Abstract
In rodent species, the expression of reproductive behavior relies heavily on the perception of social odors, as well as the presence of circulating steroid hormones. In the Syrian hamster, chemosensory and hormonal cues are processed within an interconnected network of ventral forebrain nuclei that regulates many aspects of social behavior. Within this network, the posteromedial cortical amygdala (PMCo) receives direct projections from the accessory olfactory bulbs and contains a dense population of steroid receptor-containing neurons. Consequently, the PMCo may be important for generating odor-guided aspects of reproductive behavior, yet little is known regarding the role of this nucleus in regulating these behaviors. Thus, the present study tested male hamsters with site-specific electrolytic lesions of the PMCo for their (a) sexual odor preference in a Y-maze apparatus (b) sexual odor discrimination in a habituation-dishabituation task and (c) copulatory behavior when paired with a sexually receptive female. PMCo-lesioned males preferred to investigate female odors over male odors and were able to discriminate between these odor sources. However, PMCo lesions were associated with several alterations in the male copulatory pattern. First, PMCo-lesioned males displayed increased investigation of the female’s non-anogenital region, suggesting that the PMCo may be involved in directing appropriate chemosensory investigation during mating. Second, PMCo lesions altered the temporal pattern of the mating sequence, as PMCo-lesioned males took longer than Sham-lesioned males to reach sexual satiety, as indicated by the delayed expression of long intromissions. This delayed onset of satiety was associated with an increased number of ejaculations compared to Sham-lesioned males. Importantly, these data provide the first direct evidence for a functional role of the PMCo in regulating male reproductive behavior.
Keywords: Sexual behavior, Reproductive behavior, Sexual satiety, Olfaction, Lesions
In many rodent species, including the Syrian hamster, male reproductive behavior relies heavily on the perception of sexual odors (Hull et al., 2002). Male hamsters are highly attracted to female odors, and these chemosignals stimulate the expression of copulatory behaviors (Johnston, 1974, 1975, 1986) via both the main and accessory olfactory systems (Murphy and Schneider, 1970, Winans and Powers, 1977, Meredith, 1991, Restrepo et al., 2004). In addition to chemosensory processing, male hamster sexual behavior also requires the presence of circulating gonadal steroid hormones (Morin and Zucker, 1978, Powers and Bergondy, 1983, Powers et al., 1985, Petrulis and Johnston, 1995). Consequently, the expression of male reproductive behavior in the hamster involves the integration of chemosensory and hormonal cues (Wood, 1998), and this integration likely occurs within the extended network of ventral forebrain nuclei known to regulate mating behavior (Wood and Newman, 1995, Wood and Coolen, 1997).
Previous research has identified several critical brain areas within this network, including the medial preoptic areas (MPOA), bed nucleus of the stria terminalis (BNST) and medial amygdala (MEA), which regulate various aspects of male reproductive behavior (Lehman et al., 1980, Lehman et al., 1983, Powers et al., 1987, Maras and Petrulis, 2006). Although the posteromedial cortical nucleus of the amygdala (PMCo) is also part of the ventral forebrain circuit (Wood, 1997), the function of this nucleus in guiding male sexual behavior remains largely unknown. However, several lines of evidence suggest that it may be involved in reproductive behavior. First, the PMCo has reciprocal connections with BNST and MEA (Kevetter and Winans, 1981a, Gomez and Newman, 1992, Coolen and Wood, 1998, Wood and Swann, 2005), as well as strong connections with chemosensory circuitry. Specifically, the PMCo receives direct projections from the accessory olfactory bulbs (Scalia and Winans, 1975) and indirect connections from the main olfactory bulbs via the anterior and posterolateral cortical nuclei of the amygdale (Kevetter and Winans, 1981b). Second, the PMCo is sensitive to gonadal steroids, as it contains dense populations of steroid receptor-containing neurons (Simerly et al., 1990, Wood et al., 1992, Wood and Newman, 1993, Shughrue et al., 1997). In rats, this nucleus is sexually dimorphic (Vinader-Caerols et al., 1998) and is masculinzed by perinatal estradiol treatment (Vinader-Caerols et al., 2000). Finally, in male hamsters, neurons within the PMCo display elevated levels of c-fos expression, an indirect measure of neuronal activity, following either copulatory behavior or exposure to female odors (Kollack and Newman, 1992, Wood and Newman, 1993, Kollack-Walker and Newman, 1995, Fernandez-Fewell and Meredith, 1998, Fewell and Meredith, 2002).
Together, these data indicate that the PMCo may function to regulate odor-guided aspects of male reproductive behavior, yet, to our knowledge, this hypothesis has never been directly tested. Consequently, we assessed the effects of electrolytic lesions of the PMCo on both appetitive and consummatory aspects of reproductive behavior in male Syrian hamsters. Males were tested first for their preference to investigate female odors over male odors in a Y-maze apparatus, as well as for their ability to discriminate between these odors in a habituation-dishabituation task. Males were then tested for their copulatory behavior to satiety when paired with a receptive female. Due to the PMCo’s substantial projections from the accessory olfactory bulbs (Scalia and Winans, 1975), we hypothesized that this nucleus would regulate male reproductive behavior primarily through its processing of vomeronasal information. In male hamsters, the role of the vomeronasal organ in regulating sexual behavior changes with sexual experience. Specifically, although the vomeronasal organ is critical for the expression of sexual behavior in naïve males, the main olfactory system can compensate for a lack of vomeronasal processing in sexually experienced males (Meredith, 1986). As the current study is an initial attempt to identify a functional role for the PMCo in regulating these behaviors, we wanted experimental conditions that specifically rely on accessory olfactory processing and therefore used sexually naïve male subjects. Our results show that the PMCo regulates distinct aspects of male hamster copulatory behavior, although this nucleus is not critical for the expression of sexual odor preferences.
EXPERIMENTAL PROCEDURES
Subjects
All animals in this study were Syrian hamsters (Mesocricetus auratus) purchased from Charles River Laboratory at three weeks of age and singly-housed until the age of behavioral testing (3–6 months). Subjects were sexually naïve males that had been gonadectomized and implanted subcutaneously with testosterone Silastic capsules prior to lesion surgery (see below). Ovariectomized, hormone-primed female hamsters served as stimulus animals for the copulatory behavior tests (see below). A separate group of gonadally-intact male and female hamsters were used to provide social odor stimuli. Subjects were unrelated to, and had no previous contact with, stimulus females or odor donors. All animals were housed in solid-bottom Plexiglas cages (36 cm × 30 cm × 16 cm) and maintained on a reversed 14-h light/ 10-h dark photoperiod (lights off/on at 9 am/7 pm). Food and water were available ad libitum. All animal experiments were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23; revised 1996) and were approved by the Georgia State University Institutional Animal Care and Use Committee. All efforts were made to minimize the number of animals used and their suffering.
Surgical procedures
Gonadectomy and testosterone implants in male subjects
In male hamsters, exposure to female odors causes a rapid increase in serum testosterone levels (Macrides et al., 1974, Pfeiffer and Johnston, 1992), and it is possible that lesions of the PMCo may alter this testosterone surge. Thus, in order to equalize steroid hormone levels between experimental groups, all subjects were gonadectomized and provided with physiological levels of exogenous testosterone (Maras and Petrulis, 2006). Males were anesthetized with 1–2% isoflurane (mixed with 100% oxygen). Following a midline abdominal incision, testicles were bilaterally removed via cauterization of the ductus deferens and blood vessels. Immediately following gonadectomy, males were given chronic testosterone (Sigma Chemical Co., St. Louis, MO, USA) replacement via a 20 mm Silastic capsule (i.d. 1.57 mm, o.d. 2.41 mm, Dow Corning, Midland, MI) that was implanted subcutaneously between the scapulae.
Ovariectomy and hormone priming of stimulus female
Stimulus females for copulatory behavior tests were anesthetized with 1–2% isoflurane (mixed with 100% oxygen) and ovariectomized via bilateral flank incisions. Immediately following ovariectomy, stimulus females were given chronic estradiol (Sigma Chemical Co., St. Louis, MO, USA) treatment via a 5 mm Silastic capsule (i.d. 1.57 mm, o.d. 2.41 mm, Dow Corning, Midland, MI, USA) that was implanted subcutaneously between the scapulae. Females were allowed at least two weeks for recovery prior to being used as stimulus animals in copulatory behavior tests. To induce behavioral receptivity, females were given a subcutaneous injection of 0.25 mg of progesterone (dissolved in sesame oil, 2.5 mg/ml) (Sigma Chemical Co., St. Louis, MO, USA) four hours prior to copulatory behavior tests.
