Summary
In sensory biology, a major outstanding question is how sensory receptor cells minimize noise while maximizing signal to set the detection threshold. This optimization could be problematic because the origin of both the signals and the limiting noise in most sensory systems is believed to lie in stimulus transduction. Signal processing in receptor cells can improve the signal-to-noise ratio. However, neural circuits can further optimize detection threshold by pooling signals from sensory receptor cells and processing them using a combination of linear and nonlinear filtering mechanisms. In the visual system, the noise limiting light detection has been assumed to arise from stimulus transduction in rod photoreceptors. In this context the evolutionary optimization of the signal-to-noise ratio in the retina has proven critical in allowing visual sensitivity to approach the limits set by the quantal nature of light. Here we discuss how noise in the mammalian retina is mitigated to allow for highly sensitive night vision.
Keywords: linear filtering, nonlinear threshold, retinal circuitry, signal-to-noise ratio, signal transfer
Introduction
Key to the survival of any organism is the ability to extract sensory information about its environment in the form of light, odors, chemicals, sounds, etc. These cues are essential for directing organisms to nourishment, in evading predators, and in reproduction. Common to all senses are specialized receptor cells that interact with and transduce stimuli into a change in the cell's neurotransmitter release, allowing stimuli to be encoded in neural circuitry. The signals and noise of the sensory receptors themselves set the fundamental limits for any sensation, and many sensory systems have consequently evolved receptors and neural pathways to encode information that approaches the limitations set by the physical nature of the stimulus (i.e. a quantum of light).
Near absolute threshold, sensory systems face the problem of maximizing their sensitivity under conditions where the stimulus is sparse or weak, and the amplification of the signal becomes essential (e.g. if the response of a single absorbed photon in a few rod photoreceptors is to be reliably transmitted to the retinal output). However, amplification is only beneficial if the signal can be amplified more efficiently than the noise. The convergence of the signal in neural circuits improves sensitivity for downstream signals, but will also result in the pooling of intrinsic noise from receptor cells that are not detecting the stimulus. Thus, by virtue of how the system is constructed, the intrinsic noise within the receptor cells and the neural circuitry remain important in setting the detection limit for any sensory system. One of the important tasks in sensory processing then becomes how to minimize intrinsic noise while maximizing the signal, a task complicated by the fact that stimulus transduction also produces noise.
The detection of light near absolute threshold for the visual system provides an opportunity to study how the processing of signals and noise is optimized. Since both signals and noise can be reliably measured within rod photoreceptors and defined rod pathways, and the light stimulus can be controlled accurately, it is possible to evaluate how the magnitude of signals evolves with respect to intrinsic noise. Here we describe mechanisms in the mammalian retina that allow the optimization of signal-to-noise ratio (SNR) for the detection of light.
Retinal control of signals and noise near visual threshold
Dark-adapted behavioral threshold of human observers requires a light flash delivering only a few photons, activating a small fraction of the rod photoreceptors in the retina [1, 2]. Such exquisite sensitivity requires that rod photoreceptors reliably signal the absorption of single photons, and that this signal is reliably transmitted to the retinal output (reviewed in [3]). Rod photoreceptors across all studied mammalian species including mice, rats, cats, guinea pigs, rabbits, and monkeys can signal the absorption of single photons [4-7], although their SNR may vary. How the retina establishes this exquisite performance despite these variations in SNR is informative about how stimulus transduction and neural processing shape the retinal output and visual performance.
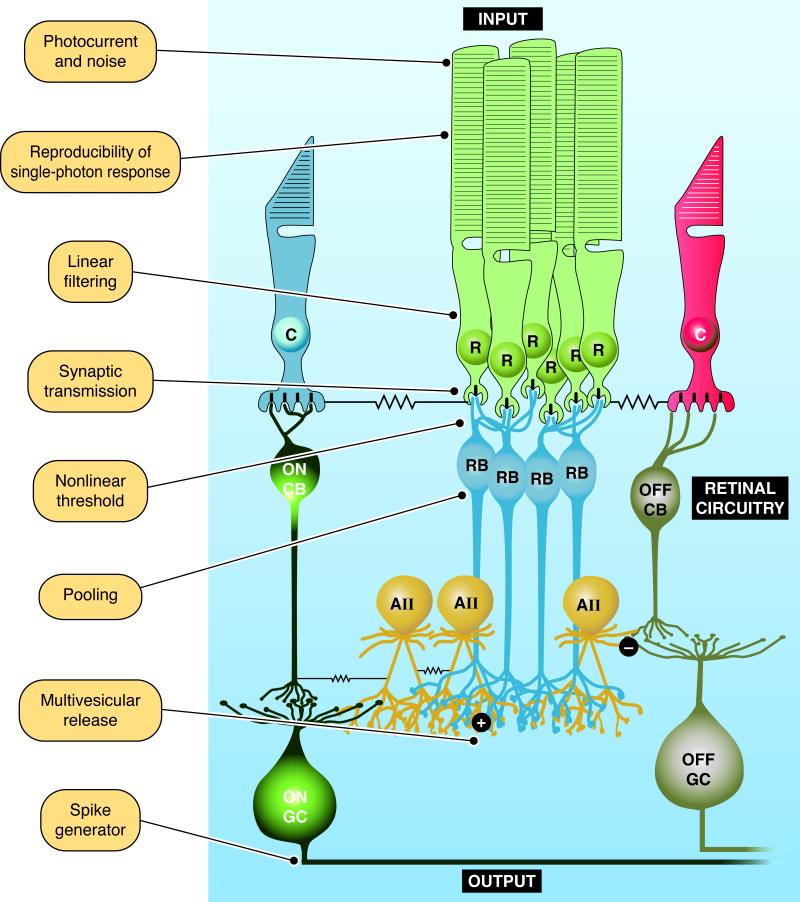
Signals from individual rod photoreceptors are conveyed through a highly conserved pathway in the mammalian retina called the rod bipolar pathway (Fig. 1) [8, 9]. In this pathway rod outputs are pooled by a rod ON bipolar cell, whose outputs are in turn pooled by a depolarizing AII amacrine cell. These signals are then relayed through cone photoreceptor circuits to the retinal output, the ganglion cells, which may ultimately pool the output of thousands of rods [10].
Figure 1.
The rod bipolar pathway and mechanisms optimizing the signal-to-noise ratio of single-photon responses. Near visual threshold a retinal pathway conserved across mammalian species, called the rod bipolar pathway, conveys single-photon responses generated in rod photoreceptors (R) to retinal ganglion cells (GCs). In this pathway many rods converge on a rod ON bipolar cell (RB), which in turn send their excitatory output through glutamatergic synapses (+) to depolarizing AII amacrine cells (AII). In turn these signals are relayed to ON and OFF cone bipolar cells (CBs) through gap junctions and glycinergic synapses (-), respectively. ON and OFF CBs in turn feed into ON and OFF GCs. Cone photoreceptors (C) are also depicted. Functional specializations throughout this pathway improve the detection of single-photon responses, and are identified. The challenges faced by the visual system as the signal sequentially proceeds through this circuitry are espoused in the text.
