Abstract
Heparin-induced thrombocytopenia is a rare and serious reaction to unfractionated heparin and low-molecular-weight heparins in children. Quick recognition, discontinuation of heparin, and subsequent treatment with an alternative anticoagulant are essential steps to prevent serious complications such as thrombus and limb amputation. The purpose of this review is to describe the clinical features of heparin-induced thrombocytopenia in children and to summarize the data available for its management. This paper summarizes data and relates the use of direct thrombin inhibitors with clinical outcomes. A literature search was conducted with Ovid, using the key terms argatroban, bivalirudin, hirulog, danaparoid, lepirudin, direct thrombin inhibitor, heparin-induced thrombocytopenia, thrombosis, warfarin, and fondaparinux. Articles were excluded if they were classified as editorials, review articles, or conference abstracts or if they involved patients 18 years of age or older or described disease states not related to thrombosis. Nineteen articles containing 33 case reports were identified and evaluated for this review. Of the 33 cases, 14, 10, 4, and 2 cases described the use of lepirudin, danaparoid, argatroban, and bivalirudin, respectively. Two cases did not report the type of anticoagulant used, and 1 case used aspirin. The most commonly reported complication was bleeding.
INDEX TERMS: children, direct thrombin inhibitor, heparin-induced thrombocytopenia, low-molecular-weight heparin, unfractionated heparin
INTRODUCTION
Heparin-induced thrombocytopenia (HIT) is a severe, life-threatening, immunological drug reaction that carries a high risk of morbidity and mortality. In the adult population, HIT affects approximately 5% of patients exposed to heparin.1 In children, the frequency is reported to be 2.3% to 3.7% with a 1% to 3% prevalence in children undergoing cardiac surgery with the use of unfractionated heparin (UFH). The paucity of data available and the limited extent of reporting may be contributing to the low incidence. Nonetheless, the incidence in children is increasing, most likely due to increased awareness of the condition.2–9 The purpose of this review article is to describe the clinical features of HIT in children and to summarize the data available for its treatment.
METHODS
A comprehensive literature search was performed using Medline (1950 to the second week of June 2010), International Pharmaceutical Abstracts (1970 to May 2010), and Embase (1980- 2010 Week 22). Keywords used in the search included argatroban, bivalirudin, hirulog, danaparoid, lepirudin, direct thrombin inhibitor, heparin-induced thrombocytopenia, thrombosis, warfarin, and fondaparinux. Search results were limited to children 17 years of age and younger, English language, and human. Inclusion criteria were studies with patients less than or equal to 17 years of age with a confirmed diagnosis of HIT and case reports. Exclusion criteria were patients 18 years of age of older, diseases not related to thrombosis, editorials, review articles, and conference abstracts.
PATHOPHYSIOLOGY
HIT is immunological in nature and can occur after any exposure to heparin, including heparin flushes and heparin-coated catheters.10,11 UFH is often the anticoagulant of choice in children because of its efficacy, ease of monitoring, and reversibility, and clinical experience is extensive.12 Thus, clinicians should be aware of how HIT occurs and that it can result as a serious consequence following the use of UFH.13
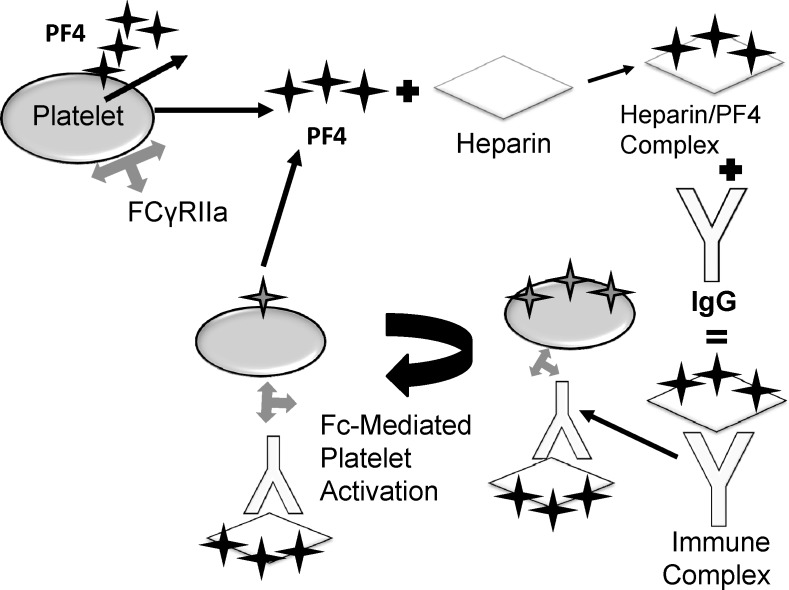
Pathologically, HIT results from the formation of antibodies against heparin, leading to platelet activation and consequent thrombin production.14 Platelet factor 4 (PF4) is a highly positive protein present in the α-granules of platelets. Following heparin exposure, PF4 quickly binds and neutralizes heparin, The PF4-heparin complex then serves as the primary antigen for antibodies, typically of the immunoglobulin G (IgG) class, which recognize and bind to exposed epitopes on PF4.15–21 The 3-component immune complex, composed of IgG, PF4, and heparin, then binds to and activates platelets via the Fc receptor (FcγRII). Following activation, platelets release more PF4 and other prothrombotic microparticles, propagating the cycle of platelet generation and thrombosis (Figure).22,23 Platelets then aggregate and are removed by the reticuloendothelial system, leading to thrombocytopenia.24 Thrombocytopenia may also be explained by increased platelet consumption due to extensive thrombosis.25 Additionally, the antibody-antigen complex induces endothelial injury by binding to FcγRII receptors on monocytes, leading to tissue factor and thrombin production and promulgation.26,27 Therefore, thrombin plays a central role in the pathogenesis of HIT and represents a target for treatment via direct thrombin inhibitors (DTIs).22,28,29
Figure.
The pathophysiology of heparin-induced thrombocytopenia. FCγRIIa, Fc receptor; PF4, Platelet factor 4.
Other negatively charged polysaccharides or polyanions can bind to PF4 and induce conformational changes depending on the chain length and degree of sulfation.21,30 Because low-molecular-weight heparins (LMWH) are prepared from depolymerization of UFH, they bind weakly to PF4. This decreased affinity for PF4 may explain the lower antigenic potential of LMWH in causing HIT.31–35 The incidence of HIT in patients receiving LMWH is 1%, whereas with UFH it is estimated at 3% to 5%.34,36,37
Risk Factors
The risk of children developing HIT is related to the extent of UFH exposure. Pediatric patients may receive UFH for various indications including prophylaxis and treatment of thromboembolic disorders, maintenance of indwelling arterial and venous cannulae, cardiac catheterization, cardiopulmonary bypass (CPB), extracorporeal membrane oxygenation (ECMO), dialysis, and perioperative anticoagulation or in cases of antiphospholipid and anticardiolipin antibodies or protein S deficiency.38–41 The greatest frequency of HIT has been reported in children with prolonged UFH exposure or those receiving larger cumulative doses of UFH.3,42
In the pediatric population, a bimodal frequency for HIT occurrence is observed in neonates and adolescents, with its respective roles in cardiac surgery and umbilical lines or treatment of venous thromboembolism and surgery.42,43 In both populations, underlying thrombosis may additionally contribute to further thrombotic complications once HIT antibodies are formed.44
Diagnosis
HIT may be observed following several clinical scenarios: when platelets decrease by more than 50% from baseline within 4 to 14 days after initiation of heparin; with or without the presence of thromboembolic complications or bleeding; or in the presence of heparin-dependent antibodies.2,13 Diagnosis is based on interpretation of clinical findings with laboratory confirmation of HIT antibodies.5 Diagnosis should be considered in light of recent platelet transfusion or differentiated against other causes of thrombocytopenia.45–49
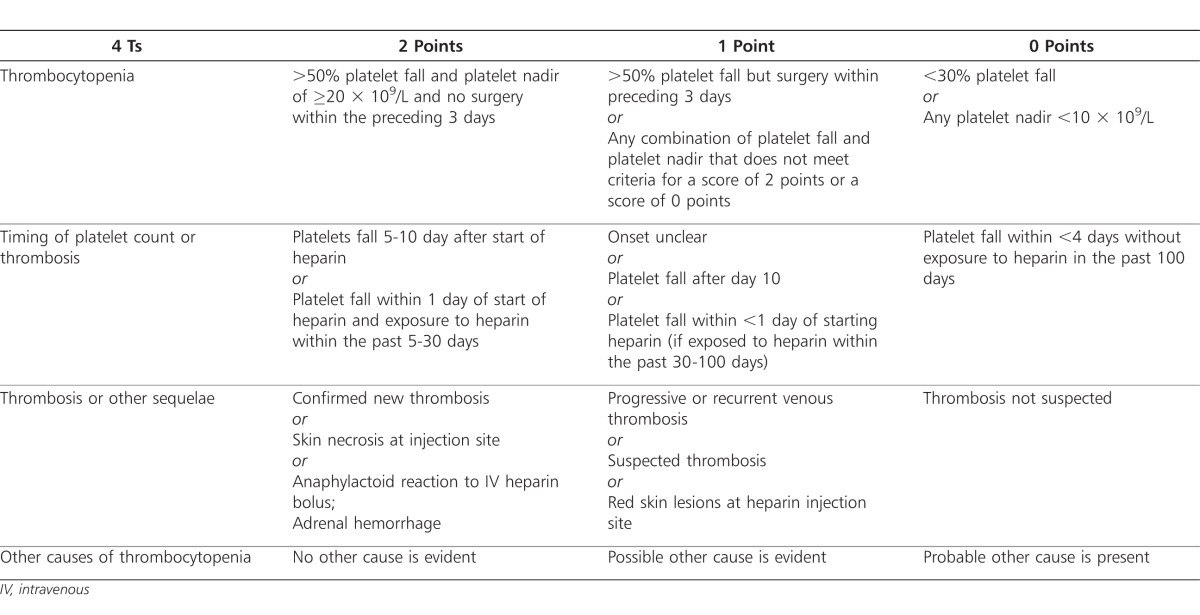
The use of pretest probability scales can be helpful in determining the likelihood of HIT diagnosis. While no scale has been validated in children, the “4T” pretest probability scale developed by Warkentin and colleagues has been used in some pediatric institutions.51–53,86 With this scale, patients are assigned points based on the following criteria: thrombocytopenia, timing of platelet count fall or other sequelae, thrombosis or other complications, and other causes of thrombocytopenia. Points from each criterion are totaled and used to classify a patient as low, possible, or highly probable of positive HIT diagnosis (Table 1).53–55,86
Table 1.
