Abstract
Biglycan is a proteoglycan ubiquitously present in extracellular matrix of a variety of organs including heart and has been reported to be overexpressed in myocardial infarction. Myocardial infarction may be complicated by perimyocarditis through to date unclear mechanisms. Our aim was to investigate the capacity of TLR2/TLR4 ligand biglycan to enhance the presentation of specific Ags released upon cardiomyocyte necrosis. In vitro, Ova-pulsed bone-marrow derived dendritic cells from WT (C57BL/6), TLR2-, TLR4-, MyD88- or TRIF- deficient mice were co-treated with LPS, biglycan or vehicle and incubated with Ova-recognizing MHCI- or MHCII-restricted T cells. Biglycan enhanced Ova-specific cross-priming by more than 80% to MHCI-restricted T cells in both TLR2 - and TLR4 – pathway dependent manner. Accordingly, biglycan-induced cross-priming by both MyD88- and TRIF-deficient DCs was strongly diminished. Ova-specific activation of MHCII-restricted T cells was predominantly TLR4-dependent. Our first in vivo correlate was a model of experimental autoimmune perimyocarditis triggered by injection of cardiac-Ag pulsed DCs (BALB/c). Biglycan-treated DCs triggered perimyocarditis in extent and intensity comparable to LPS-treated DCs (average scores 1.3±0.3 and 1.5±0.4, respectively). Substitution with TLR4-deficient DCs abolished this effect. In a second in vivo approach, WT and biglycan-deficient mice were followed two weeks after induction of myocardial infarction. WT mice demonstrated significantly higher myocardial T-lymphocyte infiltration in comparison to biglycan-deficient animals. We conclude that TLR2/4 ligand biglycan, a component of the myocardial matrix, may enhance Ag-specific T cell priming via MyD88 and TRIF and stimulate autoimmune perimyocarditis.
Introduction
Biglycan is a small leucine-rich proteoglycan characterized by a protein core highly preserved across species (1–4). Together with another proteoglycan, decorin, biglycan has been found in extracellular matrix of a variety of organs where it may interact with collagens, cytokines or signalling membrane receptors (5–10). Biglycan within the tissue is physiologically distributed pericellulary and on the cell surface, suggesting its involvement in signal transduction (11). Originally detected in the context of connective tissue remodelling, diverse biological functions of biglycan have been now explored in several physiologic and pathophysiologic settings (8, 12–18). Biglycan and decorin have been shown to mediate osteoblast differentiation and bone mineralization (19–25). In kidney, biglycan has been found to participate in renal inflammation induced by obstructive nephropathy acting via stimulation of fibrillin-1 expression, prevention of mesangial cell apoptosis in a NO-dependent manner and strong upregulation of mononuclear cell infiltration (7, 26–29). The role of biglycan in cardiovascular pathology has also been explored (11, 30–33). It affects apolipoprotein retention in atherosclerosis, exerts a cardioprotective role via upregulation of TGF-β and NO synthases to promote cardiac remodelling and plays a role in cell signalling through regulation of MAPK, TGF-β and calmodulin expression (11, 31, 33, 34). Biglycan has been recently described as being involved in stable scar formation after myocardial infarction (35, 36).
Recently, biglycan has been described as an effective proinflammatory cytokine-like molecule (37). During tissue injury and inflammation, biglycan may be released from the extracellular matrix or secreted from infiltrating monocytes/macrophages. It may act as a strong endogenous ligand for TLR2, TLR4 and purinergic P2X receptors to trigger TLR- and inflammasome-pathways: TLR-dependent p38/ERK/NF-κB – mediated TNF-α and MIP-2 secretion; TLR-dependent NLRP3- and pro-IL-1β-mRNA expression; P2X-dependent activation of NLRP3/ASC inflammasome as well as caspase-1 activation and release of mature IL-1β (29, 37, 38). The role of TLR2 and TLR4 signalling adaptors (TRIF, MyD88 and TIRAP) in this regard has not yet been explored. Biglycan, thus, represents a potent and ubiquitously expressed endogenous ‘danger signal’ acting through complex and to-date not fully elucidated mechanisms. Dendritic cells (DC) and macrophages (MΦ) are major components of the innate immune system and an obligatory part of an immune response to tissue damage. They exhibit strong capacity to ingest external antigens via receptor-independent pinocytosis or receptor-mediated endocytosis, process them through MHC class II compartments and present the antigen to induce CD4+ T cell responses (39, 40). Presentation of peptide antigen within MHC class I molecules will lead to recognition by naive CD8+ T cells. A specific subset of dendritic cells characterized by CD11c+ CD8α+ phenotype demonstrates a capacity to present phagocytosed antigens within MHC class I molecules, thus permitting an effective cytotoxic T lymphocyte (CTL) response to tumor and viral antigens of extracellular origin (41–48). The phenomenon of CTL priming with externally acquired antigen has been originally termed cross-priming and the matching antigen processing pathway in the dendritic cell, cross-presentation (49, 50). Maturation and activation of MΦ and DC, classically coupled to the ingestion of microbial particles or endogenous danger signals, may trigger a set of immune defense pathways. These include TLR signalling, activation of Mincle (macrophage-inducible C-type lectin) and CLEC9A (C-type lectin domain family 9 member A) pathway through cell damage-associated molecules released from necrotic cells (38, 51–55). Here we hypothesised that biglycan acts as TLR2/4 ligand to trigger and exacerbate an autoimmune response. The aim of this study was to evaluate the role of biglycan in Ag-specific T cell activation. We analyzed whether biglycan had capacity to induce experimental autoimmune perimyocarditis (EAP) and influence T cell inflammation after myocardial infarction. Our in vitro results have shown that biglycan clearly facilitated MHCI and MHCII (OTI and OTII) restricted T cell priming, without having an effect on Ova uptake. The increase in cross-priming was TLR2- and TLR4- dependent and required both TLR2 and TLR4 adaptors MyD88, TRIF and TIRAP, whereas MHCII mediated Ag-specific T cell activation was mainly TLR4-mediated. In an in vivo EAP model, biglycan triggered MyHC-α (myosin heavy chain alpha) and TnI (troponin I)- dependent experimental autoimmune perimyocarditis comparable to the severity to LPS-triggered disease, acting in a TLR4-dependent manner. In a myocardial infarction model, pericardial and myocardial T cell infiltration two weeks after induction of myocardial infarction was significantly stronger in control mice (C57BL/6), in comparison to biglycan-deficient mice. Both approaches strongly indicate that biglycan, a known endogenous TLR2/TLR4 ligand, may act as an inducer of pericardial and myocardial inflammation in injured heart tissue.
Material and methods
Mice
C57BL/6 and BALB/cJ wild type mice were purchased from Charles River Germany. MyD88−/−, TRIF−/− and OTI mice (C57BL/6) were bred and obtained from German Cancer Research Center animal facility, Heidelberg, Germany. TLR2−/− in C57BL/6 and TLR4−/− mice in C57BL/10 background (C57BL/6 and C57BL/10 share the same MHC H-2b haplotype) were provided by Liliana Schaefer, Frankfurt am Main, Germany. Biglycan −/− mice (C57BL/6) were obtained from Marian F. Young, Bethesda, Maryland, USA. OTII mice (C57BL/6) were obtained from Sven Burgdorf, Bonn, Germany. TLR4−/− (BALB/cAnPt) and corresponding wild type control (BALB/cByJ) were purchased from The Jackson Laboratory, U.S.A. All mice were kept in specific pathogen-free conditions and used in accordance with local experimental guidelines.
