Abstract
Purpose.
Effects of low vision on peripheral visual function are poorly understood, especially in children whose visual skills are still developing. The aim of this study was to measure both central and peripheral visual functions in youths with typical and low vision. Of specific interest was the extent to which measures of foveal function predict performance of peripheral tasks.
Methods.
We assessed central and peripheral visual functions in youths with typical vision (n = 7, ages 10–17) and low vision (n = 24, ages 9–18). Experimental measures used both static and moving stimuli and included visual crowding, visual search, motion acuity, motion direction discrimination, and multitarget motion comparison.
Results.
In most tasks, visual function was impaired in youths with low vision. Substantial differences, however, were found both between participant groups and, importantly, across different tasks within participant groups. Foveal visual acuity was a modest predictor of peripheral form vision and motion sensitivity in either the central or peripheral field. Despite exhibiting normal motion discriminations in fovea, motion sensitivity of youths with low vision deteriorated in the periphery. This contrasted with typically sighted participants, who showed improved motion sensitivity with increasing eccentricity. Visual search was greatly impaired in youths with low vision.
Conclusions.
Our results reveal a complex pattern of visual deficits in peripheral vision and indicate a significant role of attentional mechanisms in observed impairments. These deficits were not adequately captured by measures of foveal function, arguing for the importance of independently assessing peripheral visual function.
Peripheral visual processing in observers with low vision is characterized by a complex pattern of visual impairments. These deficits are not adequately captured by measures of foveal function, indicating the importance of independently assessing peripheral visual function.
Introduction
Clinical assessments of perceptual abilities in persons with visual impairments typically measure central acuity and the extent of the visual field. These two measures guide educational and training strategies such as those involving orientation and mobility skills. Such assessments, however, are hampered by insufficient knowledge about relationships between measured visual impairments and visual function.1,2 Acuity and visual field integrity are undoubtedly important, but these two assessments alone do not predict visual function across variations under viewing conditions (e.g., low illumination, glare, moving objects, crowded complex scenes), task requirements (e.g., reading, mobility, spatial perception, visual search), and demands for correlated cognitive and motor skills.3 Simply stated, visual ability is not one-dimensional. Inclusion of additional measurements, such as color vision, contrast sensitivity, and eye movement assessments, provides a broader characterization of visual ability. However, even these measurements provide a very limited description of overall visual function. The key question is to what extent standard clinical measures of visual impairments predict visual function. A better understanding of how key visual functions are affected by low vision is especially important for children, whose visual skills are still developing and who may be more amenable to clinical interventions.
Our present focus is on peripheral visual function in low vision. Because acuity is much lower in the periphery than the fovea, the periphery is sometimes thought to have a diminished role in visual function. Refractive corrections are usually based on measures of central acuity; peripheral acuity is often thought to be too low to benefit from refractive correction. Gustafsson,4 however, demonstrated that some adults with central field losses benefit from eccentric corrections. Importantly, peripheral and foveal functions are not independent: impairment of either the central or the peripheral field often reduces visual performance in other regions.5 Moreover, considerable evidence demonstrates that peripheral vision is particularly important for mobility.6–16 Reduced visual fields hinder several aspects of visual-motor performance, including postural stability,17–20 locomotion,21 reach-and-grasp movements,22 and driving.23 Field defects, however, are assessed with a simple, stationary detection task,24 and, consequently, might not reveal impairments in more complex visual functions, such as motion perception and visual search.25 Indeed, low vision may cause reduced use of the peripheral field even without a known visual field restriction (Charles Huss, personal communication, March 2003).26,27 Studies by Ball et al.25 and Ball and Owsley28 found that individuals differ in their use of peripheral vision: estimates of “useful field of view” were found to decrease with age in the absence of any known visual pathology. This underutilization of visual periphery was found to predict risk of automobile crashes by older drivers.29,30
Here we investigated effects of low vision on a range of visual functions in 24 students with diverse disease origins (see Methods and Table 1). This heterogeneous sample allowed us to (1) identify visual characteristics that are shared by different groups with low vision and (2) use an individual differences approach to investigate relationships between various functional impairments. We examined both central and peripheral fields, but the focus was on understanding peripheral visual function. An additional emphasis was placed on motion perception, a key perceptual ability that is often better in the periphery than in the fovea31,32 and consequently may be disproportionately affected by limitations in peripheral visual performance. The full battery included five experiments: (1) central acuity for moving objects; (2) single-target motion discrimination at central and peripheral locations (0°, 12°, and 25° eccentricity); (3) comparison of motion directions across multiple targets spread over ±12° or ±25°; (4) effects of visual crowding on peripheral form discrimination (at 8° and 16° eccentricity); and (5) visual search of large, cluttered visual scenes.
Table 1. .
Observers with Low Vision: Demographics and Causes
|
Age |
Binocular Acuity |
Clinical Diagnosis |
Nystagmus |
| 14 | 20/200 | Aphakia, chronic blepharitis, juvenile glaucoma | No |
| 15 | 20/200 | Bilateral iris, retinal colobomas | No |
| 16 | 20/200 | Congenital cataracts, aphakia | Yes |
| 15 | 20/200 | Congenital cataracts, aphakia | Yes |
| 10 | 20/300 | Esotropia | Yes |
| 15 | 20/200 | Hyperopia | Yes |
| 14 | 20/400 | Nystagmus | Yes |
| 16 | 20/800 | Ocular albinism | Yes |
| 14 | 20/200 | Ocular albinism | Yes |
| 14 | 20/200 | Ocular albinism | Yes |
| 17 | 20/400 | Ocular albinism | Yes |
| 10 | 20/800 | Ocular albinism | Yes |
| 16 | 20/400 | Ocular albinism, exotropia | Yes |
| 16 | 20/200 | Ocular albinism, photophobia | Yes |
| 13 | 20/200 | Ocular albinism, photophobia | Yes |
| 16 | 20/400 | Retinitis pigmentosa | No |
| 9 | 20/200 | Retinopathy of prematurity | No |
| 15 | 20/400 | Retinopathy of prematurity | No |
| 15 | 20/800 | Retinopathy of prematurity | No |
| 13 | 20/200 | Septo-optic dysplasia | No |
| 10 | 20/200 | Stargardt's macular dystrophy | No |
| 15 | 20/200 | Stargardt's macular dystrophy | No |
| 18 | 20/200 | Stargardt's macular dystrophy | No |
| 14 | 20/60 | Stargardt's macular dystrophy | No |
Methods
Participants
Twenty-four observers with low vision (ages 9–18; mean, 14.2 years) were recruited and tested at two residential schools (the Tennessee and Oklahoma Schools for the Blind). Participants' best corrected binocular acuities were between 20/60 and 20/800 (mean logarithm of the minimum angle of resolution [logMAR] = 1.12). All participants had visual fields of at least 35° in both visual hemifields and no history of cognitive impairments. Based on these criteria, participants were first screened by appropriate staff at the schools from a larger population of possible subjects (∼300 students). The schools identified approximately 50 potential participants, from which we selected 24 students based on the vision reports provided by the schools. All 24 participants completed the entire study. Background information and causes are given in Table 1. Thirteen subjects had nystagmus; 11 did not. A comparison group of 7 adolescents with normal or corrected-to-normal acuity and normal visual fields (ages 10–17; mean, 14.6 years) was tested at Vanderbilt University. The study was conducted in accordance with the tenets of the Declaration of Helsinki and was approved by Institutional Review Board at Vanderbilt University. All participants provided informed consent.
