Abstract
BACKGROUND
The authors conducted exploratory phase 1–2 clinical trials vaccinating breast cancer patients with E75, a human leukocyte antigen (HLA) A2/A3–restricted HER-2/neu (HER2) peptide, and granulocyte-macrophage colony-stimulating factor. The vaccine is given as adjuvant therapy to prevent disease recurrence. They previously reported that the vaccine is safe and effective in stimulating expansion of E75-specific cytotoxic T cells. Here, they report 24-month landmark analyses of disease-free survival (DFS).
METHODS
These dose escalation/schedule optimization trials enrolled lymph node-positive and high-risk lymph node-negative patients with HER2 (immunohistochemistry [IHC] 1-3+) expressing tumors. HLA-A2/A3+ patients were vaccinated; others were followed prospectively as controls for recurrence. DFS was analyzed by Kaplan-Meier curves; groups were compared using log-rank tests.
RESULTS
Of 195 enrolled patients, 182 were evaluable: 106 (58.2%) in the vaccinated group and 76 (41.8%) in the control group. The 24-month landmark analysis DFS was 94.3% in the vaccinated group and 86.8% in the control group (P = .08). Importantly, because of trial design, 65% of patients received a lower than optimal vaccine dose. In subset analyses, patients who benefited most from vaccination (vaccinated group vs control group) had lymph node-positive (DFS, 90.2% vs 79.1%; P = .13), HER2 IHC 1+-2+ (DFS, 94.0% vs 79.4%; P = .04), or grade 1 or 2 (DFS, 98.4% vs 86.0%; P = .01) tumors and were optimally dosed (DFS, 97.3% vs 86.8%; P = .08). A booster program has been initiated; no patients receiving booster inoculations have recurred.
CONCLUSIONS
The E75 vaccine has clinical efficacy that is more prominent in certain patients. A phase 3 trial enrolling lymph node-positive patients with HER2 low-expressing tumors is warranted.
Keywords: breast cancer, HER2/neu, E75, immunotherapy, cancer vaccines
INTRODUCTION
The identification of tumor-associated antigens that can be targeted therapeutically has led to the development of immune-based therapies, including cancer vaccines. Using cancer vaccines to enlist the patient’s immune system to recognize and target tumor cells is an appealing strategy because it represents a specific therapeutic modality with minimal toxicity. Because vaccines stimulate the adaptive immune system, T-cell memory responses are generated, which can potentially lead to a long-term benefit.
The most studied tumor-associated antigen in breast cancer is HER-2/neu (HER2), and several immunogenic peptides derived from HER2 have been shown to elicit a specific immune response. Among these peptides, the most studied in the laboratory and in clinical studies is E75 (KIFGSLAFL, HER2:369-377; reviewed by Mittendorf et al).1 Our group conducted exploratory phase 1-2 clinical trials of a peptide-based vaccine strategy of intradermally administering E75 mixed with granulocyte-macrophage colony-stimulating factor (GM-CSF) immunoadjuvant to disease-free lymph node-positive or high-risk lymph node-negative breast cancer patients in the adjuvant setting. The goal of our strategy is to prevent disease recurrence in patients at high risk for relapse. The goal of these exploratory trials was to determine whether further investigation of E75 + GM-CSF was warranted in a phase 3 trial and to determine the appropriate patient population to enroll on such a study.
We previously reported a primary analysis of these trials initiated at a median follow-up of 18 months per protocol design.2 The vaccine was demonstrated to be safe and effective in stimulating HER2-specific immunity. Importantly, at the time of that planned analysis, the vaccine had clinical efficacy, with the vaccinated group having a breast cancer recurrence rate of only 5.6% compared with 14.2% for the observation (control) group (P = .04). Trial follow-up was extended to 5 years, and patients provided consent for the additional participation period.
Later, it was observed that late recurrences in the vaccinated group corresponded to waning immunity, as demonstrated by decreased levels of E75-specific cytotoxic T lymphocytes (CTLs). This finding suggested that a booster inoculation may be necessary to maintain significant immunity. Toward that end, we instituted a booster program and recently reported the booster to be safe and effective in restimulating E75-specific immunity in patients who had failed to maintain significant residual immunity after initial vaccination.3
When the length of follow-up for the trials was extended to 5 years, additional analyses were incorporated to include the evaluation of disease-free survival (DFS) at 24 and 60 months. Twenty-four–month follow-up has now been completed in all patients, and here we report the 24-month landmark analyses as well as outcomes data for study patients who received booster inoculations.
MATERIALS AND METHODS
Patient Characteristics and Clinical Protocols
The trials were approved by local institutional review boards and conducted under an investigational new drug application (BB-IND#9187). Trial details were previously reported.2,4 Briefly, all patients had histologically confirmed lymph node-positive or high-risk lymph node-negative breast cancer. High-risk lymph node-negative was defined as the presence of any of the following: T2 tumor, grade 3, presence of lymphovascular invasion, estrogen receptor (ER) or progesterone receptor (PR) negative, HER2 ICH 3−, or pN0(i+). All patients completed a standard course of surgery, chemotherapy, and radiation therapy (as required) before enrollment. All patients were determined to be disease free at the time of enrollment based on standard of care evaluation performed by their treating physicians. Patients who had been receiving hormonal therapy were continued on their prescribed regimens. Because E75 binds to the human leukocyte antigen (HLA) A2 and A3 alleles, enrolled patients were HLA typed. HLA-A2/A3+ patients were vaccinated, and HLA-A2/A3− patients were observed prospectively for recurrence. Before vaccination, all patients were determined to be immunocompetent using a recall antigen panel.
