Abstract
Endotoxin administration recapitulates many of the host responses to sepsis. Inhibitors of the cysteine protease caspase 1 have long been sought as a therapeutic because mice lacking caspase 1 are resistant to LPS-induced endotoxic shock. According to current thinking, caspase 1-mediated shock requires the proinflammatory caspase 1 substrates IL-1β and IL-18. We show, however, that mice lacking both IL-1β and IL-18 are normally susceptible to LPS-induced splenocyte apoptosis and endotoxic shock. This finding indicates the existence of another caspase 1-dependent mediator of endotoxemia. Reduced serum high mobility group box 1 (HMGB1) levels in caspase 1-deficient mice correlated with their resistance to LPS. A critical role for HMGB1 in endotoxemia was confirmed when mice deficient for IL-1β and IL-18 were protected from a lethal dose of LPS by pretreatment with HMGB1-neutralizing Abs. We found that HMGB1 secretion from LPS-primed macrophages required the inflammasome components apoptotic speck protein containing a caspase activation and recruitment domain (ASC), caspase 1 and Nalp3, whereas HMGB1 secretion from macrophages infected in vitro with Salmonella typhimurium was dependent on caspase 1 and Ipaf. Thus, HMGB1 secretion, which is critical for endotoxemia, occurs downstream of inflammasome assembly and caspase 1 activation.
Lethal endotoxemia in mice models mimics many features of septic shock in patients, including elevated cytokine production and extensive leukocyte apoptosis (1). The molecular mechanisms underlying lethal endotoxemia are not understood completely, but one important mediator is high mobility group box 1 (HMGB1) (2–4), which was identified originally as a highly conserved DNA-binding factor in the nucleus with roles in DNA transcription, replication, and repair (5). Subsequent studies showed HMGB1 at high levels in the serum of septic humans and animals (2, 6). A role for HMGB1 in shock-associated lethality was indicated when passive immunization with HMGB1-neutralizing Abs prevented organ damage in animal sepsis models (3).
Macrophage cell lines have been shown to release HMGB1 in response to LPS and during necrosis, but not when exposed to apoptotic stimuli (7). These observations fueled the concept that HMGB1 is a prototypical endogenous danger signal or alarmin, which is released from activated macrophages and necrotic cells. Secreted HMGB1 binds immune receptors, including TLRs and the receptor for advanced glycation end products (RAGE), to elicit proinflammatory responses (8). HMGB1 lacks a classic secretion signal, so the mechanism by which HMGB1 is released from cells remains unclear. Proinflammatory cytokines IL-1β and IL-18 also lack a signal peptide for the classic endoplasmic reticulum–Golgi exocytosis pathway. These cytokines are both produced as inactive precursors in the cytosol and are released in an ill-defined manner following their maturation by the cysteine protease caspase 1. The cytosolic zymogen form of caspase 1 consists of an N-terminal prodomain, followed by two subunits that together form the protease (9). Procaspase 1 is processed and activated within large protein complexes termed inflammasomes, which are assembled on nucleotide-binding oligomerization domain-like receptor (NLR) protein scaffolds (10).
NLR family members are believed to recognize conserved microbial and viral components called pathogen-associated molecular patterns (PAMPs), as well as endogenous, intracellular danger-associated molecular patterns (10). The N terminus of an NLR generally contains a homotypic interaction motif, such as the caspase activation and recruitment domain (CARD) or pyrin domain. A central nucleotide-binding oligomerization domain is thought to be involved in self-oligomerization, whereas the C-terminal leucine-rich repeats sense specific PAMPs and autoregulate NLR activity. Adaptor protein apoptosis-associated speck-like protein containing a CARD (ASC) bridges the interaction between NLR proteins and caspase 1, having an N-terminal pyrin domain and C-terminal CARD. Whereas ASC is always essential for activation of caspase 1 (11), the NLR protein Ipaf is only required for caspase 1 activation in macrophages infected with bacterial pathogens Salmonella (11–13), Pseudomonas (14–16), Legionella (17), and Shigella (18). The related NLR family member Nalp3 mediates caspase 1 activation in response to bacterial PAMPs such as LPS when combined with a second stimulus, such as the P2X7 receptor ligand ATP or the cation ionophore nigericin (19–23). Nalp3 also activates caspase 1 in LPS-primed macrophages exposed to uric acid, silica, and asbestos crystals (24–26) or when macrophages are infected with Staphylococcus aureus (19, 27) or Klebsiella pneumonia (28).
Caspase 1-deficient mice are highly resistant to LPS-induced endotoxemia (29), but the contributions of caspase 1 substrates IL-1β and IL-18 to endotoxic shock are less clear. Mice lacking IL-1β are normally susceptible to LPS-induced endotoxemia (30). We compared the response of caspase 1-deficient mice to that of animals lacking both IL-1β and IL-18. Unlike mice lacking caspase 1, animals lacking both IL-1β and IL-18 were normally susceptible to LPS-induced splenocyte apoptosis and endotoxic shock. We show that caspase 1 and its upstream activators are required for secretion of a third proinflammatory mediator called HMGB1, and when this is neutralized in vivo, IL-1β/IL-18 doubly deficient mice are protected from endotoxemia.
Materials and Methods
Mice
Nalp3−/−, Ipaf−/−, ASC−/−, caspase 1−/−, caspase 7−/−, and IL-1β−/−/IL-18−/− mice have been described (11, 19, 31–33). All strains were generated with C57BL/6 embryonic stem cells or backcrossed to C57BL/6 mice for at least 10 generations. Studies complied with the National Institutes of Health Guide for the Care and Use of Laboratory animals and were approved by the Genentech Institutional Animal Care and Use Committee (South San Francisco, CA).
Macrophages
Bone marrow-derived macrophages (BMDMs) were prepared as described (33). Briefly, bone marrow from femurs of 6–12-wk-old mice were cultured in IMDM containing 10% heat-inactivated FBS, 20% L cell-conditioned medium, 100 U/ml penicillin, and 100 mg/ml streptomycin at 37°C in a humidified atmosphere containing 5% CO2. Adherent cells after 5–7 d of culture were replated in 6-well or 24-well dishes in IMDM containing 10% heat-inactivated FBS and antibiotics.
Endotoxemia
Male mice (6–8 wk old) were maintained in microisolator cages and received food and water ad libitum according to American Association of Laboratory Animal Care guidelines. Lethal endotoxemia was induced by i.p. injection of 40 mg/kg LPS (Escherichia coli serotype 0111:B4; Sigma-Aldrich, St. Louis, MO). The spleen was harvested after 24 h, and formalin-fixed, paraffin-embedded sections were stained with H&E. IL-1β−/−/IL-18−/− mice were injected i.p. with 100 µg/kg rabbit polyclonal HMGB1-neutralizing Abs (34) or control rabbit IgGs (I5006; Sigma-Aldrich) 1 h prior to i.p. injection of 20 mg/kg LPS. Group survival was analyzed with the Kaplan-Meier test in Prism5 (GraphPad, San Diego, CA). A p value < 0.05 was considered statistically significant.
Bacterial infection and stimulation with microbial ligands
Salmonella enterica serovar typhimurium strain SL1344 and the isogenic orgA mutant strain BJ66 (SipB−) were kindly provided by D. Monack (Stanford University, Stanford, CA). The fljB−/fliC−S. typhimurium strain was a generous gift of A. Aderem (Institute for Systems Biology, Seattle, WA). Single colonies were inoculated into 3 ml BHI medium and grown overnight at 30°C with shaking. Macrophages were infected at a multiplicity of infection (MOI) of 10 for 1 h. Other stimuli included ultrapure LPS (Invivogen, San Diego, CA), 5 mM ATP (Roche, Basel, Switzerland), and 20 µM nigericin (Sigma-Aldrich).
Plasmids and transfection
pCMV-Sport6–pro-IL-1β and pCMV-Sport6-HMGB1 plasmids encoding mouse HMGB1 and pro-IL-1β were purchased from American Type Culture Collection (Manassas, VA), and cDNA inserts were verified by DNA sequencing. 293T cells were grown in DMEM supplemented with 10% FBS, 100 U/ml penicillin, 100 mg/ml streptomycin, and 2 mM l-glutamine at 37°C in a humidified atmosphere containing 5% CO2. Cells were transfected with lipofectamine (Invitrogen, Carlsbad, CA) for 12 h, and then lysates were prepared in 1% Nonidet P-40, 10 mM Tris-HCl (pH 7.4), 200 mM NaCl, 5 mM EDTA, and 10% glycerol.
