Abstract
It has been established that typically developing individuals have a bias to attend to facial information in the left visual field (LVF) more than in the right visual field (RVF). This bias is thought to arise from the right hemisphere’s advantage for processing facial information, with evidence suggesting it to be driven by the configural demands of face processing. Considering research showing that individuals with autism have impaired face processing abilities, with marked deficits in configural processing, it was hypothesized that they would not demonstrate a LVF bias for faces. Eye-tracking technology was used to show that, individuals with autism were not spontaneously biased to facial information in the LVF, in contrast to a control group, while discriminating facial gender.
Keywords: Autism, face processing, left visual field (LVF) bias
Since the first chimeric face experiments (Wolff, 1933) using stimuli composed of two different halves of faces fused at the vertical midline, it has been well established that when perceiving faces, individuals have a bias to pay attention to the right side of the face more than the left. While this phenomenon was originally thought to be the result of heightened right-side facial cues, research by Gilbert and Bakan (1973) revealed that a left visual field (LVF) perceptual advantage was responsible for the looking bias. Studies have shown that this LVF bias is seen with a variety of facial perception tasks using chimeric faces, including the discrimination of emotion, gender, age, and judgments of attractiveness (Burt & Perret, 1997; Luh et al., 1991)
More recent research suggests that the LVF bias is the result of brain hemispheric specialization. Information from the LVF is sent to the right hemisphere (RH) of the brain which has long been associated with face processing abilities and numerous studies using fMRI have shown a consistent and heightened activation in the right fusiform gyrus when processing facial information (for review, see Haxby, Hoffman, & Gobbini, 2000). Similarly, research with populations who have difficulties perceiving faces have found diminished RH processing. For example, prosopagnosic individuals with damage to temporal regions, particularly in the RH, have an impaired ability to recognize faces (De Renzi, 1989). Individuals with autism also demonstrate face processing deficits that are associated with diminished activation of the right Face Fusiform Area (FFA) (for review, see Curby, Willenbockel, Tanaka, & Schultz; 2010).
The direct relation between RH specialization for face processing and the LVF bias during face processing was established by De Renzi and colleagues (1994) when they found that individuals with RH damage who demonstrated a decline in face recognition abilities. In contrast to healthy participants, these patients did not display any recognition advantage for stimuli presented in the LVF. Yovel, Tambini, and Brandman (2008) conducted a longitudinal fMRI and eye-tracking study that showed the extent of an individual’s LVF bias is correlated with the magnitude of lateralized activity in the RH during face processing tasks. They also found the degree of individual lateralization to be a stable measure over time.
More recently, studies have employed the use of eye tracking technology to confirm the presence of a LVF bias. Butler et al. (2005) showed that individuals make more fixations in the LVF when determining the gender of both chimeric and natural face stimuli. While this study found that the bias is only reliably measured in the first saccade, others have since found the bias to extend to an increase in looking time to the LVF over the duration of stimulus presentation (Butler & Harvey, 2006; Guo, Meints, Hall, Hall & Mills, 2009). In fact, Guo et al. (2009) found the increase in LVF looking time to be seen only for upright for upright human faces, with the first saccade to the LVF also present for the viewing of inverted human faces, and upright and inverted monkey faces.
A leftward gaze bias may seem counter-intuitive to the idea of a left visual field superiority for faces, because looking to the left does in fact put more of the face in the right visual field. Despite this, however, the left gaze has been found to be consistent with the LVF bias (Butler et al., 2005; Butler & Harvey, 2006), and increased leftward fixations during face processing is correlated with heightened right hemisphere activation (Yovel, Tambini, and Brandman, 2008). A possible explanation for this is that with the development of face expertise and specialization we come to process faces in right hemisphere. This leads to a tendency to pay more attention to facial information in the LVF, which is mostly being projected to the RH. As a result, we have a bias to gaze to the left and to continue to explore the left side facial information more than right. Essentially, the argument is that RH specialization leads to more interest and exploration of the facial information on the left, which leads to a bias to turn toward and explore that side of a person’s face. Thus, the argument is the LVF bias comes from specialization and the development of expertise is in the perception of faces.
Processing faces requires perception of subtle configural or spatial relations among the facial features (Diamond & Carey, 1986). Support for this is found in the face inversion effect, where the ability to recognize faces is diminished when a face is presented upside-down. Inverting a face disrupts configural arrangement without changing the face’s featural properties (Maurer, Le Grand, & Mondloch, 2002).