Cells, culture media, antibodies and reagents
B3Z cells were obtained from the Tumor Bank of the German Cancer Research Center (56). OTI and OTII cells were magnetically isolated from the spleens of OTI and OTII mice using MACS technology and magnetically labeled anti-CD90 antibodies according to manufacturer’s protocol (Milteny Biotec, Germany). The DC culture medium was complete IMDM medium (Invitrogen, Germany) conditioned with 30% medium from GM-CSF-producing NIH 3T3 cells (R1). The B3Z, OTI and OTII cells were cultured in RPMI-1640 (Sigma, Germany). Both media were conditioned with 10% heat-inactivated FCS, 100 IU/ml penicillin, 100 mg/ml streptomycin, 2 mM L-glutamine, 10 mM HEPES and 50 mM 2-mercaptoethanol (all from Invitrogen, Germany). Endotoxin-free Ova was purchased from Hyglos, Germany. LPS (E. coli, 0127.B6) was from Sigma, Germany. TIRAP inhibitory peptide set was purchased from Imgenex, USA. Purified rat anti-mouse CD40 antibody (clone 3/23), APC hamster anti-mouse CD11c (clone HL3), BD flex-sets (IL-2, IFN-γ) and BD Inflammation Kit were from BD Pharmingen, Germany. FITC rat anti-mouse MHC class II (clone M5/114.15.2) was purchased from NatuTec, Germany. CFSE, Alexa 647 Ova and Alexa 546 phalloidin were from Invitrogen, Germany. Goat anti-mouse CD3-ε (M-20) antibody was from Santa Cruz Biotechnology, Germany. Rat anti-mouse ER-HR3 antibody was purchased from Acris Antibodies GmbH, Germany. Immunogenic mouse myosin heavy chain-α (MyHC-α) and troponin I (TnI) peptide syntheses were provided by Genomics & Proteomics core facilities of German cancer Research Center and University Clinic Heidelberg, Germany.
Purification of human biglycan
Human biglycan was expressed in 293 HEK cells as described previously (57). For purification of native biglycan the conditioned media were supplemented with proteinase inhibitors (0.1 M ε-amino-n-caproic acid, 10 mM ethylenediaminetetraacetic acid, 5 mM benzamidine, 10 mM N-ethyl-maleimide, 1 mM phenylmethyl sulfonyl fluoride) and passed over a DEAE-Trisacryl-M (Pall) column followed by elution with 20 mM Tris/HCl, pH 7.4, containing 1 M NaCl. After concentrating the relevant fractions with Aquacide I, as instructed by the manufacturer (Calbiochem), biglycan was dialysed for 2 h against 20 mM Tris-HCl, pH 7.4, containing 150 mM NaCl and separated by high performance liquid chromatography (Prominence LC, Shimadzu) on a TSK-GEL-DEAE-5PW, 7.5 mm ID × 7.5cm, 10 µm column (Tosoh Bioscience) by a discontinuous binary NaCl gradient. The purity of intact biglycan and its protein core was verified by silver staining after SDS gel electrophoresis. After each purification, endotoxin contamination of biglycan was tested with the LAL kinetic chromogenic assay using the PTS™ Endosafe system (Charles River). Endotoxin contamination of biglycan (1 µg/ml) corresponded to 2.34 ± 0.74 pg/ml (n=4) of LPS. It was additionally controlled by boiling and trypsin digestion of biglycan and by co-incubation with Polymyxin B (Sigma-Aldrich, 25–50 µg/ml). Contaminations of biglycan with IL-6, IL-1β, TGF-1β, TNF-α, and MIP-2 were tested with respective ELISAs (R&D Systems). The presence of selective TLR2 and TLR4 ligands was excluded by dot blots using 5–10 µg biglycan followed by immunodetection using mouse anti-fibronectin extra domain A (Harlan Sera-Lab) and mouse anti-human heat shock proteins 60 and 70 (StressGen Biotechnologies).
Generation of bone marrow – derived DCs
BMDCs were generated using GMCSF-rich medium from R1 cells as described (58, 59). At day 7, the DCs were checked for CD11c and MHCII expression (FACS analysis detected > 90% of cells CD11c+ MHCII+) and used in the experiments.
Light microscopy, morphometric analyses, confocal microscopy and flow cytometry
Image acquisition and analysis was done using a Leica TCS-SL microscope and Leica confocal software (v. 2.61). Morphometry in acute experimental perimyocarditis model was performed on hematoxylin / eosin (H/E) stained 5 µm heart sections (3 sections per mouse). Degree of inflammation was assessed using an 0–4 scoring scale (0 – no inflammation; 0.5 – focal inflammation; 1 – <10%; 2 – 11%–30%; 3 – 31%–50%; 4 – 51%–90% leukocyte infiltration within a complete heart section excluded from atriums and vascular bead) from previously published models (60–62). Morphometric analysis in myocardial infarction model was performed using a semiautomatic image analysing system (Leica Q600 Qwin, Cambridge, England) (63). Post-infarction damage area (fibrosis and granulation tissue) was determined on hematoxylin / eosin (H/E) and Masson’s trichrom stained 5 µm heart sections. Five heart sections per mouse were evaluated. Results were expressed as a percent damage of the total heart tissue present on the section, obtained after exclusion of atriums and major blood vessels. Analyses were performed independently by two morphologists (HJG and ZVP) in a blinded manner. Flow cytometric acquisition and analysis was performed using FACScalibur flow cytometer and BD CellQuesttm Pro v.5.2.1 and FCAP Array v1.0.1. softwares (BD Biosciences, Germany).
Coculture of DCs with B3Z, OT-I or OT-II T cells
For in vitro experiments, BMDCs from wild type, TLR2 −/−, TLR4 −/−, MYD88 −/− and TRIF −/− mice were incubated with 1 mg/ml Ova together with either biglycan (2 µg/ml), LPS (100 ng/ml) or vehicle (PBS) for 2 h and subsequently cocultured with B3Z, OT-I or OT-II T cells (64). In some experiments, DCs were pretreated with TIRAP inhibitory peptide (DRQIKIWFQNRRMKWKKLQLRDAAPGGAIVS) as described (65). To quantify T cell activation, supernatants were removed after 18h of coculture and assayed by BD CBA Flex-Set for IL-2 (B3Z) or IFN-γ (OT-I and OT-II) using FACSCalibur. To directly evaluate T cell proliferation, OT-I and OT-II T cells were labeled for 10 min with 5 µM CFSE, thoroughly washed and cocultured with BM-DCs for 48h or 96h. Proliferation was determined by flow cytometric analysis of the CFSE dilution (66).
In vivo murine experimental autoimmune perimyocarditis assay and myocardial infarction model
Immature BMDCs from WT or TLR4−/− mice (BALB/c) were pulsed for 1 hour with 10µg/ml of mouse MyHC-α (614–629), 5µg/ml of CD40 stimulating antibody (CD40L) and 1µg/ml LPS (positive control mice) (67). Alternatively, MyHC-α was replaced with 10µg/ml of mouse TnI peptide (61, 62). In some experiments, biglycan (2 µg/ml) was applied either instead of LPS (as a TLR ligand) or instead of MyHC-α/TnI (to substitute the antigen). Control BMDCs were pulsed with biglycan and CD40L only. Activated DCs were immediately i.p. injected in WT or TLR4−/− mice at a concentration of 0.25 × 106 / 0.5ml medium / mouse (day 0). Remaining DCs were frozen at −80°C and thawed at days 2 and 4 for re-injection. The mice were euthanized and hearts, spleens, kidneys and lungs were removed for analyses at day 8. Myocardial infarction was achieved by permanent ligation of the left anterior descending artery (LAD), as described (68). Animals that survived the acute infarction phase were sacrificed on day 14 and hearts were removed for analysis.
Histology and immunohistology
5 µM serial sections were done on paraffin-embedded hearts and stained with hematoxylin/eosin (H/E) to detect the inflammatory mononuclear cell infiltration or Masson’s trichrome staining to determine the fibrosis, as described. Immunohistochemical staining of mouse heart tissue sections for biglycan with chicken anti-rat MAY-01 antibody (1:500) was performed in 2 µm thick heart tissue sections fixed with 4% formaldehyde in PBS as described previously (69). Immunohistochemical stainings of formalin-fixed paraffin embedded heart sections with purified rat anti-mouse CD4 (L3T4, clone 129.19), CD3 and ER-HR3 antibodies was done according to manufacturer’s protocol.
Statistical analyses
Statistical analyses were performed using GraphPad Prism v2.01, CellQuesttm v3.3, BD CellQuesttm Pro v.5.2.1 and FCAP Array v1.0.1. softwares. All data are reported as mean ± SEM. Statistical differences were calculated using Student’s paired t-test.