Data Analysis
Nonparametric statistical tests (Wilcoxon-Mann-Whitney, Wilcoxon signed ranks, and Friedman's tests and Spearman rank order correlation) were used to test statistical significance because of skewed distributions and unequal variances.
Visual Displays
Stimuli were created using the Psychophysics Toolbox in MATLAB software (The MathWorks, Inc., Natick, MD).33,34 All stimuli were presented at peak contrast of 99%. Stimuli for the central acuity task were displayed on a linearized 19 inch liquid crystal display monitor (model VX924; 1024 × 768 resolution at 85 Hz; ViewSonic). Viewing was binocular at a distance of 77 cm; individual pixels subtended 1.64 arcmin2. Ambient and background illumination levels were 0.13 and 33.5 cd/m2, respectively. Stimuli used in other tasks were projected onto a matte screen (174 × 130 cm ≈ 58° × 45°) by a linearized projector (model WT610; 1024 × 768 resolution at 120 Hz; NEC). Viewing distance was 156 cm; individual pixels subtended 3.75 arcmin2. Ambient and background illumination levels were 0.04 and 46.3 cd/m2, respectively.
Procedure
Visual tasks were given in the same order for all observers. The sequential order was always (1) multitarget motion comparison, (2) crowding, (3) visual search, (4) single-target motion discrimination, and (5) central acuity for motion. This consistency in task sequence was intended to reduce the effects of changing stimulus-response mappings between tasks, as such effects had been observed in earlier pilot work. Specifically, when the comparison task followed blocks of motion direction discriminations, we found that observers would sometimes erroneously respond based on motion direction. For the same reason, we placed search and crowding tasks between two motion tasks to provide an additional “buffer.” In all tasks, except for the visual search, a large fixation cross was used before each trial (2.5° width/height) (Fig. 1C). Eye movements were not recorded. Fixation compliance was informally verified by the experimenter. For tasks that included peripheral stimulus presentation (task nos. 1, 2, and 4), varied eccentricities were randomly intermixed, making exact stimulus position unpredictable and, consequently, precluding anticipatory eye movements. Given the inherent fixation difficulty associated with nystagmus, for each experiment, we first examined the effects of nystagmus. In all but one task no statistically significant differences were found between low-vision observers with and without nystagmus (see below for details).
Figure 1. .
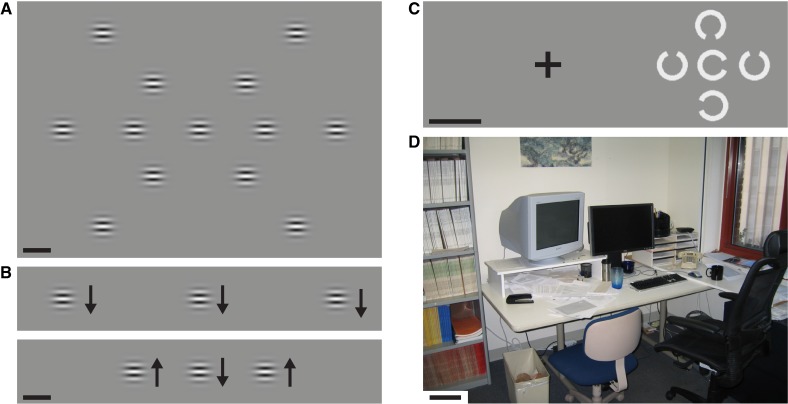
Illustrations of tasks. All scale bars are 5°. (A) Arrangement of 13 stimulus locations in the single-target motion direction discrimination task. On each trial, a single target stimulus appeared with equal likelihood at one of three eccentricities (0°, 12.5°, or 25°) and one of three radial axes (horizontal, 45°/225°, 135°/315°). The observer's task was to identify stimulus motion direction, which was either upward or downward. (B) An example of stimuli used in the multitarget direction comparison task. The flanking stimuli were presented either at 12.5° (bottom image) or 25° (top). The observer's task was to determine whether all stimuli moved in the same direction (top) or whether one was different (bottom). (C) An example of the crowding stimulus at 16° eccentricity. The fixation cross was not visible during the crowding stimulus presentation (it disappeared immediately before the crowding stimulus presentation). The observer's task was to identify the orientation of the middle stimulus' gap. (D) An example of stimuli used for the visual search task. In this image, observers were asked to localize the coffee mug.
Observers practiced each task until sequential thresholds did not vary by more than 15% (usually requiring 3–4 blocks of trials). Following practice, threshold estimates (82% correct) were obtained from three to five 25-trial blocks, using the QUEST staircase.35 Auditory feedback signaled correct responses. Individual trials were self-paced, with the observer initiating the stimulus sequence by a key press.
Visual Tasks
Central Acuity for Moving Stimuli (High Spatial Frequency Cutoff).