There is evidence to suggest that the LVF bias is used to process configural information from faces. When faces are inverted and configural processing is disrupted, the amount of lateralized activity in the RH is reduced (Yovel & Kanwisher, 2005; Leehey et al., 1978; Levine, Banich, & Koch-Weser, 1988) and the bias to the LVF is significantly reduced (Luh, 1998) or eliminated (Collican et al., 2008). Configural processing can increasingly enhance the processing of stimuli according to the amount of expertise a person develops for that stimulus category, a process that occurs developmentally with respect to faces (Diamond & Carey, 1986; Gauthier & Tarr, 1996; Gauthier, Williams, Tarr, & Tanaka, 1998). This expertise (or lack of expertise) can be demonstrated in adults when processing faces of an unfamiliar ethnicity, where there is a reduction of LVF bias (Rhodes, 1993) and disruption of the face inversion effect (Gajewski, Schlegel, & Stoerig, 2008). Also, Hsiao and Cottrell (2009) recently showed that a LVF bias exists for fluent readers of Chinese when viewing Chinese characters; however, this LVF bias was not present for nonreaders of Chinese.
It is well established that individuals with autism have impaired facial processing, including but not limited to gender discrimination (for review see Newell et al., 2010). Many believe that this reduced ability in face processing can be attributed to individuals with autism relying more on featural processing rather than configural processing when discriminating facial information (e.g., Curby et al., 2010). Evidence for this comes from several studies showing that individuals with autism spectrum disorder (ASD) are less affected by the face inversion effect than typically developing individuals (Boucher & Lewis, 1992; Boucher, Lewis, & Collis, 1998; Davies, Bishop, Manstead, & Tantam, 1994; Klin et al., 1999). That is to say that by showing no impairment by the disruption of configural information when processing faces, they are presumed to be focusing more on features of the face that are not disrupted when presented upside-down.
Impairments to face processing have also been attributed to potential differences in the way individuals with autism distribute their attention to various facial features. Clinically, it is recognized that individuals with autism engage in limited eye contact when involved in social interactions (American Psychiatric Association, 2000). As a result, a number of studies have focused on how individuals with autism might differ from typically developing individuals in their attentional allocation to primary facial features such as the eyes and mouth. Pelphrey and colleagues (2002) measured how adults with high-functioning autism visually scan static face images. Compared to typically developing controls, individuals with high-functioning autism looked less at the internal features of the face (e.g., the mouth, eyes and nose region) and particularly at the eye region of faces. A second eye-tracking study, conducted by Klin, Jones, Schultz, Volkmar and Cohen (2002) investigated the visual scanning during passive viewing of naturalistic, social scenes. In comparison to controls, individuals with autism looked less at the eyes and more at the mouth region of faces. Additionally, they looked more at non-face body regions and objects.
Recently, a number of eye-tracking studies have investigated how individuals with ASD attend to different regions of the face. These studies have almost exclusively focused on the extent to which attention is given to the eye region of the face versus the mouth regions. A number of studies suggest that individuals with autism attend less to the eyes and more to the mouth than do typically developing individuals (Corden, Chilvers, & Skuse, 2008; Hernandez, 2009; Neuman, Spezio, Piven, & Adolphs, 2006; Norbury et al., 2009; Spezio, Adolphs, Hurley & Piven, 2006). However, this finding has not always been replicated (Fletcher-Watson, Leekam, Benson, Frank, & Findlay, 2009; van der Geest, Kemner, Camfferman, Verbaten, & Engeland, 2002; van der Geest, Kemner, Verbaten, & Engeland, 2002), and a recent study suggests that the extent to which individuals with autism attend to the eyes or mouth may vary with the demands of the facial task (Hannigen et al, 2009). Furthermore, a study by Best, Minshew, and Strauss (2010) demonstrated that individuals with ASD, similar to controls, are better at discriminating facial gender from isolated eye region information than mouth information.
Given all of the research that has focused on potential differences in fixating to eye versus mouth regions, it is surprising that there are no studies exploring possible eye-tracking differences in attending to the right or left halves of the face. Considering the research on differences in configural processing among individuals with autism, there is reason to believe that individuals with autism are less likely to demonstrate a LVF bias while performing face processing tasks. Importantly, a behavioral study using chimeric faces by Ashwin, Wheelwright & Baron-Cohen (2005) found that adults with Asperger’s syndrome showed a reduced bias to the LVF compared to the control group. Thus, the current study was designed to examine the nature of this reduced bias with the use of eye tracking technology to see if there were LVF bias differences in individuals with high functioning autism in contrast to matched controlled participants.
Methods
Participants
Participants consisted of 34 typically developing adolescents and adults, mean age 21.59 years, and 29 adolescents and adults diagnosed with autism, mean age 18.90 years. Participants were matched on Verbal IQ, Performance IQ, Full Scale IQ, and chronological age (Table 1). Participants were recruited through public advertisements. For the autism group, participants autism diagnoses were confirmed using the Autism Diagnostic Observation Schedule (ADOS; Lord, Rutter, DiLavore, & Risi, 2003), the Autism Diagnostic Interview-Revised (ADI-R; Rutter, LeCouteur, & Lord, 2003), and clinical opinion. Participants with Asperger’s disorder or PDD-NOS were excluded. Control participants were volunteers recruited from the community. Control participants were required to have a negative family history of first degree relatives with major psychiatric disorders and of first and second degree relatives with autism spectrum disorder. Control participants were also excluded if they had a history of poor school attendance or evidence of a disparity between general level of ability and academic achievement suggesting of a learning disability. Additionally, the Wide Range Achievement Test-Fourth Edition (WRAT4; Wilkinson & Robertson, 2006) was administered to all participants to identify participants with a diagnosable learning disability. All participants were healthy, free of seizures, had a negative history of traumatic brain injury, and had an IQ greater than 80 as determined by the Wechsler Abbreviated Scale of Intelligence (WASI; Wechsler, 1999).
