Abstract
OBJECTIVE
This study evaluates insulin sensitivity, pancreatic β-cell function (BCF), and the balance between the two in youth with type 2 diabetes and assesses the relationship of diabetes duration and HbA1c to insulin sensitivity and BCF.
RESEARCH DESIGN AND METHODS
The subjects were 14 adolescents with type 2 diabetes and 20 obese control subjects of comparable age, BMI, body composition, and puberty. Insulin sensitivity was evaluated with a 3-h hyperinsulinemic (80 mU · m−2 · min−1) euglycemic clamp. First-phase insulin secretion (FPIS) and second-phase insulin secretion (SPIS) were evaluated with a 2-h hyperglycemic (12.5 mmol/l) clamp. Fasting glucose rate of appearance was determined with the use of [6,6-2H2]glucose.
RESULTS
Fasting glucose rate of appearance was higher in type 2 diabetic patients than in obese control subjects (16.5 ± 1.1 vs. 12.3 ± 0.5 µmol · kg−1 · min−1; P = 0.002). Insulin sensitivity was lower in type 2 diabetic patients than in obese control subjects (1.0 ± 0.1 vs. 2.0 ± 0.2 µmol · kg−1 · min−1 per pmol/l; P = 0.001). Fasting insulin was higher in type 2 diabetic patients than in obese control subjects (289.8 ± 24.6 vs. 220.2 ± 18.0 pmol/l; P = 0.007), and FPIS and SPIS were lower (FPIS: 357.6 ± 42.0 vs. 1,365.0 ± 111.0 pmol/l; SPIS: 652.2 ± 88.8 vs. 1,376.4 ± 88.8 pmol/l; P < 0.001 for both). The glucose disposition index (GDI = insulin sensitivity × FPIS) was ~86% lower in type 2 diabetic patients than in obese control subjects. HbA1c correlated with FPIS (r = −0.61, P = 0.025) with no relationship to insulin sensitivity.
CONCLUSIONS
Despite the impairment in both insulin sensitivity and BCF in youth with type 2 diabetes, the magnitude of the derangement is greater in BCF than insulin sensitivity when compared with that in obese control subjects. The inverse relationship between BCF and HbA1c may either reflect the impact of deteriorating BCF on glycemic control or be a manifestation of a glucotoxic phenomenon on BCF. Future studies in youth type 2 diabetes should target the natural course of β-cell failure and means of retarding and/or preventing it.
Despite the increasing rate of type 2 diabetes in youth, the information on its pathophysiology is mostly derived from adult studies (1). Decreased insulin sensitivity and impaired β-cell function (BCF) are the two key components in type 2 diabetes pathogenesis (2–4). The development sequence of these abnormalities has been long debated. Several studies in adults proposed that insulin resistance with compensatory hyperinsulinemia is the initial step in type 2 diabetes pathogenesis (2,4). This is an implication of the hyperbolic relationship between insulin sensitivity and BCF, which calls for an increase in insulin secretion when insulin sensitivity decreases (4). The subsequent step in type 2 diabetes pathogenesis is impaired early insulin secretion, leading to postprandial and, later, fasting hyperglycemia (at which time clinical diabetes becomes evident). This sequence has also been documented by longitudinal studies in populations at high risk for developing type 2 diabetes, such as the Pima Indians of Arizona (3). In the former study, the progression from normal to impaired glucose tolerance (IGT) was associated with increased body weight, decreased insulin sensitivity, and a decline in the acute insulin secretory response to intravenous glucose. Progression from IGT to diabetes required a further increase in body weight and impairment in insulin sensitivity and BCF, as well as an increase in basal endogenous glucose output (3). Similar observations were reported in insulin-sensitive and insulin-resistant African-American adults with type 2 diabetes who showed a marked decrease in insulin secretion as they progressed from near normoglycemia to frank hyperglycemia (5).
Metabolic studies of insulin sensitivity and secretion in type 2 diabetes in youth are almost nonexistent. Therefore, the objectives of this study were 1) to investigate in vivo insulin sensitivity and BCF, using the hyperinsulinemic-euglycemic and hyperglycemic clamp methods in adolescents with type 2 diabetes in comparison with obese, nondiabetic control subjects, and 2) to assess the relationship of diabetes duration and HbA1c to insulin sensitivity and BCF.
RESEARCH DESIGN AND METHODS
We studied 14 adolescents with type 2 diabetes in comparison with 20 obese control subjects who were otherwise healthy. Some of the obese control subjects were reported previously (6,7). The duration of diabetes was 1.5 ± 0.5 years (median 1 year), with the exception of one subject who had a diabetes duration of 6.3 years. Because results were not different when analysis was performed with and without this subject, we report our results including this subject. The characteristics of the study population are summarized in Table 1.
Table 1.
