Abstract
Adipose tissue is the primary energy reservoir in the body and an important endocrine organ that plays roles in energy homeostasis, feeding, insulin sensitivity and inflammation. While it was tacitly assumed that fat in different anatomical locations had a common origin and homogenous function, it is now clear that regional differences exist in adipose tissue characteristics and function. This is exemplified by the link between increased deep abdominal or visceral fat, but not peripheral adipose tissue, and the metabolic disturbances associated with obesity. Regional differences in fat function are due in large part to distinct adipocyte populations that comprise the different fat depots. Evidence accrued primarily in the last decade indicate that the distinct adipocyte populations are generated by a number of processes during and after development. These include the production of adipocytes from different germ cell layers, the formation of distinct preadipocyte populations from mesenchymal progenitors of mesodermal origin, and the production of adipocytes from hematopoietic stem cells from the bone marrow. This review will examine each of these process and their relevance to normal adipose tissue formation and contribution to obesity-related diseases.
Keywords: stem cells, progenitor cells, preadipocytes, adipocytes, obesity, adipose depot heterogeneity
Once considered merely an energy reservoir, adipose tissue is now also recognized as an endocrine organ that plays central roles in energy homeostasis, feeding behavior, energy expenditure, insulin sensitivity and inflammation. These activities are manifested through the regulated storage of dietary fuels and release of fatty acids into the circulation. Adipocytes also produce several inflammatory cytokines (e.g. interleukin-6 and tumor necrosis factor-α) and “adipokines” including leptin, which suppresses appetite and increases metabolic rate and fat oxidation 1, and adiponectin, which enhances insulin sensitivity and diminishes inflammation 2. In spite of the pivotal role of adipose tissue in metabolism, the processes that promote its development, expansion and maintenance are poorly understood. Limitations in our understanding of adipose development are especially disconcerting due to the alarming increase in obesity in the United States and other developed nations. It is now estimated that two thirds of Americans are overweight or obese, with similar levels reported in other developed nations 3, 4.
Excess adiposity is linked to type 2 diabetes, cardiovascular disease (hypertension and atherosclerosis), pulmonary, liver and kidney disorders and certain forms of cancer 5–7. Increases in visceral adipose tissue (VAT) are in particular linked to these diseases whereas, storage of fat in subcutaneous adipose tissue (SAT) is linked to a lower risk for these conditions 8–10.
Fat cells come in two general types, white and brown. White adipocytes are the most numerous fat cells in the major adipose depots of the body. They have a single large lipid drop-let (unilocular appearance) and their primary function is to regulate energy storage and release. Brown adipocytes have a more limited distribution in the body, contain several smaller lipid droplets (multilocular appearance), and oxidize fatty acids for heat production.
The differential impact of visceral versus subcutaneous fat on health and the distinction between white and brown fat highlight an important concept in adipose tissue biology, namely that fat in different body locations exhibits distinct features and functional characteristics. Regional differences in adipose tissue composition and function include variations in non-adipocyte cell populations (endothelial cells, myeloid cells, lymphocytes, etc.), differences in the microvasculature of each depot, or differences in innervation 11. In addition, different regional depots harbor distinct adipocyte populations.
Differences between adipocytes from visceral and subcutaneous depots have been recognized for many years. For example, VAT adipocytes exhibit higher rates of fatty acid turnover and lipolysis, and are less responsive to the antilipolytic affects of insulin than SAT adipocytes12, leading to greater release of free fatty acids into the circulation from VAT. Likewise, VAT adipocytes produce more interleukin-6 (an inflammatory cytokine that suppresses insulin responsiveness) but less adiponectin and leptin than SAT fat cells 13. Such differences provide a link between increased visceral adiposity and the insulin resistance and inflammation that characterize obesity.
Are differences in adipocyte function the result of distinct developmental pathways for producing fat cells in different body locations, or do adipocytes in different depots share a common origin but become “programmed” towards depot-specific functions? This review will explore evidence, garnered primarily within the last decade, demonstrating that 1) adipocytes can be generated from both mesoderm and neuroectoderm, 2) fat in different body locations contains discrete adipocyte progenitors whose distinctive features are programmed early in development, 3) some adipocytes arise from non-resident progenitors, in particular, bone marrow hematopoietic stem cells, and 4) mature adipocytes exercise phenotypic plasticity.
White Adipocyte Progenitors Are Present in Adipose Stroma
A long-standing paradigm in adipose biology was that all adipocytes are generated from mesenchymal progenitor cells of mesodermal origin. Such progenitors were believed to localize to vascular networks where the regional fat depots eventually form, and give rise to a resident self-renewing population of adipocyte progenitors. Adipose lineage-committed preadipocytes and stem cells capable of multilineage differentiation (endothelial, adipose, cartilage and bone) can be isolated from fat stroma 14. These cells are apparently present throughout life to replace dying adipocytes (in humans approximately 10% of adipocytes are regenerated each year 15) and for expansion of adipose tissue during times of excess energy availability. However, the identity of these adipocyte progenitors remained unclear because of the heterogeneous population of cells that comprise adipose stroma and absence of markers to distinguish progenitors from other stromal cells.
Initial studies to identify and localize adipose-resident progenitors were performed by Graff and colleagues 16 who generated lineage analysis models based on the rationale that the transcription factor PPARγ is the central regulator of adipogenesis. Accordingly, they analyzed the timeline of PPARγ induction during postnatal white adipose tissue development via a triple transgenic system, in which the Pparγ locus drives cre-mediated lineage marking of cells, which can be turned off by doxycyclin (dox) at any time. Their surprising finding was that PPARγ-driven lineage marking of would-be adipocytes could not be prevented after the second postnatal day, suggesting that PPARγ-expressing progenitors committed to the white adipocyte lineage were in place as early as the pre- or perinatal period. Furthermore, the Graff team were able to isolate cells expressing GFP under the control of the PPARγ gene promoter from adipose stroma and demonstrate that these cells proliferated both in vivo and in culture, underwent spontaneous adipogenesis in vitro and formed ectopic fat pads in nude mice. The PPARγ-GFP cells resided in the adipose vasculature and expressed the mural-endothelial cell markers PDGFRβ, SM-actin and NG2. These cells may be related to mesoangioblasts, mesenchymal-like stem cells derived from pericytes associated with vessel walls17. Adult mesoangioblasts spontaneously differentiate into cell types of their tissue-of-origin, and can differentiate into other mesodermal cell types.