Noise in rod phototransduction, or at any subsequent stage in this pathway, can potentially reduce the discriminability of the signal and elevate visual threshold. We discuss the constraints noise places on signal detection and the retinal mechanisms that maximize signals and minimize noise in the context of the retinal circuitry. Emphasis will be placed on how noise at each stage of processing (as annotated in Fig. 1) is mitigated to improve the fidelity of light-driven signals.
Noise in the rod photocurrent obscures the single-photon response
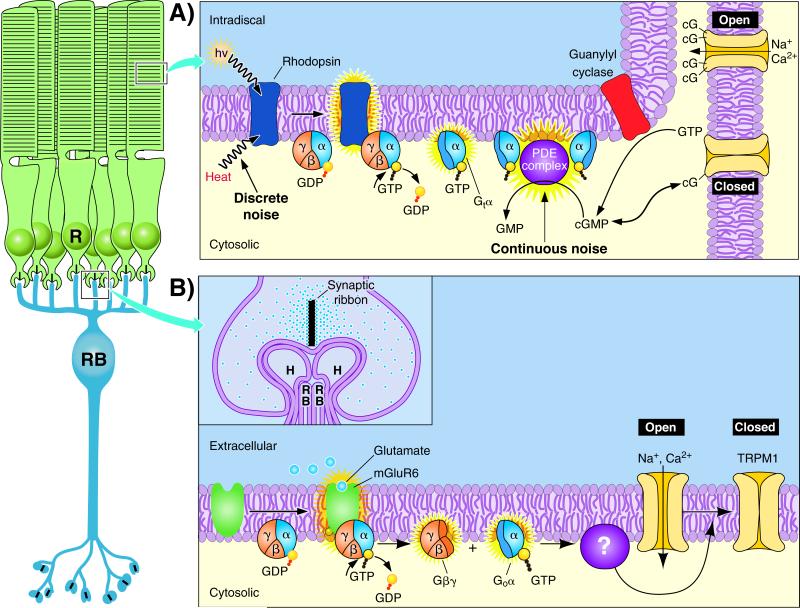
Vision is initiated in the outer segments of rod photoreceptor cells. When a rhodopsin molecule absorbs a photon of light a G-protein coupled signaling cascade is triggered leading to the closure of cGMP-gated channels (Fig. 2A). The closure of cGMP-gated channels, which are normally open in darkness and depolarize the cell's membrane potential, causes a small graded hyperpolarization in membrane potential of approximately 1 mV [11]. This light-evoked hyperpolarization in turn reduces the release of glutamate from the rod's synaptic terminal, or spherule, and constitutes the signal passed on to the second order rod bipolar cells. The high amplification of signals by the rod phototransduction cascade is required to allow the magnitude of the single-photon response to exceed the cell's intrinsic noise. However, amplification in the transduction machinery inevitably introduces noise into the system, as stochastic events unrelated to the signal are also amplified. This noise is passed forward in the retinal circuitry and threatens to obscure the detection of signals. Under circumstances where the rods do not generate noise in darkness, visual threshold may theoretically reach limits imposed by the Poisson statistics of light absorption. In reality, however, two major forms of intrinsic noise in the rod photocurrent (discrete and continuous noise) must ultimately be handled by the neural circuits that relay light-evoked signals to higher visual centers.
Figure 2.
The signal transduction cascade in rods and rod bipolar cells. The functional properties of the rods (R) and rod ON bipolar cells (RB) are embodied in signal transduction cascades that encode the light response. A: Phototransduction in the outer segments of rod photoreceptors is initiated when rhodopsin absorbs a photon (hν) and triggers the exchange of GTP for GDP on the G-protein, transducin (Gtα), which leads to an increase in cGMP (or cG) hydrolysis by a cGMP phosphodiesterase complex (PDE complex). Reduced cGMP concentration closes cGMP-gated channels, which are normally open in darkness and depolarize the cell's membrane potential due to the influx of Na+ and Ca2+. Thus photon absorption leads to a small graded hyperpolarization in membrane potential. Recovery of the dark current is dependent on the shutoff of the phototransduction cascade (not shown), and the synthesis of cGMP from GTP by guanylyl cyclase. Noise within rod phototransduction can limit the detection of light, and the mechanisms that generate the two main forms of noise in the rod photocurrent, discrete and continuous noise, are denoted. B: Glutamate released from rods is sensed on rod bipolar cell dendrites by mGluR6 receptors, which activate heterotrimeric G-protein subunits, Gαo and Gβγ, whose activity through unknown mechanisms (?) close TRPM1 cation channels. The threshold that separates rod single-photon responses from noise resides in this signaling cascade downstream of mGluR6 (see also Fig. 3), and probably also downstream of Gαo [58]. Inset is an expanded view of the rod-to-rod bipolar synapse. This triad synapse is formed as the dendrites of two rod bipolar cells (RB) and two horizontal cells (H) are inserted into the invagination of the rod spherule opposite the synaptic ribbon, from which glutamate release is initiated.
In total darkness recordings of the rod photocurrent reveal discrete noise events that have an amplitude and time course identical to the single-photon response, and are believed to arise from the spontaneous thermal activation of rhodopsin (Fig. 2A) [12]. Since these events cannot be distinguished from the single-photon response they produce a ‘dark light’ [13, 14] that has been proposed set the lower limits for light detection [15, 16]. In principle other forms of noise can be discriminated from light-evoked signals in latter stages of retinal processing using linear and nonlinear mechanisms (see below), but this is not possible when the noise and signal are indistinguishable. The only recourse for this system is to minimize the number of discrete noise events that occur, and consequently rhodopsin has evolved to maintain great stability in darkness. A mammalian rod contains on the order of 100,000,000 rhodopsin molecules and discrete noise events at 37°C occur at a rate of ~ 0.006 s-1 in primate rods [4] and ~ 0.01 s-1 in mouse rods [17]. On the basis of an individual rhodopsin molecule this means that spontaneous discrete noise events occur on average once every several hundreds of years. In amphibian retinas, both ganglion cell recordings and behavioral experiments have suggested that visual threshold is limited by the discrete noise [18, 19], consistent with interpretations from behavioral experiments on humans [1] and mice [20]. It should be noted, however, that the high sensitivity of vision near absolute threshold could be explained equally well with a low or high threshold number of absorbed photons depending on how much noise in the rods is assumed [3]. Thus historical notions that discrete noise solely limits detection threshold are arguable (see also [21]).