Physical characteristics and fasting metabolic data of the study subjects
| Obese control subjects |
Type 2 diabetic patients |
P | |
|---|---|---|---|
| n | 20 | 14 | |
| Age (years) | 14.6 ± 0.4 | 15.5 ± 0.5 | 0.12 |
| Sex (M/F) | 7/13 | 4/10 | 1.00 |
| Ethnicity (African American/white) | 16/4 | 10/4 | 0.69 |
| BMI (kg/m2) | 37.5 ± 1.3 | 35.9 ± 1.8 | 0.47 |
| Percent body fat | 43.5 ± 1.0 | 41.3 ± 1.5 | 0.21 |
| Visceral adipose tissue (cm2) | 68.2 ± 6.2 | 90.6 ± 15.0 | 0.27 |
| Subcutaneous adipose tissue (cm2) | 608.7 ± 35.5 | 521.8 ± 39.2 | 0.12 |
| HbA1c (%) | 5.4 ± 0.1 | 6.9 ± 0.3 | <0.001 |
| Glucose (mmol/l) | 5.4 ± 0.1 | 7.5 ± 0.3 | <0.001 |
| Insulin (pmol/l) | 220.2 ± 18.0 | 289.8 ± 24.6 | 0.007 |
| C-peptide (nmol/l) | 0.87 ± 0.08 | 1.43 ± 0.17 | 0.003 |
| Proinsulin (pmol/l) | 36.8 ± 5.1 | 93.8 ± 13.1 | <0.001 |
| Proinsulin-to-insulin ratio | 0.16 ± 0.02 | 0.34 ± 0.04 | 0.002 |
| Adiponectin (µg/ml) | 7.5 ± 1.0 | 3.6 ± 0.3 | 0.001 |
| Hepatic glucose production (µmol · kg−1 · min−1) |
12.3 ± 0.5 | 16.5 ± 1.1 | 0.002 |
| Triglycerides (mmol/l) | 1.07 ± 0.09 | 1.63 ± 0.24 | 0.042 |
| VLDL (mmol/l) | 0.21 ± 0.02 | 0.33 ± 0.05 | 0.046 |
Data are means ± SEM. Cholesterol, LDL, and HDL values are not statistically different between the two groups (data not shown).
Participants with type 2 diabetes were recruited from the Diabetes Center of Children’s Hospital of Pittsburgh. The diagnosis of type 2 diabetes was established based on the American Diabetes Association criteria for diabetes (1) and absence of pancreatic autoimmune markers including GAD antibodies and islet cell antibodies (ICAs). The obese control subjects were recruited through local advertisements. The treatment of type 2 diabetic patients consisted of metformin (seven patients), insulin (one patient), and insulin and metformin (one patient), in addition to recommendations for lifestyle modification of nutrition and physical activity in all. Five patients were receiving no medication for diabetes treatment at the time of the study. The eligibility criteria for the type 2 diabetic subjects included an HbA1c level <8.5% and absence of other significant diseases or medications, particularly those with a known impact on blood glucose regulation (i.e., systemic glucocorticoids). One female with type 2 diabetes was receiving oral contraceptive pills. Two of the 10 females with type 2 diabetes had polycystic ovary syndrome (PCOS) before the diagnosis of type 2 diabetes. Pubertal development was assessed by physical examination according to Tanner criteria (8). All control subjects were in good health, as assessed by history, physical examination, and routine hematological and biochemical tests. No subjects were receiving any medication, including contraceptive pills.
This study was approved by the Institutional Review Board of Children’s Hospital of Pittsburgh. Informed consent and assent were obtained from each subject and their legal guardians.
Each subject underwent a hyperinsulinemic-euglycemic clamp experiment to assess in vivo insulin sensitivity and a hyperglycemic clamp to assess in vivo insulin secretion, in random order within a 1- to 3-week interval. All subjects were admitted to the General Clinical Research Center on the afternoon before the day of clamp study. Each clamp study was performed after a 10- to 12-h overnight fast. Patients with type 2 diabetes were instructed to discontinue insulin and/or metformin 48 h before each clamp study. For each study, two intravenous catheters were inserted after the skin and subcutaneous tissues were anesthetized with Emla cream (Astra Pharmaceutical Products, West Borough, MA). One catheter was placed in a vein on the forearm for administration of insulin, glucose, and stable isotopes; the second catheter was placed in a vein on the dorsum of the contralateral heated hand for sampling of arterialized venous blood (9).
In vivo glucose metabolism and insulin sensitivity
Fasting endogenous glucose production (hepatic glucose production) was measured with a primed constant-rate infusion of [6,6-2H2]glucose (0.306 ± 0.009 µmol · kg−1 · min−1 in obese control subjects and 0.333 ± 0.01 µmol · kg−1 · min−1 in type 2 diabetic patients) (Isotech, Miamisburg, OH) from 7:30 a.m. to 9:30 a.m., as described by us previously (7). Blood was sampled at the start of the stable isotope infusion (−120 min) and every 10 min from −30 min to time 0 (basal period) for determination of plasma glucose, insulin, proinsulin, adiponectin, and isotopic enrichment of glucose. Fasting turnover calculations were made during the last 30 min (−30 to 0 min) of the basal 2-h infusion period.