Adipocyte progenitors in adipose stroma were also identified by Friedman and colleagues 18 using sequential flow cytometry fractionation of stromal cells based on their expression of stem cell markers. They identified a Lin-:CD29+:CD34+:Sca-1+:CD24+ stromal population capable of spontaneous adipogenic differentiation in vitro. While these cells were unable to restore white adipose tissue formation in chow-fed wild type mice, they could reconstitute white adipose tissue in either lipodystrophic mice or wild-type mice fed a high-fat diet. The investigators surmised that successful proliferation of the transplanted progenitors required a microenvironment enriched with factors that support proliferation and differentiation of preadipocytes; such conditions conceivably exist in both the continual turn over of lipodystrophic tissue and the rapidly expanding adipose pads of high-fat fed animals, but not in the fat pads of normal mice on a normal diet. Moreover, in lipodystrophic recipients, the transplanted wild type cells clearly possess a selective genetic advantage over the host cells, and thus may stably reconstitute the otherwise unstable tissue. Such advantages are often necessary for long-term engraftment of progenitor cells when the recipient niche is occupied as demonstrated in liver studies using bone marrow transplantation into wild type and FAH knockout mice 19.
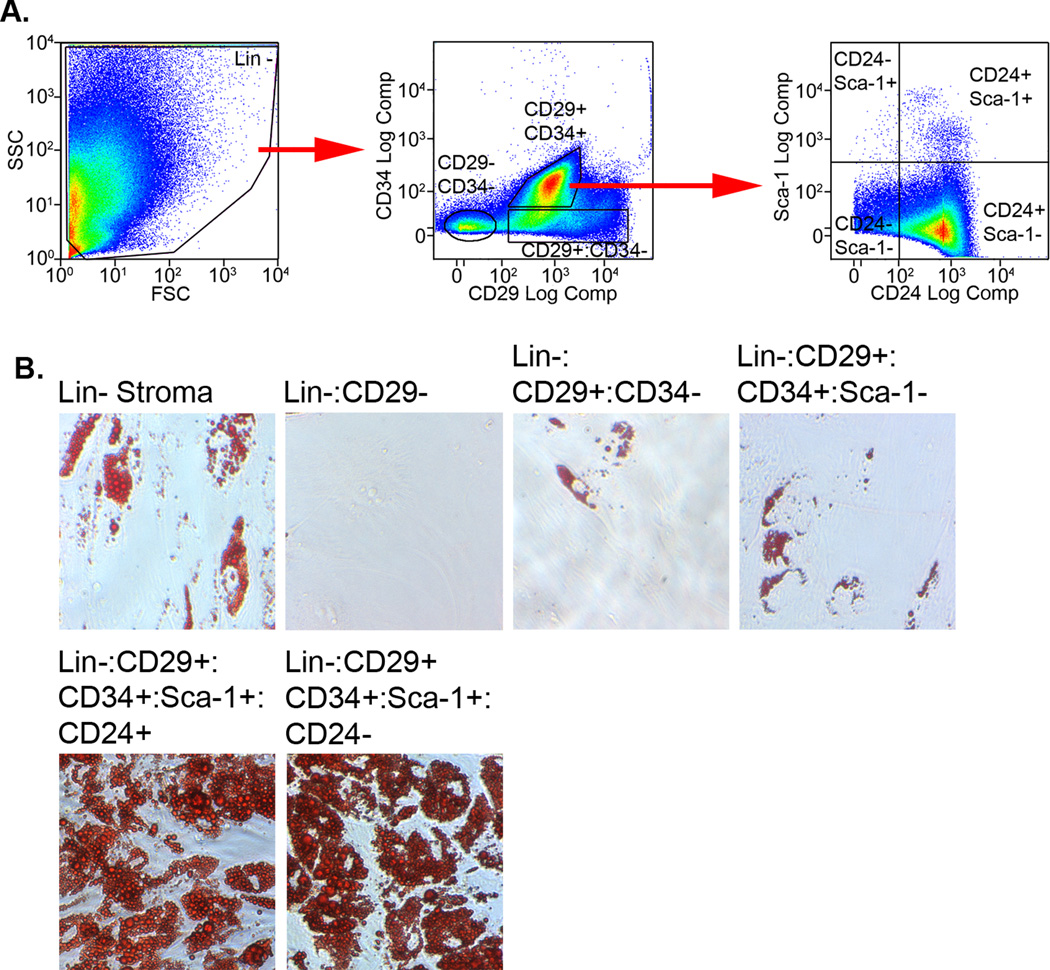
The corresponding CD24 negative stromal population (Lin-:CD29+:CD34+:Sca-1+:CD24−), which constitutes over 50% of Lin- stroma, was also able to produce adipocytes in vitro, but not in lipodystrophic or high fat fed mice. This crucial observation indicates that the bulk of stromal cells, while capable of in vitro adipogenesis, are likely functionally distinct from the CD24+ population in vivo. The numerous previous studies that have used mixed adipose stroma to explore fat tissue formation have likely overestimated the adipogenic potential of the mixed population. We have confirmed the in vitro portion of the Friedman studies by simultaneously sorting CD29+:CD34+:Sca-1+:CD24+ and corresponding CD24- cells from Lin- adipose stroma (Fig. 1A and B). These populations were indeed capable of robust adipogenic differentiation compared to unsorted stroma or cells negative for CD29, CD34 or Sca-1 expression.
Figure 1. Flow cytometry isolation of resident adipocyte progenitors from adipose stroma.
