There is an array of conditions, both inherited and acquired, which may be responsible for thrombocytopenia in the pediatric population. The conditions leading to thrombocytopenia may vary from relatively benign with the only risk being bleeding from defective coagulation to life-threatening conditions such as sepsis and multisystem organ failure that result not only in thrombocytopenia but severe end-organ dysfunction. Of the many medications used in the hospital setting, the potential role of heparin in inducing thrombocytopenia is being recognized more frequently. In a subset of patients exposed to heparin, there may be the development of antibodies that induce thrombocytopenia and, at times, thrombosis. Given the potential for morbidity if unrecognized, heparin-induced thrombocytopenia (HIT) should be considered in the differential diagnosis of thrombocytopenia in the pediatric patient. In this issue of the Journal of Pediatric Pharmacology and Therapeutics, Dr. Vakil et al1 present a comprehensive and informative review of what is recognized as one of the more serious and potentially life-threatening causes of a falling platelet count, HIT. Although originally described in the adult population, with increased awareness of the entity, heightened vigilance, and improved diagnostic laboratory tests, HIT is being recognized with increased frequency in the pediatric population. Timely and comprehensive reviews such as the one presented herein serve to inform and educate all of us involved in the care of pediatric patients.
Heparin is one of the oldest medications in widespread clinical use. Although its discovery in 1916 predates the establishment of the Food and Drug Administration, it did not enter clinical trials until 1935.2 Heparin was originally isolated from canine liver cells, hence its name hepar from the Greek meaning “liver.” Native heparin is a polymer with a molecular weight ranging from 3 to 30 kDa, although the average molecular weight of most commercial heparin preparations varies from 12 to 15 kDa. Heparin is a member of the glycosaminoglycan family of carbohydrates (which includes the closely related molecule heparin sulfate). It is composed of a variably sulfated repeating disaccharide unit. Heparin is a naturally occurring anticoagulant produced by basophils and mast cells. It has been estimated that approximately 12 million individuals or one-third of hospitalized patients have some type of exposure to heparin yearly.
HIT, sometimes referred to as the “white clot syndrome,” is a potentially fatal disorder. Although significant attention has been given to this disorder in the adult population, there have been limited reports in pediatric-aged patients.3–5 Shortly after the introduction of heparin for clinical use in the 1930s, reports of thrombocytopenia began to appear, although its clinical implications were not truly appreciated.6–8 In 1958, two vascular surgeons reported arterial thrombosis in 10 patients receiving heparin.9 This was followed by a report of 11 additional patients in 1964 and the suggestion that the process resulted from an autoimmune (antigen–antibody) interaction.10 This theory was later proven in 1973 by Rhodes et al.11 Diagnostic tests for HIT became available in the 1980s, while the 1990s saw the introduction of several non-heparin agents to provide anticoagulation.12
The exact incidence of HIT is unknown, but studies have suggested that it ranges from as low as 1% to as high as 30%.13–16 HIT can develop after exposure to either unfractionated or low-molecular-weight heparin, although the incidence is lower with the second type. Exposure may be from any method of administration, including a bolus, infusion, intravenous, or subcutaneous route, as a flush solution, or on heparin-impregnated catheters.17–21 In most scenarios, the patient's platelet count falls 5 to 14 days after heparin is first given; however, if circulating anti-heparin antibody (immunoglobulin G [IgG]) from previous heparin exposure is already present, the platelet count may fall immediately on reexposure. The most common symptom of HIT is enlargement or extension of a previously diagnosed thrombus or the development of a new thrombus elsewhere in the body. In the adult population, thrombolytic complications are primarily arterial and tend to involve the extremities, although there are reports of cerebral, myocardial, and mesenteric thrombosis.22–24 Venous thromboses, especially deep venous thrombosis (DVT), and their pulmonary embolic complications are less frequent, although a strong index of suspicion should be maintained when HIT is suspected. Thrombotic complications may occur at multiple anatomic sites and can result in significant morbidity including loss of limb and mortality rates of up to 30% in adult patients.24–26 The location, incidence, and risk of morbidity and mortality from HIT is less well defined in pediatric patients. Makhoul et al27 reported that arterial and venous thrombotic events occurred in previously catheterized vessels in 19 of their 25 patients when anticoagulation was administered for cardiopulmonary bypass and thrombocytopenia was noted postoperatively. The latter condition may suggest a propensity to develop thrombotic complications in vessels with previous endothelial damage.