After the 2-h baseline isotopic infusion period, insulin-mediated glucose metabolism and in vivo insulin sensitivity were measured during a 3-h hyperinsulinemic-euglycemic clamp, in conjunction with indirect calorimetry. Intravenous crystalline insulin (Humulin; Lilly, Indianapolis, IN) was infused at a constant rate of 80 mU · m−2 · min−1. Plasma glucose was clamped at ~5.5 mmol/l, with a variable rate infusion of 20% dextrose in water. Blood was sampled every 10–15 min for determination of insulin and every 5 min for glucose levels. Insulin-stimulated glucose metabolism was calculated during the last 30 min of the clamp.
Continuous indirect calorimetry by a ventilated hood system (Deltatrac Metabolic Monitor; Sensormedics, Anaheim CA) was performed to measure carbon dioxide production, oxygen consumption, and respiratory quotient (10).
In vivo insulin secretion
In vivo insulin secretion was assessed during a 2-h hyperglycemic clamp. Plasma glucose was increased rapidly to 12.5 mmol/l by a bolus infusion of dextrose given over a 2-min period and maintained at that level by variable rate infusion of 20% dextrose solution for 120 min. Glucose, insulin, and C-peptide concentrations were measured every 2.5 min (at 2.5, 5, 7.5, 10, and 12.5 min) and then every 5 min for glucose and every 15 min for insulin and C-peptide.
Body composition and abdominal fat
Body composition was determined by dual-energy X-ray absorptiometry (DEXA). Subcutaneous adipose tissue and visceral adipose tissue were examined by a single-slice computed tomography scan at intervertebral space L4–L5, as we reported before (7).
Biochemical measurements
Plasma glucose was measured by the glucose oxidase method with a glucose analyzer (Yellow Springs Instruments, Yellow Springs, OH), and the insulin concentration by radioimmunoassay using guinea pig antihuman insulin antibodies (Linco cat. no. 1011, Lot H15–30P [2T]; Linco Research) as reported by us previously (9). HbA1c was measured by high-performance liquid chromatography (A1c 2.2 Plus Glycohemoglobin Analyzer; Tosoh Medics, San Francisco, CA). Deuterium enrichment of glucose in the plasma was determined on a Hewlett Packard 5971 mass spectrometer (Hewlett Packard, Palo Alto, CA) coupled with a 5890 series II gas chromatograph, as reported previously (11). GAD antibodies and ICAs were measured by an immunoprecipitation assay at Quest Diagnostics Nichols Institute (San Juan Capistrano, CA). Plasma C-peptide and proinsulin levels were analyzed by immunochemiluminescent assay (Esoterix Endocrinology Laboratory, Calabasas Hills, CA). Fasting adiponectin was measured using a commercially available radioimmunoassay kit (Linco Research) as before (7). The intra- and interassay coefficients of variation were 3.6 and 9.3% for low serum concentrations and 1.8 and 9.3% for high serum concentrations, respectively. Lipid levels were measured in the Nutrition Laboratory of the University of Pittsburgh Graduate School of Public Health, certified by the Centers for Disease Control National Heart, Lung, and Blood Institute standardization program.
Calculations
Basal rate of appearance of glucose or hepatic glucose production was calculated during the last 30 min of the fasting 2-h isotope infusion period, according to steady-state tracer dilution equations as reported by us previously (11,12).
During the steady state of the 80 mU · m−2 · min−1 hyperinsulinemic-euglycemic clamp, insulin-stimulated glucose metabolism was calculated during the last 30 min to be equal to the exogenous glucose infusion rate (micromoles per kilogram per minute). In vivo insulin sensitivity (micromoles per kilogram per minute per picomoles per liter × 100) was calculated by dividing insulin-stimulated glucose metabolism (micromoles per kilogram per minute) by the steady-state plasma insulin concentration (picomoles per liter) as described previously (13).
Insulin-stimulated carbohydrate oxidation rates were calculated from indirect calorimetry data by averaging the data over the last 30 min of the hyperinsulinemic-euglycemic clamp according to Frayn formulas (12). Glucose storage or nonoxidative glucose disposal during hyperinsulinemia was estimated by subtracting glucose oxidation from total glucose disposal.
During the hyperglycemic clamp, the first-phase insulin secretion (FPIS) (picomoles per liter) was calculated as the mean of five insulin determinations at times 2.5, 5.0, 7.5, 10.0, and 12.5 min of the clamp. The second-phase insulin secretion (SPIS) (picomoles per liter) was calculated as the mean of eight determinations from 15–120 min (9).
Statistical analysis
Differences in continuous variables between the type 2 diabetic patients and obese control subjects groups were tested with either Student’s t test or the nonparametric equivalent, based on the nonviolation of statistical assumptions. Pearson or Spearman correlation analysis was used when applicable to examine bivariate relationships. All statistical assumptions were met. Data are presented as means ± SEM unless otherwise indicated. Statistical significance was set at P ≤ 0.05.
