusively by C8-SLPI2 tumours (11%). To perform a size-matched comparison, nine small tumours (<5 g in weight) were selected from C8-SLPI2 tumours. They also yielded lung metastases in higher incidence (56%) and greater colony number (0–8; p < 0.05) than C8-control tumours.
SLPI induces sinusoidal angiogenesis and invasion-independent metastasis
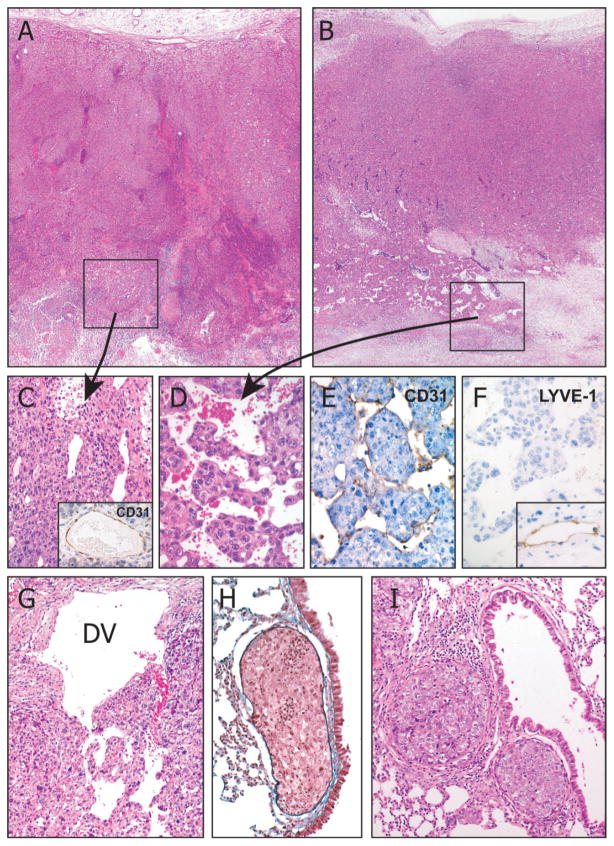
To elucidate the mechanism by which an anti-invasive molecule can promote metastasis, we observed the metastatic process morphologically. A marked difference was found in the vascular architecture, which would induce tumour intravasation as a initial step of cancer metastasis within the inoculated tumours. In the periphery of the primary tumour mass, poor vascularity was formed in all tumours (Figure 2A, B). However, the perinecrotic area in the centre of the tumour of C8-SLPI2 and -5 (high SLPI expressors) formed well-developed sinusoidal vessels, which closely surrounded tumour nests (Figure 2B, D), whereas C8-SLPI5 and C8-control (low SLPI expressors) had simply dilated vessels, with no evidence of sinusoidal vasculature (Figure 2C). Immunohistochemical examination revealed that endothelial cells of the sinusoidal vessels expressed CD31 antigen (Figure 2E) but not prox-1 or LYVE-1 (Figure 2F), which are markers for lymphatics, indicative of blood vessel origin. C8-SLPI2 induced a sequential metastatic process of the invasion-independent pathway, including intravasation of endothelial-coated tumour emboli in the drainage veins (Figure 2G), intravascular growth in the pulmonary arterioles (Figure 2H), extravasation (Figure 2I) and colony formation in the lung.
Figure 2.
