Abstract
As physical barriers that separate teleost fish from the external environment, mucosae are also active immunological sites that protect them against exposure to microbes and stressors. In mammals, the sites where antigens are sampled from mucosal surfaces and where stimulation of naive T and B lymphocytes occurs are known as inductive sites and are constituted by mucosa-associated lymphoid tissue (MALT). According to anatomical location, the MALT in teleost fish is subdivided into gut-associated lymphoid tissue (GALT), skin-associated lymphoid tissue (SALT), and gill-associated lymphoid tissue (GIALT). All MALT contain a variety of leukocytes, including, but not limited to, T cells, B cells, plasma cells, macrophages and granulocytes. Secretory immunoglobulins are produced mainly by plasmablasts and plasma cells, and play key roles in the maintenance of mucosal homeostasis. Until recently, teleost fish B cells were thought to express only two classes of immunoglobulins, IgM and IgD, in which IgM was thought to be the only one responding to pathogens both in systemic and mucosal compartments. However, a third teleost immunoglobulin class, IgT/IgZ, was discovered in 2005, and it has recently been shown to behave as the prevalent immunoglobulin in gut mucosal immune responses. The purpose of this review is to summarise the current knowledge of mucosal immunoglobulins and B cells of fish MALT. Moreover, we attempt to integrate the existing knowledge on both basic and applied research findings on fish mucosal immune responses, with the goal to provide new directions that may facilitate the development of novel vaccination strategies that stimulate not only systemic, but also mucosal immunity.
Keywords: mucosal immunity, MALT, IgT, immunoglobulins, B cells, CMIS, teleost, vaccines
1. Introduction
Higher metazoans have different barriers that separate themselves from the surrounding environment. Whereas some animal species evolved non-mucosal barriers (i.e. the cuticle of arthropods), others, such as teleost fish, developed mucosal surfaces as their strategy to protect themselves from the aggressions of the environment. In addition to being physical barriers, mucosal surfaces are also active immunological sites armed with cellular and humoral defences. Since these surfaces represent the interface between each animal and the external environment, they are exposed more than any other site, to a continuous bombardment of microbes and stressors. The mucosa-associated lymphoid tissue (MALT) contains B cells and immunoglobulins, which play a pivotal role in the maintenance of mucosal homeostasis (reviewed by (Brandtzaeg, 2009)). In higher vertebrates, secretory immunoglobulins (sIg) as well as their importance in innate and adaptive immunity, are fairly well characterised. Despite the fact that sIg in mammals has been classically associated with IgA and to a lesser degree with IgM, there is a growing appreciation that all immunoglobulin classes are in fact relevant at mucosal sites (Baker et al., 2010).
In lower vertebrates, and in particular teleost fish, the presence of immunoglobulins in mucosal secretions was first reported in plaice (Pleuronectes platessa) in the late 1960’s (Fletcher and Grant, 1969). Until recently, there has been a general belief that IgM was the only functional immunoglobulin in teleosts, both in systemic and mucosal compartments. Recent breakthroughs in the field of fish immunoglobulins have added two new players to the scene, IgD (Edholm et al., 2010a) and IgT/IgZ (Danilova et al., 2005; Hansen et al., 2005). Significantly, IgT has been reported to be an immunoglobulin specialized in gut mucosal immunity (Zhang et al., 2010), a novel finding that makes the field of mucosal immunoglobulins and mucosal B cells in fish even more appealing. As commented by Flajnik (Flajnik, 2010) in reference to recent findings on IgT, all GOD’s (Generation Of Diversity) creatures, even fish!, appear to have dedicated mucosal immunoglobulins. To our delight, there is still much to discover about these immunoglobulins and their immune functions. The present review summarises our current knowledge on teleost B cells and immunoglobulins found in mucosal surfaces. It also examines, with an evolutionary and comparative eye, the parallelisms and dissimilarities of sIg in bony fish versus higher vertebrates. Moreover, this review attempts to integrate past and current basic and applied research findings of fish mucosal immune responses as a platform to provide new directions that facilitate the future development of novel vaccination strategies. These strategies should target stimulation not only of systemic, but also of mucosal immunity.
2. Gross anatomy of MALT
Obvious physiological, anatomical and histological differences exist between terrestrial and aquatic vertebrates, which clearly translate into the presence of distinct MALT in fish and mammals. It is accepted that the mucosal immune system is more complex than its systemic counterpart both in terms of effectors and anatomy (Brandtzaeg, 2009; Cerutti and Rescigno, 2008; Fagarasan, 2008; Macpherson et al., 2008) and, as a consequence, its nomenclature also becomes more intricate. The Nomenclature Committee of the Society of Mucosal Immunology proposed a standard nomenclature for both secretory immune-function molecules and mucosa-associated immune-cell compartments. Table 1 includes the nomenclature for mucosa-associated immune-cell compartments accepted in mammals (summarised in (Brandtzaeg et al., 2008)) as well as those thus far used in fish. It is important to note that no standard nomenclature has yet been proposed for fish MALTs and therefore, we recommend adopting the mammalian one in those cases where it is applicable. MALT uniquely present in teleosts has its own terminology but unfortunately there has not been a general agreement on it by the scientific community. The three main mucosal immune compartments found in bony fish are: 1) the gut-associated lymphoid tissue (GALT) with the lamina propria (LP) and intraepithelial (IEL) compartments; 2) the skin-associated lymphoid tissue (SALT); 3) the gill-associated lymphoid tissue (we propose to abbreviate it as GIALT) which includes the gills and the interbranchial immune tissue (ILT).
Table 1.
Nomenclature for vertebrate mucosa-associated immune-cell compartments
| Abbreviation | Taxa | Tissue area |
|---|---|---|
| MALT* | All vertebrates | Mucosa-associated lymphoid tissue (principal inductive sites for mucosal immune responses, subdivided according to anatomical location) |
| GALT* | All vertebrates | Gut-associated lymphoid tissue |
| PP* | Birds, mammals | Peyer’s patch |
| MLN* | Birds, mammals | Mesenteric lymph node |
| LP* | All vertebrates | Lamina propria |
| IEL compartment* | All vertebrates | Surface epithelium where intraepithelial lymphocytes are localised |
| NALT* | Birds, mammals | Nasopharynx (or nose)-associated lymphoid tissue |
| BALT* | Birds, mammals | Bronchus-associated lymphoid tissue |
| SALT | Fish, amphibians | Skin-associated lymphoid tissue |
| GIALT | Fish | Gill-associated lymphoid tissue (includes gill filaments and interbranchial lymphoid tissue) |
Abbreviations proposed in (Brandtzaeg et al., 2008).
2.1. The gut-associated lymphoid tissue (GALT)
Herbivorous, detritivorous, omnivorous and carnivorous fish species differ from each other in terms of the presence or absence of a stomach, the length of the intestine (from 1 to more than 20 times the body length), and the presence and number of pyloric caeca, intestinal loops and valves (Evans, 1998). The GALT is strikingly diverse across vertebrate groups. For instance, chickens have caecal tonsils not present in mammals, and even within mammals, their GALT exhibit a significant structural diversity (Fagarasan, 2008; Finke and Meier, 2006). Generally speaking, the GALT of higher vertebrates consists of both scattered and organised lymphoid tissue. Fish, however, lack an organised GALT, and thus, have no PP or MLNs (Rombout et al., 2010), whereas the presence of PP or MLN in amphibians remains to be demonstrated, although lymphoid accumulations were demonstrated in the lamina propria of the amphibian urodele, Pleurodeles waltlii (Ardavin et al., 1982; Zapata and Amemiya, 2000). In fish, lymphoid cells are present in a scattered manner along the alimentary canal. The LP and IEL compartments are nevertheless identified. An updated review on the teleost fish GALT, including the description of all the immune cell types therein present has been recently compiled (Rombout et al., 2010) and additional details among different cartilaginous and bony fish are reviewed in (Hart et al., 1988; Zapata and Amemiya, 2000). Generally speaking, teleost gut LP harbours a variety of immune cells including, but not limited to macrophages, granulocytes, lymphocytes and plasma cells, whereas the IEL compartment is mainly composed by T cells and few B cells. One exception is the halibut (Hippoglossus hippoglossus), where a great diversity of leukocytes is observed in the epithelium of the second segment of the gut (Grove et al., 2006a).
Immunological differences are recognised along the different segments of the fish gastrointestinal (GI) tract as it is the case in mammals. Most of our knowledge in that area relates to the differential uptake of particles in the anterior gut (also known as foregut or first segment) compared to the posterior gut (hindgut or second segment). More details about particle uptake in the gut can be found in (Rombout et al., 2010). Another example is found in cod (Gadus morhua L.), where clear immunological differences between the second segment of the gut and the rectum exist (Inami et al., 2009). The geographical map of teleost gut immune cell populations is however far from complete. In that regard, very little is known in particular about the distribution of sIg classes and B cell subsets in different portions of the GI tract. It is worth mentioning that the pH conditions along the fish GI tract change drastically. For instance, catfish (Ictalurus punctatus) has a pH between 2 and 4 in the stomach, then becomes alkaline below the pylorus (pH = 7–9). In the foregut the pH is 8.3 and it is near neutral in the hindgut (Pillay and Kutty, 2005). Thus the question arises, how do pH changes affect antigen uptake, antigen-antibody interactions, or other immune processes?
There is still considerable debate regarding which cells are the main antigen collecting cells in the teleost GI tract. Whereas some authors claim that enterocytes uptake certain antigens such as ferritin (Rombout et al., 1985), others suggest the presence of M-like cells in the posterior gut of salmonids (Fuglem et al., 2010). Some but no conclusive evidence has shown that certain fish GALT epithelial cells display morphological similarities with mammalian M cells and sample luminal antigens (Fuglem et al., 2010). In cyprinids like carp (Cyprinus carpio L.) (Rombout et al., 1985) and goldfish (Carassius auratus auratus) (Temkin and McMillan, 1986), large intraepithelial macrophages containing phagocytosed material have been observed and therefore these are thought to be the main antigen presenting cells. However, no macrophages were found in the gut of seabream (Sparus aurata) (Mulero et al., 2008). One study measured the respiratory burst response of GALT leukocyte suspensions from the seabream and found very few phagocytic cells (Salinas et al., 2007). It is obvious that we know very little about how antigens are uptaken and presented in fish GALT and once again we can appreciate that the gut of different teleost species is immunologically speaking as variable as their physiology.
The microbiota associated to the gut of several teleost species has received a fair amount of attention, mainly due to its application as probiotics in the fish farming industry (Nayak, 2010). It is apparent that commensal microorganisms associated with the gut of fish are more transient and variable in their composition than those of terrestrial animals, probably because of the microbiology of the diverse aquatic environments in which fish live. Initial colonisation of the gut by different microbial species determines GALT early development as well as the total B cell repertoire in different animal models (Fagarasan, 2008; Lanning et al., 2005; Rawls et al., 2004). Moreover, the development of epithelial barrier function and gut innate immunity in gnotobiotic zebrafish appears to be determined by bacterial colonisation in the gut (Rawls et al., 2004). Thus, similar to the situation in higher vertebrates, gut commensal microorganisms are thought to modulate immune responses in the fish gut (Nayak, 2010; Perez et al., 2010; Rawls et al., 2004). In the context of the present review, it is essential to mention the interaction between fish sIg in the gut and their commensal bacteria. Interestingly, IgT and, to a lesser extent, IgM from the gut mucus of rainbow trout (Oncorhynchus mykiss) coat a high percentage of the bacteria present in the gut (Zhang et al., 2010). Thus, bony fish sIg are likely to exert immune exclusion on their commensal bacteria, in a similar manner mammalian sIgA and sIgM do (Brandtzaeg, 2009), thereby providing a means for controlling gut homeostasis.
2.2. The skin-associated lymphoid tissue (SALT)
The SALT (also known as skin immune system (SIS) or cutaneous immune system) in fish is a mucosal lymphoid tissue, whereas in terrestrial vertebrates is not strictly so. Teleost skin is histologically very different to that of mammals since in fish the outermost layer of cells is alive and it retains the capacity to divide. In that regard, teleost epidermis is a stratified but non-keratinized epithelium of variable thickness. Among the epithelial cells, abundant secretory cells are present and produce altogether a complex mucus secretion with ample biological functions. Four types of secretory cells can be found in different fish epidermis including malpighian cells, goblet cells, sacciform cells and club cells (for more details on the structure of fish epidermis refer to the review by (Zaccone et al., 2001)). Once again, teleost diversity is reflected in a variety of skin morphologies, cell composition, mucus characteristics and molecules present in the mucosal secretions. One example of this diversity is found in the work by Fast (Fast et al., 2002), where the skin of three salmonid species was shown to be biochemically and histologically different.
Both malpighian and goblet cells have been proposed to be phagocytic in fish skin (Asbakk, 2001; Iger and Abraham, 1990). Moreover, the presence of IgM was detected in the goblet cells of trout skin (Peleteiro and Richards, 1988). In addition to secretory cells, leukocytes such as granulocytes, macrophages and lymphocytes (Davidson et al., 1993a; Herbomel et al., 2001; Iger et al., 1988; Peleteiro and Richards, 1990) have been observed in the skin of different teleost species. Very little information is however available on the function of each cell type during the course of an immune response. The importance of the skin as an immune organ was clearly demonstrated when transcript analysis of common carp revealed 82 orthologues of genes of immune relevance previously described in other organisms (61 of them had never been described before in carp) (Gonzalez et al., 2007).
Finally, fish skin mucus harbours an apparently abundant and diverse microbial community including bacteria and fungi (Austin, 2006; Liu et al., 2008). The modulation of SALT by this microbial community is unknown and further studies are clearly required in that area.
2.3 The gill-associated lymphoid tissue (GIALT)
Teleost generally have four pairs of gill arches supported by cartilage or bone tissue. Gill arches contain gill filaments (or primary lamellae), which are subdivided into gill lamellae (or secondary lamellae). These comprise the main respiratory surfaces of the fish. A comprehensive review on fish gill morphology can be found in (Wilson and Laurent, 2002). Recent morphological studies on nile tilapia (Oreochromis niloticus) have described the branchial filament as a multilayered epithelium, such as in other teleostean species. The filament epithelium, on the other hand, was shown to consist of two distinct regions, a superficial and a deep layer, the latter characterized by a network of undifferentiated cells, wide intercellular spaces where cells from the neuroendocrine and immune systems reside (Monteiro et al., 2010).
Small and large lymphocytes (Grove et al., 2006a; Lin et al., 1998), macrophages (Lin et al., 1998; Mulero et al., 2008), neutrophils (Lin et al., 1998), eosinophilic granulocytes (Barnett et al., 1996; Lin et al., 1998; Mulero et al., 2007) and antibody-secreting cells (ASC) (Davidson et al., 1997; dos Santos et al., 2001a) have been observed in the GIALT of different fish species (below in sections 4.4 and 5.3 we review in more depth GIALT B cells and ASCs). Gill cell suspensions from the dab (Limanda limanda) were exposed to LPS and PHA and the produced mitogenic responses indicated the presence of few B-cells and a preponderance of T-cells (Lin et al., 1999). Secondary lamellae are formed by a very thin epithelium that is supported by pillar cells. This creates a capillary space for erythrocytes to flow. Thus, it is not common to see lymphoid cells in this area (Monteiro et al., 2010). Many of the anatomical characteristics of interlamellar vessels are strikingly similar to those of mammalian lymphatic capillaries and they have been suggested to be physiologically, if not embryologically, equivalent (Olson, 2002). Interestingly, it has recently been reported that the basal chordate Amphyoxus also possess a gill-associated lymphoid tissue (Han et al., 2010).
In addition to the lymphoid tissue found within the gill lamellae, an interbranchial lymphoid tissue (ILT) has been recently described in salmonids (Haugarvoll et al., 2008; Koppang et al., 2010). The organization of this lymphoid tissue resembles that of the thymus: it is covered by an epithelial layer and traversed by trabecular walls. These studies also showed the predominant presence of T cells in salmon ILT. Therefore, at least salmonid GIALT consists both of dispersed leukocytes within the lamellar epithelium and organised lymphoid areas between gill arches.
Mucus production is proven to be higher in the area surrounding the gill cover than in any other skin sites (Shephard, 1994). Additionally, fish gills have an associated microbial community (Ringo and Holzapfel, 2000) which, in the case of the gibel carp (Carassius auratus gibelio) and bluntnose black bream (Megalobrama amblycephala), is less diverse than that of the skin (Wang et al., 2010).
3. Presence and transport of immunoglobulins in mucosal sites
3.1. Teleost serum immunoglobulins
Immunoglobulins are mainly produced by plasmablasts and plasma cells, and are found secreted into body fluids (including serum and mucosal secretions) as antibodies (soluble form), or on the surface of B cells as B cell receptors (BCR) (membrane-bound form). Ig molecules are typically composed of two identical heavy (H) chains and two identical light (L) chains which provide two identical antigen-binding sites by the amino-terminal variable (V) domains of both H and L chains. The H chain carboxyl-terminal constant (C) domains define the Ig isotypes (classes) and the effector functions of the Ig through binding to their receptors on effector cells. The H and L chains are encoded by separate genomic loci, igh and igl respectively, and their V and C domains are each encoded by independent elements: the variable (V), diversity (D, only for H chains), and joining (J) gene segments for the V domain, and individual constant (C) gene segments for the C domains.
Up to date, three major Ig isotypes have been reported in teleost fish, among which IgM was the first one discovered decades ago, while IgD and IgT/IgZ were discovered later in 1997 (Wilson et al., 1997) and 2005 (Danilova et al., 2005; Hansen et al., 2005), respectively. Generally, in an igh locus of teleost, for example in rainbow trout, the gene segments (VHDτJτCτ) encoding for the H chain (τ) of IgT are located upstream of those (VHDμJμCμCδ) encoding for the H chains (μ and δ) of IgM and IgD (Hansen et al., 2005). In such a locus, the upstream V segments were predicted to rearrange either to DτJτCτ to encode τ chain or to DμJμCμ to encode μ chain, and consequently, B cells of this species were predicted to express either IgT or IgM (Flajnik, 2005). Confirming the aforementioned prediction, in 2010 it was reported that rainbow trout contained a new B lineage uniquely expressing surface IgT, whereas IgM+ B cells were found devoid of IgT expression (Zhang et al., 2010). For further information on the genomic organization of teleost igh and igl loci, see recent reviews (Edholm et al., 2011; Hikima et al., 2010; Solem and Stenvik, 2006; Sun et al., 2011; Zhang et al., 2011).
In general, the prevalent serum Ig in most teleost is a high molecular weight (HMW) Ig (600–850 kilodaltons (kDa)), corresponding to tetrameric IgM, which is stable under physiological conditions, but under denaturing conditions exists as various redox forms that differ in the degree of inter-heavy chain disulfide polymerization (Bromage et al., 2004b; Kaattari et al., 1998). Recently, an association between the increased disulfide polymerization and the greater affinity of trout IgM to antigen has been reported (Ye et al., 2010). The concentration of teleost serum IgM may vary (0.6–16 mg ml−1) between species and may change depending on fish size, environment temperature, water quality, season of the year, as well as stress, stimulation, or immunisation (reviewed in (Solem and Stenvik, 2006)). Besides the HMW Ig, a low molecular weight (LMW) Ig was also described in the serum of giant grouper (Epinephelus itaira) (Clem, 1971), margate (Haemulon album) (Clem and McLean, 1975), sheepshead (Archosargus probatocephalus) (Lobb and Clem, 1981d), rainbow trout (Elcombe et al., 1985), European perch (Perca fluviatilis L.) (Whittington, 1993), flounder (Paralichthys olivaceus) (Bang et al., 1996), and Southern bluefin tuna (Thunnus maccoyii Castelnau) (Watts et al., 2001). Some of the LMW Ig (160–180 kDa) contains H chain with slightly smaller size (50–75 kDa) than those (60–80 kDa) of the HMW Ig (Clem and McLean, 1975; Elcombe et al., 1985; Watts et al., 2001), while some of the serum LMW Ig (120–140 kDa) has a significantly shorter H chain (40–45 kDa) (Clem, 1971; Lobb and Clem, 1981d). As shown in Table 2, in the case of the sheepshead (Lobb and Clem, 1981a; Lobb and Clem, 1981d), the serum HMW Ig (~700 kDa) contains two subpopulations, one is a disulfide linked (covalent) form, and the other one is assembled with two non-covalent subunits of disulfide linked dimmers (~350 kDa), while the LMW Ig (~140 kDa) is composed of two subunits of halfmers (H1L1) (~70 kDa) associated to one another non-covalently. Notably, the H chain (~45 kDa) of the LMW Ig could not be recognised by the polyclonal antibody (pAb) developed against the HMW Ig. In early studies, the half-lives of both HMW and LMW Ig of teleost serum were found to be around 12–16 days (Avtalion et al., 1973; Lobb and Clem, 1981a). In a recent study, the high-affinity, highly polymerized trout IgM was shown to have longer half-lives than the lower-affinity, lightly polymerized IgM (Ye et al., 2010).