Several tasks described below involved moving stimuli. Given the fact that acuity limitations for moving stimuli differ from those for stationary stimulus acuity,36 it was important to determine resolution limits for such stimuli. Here, we measured the upper spatial frequency (SF) cutoff for motion discriminations of 10 Hz drifting gratings. In our pilot work with 9 low-vision subjects (mean logMAR = 0.82; mean age = 14.9 years), we found, as expected, that SF cutoff measured with stationary gratings strongly correlated with logMAR acuity (rs = 0.94; P = 0.0002). However, the SF cutoffs for the same subjects for moving stimuli were not well predicted by logMAR acuity (rs = 0.41; P = 0.27). Finally, SF cutoffs for moving and static gratings correlated only weakly in both the fovea (rs = 0.49, P = 0.09) and at 30° eccentricity (rs = 0.37, P = 0.33). These findings underscore the importance of separately assessing acuity limits for moving stimuli.
Specifically, we measured the highest SF at which motion discrimination was possible (i.e., cut-off SF). In this task, observers simply identified perceived motion direction on each trial (up or down). We used vertical motions because our pilot experiments showed that nystagmus subjects have better acuity for vertical than for horizontal motions (by approximately 25%). Stimulus size was set by a raised cosine window whose diameter was adjusted to contain 2.25 grating cycles (i.e., stimuli with higher SF were shown in smaller spatial envelopes). Stimuli were presented for 150 ms (square-wave envelope) with 10-Hz temporal frequency.
Single-Target Motion Direction Discrimination.
Here, we used a simple visual task where participants identified motion direction (up or down) of briefly presented stimuli in randomly varied locations (this task resembles the Useful Field of View test28). We adaptively adjusted stimulus duration to estimate the shortest exposure durations sufficient for participants to accurately perceive stimulus motion direction. Previous studies have documented the validity and power of temporal duration thresholds for measuring motion sensitivity in both typical and low-vision observers.31,32 Stimuli were gratings presented in a Gaussian spatial envelope (2σ width = 3.2°) and moved either upward or downward (13.3°/second, 10 Hz). Low SF (0.75 cycles/deg) ensured stimulus visibility. These stimuli appeared at 1 of 13 possible locations (Fig. 1A). Stimulus location was equally likely at each of the three eccentricities (0°, ±12°, ±25°) and equally likely on each of the three radial axes (horizontal, 45°/225°, 135°/315°). Stimulus duration was determined by a hybrid Gaussian envelope37 whose width was adjusted to estimate duration thresholds (defined as the width at half height of the temporal envelope). A separate threshold was estimated for each eccentricity, collapsing across radial axes (pilot measurements confirmed that isoeccentric thresholds along different radial axes were similar). Each trial started with the observer fixating on a centrally presented cross (2.5° width/height) and initiating the stimulus sequence by a key press (Fig. 1).
Multi-Target Motion Direction Comparison.
Here, we measured the ability to discriminate three spatially separate and simultaneous motions in the left, central, and right visual fields (i.e., the ability to perceptually compare spatially separate stimuli). The three motion directions were either the same (i.e., up or down were equally likely), or, for 50% of the trials, one direction was different (Fig. 1B). The observers' task was to indicate whether all three directions were the same or one was different (i.e., oddball detection). As in the above-described direction discrimination experiment, duration thresholds were measured. There were two randomly interleaved display conditions that differed in the location of two peripheral stimuli along the horizontal meridian: either ±12° or ±25° eccentricity (Fig. 1B). Other stimulus parameters were as described in the single-target experiment.
Visual Crowding.
When several neighboring objects are presented in the periphery, surrounding objects interfere with discriminations of a central target, an effect known as “crowding.”38–42 As eccentricity increases, larger spatial separation between targets is required to avoid crowding. Thus, crowding constrains effective spatial resolution of vision in cluttered (i.e., naturalistic) environments, limiting critical visual tasks such as object recognition and reading.38–42
Stimuli were composed of five adjacent C shapes (3° diameter, with a 45° opening) (Fig. 1C), with the target shape in the center and four equidistant distracter shapes located around the target. Each trial started with the observer fixating on a centrally presented cross (2.5° width/height) and initiating the stimulus sequence by a key press. The stimulus then briefly (150 ms) appeared at one of four locations (±8° or ±16° eccentricity, randomly selected) (Fig. 1C). The observer's task was to identify the direction of the gap in the C-shaped central target (up, down, left, right). Each distracter had a randomly chosen gap direction (up, down, left, right). Thresholds were measured as the smallest center-to-center separation between target and surrounding distracters that permitted 82% correct task performance. Smaller spacing thresholds indicated better performance and greater tolerance of crowding.
Visual Search.
Children with low vision often indicate difficulties in visually locating objects in crowded natural scenes. Visual search involves a host of visual functions ranging across spatial acuity, object recognition, attention, and eye movement control. Thus, visual search skill seems pertinent to overall visual function. Here, we measured search speeds using photographs of common objects in cluttered scenes (Fig. 1D). Thirty-six photographs displayed nine target objects (e.g., stapler, medicine bottle) in four different background scenes (office rooms). The size of the target objects ranged from 3° to 9° (median, ∼5°). Each target-scene pair was displayed on the large projection screen described above (58° × 45°) and presented once in a set of 36 trials.
Each trial began with a central presentation of the target object (5 seconds) that was verbally identified by the experimenter. Next, the search photograph appeared. The observer's task was to visually locate the target object (standard visual search) and then use a laser pointer to identify its location. The pointing action was almost always rapid, direct, and accurate (i.e., differences in search times reflect visual search variability, and not the pointing action differences). Once the target had been localized, the experimenter stopped the timer. If the observer pointed to an incorrect target, the experimenter simply said “No,” which informed the observer to keep searching. If the target was not located in 30 seconds, the experimenter would say “Keep looking, you'll find it.” If a full minute elapsed, the experimenter repeated the name of the target.
Results
Results for each of five tasks are shown in Figures 2 to 5. Differences between low-vision observers with and without nystagmus were not statistically significant (all z < 1.36, P > 0.17), except for the visual crowding task (discussed below). The key results are described as follows.
Figure 2. .