Table 1.
Matched groups
| Autism (N = 29)
|
Control (N =34)
|
|||
|---|---|---|---|---|
| M | SD | M | SD | |
|
| ||||
| Age (years) | 18.90 | 5.77 | 21.59 | 7.08 |
| VIQ | 105.79 | 11.58 | 110.06 | 6.28 |
| PIQ | 110.34 | 10.76 | 112.14 | 7.39 |
| FSIQ | 109.03 | 10.10 | 112.59 | 6.48 |
| ADOS | 14.14 | 3.08 | ||
| Gender (M/F) | (26/3) | (34/0) | ||
| Ethnicity | 27 Caucasian 1 more than one race 1 Other |
34 Caucasian | ||
Stimuli
A gender identification task was used as a measure of face processing ability. Gender identification is a task that has consistently be used to demonstrate the LVF bias. In order to increase the configural processing demands of the task, the task was made more difficult by using a commercial software morphing program (FantaMorph 3.0, Abrosoft, www.fantamorph.com) to create stimuli that were blends of the two genders. Each stimulus consisted of a male and female face morphed together, creating more androgynous looking faces that lacked strong featural cures for gender identification. The morphed “female” face contained between 55% and 75% of the natural female face with the remaining 45% to 25% being that of a natural male face. The morphed “male” face was comprised of the reverse proportions. The faces were shown in grayscale and cropped so that only the internal facial features could be seen. The gender and difficulty of gender discrimination were assigned to the faces using an agreement rating done by a large class of undergraduate students. Sixty faces were selected as stimuli with equal numbers of male and female faces across three levels of difficulty: easy, medium, and hard. An example of a male and female faces of each difficulty level can be seen in Figure 1.
Figure 1.
Example of morphed stimuli
Procedure
Stimuli were presented approximately 5 feet in front of participants on a large screen using rear projection. Stimuli were 10 inches in width and 14.5 inches in height, subtending a visual angle of 13.78 degrees in height by 7 degrees in width. A free standing Tobii X120 eye tracker was positioned 27 inches in front and 30 degrees below participants. Eye movements were recorded at a speed of 60 Hz per eye. A fixation was defined as a 35 pixel diameter region.
Participants were asked to determine the gender of each face and respond by pressing one of two buttons on a keypad that were labeled male and female. Each stimulus was preceded by a fixation oval which was advanced by the experimenter when the participant was attending to the screen. Immediately following the fixation oval, a face was presented and remained on the screen until the participant made a key response. Participants responded to a total of 60 faces, half male and half female, with an equal number of easy, medium, and hard faces for both genders. The accuracy of the eye tracking was confirmed at three equally distributed intervals during the experiment by asking participants to look at targets presented on the screen. Data from participants who demonstrated tracking that was shifted from calibration targets were disqualified. The Tobii Studio software collected responses and reaction times. All subjects were tested with the same equipment, in the same testing room, and under similar testing conditions.
Data Reduction
The number of fixations made to the left or right visual fields was analyzed by creating regions of interest (ROI) that included the entire right or left sides of the faces (Figure 2). The proportion of fixations made in the left visual field was calculated by taking the number of fixations in the left side of the faces divided by the total number of fixations made to both the right and left sides of the face. The number of fixations made to the eye or mouth regions was also analyzed. The eye ROI included the highest point of the eyebrow to the top of the orbital bone. The mouth ROI was a region starting half way between the bottom of the nose and the top of the mouth and ending an equal distance below the mouth. Percentage of fixations to the eye region was calculated by taking the number of fixations to the eye region over the total number of fixations made to both the eye and mouth regions.
Figure 2.