Table 2.
Main features of immunoglobulins characterized from representative teleost fish
| Body fluid | Species | References | Ig isotype | Native form (based on GF) |
GF/SDS-PAGE size (kDa) |
H/L chain size (kDa) |
Concentration (mg ml−1) |
|---|---|---|---|---|---|---|---|
| Serum | Rainbow trout (O. mykiss) |
(Elcombe et al., 1985),(Zhang et al., 2010) | HMW/IgM LMW/IgT |
Tetramer Monomer |
~700/700 ~180/180 |
~75/25 ~70/25 |
2.52 0.004 |
| Common carp (C. carpio) |
(Rombout et al., 1986),(Rombout et al., 1993a) | HMW | Tetramer | ~/760,380,190 | ~70/25 | 1.73 | |
| Sheepshead (A. probatocephalus) |
(Lobb and Clem, 1981d),(Lobb and Clem, 1981a) | HMW LMW |
Tetramer Monomer |
~700/700,350 ~140/70 |
~70/25 ~45/25 |
2.90 0.17 |
|
| Gut mucus or Bile |
Rainbow trout (O. mykiss) |
(Zhang et al., 2010) | IgM IgT |
Tetramer Tetramer |
~700/700 ~700/180 |
~75/25 ~70/25 |
0.075 0.007 |
| Sheepshead (A. probatocephalus) |
(Lobb and Clem, 1981b),(Lobb and Clem, 1981a) | Bile Ig | Dimer | ~320/160 | ~55/25 | 0.09 | |
| Skin mucus | Rainbow trout (O. mykiss) |
(Bromage et al., 2006) | IgM | Tetramer | ~800/800,600,400, 200,100 |
~72/25 | N/A |
| Common carp (C. carpio) |
(Rombout et al., 1986),(Rombout et al., 1993a) | HMW | Tetramer | ~/760,380,190 | ~70/25 | 0.008 | |
| Sheepshead (A. probatocephalus) |
(Lobb and Clem, 1981c),(Lobb and Clem, 1981a) | HMW Dimeric Ig |
Tetramer Dimer |
~700/700 ~400/400, 350/175 |
~70/25 ~70/25 |
0.09 0.024 |
Abbreviations: HMW = high molecular weight Ig, LMW = low molecular weight Ig, C = covalent, NC = noncovalent, GF = gel filtration, Ab = antibody, mAb = monoclonal antibody, pAb = polyclonal antibody.
Up until recently, it has been widely accepted that the HMW Ig in the serum of teleost is tetrameric IgM. However, the molecular nature of the LMW Ig has been a mystery since its identification 40 years ago. One could suggest, as other authors did in previous studies (Clem, 1971; Clem and McLean, 1975), that the LMW Ig is the in vivo precursor/redox form or the in vitro degradation product of the HMW Ig. Alternatively, the monomeric LMW Ig may represent an unknown Ig isotype different from IgM, based on the fact that the H chain of LMW Ig was, in some cases, structurally and/or antigenically distinct from that of HMW Ig. For example, although the HMW and LMW Ig forms of rainbow trout had similar binding affinities for a hapten, significant differences were revealed in the size, peptide maps, immunoreactivities of their H chains with the pAb against their corresponding H chains. More importantly, the abilities to activate complement between the HMW and LMW Ig forms differed markedly (Elcombe et al., 1985). Furthermore, in sheepshead the HMW and LMW Igs appeared not to be metabolic products of one another, which suggested that they are the products of separate genes (Lobb and Clem, 1981a). However, it was difficult at that time to clarify the relationship between the HMW and LMW Igs and to identify the exact Ig isotype of the LMW Ig due to limited genetic information available for Ig isotypes in teleost. One of the two Ig isotypes (IgD and IgT/IgZ) discovered later in teleost might solve the mystery on the identification of the LMW Ig. Since 1997, the H chain gene (ighδ) of the second Ig isotype (IgD) has been identified in all studied teleost, which has shown variable genomic structure and splicing pattern among species (Hordvik, 2002; Saha et al., 2004; Srisapoome et al., 2004; Wilson et al., 1997). Surprisingly, the gene encoding a secretory δ molecule was only found in the genome of channel catfish (Bengten et al., 2002), and interestingly, in catfish serum the secreted δ molecule (~105, 150 or 180 kDa) lacked the V region and the Cμ1 domain, instead it contained only the Fc portion (Cδ1δ2δ3δ4δ5δ6δ7δsec or Cδ1(δ2δ3δ4)2δ5δ6δ7δsec) (Bengten et al., 2002; Edholm et al., 2010a). Furthermore, there was no evidence that the catfish secretory δ molecules associated with L chains form a typical Ig containing equimolar H and L chains (see the review by Bengten et al., in this issue). Thus it would seem unlikely that the LMW Ig is a secretory IgD, however, since the gene organization of IgD in teleosts is very variable, the possibility that the LMW Ig corresponds to an IgD in some species cannot be completely overruled. Excitingly, the gene (ighτ/ighζ) encoding the H chain of a third teleost Ig isotype (IgT/IgZ) was discovered in the genomes of rainbow trout (Hansen et al., 2005), zebrafish (Danio rerio) (Danilova et al., 2005), fugu (Takifugu rubripes) (Savan et al., 2005b), and carp (Savan et al., 2005a) in 2005. After that, similar genes have been identified in almost all studied species belonging to the main orders of teleost fish (reviewed in (Zhang et al., 2011)) except catfish (Bengten et al., 2006); the completion of the catfish genome may finally reveal whether or not IgT is present in this species. So far, the physicochemical features and functional roles of IgT/IgZ have not been widely investigated except for the case of trout IgT (Zhang et al., 2010). Trout serum IgT is a monomer with a molecular mass similar to those of the above mentioned LMW Ig described in the same species (Elcombe et al., 1985) as well as in other teleosts (Clem and McLean, 1975; Watts et al., 2001). Thus, if the serum LMW Ig is actually a monomeric IgT/IgZ, its H chains (70–75 kDa) in margate (Clem and McLean, 1975) and tuna (Watts et al., 2001) may be predicted to have four C domains like that of rainbow trout (Hansen et al., 2005) and most other teleost, while the H chains (~45 kDa) of putative serum IgT/IgZ in giant grouper (Clem, 1971) and sheepshead (Lobb and Clem, 1981d) would presumably contain two C domains like that of fugu IgT (Savan et al., 2005b) or carp chimeric IgM-IgT (Savan et al., 2005a). It is clear that to demonstrate the identity of the LMW Ig in teleost, more detailed molecular and biochemical analyses are required. For example, the genomic information of each igh locus needs to be completed at least in the most important model species; the mono-specific Abs against each Ig isotype (IgM, IgT/IgZ, and IgD) should be developed; and the physicochemical features and functional roles of each Ig isotype need to be investigated globally. Moreover, we anticipate that new conclusive data could be obtained by re-analysing previous LMW Ig studies.
Besides H chain heterogeneities in teleost serum Ig, several L chain isotypes/variants have also been identified. At the protein level, IgL masses are available from Southern bluefin tuna (~28, 29 kDa) (Watts et al., 2001), rainbow trout (~24, 26 kDa) (Sanchez and Dominguez, 1991), Atlantic salmon (Salmo salar) (~25, 27 kDa) (Havarstein et al., 1988), European perch (~27–30 kDa) (Whittington, 1993), and channel catfish (F, ~22/24 kDa; G, ~26 kDa; σ, ~27 kDa; λ, unknown size) (Edholm et al., 2010a; Edholm et al., 2009; Lobb et al., 1984). Such differences were identified based both on molecular size and structural/antigenic analyses. Interestingly, up to four L chain variants (~27–30 kDa) were found in perch HMW Ig preparations, while only the lightest two L chains (~27, 28 kDa) were found in the LMW Ig population (Whittington, 1993). This bias raises the questions of whether certain L chain isotypes preferentially associate to a particular H chain isotype (for example, τ, μ, or δ in trout) and whether that happens in specific tissues or at different developmental stages in response to different types of pathogens. In teleosts, Igκ genes (F and G in catfish) are the most abundantly expressed in PBLs, with only 2% of IgM+ cells expressing Ig λ and Ig σ (Edholm et al., 2009). However, with the exception of the catfish (Edholm et al., 2010a; Edholm et al., 2010b; Edholm et al., 2009; Lobb et al., 1984) very little is known with regards to which particular L chain isotypes correspond the protein bands detected by SDS-PAGE, and thus, specific antibodies against these light chains are required to solve this issue. For further details on the molecular and genomic characterization of teleost L chains please refer to (Hikima et al., 2010; Pilstrom, 2002) (see also the review by Edholm et al. in this issue (Edholm et al., 2011)).
3.2. Immunoglobulins in intestine and bile
Until now Ig present in the gut mucus of telesots has been poorly studied, in part due to methodological challenges imposed by the large amounts of proteolytic enzymes in the collected gut mucus, as reported in Atlantic salmon (Hatten et al., 2001). Nonetheless, differences between gut mucus/bile and serum Ig have long been acknowledged. In the bile of sheepshead, a dimeric Ig (~320 kDa) was described with H chains of 55 kDa, which appeared to be different from either the serum tetrameric Ig or the skin mucus dimeric Ig, based on differences in its antigenicity and H chain size (Lobb and Clem, 1981b). In rainbow trout, when analysed by gel filtration, the IgM is tetrameric in both serum and gut mucus, while the IgT in serum and gut mucus is chiefly monomeric and polymeric respectively. Interestingly, by SDS-PAGE under nonreducing conditions, the polymeric gut mucus IgT migrates as a monomer, thus indicating that the monomeric subunits of gut polymeric IgT are associated by non-covalent interactions (Zhang et al., 2010). This situation in gut polymeric IgT differs from that of IgM in which its monomers are for the most part associated by covalent (disulfide) bonds (Kaattari et al., 1998). Moreover, while the concentration of IgM in serum (2.5 mg ml−1) was found to be much higher than that in gut mucus (0.075 mg ml−1), the concentration of IgT in gut mucus (0.007 mg ml−1) was double to that of serum. This suggested that teleost IgT could have an important role in gut mucosal immunity as demonstrated in the same study (Zhang et al., 2010). Interestingly, in sheepshead, the concentration (0.09 mg ml−1) of total bile Ig (HMW plus LMW Ig) detected with a polyclonal antibody (Lobb and Clem, 1981a) was comparable with that (0.082 mg ml−1) of trout gut mucus Ig (IgM plus IgT) (Zhang et al., 2010). Further details on the biochemical structure of gut mucus IgT and IgM are provided in (Rombout et al., 2010; Zhang et al., 2010; Zhang et al., 2011).
3.3. Immunoglobulins in skin and gill
Compared with the high concentration of serum Ig in teleost, Ig is present at a very low concentration (8–90 μg ml−1) in the skin mucus (Hatten et al., 2001; Lobb and Clem, 1981a; Rombout et al., 1986). In terms of spatial distribution, Ig levels in channel catfish were found to be highest on lateral skin, between the gill cover to the dorsal fin, lower between pectoral to anal fins, and lowest on the tail and ventral skin (Zilberg and Klesius, 1997). In the skin mucus of sheepshead, two Igs of different molecular weights were observed (Lobb and Clem, 1981c), one was tetrameric (~700 kDa), similar to the serum HMW Ig, and the other one was dimeric. A portion (~350 kDa) of the dimeric Ig consisted of monomeric units associated by non-covalent bonds, while the other portion (400–500 kDa) was covalently linked and associated with a protein (~95 kDa). This protein was presumed to be a secretory component (SC) of the polymeric Ig receptor (pIgR), but its size is in disagreement with that of the SC recently identified in fugu (~60 kDa) (Hamuro et al., 2007) and trout (~35 kDa) (Zhang et al., 2010). The ratio of LMW to HMW Ig was 3 times higher in the skin mucus than serum in sheepshead (Lobb and Clem, 1981a). In olive flounder (Paralichthys olivaceus), a monomeric Ig was detected in the cutaneous mucus with a monoclonal antibody (mAb) against a L chain of serum Ig purified with mannan-binding protein (MBP) affinity column (Palaksha et al., 2008; Shin et al., 2007). In carp, the Ig from cutaneous mucus had different protein/carbohydrate composition and antigenicity from that of serum IgM, and the electophoretic analysis revealed that the majority of both Igs were tetrameric (Rombout et al., 1993b). One could speculate that this tetrameric cutaneous Ig in carp may correspond to one of the two recently identified carp IgZ (IgZ1 and IgZ2 (Ryo et al., 2010; Savan et al., 2005a). In rainbow trout skin mucus, besides the homogeneous redox forms of tetrameric IgM (~800 kDa) found in serum, a unique redox form consisting of halfmeric constituents (H1L1, ~100 kDa) has been reported (Bromage et al., 2006). Studies in catfish, on the other hand, did not reveal apparent differences between skin mucus and serum Igs, that is, denaturation of the tetrameric IgM produced eight redox forms, the smallest being a halfmer and the largest a fully linked tetramer (Lobb, 1987).
Thus far biochemical analyses on gill mucus immunoglobulins are lacking, although specific IgM responses have been described in the gill mucus from a very small number of teleost species ((Lumsden et al., 1993; Lumsden et al., 1995) and see section 5.3 of this review). Thus, a detailed biochemical characterization of gill immunoglobulins is required to have a better understanding of mucosal immune responses in these areas. In that regard, it will be interesting to ascertain whether IgT/IgZ is the prevalent immunoglobulin in gill and skin mucus, as it has been found to be the case in the gut mucus of rainbow trout (Zhang et al., 2010).
3.4. Synthesis and transport of immunoglobulins in mucosal sites
Once structural differences between mucosal and systemic Igs in teleost were found, the question “where do mucosal antibodies come from?” arose. Early studies in sheepshead addressed the metabolic relationships of the Igs found in the serum, skin mucus, and bile by monitoring intravenously injected radiolabeled serum Ig. The authors concluded that the Igs in skin mucus and bile were not derived from the HMW or LMW Ig in serum through simple transudation or active transport, and therefore the Ig in mucosal secretions must have been the result of local synthesis (Lobb and Clem, 1981a). In other teleost species, Ig found in liver extracts suggested that sIg could be transported across the hepatocytes, to be secreted into the bile (Abelli et al., 2005; Jenkins et al., 1994; Rombout et al., 1986). In that regard, it is well known that mammalian sIg can reach the luminal area of the gut through the bile, which contains sIg taken up from plasma by liver hepatocytes that use pIgR for the transcytosis process (Brown and Kloppel, 1989). In carp, skin mucus IgM specific mAbs were reactive with bile capillaries and ducts but not with the intestinal epithelium, thus suggesting that hepatobiliary transport might be the main route used by IgM found in the intestine of this species (Rombout et al., 1993b). In the same species, the above mAbs were immunoreactive with the skin epithelium and the H chain of mucus Ig but not or hardly with the H chain of serum Ig, indicating differences in the composition of the H chains of both molecules and therefore the IgM in skin mucus must have been produced locally (Rombout et al., 1993b). However, in naïve rainbow trout, a number of both IgM+ and IgT+ B cells could be observed in the LP and epithelium of the gut, an observation that suggested a role of some of these cells in producing Ig locally (Zhang et al., 2010). Local production of immunoglobulins in the GALT probably occurs in other species as a number of studies have found B cells in the GALT of fish (see below in section 4.2),
In mammals, sIgs (secretory pentameric IgM or dimeric IgA) are synthesized and secreted by the plasma cells localized at either the MALT or the liver, and then transcytosed through the epithelial layer into the gut lumen or other mucosal sites by the pIgR expressed on the surface of epithelial cells. After transport of the sIg-pIgR complex into the apical pole of the cells, the complex is cleaved off and released into the luminal area. At that point, the sIg remains covalently bound to a portion of the pIgR designated as the secretory component (SC) (reviewed in (Brandtzaeg, 2009; Brandtzaeg et al., 2008; Rojas and Apodaca, 2002)). The pIgR seems to play a pivotal role in the secretory system of fish. Thus far, pIgR orthologues have been identified in fugu (Hamuro et al., 2007), common carp (Rombout et al., 2008), orange-spotted grouper (Epinephelus coioides) (Feng et al., 2009), and rainbow trout (Zhang et al., 2010). Functional studies have shown that teleost pIgR binds to IgM (Feng et al., 2009; Hamuro et al., 2007; Zhang et al., 2010), as well as to IgT (Zhang et al., 2010). In rainbow trout, gut mucus IgM and IgT were shown to be associated to the SC of the pIgR, whereas serum Ig was devoid of this association, thus implying a role of trout pIgR for the transport of sIg into the gut luminal area (Zhang et al., 2010). These data resembled that of the report on fugu pIgR, in which skin mucus IgM was found associated to a fugu pIgR fragment (Hamuro et al., 2007). The identification of pIgR orthologues in common carp (Rombout et al., 2008) and orange-spotted grouper (Epinephelus coioides) (Feng et al., 2009) further indicated that the mucosal Igs of teleost, like mammalian polymeric Igs, need to be transported by a pIgR, although the teleost pIgR only consists of two Ig-like domains, which correspond to the first and fifth domains of mammalian pIgR, respectively (for more extensive reviews on fish pIgR see (Rombout et al., 2010; Zhang et al., 2011)).
4. B cells in mucosal sites
To define teleost B cells and to investigate their lymphogenesis, specific mAbs against fish IgM have been developed over last few decades. Thus, anti-IgM mAbs have been developed in many teleost species (e.g., channel catfish (Lobb and Clem, 1982), rainbow trout (DeLuca et al., 1983), carp (Koumans-van Diepen et al., 1995; Secombes et al., 1983), and sea bass (Dicentrarchus labrax) (Scapigliati et al., 1996)). However, a full characterization of the additional teleost B cell subsets has been missing until recently. The production of mAbs against rainbow trout IgT (Zhang et al., 2010) and channel catfish IgD (Edholm et al., 2010a) has unraveled new knowledge on B cell subsets from different lymphoid organs. Using these new tools, three B cell subsets were identified in catfish: IgM+/IgD−, IgM+/IgD+, IgM−/IgD+ (Edholm et al., 2010a), while two B cell subsets were identified in rainbow trout: IgM+/IgD+/IgT− and IgM−/IgD−/IgT+ (Zhang et al., 2010). Thus, a total of four B cell subsets have been described thus far in teleosts, three subsets solely express surface IgM, IgD or IgT, and a subset coexpresses surface IgM and IgD. However, less is known in terms of the distribution of these different subsets in systemic and mucosal sites (see sections 4.2–4.4).
In teleost fish, the head kidney (HK) or pronephros (the bone marrow equivalent) is considered the primary lymphoid tissue, a key hematopoetic organ, and an important source of B cells, while the thymus is the primary lymphoid tissue for T cells (Hansen and Zapata, 1998; Rombout et al., 2005; Zapata et al., 2006). The spleen contains a large number of B cells in adult teleost and, like the HK, it also serves as a secondary lymphoid tissue. From trout studies, it appears that the spleen is a site for B cell activation and plasmablast formation and differentiation into plasma cells. Plasma cells migrate to the HK and therefore the spleen harbours far fewer Ig-secreting cells than the HK (Bromage et al., 2004a) (reviewed in this issue by Ye et al. (Ye et al.)). In addition, the spleen is involved in trapping antigens from the bloodstream, and is also an organ in which activation and differentiation of B cells occur (see reviews by (Solem and Stenvik, 2006) and in this issue by Ye et al. (Ye et al.)). Besides the above mentioned major sources of B lymphocytes, the MALT of teleosts also contain B cells (see sections 4.2–4.4), although very little is known with regards to their origin, activation and differentiation into plasmablasts and plasma cells. Thus, further studies will have to address whether B cells found in the fish MALT originate locally or migrate there from other lymphoid tissues, or both. Moreover, the presence of memory cells and long-lived plasma cells from mucosal tissues has never been investigated in fish thus far, in part due to the lack of suitable reagents. It is worthwhile mentioning that B cells from teleost fish have been reported to be highly phagocytic and to display microbicidal properties (Li et al., 2006; Overland et al., 2009; Zhang et al., 2010). Thus far, such phagocytic and microbicidal capacities have only been reported for B cells of systemic lymphoid organs; whether B cells from mucosal sites have similar activities, remains to be investigated.
4.1. Ontogeny of teleost fish B cells
Ontogenic studies in teleost fish are of key importance for determining the onset of immunocompetence and the time for effective vaccination (Zapata et al., 2006). To date, ontogenic studies on teleost B cells and plasma cells have mostly investigated the presence of IgM-bearing and IgM-secreting cells, but very little is known about IgD- or IgT/IgZ-bearing or secreting cells with regards to their ontogeny or their relative tissue distribution and population dynamics in bony fish. The appearance and differentiation of B cell varies considerably in different teleost species due to important differences in egg size and developmental status at hatch (Solem and Stenvik, 2006). Additionally, the methods (immunohistochemistry, flow cytometry, ELISPOT, in situ hybridization or RT-PCR) used to detect the B cells are not always comparable (Magnadottir et al., 2005).