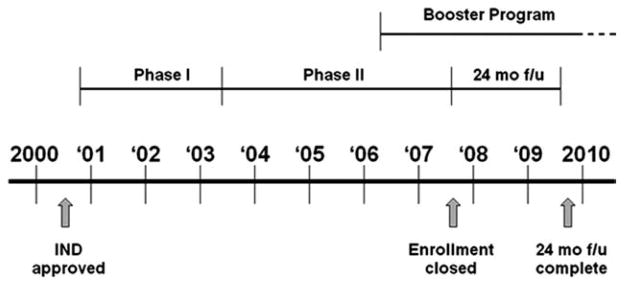
The initial lymph node-positive trial was a phase 1 two-stage safety trial of the vaccine designed with escalating doses of E75 peptide in the initial stage followed by schedule alterations.4 Groups were expanded to determine and confirm optimal dosing in lymph node-positive patients (Table 1). The lymph node-negative trial was designed to further delineate optimal biologic dosing of the vaccine by varying the GM-CSF dose and altering the inoculation schedule (Table 1). Both trials transitioned to phase 2 with disease recurrence as the primary efficacy endpoint. Because the lymph node-positive and lymph node-negative trials were run concurrently and the protocols were identical except for overlapping doses/schedules, the results were merged for analysis as previously described.2 The trial timeline is outlined in Figure 1. Once the booster program was initiated, patients were offered an optional booster dose if they were ≥6 months from completion of their primary vaccination series.
Table 1.
E75 Dosing Regimens for Breast Cancer Node-Positive and Node-Negative Patient Groups by Trial Design
| Patient Group | Patients, No. | Peptide Dose, μg | GM-CSF Dose, μg | Months Vaccinated |
|---|---|---|---|---|
| Node positive | ||||
| 100.250.6a | 2b | 100 | 250 | 0, 1, 2, 3, 4, 5 |
| 500.250.4 | 6 | 500 | 250 | 0, 1, 2, 5 |
| 500.250.6 | 5 | 500 | 250 | 0, 1, 2, 3, 4, 5 |
| 1000.250.4 | 11 | 1000 | 250 | 0, 1, 2, 5 |
| 1000.250.6 | 27c | 1000 | 250 | 0, 1, 2, 3, 4, 5 |
| Node negative | ||||
| 500.125.3 | 10 | 500 | 125 | 0, 1, 5 |
| 500.125.4 | 9 | 500 | 125 | 0, 1, 2, 5 |
| 500.250.4 | 12 | 500 | 250 | 0, 1, 2, 5 |
| 500.250.6 | 13 | 500 | 250 | 0, 1, 2, 3, 4, 5 |
| 1000.250.6 | 11 | 1000 | 250 | 0, 1, 2, 3, 4, 5 |
| Total | 106 |
Abbreviation: GM-CSF, granulocyte-macrophage colony-stimulating factor.
Nomenclature signifies peptide dose, GM-CSF dose, and number of inoculations (ie, 100.250.6 describes 100 μg E75 mixed with 250 μg GM-CSF administered in 6 monthly inoculations).
One patient assigned to the 100.6 group withdrew, and no replacement was designated.
One patient assigned to the 1000.6 group received a single vaccine inoculation but was excluded secondary to a hepatitis C infection. She was followed in the vaccine arm on a per-treatment basis but not included in the optimal dose analysis.
Figure 1.
E75 vaccine trial schema. IND = Investigational New Drug.
Vaccine
The E75 peptide was commercially produced in good manufacturing practices grade by NeoMPS (San Diego, Calif). The peptide was purified to >95%. The amino acid content was determined by amino acid analysis. Sterility and general safety testing was carried out by the manufacturer. Lyophilized peptide was reconstituted at the appropriate dose in 0.5 mL sterile saline. The peptide was mixed with GM-CSF (Berlex, Seattle, Wash) in 0.5 mL, the 1.0-mL inoculation was split, and 0.5 mL was given intradermally at 2 sites 5 cm apart in the same extremity.
Clinical Recurrences of Disease
All patients were evaluated for clinical recurrence of disease per standard of care cancer screening dictated by their oncologists. Imaging studies were obtained based on symptomatology; there were no protocol-mandated imaging studies required. A patient was considered to have a breast cancer recurrence if the recurrence was biopsy proven or if the patient was treated for recurrence.