In vitro caspase 1 cleavage assay
pCMV-Sport6–pro-IL-1β and pCMV-Sport6-HMGB1 (250 ng each) were transcribed and translated in vitro with a SP6-promoter-driven wheat germ-extract protein expression system (Promega, Madison, WI) and [35S]methionine. Translation products (2 µl) were incubated with 1 U recombinant caspase 1 (BioVision, Mountain View, CA) in 23 µl cell-free system buffer [10 mM HEPES (pH 7.4), 220 mM mannitol, 68 mM sucrose, 2 mM NaCl, 2.5 mM KH2PO4, 0.5 mM EGTA, 2 mM MgCl2, 5 mM sodium pyruvate, and 1 mM DTT] for up to 1 h at 37°C. Where indicated, recombinant caspase 1 was pretreated with 1 µM YVAD-cmk (Calbiochem, San Diego, CA) for 10 min.
Fluorescence microscopy
A total of 100,000 BMDMs were plated on glass cover slips (BD Biosciences, San Jose, CA), and 24 h later, cells were stimulated, washed in PBS, and fixed in 4% paraformaldehyde for 10 min. Cells were permeabilized with 0.1% Triton X-100 for 5 min. Blocking was with Image-iT FX Signal enhancer (Invitrogen) and then blocking buffer (10% goat serum in PBS, 0.1% Triton X-100, 0.1% saponin) for 60 min. Cells were stained overnight with 1 µg/ml HMGB1 Ab (Abcam, Cambridge, MA) in blocking buffer. After washing, bound Ab was revealed with 1 mg/ml Texas Red-conjugated goat anti-rabbit Ab (Jackson ImmunoResearch Laboratories, West Grove, PA) for 1 h. Slides were mounted with Prolong gold-DAPI Mounting Media (Invitrogen) and analyzed at room temperature under a Zeiss LD Plan-NEOFLUAR 20×/0.4 Ph2 korr objective on a Zeiss Axiovert 200M microscope equipped with a Zeiss Axiocam CCD digital camera and Axiovision Rel.4.6 software (Zeiss, Oberkochen, Germany). Fluorescence signals in digital black-and-white fluorographs were artificially colored with Adobe Photoshop (Adobe Systems, San Jose, CA).
Abs
Caspase 1 Ab was a generous gift of Dr. P. Vandenabeele (Ghent University, Ghent, Belgium). The HMGB1 Ab was purchased from Abcam (catalog number ab18256).
HMGB1 and cytokine release
HMGB1 in serum and culture supernatants was quantified with an HMGB1 ELISA (Shino test). Mouse cytokines were measured by Luminex assay (Bio-Rad, Hercules, CA). Data were analyzed with the Student t test. A p value < 0.05 was considered statistically significant.
Results
Caspase 1-dependent release of HMGB1 is essential for LPS-induced endotoxemia
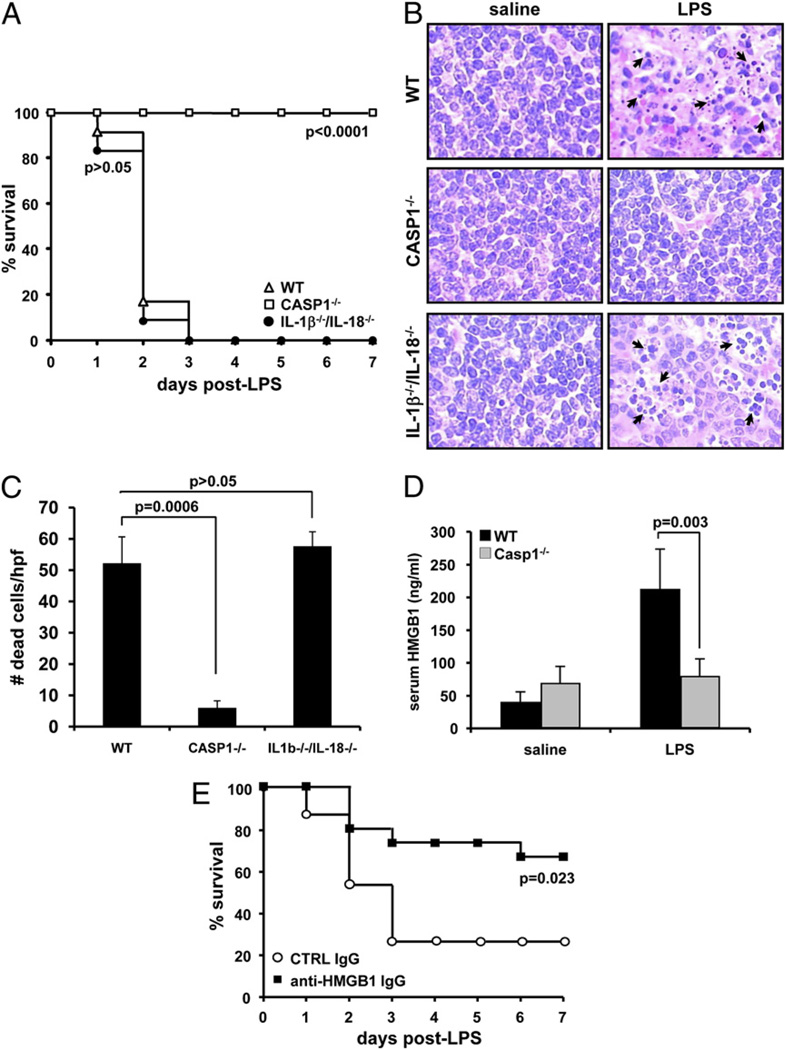
Although many of the downstream proinflammatory effects of caspase 1 activation are mediated by caspase 1 substrates IL-1β and IL-18 (35), it still is not clear why mice lacking caspase 1 are resistant to LPS-induced splenocyte apoptosis and lethality (29). IL-1β–deficient mice, unlike caspase 1-deficient mice, are susceptible to LPS-induced endotoxemia (30), so we determined whether this is because they still produce the proinflammatory cytokine IL-18. Wild-type (WT), caspase 1−/−, and il-1β−/−/il-18−/− mice were given a lethal dose of LPS. All WT and il-1β−/−/il-18−/− mice had succumbed by 72 h (Fig. 1A). By contrast, the caspase 1−/− mice remained viable after 7 d. Consistent with these data, significant endotoxin-induced lymphocyte apoptosis (1, 36) was evident at 24 h in the spleens of WT and il-1β−/−/il-18−/− mice, but this was markedly reduced in caspase 1−/− spleens (Fig. 1B). Splenocyte apoptosis in WT and il-1β−/−/il-18−/− mice was due to the LPS treatment, because apoptosis was not seen in mice receiving saline alone. We counted apoptotic cells in the splenic white pulp of 4 mice of each genotype using five randomly chosen high-power fields (×400), and this confirmed that caspase 1−/− mice contained significantly fewer apoptotic lymphocytes than WT or il-1β−/−/il-18−/− mice (p = 0.0006 and p = 0.00002, respectively) (Fig. 1C). These results indicate that neither IL-1β nor IL-18 is essential for LPS-induced splenocyte apoptosis and lethal endotoxic shock. Given that HMGB1 is an important mediator of shock (2, 3, 6), we measured its levels in the serum of LPS-treated WT and caspase 1−/− mice. In agreement with published data (2), LPS treatment increased serum HMGB1 levels in WT mice >5-fold (Fig. 1D). In stark contrast, serum HMGB1 levels in caspase 1−/− mice differed little from vehicle-treated WT or caspase 1−/− mice. This caspase 1-dependent HMGB1 release was crucial for endotoxemia because HMGB1-neutralizing Abs, but not control Igs, protected against LPS-induced lethality in il-1β−/−/il-18−/− mice (Fig. 1E).
Figure 1.
