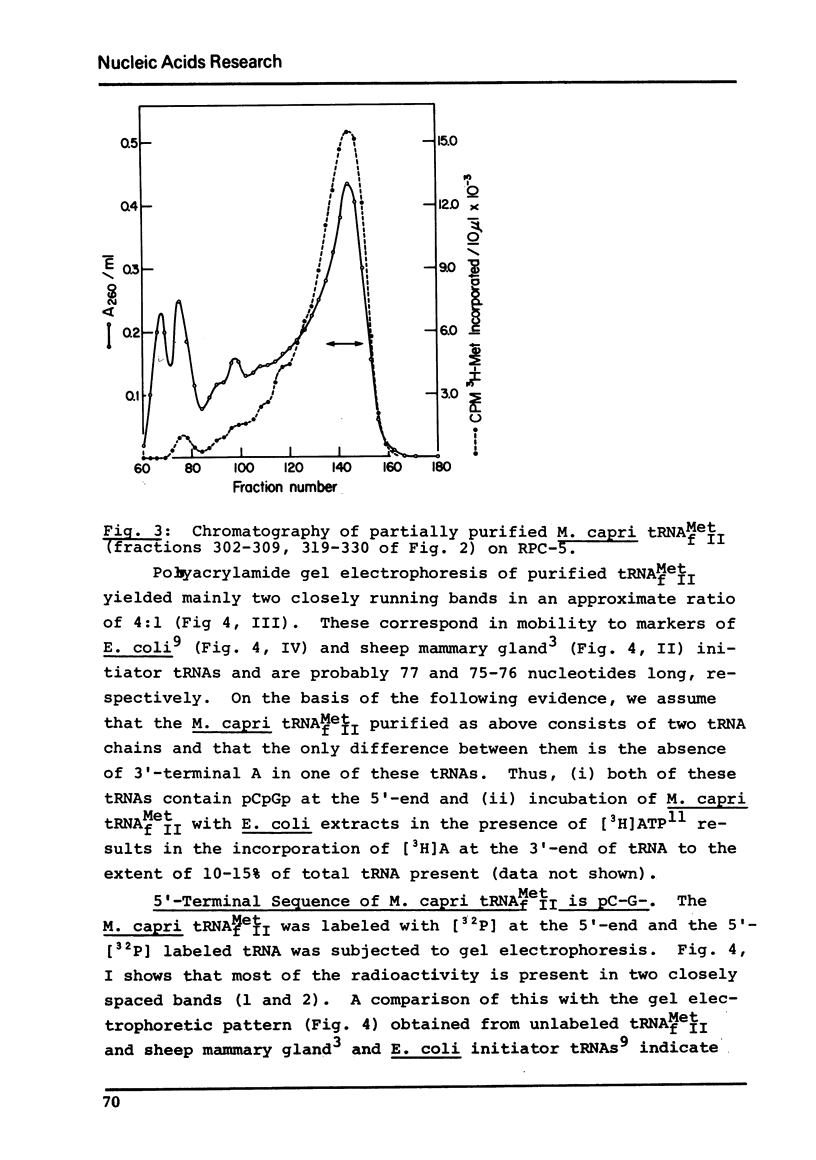
Abstract
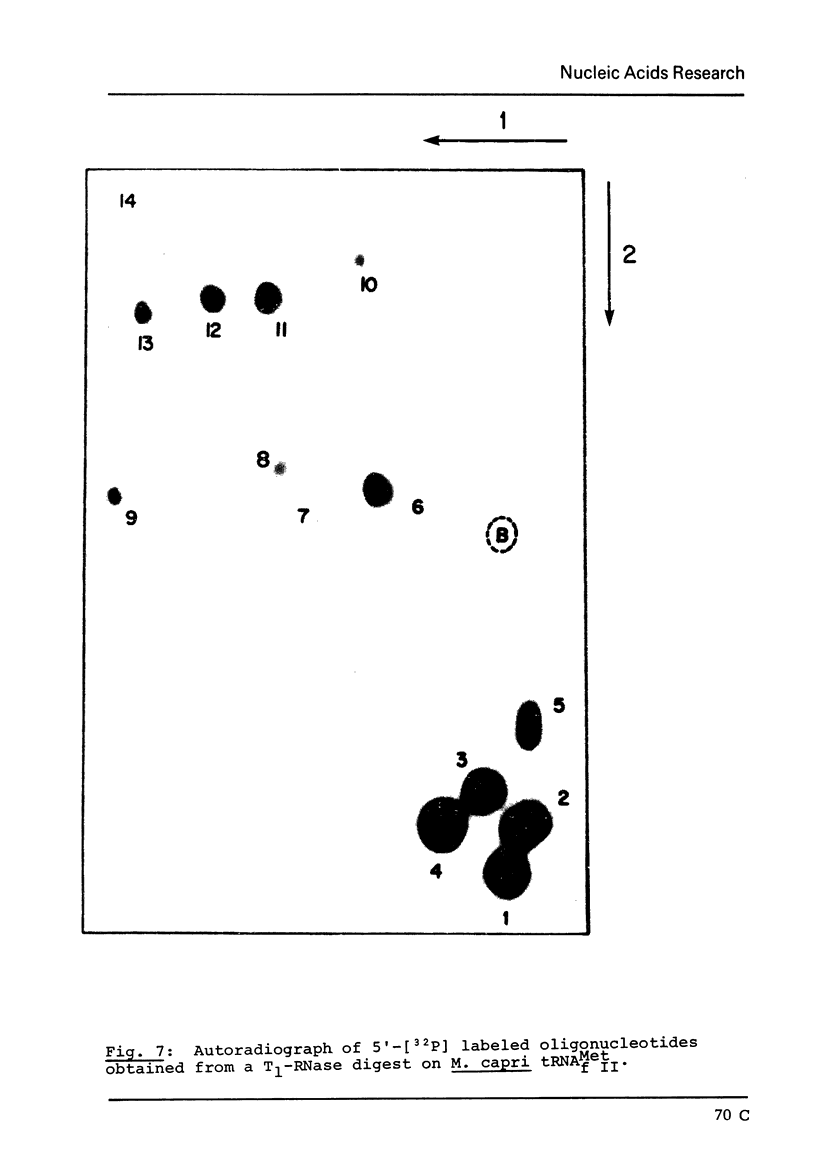
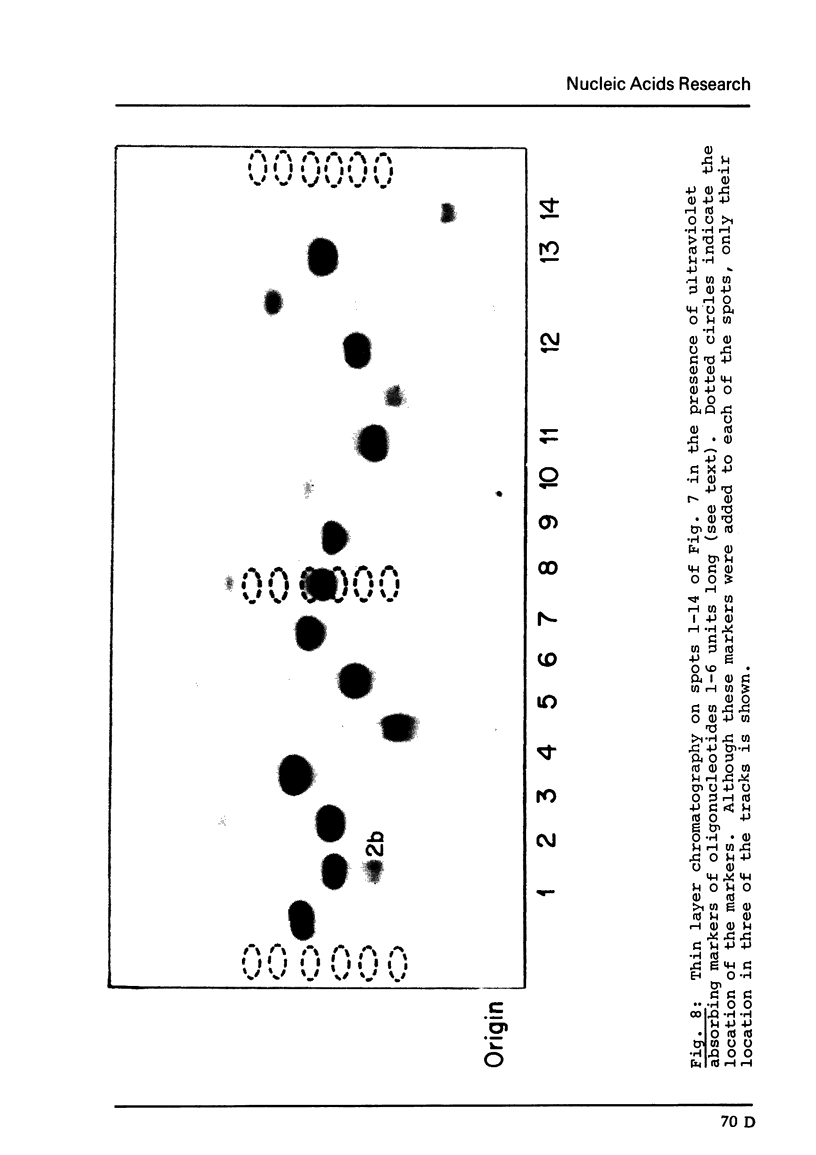
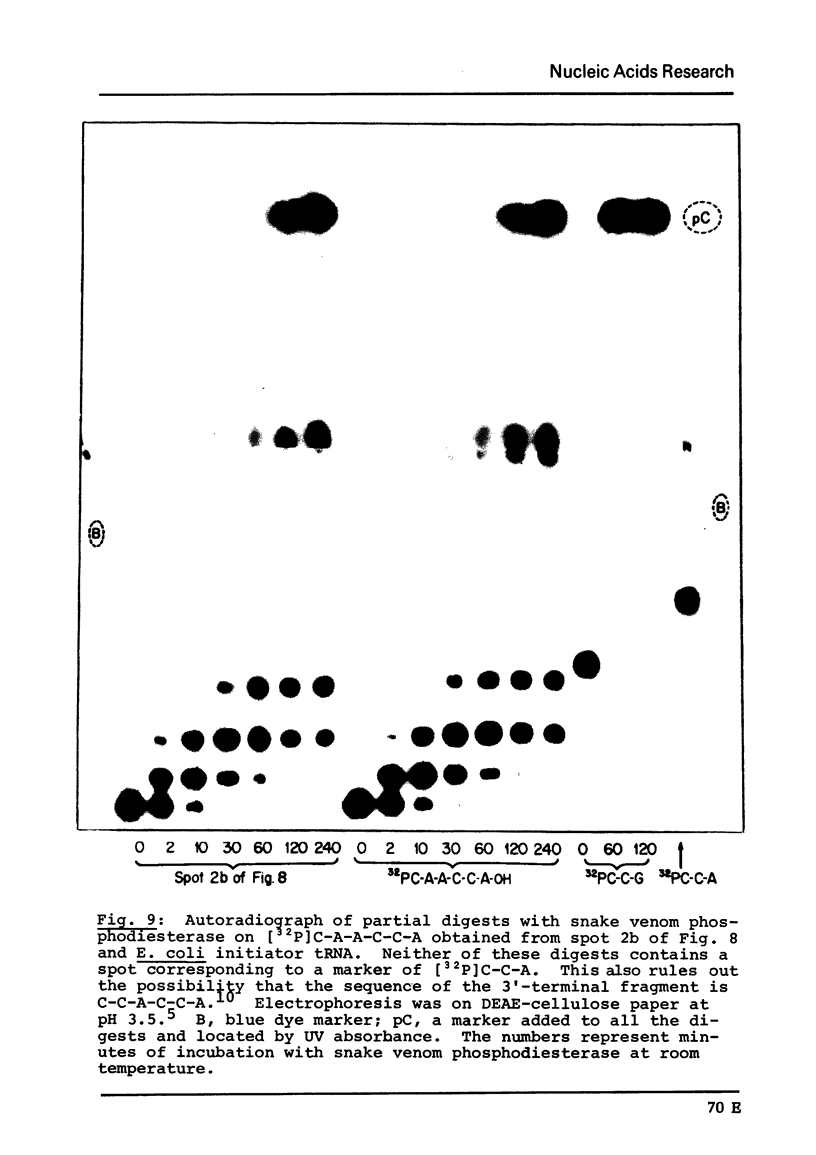
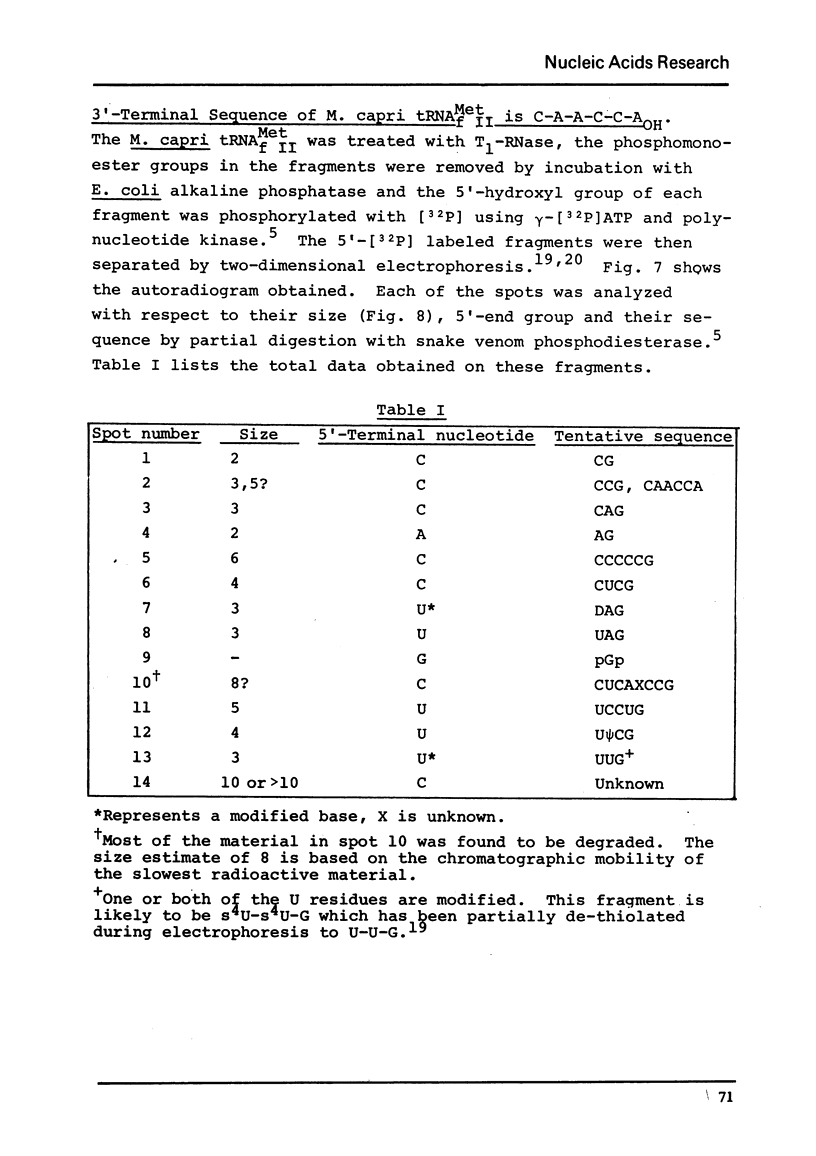
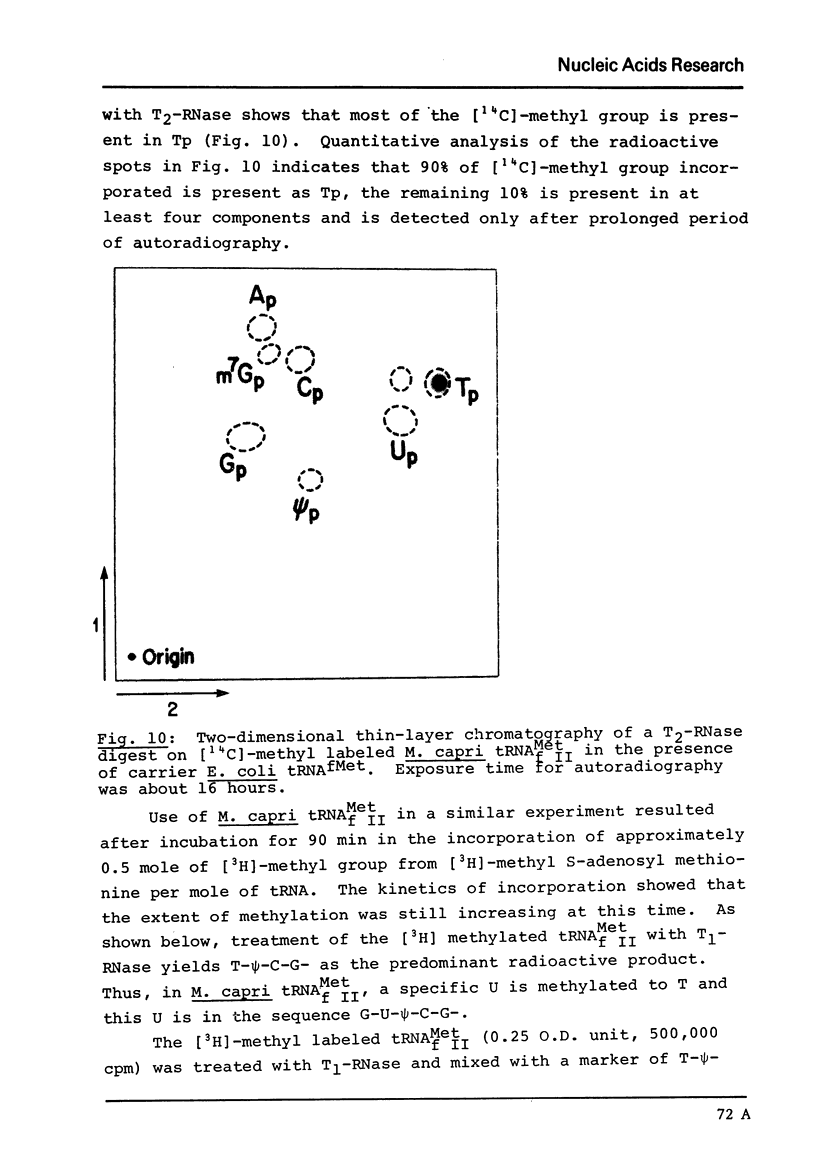
The major species of the formylatable methionine tRNA from Mycoplasma mycoides var capri has been purified. The 5'- and 3'-terminal sequences of the purified tRNA are pC-G- and C-A-A-C-C-AOH, respectively. Thus, this tRNA also contains the unique structural feature found in two other prokaryotic initiator tRNAs in that the first nucleotide at the 5'-end cannot form a Watson-Crick type of base-pair to the fifth nucleotide from the 3'-end. The Mycoplasma tRNA does not contain ribothymidine; however, a specific uridine residue in the sequence G-U-psi-C-G- can be enzymatically methylated by E. coli extracts to yield G-T-psi-C-G. Since ribothymidine is absent in crude tRNA from this strain of Mycoplasma, the absence of T is probably due to the lack of a U yields T modifying enzyme.