Laboratory Tests
While laboratory tests are supportive in the clinical diagnosis, neither functional nor serologic assays are 100% sensitive or specific because of their inherent design.25,57 Diagnosis is primarily clinical in approximately 20% of cases, and negative laboratory results do not exclude the diagnosis of HIT.56–58
Functional assays detect HIT based on the ability of the heparin-IgG-PF4 complex to activate platelets via FcγRII receptors.59–62 The serotonin release assay is considered the gold standard in HIT functional assays, based on high specificity and sensitivity results.64,65 However, a less sensitive assay (heparin-induced platelet aggregation test) was more commonly used in observed case reports.3,9,44,68–70
While functional assays offer higher specificity and sensitivity, they are less sensitive than serologic assays. Serological or nonfunctional assays are immunological tests that detect antibody binding to PF4-heparin but not the ability of the complex to activate platelets. Those tests detect all subtypes of antibodies, including IgG, IgA, and IgM.59–60 Examples of serological assays include fluid-phase anti-PF4-heparin enzyme immunoassay, particle gel immunoassay, and enzyme-linked immunosorbent assay (ELISA).70–72 The serological assay most commonly used in children is the ELISA test.43,48,68,69,74–83
Immunoassays are available at most clinical laboratories and are technically less demanding than functional assays.6,84,85 Additional advantages include their increased sensitivity (>97%) compared to functional assays and the fact that results are unaffected by platelet or plasma factors.6,57,84,85 However, they have lower specificity in diagnosing HIT (74%-86%) as they detect antibody binding ability only. Because they detect HIT antibodies in patents who do not have thrombocytopenia or complications, the patients test positive in cases of both pathogenic and nonpathogenic HIT.6,12
While the level of concordance between functional and immunological assays is high, 10% to 20% of cases have discordant results.61 A negative ELISA result generally rules out HIT. If the ELISA is positive, a functional assay may be performed to confirm diagnosis or to invalidate any chance of false positive with ELISA.84,86 Using a second assay following a negative functional or serological is especially important if the patient is at high risk for bleeding with a DTI or if clinical suspicion of HIT is low.52 The false-negative rate for both functional and antigen assays is approximately 5%.87
Clinical Manifestations
Timing of Thrombocytopenia
The time to onset of thrombocytopenia, defined as a platelet count of less than 150 × 109/L, varies based on history of heparin exposure. In two-thirds of children with HIT, the platelet count begins to fall 5 to 10 days after heparin initiation and is known as typical onset.2 In more than half of pediatric cases, children experienced a drop in platelets 5 to 15 days following initial therapy with UFH or enoxaparin.43,48,68,69,74,76,77,81,82,88 Conversely, some children may present with rapid-onset HIT. In this scenario, platelets decrease within minutes or hours of heparin initiation. This often occurs due to recent heparin exposure (within the last 100 days) that sensitizes the patient with residual and reactive HIT antibodies.57,89,90–92 Another small subset of patients may rarely experience delayed-onset HIT, which is observed days after heparin has been discontinued. This atypical presentation validates the autoimmune nature of HIT, demonstrating that PF4/heparin-reactive antibodies can activate platelets even in the absence of heparin.93–95
Characteristics of Thrombocytopenia
Platelet counts greater than 150 × 109/L have been observed in children with HIT.35 Because the individual platelet count may vary, a more accurate indicator of HIT is a decrease of >50% in platelet count from baseline.34,96,97 Usually, platelet counts fall to a nadir of 50 × 109/L, although for 10% of children, their lowest platelet count is reached while it is still in the normal range.42
Thrombosis and Other Complications
In children, a new thromboembolic complication (TEC) may be the first presenting symptom of HIT. In all pediatric cases identified, at least one TEC was observed prior to HIT diagnosis.3,9,43,44,48,68–70,74–83,98,99 Studies in humans suggest that the thrombotic risk is higher in patient with higher levels of PF4-heparin antibody.100 While the thromboembolic events may be arterial or venous in origin, venous thromboses are more commonly observed and include thrombi in the subclavian and femoral veins42,97 Pulmonary embolism, a common complication in adult patients with HIT, is rarely seen in young children (<12 years old).3
HIT should be suspected in children with normal platelet counts suffering from unexplained thrombosis.42 In those cases, early recognition of HIT is essential, as thromboembolism can result in loss of limb, myocardial infarction, stroke, and death.101 However, bleeding is a relatively rare complication in children and adults with HIT.4,42 Other rare complications include acute thoracic pain, respiratory distress, anaphylactic shock, prolonged fever.42,43,102 Symptoms observed in adults that have not yet been reported in children include cerebral venous thrombosis, adrenal infarction, and transient global amnesia.42,97
PHARMACOTHERAPY
Direct Thrombin Inhibitors
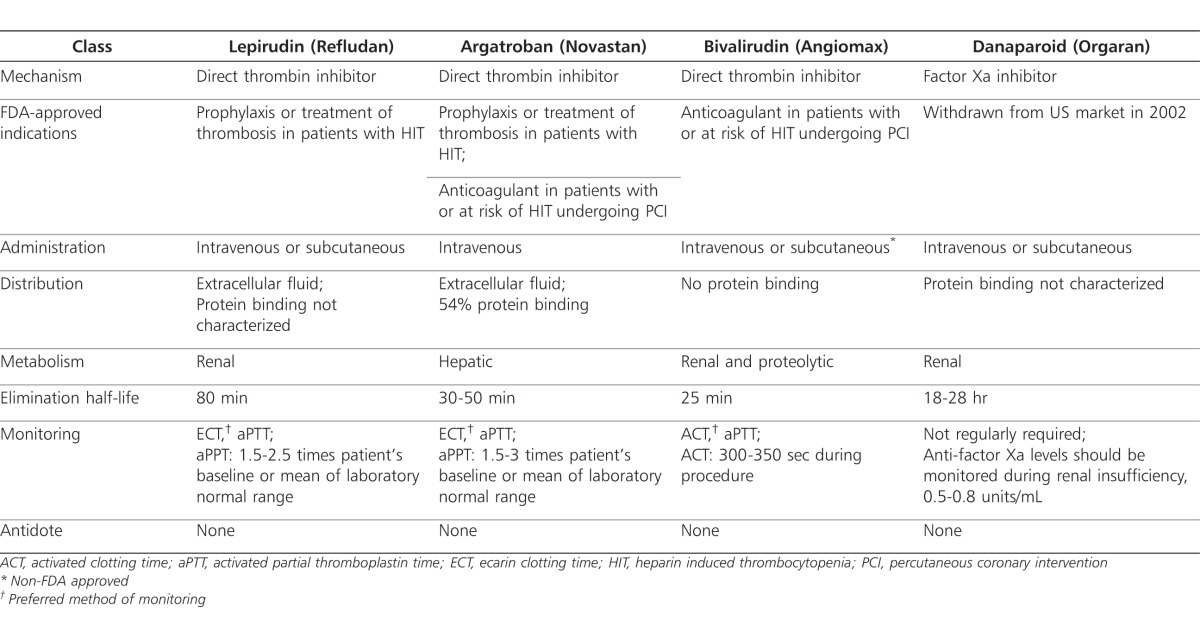
Direct thrombin inhibitors bind to 3 active sites and 2 exosites on thrombin and inhibit fibrin formation. Exosite 1 serves primarily as a dock for fibrin, while exosite 2 allows for heparin binding.103 Based on the DTI's affinity for either the active site or exosite 1, it may be classified as bivalent or univalent. Bivalent DTIs bind with equal affinity to the active site and exosite and include lepirudin (Refludan; Bayer Healthcare Pharmaceuticals, Robinson, PA) and bivalirudin (Angiomax; The Medicines Company, Parsippany, NJ). Univalent agents bind only to the active site and include argatroban (Novastan; GlaxoSmithKline, Zebulon, NC).104 Because DTIs act independently of antithrombin, they have an enhanced capacity to inhibit thrombin bound to fibrin, offering a unique advantage over heparins.105–107 Additionally, they do not bind to plasma proteins, offering a more predictable response over UFH and greater efficacy than LMWH.108 DTIs also possess antiplatelet effects due to thrombin's role in platelet activation.109,110
Lepirudin
Lepirudin is approved by the US Food and Drug Administration (FDA) for anticoagulation in adult patients with HIT and associated thromboembolic disease to prevent further TEC (Table 2).110 However, its safety and efficacy have not been established in children. Lepirudin is a recombinant protein analog of the leech hirudin protein. It is highly specific in its ability to irreversibly bind to both the catalytic and enzymatic sites of thrombin.110–112 Mechanistically, 1 molecule of lepirudin forms a tight complex with 1 molecule of thrombin, neutralizing the actions of thrombin, including those molecules already entrapped in clots.112 Lepirudin is eliminated renally and requires dose modification in patients with renal insufficiency or dialysis. Approximately 50% of a lepirudin dose is excreted in the urine and most of it remains unchanged. Lepirudin follows a 2-compartment model with an initial distributive half-life of 10 minutes and an elimination half-life of 80 minutes.110
Table 2.