LPS-induced endotoxemia requires caspase 1-dependent HMGB1 release, but not the caspase 1 substrates IL-1β and IL-18. A, WT (n = 12), caspase 1−/− (n = 10), and IL-1β−/−/IL-18−/− (n = 12) mice were injected i.p. with 40 mg/kg LPS, and their survival was monitored. Results shown are from a single experiment and representative for two independent experiments. Data were compared with WT mice and analyzed with the Kaplan-Meier test. B, WT (n = 4), caspase 1−/− (n = 4), and IL-1β−/−/IL-18−/− (n = 4) mice were injected i.p. with either saline or 40 mg/kg LPS for 24 h before spleens were collected, and sections were stained with H&E. Arrows indicate selected apoptotic splenocytes in the white pulp of the spleen. Original magnification ×400. C, The number of apoptotic cells in five high-power fields (×400) from the white pulp of the spleen of each animal was quantified. Results represent mean ± SD for each genotype. Data were analyzed by Student t test. D, WT and caspase 1−/− mice were injected i.p. (n = 4/group) with either saline or 40 mg/kg LPS for 24 h before serum was collected to measure secreted HMGB1. Results are expressed in ng/ml and represent mean ± SD. Data were analyzed with Student t test. E, One hour before endotoxemia was induced by i.p. injection of 20 mg/kg LPS, IL-1β−/−/IL-18−/− mice (n = 15/group) received an i.p. injection of 100 µg/kg control IgG or HMGB1-neutralizing IgG. Survival was monitored daily for 7 d. Results shown are from a single experiment and representative for two independent experiments. Data were analyzed with the Kaplan-Meier test.
HMGB1 release from LPS-primed macrophages requires the NALP3 inflammasome
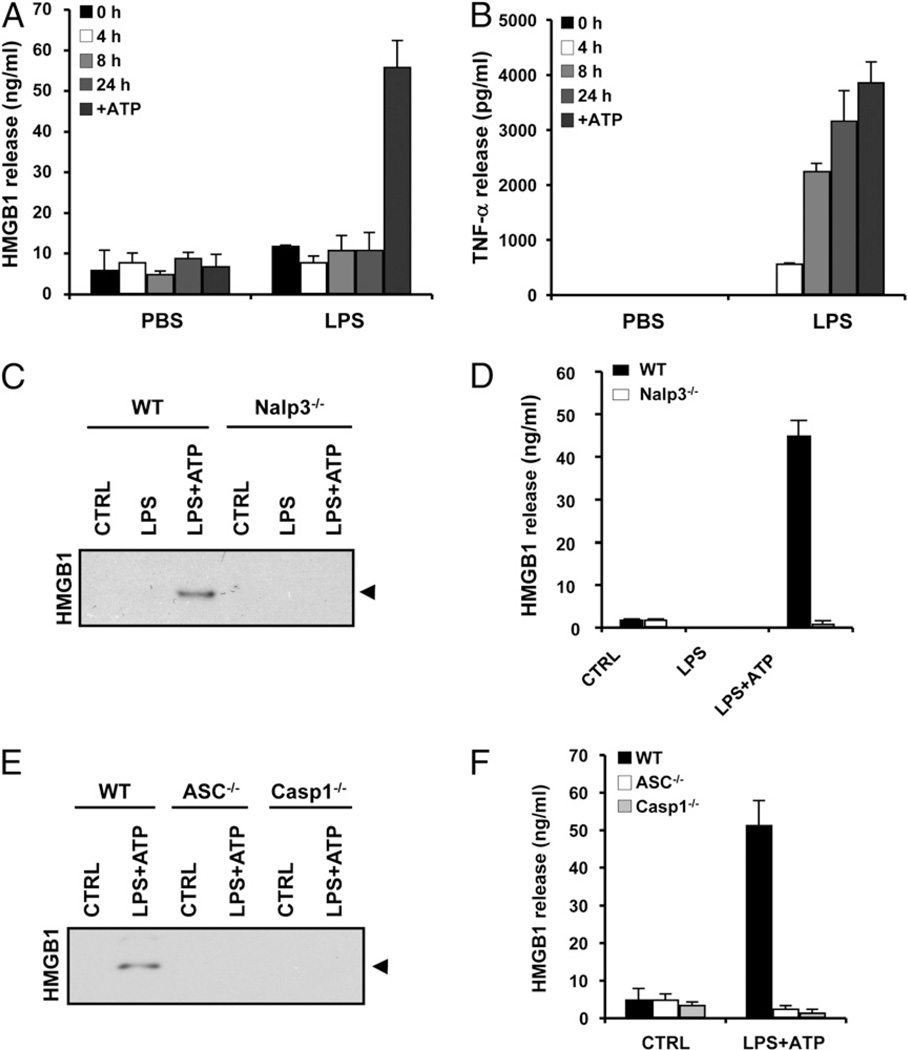
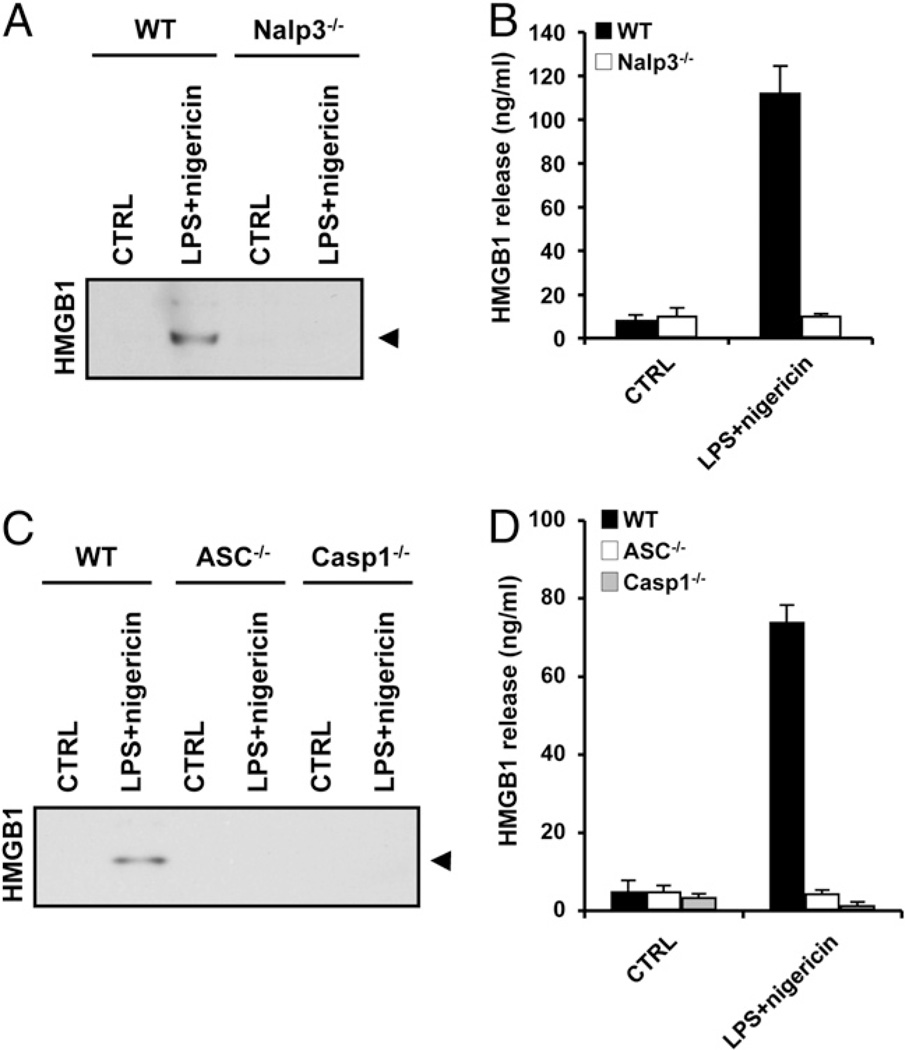
Next we sought direct evidence that inflammasome components are required for HMGB1 release from primary mouse macrophages. Although monocytes and macrophage-like cell lines such as RAW264.7 release HMGB1 (2) and IL-1β (37) in response to LPS stimulation alone, it is well established that mouse BMDMs activate caspase 1 and release IL-1β only when LPS priming is accompanied by a second stimulus such as ATP (10, 11, 33, 37). We observed a similar phenomenon for caspase 1-dependent HMGB1 release: BMDMs cultured in 10 µg/ml LPS alone for up to 24 h did not release HMGB1 (Fig. 2A). Only BMDMs that were primed with LPS and then exposed to 5 mM ATP for 30 min secreted significant amounts of HMGB1. ATP stimulation of BMDMs in the absence of LPS-priming did not cause HMGB1 release (Fig. 2A). As a control for LPS functionality, proinflammatory TNF-α was released from BMDMs in response to LPS alone (Fig. 2B). Release of HMGB1 from BMDMs treated with LPS plus ATP was dependent on Nalp3 (Fig. 2C, 2D), ASC (Fig. 2E, 2F), and caspase 1 (Fig. 2E, 2F), further extending the similarities between HMGB1 and IL-1β secretion from BMDMs. The ionophore nigericin can substitute for ATP in Nalp3-dependent IL-1βsecretion from BMDMs (19, 33). We found that BMDMs primed with 10 µg/ml LPS for 3 h and then treated with 20 µM nigericin for 30 min also released significant amounts of HMGB1 in a Nalp3- (Fig. 3A, 3B), ASC- (Fig. 3C, 3D), and caspase 1-dependent manner (Fig. 3C, 3D). In agreement, the caspase 1 inhibitor Ac-YVAD-cmk prevented the release of HMGB1 from LPS plus ATP and LPS plus nigericin-treated BMDMs (Supplemental Fig. 1). In contrast, HMGB1 release was not appreciably affected by the cathepsin B inhibitor CA-074-OMe (Supplemental Fig. 2).
