Abstract
Non-pharmacological and non-surgical transcranial modulation of the nerve function may provide new opportunities in evaluation and treatment of cranial nerve diseases. This study investigates the possibility of using low-intensity transcranial focused ultrasound (FUS) to selectively stimulate the rat abducens nerve located above the base of the skull. FUS (frequencies of 350 kHz and 650 kHz) operating in a pulsed mode was applied to the abducens nerve of Sprague-Dawley rats under stereotactic guidance. The abductive eyeball movement ipsilateral to the side of sonication was observed at 350 kHz, using the 0.36 msec tone burst duration (TBD), 1.5 kHz pulse repetition frequency (PRF), and the overall sonication duration of 200 msec. Histological and behavioral monitoring showed no signs of disruption in the blood brain barrier (BBB) as well as no damage to the nerves and adjacent brain tissue resulting from the sonication. As a novel functional neuro-modulatory modality, the pulsed application of FUS has potential in diagnostic and therapeutic applications in diseases of the peripheral nervous system.
Keywords: focused ultrasound, abducens nerve, cranial nerve VI, brain, neuromodulation, peripheral nervous system
Introduction
The cranial nerves, as part of the peripheral nervous system, dictate important sensory motor functions of the head ranging from vision, audition, and olfaction, to the movement of ocular as well as facial/vocal muscles. In addition, cranial nerves sub-serve autonomic nervous functions, such as innervations of abdominal viscera in the case of the vagus nerve. Aberrant or disrupted cranial nerve function ramifies into pathological sensory conditions (e.g., trigeminal neuralgia), impaired facial expression, or abnormal eye movement (e.g., eye movement disorders associated with cerebral nerve palsy) (Wilson-Pauwels 2010).
Progress has been made in the attempt to gain control over aberrant cranial neural transmissions, mainly through surgical or pharmacological interventions. Electrical neuromodulation of cranial nerves, such as vagus nerves stimulation (VNS) and trigeminal nerve stimulation (TNS), have been applied using surgically-implanted electrodes to treat epilepsy and trigeminal neuralgia (DeGiorgio et al. 2003; Uthman et al. 2004). Direct electrical stimulation of optic and auditory nerves has also been proposed to augment corresponding impaired sensory functions (Simmons et al. 1965; Killian and Fromm 1968; Humayun et al. 1999). Pharmacological treatments aiming for the modulation of neural excitability, such as administration of anticonvulsants for trigeminal neuralgia, have been deployed (Blom 1962); however, they lack selectivity toward the specific neural tissue-of-interest and have potential adverse effects (e.g., aplastic anemia) (Donaldson and Graham 1965; Dyer et al. 1966). Therefore, non-invasive and non-pharmacological means to harness cranial nerve function, with the spatial specificity to target the anatomy-of-interest, have been warranted.
As a non-invasive alternative to the surgical interventions, transcranial magnetic stimulation (TMS) of cranial nerves has been proposed (Wessel and Kompf 1991; Roth et al. 1994). TMS relies on inducing electrical current on biological tissue by applying strong time-variant magnetic fields over the skin surface, and has been used for non-invasive stimulation of peripheral nerves as well as brain cortices (Roth et al. 1994). However, the inductive nature of TMS lacks sufficient penetration depth to reach the deep brain area (due to the rapid reduction of the magnetic field away from the coil) and also lacks spatial specificity during the stimulation (i.e., the modulatory area is wide, on the order of a few centimeters in diameter) (Kobayashi and Pascual-Leone 2003).
With recent advancements in focused ultrasound (FUS) technique, highly-focused acoustic energy can be delivered to specific areas of biological tissues, as small as a few millimeters in diameter. The acoustic energy is deposited to the tissues at the focus as thermal or mechanical energy, which can be used to ablate tumorous tissue via hyperthermia, or to break down crystalline structures such as kidney stones. Ultrasound, typically operating at a frequency under 1 MHz, can also be delivered through the skull to specific areas of the brain, such as the thalamus, in a focused manner using helmet-like multi-array ultrasound transducers (Clement et al. 2005) or through thin temporal bone (Gavrilov et al. 1996). Most of the acoustic energy given above 1MHz is attenuated during the transmission through the skull. The lower acoustic frequency (compared to the one used in diagnostic imaging, i.e., on the order of 1–15MHz) (O'Brien 2007) favors the transcranial application of the FUS due to longer wavelength, for example, only mild attenuation (22.5%) of the acoustic intensity has been observed at 120 kHz through a human skull (Coussios et al. 2002). For these reasons, commercially-available transcranial FUS equipments (for human use) utilize the frequencies in the range of 200–700 kHz, and have been used in hyperthermic ablative treatments for brain tumors (McDannold and Jolesz 2000) and functional neurosurgery (Martin et al. 2009). Regarding its extended potential in modulating peripheral nerve function, Colluci and colleagues have shown that pulsed high-intensity (high enough to raise the temperatue of the nerve bundle) FUS, applied to excised frog sciatic nerves, supressed the magnitude and latencies in action potential propagation (Colucci et al. 2009).
Pulsed application of the ultrasound at low acoustic intensity, under the threshold for heat generation or mechanical damage in biological tissue, has been shown to modulate the excitability of the brain tissue both ex vivo (Gavrilov et al. 1996; Bachtold et al. 1998) as well as in vivo (Tufail et al. 2010). We have also recently demonstrated that FUS, when applied in short bursts of pulses, modulates neural tissue excitability in the motor and visual cortices of rabbits without changing tissue temperature (Yoo et al. 2011). This exciting feature has also been applied to decrease the electrographic seizure activites from chemically-induced epileptic rats (Min et al. 2011a), and to modify the extracelluar level of neurotransmitters (Min et al. 2011b; Yang et al. 2012). Converging evidences from these studies indicate that application of FUS at low acoustic intensity, typically under the FDA-limit for diagnostic imaging (720 mW/cm2 Ispta; spatial-peak temporal-average intensity) (AIUM Clinical Standards Committee 2004), temporarily and reversibly modified neural function in vivo.