Some general features can be highlighted regarding the ontogeny of B cells: i) Ig-producing cells appear first in HK, followed by the spleen, and finally in the MALT; ii) surface Ig expression occurs earlier than cytoplasmic Ig (cIg) in Ig-producing cells (this however maybe due to differences of interpretation depending on the detection methods used to identify surface or cytoplasmatic Ig); and iii) Ig-producing cells appear earlier in freshwater species than marine species (reviewed in (Rombout et al., 2010; Rombout et al., 2005; Solem and Stenvik, 2006; Zapata et al., 2006)). This may be explained by the very distinct ecological strategies adopted by freshwater versus marine fish. We speculate that the greater size of the eggs, and earlier the developmental stage of freshwater fish larvae at the time of hatch may point out to an early differentiation of tissues and organs when compared to marine fish. This hypothesis however needs to be experimentally addressed.
In carp, for example, surface IgM+ cells were first detected in HK and spleen at 2 weeks post-fertilization (wpf), whereas the first cytoplasmic IgM+ plasma cells were detected from 4 wpf, then the B cells departed from kidney and spleen at 5 wpf to populate peripheral organs like the gut, indicating that mucosal immunity does not develop before this stage in carp (Koumans-van Diepen et al., 1994; Romano et al., 1997b; Rombout et al., 2005). In a similar study, carp WCI12+ B cells were found in the intestine and gills at 6–7 wpf and putative IEL T cells occurred much earlier. (Huttenhuis et al., 2006). Plasma cells seem to follow a similar developmental pattern as that of IgM+ B cells since they were first found in the HK of wolfish followed by the spleen and later in the gut. At the end of the study (21 weeks post hatch) neither the gills nor the skin contained any IgM plasma cells (Grontvedt and Espelid, 2003). It is possible that antigenic stimulation triggers plasma cell development at mucosal sites since 0.1 g seabass fry showed IgM production in the gills following immersion with Photobacterium damselae bacterin (dos Santos et al., 2001a).
In zebrafish, the expression order of IgM during the ontogeny of immune system was detected as follows: transcript of surface Ig (7 days post-fertilization, 7 dpf), transcript of Ig H chain in pancreas (10 dpf), transcript of secretory Ig (13 dpf), transcript of Ig H chain in HK (19 dpf), and detectable humoral Ig (28 dpf), which was consistent with the time when carp plasma cells could be detected (4 wpf) (Danilova et al., 2005; Danilova and Steiner, 2002; Lam et al., 2004). During the ontogenesis of mandarin fish (Siniperca chuatsi), transcripts of IgM were initially detected by in situ hybridization in HK (20 dph), then in spleen (26 dph) and thymus (39 dph), and much later in intestine (87 dph) and gill (90 dph) (Tian et al., 2009b).
In carp, a recent study measured the expression of IgM, IgZ1 and IgZ2 transcripts. Whole embryos showed constitutive expression of all three isotypes at 4 dpf, with IgM being the predominant form (Ryo et al., 2010). Regarding MALT, IgM was first detected in the gut after 4 weeks, and IgZ1 and IgZ2 after 16 weeks. In the same study, gut from juvenile carp (16 and 33 wpf) showed higher levels of IgZ2 expression than IgM and IgZ1. Further information on the ontogeny of teleost intestinal B cells can be found in the recent review (Rombout et al., 2010).
4.2. B cells in intestine
Although teleosts have a more dispersed GALT, which is morphologically and functionally different from that of mammals (e.g., teleost lack PPs and MLNs), most immune cells necessary for a local immune response are abundantly present in the LP and the intestinal epithelium of the studied species (Rombout et al., 2010). The proportion of B cells in isolated gut cell suspensions has generally been reported to be low and variable among the studied teleost species. For instance, within the GALT of sea bass (dos Santos et al., 2000; dos santos et al., 1997; Romano et al., 1997a), carp (Rombout et al., 1998), and rainbow trout (Zhang et al., 2010) about 2–12% of the isolated leukocytes were surface IgM-positive as detected by flow cytometry. In carp (immunohistochemistry) (Rombout et al., 1993a), sea bass (immunohistochemistry) (Abelli et al., 1997), Atlantic cod (in situ hybridization) (Schrøder et al., 1998), turbot (Scophthalmus maximus) (immunohistochemistry) (Fournier-Betz et al., 2000), zebrafish (in situ hybridization) (Danilova and Steiner, 2002), spotted wolfish (Anarhichas minor Olafsen) (in situ hybridization) (Grontvedt and Espelid, 2003), and rainbow trout (immunohistochemistry) (Zhang et al., 2010), IgM+ cells were found mainly in the LP of both anterior and posterior intestine, although these cells could also be detected in the epithelium, albeit to a lesser degree. In Atlantic halibut, by immunohistochemical analysis, IgM+ cells were distributed more commonly within the epithelium (particularly of the posterior intestine) than within the LP (Grove et al., 2006a). Notably, a gradually increasing number of IgM+ cells was established from the anterior and middle to the posterior part of the intestine of sea bass, which suggested a higher immunological relevance for the posterior gut (Abelli et al., 1997), where absorption of antigens has been reported in Atlantic halibut (Strand and Dalmo, 1997). In a recent report on the distribution of IgM, IgD, and IgZ in the lymphoid tissues of mandarin fish, in situ hybridization studies showed that IgM-producing cells could only be detected at the submucosa and LP of posterior intestine, while no IgZ or IgD positive cells were present in intestine (Tian et al., 2009a). Studies in rainbow trout using anti-IgM- and -IgT-specific Abs, both IgM+ and IgT+ cells were detected in the LP and to lesser degree, within the epithelium (Zhang et al., 2010). Using in situ hybridization, fugu, IgZ+ cells with strong signals could also be detected in the intestinal epithelium (Savan et al., 2005b).
4.3. B cells in skin
Adaptive immune elements in skin are detectable both in cartilaginous and teleost fish (Wolfle et al., 2009). In carp skin, B cells have been detected only in the epithelium. Using mAbs against skin mucus Ig, a decreasing immunostaining reaction was observed from the basal cells layer towards the surface (Rombout et al., 1993b). Similarly, in rainbow trout skin, strong staining of the basement membrane area, less bright staining of the epithelial layer, and occasional or clusters of cytoplasmic staining cells (plasma cells) in the dermis or subepidermal layer, were shown when using pAb against serum IgM (St Louis-Cormier et al., 1984). However, it is possible that the pAb anti-IgM used in the aforementioned study might have cross-reacted with skin IgT as the first constant domain of IgT bears a striking resemblance to that of IgM, and in addition, light chains of IgT and IgM are probably shared. In spotted wolffish, by using in situ hybridization, IgM+ cells were found both in the epithelium near the basal membrane and further out in the epidermis (Grontvedt and Espelid, 2003). The subepidermal plasma cells may be responsible for the production and transport of at least a part of the Ig to skin mucus. Within the skin epidermis of channel catfish numerous lymphocytes were predominantly associated with the basal layer (Lobb, 1987), albeit only a few were IgM-secreting cells. However, the numbers of IgM-secreting cells (~160/cm2 skin) increased 20-fold following immunisation of channel catfish with the protozoan parasite Ichthyophthirius multifiliis (Zhao et al., 2008).
Altogether, the studies highlighted in this section support the idea that fish have a secretory immune system associated with their skin and that it is likely to play a key role against pathogens (St Louis-Cormier et al., 1984). The presence and role of non-IgM+ B cells (i.e., IgT/IgZ+ or IgD+) in teleost SALT has not yet been investigated. Future studies on these skin B cell subsets are required to understand further the specific contribution of all teleost Ig isostypes in skin immunity.
4.4. B cells in gill
The gills are large mucosal surfaces and very important portals for pathogen entry in fish (Grove et al., 2006a; Holzer et al., 2003). To date, very few studies have examined the distribution and role of B cells and plasma cells in the gills of teleost fish. In Atlantic halibut (Grove et al., 2006a), IgM+ cells were found abundantly in the stratified epithelium of the gill arch and filaments, whereas in spotted wolffish (Grontvedt and Espelid, 2003), IgM+ cells were detected in the primary gill lamellae and in gill filaments along blood vessels. Moreover, both IgM+ and IgZ+ B cells are present along the gill filaments of mandarin fish as shown by in situ hybridization (Tian et al., 2009a). In fugu, IgZ+ cells with strong signals could also be detected in gill by using in situ hybridization (Savan et al., 2005b). In a recent report, immunohistochemistry was used to show the presence of IgT+ B cells in the gills of rainbow trout, using an anti-trout IgT antibody. IgT+ B cells appeared located in the epithelium of the gill lamellae. IgM+ B cells, in turn, were found in gill arterioles and lamellar capillaries. However, the reactivity and specificity of this antibody for trout IgT remains to be shown (Olsen et al.). In dab, by using the ELISPOT assay, a mean of 4227 'constitutive' IgM-secreting cells/106 cells were detected in the gills, which were fewer than those in HK but more numerous than those in peripheral blood leukocytes (Davidson et al., 1997). In the same study, the number of specific IgM-secreting cells in the gills against human gamma globulin (HGG) following intraperitoneal (i.p.) or oral administration of HGG was also determined. Few anti-HGG cells were detected following i.p. immunisation, and even less after oral immunisation, which indicated that the contribution of 'constitutive' IgM-secreting cells in the gills of dab was more substantial than that of elicited specific ones (Davidson et al., 1997). Interestingly, in the recently identified ILT, very few IgM+ cells were detected (Haugarvoll et al., 2008; Koppang et al., 2010), however this cannot exclude the possibility that other B cell subsets (IgT+ and IgD+) are present in the ILT.
5. Mucosal immune responses
In this section we will exclusively focus on immunoglobulins, B cells and ASCs since other cellular and humoral immune defences present in the teleost mucosal immune system are beyond the scope of this review. Tables 3–5 summarise most of the available literature regarding the teleost mucosal secretory system.
Table 3.
Summary of teleost gut mucosal and systemic antibody specific responses following oral, immersion (imm.), peroral (p.o.), bath or intraperitoneal (i.p.) immunisations, experimental infection (exp.inf.) or natural infection (n.i.)
| Species | Route | Antigen | Responsea | Reference | |
|---|---|---|---|---|---|
| Gut | Systemic | ||||
| Carp | oral |
A.hydrophila ghosts A. hydrophila formalin killed |
+++ ++ |
++ + |
(Tu et al., 2010) |
| Rainbow trout | n.i. | Ceratomyxa Shasta | + (IgT only) | ++ (IgM only) | (Zhang et al., 2010) |
| Carp | oral | BSA-liposomes | + | + | (Irie et al., 2003) |
| Seabass (ELISPOT) | i.p. + i.p. |
P. damselae spp. Piscicida V. anguillarum Vibrogen DNP-KLH |
ns + (boost) ns |
+ (primary & boost) ++ (boost) + |
(dos Santos et al., 2001b) |
| Rainbow trout (ELISPOT) | p.o. i.p. |
A. salmonicida | + + |
+ + |
(Davidson et al., 1993b) |
| Chub | exp.inf. | Pomphorhynchus laevis | + | + | (Harris, 1972) |
Responses are shown as positive or negative specific IgM levels measured by ELISA unless otherwise stated. Responses were scored: + (significant but low), ++ (intermediate), +++ (highest), or ns (not statistically significant). These scores are not comparable across different studies.
Table 5.
Summary of teleost mucosal and systemic antibody responses following oral, immersion (imm.), peranal (p.a.), peroral (p.o.), bath or intraperitoneal (i.p.) immunisations or horizontal infection (h.i.)
| Species | Route | Antigen | Responsea | Reference | |||
|---|---|---|---|---|---|---|---|
| Skin | Gil | Gut | Systemic | ||||
| Yellow croacker (ELISA and ELISPOT) |
oral i.p. imm. |
Vibrio harveyi | − − + |
− + + |
NM | + + + |
(Xu et al., 2009b) |
| Rainbow trout (ELISA fluids and tissue explants) |
i.p. p.a |
FITC/KLH±FCA | + − |
+ − |
+ − |
+ − |
(Swan et al., 2008) |
| African catfish | oral p.a. imm. i.p. |
V. anguillarum 02 | + + + − |
NM | + + + − |
+ ++ + ++ |
(Vervarcke et al., 2005) |
| European eel (ELISA and in situ dot blot) |
imm. | V. vulnificus | + | + | NM | + | (Esteve-Gassent et al., 2003) |
| European eel | i.p. p.a. oral imm. |
V. vulnificus bivalent | + + + + |
NM | ++ ++ ++ ++ |
+++ +++ ++ ++ |
(Esteve-Gassent et al., 2004b) |
| Sea bass (ELISPOT) |
imm. |
Photobacterium damselae spp. piscicida |
NM | +++ | + | + | (dos Santos et al., 2001a) |
| Spotted sand bass (ELISA and ELISPOT) |
oral | A. veronii lectin | + | + | + | + | (Merino-Contreras et al., 2001) |
| Dab (ELISA and ELISPOT) | bath p.o. p.o p.a. i.p. |
HGG-lipid emulsion HGG-lipid emulsion HGG HGG HGG |
NM | − + − − ++ |
− + − + + |
− − − − +++ |
(Lin et al., 2000) |
| Rainbow trout | imm. i.p. |
IHNV | − − |
NM | − − |
+ + |
(Cain et al., 1996) |
| Brook trout | h.i. bath |
F. branchiophilum | NM NM |
ns ++ |
NM NM |
ns + |
(Lumsden et al., 1993) |
Responses are shown as positive or negative specific IgM levels measured by ELISA unless otherwise stated. Responses are scored: + (significant but low), ++ (intermediate), +++ (highest), ns (not statistically significant), orNM (not measured). These scores are not comparable across different studies.
5.1 Responses in the gut
Table 3 shows the available literature regarding systemic and gut antibody and B cell responses in fish. Additionally, studies where GALT and at least another MALT were measured appear in Table 5.
Oral immunisation delivers antigen in the feed. This route has disadvantages due to the strong physiological conditions imposed in the first portions of the GI tract, and thus, it typically results in weak immune responses (Ellis, 1995; Sun et al., 2010). A variety of vaccine formulations and delivery methods have been and still are devised to overcome antigenic degradation in the stomach (Ellis, 1995) (see the review by LaPatra et al. in this issue).
Oral and anal intubations have been experimentally used to deliver antigens to the GI tract. Generally speaking, anal intubation results in greater immune responses than oral intubation due to: i) avoidance of antigen degradation in the anterior portion of the GI tract where very low pH levels may be present, and ii) arrival of intact antigen to the second portion of the gut, which at least in some species, has a great antigen uptake capacity (Strand and Dalmo, 1997). Most of the peroral and peranal studies have either used particulate antigens or bacterial formulations. For example, carp orally immunised with BSA-containing liposomes induced significant anti-BSA IgM in serum as well as in intestinal mucus and bile but the same dose of BSA-containing unstable liposomes or BSA alone failed to do so. BSA-specific ASCs were also detected in the spleen and HK of immunised fish (Irie et al., 2003). Despite the abundance of oral vaccination studies most of them only measure systemic IgM levels and thus mucosal IgT and IgM responses remain unknown.
5.1.1 Gut Ig responses against Myxozoan parasites
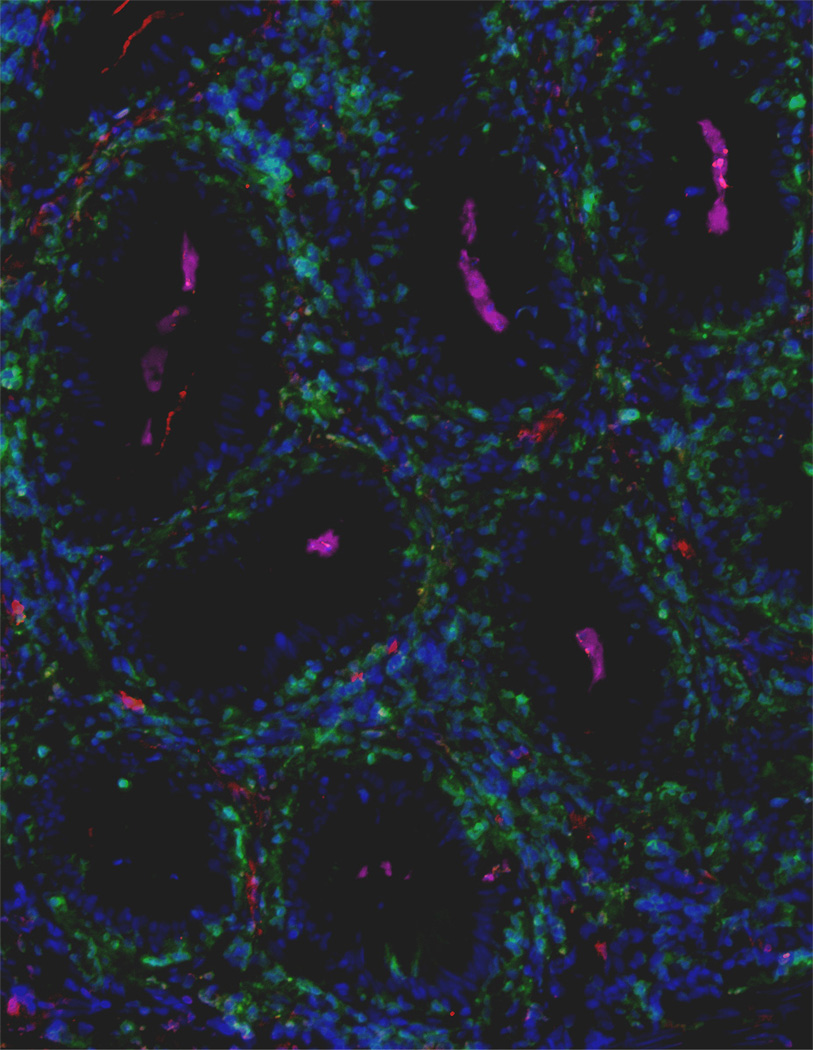
For a review on innate and adaptive immune responses in fish against parasites see (Alvarez-Pellitero, 2008). Among parasitic models, myxozoans are particularly relevant because many of them specifically infect the gut of both freshwater and marine teleosts. Cohabitation challenge of turbot with Enteromyxum scophthalmi results in a leukocyte infiltration in the intestine. The infiltration, consisting mainly of lymphocytes, was only assessed by light microscopy, and no specific IgM could be detected in serum (Sitja-Bobadilla et al., 2006). Using the same parasite-host model, experimental infection by effluent transmission resulted in an increase in the number of IgM+ cells present in the gut of recipient fish 78 days post-exposure (Bermudez et al., 2006). Earlier studies also recorded similar results in carp experimentally infected with the enteric protozoan parasite Goussia carpelli (Steinhagen and Rombout, 1994). Different conclusions were recently obtained in trout surviving Ceratomyxa shasta natural infections, another myxosporidian parasite (Zhang et al., 2010). In that study, numbers of IgM+ B cells in the gut of survivor fish remain unchanged when compared to control animals, while parasite-specific IgM titers were detected only in the serum of these animals. Conversely, significant accumulations of IgT+ B cells were detected in the gut (Fig. 1), a result that correlated with elevated C. shasta-specific IgT titers in gut mucus. In contrast, no IgT titers could be detected in serum of survivor fish, thus revealing a compartmentalization of IgT and IgM responses in mucosal versus systemic compartments respectively (Zhang et al., 2010). Thus far, the aforementioned study is the only one that has evaluated both IgM and IgT responses in a gut infection model in teleost. It is possible that natural infections stimulate gut B cells in a different manner than experimental ones, such as effluent transmission with E. scophthalmi used by Bermudez (Bermudez et al., 2006) to infect turbot in order to reproduce natural conditions. Alternatively, E. scophthalmi and C. shasta may elicit different B cell and antibody responses in the gut since they belong to two different genera of myxosporidians. Another possibility is that different fish species respond differently to these parasites. Clearly more studies are required to evaluate all these possibilities. In that regard, it is worthy mentioning that in mammalian models, even different strains of Giardia induce high or low IgA responses in the gut (Langford et al., 2002), thus it is likely that IgT and IgM responses in fish may be dependent not only on the myxosporidian parasite species but also on the specific strain of the parasite used. In that respect, the existence of host-specific C. shasta genotypes infecting rainbow trout and Chinook salmon (Oncorhynchus tshawytscha) has recently been shown (Atkinson and Bartholomew, 2010). It is anticipated that future work is required to evaluate whether these different strains induce different IgT and IgM responses in the same host.
Figure 1. Extensive accumulations of IgT+ B cells are observed in the gut lamina propria and epithelium of rainbow trout surviving infection with the parasite Ceratomyxa Shasta.
Immunofluorescence staining of a gut cryosection from rainbow trout, three month post-infection with C. Shasta. Cryosection was stained for IgM (red), IgT (green) and C. Shasta (Magenta); nuclei are stained with DAPI (blue). Parasites (indicated by pink arrows) are localised within the gut lumen (dark area).