Lin-stroma was prepared from mouse gonadal fat by magnetic bead separation. A) Lin- cells were simultaneously labeled with anti-CD29-PE/Cy7, anti-CD34-PE, anti-Sca-1-PerCP/Cy5.5 and anti-CD24-APC/Cy7 antibodies. The cells were sorted into the indicated subpopulations on a MoFlo XDP using the isolation scheme reported by Friedman and colleagues 18. B) Cells from the indicated populations were plated under colony forming unit-fibroblast conditions until they reached confluence. They were then treated with adipogenic inducers for 10 days and stained with oil red O to reveal triglyceride droplets. Longer periods of induction did not increase the number of lipid-containing cells. Our results substantiate the ability of Lin-:CD29+:CD34+:Sca-1+:CD24+/− stromal cells to undergo adipogenic conversion in culture as described by the Friedman group.
Although both the Graff and Friedman groups make a strong case that their adipocyte progenitors are adipose resident, other interpretations remain viable. For example, the location of the cells described by Graff and coworkers adjacent to adipose vessels may equally reflect precursors that migrated from an external niche through the bloodstream and were caught during the slow, rate-limiting step of extravasation across the endothelium. While these cells exhibit the proliferative capacity one would expect for resident, self-renewing progenitors, migrating cells which have committed to the adipocyte lineage, but are in the ensuing mitotic expansion stage 20, would also display proliferative capacity. Moreover, while preadipocyte proliferation has been reported in high-fat fed rats 21, expansion of the preadipocyte pool in humans is not changed with overfeeding 22 and preadipocyte numbers decrease with increasing adiposity and humans 23. Thus, “resident” preadipocytes in humans may have limited proliferative capacity and require replacement from non-resident sources.
Non-Adipose Tissue-Resident Progenitors Contribute to the White Adipocyte Pool
Tissues throughout the body contain progenitor cells capable of adipogenic differentiation. This includes bone marrow, which is a rich source for both mesenchymal and hematopoietic stem cells. Although marrow mesenchymal cells do not enter the circulation, hematopoietic cells with mesenchymal characteristics do leave the marrow and traffic to other tissues.
We therefore proposed that some adipocytes may arise from marrow progenitors that home to fat tissue and subsequently undergo adipogenic conversion. This hypothesis was confirmed by transplantation of GFP-labeled bone marrow into wild type recipient mice 24. GFP-expressing adipocytes were detected by fluorescence microscopy and flow cytometry in the major adipose depots. Similar results were reported by Sera, et al 25 and Tomiyama, et al 26, although other laboratories 27 have failed to detect these cells perhaps due to low marker expression in adipocytes arising from bone marrow. The number of bone marrow progenitor (BMP)-derived adipocytes was elevated in mice treated with a thiazolidinedione or high fat diet, treatments that increase adipogenesis.
Bone marrow reconstitution studies with GFP-labeled mesenchymal versus hematopoietic cells revealed that the BMP-derived adipocytes arise from hematopoietic cells of the myeloid lineage rather than mesenchymal cells 28. This finding was confirmed with a non-transplant model using mice in which LacZ expression is restricted to the myeloid lineage. A significant number of LacZ+ adipocytes were detected in these mice, confirming a myeloid origin for a subset of adipocytes in adipose tissue. Non-LacZ+ adipocytes were also present in these mice indicating that not all adipocytes were generated from myeloid progenitors allowing for other resident or non-resident sources for the remainder of the adipocyte pool. Additionally, the lineage marking model demonstrated that BMP-derived adipocytes were not an artifact of myeloablative injury. Ogawa and colleagues 25 also reported the generation of adipocytes in vivo from clonally derived marrow hematopoietic stem cells in transplant studies.
Our studies also identified a population of myeloid-derived adipocyte progenitors in adipose stroma that lack expression of hematopoietic (CD45) or myeloid (CD11b) markers, presumably due to downregulation of these markers in a tissue and function-specific manner. In this respect these cells mirrored the dual hematopoietic/mesenchymal nature of fibrocytes. Strieter and colleagues 29 have demonstrated that circulating fibrocytes isolated from the circulation are capable of adipogenic differentiation in vitro and in vivo. We speculate that fibrocytes are precursors for the myeloid-derived cell population that we observed in adipose stroma.
Two additional aspects of our studies are important for consideration. First, BMP-derived adipocytes preferentially accumulated in visceral fat depots, and were less numerous in subcutaneous adipose tissue. Accumulation was also higher in adipose tissue of female mice than in males. Second, global gene expression analysis indicated that BMP-derived adipocytes differ from conventional white adipocytes in increased expression of inflammatory cytokines and decreased expression of leptin. Expression of genes involved in mitochondrial biogenesis and fuel oxidation was also lower in BMP-derived adipocytes. It is interesting to note that BMP-derived adipocytes accumulated in depots noted for lower leptin and greater inflammatory cytokine production, which is linked to insulin resistance and other comorbidities 30. We propose that accumulation of BMP-derived adipocytes may in part account for adipose depot heterogeneity, especially as individuals age and/or gain weight. The higher numbers of BMP-derived adipocytes detected in female mice may also have important inferences for human biology. Women generally have a higher percentage of body fat than men, but tend to store fat in subcutaneous depots before menopause, whereas men tend to accumulate fat in visceral depots throughout life 31. However, following menopause women exhibit disproportionate gains in visceral fat mass, which likely contributes to the increased risk of metabolic disturbances associated with the post-menopausal state 32. This pattern of fat deposition coupled with the preferential accumulation of BMP-derived adipocytes in visceral fat may provide an unorthodox explanation for the relative low risk for adipose-related disease in premenopausal women.
Preadipocyte Populations Differ Between Depots
Regardless of resident or non-resident origin, differences in adipocyte phenotype have been recognized for many years. However, convincing evidence for distinct adipocyte progenitor populations has only been described in the last decade. Initial studies of human primary adipose stromal cells by Kirkland and colleagues 33 revealed that abdominal subcutaneous stromal cells accumulated more lipid and expressed adipocyte markers (including PPARγ2, C/EBP α and aP2) earlier and at higher levels than omental (visceral) stromal cells isolated from the same person.