Statistical Analysis
A prespecified 24-month landmark analysis was conducted using each patient’s data at the 24-month time point after enrollment onto the trial. This method of analysis allowed for each patient’s data regarding disease recurrence to be recorded as a binary outcome, that is, either yes or no at that 24-month time point. Categorical variables between groups were compared using Fisher exact test. Continuous data are presented as means ± standard deviations and compared using a Wilcoxon rank sum test. Data were also summarized using the median and range, and groups were compared using the Wilcoxon rank sum test. Kaplan-Meier curves were used to quantify DFS over time; formal comparison between groups was conducted using the simple log-rank test. A P value <.05 was considered significant. Statistical analyses were performed using SPSS software (IBM, Armonk, NY).
RESULTS
Patients and Study Design
The combined clinical trials enrolled 195 patients (lymph node positive, 100; lymph node negative, 95). Six patients withdrew, 1 was lost to follow-up, and 1 was excluded for failure to receive standard of care surgical therapy. At the time trial follow-up was extended to 5 years, 5 patients did not sign informed consent for follow-up beyond the original 18 months. No recurrences were documented among these 5 patients at that time point; however, they were not included in this analysis because they did not complete the full 24 months of follow-up. This left a cohort of 182 evaluable patients (lymph node positive, 94; lymph node negative, 88) for the 24-month analysis. HLA-A2/A3+ patients (n = 106) were vaccinated (lymph node positive, 51; lymph node negative, 55), whereas the remaining 76 (lymph node positive, 43; lymph node negative, 33) were assigned to observation. Table 2 details patient clinicopathologic characteristics by treatment group.
Table 2.
Clinicopathologic Characteristics of Evaluable Patients in the E75 Vaccine Trials by Treatment Group at 24-Month Landmark Analysis
| Characteristics | Vaccinated, n = 106, No. (%) | Controls, n = 76, No. (%) | P |
|---|---|---|---|
| Age, y | .38 | ||
| Median | 57 | 53 | |
| Range | (28–78) | (32–83) | |
| Race | .19 | ||
| White | 95 (89.6%) | 64 (81.2%) | |
| Black | 5 (4.7%) | 10 (13.2%) | |
| Other | 6 (5.7%) | 2 (2.6%) | |
| Time to enrollment in trial in days | |||
| Median | 472 | 435 | .25 |
| Tumor size | .45 | ||
| T1 | 71 (67.0%) | 46 (60.5%) | |
| T2 | 26 (24.5%) | 18 (23.7%) | |
| T3 | 7 (6.6%) | 8 (10.5%) | |
| T4 | 2 (1.9%) | 4 (5.3%) | |
| Nodal status | .13 | ||
| N0 | 55 (51.9%) | 33 (43.4%) | |
| N1 | 39 (36.8%) | 25 (32.9%) | |
| N2 | 9 (8.5%) | 11 (14.5%) | |
| N3 | 3 (2.8%) | 7 (9.2%) | |
| Other tumor characteristics | .45 | ||
| Histologic grade 3 | 40 (38.8%) | 30 (41.1%) | .88 |
| ER and PR negative | 33 (31.7%) | 14 (18.4%) | .06 |
| HER2 overexpressiona | 30 (30.3%) | 18 (26.5%) | .61 |
| Trastuzumab | 12 | 3 | |
| Treatment | .13 | ||
| Hormonal therapy | 70 (66.0%) | 57 (76.0%) | .19 |
| Chemotherapy | 79 (74.5%) | 54 (71.1%) | .62 |
| Radiation therapy | 77 (72.6%) | 62 (81.6%) | .22 |
| Received optimal dose of vaccineb | 37 (34.9%) | N/A | |
| Yes | 37 (34.9%) | N/A | |
| No | 69 (65.1%) | N/A |
Abbreviations: ER, estrogen receptor; HER2, human epidermal growth receptor 2; N/A, not applicable; PR, progesterone receptor.
HER2 status was not determined in 7 vaccinated patients and 8 control patients.
The optimal dose was determined to be 1000 μg E75 mixed with 250 μg granulocyte-macrophage colony-stimulating factor administered in 6 inoculations.
DFS
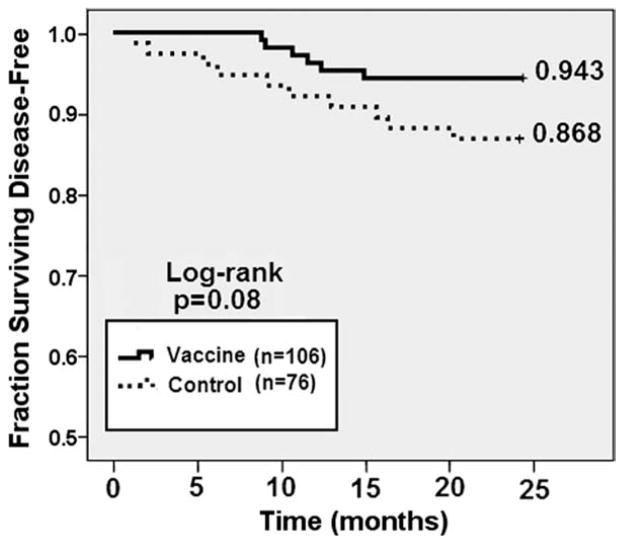
The 24-month analysis demonstrated disease recurrence in 5.6% of the vaccinated patients and 13.1% of the controls for DFS rates of 94.3% and 86.8%, respectively (P = .08) (Fig. 2). At 24 months, the vaccine was associated with a 57% reduction in relative risk of recurrence.