5.1.2 Gut Ig responses against other parasites
A very early study experimentally infected dace (Leuciscus leuciscus) with larvae of the intestinal cestode parasite Caryophyllaeus laticeps. Parasites were recovered from the fish intestine but serum antibodies could not be detected (Kennedy and Walker, 1969). Cyprinid fish (chub) naturally and experimentally infected with the acanthocephalan parasite Pomphorhynchus laevis produced specific antibody titers both in the serum and intestinal mucus, suggesting the presence of a secretory antibody system in this fish (Table 3). The author stated that the precipitins appeared to have similar chemical characteristics to IgM-type antibody (Harris, 1972).
Oral administration of the microsporidian Glugea plecoglossi spores to ayu (Plecoglossus altivelis) resulted in specific IgM reponses against the intact spores 10 days post-immunisation and no more significant increases in the antibody levels were observed (Kim et al., 1996). Naturally infected ayu contained variable IgM levels not different from uninfected fish. Because the intensity of infection (number of cysts) was not related to the antibody levels both in the artificially and naturally infected fish, authors concluded that antibody production against the intact spores of G. plecoglossi played no protective role against G. plecoglossi infection. However, it is unknown whether gut mucosal IgT/IgZ responses were induced in this study.
5.1.3 Gut Ig responses against bacteria
Several studies have evaluated the induction of antibody responses in teleost following bacterial immunisations, however, very little is known in the case of natural infections. Several common fish bacterial pathogens such as Aeromonas salmonicida and Vibrio anguillarum, are known to disrupt the gut epithelial barrier of Atlantic salmon and induce mobilization of leucocytes in the gut (Ringo et al., 2007).
The identification of IgM ASCs in trout gut was first reported by Davidson (Davidson et al., 1993b). In this study, i.p. injection and peroral intubation were compared. The magnitude of the responses in the gut was comparable to that of the HK in both immunisation routes, but the kinetics of the responses was clearly different. Systemic immunisation resulted in a peak of ASCs in the HK at week 3, and gut responses did not initiate until week 7. When gut mucosa was targeted, both gut and HK ASCs peaked at week 3 only (Davidson et al., 1993b) (Table 3).
Feeding Aeromonas hydrophila ghosts to carp (C. auratus gibelio) resulted in higher IgM titers in the intestine and serum (both peaking at weeks 5 and 6 post-immunisation) (Table 3) than the formalin-killed bacteria. It is interesting that in this study gut mucus IgM titers were twice as high as those found in serum. Up to 80% protection to homologous injection challenge was recorded in the ghost vaccinated group whereas the formalin-killed formulation conferred about 60% protection (Tu et al., 2010).
V. anguillarum but not P. damselae ssp. piscicida i.p. injection significantly increased specific gut ASC numbers compared to controls in seabass, especially during the secondary response. ASC numbers were highest at 7–9 days post-boost (~1000 ASC/106 gut leukocytes) and quite similar to those found in HK, which peaked about 3 days later. DNP-KLH injection resulted in non-significant elevation of gut ASC during both primary and secondary responses, with the spleen reaching the highest ASC numbers (dos Santos et al., 2001b).
In higher vertebrates, colonisation of the mucosal epithelium by commensal bacteria greatly affects the development of GALT, B cell responses and antibody repertoire (Lanning et al., 2005). We currently ignore if or how the gut microbiota shapes the onset of B cell responses in the gut of teleosts. However, there seems to be a conserved response pattern to gut microbiota colonisation in zebrafish and mice. In that regard, DNA microarray studies on gnotobiotic zebrafish have shown that microbial colonisation influences for instance epithelial proliferation and innate immune responses (Rawls et al., 2004).
Few studies have evaluated the total (non-specific) levels of IgM in the gut mucus (Salinas et al., 2008) or IgM+ cells (Picchietti et al., 2007) of fish fed probiotic-supplemented diets. For a review of fish probiotics and immunity see (Nayak, 2010). Generally speaking, delivery of probiotic bacteria results in greater numbers of IgM+ B cells in the gut lamina propria both in juveniles and developing larvae (Abelli et al., 2009). Similar studies are still to be conducted on IgT/IgZ but we anticipate exciting results in this area given the involvement demonstrated of this Ig class in gut immune responses (Zhang et al., 2010).
5.1.4 Gut Ig responses against viruses
Few studies have examined the use of oral vaccines against viral diseases of fish and thus, antiviral immune responses of fish elicited by oral immunisation remain largely uncharacterized (Sato and Okamoto, 2010). Most studies that have used oral delivery of viral antigens in fish (Adelmann et al., 2008; Yasumoto et al., 2006) have not looked into the local production of Igs in the different MALT. Hence, we have very little evidence that teleosts produce virus-specific antibodies in their skin, gut or gills.
Cain (Cain et al., 1996) was able to detect low anti-IHNV (infectious hematopoietic necrosis virus) activity in gut mucus of juvenile rainbow trout following immersion with this virus. Interestingly, neutralising activity was also present in gut mucus following injection challenge but titers were lower than in the waterborne challenge. However, titers were higher in control fish than in infected fish and authors concluded that mucosal antibodies were not involved in preventing infection or virus clearance. The same study found even lower amounts of anti-IHNV activity in cutaneous mucus (Table 5). It is worth noting that IHNV challenged fish had neutralising antibodies (IgM) in serum but not in mucosal secretions as detected by ELISA. So, was IgT responsible for the mucosal antiviral activity recorded? In a more recent study, ginbuna crucian carp (Carassius auratus langsdorfii) orally intubated with inactivated crucian carp hematopoietic necrosis virus (CHNV) showed some serum neutralising antibodies (mucosal Ig titers were not measured) but the responses were weak, slow and transient (Sato and Okamoto, 2010). On the other hand, i.m. injection of nodavirus in halibut did not change the relative numbers or the distribution of gut IgM+ cells (Grove et al., 2006b). There is no doubt that antiviral oral vaccines are still at their infancy and more efforts should be devoted to analysing the type of systemic and mucosal responses they may elicit.
5.2 Responses in the skin
Table 4 shows the available literature regarding systemic and skin antibody and B cell responses in fish. Additionally, studies where skin and at least another MALT were measured appear in Table 5.
Table 4.
Summary of teleost skin mucosal and systemic antibody responses following oral, immersion (imm.), bath, intraperitoneal (i.p.) or surface exposure (s.e.) immunizations
| Species | Route | Antigen | Responsea | Reference | |
|---|---|---|---|---|---|
| Skin | Systemic | ||||
| Channel catfish | i.p. |
I.multifiliis sonicated trophonts I. multifiliis formalin killed I. multifiliis freeze-thawed trophonts |
++ − − |
++ − − |
(Xu et al., 2009a) |
| Grass carp | i.p. | SMRV | + | ++ | (Lu et al., 2008) |
| Olive flounder | oral | E. tarda ghosts | + | + | (Kwon et al., 2007) |
| White sturgeon | i.p. | WSIV FITC:KLH±FCA |
+ + |
+ + |
(Drennan et al., 2007) |
| Grouper | s.e. i.p. |
Cryptocarion irritans | + + |
+ + |
(Luo et al., 2007) |
| Barramundi | oral | Streptococcus iniae | + | + | (Delamare-Deboutteville et al., 2006) |
| Channel catfish (ELISA from skin explants) |
i.p. i.p. + i.p. i.p. + bath |
F. columnare | + | NM | (Shoemaker et al., 2005) |
| Tilapia | imm. i.p. |
F. columnare | ns + |
ns ++ |
(Grabowski et al., 2004) |
| European eel | imm. (x3) | V. vulnificus | + | ++ | (Esteve-Gassent et al., 2004b) |
| Rainbow trout (Plasmon resonance) |
i.p. | FITC-KLH | + | ++ | (Cain et al., 2002) |
| European eel (ELISA and in situ dot blot) |
oral | V. anguillarum enteric coated | + | + | (Wong et al., 1992) |
Responses are shown as positive or negative specific IgM levels measured by ELISA unless otherwise stated. Responses were scored: + (significant but low), ++ (intermediate), +++ (highest), ns (notstatistically significant), or NM (not measured). These scores are not comparable across different studies.
Early studies conducted on the sheepshead demonstrated that the presence of Ig in skin mucus is due to local production and not transudation or transport from serum Ig (Lobb and Clem, 1981a). Next, the presence of Ig-containing plasma cells in cutaneous dermis of trout was demonstrated (St Louis-Cormier et al., 1984). Later work has identified the presence of IgM+ B cells and IgM ASCs in the epidermis of catfish (Zhao et al., 2008) and their capacity to proliferate in vitro upon LPS stimulation. Functional studies on the skin secretory system are nevertheless scarce and always refer to IgM levels only.
Many fish parasites are ectodermic or cause skin lesions and therefore Ig responses in skin mucus during parasitic infections are an excellent model for the study of cutaneous sIg. As a consequence, skin sIg studies against parasites are more numerous than in the case of bacteria or viruses.
5.2.1 Skin Ig responses against Ciliophora parasites
Previous studies on the common parasitic ciliate Ichtyophthirius multifiliis have provided abundant data concerning mucosal IgM responses in teleost skin. For reviews on this parasite please see (Buchmann et al., 2001; Matthews, 2005).
Both Ig H and L chain transcripts were up-regulated in carp skin infected with I. multifiliis (Gonzalez et al., 2007). Catfish immunised by surface exposure to the theronts of I. multifiliis produced transient IgM in cutaneous mucus around the time of infection resolution and the production of mucus and serum antibodies was not synchronised (Maki and Dickerson, 2003). Anti-Ich cutaneous IgM were shown effective at reducing theront infectivity in catfish (Xu and Klesius, 2003). Moreover, I. multifiliis infection resulted in an increase of IgM+ B cells and both nonspecific and specific IgM ASCs in the epidermis of catfish (Zhao et al., 2008). I.p. injection of I. multifiliis sonicated trophonts (but not formalin-killed or freeze-thawed formulations) did elicit specific serum (titers=210–480) and skin mucus (titers ~50) IgM (both peaking 22 days post-injection) as well as 60% protection against challenge in channel catfish (Xu et al., 2009a) (Table 4). Another ciliate parasite, Cryptocaryon irritans, also induced specific IgM responses in the skin of grouper (Epinephelus coioides) after immunisation by surface exposure or i.p. injection (Luo et al., 2007). Interestingly, serum IgM titers were higher in the i.p. group, especially after 4 weeks, but surface exposure resulted in higher skin IgM titers than i.p. injection, particularly after 6 weeks. In a similar study, vaccination of grouper was carried out by a low level exposure to live C. irritans theronts followed by i.p. injection of a vaccine consisting of formalin-killed theronts. This immunisation effectively induced specific skin IgM (serum IgM titers were not measured) and protected between 62.5% and 100% of the grouper from C. irritans infection (Yambot and Song, 2006).
5.2.2 Skin Ig responses against other parasites
Less obvious responses have been observed in other studies. Only some eel (Anguilla japonica) individuals infected with the microsporidian Pleistophora anguillarum produced specific skin mucus IgM (Hung et al., 1997). Similarly, specific IgM against the dinoflagelate ectoparasite Amyloodinium ocellatum was rarely detected in skin mucus and in the serum of some but not all infected clownfish (Amphiprion frenatus) (Cobb et al., 1998a; Cobb et al., 1998b). Specific cutaneous IgM against the molluscan parasite Utterbackia imbecillis was detected in bluegill sunfish (Lepomis macrochirus) following multiple infections by immersion challenge. Low but significant anti-glochidia antibodies were measured in the skin mucus during the first two infections. Interestingly, a more pronounced increase was observed after the third infection (day 60) (Rogers-Lowery et al., 2007).
The monogenean Neobenedemia girellae oncomiracidia expresses a ciliary antigen that induces serum and skin mucus antibodies but no correlation was found between both responses (Hatanaka et al., 2005). I.p. injection of sonicated monogenean Heterobothrium okamotoi oncomiracidia or their cilia induced specific skin mucus IgM in tiger puffer Takifugu rubripes 75 days after a booster immunisation (Umeda et al., 2007). No differences in IgT or IgM transcript expression were found in the skin of trout infected with Gyrodactylus salaries compared to uninfected controls (Jorgensen et al., 2009).
5.2.3 Skin Ig responses against bacteria
With regards to bacterial models, Flavobacterium columnare was able to induce specific cutaneous IgM both in catfish (Shoemaker et al., 2005) and in tilapia (Grabowski et al., 2004) as shown in Table 4. In catfish, experimental infection was conducted by i.p. injection, followed by a boost by immersion or i.p. injection. Specific titers were measured from in vitro supernatants of skin explants. I.p. infected fish showed the highest skin IgM levels at day 16 (mean titer=44) and neither of the boosting protocols resulted in higher cutaneous IgM (mean titers=4–16) (Shoemaker et al., 2005). In contrast, when catfish were experimentally infected (immersion) with Edwardsiella tarda, no specific IgM could be detected in the skin mucus (Zilberg and Klesius, 1997). In tilapia i.p. injection but not immersion vaccination with F. columnare elicited specific cutaneous IgM (Grabowski et al., 2004). In a different study, rainbow trout were immunised with 8 different F. psychrophilum formulations by i.p. injection or immersion. IgM responses were measured in serum and skin mucus after first and boost immunisations followed by bacterial challenge (LaFrentz et al., 2002). Again, i.p. injection of killed F. psychrophilum, but not immersion with the killed bacteria, induced serum IgM from week 6 and skin mucus IgM at week 9 (titres about 3 times lower than in serum).
Thus, it appears that immersion vaccination with killed Flavobacterium spp. is not an effective way to stimulate skin IgM responses. Does IgT play a role in these models?
Other studies testing immersion vaccination with other bacterial pathogens have revealed different results. Eels immunised by immersion with Vibrio vulnificus vaccine (Esteve-Gassent et al., 2003). In that case, significant differences were found between vaccinated and unvaccinated elvers from day 4 until day 11 (maximal titers of 65 at day 4). At this time, anti-V. vulnificus IgM titers in serum were not significantly different from that of the control but the response was still relatively rapid, with peak titers of 25,000 at day 7. Thus, the authors concluded that mucosal lymphocytes must be immediately stimulated upon immersion, while the antigen has to get to the kidney to stimulate lymphocytes responsible for serum antibody production. In a later study, primed eels (immunised by prolonged immersion with V. vulnificus vaccine at the elver stage) received or not, a low dose immersion boost two years later. In the boosted group skin mucus titers peaked at day 12 (titer=127) and were consistently higher until the end of the experiment. Serum titers, on the other hand, peaked at day 20 (titer~21,000) and then dropped. Boosted-eels were 100% protected against posterior bath challenge (Esteve-Gassent et al., 2004a). Bivalent Vulnivaccine was administered to eels by four different routes of immunisation. All four resulted in specific IgM responses in serum, gut mucus and skin mucus (see Table 5) (Esteve-Gassent et al., 2004b).
Finally, significant specific IgM levels were recorded in the cutaneous mucus from freshwater or seawater acclimated barramundi (Lates calcarifer) after immersion or i.p. injection of inactivated Streptoccocus iniae (Delamare-Deboutteville et al., 2006). Interestingly, both routes resulted in higher specific IgM titers in barramundi kept in seawater compared to freshwater. Within the seawater group, fish immersion was more effective in two fish and i.p. injection was more effective in six fish. While larger numbers of fish would need to be evaluated to reach any definitive conclusions, the aforementioned findings may reflect the higher viscosity in the skin mucus in seawater compared to freshwater which, in turn, would result in higher antibody retention in the skin mucus in seawater as already demonstrated in salmonids (Roberts and Powell, 2005). In the same study, simultaneous measurement of barramundi serum responses 21 days post-immunisation revealed low specific IgM titers in the immersion group compared to the i.p. group (around 5 times lower) (Table 4).
Skin IgM production is also induced by oral delivery of antigen. For instance, coho salmon (Oncorhynchus kisutch) fed enteric coated lyophilised Vibrio anguillarum showed significantly higher skin mucus and serum antibody levels than controls (Table 4). However, challenge of the fish with live bacteria did not reveal any significant differences in survival (Wong et al., 1992). Olive flounder (P. olivaceus) fed with E. tarda ghosts produced specific IgM levels in skin mucus and serum more effectively than flounder fed with the formalin-killed bacterium (Kwon et al., 2007) (Table 4). In the same study, serum IgM responses were less than two times of those recorded in the skin. In addition, following challenge with E. tarda either by bath or i.p. injection, flounder orally vaccinated with the ghosts showed the greatest protection (85–90%) whereas the formalin-killed vaccine conferred lower protection (60–75%).
5.2.4 Skin Ig responses against viruses
In the skin of halibut, numerous IgM+ cells could be observed in the epithelium of both nodavirus-challenged (i.m.) and control fish with no significant differences between both groups (Grove et al., 2006b). I.p. injection of white sturgeon (Acipenser transmontanus) with white sturgeon iridovirus (WSIV) resulted in anti-WSIV IgM titers of 8 and 16 in the skin mucus of two fish from the WSIV group at week 12 and four different fish at week 15 (average titer=4). Another group received an i.p. WSIV-FCA injection but no specific IgM was detected in the skin mucus of any fish (Drennan et al., 2007). Some but not all fish responded to the immunisations by producing serum anti-WSIV IgM. Generally speaking, serum responses began at week 9 and skin mucosal responses were first recorded at week 12 (a 3 week delay period) resembling the results obtained by LaFrentz et al. (2002) explained above. Finally, excised skin explants of grass carp (Ctenopharyngodon idella) i.p. immunised with Scophthalmus maximus rhabdovirus (SMRV) showed that cutaneous antibody titers were much lower (12) than serum titers (1,458) (see Table 4) (Lu et al., 2008).
5.2.5 Additional reported Ig skin responses
One study addressed the issue of affinity maturation in skin-derived mucus from rainbow trout immunised i.p. with FITC-KLH (Cain et al., 2002). The antibody-antigen binding kinetics from serum and skin mucus was very similar and it was concluded that affinity maturation of both systemic and cutaneous IgM is low in teleosts.
It has been shown that a majority of gut commensal bacteria are prevalently coated with sIgT and to a lesser degree with sIgM (Zhang et al., 2010). However, whether skin commensal bacteria are also coated by these sIgs is unknown to this date, although it is well established that the skin mucus of fish harbours a diverse population of commensals (Liu et al., 2008; Wang et al., 2010). Thus far, IgD and IgT/IgZ responses in skin have never been investigated neither at the gene nor at the protein level. This implies that we currently have a small picture of the skin secretory immune system in fish. Furthermore, how sIgs interact with antigens at a mucosal surface, which is constantly exposed to water and swimming forces is completely unknown. One would expect possible biological adaptations in the antigen-antibody interactions taking place in such a particular mucosal environment.
5.3 Responses in the gills
Teleost gill is not only a point of entry of pathogens but also as a tissue capable of mounting an immune response (Campos-Perez et al., 2000). It has been suggested that immersion vaccination is effective, to a great extent, thanks to the active role of the gills and the local presence of B cells and ASCs (dos Santos et al., 2001a; Wong et al., 1992). However, to date the information on B cell and antibody responses occurring in the gills is very limited.
5.3.1 Gill Ig responses against parasites
In carp, IgZ1 but not IgZ2 or IgM expression has been shown to be up-regulated at the gene level in the gills of Trypanosoma borreli-infected animals (Ryo et al., 2010). In the case of gill B cells from trout, experimental infections with I. multifiliis did not result in a redistribution or increase of IgT+ or IgM+ B cells as assessed by immunohistochemistry (Olsen, 2011). Plasma-like cells as identified by transmission electron microscope were reported in the gills of chinook salmon (Oncorhynchus tshawytscha) experimentally infected with Loma salmonae, which may cause microsporidial gill disease, although antibody responses were not evaluated (Lovy et al., 2007). Clearly more studies are required to evaluate the specific responses, both IgT/IgZ and IgM, in fish gills and to unravel whether IgT/IgZ plays a pivotal role in gill mucosal responses like in the gut (Zhang et al., 2010).
5.3.2 Gill Ig responses against bacteria
Very interesting and conclusive studies have been conducted on the European eel (Anguilla anguilla) using immersion vaccination against V. vulnificus (Esteve-Gassent et al., 2003). Here, a novel method was employed to detect secreted IgM levels around the gill area by applying in situ dot blots. Vaccinated eels had significantly higher specific IgM levels than unvaccinated fish from day 1 until day 5 post-vaccination, with a maximal titer of 26 at day 3. At this time, there were no significant differences in antibody titers in serum. The authors concluded that the early local response detected in the gill could contribute to protection by reducing colonisation. Whether or not the IgM secreted at those very early time points is truly the result of an adaptive immune response or the result of natural antibody secretion with polyreactive capacity, remains to be demonstrated. Further evidence supporting the early stimulation of IgM responses in the gill is found in a recent study in large yellow croaker (Pseudosciaena crocea) vaccinated with V. harveyi (Xu et al., 2009b). At day 7, specific IgM ASCs were observed in the gills after immersion vaccination but not after i.p. or oral immunisations. At this point, the gill IgM ASC response was parallel with a peak in skin mucus IgM titers. I.p. immunisation induced gill ASCs 3 weeks after injection. Serum IgM was highest in the i.p. group at all sampling times. Importantly, immersion and i.p. vaccination resulted in 60% protection to i.p. challenge whereas the oral vaccination protected 40% of the croaker fish.