Electrolytic lesions
One to two weeks following gonadectomy, male subjects were randomly assigned to either PMCo lesion (PMCoX, n = 22) or sham lesion (SHAM, n = 7) group. Subjects were anesthetized with 1–2% isoflurane (mixed with 7:3 oxygen: nitrous oxide) and positioned in a stereotaxic apparatus so that the skull was flat. The temporal muscles were retracted from the skull and small holes were drilled to expose the dura. Bilateral electrolytic lesions were made using a platinum/iridium electrode (0.25 mm diameter, 0.45 mm uninsulated tip, Frederick Haer & Co., Bowdoinham, ME, USA) and by passing 1 mA of anodal current from a lesion-making device (Ugo Basile, Comerio, VA, Italy). As the PMCo extends over 2 mm in the rostral-caudal direction, and the size, shape and location of the nucleus varies along its length, we used a combination of multiple small lesions in order to generate maximal damage of the PMCo while limiting collateral damage to nearby nuclei. Therefore, each PMCoX male received a total of five bilateral penetrations, and the current duration varied across penetrations (Table 1). Sham surgeries were identical to lesion surgeries except that the electrode was lowered 1.5 mm above the target coordinate and no current was passed. Gel foam (Pharmacia & Upjohn Co., Kalamazoo, MI, USA) was used to pack the holes, and the incision was closed with wound clips.
Table 1. Surgical coordinates for electrolytic lesions of the PMCo.
PMCo lesions were generated using a combination of 5 bilateral coordinates (total of 10 penetrations/animal). All coordinates are in millimeters. Anterior-posterior (AP) and medial-lateral (ML) coordinates are relative to bregma, whereas dorsal-ventral (DV) coordinates are relative to the level of dura at each coordinate. Seconds indicate the duration of anodal current (1mA) passed at each coordinate.
| AP | ML | DV | Seconds |
|---|---|---|---|
| +1.00 | ±2.75 | −8.05 | 5 |
| +1.40 | ±3.45 | −8.30 | 5 |
| +1.85 | ±3.50 | −7.95 | 9 |
| +1.85 | ±4.00 | −8.10 | 12 |
| +2.20 | ±3.75 | −7.95 | 6 |
Behavioral testing
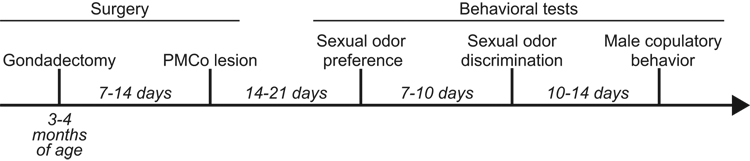
To determine the role of the PMCo in generating responses to sexual odors, as well as male copulatory behavior, subjects were given a series of behavioral tests beginning 2 to 3 weeks after lesion surgery (Figure 1). First, subjects were tested for their preference to investigate female odors over male odors in a Y-maze apparatus (Sexual odor preference). Subjects were then tested for their ability to discriminate between these odor sources in habituation-dishabituation task (Sexual odor discrimination). Finally, subjects were tested for their copulatory behavior when paired with a sexually receptive female (Male copulatory behavior). All testing was done during the first four hours of the dark phase of the photoperiod and under dim light.
Figure 1.
Timeline of surgeries and behavioral testing in experiment.
Odor Stimuli
Male and female odor stimuli used for sexual odor preference and sexual odor discrimination tests were collected from cages that had housed a single odor donor and had not been changed for 10–13 days. Odor stimuli consisted of 12 g of soiled cotton bedding (4 Nestlets, ANCARE, Bellmore, NY); 50 ml of soiled corncob litter; one damp cotton gauze pad that was used to wipe along the inner walls of the odor donor cage; and an additional damp gauze pad that was used to wipe the odor donor’s bilateral flank and anogenital regions. For female odor stimuli, vaginal secretion was collected onto an additional gauze pad by inducing an estrous donor female into lordosis and gently palpating the vaginal area with a disposable probe. Clean odor stimuli consisted of unsoiled components identical to those of the social odor stimuli. All odor stimuli were stored in plastic bags at 4°C until 30 minutes prior to use. Odor samples older than one month were discarded, and care was taken to ensure that subjects were not tested with the same individual’s odor more than once. Clean latex gloves were worn while collecting odor samples to prevent contamination of odor cues. To conserve stimulus odors, each odor was used for two consecutive sexual odor preference or discrimination tests.
Sexual odor preference
Subjects were tested for their preference to investigate female over male odor stimuli when presented in a Y-maze apparatus (Maras and Petrulis, 2006). The Y-maze consisted of a stem arm (61 cm long) and two side arms (68 cm long). All arms of the maze were 10 cm wide, with walls 10 cm high. Each side arm had a stimulus chamber (20 cm long) at its distal end, in which odor stimuli (see above) were placed. Stimulus chambers had perforated doors that allowed airflow, but prevented contact with the odor stimuli. Thus, subjects were exposed to only the volatile components of the odor stimuli. A start chamber (20 cm long), with a removable, perforated door, was located at the distal end of the stem arm. An electric fan located behind the start chamber pulled air from the stimulus chambers through the entire length of the Y-maze (airflow rate of 2.0 km/hr, measured at the start box). The top of the Y-maze was secured with a clear Plexiglas top to allow for overhead video recording of the subject’s behavior.
Subjects were tested in a sequence of two Y-maze tests, separated by 24 hours. First, to habituate the subjects to the Y-maze and obtain baseline behavioral data, subjects were tested with clean odor stimuli in both stimulus chambers of the Y-maze (Clean). Subjects were then tested for their sexual odor preference by placing male and female odors in opposite stimulus chambers (Preference). For all tests, subjects were placed in the start chamber for one minute, after which, the door was removed and subjects were allowed nine minutes to explore the Y-maze. All surfaces of the Y-maze were thoroughly cleaned with 50% alcohol and allowed to dry between subjects.
Video recordings of Y-maze tests were digitized onto a computer and scored using the Observer for Windows, version 5.0 (Noldus Information Technology B.V., Wageningen, The Netherlands). All observers were blind to the condition of the subject, and different observers reached at least an 85% inter-observer reliability score prior to coding behavior. Both the time spent investigating the stimulus chambers and the numbers of entries into each arm of the Y-maze were scored. Investigation of the stimulus chamber was coded when the subject made contact with, or directed its nose within 1 cm of, the stimulus chamber door. Arm entry was coded when the front half of the subject’s body crossed into that arm.
Sexual odor discrimination
A habituation-dishabituation model was used to test discrimination between male and female odors. This approach involves repeated presentations of the same odor source followed by a test presentation of a novel odor source. A decrease in investigation during the repeated presentations indicates a perception of the odors as being the same or familiar. An increase in investigation of the novel odor compared to the last presentation of the habituated odor indicates an ability to discriminate between the two odors (Johnston, 1993, Baum and Keverne, 2002).
The testing sequence consisted of four, 3-minute presentations of repeated odors (habituation) followed by a fifth, 3-minute presentation of a novel odor (test). Five-minute inter-trial intervals separated each odor presentation. As we have previously shown that male hamsters do not consistently habituate to repeated presentations of female odors (Maras and Petrulis, 2006), all subjects were tested using male odors as the habituation stimuli and female odors as the test stimuli. Subjects were presented with a different male’s odor on each of the habituation trials so that subjects were habituated to the sexual identity of the repeated odor, rather than to the individual identity of an odor donor.
Odor stimuli were presented in modified 50 ml polypropylene collection tubes, with ½ cm holes drilled 1 cm apart along the surface of the tube. Wire mesh lined the inner surface of the odor container to prevent contact with the odor stimulus. Thus, subjects were exposed to only the volatile components of the odor stimuli during these tests. Odor containers were placed in the center of the subject’s home cage and investigation was scored when the subject’s nose contacted, or came within 1cm of, the odor container. Total investigation times were measured using a stopwatch. Odor containers were cleaned with 50% alcohol and allowed to air dry for 24 hours prior to re-use.
Male copulatory behavior
Subjects were tested for their copulatory behavior when paired with a receptive stimulus female hamster in a clear, Plexiglas arena. An angled mirror was placed under the testing arena to provide a view of the ventral surface of the animals (in addition to the side view). Males were placed into the empty testing arena for five minutes prior to the addition of the stimulus female. Copulatory tests lasted 30 minutes, at which time the stimulus female was removed.
Copulatory behavior tests were video-recorded and the male’s behavior was later scored using the Observer for Windows, version 5.0 (Noldus Information Technology B.V., Wageningen, The Netherlands). The total number and latencies (from test onset) of several behavioral measures were scored: mounts without intromissions (mounts), mounts with intromissions (intromissions), ejaculations, and long intromissions. Long intromissions are distinguished from regular intromissions in that the male does not quickly dismount the female following vaginal penetration, but instead displays a repetitive thrusting pattern (Bunnell et al., 1977, Parfitt and Newman, 1998). Importantly, the expression of long intromissions is associated with the onset of sexual satiety in this species (Bunnell et al., 1977, Parfitt and Newman, 1998). In addition, the total durations of time the male spent investigating the female’s anogenital region, investigating the female’s head or body region (non-anogenital) and self-grooming were also scored. Finally, several derived measures of copulatory behavior were also analyzed: Post-ejaculatory interval (latency to display a mount or intromission after each ejaculation), the number of intromissions to reach each ejaculation, and mounting efficiency (the total number of intromissions divided by the total number of mounts + intromissions).