A single-photon response must not only be distinguished from discrete noise events, but also from continuous noise in the rod photocurrent. Continuous noise can be observed as continuous, low amplitude fluctuations in the rod photocurrent [22], with a probability of reaching the amplitude of the average single-photon response of ~ 0.005 s-1 in mouse rods [23]. This constantly present noise arises in the phototransduction cascade downstream of rhodopsin, which in toad rods has been shown as fluctuations in cGMP concentration caused by the spontaneous activation of the cGMP phosphodiesterase (PDE complex; Fig. 2A) [24]. Thus with low probability continuous noise could potentially contribute to the ‘dark light’ and limit visual threshold. Moreover, as opposed to the infrequently occurring discrete noise events, the continuous noise is constantly present in all rods. This poses problems for the pooling of the rod output by rod bipolar cells (see below). Although the higher probability of discrete compared to continuous noise events in generating false positive signals might seem to limit threshold at the level of the rods, both forms of noise will affect behavioral threshold by virtue of nonlinear processing of the rod output and rod convergence downstream in the retina. Thus, the relative contributions of these two forms of rod noise in setting visual threshold remains unresolved.
When considering noise in rod photoreceptors, a fundamental constraint is that cGMP-gated channels remain open in darkness allowing rods to maintain a relatively depolarized resting membrane potential (Fig. 2A). This arrangement may be beneficial for optimizing energy expenditure [25] and for the downstream processing of single-photon responses (see below), but the open channels will report fluctuations in cGMP concentration not associated with the light-driven signal. To some extent this noise is minimized as rods maintain their dark current using a small fraction of the available cGMP-gated channels [26] with a low unitary conductance [27, 28] due to a divalent permeating channel block [29, 30]. Such mechanisms could allow fine control of membrane potential by the phototransduction cascade, and thus attenuate noise in the gating of cGMP-gated channels.
Reproducibility reduces noise in the rod single-photon response
The single-photon response in rod photoreceptors by definition is initiated by the activation of a single rhodopsin molecule. Variations in the magnitude or time course of the single-photon response will degrade the ability to detect light. One might expect that a macroscopic response initiated by a single molecule would be subject to considerable noise due to the stochastic nature of interactions between molecules. However, the single-photon response of rod photoreceptors in both amphibians and mammals displays remarkable reproducibility, or a low coefficient of variation [5, 31-33]. Such reproducibility is believed to arise from the stereotyped shutoff of rhodopsin following its activation [5, 31, 32, 34], and will reduce noise in the signal itself. Such reproducibility is believed to allow the visual system to identify accurately the timing of arrival of single photons [35] and thus to optimize the temporal properties of signal transfer.
Linear filtering removes noise not associated with the single-photon response
It has been shown theoretically that linear bandpass filtering of the rod photocurrent can improve the fidelity of signals [36, 37], by removing noise with temporal frequencies not associated with the single-photon response [38]. Bandpass filtering would remove low temporal frequencies for events slower than the single-photon response and high temporal frequencies not associated with phototransduction. It should be noted that linear filtering of these signals can attenuate some continuous noise [22], but cannot be the dominant mechanism that removes noise from the rod photocurrent. This is because single-photon responses and continuous noise originate in the phototransduction cascade and are both controlled by the rate of cGMP turnover. Thus signals and noise are largely composed of similar temporal frequencies and linear filtering would consequently act on both, highlighting the need for a nonlinear mechanism to separate the two downstream [4]. However, some high-pass filtering is known to occur by virtue of the speeded responses in mammalian rod bipolar cells compared to rod photoresponses [39, 40], and recent evidence suggests this occurs in the in rods themselves in the photocurrent to photovoltage conversion [41]. Since bipolar cells preferentially report the rising phase of the rod photoresponse [37, 42], this type of linear filtering may improve the system's temporal properties, but for the detection limit this is of lesser importance.
Synaptic transmission between rods and rod bipolar cells is tuned for the transmission of small signals
Phototransduction in retinal photoreceptors generates graded changes in membrane potential following the absorption of photons. The ~ 1 mV hyperpolarization produced by photon absorption [11] needs to produce a change in glutamate release from the rod spherule that exceeds the noise associated with synaptic transmission. While open cGMP-gated channels in darkness might seem a poor strategy for minimizing sensory receptor cell noise, it is a ‘necessary evil’ for allowing robust synaptic transfer for small signals. By employing non-desensitizing Cav1.4 L-type Ca2+ channels [43, 44] along with the Ca2+-binding protein CABP4 to tune voltage sensitivity [45], the rod spherule can modulate finely the Ca2+ current with small graded changes in the membrane potential. Such modulation of the Ca2+ current, and vesicle release by proxy, is difficult if the membrane potential does not exceed the voltage threshold for activation for Ca2+ channels, or if Ca2+ channels are subject to voltage-dependent or Ca2+-dependent desensitization [46]. Thus, the dark membrane potential of rods is positioned for the Ca2+ channels to respond robustly for small changes in membrane potential. Moreover, assuming vesicle release from the rod spherule is a Poisson process (cf. [47]) the relatively depolarized dark membrane potential allows a high synaptic release rate, which will minimize relative fluctuations in vesicle release compared to lower mean rates of release.
A nonlinear threshold in rod bipolar cells eliminates noise from the array of rods
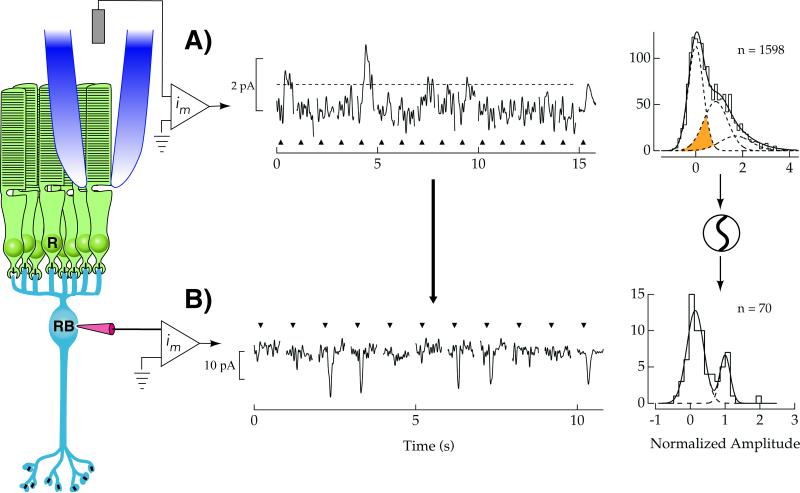
Rod bipolar cells in the mammalian retina serve as the first point for pooling of rod outputs, allowing convergence of 20-100 rods [48, 49]. Near visual threshold perhaps one rod in this pool might carry a single-photon response, while the remainder will report the transduction noise described above. It has long been appreciated that linearly pooling rod outputs will obscure the single-photon response carried in few rods by pooling continuous noise carried by the vast majority of rods [4]. Thus some nonlinear mechanism is required to maximize the SNR. A nonlinear threshold (henceforth ‘the threshold’) was suggested to improve SNR by eliminating noise from rods not absorbing photons [50]. As shown in Fig. 3, the relatively noisy rod photocurrent makes the discrimination of single-photon responses above the dark noise difficult (Fig. 3A, histogram). However if rod bipolar cells pool responses only from rods whose signals exceed threshold amplitude (Fig. 3A, dashed), their single-photon responses would become more discernable (Fig. 3B). The presence of such a threshold has been demonstrated in both mouse and rabbit retinas [7, 40, 51, 52]. While such a threshold would lead to the loss of more than half of single-photon responses, the elimination of the continuous rod noise from rods not absorbing photons (Fig. 3A, below dashed line) more than compensates for this lost signal. The suggested improvement in SNR for rod bipolar cells in the mouse retina may be more than 300-fold over the linear pooling of rod outputs [40].