Results
Efficient uptake of Ova by BMDCs is not altered by biglycan or LPS stimulation
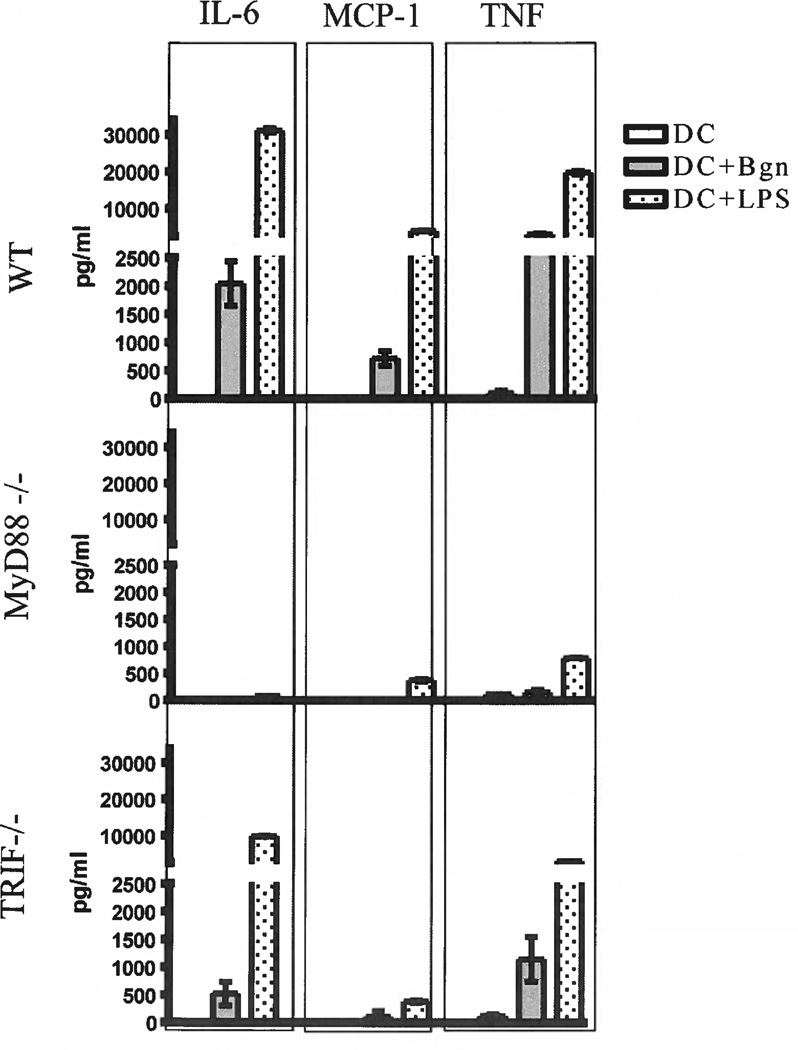
To analyze the uptake of Ova by BM-derived DCs, we incubated the DCs from C57BL/6 mice with fluorescence-labeled Ova (Alexa647 Ova) together with biglycan, LPS or vehicle. After 15 min of co-incubation, DCs were subjected to confocal (Fig. 1a) and flow cytometric (Fig. 1b) evaluation of intracellular fluorescence intensity. Statistical analyses (Fig. 1c) did not show significant differences in the intensity of Ova uptake between the control, biglycan-treated and LPS-treated groups. To confirm previously described TLR2 and TLR4 receptor activation by biglycan, we incubated BM-derived DCs with biglycan, LPS or vehicle for 12h and analyzed the supernatant for TLR-dependent inflammatory cytokines. Our results showed abundant TNF, IL-6 and MCP-1 secretion under stimulation with biglycan or LPS. Blockade of the MyD88 pathway nearly completely annulled secretion of proinflammatory cytokines, while TRIF pathway blockade induced significant reduction of biglycan and LPS induced cytokine secretion (Figure 2).
Figure 1. Uptake of ovalbumin by murine bone marrow-derived DCs.
A–C. Intensity of Alexa-647-Ova uptake by DCs. A. Confocal photomicrographs of BMDCs after endocytosis of Ova (1200×). BMDCs previously plated on cover slips were coincubated with 10 µg/ml Ova:Alexa 647 together with vehicle (PBS), biglycan (1 µg/ml) or LPS (100 ng/ml) for 15 min. The DCs were thoroughly washed from non-ingested Ova, stained with Alexa 546 phalloidin (actin) and visualised using confocal microscopy. Data are presented as merge of red and blue channel (Alexa 546 and Alexa 647, respectively). B. Histograms of CD11c+-gated BMDCs analyzed by flow cytometry after co- incubation with OVA:Alexa 647 as previously described. C. Statistical analyses of confocal (left) and flow cytometry (right) experiments. Data are presented as mean ± SEM of 3 experiments in duplicates. Statistical differences were evaluated by paired t-test. Bgn – Biglycan.
Figure 2. Cytokine profile of biglycan-stimulated DCs.
BMDCs from C57BL/6 WT, MyD88−/− and TRIF−/− mice were stimulated with LPS (100 ng/ml), biglycan (1 µg/ml) or vehicle for 12 h at standard cell culture conditions. The supernatants were collected, measured for IL-6, MCP-1 and TNF using mouse CBA assays and analyzed by FCAP Array software. Data are presented as mean ± SEM of 3 experiments in triplicates.
Biglycan enhances Ova-specific activation of B3Z, OTI and OTII cells
To further investigate the effect of biglycan on antigen-specific T cell activation, we incubated Ova-loaded DCs with Ova-recognizing MHCI-restricted T cell hybridoma (B3Z) and ex-vivo isolated MHCI and MHCII (OTI and OTII) T cells. B3Z activation was evaluated by measurement of secreted IL-2 from the cell supernatant, while OTI and OTII cell proliferation were determined using CFSE labeling. Our results showed that co-stimulation of DCs with Ova and biglycan significantly increased Ova-specific activation of B3Z cells (Fig 3a). Accordingly, cross-priming of OTI cells was strongly induced in biglycan and LPS treated groups in an increasing manner (after 48h and 96h of DC/OTI co-incubation) (Fig 3b). We further investigated whether biglycan modulates MHCII-mediated antigen presentation as well. Both biglycan and LPS induced strong enhancement of OTII cell proliferation, in comparison to the control (Fig 3c).
Figure 3. Cross-priming and MHCII-mediated T cell priming.
After coincubation with Ova (1 mg/ml), Ova.Bgn or Ova.LPS for 2 hours, BMDCs were washed and co-cultured with B3Z, OTI or OTII cells. A. Cross-priming of B3Z cells by Ova. Statistical analysis of IL-2 secretion by B3Z cells after overnight co-culture with BMDCs stimulated with control Ova (+vehicle), Ova.Bgn or Ova.LPS. B, C. Cross-priming and MHCII-mediated priming of OTI and OTII cells by Ova. T cells were isolated from the spleens of OTI and OTII mice, sorted by CD90.2 magnetic beads and labeled by CFSE as described. T cells were co-incubated with Ova-presenting BMDCs for 48h and 96h (OTI, B) or 96h (OTII, C). Data are presented as mean ± SEM from 3 experiments in triplicates. Statistical differences were evaluated by paired t-test. **, p<0,01. For B. and C., representative histogram plots from two experiments in duplicates are shown.
Biglycan modulates cross-priming via TLR2- and TLR4-pathways involving both MyD88 and TRIF adaptors and triggers MHCII-mediated antigen presentation preferentially via TLR4-pathway
To investigate the influence of TLR2 and TLR4 on biglycan-mediated enhancement of antigen presentation, BMDCs from TLR2 −/− and TLR4 −/− mice were further included in the experiments (Fig 4). Biglycan-induced cross-priming of Ova to B3Z cells was completely abrogated in TLR2 and TLR4 deficient DCs (Fig 4a). As expected, LPS-triggered antigen presentation to B3Z cells was blocked in TLR4 −/− and remained nearly intact in TLR2 −/− DCs (Fig 3a). The same phenomenon could be observed in biglycan- or LPS- induced cross-priming of OTI cells (Fig 4b). However, biglycan-induced MHCII-mediated priming of OTII cells appeared to be predominantly TLR4 dependent (Fig 4c). To investigate the influence of TLR2 and TLR4 adaptors MyD88, TRIF and TIRAP, we isolated BMDCs from MyD88−/− and TRIF−/− mice. Additionally, TIRAP blockade in DCs was achieved using a TIRAP inhibitory peptide. In both MyD88 and TRIF deficient DCs the effect of biglycan on cross-priming was diminished. Interestingly, although TIRAP inhibition in wild type DCs succeeded to abrogate the effect of LPS, it was not sufficient to block the biglycan-induced cross-priming. TIRAP blockade in MyD88 and TRIF deficient DCs exhibited an additive suppression of cross-priming for both biglycan and LPS (Fig 4d).
Figure 4. Ova presentation by BMDCs from WT, TLR2 −/− and TLR4 −/− mice to B3Z, OTI and OTII cells and cross-presentation of Ova by BMDCs from WT, MyD88 −/− and TRIF −/− mice under influence of TIRAP-specific inhibitory peptide.