Histology of orthotopically inoculated tumours derived from C8-SLPI2 cells. C8-control (A, ×20), and C8-SLPI2 (B, ×20) tumours showed similar morphology, characterized by a solid sheet pattern at the periphery. In the perinecrotic regions, C8-control tumours induced simply dilated blood vessels (C, ×200), which were surrounded by CD31-positive endothelial cells (inset, ×100). In contrast, C8-SLPI2 formed a well-developed sinusoidal vasculature which closely surrounded the tumour nests (D, ×400). The sinusoidal vessels were lined by CD31-positive (E, ×400) and LYVE-1-negative (F, ×400) endothelial cells, indicative of vascular origin. Inset of (F) shows subcutaneous lymphatics as a positive control for LYVE-1 (×100). Tumour emboli were released in the drainage vein (DV) from the primary tumour (F, ×100) and arrested in the pulmonary arteriole, followed by intravascular growth (G, ×100) and the formation of metastatic nodules in the lung (H, ×100). H&E stain (A–D, G, I); Elastica Masson stain (H)
Effects of SLPI over-expression on tumour angiogenesis in vivo
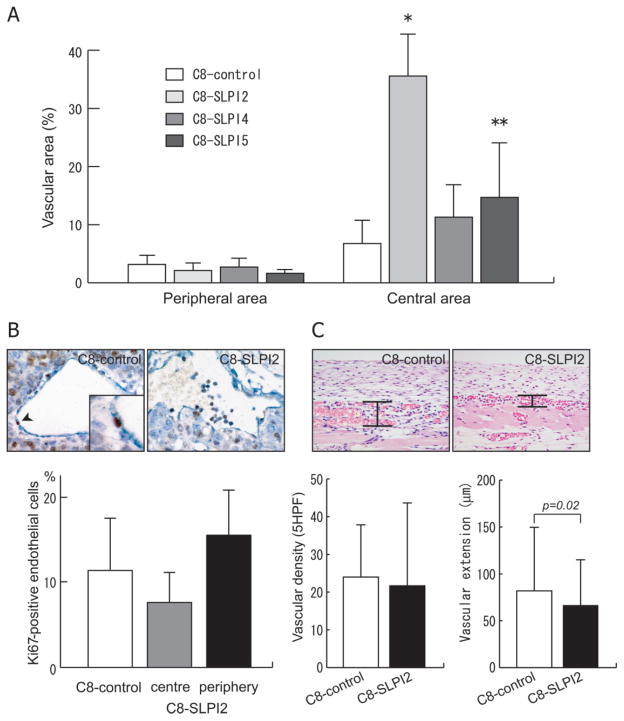
To assess the development of sinusoidal vasculature within the inoculated tumours, intratumoural vascular area was measured using an image analyser. Whereas the proportions of vascular areas in the periphery of the tumours were equally low in all transfectants with SLPI and empty vector, those in the central regions were significantly higher in C8-SLPI2 and -5 than in the C8-control (p < 0.01 and p < 0.05, respectively; Figure 3A). The increased vascular areas in these SLPI-transfectants are in line with the histological findings indicating the induction of sinusoidal blood vessels. In contrast with the sinusoidal vascular development, the double immunostaining technique for CD31 and Ki67 demonstrated that the endothelial proliferation activity of the sinusoidal vessels in the C8-SLPI2 tumours was not higher than that of vessels with normal structure in the same tumours or C8-control tumours (Figure 3B). We evaluated the effect of SLPI over-expression on the steps of the angiogenesis process; vascular proliferation and vascular extension, using the dorsal air sac assay. SLPI over-expression did not affect vascular proliferation, as evaluated by vascular density, but significantly reduced the extension of newly-formed blood vessels (p = 0.02; Figure 3C). These data suggest that SLPI may also have an anti-migration effect on blood vessels, which can induce vascular remodelling to form the sinusoidal architecture.
Figure 3.
The effects of SLPI on tumour angiogenesis. (A) The sinusoidal development of tumour vessels was assessed by means of measuring the proportion of vascular area at the periphery and the centre of tumours transfected with SLPI and control vector (*p < 0.01 and **p < 0.05 compared with C8-control). (B) Endothelial proliferation of tumour vessels induced by C8-control and C8-SLPI2 cells was quantified, using a double immunohistochemical technique to stain the endothelial cells for CD31 expression (dark blue) and stain proliferating cells for Ki67 (brown). The sections were counterstained with haematoxylin. The proportion of Ki67-positive cells in sinusoidal vessels in the centre of the C8-SLPI2 tumour was not significantly different from the small vessels in the peripheral area or C8-control tumour. (C) Angiogenesis activities of the transfectant cells with SLPI using the dorsal air sac assay. Vascular density was assessed by counting the vascular number per five high-power fields in the subcutaneous tissue of a vertical histological section. Vascular extension was estimated by measuring the distance of vascular sprouts from the subcutaneous muscle. Each value in (A–C) represents mean ± SD
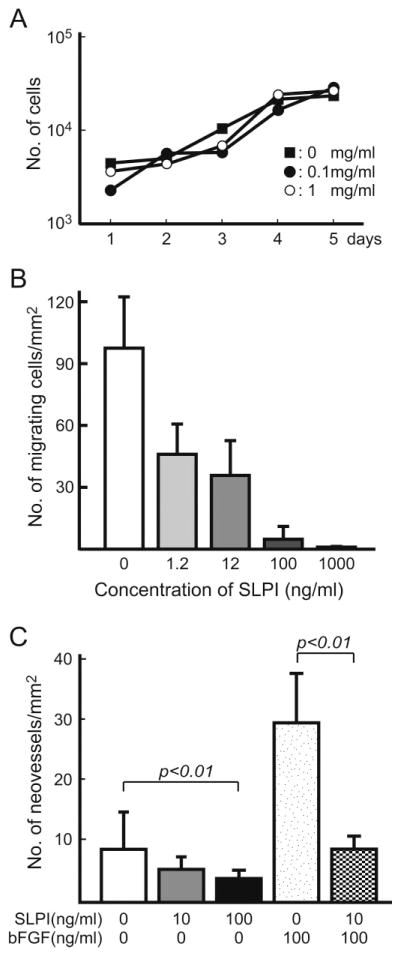
SLPI suppresses vascular migration
We examined the effect of SLPI directly on host vascular formation, including endothelial cell growth and vascular migration. In in vitro studies using rSLPI, the protein did not affect endothelial cell proliferation (Figure 4A). By contrast, rSLPI inhibited human endothelial cell migration in a dose-dependent manner in the in vitro migration assay using Matrigel (p < 0.01 for dose effect by one-way ANOVA, followed by Fisher’s PLSD procedure; Figure 4B). An in vivo study using the Matrigel plug assay showed that rSLPI also caused a dose-dependent reduction in the number of newly formed blood vessels migrating into the Matrigel (p < 0.01 for dose effect by one-way ANOVA, followed by Fisher’s PLSD procedure; Figure 4C). This inhibition was maintained by rSLPI even in the presence of the angiogenesis-inducing factor bFGF (p < 0.01).