Thus, it seems clear that sIgM and B cells responses take place in the teleost gills during the early immune responses following immersion with different species of the genus Vibrio. Moreover, the gill may also play a significant role later in the immune response. In that regard, levels of anti-F.branchiophilum specific IgM in the gill of brook trout (Salvelinus fontinalis) were recorded 57 days after bath vaccination with F. branchiophilum (Lumsden et al., 1993). Interestingly, gill and serum antibody levels were not correlated in individual fish (Table 5). In addition, oral antigen delivery also stimulated specific ASCs in the gills. In a different study, high numbers of specific mucosal IgM plasma cells appeared in gills and gut of carp orally vaccinated with encapsulated V. anguillarum bacterin although they were absent after i.m. injection (Joosten et al., 1997). Conversely, orally vaccinated carp showed very low numbers of IgM ASCs in PBLs and HKLs. I.p. vaccination of spotted wolfish juveniles with A. salmonicida bacterin resulted in higher numbers of IgM plasma cells in spleen, HK and, to a lesser extent, in gut but not in gills (Grontvedt and Espelid, 2004).
One study has looked at transcript levels of all three Ig classes in the gills of mandarin fish i.p. immunised once or twice with F. columnare. The kinetics of the different Ig classes differed from organ to organ. IgZ expression in the gills was different from that of systemic organs. In the single-immunised group, IgZ and IgD expression were up-regulated at weeks 1 and 2 in the gill but not in the HK, blood or spleen. The gill response lasted until week 4 in the case of IgZ and until week 2 for IgD. From week 3 on, up-regulation of IgZ and IgD was observed in at least one of the systemic compartments studied. Gill IgM expression peaked after 1 week in the single-immunised group and at week 5 in the boosted group, which was concomitant with high IgM expression in all systemic organs (Tian et al., 2009a). While these changes in gene expression are interesting, it remains to be demonstrated whether the different antibody isotypes were secreted and whether they specifically recognised F. columnare.
5.3.3 Gill Ig responses against viruses
Not many studies have addressed gill Ig responses to viruses. In a 2006 study, halibut gill IgM+ cells were predominantly found in the thick stratified epithelium covering the gill arch, the gill rakers and parts of the filaments. Only a few IgM+ cells were seen in the simple epithelium covering the interlamellar parts of the filament, and IgM+ cells were only rarely observed in lamellar epithelium. This IgM+ B cell distribution and numbers did not differ in control and nodavirus (i.m.) immunised halibut (Grove et al., 2006b).
5.3.4 Additional reported gill Ig responses
IgM ASCs were quantified following oral intubation or i.p. injection with human gamma globulin (HGG) in the dab. In this case, specific ASCs in the gills were higher in the i.p. group (153×104/106 cells) than in the oral group (1.5×103/106 cells). Gill mucus IgM levels were not measured but serum titers in the i.p. group peaked at week 7 and correlated with HK specific ASC numbers, whereas in the oral group serum IgM was at least 100 times lower and it resembled the ASC response in PBLs (Davidson et al., 1997). In a similar experiment, dab gill contained specific IgM ASCs 2 weeks after receiving peroral HGG in lipid emulsion and from week 3 to 10 after i.p. injection. Bath delivery failed to induce any gill responses (Lin et al., 2000). In conclusion, from these two studies, it would appear that gill IgM antibody responses are better stimulated by systemic than mucosal delivery, at least in a HGG model.
IgM expression in gills is significantly affected by heat shock, with severe impairment of the expression occurring between 12 and 8 h in orange spotted grouper (Cui et al., 2010). Whether other environmental stressors may induce such detrimental changes in the sIg response will need further research.
No functional studies have yet analysed the responses in ILT, where large amounts of T cells have recently been found (Haugarvoll et al., 2008; Koppang et al., 2010). As explained above, few IgM+ cells were found in this tissue but this does not rule out that IgT, IgD or even IgM responses are elicited during infection or vaccination.
6. A common-mucosal immune system (CMIS)?
The CMIS is a concept that acknowledges the presence of an integrated immune system that communicates all mucosal inductive and effector sites (Iijima H, 2001). This concept implies that antigens encountered at one mucosal inductive site will disseminate and home in a way that the mucosal surfaces where the infection actually occurs become protected. Nowadays, many consider CMIS an obsolete term that can be simply explained by the shared expression of adhesion and chemokine receptor pairs. Extensive regionalization and compartmentalization exists, in fact, within the mucosal immune system (Brandtzaeg, 2010; Macpherson et al., 2008). As a consequence, the regionalization of mucosal immune compartments makes effective vaccination strategies not as straightforward as originally anticipated. For instance, nasal immunisation results in respiratory and urogenital antigen-specific immunity and vaccines that target GALT predominantly elicit responses in the GI tract (reviewed by (Chen and Cerutti, 2010)). Such associations are less clear in the case of teleosts but future studies will further shed light onto these aspects of CMIS.
Thus, a key question is, can we immunise fish by one mucosal route and induce protection across all mucosal sites? Multiple studies have demonstrated the importance of the route of immunisation in fish vaccination (Davidson et al., 1997; Ellis, 1988; Rombout et al., 2010). The idea of a CMIS in fish was first suggested by Rombout (Rombout et al., 1986). In this study, carp were either orally or anally intubated with V. anguillarum. Anally immunised fish developed specific IgM responses in the gut, skin, bile and serum. However, oral administration failed to induce serum responses. However, other studies have reported specific plasma antibody responses following oral immunisations as summarised in Tables 3–5. For instance, tilapia orally and anally immunised with HGG produced specific IgM responses in plasma, bile and cutaneous mucus (Jenkins et al., 1994). Interestingly, in this study, the immune responses in the three secretions were short-lived (about 21 days in duration) and mirrored each other. Gut and gill antibody levels were not measured.
As shown in Table 5, in most fish studies, when one mucosal immune site secretes specific IgM other MALT sites are also stimulated (Esteve-Gassent et al., 2003; Lin et al., 2000; Merino-Contreras et al., 2001; Swan et al., 2008; Vervarcke et al., 2005; Xu et al., 2009a). The kinetics and potency of the response of each compartment are however different in different experimental models. Thus, there is some disparity on how the integration of the responses takes place. Some authors recognise that systemic immunisation results in a delayed appearance of mucosal sIg compared to mucosa-targeted stimulations, but others have reported simultaneous responses in MALT and serum regardless of the route. This disparity is in agreement with mammalian studies that have acknowledged disparity with regard to migration of memory/effector cells from mucosal inductive sites to secretory-effector sites and systemic immune organs (Brandtzaeg, 2007).
To date, comprehensive studies that have analysed production of all Ig classes in skin, gills, gut and serum following mucosal immunisation are lacking. In addition, as Table 5 shows, little data is available for all four compartments measured during the same study. Our patchy knowledge becomes even blurrier since antigen-specific IgT/IgZ responses are missing from all these studies, except in a recent report where IgT was found to behave as a mucosal gut Ig (Zhang et al., 2010). We ignore for instance, if stimulating gut IgT responses results in seeding of other MALT with IgT-secreting cells.
At present we know that many of the components involved in mammalian mucosal immune responses, including PP and MLN are missing in fish. For this reason, some authors have recently ruled out the original idea that a CMIS exists in teleost (Rombout et al., 2010). Recent advances in mammalian mucosal immunology have revealed the presence of compartmentalised responses within MALT. The very different teleost MALT architecture may utilise other mechanisms to integrate (or not) mucosal immune responses, but it is possible that the lack of organised MALT facilitates such integration. What we do know is that even if IgA is not present in fish, there appears to be a functional equivalent, IgT, at least in rainbow trout (Zhang et al., 2010). Intriguingly, the J chain in teleosts is lacking, but teleost pIgR appear to be involved in the transport of IgM and IgT through mucosal epithelia (Zhang et al., 2010).
One of the most important areas to investigate in order to address the presence of a teleost CMIS is the selective homing of B and T cells to systemic and mucosal lymphoid organs during the course of infection or the development of an immune response. To this end, it will be crucial to elucidate the involvement of chemokines and chemokine receptors in the homing of lymphocytes to mucosal and systemic sites (see in this issue the review on fish chemokines and chemokine receptors (Alejo and Tafalla)). Thus far, very little is known about the homing of T and B cells to teleost lymphoid organs. In that regard, one study has shown that trout gut intraepithelial T lymphocytes appear to be phenotypically and functionally identical to systemic T cells (Bernard et al., 2006). Clearly much work is required in understanding the homing and role of teleost lymphocytes into systemic and mucosal compartments.
Finally, fish may possess alternative mechanisms that integrate and enable cross-communication among MALTs. We expect that fresh data will in a near future be available on a variety of teleost species and, that responses of all Ig classes and B cell subsets from all mucosal and systemic sites will be integrated, thus revealing whether fish have a CMIS.
7. Concluding remarks and future directions
This review attempts to gather past as well as recent information available regarding mucosal antibody and B cell responses in teleost fish. Looking at mucosal responses from mammalian species, and from the limited data available in teleosts, it is clear that mucosal immune responses are significantly more complex to study and understand than systemic ones. Thus, much work remains to be done in order to achieve a holistic view of systemic and mucosal antibody and B cell responses in teleosts.
The delivery method of one given pathogen or immunogen is determinant to elicit or not mucosal Ig responses, especially in oral immunisation. It is clear that systemic immunisation results in specific IgM responses at mucosal sites but the kinetics, intensity and duration of the response may significantly differ depending on the antigen (Davidson et al., 1997; Drennan et al., 2007; Lin et al., 2000).
As a general rule of thumb, stimulation of skin IgM by i.p. delivery seems to occur later (about 3 weeks) than in serum. Responses in the gill appear to support the skin data since i.p. injection is a more effective inducing route than mucosal ones. This would imply that IgM ASCs in the skin and gills are seeded from the blood or that differentiation of resident mucosal B cells into plasma cells takes place at a mucosal site even when we stimulate systemically.
As we have already discussed, the intensity of the mucosal responses greatly depends on the species and immunisation route. In terms of the kinetics, some authors have reported antibody levels in mucus peak sooner than those in serum in the case of mucosal immunisation routes (Rombout et al., 1989; Rombout et al., 1986) (Rombout et al., 1993b) whereas others found still that mucosal IgM appears later than serum IgM even if fish are infected by a mucosal route (Maki and Dickerson, 2003; Zilberg and Klesius, 1997). We cannot therefore withdraw conclusive remarks in this respect and we await further investigations in order to clarify this point.
On the other hand, serum IgM production is often much higher when antigens are systemically injected than when delivered by any of the mucosal routes (Crosbie and Nowak, 2004; Delamare-Deboutteville et al., 2006; Santos et al., 2005; Xu et al., 2009a). One consideration to bear in mind is that i.p. injections are often delivered with adjuvants such as FCA and thus there is no direct comparison with mucosal immunisations such as immersion protocols in which no adjuvants are added. Nevertheless, exceptions remain. First, anal intubation of African catfish with V. anguillarum elicited similar serum IgM titers to i.p. injection after 2 weeks (Vervarcke et al., 2005). Second, live attenuated E. tarda formulations recently tested in flounder (Sun et al., 2010) showed that i.p. vaccination resulted in similar serum IgM levels as immersion and oral routes, whilst the oral vaccine was slightly less effective (mucosal responses were not measured in this study). Third, immersion infection of channel catfish with Ich theronts produced a similar serum response as i.p. injection of the purified Ich -antigen (Maki and Dickerson, 2003). Thus, it is possible in some instances to induce equally strong mucosal IgM responses by using mucosal or systemic immunisations.
Live microorganisms (infections) or vaccines that use live attenuated pathogens are more effective inducers of mucosal responses than their inactivated counterparts in mammalian studies (Cox et al., 2004; Levine and Kaper, 1995). Thus, the lack of strong mucosal Ig responses in fish under different mucosal immunisation set ups may be the consequence of inadequate stimulation of the local immune system by non-viable pathogens or particulate antigens. The latter may also explain why in fish, vaccine formulations using bacterium ghosts where surface proteins are intact, are more effective at stimulating sIg than the formalin killed formulations (Kwon et al., 2007).
It is possible that teleost mucosal surfaces require exposure to live pathogens or even multiple exposures or chronic infections in order to mount robust mucosal antibody responses (Maki and Dickerson, 2003; Rogers-Lowery et al., 2007; Rombout et al., 1989; Zhang et al., 2010). Unfortunately, very few studies have analysed mucosal responses of fish in which pathogens specifically infect or stimulate a mucosal surface and, no direct comparisons between live and killed formulations have been carried out.
The importance of mucosal Ig in protection remains unclear at this point, and it will likely be hard to evaluate, as it has been the case with sIgA in mammalian disease models (Brandtzaeg, 2007). To test whether induced specific sIg mucosal responses in fish are protective, transfer experiments using immune secretory IgT/IgZ or IgM will be required. However, the question will be how to transfer or deliver immune sIg to naïve fish to ensure that it reaches the right mucosal surface without being degraded and in sufficient concentrations. So far we have evidence that specific anti-C. shasta IgT titers are present in survivor trout (Zhang et al., 2010). Whether the IgT produced is responsible for protecting fish is a point that remains to be addressed. Future work lies ahead to find the correct strategy to demonstrate the protective effect of this parasite-specific IgT. Conversely, another important question that needs to be addressed is whether mucosal antibody responses elicited by systemic immunisation can elicit protective responses.
It is important to note that the evaluation of antibody responses in fish skin, gills and gut is likely to be variable among different labs since the collection procedures of fish mucosal secretions are not standardized, a significant problem also acknowledged in the case of mammalian studies. Additional inconsistencies in the results may be due to subsequent sample processing and assays used to measure sIg. Moreover, the analysis of sIg content and titers is further complicated by the presence of proteases and microbial contamination in the mucus samples. Studies on the comparative susceptibility of teleost sIgs to proteases are missing but it is likely that differences exist among the different Ig classes, as it is the case in mammalian sIgs where IgA2 has an increased resistance to bacterial proteases compared to IgA1 (reviewed by (Cerutti and Rescigno, 2008).
Another point to take into account is the possibility that mucosal Ig plays an important role in the innate and early antibody responses during infection. In that regard, the role of sIg may have been overlook in many of the studies. The evidence provided in few studies for an early gill sIg production supports this idea. Thus, the role of mucosal Igs in innate immunity deserves further investigation.
It is important to point out that mammalian B cells are known to play an important role in the regulation of inflammation, particularly as regulatory cells that secrete Il-10, a potent anti-inflammatory cytokine (Fillatreau et al., 2008; Fillatreau et al., 2002; Mauri). For example, chronic intestinal inflammatory conditions such as experimental colitis prompt the appearance of a B cell subset that produces IL-10 and suppresses progression of intestinal inflammation (Mizoguchi et al., 2002). Whether or not B cells of teleost fish play a role in the regulation of inflammation is an important subject that remains to be investigated. In that context, it is worth mentioning that teleost B cells express C3a and C5a receptors (Boshra et al., 2004; Boshra et al., 2005), both of which are known to play important pro-inflammatory roles (Sunyer et al., 2005). Thus, it will be interesting to decifer the specific contribution of mucosal B cell subsets both in the induction and regulation of inflammation during the course of infection.
Another fascinating point that we need to consider and understand is the capacity of mucosal B cells to take up and present antigen. In that regard, B cells from several fish species have been shown to be highly phagocytic (Li et al., 2006; Overland et al., 2009; Zhang et al., 2010). Moreover, it has recently been shown that trout B cells express very high levels of CD80/86, a crucial co-stimulatory molecule (Zhang et al., 2009). In addition catfish B cells have been reported to express high levels of surface MHC-Class II molecules (Moulana et al., 2008). Thus, the potential phagocytic capacity of mucosal B cells, combined with their potential high expression of CD80/86 and MHC-Class II molecules, could turn these cells into important antigen-presenting cells in mucosal surfaces. While this hypothesis needs to be investigated, its substantiation would have important implications for the design of vaccines that induce mucosal immunity.
In conclusion, this review has gathered a vast body of existing data that illustrates our knowledge of antibody and B cell responses occurring in mucosal surfaces. We have highlighted the complexity of teleostean and mammalian MALT as well as the necessity for holistic studies that concomitantly measure fish B cell dynamics and Ig responses in the systemic and mucosal compartments. The intricacy of the fish MALT picture reaches higher levels of complexity when trying to understand the relationship among different mucosal compartments, inductive and effector sites. We currently know very little about IgT+/IgZ+, and IgD+ B cell populations and the immunoglobulins they produce in fish mucosae and we know even less about the presence and dynamics of plasma cells, plasmablasts and long-lived plasma cells secreting these Ig classes in these mucosal sites. Until now IgM was thought to be the only Ig class responding to pathogenic challenge in teleosts, both in systemic and mucosal compartments. The recent discovery of IgT/IgZ predominant role in gut mucosal immunity opens up a new avenue for the study of fish mucosal immune responses as well as for the evaluation and design of novel fish vaccines and immunotherapies that stimulate not only systemic, but also mucosal immune responses.