Figure 2.
24-month disease-free survival for all vaccinated patients compared with unvaccinated control patients.
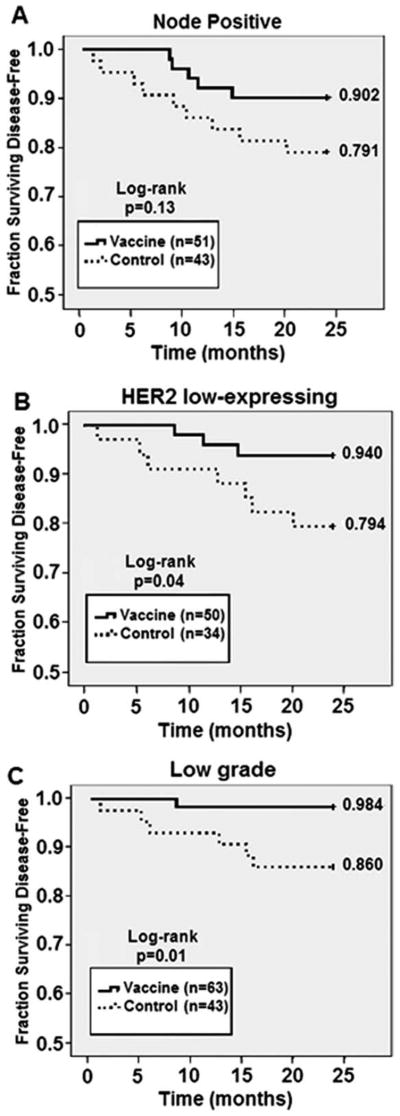
We performed subgroup analyses to determine whether there were specific factors that could identify patients more likely to benefit from vaccination. We also analyzed the lymph node-positive and lymph node-negative trials separately. Among lymph node-positive patients, we found no statistically significant differences with respect to clinicopathologic factors between vaccinated patients (n = 51) and controls (n = 43). The 24-month DFS rate for lymph node-positive patients was 90.2% for vaccinated patients and 79.1% for controls (P = .13) (Fig. 3A). This equates to a 53% relative risk reduction in recurrence. Among lymph node-negative patients, we found no statistically significant differences with respect to clinicopathologic factors between vaccinated patients (n = 55) and controls (n = 33). The 24-month DFS rate for lymph node-negative patients was 98.2% for vaccinated patients and 97.0% for controls (P = 1.0) The individual analyses demonstrated a reduction in recurrences in both lymph node-positive and lymph node-negative trials that was more pronounced in the lymph node-positive group, likely because of the higher event rate.
Figure 3.
24-month disease-free survival (DFS) determined for clinicopathologic subgroups. DFS was compared between vaccinated patients and unvaccinated controls in patients with (A) node-positive breast cancer, (B) HER2 low-expressing (IHC 1+ or 2+ or FISH < 2.0) breast cancer, and (C) low-grade (grade 1 or 2) breast cancer.
An important aspect of our clinical trials was that they enrolled patients with breast tumors expressing all levels of HER2. Previously, we reported on the impact of HER2 expression levels on the response to vaccination and showed that patients with breast tumors that expressed low levels of HER2 (immunohistochemistry [IHC] 1+ or 2+ or fluorescent in situ hybridization [FISH] <2.0) had more robust immunologic responses than did patients whose tumors overexpressed HER2.5 Given that observation, we assessed DFS in patients with low HER2-expressing (IHC 1+ or 2+) tumors. We found no statistically significant differences with respect to clinicopathologic factors between vaccinated patients with HER2 1+ or 2+ tumors and controls. Vaccinated patients had a DFS rate of 94% versus 79.4% for controls (P = .04; Fig. 3B). In contrast, in patients whose tumors overexpressed HER2, the DFS rate was 90.3% for vaccinated patients versus 83.3% for controls (P = .44). Our trials began enrolling patients before trastuzumab became the standard of care therapy for HER2-overexpressing breast cancer in the adjuvant setting; therefore, the majority (68.8%) of patients with HER2-overexpressing tumors did not receive trastuzumab. Of 30 vaccinated patients who had HER2-overexpressing tumors, 12 patients received trastuzumab before vaccination, and none of these patients have had recurrences. Of 18 patients who did not receive trastuzumab, 3 (16.7%) patients have had disease recurrence within 24 months of enrollment.
Finally, we looked at the effect of tumor grade on clinical response to vaccination. Patients were divided into those with grade 1 or 2 versus grade 3 tumors. We observed that among patients with grade 1 and 2 tumors, vaccinated patients (n = 63) were less likely to have T2-T4 tumors than controls (n = 43; 21.0% vs 39.5%, respectively; P = .05). Conversely, vaccinated patients were more likely to have ER− and PR− tumors than controls (17.7% vs 2.3%, respectively; P = .03). Among patients with grade 1 and 2 tumors, there was a significant difference in DFS rates between vaccinated patients (98.4%) and controls (86.0%) (P = .01; Fig. 3C). Among the cohort with grade 3 tumors, there was no difference in the DFS rate between vaccinated patients (12.5%) and controls (13.3%; P = .90).