In the present study, we were motivated to extend the applicability of FUS in stimulating the peripheral nervous system, specifically the cranial nerves. The ability to stimulate intracranial nerves using focused ultrasound was explored at two different frequencies (350 kHz and 650 kHz), falling within the ranges of frequencies adopted in human transcranial FUS systems. We hypothesized that the successful stimulation of the abducens nerve by pulsed FUS sonication would induce corresponding abductive eyeball movements.
Materials and Methods
All experiments were conducted under institutional review and approval by the Harvard Medical Area Standing Committee on Animals. Male Sprague–Dawley rats (weight = 290–405g; n=22) were used in this study. All animals were anesthetized with an intraperitoneal injection of a ketamine/xylazine mixture of 80:10 mg/kg prior to sonication.
Focused ultrasound sonication
After anesthetizing the rat, the head was depilated, and the rat was placed in a magnetic resonance imaging (MRI) compatible stereotactic frame (SRP-AR, Narishige, Japan), as shown in Fig. 1A. Then, an air-backed, segmented spherical ultrasound transducer (6 cm in outer diameter; 7 cm in radius-of-curvature; fundamental frequency of 350 kHz or 650 kHz) was coupled to the rat’s head via a degassed water bag, while hydrogel was applied between the water bag and the scalp.
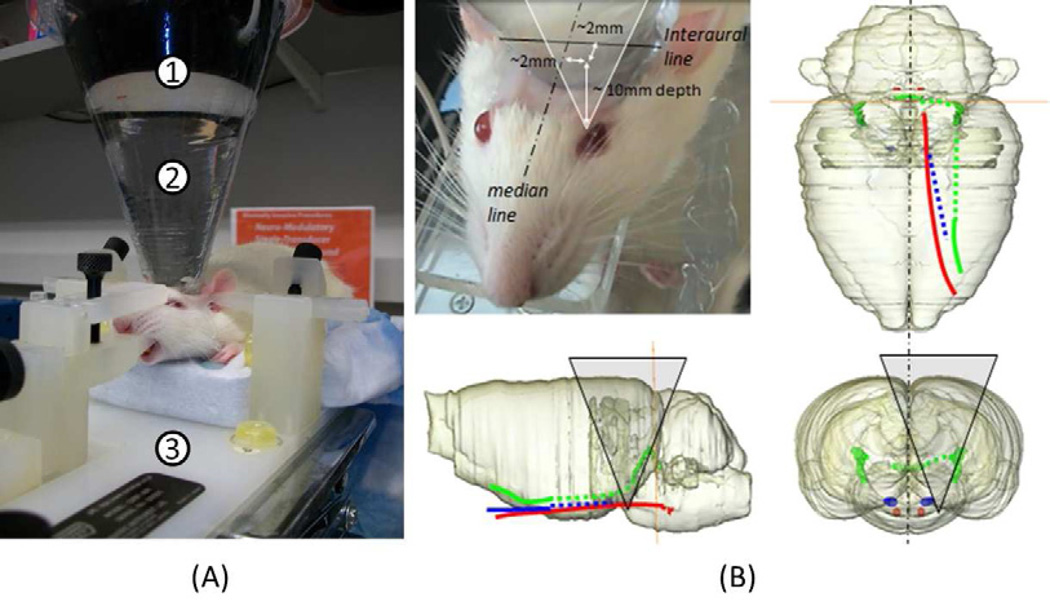
Figure 1.
(A) An experimental set-up. The FUS transducer (1) coupled to the rat’s head via a degassed water bag (2) was powered by two function generators and a linear power amplifier (not shown). The rodent was placed on the MRI-compatible fixation head-frame (3). (B) Estimated sonication path (triangle) on 3-D reconstruction of the rat atlas (Watson 2004); abducens nerve (red), trochlear nerve (green), oculomotor nerve (blue); The cranial nerve paths were traced in solid line while overlaying onto the rat anatomy (Greene 1968), and the rest of the approximated nerve paths were depicted in dashed lines.
The input signal to the FUS transducer was generated by two waveform generators (33210A, Agilent, Santa Clara, CA). The first waveform generator was used to define the pulse repetition frequency (PRF) at 1.5 kHz, the overall sonication duration of 200 msec, and the timing of each sonication. This waveform generator triggered the operation of the second waveform generator that sent out sinusoidal waves at each fundamental frequency having a tone-burst duration (TBD) of 0.36 msec. The choice of the pulsing parameters, except the acoustic intensity, was based on the previous work that elicited neural excitation in motor areas of rodents (Tufail et al. 2010). The resulting signals were amplified by a linear power amplifier (240L, ENI Inc., Rochester, NY) and then transmitted to the FUS transducer. According to the calibration of acoustic pressure distribution by the hydrophone in a previous study (Yoo et al. 2011), the acoustic focus was roughly cigar-shaped (3.5 mm in diameter, 6.2 mm in length for 650 kHz; 6.5 mm in diameter, 11.5 mm in length for 350 kHz) at the full-width-at-half maximum (FWHM) of the acoustic pressure field. The pressure amplitude at the focus was estimated after taking into account ultrasound attenuation through the rodent skull in situ (~87% of the incoming sonication pressure) (Kinoshita et al. 2006).