Ova, Ova.Bgn or Ova.LPS was offered to BMDCs derived from WT, TLR2−/− (C57BL/6) and TLR4 −/− (C57BL/10) mice as earlier described. Statistical analyses of (A) levels of IL-2 secreted by B3Z and (B–C) IFNγ measured in the supernatant of OTI and OTII cells after overnight coculture with BMDCs. D. BMDC were isolated from WT, MyD88 −/− and TRIF −/− mice. 12 hours before co-incubation with Ova, Ova.Bgn or Ova.LPS, the DCs were incubated with TIRAP inhibitory peptide (100 µM), extensively washed and subjected to cross-presentation experiments. IL-2 secreted by B3Z cells was used as readout of intensity of cross-presentation. Data are presented as mean ± SEM of 3 experiments in triplicates. Statistical differences were evaluated by paired t-test. *, p<0,05; **, p<0,01 and ***, p<0,001.
Biglycan triggers in vivo experimental autoimmune perimyocarditis and exacerbates myocardial T cell inflammation after myocardial infarction
In order to show distribution of biglycan within normal and inflamed cardiac tissue, we performed immunolabeling of corresponding heart sections. As expected, biglycan showed predominantly perivascular and interstitial distribution within normal (placebo-treated) hearts and additionally a strong peri-leukocytic expression in experimental autoimmune perimyocarditis hearts (Fig 5). To investigate a potential effect of biglycan on antigen-specific T cell activation in vivo with consecutive inflammation, we applied two independent experimental heart injury models. In an experimental autoimmune perimyocarditis model in BALB/c mice (Fig 6), we performed immunization of this susceptible mouse strain with purified cardiac MyHCα or TnI peptides in combination with biglycan or LPS (Fig 6a, b) to address a potential role of biglycan in the pathogenesis of myocarditis and pericarditis induced by autoimmune mechanisms. Expression of CD3 and CD4 was detected in heart sections. There was no significant difference in the intensity of CD3+-cell infiltration between groups injected by biglycan- or LPS-treated DCs (Fig 6c). Control animals (Fig 6d) did not show any significant inflammatory activity. Within this model we further included TLR4-deficient mice – both as donors and acceptors of biglycan-stimulated DCs. In the case of BMDCs from WT mice administered to WT mice, pattern and intensity of biglycan-induced myocarditis was fully comparable to the LPS induction. TLR4-deficient BMDCs, injected to either WT or TLR4−/− mice, were not able to trigger cardiac inflammation. Vice versa, biglycan-stimulated DCs from WT mice were fully capable of inducing perimyocarditis in TLR4−/− mice, together indicating that biglycan-TLR4 signalling plays here an important role at the stage of Ag-processing within a DC, yet has no significant influence on consecutive priming of T cells (Fig 7). In a second approach, we induced myocardial infarction by ligation of the left anterior descending coronary artery in WT (C57BL/6) and biglycan-deficient mice to assess the influence of biglycan on T cell infiltration in infarction-affected myocardium. A significantly reduced T cell infiltration was seen in biglycan knockout mice two weeks after induction of infarction, in comparison to controls (fig 8). Myocardial infarction area and infiltration of professional antigen-presenting (HR3+) cells did not significantly differ between the two groups, indicating that diminished T cell infiltration in biglycan-deficient mice does not result from reduced tissue damage or decreased monocyte infiltration, but from a decrease in DC-T cell interaction.
Figure 5. Biglycan expression in murine experimental autoimmune perimyocarditis.
Murine heart tissue was fixed with 4% formaldehyde in PBS. Paraffin sections (3 µm) were subjected to immunohistochemical staining with chicken anti-rat biglycan antibody (MAY-1) as previously described. Biglycan expression in normal non-treated mouse heart (middle) and in severe experimental autoimmune perimyocarditis (EAP; right two lanes). The specificity of immunolabeling was tested by omitting the primary antibody (negative control, left). Representative micrographs from 3 experiments in triplicates are shown.
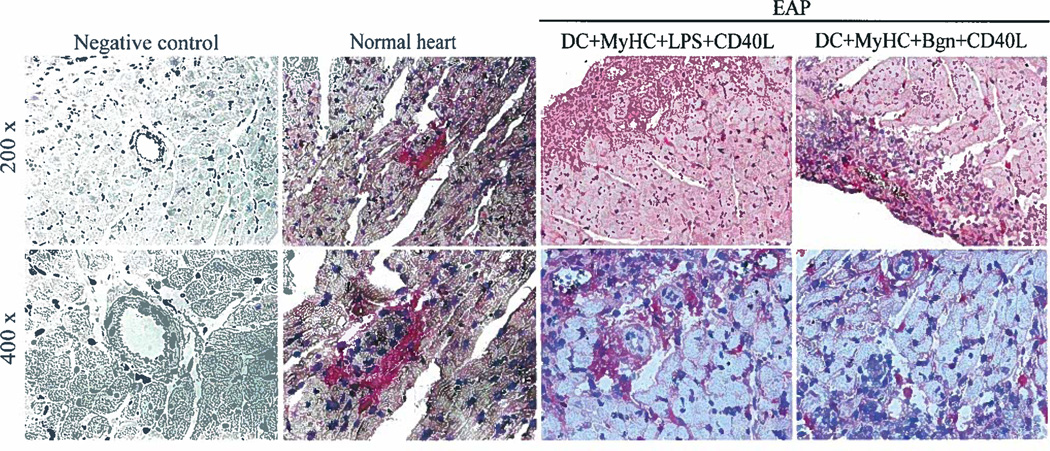
Figure 6. In vivo induction of experimental autoimmune perimyocarditis.
Photomicrographs of BALB/c mice heart sections. A. BMDCs developed from BALB/c mice were incubated with following cocktails: TnI+LPS+CD40L (up left) or TnI+biglycan+CD40L (up right); MyHC-α+LPS+CD40L (low left) or MyHC-α+biglycan+CD40L (low right) (100×); B. Heart inflammation was scored 0–4 based on the percentage of heart section affected by leukocyte infiltration (0 – no inflammation; 0.5 – focal inflammation; 1 – <10%; 2 – 11%–30%; 3 – 31%–50%; 4 – 51%–90%). C. Stained heart sections were analyzed for CD3+ and CD4+ T cell infiltration (200×). CD3+ cells were counted in 3 high-power fields (HPF, 200×) per mouse. Data in B. and C. are presented as mean ± SEM of 3 experiments in triplicates. Statistical differences were evaluated by paired t-test. D. Photomicrographs of heart sections from control mice (left to right): injection of DCs stimulated by cardiac-specific Ag without TLR ligand; Bgn without cardiac Ag; and Bgn and LPS without cardiac Ag (H/E, 200×). Representative micrographs from 3 experiments in triplicates are shown.
Figure 7. In vivo induction of EAP in TLR4-deficient mice.
BMDCs developed from BALB/cByJ and TLR4−/− (BALB/cAnPt) were used in EAP model as described. Photomicrographs from heart section of: WT mice injected with WT-DCs (up, left); WT mice injected with TLR4-deficient DCs (up, right); TLR4-deficient mice injected with WT DCs (low, left); and TLR4-deficient mice injected with TLR4-deficient DCs (low, right). Representative micrographs from 3 experiments in triplicates are shown. Data are presented as mean ± SEM. Statistical differences were evaluated by unpaired t-test. *, p<0,05; **, p<0,01.
Figure 8. In vivo induction of myocardial infarction in WT and biglycan-deficient mice.
WT and biglycan −/− mice (C57BL/6) were used in a myocardial infarction model. Two weeks after induction of infarction by a ligation of the left anterior descending artery, mouse heart sections were analyzed for biglycan expression (upper lane, 400×), size of post-myocardial infarction (MI) fibrosis (evaluated as percentage of ischemic damage within heart section from 5 sections per mouse; H/E, 200×), intensity of HR3+ cell infiltration (200×) and intensity of CD3+ cell infiltration (lower lane, 200×). Cells were counted in (total) 10 HPF from 5 sections per mouse. Data are presented as mean ± SEM from 3 experiments in duplicates. Statistical differences were evaluated by unpaired t-test. **, p<0,01.
