Abstract
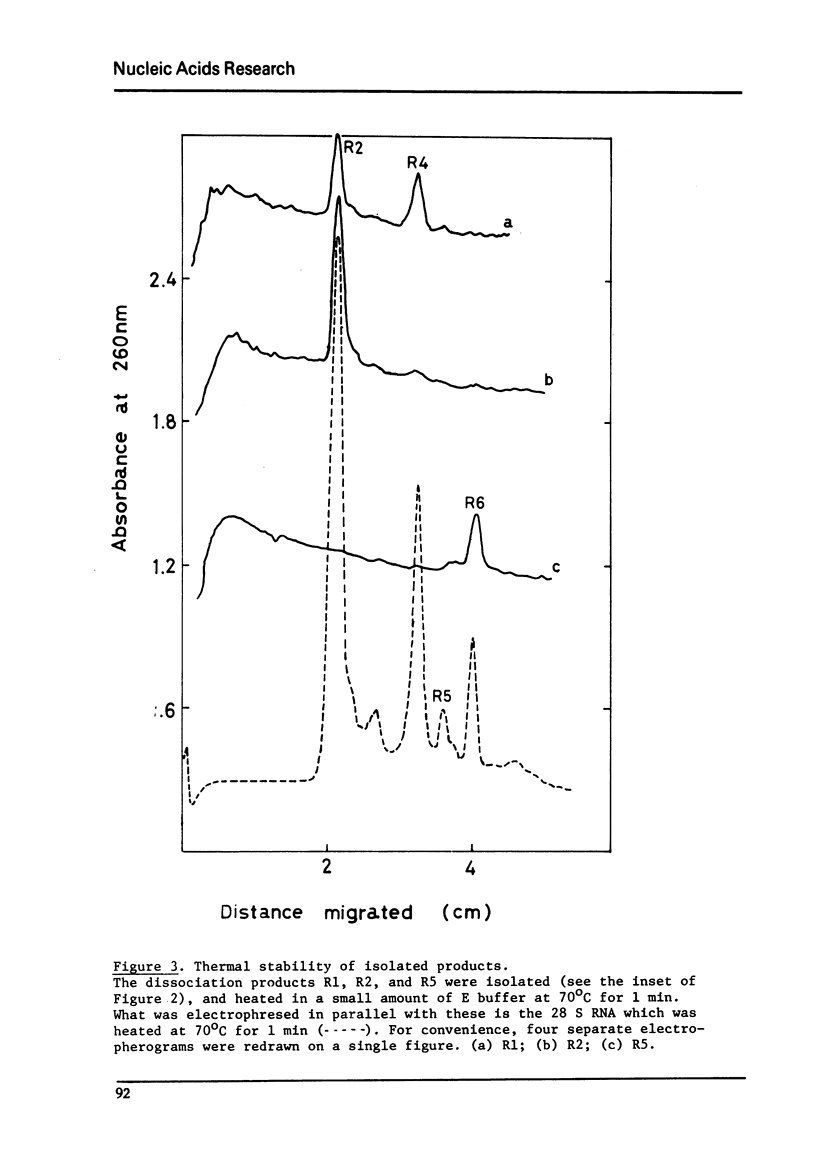
If RNA is extracted from the ribosomes which had been isolated from frozen-thawed tissue of Galleria mellonella, the 28 S RNA, when heated or treated with urea, dissociates into seven different species of polynucleotide fragments. They were designated as R1, R2, R3, R4, R5, R6, and R7, whose molecular weights were estimated to be 1.15x10-6, 0.75x10-6, 0.55x10-6, 0.40x10-6, 0.30x10-6, 0.25x10-6, 0.20x10-6 daltons, respectively. It is likely that R1 and R5 arise from a single nick in original 38 S rRNA. Experiments with isolated R1 suggest that it is made up of a hydrogen-bonded complex of R2 and R4. R5 is a complex of R6 and an unidentified species, X. It is suggested that these fragments result from nicks which are introduced, secondarily, in the phosphodiester bonds by an endogenous endonuclease(s). Since the secondary nicks are limited in number and located in specific points of the molecule, it appears that the reaction is quite specific. It was also shown that the 28 S aphid RNA, which apparently lacks the primary nick, is susceptible to nicking.