Figure 2.
HMGB1 release from LPS and ATP-stimulated BMDMs requires the Nalp3 inflammasome. A and B, BMDMs were stimulated with PBS or LPS (100 ng/ml) for the indicated durations. In one setup, BMDMs were stimulated with 100 ng/ml LPS for 24 h, the last 30 min of which in the presence of 5 mM ATP (+ATP). Culture medium was collected and analyzed for secreted HMGB1 (A) and TNF-α (B). C and D, BMDMs from WT and Nalp3−/− mice were left untreated (CTRL), stimulated with 10 µg/ml LPS for 3 h (LPS), or treated with 10 µg/ml LPS for 3 h followed by 5 mM ATP for 30 min (LPS+ATP). Culture supernatants were collected and analyzed for secreted HMGB1 by Western blotting (C) and ELISA (D). E and F, BMDMs from WT, ASC−/−, and caspase 1−/− mice were left untreated (CTRL) or stimulated with 10 µg/ml LPS for 3 h and 5 mM ATP for 30 min (LPS+ATP). Culture supernatants were collected and analyzed for secreted HMGB1 by Western blotting (E) and ELISA (F). Black arrowhead on Western blots marks HMGB1 (29 kDa). ELISA and Luminex data represent the mean ± SD of triplicate samples from a single experiment, and all results are representative of at least three independent experiments.
Figure 3.
Nalp3 inflammasome-dependent release of HMGB1 from LPS plus nigericin-stimulated macrophages. A and B, BMDMs from WT and Nalp3−/− mice were left untreated (CTRL) or stimulated with 10 µg/ml LPS for 3 h and 20 µM nigericin for 30 min (LPS+nigericin). Culture supernatants were collected and analyzed for secreted HMGB1 by Western blotting (A) and ELISA (B). C and D, BMDMs from WT, ASC−/−, and caspase 1−/− mice were left untreated (CTRL) or stimulated with 10 µg/ml LPS for 3 h and 20 µM nigericin for 30 min (LPS+nigericin). Culture supernatants were collected and analyzed for secreted HMGB1 by Western blotting (C) and ELISA (D). Black arrowhead on Western blots marks HMGB1 (29 kDa). ELISA data represent the mean ± SD of triplicate samples from a single experiment, and all results are representative of three independent experiments.
Salmonella-induced HMGB1 release requires the Ipaf inflammasome
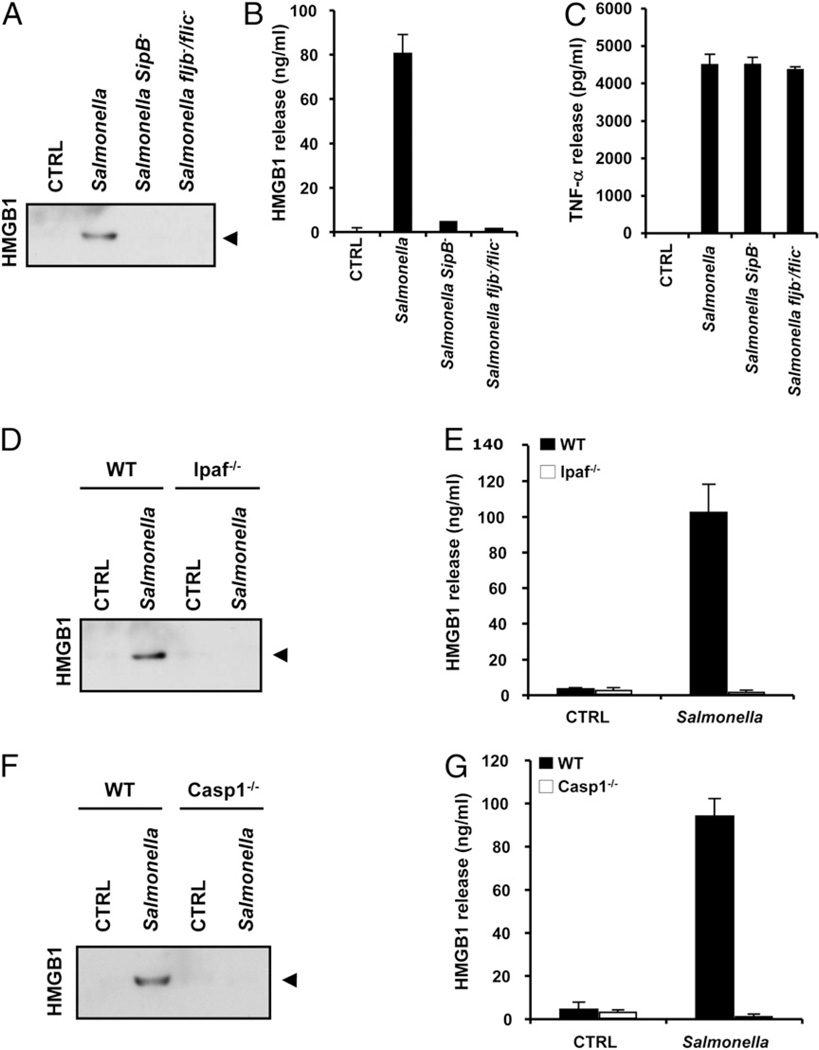
Whereas LPS/ATP and LPS/nigericin induce caspase 1 activation via the Nalp3 inflammasome, macrophages infected with the Gram-negative bacterial pathogen Salmonella typhimurium (Salmonella) respond by activating caspase 1 through the Ipaf inflammasome (11–13). Ipaf, in a manner yet to be determined, senses bacterial flagellin, which appears to access the cytosol using the bacterial type III secretion system (11–13). We used Salmonella infection of BMDMs to determine whether HMGB1 secretion was linked to the Nalp3 inflammasome specifically, or was a general outcome of caspase 1 activation. BMDMs infected with WT Salmonella, but not flagellin-mutant fljb−/flic− or type III secretion system mutant SipB−, activated caspase 1 (Supplemental Fig. 3) and released significant amounts of HMGB1 into the culture medium, as determined by HMGB1 immunoblotting (Fig. 4A) and HMGB1-specific ELISA (Fig. 4B). TNF-α released in response to the two mutant bacteria, however, was comparable to that induced by WT Salmonella (Fig. 4C), demonstrating that impaired HMGB1 release was not due to a general lack of macrophage stimulation. The correlation between caspase 1 activation and HMGB1 secretion in macrophages infected with WT Salmonella suggested that Ipaf inflammasome activation was upstream of HMGB1 secretion. Indeed, BMDMs from ipaf−/− and caspase 1−/− mice failed to secrete HMGB1 in response to WT Salmonella (Fig. 4D–G), although they released normal amounts of TNF-α (11) (Supplemental Fig. 4). In agreement, the caspase 1 inhibitor Ac-YVAD-cmk prevented the release of HMGB1 from Salmonella-infected BMDMs (Supplemental Fig. 1), whereas the cathepsin B inhibitor CA-074-OMe had no affect (Supplemental Fig. 2). These results demonstrate that the Ipaf inflammasome is essential for HMGB1 release from Salmonella-infected macrophages and that HMGB1 release is a general consequence of caspase 1 activation.