Discussion
In vivo mice and rat myocardial infarction models have shown strong overexpression of biglycan mRNA and protein in infarction-affected areas (33, 70). Clinical studies report occurrence of post myocardial infarction pericarditis in a relevant proportion of patients (71–78). However, molecular pathomechanisms of the infarction-related myocarditis syndrome remained unclear. It is probable that DCs and infiltrating T cells play an important role. DCs have a capacity to regulate T cell responses, leading to either tolerance or immunity (79–81). Current data support the view that overall DC activation state, rather than surface receptor phenotype only, orchestrates the final ‘immunity or tolerance’ decision (82, 83). We have shown here that biglycan may act as an endogenous TLR2- and TLR4- ligand in tissue injury to activate BMDCs and to potently trigger T cell priming leading to an enhancement of the immune response.
It is known that TLR ligands trigger signal transduction that requires recruitment of specific adaptor proteins. These adaptors associate with the cytoplasmatic domains of TLRs through specific interactions between Toll/IL-1 receptor domains of TLR and corresponding adaptor. All TLR family members recruit the MyD88 adaptor, except TLR3, which uses exclusively TRIF (84). Our in vitro assays have shown that the biglycan-mediated boost of cross-presentation requires TLR2 and TLR4 and involves MyD88, TRIF and TIRAP adaptors. In experiments with TLR2 −/−, TLR4 −/−, MyD88 −/− and TRIF −/− DCs, biglycan apparently acted through both receptors with corresponding adaptors to enhance T cell priming; absence of either TLR2 or TLR4 as well as absence of TRIF (involved in TLR4, but not TLR2 signalling) has lead to almost complete inhibition of the effect. A similar outcome has been achieved by a synchronous blockade of both TLR2 and TLR4 pathways (MyD88−/−; TIRAP blockade). Thereby, our data support the assumption that biglycan-induced priming of T cells may require co-action of TLR2 and TLR4 pathways (28, 37, 85–87). This observation is in line with our previous study showing that biglycan acts as a strong autocrine and paracrine activator of both TLR2 and TLR4 signalling in MΦs (37). Experimental autoimmune perimyocarditis (EAP; also referred to in the literature as ‘experimental autoimmune myocarditis’) is a model originally applied to mimic post-infectious myocarditis. EAP can be induced in susceptible mouse strains (BALB/c, A/J) by immunizing with self-peptides derived from the myosin α heavy chain (MyHC-α) or Troponin I (TnI) together with a strong adjuvant, or by injecting peptide-loaded DCs (62, 67, 88). It has been demonstrated that EAP is a T cell mediated autoimmune disease that requires a major effector role of CD4+ T cells and an initiating role of CD8+ T (67, 89). In other organ-specific autoimmune models, such as experimental autoimmune encephalitis (EAE) and multiple sclerosis, it has been shown that CD8+ T cells may as well be important in the chronic phase of the disease (90, 91). Collectively, these findings indicated that immune tolerance can be broken by damage during infection, resulting in the release of self-antigens and the activation of DCs followed by the priming of autoreactive T cells. Biglycan is ubiquitously distributed in the heart (92). We have now shown that biglycan may induce experimental autoimmune pericarditis and myocarditis via potent TLR4-mediated stimulation of specific cardiac peptide presentation; hence enabling an autoimmune response to develop without infection. Via induction of myocardial infarction in WT and biglycan deficient mice, we have shown that biglycan can play an important role in T cell activation in infarction-affected cardiac tissue, without influencing post-infarction fibrosis or infiltration of antigen presenting cells. Based on our data, we propose that biglycan acts in myocardial lesions as a potent amplifier of specific cardiomyocyte antigen (TnI, MyHCα) presentation by infiltrating DCs and MΦs to successfully prime T cells and cause an immune autoreactive response. Hence, our study here is the first line of evidence to identify the extracellular matrix protein, biglycan, as a potential link between peptide antigen presentation and TLR receptor pathways and as an endogenous enhancer of autoimmune response in damaged heart tissue.
Acknowledgments
We thank Bernd Arnold and Thilo Oelert for helpful discussions and comments; Claudia Schmidt and Gabi Schmidt for expert technical assistance. This work was supported by the Deutsche Forschungsgemeinschaft (SFB 953 to H.-J.G. and SCHA 1082/2-1 to L.S. and H.-J.G.) and by the Division of Intramural Research, NIDCR, of the Intramural Research Program, NIH, DHHS (M.F.Y.)
References
- 1.Bianco P, Fisher LW, Young MF, Termine JD, Robey PG. Expression and localization of the two small proteoglycans biglycan and decorin in developing human skeletal and non-skeletal tissues. J Histochem Cytochem. 1990;38:1549–1563. doi: 10.1177/38.11.2212616. [DOI] [PubMed] [Google Scholar]
- 2.Dreher KL, Asundi V, Matzura D, Cowan K. Vascular smooth muscle biglycan represents a highly conserved proteoglycan within the arterial wall. Eur J Cell Biol. 1990;53:296–304. [PubMed] [Google Scholar]
- 3.McBride OW, Fisher LW, Young MF. Localization of PGI (biglycan, BGN) and PGII (decorin, DCN, PG-40) genes on human chromosomes Xq13-qter and 12q, respectively. Genomics. 1990;6:219–225. doi: 10.1016/0888-7543(90)90560-h. [DOI] [PubMed] [Google Scholar]
- 4.Neame PJ, Choi HU, Rosenberg LC. The primary structure of the core protein of the small, leucine-rich proteoglycan (PG I) from bovine articular cartilage. J Biol Chem. 1989;264:8653–8661. [PubMed] [Google Scholar]
- 5.Iozzo RV. The biology of the small leucine-rich proteoglycans. Functional network of interactive proteins. J Biol Chem. 1999;274:18843–18846. doi: 10.1074/jbc.274.27.18843. [DOI] [PubMed] [Google Scholar]
- 6.Wegrowski Y, Pillarisetti J, Danielson KG, Suzuki S, Iozzo RV. The murine biglycan: complete cDNA cloning, genomic organization, promoter function, and expression. Genomics. 1995;30:8–17. doi: 10.1006/geno.1995.0002. [DOI] [PubMed] [Google Scholar]
- 7.Schaefer L, Macakova K, Raslik I, Micegova M, Grone HJ, Schonherr E, Robenek H, Echtermeyer FG, Grassel S, Bruckner P, Schaefer RM, Iozzo RV, Kresse H. Absence of decorin adversely influences tubulointerstitial fibrosis of the obstructed kidney by enhanced apoptosis and increased inflammatory reaction. Am J Pathol. 2002;160:1181–1191. doi: 10.1016/S0002-9440(10)64937-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zanotti S, Negri T, Cappelletti C, Bernasconi P, Canioni E, Di Blasi C, Pegoraro E, Angelini C, Ciscato P, Prelle A, Mantegazza R, Morandi L, Mora M. Decorin and biglycan expression is differentially altered in several muscular dystrophies. Brain. 2005;128:2546–2555. doi: 10.1093/brain/awh635. [DOI] [PubMed] [Google Scholar]
- 9.Merline R, Lazaroski S, Babelova A, Tsalastra-Greul W, Pfeilschifter J, Schluter KD, Gunther A, Iozzo RV, Schaefer RM, Schaefer L. Decorin deficiency in diabetic mice: aggravation of nephropathy due to overexpression of profibrotic factors, enhanced apoptosis and mononuclear cell infiltration. J Physiol Pharmacol. 2009;60(Suppl 4):5–13. [PMC free article] [PubMed] [Google Scholar]