Dosing and Effect on DFS
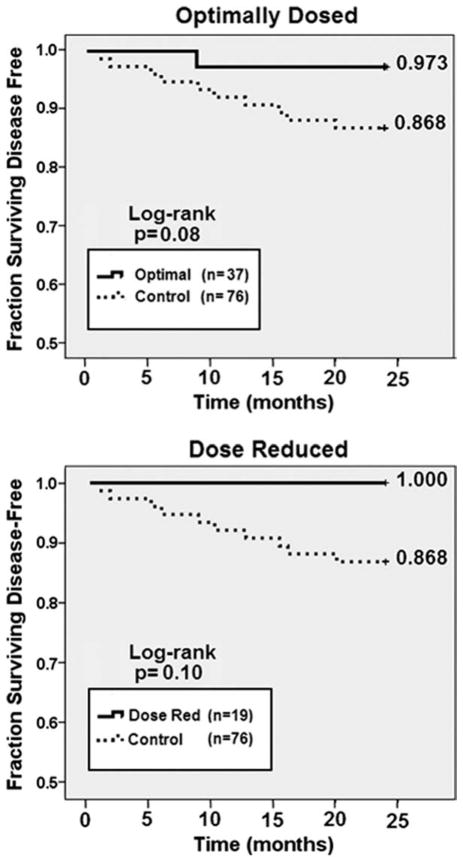
Because these trials began as dose- and schedule-finding trials, not all patients received the vaccine dose that was eventually determined to be optimal (1000 μg E75 + 250 μg GM-CSF).6 Therefore, we sought to determine the effect of dosing on DFS by comparing the DFS of patients who received the optimal dose (n = 37) with that of controls (n = 76). The 24-month DFS for patients who received the optimal dose of vaccine was 97.3% versus 86.8% for the controls (P = .08; Fig. 4, Top). Patients receiving the optimal dose of vaccine were more likely to have received chemotherapy in the adjuvant setting (89.2% vs 71.1% for controls; P = .03), a finding attributable to the finding that vaccinated patients were more likely to have lymph node-positive disease (70.3% vs 56.6% for controls; P = .22). In addition, because patients receiving the optimal dose were enrolled later in the trial after trastuzumab had become standard of care therapy for HER2+ breast cancer in the adjuvant setting, optimally dosed patients were more likely to have received trastuzumab (24.3%) than were controls (2.7%).
Figure 4.
24-month disease-free survival (DFS) based on dosing regimen. (A) patients who received the optimal dose were compared with unvaccinated controls. (B) patients who required dose reductions due to significant local reactions or ≥ grade 2 systemic toxicity were compared with unvaccinated contols. To date, no patient requiring a dose reduction has had disease recurrence.
As required by trial design, local reactions at the inoculation site as well as systemic toxicity were monitored. If a patient experienced a grade >2 systemic toxicity or if local reaction at the 2 inoculation sites merged and measured >100 mm in diameter, the GM-CSF dose was reduced by 50%. Dose reductions were required in 19 (17.9%) vaccinated patients, with all dose reductions occurring in the 2 highest-dose groups.6 Importantly, there were no recurrences among those patients demonstrating these robust reactions to the inoculations (Fig. 4, Bottom).
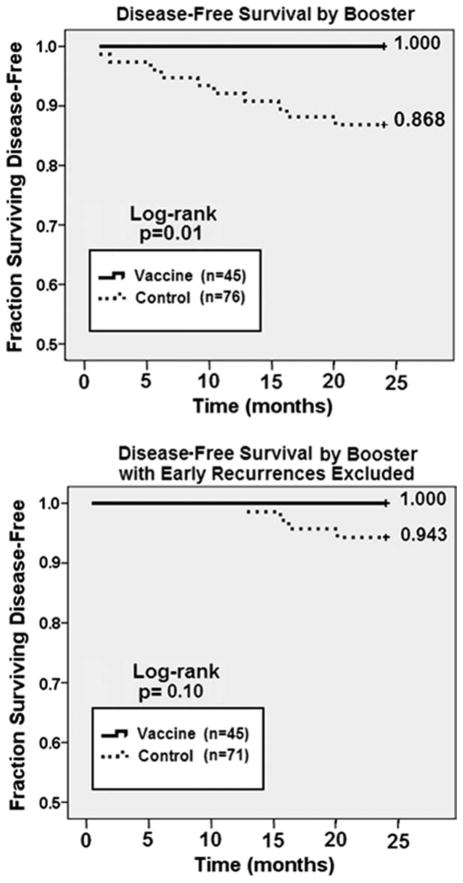
Booster Inoculations Outcomes
We instituted a voluntary vaccine booster program after observing late disease recurrences in vaccinated patients that corresponded with decreasing E75-specific immunity. Forty-five (42.5%) vaccinated patients received booster inoculations. Compared with controls, booster patients were more likely to be ER/PR negative (37.8% vs 18.4%, P = .03) and were more likely to have received trastuzumab (24.4% vs 2.7%, P < .01). Among patients who received booster inoculations, none has recurred, compared with a recurrence rate of 13.2% among controls (P = .01; Fig. 5, Top). Because patients whose disease recurred early after vaccination would not have been eligible for a booster inoculation, we also compared booster patients with controls, excluding control patients whose disease recurred early (<12 months). Again, booster patients had longer DFS times (Fig. 5, Bottom).