Data on visceral adiposity were missing in one type 2 diabetic subject and one obese control subject, and DEXA measurements were not possible in four subjects with type 2 diabetes and four obese control subjects with severe obesity whose weight exceeded the DEXA limit of 250 lb. Therefore, metabolic data are expressed per kilogram of body weight. One subject with type 2 diabetes did not participate in the hyperinsulinemic-euglycemic clamp study. Insulin levels were not available for analysis in another subject with type 2 diabetes whose serum revealed high nonspecific binding due to insulin antibodies. This subject had been receiving insulin therapy since diagnosis of diabetes.
RESULTS
Clinical and biochemical characteristics of the study subjects are presented in Table 1. The type 2 diabetic and obese control groups were comparable with respect to age, sex, ethnicity, pubertal development, BMI, percent body fat, and visceral adiposity. ICAs and GAD antibodies were undetectable in all type 2 diabetic patients, except for one patient with low-titer GAD antibodies but undetectable ICAs.
Fasting metabolic data
Fasting plasma glucose, insulin, proinsulin-to-insulin ratio, and HbA1c were significantly higher in type 2 diabetic patients than in obese control subjects (Table 1). Fasting hepatic glucose production was higher in type 2 diabetic patients than in obese control subjects (Table 1). Among the fasting lipids, only triglycerides and VLDL were significantly higher in type 2 diabetic patients. Adiponectin was significantly lower in type 2 diabetic patients compared with obese control subjects (Table 1).
Insulin sensitivity
Steady-state plasma glucose and insulin concentrations during the hyperinsulinemic-euglycemic clamp were not different between the type 2 diabetic and obese control groups (5.6 ± 0.03 mmol/l in both groups; 1,883.0 ± 215.7 and 1,787.0 ± 95.0 pmol/l, respectively).
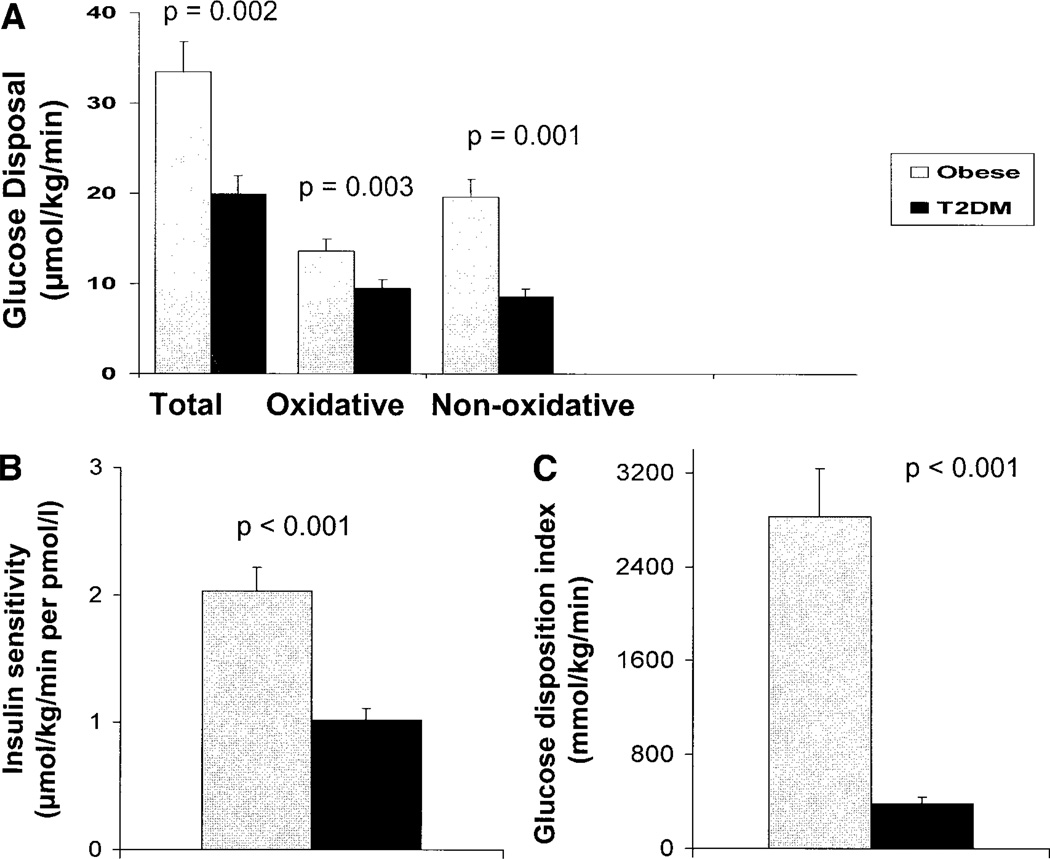
Insulin-stimulated total glucose disposal and oxidative and nonoxidative glucose disposal were significantly lower in type 2 diabetic patients than in obese control subjects (Fig. 1). Insulin sensitivity was 50% lower in type 2 diabetic patients compared with obese control subjects (Fig. 1A and B).
Figure 1.
Insulin-stimulated total, oxidative, and nonoxidative glucose disposal (A) and insulin sensitivity (B) during a 3-h hyperinsulinemic (80 mU · m−2 · min−1)-euglycemic clamp in adolescents with type 2 diabetes (T2DM) versus obese control subjects. C: GDI in adolescents with type 2 diabetes and obese control subjects.