Since adipose stroma is heterogeneous, the investigators examined the response of preadipocytes derived from single stromal cells. They found that cloned subcutaneous preadipocytes differentiated more rapidly than cloned omental cells, and a higher percentage of subcutaneous cells underwent adipogenic conversion than omental preadipocytes. The reduced differentiation capacity of omental preadipocytes may in part explain the accumulation of fat in extant adipocytes and the resulting enlargement of lipid droplets. Likewise, the higher differentiation capacity of subcutaneous preadipocytes would result in the deposition of lipid in new adipocytes with smaller fat droplets. The size of lipid droplets in visceral adipocytes correlates with circulating lipid levels, while hyperplasia and droplet size in subcutaneous adipocytes links to insulin sensitivity 34.
Interestingly, differences in preadipocyte characteristics observed by the Kirkland group were stable and heritable over many population doublings. This was demonstrated via stable ectopic expression of telomere reverse transcriptase in cloned preadipocytes from different fat depots 35. This results in telomere elongation, which allowed for repeated subculturing of the cells without loss of proliferative capacity and maintenance of their ability to differentiate. With this system, subcutaneous preadipocytes retained their high lipid accumulation and adipose-specific transcription factor expression compared to omental preadipocytes over 40 population doublings. The heritable nature of regional preadipocyte traits was also observed in global gene expression patterns, which were different between human preadipocytes from subcutaneous, mesenteric and omental fat 11, and epididymal and perirenal preadipocytes from rats 36.
It is unclear how and when the depot-specific differences in preadipocyte phenotype are established during development. A potential clue emerges from the gene array analysis performed by the Kirkland group, which revealed extensive changes in genes that regulate early development including a number of homeotic genes 36. Kahn and colleagues have reported similar depot-related differences in the expression of genes involved in embryonic development and pattern specification in both mouse and human preadipocytes 37, 38. These genetic differences were maintained during in vitro culture and differentiation and, importantly, were largely unaffected by nutritional status, suggesting that each depot has a unique intrinsic, stable gene expression signature. The data also suggest that differences between adipocyte progenitors are specified early in development, implicating epigenetic regulation.
Distinct Developmental Pathways Generate Distinct Subpopulations of White Adipocytes
It has been assumed that adipocytes arise entirely from mesoderm. However there is evidence that certain subsets of white adipocytes are produced from neuroectoderm as well. Early work by Billon, et al 39 demonstrated that neuroepithelial cells selected from mouse embryonic stem cells have the capacity to generate mature adipocytes. This indicated that neuroectoderm could function as a source of adipocytes. This was confirmed by Takashima, et al 40 who derived mesodermal and neuroepithelial cells from mouse embryonic stem cells and mouse embryos and showed both were capable of adipogenic differentiation in culture. To determine whether this phenomenon occurred in vivo Billon, et al 41 employed mice in which the Sox10 (strongly expressed in neural crest but not mesoderm) gene promoter drove cre recombinase expression to irreversibly label neural crest-derived cells with yellow fluorescent protein (YFP). YFP-labeled adipocytes were detected in cephalic adipose tissue between the salivary gland and ear. Adipocytes in other body locations were YFP negative. These results show that a subset of facial adipocytes indeed originate from neural crest and not mesoderm.
Brown Adipogenesis
Interest in the origin of brown fat has grown as a potential means to address the epidemic of obesity. Therapies to increase brown adipogenesis and, thus, fatty acid oxidation could potentially “tip the scales” away from fuel storage and prevent accumulation of body fat. While it was long thought that adult humans had only a modest amount of brown fat, studies using positron emission tomography to identify fluorodeoxyglucose uptake by metabolically active cells revealed a significant amount of brown adipose tissue located in the neck, supraclavicular, mediastinal, suprarenal and paravertebral regions 42.
Currently, two mechanistic explanations account for the generation of brown adipocytes. First, Timmons, et al 43 used microarray analysis to examine global gene expression patterns in white versus brown preadipocytes. To their surprise, brown preadipocytes demonstrated a myogenic transcriptional signature suggesting a distinct origin than that of white adipocytes. This theory was confirmed by Spiegelman and colleagues 44 who used a lineage-tracing model in which expression of YFP was dependent on Myf5 (myogenic lineage specific)-promoter driven cre recombinase expression. Skeletal muscle cells and brown adipocytes in intrascapular or perirenal adipose tissue expressed YFP whereas white adipocytes were YFP negative in all other depots illustrating a myogenic lineage origin for brown adipocytes.
The second mechanism entails the direct differentiation or transdifferentiation of mature unilocular white adipocytes to multilocular brown adipocytes. This phenomenon was originally observed in rodent models exposed to chronic low temperature 45 or treatment with β3-adrenergic agonists 46, 47. These treatments increased the number of multilocular cells expressing UCP1 and decreased unilocular cell numbers while total fat cell numbers remained constant 48. The appearance of brown adipocytes and loss of white fat cells occurred in the absence of white adipocyte death or an increase in adipocyte progenitors in fat tissue. Thus, it was presumed that the brown adipocytes arose via transdifferentiation from white adipocytes. More detailed studies by Nedergaard and colleagues 49 showed that although these brown adipocytes are functionally thermogenic, they failed to express classic brown adipocyte genes including Zic1, Lhx8, Meox2 and PDRM16, and continue to express white adipocyte markers like Hoxc9. Therefore, it appears that this class of “brown” adipocytes is distinct from those originating from myogenic precursors. This idea was confirmed by Spiegelman and colleagues 44 who showed that brown adipocytes in white adipose tissue generated by chronic β-adrenergic stimulation did not arise from Myf5-expressing precurors.