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Applebaum S. W., Ebstein R. P., Wyatt G. R. Dissociation of ribonucleic acid from silkmoth pupae by heat and dimethylsulfoxide: evidence for specific cleavage points. J Mol Biol. 1966 Oct 28;21(1):29–41. doi: 10.1016/0022-2836(66)90077-5. [DOI] [PubMed] [Google Scholar]
- Attardi G., Amaldi F. Structure and synthesis of ribosomal RNA. Annu Rev Biochem. 1970;39:183–226. doi: 10.1146/annurev.bi.39.070170.001151. [DOI] [PubMed] [Google Scholar]
- Bishop D. H., Claybrook J. R., Spiegelman S. Electrophoretic separation of viral nucleic acids on polyacrylamide gels. J Mol Biol. 1967 Jun 28;26(3):373–387. doi: 10.1016/0022-2836(67)90310-5. [DOI] [PubMed] [Google Scholar]
- Cox R. A. A spectrophotometric study of the secondary structure of ribonucleic acid isolated from the smaller and larger ribosomal subparticles of rabbit reticulocytes. Biochem J. 1970 Mar;117(1):101–118. doi: 10.1042/bj1170101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahnestock S., Erdmann V., Nomura M. Reconstitution of 50S ribosomal subunits from protein-free ribonucleic acid. Biochemistry. 1973 Jan 16;12(2):220–224. doi: 10.1021/bi00726a007. [DOI] [PubMed] [Google Scholar]
- Fellner P., Ehresmann C., Ebel J. P. Nucleotide sequences present within the 16S ribosomal RNA of Escherichia coli. Nature. 1970 Jan 3;225(5227):26–29. doi: 10.1038/225026a0. [DOI] [PubMed] [Google Scholar]
- Greenberg J. R. Synthesis and properties of ribosomal RNA in Drosophila. J Mol Biol. 1969 Nov 28;46(1):85–98. doi: 10.1016/0022-2836(69)90059-x. [DOI] [PubMed] [Google Scholar]
- Howells A. J., Wyatt G. R. The apparent template activity of RNA from developing wings of the cecropia silkmoth. Biochim Biophys Acta. 1969 Jan 21;174(1):86–98. doi: 10.1016/0005-2787(69)90231-7. [DOI] [PubMed] [Google Scholar]
- Ilan J. Amino acid incorporation and aminoacyl transfer in an insect pupal system. J Biol Chem. 1968 Nov 25;243(22):5859–5866. [PubMed] [Google Scholar]
- Ishikawa H. Comparative studies on the thermal stability of animal ribosomal RNA's. Comp Biochem Physiol B. 1973 Oct 15;46(2):217–227. doi: 10.1016/0305-0491(73)90312-x. [DOI] [PubMed] [Google Scholar]
- Ishikawa H., Newburgh R. W. A rapidly-labeled RNA species from the silkgland of the wax moth, Galleria mellonella (L.). Biochem Biophys Res Commun. 1970 Aug 11;40(3):654–660. doi: 10.1016/0006-291x(70)90954-x. [DOI] [PubMed] [Google Scholar]
- Ishikawa H., Newburgh R. W. Studies of the thermal conversion of 28 S RNA of Galleria mellonella (L.) to an 18 S product. J Mol Biol. 1972 Feb 28;64(1):135–144. doi: 10.1016/0022-2836(72)90325-7. [DOI] [PubMed] [Google Scholar]
- Ishikawa H. Primary and secondary nicks in the ribosomal ribonucleic acid of insects. Biochem Biophys Res Commun. 1973 Sep 5;54(1):301–307. doi: 10.1016/0006-291x(73)90923-6. [DOI] [PubMed] [Google Scholar]
- Kedes L. H., Gross P. R. Synthesis and function of messenger RNA during early embryonic development. J Mol Biol. 1969 Jun 28;42(3):559–575. doi: 10.1016/0022-2836(69)90243-5. [DOI] [PubMed] [Google Scholar]
- King H. W., Gould H. Low molecular weight ribonucleic acid in rabbit reticulocyte ribosomes. J Mol Biol. 1970 Aug;51(3):687–702. doi: 10.1016/0022-2836(70)90017-3. [DOI] [PubMed] [Google Scholar]