Lepirudin is monitored every 4 hours, based on its terminal half-life, using activated partial thromboplastin time (aPTT) (Table 2).5,110 Similar to other agents in its class, lepirudin does not interact with HIT antibodies. However, in 30% of patients, antibodies develop against lepirudin after the first exposure and in 70% of patients upon second exposure. Severe and in some cases fatal reactions have been reported following lepirudin sensitization. For this reason, lepirudin should be used only once during a patient's lifetime.5,110 As a result of antibody formation, renal clearance of lepirudin is delayed, leading to accumulation, which may place the patient at increased risk of bleeding.113 Among the DTIs, the incidence of bleeding is highest with lepirudin, at approximately 17% in patients receiving therapeutic doses. Lepirudin antibodies also display cross-sensitivity to bivalirudin. Therefore, it is recommended that bivalirudin be avoided in patients who previously received lepirudin.112
Lepirudin is typically administered intravenously for HIT management; however, subcutaneous administration without a bolus dose has also been reported in a few instances.114 For example, a patient with a history of HIT who is not able to tolerate warfarin would not be a candidate for bridge therapy from intravenous (IV) lepirudin to warfarin. In this patient, bridging therapy to subcutaneous lepirudin may be an option.115
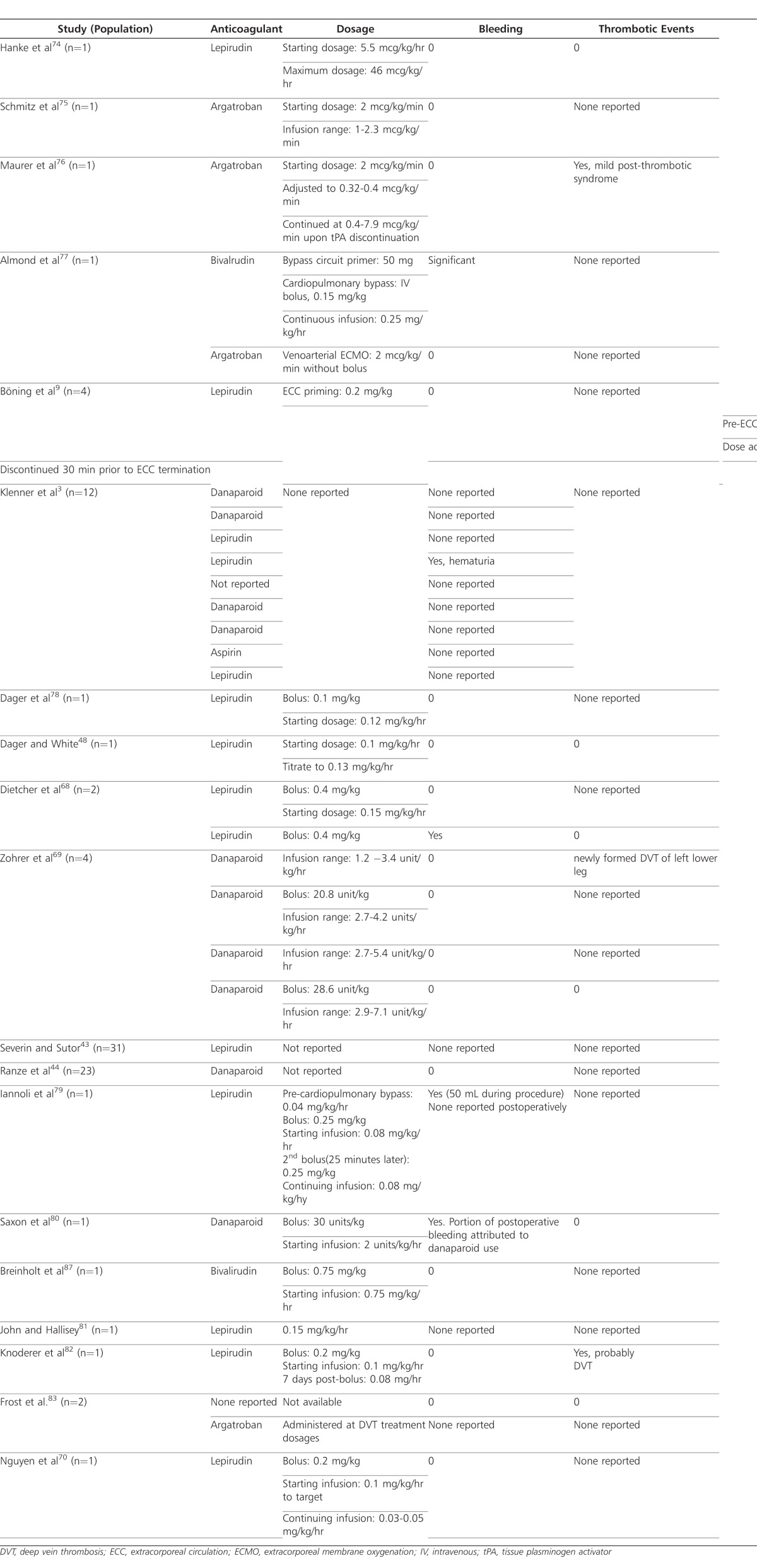
Of the 33 pediatric HIT cases reported in the literature, lepirudin was used in 14 of them (Table 3).3,9,43,48,68,70,74,78,79,81,82 In 6 of those cases, 14 children were male and 7 were female; 1 case did not report the gender of the child. Children ranged in age from 28 weeks to 17 years. Indications for anticoagulation included spontaneous deep vein thrombosis (DVT), thrombus following various cardiac surgeries, cardiac catheterization, and ECMO. Patient's concomitant medications were not mentioned in any of the cases. Bolus doses ranged from 0.25 to 0.4 mg/kg, compared to the usual adult bolus dose of 0.4 mg/kg. Starting infusion rates were reported in 6 of the 14 cases and ranged from 0.0055 to 0.15 mg/kg/hr compared to an adult infusion rate of 0.15 mg/kg/hr.
Table 3.
Anticoagulants and Bleeding Complications in Selected Case Reports
Bleeding outcomes were reported in 10 of these 14 cases.3,68,78,79,81 Three of these 10 cases were positive for a bleeding complication, while the other 7 reported no bleeding complications. The first case with bleeding complications included an 11-year-old female who was diagnosed with HIT following anticoagulation with UFH for treatment of DVT in the pelvis and leg.3 The patient experienced hematuria while receiving lepirudin; however, the bolus dose and rate of infusion were not reported.
The second patient, a 4-year-old female weighing 12 kg, received a 4.8-mg loading dose (0.4 mg/kg) and 3.37-mg priming dose (0.28 mg/kg) of lepirudin following a newly discovered clot in the ECMO circuit.68 Three days prior to starting ECMO, the patient was diagnosed with HIT using supported by the use of PF4-heparin antibody assay. Despite this diagnosis, the ECMO circuit was primed and maintained using heparin, which resulted in clot formation and platelet drop within 24 hours. While no bleeding and thrombolytic complications were reported while using lepirudin, postoperative bleeding was not well controlled. The patient died 5 days after heart transplantation.
The third patient, a 4-month-old patient weighing 5.7 kg, lost 50 mL of blood during a surgical procedure using lepirudin boluses of 0.25 mg/kg and infusion doses of 0.04 mg/kg/hr, initially with a continuing dose of 0.08 mg/kg/hr for use during CPB.79 The patient had been identified as HIT-positive during a previous hospitalization at which time she underwent a cardiac procedure using heparin. During the second hospitalization, this patient underwent a bidirectional Glenn shunt procedure. In anticipation of CPB, an infusion of lepirudin was initiated. A bolus dose for the procedure was 0.25 mg/kg, followed by another bolus of 0.25 mg/kg 25 minutes later. Starting and continuing infusions were 0.08 mg/kg/hr. Monitoring using aPTT was done at baseline and 10 minutes following each bolus dose. Values were reported at 51.9, 83.1, and 105.2 seconds, respectively, with a normal reference value of 25 seconds. The higher aPTT values were anticipated in the vascular surgery; however, lepirudin infusion was stopped for 1 hour following the procedure to allow the aPTT value to decrease to the preoperative therapeutic range.
In most cases, thrombolytic complications directly resulting from the use of lepirudin were not reported.3,9,43,68,70,78,78,81 One case, however, discussed a probable DVT well after lepirudin had been discontinued.8 A 21-month-old male patient with a history of hypoplastic left heart syndrome experienced decreasing platelet counts over 7 days despite transfusion, and test results indicated the presence of HIT antibodies. Heparin was discontinued on the eighth day following surgery, and lepirudin was initiated as a 0.2 mg/kg bolus dose, followed by an infusion at 0.1 mg/kg/hr and was adjusted to maintain the aPTT at 1.5 to 2 times the baseline value. Suspicious thrombus formation was discovered upon echocardiogram 24 days after surgery and 8 days after lepirudin had been discontinued. While computed topography scans suggested thrombus formation in extracardiac conduit and probable DVT in the right femoral and external iliac vein, warfarin was continued for 26 more days. The aPTT value before discontinuation of lepirudin was therapeutic, 45 to 60 seconds (1.5-2 times baseline aPTT), and the infusion rate was decreased to 0.08 mg/kg/hour 1 day before the drug's discontinuation due to decreased renal function.