Acoustic intensities from 0.5 up to 20 W/cm2 spatial-peak pulse-average intensity (Isppa), with incremental steps of 0.5 W/cm2, were used to sonicate the rats (n=3) in the 650 kHz. For the 350 kHz, the same range of acoustic intensities were applied to the separate group of animals (n=3), and Isppa of 8.6 W/cm2, the minimum intensity that resulted in the successful stimulation, was used throughout the rest of the experiment (n=16). The resulting Ispta was calculated as 4.6 W/cm2 considering the duty factor (54%) with the corresponding mechanical index (MI) of 0.9 (peak negative pressure - 0.53 MPa). As the sonication focus lies close to the cranial vault, potential bone heating due to sonication was assessed using bone thermal index (TIB), which was estimated for the rats. We estimated that a fraction (0.132 according to Kinoshita et al. (2006)) of incident acoustic intensity is absorbed at the thin rat skull beneath the acoustic focus, resulting in the corresponding TIB of 0.99 (IsptaAα/Wdeg = 4600 mW/cm2 × 0.33 cm2 × 0.132/26 mW, where A is focal area in cm2, α is absorption fraction, Wdeg is power required to cause 1°C temperature rise (Abbott 1999)).
The sonication was given in one second intervals ten times. The time interval between the sonication resulted in the discrete and accumulative eyeball movement detectable by the video analysis (refer to Quantitative evaluation of eyeball movement section). In the selected sonication setting, the maximal abduction of the eyeball was achieved within ten FUS stimulations. The sonication parameters did not cause any muscle contractions based on our previous experiences on the sonication of neck and leg muscles and recordings of corresponding electromyography (not reported).
Determination of sonication site
The transcranial FUS was unilaterally applied to the abducens nerve (cranial nerve VI) en route to the eye socket. The abducens nerve was targeted due to the simplicity of detection of successful stimulation via abductive eyeball movement (mainly via lateral rectus of the eye muscles), whereas the other cranial nerves, such as the oculomotor and trochlear nerves, may create complex eyeball movements that are difficult to identify (e.g., oculomotor nerves innervate multiple eye muscles, whereas trochlear nerves innervate superior oblique muscles resulting in depression/intortion of the eyes).
The sonication site was determined based on the stereotactic atlas of the rat (Watson 2004) and the cranial nerve trajectories of the rat anatomy (Greene 1968) (Fig. 1B). According to the origins and trajectory information on the cranial nerves on the atlas, the sonication focus was targeted at the trunk of the abducens nerve (~2mm off median; ~7mm posterior to Bregma; ~10mm depth from the skull surface). The root of the oculomotor nerve (from the midbrain) was away from the sonication focus. The sonication trajectory passed through the posterior part of the trochlear nerve (which exited from behind the midbrain and traveled more laterally toward the eye), but the sonication focus was clearly away from the nerve path depositing less than 2% of the incident acoustic pressure. The sonication focus was spatially guided by an optical image-guidance system (Sonomo, SensMed, Framingham, MA), where the virtual location of the acoustic focus and its sonication path can be overlaid on a pre-operative MRI of the rat.
Quantitative evaluation of eyeball movement
In vivo electrophysiological studies on selective rodent eye muscle are difficult due to small anatomical structure. Instead, the abductive motion of the eyes was used as an indirect indication on the consequence of abducens nerve stimulation. The eyeball movement was recorded with a video camera (DCR-SX41, Sony, Japan), and subjected to retrospective frame-by-frame analysis on the recorded video data. Since the sonication was given every second, 11 video frames (including baseline) were sampled at the end of each sonication in one second intervals, and the movement distance of the lateral edge of the iris along the horizontal axis was measured for subsequent quantification.
In-vitro evaluation of temperature change by sonication
Additionally, potential temperature changes at the sonication focus were evaluated using a thermocouple probe (TT-T- 40-SLE-100, Omega, Stamford, CT, and matching thermometer unit - HH2000, Omega, Stamford, CT) placed on the sonication focus within an acrylamide-based gel phantom with similar acoustic properties of the soft tissue. The composition of the gel phantom was distilled water (65% volume), Bovine serum albumin (9% weight/volume), 1 Mol tris (hydroxymethyl) aminomethane (pH 8; 10% volume), acrylamide (prepared in 40% weight/volume; 25% volume), ammonium persulfate (prepared in 10% weight/volume; 0.84 % volume), and Tetramethylethylenediamine (0.05% volume) as described elsewhere (Lafon et al. 2005) (all material purchased from Sigma Aldrich, St Louis, MO). The temperature was also measured at the sonication site using the excised rat cranial structure, with intact tissue and skull around the basis of the cranium.
Histological assessment
The acute effects due to the sonication were examined by sacrificing the animal immediately after the sonication (n=9). Among the animals, the presence of blood-brain barrier (BBB) disruption was examined by systemic circulation of Trypan blue dye injected via tail vein (n=4). Systemic circulation of formalin (10%) right after the sacrifice fixed the brain tissues, and the skull and tissue containing the cranial nerves were extracted. The extracted tissues were immersed in 10% formalin solution for an additional 1–2 weeks before sectioning. The remainder of the animals (n=10) were allowed to survive (1, 7, 21 and 49 days, n=3, 2, 4 and 1 respectively) after the experiment and then sacrificed and fixated. Serial sections perpendicular to the sonication beam were obtained, and underwent histological analysis using hematoxylin and eosin (H&E) stain to examine the presence of hemorrhaging or tissue damage.