Figure 3.
A threshold in rod bipolar cells improves the signal-to-noise ratio of the rod photoresponse. A: The delivery of flashes of light (upward triangles) yielding on average less than 1 photon absorbed per trial generates individual rod photocurrents of variable size in the mouse retina. These responses result from 0, 1, or 2 absorbed photons with the smallest responses remaining difficult to discern from the rod continuous noise. A histogram of response amplitudes demonstrates this difficulty in discerning signals from noise, as the Gaussian distributions of single-photon response and continuous noise amplitudes overlap considerably (shaded). To improve the detection of single-photon responses in rod bipolar cells, a threshold is applied to the rod output (dashed line), after which rod bipolar cells pool these signals. B: Individual rod bipolar currents in response to a flash of light (triangles) that produce photon absorptions in a small fraction of the rods. Single-photon responses are more discernable compared to trials where no response was observed. A histogram of response amplitudes reveals that Gaussian distributions of single-photon response and noise amplitudes are more separated compared to the rods, indicating a higher SNR compared to the rods. Data shown from [23].
The mechanism setting the position of the threshold is manifested in the dendrites of rod bipolar cells, which are unusual in that they employ a G-protein signaling cascade to invert the sign of the rod photoresponse into an ON signal (Fig. 2B). Despite the functional importance of ON responses for defining the receptive fields of neurons in higher visual areas, the components of this signaling cascade are still not fully elucidated. In darkness mGluR6 receptors on rod bipolar dendrites sense glutamate release from rods and activate a heterotrimeric G-protein, Gαo. Through unknown mechanisms Gαo and/or Gβγ close non-selective cation channels identified recently to be at least in part TRPM1 [53-55]. It should be noted that the effector molecule and the gating particle controlling the opening of TRPM1 remain unidentified, as are the major modulatory proteins controlling the sensitivity and time course of rod bipolar light responses (but see [56]). To separate single-photon responses from the continuous noise in rods the threshold position is set by saturation downstream of the mGluR6 receptors within this G-protein signaling cascade [57]. The activity of the mGluR6 cascade in darkness is sufficient to hold most of the TRPM1 channels closed, preventing noise in rod phototransduction or synaptic transmission from altering significantly the number of open transduction channels. This arrangement only allows sufficiently large single-photon responses to reduce glutamate release from rods enough to relieve saturation and open transduction channels, thereby acting as a threshold.
The location of saturation in the mGluR6 signaling pathway that sets the position of the threshold remains undefined due to the unknown identity of components downstream of Gαo. However, saturation probably resides downstream of Gαo and may potentially be set in the activity of the effector molecule that controls the gating particle of TRPM1, or in the open probability of TRPM1 itself [57, 58]. For the threshold position to separate optimally single-photon responses from noise, the amount of saturation within the mGluR6-signaling pathway needs to be set precisely. With respect to the probability distribution of single-photon response amplitudes and continuous noise [23, 40], saturation must produce a threshold position that is poised to separate signals and noise near the amplitude where the probability of observing either is equal (Fig. 4A). If the amount of saturation in mGluR6 signaling is too high, the high threshold position will exclude too much of the signal. If the amount of saturation in mGluR6 signaling is too low, the low threshold position will introduce too much noise. Thus postsynaptic processing in rod bipolar cells should be optimally tuned to the probability distributions of signals and noise in the rods for visual threshold to be optimized (see [23]).
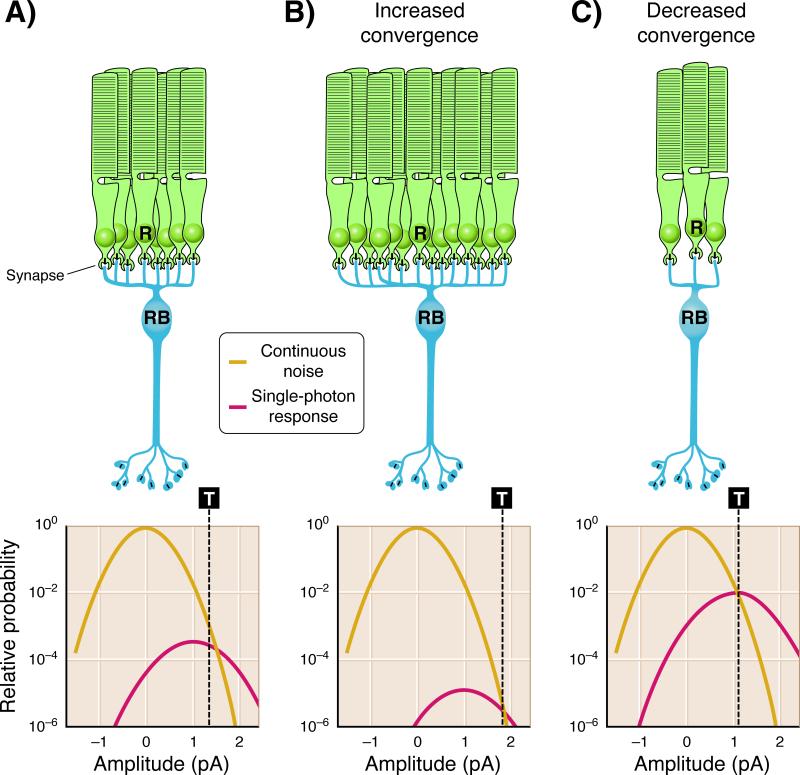
Figure 4.
Optimal position of the threshold in rod bipolar cells is dependent on convergence. The extent of convergence of rods onto rod bipolar cells will alter the optimal position of the threshold (T - dashed line) that separates single-photon responses from continuous noise. A: In the mouse retina ~ 20 rods converge on downstream rod bipolar cells [49]. The task for rod bipolar cells is to distinguish single-photon responses (Gaussian distribution with mean amplitude ~ 1 pA) from the rod continuous noise (Gaussian distribution with mean amplitude ~ 0 pA). Previous studies have shown that the optimal position of the threshold (T ~ 1.3 pA) is close to the crossing point between these Gaussian distributions [40, 50]. Events larger than threshold are more likely single-photon responses and are preserved, while those smaller than threshold are more likely noise and are discarded. B: The optimal position of the threshold will change with the extent of rod convergence onto rod bipolar cells. As convergence increases, the probability of observing continuous noise increases with respect to the probability of observing single-photon responses, since near visual threshold a small minority of the rods receive a photon. Consequently the threshold would need to be positioned at higher amplitudes (T ~ 2 pA) to allow the optimal separation of single-photon responses from continuous noise. C: As convergence decreases, the probability of observing single-photon responses increases with respect to the probability of observing continuous noise, requiring the threshold to be positioned at a lower amplitude (T ~ 1 pA) to allow the optimal separation of single-photon responses from continuous noise. Adapted from [23].