Acknowledgements
Supported by the National Science Foundation (NSF-MCB-0719599 to J.O.S.), the National Institutes of Health (R01GM085207-01 to J.O.S.) and the United States Department of Agriculture (USDA-NRI 2006-01619 and USDA-NRI 2007-01719 to J.O.S.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abelli L, Coscia MR, De Santis A, Zeni C, Oreste U. Evidence for hepato-biliary transport of immunoglobulin in the antarctic teleost fish Trematomus bernacchii. Dev Comp Immunol. 2005;29:431–442. doi: 10.1016/j.dci.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Abelli L, Picchietti S, Romano N, Mastrolia L, Scapigliati G. Immunohistochemistry of gut-associated lymphoid tissue of the sea bass Dicentrarchus labrax (L) Fish Shellfish Immunol. 1997;7:235–245. [Google Scholar]
- Abelli L, Randelli E, Carnevali O, Picchietti S. Stimulation of gut immune system by early administration of probiotic strains in Dicentrarchus labrax and Sparus aurata. Ann N Y Acad Sci. 2009;1163:340–342. doi: 10.1111/j.1749-6632.2008.03670.x. [DOI] [PubMed] [Google Scholar]
- Adelmann M, Kollner B, Bergmann SM, Fischer U, Lange B, Weitschies W, Enzmann PJ, Fichtner D. Development of an oral vaccine for immunisation of rainbow trout (Oncorhynchus mykiss) against viral haemorrhagic septicaemia. Vaccine. 2008;26:837–844. doi: 10.1016/j.vaccine.2007.11.065. [DOI] [PubMed] [Google Scholar]
- Alejo A, Tafalla C. Chemokines in teleost fish species. Dev Comp Immunol. doi: 10.1016/j.dci.2011.03.011. [DOI] [PubMed] [Google Scholar]
- Alvarez-Pellitero P. Fish immunity and parasite infections: from innate immunity to immunoprophylactic prospects. Veterinary Immunology and Immunopathology. 2008;126:171–198. doi: 10.1016/j.vetimm.2008.07.013. [DOI] [PubMed] [Google Scholar]
- Ardavin CF, Zapata A, Garrido E, Villena A. Ultrastructure of gut-associated lymphoid tissue (GALT) in the amphibian urodele, Pleurodeles waltlii. Cell Tissue Res. 1982;224:663–671. doi: 10.1007/BF00213761. [DOI] [PubMed] [Google Scholar]
- Asbakk K. Elimination of foreign material by epidermal malpighian cells during wound healing in fish skin. J Fish Biol. 2001;58:953–966. [Google Scholar]
- Atkinson SD, Bartholomew JL. Disparate infection patterns of Ceratomyxa shasta (Myxozoa) in rainbow trout (Oncorhynchus mykiss) and Chinook salmon (Oncorhynchus tshawytscha) correlate with internal transcribed spacer-1 sequence variation in the parasite. International Journal for Parasitology. 2010;40:599–604. doi: 10.1016/j.ijpara.2009.10.010. [DOI] [PubMed] [Google Scholar]
- Austin B. The bacterial microflora of fish, revised. ScientificWorldJournal. 2006;6:931–945. doi: 10.1100/tsw.2006.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avtalion RR, Wojdani A, Malik Z, Shahrabani R, Duczyminer M. Influence of environmental temperature on the immune response in fish. Curr Top Microbiol Immunol. 1973;61:1–35. doi: 10.1007/978-3-642-65531-9_1. [DOI] [PubMed] [Google Scholar]
- Baker K, Lencer WI, Blumberg RS. Beyond IgA: the mucosal immunoglobulin alphabet. Mucosal Immunol. 2010;3:324–325. [Google Scholar]
- Bang J-D, Kim J-W, Lee S-D, Park S-I, Chun S-G, Jeong C-S, Park J-W. Humoral immune response of flounder to Edwardsiella tarda: The presence of various sizes of immunoglobulins in flounder. Dis Aquat Org. 1996;26:197–203. [Google Scholar]
- Barnett RR, Akindele T, Orte C, Shephard KL. Eosinophilic granulocytes in the epidermis of Oreochromis mossambicus gill filaments studied in situ. Journal of Fish Biology. 1996;49:148–156. [Google Scholar]
- Bengten E, Quiniou S, Hikima J, Waldbieser G, Warr GW, Miller NW, Wilson M. Structure of the catfish IGH locus: analysis of the region including the single functional IGHM gene. Immunogenetics. 2006;58:831–844. doi: 10.1007/s00251-006-0139-9. [DOI] [PubMed] [Google Scholar]
- Bengten E, Quiniou SM, Stuge TB, Katagiri T, Miller NW, Clem LW, Warr GW, Wilson M. The IgH locus of the channel catfish, Ictalurus punctatus, contains multiple constant region gene sequences: different genes encode heavy chains of membrane and secreted IgD. J Immunol. 2002;169:2488–2497. doi: 10.4049/jimmunol.169.5.2488. [DOI] [PubMed] [Google Scholar]
- Bermudez R, Vigliano F, Marcaccini A, Sitja-Bobadilla A, Quiroga MI, Nieto JM. Response of Ig-positive cells to Enteromyxum scophthalmi (Myxozoa) experimental infection in turbot, Scophthalmus maximus (L.): A histopathological and immunohistochemical study. Fish & Shellfish Immunology. 2006;21:501–512. doi: 10.1016/j.fsi.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Bernard D, Six A, Rigottier-Gois L, Messiaen S, Chilmonczyk S, Quillet E, Boudinot P, Benmansour A. Phenotypic and functional similarity of gut intraepithelial and systemic T cells in a teleost fish. J Immunol. 2006;176:3942–3949. doi: 10.4049/jimmunol.176.7.3942. [DOI] [PubMed] [Google Scholar]
- Boshra H, Li J, Peters R, Hansen J, Matlapudi A, Sunyer JO. Cloning, expression, cellular distribution, and role in chemotaxis of a C5a receptor in rainbow trout: the first identification of a C5a receptor in a nonmammalian species. Journal of Immunology. 2004;172:4381–4390. doi: 10.4049/jimmunol.172.7.4381. [DOI] [PubMed] [Google Scholar]
- Boshra H, Wang T, Hove-Madsen L, Hansen J, Li J, Matlapudi A, Secombes CJ, Tort L, Sunyer JO. Characterization of a C3a receptor in rainbow trout and Xenopus: the first identification of C3a receptors in nonmammalian species. J. Immunol. 2005;175:2427–2437. doi: 10.4049/jimmunol.175.4.2427. [DOI] [PubMed] [Google Scholar]
- Brandtzaeg P. Induction of secretory immunity and memory at mucosal surfaces. Vaccine. 2007;25:5467–5484. doi: 10.1016/j.vaccine.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Brandtzaeg P. Mucosal immunity: induction, dissemination, and effector functions. Scand J Immunol. 2009;70:505–515. doi: 10.1111/j.1365-3083.2009.02319.x. [DOI] [PubMed] [Google Scholar]
- Brandtzaeg P. Function of mucosa-associated lymphoid tissue in antibody formation. Immunol Invest. 2010:303–355. doi: 10.3109/08820131003680369. [DOI] [PubMed] [Google Scholar]
- Brandtzaeg P, Kiyono H, Pabst R, Russell MW. Terminology: nomenclature of mucosa-associated lymphoid tissue. Mucosal Immunol. 2008;1:31–37. doi: 10.1038/mi.2007.9. [DOI] [PubMed] [Google Scholar]
- Bromage ES, Kaattari IM, Zwollo P, Kaattari SL. Plasmablast and plasma cell production and distribution in trout immune tissues. J Immunol. 2004a;173:7317–7323. doi: 10.4049/jimmunol.173.12.7317. [DOI] [PubMed] [Google Scholar]
- Bromage ES, Ye J, Kaattari SL. Antibody structural variation in rainbow trout fluids. Comp Biochem Physiol B Biochem Mol Biol. 2006;143:61–69. doi: 10.1016/j.cbpb.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Bromage ES, Ye J, Owens L, Kaattari IM, Kaattari SL. Use of staphylococcal protein A in the analysis of teleost immunoglobulin structural diversity. Dev Comp Immunol. 2004b;28:803–814. doi: 10.1016/j.dci.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Brown WR, Kloppel TM. The role of the liver in translocation of IgA into the gastrointestinal tract. Immunol Invest. 1989;18:269–285. doi: 10.3109/08820138909112242. [DOI] [PubMed] [Google Scholar]
- Buchmann K, Sigh J, Nielsen CV, Dalgaard M. Host responses against the fish parasitizing ciliate Ichthyophthirius multifiliis. Veterinary Parasitology. 2001;100:105–116. doi: 10.1016/s0304-4017(01)00487-3. [DOI] [PubMed] [Google Scholar]
- Cain KD, Jones DR, Raison RL. Antibody-antigen kinetics following immunization of rainbow trout (Oncorhynchus mykiss) with a T-cell dependent antigen. Dev Comp Immunol. 2002;26:181–190. doi: 10.1016/s0145-305x(01)00063-5. [DOI] [PubMed] [Google Scholar]
- Cain KD, LaPatra SE, Baldwin TJ, Shewmaker B, Jones J, Ristow SS. Characterization of mucosal immunity in rainbow trout Oncorhynchus mykiss challenged with infectious hematopoietic necrosis virus: Identification of antiviral activity. Diseases of Aquatic Organisms. 1996;27:161–172. [Google Scholar]
- Campos-Perez JJ, Ward M, Grabowski PS, Ellis AE, Secombes CJ. The gills are an important site of iNOS expression in rainbow trout Oncorhynchus mykiss after challenge with the Gram-positive pathogen Renibacterium salmoninarum. Immunology. 2000;99:153–161. doi: 10.1046/j.1365-2567.2000.00914.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerutti A, Rescigno M. The biology of intestinal immunoglobulin A responses. Immunity. 2008;28:740–750. doi: 10.1016/j.immuni.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K, Cerutti A. Vaccination strategies to promote mucosal antibody responses. Immunity. 2010;33:479–491. doi: 10.1016/j.immuni.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clem LW. Phylogeny of immunoglobulin structure and function. IV. Immunoglobulins of the giant grouper, Epinephelus itaira. J Biol Chem. 1971;246:9–15. [PubMed] [Google Scholar]
- Clem LW, McLean WE. Phylogeny of immunoglobulin structure and function. VII. Monomeric and tetrameric immunoglobulins of the margate, a marine teleost fish. Immunology. 1975;29:791–799. [PMC free article] [PubMed] [Google Scholar]
- Cobb CS, Levy MG, Noga EJ. Acquired immunity to amyloodiniosis is associated with an antibody response. Dis Aquat Organ. 1998a;34:125–133. doi: 10.3354/dao034125. [DOI] [PubMed] [Google Scholar]
- Cobb CS, Levy MG, Noga EJ. Development of immunity by the tomato clownfish Amphiprion frenatus to the dinoflagellate parasite Amyloodinium ocellatum. J Aquat Anim Health. 1998b;10:259–263. [Google Scholar]
- Cox RJ, Brokstad KA, Ogra P. Influenza Virus: Immunity and Vaccination Strategies. Comparison of the Immune Response to Inactivated and Live, Attenuated Influenza Vaccines. Scand J Immunol. 2004;59:1–15. doi: 10.1111/j.0300-9475.2004.01382.x. [DOI] [PubMed] [Google Scholar]
- Crosbie PBB, Nowak BF. Immune responses of barramundi, Lates calcarifer (Bloch), after administration of an experimental Vibrio harveyi bacterin by intraperitoneal injection, anal intubation and immersion. J Fish Dis. 2004;27:623–632. doi: 10.1111/j.1365-2761.2004.00575.x. [DOI] [PubMed] [Google Scholar]
- Cui M, Zhang Q, Yao Z, Zhang Z, Zhang H, Wang Y. Immunoglobulin M gene expression analysis of orange-spotted grouper, Epinephelus coioides following heat shock and Vibrio alginolyticus challenge. Fish Shellfish Immunol. 2010;29:1060–1065. doi: 10.1016/j.fsi.2010.08.018. [DOI] [PubMed] [Google Scholar]
- Danilova N, Bussmann J, Jekosch K, Steiner LA. The immunoglobulin heavy-chain locus in zebrafish: identification and expression of a previously unknown isotype, immunoglobulin Z. Nat Immunol. 2005;6:295–302. doi: 10.1038/ni1166. [DOI] [PubMed] [Google Scholar]
- Danilova N, Steiner LA. B cells develop in the zebrafish pancreas. Proc Natl Acad Sci U S A. 2002;99:13711–13716. doi: 10.1073/pnas.212515999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson GA, Ellis AE, Secombes CJ. Novel cell types isolated from the skin of rainbow trout. J Fish Dis. 1993a;42:301–306. [Google Scholar]
- Davidson GA, Ellis AE, Secombes CJ. Route of immunization influences the generation of antibody secreting cells in the gut of rainbow trout (Oncorhynchus mykiss) Dev Comp Immunol. 1993b;17:373–376. doi: 10.1016/0145-305x(93)90008-e. [DOI] [PubMed] [Google Scholar]
- Davidson GA, Lin SH, Secombes CJ, Ellis AE. Detection of specific and 'constitutive' antibody secreting cells in the gills, head kidney and peripheral blood leucocytes of dab (Limanda limanda) Vet Immunol Immunopathol. 1997;58:363–374. doi: 10.1016/s0165-2427(97)00017-2. [DOI] [PubMed] [Google Scholar]
- Delamare-Deboutteville J, Wood D, Barnes AC. Response and function of cutaneous mucosal and serum antibodies in barramundi (Lates calcarifer) acclimated in seawater and freshwater. Fish Shellfish Immunol. 2006;21:92–101. doi: 10.1016/j.fsi.2005.10.005. [DOI] [PubMed] [Google Scholar]
- DeLuca D, Wilson M, Warr GW. Lymphocyte heterogeneity in the trout, Salmo gairdneri, defined with monoclonal antibodies to IgM. Eur J Immunol. 1983;13:546–551. doi: 10.1002/eji.1830130706. [DOI] [PubMed] [Google Scholar]
- dos Santos NM, Romano N, de Sousa M, Ellis AE, Rombout JH. Ontogeny of B and T cells in sea bass (Dicentrarchus labrax, L.) Fish Shellfish Immunol. 2000;10:583–596. doi: 10.1006/fsim.2000.0273. [DOI] [PubMed] [Google Scholar]
- dos Santos NM, Taverne-Thiele JJ, Barnes AC, van Muiswinkel WB, Ellis AE, Rombout JH. The gill is a major organ for antibody secreting cell production following direct immersion of sea bass (Dicentrarchus labrax, L.) in a Photobacterium damselae ssp. piscicida bacterin: an ontogenetic study. Fish Shellfish Immunol. 2001a;11:65–74. doi: 10.1006/fsim.2000.0295. [DOI] [PubMed] [Google Scholar]
- dos Santos NMS, Taverne-Thiele JJ, Barnes AC, Ellis AE, Rombout JHWM. Kinetics of juvenile sea bass (Dicentrarchus labrax L.) systemic and mucosal antibody secreting cell response to different antigens (Photobacterium damselae spp. piscicida, Vibrio anguillarum and DNP) Fish Shellfish Immunol. 2001b;11:317–331. doi: 10.1006/fsim.2000.0320. [DOI] [PubMed] [Google Scholar]
- dos santos NMS, Taverne N, Taverne-Thiele AJ, De Sousa M, Rombout JHWM. Characterisation of monoclonal antibodies specific for sea bass (Dicentrarchus labraxL.) IgM indicates the existence of B cell subpopulations. Fish Shellfish Immunol. 1997;7:175–191. [Google Scholar]
- Drennan JD, LaPatra SE, Swan CA, Ireland S, Cain KD. Characterization of serum and mucosal antibody responses in white sturgeon (Acipenser transmontanus Richardson) following immunization with WSIV and a protein hapten antigen. Fish Shellfish Immunol. 2007;23:657–669. doi: 10.1016/j.fsi.2007.01.016. [DOI] [PubMed] [Google Scholar]
- Edholm ES, Bengten E, Stafford JL, Sahoo M, Taylor EB, Miller NW, Wilson M. Identification of two IgD+ B cell populations in channel catfish, Ictalurus punctatus. J Immunol. 2010a;185:4082–4094. doi: 10.4049/jimmunol.1000631. [DOI] [PubMed] [Google Scholar]
- Edholm ES, Bengten E, Wilson M. Insights into the function of IgD. Dev Comp Immunol. 2011 doi: 10.1016/j.dci.2011.03.002. [DOI] [PubMed] [Google Scholar]
- Edholm ES, Hudgens ED, Tompkins D, Sahoo M, Burkhalter B, Miller NW, Bengten E, Wilson M. Characterization of anti-channel catfish IgL sigma monoclonal antibodies. Vet Immunol Immunopathol. 2010b;135:325–328. doi: 10.1016/j.vetimm.2010.01.004. [DOI] [PubMed] [Google Scholar]
- Edholm ES, Wilson M, Sahoo M, Miller NW, Pilstrom L, Wermenstam NE, Bengten E. Identification of Igsigma and Iglambda in channel catfish, Ictalurus punctatus, and Iglambda in Atlantic cod, Gadus morhua. Immunogenetics. 2009;61:353–370. doi: 10.1007/s00251-009-0365-z. [DOI] [PubMed] [Google Scholar]
- Elcombe BM, Chang RJ, Taves CJ, Winkelhake JL. Evolution of antibody structure and effector functions: comparative hemolytic activities of monomeric and tetrameric IgM from rainbow trout, Salmo gairdnerii. Comp Biochem Physiol B. 1985;80:697–706. doi: 10.1016/0305-0491(85)90448-1. [DOI] [PubMed] [Google Scholar]
- Ellis AE. Fish Vaccination. Academic Press Inc.; 1988. [Google Scholar]
- Ellis AE. Recent development in oral vaccine delivery systems. Fish Pathology. 1995;30:293–300. [Google Scholar]
- Esteve-Gassent MD, Barrera R, Amaro C. Vaccination of market-size eels against vibriosis due to Vibrio vulnificus serovar E. Aquaculture. 2004a;241:9–19. [Google Scholar]
- Esteve-Gassent MD, Fouz B, Amaro C. Efficacy of a bivalent vaccine against eel diseases caused by Vibrio vulnificus after its administration by four different routes. Fish Shellfish Immunol. 2004b;16:93–105. doi: 10.1016/S1050-4648(03)00036-6. [DOI] [PubMed] [Google Scholar]
- Esteve-Gassent MD, Nielsen ME, Amaro C. The kinetics of antibody production in mucus and serum of European eel (Anguilla anguilla L.) after vaccination against Vibrio vulnificus: development of a new method for antibody quantification in skin mucus. Fish & Shellfish Immunology. 2003;15:51–61. doi: 10.1016/s1050-4648(02)00138-9. [DOI] [PubMed] [Google Scholar]
- Evans DH. The physiology of fishes. CRC Press; 1998. [Google Scholar]
- Fagarasan S. Evolution, development, mechanism and function of IgA in the gut. Curr Opin Immunol. 2008;20:170–177. doi: 10.1016/j.coi.2008.04.002. [DOI] [PubMed] [Google Scholar]
- Fast MD, Sims DE, Burka JF, Mustafa A, Ross NW. Skin morphology and humoral non-specific defence parameters of mucus and plasma in rainbow trout, coho and Atlantic salmon. Comp Biochem Physiol A Mol Integr Physiol. 2002;132:645–657. doi: 10.1016/s1095-6433(02)00109-5. [DOI] [PubMed] [Google Scholar]
- Feng LN, Lu DQ, Bei JX, Chen JL, Liu Y, Zhang Y, Liu XC, Meng ZN, Wang L, Lin HR. Molecular cloning and functional analysis of polymeric immunoglobulin receptor gene in orange-spotted grouper (Epinephelus coioides) Comp Biochem Physiol B Biochem Mol Biol. 2009;154:282–289. doi: 10.1016/j.cbpb.2009.07.003. [DOI] [PubMed] [Google Scholar]
- Fillatreau S, Gray D, Anderton SM. Not always the bad guys: B cells as regulators of autoimmune pathology. Nat Rev Immunol. 2008;8:391–397. doi: 10.1038/nri2315. [DOI] [PubMed] [Google Scholar]
- Fillatreau S, Sweenie CH, McGeachy MJ, Gray D, Anderton SM. B cells regulate autoimmunity by provision of IL-10. Nat Immunol. 2002;3:944–950. doi: 10.1038/ni833. [DOI] [PubMed] [Google Scholar]
- Finke D, Meier D. Molecular networks orchestrating GALT development. Curr Top Microbiol Immunol. 2006;308:19–57. doi: 10.1007/3-540-30657-9_2. [DOI] [PubMed] [Google Scholar]
- Flajnik MF. The last flag unfurled? A new immunoglobulin isotype in fish expressed in early development. Nat Immunol. 2005;6:229–230. doi: 10.1038/ni0305-229. [DOI] [PubMed] [Google Scholar]
- Flajnik MF. All GOD's creatures got dedicated mucosal immunity. Nat Immunol. 2010;11:777–779. doi: 10.1038/ni0910-777. [DOI] [PubMed] [Google Scholar]
- Fletcher TC, Grant PT. Immunoglobulins in the serum and mucus of the plaice (Pleuronectes platessa) Biochem J. 1969;115:65P. doi: 10.1042/bj1150065p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier-Betz V, Quentel C, Lamour F, LeVen A. Immunocytochemical detection of Ig-positive cells in blood, lymphoid organs and the gut associated lymphoid tissue of the turbot (Scophthalmus maximus) Fish Shellfish Immunol. 2000;10:187–202. doi: 10.1006/fsim.1999.0235. [DOI] [PubMed] [Google Scholar]
- Fuglem B, Jirillo E, Bjerkas I, Kiyono H, Nochi T, Yuki Y, Raida M, Fischer U, Koppang EO. Antigen-sampling cells in the salmonid intestinal epithelium. Dev Comp Immunol. 2010;34:768–774. doi: 10.1016/j.dci.2010.02.007. [DOI] [PubMed] [Google Scholar]
- Gonzalez SF, Chatziandreou N, Nielsen ME, Li W, Rogers J, Taylor R, Santos Y, Cossins A. Cutaneous immune responses in the common carp detected using transcript analysis. Mol Immunol. 2007;44:1664–1679. doi: 10.1016/j.molimm.2006.08.016. [DOI] [PubMed] [Google Scholar]