In two case reports, lepirudin was used during surgical procedures.9,78 In the first case, lepirudin was used for extracorporeal circulation (ECC) priming and prior to ECC initiation with bolus doses of 0.2 mg/kg and 0.25 mg/kg, respectively.9 The infusion dose was maintained to keep blood concentrations between 3.5 and 5 mg/L and was discontinued 30 minutes prior to ECC termination. No bleeding or thrombotic complications resulting from lepirudin or surgery were discussed. In the second case, lepirudin was used prior to, during, and after a bidirectional Glenn shunt procedure.78 While the duration of therapy was variable in these two case reports, patients were treated for an average length of 9 days.9,78
Extended use of lepirudin was described by Hanke and colleagues74 in a 6-year-old patient who went through a surgical intervention for tetralogy of Fallot using UFH. Two days after surgery, the patient began receiving continuous renal replacement therapy (CRRT). Seven days after surgery, an ELISA for heparin-induced antibodies was positive. Anticoagulation therapy was changed to lepirudin, and monitoring was performed using the ecarin clotting time test with a target range of 0.1 to 0.2 mg/L. The only reported complication occurred 12 days after the patient began lepirudin when platelet transfusion led to a catheter-related thrombosis and increased D-dimer. Consequently, CRRT was discontinued, the patient's platelet count improved steadily, and lepirudin was continued successfully for a total of 6 weeks.
Argatroban
Argatroban is FDA approved for prophylaxis and treatment of thrombosis in adult patients with HIT. It is also used as an adjunct therapy for percutaneous coronary intervention (PCI) in patients who have or are at risk for thrombosis due to HIT (Table 2). Argatroban is a small molecule synthetic compound derived from l-arginine that binds selectively and reversibly to the catalytic site of thrombin, competitively inhibiting the action of thrombin, including fibrin generation and platelet aggregation.111,112 Following intravenous administration, the volume of distribution of argatroban in healthy subjects is 180 mL/kg, with a half-life of 30 to 50 minutes. Unlike lepirudin, argatroban is cleared hepatically and excreted in bile and must be dose-adjusted in liver dysfunction.116 Bleeding with argatroban has been reported in 6% to 7% of adult and child patients. Contrary to the mechanism of lepirudin, the development of antibodies against argatroban has not been reported.112 Additionally, argatroban falsely increases International Normalized Ratio (INR) value. While all DTIs have the ability to inaccurately increase INR values, argatroban has the greatest tendency to do so because of the high molar concentrations of argatroban required to achieve thrombin inhibition. In vitro, this results in greater thrombin inhibition and, subsequently, higher prothrombin values. While this interaction constitutes a drug-laboratory and not a pharmacodynamic interaction, it is of particular importance when transitioning to warfarin therapy.117,118
Children treated with argatroban ranged in age between 2 months and 15 years.75–77,83 Two children were female and the other 2 were male. Indications of anticoagulation with heparin included flushing of central catheter, DVT prophylaxis and treatment, bilateral pulmonary embolism treatment, left-ventricular assist device (LVAD) placement, ECMO, and CPB. Concomitant medications were not discussed in any of the cases; however, in the case of a 15-year-old male with Gitelman syndrome, the patient had received digoxin, furosemide, captopril, and aspirin at home.75 As his conditioned worsened, milrinone was also added to his medication regimen. In the 4 cases that used argatroban, the starting infusion rate was 2 mcg/kg/min without a bolus dose, similar to the recommended dose used in the adult population.75–77,83 Continuing doses were adjusted based on aPTT values and administered at a wide range of doses (0.32-8 mcg/kg/min)
In 2 pediatric HIT cases, argatroban was used after an alternative anticoagulant failed.76,77 The first case report was in a previously healthy, obese 11-year-old 68-kg female treated for lobar pneumonia.76 Immobilization, obesity, and May-Thurner syndrome contributed to development of DVT and bilateral pulmonary emboli, which were successfully treated with heparin. Sixteen days later, the patient experienced recurrent DVT in both legs, and ELISA results were reported positive. Consequently, bivalirudin was initiated at a 125 mcg/kg bolus, followed by 125 mcg/kg/hr infusion to maintain an aPTT goal of 1.5 to 2 times baseline. However, 12 days after the patient began taking bivalirudin, rethrombosis occurred, and site-directed alteplase was used concurrently for 12 days to successfully lyse the clot. Because of renal compromise, argatroban was initiated at 2 mcg/kg/min. Nineteen days later, therapy with argatroban was switched to fondaparinux, 7.5 mg daily, in preparation for discharge. The patient, however, experienced recurrent pulmonary emboli and bilateral vein occlusion upon transition to fondaparinux. Argatroban at infusion rates of up to 17.5 mcg/kg/hr and alteplase were used. After 3 months in the hospital, the patient was transitioned from argatroban to warfarin and discharged on warfarin for 21 more days.
In the second case, a 5-year-old female heart transplant patient was on ECMO for 14 days before being diagnosed with HIT.77 When a donor heart became available, bivalirudin was used during CPB. Significant bleeding occurred once CBP was discontinued. Following the bleed, argatroban was resumed at 2 mcg/kg/min and continued until her transfer to a different ward, 33 days later.
In the 2 remaining cases, argatroban was used as the primary anticoagulant following diagnosis of HIT. The first case, described by Schmitz and colleagues, 75 involved a 15-year-old male with Gitelman syndrome and asthma, in which heparin was used as thromboprophylaxis for 4 weeks. An LVAD was placed using additional heparin bolus and infusion doses. In addition, heparin flushes were used to maintain patency of a central catheter. However, heparin was discontinued shortly thereafter when the ELISA result came back positive. Warfarin and argatroban were started 24 hours after LVAD placement. Two days later, warfarin was gradually increased as argatroban was decreased while targeting an INR value of 3. Argatroban was discontinued 17 days postoperatively, and the patient was continued on warfarin, aspirin, and dipyridamole.
The remaining case discussed a 37-week-old gestational age male infant with a diagnosis of primary pulmonary hypertension and seizure disorder.83 A total of 200 units of heparin were used during the patient's first 10 days of life. At 2 months of age, a routine check-up revealed a platelet count of 21,000, and the patient was admitted for anemia, thrombocytopenia, and infection soon after. A central line was flushed with 10 units of heparin. Eight days after admission, the patient experienced a DVT that was treated with heparin. Further testing revealed positive heparin antibodies. Heparin was immediately discontinued, and argatroban therapy was initiated at 2 mcg/kg/min. Later, argatroban was used for triscuspid valve excision, although the doses during the procedure were not reported. None of the patients receiving argatroban experienced bleeding. One patient, however, experienced mild post-thrombotic syndrome which was not described further by the authors.75
Bivalirudin
Bivalirudin is FDA approved for anticoagulation in adult patients undergoing PCI with or at risk of HIT or heparin-induced thrombocytopenia-thrombosis syndrome. Like other DTIs, bivalirudin is not approved in children. A molecule of this 20 amino acid polypeptide binds bivalently to thrombin, inhibiting its immediate and downstream actions. Unlike lepirudin, bivalirudin offers only transient thrombin inhibition; once bound, bivalirudin is cleaved by thrombin, which restores thrombin's function.107 Bivalirudin may be administered intravenously or subcutaneously, although the latter is not FDA-approved.
Bivalirudin displays linear pharmacokinetics and is eliminated from plasma by a combination of renal and enzymatic mechanisms. Because 80% of the drug's metabolism occurs through proteolytic enzymes, bivalirudin offers a unique advantage in patients who experience renal and/or hepatic insufficiency. In a study by Steinberg,119 115 patients were examined for effects of renal function on bleeding risk from bivalirudin. Patients with creatinine clearance less than 60 mL/min were approximately 3.3 times more likely to experience bleeding complication than those with creatinine clearance greater than 60 mL/min.119 Elimination half-life of this agent is the shortest of all direct thrombin inhibitors, at approximately 25 minutes in patients with normal renal function. Bleeding incidence is approximately 2.4% in patients undergoing PCI with a history of HIT.107 Despite its metabolism, bivalirudin doses should be decreased in cases of moderate and severe renal dysfunction to protect the patient from over-anticoagulation.
Bivalirudin use was reported in two cases. Almond and colleagues77 reported using a 50-mg loading dose and a 0.15 mg/kg bolus for bypass circuit primer and CPB, respectively, followed by an intravenous infusion of 0.25 mg/kg/hr in a 5-year-old female (weight unknown). Repeat bolus doses were administered, and the infusion rate was titrated to maintain activated clotting time >400 seconds over the next first 30 minutes of therapy. The patient experienced significant bleeding following separation from bypass, although the parameters qualifying the bleed as significant were not provided. While the causality between bivalirudin and bleeding incidence was not clear, bleeding resolved in 12 hours with the administration of fresh-frozen plasma, recombinant Factor VIIa, and ultrafiltration. No thrombotic complications were reported.
In the second case report, a bolus dose of 0.75 mg/kg, followed by a starting infusion of 0.75 mg/kg/hr for cardiac catheterization was used in a 2-year-old boy with complex congenital heart disease. The patient had been diagnosed with HIT following a bidirectional Glenn procedure at 6 months of age.87 Unlike the first case, bivalirudin did not result in bleeding complications. Thrombotic complications as a result of bivalirudin were reported in either case (Table 3).77,87
Danaparoid
Danaparoid (Orgaran; Schering-Plough Corp., Memphis, TN), while not FDA approved for HIT in either adults or children in the United States (US), has been used in other countries.111 Danaparoid was voluntarily withdrawn from the US market in 2002 with the introduction of fondaparinux. It is a low-molecular-weight heparinoid consisting of a mixture of heparin, dermatan, and chondroitin sulfates. Derived from porcine gut mucosa, danaparoid selectively inhibits Factor Xa, similar to the actions of fondaparinux. Unlike the direct thrombin inhibitors, danaparoid may be administered subcutaneously or intravenously and possesses a long half-life, ranging from 18-28 hours.120 Danaparoid is renally cleared and requires dose adjustment in renal dysfunction. While routine monitoring is not required, anti-factor Xa levels may be assessed in patients with renal failure, dialysis, or in body habitus extremes. Incidence of bleeding has been reported at approximately 8% in the general population.112
Danaparoid was used in 10 of the 33 identified case reports. Three cases reported use of bolus doses, ranging from 20 to 30 units/kg.69,80 Infusion rates were variable and ranged from 2 to 7.1 units/kg/hr.69–80 In most cases, danaparoid did not result in bleeding complications; however, Saxon and colleagues attributed a portion of postoperative bleeding to the use of danaparoid.80 One case report, described by Zohrer and colleagues,69 resulted in a complication of thromboembolic origin; the patient had a newly formed DVT in the left lower leg. Cross-sensitivity to heparin antibodies was suspected, and danaparoid was replaced with hirudin for 2 days with no further progression of the thrombus (Table 3).