How the threshold is set with respect to the probability distributions of single-photon response and dark noise amplitudes remains an important problem for retinal processing near visual threshold, especially given variations in the SNR of the rod photocurrent in different mammalian species. The SNR of macaque rod single-photon responses has been measured to be ~ 2-fold higher than for mouse rods [5, 23]. This greater separation of single-photon responses from continuous noise suggests reduced saturation in mGluR6 signaling compared to mouse rods, possibly allowing the macaque visual system to retain a greater fraction of single-photon responses near threshold. Recent evidence suggests that such optimization of saturation may be hard-wired into the mGluR6-signaling pathway, as the amount of saturation in rod bipolar cells of transgenic mice with altered probability distributions of rod single-photon response and dark noise amplitudes appears unaffected [23]. Identifying how saturation of the mGluR6 signaling pathway, and thus threshold position, is set will be important for understanding how mammalian species utilize common retinal circuitry to optimize visual threshold.
Pooling signals after removal of noise improves sensitivity
The high sensitivity of rod-mediated vision is achieved through amplification of the single-photon response in rods and the pooling of rod outputs by the retinal circuitry. Indeed, through the rod bipolar pathway a ganglion cell may sample the output of thousands of rod photoreceptors [10]. Convergence will ultimately improve a sensory system's sensitivity, but such a mechanism will pool both signals and noise. Thus the elimination of noise at the earliest point in signal processing before considerable pooling is critical, since once signal and noise with similar temporal characteristics are combined their separation becomes more difficult [40]. Consequently a threshold is applied at the dendritic tips of rod bipolar cells by virtue of saturation in the mGluR6 signaling cascade [57], and only then do rod bipolar cells pool the signal [40]. The amount of pooling will also influence the need for a threshold. As the number of pooled rods increases (assuming a fixed rod SNR), the probability distribution of the noise will increase more than the signal since a small fraction of the rods carry single-photon responses near visual threshold. As shown in Fig. 4 the rescaling of these distributions with respect to one another alters the optimal position for their separation. Thus, the optimal separation of the signal from the noise as the number of pooled rods increases requires a threshold with a higher position to attenuate the added noise (Fig. 4B). For instance estimates of rod convergence in rabbit retina are higher by ~ 5-fold compared to the mouse retina, and consequently the threshold in rabbits discards almost 90 % of the single-photon responses [7]. Similarly a threshold with a lower position would be required to separate optimally the signals from noise for fewer pooled rods (Fig. 4C). Such adjustments in saturation within the mGluR6 pathway would be expected to improve the detection of signals near threshold, and thus might be controlled differentially based on the SNR of the rod photocurrent in various mammalian species.
Multivesicular release at rod bipolar synaptic terminals selectively amplifies signals in AII amacrine cells
The signal from rod bipolar cells is passed on to AII amacrine cells, which receive convergent input from ~ 25 rod bipolar cells [10]. This convergence enhances signal detection, just as does convergence between rods and rod bipolar cells (see above). However the added noise from synaptic transmission will once again interfere with detection [59-61]. To overcome synaptic noise, the light-evoked signals in AII amacrine cells are boosted by coordinated multivesicular release (MVR) from the rod bipolar synaptic terminal. MVR will produce responses in the AII amacrine cells that are larger than the noise arising from the univesicular basal level of glutamate release [61]. Thus just as for rod-to-rod bipolar signal transmission, AII amacrine cells pool rod signals after noise is removed. Similar coordinated multivesicular release sites are also found at hair cell ribbon synapses [62, 63], suggesting MVR could impose a similar threshold to improve signal detection elsewhere. It should be noted that noise in AII amacrine cells is also partially mitigated by the electrical coupling of these cells with connexin 36 gap junctions [64]. Such a coupled network of cells will effectively reduce noise by providing the system with inertia against voltage fluctuations in individual AII amacrine cells [65-67].
A threshold for action potential generation in ganglion cells removes noise in their synaptic input
Ganglion cells serve as the conduit between light-evoked signals generated in the retina and higher visual centers, and are the main neurons in the retina that generate action potentials (or spikes). These cells respond to light by either increasing (ON cells) or decreasing (OFF cells) the number of spikes generated at their axon hillock (Fig. 1). It is appreciated that spike timing in ganglion cells is reproducible from trial to trial [68, 69], limited by variability in the light-evoked signals feeding into the ganglion cells [70]. Such variability in the spike generator limits the temporal precision of spikes, introducing ‘jitter’ into ganglion cell responses. However, relatively little is known about how control of spike generation influences signals near visual threshold (cf. [71, 72]). Control of spike generation could effectively introduce another threshold in the system, if subthreshold fluctuations are eliminated thereby enhancing SNR in the spike output [73]. By analogy to the threshold in rod bipolar cells and MVR at the rod bipolar-AII amacrine cell synapse (see above), following nonlinearity in spike generation there is another good opportunity to pool rod-driven signals.
Reducing receptor noise: Similarities and differences between sensory systems
Neural processing in most sensory systems requires the extraction of salient information from a sparse set of receptor cell inputs. The noise limiting stimulus detection has been suggested to arise from the transduction processes in sensory receptor cells themselves, since a physical stimulus interacting with proteins within sensory receptor cell specializations is inherently stochastic. Noise in transduction is ultimately reported through open channels in the cell membrane, thus the minimization of noise requires that sensory receptor cells maintain the fewest open channels possible at rest.
Rod photoreceptors may differ from other sensory receptor cells since in the absence of light transduction channels are open (although the properties of the open channels attempt to minimize noise). As described previously such an organization may ultimately improve the efficiency of signal transmission to rod bipolar cells. However, one general issue with the study of sensory systems is that our personal views bias us toward assumptions of what a system's output is optimized for. For instance, if rod phototransduction were optimized purely for light detection, it is curious why rods would be electrically-coupled to one another. Coupling would attenuate the signals in the minority of the rods absorbing a photon near visual threshold without impacting noise [74]. Perhaps mammalian rods and their circuitry are organized to attempt to optimize both their sensitivity and speed. Temporal resolution within the rod phototransduction cascade is improved by increasing the rate at which cGMP molecules are turned over [24, 75], leading to fluctuations in cGMP concentration (i.e. continuous noise) observed in the rod photocurrent. However, this noise can be largely eliminated in the retinal circuitry using a threshold in the rod bipolar cell dendrites [40, 50, 51]. Thus the circuitry carrying rod photoresponses to higher visual centers might allow the simultaneous optimization of both sensitivity and the temporal properties of stimulus transduction.