Figure 5.
24-month disease-free survival (DFS) comparing vaccinated patients who received booster inoculation with unvaccinated controls. (A) DFS comparing all vaccinated patients who received at least one booster inoculation prior to 24 months with unvaccinated controls. (B) DFs comparing vaccinated patients who received a booster 6 months after completing the primary vaccine series with unvaccinated controls, with patients having early recurrences (<12 months) excluted.
DISCUSSION
This study represents the largest breast cancer adjuvant vaccine trial conducted to date and the only 1 using the E75 + GM-CSF formulation. We previously reported the initial results of these exploratory E75 vaccine trials, demonstrating the vaccine to be safe and effective in eliciting HER2-specific immunity.2 Those early results suggested that the vaccine may have clinical efficacy in preventing or delaying disease recurrence in patients at high risk for relapse. The findings in this 24-month landmark analysis provide important information regarding which patients may benefit most from vaccination and have served to identify the population of patients to be targeted for enrollment onto a phase 3 trial. On the basis of these data, such a phase 3 trial investigating the efficacy of E75 in the adjuvant setting is warranted.
An important result of the current analysis is that it has further elucidated the population of patients that may benefit the most from E75 vaccination. Interestingly, despite the finding that this vaccine targets HER2, our previously reported data suggest that patients whose tumors have low HER2 expression mount a more robust immunologic response after vaccination than do patients whose tumors overexpress HER2. Patients with HER2 low-expressing tumors demonstrated significantly larger delayed-type hypersensitivity responses after completion of the vaccination series as well as more sustained long-term E75-specific CTL responses.5 This 24-month analysis provides further evidence suggesting that patients whose tumors have low HER2 expression benefit from vaccination, showing a statistically significant improvement in DFS rates (P = .04) compared with controls. These results suggest that the E75 vaccine could represent targeted therapy for patients whose tumors have some degree of HER2 expression but who do not meet current clinical criteria to receive trastuzumab.
We have previously hypothesized that patients with HER2-overexpressing tumors have some element of immunologic tolerance.5 However, despite the finding that these patients do not mount as robust an immune response as do patients with low HER2-expressing tumors, the vaccine still augments their HER2-specific immunity. It appears that patients with HER2-overexpressing tumors benefit from vaccination in addition to trastuzumab; we did not observe any disease recurrences in 11 patients who received both compared with recurrences in almost 20% of patients with HER2-overexpressing tumors vaccinated in the absence of trastuzumab. Because only 3 patients with HER2-overexpressing tumors received trastuzumab without vaccination, we are limited in the conclusions that can be drawn regarding the benefit of adding vaccination to standard of care trastuzumab therapy. However, data from the combined analysis of the National Surgical Adjuvant Breast and Bowel Project B-31 and North Central Cancer Treatment Group N9831 trials can be used to put our data into context. These trials evaluated the efficacy of trastuzumab in the adjuvant setting. At 3 years, the DFS in patients with HER2-overexpressing tumors who received trastuzumab was 87%.7 The number of patients in our trial is small; therefore, the data must be interpreted with caution. However, taken together with the results of the adjuvant trastuzumab trials, these data suggest that patients with HER2-overexpressing tumors who receive trastuzumab might derive additional benefit from vaccination. These findings are consistent with preclinical data published by our group and others demonstrating that treatment with trastuzumab enhanced the sensitivity of HER2-expressing tumor targets to HER2-specific CTLs.8–10 These findings are also consistent with previously published data from these vaccine trials demonstrating that patients who received the vaccine after trastuzumab were more likely to maintain long-term specific immunity, as quantified by the percentage of E75-specific CTLs.5 We are currently conducting a randomized phase 2 trial (NCT00524277; principal investigator, E.A.M.) investigating GP2, another HER2-derived HLA class I peptide that can stimulate the immune system. Because trastuzumab is now the standard of care for patients with HER2-overexpressing breast tumors, all patients with HER2-overexpressing tumors in that trial are receiving trastuzumab before vaccination. We anticipate that data from that trial will yield additional insight into this combination immunotherapeutic strategy of trastuzumab plus a CTL-eliciting vaccine.