The mechanism by which heparin causes thrombocytopenia has not been completely elucidated. Heparin antibodies form an antigenic compound by binding to platelet factor 4. Platelet factor 4 is a protein stored in the α-granule of the platelet. The platelet factor 4-heparin complex binds additional heparin antibodies and activates platelets, leading to their clearance from the circulation and subsequently thrombocytopenia.29,30 Although the process generally takes 5 to 14 days, the decline in the platelet count may be more rapid in patients who have been previously exposed to heparin and have already formed antibody.
HIT can be divided into two subgroups, HIT type 1 and HIT type 2. It is unknown whether these are two distinct pathophysiological processes or different manifestations of the same process. Type 1 HIT is a less severe form of the disease. There is a mild and early decrease in platelet count that improves even if heparin administration is continued. Thrombotic complications generally do not occur with HIT type 1. HIT type 2 results in a more severe degree of thrombocytopenia, which does not resolve without cessation of heparin therapy. Thrombotic complications occur with HIT type 2. Current laboratory testing cannot differentiate between the two subgroups of HIT. Given the severity of HIT type 2 and its associated morbidity and mortality, clinicians should assume a diagnosis of HIT type 2.
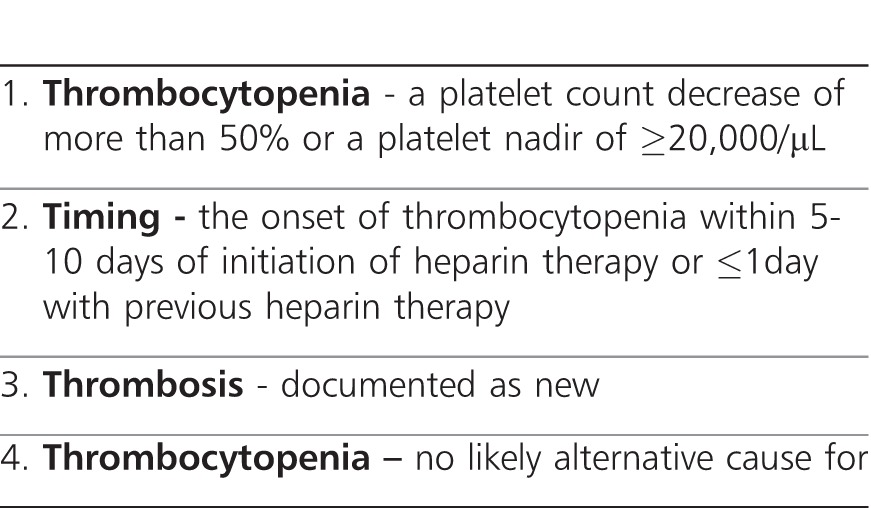
In patients exposed to heparin with a declining platelet count, there are different laboratory tests, which may be used to aid in the diagnosis of HIT. These tests include either identification of the antibody in the patient's serum or demonstration of platelet aggregation and activation when incubated with the patient's serum. An enzyme-linked immunosorbent assay (ELISA) for heparin-platelet factor 4 has been shown to have better sensitivity than the platelet aggregation test. Sensitivity is increased because ELISA can detect antibodies to IgM and IgA (which are typically not detected by the aggregation assays) in addition to the more common IgG antibodies.31,32 However, there is a relatively high false-positive rate as the presence of the antibody does not demonstrate that it actually results in platelet activation. Given this problem, other tests that demonstrate platelet activation have been shown to have a higher specificity, although they may not be routinely available in most institutions. One such test is the heparin-induced platelet aggregation study in which donor platelets are mixed with the patient's plasma. Aggregation of platelets results in a change in the optical density of the solution. The test is considered positive if aggregation results in a change of optical density greater than 20%. Results are reported as “negative,” “weakly positive,” “positive,” or “strongly positive.” The test offers a sensitivity and specificity rate of more than a 90%.33 Other tests assess platelet aggregation by identifying the release of a specific substance from platelets during activation, such as adenosine diphosphate (identified by chemiluminescence) or a 14C-labeled serotonin release assay.31 These tests are considered the “gold standard” but are also more cumbersome and time consuming and are likely available only in research laboratories. Given the issues outlined above with the laboratory diagnosis of HIT, attention has been focused on clinical signs and symptoms.34,35 The authors suggest that patients with a high score may need to be treated with an alternative drug while more sensitive and specific tests for HIT are performed, whereas those patients with a low score can safely continue receiving heparin as the likelihood that they have HIT is extremely low. The 4T scoring system can be found in the Table.