- 10.Schaefer L, Iozzo RV. Biological functions of the small leucine-rich proteoglycans: from genetics to signal transduction. J Biol Chem. 2008;283:21305–21309. doi: 10.1074/jbc.R800020200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bereczki E, Santha M. The role of biglycan in the heart. Connect Tissue Res. 2008;49:129–132. doi: 10.1080/03008200802148504. [DOI] [PubMed] [Google Scholar]
- 12.Hardingham TE, Fosang AJ. Proteoglycans: many forms and many functions. FASEB J. 1992;6:861–870. [PubMed] [Google Scholar]
- 13.Stanescu V. The small proteoglycans of cartilage matrix. Semin Arthritis Rheum. 1990;20:51–64. doi: 10.1016/0049-0172(90)90047-j. [DOI] [PubMed] [Google Scholar]
- 14.Sjoberg AP, Manderson GA, Morgelin M, Day AJ, Heinegard D, Blom AM. Short leucine-rich glycoproteins of the extracellular matrix display diverse patterns of complement interaction and activation. Mol Immunol. 2009;46:830–839. doi: 10.1016/j.molimm.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stokes MB, Holler S, Cui Y, Hudkins KL, Eitner F, Fogo A, Alpers CE. Expression of decorin, biglycan, and collagen type I in human renal fibrosing disease. Kidney Int. 2000;57:487–498. doi: 10.1046/j.1523-1755.2000.00868.x. [DOI] [PubMed] [Google Scholar]
- 16.Shimizu-Hirota R, Sasamura H, Kuroda M, Kobayashi E, Hayashi M, Saruta T. Extracellular matrix glycoprotein biglycan enhances vascular smooth muscle cell proliferation and migration. Circ Res. 2004;94:1067–1074. doi: 10.1161/01.RES.0000126049.79800.CA. [DOI] [PubMed] [Google Scholar]
- 17.Schaefer L, Grone HJ, Raslik I, Robenek H, Ugorcakova J, Budny S, Schaefer RM, Kresse H. Small proteoglycans of normal adult human kidney: distinct expression patterns of decorin, biglycan, fibromodulin, and lumican. Kidney Int. 2000;58:1557–1568. doi: 10.1046/j.1523-1755.2000.00317.x. [DOI] [PubMed] [Google Scholar]
- 18.O'Brien KD, Olin KL, Alpers CE, Chiu W, Ferguson M, Hudkins K, Wight TN, Chait A. Comparison of apolipoprotein and proteoglycan deposits in human coronary atherosclerotic plaques: colocalization of biglycan with apolipoproteins. Circulation. 1998;98:519–527. doi: 10.1161/01.cir.98.6.519. [DOI] [PubMed] [Google Scholar]
- 19.Wallace JM, Rajachar RM, Chen XD, Shi S, Allen MR, Bloomfield SA, Les CM, Robey PG, Young MF, Kohn DH. The mechanical phenotype of biglycan-deficient mice is bone- and gender-specific. Bone. 2006;39:106–116. doi: 10.1016/j.bone.2005.12.081. [DOI] [PubMed] [Google Scholar]
- 20.Wallace JM, Golcuk K, Morris MD, Kohn DH. Inbred strain-specific response to biglycan deficiency in the cortical bone of C57BL6/129 and C3H/He mice. J Bone Miner Res. 2009;24:1002–1012. doi: 10.1359/jbmr.081259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roberts HC, Moseley R, Sloan AJ, Youde SJ, Waddington RJ. Lipopolysaccharide alters decorin and biglycan synthesis in rat alveolar bone osteoblasts: consequences for bone repair during periodontal disease. Eur J Oral Sci. 2008;116:207–216. doi: 10.1111/j.1600-0722.2008.00535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kamiya N, Shigemasa K, Takagi M. Gene expression and immunohistochemical localization of decorin and biglycan in association with early bone formation in the developing mandible. J Oral Sci. 2001;43:179–188. doi: 10.2334/josnusd.43.179. [DOI] [PubMed] [Google Scholar]
- 23.Corsi A, Xu T, Chen XD, Boyde A, Liang J, Mankani M, Sommer B, Iozzo RV, Eichstetter I, Robey PG, Bianco P, Young MF. Phenotypic effects of biglycan deficiency are linked to collagen fibril abnormalities, are synergized by decorin deficiency, and mimic Ehlers-Danlos-like changes in bone and other connective tissues. J Bone Miner Res. 2002;17:1180–1189. doi: 10.1359/jbmr.2002.17.7.1180. [DOI] [PubMed] [Google Scholar]
- 24.Chen XD, Shi S, Xu T, Robey PG, Young MF. Age-related osteoporosis in biglycan-deficient mice is related to defects in bone marrow stromal cells. J Bone Miner Res. 2002;17:331–340. doi: 10.1359/jbmr.2002.17.2.331. [DOI] [PubMed] [Google Scholar]
- 25.Douglas T, Heinemann S, Hempel U, Mietrach C, Knieb C, Bierbaum S, Scharnweber D, Worch H. Characterization of collagen II fibrils containing biglycan and their effect as a coating on osteoblast adhesion and proliferation. J Mater Sci Mater Med. 2008;19:1653–1660. doi: 10.1007/s10856-007-3250-z. [DOI] [PubMed] [Google Scholar]
- 26.Schaefer L, Mihalik D, Babelova A, Krzyzankova M, Grone HJ, Iozzo RV, Young MF, Seidler DG, Lin G, Reinhardt DP, Schaefer RM. Regulation of fibrillin-1 by biglycan and decorin is important for tissue preservation in the kidney during pressure-induced injury. Am J Pathol. 2004;165:383–396. doi: 10.1016/S0002-9440(10)63305-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leemans JC, Butter LM, Pulskens WP, Teske GJ, Claessen N, van der Poll T, Florquin S. The role of Toll-like receptor 2 in inflammation and fibrosis during progressive renal injury. PLoS One. 2009;4:e5704. doi: 10.1371/journal.pone.0005704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schaefer L, Beck KF, Raslik I, Walpen S, Mihalik D, Micegova M, Macakova K, Schonherr E, Seidler DG, Varga G, Schaefer RM, Kresse H, Pfeilschifter J. Biglycan, a nitric oxide-regulated gene, affects adhesion, growth, and survival of mesangial cells. J Biol Chem. 2003;278:26227–26237. doi: 10.1074/jbc.M210574200. [DOI] [PubMed] [Google Scholar]
- 29.Schaefer L. Extracellular matrix molecules: endogenous danger signals as new drug targets in kidney diseases. Curr Opin Pharmacol. 2010;10:185–190. doi: 10.1016/j.coph.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 30.Sousa MM, do Amaral JB, Guimaraes A, Saraiva MJ. Up-regulation of the extracellular matrix remodeling genes, biglycan, neutrophil gelatinase-associated lipocalin, and matrix metalloproteinase-9 in familial amyloid polyneuropathy. FASEB J. 2005;19:124–126. doi: 10.1096/fj.04-2022fje. [DOI] [PubMed] [Google Scholar]
- 31.Bereczki E, Gonda S, Csont T, Korpos E, Zvara A, Ferdinandy P, Santha M. Overexpression of biglycan in the heart of transgenic mice: an antibody microarray study. J Proteome Res. 2007;6:854–861. doi: 10.1021/pr060571b. [DOI] [PubMed] [Google Scholar]
- 32.Ayada Y, Kusachi S, Murakami T, Hirohata S, Takemoto S, Komatsubara I, Hayashi J, Iwabu A, Ninomiya Y, Tsuji T. Increased expression of biglycan mRNA in pressure-overloaded rat heart. Clin Exp Hypertens. 2001;23:633–643. doi: 10.1081/ceh-100107393. [DOI] [PubMed] [Google Scholar]
- 33.Ahmed MS, Oie E, Vinge LE, Yndestad A, Andersen GG, Andersson Y, Attramadal T, Attramadal H. Induction of myocardial biglycan in heart failure in rats--an extracellular matrix component targeted by AT(1) receptor antagonism. Cardiovasc Res. 2003;60:557–568. doi: 10.1016/j.cardiores.2003.08.017. [DOI] [PubMed] [Google Scholar]
- 34.Derbali H, Bosse Y, Cote N, Pibarot P, Audet A, Pepin A, Arsenault B, Couture C, Despres JP, Mathieu P. Increased biglycan in aortic valve stenosis leads to the overexpression of phospholipid transfer protein via Toll-like receptor 2. Am J Pathol. 2010;176:2638–2645. doi: 10.2353/ajpath.2010.090541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Westermann D, Mersmann J, Melchior A, Freudenberger T, Petrik C, Schaefer L, Lullmann-Rauch R, Lettau O, Jacoby C, Schrader J, Brand-Herrmann SM, Young MF, Schultheiss HP, Levkau B, Baba HA, Unger T, Zacharowski K, Tschope C, Fischer JW. Biglycan is required for adaptive remodeling after myocardial infarction. Circulation. 2008;117:1269–1276. doi: 10.1161/CIRCULATIONAHA.107.714147. [DOI] [PubMed] [Google Scholar]