Previous data reporting on the recurrences seen in these trials demonstrated that overall, vaccinated patients were less likely than controls to have recurrences and did not have recurrence of bone-only disease compared with controls, of whom 50% had bone-only disease.11 These data suggest that patients with biologically less aggressive disease may respond better to vaccination. Because tumor grade is a marker of disease aggressiveness, we compared DFS by grade. Patients with low-grade tumors who were vaccinated were more likely to have T1 tumors but were less likely to have ER+ or PR+ disease than were controls. These are competing risk factors, as it is known that smaller tumors are less likely to recur; however, ER− tumors are more likely to recur, in part because these patients do not receive the benefit of hormonal therapy.12–14 Regardless, among patients who had low-grade tumors, those who were vaccinated achieved a significant improvement in DFS over unvaccinated controls.
When evaluating these data, an important consideration is that these trials began as phase 1 trials designed to determine the safety and optimal dose/schedule of the vaccine; therefore, not all patients received the optimal dose. This is true for the overall DFS analysis as well as for subset analyses. We previously reported that patients who received the optimal dose of E75 had similar toxicity and enhanced immune responses compared with patients who received a lower dose. Importantly, there was a trend toward decreased recurrences in optimally dosed patients.6 Data presented in the current report confirm that finding. It also appears that incorporating a booster inoculation will be important in the E75 vaccine strategy. The decrease in E75-specific CTLs that we demonstrated in vaccinated patients in this trial is consistent with what is known about T-cell biology and response to antigenic stimulation. Immediately after stimulation, CTLs undergo a dramatic expansion phase, after which they contract and plateau during the death phase, when many CTLs undergo apoptosis. The remaining cells are antigen-specific central and effector memory CTLs that are critical for the maintenance of active immunologic memory. Levels of antigen-specific memory CTL have been observed to decline in this and other vaccine studies.3,15–17 Importantly, booster inoculations are effective in restimulating E75-specific immunity,3 and data in this report suggest that this restimulation may contribute to the prevention of disease recurrence.
On the basis of the data obtained in these exploratory trials, a phase 3 trial evaluating efficacy of the vaccine is warranted. In designing that trial, we used data from this study to define the appropriate patient population to enroll. The planned trial will enroll lymph node-positive patients, as they are at higher risk for disease recurrence. Only patients with HER2 low-expressing tumors defined as IHC 1+ or 2+ or FISH <2.2 but >1.4 will be eligible, as these patients have the most robust response to vaccination. All patients will receive the optimal dose plus booster inoculations. Importantly, HLA-A2/A3+ patients who meet eligibility criteria will be randomized to 1 of 2 arms: E75 + GM-CSF or GM-CSF alone. This double-blind trial design will address weaknesses of our phase 1–2 trials. First, our initial trials vaccinated all HLA-A2/A3+ patients and used HLA-A2/A3− patients as controls. It is unknown whether HLA status itself had a prognostic effect in these patients; however, direct comparisons between the vaccinated and control groups did not reveal significant clinically relevant differences in prognostic factors. Second, the completed trials had no immunoadjuvant-only arm; therefore, one could question whether the responses seen were because of GM-CSF alone, although this is unlikely based on our current randomized phase 2 GP2 trial with a GM-CSF–only arm.
By using the criteria proposed for the phase 3 trial, we identified 18 vaccinated and 27 control patients from the current cohort who were lymph node positive, had HER2 low-expressing tumors, and received optimal doses of the vaccine. There were no differences with respect to clinicopathologic characteristics between the 2 groups. Among these patients, the 24-month DFS rate was 100% for the vaccinated patients and 77.8% for controls (P = .04). There are limitations to this analysis. Specifically, unlike the strategy in the proposed phase 3 trial, the controls in these trials were not HLA-A2/A3+ and did not receive GM-CSF alone. Nevertheless, the significant difference in DFS noted between vaccinated and control patients suggests that the patient population we identified based on analysis of our phase 1–2 trials is the appropriate population to study in the next trial.
In summary, these analyses have demonstrated that the E75 vaccine may have clinical efficacy when optimally dosed and administered to appropriate patients. A phase 3 trial targeting the specific population of patients with lymph node-positive, HER2 low-expressing tumors is warranted.
Acknowledgments
FUNDING SOURCES
This work was supported by the United States Military Cancer Institute, Department of Surgery, Uniformed Services University of the Health Sciences; Clinical Breast Care Project; and Department of Clinical Investigation, Walter Reed Army Medical Center. Funding sources were not involved with study design; in collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication. Additional support was provided by the National Cancer Institute (4R00CA133244-03 to E.A.M.).
Footnotes
The first 2 authors contributed equally to this article.
The opinions or assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of the Department of the Army, the Department of the Navy, or the Department of Defense.
CONFLICT OF INTEREST DISCLOSURES
G.E.P. has partial inventor rights to E75 (owned jointly by The University of Texas MD Anderson Cancer Center and the US Government), and the patent was licensed to Apthera, Inc., Scottsdale, Arizona, after completion of the described trials. Under the terms of the license, G.E.P. is entitled to licensing revenues, and he also serves as a consultant to Apthera.