Although HIT is well described in the adult population, there is a paucity of reports of HIT in infants and children. Ranze et al4 reviewed the literature and identified a total of 9 pediatric patients, ranging in age from 3 months to 15 years, who developed HIT. All of these patients developed HIT following unfractionated heparin administration. In 8 of the 9 patients, there were thrombotic complications. The majority of the thrombotic complications included the venous system. Five of the 6 venous thromboses affected the inferior vena cava or the lower extremity venous system. Four patients had arterial involvement, including 2 patients with evidence of central nervous system involvement (hemiparesis, amaurosis fugax), an intracardiac thrombus in 1 patient, and thrombosis of the femoral/tibial artery in another. Morbidity was noted in 3 patients, including residual hemiparesis, gangrene of the left forefoot that required amputation, and the need for mitral valve replacement.
Spadone et al5 evaluated 34 neonates with a mean gestational age of 29 weeks for HIT during their neonatal intensive care unit courses. None of the infants had received therapeutic anticoagulation, as heparin was administered only to maintain patency of invasive vascular catheters. Screening for HIT was initiated because of a platelet count of <70,000/μL (n=23), a precipitous 30% to 50% decrease of the platelet count (n=5), or an unexplained thrombotic event (n=6). HIT was diagnosed in 14 newborns in the cohort of 34. This finding resulted in an overall incidence of 1.5% of neonates exposed to heparin during the study period. Aortic thrombosis was diagnosed by ultrasonography in 11 of the 14 patients (85%) compared with 5 of the 20 (25%) neonates with negative laboratory test results for HIT. Overall mortality in the neonates with HIT was 21% vs. 35% (p=not significant) in neonates with thrombocytopenia of other causes.
Of primary importance when dealing with either the adult or pediatric patient with HIT is immediate cessation of heparin administration via any route. There are several alternative anticoagulants for patients with HIT who require ongoing anticoagulation. Warfarin therapy is generally not suggested as there has been an association between warfarin and an increased risk of microvascular thrombosis during HIT.32 If warfarin is used, treatment should be delayed until the platelet count is at least 150,000/μL. Although cross-reactivity occurs with low-molecular-weight heparin in 80% to 90% of cases, these medications have been safely used provided that in vitro testing fails to demonstrate platelet aggregation.25 A lower incidence of cross-reactivity (10%-20%) is seen with danaparoid, a heparinoid with factor Xa-inhibiting properties.36 Other non-heparin anticoagulants include the glycoprotein (GP) IIb/IIIa inhibitors, the direct thrombin inhibitors (hirudin, lepirudin, bivalirudin, and argatroban), and the defibrinogenating enzyme (ancrod).3,37–49 The synthetic direct thrombin inhibitor argatroban is not immunogenic, as opposed to danaparoid sodium and lepirudin, which have some immunogenicity. Argatroban is approved in the United States for treatment of patients with HIT. It is given intravenously as a continuous infusion with a half-life of elimination of about 45 minutes. The dose of argatroban does not need to be adjusted in the presence of renal insufficiency; however, it does need to be lowered in case of hepatic injury or insufficiency. The therapeutic effect of argatroban can be followed by serial activated partial thromboplastin time examinations.