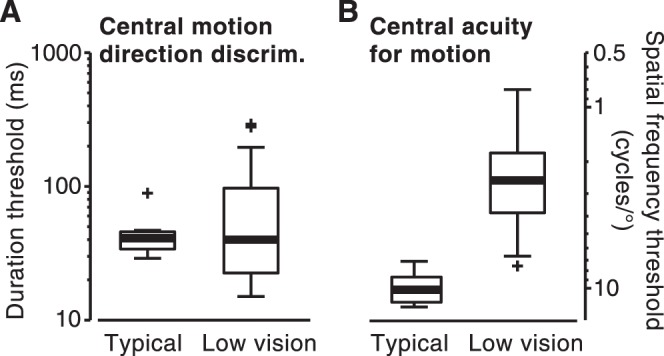
Effects of low vision on foveal motion perception. (A) Motion direction discrimination and (B) acuity for moving stimuli. (A) Data are the same as those described under the condition of 0° in Figure 3A and are plotted here for comparison with data in (B). All plots show the median threshold (bold horizontal lines), interquartile range (box), standard range (whiskers), and outliers (+, points whose distance from box edges is more than 1.5 times the interquartile range).
Figure 5. .
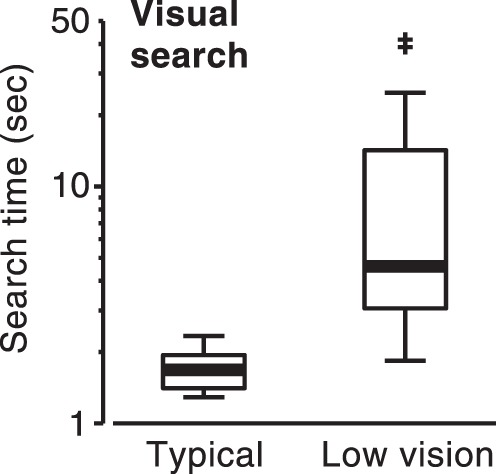
Effects of low vision on visual search speed.
Impaired Motion Acuity but Typical Motion Sensitivity in Central Vision
We assessed motion sensitivity by measuring duration thresholds, effectively estimating the amount of stimulus information that is needed for observers to perceive stimulus motion direction.31,32 For 0.75 cycles/deg stimuli, the sensitivities of observers with low vision to motion direction in the central field did not differ from those of the group with typical vision (Fig. 2A; z = 0, P = 1). Interestingly, 9 observers with low vision had lower motion discrimination thresholds than any of the observers with typical vision (7 observers with low vision had thresholds higher than that of the worst observer with typical vision). This result indicates that on average, low vision had no detrimental effects on perceiving low SF motion stimuli.
The low-vision group's good motion sensitivities in the central field contrasted with their low acuities for similar moving stimuli (defined as maximum SF for motion discrimination). Motion acuities of the two groups with low vision were only approximately one-fourth those of the observers with typical vision (Fig. 2B; z = 3.39, P < 0.001). Consistent with the different results in motion sensitivity and acuity tasks, performance of the observers with low vision in these two tasks correlated relatively weakly (rs = 0.59 [see below for additional discussion]) (Fig. 2).
Motion Sensitivity of Observers with Low Vision Deteriorates in Periphery
Despite typical motion sensitivity in central vision, motion discriminations for observers with low vision were considerably impaired in the periphery, with duration thresholds increasing approximately 5-fold as eccentricity increased from 0° to 25° (Fig. 3A; χ2(2) = 41.4, P = 0.0001). This result contrasts starkly with that of the group with typical vision, where motion discriminations improved with increasing eccentricity (Fig. 3A; χ2(2) = 10.3, P = 0.006). Notably, in a task where no group differences were found in the fovea, the two groups differed by more than 6-fold at 25° eccentricity (z = 3.66, P < 0.001).
Figure 3. .
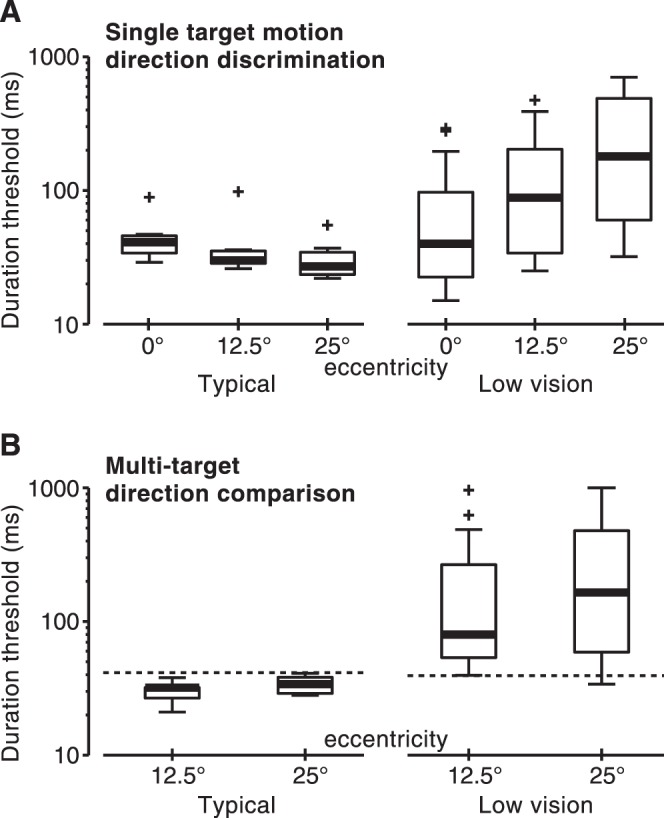
Effects of low vision on motion perception across eccentricity. (A) Single-target motion direction discrimination and (B) multitarget direction comparison. For comparison purposes, thresholds from the 0° condition in (A) are replotted in (B) as dashed lines. Other conventions are as described in the legend to Figure 2.
We found similar results when we compared observers' simultaneous central and peripheral motion detections (Fig. 3B). At 12.5° eccentricity, observers with typical vision performed better at the comparison task than at perceiving motion of a single centrally presented stimulus (at 12.5°, W = 28, P = 0.016; at 25°, W = 19, P = 0.12) (Fig. 3B, dashed line). In contrast, observers with low vision performed worse in the multitarget comparison task (at 12.5°, z = −3.07, P = 0.002; at 25°, z = −4.29, P < 10−4). Performance on the multitarget task, evidently, was limited by deficits in peripheral motion perception (Fig. 3A). Thresholds were similar to and highly correlated with those for peripheral single-target motions (Table 2; rs = 0.93 [see below for additional discussion]) (Fig. 3).
Table 2. .