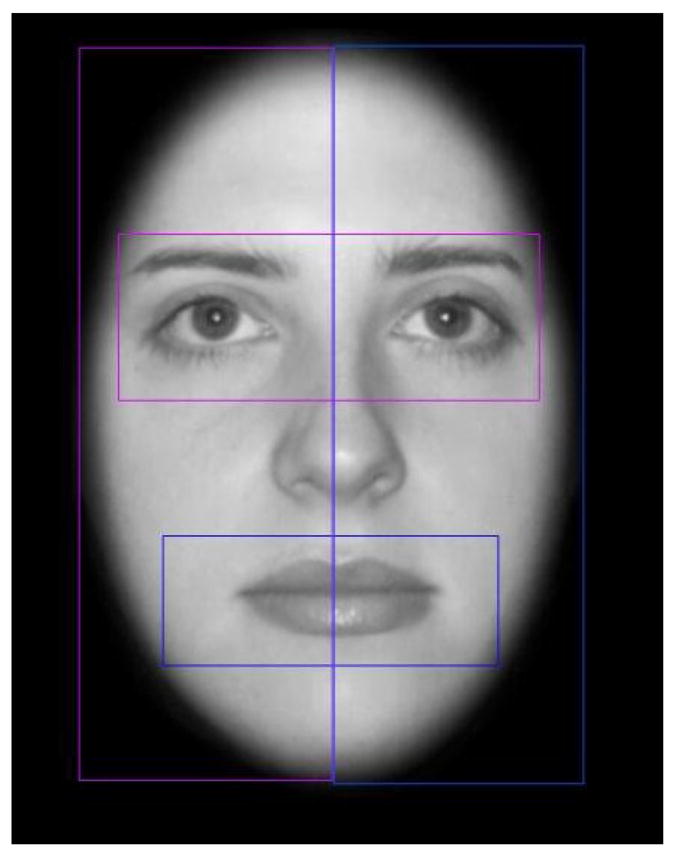
Example of ROIs
The participants’ accuracy at identifying the gender of the faces was also calculated by taking the total number correct responses divided by the total number of trials.
Results
We first established that there was no difference in the total number of fixations between the control (M = 294, SD = 113) and autism (M = 299, SD = 145) groups, F(1, 62) = 0.162, p > .05, to rule out a possible confounding explanation for other group differences. We then examined the relationship between the count of fixations and the duration of looking time and found the two measures to be highly correlated, r = .866, n = 60, p < .0001. In order to reduce redundancy, we only report data in the form of fixation count.
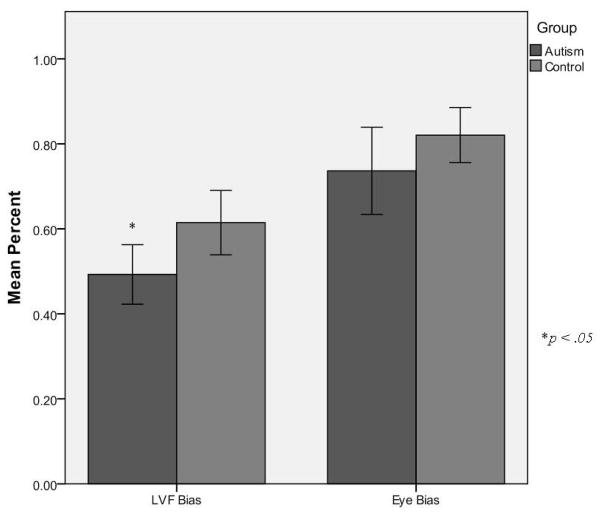
To analyze our primary interest, a left visual field bias, a one-way analysis of variance (ANOVA) was conducted on the proportion of the left visual field fixations with group (controls vs. autism) as the between measure. This analysis demonstrated a significant difference in the two groups’ proportion of fixations to the LVF, F(1, 62) = 5.45, p < .05. Comparing the proportion of fixations to the LVF to a non-biased response of .5 indicated that the control group demonstrated a significant LVF bias (M = .61, SD = .039), t(34) = 3.03, p < .01). In contrast, the autism group did not show a bias in either direction (M = .49, SD = .035), t(29) = −.21, p > .05 (Figure 3).
Figure 3.
LVF and Eye bias by group
We also examined the eye vs. mouth bias because it is an area of autism research that has yielded mixed results. To analyze whether there were any group differences in the proportion of fixations that were made to the eye versus mouth regions, a one-way ANOVA comparing the groups was calculated for the proportion of fixations that were made to the eye region in contrast to the mouth region. This analysis indicated that there was not a significant difference between the groups’ biases to the eye region, F(1, 62) = 2.03, p > .05. That is, both the control group (M = .82, SD = .032, t(34) = 9.874 ) and the autism group (M = .74, SD = .051, t(29) = 4.61, p < .001) spent a greater proportion of time attending to the eye region than the mouth region. Analyses were conducted to determine participants accuracy to discriminate gender and their reaction times to make this discrimination. The control group was more accurate in identifying the gender of the faces (M = .72, SD = .072), than the autism group (M = .68, SD = .060), F(1,62) = 4.33, p < .05.
Analyses were also conducted to determine the participant’s behavioral performance in the gender discrimination task. The control group was slightly more accurate in identifying the gender of the faces (M = .72, SD = .072) than was the autism group (M = .68, SD = .060), F(1,62) = 4.33, p = .042. The autism group did, however, perform significantly better than chance, t(28) = 16.74, p < .0001. The autism group was also significantly slower at identifying the gender of the faces (control: M = 1481.46, SD = 575.52; autism: M = 1837.69, SD = 819.95) F(1,59) = 3.893, p = .05. An outlier was removed from each group for taking significantly longer than the group mean.