BCF and insulin secretion
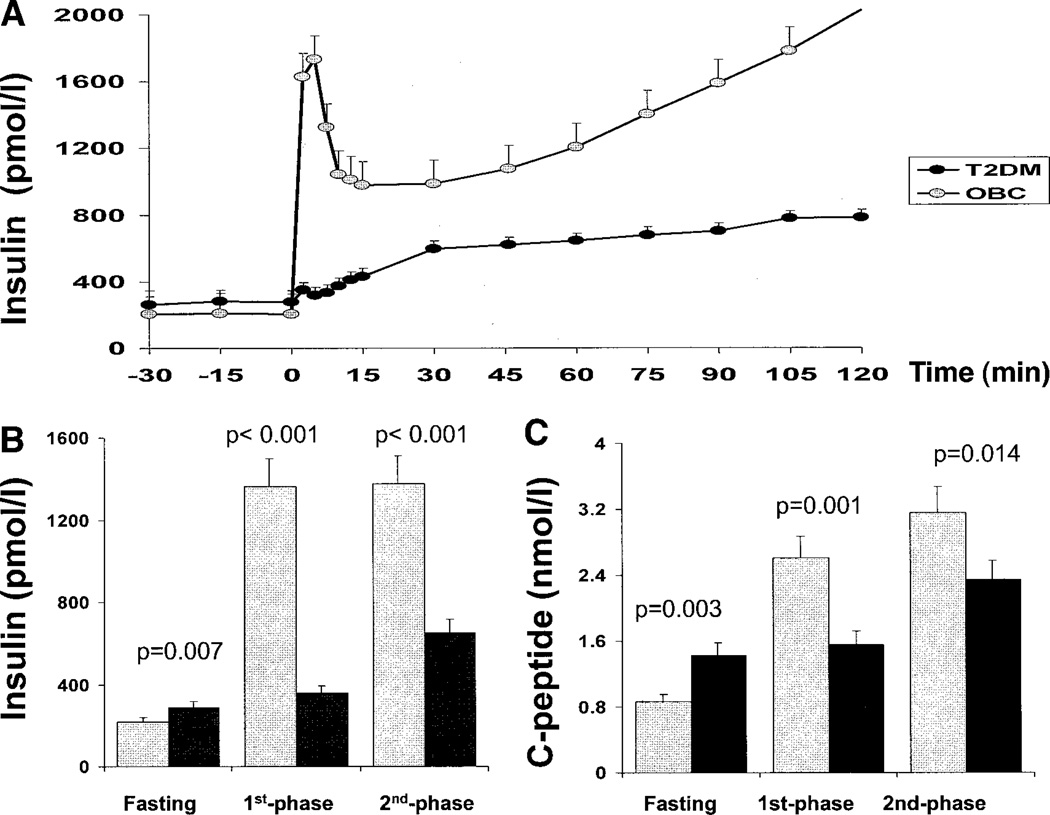
FPIS and SPIS responses, as well as C-peptide responses, were significantly lower in type 2 diabetic subjects compared with obese control subjects (Fig. 2).
Figure 2.
Insulin levels during the hyperglycemic clamp (A), mean of fasting, first-phase, and second-phase insulin levels (B), and mean of fasting, first-phase, and second-phase C-peptide levels (C) during the hyperglycemic (12.5 mmol/l) clamp in type 2 diabetes (T2DM) patients versus obese control (OBC) subjects.
Glucose disposition index
Glucose disposition index (GDI) was ~86% lower in type 2 diabetic patients compared with obese control subjects (379.7 ± 60.5 vs. 2,831.0 ± 410.2 mmol · kg−1 · min−1; P < 0.001) (Fig. 1C). When the African-American obese and type 2 diabetic subjects were compared without including the white subjects, data were consistent with those for the total group, demonstrating significantly low insulin sensitivity, insulin secretion, and GDI in the type 2 diabetic group. There were no significant differences in GDI between African-American and white diabetic subjects. However, the number of subjects is limited for statistical significance (data not shown).
Correlations
In youth with type 2 diabetes, HbA1c correlated inversely with FPIS (r = −0.61, P = 0.025) with no relationship to insulin sensitivity. Duration of diabetes did not correlate with insulin sensitivity or FPIS. GDI correlated negatively with HbA1c (r = −0.77, P = 0.004) but did not correlate with duration of diabetes.
In the total group, adiponectin correlated positively with insulin sensitivity (r = 0.52, P = 0.003) and FPIS (r = 0.38, P = 0.033) and negatively with proinsulin-to-insulin ratio (r = −0.45, P = 0.013).
CONCLUSIONS
This is the first study in youth demonstrating that both insulin sensitivity and insulin secretion are impaired in adolescents with type 2 diabetes compared with matched obese control subjects. Even though our findings are in agreement with the literature on adults with type 2 diabetes (2–4), the disconcerting observation is the severe impairment in insulin secretion (FPIS ~75% lower; SPIS ~50% lower) at a very young age with a relatively short duration of diabetes. The implication of such findings could potentially be the early need for insulin replacement therapy to maintain glycemic control. Moreover, the present investigation shows an inverse relationship between HbA1c and FPIS. This relationship may either reflect the impact of deficient insulin secretion on the outcome of glycemic control or may be viewed as a glucotoxic phenomenon of poor glycemic control on insulin secretion.