- Grabowski LD, LaPatra SE, Cain KD. Systemic and mucosal antibody response in tilapia, Oreochromis niloticus (L.), following immunization with Flavobacterium columnare. Journal of Fish Diseases. 2004;27:573–581. doi: 10.1111/j.1365-2761.2004.00576.x. [DOI] [PubMed] [Google Scholar]
- Grontvedt RN, Espelid S. Immunoglobulin producing cells in the spotted wolffish (Anarhichas minor Olafsen): localization in adults and during juvenile development. Dev Comp Immunol. 2003;27:569–578. doi: 10.1016/s0145-305x(03)00028-4. [DOI] [PubMed] [Google Scholar]
- Grontvedt RN, Espelid S. Vaccination and immune responses against atypical Aeromonas salmonicida in spotted wolffish (Anarhichas minor Olafsen) juveniles. Fish & Shellfish Immunology. 2004;16:271–285. doi: 10.1016/S1050-4648(03)00109-8. [DOI] [PubMed] [Google Scholar]
- Grove S, Johansen R, Reitan LJ, Press CM. Immune- and enzyme histochemical characterisation of leukocyte populations within lymphoid and mucosal tissues of Atlantic halibut (Hippoglossus hippoglossus) Fish Shellfish Immunol. 2006a;20:693–708. doi: 10.1016/j.fsi.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Grove S, Johansen R, Reitan LJ, Press CM, Dannevig BH. Quantitative investigation of antigen and immune response in nervous and lymphoid tissues of Atlantic halibut (Hippoglossus hippoglossus) challenged with nodavirus. Fish Shellfish Immunol. 2006b;21:525–539. doi: 10.1016/j.fsi.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Hamuro K, Suetake H, Saha NR, Kikuchi K, Suzuki Y. A teleost polymeric Ig receptor exhibiting two Ig-like domains transports tetrameric IgM into the skin. J Immunol. 2007;178:5682–5689. doi: 10.4049/jimmunol.178.9.5682. [DOI] [PubMed] [Google Scholar]
- Han Y, Huang GH, Zhang QF, Yuan SC, Liu JZ, Zheng TT, Fan LF, Chen SW, Xu AL. The primitive immune system of amphioxus provides insights into the ancestral structure of the vertebrate immune system. Developmental and Comparative Immunology. 2010;34:791–796. doi: 10.1016/j.dci.2010.03.009. [DOI] [PubMed] [Google Scholar]
- Hansen JD, Landis ED, Phillips RB. Discovery of a unique Ig heavy-chain isotype (IgT) in rainbow trout: Implications for a distinctive B cell developmental pathway in teleost fish. Proc Natl Acad Sci U S A. 2005;102:6919–6924. doi: 10.1073/pnas.0500027102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen JD, Zapata AG. Lymphocyte development in fish and amphibians. Immunol Rev. 1998;166:199–220. doi: 10.1111/j.1600-065x.1998.tb01264.x. [DOI] [PubMed] [Google Scholar]
- Harris JE. The immune response of a Cyprinid fish to infections of the acanthocephalan Pomphorhynchus laevis. Int J Parasitol. 1972;2:459–469. doi: 10.1016/0020-7519(72)90091-4. [DOI] [PubMed] [Google Scholar]
- Hart S, Wrathmell AB, Harris JE, Grayson TH. Gut immunology in fish: a review. Dev Comp Immunol. 1988;12:453–480. doi: 10.1016/0145-305x(88)90065-1. [DOI] [PubMed] [Google Scholar]
- Hatanaka A, Umeda N, Yamashita S, Hirazawa N. A small ciliary surface glycoprotein of the monogenean parasite Neobenedenia girellae acts as an agglutination/immobilization antigen and induces an immune response in the Japanese flounder Paralichthys olivaceus. Parasitology. 2005;131:591–600. doi: 10.1017/S0031182005008322. [DOI] [PubMed] [Google Scholar]
- Hatten F, Fredriksen A, Hordvik I, Endresen C. Presence of IgM in cutaneous mucus, but not in gut mucus of Atlantic salmon, Salmo salar. Serum IgM is rapidly degraded when added to gut mucus. Fish Shellfish Immunol. 2001;11:257–268. doi: 10.1006/fsim.2000.0313. [DOI] [PubMed] [Google Scholar]
- Haugarvoll E, Bjerkas I, Nowak BF, Hordvik I, Koppang EO. Identification and characterization of a novel intraepithelial lymphoid tissue in the gills of Atlantic salmon. J Anat. 2008;213:202–209. doi: 10.1111/j.1469-7580.2008.00943.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havarstein LS, Aasjord PM, Ness S, Endresen C. Purification and partial characterization of an IgM-like serum immunoglobulin from Atlantic salmon (Salmo salar) Dev Comp Immunol. 1988;12:773–785. doi: 10.1016/0145-305x(88)90052-3. [DOI] [PubMed] [Google Scholar]
- Herbomel P, Thisse B, Thisse C. Zebrafish early macrophages colonize cephalic mesenchyme and developing brain, retina, and epidermis through a M-CSF receptor-dependent invasive process. Dev Biol. 2001;238:274–288. doi: 10.1006/dbio.2001.0393. [DOI] [PubMed] [Google Scholar]
- Hikima JI, Jung TS, Aoki T. Immunoglobulin genes and their transcriptional control in teleosts. Dev Comp Immunol. 2010;35:924–936. doi: 10.1016/j.dci.2010.10.011. [DOI] [PubMed] [Google Scholar]
- Holzer AS, Sommerville C, Wootten R. Tracing the route of Sphaerospora truttae from the entry locus to the target organ of the host, Salmo salar L., using an optimized and specific in situ hybridization technique. J Fish Dis. 2003;26:647–655. doi: 10.1046/j.1365-2761.2003.00501.x. [DOI] [PubMed] [Google Scholar]
- Hordvik I. Identification of a novel immunoglobulin delta transcript and comparative analysis of the genes encoding IgD in Atlantic salmon and Atlantic halibut. Mol Immunol. 2002;39:85–91. doi: 10.1016/s0161-5890(02)00043-3. [DOI] [PubMed] [Google Scholar]
- Hung HW, Lo CF, Tseng CC, Kou GH. Antibody response of glass eels, Anguilla japonica Temminck & Schlegel, to Pleistophora anguillarum Hoshina (Microspora) infection. Journal of Fish Diseases. 1997;20:237–239. [Google Scholar]
- Huttenhuis HB, Romano N, Van Oosterhoud CN, Taverne-Thiele AJ, Mastrolia L, Van Muiswinkel WB, Rombout JH. The ontogeny of mucosal immune cells in common carp (Cyprinus carpio L.) Anat Embryol (Berl) 2006;211:19–29. doi: 10.1007/s00429-005-0062-0. [DOI] [PubMed] [Google Scholar]
- Iger Y, Abraham M. The process of skin healing in experimentally wounded carp. J Fish Biol. 1990;36:421–437. [Google Scholar]
- Iger Y, Abraham M, Dotan A, Fattal B, Rahamim E. Cellular responses in the skin of carp maintained in organically fertilized water. J Fish Biol. 1988;33:711–720. [Google Scholar]
- Iijima HTI, Kiyono H. Mucosal immune network in the gut for the control of infectious diseases. Rev Med Virol. 2001;11:117–133. doi: 10.1002/rmv.307. [DOI] [PubMed] [Google Scholar]
- Inami M, Taverne-Thiele AJ, Schroder MB, Kiron V, Rombout JH. Immunological differences in intestine and rectum of Atlantic cod (Gadus morhua L.) Fish Shellfish Immunol. 2009;26:751–759. doi: 10.1016/j.fsi.2009.03.007. [DOI] [PubMed] [Google Scholar]
- Irie T, Watarai S, Kodama H. Humoral immune response of carp (Cyprinus carpio) induced by oral immunization with liposome-entrapped antigen. Developmental and Comparative Immunology. 2003;27:413–421. doi: 10.1016/s0145-305x(02)00137-4. [DOI] [PubMed] [Google Scholar]
- Jenkins PG, Wrathmell AB, Harris JE, Pulsford AL. Systemic and mucosal immune responses to enterically delivered antigen in Oreochromis mossambicus. Fish Shellfish Immunol. 1994;4:255–271. [Google Scholar]
- Joosten PHM, Tiemersma E, Threels A, Caumartin-Dhieux C, Rombout JHWM. Oral vaccination of fish against Vibrio anguillarum using alginate microparticles. Fish Shellfish Immunol. 1997;7:471–485. [Google Scholar]
- Jorgensen TR, Raida MK, Kania PW, Buchmann K. Response of rainbow trout (Oncorhynchus mykiss) in skin and fin tissue during infection with a variant of Gyrodactylus salaris (Monogenea: Gyrodactylidae) Folia Parasitologica. 2009;56:251–258. doi: 10.14411/fp.2009.029. [DOI] [PubMed] [Google Scholar]
- Kaattari S, Evans D, Klemer J. Varied redox forms of teleost IgM: an alternative to isotypic diversity? Immunol Rev. 1998;166:133–142. doi: 10.1111/j.1600-065x.1998.tb01258.x. [DOI] [PubMed] [Google Scholar]
- Kennedy CR, Walker PJ. Evidence for an immune response by dace, Leuciscus Leuciscus, to infection by cestode Caryophyllaeus laticeps. Journal of Parasitology. 1969;55:579–582. [PubMed] [Google Scholar]
- Kim JH, Yokoyama H, Ogawa K, Takahashi S, Wakabayashi H. Humoral immune response of ayu, Plecoglossus altivelis to Glugea plecoglossi (Protozoa: Microspora) Fish Pathology. 1996;31:215–220. [Google Scholar]
- Koppang EO, Fischer U, Moore L, Tranulis MA, Dijkstra JM, Kollner B, Aune L, Jirillo E, Hordvik I. Salmonid T cells assemble in the thymus, spleen and in novel interbranchial lymphoid tissue. J Anat. 2010;217:728–739. doi: 10.1111/j.1469-7580.2010.01305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koumans-van Diepen JC, Egberts E, Peixoto BR, Taverne N, Rombout JH. B cell and immunoglobulin heterogeneity in carp (Cyprinus carpio L.); an immuno(cyto)chemical study. Dev Comp Immunol. 1995;19:97–108. doi: 10.1016/0145-305x(94)00061-j. [DOI] [PubMed] [Google Scholar]
- Koumans-van Diepen JCE, Taverne-Thiele JJ, van Rens BTTM, Rombout JHMW. Immunocytochemical and flow cytometric analysis of B cells and plasma cells in carp (Cyprinus carpio L.); an ontogenetic study. Fish Shellfish Immunol. 1994;4:19–28. [Google Scholar]
- Kwon SR, Lee EH, Nam YK, Kim SK, Kim KH. Efficacy of oral immunization with Edwardsiella tarda ghosts against edwardsiellosis in olive flounder (Paralichthys olivaceus. Aquaculture. 2007;269:84–88. [Google Scholar]
- LaFrentz BR, LaPatra SE, Jones GR, Congleton JL, Sun B, Cain KD. Characterization of serum and mucosal antibody responses and relative per cent survival in rainbow trout, Oncorhynchus mykiss (Walbaum), following immunization and challenge with Flavobacterium psychrophilum. J Fish Dis. 2002;25:703–713. [Google Scholar]
- Lam SH, Chua HL, Gong Z, Lam TJ, Sin YM. Development and maturation of the immune system in zebrafish, Danio rerio: a gene expression profiling, in situ hybridization and immunological study. Dev Comp Immunol. 2004;28:9–28. doi: 10.1016/s0145-305x(03)00103-4. [DOI] [PubMed] [Google Scholar]
- Langford TD, Housley MP, Boes M, Chen JZ, Kagnoff MF, Gillin FD, Eckmann L. Central importance of immunoglobulin a in host defense against Giardia spp. Infection and Immunity. 2002;70:11–18. doi: 10.1128/IAI.70.1.11-18.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanning DK, Rhee KJ, Knight KL. Intestinal bacteria and development of the B-lymphocyte repertoire. Trends Immunol. 2005;26:419–425. doi: 10.1016/j.it.2005.06.001. [DOI] [PubMed] [Google Scholar]
- Levine MM, Kaper JB. Live oral cholera vaccine : from principle to product. Bulletin de l'Institut Pasteur. 1995;93:243–253. [Google Scholar]
- Li J, Barreda DR, Zhang YA, Boshra H, Gelman AE, Lapatra S, Tort L, Sunyer JO. B lymphocytes from early vertebrates have potent phagocytic and microbicidal abilities. Nat Immunol. 2006;7:1116–1124. doi: 10.1038/ni1389. [DOI] [PubMed] [Google Scholar]
- Lin SH, Davidson GA, Secombes CJ, Ellis AE. A morphological study of cells isolated from the perfused gill of dab and Atlantic salmon. Journal of Fish Biology. 1998;53:560–568. [Google Scholar]
- Lin SH, Davidson GA, Secombes CJ, Ellis AE. Use of a lipid-emulsion carrier for immunisation of dab (Limanda limanda) by bath and oral routes: an assessment of systemic and mucosal antibody responses. Aquaculture. 2000;181:11–24. [Google Scholar]
- Lin SH, Ellis AE, Davidson GA, Secombes CJ. Migratory, respiratory burst and mitogenic responses of leucocytes isolated from the gills of rainbow trout (Oncorhynchus mykiss) Fish & Shellfish Immunology. 1999;9:211–226. [Google Scholar]
- Liu Y, Zhou Z, Shi P, He S, Yao B. The comparative analysis of the adherent bacterial flora in the gill and skin surface of Epinephelus awoara in cages by PCR-DGGE. J Agric Sc Technol. 2008;10:81–86. [Google Scholar]
- Lobb CJ. Secretory immunity induced in catfish, Ictalurus punctatus, following bath immunization. Dev Comp Immunol. 1987;11:727–738. doi: 10.1016/0145-305x(87)90060-7. [DOI] [PubMed] [Google Scholar]
- Lobb CJ, Clem LW. The metabolic relationships of the immunoglobulins in fish serum, cutaneous mucus, and bile. J Immunol. 1981a;127:1525–1529. [PubMed] [Google Scholar]
- Lobb CJ, Clem LW. Phylogeny of immunoglobulin structure and function-XII. Secretory immunoglobulins in the bile of the marine teleost Archosargus probatocephalus. Mol Immunol. 1981b;18:615–619. doi: 10.1016/0161-5890(81)90032-8. [DOI] [PubMed] [Google Scholar]
- Lobb CJ, Clem LW. Phylogeny of immunoglobulin structure and function. XI. Secretory immunoglobulins in the cutaneous mucus of the sheepshead, Archosargus probatocephalus. Dev Comp Immunol. 1981c;5:587–596. doi: 10.1016/s0145-305x(81)80033-x. [DOI] [PubMed] [Google Scholar]
- Lobb CJ, Clem LW. Fish lymphocytes differ in the expression of surface immunoglobulin. Dev Comp Immunol. 1982;6:473–479. doi: 10.1016/s0145-305x(82)80033-5. [DOI] [PubMed] [Google Scholar]
- Lobb CJ, Clem W. Phylogeny of immunoglobulin in structure and function-x. Humoral immunoglobulins of the sheepshead, Archosargus probatocephalus. Dev Comp Immunol. 1981d;5:271–282. doi: 10.1016/0145-305x(81)90034-3. [DOI] [PubMed] [Google Scholar]
- Lobb CJ, Olson MO, Clem LW. Immunoglobulin light chain classes in a teleost fish. J Immunol. 1984;132:1917–1923. [PubMed] [Google Scholar]
- Lovy J, Wright GM, Speare DJ. Ultrastructural examination of the host inflammatory response within gills of netpen reared chinook salmon (Oncorhynchus tshawytscha) with Microsporidial Gill Disease. Fish Shellfish Immunol. 2007;22:131–149. doi: 10.1016/j.fsi.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Lu AJ, Li ZQ, Zhang QY. Detection of cutaneous antibodies in excised skin explants from grass carp, Ctenopharyngodon idella (Valenciennes), immune to Scophthalmus maximus rhabdovirus. J Fish Dis. 2008;31:559–565. doi: 10.1111/j.1365-2761.2008.00919.x. [DOI] [PubMed] [Google Scholar]
- Lumsden JS, Ostland VE, Byrne PJ, Ferguson HW. Detection of a distinct gill-surface antibody-response following horizontal infection and bath challenge of brook trout Salvelinus fontinalis with Flavobacterium branchiophilum, the causative agent of bacterial gill disease. Dis Aquat Org. 1993;16:21–27. [Google Scholar]
- Lumsden JS, Ostland VE, MacPhee DD, Ferguson HW. Production of gill-associated and serum antibody by rainbow trout (Oncorhynchus mykiss) following immersion immunization with acetone-killed Flavobacterium branchiophilum and the relationship to protection from experimental challenge. Fish Shellfish Immunol. 1995;5:151–165. [Google Scholar]
- Luo XC, Xie MQ, Zhu XQ, Li AX. Protective immunity in grouper (Epinephelus coioides) following exposure to or injection with Cryptocaryon irritans. Fish & Shellfish Immunology. 2007;22:427–432. doi: 10.1016/j.fsi.2006.04.011. [DOI] [PubMed] [Google Scholar]
- Macpherson AJ, McCoy KD, Johansen F-E, Brandtzaeg P. The immune geography of IgA induction and function. Mucosal Immunol. 2008;1:11–22. doi: 10.1038/mi.2007.6. [DOI] [PubMed] [Google Scholar]
- Magnadottir B, Lange S, Gudmundsdottir S, Bogwald J, Dalmo RA. Ontogeny of humoral immune parameters in fish. Fish Shellfish Immunol. 2005;19:429–439. doi: 10.1016/j.fsi.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Maki JL, Dickerson HW. Systemic and cutaneous mucus antibody responses of channel catfish immunized against the protozoan parasite Ichthyophthirius multifiliis. Clinical and Diagnostic Laboratory Immunology. 2003;10:876–881. doi: 10.1128/CDLI.10.5.876-881.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews RA. Advances in Parasitology, Vol 59. Vol. 59. San Diego: Elsevier Academic Press; 2005. Ichthyophthirius multifiliis Fouquet and ichthyophthiriosis in freshwater teleosts; pp. 159–241. [DOI] [PubMed] [Google Scholar]
- Mauri C. Regulation of immunity and autoimmunity by B cells. Curr Opin Immunol. 22:761–767. doi: 10.1016/j.coi.2010.10.009. [DOI] [PubMed] [Google Scholar]
- Merino-Contreras ML, Guzman-Murillo MA, Ruiz-Bustos E, Romero MJ, Cadena-Roa MA, Ascencio F. Mucosal immune response of spotted sand bass Paralabrax maculatofasciatus (Steindachner 1868) orally immunised with an extracellular lectin of Aeromonas veronii. Fish Shellfish Immunol. 2001;11:115–126. doi: 10.1006/fsim.2000.0299. [DOI] [PubMed] [Google Scholar]
- Mizoguchi A, Mizoguchi E, Takedatsu H, Blumberg RS, Bhan AK. Chronic intestinal inflammatory condition generates IL-10-producing regulatory B cell subset characterized by CD1d upregulation. Immunity. 2002;16:219–230. doi: 10.1016/s1074-7613(02)00274-1. [DOI] [PubMed] [Google Scholar]
- Monteiro SM, Oliveira E, Fontainhas-Fernandes A, Sousa M. Fine Structure of the Branchial Epithelium in the Teleost Oreochromis niloticus. Journal of Morphology. 2010;271:621–633. doi: 10.1002/jmor.10821. [DOI] [PubMed] [Google Scholar]
- Moulana M, Evenhuis J, Albertino M, Godwin U, Kountikov EI, Stuge TB, Wilson M, Bengten E, Miller NW, McConnell TJ. Characterization of anti-channel catfish MHC class IIbeta monoclonal antibodies. Vet Immunol Immunopathol. 2008;126:120–130. doi: 10.1016/j.vetimm.2008.06.012. [DOI] [PubMed] [Google Scholar]
- Mulero I, Pilar Sepulcre M, Roca FJ, Meseguer J, Garcia-Ayala A, Mulero V. Characterization of macrophages from the bony fish gilthead seabream using an antibody against the macrophage colony-stimulating factor receptor. Dev Comp Immunol. 2008;32:1151–1159. doi: 10.1016/j.dci.2008.03.005. [DOI] [PubMed] [Google Scholar]
- Mulero I, Sepulcre MP, Meseguer J, Garcia-Ayala A, Mulero V. Histamine is stored in mast cells of most evolutionarily advanced fish and regulates the fish inflammatory response. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:19434–19439. doi: 10.1073/pnas.0704535104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayak SK. Probiotics and immunity: a fish perspective. Fish Shellfish Immunol. 2010;29:2–14. doi: 10.1016/j.fsi.2010.02.017. [DOI] [PubMed] [Google Scholar]
- Olsen MM, Kania PW, Heinecke RD, Skjoedt K, Rasmussen KJ, Buchmann K. Cellular and humoral factors involved in the response of rainbow trout gills to Ichthyophthirius multifiliis infections: molecular and immunohistochemical studies. Fish Shellfish Immunol. 2011;30:859–869. doi: 10.1016/j.fsi.2011.01.010. [DOI] [PubMed] [Google Scholar]
- Olson KR. Vascular anatomy of the fish gill. Journal of Experimental Zoology. 2002;293:214–231. doi: 10.1002/jez.10131. [DOI] [PubMed] [Google Scholar]
- Overland HS, Pettersen EF, Ronneseth A, Wergeland HI. Phagocytosis by B-cells and neutrophils in Atlantic salmon (Salmo salar L.) and Atlantic cod (Gadus morhua L.) Fish Shellfish Immunol. 2009 doi: 10.1016/j.fsi.2009.10.021. [DOI] [PubMed] [Google Scholar]
- Palaksha KJ, Shin GW, Kim YR, Jung TS. Evaluation of non-specific immune components from the skin mucus of olive flounder (Paralichthys olivaceus) Fish Shellfish Immunol. 2008;24:479–488. doi: 10.1016/j.fsi.2008.01.005. [DOI] [PubMed] [Google Scholar]