The Clinical Perspective
While we are far from having a comprehensive understanding of adipose tissue development, the study of adipocyte origins is beginning to illustrate how regionally distinct adipocyte populations are produced and ultimately impact health. First, we have learned that adipocytes in different body locations possess distinct phenotypes and exert differential effects on body wide energy metabolism and inflammation via differences in their ability to store and release lipids and to produce adipokines (e.g. leptin, adiponectin) and inflammatory cytokines. Second, these regionally distinct adipocyte populations are likely produced from discrete progenitor cells, which are generated by distinct developmental processes. These processes may be key targets for individualized strategies to counter obesity and its negative metabolic consequences. Third, apart from generating distinct progenitor populations, the developmental processes may themselves stipulate features of terminal adipocyte function. For example, the work by Kirkland’s group suggests that the detrimental phenotype of visceral adipocytes may derive in part from the slow proliferation and poor differentiation capacity of visceral preadipocytes. The slow production of new visceral fat cells presumably contributes to the accumulation of lipid, and hence hypertrophy, in extant adipocytes. Insulin responsiveness is blunted in large adipocytes 50 while inflammatory cytokine secretion is elevated 51. Thus, the differential capacity of distinct progenitor populations to proliferate and differentiate can influence the insulin sensitivity and cytokine production of mature adipocytes.
Our understanding of adipocyte origins is likely to impact the clinical picture of obesity in several other ways. First, it may provide clues to specific syndromes linked to adipose tissue function. For example, maternal obesity during gestation is linked to increased adiposity and elevated risk for obesity comorbidities in offspring 52. This may occur as the results of genetic and/or metabolic imprinting of the fetus in utero 53. Such imprinting could potentially alter adipocyte developmental programs altering the overall rate of adipocyte production or, more importantly, the generation of specific subsets of adipocytes. Lipodystrophic disorders are another area where progenitor studies may yield important insights. Most lipodystrophies are characterized by loss of fat in certain body locations while fat in other areas is spared or even expands 54. These regional differences in fat loss and expansion may reflect the impact of genetic factors or environmental insults on discrete progenitor populations or developmental programs. Studies of defined progenitors in the aforementioned cases and induced pluripotent stem cells (iPS) from rodent models and patient samples may provide clues regarding the epigenetic variability and regulation of these developmental programs.
Second, there is keen interest in using adipose stromal progenitor cells in reconstructive surgery and regenerative medicine applications. Given our current understanding, it seems likely that progenitors from certain body locations may be more suitable than others. Thus, careful assessment of well-defined progenitor populations may be key to successful use of adipose stromal cells in regenerative medicine applications.
Finally, it is tempting to speculate that in the future it may be possible to alter an individual’s adipocyte repertoire by modifying adipocyte developmental pathways. For instance, as noted earlier, investigators are pursuing means to increase the production of brown adipocytes thereby increasing thermogenic fuel oxidation rather than fuel storage as fat. Conversely, we are exploring the mechanisms by which BMP cells are recruited to adipose tissue and differentiate into adipocytes. By blocking these processes it may be possible to suppress the generation of BMP-derived adipocytes and mitigate their putative negative affects on health.
The Future
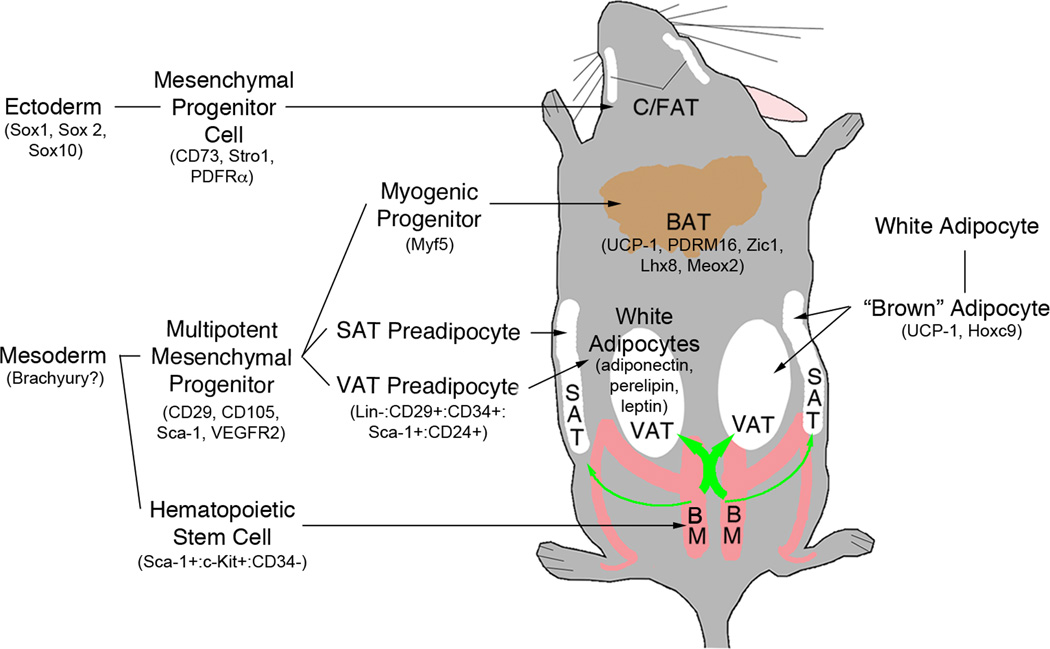
The origin of adipocytes has become a focal point in adipose biology. It is now clear that more than one lineage contributes to the genesis of adipocytes (Fig.2), highlighting the functional and developmental complexity of what was once thought to be merely an energy reservoir. In spite of impressive progress in this field there is much to learn. Can external stimuli alter the various mechanisms for generating distinct progenitor and adipocyte populations? Is the slow rate of resident progenitor renewal sufficient for the production of new adipocytes or are other sources required? Are there non-resident sources for adipocytes other than bone marrow? Unfortunately, definitive models to address these questions are lacking in many instances. For example, while Billon et al 41 demonstrated that some adipocytes in the craniofacial region derive from neuroectoderm using a lineage analysis model based on Sox10, no such model exists for mesoderm, the likely source of other adipocytes. (Brachyury is often proposed as a marker of definitive mesoderm, but it is also transiently expressed during endoderm formation prior to expression of FoxA2.) In figure 2 we have indicated some potential biomarkers for various pathways and stages of adipocyte production. These and other markers should be applied with caution until more definitive results are available.