DISCUSSION
Summary of Findings
This review summarizes the most recent case reports of HIT in children, with a focus on available anticoagulants and associated complications. Earlier reviews have focused on identifying age groups in which HIT is most commonly observed or identifying differences in clinical presentation; however, they have not focused on doses used in available cases. Previous literature has focused on determining the frequency of DTI and Factor Xa inhibitor use or risk factors for HIT in children.
Pediatric patients exposed to heparin are at risk for side effects and complications, including HIT. Based on the limited number of case reports available, the occurrence of HIT in children appears rare. However, the true incidence of HIT is unknown, as all cases of HIT in children may not have been reported and/or published. In most cases, the offending agent in identified pediatric patients was UFH; in 1 case, the agent was enoxaparin. Current data in children do not compare incidence rates of HIT with heparin versus LMWH. Furthermore, prospective clinical trials do not currently exist to offer guidance on the diagnosis and management of this condition in children. Most patients are successfully treated with one of these agents. In two cases, failure with bivalirudin and fondaparinux prompted the use of argatroban.
The demographics of the children in these cases are diverse, with higher incidence in neonates and adolescents. The cases represent myriad ages and include an equal distribution of male and female patients. Patients treated with lepirudin ranged from 4 months to 11 years of age, while those treated with argatroban varied from a neonate to a 15-year-old. Bivalirudin was used in 2- and 5-year-old children.
While all direct thrombin inhibitors and danaparoid have been used with success for treatment of HIT in children, none has FDA approval for this indication in this population. Anticoagulants used in these cases include the direct thrombin inhibitors, lepirudin, argatroban, bivalirudin, and the factor Xa inhibitor, danaparoid. The most commonly used anticoagulant was lepirudin, followed by danaparoid, argatroban, and bivalirudin. All agents, regardless of its metabolic pathway, displayed similar bleeding incidences, and none seemed to carry a greater risk. Since there were an unequal proportion of cases represented by each agent, it is not possible to conclude which agent is the safest in this population. Prospective studies providing evidence for the efficacy and safety of these agents are needed.
Lepirudin doses were lower or equal to recommended adult doses, while starting doses for argatroban were identical to adult dosing. However, a wide range of infusion rates with argatroban were reported. This may reflect the wide range of ages represented in these cases, which represents various degrees of hepatic maturity. For the cases that reported danaparoid doses, the infusion rates varied and were patient-specific.
Implications for Pharmacy Practice
Dosing recommendations for the direct thrombin inhibitors and danaparoid are often extrapolated from adult dosing. While dosing formulas and allometric scaling may be appropriate for children older than 8 years, whose body composition and organ function approximates to an adult, these approaches have limited merit in young children and neonates. The developmental changes that occur during childhood may alter the pharmacokinetics of the anticoagulants.121 The metabolism route, ease of monitoring, and relative risk of bleeding of each anticoagulant should be considered in choosing appropriate therapy. An understanding of the extent organ maturity may help to predict the pharmacokinetics of the agent in the child.
CONCLUSIONS
Heparin-induced thrombocytopenia remains a life-threatening condition in the pediatric population, and proper understanding of its risks and management are essential. Tools such as assays and probability tests can aid in proper diagnosis. The limited data and lack of guidelines in the pediatric population makes pharmacotherapeutic decisions challenging. Following diagnosis of HIT, treatment with direct thrombin inhibitors and danaparoid have been described using case reports; however, use has not been evaluated in large studies of children with HIT. Further knowledge of developmental pharmacology and pharmacokinetics can guide pharmacotherapeutic decisions and individualize dosing in children in the future.
ACKNOWLEDGEMENT
The authors thank Kimberley Pesaturo, PharmD, BCPS, who made significant contributions to the work reported in the manuscript.
ABBREVIATIONS
- aPTT
activated partial thromboplastin time
- CPB
cardiopulmonary bypass
- CRRT
continuous renal replacement therapy
- DTI
direct thrombin inhibitor
- DVT
deep vein thrombosis
- ECC
extracorporeal circulation
- ECMO
extracorporeal membrane oxygenation
- ELISA
enzyme-linked immunosorbent assay
- FcγRII
Fc receptor
- FDA
Food and Drug Administration
- HIT
Heparin-induced thrombocytopenia
- INR
international normalized ratio
- LMWH
low-molecular weight heparin
- LVAD
left-ventricular assist device
- PF4
Platelet factor 4
- PCI
percutaneous coronary intervention
- UFH
unfractionated heparin
DISCLOSURE The authors declare no conflicts or financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employment, gifts, and honoraria.
See related editorial on page 2
REFERENCES
- 1.Kelton JG. The clinical management of heparin-induced thrombocytopenia. Semin Hematol. 1999;36(suppl 1):S17–S21. [PubMed] [Google Scholar]
- 2.Risch L, Huber AR, Schmugge M. Diagnosis and treatment of heparin-induced thrombocytopenia in neonates and children. Thromb Res. 2006;18(1):123–125. doi: 10.1016/j.thromres.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 3.Klenner AF, Lubenow N, Raschke R, Greinacher A. Heparin-induced thrombocytopenia in children: 12 new cases and review of the literature. Thromb Haemost. 2004;91(4):719–724. doi: 10.1160/TH03-09-0571. [DOI] [PubMed] [Google Scholar]
- 4.Schmugge M, Risch L, Huber AR, et al. Heparin-induced thrombocytopenia-associated thrombosis in pediatric intensive care patients. Pediatrics. 2002;109(1):e10. doi: 10.1542/peds.109.1.e10. [DOI] [PubMed] [Google Scholar]
- 5.Warkentin TE, Greinacher A. Heparin-induced thrombocytopenia: recognition, treatment, and prevention: the seventh ACCP conference on antithrombotic and thrombolytic therapy. Chest. 2004;1263(suppl 3):311S–337S. doi: 10.1378/chest.126.3_suppl.311S. [DOI] [PubMed] [Google Scholar]
- 6.Warentin TE, Sheppard JI, Horsewood P, et al. Impact of the patient population on the risk for heparin-induced thrombocytopenia. Blood. 2000;96(5):1703–1708. [PubMed] [Google Scholar]
- 7.Pouplard C, May MA, Lochmann S, et al. Antibodies to platelet factor 4-heparin after cardiopulmonary bypass in patients anticoagulated with unfractionated heparin or a low-molecular weight heparin. Circulation. 1999;99(19):2530–2536. doi: 10.1161/01.cir.99.19.2530. [DOI] [PubMed] [Google Scholar]
- 8.Warkentin TE, Greinacher A. Heparin-induced thrombocytopenia and cardiac surgery. Ann Thorac Surg. 2003;76(2):638–648. doi: 10.1016/s0003-4975(03)00756-2. [DOI] [PubMed] [Google Scholar]
- 9.Böning A, Morschheuser T, Bläse U, et al. Incidence of heparin-induced thrombocytopenia and therapeutic strategies in pediatric cardiac surgery. Ann Thorac Surg. 2005;79(1):62–65. doi: 10.1016/j.athoracsur.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 10.Nand S, Wong W, Yuen B, et al. Heparin-induced thrombocytopenia with thrombosis: incidence, analysis of risk factors, and clinical outcomes in 108 consecutive patients treated at a single institution. Am J Hematol. 1997;56(1):12–16. doi: 10.1002/(sici)1096-8652(199709)56:1<12::aid-ajh3>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 11.Kadidal VV, Mayo DJ, Horne MK. Heparin-induced thrombocytopenia (HIT) due to heparin flushes: a report of three cases. J Intern Med. 1999;246(3):325–329. doi: 10.1046/j.1365-2796.1999.00527.x. [DOI] [PubMed] [Google Scholar]
- 12.Alsoufi B, Boshkov LK, Kirby A, et al. Heparin-induced thrombocytopenia (HIT) in pediatric cardiac surgery: an emerging cause of morbidity and mortality. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2004;7:155–171. doi: 10.1053/j.pcsu.2004.02.024. [DOI] [PubMed] [Google Scholar]