- Loening U. E. Molecular weights of ribosomal RNA in relation to evolution. J Mol Biol. 1968 Dec;38(3):355–365. doi: 10.1016/0022-2836(68)90391-4. [DOI] [PubMed] [Google Scholar]
- Loening U. E. The determination of the molecular weight of ribonucleic acid by polyacrylamide-gel electrophresis. The effects of changes in conformation. Biochem J. 1969 Jun;113(1):131–138. doi: 10.1042/bj1130131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. V., Sypherd P. S. Topography of the Escherichia coli 30 S ribosome revealed by the modification of ribosomal proteins. J Mol Biol. 1973 Aug 15;78(3):539–550. doi: 10.1016/0022-2836(73)90474-9. [DOI] [PubMed] [Google Scholar]
- Nomura M. Bacterial ribosome. Bacteriol Rev. 1970 Sep;34(3):228–277. doi: 10.1128/br.34.3.228-277.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne P. I., Loening U. E. RNA breakdown accompanying the isolation of pea root microsomes. An analysis by polyacrylamide gel electrophoresis. Biochim Biophys Acta. 1970 Nov 12;224(1):128–135. doi: 10.1016/0005-2787(70)90626-x. [DOI] [PubMed] [Google Scholar]
- Pene J. J., Knight E., Jr, Darnell J. E., Jr Characterization of a new low molecular weight RNA in HeLa cell ribosomes. J Mol Biol. 1968 May 14;33(3):609–623. doi: 10.1016/0022-2836(68)90309-4. [DOI] [PubMed] [Google Scholar]
- Revzin A., Neumann E., Katchalsky A. Metastable secondary structures in ribosomal RNA molecular hysteresis in the acid-base titration of Escherichia coli ribosomal RNA. J Mol Biol. 1973 Sep 5;79(1):95–114. doi: 10.1016/0022-2836(73)90272-6. [DOI] [PubMed] [Google Scholar]
- Rubin G. M. The nucleotide sequence of Saccharomyces cerevisiae 5.8 S ribosomal ribonucleic acid. J Biol Chem. 1973 Jun 10;248(11):3860–3875. [PubMed] [Google Scholar]
- Shine J., Dalgarno L. Occurrence of heat-dissociable ribosomal RNA in insects: the presence of three polynucleotide chains in 26 S RNA from cultured Aedes aegypti cells. J Mol Biol. 1973 Mar 25;75(1):57–72. doi: 10.1016/0022-2836(73)90528-7. [DOI] [PubMed] [Google Scholar]
- Stanley W. M., Jr, Bock R. M. Isolation and physical properties of the ribosomal ribonucleic acid of Escherichia coli. Biochemistry. 1965 Jul;4(7):1302–1311. doi: 10.1021/bi00883a014. [DOI] [PubMed] [Google Scholar]
- TASHIRO Y., SIEKEVITZ P. ULTRACENTRIFUGAL STUDIES ON THE DISSOCIATION OF HEPATIC RIBOSOMES. J Mol Biol. 1965 Feb;11:149–165. doi: 10.1016/s0022-2836(65)80047-x. [DOI] [PubMed] [Google Scholar]
- Tanaka S., Shimura K. Stimulation of amino acid incorporation in an Escherichia coli cell-free system by silkgland RNA. J Biochem. 1965 Aug;58(2):145–152. doi: 10.1093/oxfordjournals.jbchem.a128176. [DOI] [PubMed] [Google Scholar]
- Tinoco I., Jr, Uhlenbeck O. C., Levine M. D. Estimation of secondary structure in ribonucleic acids. Nature. 1971 Apr 9;230(5293):362–367. doi: 10.1038/230362a0. [DOI] [PubMed] [Google Scholar]
- Wellauer P. K., Dawid I. B. Secondary structure maps of RNA: processing of HeLa ribosomal RNA. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2827–2831. doi: 10.1073/pnas.70.10.2827. [DOI] [PMC free article] [PubMed] [Google Scholar]