Histology and lesion verification
Following the last behavioral test, subjects were injected with an overdose of sodium pentobarbital (Nembutal, 100 mg/kg) and transcardially perfused with 200 ml of 0.1M phosphate-buffered saline (PBS, pH 7.4) followed by 200 ml of phosphate-buffered formalin (10%). Brains were post-fixed in phosphate-buffered formalin (10%) overnight and then cryoprotected for 48–72 hours in 30% sucrose in PBS solution. Coronal sections (40-µm) of brain tissue were sectioned on a cryostat (−20°C) and stored in PBS until mounting. Every third section was mounted onto glass slides using a 1% gelatin mounting solution and stained with cresyl violet.
Sections were examined under a light microscope for the location and extent of lesion damage as compared with published hamster neuroanatomical plates (Morin and Wood, 2001). Brain sections from subjects with minimum- and maximum-sized lesions were captured at 5X magnification by a Zeiss Axiocam using Axiovision 4.0 software (2002). These lesions were traced onto anatomical plates using Adobe Illustrator CS 11.0 software (2003).
Blood collection and radioimmunoassay
Blood samples were collected from the inferior vena cava immediately prior to perfusion and stored in vacutainer collection tubes (VWR, West Chester, PA., 4 ml draw, red/gray) on ice until centrifuging. Samples were centrifuged at 3200 rpms, at 4°C for 20 minutes and serum was stored in 200µl aliquots at −20°C until assay. Testosterone levels (ng/ml) were measured by radioimmunoassasy kits from Diagnostics System Laboratories (DSL 4000 Testosterone), with a sensitivity range of 0.05–22.92 ng/ml and an inter-assay variance of 6%, previously validated for hamster serum (Cooper et al., 2000).
Data analysis
All data were analyzed using SPSS 11.0 (SPSS Inc., Chicago, IL, USA) for Windows and are reported as mean ± SEM. To establish investigatory preferences in each Y-maze test (Clean, Preference), 2 (Lesion group: PMCoX, SHAM) × 2 (Stimulus; Clean test: left, right; Preference: female, male odor) ANOVAs were performed. In addition, independent t-tests were used to detect group differences in the total number of arm entries made during each Y-maze test.
For the sexual odor discrimination tests, data were analyzed using a 2 (Lesion group) × 5 (Odor presentation: Male 1–4, Female) ANOVA. For post-hoc analysis, pairwise comparisons with Bonferroni corrections were used to compare investigation times between Male 1 vs. Male 4 and Male 4 vs. Female presentations.
Group differences in all copulatory measures were detected using independent t-tests. Furthermore, to detect changes in post-ejaculatory intervals or the number of intromissions to reach each ejaculation across the duration of the copulatory test, separate 2 (Lesion group) × 2 (First, Last ejaculatory series) ANOVAs were performed.
RESULTS
Lesion verification
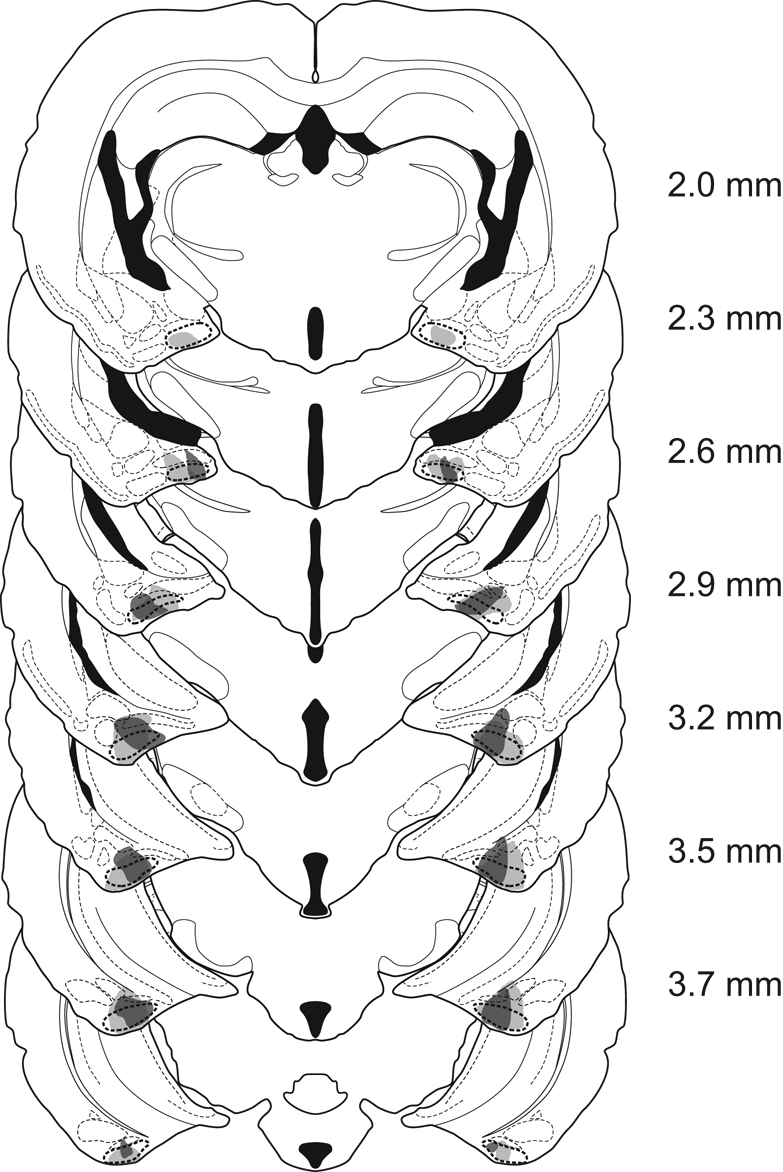
Males were included in the PMCoX lesion group (n = 11) only if they had extensive bilateral damage of the PMCo. Specifically, all males in the PMCoX group had at least 50% bilateral damage that included the middle three sections of the PMCo (Figure 2). In four of these males, damage extended into the rostral sections of the PMCo, whereas in seven subjects, damage extended into caudal PMCo. However, there were no differences in either the preference or copulatory behavior between males with rostral or caudal spread of lesion damage (all p > .05). Males were excluded from the PMCoX group if there was any damage to the posterior medial amygdala (n = 2) or if there was substantial sparing of the PMCo (n = 9).
Figure 2.
Reconstruction of coronal sections of the largest (light gray) and smallest (dark gray) electrolytic lesions in PMCoX males (n = 11). Sections proceed from anterior (top) to posterior (bottom) levels, with the numbers representing the distance posterior to bregma.
In addition to damage of the PMCo, a subset of PMCoX males also had minimal damage (< 10% in any section) to adjacent nuclei, including the posterior basomedial (BMP, n = 5), posterior basolateral (n = 6) and posterolateral cortical amygdala (n = 6), and the amygdalopiriform transition area (n = 3). Importantly, damage to these regions was mostly unilateral and never complete. All PMCoX males also had some lesion damage to the amygdalohippocampal area (AHi). In four males, this damage was minimal (< 10%), whereas in seven males, damage to the AHi was moderate (≤ 50%). There were no differences in either the preference or copulatory behavior between males with minimal or moderate damage to the AHi (all p > .05).
Behavioral measures
Sexual odor preference
In the Clean test, there were no significant differences between investigation levels of the two sides of the Y-maze, F(1, 16) = 1.139, p > .05, or between experimental groups, F(1, 16) = .228, p > .05; there was also no significant interaction between these factors, F(1, 45) = .001, p > .05 (Table 2). Furthermore, when the investigation times were summed for the left and right arms, PMCoX and SHAM males did not differ in their total duration of investigation of the clean stimulus chambers, t(16) = .478, p > .05. Levels of activity, as measured by the total number of arm entries, were also not different between PMCoX and SHAM males, t(16) = 1.088, p > .05 (Table 2).
Table 2. Summary of behavioral measures from Clean Y-maze tests.