Metabolic studies in type 2 diabetes of youth are scarce in the literature. A Japanese study (14) using the frequently sampled intravenous glucose tolerance test revealed that obese nondiabetic adolescents and obese adolescents with type 2 diabetes were equally insulin resistant. This is in contrast to our findings of ~50% lower insulin sensitivity in the type 2 diabetic group compared with obese control subjects. The former study, however, did not match the groups with respect to body composition and body fat distribution. Our finding of increased insulin resistance in youth with type 2 diabetes is in agreement with various cross-sectional and longitudinal studies in adult populations with (or at risk for developing) type 2 diabetes that identified insulin resistance as a key component of type 2 diabetes pathophysiology (3,15–17). In further support of the lower insulin sensitivity in type 2 diabetes is our finding of significantly lower adiponectin levels in type 2 diabetic patients, despite similar body composition and visceral adiposity to obese control subjects. Hotta et al. (18) demonstrated decreased plasma adiponectin levels in adults with type 2 diabetes compared with obese control subjects. Weyer et al. (19) reported lower plasma adiponectin levels in Pima Indians with IGT and type 2 diabetes than in those with normal glucose tolerance (NGT). In a recent investigation of nondiabetic youth, we demonstrated that hypoadiponectinemia is a strong and independent correlate of insulin resistance (7). Our findings with respect to lower FPIS and GDI in adolescents with type 2 diabetes are in agreement with the Japanese study in adolescents (14).
Another study in 9- to 20-year-old type 2 diabetic patients revealed relative hypoinsulinemia and hyperglucagonemia in response to a mixed liquid meal tolerance test (20). However, the insulin deficiency was not expressed relative to the degree of insulin resistance (not measured).
The increased fasting hepatic glucose production in adolescents with type 2 diabetes in our study (Table 1) is also in accordance with the longitudinal study by Weyer et al. (3), who identified an increase in basal endogenous glucose output as a critical component of transition from IGT to type 2 diabetes in adult Pima Indians who already had impairment in insulin sensitivity and BCF.
Impairments in the insulin biosynthetic process have been described in adults with type 2 diabetes. Levels of circulating proinsulin and its cleavage intermediate des-31,32-proinsulin are disproportionately elevated (4). The fasting proinsulin-to-insulin ratio was significantly higher in our youth with type 2 diabetes compared with obese control subjects, similar to the findings of Roder et al. (21) in adults with type 2 diabetes and obese control subjects. This is another metabolic phenotype of impaired BCF in youth with type 2 diabetes.
There are no reported longitudinal studies in pediatric subjects with type 2 diabetes assessing the evolution of the disease. PCOS is a condition characterized by severe insulin resistance and is a major risk factor for type 2 diabetes (1). Our previous cross-sectional studies in this high-risk group demonstrated that PCOS adolescents with NGT are insulin resistant and hyperinsulinemic compared with matched obese girls (13). However, PCOS adolescents with IGT have impaired FPIS with no derangement in SPIS (11). Our current study in type 2 diabetic adolescents shows severe impairments in both FPIS and SPIS. These studies, although cross-sectional, collectively suggest that the essential metabolic determinant of the progression from NGT to IGT to type 2 diabetes is BCF. Our cross-sectional observations are in agreement with longitudinal data in insulin-resistant Pima Indian adults demonstrating progressive loss of acute insulin response to intravenous glucose throughout transition from NGT to IGT to type 2 diabetes (3). The Botnia study of type 2 diabetes pathogenesis in at-risk European populations depicted a lower insulin sensitivity when comparing NGT with IGT and IGT with type 2 diabetes, cross-sectionally with the homeostasis model assessment (HOMA) approach (17). This study further demonstrated an inverted U-shaped insulin secretion pattern (determined with the oral glucose tolerance test) such that subjects with type 2 diabetes showed markedly impaired insulin secretion that could no longer compensate for insulin resistance and elevated glucose levels (17).
The U.K. Prospective Diabetes Study found that BCF was 50% of normal at the time of clinical diagnosis of type 2 diabetes (22). The clinically recognized progressive nature of adult type 2 diabetes as an ongoing decline in BCF without a change in insulin sensitivity has been reaffirmed by the U.K. Prospective Diabetes Study (22–23) and the Belfast diet intervention study (24) with the HOMA approach. As our study is a cross-sectional evaluation, we are unable to comment on the progressive nature of β-cell failure in type 2 diabetes of youth. However, in a recent case study we detected an ~15% decline in BCF per year over the 6-year duration of diabetes, with no substantial changes in insulin sensitivity (25). Further studies are needed to explore whether this observation of an accelerated loss in BCF with increasing duration of diabetes can be generalized to all youth with type 2 diabetes. If the early and severe impairment in pancreatic BCF is indeed coupled with an accelerated pace of BCF deterioration in youth with type 2 diabetes, then early insulin therapy rather than “insulin as the last resort” should carefully be considered.