Correlations between Different Measures of Visual Function
|
Foveal Motion Acuity |
Foveal Motion Acuity |
Motion Discrimination in Fovea |
Motion Discrimination in Periphery |
Multitarget Motion Comparison |
Visual Crowding |
Visual Search |
| Motion discrimination in fovea | 0.59† | |||||
| Motion discrimination in periphery | 0.54 | 0.84§ | ||||
| Multitarget motion comparison | 0.56* | 0.77§ | 0.93§ | |||
| Visual crowding | 0.35 | 0.42 | 0.52 | 0.50 | ||
| Visual search | 0.65‡ | 0.38 | 0.66‡ | 0.76§ | 0.42 | |
| logMAR acuity | 0.49 | 0.50 | 0.44 | 0.50 | 0.01 | 0.49 |
values were Bonferroni corrected. For comparison, all correlations over 0.40 are significant at an uncorrected α of 0.05. Snellen acuity correlations were adjusted (increased) to account for the upper limit (r = 0.89) set by the semicategorical distribution of acuities (Table 1). Terms *, †, ‡, and § indicate corrected P values of 0.1, 0.05, 0.01, and 0.001, respectively.
Reduced Peripheral Crowding for the Group with Nystagmus
Low-vision observers with nystagmus exhibited smaller crowding than the group without nystagmus. This effect was significant at 8° (z = 2.00, P = 0.045) and marginally significant at 16° (z = 1.77, P = 0.077). No such group differences were found in other tasks presented here (all, P > 0.17). The largest absolute advantage of observers with nystagmus was at 16° eccentricity: the median spatial separation required to avoid crowding was lower for the group with nystagmus (2.9°) than for the groups with typical vision (4.4°) and without nystagmus (5.5°). Due to the high variance of the group with nystagmus, differences between the groups with nystagmus and typical vision were not significant (P = 0.15). Nevertheless, the seven best-performing observers were all from the group with nystagmus. Low-vision observers without nystagmus exhibited greater crowding than the group with typical vision at 8° (z = 2.32, P < 0.02) but not at 16° (P = 0.32) (Fig. 4).
Figure 4. .
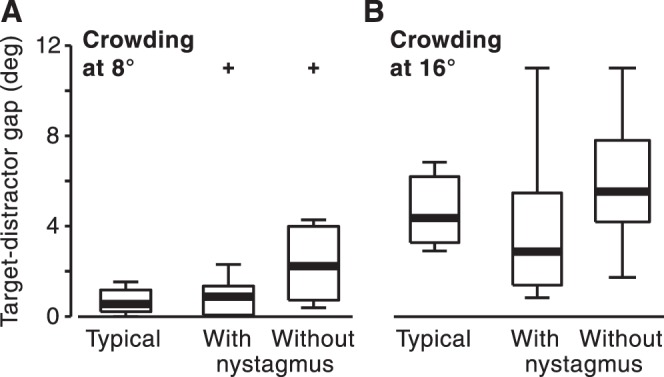
Effects of low vision on visual crowding. Crowding stimuli were presented at either (A) 8° or (B) 16° eccentricity. Observers with low vision were split into two groups: those with and without nystagmus. Other conventions are as described in Figure 2 legend.
Substantial Impairments in Visual Search
Figure 5 shows visual search times for objects presented in wide-field naturalistic displays (58° × 45°). Observers with low vision required considerably more time to locate target objects than did the group with typical vision (z = 3.82, P = 0.0001). Search times for the low-vision groups were approximately 4 seconds longer (Fig. 5).
Relationships among Visual Skills
To determine the relationships between individual differences in observers with low vision, we correlated the results described above with each other and logMAR acuity (Table 2). To reduce the number of comparisons, results from two peripheral locations were averaged for single-target motion discrimination (12.5° and 25°, correlated at rs = 0.91), motion comparison (12.5° and 25°, correlated at rs = 0.95), and visual crowding conditions (8° and 16°, correlated at rs = 0.78). These results and those from other experiments were correlated with each other and with logMAR acuity for all observers with low vision. All correlations were positive, such that better performance on one task predicted better performance on other tasks. Without correction for multiple comparisons, 18 of 21 correlations were significant at an α value of 0.05 (Table 2). However, correlation magnitudes were not high, explaining, on average, only 29% of variance. The strongest relationships were, unsurprisingly, between motion discrimination and motion comparison tasks (mean rs = 0.85). On average, visual search and crowding correlated with other experimental tasks at 0.58 and 0.44, respectively. Similar correlations were observed for foveal motion acuity (mean rs = 0.55). Overall, logMAR acuity correlated reasonably well with most other measures (all but one rs > 0.42). The exception was a lack of correlation with visual crowding (rs = 0.01). As elaborated in Discussion below, it is evident that standard foveal acuity measurements are a reliable but moderate predictor of motion perception and peripheral visual ability.
This result, however, should not be taken as indicating that standard acuity tests cannot be a strong predictor of psychophysical performance. With 9 pilot observers, we found a strong correlation between logMAR and SF cutoff as measured with stationary gratings (rs = 0.94; P = 0.0002). For the same observers, however, measurements of the SF cutoff with moving gratings (i.e., as in the foveal motion acuity task presented here) yielded a much weaker correlation (rs = 0.41, P = 0.27; note that this correlation is very similar to that reported in Table 2).
Discussion
Low vision is defined primarily by low and noncorrectable acuity.43 We show, however, that low acuity is insufficient for describing visual function in low vision. Our findings indicate a complex pattern of visual impairments in observers with low vision, ranging from normal motion discriminations in the fovea to greatly impaired visual search ability. These results exhibited a modest relationship with the standard measure of visual acuity: logMAR acuity accounted for only 24% (median) of the variance in six experimental tasks (Table 2). Acuity measurements with moving stimuli had a slightly better predictive power but still explained only 31% of the variance in other experimental measures (Table 2). The range of visual skills tested in this study certainly does not provide a comprehensive account of visual function, but the observed heterogeneity has implications for both the assessment of visual function and the development of training strategies.