Following identification and treatment of an episode of HIT, education of the patient and family is critical to avoiding reexposure. Heparin must be clearly flagged as a medication to be avoided in the patient's chart. Medical alert bracelets may aid in preventing future exposure to heparin. Education of healthcare professionals is also crucial to successful prevention of reexposure. Healthcare professionals must be educated to check for contraindications to heparin. Careful attention must be paid to avoid heparin-containing flushes and heparin-impregnated catheters as even a small exposure to heparin can trigger another acute thrombotic event.
In summary, HIT should be considered in any patient presenting with thrombocytopenia. Although HIT is diagnosed most commonly in the adult cardiac surgical patient, even minor exposures to heparin via flush solutions for arterial and venous cannulae can precipitate HIT. Diagnosis is based on clinical findings in association with available laboratory testing. With increased awareness and vigilance, HIT is now being diagnosed in pediatric patients of all ages. Given the risk of significant morbidity related to arterial and venous thrombolytic disease, prompt diagnosis and cessation of heparin therapy are necessary.
ABBREVIATIONS
- DVT
deep venous thrombosis
- ELISA
enzyme-linked immunosorbent assay
- HIT
heparin-induced thrombocytopenia
- kDa
kiloDaltons
See related article on page 12
DISCLOSURE The authors declare no conflicts or financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employment, gifts, and honoraria.
Table.
Criteria Included in the 4Ts Scoring System for Heparin-Induced Thrombocytopenia
REFERENCES
- 1.Vakil NH, Kanaan AO, Pesaturo KA, Donovan JL. Treatment of heparin-induced thrombocytopenia in the pediatric population: a review of current literature. J Pediatr Pharm Ther. 2012;17(1):12–30. doi: 10.5863/1551-6776-17.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Linhardt RJ. Heparin: an important drug enters its seventh decade. Chem Indust. 1991;2(1):45–50. [Google Scholar]
- 3.Dyke PC, II, Russo P, Mureebe L, et al. Argatroban for anticoagulation during cardiopulmonary bypass in an infant. Paediatr Anaesth. 2005;15(4):328–333. doi: 10.1111/j.1460-9592.2005.01417.x. [DOI] [PubMed] [Google Scholar]
- 4.Ranze O, Ranze P, Magnani HN, et al. Heparin-induced thrombocytopenia in paediatric patients: a review of the literature and a new case treated with danaparoid sodium. Eur J Pediatr. 1999;158(suppl 3):S130–S133. doi: 10.1007/pl00014338. [DOI] [PubMed] [Google Scholar]
- 5.Spadone D, Clark F, James E, et al. Heparin-induced thrombocytopenia in the newborn. J Vasc Surg. 1992;15(2):306–312. doi: 10.1067/mva.1992.33807. [DOI] [PubMed] [Google Scholar]
- 6.Murdoch IA, Beattie RM, Silver DM. Heparin-induced thrombocytopenia in children. Acta Paediatr. 1993;82(5):495–497. doi: 10.1111/j.1651-2227.1993.tb12732.x. [DOI] [PubMed] [Google Scholar]