- 13.Chong BH, Chong JH. Heparin-induced thrombocytopenia. Expert Rev Cardiovasc Ther. 2004;2(4):547–559. doi: 10.1586/14779072.2.4.547. [DOI] [PubMed] [Google Scholar]
- 14.Reily RF. The pathophysiology of immune-mediated heparin-induced thrombocytopenia. Semin Dial. 2003;16(1):54–60. doi: 10.1046/j.1525-139x.2003.03013.x. [DOI] [PubMed] [Google Scholar]
- 15.Arepally GM, Mayer IM. Antibodies from patients with heparin-induced thrombocytopenia stimulate monocytic cells to express tissue factor and secrete interleukin 8. Blood. 2001;98(4):1252–1254. doi: 10.1182/blood.v98.4.1252. [DOI] [PubMed] [Google Scholar]
- 16.Dawes J, Pumphrey CW, McLaren KM, et al. The in vivo release of human platelet factor 4 by heparin. Thromb Res. 1982;27(1):65–76. doi: 10.1016/0049-3848(82)90279-1. [DOI] [PubMed] [Google Scholar]
- 17.Grewitz AM, Calabretta B, Rucinski B, et al. Inhibition of human megakaryocytopoiesis in vitro by platelet factor (PF4) and a synthetic COOH-terminal PF4 peptide. J Clin Invest. 1989;83(5):1477–1486. doi: 10.1172/JCI114041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maione TE, Gray GS, Petro J, et al. Inhibition of angiogenesis by recombinant human platelet factor-4 and related peptides. Science. 1990;247(4938):77–79. doi: 10.1126/science.1688470. [DOI] [PubMed] [Google Scholar]
- 19.Sharpe RJ, Byers HR, Scott CF, et al. Growth inhibition of murine melanoma and human colon carcinoma by recombinant human platelet factor 4. J Natl Cancer Inst. 1990;82(10):848–853. doi: 10.1093/jnci/82.10.848. [DOI] [PubMed] [Google Scholar]
- 20.Sato Y, Abe M, Takaki R. Platelet factor 4 blocks the binding of basic fibroblast growth factor to the receptor and inhibits the spontaneous migration of vascular endothelial cells. Biochem Biophys Res Commun. 1990;172(2):595–600. doi: 10.1016/0006-291x(90)90715-y. [DOI] [PubMed] [Google Scholar]
- 21.Visentin GP, Moghaddam M, Collins JL, et al. Antibodies associated with heparin-induced thrombocytopenia (HIT) report conformational changes in platelet Factor 4 (PF4) induced by linear polyanionic compounds. Blood. 1997;90(suppl 1):460a. [Google Scholar]
- 22.Aster RH. Heparin-induced thrombocytopenia and thrombosis. N Engl J Med. 1995;332(5):1374–1376. doi: 10.1056/NEJM199505183322011. [DOI] [PubMed] [Google Scholar]
- 23.Cines DB, Tomaski A, Tannenbaum S. Immune endothelial-cell injury in heparin-associated thrombocytopenia. N Eng J Med. 1987;316(1):581–589. doi: 10.1056/NEJM198703053161004. [DOI] [PubMed] [Google Scholar]
- 24.Dager WE, Dougherty JA, Nguyen PH, et al. Heparin-induced thrombocytopenia: treatment options and special considerations. Pharmacotherapy. 2007;27(4):564–587. doi: 10.1592/phco.27.4.564. [DOI] [PubMed] [Google Scholar]
- 25.Chong BH. Heparin-induced thrombocytopenia. Br J Haematol. 1995;89(3):431–439. doi: 10.1111/j.1365-2141.1995.tb08346.x. [DOI] [PubMed] [Google Scholar]
- 26.Pouplard C, Iochmann S, Renard B, et al. Induction of monocyte tissue factor expression by antibodies to heparin-platelet Factor 4 complexes developed by heparin-induced thrombocytopenia. Blood. 2001;97(1):3300–3302. doi: 10.1182/blood.v97.10.3300. [DOI] [PubMed] [Google Scholar]
- 27.Arepally GM, Mayer IM. Antibodies from patients with heparin-induced thrombocytopenia stimulate monocytic cells to express tissue factor and secrete interleukin 8. Blood. 2001;98(4):1252–1254. doi: 10.1182/blood.v98.4.1252. [DOI] [PubMed] [Google Scholar]
- 28.Verstraete M. Direct thrombin inhibitors: appraisal of the antithrombotic/hemorrhagic balance. Thromb Haemost. 1997;78(1):357–363. [PubMed] [Google Scholar]
- 29.Fenton JW, II, Ofosu FA, Brezniak DV, Hassouna HI. Thrombin and antithrombotics. Semin Thromb Hemost. 1998;316(2):581–589. doi: 10.1055/s-2007-995828. [DOI] [PubMed] [Google Scholar]
- 30.Greinacher A, Alban S, Dummel V, et al. Characterization of the structural requirements for a carbohydrate based anticoagulant with a reduced risk of inducing the immunological type of heparin-induced thrombocytopenia. Thromb Haemost. 1995;74(3):886–892. [PubMed] [Google Scholar]
- 31.Warkentin TE, Levine MN, Hirsh J, et al. Heparin-induced thrombocytopenia in patients treated with low-molecular-weight heparin or unfractionated heparin. N Engl J Med. 1995;332(20):1330–1335. doi: 10.1056/NEJM199505183322003. [DOI] [PubMed] [Google Scholar]
- 32.Ahmad S, Haas S, Hoppensteadt DA, et al. Differential effects of clivarin and heparin in patients undergoing hip and knee surgery for generation of anti-heparin Factor 4 antibodies. Thromb Res. 2002;108(1):49–55. doi: 10.1016/s0049-3848(02)00397-3. [DOI] [PubMed] [Google Scholar]
- 33.Greinacher A. Antigen generation in heparin-associated thrombocytopenia: the nonimmunologic type and the immunologic type are closely linked in their pathogenesis. Semin Throm Hemost. 1995;21(1):106–116. doi: 10.1055/s-2007-1000384. [DOI] [PubMed] [Google Scholar]
- 34.Gruel Y, Pouplard C, Nguyen P, et al. Biological and clinical features of low-molecular-weight-heparin-induced thrombocytopenia. Br J Hematol. 2003;121(5):786–792. doi: 10.1046/j.1365-2141.2003.04363.x. [DOI] [PubMed] [Google Scholar]
- 35.Visentin GP, Moghaddam M, Beery SE, et al. Heparin is not required for detection of antibodies associated with heparin-induced thrombocytopenia/thrombosis. J Lab Clin Med. 2001;138(1):22–31. doi: 10.1067/mlc.2001.115525. [DOI] [PubMed] [Google Scholar]
- 36.Arepally GM, Becker RC. Antithrombotic therapy. In: RS Irwin, Rippe JM., editors. Irwine and Rippe's Intensive Care Medicine. 6th ed. Philadelphia: Lippincott Williams & Wilkins;; 2008. pp. 1361–1367. In. eds. [Google Scholar]
- 37.Warkentin TE. Heparin-induced thrombocytopenia: part 1: the diagnostic clues. J Crit Illn. 2002;17(5):172–178. [Google Scholar]
- 38.Garrelts JC, LaRocca J, Ast D, et al. Comparison of heparin and 0.9% sodium chloride injection with and without heparin and 0.9% sodium chloride injection in the maintenance of indwelling i.v. devices. Clin Pharm. 1989;8(1):34–39. [PubMed] [Google Scholar]
- 39.Lesko SM, Mitchell AA, Epstein MF, et al. Heparin use as a risk factor for intraventricular hemorrhage in low-birth-weight infants. N Engl J Med. 1986;314(18):1156–1160. doi: 10.1056/NEJM198605013141805. [DOI] [PubMed] [Google Scholar]
- 40.Rajani K, Goetzman BM, Wennberg RP, et al. Effect of heparinization fluids infused through an umbilical artery catheter on catheter patency and frequency of complications. Pediatrics. 1979;63(4):552–556. [PubMed] [Google Scholar]
- 41.Sutor AH, Massicote P, Leaker M, et al. Heparin therapy in pediatric patients. Semin Thromb Hemost. 1997;23(3):303–319. doi: 10.1055/s-2007-996103. [DOI] [PubMed] [Google Scholar]
- 42.Risch L, Fischer JE, Herklotz R, et al. Heparin-induced thrombocytopenia in pediatrics: clinical characteristics, therapy, outcomes. Intensive Care Med. 2004;30(8):1615–1624. doi: 10.1007/s00134-004-2315-4. [DOI] [PubMed] [Google Scholar]
- 43.Severin T, Sutor AH. Heparin-induced thrombocytopenia in pediatrics. Semin Thromb Hemost. 2001;27(3):293–299. doi: 10.1055/s-2001-15259. [DOI] [PubMed] [Google Scholar]
- 44.Ranze O, Ranze P, Magnani HN, et al. Heparin-induced thrombocytopenia in paediatric patients: a review of the literature and a new case treated with danaparoid sodium. Eur J Pediatr. 1999;158(suppl 3):S130–S133. doi: 10.1007/pl00014338. [DOI] [PubMed] [Google Scholar]
- 45.Sutor AH, Massicotte P, Leaker M, et al. Heparin therapy in pediatric patients. Semin Thromb Hemost. 1997;23(3):303–319. doi: 10.1055/s-2007-996103. [DOI] [PubMed] [Google Scholar]
- 46.Sutor AH, Uhl M. Diagnosis of thromboembolic disease during infancy and childhood. Semin Thromb Hemost. 1997;23(3):237–246. doi: 10.1055/s-2007-996096. [DOI] [PubMed] [Google Scholar]
- 47.Lombardi TP, Gundersen B, Zammett LO, et al. Efficacy of 0.9% sodium chloride injection with and without heparin sodium for maintain patency of intravenous catheters in children. Clin Pharm. 1988;7(11):832–836. [PubMed] [Google Scholar]
- 48.Dager WE, White RH. Low-molecular-weight-heparin-induced thrombocytopenia in a child. Ann Pharmacother. 2004;38(2):247–250. doi: 10.1345/aph.1D308. [DOI] [PubMed] [Google Scholar]