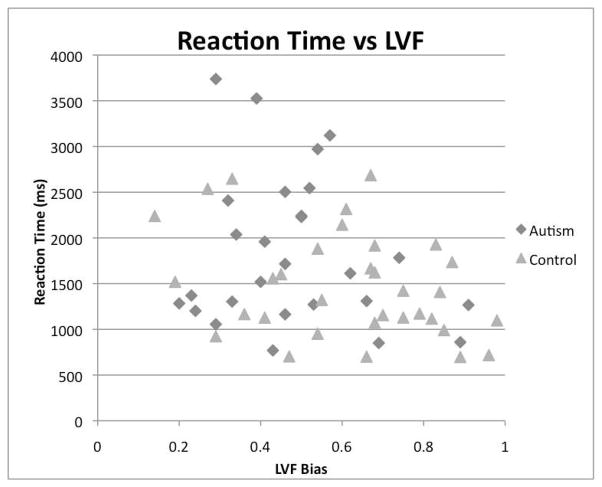
Finally, the relationship between performance on discrimination task (with respect to accuracy and reaction time) and the extent of LVF bias was examined. Correlations were calculated for each group to determine if there was a relation between the participant’s LVF proportion scores and accuracy scores. Results indicated that there was not a reliable association between LVF bias and accuracy for either the control group, r = .263, n = 34, p > .05, or the autism group, r = −.332, n = 29, p >.05. Correlations calculated to determine if there was a relationship between LVF proportion scores and reaction time determined that there was no significant correlation between reaction time and degree of LVF for the autism group (r = −.175, n = 27, p > .05), while there was a significant correlation in the control group (r = −.340, n = 33, p < .05). When the two groups were combined there was a moderate but significant relation (r = −.308, n = 58, p <. 05, Figure 4).
Figure 4.
LVF and Reaction Time
Discussion
This study confirms the results presented by Butler et al. (2005), showing that typically developing individuals demonstrate a LVF bias by looking to the left side of the face. For controls, this tendency to look to the left side of the face was related to faster processing speed in gender discrimination. In contrast, the current study found that individuals with autism do not demonstrate a LVF bias, which supports the previous findings of Ashwin, Wheelwright & Baron-Cohen (2005). However, the current study was the first study to directly measure visual field preference by recording online eye movements.
The autism group did demonstrate a significant bias to look at the eye region over the mouth region. This supports recent studies that show no difference between eye and mouth looking behavior between autism and control groups (Best, Minshew & Strauss, 2010; Fletcher-Watson, Leekam, Benson, Frank, & Findlay, 2009; Hannigen et al., 2009; van der Geest, Kemner, Camfferman, Verbaten, & Engeland, 2002; van der Geest, Kemner, Verbaten, & Engeland, 2002).
As expected, the autism group was less accurate at discriminating facial gender. These results support previous research demonstrating that individuals with autism are poorer at gender discrimination than typically developing individuals (e.g., Newell et al., 2010). In line with previous research (Sasson, 2006), this study also found that the autism group took significantly longer to make their gender judgments.
The study did not find an association between LVF bias and accuracy for individual or combined groups. It could be that with more participants, or a greater variability in accuracy, a significant correlation could be established between a greater LVF bias and better discrimination. Though it was not found in this study, studies showing the lateralized RH activity occurring with the development of facial expertise (Aylward et al., 2005) suggest that a positive LVF bias and accuracy correlation is possible.
The control data did suggest a significant negative correlation between reaction time and LVF bias. This relationship remained significant when groups were combined, indicating a greater LVF bias is related to faster response time. It has been proposed that a longer response time eliminates the bias because it allows more time for subjects to equally distribute their gaze. However, these correlational data suggest this is not the case, because participants with longer response times were more biased to the right visual field (RVF). This finding supports the idea that the LVF bias is present for face processing to increase the efficiency of processing, possibly directly providing information to the right hemisphere.
While much of the research on face processing in autism has been focused on eye and mouth looking behavior, this study suggests that when it comes to the processing of internal face features, the lack of a visual field bias may be more indicative of the disorder. It is possible that individuals with autism are looking to the eye region, but cannot extract the information needed for discrimination, because they have failed to develop face processing expertise. However, considering that the LVF bias aids in processing faces more effectively and efficiently, there is reason to believe that it could be related to the deficit seen in face processing abilities of those with autism.
Since this study was limited to the processing faces, it would be interesting to see if individuals with autism also lack visual field biases for other stimuli that are known to exist in neurotypical populations. If this is the case, it would strongly support the idea that the lack of a visual field bias is representative of a disruption in development of configural processing expertise.
What remains unanswered is how the visual processing bias and hemispheric specialization for faces are developing within neurotypical populations. Little is known about the exact nature of what is causing the emergence of both laterality biases. There is some evidence that traces of the bias exist during infancy (Guo et al., 2009) with other work suggesting a prolonged developmental trajectory over the course of childhood and adolescence (Aylward et al., 2005). There is however, no work relating the developmental of the gaze bias and hemispheric specialization to each other in individual children. It could be that the development of one of these laterality biases is driving the manifestation of the other, or that both are presenting simultaneously. Further investigation of this process could offer insight not only into how face processing abilities are developing, but also how they are disrupted in autism.