Full text
PDF







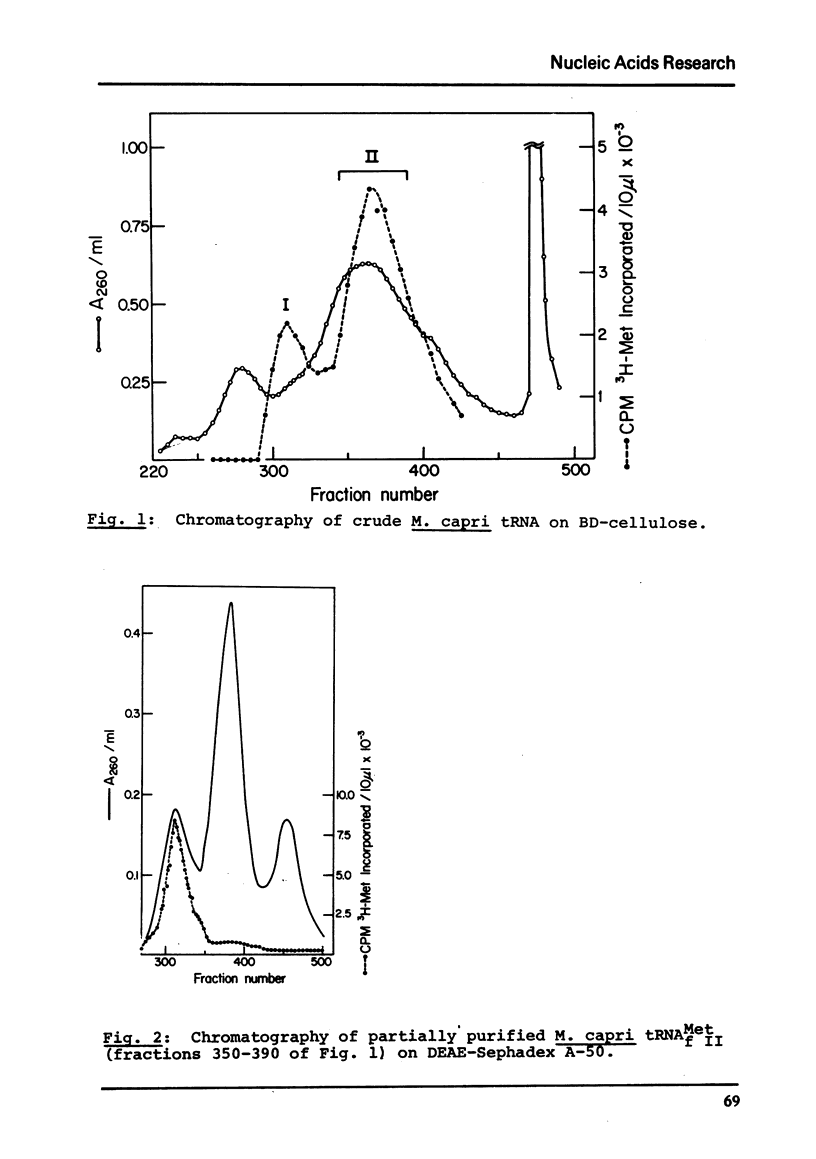
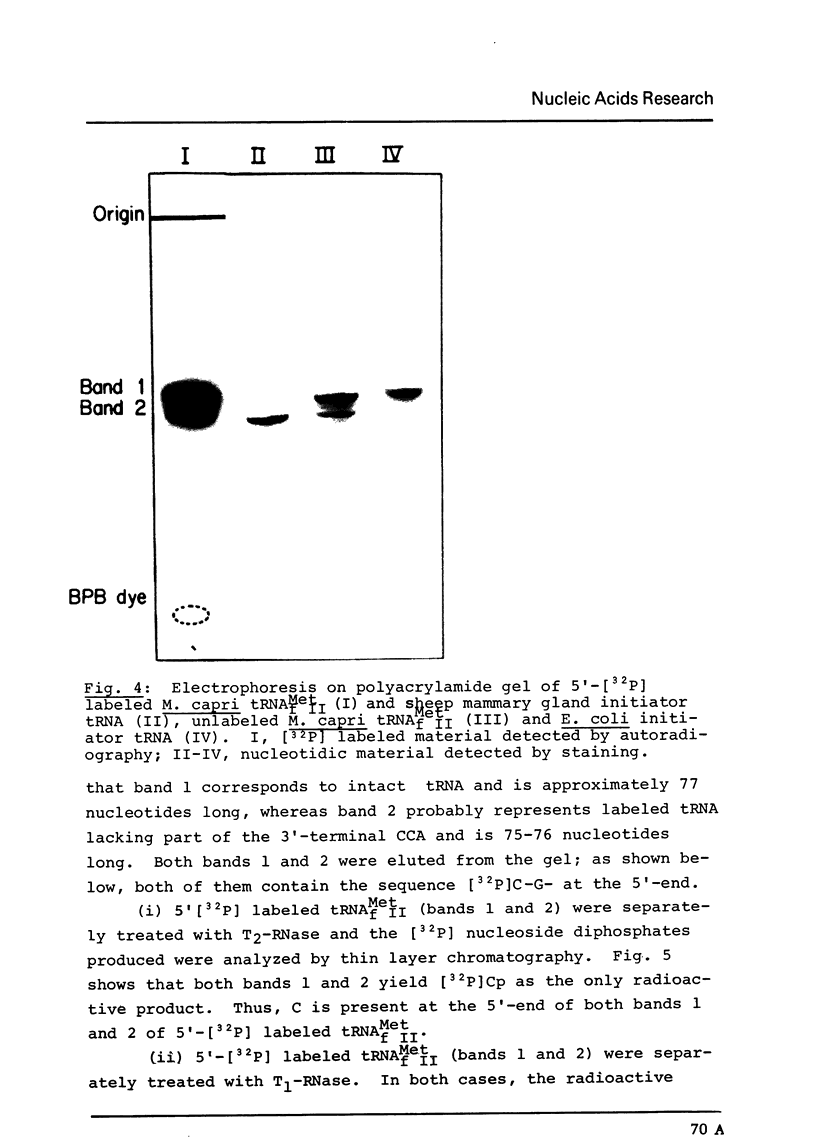


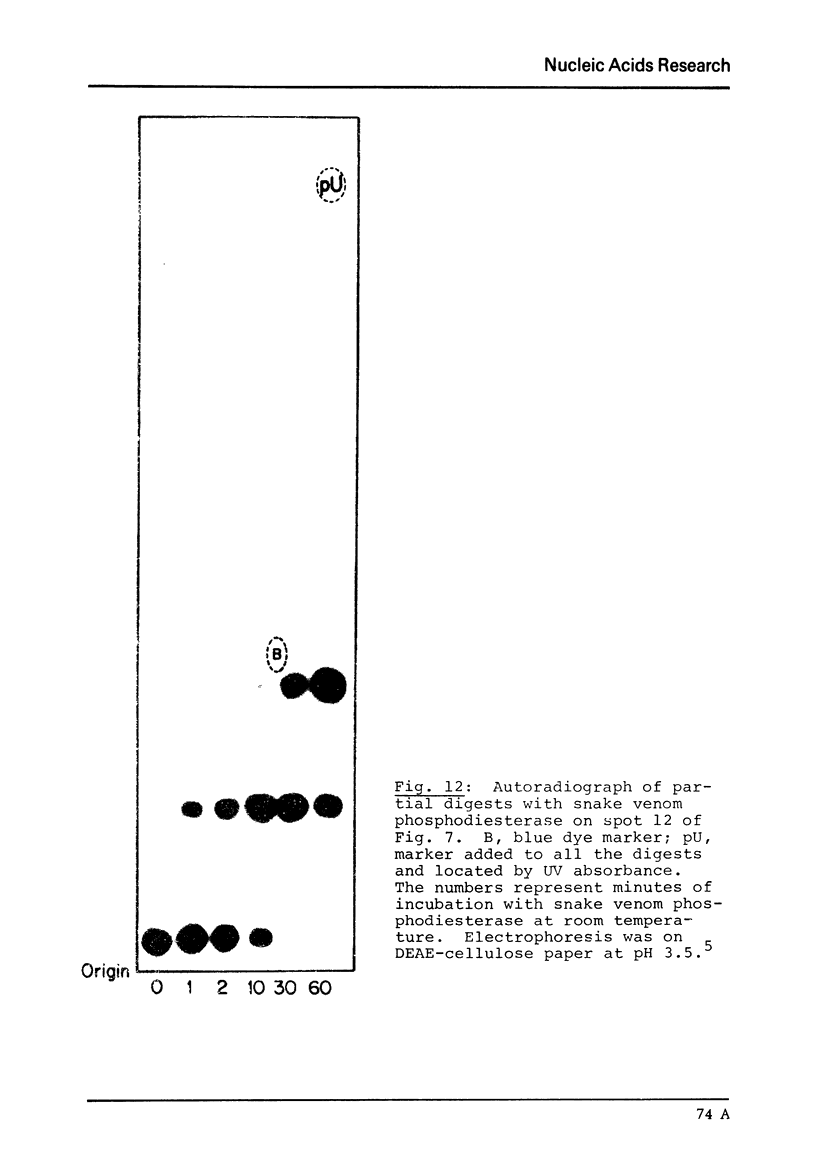




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dube S. K., Marcker K. A., Clark B. F., Cory S. Nucleotide sequence of N-formyl-methionyl-transfer RNA. Nature. 1968 Apr 20;218(5138):232–233. doi: 10.1038/218232a0. [DOI] [PubMed] [Google Scholar]
- Ecarot B., Cedergren R. J. Structure-function correlations of formylmethionine transfer RNA. Biochem Biophys Res Commun. 1974 Jul 10;59(1):400–405. doi: 10.1016/s0006-291x(74)80220-2. [DOI] [PubMed] [Google Scholar]
- Egan B. Z. Separation of oligonucleotides by reversed-phase chromatography. Biochim Biophys Acta. 1973 Mar 19;299(2):245–252. doi: 10.1016/0005-2787(73)90347-x. [DOI] [PubMed] [Google Scholar]
- Feldmann H., Falter H. Transfer ribonucleic acid from Mycoplasma laidlawii A. Eur J Biochem. 1971 Feb;18(4):573–581. doi: 10.1111/j.1432-1033.1971.tb01278.x. [DOI] [PubMed] [Google Scholar]
- Ghosh K., Ghosh H. P., Simsek M., Raj Bhandary U. L. Initiator methionine transfer ribonucleic acid from wheat embryo. Purification, properties, and partial nucleotide sequences. J Biol Chem. 1974 Aug 10;249(15):4720–4729. [PubMed] [Google Scholar]
- Hayashi H., Fisher H., Söll D. Transfer ribonucleic acid from Mycoplasma. Biochemistry. 1969 Sep;8(9):3680–3686. doi: 10.1021/bi00837a028. [DOI] [PubMed] [Google Scholar]
- JONES A. S., WALKER R. T. Isolation and analysis of the deoxyribonucleic acid of Mycoplasma mycoides var. Capri. Nature. 1963 May 11;198:588–589. doi: 10.1038/198588a0. [DOI] [PubMed] [Google Scholar]