Figure 2. There’s more than one way to make an adipocyte.
The general location of visceral (VAT), subcutaneous (SAT), craniofacial (C/FAT) and brown (BAT) adipose tissue in the mouse is indicated. Adipocytes are generated from mesoderm and neuroectoderm, the latter limited to the craniofacial region. Other adipocytes including white adipocytes, bone marrow progenitor-derived white adipocytes and brown adipocytes have a mesodermal origin. Adipose depot-specific preadipocytes emerge during development and give rise to mature adipocytes with distinct phenotypes. Brown adipocytes are produced from either myogenic progenitors or through transdifferentiation of white adipocytes. Finally, some white adipocytes arise de novo from bone marrow-derived hematopoietic progenitors. This process contributes more to visceral than subcutaneous adipose development (green arrow thickness). Potential biomarkers for the various paths and stages are indicated inside parentheses.
Another key concern is the use of mixed stromal populations in experiments addressing adipocyte development. Adipose stromal cells have been routinely isolated based on their adherence to plastic substrates and in some cases termed multipotent stem cells while lacking well-defined markers or clonal differentiation analyses. Adipose stroma contains a surfeit of cell types at various developmental stages including cells that exert an inhibitory effect on adipogenesis 55. Even when studies have employed clonally isolated progenitors, the provenance of the cells with respect to developmental lineage and stage, as well as, marker expression has often not been evaluated. Moreover, the use of different surface markers by various research groups has made it difficult to compare results from these studies and draw definitive conclusions.
While these issues may prove difficult in the near term, they are unlikely to remain roadblocks for long. Novel models, markers and techniques become increasingly available, and collaborations between adipocyte biologists and stem cell and developmental biologists will improve the application of these new resources. The next decade should be as exciting in the field of adipocyte origins as the last. We look forward to weighing the new possibilities.
Acknowledgments
Supported by National Institutes of Health Grant DK078966 to DJK.
Abbreviations
- VAT
visceral adipose tissue
- SAT
subcutaneous adipose tissue
- PPARγ
peroxisome proliferator-activated receptor γ
- GFP
green fluorescent protein
- YFP
yellow fluorescent protein
- Dox
doxycycline
- PDGFRβ
platelet-derived growth factor receptor β
- SM-actin
smooth muscle actin
- Lin
hematopoietic lineage
- BMP
bone marrow progenitor
- LacZ
product of LacZ gene, β-galactosidase
- C/EBP α
CCAAT/enhancer binding protein α
- aP2
adipocyte fatty acid binding protein
- UCP-1
uncoupling protein-1
Footnotes
Disclosure of Potential Conflicts of Interest
The authors indicate no potential conflicts of interest.
References
- 1.Pelleymounter MA, Cullen MJ, Baker MB, et al. Effects of the obese gene product on body weight regulation in ob/ob mice. Science. 1995;269:540–543. doi: 10.1126/science.7624776. [DOI] [PubMed] [Google Scholar]
- 2.Brochu-Gaudreu K, Rehfeldt C, Blouin R, et al. Adiponectin action from head to toe. Endocrine. 2010;37:11–32. doi: 10.1007/s12020-009-9278-8. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention, and Department of Human Services. Overweight and Obesity. Available at: www.cdc.gov/nccdphp/dpna/obesity/indes.htm.
- 4.National Institute of Diabetes & Digestive & Kidney Diseases. Prevalence statisitics related to overwight and obesity. Available at: www.win.niddk.nih.gov/statistics/index.htm.
- 5.Bays H, Ballantyne C. Adiposopathy: why do adiposity and obesity cause metabolic disease. Future Lipidol. 2006;1:389–420. [Google Scholar]
- 6.Brown WV, Fujioka K, Wilson PW, et al. Obesity: why be concerned? Am J Med. 2009;122:S4–S11. doi: 10.1016/j.amjmed.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 7.Guh DP, Zhang W, Bansback N, et al. The incidence of co-morbiditites related to obesity and overweight: a systematic review and meta-analysis. BMC Public Health. 2009;9:88–108. doi: 10.1186/1471-2458-9-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bays HE, Gonzalez-Campoy JM, Bray GA, et al. Pathogenic potential of adipose tissue and metabolic consequences of adipocyte hypertrophy and increased visceral adiposity. Expert Rev. Cardiovasc. Ther. 2008;6:343–368. doi: 10.1586/14779072.6.3.343. [DOI] [PubMed] [Google Scholar]
- 9.Despres JP. Is visceral obesity the cause of the metabolic syndrome? Ann Med. 1998;38:52–63. doi: 10.1080/07853890500383895. [DOI] [PubMed] [Google Scholar]
- 10.Wajchenberg BL, Giannella-Neto D, da Silva MER, et al. Depot-specific hormonal characterization of subcutaneous and visceral adipose tissue and their relation to the metabolic syndrome. Horm Metab Res. 2002;34:616–621. doi: 10.1055/s-2002-38256. [DOI] [PubMed] [Google Scholar]
- 11.Tchkonia T, Lenburg M, Thomou T, et al. Identification of depot-specific human fat cell progenitors through distinct expression profiles and developmental gene patterns. American Journal of Physiology Endocrinology Metabolism. 2007;292:E298–E307. doi: 10.1152/ajpendo.00202.2006. [DOI] [PubMed] [Google Scholar]