Figure 4.
The effect of SLPI on in vitro growth and migration of HUVECs. (A) 1 × 104 cells/well were seeded in triplicate in 12-well plates and cultivated in growth medium containing recombinant SLPI at the indicated concentration. Cell numbers were determined by direct counting, using a haemocytometer, after detachment of adherent cells. (B) Effect of SLPI on endothelial cell migration. A total of 1 × 105 HUVECs were seeded in the upper chamber of a culture insert coated with Matrigel containing rSLPI (0, 0.1 and 1 μg/ml). After 6 h incubation, the migrating cells were counted. (C) Effect of SLPI on an in vivo Matrigel plug assay. Various doses of rSLPI were mixed with Matrigel, with or without bFGF (100 ng/ml). A total of 0.5 ml Matrigel was implanted into the mouse. After 7 days, the Matrigel plug was removed and neovessel formation was quantified by calculating the vascular density. The dose-dependent effects of rSLPI in (B) and (C) were validated by one-way ANOVA, followed by Fisher’s PLSD procedure. Each value in (A–C) represents mean ± SD of triplicate measurements
Discussion
This study indicates a new relationship between cancer invasion and metastasis, where a molecule such as SLPI, which suppresses the invasive activity of cancer cells, can concomitantly promote increased metastatic potential. Current hypotheses propose that the invasion of cancer cells into the stroma and through the vascular wall is an essential step in the metastatic process. In contrast, we have previously reported data supportive of an alternative metastatic pathway, independent of cancer invasion, in a mouse mammary tumour model [4], and we have presented evidence for the existence of the same pathway in human cancers [5]. However, little was known about the molecular mechanism regulating this alternative metastatic pathway. This study provides a potential mechanism for this pathway and suggests that it is dependent on anti-invasive activity, rather than being independent of invasiveness.
Our study revealed that SLPI can inhibit the invasive activity of cancer cells. SLPI is a kazal-type serine protease inhibitor of neutrophil elastase, cathepsin G, chymotrypsin and trypsin. Most of these enzymes are known to promote cancer invasion and progression [19,20]. In addition, SLPI can suppress the production of matrix metalloproteinases (MMP1 and -9), which are important for cancer invasion, independent of its anti-protease activity in monocytes [21]. These data on the biological activities of SLPI suggest that this molecule may function as a potential anti-invasive agent for cancer cells. In our system, over-expression of SLPI by transfection and/or the addition of exogenous SLPI inhibited cancer invasion in vitro. Further studies on other tumour cell lines will be needed to confirm the correlation of SLPI expression with cancer invasion and aggressiveness.
Our data demonstrate that SLPI promoted in vivo growth and spontaneous metastasis in a mouse mammary tumour model system. There have been reports that SLPI can enhance tumour growth and aggressiveness [11,13,15]. In the present study, over-expression of SLPI did not directly affect cell proliferation in vitro but increased the in vivo growth of mouse mammary tumour cells. The discrepancy between the in vitro and the in vivo cell proliferation may be explained by angiogenic activity, because SLPI transfectants induced well-developed vasculature in resulting tumours when orthotopically inoculated into the mammary fat pad, while controls did not.