In conclusion, when type 2 diabetes is clinically present in the pediatric population, both insulin sensitivity and BCF are impaired and hepatic glucose output is increased. The impairment in BCF appears to be of greater magnitude relative to that of insulin sensitivity compared with a nondiabetic obese group. Further studies are needed to investigate not only the natural history of BCF in youth with type 2 diabetes, but also strategies to retard and/or prevent its progressive failure.
Supplementary Material
Acknowledgments
This work was supported by U.S. Public Health Service Grant RO1 HD27503 (to S.A.), The Pittsburgh Foundation (to N.G.), Grant K24 HD01357 (to S.A.), General Clinical Research Center Grant MO1-RR00084 (to S.A. and N.G.), The University of Pittsburgh Obesity and Nutrition Research Center (to N.G.), the Cochrane-Weber Endowed Fund, and the Renziehausen Trust Fund.
We thank the nurses of the general clinical research center for expert nursing assistance, Pat Antonio and Kathy Wypychowski for secretarial assistance, and Sandy Stange and Jill Landsbaugh for recruitment efforts. We also thank our colleagues from the Children’s Hospital of Pittsburgh Diabetes Clinic for patient referrals. Last but not least, we express our sincere thanks to the volunteer adolescents and their parents.
Abbreviations
- BCF
β-cell function
- DEXA
dual-energy X-ray absorptiometry
- FPIS
first-phase insulin secretion
- GDI
glucose disposition index
- HOMA
homeostasis model assessment
- ICA
islet cell antibody
- IGT
impaired glucose tolerance
- NGT
normal glucose tolerance
- PCOS
polycystic ovary syndrome
- SPIS
second-phase insulin secretion.
Footnotes
A table elsewhere in this issue shows conventional and Système International (SI) units and conversion factors for many substances.
References
- 1.American Diabetes Association. Type 2 diabetes in children and adolescents. Diabetes Care. 2000;23:381–389. doi: 10.2337/diacare.23.3.381. [DOI] [PubMed] [Google Scholar]
- 2.Saad MF, Knowler WC, Pettitt DJ, Nelson RG, Charles MA, Bennett PH. A two-step model for development of non-insulindependent diabetes. Am J Med. 1991;90:229–235. [PubMed] [Google Scholar]
- 3.Weyer C, Bogardus C, Mott DM, Pratley RE. The natural history of insulin secretory dysfunction and insulin resistance in the pathogenesis of type 2 diabetes mellitus. J Clin Invest. 1999;104:787–794. doi: 10.1172/JCI7231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kahn SE. The importance of β-cell failure in the development and progression of type 2 diabetes. J Clin Endocrinol Metab. 2001;86:4047–4058. doi: 10.1210/jcem.86.9.7713. [DOI] [PubMed] [Google Scholar]
- 5.Banerji MA, Lebovitz HE. Insulin action in black Americans with NIDDM. Diabetes Care. 1992;15:1295–1302. doi: 10.2337/diacare.15.10.1295. [DOI] [PubMed] [Google Scholar]
- 6.Bacha F, Saad R, Gungor N, Janosky J, Arslanian SA. Obesity, regional fat distribution and syndrome X in obese black versus white adolescents: race differential in diabetogenic and atherogenic risk factors. J Clin Endocrinol Metab. 2003;88:2534–2540. doi: 10.1210/jc.2002-021267. [DOI] [PubMed] [Google Scholar]
- 7.Bacha F, Saad R, Gungor N, Arslanian S. Adiponectin in youth: relationship to visceral adiposity, insulin sensitivity, and β-cell function. Diabetes Care. 2004;27:547–552. doi: 10.2337/diacare.27.2.547. [DOI] [PubMed] [Google Scholar]
- 8.Tanner JM. Growth and maturation during adolescence. Nutr Rev. 1981;39:43–55. doi: 10.1111/j.1753-4887.1981.tb06734.x. [DOI] [PubMed] [Google Scholar]
- 9.Gungor N, Saad R, Janosky J, Arslanian S. Validation of surrogate estimates of insulin sensitivity and insulin secretion in children and adolescents. J Pediatr. 2004;144:47–55. doi: 10.1016/j.jpeds.2003.09.045. [DOI] [PubMed] [Google Scholar]
- 10.Danadian K, Balasekaran G, Lewy V, Meza MP, Robertson R, Arslanian SA. Insulin sensitivity in African-American children with and without family history of type 2 diabetes. Diabetes Care. 1999;22:1325–1329. doi: 10.2337/diacare.22.8.1325. [DOI] [PubMed] [Google Scholar]