Both PMCoX and SHAM males investigated the left and right stimulus sides equally. There were no differences in general activity levels or total investigation levels between SHAM and PMCoX males. All investigation times are in seconds.
| Total number of arm entries | Investigation time (left) | Investigation time (right) | |
|---|---|---|---|
| SHAM | 21.3 ± 2.8 | 53.8 ± 8.1 | 60.6 ± 9.9 |
| PMCoX | 19.1 ± 1.5 | 50.2 ± 8.9 | 64.2 ± 10.1 |
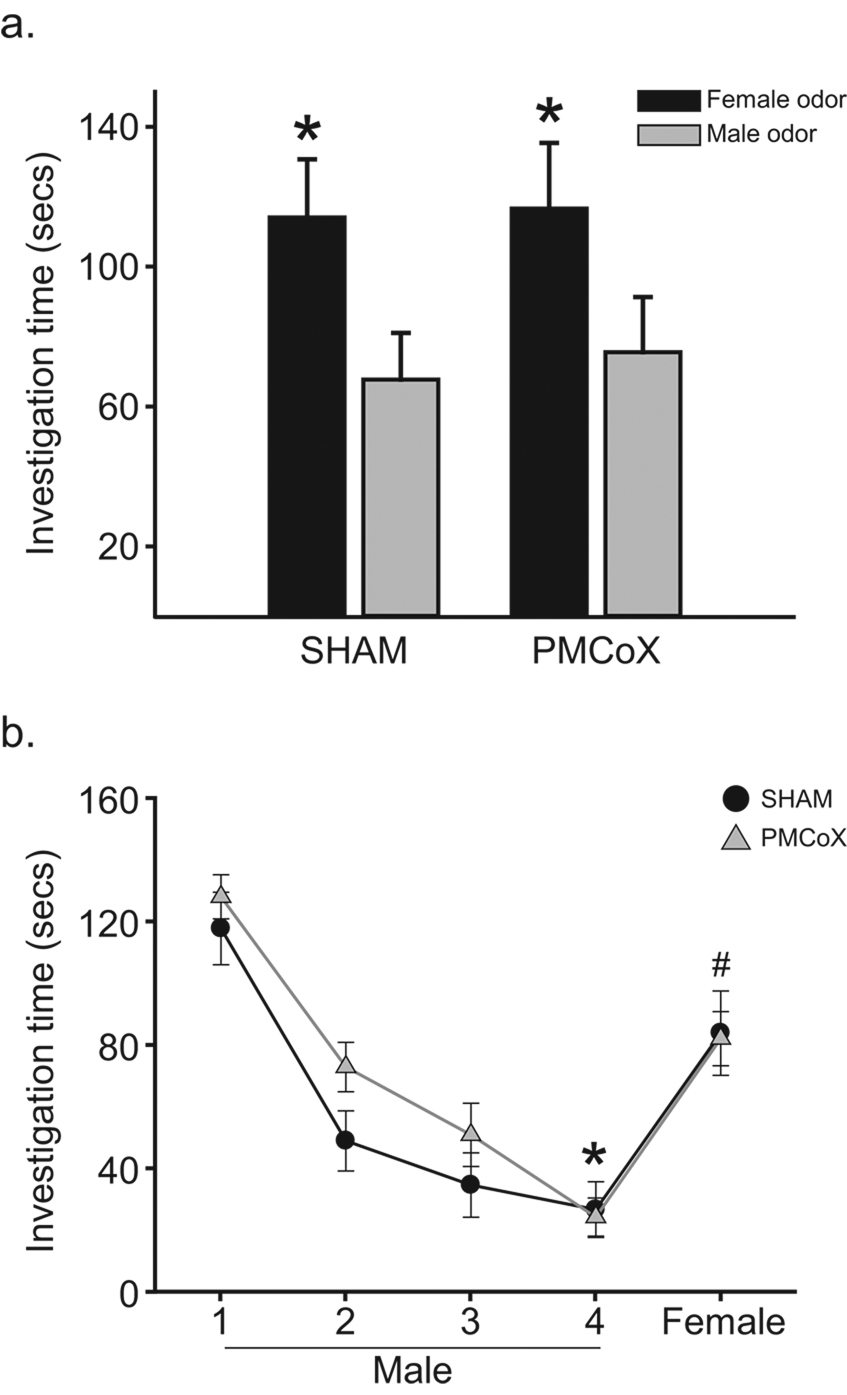
In the Preference test, subjects investigated female odors longer than male odors, F(1, 16) = 7.760, p < .05, with no difference in investigation between experimental groups, F(1, 16) = .029, p > .05, or significant interaction between odor stimulus and experimental group, F(1, 16) = .019, p > .05 (Figure 3a). In addition, PMCoX and SHAM males spent similar amounts of time investigating female odors, t(16) =−.054, p > .05, and male odors, t(16) =−.248, p > .05.
Figure 3.
(a) Odor investigation times from the sexual odor preference test in the Y-maze. Both PMCoX (n = 11) and SHAM (n = 7) males preferred to investigate female odors longer than male odors, * p < .05 relative to investigation of male odor. (b) Odor investigation times during the sexual odor discrimination test. Both groups displayed decreased investigation during the fourth presentation of the male odor compared to the first presentation of the male odor, * p < .05. Both groups also displayed increased investigation during the test presentation of the female odor compared to the fourth presentation of the male odor, # p < .05.
Sexual odor discrimination
There was a significant difference in investigation times across stimulus presentations, F(4, 16) = 32.359, p < .05 (Figure 3b). There was no significant difference between lesion groups, F(1, 16) = 2.908, p > .05, nor was there a significant interaction between stimulus presentation and lesion group, F(4, 16) = 2.294, p > .05. Post-hoc pairwise comparisons detected significant differences in investigation times between the first and fourth presentations of male odors, t(16) = 10.120, p < .05 , as well as between the fourth presentation of the male odor and the test presentation of the female odor, t(16) = −11.151, p < .05.
Male copulatory behavior
When tested with a sexually receptive female, all male subjects ejaculated and all, except one PMCoX male, reached sexual satiety, as indicated by the expression of long intromissions. However, PMCoX and SHAM males did differ in the expression of several aspects of the male copulatory sequence.
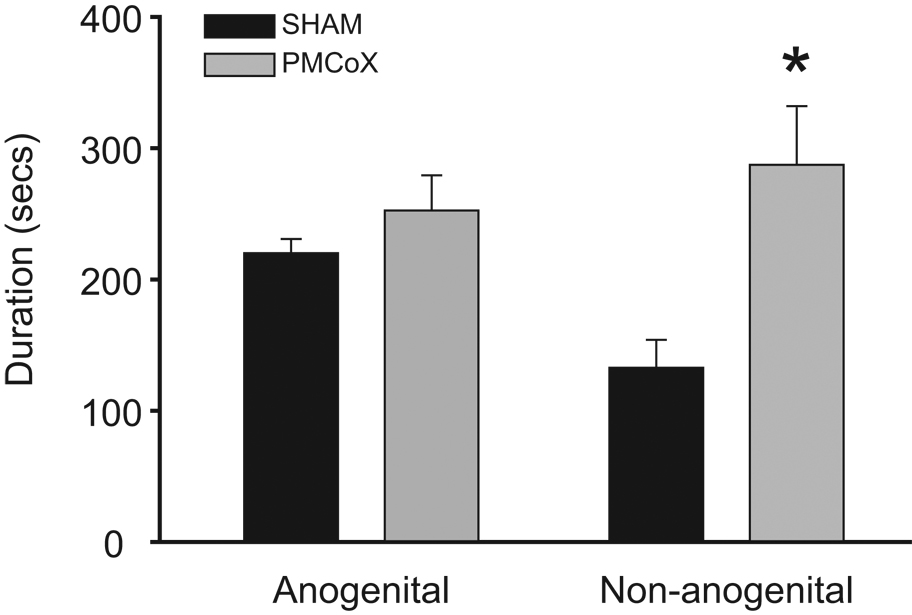
PMCoX males investigated the female’s non-anogenital region significantly longer than SHAM males, t(16) = −2.309, p < .05, although groups did not differ in their duration of anogenital investigation, t(16) = −1.218, p > .05 (Figure 4). PMCoX males also displayed less self-grooming than SHAM males, t(16) = 2.953, p < .05 (PMCoX = 454 ± 29.0 seconds; SHAM = 580 ± 28.2 seconds).
Figure 4.
Total durations of investigation behavior during the male copulatory behavior test. Although groups did not differ in the duration of anogenital investigation, PMCoX males (n = 11) increased investigation of the female’s non-anogenital region compared to SHAM males (n = 7). * p < .05 relative to SHAM group.
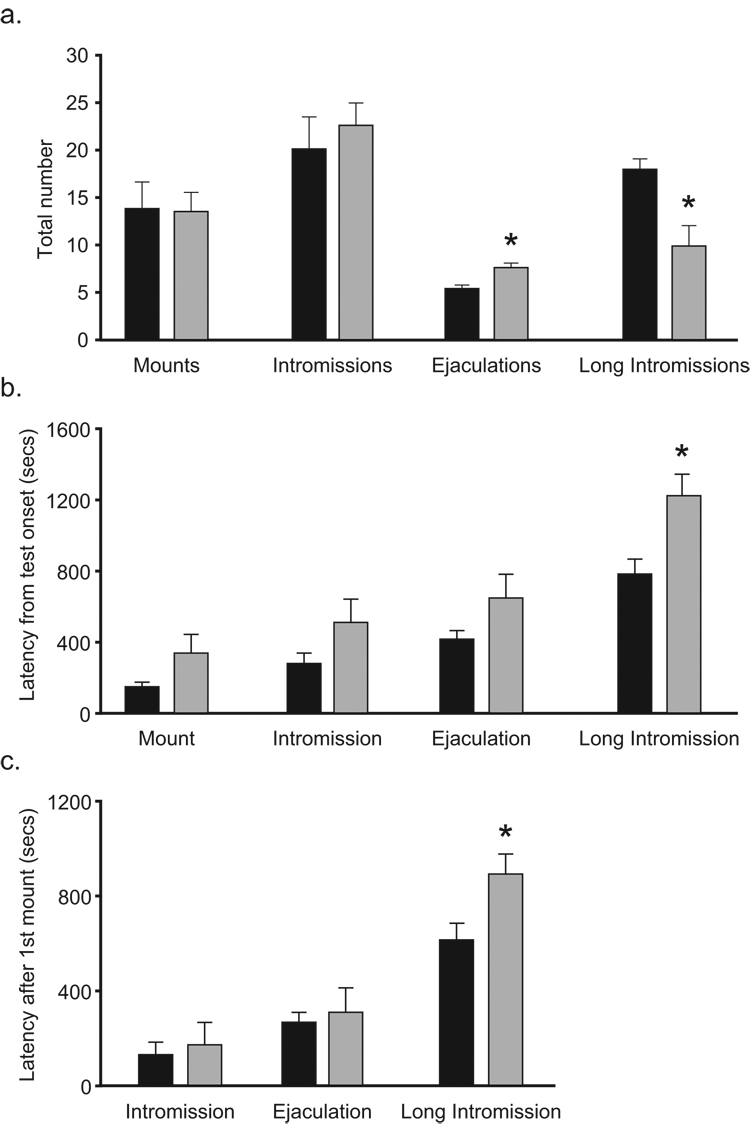
Although PMCoX and SHAM males displayed equal numbers of mounts, t(16) = .093, p > .05, and intromissions, t(16) = −.626, p > .05, PMCoX males displayed more ejaculations, t(16) = −3.320, p < .05, and less long intromissions, t(16) = 2.831 p < .05, compared to SHAM males (Figure 5a). PMCoX males also took slightly longer than SHAM males to express mounts, intromissions and ejaculations from test onset, although these differences were not statistically significant (all p > .05; Figure 5b). PMCoX males, however, took significantly longer than SHAM males to express long intromissions, t(16) = −2.634, p < .05 (Figure 5b).