- 36.Csont T, Ferdinandy P. Letter by Csont and Ferdinandy regarding article, "Biglycan is required for adaptive remodeling after myocardial infarction". Circulation. 2008;118:e521. doi: 10.1161/CIRCULATIONAHA.108.791699. author reply e522. [DOI] [PubMed] [Google Scholar]
- 37.Schaefer L, Babelova A, Kiss E, Hausser HJ, Baliova M, Krzyzankova M, Marsche G, Young MF, Mihalik D, Gotte M, Malle E, Schaefer RM, Grone HJ. The matrix component biglycan is proinflammatory and signals through Toll-like receptors 4 and 2 in macrophages. J Clin Invest. 2005;115:2223–2233. doi: 10.1172/JCI23755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Babelova A, Moreth K, Tsalastra-Greul W, Zeng-Brouwers J, Eickelberg O, Young MF, Bruckner P, Pfeilschifter J, Schaefer RM, Grone HJ, Schaefer L. Biglycan, a danger signal that activates the NLRP3 inflammasome via toll-like and P2X receptors. J Biol Chem. 2009;284:24035–24048. doi: 10.1074/jbc.M109.014266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Montfoort N, Camps MG, Khan S, Filippov DV, Weterings JJ, Griffith JM, Geuze HJ, van Hall T, Verbeek JS, Melief CJ, Ossendorp F. Antigen storage compartments in mature dendritic cells facilitate prolonged cytotoxic T lymphocyte cross-priming capacity. Proc Natl Acad Sci U S A. 2009;106:6730–6735. doi: 10.1073/pnas.0900969106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yewdell JW. Plumbing the sources of endogenous MHC class I peptide ligands. Curr Opin Immunol. 2007;19:79–86. doi: 10.1016/j.coi.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 41.Chtanova T, Mackay CR. T cell effector subsets: extending the Th1/Th2 paradigm. Adv Immunol. 2001;78:233–266. doi: 10.1016/s0065-2776(01)78005-4. [DOI] [PubMed] [Google Scholar]
- 42.Hochrein H, Shortman K, Vremec D, Scott B, Hertzog P, O'Keeffe M. Differential production of IL-12, IFN-alpha, and IFN-gamma by mouse dendritic cell subsets. J Immunol. 2001;166:5448–5455. doi: 10.4049/jimmunol.166.9.5448. [DOI] [PubMed] [Google Scholar]
- 43.Maldonado-Lopez R, De Smedt T, Pajak B, Heirman C, Thielemans K, Leo O, Urbain J, Maliszewski CR, Moser M. Role of CD8alpha+ and CD8alpha− dendritic cells in the induction of primary immune responses in vivo. J Leukoc Biol. 1999;66:242–246. doi: 10.1002/jlb.66.2.242. [DOI] [PubMed] [Google Scholar]
- 44.O'Shea JJ, Paul WE. Regulation of T(H)1 differentiation--controlling the controllers. Nat Immunol. 2002;3:506–508. doi: 10.1038/ni0602-506. [DOI] [PubMed] [Google Scholar]
- 45.Parajuli P, Mosley RL, Pisarev V, Chavez J, Ulrich A, Varney M, Singh RK, Talmadge JE. Flt3 ligand and granulocyte-macrophage colony-stimulating factor preferentially expand and stimulate different dendritic and T-cell subsets. Exp Hematol. 2001;29:1185–1193. doi: 10.1016/s0301-472x(01)00722-6. [DOI] [PubMed] [Google Scholar]
- 46.Paul WE, Seder RA. Lymphocyte responses and cytokines. Cell. 1994;76:241–251. doi: 10.1016/0092-8674(94)90332-8. [DOI] [PubMed] [Google Scholar]
- 47.Burgdorf S, Kautz A, Bohnert V, Knolle PA, Kurts C. Distinct pathways of antigen uptake and intracellular routing in CD4 and CD8 T cell activation. Science. 2007;316:612–616. doi: 10.1126/science.1137971. [DOI] [PubMed] [Google Scholar]
- 48.Dudziak D, Kamphorst AO, Heidkamp GF, Buchholz VR, Trumpfheller C, Yamazaki S, Cheong C, Liu K, Lee HW, Park CG, Steinman RM, Nussenzweig MC. Differential antigen processing by dendritic cell subsets in vivo. Science. 2007;315:107–111. doi: 10.1126/science.1136080. [DOI] [PubMed] [Google Scholar]
- 49.Carbone FR, Kurts C, Bennett SR, Miller JF, Heath WR. Cross-presentation: a general mechanism for CTL immunity and tolerance. Immunol Today. 1998;19:368–373. doi: 10.1016/s0167-5699(98)01301-2. [DOI] [PubMed] [Google Scholar]
- 50.Bevan MJ. Cross-priming for a secondary cytotoxic response to minor H antigens with H-2 congenic cells which do not cross-react in the cytotoxic assay. J Exp Med. 1976;143:1283–1288. doi: 10.1084/jem.143.5.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 52.Brown GD. Sensing necrosis with Mincle. Nat Immunol. 2008;9:1099–1100. doi: 10.1038/ni1008-1099. [DOI] [PubMed] [Google Scholar]
- 53.Yamasaki S, Ishikawa E, Sakuma M, Hara H, Ogata K, Saito T. Mincle is an ITAM-coupled activating receptor that senses damaged cells. Nat Immunol. 2008;9:1179–1188. doi: 10.1038/ni.1651. [DOI] [PubMed] [Google Scholar]
- 54.Zhou R, Tardivel A, Thorens B, Choi I, Tschopp J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat Immunol. 11:136–140. doi: 10.1038/ni.1831. [DOI] [PubMed] [Google Scholar]
- 55.Cambi A, Figdor C. Necrosis: C-type lectins sense cell death. Curr Biol. 2009;19:R375–R378. doi: 10.1016/j.cub.2009.03.032. [DOI] [PubMed] [Google Scholar]
- 56.Karttunen J, Sanderson S, Shastri N. Detection of rare antigen-presenting cells by the lacZ T-cell activation assay suggests an expression cloning strategy for T-cell antigens. Proc Natl Acad Sci U S A. 1992;89:6020–6024. doi: 10.1073/pnas.89.13.6020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kresse H, Seidler DG, Muller M, Breuer E, Hausser H, Roughley PJ, Schonherr E. Different usage of the glycosaminoglycan attachment sites of biglycan. J Biol Chem. 2001;276:13411–13416. doi: 10.1074/jbc.M009321200. [DOI] [PubMed] [Google Scholar]
- 58.Lutz MB, Kukutsch N, Ogilvie AL, Rossner S, Koch F, Romani N, Schuler G. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999;223:77–92. doi: 10.1016/s0022-1759(98)00204-x. [DOI] [PubMed] [Google Scholar]
- 59.Villadangos JA, Cardoso M, Steptoe RJ, van Berkel D, Pooley J, Carbone FR, Shortman K. MHC class II expression is regulated in dendritic cells independently of invariant chain degradation. Immunity. 2001;14:739–749. doi: 10.1016/s1074-7613(01)00148-0. [DOI] [PubMed] [Google Scholar]
- 60.Afanasyeva M, Wang Y, Kaya Z, Park S, Zilliox MJ, Schofield BH, Hill SL, Rose NR. Experimental autoimmune myocarditis in A/J mice is an interleukin-4-dependent disease with a Th2 phenotype. Am J Pathol. 2001;159:193–203. doi: 10.1016/S0002-9440(10)61685-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Goser S, Andrassy M, Buss SJ, Leuschner F, Volz CH, Ottl R, Zittrich S, Blaudeck N, Hardt SE, Pfitzer G, Rose NR, Katus HA, Kaya Z. Cardiac troponin I but not cardiac troponin T induces severe autoimmune inflammation in the myocardium. Circulation. 2006;114:1693–1702. doi: 10.1161/CIRCULATIONAHA.106.635664. [DOI] [PubMed] [Google Scholar]
- 62.Kaya Z, Goser S, Buss SJ, Leuschner F, Ottl R, Li J, Volkers M, Zittrich S, Pfitzer G, Rose NR, Katus HA. Identification of cardiac troponin I sequence motifs leading to heart failure by induction of myocardial inflammation and fibrosis. Circulation. 2008;118:2063–2072. doi: 10.1161/CIRCULATIONAHA.108.788711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Adams J, Kiss E, Arroyo AB, Bonrouhi M, Sun Q, Li Z, Gretz N, Schnitger A, Zouboulis CC, Wiesel M, Wagner J, Nelson PJ, Grone HJ. 13-cis retinoic acid inhibits development and progression of chronic allograft nephropathy. Am J Pathol. 2005;167:285–298. doi: 10.1016/S0002-9440(10)62973-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Burgdorf S, Scholz C, Kautz A, Tampe R, Kurts C. Spatial and mechanistic separation of cross-presentation and endogenous antigen presentation. Nat Immunol. 2008;9:558–566. doi: 10.1038/ni.1601. [DOI] [PubMed] [Google Scholar]