Figure 4.
HMGB1 release from Salmonella-infected macrophages requires bacterial flagellin, a functional type III secretion and the Ipaf inflammasome. A–C, BMDMs were left untreated (CTRL), infected with WT Salmonella (MOI 10), or with the type III secretion system-deficient (SipB−) or flagellin-deficient (fljB−/fliC−) mutants for 1 h. Culture supernatants were collected and analyzed for secreted HMGB1 by Western blotting (A) and ELISA (B) and for secreted TNF-α by Luminex assay (C). D and E, BMDMs from WT and Ipaf−/− mice were left untreated (CTRL) or infected with WT Salmonella (MOI 10) for 1 h. Culture supernatants were collected and analyzed for secreted HMGB1 by Western blotting (D) and ELISA (E). F and G, BMDMs from WT and caspase 1−/− mice were left untreated (CTRL) or infected with WT Salmonella (MOI 10) for 1 h. Culture supernatants were analyzed for secreted HMGB1 by Western blotting (F) and ELISA (G). Black arrowhead on Western blots marks HMGB1 (29 kDa). ELISA and Luminex data represent the mean ± SD of triplicate samples from a single experiment, and all results are representative of three independent experiments.
Inflammasome-mediated translocation and release of full-length HMGB1 is independent of IL-1β, IL-18, and caspase-7
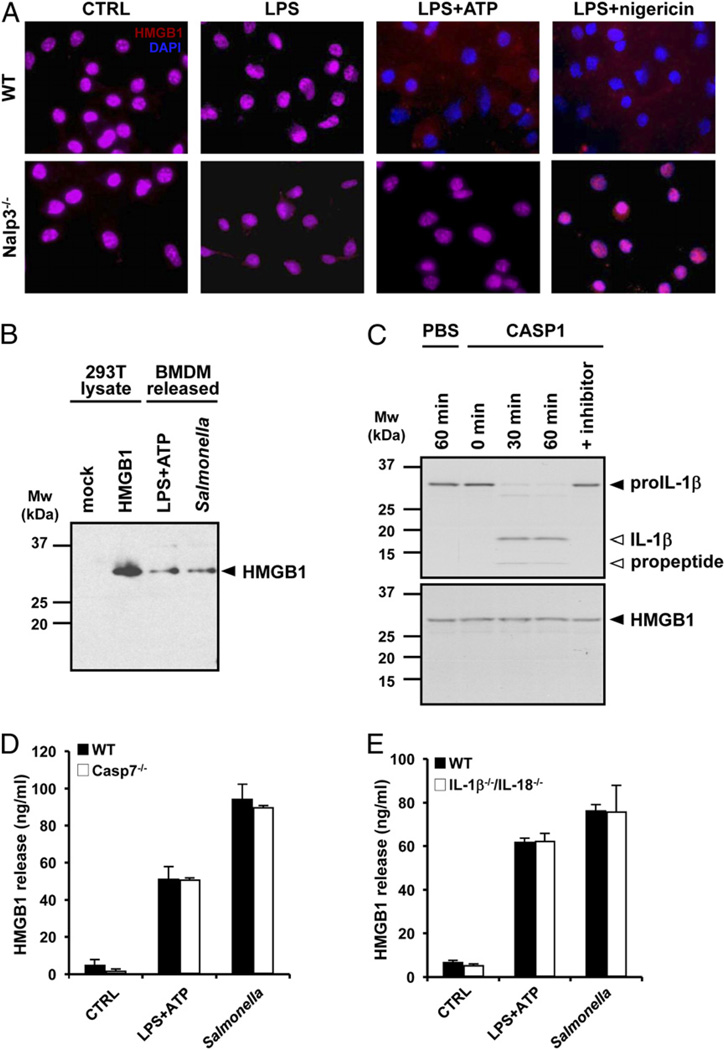
HMGB1 exists in the nucleus of resting cells, where it is linked to DNA transcription, replication, and repair (5). We made use of immunofluorescence microscopy to determine the role of the inflammasome in the subcellular localization of HMGB1 in LPS-primed BMDMs. WT and nalp3−/− BMDMs contained nuclear HMGB1 both before (Fig. 5A, leftmost panels) and after LPS priming (Fig. 5A, second to left panels), the latter observation consistent with a second stimulus being required for HMGB1 release (Fig. 2A). Subsequent exposure to either ATP or nigericin resulted in almost complete depletion of the nuclear pool of HMGB1 in WT BMDMs (Fig. 5A, second to right and right upper panels). By contrast, nuclear HMGB1 levels were not reduced in LPS/ATP- and LPS/nigericin-treated nalp3−/− BMDMs (Fig. 5A, second to right and right lower panels). These results demonstrate that LPS priming is not sufficient for Nalp3-dependent translocation of nuclear HMGB1 out of macrophages.
Figure 5.
The inflammasome is essential for nuclear translocation and secretion of full-length HMGB1 independently of caspase 1 processing and substrates. A, BMDMs from WT and Nalp3−/− mice were plated on cover slips and left untreated (CTRL), stimulated with 10 µg/ml LPS for 3 h (LPS), or treated with 10 µg/ml LPS for 3 h followed by 5 mM ATP (LPS+ATP) or 20 µM nigericin for 30 min. Cells were immunostained for HMGB1, and nuclei were counterstained with DAPI. Original magnification ×20. B, Cell extracts of mock-transfected and HMGB1-overexpressing 293T cells were immunoblotted for HMGB1 together with culture supernatants of BMDMs treated with 10 µg/ml LPS and 5 mM ATP or infected with Salmonella (MOI 10). Black arrowhead on the Western blot marks full-length HMGB1 (29 kDa). C, [35S]-labeled pro-IL-1βand HMGB1 were incubated with PBS or 1 U recombinant caspase 1 for the indicated durations, and cleavage fragments were analyzed by SDS-PAGE and autoradiography. In one setup, recombinant caspase 1 was pretreated with 1 µM YVAD-cmk preincubation with pro-IL-1βand HMGB1 for 60 min. Black arrowhead marks full-length pro-IL-1β and HMGB1, and white arrowheads mark IL-1β cleavage fragments. BMDMs from WT and caspase 7−/− mice (D) and IL-1β−/−/IL-18−/− mice (E) were left untreated (CTRL), stimulated with 10 µg/ml LPS for 3 h followed by 5 mM ATP for 30 min (LPS+ATP), or infected with Salmonella (MOI 10) for 1 h. Culture supernatants were collected and analyzed for secreted HMGB1 by ELISA. Data represent the mean ± SD of triplicate samples from a single experiment, and all results are representative of three independent experiments.
Secreted IL-1β and IL-18 undergo caspase 1-dependent maturation (35), but we found no evidence of HMGB1 cleavage upon release from BMDMs. HMGB1 recovered from the culture medium of BMDMs exposed to either LPS/ATP or Salmonella showed comparable migration by SDS-PAGE to full-length HMGB1 over-expressed in 293T cells (Fig. 5B). In addition, recombinant caspase 1 converted pro-IL-1β to mature IL-1β within 30 min, but under the same conditions, recombinant caspase 1 did not cleave in vitrotranslated HMGB1, even after 60 min (Fig. 5C). Caspase 7 also is activated downstream of caspase 1 (38). However, caspase 7 does not mediate caspase 1-dependent release of HMGB1 because caspase 7−/− BMDMs stimulated with LPS/ATP or infected with Salmonella secreted normal levels of HMGB1 (Fig. 5D). As expected from our in vivo studies (Fig. 1A), release of HMGB1 was not compromised in IL-1β−/−/IL-18−/− BMDMs (Fig. 5E). Therefore, HMGB1 does not undergo proteolytic processing and is released independently of the known caspase 1 substrates IL-1β, IL-18, and caspase 7.