Figure 5.
The expression of mating events during the male copulatory behavior test. (a) Although groups displayed equal numbers of mounts and intromissions, PMCoX males (n = 11) displayed more ejaculations, and less long intromissions, compared to SHAM males (n = 7). (b) PMCoX males took longer (from test onset) than SHAM males to display long intromissions. (c) After mating began, PMCoX males still took longer than SHAM males to display long intromissions. All * p < .05, relative to SHAM group.
Because the latencies to initiate mating (display the first mount) were slightly different between experimental groups, and this difference could affect the latencies to display subsequent mating behaviors, we also analyzed the latencies to display intromissions, ejaculations, and long intromissions after correcting for the latency to first mount (Figure 5c). After mating began, PMCoX and SHAM males had comparable latencies to display intromissions, t(16) = −.329, p > .05, and ejaculations, t(16) = −.314, p > .05. PMCoX males, however, still took longer than SHAM males to display long intromissions, t(16) = −2.314, p < .05.
PMCoX and SHAM males did not differ in the duration of their first post-ejaculatory interval, t(16) = .075, p > .05. In both PMCoX and SHAM males, post-ejaculatory interval durations increased across the ejaculatory series, F(1,16) = 26.155, p < .05, and there was no difference between groups in the pattern of this increase, F(1,16) = 2.789, p > .05. PMCoX and SHAM males also did not differ in the number of intromissions to reach the first ejaculation, t(16) = .358, p > .05. In both groups, fewer intromissions were required to reach the last ejaculation compared to the first ejaculation, F(1,16) = 26.807, p < .05, and there was no difference between groups in the pattern of this decline, F(1,16) = .309, p > .05. Finally, PMCoX and SHAM males were comparable in their mounting efficiency, t(16) = −.407, p > .05. Table 3 summarizes these derived measures of male copulatory behavior.
Table 3. Summary of derived behavioral measures from male copulatory tests.
In both PMCoX and SHAM males, the post-ejaculatory intervals increased, whereas the number of intromissions to ejaculation decreased, across the first to last ejaculatory series within the copulatory test
| Post-ejaculatory interval | Number of intromissions to ejaculation |
Mounting efficiency |
|||
|---|---|---|---|---|---|
| First | Last | First | Last | ||
| SHAM | 30.6 ± 4.6 | 47.7 ± 5.4* | 7.3 ± 1.1 | 2.6 ± 0.6* | .59 ± .06 |
| PMCoX | 30.2 ± 4.0 | 55.6 ± 4.9* | 6.7 ± 1.2 | 2.9 ± 0.6* | .62 ± .04 |
p < .05 compared to first ejaculatory series within each experimental group. Mounting efficiencies were not different between PMCoX and SHAM males.
Males with damage primarily outside the PMCo
For an additional comparison, we also analyzed the copulatory behavior of a subset of males that were excluded from the PMCoX group (Non-PMCoX, n = 6). In this subset, males had less than 20% damage of the PMCo and moderate to substantial damage of the AHi and/or BMP. Importantly, males with damage primarily outside the PMCo did not differ from SHAM males in any of the copulatory behavior measures analyzed (all p > .05, Table 4).
Table 4. Comparison of copulatory behavior between SHAM males and males with damage primarily outside the PMCo (Non-PMCoX).
The copulatory behavior of males with damage primarily outside the PMCo (Non-PMCoX, n = 6) was compared to that of SHAM males. In Non-PMCoX males, damage targeted the amygdalohippocampal area (AHi) and posterior basomedial amygdala (BMP). Non-PMCoX males did not differ from SHAM males in any of the measures of copulatory behavior analyzed (all p > .05). Investigation times and latencies are in seconds.
| Investigation times | SHAM | Non-PMCoX |
|---|---|---|
| Anogenital | 210.2 ± 10.2 | 256.4 ± 34.9 |
| Non-anogenital | 132.8 ± 24.2 | 149.2 ± 24.4 |
| Self-groom | 580.0 ± 28.2 | 547.0 ± 32.2 |
| Number | Latency | |||
|---|---|---|---|---|
| Mating events | SHAM | Non-PMCoX | SHAM | Non-PMCoX |
| Mount | 16.6 ± 3.1 | 15.3 ± 3.0 | 150.8 ± 25.5 | 286.7 ± 94.4 |
| Intromission | 20.1 ± 3.4 | 28.7 ± 3.9 | 280.3 ± 58.6 | 340.2 ± 75.8 |
| Ejaculation | 5.5 ± 0.4 | 5.7 ± 0.4 | 417.1 ± 48.1 | 453.4 ± 73.5 |
| Long intromission | 18.0 ± 1.1 | 15.2 ± 1.7 | 783.5 ± 83.5 | 876.3 ± 76.9 |
Testosterone assay
There was no difference in testosterone levels (ng/ml) between PMCoX and SHAM males, F(1,16) = .006, p > .05 (PMCoX = 5.972 ± 0.699; SHAM = 5.880 ± 1.043). The ranges of testosterone levels in both groups (PMCoX = 2.82 – 8.05 ng/ml; SHAM = 2.66 – 7.70 ng/ml) were within the physiological range reported for this species (Moore et al., 2004).
DISCUSSION
The present results demonstrate that the PMCo regulates two distinct aspects of the mating sequence in male Syrian hamsters. First, the PMCo may be involved in directing appropriate chemosensory investigation during mating, as males with lesions of the PMCo displayed increased investigation of the female’s non-anogenital region compared to SHAM males. Second, the PMCo may regulate sexual satiety, as PMCo-lesioned males took longer than SHAM males to display long intromissions, an indication of the onset of sexual satiety in this species (Bunnell et al., 1977, Parfitt and Newman, 1998). This delayed onset of satiety was associated with both a decreased number of long intromissions and an increased number of ejaculations, compared to SHAM males. In contrast to these effects on copulatory behavior, males with lesions of the PMCo preferred to investigate female odors over male odors, as did SHAM males, and were able to discriminate between male and female odors in a habituation-dishabituation task.
Electrolytic lesion technique
This study used multiple, small electrolytic lesions to generate discrete damage targeted at the PMCo. One limitation of this technique is that damage is not restricted to neuronal cell bodies but also includes fibers of passage. Consequently, it is possible that PMCo lesions disrupted anatomical connections of nearby brain areas. However, the primary fiber tracts associated with the PMCo are the accessory olfactory bulb efferents traveling along the ventral surface of the brain to the PMCo itself (Kevetter and Winans, 1981a, Kemppainen et al., 2002). As the PMCo is the most caudal target of these fibers (Scalia and Winans, 1975), damage to the ventral surface does not disconnect the accessory olfactory bulb from other brain areas. Furthermore, males with lesion damage primarily to nuclei outside the PMCo, including the AHi and/or BMP, displayed copulatory behavior similar to that of SHAM males, suggesting that the behavioral deficits observed in PMCo-lesioned males do not simply reflect a disconnection of nearby brain areas. The use of excitotoxins for making lesions would reduce many of these concerns, as they spare fibers of passage, but we have found that they do not produce reliable, controllable lesion damage in this nucleus (unpublished observations).
The role of the PMCo in male copulatory behavior
Unlike lesions of the MPOA or MEA, which eliminate male copulatory behavior in many rodent species (Lehman et al., 1980, Powers et al., 1987, Kondo, 1992, Paredes and Baum, 1997, Heeb and Yahr, 2000), PMCo-lesioned males displayed all components of the mating sequence. PMCo lesions were associated, however, with critical alterations in the pattern of mating behavior, and these changes can be partitioned into two functional categories: inappropriate direction of chemosensory investigation and delayed onset of sexual satiety.