Results
Effect of sonication on abductive eye movement
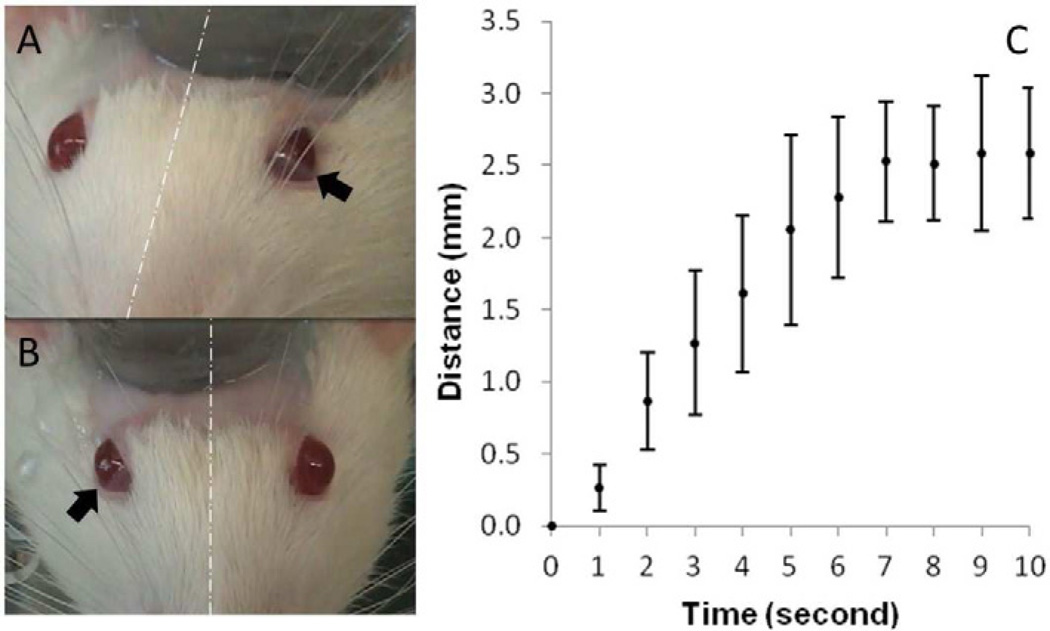
The application of 650 kHz FUS to the same area, across the ranges of acoustic intensities, from 0.5 up to 20 W/cm2 Isppa, did not elicit eye movement in any of the tested animals (n=3). On the other hand, abductive eyeball movement, ipsilateral to the site of sonication was observed during the sonication at 350 kHz, as shown in Figure 2A and 2B (and Supplemental Movie S1). The eyeball movement was discrete, and occurred upon each sonication, which was given at one second intervals. Figure 2C shows the mean values and standard deviations of eyeball movement (n=2 for left eyeball, n=5 for right eyeball), which were quantified by the frame-by-frame video analysis. The movement increased stepwise during the repetitive sonication, and reached its maximum around 7 seconds after the onset of sonication. As soon as the sonication stopped, the eyeball rolled back to its original position in 6.9 ± 0.4 seconds. In vitro temperature assessment study also confirmed that the tested sonication parameters did not cause any temperature changes beyond the variations of instrumental fluctuations (<0.1°C).
Figure 2.
Ipsilateral abductive eye movement induced by sonication: (A) left eye or (B) right eye in movement indicated by the arrows at the same side of sonication. (C) Quantitative estimation of eyeball movement based on video analysis: each marker indicates mean value, each vertical error bar shows standard deviation (n=7). The link to the related video is also submitted.
Post-sonication histological analysis
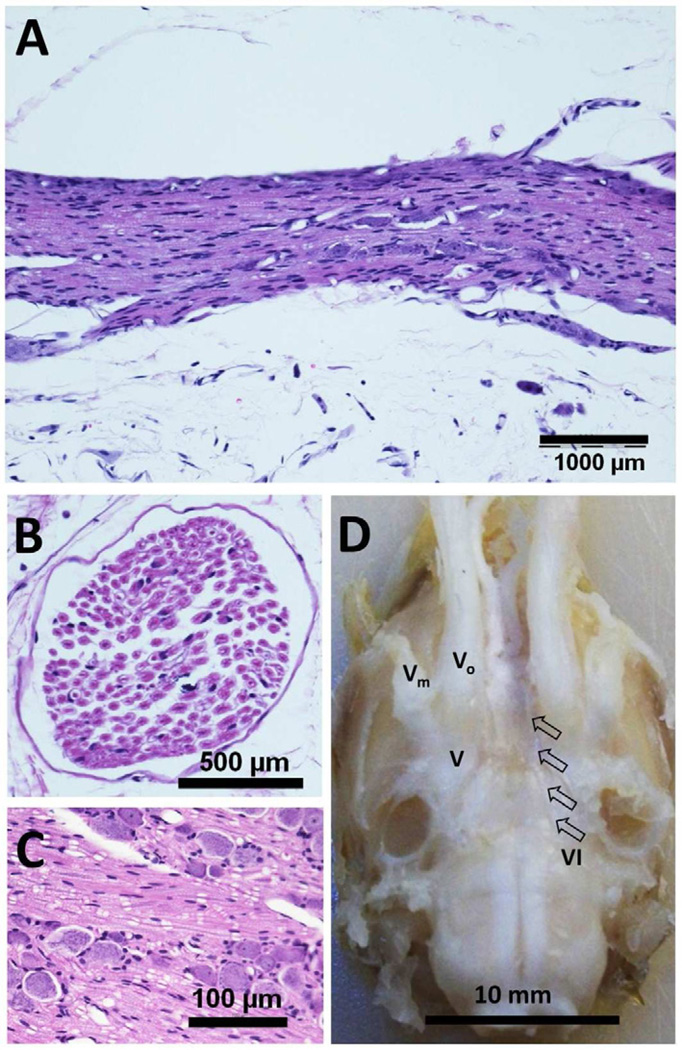
We did not find any evidence of BBB disruption (based on Trypan blue injection) or acute damages to the nerves and surrounding neural tissues after the sonication. The behavior of the rats, which were allowed to survive after the sonication experiment, was monitored once every two days until they were sacrificed. All of them, across the survival period, showed normal behavior including normal eye tracking of food in the visual field of both eyes. Histological analysis did not show the presence of nerve tissue damage associated with the sonication in any of the animals, as an example shown in Figure 3. The brain tissue above the sonication focus did not show any evidence of neural tissue damage or hemorrhage.