Discussion
The evolutionarily conserved cysteine protease caspase 1 is the effector protein of large (700 kDa) cytosolic protein complexes called inflammasomes (10). The stimulus-dependent oligomerization of certain NLR family members triggers inflammasome assembly and the proximity-induced autoproteolytic activation of caspase 1 (10, 39–41). Activated caspase 1 subsequently processes its substrates, which include caspase 7 (38) and the proinflammatory cytokines IL-1βand IL-18 (29, 42, 43). Caspase 1 also mediates a specialized form of programmed cell death in myeloid cells that is often referred to as pyroptosis, as it differs from apoptosis and programmed necrosis (44). The molecular mechanisms of pyroptosis remain elusive.
Mice lacking inflammasome components Nalp3 and caspase 1 are resistant to LPS-induced endotoxemia (19, 21, 29, 43), but the roles of the caspase 1 substrates IL-1βand IL-18 in endotoxic shock are less clear. Whereas il-1β−/− mice are susceptible to LPS-induced endotoxemia (30), the response of il-18−/− mice has not been reported. Our finding that il-1β−/−/il-18−/− mice are normally susceptible to LPS-induced endotoxic shock indicates that caspase 1 elicits endotoxemia through other effectors. il-1β−/−/il-18−/− mice also succumbed to Escherichia coli-induced sepsis, whereas caspase 1-deficient mice cleared the infection (32). HMGB1 is a nuclear protein that is believed to cause inflammation when released into the extracellular space (2, 7). rHMGB1 produced in E. coli is a potent inducer of proinflammatory cytokines when administered to cells or injected in mice (2, 45–49), but studies using HMGB1 purified from eukaryotic sources such as rat brain (49) or calf thymus (50) suggested a requirement for endogenous or microbial cofactors for HMGB1 to potently activate RAGE and induce cytokine production. Thus, HMGB1 that is released in the bloodstream during infection might bind microbial components or substances released from injured tissue to induce or augment the production of inflammatory mediators. The assembly of such immunostimulatory complexes has been demonstrated for RAGE receptor activation by an HMGB1–CpG DNA complex (50). In addition to RAGE, HMGB1 was suggested to induce NF-ΚB signaling through TLR2 and TLR4 activation (51, 52), but this has been contested by others (50).
We showed in this study that serum HMGB1 levels are markedly reduced in LPS-challenged caspase 1−/− mice. The pan-caspase inhibitor zVAD-fmk was shown previously to both reduce serum HMGB1 levels and suppress apoptosis in the spleen and thymus of septic mice, but the critical caspase associated with HMGB1 release was not evident (53). HMGB1-neutralizing Abs protected il-1β−/−/il-18−/− mice from a normally lethal dose of LPS, underscoring the critical role of HMGB1 release in endotoxemia. Because HMGB1 neutralization is being evaluated in clinical trials as a treatment for sepsis, bacteremia, and ARDS (4, 54), our finding that HMGB1 release is dependent on inflammasome assembly and caspase 1 activation provides important insight into the upstream mechanisms of HMGB1 release. Given that we saw no evidence of HMGB1 cleavage by caspase 1 and that caspase 1 substrates caspase 7, IL-1β, and IL-18 were dispensable for HMGB1 release from macrophages exposed to LPS/ATP or Salmonella, the caspase 1 substrate that promotes HMGB1 release remains to be identified. Caspase 1 has been suggested to allow secretion of leaderless cytokines, such as IL-1β and IL-18, through proteolytic activation of a secretion apparatus of unknown identity (55). One possibility is that HMGB1 secretion also occurs through this mechanism. Another possibility is that HMGB1 release is linked to the caspase 1-dependent cell death process that occurs in splenocytes during endotoxemia and in isolated macrophages exposed to LPS plus ATP or Salmonella (33, 56, 57). The answer to these questions awaits the molecular characterization of the mechanisms by which caspase 1 mediates secretion of leaderless cytokines and cell death in immune cells.
In summary, our results show that HMGB1 release is a new mechanism by which caspase 1 contributes to inflammation and endotoxemia. Caspase 1-mediated HMGB1 release occurred in the absence of IL-1β and IL-18, demonstrating that inflammasomes engage various independent effector mechanisms to promote inflammation.
Supplementary Material
Acknowledgments
We thank Andres Paler Martinez and Natacha Rosseel for technical support and Dr. Peter Vandenabeele (Ghent University, Ghent, Belgium) for caspase 1 Ab.
This work was supported in part by National Institutes of Health Grant AR056296 and the American Lebanese and Syrian Associated Charities (to T.-D.K.) and by National Institutes of Health Grant R01 HL076278 (to M.D.W.). M.L. and L.V.W. are supported by the Fonds voor Wetenschappelijk Onderzoek-Vlaanderen.
Abbreviations used in this paper
- ASC
apoptotic speck protein containing a caspase activation and recruitment domain
- BMDM
bone marrow-derived macrophage
- CARD
caspase activation and recruitment domain
- CTRL
control
- HMGB1
high mobility group box 1
- MOI
multiplicity of infection
- NLR
nucleotide-binding oligomerization domain-like receptor
- PAMP
pathogen-associated molecular pattern
- RAGE
receptor for advanced glycation end products
- WT
wild-type
Footnotes
Disclosures
M.L., A.C.V., and V.M.D. are/were employees and/or shareholders of Genentech.
The online version of this article contains supplemental material.
References
- 1.Gautier EL, Huby T, Saint-Charles F, Ouzilleau B, Chapman MJ, Lesnik P. Enhanced dendritic cell survival attenuates lipopolysaccharide-induced immunosuppression and increases resistance to lethal endotoxic shock. J. Immunol. 2008;180:6941–6946. doi: 10.4049/jimmunol.180.10.6941. [DOI] [PubMed] [Google Scholar]
- 2.Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, Che J, Frazier A, Yang H, Ivanova S, Borovikova L, et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285:248–251. doi: 10.1126/science.285.5425.248. [DOI] [PubMed] [Google Scholar]
- 3.Yang H, Ochani M, Li J, Qiang X, Tanovic M, Harris HE, Susarla SM, Ulloa L, Wang H, DiRaimo R, et al. Reversing established sepsis with antagonists of endogenous high-mobility group box 1. Proc. Natl. Acad. Sci. USA. 2004;101:296–301. doi: 10.1073/pnas.2434651100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abraham E, Arcaroli J, Carmody A, Wang H, Tracey KJ. HMG-1 as a mediator of acute lung inflammation. J. Immunol. 2000;165:2950–2954. doi: 10.4049/jimmunol.165.6.2950. [DOI] [PubMed] [Google Scholar]
- 5.Bustin M. Regulation of DNA-dependent activities by the functional motifs of the high-mobility-group chromosomal proteins. Mol. Cell. Biol. 1999;19:5237–5246. doi: 10.1128/mcb.19.8.5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sundén-Cullberg J, Norrby-Teglund A, Rouhiainen A, Rauvala H, Herman G, Tracey KJ, Lee ML, Andersson J, Tokics L, Treutiger CJ. Persistent elevation of high mobility group box-1 protein (HMGB1) in patients with severe sepsis and septic shock. Crit. Care Med. 2005;33:564–573. doi: 10.1097/01.ccm.0000155991.88802.4d. [DOI] [PubMed] [Google Scholar]