The molecular mechanisms involved in the promotion of metastasis by SLPI have not previously been clarified, primarily because of a lack of models for cancer metastasis without invasion [22]. According to our concept of the invasion-independent metastasis pathway, it would not be surprising that an anti-protease, or anti-invasive factors, can participate in enhancing metastatic potential. In our system, over-expression of SLPI by transfection promoted spontaneous lung metastasis of MCH66C8 via the invasion-independent pathway, where tumour nests are enveloped by sinusoidal vessels and subsequently released into the bloodstream. In this pathway, the formation of an organized tissue complex composed of tumour nests and surrounding sinusoidal blood vessels seems to be a key event. Our previous study demonstrated that tumour nests within mouse mammary tumours displayed non-invasive features, such as conserving a continuous basement membrane, even during the metastatic process [3]. An anti-invasive effect of SLPI may play an important role in forming and conserving these well-organized tumour nests in the metastatic process.
Furthermore, SLPI can affect not only tumour cell behaviour but also blood vessel formation. Recombinant SLPI inhibited the migration activities of vascular endothelial cells, both in vitro and in vivo, whereas it did not affect the in vitro cell growth. At first sight, it seems counter-intuitive that a so-called ‘anti-angiogenic’ molecule, SLPI, can induce a well-developed sinusoidal vasculature in primary tumours. This is likely due to the morphogenesis of the sinusoidal vasculature, which seems to be generated not in the usual angiogenic process, which involves endothelial growth, vascular extension and tube formation, but in a specific remodelling process of newly formed blood vessels with other constituent steps. The sinusoidal vessels were localized to the perinecrotic area in the tumours of SLPI transfectants, although SLPI proteins were uniformly over-expressed in the whole area. This observation indicates that the collaboration of other molecules, such as hypoxia-related angiogenesis factors and necrosis-induced cytokines, may be necessary for sinusoidal angiogenesis and subsequent blood-borne metastasis.
In conclusion, this study provides a new model explaining why SLPI, an anti-invasive molecule, can promote cancer metastasis. The over-expression of SLPI-induced sinusoidal tumour vasculature, which is an initial step of the invasion-independent pathway of cancer metastasis. The inhibitory effects of SLPI on both cancer cells and the cells of the host vasculature may influence the interactions between them, and thus lead to the formation and conservation of well-organized tumour emboli during the invasion-independent metastatic process.
Acknowledgments
This study was funded by a Grant-in-Aid for Scientific Research (C) from the Ministry of Education, Science, Sports and Culture, Japan (No. 14570126).
Footnotes
No conflicts of interest were declared.
References
- 1.Fidler IJ, Balch CM. The biology of cancer metastasis and implications for therapy. Curr Probl Surg. 1987;24:129–209. doi: 10.1016/0011-3840(87)90002-5. [DOI] [PubMed] [Google Scholar]
- 2.Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer. 2002;2:563–572. doi: 10.1038/nrc865. [DOI] [PubMed] [Google Scholar]
- 3.Sugino T, Kawaguchi T, Suzuki T. Sequential process of blood-borne lung metastases of spontaneous mammary carcinoma in C3H mice. Int J Cancer. 1993;55:141–147. doi: 10.1002/ijc.2910550125. [DOI] [PubMed] [Google Scholar]
- 4.Sugino T, Kusakabe T, Hoshi N, Yamaguchi T, Kawaguchi T, Goodison S, et al. An invasion-independent pathway of blood-borne metastasis: a new murine mammary tumor model. Am J Pathol. 2002;160:1973–1980. doi: 10.1016/S0002-9440(10)61147-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sugino T, Yamaguchi T, Ogura G, Saito A, Hashimoto T, Hoshi N, et al. Morphological evidence for an invasion-independent metastasis pathway exists in multiple human cancers. BMC Med. 2004;2:9. doi: 10.1186/1741-7015-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thompson RC, Ohlsson K. Isolation, properties, and complete amino acid sequence of human secretory leukocyte protease inhibitor, a potent inhibitor of leukocyte elastase. Proc Natl Acad Sci USA. 1986;83:6692–6696. doi: 10.1073/pnas.83.18.6692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Franken C, Meijer CJ, Dijkman JH. Tissue distribution of antileukoprotease and lysozyme in humans. J Histochem Cytochem. 1989;37:493–498. doi: 10.1177/37.4.2926127. [DOI] [PubMed] [Google Scholar]