Figure 3.
An example of histological analysis of the sonicated rat cranial nerve (H&E Staining): (A) longitudinal, (B) transversal, and (C) magnified longitudinal section, along with (D) an extracted tissue sample (brain and cranial nerves) for histology analysis. Arrow indicates the abducens nerve (noted as ‘VI’, unilateral side) and the trigeminal nerve (noted as ‘V’, its ophthalmic and maxillary branches are denoted as ‘Vo’ and ‘Vm’ respectively) are also shown.
Discussion
The application of low-intensity FUS, transcranially delivered to the abducens nerve, elicited the corresponding abductive ipsilateral eyeball movement in rats. The acoustic intensity used in the present study for successful stimulation (4.6 W/cm2 Ispta) was comparable to the intensity used in a previous study on excitation of the motor area of the rabbit brain (6.3 W/cm2 Ispta) (Yoo et al. 2011), suggesting that acoustic intensity, on the order of 4.6–6.3 W/cm2 Ispta, successfully stimulates both brain tissue as well as the cranial nerves. It is notable that both have similar duty factors (~50%), although the pulsing parameters were significantly different (i.e., 0.36 msec TBD and 1.5 kHz PRF in this study, compared to 50 msec TBD and 10 Hz PRF for the excitation of the brain tissue). This may lead to the conjecture that temporal averaged deposition of the acoustic energy (represented in Ispta), in addition to the specific pulsing parameters, may play an important role in inducing desired neuromodulation. However, it should be noted that the acoustic intensity used in the present study was significantly higher than that of the previous work on mice using a non-focused collimated ultrasound transducer (36.2 mW/cm2 Ispta) (Tufail et al. 2010). This discrepancy may be caused by the differences in the choice of animal species, method of ultrasound delivery (i.e., focused versus collimated beam), method of transducer calibration, and experimental design, which requires further investigation.
We observed that the use of higher fundamental frequency (650 kHz) at the same sonication parameters did not induce the desired eyeball movement, even with the higher acoustic intensity of 20 W/cm2 Isppa. The tendency of superior stimulatory efficiency at lower frequency is in good agreement with the previous study on unfocused ultrasound stimulation of the brain to evoke motor activity (Tufail et al. 2010). We conjecture this differential stimulation sensitivity toward frequencies is linked to the potential mechanism underlying the neuronal activation. Since the delivered acoustic energy level is low and non-thermal, it is reasonable to believe the stimulation might be from the effects of mechanical origin, for example, acoustic streaming and/or radiation force on ion channels of the neurons (Tyler et al. 2008; Bystritsky et al. 2011). Therefore, mechanical index, burst length, pulse repetition frequency, and ultrasound frequency, etc. of the sonication may play the key roles in determining the efficacy of the stimulation. The higher stimulation efficiency at lower frequency should be approached with caution for human translation due to the increased risks of imposing mechanical damages.
The acoustic intensity that elicited the stimulation (4.6 W/cm2 Ispta) in the present study was greater than the upper limit of FDA guidelines on ultrasound imagers (720 mW/cm2 Ispta) (Food 1997). However, the overall acoustic dose of the proposed method, due to its operation in pulse mode, would be considerably lower than that of an ultrasound imager that uses continuous sonication. The mechanical index (MI), one of the important indices to estimate the mechanical bioeffect, of this study (0.9) was much lower than the upper limit of clinical diagnostic imaging applications (1.9) (Food 1997). The peak negative pressure used in this study (0.54 MPa) was also far lower than the intensity that could cause any cavitation-related brain tissue damage (i.e., acoustic pressure of 40 MPa in the absence of air bubbles) (Dalecki 2004). Histological analysis and behavioral monitoring also indicated that pulsed application of the FUS did not damage the neural tissues at the acoustic focus, suggesting initial safety of the proposed method.
However, for potential human translation of the technique, one should pay attention to potential heating of the skull near the cranial nerve (i.e., sonication target), which is particularly problematic during the use of high-intensity FUS (McDannold et al. 2004). Although thin rat skull did not absorb much acoustic energy (thus no temperature elevation), it is notable that the acoustic intensity in the present study (i.e., 350kHz, 4.6 W/cm2 Ispta) may result in TIB (for human application) as high as 68.8 at the focus, which would be a concern if it is given in continuous mode. Although the TIB tends to overestimate the possibility of actual temperature elevation (Abbott 1999), it is still plausible that heat may be generated at the cranial vault near the acoustic focus. Real-time monitoring of tissue temperature, for example through the use of magnetic resonance thermometry (Ishihara et al. 1995), or administration of the FUS in low duty factor for a short period of time (i.e., on the order of few 100 msec) with sufficient intervals, will also help to minimize any risk for skull heating.