While rod photoreceptors maintain a standing current based on open cGMP-gated channels in darkness, this doesn't appear to be the strategy used by other sensory receptor cells. Olfactory receptor cells [76], taste receptor cells [77], hair cells [78], and the rhabdomeric photoreceptors of Drosophila [79] and Limulus [80] all appear to operate with few open channels at rest. Thus regardless of whether stimulus transduction requires a G-protein signaling or direct control of channel gating by the stimulus, or whether the stimulus produces graded potentials or action potentials, the resting state in these sensory receptor cells is more quiet.
It should be noted that the mechanisms by which signals from these sensory receptor cells are interpreted by their neural circuitries remain less well elucidated than for the visual system. The accessibility of retinal tissue and the relative precision with which the natural stimulus (light) can be delivered provides distinct advantages for the study of sensory processing. However the general principles of linear/ nonlinear filtering followed by pooling of receptor outputs could guide investigation into sensory processing for these systems.
Conclusions
While the remarkable sensitivity of stimulus transduction in sensory receptor cells has received much attention [12, 78, 81], the control of noise in sensory receptor cells, and within the neural circuits that carry sensory information to higher centers, is equally influential in setting a system's threshold. Such sensitivity is improved as sensory systems pool information from many receptor cells, but this pooling will also combine noise with the signals. Sensory systems can optimize signal detection through linear filtering to eliminate noise not associated with the signal and by applying thresholds at crucial (and preferably early) synapses in the pathway. The pooling of signals by the circuitry following the removal of noise will provide the cleanest representation of the signal for subsequent processing. The optimal removal of intrinsic noise is ultimately required for a sensory system to lower its threshold to reach the detection limit defined by the receptor SNR [23].
Acknowledgments
We thank Drs. Petri Ala-Laurila and Greg Field for helpful comments on the manuscript. APS is supported by NIH Grant EY17606 and a McKnight Scholar Award from the McKnight Endowment Fund for Neurosciences.
Abbreviations
- cGMP
cyclic guanosine monophosphate
- MVR
multivesicular release
- PDE
phosphodiesterase
- SNR
signal-to-noise ratio
References
- 1.Hecht S, Schlaer S, Pirenne MH. Energy, quanta, and vision. J Gen Physiol. 1942;25:819–40. doi: 10.1085/jgp.25.6.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van der Velden HA. The number of quanta necessary for the perception of light in the human eye. Opthalmologica. 1946;111:321–31. doi: 10.1159/000300352. [DOI] [PubMed] [Google Scholar]
- 3.Field GD, Sampath AP, Rieke F. Retinal processing near absolute threshold: from behavior to mechanism. Annu Rev Physiol. 2005;67:491–514. doi: 10.1146/annurev.physiol.67.031103.151256. [DOI] [PubMed] [Google Scholar]
- 4.Baylor DA, Nunn BJ, Schnapf JL. The photocurrent, noise and spectral sensitivity of rods of the monkey Macaca fascicularis. J Physiol. 1984;357:575–607. doi: 10.1113/jphysiol.1984.sp015518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Field GD, Rieke F. Mechanisms regulating variability of the single photon responses of mammalian rod photoreceptors. Neuron. 2002;35:733–47. doi: 10.1016/s0896-6273(02)00822-x. [DOI] [PubMed] [Google Scholar]
- 6.Nakatani K, Tamura T, Yau KW. Light adaptation in retinal rods of the rabbit and two other nonprimate mammals. J Gen Physiol. 1991;97:413–35. doi: 10.1085/jgp.97.3.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trexler EB, Casti AR, Zhang Y. Nonlinearity and noise at the rod-rod bipolar cell synapse. Vis Neurosci. 2011;28:61–8. doi: 10.1017/S0952523810000301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dacheux RF, Raviola E. The rod pathway in the rabbit retina: a depolarizing bipolar and amacrine cell. J Neurosci. 1986;6:331–45. doi: 10.1523/JNEUROSCI.06-02-00331.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith RG, Freed MA, Sterling P. Microcircuitry of the dark-adapted cat retina: functional architecture of the rod-cone network. J Neurosci. 1986;6:3505–17. doi: 10.1523/JNEUROSCI.06-12-03505.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sterling P, Freed MA, Smith RG. Architecture of rod and cone circuits to the on-beta ganglion cell. J Neurosci. 1988;8:623–42. doi: 10.1523/JNEUROSCI.08-02-00623.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schneeweis DM, Schnapf JL. Photovoltage of rods and cones in the macaque retina. Science. 1995;268:1053–6. doi: 10.1126/science.7754386. [DOI] [PubMed] [Google Scholar]
- 12.Baylor DA, Lamb TD, Yau KW. Responses of retinal rods to single photons. J Physiol. 1979;288:613–34. [PMC free article] [PubMed] [Google Scholar]
- 13.Aguilar M, Stiles WS. Saturation of the rod mechanism of the retina at high light levels of stimulation. Optica Acta. 1954;1:59–65. [Google Scholar]
- 14.Barlow HB. Retinal noise and absolute threshold. J Opt Soc Am. 1956;46:634–9. doi: 10.1364/josa.46.000634. [DOI] [PubMed] [Google Scholar]
- 15.Autrum H. Uber Kleinste Rieze bei Sinnesorganen. Biologisches Zentralblatt. 1943;66:209–36. [Google Scholar]
- 16.Barlow HB. Purkinje shift and retinal noise. Nature. 1957;179:255–6. doi: 10.1038/179255b0. [DOI] [PubMed] [Google Scholar]
- 17.Burns ME, Mendez A, Chen J, Baylor DA. Dynamics of cyclic GMP synthesis in retinal rods. Neuron. 2002;36:81–91. doi: 10.1016/s0896-6273(02)00911-x. [DOI] [PubMed] [Google Scholar]
- 18.Aho AC, Donner K, Hyden C, Larsen LO, et al. Low retinal noise in animals with low body temperature allows high visual sensitivity. Nature. 1988;334:348–50. doi: 10.1038/334348a0. [DOI] [PubMed] [Google Scholar]