- 7.Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–195. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- 8.van Zoelen MA, Yang H, Florquin S, Meijers JC, Akira S, Arnold B, Nawroth PP, Bierhaus A, Tracey KJ, van der Poll T. Role of toll-like receptors 2 and 4, and the receptor for advanced glycation end products in high-mobility group box 1-induced inflammation in vivo. Shock. 2009;31:280–284. doi: 10.1097/SHK.0b013e318186262d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lamkanfi M, Declercq W, Kalai M, Saelens X, Vandenabeele P. Alice in caspase land. A phylogenetic analysis of caspases from worm to man. Cell Death Differ. 2002;9:358–361. doi: 10.1038/sj.cdd.4400989. [DOI] [PubMed] [Google Scholar]
- 10.Lamkanfi M, Dixit VM. Inflammasomes: guardians of cytosolic sanctity. Immunol. Rev. 2009;227:95–105. doi: 10.1111/j.1600-065X.2008.00730.x. [DOI] [PubMed] [Google Scholar]
- 11.Mariathasan S, Newton K, Monack DM, Vucic D, French DM, Lee WP, Roose-Girma M, Erickson S, Dixit VM. Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature. 2004;430:213–218. doi: 10.1038/nature02664. [DOI] [PubMed] [Google Scholar]
- 12.Miao EA, Alpuche-Aranda CM, Dors M, Clark AE, Bader MW, Miller SI, Aderem A. Cytoplasmic flagellin activates caspase-1 and secretion of interleukin 1beta via Ipaf. Nat. Immunol. 2006;7:569–575. doi: 10.1038/ni1344. [DOI] [PubMed] [Google Scholar]
- 13.Franchi L, Amer A, Body-Malapel M, Kanneganti TD, Ozören N, Jagirdar R, Inohara N, Vandenabeele P, Bertin J, Coyle A, et al. Cytosolic flagellin requires Ipaf for activation of caspase-1 and interleukin 1beta in salmonella-infected macrophages. Nat. Immunol. 2006;7:576–582. doi: 10.1038/ni1346. [DOI] [PubMed] [Google Scholar]
- 14.Sutterwala FS, Mijares LA, Li L, Ogura Y, Kazmierczak BI, Flavell RA. Immune recognition of Pseudomonas aeruginosa mediated by the IPAF/NLRC4 inflammasome. J. Exp. Med. 2007;204:3235–3245. doi: 10.1084/jem.20071239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miao EA, Ernst RK, Dors M, Mao DP, Aderem A. Pseudomonas aeruginosa activates caspase 1 through Ipaf. Proc. Natl. Acad. Sci. USA. 2008;105:2562–2567. doi: 10.1073/pnas.0712183105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Franchi L, Stoolman J, Kanneganti TD, Verma A, Ramphal R, Núñez G. Critical role for Ipaf in Pseudomonas aeruginosa-induced caspase-1 activation. Eur. J. Immunol. 2007;37:3030–3039. doi: 10.1002/eji.200737532. [DOI] [PubMed] [Google Scholar]
- 17.Amer A, Franchi L, Kanneganti TD, Body-Malapel M, Ozören N, Brady G, Meshinchi S, Jagirdar R, Gewirtz A, Akira S, Núñez G. Regulation of Legionella phagosome maturation and infection through flagellin and host Ipaf. J. Biol. Chem. 2006;281:35217–35223. doi: 10.1074/jbc.M604933200. [DOI] [PubMed] [Google Scholar]
- 18.Suzuki T, Franchi L, Toma C, Ashida H, Ogawa M, Yoshikawa Y, Mimuro H, Inohara N, Sasakawa C, Nuñez G. Differential regulation of caspase-1 activation, pyroptosis, and autophagy via Ipaf and ASC in Shigella-infected macrophages. PLoS Pathog. 2007;3:e111. doi: 10.1371/journal.ppat.0030111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mariathasan S, Weiss DS, Newton K, McBride J, O’Rourke K, Roose-Girma M, Lee WP, Weinrauch Y, Monack DM, Dixit VM. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440:228–232. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- 20.Kanneganti TD, Lamkanfi M, Kim YG, Chen G, Park JH, Franchi L, Vandenabeele P, Núñez G. Pannexin-1-mediated recognition of bacterial molecules activates the cryopyrin inflammasome independent of Toll-like receptor signaling. Immunity. 2007;26:433–443. doi: 10.1016/j.immuni.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 21.Sutterwala FS, Ogura Y, Szczepanik M, Lara-Tejero M, Lichtenberger GS, Grant EP, Bertin J, Coyle AJ, Galán JE, Askenase PW, Flavell RA. Critical role for NALP3/CIAS1/Cryopyrin in innate and adaptive immunity through its regulation of caspase-1. Immunity. 2006;24:317–327. doi: 10.1016/j.immuni.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 22.Kanneganti TD, Ozören N, Body-Malapel M, Amer A, Park JH, Franchi L, Whitfield J, Barchet W, Colonna M, Vandenabeele P, et al. Bacterial RNA and small antiviral compounds activate caspase-1 through cryopyrin/Nalp3. Nature. 2006;440:233–236. doi: 10.1038/nature04517. [DOI] [PubMed] [Google Scholar]
- 23.Kanneganti TD, Body-Malapel M, Amer A, Park JH, Whitfield J, Franchi L, Taraporewala ZF, Miller D, Patton JT, Inohara N, Núñez G. Critical role for Cryopyrin/Nalp3 in activation of caspase-1 in response to viral infection and double-stranded RNA. J. Biol. Chem. 2006;281:36560–36568. doi: 10.1074/jbc.M607594200. [DOI] [PubMed] [Google Scholar]
- 24.Hornung V, Bauernfeind F, Halle A, Samstad EO, Kono H, Rock KL, Fitzgerald KA, Latz E. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat. Immunol. 2008;9:847–856. doi: 10.1038/ni.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cassel SL, Eisenbarth SC, Iyer SS, Sadler JJ, Colegio OR, Tephly LA, Carter AB, Rothman PB, Flavell RA, Sutterwala FS. The Nalp3 inflammasome is essential for the development of silicosis. Proc. Natl. Acad. Sci. USA. 2008;105:9035–9040. doi: 10.1073/pnas.0803933105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dostert C, Pétrilli V, Van Bruggen R, Steele C, Mossman BT, Tschopp J. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science. 2008;320:674–677. doi: 10.1126/science.1156995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller LS, Pietras EM, Uricchio LH, Hirano K, Rao S, Lin H, O’Connell RM, Iwakura Y, Cheung AL, Cheng G, Modlin RL. Inflammasome-mediated production of IL-1beta is required for neutrophil recruitment against Staphylococcus aureus in vivo. J. Immunol. 2007;179:6933–6942. doi: 10.4049/jimmunol.179.10.6933. [DOI] [PubMed] [Google Scholar]
- 28.Willingham SB, Allen IC, Bergstralh DT, Brickey WJ, Huang MT, Taxman DJ, Duncan JA, Ting JP. NLRP3 (NALP3, Cryopyrin) facilitates in vivo caspase-1 activation, necrosis, and HMGB1 release via inflammasome-dependent and -independent pathways. J. Immunol. 2009;183:2008–2015. doi: 10.4049/jimmunol.0900138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuida K, Lippke JA, Ku G, Harding MW, Livingston DJ, Su MS, Flavell RA. Altered cytokine export and apoptosis in mice deficient in interleukin-1 beta converting enzyme. Science. 1995;267:2000–2003. doi: 10.1126/science.7535475. [DOI] [PubMed] [Google Scholar]
- 30.Fantuzzi G, Zheng H, Faggioni R, Benigni F, Ghezzi P, Sipe JD, Shaw AR, Dinarello CA. Effect of endotoxin in IL-1 beta-deficient mice. J. Immunol. 1996;157:291–296. [PubMed] [Google Scholar]