- Johnson L., Hayashi H., Söll D. Isolation and properties of a transfer ribonucleic acid deficient in ribothymidine. Biochemistry. 1970 Jul 7;9(14):2823–2831. doi: 10.1021/bi00816a011. [DOI] [PubMed] [Google Scholar]
- Jones A. S., Tittensor J. R., Walker R. T. The chemical composition of the nucleic acids and other macromolecular constituents of Mycoplasma mycoides var. capri. J Gen Microbiol. 1965 Sep;40(3):405–411. doi: 10.1099/00221287-40-3-405. [DOI] [PubMed] [Google Scholar]
- Kelmers A. D., Heatherly D. E. Columns for rapid chromatographic separation of small amounts of tracer-labeled transfer ribonucleic acids. Anal Biochem. 1971 Dec;44(2):486–495. doi: 10.1016/0003-2697(71)90236-3. [DOI] [PubMed] [Google Scholar]
- Kössel H., RajBhandary U. L. Studies on polynucleotides. LXXXVI. Enzymic hydrolysis of N-acylaminoacyl-transfer RNA. J Mol Biol. 1968 Aug 14;35(3):539–560. doi: 10.1016/s0022-2836(68)80013-0. [DOI] [PubMed] [Google Scholar]
- Nishimura S., Harada F., Narushima U., Seno T. Purification of methionine-, valine-, phenylalanine- and tyrosine-specific tRNA from Escherichia coli. Biochim Biophys Acta. 1967 Jun 20;142(1):133–148. doi: 10.1016/0005-2787(67)90522-9. [DOI] [PubMed] [Google Scholar]
- Ono Y., Skoultchi A., Klein A., Lengyel P. Peptide chain elongation: discrimination against the initiator transfer RNA by microbial amino-acid polymerization factors. Nature. 1968 Dec 28;220(5174):1304–1307. doi: 10.1038/2201304a0. [DOI] [PubMed] [Google Scholar]