- Peleteiro MC, Richards RH. Immunocytochemical studies on immunoglobulin-containing cells in the epidermis of rainbo trout Salmo gairdneri Richardson: influence of bath vaccination. J Fish Biol. 1988;32:845–858. [Google Scholar]
- Peleteiro MC, Richards RH. Phagocytic cells in the epidermis of rainbow trout, Salmo gairdneri Richardson. J Fish Dis. 1990;13:225–232. [Google Scholar]
- Perez T, Balcazar JL, Ruiz-Zarzuela I, Halaihel N, Vendrell D, de Blas I, Muzquiz JL. Host-microbiota interactions within the fish intestinal ecosystem. Mucosal Immunol. 2010;3:355–360. doi: 10.1038/mi.2010.12. [DOI] [PubMed] [Google Scholar]
- Picchietti S, Mazzini M, Taddei AR, Renna R, Fausto AM, Mulero V, Carnevali O, Cresci A, Abelli L. Effects of administration of probiotic strains on GALT of larval gilthead seabream: Immunohistochemical and ultrastructural studies. Fish & Shellfish Immunology. 2007;22:57–67. doi: 10.1016/j.fsi.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Pillay TVR, Kutty MN. Aquaculture: principles and practices. Blackwell Publishing Ltd.; 2005. [Google Scholar]
- Pilstrom L. The mysterious immunoglobulin light chain. Dev Comp Immunol. 2002;26:207–215. doi: 10.1016/s0145-305x(01)00066-0. [DOI] [PubMed] [Google Scholar]
- Rawls JF, Samuel BS, Gordon JI. Gnotobiotic zebrafish reveal evolutionarily conserved responses to the gut microbiota. Proc Natl Acad Sci U S A. 2004;101:4596–4601. doi: 10.1073/pnas.0400706101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringo E, Holzapfel W. Identification and characterization of carnobacteria associated with the gills of Atlantic salmon (Salmo salar L.) Systematic and Applied Microbiology. 2000;23:523–527. doi: 10.1016/s0723-2020(00)80026-0. [DOI] [PubMed] [Google Scholar]
- Ringo E, Salinas I, Olsen RE, Nyhaug A, Myklebust R, Mayhew TM. Histological changes in intestine of Atlantic salmon (Salmo salar L.) following in vitro exposure to pathogenic and probiotic bacterial strains. Cell Tissue Res. 2007;328:109–116. doi: 10.1007/s00441-006-0323-0. [DOI] [PubMed] [Google Scholar]
- Roberts SD, Powell MD. The viscosity and glycoprotein biochemistry of salmonid mucus varies with species, salinity and the presence of amoebic gill disease. J Comp Physiol B. 2005;175:1–11. doi: 10.1007/s00360-004-0453-1. [DOI] [PubMed] [Google Scholar]
- Rogers-Lowery CL, Dimock RV, Kuhn RE. Antibody response of bluegill sunfish during development of acquired resistance against the larvae of the freshwater mussel Utterbackia imbecillis. Dev Comp Immunol. 2007;31:143–155. doi: 10.1016/j.dci.2006.05.011. [DOI] [PubMed] [Google Scholar]
- Rojas R, Apodaca G. Immunoglobulin transport across polarized epithelial cells. Nat Rev Mol Cell Biol. 2002;3:944–955. doi: 10.1038/nrm972. [DOI] [PubMed] [Google Scholar]
- Romano N, Abelli L, Mastrolia L, Scapigliati G. Immunocytochemical detection and cytomorphology of lymphocyte subpopulations in a teleost fish Dicentrarchus labrax. Cell Tissue Res. 1997a;289:163–171. doi: 10.1007/s004410050862. [DOI] [PubMed] [Google Scholar]
- Romano N, Taverne-Thiele JJ, Van Maanen JC, Rombout JHMW. Leucocyte subpopulations in developing carp (Cyprinus carpioL.): immunocytochemical studies. Fish Shellfish Immunol. 1997b;7:439–453. [Google Scholar]
- Rombout JH, Abelli L, Picchietti S, Scapigliati G, Kiron V. Teleost intestinal immunology. Fish Shellfish Immunol. 2010 doi: 10.1016/j.fsi.2010.09.001. [DOI] [PubMed] [Google Scholar]
- Rombout JH, Huttenhuis HB, Picchietti S, Scapigliati G. Phylogeny and ontogeny of fish leucocytes. Fish Shellfish Immunol. 2005;19:441–455. doi: 10.1016/j.fsi.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Rombout JH, Joosten PH, Engelsma MY, Vos AP, Taverne N, Taverne-Thiele JJ. Indications for a distinct putative T cell population in mucosal tissue of carp (Cyprinus carpio L.) Dev Comp Immunol. 1998;22:63–77. doi: 10.1016/s0145-305x(97)00048-7. [DOI] [PubMed] [Google Scholar]
- Rombout JH, Lamers CH, Helfrich MH, Dekker A, Taverne-Thiele JJ. Uptake and transport of intact macromolecules in the intestinal epithelium of carp (Cyprinus carpio L.) and the possible immunological implications. Cell Tissue Res. 1985;239:519–530. doi: 10.1007/BF00219230. [DOI] [PubMed] [Google Scholar]
- Rombout JH, Taverne-Thiele AJ, Villena MI. The gut-associated lymphoid tissue (GALT) of carp (Cyprinus carpio L.): an immunocytochemical analysis. Dev Comp Immunol. 1993a;17:55–66. doi: 10.1016/0145-305x(93)90015-i. [DOI] [PubMed] [Google Scholar]
- Rombout JH, Taverne N, van de Kamp M, Taverne-Thiele AJ. Differences in mucus and serum immunoglobulin of carp (Cyprinus carpio L.) Dev Comp Immunol. 1993b;17:309–317. doi: 10.1016/0145-305x(93)90003-9. [DOI] [PubMed] [Google Scholar]
- Rombout JH, van der Tuin SJ, Yang G, Schopman N, Mroczek A, Hermsen T, Taverne-Thiele JJ. Expression of the polymeric Immunoglobulin Receptor (pIgR) in mucosal tissues of common carp (Cyprinus carpio L.) Fish Shellfish Immunol. 2008;24:620–628. doi: 10.1016/j.fsi.2008.01.016. [DOI] [PubMed] [Google Scholar]
- Rombout JHWM, van den Berg AA, van den Berg CTGA, Witte P, Egberts E. Immunological importance of the second gut segment of carp. III. Systemic and/or mucosal immune responses after immunization with soluble or particulate antigen. J Fish Biol. 1989;35:179–186. [Google Scholar]
- Rombout JW, Blok LJ, Lamers CH, Egberts E. Immunization of carp (Cyprinus carpio) with a Vibrio anguillarum bacterin: indications for a common mucosal immune system. Dev Comp Immunol. 1986;10:341–351. doi: 10.1016/0145-305x(86)90024-8. [DOI] [PubMed] [Google Scholar]
- Ryo S, Wijdeven RH, Tyagi A, Hermsen T, Kono T, Karunasagar I, Rombout JH, Sakai M, Kemenade BM, Savan R. Common carp have two subclasses of bonyfish specific antibody IgZ showing differential expression in response to infection. Dev Comp Immunol. 2010;34:1183–1190. doi: 10.1016/j.dci.2010.06.012. [DOI] [PubMed] [Google Scholar]
- Saha NR, Suetake H, Kikuchi K, Suzuki Y. Fugu immunoglobulin D: a highly unusual gene with unprecedented duplications in its constant region. Immunogenetics. 2004;56:438–447. doi: 10.1007/s00251-004-0693-y. [DOI] [PubMed] [Google Scholar]
- Salinas I, Abelli L, Bertoni F, Picchietti S, Roque A, Furones D, Cuesta A, Meseguer J, Esteban MA. Monospecies and multispecies probiotic formulations produce different systemic and local immunostimulatory effects in the gilthead seabream (Sparus aurata L.) Fish Shellfish Immunol. 2008;25:114–123. doi: 10.1016/j.fsi.2008.03.011. [DOI] [PubMed] [Google Scholar]
- Salinas I, Meseguer J, Esteban MA. Assessment of different protocols for the isolation and purification of gut associated lymphoid cells from the gilthead seabream (Sparus aurata L.) Biol Proced Online. 2007;9:43–55. doi: 10.1251/bpo132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez C, Dominguez J. Trout immunoglobulin populations differing in light chains revealed by monoclonal antibodies. Mol Immunol. 1991;28:1271–1277. doi: 10.1016/0161-5890(91)90014-b. [DOI] [PubMed] [Google Scholar]
- Santos Y, Garcia-Marquez S, Pereira PG, Pazos F, Riaza A, Silva R, El Morabit A, Ubeira FM. Efficacy of furunculosis vaccines in turbot, Scophthalmus maximus (L.): evaluation of immersion, oral and injection delivery. J Fish Dis. 2005;28:165–172. doi: 10.1111/j.1365-2761.2005.00610.x. [DOI] [PubMed] [Google Scholar]
- Sato A, Okamoto N. Induction of virus-specific cell-mediated cytotoxic responses of isogeneic ginbuna crucian carp, after oral immunization with inactivated virus. Fish Shellfish Immunol. 2010;29:414–421. doi: 10.1016/j.fsi.2010.04.017. [DOI] [PubMed] [Google Scholar]
- Savan R, Aman A, Nakao M, Watanuki H, Sakai M. Discovery of a novel immunoglobulin heavy chain gene chimera from common carp (Cyprinus carpio L.) Immunogenetics. 2005a;57:458–463. doi: 10.1007/s00251-005-0015-z. [DOI] [PubMed] [Google Scholar]
- Savan R, Aman A, Sato K, Yamaguchi R, Sakai M. Discovery of a new class of immunoglobulin heavy chain from fugu. Eur J Immunol. 2005b;35:3320–3331. doi: 10.1002/eji.200535248. [DOI] [PubMed] [Google Scholar]
- Scapigliati G, Romano N, Picchietti S, Mazzini M, Mastrolia L, Scalia D, Abelli L. Monoclonal antibodies against sea bass Dicentrarchus labrax (L) immunoglobulins: Immunolocalisation of immunoglobulin-bearing cells and applicability in immunoassays. Fish Shellfish Immunol. 1996;6:383–401. [Google Scholar]
- Schrøder MB, Flaño E, Pilström L, Jørgensen TØ. Localisation of Ig heavy chain mRNA positive cells in Atlantic cod (Gadus morhuaL.) tissues; identified byin situhybridisation. Fish Shellfish Immunol. 1998;8:565–576. [Google Scholar]
- Secombes CJ, van Groningen JJ, Egberts E. Separation of lymphocyte subpopulations in carp Cyprinus carpio L. by monoclonal antibodies: immunohistochemical studies. Immunology. 1983;48:165–175. [PMC free article] [PubMed] [Google Scholar]
- Shephard KL. Functions for fish mucus. Reviews in Fish Biology and Fisheries. 1994;4:401–429. [Google Scholar]
- Shin GW, Kim YR, Shin YS, Lee EG, Oh MJ, Yoshida T, Jung TS. Purification of two different immunoglobulins (Igs) from olive flounder Paralichthys olivaceus and analysis of Lactococcus garvieae antigens by the Igs. Fish Pathol. 2007;42:19–28. [Google Scholar]
- Shoemaker CA, Xu DH, Shelby RA, Klesius PH. Detection of cutaneous antibodies against Flavobacterium columnare in channel catfish, Ictalurus punctatus (Rafinesque) Aquaculture Research. 2005;36:813–818. [Google Scholar]
- Sitja-Bobadilla A, Redondo MJ, Bermudez R, Palenzuela O, Ferreiro I, Riaza A, Quiroga I, Nieto JM, Alvarez-Pellitero P. Innate and adaptive immune responses of turbot, Scophthalmus maximus (L.), following experimental infection with Enteromyxum scophthalmi (Myxosporea : Myxozoa) Fish & Shellfish Immunology. 2006;21:485–500. doi: 10.1016/j.fsi.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Solem ST, Stenvik J. Antibody repertoire development in teleosts--a review with emphasis on salmonids and Gadus morhua L. Dev Comp Immunol. 2006;30:57–76. doi: 10.1016/j.dci.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Srisapoome P, Ohira T, Hirono I, Aoki T. Genes of the constant regions of functional immunoglobulin heavy chain of Japanese flounder, Paralichthys olivaceus. Immunogenetics. 2004;56:292–300. doi: 10.1007/s00251-004-0689-7. [DOI] [PubMed] [Google Scholar]
- St Louis-Cormier EA, Osterland CK, Anderson PD. Evidence for a cutaneous secretory immune system in rainbow trout (Salmo gairdneri) Dev Comp Immunol. 1984;8:71–80. doi: 10.1016/0145-305x(84)90011-9. [DOI] [PubMed] [Google Scholar]
- Steinhagen D, Rombout J. Response of Ig-positive cells to Goussia carpelli (Protozoa, Apicomplexa) infection in carp (Cyprinus carpio L) Folia Parasitologica. 1994;41:173–176. [PubMed] [Google Scholar]
- Strand HK, Dalmo RA. Absorption of immunomodulating beta(1,3)- glucan in yolk sac larvae of Atlantic halibut, Hippoglossus hippoglossus (L) J Fish Dis. 1997;20:41–49. [Google Scholar]
- Sun Y, Liu CS, Sun L. Isolation and analysis of the vaccine potential of an attenuated Edwardsiella tarda strain. Vaccine. 2010;28:6344–6350. doi: 10.1016/j.vaccine.2010.06.101. [DOI] [PubMed] [Google Scholar]
- Sun Y, Wei Z, Hammarstrom L, Zhao Y. The immunoglobulin delta gene in jawed vertebrates: A comparative overview. Dev Comp Immunol. 2011;35:975–981. doi: 10.1016/j.dci.2010.12.010. [DOI] [PubMed] [Google Scholar]
- Sunyer JO, Boshra H, Li J. Evolution of anaphylatoxins, their diversity and novel roles in innate immunity: insights from the study of fish complement. Vet Immunol Immunopathol. 2005;108:77–89. doi: 10.1016/j.vetimm.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Swan CM, Lindstrom NM, Cain KD. Identification of a localized mucosal immune response in rainbow trout, Oncorhynchus mykiss (Walbaum), following immunization with a protein-hapten antigen. J Fish Dis. 2008;31:383–393. doi: 10.1111/j.1365-2761.2008.00918.x. [DOI] [PubMed] [Google Scholar]
- Temkin RJ, McMillan DB. Gut-associated lymphoid tissue (GALT) of the goldfish, Carassius auratus. J Morphol. 1986;190:9–26. doi: 10.1002/jmor.1051900103. [DOI] [PubMed] [Google Scholar]
- Tian JY, Sun BJ, Luo YP, Zhang YA, Nie P. Distribution of IgM, IgD and IgZ in mandarin fish, Siniperca chuatsi lymphoid tissues and their transcriptional changes after Flavobacterium columnare stimulation. Aquaculture. 2009a;288:14–21. [Google Scholar]
- Tian JY, Xie HX, Zhang YA, Xu Z, Yao WJ, Nie P. Ontogeny of IgM-producing cells in the mandarin fish Siniperca chuatsi identified by in situ hybridisation. Vet Immunol Immunopathol. 2009b;132:146–152. doi: 10.1016/j.vetimm.2009.05.018. [DOI] [PubMed] [Google Scholar]
- Tu FP, Chu WH, Zhuang XY, Lu CP. Effect of oral immunization with Aeromonas hydrophila ghosts on protection against experimental fish infection. Letters in Applied Microbiology. 2010;50:13–17. doi: 10.1111/j.1472-765X.2009.02746.x. [DOI] [PubMed] [Google Scholar]
- Umeda N, Hatanaka A, Hirazawa N. Immobilization antibodies of tiger puffer Takifugu rubripes induced by i.p. injection against monogenean Heterobothrium okamotoi oncomiracidia do not prevent the infection. Parasitology. 2007;134:853–863. doi: 10.1017/S0031182006002174. [DOI] [PubMed] [Google Scholar]
- Vervarcke S, Ollevier F, Kinget R, Michoel A. Mucosal response in African catfish after administration of Vibrio anguillarum O2 antigens via different routes. Fish Shellfish Immunol. 2005;18:125–133. doi: 10.1016/j.fsi.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Wang WW, Zhou ZG, He SX, Liu YC, Cao YN, Shi PJ, Yao B, Ringo E. Identification of the adherent microbiota on the gills and skin of poly-cultured gibel carp (Carassius auratus gibelio) and bluntnose black bream (Megalobrama amblycephala Yih) Aquaculture Research. 2010;41:e72–e83. [Google Scholar]
- Watts M, Munday BL, Burke CM. Isolation and partial characterisation of immunoglobulin from southern bluefin tuna Thunnus maccoyii Castelnau. Fish Shellfish Immunol. 2001;11:491–503. doi: 10.1006/fsim.2000.0329. [DOI] [PubMed] [Google Scholar]
- Whittington RJ. Purification and partial characterisation of serum immunoglobulin of the European perch (Perca fluviatilis L.) Fish Shellfish Immunol. 1993;3:331–343. [Google Scholar]
- Wilson JM, Laurent P. Fish gill morphology: inside out. Journal of Experimental Zoology. 2002;293:192–213. doi: 10.1002/jez.10124. [DOI] [PubMed] [Google Scholar]
- Wilson M, Bengten E, Miller NW, Clem LW, Du Pasquier L, Warr GW. A novel chimeric Ig heavy chain from a teleost fish shares similarities to IgD. Proc Natl Acad Sci U S A. 1997;94:4593–4597. doi: 10.1073/pnas.94.9.4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfle U, Martin S, Emde M, Schempp C. Dermatology in the Darwin anniversary. Part 2: Evolution of the skin-associated immune system. J Dtsch Dermatol Ges. 2009;7:862–869. doi: 10.1111/j.1610-0387.2009.07202.x. [DOI] [PubMed] [Google Scholar]
- Wong G, Kaattari SL, Christensen JM. Effectiveness of an oral enteric coated Vibrio vaccine for use in salmonid fish. Immunol Invest. 1992;21:353–364. doi: 10.3109/08820139209069375. [DOI] [PubMed] [Google Scholar]
- Xu DH, Klesius PH. Protective effect of cutaneous antibody produced by channel catfish, Ictalurus punctatus (Rafinesque), immune to Ichthyophthirius multifiliis Fouquet on cohabited non-immune catfish. J Fish Dis. 2003;26:287–291. doi: 10.1046/j.1365-2761.2003.00463.x. [DOI] [PubMed] [Google Scholar]
- Xu DH, Klesius PH, Shoemaker CA. Effect of immunization of channel catfish with inactivated trophonts on serum and cutaneous antibody titers and survival against Ichthyophthirius multifiliis. Fish Shellfish Immunol. 2009a;26:614–618. doi: 10.1016/j.fsi.2008.09.015. [DOI] [PubMed] [Google Scholar]
- Xu Z, Chen CF, Mao ZJ, Zhu WY. Detection of serum and mucosal antibody production and antibody secreting cells (ASCs) in large yellow croaker (Pseudosciaena crocea) following vaccination with Vibrio harveyi via different routes. Aquaculture. 2009b;287:243–247. [Google Scholar]
- Yambot AV, Song YL. Immunization of grouper, Epinephelus coioides confers protection against a protozoan parasite, Cryptocaryon irritans . Aquaculture. 2006;260:1–9. [Google Scholar]
- Yasumoto S, Kuzuya Y, Yasuda M, Yoshimura T, Miyazaki T. Oral immunization of common carp with a liposome vaccine fusing koi herpesvirus antigen. Fish Pathology. 2006;41:141–145. [Google Scholar]
- Ye J, Bromage ES, Kaattari SL. The strength of B cell interaction with antigen determines the degree of IgM polymerization. J Immunol. 2010;184:844–850. doi: 10.4049/jimmunol.0902364. [DOI] [PubMed] [Google Scholar]
- Ye J, Kaattari I, Kaattari S. Plasmablasts and plasma cells: Reconsidering teleost immune system organization. Dev Comp Immunol. 2011 doi: 10.1016/j.dci.2011.03.005. [DOI] [PubMed] [Google Scholar]
- Zaccone G, Kapoor BG, Fasulo S, Ainis L. Structural, histochemical and functional aspects of the epidermis of fishes. Adv Marine Biol. 2001;40:253–276. [Google Scholar]
- Zapata A, Amemiya CT. Phylogeny of lower vertebrates and their immunological structures. Curr Top Microbiol Immunol. 2000;248:67–107. doi: 10.1007/978-3-642-59674-2_5. [DOI] [PubMed] [Google Scholar]
- Zapata A, Diez B, Cejalvo T, Gutierrez-de Frias C, Cortes A. Ontogeny of the immune system of fish. Fish Shellfish Immunol. 2006;20:126–136. doi: 10.1016/j.fsi.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Zhang YA, Hikima J, Li J, LaPatra SE, Luo YP, Sunyer JO. Conservation of structural and functional features in a primordial CD80/86 molecule from rainbow trout (Oncorhynchus mykiss), a primitive teleost fish. J Immunol. 2009;183:83–96. doi: 10.4049/jimmunol.0900605. [DOI] [PubMed] [Google Scholar]
- Zhang YA, Salinas I, Li J, Parra D, Bjork S, Xu Z, Lapatra SE, Bartholomew J, Sunyer JO. IgT, a primitive immunoglobulin class specialized in mucosal immunity. Nat Immunol. 2010;11:827–835. doi: 10.1038/ni.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YA, Salinas I, Sunyer JO. Recent findings on the structure and function of teleost IgT. Fish Shellfish Immunol. 2011;31:627–634. doi: 10.1016/j.fsi.2011.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Findly RC, Dickerson HW. Cutaneous antibody-secreting cells and B cells in a teleost fish. Dev Comp Immunol. 2008;32:500–508. doi: 10.1016/j.dci.2007.08.009. [DOI] [PubMed] [Google Scholar]
- Zilberg D, Klesius PH. Quantification of immunoglobulin in the serum and mucus of channel catfish at different ages and following infection with Edwardsiella ictaluri. Vet Immunol Immunopathol. 1997;58:171–180. doi: 10.1016/s0165-2427(97)00033-0. [DOI] [PubMed] [Google Scholar]