- 31.Lakhani SA, Masud A, Kuida K, Porter GA, Booth CJ, Jr, Mehal WZ, Inayat I, Flavell RA. Caspases 3 and 7: key mediators of mitochondrial events of apoptosis. Science. 2006;311:847–851. doi: 10.1126/science.1115035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sarkar A, Hall MW, Exline M, Hart J, Knatz N, Gatson NT, Wewers MD. Caspase-1 regulates Escherichia coli sepsis and splenic B cell apoptosis independently of interleukin-1beta and interleukin-18. Am. J. Respir. Crit. Care Med. 2006;174:1003–1010. doi: 10.1164/rccm.200604-546OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lamkanfi M, Mueller JL, Vitari AC, Misaghi S, Fedorova A, Deshayes K, Lee WP, Hoffman HM, Dixit VM. Glyburide inhibits the Cryopyrin/Nalp3 inflammasome. J. Cell Biol. 2009;187:61–70. doi: 10.1083/jcb.200903124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang R, Harada T, Mollen KP, Prince JM, Levy RM, Englert JA, Gallowitsch-Puerta M, Yang L, Yang H, Tracey KJ, et al. Anti-HMGB1 neutralizing antibody ameliorates gut barrier dysfunction and improves survival after hemorrhagic shock. Mol. Med. 2006;12:105–114. doi: 10.2119/2006-00010.Yang. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dinarello CA. Interleukin-1 beta, interleukin-18, and the interleukin-1 beta converting enzyme. Ann. N. Y. Acad. Sci. 1998;856:1–11. doi: 10.1111/j.1749-6632.1998.tb08307.x. [DOI] [PubMed] [Google Scholar]
- 36.Grobmyer SR, Armstrong RC, Nicholson SC, Gabay C, Arend WP, Potter SH, Melchior M, Fritz LC, Nathan CF. Peptidomimetic fluoromethylketone rescues mice from lethal endotoxic shock. Mol. Med. 1999;5:585–594. [PMC free article] [PubMed] [Google Scholar]
- 37.Netea MG, Nold-Petry CA, Nold MF, Joosten LA, Opitz B, van der Meer JH, van de Veerdonk FL, Ferwerda G, Heinhuis B, Devesa I, et al. Differential requirement for the activation of the inflammasome for processing and release of IL-1beta in monocytes and macrophages. Blood. 2009;113:2324–2335. doi: 10.1182/blood-2008-03-146720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lamkanfi M, Kanneganti TD, Van Damme P, Vanden Berghe T, Vanoverberghe I, Vandekerckhove J, Vandenabeele P, Gevaert K, Núñez G. Targeted peptidecentric proteomics reveals caspase-7 as a substrate of the caspase-1 inflammasomes. Mol. Cell. Proteomics. 2008;7:2350–2363. doi: 10.1074/mcp.M800132-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Agostini L, Martinon F, Burns K, McDermott MF, Hawkins PN, Tschopp J. NALP3 forms an IL-1beta-processing inflammasome with increased activity in Muckle-Wells autoinflammatory disorder. Immunity. 2004;20:319–325. doi: 10.1016/s1074-7613(04)00046-9. [DOI] [PubMed] [Google Scholar]
- 40.Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol. Cell. 2002;10:417–426. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 41.Boatright KM, Renatus M, Scott FL, Sperandio S, Shin H, Pedersen IM, Ricci JE, Edris WA, Sutherlin DP, Green DR, Salvesen GS. A unified model for apical caspase activation. Mol. Cell. 2003;11:529–541. doi: 10.1016/s1097-2765(03)00051-0. [DOI] [PubMed] [Google Scholar]
- 42.Ghayur T, Banerjee S, Hugunin M, Butler D, Herzog L, Carter A, Quintal L, Sekut L, Talanian R, Paskind M, et al. Caspase-1 processes IFN-gamma-inducing factor and regulates LPS-induced IFN-gamma production. Nature. 1997;386:619–623. doi: 10.1038/386619a0. [DOI] [PubMed] [Google Scholar]
- 43.Li P, Allen H, Banerjee S, Franklin S, Herzog L, Johnston C, McDowell J, Paskind M, Rodman L, Salfeld J, et al. Mice deficient in IL-1 beta-converting enzyme are defective in production of mature IL-1 beta and resistant to endotoxic shock. Cell. 1995;80:401–411. doi: 10.1016/0092-8674(95)90490-5. [DOI] [PubMed] [Google Scholar]
- 44.Bergsbaken T, Fink SL, Cookson BT. Pyroptosis: host cell death and inflammation. Nat. Rev. Microbiol. 2009;7:99–109. doi: 10.1038/nrmicro2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kokkola R, Andersson A, Mullins G, Ostberg T, Treutiger CJ, Arnold B, Nawroth P, Andersson U, Harris RA, Harris HE. RAGE is the major receptor for the proinflammatory activity of HMGB1 in rodent macrophages. Scand. J. Immunol. 2005;61:1–9. doi: 10.1111/j.0300-9475.2005.01534.x. [DOI] [PubMed] [Google Scholar]
- 46.Dumitriu IE, Baruah P, Valentinis B, Voll RE, Herrmann M, Nawroth PP, Arnold B, Bianchi ME, Manfredi AA, Rovere-Querini P. Release of high mobility group box 1 by dendritic cells controls T cell activation via the receptor for advanced glycation end products. J. Immunol. 2005;174:7506–7515. doi: 10.4049/jimmunol.174.12.7506. [DOI] [PubMed] [Google Scholar]
- 47.Wang H, Yang H, Tracey KJ. Extracellular role of HMGB1 in inflammation and sepsis. J. Intern. Med. 2004;255:320–331. doi: 10.1111/j.1365-2796.2003.01302.x. [DOI] [PubMed] [Google Scholar]
- 48.Li J, Wang H, Mason JM, Levine J, Yu M, Ulloa L, Czura CJ, Tracey KJ, Yang H. Recombinant HMGB1 with cytokine-stimulating activity. J. Immunol. Methods. 2004;289:211–223. doi: 10.1016/j.jim.2004.04.019. [DOI] [PubMed] [Google Scholar]
- 49.Rouhiainen A, Tumova S, Valmu L, Kalkkinen N, Rauvala H. Pivotal advance: analysis of proinflammatory activity of highly purified eukaryotic recombinant HMGB1 (amphoterin) J. Leukoc. Biol. 2007;81:49–58. doi: 10.1189/jlb.0306200. [DOI] [PubMed] [Google Scholar]
- 50.Tian J, Avalos AM, Mao SY, Chen B, Senthil K, Wu H, Parroche P, Drabic S, Golenbock D, Sirois C, et al. Toll-like receptor 9-dependent activation by DNA-containing immune complexes is mediated by HMGB1 and RAGE. Nat. Immunol. 2007;8:487–496. doi: 10.1038/ni1457. [DOI] [PubMed] [Google Scholar]
- 51.Park JS, Gamboni-Robertson F, He Q, Svetkauskaite D, Kim JY, Strassheim D, Sohn JW, Yamada S, Maruyama I, Banerjee A, et al. High mobility group box 1 protein interacts with multiple Toll-like receptors. Am. J. Physiol. Cell Physiol. 2006;290:C917–C924. doi: 10.1152/ajpcell.00401.2005. [DOI] [PubMed] [Google Scholar]
- 52.Park JS, Svetkauskaite D, He Q, Kim JY, Strassheim D, Ishizaka A, Abraham E. Involvement of toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein. J. Biol. Chem. 2004;279:7370–7377. doi: 10.1074/jbc.M306793200. [DOI] [PubMed] [Google Scholar]
- 53.Qin S, Wang H, Yuan R, Li H, Ochani M, Ochani K, Rosas-Ballina M, Czura CJ, Huston JM, Miller E, et al. Role of HMGB1 in apoptosis-mediated sepsis lethality. J. Exp. Med. 2006;203:1637–1642. doi: 10.1084/jem.20052203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mantell LL, Parrish WR, Ulloa L. Hmgb-1 as a therapeutic target for infectious and inflammatory disorders. Shock. 2006;25:4–11. doi: 10.1097/01.shk.0000188710.04777.9e. [DOI] [PubMed] [Google Scholar]
- 55.Keller M, Rüegg A, Werner S, Beer HD. Active caspase-1 is a regulator of unconventional protein secretion. Cell. 2008;132:818–831. doi: 10.1016/j.cell.2007.12.040. [DOI] [PubMed] [Google Scholar]
- 56.Hersh D, Monack DM, Smith MR, Ghori N, Falkow S, Zychlinsky A. The Salmonella invasin SipB induces macrophage apoptosis by binding to caspase-1. Proc. Natl. Acad. Sci. USA. 1999;96:2396–2401. doi: 10.1073/pnas.96.5.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hotchkiss RS, Nicholson DW. Apoptosis and caspases regulate death and inflammation in sepsis. Nat. Rev. Immunol. 2006;6:813–822. doi: 10.1038/nri1943. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.