- 12.Engfeldt P, Arner P. Lipolysis in human adipocytes: effects of cell size, age and regional differences. Horm Metab Res Suppl. 1988;19:26–29. [PubMed] [Google Scholar]
- 13.Bahceci M, Gokalp D, Bahceci S, et al. The correlation between adiposity and adiponectin, tumor necrosis factor-a, interleukin-6 and high sensitivity C-reactive protein. Is adipocyte size associated with inflammation in adults? J Endocrinol Invest. 2007;30:210–214. doi: 10.1007/BF03347427. [DOI] [PubMed] [Google Scholar]
- 14.Guilak F, Lott KE, Awad HA, et al. Clonal analysis of the differenatiation potential of human adipose-derived stem cells. J Cell Physiol. 2006;206:229–237. doi: 10.1002/jcp.20463. [DOI] [PubMed] [Google Scholar]
- 15.Spalding KL, Arner E, Westermark PO, et al. Dynamics of fat cell turnover in humans. Nature. 2008;453:783–787. doi: 10.1038/nature06902. [DOI] [PubMed] [Google Scholar]
- 16.Tang W, Zeve D, Suh JM, et al. White Fat Progenitor Cells Reside in the Adipose Vasculature. Science. 2009;322:583–586. doi: 10.1126/science.1156232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Galli D, Innocenzi A, Staszewsky L, et al. Mesoangioblasts, vessel-associated multipotent stem cells, repair the infarcted heart by multiple cellular mechanisms. A comparison with bone marrow progenitors, fibroblasts, and endothelial cells. Arterioscler Thromb Vasc Biol. 2005;25:692–697. doi: 10.1161/01.ATV.0000156402.52029.ce. [DOI] [PubMed] [Google Scholar]
- 18.Rodeheffer MS, Birsoy K, Friedman JM. Identification of white adipocyte progenitor cells in vivo. Cell. 2008;135:240–249. doi: 10.1016/j.cell.2008.09.036. [DOI] [PubMed] [Google Scholar]
- 19.Lagasse E, Connors H, Al-Dhalimy M, et al. Purified hematopoietic stem cells can differentiate into hepatocytes in vivo. Nature Medicine. 2000;6:1229–1234. doi: 10.1038/81326. [DOI] [PubMed] [Google Scholar]
- 20.Tang QQ, Otto TC, Lane MD. Mitotic clonal expansion: a synchronous process required for adipogenesis. Proc Natl Acad Sci USA. 2003;100:44–49. doi: 10.1073/pnas.0137044100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ellis JR, McDonald RB, Stern JS. A diet high in fat stimulates adipocyte proliferation in older (22 month) rats. Exp Gerontol. 1990;25:141–148. doi: 10.1016/0531-5565(90)90045-4. [DOI] [PubMed] [Google Scholar]
- 22.Tchoukalova YD, Votruba SB, Tchkonia T, et al. Regional differences in cellular mechanisms of adipose tissue gain with overfeeding. Proc Natl Acad Sci USA. 2010;107:18226–18231. doi: 10.1073/pnas.1005259107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tchoukalova Y, Koutsari C, Jensen MD. Committed subcutaneous preadipocytes are reduced in human obesity. Diabetologia. 2007;50:151–157. doi: 10.1007/s00125-006-0496-9. [DOI] [PubMed] [Google Scholar]
- 24.Crossno JT, Jr, Majka SM, Grazia T, et al. Rosiglitazone Promotes Differentiation of Bone Marrow-Derived Circulating Progenitor Cells to Multilocular Adipocytes in Adipose Tissue. J Clin Invest. 2006;116:3220–3228. doi: 10.1172/JCI28510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sera Y, LaRue AC, Moussa O, et al. Hematopoietic stem cell origin of adipocytes. Experimental Hematology. 2009;37:1108–1120. doi: 10.1016/j.exphem.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tomiyama K, Murase N, Stolz DB, et al. Characterization of transplanted green fluorescent protein bone marrow cells into adipose tissue. Stem Cells. 2007;26:330–338. doi: 10.1634/stemcells.2007-0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koh YJ, Kang S, Lee HJ, et al. Bone marrow-derived circulating progenitor cells fail to transdifferentiate into adipocytes in adult adipose tissues in mice. Journal of Clincal Investigation. 2007;117:3684–3695. doi: 10.1172/JCI32504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Majka SM, Fox KE, Psilas JC, et al. De Novo Generation of White Adipocytes From the Myeloid Lineage Via Mesenchymal Intermediates is Age, Adipose Depot, and Gender Specific. Proceedings of the National Academy of Science USA. 2010;107:14781–14786. doi: 10.1073/pnas.1003512107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hong KM, Buridick MD, Phillips RJ, et al. Characterization of human fibrocytes as circulating adipocyte progenitors and the formation of human adipose tissue in SCID mice. FASEB J. 2005;19:2029–2031. doi: 10.1096/fj.05-4295fje. [DOI] [PubMed] [Google Scholar]
- 30.Hamdy O, Porramatikul S, Al-Ozairi E. Metabolic Obesity: The Paradox Between Visceral and Subcutaneous Fat. Curr Diabetes Rev. 2006;2:367–373. doi: 10.2174/1573399810602040367. [DOI] [PubMed] [Google Scholar]
- 31.Blaak E. Gender Differences in Fat Metabolism. Curr Opin Clin Nutr Metab Care. 2001;4:499–502. doi: 10.1097/00075197-200111000-00006. [DOI] [PubMed] [Google Scholar]
- 32.Gambacciani M, Ciaponi M, Cappagli B, et al. Prospective evaluation of body weight and body fat distribution in early postmenopausal women with and without hormone replacement therapy. Maturitas. 2001;39:125–132. doi: 10.1016/s0378-5122(01)00194-3. [DOI] [PubMed] [Google Scholar]
- 33.Tchkonia T, Giorgadze N, Pirtskhalava T, et al. Fat depot origin affects adipogenesis in primary cultured and cloned human preadipocytes. Am J Physiol Regulatory Integrative Comp Physiol. 2001;282:R1286–R1296. doi: 10.1152/ajpregu.00653.2001. [DOI] [PubMed] [Google Scholar]