Acknowledgments
This research was supported by grant from the National Institutes of Health. The authors would like to thank Dr. Charles Nelson, Dr. Giulia Righi and Dr. Marlene Behrmann for their helpful comments and discussion of the project. We also thank Kao-Wei Chua for his help in formatting the paper, and Dr. Holly Gastgeb, Dr. Keiran Rump, Desiree Wilkinson, Sarah Hannigen, Sara Green and Kao-Wei Chua for testing participants.
Contributor Information
Eva M. Dundas, Department of Psychology, University of Pittsburgh
Catherine A. Best, Department of Psychology, University of Pittsburgh
Nancy J. Minshew, Department of Psychiatry, University of Pittsburgh School of Medicine
Mark S. Strauss, Department of Psychology, University of Pittsburgh
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- Ashwin C, Wheelwright S, Baron-Cohen S. Laterality biases to chimeric faces in asperger syndrome: What is „right’ about face-processing? Journal of Autism and Developmental Disorders. 2005;35(2):183–196. doi: 10.1007/s10803-004-1997-3. [DOI] [PubMed] [Google Scholar]
- Aylward EH, Park JE, Field KM, Parson AC, Richards TL, Cramer SC, Meltzoff AN. Brain activation during face perception: Evidence of a developmental change. Journal of Cognitive Neuroscience. 2005;17:308–319. doi: 10.1162/0898929053124884. [DOI] [PubMed] [Google Scholar]
- Best CA, Minshew NJ, Strauss MS. Gender discrimination of eyes and mouth by individuals with autism. Autism Research. 2010;3(2):88–93. doi: 10.1002/aur.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher J, Lewis V. Unfamiliar face recognition in relatively able autistic children. Journal of Child Psychology and Psychiatry. 1992;33:843–859. doi: 10.1111/j.1469-7610.1992.tb01960.x. [DOI] [PubMed] [Google Scholar]
- Boucher J, Lewis V, Collis G. Familiar face and voice matching and recognition in children with autism. Journal of Child Psychology and Psychiatry. 1998;39:171–181. [PubMed] [Google Scholar]
- Burt DM, Perrett DI. Perceptual asymmetries in judgements of facial attractiveness, age, gender, speech and expression. Neuropsychologia. 1997;35:685–693. doi: 10.1016/s0028-3932(96)00111-x. [DOI] [PubMed] [Google Scholar]
- Butler S, Gilchrist ID, Burt DM, Perrett DI, Jones E, Harvey M. Are the perceptual biases found in chimeric face processing reflected in eye-movement patterns? Neuropsychologia. 2005;43:52–59. doi: 10.1016/j.neuropsychologia.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Coolican J, Eskes GA, McMullen PA, Lecky E. Perceptual biases in processing facial identity and emotion. Brain and Cognition. 2008;66:176–187. doi: 10.1016/j.bandc.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Corden B, Chilvers R, Skuse D. Emotional modulation of perception in asperger’s syndrome. Journal of Autism and Developmental Disorders. 2006;38(6):1072–80. doi: 10.1007/s10803-007-0485-y. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Daly EM, Bullmore ET, Williams CR, Van Amelsvoort T, Robertson DM, Rowe A, Phillips M, McAlonon G, Howlin P, Murphy GM. The functional neuroanatomy of social behaviour: Changes in cerebral blood flow when people with autistic disorder process facial expression. Brain. 2000;123:2203–2212. doi: 10.1093/brain/123.11.2203. [DOI] [PubMed] [Google Scholar]
- Curby K, Willenbockel V, Tanaka J, Schultz R. Face processing in autism: Insights from the perceptual expertise framework. In: Gauthier I, Tarr M, Bub D, editors. Perceptual expertise: Bridging brain and behavior. New York: Oxford University Press; 2010. [Google Scholar]
- Davies S, Bishop D, Manstead ASR, Tantam D. Face perception in children with autism and Asperger’s syndrome. Journal of Child Psychology and Psychiatry. 1994;35:1033–1057. doi: 10.1111/j.1469-7610.1994.tb01808.x. [DOI] [PubMed] [Google Scholar]
- Dawson G, Webb SJ, McPartland J. Understanding the nature of face processing impairment in autism: Insights from behavioral and electrophysiological studies. Developmental Neuropsychology. 2005;27:403–424. doi: 10.1207/s15326942dn2703_6. [DOI] [PubMed] [Google Scholar]
- De Renzi E. Current issues on prosopagnosia. In: Ellis HD, Jeeves MA, Newcombe F, Young A, editors. Aspects of face processing. Netherlands: Martinus Nijhoff; 1986. pp. 234–252. [Google Scholar]