- 49.Fahey VA. Heparin-induced thrombocytopenia. J Vasc Nurs. 1995;13(4):112–116. doi: 10.1016/s1062-0303(05)80003-2. [DOI] [PubMed] [Google Scholar]
- 50.Rice L, Nguyen PH, Vann AR. Preventing complications in heparin-induced thrombocytopenia. Alternative anticoagulants are improving patient outcomes. Postgrad Med. 2002;163(3):1849–1856. doi: 10.3810/pgm.2002.09.1307. [DOI] [PubMed] [Google Scholar]
- 51.Spliner SA, Dager W. Overview of heparin-induced thrombocytopenia. Am J Health Syst Pharm. 2003;60(suppl 5):S5–S11. doi: 10.1093/ajhp/60.suppl_5.S5. [DOI] [PubMed] [Google Scholar]
- 52.Barthelomew JR. The incidence and clinical features of heparin-induced thrombocytopenia. Semin Hematol. 2005;42(3 suppl 3):S3–S8. doi: 10.1053/j.seminhematol.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 53.Breinholt JP, Moffett BS, Texter KM, et al. Successful use of bivalirudin for superior vena cava recanalization and stent placement in a child with heparin-induced thrombocytopenia. Pediatric Cardiol. 2008;29(4):804–807. doi: 10.1007/s00246-008-9231-2. [DOI] [PubMed] [Google Scholar]
- 54.Warkentin TE. Heparin-induced thrombocytopenia. Dis Mon. 2005;51(2-3):141–149. doi: 10.1016/j.disamonth.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 55.Fabris F, Ahmad S, Cella G, et al. Pathophysiology of heparin-induced thrombocytopenia, clinical and diagnostic implications: a review. Arch Pathol Lab Med. 2000;124(11):1657–1666. doi: 10.5858/2000-124-1657-POHIT. [DOI] [PubMed] [Google Scholar]
- 56.Warkentin TE. Heparin-induced thrombocytopenia: pathogenesis, frequency, avoidance and management. Drug Saf. 1997;17(5):325–341. doi: 10.2165/00002018-199717050-00005. [DOI] [PubMed] [Google Scholar]
- 57.Daneschvar HL, Daw H. Heparin-induced thrombocytopenia (an overview) Int J Clin Pract. 2007;61(1):130–137. doi: 10.1111/j.1742-1241.2006.00874.x. [DOI] [PubMed] [Google Scholar]
- 58.Magnani NH. Orgaran (danaparoid sodium) use in the syndrome of heparin-induced thrombocytopenia. Platelets. 1997;8:74–81. [Google Scholar]
- 59.Keeling DM, Richards EM, Bagling TP. Platelet aggregation in response to four low molecular weight heparins and the heparinoid ORG 10172 in patients with heparin-induced thrombocytopenia. Br J Haematol. 1994;86(2):425–426. doi: 10.1111/j.1365-2141.1994.tb04760.x. [DOI] [PubMed] [Google Scholar]
- 60.Francis JL. Detection and significance of heparin-platelet factor 4 antibodies. Semin Hematol. 2005;42(3 suppl):S9–S14. doi: 10.1053/j.seminhematol.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 61.Greinacher A, Amiral J, Dummel V, et al. Laboratory diagnosis of heparin-associated thrombocytopenia a comparison of platelet aggregation test, heparin-induced platelet activation test, and platelet factor 4/heparin enzyme-linked immunosorbent assay. Transfusion. 1994;34(5):381–385. doi: 10.1046/j.1537-2995.1994.34594249047.x. [DOI] [PubMed] [Google Scholar]
- 62.Elalamy I, Lecrubier C, Horrelou MH, et al. Heparin-induced thrombocytopenia: laboratory diagnosis and management. Ann Med. 2000;32(suppl 1):S60–S67. [PubMed] [Google Scholar]
- 63.Chong BH, Burgess J, Ismail F. The clinical usefulness of the platelet aggregation test for diagnosis of heparin-induced thrombocytopenia. Thromb Hemost. 1993;69(4):344–350. [PubMed] [Google Scholar]
- 64.Sheridan D, Carter C, Kelton JG. A diagnostic test for heparin-induced thrombocytopenia. Blood. 1986;67(1):27–30. [PubMed] [Google Scholar]
- 65.Favaloro EJ, Bernal-Hoyos E, Exner T, et al. Heparin-induced thrombocytopenia laboratory investigation and confirmation of diagnosis. Pathology. 1992;24(3):177–183. doi: 10.3109/00313029209063169. [DOI] [PubMed] [Google Scholar]
- 66.Kelton JG, Sheridan D, Brian H, et al. Clinical usefulness of testing for a heparin-dependent platelet-aggregating factor in patients with suspected heparin-associated thrombocytopenia. J Lab Clin Med. 1984;103(4):606–612. [PubMed] [Google Scholar]
- 67.Greinacher A. Heparin-induced thrombocytopenia. J Thromb Haemost. 2009;7(suppl 1):9–12. doi: 10.1111/j.1538-7836.2009.03385.x. [DOI] [PubMed] [Google Scholar]
- 68.Dietcher SR, Topoulos AP, Bartholomew JR, et al. Lepirudin anticoagulation for heparin-induced thrombocytopenia. J Pediatr. 2002;140(2):264–266. doi: 10.1067/mpd.2002.121384. [DOI] [PubMed] [Google Scholar]
- 69.Zohrer B, Zenz W, Rettenbacher A, et al. Danaparoid (Orgaran) in four children with heparin-induced thrombocytopenia type II. Acta Paediatr. 2001;90(7):765–771. [PubMed] [Google Scholar]
- 70.Nguyen TN, Gal P, Ransom JL, et al. Lepirudin use in a neonate with heparin-induced thrombocytopenia. Ann Pharmacother. 2003;37(2):229–233. doi: 10.1177/106002800303700214. [DOI] [PubMed] [Google Scholar]
- 71.Newman P, Chong BH. Heparin-induced thrombocytopenia: the major antigenic epitope is on the modified platelet Factor 4 and not on heparin. Br J Hematol. 1999;107:303–309. [Google Scholar]
- 72.Greinacher A, Wakentin TE. Laboratory testing for heparin-induced thrombocytopenia. In: Warkentin TE, Greinacher A, editors. Heparin-Induced Thrombocytopenia. New York: Marcel Dekker;; 2004. In. eds. [Google Scholar]
- 73.Newman PM, Swanson RL, Chong BH. Heparin-induced thrombocytopenia: IgG binding to PF4-heparin complexes in the fluid phase and cross reactivity with low-molecular-weight heparin and heparinoid. Thromb Haemost. 1998;80(2):292–297. [PubMed] [Google Scholar]
- 74.Hanke CA, Barth K, Nakamura L, et al. Lepirudin treatment in a boy with suspected HIT II after surgery because of teratology of Fallot. Hämostaseologie. 2009;29(2):168–170. [PubMed] [Google Scholar]
- 75.Schmitz ML, Massicote P, Faulkner SC, et al. Management of a pediatric patient on the Berlin heart Excor ventricular assist device with argatroban after heparin-induced thrombocytopenia. ASAIO. 2008;54(5):546–547. doi: 10.1097/MAT.0b013e3181873681. [DOI] [PubMed] [Google Scholar]
- 76.Maurer SH, Wilimas JA, Wang WC, et al. Heparin-induced thrombocytopenia and re-thrombosis associated with warfarin and fondaparinux in a child. Pediatr Blood Cancer. 2009;53(3):468–471. doi: 10.1002/pbc.22067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Almond CS, Harrington J, Thiagarajan R, et al. Successful use of bivalirudin for cardiac transplantation in a child with heparin-induced thrombocytopenia. J Heart Lung Transplant. 2006;25(11):1376–1379. doi: 10.1016/j.healun.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 78.Dager WE, Gosselin RC, Yoshikawa R, et al. Lepirudin in heparin-induced thrombocytopenia and extracorporeal membranous oxygenation. Ann Pharmacother. 2004;38(4):598–601. doi: 10.1345/aph.1D436. [DOI] [PubMed] [Google Scholar]
- 79.Iannoli ED, Eaton MP, Shapiro JP. Bidirectional glenn shunt surgery using lepirudin anticoagulation in an infant with heparin-induced thrombocytopenia with thrombosis. Anesth Analg. 2005;101(1):74–76. doi: 10.1213/01.ANE.0000153019.15297.0B. [DOI] [PubMed] [Google Scholar]
- 80.Saxon BR, Black MD, Edgell D, et al. Pediatric heparin-induced thrombocytopenia: management with danaparoid (Orgaran) Ann Thorax Surg. 1999;68:1076–1078. doi: 10.1016/s0003-4975(99)00876-0. [DOI] [PubMed] [Google Scholar]
- 81.John TE, Hallisey RK. Argatroban and lepirudin requirements in a 6-year-old patient with heparin-induced thrombocytopenia after cardiac surgery in a pediatric patient. Pharmacother. 2005;25(10):1383–1388. doi: 10.1592/phco.2005.25.10.1383. [DOI] [PubMed] [Google Scholar]
- 82.Knoderer CA, Knoderer HM, Turrentine MW, et al. Lepirudin anticoagulation for heparin-induced thrombocytopenia after cardiac surgery in a pediatric patient. Pharmacother. 2006;26(5):709–712. doi: 10.1592/phco.26.5.709. [DOI] [PubMed] [Google Scholar]
- 83.Frost J, Murebee L, Russo P, et al. Heparin-induced thrombocytopenia in the pediatric intensive care unit population. Pediatric Crit Care Med. 2005;6(2):216–219. doi: 10.1097/01.PCC.0000154947.46400.17. [DOI] [PubMed] [Google Scholar]