Modulation of chemosensory investigation
Male copulatory behavior in the Syrian hamster is characterized by intense chemosensory investigation of the female throughout the mating sequence (Bunnell et al., 1977), and this investigation is critical for stimulating and maintaining other aspects of male copulatory behavior (Johnston, 1975, 1986). Most investigation is targeted toward the female’s anogenital region (Bunnell et al., 1977, Kwan and Johnston, 1980), which contains the highly attractive vaginal secretion, although other regions are also attractive to males (Johnston, 1986).
In the present study, males with PMCo lesions displayed an over-investigation of the female’s non-anogenital region, even though these males displayed normal levels of anogenital investigation. These results suggest that the PMCo is not required for attraction to, or investigation of, the female’s anogenital region. Indeed, other chemo-responsive areas, such as the MEA or BNST, mediate this aspect of mating behavior (Lehman et al., 1980, Powers et al., 1987). The PMCo may instead be critical for limiting extraneous investigation of relatively less appropriate areas of the female during mating. Non-anogenital odors, such as those produced by the flank, Harderian, and ear glands, provide different kinds of social information (Johnston and Rasmussen, 1984, Johnston, 1990) and thus may normally compete with the anogenital region for the male’s attention when investigating a female. Our data suggest that the PMCo normally functions to inhibit investigation of these non-anogenital odors. Interestingly, PMCo-lesioned males did not display increased olfactory investigation during any of the other behavioral tests (sexual odor preference or discrimination), indicating that this effect on investigation may be specific to the mating context, rather than reflecting general over investigatory behavior. Future studies are needed to determine whether the PMCo regulates non-anogenital investigation in other social contexts, such as agonistic encounters, during which investigation of non-anogenital odors may be more critical.
This proposed role of the PMCo in directing investigation behavior is supported by the PMCo’s strong chemosensory inputs. Indeed, the PMCo is reciprocally connected with the accessory olfactory bulbs (Scalia and Winans, 1975, Kevetter and Winans, 1981a, Canteras et al., 1992) and also has substantial indirect connections with the main olfactory system (Kevetter and Winans, 1981b). In hamsters, PMCo neurons display increases in c-fos expression following exposure to female vaginal secretion (Fewell and Meredith, 2002), although it is currently unclear whether the PMCo displays similar c-fos responses to other sources of female odors or whether these responses are seen in other species. Interestingly, male hamsters with damage of the stria terminalis (ST) display similar increases in non-anogenital investigation of the female during mating tests (Lehman et al., 1983). As many PMCo projections to the BNST travel via the ST (Kevetter and Winans, 1981a, Canteras et al., 1992, Wood and Swann, 2005), this particular effect of ST lesions may be due to a disconnection of the PMCo from the BNST.
Timing of sexual satiety
PMCo lesions were associated with shifts in the temporal pattern of the mating sequence. Specifically, males with PMCo lesions took longer to express long intromissions, and displayed an increased number of ejaculations, compared to SHAM males. As long intromissions and ejaculations are interdependent behaviors, it is difficult to define the underlying neural mechanism that is altered by PMCo lesions. There are, however, several possible interpretations of this pattern of results.
First, PMCo lesions may increase the expression of ejaculations independently of their effect on long intromissions. Thus, the PMCo may function as a central inhibitor of ejaculations. As the PMCo does not project to hypothalamic or brainstem nuclei known to stimulate ejaculations (Kevetter and Winans, 1981a, Canteras et al., 1992, Normandin and Murphy, 2008), a direct modulation of ejaculations by the PMCo is unlikely. It remains possible, however, that the PMCo may indirectly modulate these ejaculation centers via its connections with the MEA and BNST (Kevetter and Winans, 1981a, Canteras et al., 1992).
Second, PMCo lesions may decrease neural processing of sensory feedback from the penis. This decreased sensory processing could, in turn, cause lesioned males to require more ejaculations to reach satiety. Ejaculation-related sensory information from the penis appears to be processed via lumbar spinothalamic cells that project to the parvocellular subparafascicular nucleus of the thalamus (Coolen et al., 2004). Although other areas of the ventral forebrain, such as the MPOA BNST, and MEA, receive projections from the SPFp (Coolen et al., 1998, Coolen and Wood, 1998, Greco et al., 1998, Heeb and Yahr, 2001), it is currently unclear whether this sensory information is projected directly or indirectly to the PMCo. Future studies are therefore needed to determine whether the observed effects of PMCo lesions are due to decreased processing of penile sensory information.
Finally, we propose that the PMCo may function as a central regulator of sexual satiety. In this view, the increased ejaculations observed in PMCo-lesioned males may have occured simply as a result of the delayed onset of satiety. The concept of a central regulator of sexual satiety has been proposed previously for the posterodorsal region of the MEA, in which c-fos expression correlates specifically with the expression of long intromissions (Parfitt and Newman, 1998), and lesions of this nucleus have also been reported to delay sexual satiety (Parfitt et al., 1996). Although c-fos expression within the PMCo has not been analyzed using this sexual satiety paradigm, reciprocal connections between the PMCo and MEA (Kevetter and Winans, 1981a, Canteras et al., 1992, Coolen and Wood, 1998, Wood and Swann, 2005) suggest a possible functional relationship between these brain areas. Consistent with this interpretation, the PMCo contains a dense population of μ-opioid receptors, which are known to regulate the expression of sexual satiety in rats (Miller and Baum, 1987, Rodriguez-Manzo and Fernandez-Guasti, 1995) and hamsters (Wu and Noble, 1986). Thus, this population of neurons within the PMCo may mediate the effects of endogenous opioids on the expression of sexual satiety. Although additional studies are needed to confirm the specific role of the PMCo, our data provide initial evidence that this nucleus regulates the timing of sexual satiety.
As males in this study were sexually naïve, the effects of PMCo lesions on copulatory behavior may be specific to the first sexual encounter. Indeed, many rodent species display improvements in mating behavior after sexual experience (Dewsbury, 1969, Fleming and Kucera, 1991, Phelps et al., 1998, Hull et al., 2002). However, it is important to note that both PMCo-lesioned and SHAM males were sexually naïve and would have similar, if any, mating problems. Furthermore, most of the changes in copulatory behavior resulting from sexual experience are related to mating efficiency (ie. number of mounts, ratio of mounts to intromissions, etc.) (Hull et al., 2002). PMCo-lesioned and SHAM males, however, did not differ in any of these efficiency parameters, making it unlikely that the observed deficits were due solely to sexual inexperience. Nevertheless, the effects of PMCo lesions may be different in sexually experienced males, and future studies are needed to directly test whether sexual experience can compensate for the deficits associated with PMCo lesions.
The role of the PMCo in sexual odor preference
In contrast to its role in regulating male copulatory behavior, our results suggest that the PMCo is not critical for generating sexual odor preferences. In fact, males with lesions of the PMCo displayed robust preferences to investigate female odors over male odors when presented in a Y-maze and were able to discriminate between these odor sources in a habituation-dishabituation task. These results show that, although the PMCo receives substantial chemosensory input (Scalia and Winans, 1975, Kevetter and Winans, 1981b), other structures mediate attraction to opposite-sex odors. Specifically, we have previously demonstrated the role of the MEA in regulating opposite-sex odor preferences in male (Maras and Petrulis, 2006) and female hamsters (Petrulis and Johnston, 1999). The present results also demonstrate that the elimination of sexual odor preferences observed following MEA damage (Maras and Petrulis, 2006) was not simply due to a disconnection of the more caudal PMCo from the accessory olfactory bulbs.
Our finding that PMCo does not regulate odor preferences differs from what has been observed in a previous lesion study in female rats. Specifically, lesions of the PMCo in female rats eliminate the preference to spend time near intact males compared to castrated males (Romero et al., 1990). There are several critical differences between the previous study (Romero et al., 1990) and the current one that may explain this discrepancy. First, there may be significant sex and/or species differences in the neural regulation of odor preferences. Second, the study using female rats (Romero et al., 1990) examined a different type of odor preference (within opposite-sex, intact vs. castrated) compared to the current study (opposite- vs. same-sex). It is therefore possible that distinct brain areas regulate these qualitatively different types of odor preference.
CONCLUSIONS
The present study provides the first direct evidence for a functional role of the PMCo in regulating male reproductive behavior. Specifically, the PMCo regulates two distinct aspects of male copulatory behavior in the Syrian hamster: directing chemosensory investigation of the female and regulating the onset of sexual satiety. Importantly, the PMCo is part of an interconnected network of ventral forebrain nuclei that regulates many aspects of rodent social behavior (Wood, 1997). We hypothesize that the PMCo affects male copulatory behavior primarily through its modulation of other nuclei, such as the BNST and MEA, within this circuit. Future studies are needed to address the nature of the connections among these brain regions that may contribute to the regulation of male reproductive behavior.