- 34.Hoffstedt J, Arner E, Wahrenberg H, et al. Regional impact of adipose tissue morpholgy on the metabolic profile in morbid obesity. Diabetologia. 2010;53:2496–2503. doi: 10.1007/s00125-010-1889-3. [DOI] [PubMed] [Google Scholar]
- 35.Tchkonia T, Giorgadze N, Pirtskhalava T, et al. Fat depot-specific charactersitics are retained in strains derived from single human preadipocytes. Diabetes. 2006;55:2571–2578. doi: 10.2337/db06-0540. [DOI] [PubMed] [Google Scholar]
- 36.Cartwright MJ, Schlauch K, Lenburg ME, et al. Aging, depot origin, and preadipocyte gene expression. J Gerontol. 2010;65A:242–251. doi: 10.1093/gerona/glp213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gesta S, Bluher M, Yamamoto Y, et al. Evidence for a role of developmental genes in the origin of obesity and body fat distribution. Proc Natl Acad Sci USA. 2006;103:6676–6681. doi: 10.1073/pnas.0601752103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamamoto Y, Gesta S, Lee KY, et al. Adipose depots possess unique developmental gene signatures. Obesity (Silver Spring) 2010;18:872–878. doi: 10.1038/oby.2009.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Billon N, Jolicoeur C, Raff M. Generation and characterization ofoligodendrocytes from lineage-selectable embryonic stem cells in vitro. Methods Mol Biol. 2006;330:15–32. doi: 10.1385/1-59745-036-7:015. [DOI] [PubMed] [Google Scholar]
- 40.Takashima Y, Era T, Nakao K, et al. Neuroepithelial cells supply an initial transient wave of MSC differentiation. Cell. 2007;129:1377–1388. doi: 10.1016/j.cell.2007.04.028. [DOI] [PubMed] [Google Scholar]
- 41.Billon N, Iannarelli P, Monteiro MC, et al. The generation of adipocytes by the neural crest. Development. 2007;134:2283–2292. doi: 10.1242/dev.002642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nedergaard J, Bengtsson T, Cannon B. Unexpected evidence for active brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab. 2007;293:E444–E452. doi: 10.1152/ajpendo.00691.2006. [DOI] [PubMed] [Google Scholar]
- 43.Timmons JA, Wennmalm K, Larsson O, et al. Myogenic gene expression signature establishes that brown and white adipocytes originate from distinct cell lineages. Proc Natl Acad Sci USA. 2007;104:4401–4406. doi: 10.1073/pnas.0610615104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Seale P, Bjork B, Yang W, et al. PRDM16 controls a brown fat/skeletal muscle switch. Nature. 2008;454:961–967. doi: 10.1038/nature07182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Murano I, Zingaretti MC, Cinti S. The adipose organ of Sv129 mice contains a prevalence of brown adipocytes and shows plasticity after cold exposure. Adipocytes. 2005;1:121–130. [Google Scholar]
- 46.Granneman JG, Li P, Zhu Z, et al. Metabolic and cellular plasticity in white adipose tissue I: effects of beta3-adrenergic receptor activation. Am J Physiol Endocrinol Metab. 2005;289:E608–E616. doi: 10.1152/ajpendo.00009.2005. [DOI] [PubMed] [Google Scholar]
- 47.Himms-Hagen J, Melnyk A, Zingaretti MC, et al. Multilocular fat cells in WAT of CL-316243-treated rats derive directly from white adipocytes. Am J Physiol Cell Physiol. 2000;279:C670–C681. doi: 10.1152/ajpcell.2000.279.3.C670. [DOI] [PubMed] [Google Scholar]
- 48.Cinti S. Transdifferentiation properties of adipocytes in the adipose organ. Am J Physiol Endocrinol Metab. 2009;297:E977–E986. doi: 10.1152/ajpendo.00183.2009. [DOI] [PubMed] [Google Scholar]
- 49.Petrovic N, Walden TB, Shabalina IG, et al. Chronic peroxisome proliferator-activated receptor gamma (PPARgamma)activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1-containing adipocytes molecularly distinct from classic brown adipocytes. J Biol Chem. 2010;285:7153–7164. doi: 10.1074/jbc.M109.053942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Franck N, Stenkula KG, Ost A, et al. Insulin-induced GLUT4 translocation to the plasma membrane is blunted in large compared with small primary fat cells isolated from the same individual. Diabetologia. 2007;50:1716–1722. doi: 10.1007/s00125-007-0713-1. [DOI] [PubMed] [Google Scholar]
- 51.Skurk T, Alberti-Huber C, Herder C, et al. Relationship between adipocyte size and adipokine expression and secretion. J Clin Endocrinol Metab. 2007;92:1023–1033. doi: 10.1210/jc.2006-1055. [DOI] [PubMed] [Google Scholar]
- 52.Drake AJ, Reynolds RM. Impact of maternal obesity on offspring obesity and cardiometabolic risk. Reproduction. 2010;140:387–398. doi: 10.1530/REP-10-0077. [DOI] [PubMed] [Google Scholar]
- 53.Heerwagen MJ, Miller MR, Barbour LA, et al. Maternal obesity and fetal metabolic programming: a fertile epigenetic soil. Am J Physiol Regul Integr Comp Physiol. 2010;299:R711–R722. doi: 10.1152/ajpregu.00310.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Capeau J, Magre J, Lascois O, et al. Diseases of adipose tissue: genetic and acquired lipodystrophies. Biochem Soc Trans. 2005;33:1073–1077. doi: 10.1042/BST0331073. [DOI] [PubMed] [Google Scholar]
- 55.Rajashekhar G, Traktuev DO, Roell WC, et al. IFATS Collections: Adipose stromal cell differentiation is reduced by endothelial cell contact and paracrine communication: Role of canonical Wnt signaling. Stem Cells. 2008;26:2674–2681. doi: 10.1634/stemcells.2008-0277. [DOI] [PubMed] [Google Scholar]