- 65.Scott MJ, Billiar TR. Beta2-integrin-induced p38 MAPK activation is a key mediator in the CD14/TLR4/MD2-dependent uptake of lipopolysaccharide by hepatocytes. J Biol Chem. 2008;283:29433–29446. doi: 10.1074/jbc.M803905200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Klotz L, Hucke S, Thimm D, Classen S, Gaarz A, Schultze J, Edenhofer F, Kurts C, Klockgether T, Limmer A, Knolle P, Burgdorf S. Increased antigen cross-presentation but impaired cross-priming after activation of peroxisome proliferator-activated receptor gamma is mediated by up-regulation of B7H1. J Immunol. 2009;183:129–136. doi: 10.4049/jimmunol.0804260. [DOI] [PubMed] [Google Scholar]
- 67.Eriksson U, Ricci R, Hunziker L, Kurrer MO, Oudit GY, Watts TH, Sonderegger I, Bachmaier K, Kopf M, Penninger JM. Dendritic cell-induced autoimmune heart failure requires cooperation between adaptive and innate immunity. Nat Med. 2003;9:1484–1490. doi: 10.1038/nm960. [DOI] [PubMed] [Google Scholar]
- 68.Kolk MV, Meyberg D, Deuse T, Tang-Quan KR, Robbins RC, Reichenspurner H, Schrepfer S. LAD-ligation: a murine model of myocardial infarction. J Vis Exp. 2009 doi: 10.3791/1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schaefer L, Hausser H, Altenburger M, Ugorcakova J, August C, Fisher LW, Schaefer RM, Kresse H. Decorin, biglycan and their endocytosis receptor in rat renal cortex. Kidney Int. 1998;54:1529–1541. doi: 10.1046/j.1523-1755.1998.00149.x. [DOI] [PubMed] [Google Scholar]
- 70.Yamamoto K, Kusachi S, Ninomiya Y, Murakami M, Doi M, Takeda K, Shinji T, Higashi T, Koide N, Tsuji T. Increase in the expression of biglycan mRNA expression Co-localized closely with that of type I collagen mRNA in the infarct zone after experimentally-induced myocardial infarction in rats. J Mol Cell Cardiol. 1998;30:1749–1756. doi: 10.1006/jmcc.1998.0737. [DOI] [PubMed] [Google Scholar]
- 71.Dressler W. Flare-up of pericarditis complicating myocardial infarction after two years of steroid therapy. Am Heart J. 1959;57:501–506. doi: 10.1016/0002-8703(59)90026-2. [DOI] [PubMed] [Google Scholar]
- 72.Dressler W. The post-myocardial-infarction syndrome: a report on forty-four cases. AMA Arch Intern Med. 1959;103:28–42. doi: 10.1001/archinte.1959.00270010034006. [DOI] [PubMed] [Google Scholar]
- 73.Haiat R. Post-myocardial infarction constrictive pericarditis. Am Heart J. 1981;101:358. doi: 10.1016/0002-8703(81)90211-8. [DOI] [PubMed] [Google Scholar]
- 74.Indik JH, Alpert JS. Post-Myocardial Infarction Pericarditis. Curr Treat Options Cardiovasc Med. 2000;2:351–356. doi: 10.1007/s11936-996-0009-7. [DOI] [PubMed] [Google Scholar]
- 75.Krainin FM, Flessas AP, Spodick DH. Infarction-associated pericarditis. Rarity of diagnostic electrocardiogram. N Engl J Med. 1984;311:1211–1214. doi: 10.1056/NEJM198411083111903. [DOI] [PubMed] [Google Scholar]
- 76.Spodick DH. Decreased recognition of the post-myocardial infarction (Dressler) syndrome in the postinfarct setting: does it masquerade as "idiopathic pericarditis" following silent infarcts? Chest. 2004;126:1410–1411. doi: 10.1378/chest.126.5.1410. [DOI] [PubMed] [Google Scholar]
- 77.Stubbs DF. Post-acute myocardial infarction symptomatic pericarditis (PAMISP): report on a large series and the effect of methylprednisolone therapy. J Int Med Res. 1986;14(Suppl 1):25–29. doi: 10.1177/03000605860140S105. [DOI] [PubMed] [Google Scholar]
- 78.Turk M. Acute pericarditis in the post-myocardial infarction patient. Crit Care Nurs Q. 1989;12:34–38. doi: 10.1097/00002727-198912000-00005. [DOI] [PubMed] [Google Scholar]
- 79.Shen L, Rock KL. Priming of T cells by exogenous antigen cross-presented on MHC class I molecules. Curr Opin Immunol. 2006;18:85–91. doi: 10.1016/j.coi.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 80.Melief CJ. Mini-review: Regulation of cytotoxic T lymphocyte responses by dendritic cells: peaceful coexistence of cross-priming and direct priming? Eur J Immunol. 2003;33:2645–2654. doi: 10.1002/eji.200324341. [DOI] [PubMed] [Google Scholar]
- 81.Bevan MJ. Cross-priming. Nat Immunol. 2006;7:363–365. doi: 10.1038/ni0406-363. [DOI] [PubMed] [Google Scholar]
- 82.Mukhopadhaya A, Hanafusa T, Jarchum I, Chen YG, Iwai Y, Serreze DV, Steinman RM, Tarbell KV, DiLorenzo TP. Selective delivery of beta cell antigen to dendritic cells in vivo leads to deletion and tolerance of autoreactive CD8+ T cells in NOD mice. Proc Natl Acad Sci U S A. 2008;105:6374–6379. doi: 10.1073/pnas.0802644105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Villadangos JA, Heath WR. Life cycle, migration and antigen presenting functions of spleen and lymph node dendritic cells: limitations of the Langerhans cells paradigm. Semin Immunol. 2005;17:262–272. doi: 10.1016/j.smim.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 84.Barton GM, Kagan JC. A cell biological view of Toll-like receptor function: regulation through compartmentalization. Nat Rev Immunol. 2009;9:535–542. doi: 10.1038/nri2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kollisch G, Kalali BN, Voelcker V, Wallich R, Behrendt H, Ring J, Bauer S, Jakob T, Mempel M, Ollert M. Various members of the Toll-like receptor family contribute to the innate immune response of human epidermal keratinocytes. Immunology. 2005;114:531–541. doi: 10.1111/j.1365-2567.2005.02122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lee HK, Dunzendorfer S, Tobias PS. Cytoplasmic domain-mediated dimerizations of toll-like receptor 4 observed by beta-lactamase enzyme fragment complementation. J Biol Chem. 2004;279:10564–10574. doi: 10.1074/jbc.M311564200. [DOI] [PubMed] [Google Scholar]
- 87.Lorenz E. TLR2 and TLR4 expression during bacterial infections. Curr Pharm Des. 2006;12:4185–4193. doi: 10.2174/138161206778743547. [DOI] [PubMed] [Google Scholar]
- 88.Pummerer CL, Luze K, Grassl G, Bachmaier K, Offner F, Burrell SK, Lenz DM, Zamborelli TJ, Penninger JM, Neu N. Identification of cardiac myosin peptides capable of inducing autoimmune myocarditis in BALB/c mice. J Clin Invest. 1996;97:2057–2062. doi: 10.1172/JCI118642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hayward SL, Bautista-Lopez N, Suzuki K, Atrazhev A, Dickie P, Elliott JF. CD4 T cells play major effector role and CD8 T cells initiating role in spontaneous autoimmune myocarditis of HLA-DQ8 transgenic IAb knockout nonobese diabetic mice. J Immunol. 2006;176:7715–7725. doi: 10.4049/jimmunol.176.12.7715. [DOI] [PubMed] [Google Scholar]
- 90.Berard JL, Wolak K, Fournier S, David S. Characterization of relapsing-remitting and chronic forms of experimental autoimmune encephalomyelitis in C57BL/6 mice. Glia. 2009;58:434–445. doi: 10.1002/glia.20935. [DOI] [PubMed] [Google Scholar]
- 91.Friese MA, Fugger L. Pathogenic CD8(+) T cells in multiple sclerosis. Annals of neurology. 2009;66:132–141. doi: 10.1002/ana.21744. [DOI] [PubMed] [Google Scholar]
- 92.Latif N, Sarathchandra P, Taylor PM, Antoniw J, Yacoub MH. Localization and pattern of expression of extracellular matrix components in human heart valves. J Heart Valve Dis. 2005;14:218–227. [PubMed] [Google Scholar]