- Pearson R. L., Weiss J. F., Kelmers A. D. Improved separation of transfer RNA's on polychlorotrifuoroethylene-supported reversed-phase chromatography columns. Biochim Biophys Acta. 1971 Feb 11;228(3):770–774. doi: 10.1016/0005-2787(71)90748-9. [DOI] [PubMed] [Google Scholar]
- Petrissant G. Evidence for the absence of the G-T-psi-C sequence from two mammalian initiator transfer RNAs. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1046–1049. doi: 10.1073/pnas.70.4.1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper P. W., Clark B. F. Lack of Tp-psi-p in loop IV of a mammalian initiator transfer RNA. FEBS Lett. 1973 Mar 15;30(3):265–267. doi: 10.1016/0014-5793(73)80666-0. [DOI] [PubMed] [Google Scholar]
- Piper P. W., Clark B. F. Primary structure of a mouse myeloma cell initiator transfer RNA. Nature. 1974 Feb 22;247(5442):516–518. doi: 10.1038/247516a0. [DOI] [PubMed] [Google Scholar]
- RajBhandary U. L., Ghosh H. P. Studies on polynucleotides. XCI. Yeast methionine transfer ribonucleic acid: purification, properties, and terminal nucleotide sequences. J Biol Chem. 1969 Mar 10;244(5):1104–1113. [PubMed] [Google Scholar]
- Sanger F., Brownlee G. G., Barrell B. G. A two-dimensional fractionation procedure for radioactive nucleotides. J Mol Biol. 1965 Sep;13(2):373–398. doi: 10.1016/s0022-2836(65)80104-8. [DOI] [PubMed] [Google Scholar]
- Schulman L. H., Her M. O. Recognition of altered E. coli formylmethionine transfer RNA by bacterial T factor. Biochem Biophys Res Commun. 1973 Mar 17;51(2):275–282. doi: 10.1016/0006-291x(73)91253-9. [DOI] [PubMed] [Google Scholar]