References
- 1.Mittendorf EA, Holmes JP, Ponniah S, Peoples GE. The E75 HER2/neu peptide vaccine. Cancer Immunol Immunother. 2008;57:1511–1521. doi: 10.1007/s00262-008-0540-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peoples GE, Holmes JP, Hueman MT, et al. Combined clinical trial results of a HER2/neu (E75) vaccine for the prevention of recurrence in high-risk breast cancer patients: U.S. Military Cancer Institute Clinical Trials Group Study I-01 and I-02. Clin Cancer Res. 2008;14:797–803. doi: 10.1158/1078-0432.CCR-07-1448. [DOI] [PubMed] [Google Scholar]
- 3.Holmes JP, Clifton GT, Patil R, et al. Use of booster inoculations to sustain the clinical effect of an adjuvant breast cancer vaccine: from US Military Cancer Institute Clinical Trials Group Study I-01 and I-02. Cancer. 2011;117:463–471. doi: 10.1002/cncr.25586. [DOI] [PubMed] [Google Scholar]
- 4.Peoples GE, Gurney JM, Hueman MT, et al. Clinical trial results of a HER2/neu (E75) vaccine to prevent recurrence in high-risk breast cancer patients. J Clin Oncol. 2005;23:7536–7545. doi: 10.1200/JCO.2005.03.047. [DOI] [PubMed] [Google Scholar]
- 5.Benavides LC, Gates JD, Carmichael MG, et al. The impact of HER2/neu expression level on response to the E75 vaccine: from U.S. Military Cancer Institute Clinical Trials Group Study I-01 and I-02. Clin Cancer Res. 2009;15:2895–2904. doi: 10.1158/1078-0432.CCR-08-1126. [DOI] [PubMed] [Google Scholar]
- 6.Holmes JP, Gates JD, Benavides LC, et al. Optimal dose and schedule of an HER-2/neu (E75) peptide vaccine to prevent breast cancer recurrence: from US Military Cancer Institute Clinical Trials Group Study I-01 and I-02. Cancer. 2008;113:1666–1675. doi: 10.1002/cncr.23772. [DOI] [PubMed] [Google Scholar]
- 7.Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 8.Kono K, Takahashi A, Sugai H, et al. Dendritic cells pulsed with HER-2/neu-derived peptides can induce specific T-cell responses in patients with gastric cancer. Clin Cancer Res. 2002;8:3394–3400. [PubMed] [Google Scholar]
- 9.Mittendorf EA, Storrer CE, Shriver CD, Ponniah S, Peoples GE. Investigating the combination of trastuzumab and HER2/neu peptide vaccines for the treatment of breast cancer. Ann Surg Oncol. 2006;13:1085–1098. doi: 10.1245/ASO.2006.03.069. [DOI] [PubMed] [Google Scholar]
- 10.zum Buschenfelde CM, Hermann C, Schmidt B, Peschel C, Bernhard H. Antihuman epidermal growth factor receptor 2 (HER2) monoclonal antibody trastuzumab enhances cytolytic activity of class I-restricted HER2-specific T lymphocytes against HER2-overexpressing tumor cells. Cancer Res. 2002;62:2244–2247. [PubMed] [Google Scholar]
- 11.Amin A, Benavides LC, Holmes JP, et al. Assessment of immunologic response and recurrence patterns among patients with clinical recurrence after vaccination with a preventive HER2/neu peptide vaccine: from US Military Cancer Institute Clinical Trials Group Study I-01 and I-02. Cancer Immunol Immunother. 2008;57:1817–1825. doi: 10.1007/s00262-008-0509-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 13.Campbell HE, Gray AM, Harris AL, Briggs AH, Taylor MA. Estimation and external validation of a new prognostic model for predicting recurrence-free survival for early breast cancer patients in the UK. Br J Cancer. 2010;103:776–786. doi: 10.1038/sj.bjc.6605863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Winer EP, Hudis C, Burstein HJ, et al. American Society of Clinical Oncology technology assessment on the use of aromatase inhibitors as adjuvant therapy for postmenopausal women with hormone receptor-positive breast cancer: status report 2004. J Clin Oncol. 2005;23:619–629. doi: 10.1200/JCO.2005.09.121. [DOI] [PubMed] [Google Scholar]
- 15.Klade CS, Wedemeyer H, Berg T, et al. Therapeutic vaccination of chronic hepatitis C nonresponder patients with the peptide vaccine IC41. Gastroenterology. 2008;134:1385–1395. doi: 10.1053/j.gastro.2008.02.058. [DOI] [PubMed] [Google Scholar]
- 16.Knutson KL, Schiffman K, Cheever MA, Disis ML. Immunization of cancer patients with a HER-2/neu, HLA-A2 peptide, p369-377, results in short-lived peptide-specific immunity. Clin Cancer Res. 2002;8:1014–1018. [PubMed] [Google Scholar]
- 17.Slingluff CL, Jr, Petroni GR, Chianese-Bullock KA, et al. Immunologic and clinical outcomes of a randomized phase II trial of 2 multipeptide vaccines for melanoma in the adjuvant setting. Clin Cancer Res. 2007;13:6386–6395. doi: 10.1158/1078-0432.CCR-07-0486. [DOI] [PubMed] [Google Scholar]