- 7.Best CH. Preparation of heparin and its use in the first clinical cases. Circulation. 1959;19(1):79–86. doi: 10.1161/01.cir.19.1.79. [DOI] [PubMed] [Google Scholar]
- 8.Gollub S, Ulin AW. Heparin-induced thrombocytopenia in man. J Lab Clin Med. 1962;59(3):430–435. [PubMed] [Google Scholar]
- 9.Weismann RE, Tobin RW. Arterial embolism occurring during systemic heparin therapy. Arch Surg. 1958;76(2):219–227. doi: 10.1001/archsurg.1958.01280200041005. [DOI] [PubMed] [Google Scholar]
- 10.Roberts B, Rosato FE, Rosato EF. Heparin: a cause of arterial emboli? Surgery. 1964;55(6):803–808. [PubMed] [Google Scholar]
- 11.Rhodes GR, Dixon RH, Silver D. Heparin induced thrombocytopenia with thrombotic and hemorrhagic manifestations. Surg Gynecol Obstet. 1973;136(3):409–416. [PubMed] [Google Scholar]
- 12.Kelton JG, Warkentin TE. Heparin-induced thrombocytopenia: a historical perspective. Blood. 2008;112(7):2607–2616. doi: 10.1182/blood-2008-02-078014. [DOI] [PubMed] [Google Scholar]
- 13.Eika C, Godal HC, Laake K, et al. Low incidence of thrombocytopenia during treatment with hog mucosa and beef lung heparin. Scand J Hematol. 1980;25(1):19–24. doi: 10.1111/j.1600-0609.1981.tb01359.x. [DOI] [PubMed] [Google Scholar]
- 14.Bell WR, Tomasul PA, Alving BM, et al. Thrombocytopenia occurring during the administration of heparin. Ann Intern Med. 1976;85(2):155–160. doi: 10.7326/0003-4819-85-2-155. [DOI] [PubMed] [Google Scholar]
- 15.Bell WR, Royall RM. Heparin-associated thrombocytopenia: a comparison of three heparin preparations. N Engl J Med. 1980;303(16):902–907. doi: 10.1056/NEJM198010163031602. [DOI] [PubMed] [Google Scholar]
- 16.Powers PJ, Kelton JG, Carter CJ. Studies on the frequency of heparin-associated thrombocytopenia. Thromb Res. 1984;33(4):439–443. doi: 10.1016/0049-3848(84)90083-5. [DOI] [PubMed] [Google Scholar]
- 17.Johnson RA, Lazarus KH, Henry DH. Heparin-induced thrombocytopenia: a prospective study. Am J Hematol. 1984;17(4):349–353. doi: 10.1002/ajh.2830170404. [DOI] [PubMed] [Google Scholar]
- 18.Hrushesky WJ. Subcutaneous heparin-induced thrombocytopenia. Arch Intern Med. 1978;138(10):1489–1491. [PubMed] [Google Scholar]
- 19.Galle PC, Muss HB, McGrath K, et al. Thrombocytopenia in two patients treated with low-dose heparin. Obstet Gynecol. 1978;52(suppl 1):9S–11S. [PubMed] [Google Scholar]
- 20.Heeger PS, Backstrom JT. Heparin flushes and thrombocytopenia. Ann Intern Med. 1986;105(1):143–146. doi: 10.7326/0003-4819-105-1-143_1. [DOI] [PubMed] [Google Scholar]
- 21.Laster J, Silver DS. Heparin-coated catheters and heparin-induced thrombocytopenia. J Vasc Surg. 1988;7(5):667–672. doi: 10.1067/mva.1988.avs0070667. [DOI] [PubMed] [Google Scholar]
- 22.Baird RA, Convery FR. Arterial thromboembolism in patients receiving systemic heparin therapy: a complication associated with heparin-induced thrombocytopenia. J Bone Joint Surg. 1977;59(8):1061–1064. [PubMed] [Google Scholar]
- 23.Becker PS, Miller VT. Heparin-induced thrombocytopenia. Stroke. 1989;20(11):1449–1459. doi: 10.1161/01.str.20.11.1449. [DOI] [PubMed] [Google Scholar]