- 84.Warkentin TE, Sheppard J-AI, Moore JC, et al. Laboratory testing for the antibodies that cause heparin-induced thrombocytopenia: how much class do we need? J Lab Clin Med. 2005;146:341–346. doi: 10.1016/j.lab.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 85.Pouplard C, Admiral J, Borg JY, et al. Decision analysis for use of platelet aggregation test, carbon 14-serotonin release assay, and heparin-platelet factor 4 enzyme-linked immunosorbent assay for diagnosis of heparin-induced thrombocytopenia. Am J Clin Pathol. 1999;111:700–706. doi: 10.1093/ajcp/111.5.700. [DOI] [PubMed] [Google Scholar]
- 86.Lo GK, Juhl D, Warkentin TE, et al. Evaluation of pretest clinical score (4 T's) for the diagnosis of heparin-induced thrombocytopenia in two clinical settings. J Thromb Haemost. 2006;4:759–765. doi: 10.1111/j.1538-7836.2006.01787.x. [DOI] [PubMed] [Google Scholar]
- 87.Breinholt JP, Moffett BS, Texter KM, et al. Successful use of bivalirudin for superior vena cava recanalization and stent placement in a child with heparin-induced thrombocytopenia. Pediatric Cardiol. 2008;29(4):804–807. doi: 10.1007/s00246-008-9231-2. [DOI] [PubMed] [Google Scholar]
- 88.Warentin TE, Kelton JG. Temporal aspects of HIT. N Eng J Med. 2001;344:1286–1292. doi: 10.1056/NEJM200104263441704. [DOI] [PubMed] [Google Scholar]
- 89.Fitton A. Heparin-induced thrombocytopenia and the role of lepirudin. Reactions. 2003;933:3. [Google Scholar]
- 90.Tomer A, Masalunga C, Abshire T. Determination of heparin-induced thrombocytopenia: management with danaparoid. Ann Thorac Surg. 1999;61:53–61. [Google Scholar]
- 91.Weigel B, Lasky A, Krishnamurti L, et al. Danaparoid (Orgaran) anticoagulation of pediatric patients with heparin-induced thrombocytopenia (HIT) J Pediatr Hematol Oncol. 1999;21:327a. [Google Scholar]
- 92.Warkentin TE, Kelton JG. Delayed-onset heparin-induced thrombocytopenia and thrombosis. Ann Intern Med. 2001;135:502–506. doi: 10.7326/0003-4819-135-7-200110020-00009. [DOI] [PubMed] [Google Scholar]
- 93.Rice L, Attisha WK, Drexler A, et al. Delayed-onset heparin-induced thrombocytopenia. Ann Intern Med. 2002;136:210–215. doi: 10.7326/0003-4819-136-3-200202050-00009. [DOI] [PubMed] [Google Scholar]
- 94.Warkentin TE, Bernstein RA. Delayed-onset heparin-induced thrombocytopenia and cerebral thrombosis after a single administration of unfractionated heparin [letter] N Engl J Med. 2003;348:1067–1069. doi: 10.1056/NEJM200303133481120. [DOI] [PubMed] [Google Scholar]
- 95.Warkentin TE, Greinacher A, Koster A, et al. Treatment and prevention of heparin-induced thrombocytopenia: American College of Chest Physicians evidence-based clinical practice guidelines (8th edition) Chest. 2008;133(suppl 6):S340–S380. doi: 10.1378/chest.08-0677. [DOI] [PubMed] [Google Scholar]
- 96.Warkentin TE. Clinical picture of HIT. In: Warkentin TE, Greinacher A, editors. Heparin-Induced Thrombocytopenia. 3 ed. New York: Marcel Dekker;; 2004. pp. 53–106. In. eds. [Google Scholar]
- 97.Arepally G, Cines DB. Heparin-induced thrombocytopenia and thrombosis. Clin Rev Allergy Immunol; 1998;16:237–247. doi: 10.1007/BF02737634. [DOI] [PubMed] [Google Scholar]
- 98.King DJ, Kelton JG. Heparin-associated thrombocytopenia. Ann Intern Med. 1984;100:535–540. doi: 10.7326/0003-4819-100-4-535. [DOI] [PubMed] [Google Scholar]
- 99.Fabris F, Luzzatto G, Soni B, et al. Risk factors for thrombosis in patients with immune mediated heparin-induced thrombocytopenia. J Intern Med. 2002;252:149–154. doi: 10.1046/j.1365-2796.2002.01021.x. [DOI] [PubMed] [Google Scholar]
- 100.Rice L. Heparin-induced thrombocytopenia. Myths and misconceptions (that will cause trouble for you and your patients) Arch Intern Med. 2004;164(18):1961–1964. doi: 10.1001/archinte.164.18.1961. [DOI] [PubMed] [Google Scholar]
- 101.Gatti L, Carnelli V, Rusoni R, et al. Heparin-induced thrombocytopenia and warfarin-induced skin necrosis in a child with severe protein C deficiency: successful treatment with dermatan sulfate and protein C concentrate. J Thromb Haemost. 2003;1:1378–1388. doi: 10.1046/j.1538-7836.2003.00057.x. [DOI] [PubMed] [Google Scholar]
- 102.Tullinsky A. Molecular interactions of thrombin. Semin Thromb Hemost. 1996;22:117–124. doi: 10.1055/s-2007-998998. [DOI] [PubMed] [Google Scholar]
- 103.Rydel TJ, Ravichandran KG, Tullinsky A, et al. The structure of a complex of recombinant hirudin and human α-thrombin. Science. 1990;249:277–280. doi: 10.1126/science.2374926. [DOI] [PubMed] [Google Scholar]
- 104.Weitz JI, Hudoba M, Massel D, et al. Clot-bound thrombin is protected from the inhibition of heparin-antithrombin III but is susceptible to inactivation by antithrombin III-independent inhibitors. J Clin Invest. 1990;86:385–391. doi: 10.1172/JCI114723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Weitz JI, Leslie B, Hudoba M. Thrombin binds to soluble fibrin degradation products where it is protected from inhibition by heparin-antithrombin but susceptible to inactivation by antithrombin-independent inhibitors. Circulation. 1998;97:544–552. doi: 10.1161/01.cir.97.6.544. [DOI] [PubMed] [Google Scholar]
- 106.Bates SM, Weitz JI. The mechanism of action of thrombin inhibitors. J Invasive Cardiol. 2000;12(suppl F):27F–32F. [PubMed] [Google Scholar]
- 107.Di Nisio MD, Middledorp S, Buller HR. Direct thrombin inhibitors. N Engl J Med. 2005;353:1028–1038. doi: 10.1056/NEJMra044440. [DOI] [PubMed] [Google Scholar]
- 108.Xiao Z, Theroux P. Platelet activation with unfractionated heparin at therapeutic concentrations and comparisons with a low-molecular-weight heparin and with a direct thrombin inhibitor. Circulation. 1998;97:251–256. doi: 10.1161/01.cir.97.3.251. [DOI] [PubMed] [Google Scholar]
- 109.Sarich TC, Wolzt M, Eriksson UG, et al. Effects of ximelagatran, an oral direct thrombin inhibitor, r-hirudin and enoxaparin on thrombin generation and platelet activation in healthy male subjects. J Am Coll Cardiol. 2003;41:557–564. doi: 10.1016/s0735-1097(02)02868-1. [DOI] [PubMed] [Google Scholar]
- 110.Refludan (lepirudin [rDNA] for injection) prescribing information [package insert] Wayne, NJ: Bayer Healthcare Pharmaceuticals, Inc;; Dec, 2006. [Google Scholar]
- 111.Gupta S, Gupta MM. Heparin-induced thrombocytopenia. J Assoc Physicians India. 2008;56:622–627. [PubMed] [Google Scholar]
- 112.Arepally GM, Ortel TL. Heparin-induced thrombocytopenia. N Engl J Med. 2006;355(8):809–817. doi: 10.1056/NEJMcp052967. [DOI] [PubMed] [Google Scholar]
- 113.Deitcher SR. Clinical utility of subcutaneous hirudins. Am J Health Sys Pharm. 2003;60(Suppl 5):S27–S31. doi: 10.1093/ajhp/60.suppl_5.S27. [DOI] [PubMed] [Google Scholar]
- 114.Deitcher SR, Ngengwe R, Kaplan R, et al. Subcutaneous lepirudin for heparin-induced thrombocytopenia and when other anticoagulants fail: illustrative cases. Clin Adv Hematol Oncol. 2004;2(6):382–384. [PubMed] [Google Scholar]
- 115.Huhle G, Hoffman U, Hoffman I, et al. A new therapeutic option by subcutaneous hirudin in patients with heparin-induced thrombocytopenia type II: a pilot study. Thromb Res. 2000;99(4):325–334. doi: 10.1016/s0049-3848(00)00253-x. [DOI] [PubMed] [Google Scholar]
- 116.Argatroban injection [package insert]. Prescribing Information. Research Triangle Park, NC: GlaxoSmithKline;; May, 2008. [Google Scholar]
- 117.Warkentin TE, Greinacher A, Craven S, et al. Differences in clinical effective molar concentrations of four direct thrombin inhibitors explain their variable prothrombin time prolongation. Thromb Haemost. 2005;94(5):958–964. doi: 10.1160/TH05-03-0154. [DOI] [PubMed] [Google Scholar]
- 118.Brown PM, Hursting MJ, et al. Lack of pharmacokinetic interactions between argatroban and warfarin. Am J Health Sys Pharm. 2002;59(21):2078–2083. doi: 10.1093/ajhp/59.21.2078. [DOI] [PubMed] [Google Scholar]
- 119.Roguin A, Steinberg BA, Watkins SP, et al. Safety of bivalirudin during PCIs in patients with abnormal renal function. Int J Cardiovasc Intervent. 2005;7(2):88–92. doi: 10.1080/14628840510011298. [DOI] [PubMed] [Google Scholar]
- 120.Stewart CF, Hampton EM. Effect of maturation on drug disposition in children. Clin Pharm. 1987;6(7):548–564. [PubMed] [Google Scholar]
- 121.Kearns GL, Abdel-Rahman SM, Lander SW, et al. Drug therapy: developmental pharmacology-drug disposition, action, and therapy in infants and children. N Engl J Med. 2003;349(12):1157–1167. doi: 10.1056/NEJMra035092. [DOI] [PubMed] [Google Scholar]