- 19.Copenhagen DR, Donner K, Reuter T. Ganglion cell performance at absolute threshold in toad retina: effects of dark events in rods. J Physiol. 1987;393:667–80. doi: 10.1113/jphysiol.1987.sp016847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Naarendorp F, Esdaille TM, Banden SM, Andrews-Labenski J, et al. Dark light, rod saturation, and the absolute and incremental sensitivity of mouse cone vision. J Neurosci. 2010;30:12495–507. doi: 10.1523/JNEUROSCI.2186-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chichilnisky EJ, Rieke F. Detection sensitivity and temporal resolution of visual signals near absolute threshold in the salamander retina. J Neurosci. 2005;25:318–30. doi: 10.1523/JNEUROSCI.2339-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baylor DA, Matthews G, Yau KW. Two components of electrical dark noise in toad retinal rod outer segments. J Physiol. 1980;309:591–621. doi: 10.1113/jphysiol.1980.sp013529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Okawa H, Miyagishima KJ, Arman AC, Hurley JB, et al. Optimal processing of photoreceptor signals is required to maximize behavioural sensitivity. J Physiol. 2010;588:1947–60. doi: 10.1113/jphysiol.2010.188573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rieke F, Baylor DA. Molecular origin of continuous dark noise in rod photoreceptors. Biophys J. 1996;71:2553–72. doi: 10.1016/S0006-3495(96)79448-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Okawa H, Sampath AP, Laughlin SB, Fain GL. ATP consumption by mammalian rod photoreceptors in darkness and in light. Curr Biol. 2008;18:1917–21. doi: 10.1016/j.cub.2008.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yau KW, Nakatani K. Light-suppressible, cyclic GMP-sensitive conductance in the plasma membrane of a truncated rod outer segment. Nature. 1985;317:252–5. doi: 10.1038/317252a0. [DOI] [PubMed] [Google Scholar]
- 27.Bodoia RD, Detwiler PB. Patch-clamp recordings of the light-sensitive dark noise in retinal rods from the lizard and frog. J Physiol. 1985;367:183–216. doi: 10.1113/jphysiol.1985.sp015820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gray P, Attwell D. Kinetics of light-sensitive channels in vertebrate photoreceptors. Proc R Soc Lond B Biol Sci. 1985;223:379–88. doi: 10.1098/rspb.1985.0007. [DOI] [PubMed] [Google Scholar]
- 29.Haynes LW, Kay AR, Yau KW. Single cyclic GMP-activated channel activity in excised patches of rod outer segment membrane. Nature. 1986;321:66–70. doi: 10.1038/321066a0. [DOI] [PubMed] [Google Scholar]
- 30.Zimmerman AL, Baylor DA. Cyclic GMP-sensitive conductance of retinal rods consists of aqueous pores. Nature. 1986;321:70–2. doi: 10.1038/321070a0. [DOI] [PubMed] [Google Scholar]
- 31.Hamer RD, Nicholas SC, Tranchina D, Liebman PA, et al. Multiple steps of phosphorylation of activated rhodopsin can account for the reproducibility of vertebrate rod single-photon responses. J Gen Physiol. 2003;122:419–44. doi: 10.1085/jgp.200308832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rieke F, Baylor DA. Origin of reproducibility in the responses of retinal rods to single photons. Biophys J. 1998;75:1836–57. doi: 10.1016/S0006-3495(98)77625-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Whitlock GG, Lamb TD. Variability in the time course of single photon responses from toad rods: termination of rhodopsin's activity. Neuron. 1999;23:337–51. doi: 10.1016/s0896-6273(00)80784-9. [DOI] [PubMed] [Google Scholar]
- 34.Doan T, Mendez A, Detwiler PB, Chen J, et al. Multiple phosphorylation sites confer reproducibility of the rod's single-photon responses. Science. 2006;313:530–3. doi: 10.1126/science.1126612. [DOI] [PubMed] [Google Scholar]
- 35.Rieke F, Baylor DA. Single-photon detection by rod cells of the retina. Rev Mod Phys. 1998;70:1027–36. [Google Scholar]
- 36.Bialek W. Physical limits to sensation and perception. Annu Rev Biophys Biophys Chem. 1987;16:455–78. doi: 10.1146/annurev.bb.16.060187.002323. [DOI] [PubMed] [Google Scholar]
- 37.Bialek W, Owen WG. Temporal filtering in retinal bipolar cells. Elements of an optimal computation? Biophys J. 1990;58:1227–33. doi: 10.1016/S0006-3495(90)82463-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Armstrong-Gold CE, Rieke F. Bandpass filtering at the rod to second-order cell synapse in salamander (Ambystoma tigrinum) retina. J Neurosci. 2003;23:3796–806. doi: 10.1523/JNEUROSCI.23-09-03796.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Berntson A, Taylor WR. Response characteristics and receptive field widths of on-bipolar cells in the mouse retina. J Physiol. 2000;524(Pt 3):879–89. doi: 10.1111/j.1469-7793.2000.00879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Field GD, Rieke F. Nonlinear signal transfer from mouse rods to bipolar cells and implications for visual sensitivity. Neuron. 2002;34:773–85. doi: 10.1016/s0896-6273(02)00700-6. [DOI] [PubMed] [Google Scholar]
- 41.Okawa H, Sampath AP. Temporal filtering of the single photon response in the mouse retina. Association for Research in Vision and Opthalmology; Ft. Lauderdale, FL: 2009. p. 4567. [Google Scholar]
- 42.Sampath AP, Strissel KJ, Elias R, Arshavsky VY, et al. Recoverin improves rod-mediated vision by enhancing signal transmission in the mouse retina. Neuron. 2005;46:413–20. doi: 10.1016/j.neuron.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 43.Barnes S, Kelly ME. Calcium channels at the photoreceptor synapse. Adv Exp Med Biol. 2002;514:465–76. doi: 10.1007/978-1-4615-0121-3_28. [DOI] [PubMed] [Google Scholar]
- 44.Morgans CW. Localization of the alpha(1F) calcium channel subunit in the rat retina. Invest Ophthalmol Vis Sci. 2001;42:2414–8. [PubMed] [Google Scholar]
- 45.Haeseleer F, Imanishi Y, Maeda T, Possin DE, et al. Essential role of Ca2+-binding protein 4, a Cav1.4 channel regulator, in photoreceptor synaptic function. Nat Neurosci. 2004;7:1079–87. doi: 10.1038/nn1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Koschak A, Reimer D, Walter D, Hoda JC, et al. Cav1.4alpha1 subunits can form slowly inactivating dihydropyridine-sensitive L-type Ca2+ channels lacking Ca2+-dependent inactivation. J Neurosci. 2003;23:6041–9. doi: 10.1523/JNEUROSCI.23-14-06041.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schein S, Ahmad KM. A clockwork hypothesis: synaptic release by rod photoreceptors must be regular. Biophys J. 2005;89:3931–49. doi: 10.1529/biophysj.105.070623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bloomfield SA, Dacheux RF. Rod vision: pathways and processing in the mammalian retina. Prog Retin Eye Res. 2001;20:351–84. doi: 10.1016/s1350-9462(00)00031-8. [DOI] [PubMed] [Google Scholar]
- 49.Tsukamoto Y, Morigiwa K, Ueda M, Sterling P. Microcircuits for night vision in mouse retina. J Neurosci. 2001;21:8616–23. doi: 10.1523/JNEUROSCI.21-21-08616.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van Rossum MC, Smith RG. Noise removal at the rod synapse of mammalian retina. Vis Neurosci. 1998;15:809–21. doi: 10.1017/s0952523898155037. [DOI] [PubMed] [Google Scholar]
- 51.Berntson A, Smith RG, Taylor WR. Transmission of single photon signals through a binary synapse in the mammalian retina. Vis Neurosci. 2004;21:693–702. doi: 10.1017/S0952523804215048. [DOI] [PubMed] [Google Scholar]