Motion Perception in Fovea
These varied effects of low vision across experiments are likely due to differing visual demands by different tasks. This is illustrated by motion direction discriminations in the fovea. When task difficulty increased by increasing the stimulus SF, observers with low vision showed a considerable impairment (Fig. 2B), reflecting low resolution of moving stimuli. In contrast, the performance of the same observers was indistinguishable from that of control subjects when the task difficulty was adjusted by reducing the stimulus duration (Fig. 2A). In this case, it is evident that impairments in identifying stimulus motion direction occur only if demands are placed on motion acuity. Importantly, acuity for static forms only modestly predicted observed impairments in motion acuity. This finding is consistent with separate acuity limitations for processing static and moving forms.36
Impaired Peripheral Motion Perception in Low Vision
The difficulties that observers with low vision had in discriminating peripheral motion were surprising, especially given that the same observers exhibited no difficulty at discriminating the same moving stimuli in central vision (Fig. 3A). We previously reported a similar result with a smaller sample size.31 In that study, we found that for motion speeds between 1°/second and 30°/second, observers with low vision have peripheral motion perception deficits despite normal motion perception in fovea. There are several reasons this deficit is unlikely to be due to acuity limitations in the visual periphery. First, observers with typical vision exhibited improved motion discriminations with increasing eccentricity (Fig. 3), despite the fact that visual acuity decreased in periphery.44 Second, individual differences in peripheral motion discrimination were better predicted by (unimpaired) foveal motion discriminations than by impaired foveal motion acuity (Table 2; Fisher r-to-z transformation, P = 0.005). Third, in our previous work, we showed that this advantage of peripheral motion processing occurs even for high SFs,31 further ruling out acuity as the limiting factor in this task. Finally, in the pilot study (see Methods), we measured motion acuity both in fovea and at 30° eccentricity. For observers with low vision, motion acuity was only 44% lower at 30° eccentricity, a decrease that was milder than that in control subjects, who exhibited a 79% drop in acuity. This finding also indicates that peripheral acuity limitations cannot explain a dramatic drop in peripheral motion sensitivity.
Taken together, the evidence obtained indicates that acuity limitations are not the key underlying cause of observed impairments of peripheral motion perception. An alternative possibility is the existence of a selective impairment of peripheral motion processing. However, the observed deficit might also reflect reduced functional use of the peripheral field by observers with low vision. Indeed, low vision is associated with functional impairments in field regions unaffected by known pathology.5,25–28 One speculation is that observers with low vision may develop compensatory attentional processes that adversely affect vision in regions not directly affected by underlying pathology. In other words, poor vision in one domain may draw resources from a different domain.
Implications of Impaired Peripheral Motion Perception in Low Vision
In addition to questions about its underlying causes, the observed motion perception impairments raise two additional questions. First, how do deficits in peripheral motion perception affect everyday visual functions of individuals with low vision? Peripheral motion processing plays a key role in a range of visual functions, including orienting, balance, visually guided action, and mobility.6–15,17–23 Mobility in both walking and driving are of particular interest. A comprehensive questionnaire given to a large sample of persons with low vision identified mobility as one of two primary categories of visual function (the other being acuity-demanding tasks such as reading).45 Thus, there is a clear need for increased understanding of functional consequences of these motion perception impairments. Additionally, it is imperative to develop orientation and mobility evaluations that include motion perception assessments. This is particularly important for driving with low vision, where quick perception of moving stimuli is of high significance.
A second outstanding question is whether peripheral motion perception is modifiable by training. Perceptual training is already proving to be an effective intervention for individuals with low vision.46,47 The success and speed of such interventions for peripheral motion perception impairments will likely depend on the nature of the underlying deficits. If the observed impairments are at least partially a consequence of attentional overcompensation mechanisms (see above), then it is possible that the training may be both effective and relatively expeditious.48 Moreover, recent evidence indicates that training via demanding action video games can also have broad effects on low-level visual mechanisms,49 which opens the possibility that observed impairments might be modifiable through training even if the underlying defects are low level. Additionally, both visual crowding and visual search can be improved though training interventions,46,48,50 indicating that impairments reported in this paper might be reduced through appropriate training paradigms. We are currently investigating this question.
Role of Nystagmus in Visual Crowding
A surprising finding was that many of the observers with nystagmus were less affected by peripheral crowding than were either observers with typical sight or low-vision observers without nystagmus. The explanation for this result is not yet known. One possibility is that nystagmus eye movements may place peripheral targets near the fovea, where visual crowding is reduced.42 We used a brief stimulus presentation (150 ms) to minimize the effects of nystagmus. However, a certain number of foveal and parafoveal presentations is expected to occur even with such brief presentation times. Another possibility, not necessarily incompatible with the first, is that many of these observers may develop compensatory skills for perceiving stationary forms in the periphery and may have reduced visual crowding. We are currently beginning to investigate this question by using eye tracking and gaze-dependent stimulus presentation. The effects of nystagmus were not seen in other tasks, indicating that the observed effects may be specific to crowding. It should be noted, however, that we used vertical motion directions specifically to minimize the effects of nystagmus on motion perception.51
Impaired Visual Search in Low Vision
The finding that visual search is more difficult for observers with low vision is not surprising. However, the observed 3-fold magnitude of impairment is functionally important, as such visual search deficits are likely to have a substantial effect on a wide range of visual functions.52 It is likely that large field of view (∼60°) and the use of naturalistic cluttered scenes contributed to the observed magnitude of impairment. In adults with low vision, visual search defects worsen with both increasing search field size and increasing object density.53
Underlying Mechanisms
The battery of tasks in the present study covers two broad aspects of visual function: motion perception and visual processes that are intrinsically limited by attention (crowding and visual search).38–42,54 This focus, motivated by the fundamental importance of both motion6 and attention54 processes in visual periphery, limits the generalizability of our results to other classes of visual function such as depth perception and color processing. Nevertheless, our results permit inferences, albeit speculative ones, about the underlying mechanisms of impairment. Although the origin of our sample ensures the existence of impaired retinal mechanisms, here we conjecture that significant aspects of the observed impairments are cortical in origin. Visual crowding is believed to reflect the limits in the resolution of cortical attentional mechanisms, that is, attentional acuity.38–42 This is consistent with a lack of correlation between logMAR acuity and crowding (Table 2). Similarly, attention is a key aspect of visual search,54 although visual acuity limitations would certainly have an effect on search speeds. As discussed above, key factors limiting performance in motion tasks are less obvious, but our results rule out mechanisms that uniformly affect performance across the visual field. Observed deficits in peripheral motion perception are, at least in part, consistent with the above-mentioned attentional impairments. Motion stimuli exogenously attract attention,55 a fact that likely contributes to high visual sensitivity for peripheral motion.31,32 Consequently, peripheral motion perception may be considerably affected by attentional impairments especially if stimulus location is unpredictable (as was the case in our experiments).