The formation of standing waves may occur within the skull during the application of the FUS, affecting the accuracy of sonication. The formation of the standing waves is of a particular concern, especially in high-intensity FUS application, since it can permanently damage the tissue outside of the intended target-of-interest due to aberrant heat deposition. However, the formation of the standing waves is very unlikely in the present study due to the use of short-bursts with a reduced duty factor (~50%) (O'Reilly et al. 2010). Although the presence of standing wave cannot be completely ruled out as a contributing source for the stimulation, a coincidental overlap between the standing waves and the anatomically-specific abducens nerve, is highly unlikely to occur outside of the primary sonication site. For potential human application, the possible presence of standing waves could be corrected by either using sweep frequencies (Mitri et al. 2005; Erpelding et al. 2007) or random phase modulation techniques (Tang and Clement 2009).
As to the physiological mechanism underlying the phenomena, we ruled out thermal contributions by the fact that the use of low-intensity pulsed sonication did not alter the tissue temperature as measured by de novo tissue and by the ultrasound phantom. It is a prevailing theory that mechanical energy driven by the FUS is translated to actuate the ion channels, for example, voltage-gated sodium channels or calcium channels on neural tissue membranes (Tyler et al. 2008). It has also been suggested that FUS could change the action potential in the nerve and subsequent the propagation by altering neuronal transmission. (Gavrilov et al. 1996; Bachtold et al. 1998; Colucci et al. 2009; Bystritsky et al. 2011; Yoo et al. 2011). Further study on in-vitro assessments of nerve function and the activity of ion channels (e.g., using channel blockers), combined with the measurement of trans-membrane potentials (e.g., using voltage-sensitive dyes or direct patch-clamp measurement), would be needed to reveal the detailed mechanism of neural excitation.
The possibility in inducing excitation of the nerves also warrants future extension of FUS-mediated inhibition of sensory nerve conduction, which may have great clinical implications in anesthesia and pain management. Colucci and colleagues presented that FUS induced an inhibitory effect on frog sciatic nerve in vitro (Colucci et al. 2009) at a much higher Ispta (on the order of 600–900W/cm2 at 661 kHz and 1.99 MHz frequency) compared to the present study. Further in vivo studies evaluating the effect of low-intensity FUS on blocking/inhibiting nerve function, for example, the measurement of nerve conduction velocity (NCV) or amplitude of the propagated nerve action potential is warranted. The study on the effects of prolonged administration of FUS for the possible induction of refractory periods in neural communication or for the modification of the nerve conduction velocity is also desired.
One of the limitations in the current study is that we could not completely rule out the stimulation/involvement of additional neural structures exposed to the sonication path, since the size of the FUS focus was relatively larger than the dimension of the abducens nerve. Theoretically, the minimum focus diameter could be half of the wavelength of the FUS waves (i.e., Dmin=λ/2=v/2f=1484 /2*350=2.1mm, where λ is wavelength of sound wave, v is speed of sound in water in m/s, f is fundamental frequency in kHz) at the given frequency (Moonen 2007), and a smaller area-of-interest could be sonicated by adopting different geometric configurations of the transducer, e.g., tighter focus at near-field using high ‘f-number’. The spatial accuracy of sonication can also be improved by the direct visualization of acoustic focus via imaging techniques such as acoustic radiation force imaging (ARFI) (Kaye et al. 2011).
As transcranial application of FUS to the human brain became feasible with development in multi-arrayed FUS hardware for the purpose of tumor ablation and functional neurosurgery (McDannold and Jolesz 2000; Martin et al. 2009), the pulsed operation of a similar system would enable modulation of user-controllable location of the specific cranial nerve either for diagnostic or therapeutic purposes. For example, the specific cranial nerve function and its physical representation can be probed by using low-intensity FUS, and then subsequently intervened to induce long-lasting effects (or even ablation to impose permanent effects). Further study is warranted to investigate the long-term effect of the sonication depending on the acoustic intensity or multiple sonication sessions.
The spatial specificity and the superior penetration depth of FUS enabled selective sonication of the abducens nerve, and elicited stimulation that resulted in corresponding abductive eyeball movement. Although we did not directly measure the elicitation of the action potential from the sonicated nerve, the discrete abductive eye movement that occurred upon the sonication provided strong indirect evidence of the corresponding nerve stimulation. We found that there is differential sensitivity in eliciting the nerve stimulation, favoring the use of lower frequency (350 kHz compared to 650 kHz). To our knowledge, this would be the first demonstration of transcranial excitation of the cranial motor nerve in vivo by using low-intensity pulsation of FUS.
Supplementary Material
Supplemental Movie S1 The video clip showing the ipsilateral eye movement induced by sonication on the abducens nerves (sonication on the left brain hemisphere: 7s–24s and 34s–46s, sonication on the right brain hemisphere: 55s–72s and 80s–91s).
Acknowledgements
This work was supported by grants from the National Institute of Health (R21 NS074124 to Yoo) and the National Research Foundation of Korea (Korean Ministry of Education, Science and Technology, 2010-0027294 to Park). The authors gratefully acknowledge the assistance of Drs. Yong-Zhi Zhang and Byoung-Kyong Min in data acquisition and animal preparation. The authors also thank Alan Chiu for editorial support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: All the authors have no conflict of interest to disclose.