- Simsek M., Petrissant G., Rajbhandary U. L. Replacement of the sequence G-T-phi-C-G(A)- by G-A-U-C-G- in initiator transfer RNA of rabbit-liver cytoplasm. Proc Natl Acad Sci U S A. 1973 Sep;70(9):2600–2604. doi: 10.1073/pnas.70.9.2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simsek M., RajBhandary U. L., Boisnard M., Petrissant G. Nucleotide sequence of rabbit liver and sheep mammary gland cytoplasmic initiatory transfer RNAs. Nature. 1974 Feb 22;247(5442):518–520. doi: 10.1038/247518a0. [DOI] [PubMed] [Google Scholar]
- Simsek M., RajBhandary U. L. The primary structure of yeast initiator transfer ribonucleic acid. Biochem Biophys Res Commun. 1972 Oct 17;49(2):508–515. doi: 10.1016/0006-291x(72)90440-8. [DOI] [PubMed] [Google Scholar]
- Simsek M., Ziegenmeyer J., Heckman J., Rajbhandary U. L. Absence of the sequence G-T-psi-C-G(A)- in several eukaryotic cytoplasmic initiator transfer RNAs. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1041–1045. doi: 10.1073/pnas.70.4.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel Z., Zamir A., Elson D. The specific resistance of N-substituted initiator methionyl-+RNA to enzymatic hydrolysis. Biochem Biophys Res Commun. 1968 Oct 10;33(1):94–98. doi: 10.1016/0006-291x(68)90260-x. [DOI] [PubMed] [Google Scholar]
- Walker R. T. DNA homology of two Mycoplasma species. Nature. 1967 Nov 18;216(5116):711–712. doi: 10.1038/216711a0. [DOI] [PubMed] [Google Scholar]
- Walker R. T., RajBhandary U. L. Studies on polynucleotides. CI. Escherichia coli tyrosine and formylmethionine transfer ribonucleic acids: effect of chemical modification of 4-thiouridine to uridine on their biological properties. J Biol Chem. 1972 Aug 10;247(15):4879–4892. [PubMed] [Google Scholar]