- De Renzi E, Perani D, Carlesimo GA, Silveri MC, Fazio F. Prosopagnosia can be associated with damage confined to the right hemisphere – an MRI and PET study and a review of the literature. Neuropsychologia. 1994;32:893–902. doi: 10.1016/0028-3932(94)90041-8. [DOI] [PubMed] [Google Scholar]
- Diamond R, Carey S. Why faces are and are not special: An effect of expertise. Journal of Experimental Psychology: General. 1986;115:107–117. doi: 10.1037//0096-3445.115.2.107. [DOI] [PubMed] [Google Scholar]
- Fletcher-Watson S, Leekam SR, Benson V, Frank MC, Findlay JM. Eye movements reveal attention to social information in autism spectrum disorder. Neuropsychologia. 2009;47:248–257. doi: 10.1016/j.neuropsychologia.2008.07.016. [DOI] [PubMed] [Google Scholar]
- Gajewski PD, Schlegel K, Stoerig P. Effects of human race and face inversion on the N170: A cross-race study. Federation of European Psychophysiological Societies. 2008;22:157–165. [Google Scholar]
- Gauthier I, Tarr MJ. Becoming a “greeble” expert: Exploring mechanisms for face recognition. Vision Research. 1996;37:1673–1682. doi: 10.1016/s0042-6989(96)00286-6. [DOI] [PubMed] [Google Scholar]
- Gauthier I, Williams P, Tarr MJ, Tanaka J. Training „greeble’ experts: A framework for studying expert object recognition processes. Vision Research. 1998;38:2401–2428. doi: 10.1016/s0042-6989(97)00442-2. [DOI] [PubMed] [Google Scholar]
- Gilbert C, Bakan P. Visual asymmetry in the perception of faces. Neuropsychologia. 1973;11:355–272. doi: 10.1016/0028-3932(73)90049-3. [DOI] [PubMed] [Google Scholar]
- Guo K, Meints K, Hall C, Hall S, Mills D. Left gaze bias in humans, rhesus monkeys and domestic dogs. Animal Cognition. 2009;12:409–418. doi: 10.1007/s10071-008-0199-3. [DOI] [PubMed] [Google Scholar]
- Hannigen S, Best C, Rump K, Minshew NJ, Strauss MS. An eye-tracking study: The effect of task on visual attention faces in autism. International Meetings for Autism Research; Chicago, Il. 2009. [Google Scholar]
- Haxby JV, Hoffman EA, Gobbini MI. The distributed human neural system for face perception. Trends in Cognitive Sciences. 2000;4:223–233. doi: 10.1016/s1364-6613(00)01482-0. [DOI] [PubMed] [Google Scholar]
- Hernandez N, Metzger A, Magné R, Bonnet-Brilhault F, Roux S, Barthelemy C, Martineau J. Exploration of core features of a human face by healthy and autistic adults analyzed by visual scanning. Neuropsychologia. 2009;47:1004–1012. doi: 10.1016/j.neuropsychologia.2008.10.023. [DOI] [PubMed] [Google Scholar]
- Hsiao JH, Cottrell GW. Not all visual expertise is holistic, but it may be leftist. Psychological Science. 2009;20(4):455–63. doi: 10.1111/j.1467-9280.2009.02315.x. [DOI] [PubMed] [Google Scholar]
- Hubl D, Bolte S, Feineis-Matthews S, Lanfermann H, Federspiel A, Strik W, Poustka F, Dierks T. Functional imbalance of visual pat ways indicates alternative face processing strategies in autism. Neurology. 2003;61:1232–1237. doi: 10.1212/01.wnl.0000091862.22033.1a. [DOI] [PubMed] [Google Scholar]
- Klin A, Jones W, Schultz R, Volkmar F, Cohen D. Visual fixation patterns during viewing of naturalistic social situations as predictors of social competence in individuals with autism. Archives of General Psychiatry. 2002;59:809–816. doi: 10.1001/archpsyc.59.9.809. [DOI] [PubMed] [Google Scholar]
- Klin A, Sparrow SS, de Bildt A, Cicchetti DV, Cohen DJ, Volkmar FR. A normed study of face recognition in autism and related disorders. Journal of Autism and Developmental Disorders. 1999;29(6):499–508. doi: 10.1023/a:1022299920240. [DOI] [PubMed] [Google Scholar]
- Leehey SC, Carey S, Diamond R, Cahn A. Upright and inverted faces: The right hemisphere knows the difference. Cortex. 1978;14:411–419. doi: 10.1016/s0010-9452(78)80067-7. [DOI] [PubMed] [Google Scholar]
- Levine SC, Banich MT, Koch-Weser MP. Face recognition: A general or specific right hemisphere capacity? Brain and Cognition. 1988;8:303–325. doi: 10.1016/0278-2626(88)90057-7. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore PC, Risi S. Autism diagnostic observation schedule (ADOS) Los Angeles, CA: Western Psychological Services; 2003. [Google Scholar]
- Luh KE, Rueckert LM, Levy J. Perceptual asymmetries for free viewing of several types of chimeric stimuli. Brian and Cognition. 1991;16(1):83–103. doi: 10.1016/0278-2626(91)90087-o. [DOI] [PubMed] [Google Scholar]