- 52.Robson JG, Maeda H, Saszik SM, Frishman LJ. In vivo studies of signaling in rod pathways of the mouse using the electroretinogram. Vision Res. 2004;44:3253–68. doi: 10.1016/j.visres.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 53.Koike C, Obara T, Uriu Y, Numata T, et al. TRPM1 is a component of the retinal ON bipolar cell transduction channel in the mGluR6 cascade. Proc Natl Acad Sci USA. 2010;107:332–7. doi: 10.1073/pnas.0912730107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Morgans CW, Zhang J, Jeffrey BG, Nelson SM, et al. TRPM1 is required for the depolarizing light response in retinal ON-bipolar cells. Proc Natl Acad Sci USA. 2009;106:19174–8. doi: 10.1073/pnas.0908711106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shen Y, Heimel JA, Kamermans M, Peachey NS, et al. A transient receptor potential-like channel mediates synaptic transmission in rod bipolar cells. J Neurosci. 2009;29:6088–93. doi: 10.1523/JNEUROSCI.0132-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Snellman J, Kaur T, Shen Y, Nawy S. Regulation of ON bipolar cell activity. Prog Retin Eye Res. 2008;27:450–63. doi: 10.1016/j.preteyeres.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sampath AP, Rieke F. Selective transmission of single photon responses by saturation at the rod-to-rod bipolar synapse. Neuron. 2004;41:431–43. doi: 10.1016/s0896-6273(04)00005-4. [DOI] [PubMed] [Google Scholar]
- 58.Okawa H, Pahlberg J, Rieke F, Birnbaumer L, et al. Coordinated control of sensitivity by two splice variants of Gα(o) in retinal ON bipolar cells. J Gen Physiol. 2010;136:443–54. doi: 10.1085/jgp.201010477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Petrides A, Trexler EB. Differential output of the high-sensitivity rod photoreceptor: AII amacrine pathway. J Comp Neurol. 2008;507:1653–62. doi: 10.1002/cne.21617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Singer JH. Multivesicular release and saturation of glutamatergic signalling at retinal ribbon synapses. J Physiol. 2007;580:23–9. doi: 10.1113/jphysiol.2006.125302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Singer JH, Lassova L, Vardi N, Diamond JS. Coordinated multivesicular release at a mammalian ribbon synapse. Nat Neurosci. 2004;7:826–33. doi: 10.1038/nn1280. [DOI] [PubMed] [Google Scholar]
- 62.Glowatzki E, Fuchs PA. Transmitter release at the hair cell ribbon synapse. Nat Neurosci. 2002;5:147–54. doi: 10.1038/nn796. [DOI] [PubMed] [Google Scholar]
- 63.Li GL, Keen E, Andor-Ardo D, Hudspeth AJ, et al. The unitary event underlying multiquantal EPSCs at a hair cell's ribbon synapse. J Neurosci. 2009;29:7558–68. doi: 10.1523/JNEUROSCI.0514-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Deans MR, Volgyi B, Goodenough DA, Bloomfield SA, et al. Connexin36 is essential for transmission of rod-mediated visual signals in the mammalian retina. Neuron. 2002;36:703–12. doi: 10.1016/s0896-6273(02)01046-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dunn FA, Doan T, Sampath AP, Rieke F. Controlling the gain of rod-mediated signals in the mammalian retina. J Neurosci. 2006;26:3959–70. doi: 10.1523/JNEUROSCI.5148-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Smith RG, Vardi N. Simulation of the AII amacrine cell of mammalian retina: functional consequences of electrical coupling and regenerative membrane properties. Vis Neurosci. 1995;12:851–60. doi: 10.1017/s095252380000941x. [DOI] [PubMed] [Google Scholar]
- 67.Veruki ML, Hartveit E. AII (Rod) amacrine cells form a network of electrically coupled interneurons in the mammalian retina. Neuron. 2002;33:935–46. doi: 10.1016/s0896-6273(02)00609-8. [DOI] [PubMed] [Google Scholar]
- 68.Berry MJ, Warland DK, Meister M. The structure and precision of retinal spike trains. Proc Natl Acad Sci USA. 1997;94:5411–6. doi: 10.1073/pnas.94.10.5411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Keat J, Reinagel P, Reid RC, Meister M. Predicting every spike: a model for the responses of visual neurons. Neuron. 2001;30:803–17. doi: 10.1016/s0896-6273(01)00322-1. [DOI] [PubMed] [Google Scholar]
- 70.Murphy GJ, Rieke F. Network variability limits stimulus-evoked spike timing precision in retinal ganglion cells. Neuron. 2006;52:511–24. doi: 10.1016/j.neuron.2006.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Barlow HB, Levick WR, Yoon M. Responses to single quanta of light in retinal ganglion cells of the cat. Vision Res Suppl. 1971;3:87–101. doi: 10.1016/0042-6989(71)90033-2. [DOI] [PubMed] [Google Scholar]
- 72.Mastronarde DN. Correlated firing of cat retinal ganglion cells. II. Responses of X- and Y-cells to single quantal events. J Neurophysiol. 1983;49:325–49. doi: 10.1152/jn.1983.49.2.325. [DOI] [PubMed] [Google Scholar]
- 73.Dhingra NK, Smith RG. Spike generator limits efficiency of information transfer in a retinal ganglion cell. J Neurosci. 2004;24:2914–22. doi: 10.1523/JNEUROSCI.5346-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hornstein EP, Verweij J, Li PH, Schnapf JL. Gap-junctional coupling and absolute sensitivity of photoreceptors in macaque retina. J Neurosci. 2005;25:11201–9. doi: 10.1523/JNEUROSCI.3416-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nikonov S, Lamb TD, Pugh EN., Jr The role of steady phosphodiesterase activity in the kinetics and sensitivity of the light-adapted salamander rod photoresponse. J Gen Physiol. 2000;116:795–824. doi: 10.1085/jgp.116.6.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lowe G, Gold GH. Olfactory transduction is intrinsically noisy. Proc Natl Acad Sci USA. 1995;92:7864–8. doi: 10.1073/pnas.92.17.7864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tsunenari T, Hayashi Y, Orita M, Kurahashi T, et al. A quinine-activated cationic conductance in vertebrate taste receptor cells. J Gen Physiol. 1996;108:515–23. doi: 10.1085/jgp.108.6.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hudspeth AJ, Corey DP. Sensitivity, polarity, and conductance change in the response of vertebrate hair cells to controlled mechanical stimuli. Proc Natl Acad Sci USA. 1977;74:2407–11. doi: 10.1073/pnas.74.6.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Henderson SR, Reuss H, Hardie RC. Single photon responses in Drosophila photoreceptors and their regulation by Ca2+. J Physiol 524 Pt. 2000;1:179–94. doi: 10.1111/j.1469-7793.2000.00179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Borsellino A, Fuortes MG. Responses to single photons in virual cells of limulus. J Physiol. 1968;196:507–39. doi: 10.1113/jphysiol.1968.sp008521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bhandawat V, Reisert J, Yau KW. Elementary response of olfactory receptor neurons to odorants. Science. 2005;308:1931–4. doi: 10.1126/science.1109886. [DOI] [PMC free article] [PubMed] [Google Scholar]