References
- Abbott JG. Rationale and derivation of MI and TI--a review. Ultrasound Med Biol. 1999;25:431–441. doi: 10.1016/s0301-5629(98)00172-0. [DOI] [PubMed] [Google Scholar]
- AIUM Clinical Standards Committee. How to Interpret the Ultrasound Output Display Standard for Higher Acoustic Output Diagnostic Ultrasound Devices: Version 2. J Ultrasound Med. 2004;23:723–726. [PubMed] [Google Scholar]
- Bachtold M, Rinaldi P, Jones J, Reines F, Price L. Focused ultrasound modifications of neural circuit activity in a mammalian brain. Ultrasound in Medicine & Biology. 1998;24:557–565. doi: 10.1016/s0301-5629(98)00014-3. [DOI] [PubMed] [Google Scholar]
- Blom S. Trigeminal neuralgia: its treatment with a new anticonvulsant drug (G-32883) Lancet. 1962;1:839–840. doi: 10.1016/s0140-6736(62)91847-0. [DOI] [PubMed] [Google Scholar]
- Bystritsky A, Korb AS, Douglas PK, Cohen MS, Melega WP, Mulgaonkar AP, DeSalles A, Min BK, Yoo SS. A review of low-intensity focused ultrasound pulsation. Brain Stimul. 2011;4:125–136. doi: 10.1016/j.brs.2011.03.007. [DOI] [PubMed] [Google Scholar]
- Clement GT, White PJ, King RL, McDannold N, Hynynen K. A magnetic resonance imaging-compatible, large-scale array for trans-skull ultrasound surgery and therapy. J Ultrasound Med. 2005;24:1117–1125. doi: 10.7863/jum.2005.24.8.1117. [DOI] [PubMed] [Google Scholar]
- Colucci V, Strichartz G, Jolesz F, Vykhodtseva N, Hynynen K. Focused Ultrasound Effects on Nerve Action Potential in vitro. Ultrasound in Medicine & Biology. 2009;35:1737–1747. doi: 10.1016/j.ultrasmedbio.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coussios CC, Holland CK, Shaw GJ. Transmission of a large unfocused 120-kHz and 1-MHz ultrasound beam through the human skull. The Journal of the Acoustical Society of America. 2002;112:2433. [Google Scholar]
- Dalecki D. Mechanical bioeffects of ultrasound. Annual Review of Biomedical Engineering. 2004 doi: 10.1146/annurev.bioeng.6.040803.140126. [DOI] [PubMed] [Google Scholar]
- DeGiorgio CM, Shewmon DA, Whitehurst T. Trigeminal nerve stimulation for epilepsy. Neurology. 2003;61:421–422. doi: 10.1212/01.wnl.0000073982.42650.57. [DOI] [PubMed] [Google Scholar]
- Donaldson GW, Graham JG. Aplastic anaemia following the administration of tegretol. Br J Clin Pract. 1965;19:699–702. [PubMed] [Google Scholar]
- Dyer NH, Hughes DT, Jenkins GC. Aplastic anaemia after carbamazepine. Br Med J. 1966;1:108. doi: 10.1136/bmj.1.5479.108-d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erpelding TN, Hollman KW, O’Donnell M. Bubble-based acoustic radiation force using chirp insonation to reduce standing wave effects. Ultrasound in Medicine & Biology. 2007;33:263–269. doi: 10.1016/j.ultrasmedbio.2006.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Food U. Drug Administration Information for Manufacturers Seeking Marketing Clearance of Diagnostic Ultrasound Systems and Transducers. Center for Devices and Radiological Health. 1997 [Google Scholar]
- Gavrilov L, Tsirulnikov E, Davies I. Application of focused ultrasound for the stimulation of neural structures. Ultrasound in Medicine & Biology. 1996;22:179–192. doi: 10.1016/0301-5629(96)83782-3. [DOI] [PubMed] [Google Scholar]
- Greene E. Anatomy of the rat. New York: Hafner Pub. Co.; 1968. [Google Scholar]
- Humayun MS, de Juan E, Jr, Weiland JD, Dagnelie G, Katona S, Greenberg R, Suzuki S. Pattern electrical stimulation of the human retina. Vision Res. 1999;39:2569–2576. doi: 10.1016/s0042-6989(99)00052-8. [DOI] [PubMed] [Google Scholar]
- Ishihara Y, Calderon A, Watanabe H, Okamoto K, Suzuki Y, Kuroda K, Suzuki Y. A precise and fast temperature mapping using water proton chemical shift. Magnetic Resonance in Medicine. 1995;34:814–823. doi: 10.1002/mrm.1910340606. [DOI] [PubMed] [Google Scholar]
- Kaye EA, Chen J, Pauly KB. Rapid MR-ARFI method for focal spot localization during focused ultrasound therapy. Magn Reson Med. 2011;65:738–743. doi: 10.1002/mrm.22662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killian JM, Fromm GH. Carbamazepine in the treatment of neuralgia. Use of side effects. Arch Neurol. 1968;19:129–136. doi: 10.1001/archneur.1968.00480020015001. [DOI] [PubMed] [Google Scholar]
- Kinoshita M, McDannold N, Jolesz FA, Hynynen K. Noninvasive localized delivery of Herceptin to the mouse brain by MRI-guided focused ultrasound-induced blood-brain barrier disruption. Proc Natl Acad Sci U S A. 2006;103:11719–11723. doi: 10.1073/pnas.0604318103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi M, Pascual-Leone A. Transcranial magnetic stimulation in neurology. Lancet Neurol. 2003;2:145–156. doi: 10.1016/s1474-4422(03)00321-1. [DOI] [PubMed] [Google Scholar]
- Lafon C, Zderic V, Noble M, Yuen J, Kaczkowski P, Sapozhnikov O, Chavrier F, Crum L, Vaezy S. Gel phantom for use in high-intensity focused ultrasound dosimetry. Ultrasound in Medicine & Biology. 2005;31:1383–1389. doi: 10.1016/j.ultrasmedbio.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Martin E, Jeanmonod D, Morel A, Zadicario E, Werner B. High-intensity focused ultrasound for noninvasive functional neurosurgery. Ann Neurol. 2009;66:858–861. doi: 10.1002/ana.21801. [DOI] [PubMed] [Google Scholar]