- 24.Kappa JR, Fisher CA, Berkowitz HD, et al. Heparin-induced platelet activation in sixteen surgical patients: Diagnosis and management. J Vasc Surg. 1987;5(1):101–109. doi: 10.1067/mva.1987.avs0050101. [DOI] [PubMed] [Google Scholar]
- 25.Warkentin TE, Kelton JG. Heparin-induced thrombocytopenia. Annu Rev Med. 1989;40(1):31–44. doi: 10.1146/annurev.me.40.020189.000335. [DOI] [PubMed] [Google Scholar]
- 26.Silver D, Kapsch DN, Tsoi EK. Heparin induced thrombocytopenia, thrombosis and hemorrhage. Ann Surg. 1983;198(3):301–306. doi: 10.1097/00000658-198309000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Makhoul RG, Greenberg CS, McCann RL. Heparin-associated thrombocytopenia and thrombosis: a serious clinical problem and potential solution. J Vasc Surg. 1986;4(5):522–528. doi: 10.1067/mva.1986.avs0040522. [DOI] [PubMed] [Google Scholar]
- 28.Amiral J, Bridey F, Dreyfus M, et al. Platelet factor 4 complexed to heparin is the target for antibodies generated in heparin-induced thrombocytopenia. Thromb Haemost. 1992;68(1):95–96. [PubMed] [Google Scholar]
- 29.Warkentin TE, Kelton JG. Heparin and platelets. Hematol Oncol Clin North Am. 1990;4(1):243–264. [PubMed] [Google Scholar]
- 30.Greinacher A, Potzsch B, Amiral J, et al. Heparin-associated thrombocytopenia: isolation of the antibody and characterization of a multimolecular PF4-heparin complex as the major antigen. Thromb Haemost. 1994;71(2):247–251. [PubMed] [Google Scholar]
- 31.Sheridan D, Carter C, Kelton JG. A diagnostic test for heparin-induced thrombocytopenia. Blood. 1986;67(1):27–30. [PubMed] [Google Scholar]
- 32.Warkentin TE. Heparin-induced thrombocytopenia: diagnosis and management. Circulation. 2004;110(18):e454–e458. doi: 10.1161/01.CIR.0000147537.72829.1B. [DOI] [PubMed] [Google Scholar]
- 33.Warkentin TE. Heparin-induced thrombocytopenia: pathogenesis, frequency, avoidance and management. Drug Safety. 1997;17(5):325–341. doi: 10.2165/00002018-199717050-00005. [DOI] [PubMed] [Google Scholar]
- 34.Lo GK, Juhl D, Warkentin TE, et al. Evaluation of pretest clinical score (4T's) for the diagnosis of heparin-induced thrombocytopenia in two clinical settings. J Thromb Haemost. 2006;4(4):759–765. doi: 10.1111/j.1538-7836.2006.01787.x. [DOI] [PubMed] [Google Scholar]
- 35.Demma LJ, Winkler AM, Levy JH. A diagnosis of heparin-induced thrombocytopenia with combined clinical and laboratory methods in cardiothoracic surgery intensive care unit patients. Anesth Analg. 2011;113(4):697–702. doi: 10.1213/ANE.0b013e3182297031. [DOI] [PubMed] [Google Scholar]
- 36.Magnani HN. Heparin-induced thrombocytopenia (HIT): an overview of 230 patients treated with Orgaran (Org 10172) Thromb Haemost. 1993;70(4):554–561. [PubMed] [Google Scholar]
- 37.Lubenow N, Greinacher A. Hirudin in heparin-induced thrombocytopenia. Semin Thromb Hemost. 2002;28(5):431–438. doi: 10.1055/s-2002-35283. [DOI] [PubMed] [Google Scholar]
- 38.Vasquez JC, Vichiendilokkul A, Mahmood S, et al. Anticoagulation with bivalirudin during cardiopulmonary bypass in cardiac surgery. Ann Thorac Surg. 2002;74(6):2177–2179. doi: 10.1016/s0003-4975(02)04125-5. [DOI] [PubMed] [Google Scholar]