While our results indicate a significant role of attentional impairments in low vision, they do not reveal their origin. Such impairments may reflect adaptive and/or maladaptive adjustments to primary visual impairments. For example, the presence of greatly reduced foveal acuity may require increased attentional resources, resulting in reduced attention to peripheral stimuli. Additionally, it is possible that abnormal visual processing may have direct effects on the efficiency of high-level visual processes, which typically operate on unimpaired visual inputs.
Conclusions
In sum, we report a complex pattern of visual impairments in a range of tasks that involve peripheral visual processes. Performance of these tasks had a reliable but modest relationship to foveal measures of visual function, indicating the importance of independently assessing peripheral visual ability in observers with low vision. While perimetry tests provide detailed field maps, they are based on simple detection tasks, and, thus, cannot detect deficits in more complex visual processes.24,25 Determining which visual tasks provide the most diagnostic description of peripheral visual function is an important future direction. Finally, our results are consistent with a role of higher level impairments in low vision, arguing for a broader use of interventions designed to improve high-level processes such as visual attention and crowding.46–50
Acknowledgments
The authors thank Krystel Huxlin, Seth Kaplan, and Molly Tadin for insightful comments; Doug Morse for technical assistance; and Michael Melnick for manuscript assistance.
Footnotes
Supported by National Eye Institute Grants R03 EY15558 (JL), R01 EY019295 (DT), and P30 EY08126 and P30 EY001319. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosure: D. Tadin, None; J.B. Nyquist, None; K.E. Lusk, None; A.L. Corn, None; J.S. Lappin, None
References
- 1.Colenbrander A. Guide for the Evaluation of Visual Impairment. San Francisco, CA: Pacific Vision Foundation; 1999 [Google Scholar]
- 2.Lennie P, Hemel SBV. Visual Impairments: Determining Eligibility for Social Security Benefits. Washington, DC: National Academies Press; 2002 [PubMed] [Google Scholar]
- 3.Brabyn J, Schneck M, Haegerstrom-Portnoy G, Lott L. The Smith-Kettlewell Institute (SKI) longitudinal study of vision function and its impact among the elderly: an overview. Optom Vis Sci. 2001;78:264–269 [DOI] [PubMed] [Google Scholar]
- 4.Gustafsson J. Eccentric Correction in Central Visual Field Loss. Lund, Sweden: Lund University; 2001 [Google Scholar]
- 5.Peli E. Vision multiplexing: an engineering approach to vision rehabilitation device development. Optom Vis Sci. 2001;78:304–315 [DOI] [PubMed] [Google Scholar]
- 6.Nakayama K. Biological image motion processing: a review. Vision Res. 1985;25:625–660 [DOI] [PubMed] [Google Scholar]
- 7.Geruschat D, Smith AJ. Low vision and mobility. In: Blasch WRW, Welsh RL.eds Foundations of Orientation and Mobility. New York: AFB Press; 1997:60–103 [Google Scholar]
- 8.Geruschat DR, Turano KA, Stahl JW. Traditional measures of mobility performance and retinitis pigmentosa. Optom Vis Sci. 1998;75:525–537 [DOI] [PubMed] [Google Scholar]
- 9.Greer R. Evaluation methods and functional implications: children and adults with visual impairments. In: Lueck AH.ed Functional Vision: A Practitioner's Guide To Evaluation and Intervention. New York: AFB Press; 2004:177–203 [Google Scholar]
- 10.Kuyk T, Elliott JL, Fuhr PSW. Visual correlates of mobility in real world settings in older adults with low vision. Optom Vis Sci. 1998;75:538–547 [DOI] [PubMed] [Google Scholar]
- 11.Marron JA, Bailey IL. Visual factors and orientation-mobility performance. Am J Optom Physiol Opt. 1982;59:413–426 [DOI] [PubMed] [Google Scholar]
- 12.Noe G, Ferraro JB, Lamaoureux E, Rait JF, Keefe JE. Associations between glaucomatous visual field loss and participation in activities of daily living. Clin Exp Ophthalmol. 2003;31:482–486 [DOI] [PubMed] [Google Scholar]
- 13.Rieser JJ, Hill EW, Talor CR, Bradfield A, Rosen S. Visual experience, visual field size, and the development of nonvisual sensitivity to the spatial structure of outdoor neighborhoods explored by walking. J Exp Psychol Gen. 1992;121:210–221 [DOI] [PubMed] [Google Scholar]
- 14.Turano KA, Broman AT, Bandeen-Roche K, Munoz B, Rubin GS, West S. Association of visual field loss and mobility performance in older adults: Salisbury Eye Evaluation Study. Optom Vis Sci. 2004;81:298–307 [DOI] [PubMed] [Google Scholar]
- 15.Turano KA, Rubin GS, Quigley HA. Mobility performance in glaucoma. Invest Ophthalmol Vis Sci. 1999;40:2803–2809 [PubMed] [Google Scholar]
- 16.Vargas-Martin F, Peli E. Eye movements of patients with tunnel vision while walking. Invest Ophthalmol Vis Sci. 2006;47:5295–5302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Black AA, Wood JM, Lovie-Kitchin JE, Newman BM. Visual impairment and postural sway among older adults with glaucoma. Optom Vis Sci. 2008;85:489–497 [DOI] [PubMed] [Google Scholar]
- 18.Shabana N, Cornilleau-Peres V, Droulez J, Goh JC, Lee GS, Chew PT. Postural stability in primary open angle glaucoma. Clin Exp Ophthalmol. 2005;33:264–273 [DOI] [PubMed] [Google Scholar]
- 19.Turano K, Herdman SJ, Dagnelie G. Visual stabilization of posture in retinitis pigmentosa and in artificially restricted visual fields. Invest Ophthalmol Vis Sci. 1993;34:3004–3010 [PubMed] [Google Scholar]
- 20.Turano KA, Dagnelie G, Herdman SJ. Visual stabilization of posture in persons with central visual field loss. Invest Ophthalmol Vis Sci. 1996;37:1483–1491 [PubMed] [Google Scholar]
- 21.Marigold DS. Role of peripheral visual cues in online visual guidance of locomotion. Exerc Sport Sci Rev. 2008;36:145–151 [DOI] [PubMed] [Google Scholar]