- 8.McNeely TB, Dealy M, Dripps DJ, Orenstein JM, Eisenberg SP, Wahl SM. Secretory leukocyte protease inhibitor: a human saliva protein exhibiting anti-human immunodeficiency virus 1 activity in vitro. J Clin Invest. 1995;96:456–464. doi: 10.1172/JCI118056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Song X, Zeng L, Jin W, Thompson J, Mizel DE, Lei K, et al. Secretory leukocyte protease inhibitor suppresses the inflammation and joint damage of bacterial cell wall-induced arthritis. J Exp Med. 1999;190:535–542. doi: 10.1084/jem.190.4.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu J, Nathan C, Jin W, Sim D, Ashcroft GS, Wahl SM, et al. Conversion of proepithelin to epithelins: roles of SLPI and elastase in host defense and wound repair. Cell. 2002;111:867–878. doi: 10.1016/s0092-8674(02)01141-8. [DOI] [PubMed] [Google Scholar]
- 11.Zhang D, Simmen RC, Michel FJ, Zhao G, Vale-Cruz D, Simmen FA. Secretory leukocyte protease inhibitor mediates proliferation of human endometrial epithelial cells by positive and negative regulation of growth-associated genes. J Biol Chem. 2002;277:29999–30009. doi: 10.1074/jbc.M203503200. [DOI] [PubMed] [Google Scholar]
- 12.Barker SD, Coolidge CJ, Kanerva A, Hakkarainen T, Yamamoto M, Liu B, et al. The secretory leukoprotease inhibitor (SLPI) promoter for ovarian cancer gene therapy. J Gene Med. 2003;5:300–310. doi: 10.1002/jgm.341. [DOI] [PubMed] [Google Scholar]
- 13.Ameshima S, Ishizaki T, Demura Y, Imamura Y, Miyamori I, Mitsuhashi H. Increased secretory leukoprotease inhibitor in patients with nonsmall cell lung carcinoma. Cancer. 2000;89:1448–1456. doi: 10.1002/1097-0142(20001001)89:7<1448::aid-cncr6>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 14.Koshikawa N, Nakamura T, Tsuchiya N, Isaji M, Yasumitsu H, Umeda M, et al. Purification and identification of a novel and four known serine proteinase inhibitors secreted by human glioblastoma cells. J Biochem (Tokyo) 1996;119:334–339. doi: 10.1093/oxfordjournals.jbchem.a021244. [DOI] [PubMed] [Google Scholar]
- 15.Devoogdt N, Hassanzadeh Ghassabeh G, Zhang J, Brys L, De Baetselier P, Revets H. Secretory leukocyte protease inhibitor promotes the tumorigenic and metastatic potential of cancer cells. Proc Natl Acad Sci USA. 2003;100:5778–5782. doi: 10.1073/pnas.1037154100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kluger HM, Chelouche Lev D, Kluger Y, McCarthy MM, Kiriakova G, Camp RL, et al. Using a xenograft model of human breast cancer metastasis to find genes associated with clinically aggressive disease. Cancer Res. 2005;65:5578–5587. doi: 10.1158/0008-5472.CAN-05-0108. [DOI] [PubMed] [Google Scholar]
- 17.Preuss TM, Coleman GQ. Human-specific organization of primary visual cortex: alternating compartments of dense Cat-301 and calbindin immunoreactivity in layer 4A. Cerebr Cortex. 2002;12:671–691. doi: 10.1093/cercor/12.7.671. [DOI] [PubMed] [Google Scholar]
- 18.Passaniti A, Taylor RM, Pili R, Guo Y, Long PV, Haney JA, et al. A simple, quantitative method for assessing angiogenesis and antiangiogenic agents using reconstituted basement membrane, heparin, and fibroblast growth factor. Lab Invest. 1992;67:519–528. [PubMed] [Google Scholar]
- 19.Sun Z, Yang P. Role of imbalance between neutrophil elastase and alpha 1-antitrypsin in cancer development and progression. Lancet Oncol. 2004;5:182–190. doi: 10.1016/S1470-2045(04)01414-7. [DOI] [PubMed] [Google Scholar]
- 20.Del Rosso M, Fibbi G, Pucci M, D’Alessio S, Del Rosso A, Magnelli L, et al. Multiple pathways of cell invasion are regulated by multiple families of serine proteases. Clin Exp Metast. 2002;19:193–207. doi: 10.1023/a:1015531321445. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Y, DeWitt DL, McNeely TB, Wahl SM, Wahl LM. Secretory leukocyte protease inhibitor suppresses the production of monocyte prostaglandin H synthase-2, prostaglandin E2, and matrix metalloproteinases. J Clin Invest. 1997;99:894–900. doi: 10.1172/JCI119254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mareel MM, De Baetselier P, Van Roy FM. Mechanisms of Invasion and Metastasis. CRC Press; Boca Raton, FL: 1991. [Google Scholar]