List of Abbreviations
- AHi
Amygdalohippocampal area
- BMP
Posterior basomedial amygdala nucleus
- BNST
Bed nucleus of the stria terminalis
- MEA
Medial amygdala
- MPOA
Medial preoptic area
- PMCo
Posteromedial cortical amygdala
- PMCoX
Posteromedial cortical amygdala lesion group
- ST
Stria terminalis
- SPFp
Parvocellular subparafascicular nucleus of the thalamus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baum MJ, Keverne EB. Sex difference in attraction thresholds for volatile odors from male and estrous female mouse urine. Horm Behav. 2002;41:213–219. doi: 10.1006/hbeh.2001.1749. [DOI] [PubMed] [Google Scholar]
- Bunnell BN, Boland BD, Dewsbury DA. Copulatory behavior of golden hamsters (Mesocricetus auratus) Behaviour. 1977;61:180–206. [Google Scholar]
- Canteras NS, Simerly RB, Swanson LW. Connections of the posterior nucleus of the amygdala. J Comp Neurol. 1992;324:143–179. doi: 10.1002/cne.903240203. [DOI] [PubMed] [Google Scholar]
- Coolen LM, Allard J, Truitt WA, McKenna KE. Central regulation of ejaculation. Physiol Behav. 2004;83:203–215. doi: 10.1016/j.physbeh.2004.08.023. [DOI] [PubMed] [Google Scholar]
- Coolen LM, Peters HJ, Veening JG. Anatomical interrelationships of the medial preoptic area and other brain regions activated following male sexual behavior: a combined fos and tract-tracing study. J Comp Neurol. 1998;397:421–435. doi: 10.1002/(sici)1096-9861(19980803)397:3<421::aid-cne8>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Coolen LM, Wood RI. Bidirectional connections of the medial amygdaloid nucleus in the Syrian hamster brain: simultaneous anterograde and retrograde tract tracing. J Comp Neurol. 1998;399:189–209. doi: 10.1002/(sici)1096-9861(19980921)399:2<189::aid-cne4>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Cooper TT, Clancy AN, Karom M, Moore TO, Albers HE. Conversion of testosterone to estradiol may not be necessary for the expression of mating behavior in male Syrian hamsters (Mesocricetus auratus) Horm Behav. 2000;37:237–245. doi: 10.1006/hbeh.2000.1579. [DOI] [PubMed] [Google Scholar]
- Dewsbury DA. Copulatory behaviour of rats (Rattus norvegicus) as a function of prior copulatory experience. Anim Behav. 1969;17:217–223. doi: 10.1016/0003-3472(69)90004-9. [DOI] [PubMed] [Google Scholar]
- Fernandez-Fewell GD, Meredith M. Olfactory contribution to Fos expression during mating in inexperienced male hamsters. Chem Senses. 1998;23:257–267. doi: 10.1093/chemse/23.3.257. [DOI] [PubMed] [Google Scholar]
- Fewell GD, Meredith M. Experience facilitates vomeronasal and olfactory influence on Fos expression in medial preoptic area during pheromone exposure or mating in male hamsters. Brain Res. 2002;941:91–106. doi: 10.1016/s0006-8993(02)02613-6. [DOI] [PubMed] [Google Scholar]
- Fleming AS, Kucera C. Sexual experience effects are blocked both by the protein-synthesis inhibitor, cycloheximide, and by the noncompetitive NMDA antagonist, MK-801. Behav Neural Biol. 1991;56:319–328. doi: 10.1016/0163-1047(91)90499-g. [DOI] [PubMed] [Google Scholar]
- Gomez DM, Newman SW. Differential projections of the anterior and posterior regions of the medial amygdaloid nucleus in the Syrian hamster. J Comp Neurol. 1992;317:195–218. doi: 10.1002/cne.903170208. [DOI] [PubMed] [Google Scholar]
- Greco B, Edwards DA, Zumpe D, Clancy AN. Androgen receptor and mating-induced fos immunoreactivity are co-localized in limbic and midbrain neurons that project to the male rat medial preoptic area. Brain Res. 1998;781:15–24. doi: 10.1016/s0006-8993(97)01136-0. [DOI] [PubMed] [Google Scholar]
- Heeb MM, Yahr P. Cell-body lesions of the posterodorsal preoptic nucleus or posterodorsal medial amygdala, but not the parvicellular subparafascicular thalamus, disrupt mating in male gerbils. Physiol Behav. 2000;68:317–331. doi: 10.1016/s0031-9384(99)00182-1. [DOI] [PubMed] [Google Scholar]
- Heeb MM, Yahr P. Anatomical and functional connections among cell groups in the gerbil brain that are activated with ejaculation. J Comp Neurol. 2001;439:248–258. doi: 10.1002/cne.1346. [DOI] [PubMed] [Google Scholar]
- Hull EM, Meisel RL, Sachs BD. Male Sexual Function. In: Rubin R, editor. Hormones, Brain and Behavior. vol. 1. Burlington, MA: Elsevier Science; 2002. pp. 3–137. [Google Scholar]
- Johnston RE. Sexual attraction function of golden hamster vaginal secretion. Behav Biol. 1974;12:111–117. doi: 10.1016/s0091-6773(74)91101-8. [DOI] [PubMed] [Google Scholar]
- Johnston RE. Sexual excitation function of hamster vaginal secretion. Animal learning & behavior. 1975;3:161–166. [PubMed] [Google Scholar]
- Johnston RE. Effects of female odors on the sexual behavior of male hamsters. Behav Neural Biol. 1986;46:168–188. doi: 10.1016/s0163-1047(86)90654-0. [DOI] [PubMed] [Google Scholar]
- Johnston RE. Chemical communication in golden hamsters: from behavior to molecules and neural mechanisms. In: Dewsbury DA, editor. Contemporary Issues in Comparative Psychology. Sunderland, MA: Sinauer; 1990. pp. 381–412. [Google Scholar]
- Johnston RE. Memory for individual scent in hamsters (Mesocricetus auratus) as assessed by habituation methods. J Comp Psychol. 1993;107:201–207. doi: 10.1037/0735-7036.107.2.201. [DOI] [PubMed] [Google Scholar]
- Johnston RE, Rasmussen K. Individual recognition of female hamsters by males: role of chemical cues and of the olfactory and vomeronasal systems. Physiol Behav. 1984;33:95–104. doi: 10.1016/0031-9384(84)90019-2. [DOI] [PubMed] [Google Scholar]
- Kemppainen S, Jolkkonen E, Pitkanen A. Projections from the posterior cortical nucleus of the amygdala to the hippocampal formation and parahippocampal region in rat. Hippocampus. 2002;12:735–755. doi: 10.1002/hipo.10020. [DOI] [PubMed] [Google Scholar]
- Kevetter GA, Winans SS. Connections of the corticomedial amygdala in the golden hamster. I. Efferents of the "vomeronasal amygdale". J Comp Neurol. 1981a;197:81–98. doi: 10.1002/cne.901970107. [DOI] [PubMed] [Google Scholar]
- Kevetter GA, Winans SS. Connections of the corticomedial amygdala in the golden hamster. II. Efferents of the "olfactory amygdale". J Comp Neurol. 1981b;197:99–111. doi: 10.1002/cne.901970108. [DOI] [PubMed] [Google Scholar]
- Kollack SS, Newman SW. Mating behavior induces selective expression of Fos protein within the chemosensory pathways of the male Syrian hamster brain. Neurosci Lett. 1992;143:223–228. doi: 10.1016/0304-3940(92)90270-h. [DOI] [PubMed] [Google Scholar]
- Kollack-Walker S, Newman SW. Mating and agonistic behavior produce different patterns of Fos immunolabeling in the male Syrian hamster brain. Neuroscience. 1995;66:721–736. doi: 10.1016/0306-4522(94)00563-k. [DOI] [PubMed] [Google Scholar]
- Kondo Y. Lesions of the medial amygdala produce severe impairment of copulatory behavior in sexually inexperienced male rats. Physiol Behav. 1992;51:939–943. doi: 10.1016/0031-9384(92)90074-c. [DOI] [PubMed] [Google Scholar]
- Kwan M, Johnston RE. The role of vaginal secretion in hamster sexual behavior: males' responses to normal and vaginectomized females and their odors. J Comp Physiol Psychol. 1980;94:905–913. doi: 10.1037/h0077814. [DOI] [PubMed] [Google Scholar]
- Lehman MN, Powers JB, Winans SS. Stria terminalis lesions alter the temporal pattern of copulatory behavior in the male golden hamster. Behav Brain Res. 1983;8:109–128. doi: 10.1016/0166-4328(83)90174-2. [DOI] [PubMed] [Google Scholar]
- Lehman MN, Winans SS, Powers JB. Medial nucleus of the amygdala mediates chemosensory control of male hamster sexual behavior. Science. 1980;210:557–560. doi: 10.1126/science.7423209. [DOI] [PubMed] [Google Scholar]
- Macrides F, Bartke A, Fernandez F, D'Angelo W. Effects of exposure to vaginal odor and receptive females on plasma testosterone in the male hamster. Neuroendocrinology. 1974;15:355–364. doi: 10.1159/000122326. [DOI] [PubMed] [Google Scholar]