- 22.Gonzalez-Alvarez C, Subramanian A, Pardhan S. Reaching and grasping with restricted peripheral vision. Ophthalmic Physiol Opt. 2007;27:265–274 [DOI] [PubMed] [Google Scholar]
- 23.Bowers A, Peli E, Elgin J, McGwin G Jr, Owsley C. On-road driving with moderate visual field loss. Optom Vis Sci. 2005;82:657–667 [DOI] [PubMed] [Google Scholar]
- 24.Beck RW, Bergstrom TJ, Lichter PR. A clinical comparison of visual field testing with a new automated perimeter, the Humphrey Field Analyzer, and the Goldmann perimeter. Ophthalmology. 1985;92:77–82 [DOI] [PubMed] [Google Scholar]
- 25.Ball K, Owsley C, Beard B. Clinical visual perimetry underestimates peripheral field problems in normal observers. Clin Vis Sci. 1990;5:113–125 [Google Scholar]
- 26.Ambrose GV, Corn AL. Impact of low vision on orientation: an exploratory study. RE:view. 1997;19:80–96 [Google Scholar]
- 27.Ludt R, Goodrich GL. Change in visual perception distances for low vision travelers as a result of dynamic visual assessment and training. J Vis Impair Blind. 2002;96:7–21 [Google Scholar]
- 28.Ball K, Owsley C. The useful field of view test: a new technique for evaluating age-related declines in visual function. J Am Optom Assoc. 1993;64:71–79 [PubMed] [Google Scholar]
- 29.Clay OJ, Wadley VG, Edwards JD, Roth DL, Roenker DL, Ball KK. Cumulative meta-analysis of the relationship between useful field of view and driving performance in older adults: current and future implications. Optom Vis Sci. 2005;82:724–731 [DOI] [PubMed] [Google Scholar]
- 30.Owsley C, Ball K, McGwin G, et al. Visual processing impairment and risk of motor vehicle crash among older adults. JAMA. 1998;279:1083–1088 [DOI] [PubMed] [Google Scholar]
- 31.Lappin JS, Tadin D, Nyquist JB, Corn AL. Spatial and temporal limits of motion perception across variations in speed, eccentricity, and low vision. J Vis. 2009;9:30.1–14 [DOI] [PubMed] [Google Scholar]
- 32.Tadin D, Lappin JS, Gilroy LA, Blake R. Perceptual consequences of centre-surround antagonism in visual motion processing. Nature. 2003;424:312–315 [DOI] [PubMed] [Google Scholar]
- 33.Brainard DH. The Psychophysics Toolbox. Spat Vis. 1997;10:433–436 [PubMed] [Google Scholar]
- 34.Pelli DG. The VideoToolbox software for visual psychophysics: transforming numbers into movies. Spat Vis. 1997;10:437–442 [PubMed] [Google Scholar]
- 35.Watson AB, Pelli DG. QUEST: a Bayesian adaptive psychometric method. Percept Psychophys. 1983;33:113–120 [DOI] [PubMed] [Google Scholar]
- 36.Robson JG. Spatial and temporal contrast-sensitivity functions of the visual system. J Opt Soc Am. 1966;56:1141–1142 [Google Scholar]
- 37.Tadin D, Silvanto J, Pascual-Leone A, Battelli L. Improved motion perception and impaired spatial suppression following disruption of cortical area MT/V5. J Neurosci. 2011;31:1279–1283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bouma H. Visual interference in the parafoveal recognition of initial and final letters of words. Vision Res. 1973;13:767–782 [DOI] [PubMed] [Google Scholar]
- 39.Levi DM. Crowding—an essential bottleneck for object recognition: a mini-review. Vision Res. 2008;48:635–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pelli DG. Crowding: a cortical constraint on object recognition. Curr Opin Neurobiol. 2008;18:445–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pelli DG, Tillman KA, Freeman J, Su M, Berger TD, Majaj NJ. Crowding and eccentricity determine reading rate. J Vis. 2007;720;21–36 [DOI] [PubMed] [Google Scholar]
- 42.Whitney D, Levi DM. Visual crowding: a fundamental limit on conscious perception and object recognition. Trends Cogn Sci. 2011;15:160–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pascolini D, Mariotti SP. Global estimates of visual impairment: 2010. Br J Ophthalmol. 2012;96:614–618 [DOI] [PubMed] [Google Scholar]
- 44.Anstis SM. Letter: a chart demonstrating variations in acuity with retinal position. Vision Res. 1974;14:589–592 [DOI] [PubMed] [Google Scholar]
- 45.Massof RW, Ahmadian L, Grover LL, et al. The activity inventory: an adaptive visual function questionnaire. Optom Vis Sci. 2007;84:763–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hussain Z, Webb BS, Astle AT, McGraw PV. Perceptual learning reduces crowding in amblyopia and in the normal periphery. J Neurosci. 2012;32:474–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Levi DM, Li RW. Perceptual learning as a potential treatment for amblyopia: a mini-review. Vision Res. 2009;49:2535–2549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Green CS, Bavelier D. Action-video-game experience alters the spatial resolution of vision. Psychol Sci. 2007;18:88–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li RJ, Polat U, Makous W, Bavelier D. Enhancing the contrast sensitivity function through action video game training. Nat Neurosci. 2009;12:549–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sireteanu R, Rettenbach R. Perceptual learning in visual search generalizes over tasks, locations, and eyes. Vision Res. 2000;40:2925–2949 [DOI] [PubMed] [Google Scholar]
- 51.Shallo-Hoffmann JA, Bronstein AM, Acheson J, Morland AB, Gresty MA. Vertical and horizontal motion perception in congenital nystagmus. Neuroophthalmology. 1998;19:171–183 [Google Scholar]
- 52.Liu L, Kuyk T, Fuhr P. Visual search training in subjects with severe to profound low vision. Vision Res. 2007;47:2627–2636 [DOI] [PubMed] [Google Scholar]
- 53.Dougherty BE, Martin SR, Kelly CB, Jones LA, Raasch TW, Bullimore MA. Development of a battery of functional tests for low vision. Optom Vis Sci. 2009;86:955–963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Carrasco M. Visual attention: the past 25 years. Vision Res. 2011;51:1484–1525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dick M, Ullman S, Sagi D. Parallel and serial processes in motion detection. Science. 1987;237:400–402 [DOI] [PubMed] [Google Scholar]