- 39.Davis Z, Anderson R, Short D, et al. Favorable outcome with bivalirudin anticoagulation during cardiopulmonary bypass. Ann Thorac Surg. 2003;75(1):264–265. doi: 10.1016/s0003-4975(02)04299-6. [DOI] [PubMed] [Google Scholar]
- 40.Latham P, Revelis AF, Joshi GP, et al. Use of recombinant hirudin in patients with heparin-induced thrombocytopenia with thrombosis requiring cardiopulmonary bypass. Anesthesiology. 2000;92(1):263–266. doi: 10.1097/00000542-200001000-00040. [DOI] [PubMed] [Google Scholar]
- 41.Koster A, Kuppe H, Crystal GJ, et al. Cardiovascular surgery without cardiopulmonary bypass in patients with heparin-induced thrombocytopenia type II using anticoagulation with recombinant hirudin. Anesth Analg. 2000;90(2):292–298. doi: 10.1097/00000539-200002000-00010. [DOI] [PubMed] [Google Scholar]
- 42.Koster A, Kuppe H, Hetzer R, et al. Emergent cardiopulmonary bypass in five patients with heparin-induced thrombocytopenia type II employing recombinant hirudin. Anesthesiology. 1998;89(3):777–780. doi: 10.1097/00000542-199809000-00029. [DOI] [PubMed] [Google Scholar]
- 43.Nuttall GA, WC Oliver, Santrach PJ, et al. Patients with a history of type II heparin-induced thrombocytopenia with thrombosis requiring cardiac surgery with cardiopulmonary bypass: A prospective observational case series. Anesth Analg. 2003;96(2):344–350. doi: 10.1097/00000539-200302000-00009. [DOI] [PubMed] [Google Scholar]
- 44.Gillis S, Merin G, Zahger D, et al. Danaparoid for cardiopulmonary bypass in patients with previous heparin-induced thrombocytopenia. Br J Haem. 1997;98(3):657–659. doi: 10.1046/j.1365-2141.1997.2633080.x. [DOI] [PubMed] [Google Scholar]
- 45.Saxon BR, Black MD, Edgell D, et al. Pediatric heparin-induced thrombocytopenia: management with danaparoid (Orgaran) Ann Thorac Surg. 1999;68(3):1076–1078. doi: 10.1016/s0003-4975(99)00876-0. [DOI] [PubMed] [Google Scholar]
- 46.Olin DA, Urdaneta F, Lobato E. Use of danaparoid during cardiopulmonary bypass in patients with heparin-induced thrombocytopenia. J Cardio Vasc Anesth. 2000;14(3):707–709. doi: 10.1053/jcan.2000.18531. [DOI] [PubMed] [Google Scholar]
- 47.Kanagasabay RR, Unsworth-White MJ, Robinson G, et al. Cardiopulmonary bypass with danaparoid sodium and ancrod in heparin-induced thrombocytopenia. Ann Thorac Surg. 1998;66(6):567–569. doi: 10.1016/s0003-4975(98)00511-6. [DOI] [PubMed] [Google Scholar]
- 48.Spiess BD, Gernsheimer T, Vocelka C, et al. Hematologic changes in a patient with heparin-induced thrombocytopenia who underwent cardiopulmonary bypass after ancrod defibrinogenation. J Cardiothorac Vasc Anesth. 1996;10(7):918–921. doi: 10.1016/s1053-0770(96)80057-7. [DOI] [PubMed] [Google Scholar]
- 49.Smith RE, Townsend GE, Berry BR, et al. Enoxaparin for unstable angina and ancrod for cardiac surgery following heparin allergy. Ann Pharmacother. 1996;30(5):476–480. doi: 10.1177/106002809603000508. [DOI] [PubMed] [Google Scholar]