- McDannold N, King RL, Hynynen K. MRI monitoring of heating produced by ultrasound absorption in the skull: in vivo study in pigs. Magn Reson Med. 2004;51:1061–1065. doi: 10.1002/mrm.20043. [DOI] [PubMed] [Google Scholar]
- McDannold NJ, Jolesz FA. Magnetic resonance image-guided thermal ablations. Top Magn Reson Imaging. 2000;11:191–202. doi: 10.1097/00002142-200006000-00005. [DOI] [PubMed] [Google Scholar]
- Min B-K, Bystritsky A, Jung K-I, Fischer K, Zhang Y, Maeng L-S, Park SI, Chung Y-A, Jolesz FA, Yoo S-S. Focused ultrasound-mediated suppression of chemically-induced acute epileptic EEG activity. BMC Neuroscience. 2011a;12:23. doi: 10.1186/1471-2202-12-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min B-K, Yang PS, Bohlke M, Park S, RVago D, Maher TJ, Yoo S-S. Focused ultrasound modulates the level of cortical neurotransmitters: Potential as a new functional brain mapping technique. International Journal of Imaging Systems and Technology. 2011b;21:232–240. [Google Scholar]
- Mitri FG, Greenleaf JF, Fatemi M. Chirp imaging vibro-acoustography for removing the ultrasound standing wave artifact. IEEE Trans Med Imaging. 2005;24:1249–1255. doi: 10.1109/TMI.2005.854518. [DOI] [PubMed] [Google Scholar]
- Moonen CT. Spatio-temporal control of gene expression and cancer treatment using magnetic resonance imaging-guided focused ultrasound. Clin Cancer Res. 2007;13:3482–3489. doi: 10.1158/1078-0432.CCR-07-0204. [DOI] [PubMed] [Google Scholar]
- O'Brien WD., Jr Ultrasound-biophysics mechanisms. Prog Biophys Mol Biol. 2007;93:212–255. doi: 10.1016/j.pbiomolbio.2006.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Reilly MA, Huang Y, Hynynen K. The impact of standing wave effects on transcranial focused ultrasound disruption of the blood-brain barrier in a rat model. Phys Med Biol. 2010;55:5251–5267. doi: 10.1088/0031-9155/55/18/001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth BJ, Maccabee PJ, Eberle LP, Amassian VE, Hallett M, Cadwell J, Anselmi GD, Tatarian GT. In vitro evaluation of a 4-leaf coil design for magnetic stimulation of peripheral nerve. Electroencephalography and Clinical Neurophysiology/Evoked Potentials Section. 1994;93:68–74. doi: 10.1016/0168-5597(94)90093-0. [DOI] [PubMed] [Google Scholar]
- Simmons FB, Epley JM, Lummis RC, Guttman N, Frishkopf LS, Harmon LD, Zwicker E. Auditory Nerve: Electrical Stimulation in Man. Science. 1965;148:104–106. doi: 10.1126/science.148.3666.104. [DOI] [PubMed] [Google Scholar]
- Tang SC, Clement GT. Acoustic standing wave suppression using randomized phase-shift-keying excitations. The Journal of the Acoustical Society of America. 2009;126:1667–1670. doi: 10.1121/1.3203935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tufail Y, Matyushov A, Baldwin N, Tauchmann ML, Georges J, Yoshihiro A, Tillery SIH, Tyler WJ. Transcranial Pulsed Ultrasound Stimulates Intact Brain Circuits. Neuron. 2010;66:681–694. doi: 10.1016/j.neuron.2010.05.008. [DOI] [PubMed] [Google Scholar]
- Tyler WJ, Tufail Y, Finsterwald M, Tauchmann ML, Olson EJ, Majestic C. Remote Excitation of Neuronal Circuits Using Low-Intensity, Low-Frequency Ultrasound. PLoS ONE. 2008;3:e3511. doi: 10.1371/journal.pone.0003511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uthman BM, Reichl AM, Dean JC, Eisenschenk S, Gilmore R, Reid S, Roper SN, Wilder BJ. Effectiveness of vagus nerve stimulation in epilepsy patients: a 12-year observation. Neurology. 2004;63:1124–1126. doi: 10.1212/01.wnl.0000138499.87068.c0. [DOI] [PubMed] [Google Scholar]
- Watson GPC. The Rat Brain in Stereotaxic Coordinates. 2004:1–367. [Google Scholar]
- Wessel K, Kompf D. Transcranial magnetic brain stimulation: lack of oculomotor response. Exp Brain Res. 1991;86:216–218. doi: 10.1007/BF00231056. [DOI] [PubMed] [Google Scholar]
- Wilson-Pauwels L. Cranial nerves : function and dysfunction. Shelton, Conn: People's Medical Pub. House; 2010. [Google Scholar]
- Yang PS, Kim H, Lee W, Bohlke M, Park S, Maher TJ, Yoo SS. Transcranial Focused Ultrasound to the Thalamus Is Associated with Reduced Extracellular GABA Levels in Rats. Neuropsychobiology. 2012;65:153–160. doi: 10.1159/000336001. [DOI] [PubMed] [Google Scholar]
- Yoo S-S, Bystritsky A, Lee J-H, Zhang Y, Fischer K, Min B-K, Mcdannold NJ, Pascual-Leone A, Jolesz FA. Focused ultrasound modulates region-specific brain activity. NeuroImage. 2011;56:1267–1275. doi: 10.1016/j.neuroimage.2011.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Movie S1 The video clip showing the ipsilateral eye movement induced by sonication on the abducens nerves (sonication on the left brain hemisphere: 7s–24s and 34s–46s, sonication on the right brain hemisphere: 55s–72s and 80s–91s).