- Maurer D, Le Grand R, Mondloch CJ. The many faces of configural processing. Trends in Cognitive Sciences. 2002;6(6):255–260. doi: 10.1016/s1364-6613(02)01903-4. [DOI] [PubMed] [Google Scholar]
- Neumann D, Spezio ML, Piven J, Adolphs R. Looking you in the mouth: abnormal gaze in autism resulting from impaired top-down modulation of visual attention. Social Cognitive and Affective Neuroscience. 2006;1:194–202. doi: 10.1093/scan/nsl030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell LC, Best CA, Gastgeb H, Rump KA, Strauss MS. The Development of Categorization and Facial Knowledge: Implications for the Study of Autism. In: Oakes LM, Cashon CH, Casasola M, Rakison DH, editors. The information-processing infant. New York: Oxford University Press; 2010. [Google Scholar]
- Norbury CF, Brock J, Cragg L, Einav S, Griffiths H, Nelson K. Eye-movement patterns are associated with communicative competence in autistic spectrum disorders. Journal of Child Psychology and Psychiatry. 2009;50:834–842. doi: 10.1111/j.1469-7610.2009.02073.x. [DOI] [PubMed] [Google Scholar]
- Pelphrey KA, Sasson NJ, Reznick JS, Paul G, Goldman BD, Piven J. Visual scanning of faces in autism. Journal of Autism and Developmental Disorders. 2002;32:249–261. doi: 10.1023/a:1016374617369. [DOI] [PubMed] [Google Scholar]
- Pierce K, Muller RA, Ambrose J, Allen G, Courchesne E. Face processing occurs outside the fusiform „face area’ in autism: evidence from functional MRI. Brain. 2001;124:2059–2073. doi: 10.1093/brain/124.10.2059. [DOI] [PubMed] [Google Scholar]
- Rhodes G. Configural coding, expertise, and the right hemisphere advantage for face recognition. Brain and Cognition. 1993;22:19–41. doi: 10.1006/brcg.1993.1022. [DOI] [PubMed] [Google Scholar]
- Rutter M, LeCouteur A, Lord C. Autism Diagnostic Interview-Revised (ADI-R) Los Angeles, CA: Western Psychological Services; 2003. [Google Scholar]
- Sasson NJ. The development of face processing in autism. Journal of Autism and Developmental Disorders. 2006;36:381–394. doi: 10.1007/s10803-006-0076-3. [DOI] [PubMed] [Google Scholar]
- Schultz RT, Gauthier I, Klin A, Fulbright RK, Anderson AW, Volkmar F, Skudlarski P, Lacadie C, Cohen DJ, Gore JC. Abnormal ventral temporal cortical activity during face discrimination amond individuals with autism and asperger syndrome. Archives of General Psychiatry. 2000;57:331–340. doi: 10.1001/archpsyc.57.4.331. [DOI] [PubMed] [Google Scholar]
- Senju A, Johnson MH. Atypical eye contact in autism: Models, mechanism and development. Neuroscience and Biobehavioral Reviews. 2009;33:1204–1214. doi: 10.1016/j.neubiorev.2009.06.001. [DOI] [PubMed] [Google Scholar]
- Tanaka J, Joseph RM. Holistic and part-based face recognition in children with autism. Journal of Child Psychology and Psychiatry. 2002;43(8):1–14. doi: 10.1111/1469-7610.00142. [DOI] [PubMed] [Google Scholar]
- The Interdisciplinary Council on Developmental & Learning Disorders. What is DIR/FloorTime. Retrieved April 17, 2010 from www.floortime.org/
- van der Geest JN, Kemner C, Camfferman G, Verbaten MN, van Engeland H. Eye movements, visual attention, and autism: A saccadic reaction time study using the gap and overlap paradigm. Biological Psychiatry. 2001;50:614–619. doi: 10.1016/s0006-3223(01)01070-8. [DOI] [PubMed] [Google Scholar]
- Wang AT, Dapretto M, Hariri AR, Signman M, Bookheimer SY. Neural correlates of facial affect processing in children and adolescents with autism spectrum disorder. J Am Acad Child Adolesc Psychiatry. 2003;43(4):481–490. doi: 10.1097/00004583-200404000-00015. [DOI] [PubMed] [Google Scholar]
- Wolff W. The experimental study of forms of expression. Character & Personality. 1933;2:168–176. [Google Scholar]
- Yovel G, Kanwisher N. The neural basis of the behavioral face-inversion effect. Current Biology. 2005;15:2256–2262. doi: 10.1016/j.cub.2005.10.072. [DOI] [PubMed] [Google Scholar]
- Yovel G, Tambini A, Brandman T. The asymmetry of the fusiform face area is a stable individual characteristic that underlies the left-visual-field superiority for faces. Neuropsychologia. 2008;46:3061–3068. doi: 10.1016/j.neuropsychologia.2008.06.017. [DOI] [PubMed] [Google Scholar]