- Maras PM, Petrulis A. Chemosensory and steroid-responsive regions of the medial amygdala regulate distinct aspects of opposite-sex odor preference in male Syrian hamsters. Eur J Neurosci. 2006;24:3541–3552. doi: 10.1111/j.1460-9568.2006.05216.x. [DOI] [PubMed] [Google Scholar]
- Meredith M. Vomeronasal organ removal before sexual experience impairs male hamster mating behavior. Physiol Behav. 1986;36:737–743. doi: 10.1016/0031-9384(86)90362-8. [DOI] [PubMed] [Google Scholar]
- Meredith M. Sensory processing in the main and accessory olfactory systems: comparisons and contrasts. J Steroid Biochem Mol Biol. 1991;39:601–614. doi: 10.1016/0960-0760(91)90258-7. [DOI] [PubMed] [Google Scholar]
- Miller RL, Baum MJ. Naloxone inhibits mating and conditioned place preference for an estrous female in male rats soon after castration. Pharmacol Biochem Behav. 1987;26:781–789. doi: 10.1016/0091-3057(87)90611-3. [DOI] [PubMed] [Google Scholar]
- Moore TO, Karom M, O'Farrell L. The neurobehavioral effects of phytoestrogens in male Syrian hamsters. Brain Res. 2004;1016:102–110. doi: 10.1016/j.brainres.2004.04.073. [DOI] [PubMed] [Google Scholar]
- Morin LP, Wood RI. A Stereotaxic Atlas of the Golden Hamster Brain. San Diego: Academic Press; 2001. [Google Scholar]
- Morin LP, Zucker I. Photoperiodic regulation of copulatory behaviour in the male hamster. J Endocrinol. 1978;77:249–258. doi: 10.1677/joe.0.0770249. [DOI] [PubMed] [Google Scholar]
- Murphy MR, Schneider GE. Olfactory bulb removal eliminates mating behavior in the male golden hamster. Science. 1970;167:302–304. doi: 10.1126/science.167.3916.302. [DOI] [PubMed] [Google Scholar]
- Normandin JJ, Murphy AZ. Nucleus paragigantocellularis afferents in male and female rats: organization, gonadal steroid receptor expression, and activation during sexual behavior. J Comp Neurol. 2008;508:771–794. doi: 10.1002/cne.21704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paredes RG, Baum MJ. Role of the medial preoptic area/anterior hypothalamus in the control of masculine sexual behavior. Annual review of sex research. 1997;8:68–101. [PubMed] [Google Scholar]
- Parfitt DB, Coolen LM, Newman S. Lesions of the posterior medial mucleus of the amygdala delay sexual satiety. Abstract Annual Meeting Society for Neuroscience. 1996;155:67.8. [Google Scholar]
- Parfitt DB, Newman SW. Fos-immunoreactivity within the extended amygdala is correlated with the onset of sexual satiety. Horm Behav. 1998;34:17–29. doi: 10.1006/hbeh.1998.1459. [DOI] [PubMed] [Google Scholar]
- Petrulis A, Johnston RE. A reevaluation of dimethyl disulfide as a sex attractant in golden hamsters. Physiol Behav. 1995;57:779–784. doi: 10.1016/0031-9384(94)00332-7. [DOI] [PubMed] [Google Scholar]
- Petrulis A, Johnston RE. Lesions centered on the medial amygdala impair scent-marking and sex-odor recognition but spare discrimination of individual odors in female golden hamsters. Behav Neurosci. 1999;113:345–357. doi: 10.1037//0735-7044.113.2.345. [DOI] [PubMed] [Google Scholar]
- Pfeiffer CA, Johnston RE. Socially stimulated androgen surges in male hamsters: the roles of vaginal secretions, behavioral interactions, and housing conditions. Horm Behav. 1992;26:283–293. doi: 10.1016/0018-506x(92)90048-z. [DOI] [PubMed] [Google Scholar]
- Phelps SM, Lydon JP, O'Malley BW, Crews D. Regulation of male sexual behavior by progesterone receptor, sexual experience, and androgen. Horm Behav. 1998;34:294–302. doi: 10.1006/hbeh.1998.1485. [DOI] [PubMed] [Google Scholar]
- Powers JB, Bergondy ML. Androgenic regulation of chemoinvestigatory behaviors in male and female hamsters. Horm Behav. 1983;17:28–44. doi: 10.1016/0018-506x(83)90013-2. [DOI] [PubMed] [Google Scholar]
- Powers JB, Bergondy ML, Matochik JA. Male hamster sociosexual behaviors: effects of testosterone and its metabolites. Physiol Behav. 1985;35:607–616. doi: 10.1016/0031-9384(85)90149-0. [DOI] [PubMed] [Google Scholar]
- Powers JB, Newman SW, Bergondy ML. MPOA and BNST lesions in male Syrian hamsters: differential effects on copulatory and chemoinvestigatory behaviors. Behav Brain Res. 1987;23:181–195. doi: 10.1016/0166-4328(87)90019-2. [DOI] [PubMed] [Google Scholar]
- Restrepo D, Arellano J, Oliva AM, Schaefer ML, Lin W. Emerging views on the distinct but related roles of the main and accessory olfactory systems in responsiveness to chemosensory signals in mice. Horm Behav. 2004;46:247–256. doi: 10.1016/j.yhbeh.2004.02.009. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Manzo G, Fernandez-Guasti A. Opioid antagonists and the sexual satiation phenomenon. Psychopharmacology. 1995;122:131–136. doi: 10.1007/BF02246087. [DOI] [PubMed] [Google Scholar]
- Romero PR, Beltramino CA, Carrer HF. Participation of the olfactory system in the control of approach behavior of the female rat to the male. Physiol Behav. 1990;47:685–690. doi: 10.1016/0031-9384(90)90078-i. [DOI] [PubMed] [Google Scholar]
- Scalia F, Winans SS. The differential projections of the olfactory bulb and accessory olfactory bulb in mammals. J Comp Neurol. 1975;161:31–55. doi: 10.1002/cne.901610105. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-alpha and -beta mRNA in the rat central nervous system. J Comp Neurol. 1997;388:507–525. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Simerly RB, Chang C, Muramatsu M, Swanson LW. Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: an in situ hybridization study. J Comp Neurol. 1990;294:76–95. doi: 10.1002/cne.902940107. [DOI] [PubMed] [Google Scholar]
- Vinader-Caerols C, Collado P, Segovia S, Guillamon A. Sex differences in the posteromedial cortical nucleus of the amygdala in the rat. Neuroreport. 1998;9:2653–2656. doi: 10.1097/00001756-199808030-00042. [DOI] [PubMed] [Google Scholar]
- Vinader-Caerols C, Collado P, Segovia S, Guillamon A. Estradiol masculinizes the posteromedial cortical nucleus of the amygdala in the rat. Brain Res Bull. 2000;53:269–273. doi: 10.1016/s0361-9230(00)00332-4. [DOI] [PubMed] [Google Scholar]
- Winans SS, Powers JB. Olfactory and vomeronasal deafferentation of male hamsters: histological and behavioral analyses. Brain Res. 1977;126:325–344. doi: 10.1016/0006-8993(77)90729-6. [DOI] [PubMed] [Google Scholar]
- Wood RI. Thinking about networks in the control of male hamster sexual behavior. Horm Behav. 1997;32:40–45. doi: 10.1006/hbeh.1997.1403. [DOI] [PubMed] [Google Scholar]
- Wood RI. Integration of chemosensory and hormonal input in the male Syrian hamster brain. Ann N Y Acad Sci. 1998;855:362–372. doi: 10.1111/j.1749-6632.1998.tb10594.x. [DOI] [PubMed] [Google Scholar]
- Wood RI, Brabec RK, Swann JM, Newman SW. Androgen and estrogen concentrating neurons in chemosensory pathways of the male Syrian hamster brain. Brain Res. 1992;596:89–98. doi: 10.1016/0006-8993(92)91536-n. [DOI] [PubMed] [Google Scholar]
- Wood RI, Coolen LM. Integration of chemosensory and hormonal cues is essential for sexual behaviour in the male Syrian hamster: role of the medial amygdaloid nucleus. Neuroscience. 1997;78:1027–1035. doi: 10.1016/s0306-4522(96)00629-x. [DOI] [PubMed] [Google Scholar]
- Wood RI, Newman SW. Mating activates androgen receptor-containing neurons in chemosensory pathways of the male Syrian hamster brain. Brain Res. 1993;614:65–77. doi: 10.1016/0006-8993(93)91019-o. [DOI] [PubMed] [Google Scholar]
- Wood RI, Newman SW. Integration of chemosensory and hormonal cues is essential for mating in the male Syrian hamster. J Neurosci. 1995;15:7261–7269. doi: 10.1523/JNEUROSCI.15-11-07261.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood RI, Swann JM. The bed nucleus of the stria terminalis in the Syrian hamster: subnuclei and connections of the posterior division. Neuroscience. 2005;135:155–179. doi: 10.1016/j.neuroscience.2005.05.029. [DOI] [PubMed] [Google Scholar]
- Wu FM, Noble RG. Opiate antagonists and copulatory behavior of male hamsters. Physiol Behav. 1986;38:817–825. doi: 10.1016/0031-9384(86)90048-x. [DOI] [PubMed] [Google Scholar]