- 11.Arslanian SA, Lewy VD, Danadian K. Glucose intolerance in obese adolescents with polycystic ovary syndrome: roles of insulin resistance and beta-cell dysfunction and risk of cardiovascular disease. J Clin Endocrinol Metab. 2001;86:66–71. doi: 10.1210/jcem.86.1.7123. [DOI] [PubMed] [Google Scholar]
- 12.Arslanian S, Suprasongsin C. Glucosefatty acid interactions in prepubertal and pubertal children: effects of lipid infusion. Am J Physiol. 1997;272:E523–E529. doi: 10.1152/ajpendo.1997.272.4.E523. [DOI] [PubMed] [Google Scholar]
- 13.Lewy VD, Danadian K, Witchel SF, Arslanian SA. Early metabolic abnormalities in adolescent girls with polycystic ovarian syndrome. J Pediatr. 2001;138:38–44. doi: 10.1067/mpd.2001.109603. [DOI] [PubMed] [Google Scholar]
- 14.Kobayashi K, Amemiya S, Higashida K, Ishihara T, Sawanobori E, Kobayashi K, Mochizuki M, Kikuchi N, Tokuyama K, Nakazawa S. Pathogenic factors of glucose intolerance in obese Japanese adolescents with type 2 diabetes. Metabolism. 2000;49:186–191. doi: 10.1016/s0026-0495(00)91221-6. [DOI] [PubMed] [Google Scholar]
- 15.Haffner SM, Stern MP, Mitchell BD, Hazuda HP, Patterson JK. Incidence of type II diabetes in Mexican-Americans predicted by fasting insulin and glucose levels, obesity and body fat distribution. Diabetes. 1990;39:283–288. doi: 10.2337/diab.39.3.283. [DOI] [PubMed] [Google Scholar]
- 16.Lillioja S, Mott D, Spraul M, Ferraro R, Foley JE, Ravussin E, Knowler WC, Bennett PH, Bogardus C. Insulin resistance and insulin secretory dysfunction as precursors of non-insulin-dependent diabetes mellitus: prospective studies of Pima Indians. N Engl J Med. 1993;329:1988–1992. doi: 10.1056/NEJM199312303292703. [DOI] [PubMed] [Google Scholar]
- 17.Tripathy D, Carlsson M, Almgren P, Isomaa B, Taskinen MR, Tuomi T, Groop LC. Insulin secretion and insulin sensitivity in relation to glucose tolerance: lessons from the Botnia Study. Diabetes. 2000;49:975–980. doi: 10.2337/diabetes.49.6.975. [DOI] [PubMed] [Google Scholar]
- 18.Hotta K, Funahashi T, Arita Y, Takahashi M, Matsuda M, Okamoto Y, Iwahashi H, Kuriyama H, Ouchi N, Maeda K, Nishida M, Kihara S, Sakai N, Nakajima T, Hasegawa K, Muraguchi M, Ohmoto Y, Nakamura T, Yamashita S, Hanafusa T, Matsuzawa Y. Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler Thromb Vasc Biol. 2000;20:1595–1599. doi: 10.1161/01.atv.20.6.1595. [DOI] [PubMed] [Google Scholar]
- 19.Weyer C, Funahashi T, Tanaka S, Hotta K, Matsuzawa Y, Pratley RE, Tataranni PA. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab. 2001;86:1930–1935. doi: 10.1210/jcem.86.5.7463. [DOI] [PubMed] [Google Scholar]
- 20.Umpaichitra V, Bastian W, Taha D, Banerji MA, AvRuskin TW, Castells S. C-peptide and glucagon profiles in minority children with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2001;86:1605–1609. doi: 10.1210/jcem.86.4.7415. [DOI] [PubMed] [Google Scholar]
- 21.Roder ME, Dinesen B, Hartling SG, Houssa P, Vestergaard H, Sodoyez-Goffaux F, Binder C. Intact proinsulin and β-cell function in lean and obese subjects with and without type 2 diabetes. Diabetes Care. 1999;22:609–614. doi: 10.2337/diacare.22.4.609. [DOI] [PubMed] [Google Scholar]
- 22.Matthews DR, Cull CA, Stratton IM, Holman RR, Turner RC. UKPDS 26: sulfonylurea failure in non-insulin dependent diabetic patients over six years: UK Prospective Diabetes Study (UKPDS) Group. Diabet Med. 1998;15:297–303. doi: 10.1002/(SICI)1096-9136(199804)15:4<297::AID-DIA572>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 23.Holman RR. Assessing the potential for alpha-glucosidase inhibitors in prediabetic states. Diabetes Res Clin Pract. 1998;40:S21–S25. doi: 10.1016/s0168-8227(98)00038-2. [DOI] [PubMed] [Google Scholar]
- 24.Levy J, Atkinson AB, Bell PM, McCance DR, Hadden DR. Beta-cell deterioration determines the onset and rate of progression of secondary dietary failure in type 2 diabetes mellitus: the 10-year follow-up of the Belfast Diet Study. Diabet Med. 1998;15:290–296. doi: 10.1002/(SICI)1096-9136(199804)15:4<290::AID-DIA570>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 25.Gungor N, Arslanian S. Progressive beta cell failure in type 2 diabetes mellitus of youth. J Pediatr. 2004;144:656–659. doi: 10.1016/j.jpeds.2003.12.045. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.