Abstract
The prevalence of childhood and adolescent obesity continues to rise in the United States (US). Immediate health consequences are being observed, and long-term risks are mounting within the pediatric population, secondary to obesity. The hallmark of prevention and treatment of obesity in children and adolescents includes lifestyle modification (i.e., dietary modification, increased physical activity, and behavioral modifications). However, when intensive lifestyle modification is insufficient to reach weight loss goals, adjunctive pharmacotherapy is recommended. Among the group of weight-loss medications, orlistat is the only US Food and Drug Administration (FDA)-approved prescription drug for the treatment of overweight and obese adolescents. Other medications, including metformin, need larger studies to establish their role in treatment. No single approach to management of pediatric obesity is the answer, given the complexity of the disorder and the many reasons for failure. Evidence of weight loss medications in addition to lifestyle modification supports short-term efficacy for treatment of obese children and adolescents, although long-term results remain unclear.
INDEX TERMS: adolescents, children, obese, obesity, overweight
INTRODUCTION
Overweight and obese children and adolescents continue to be a public health concern in the United States (US). Being overweight in US children 2 to 19 years of age is defined as a body mass index (BMI) between the 85th and 95th percentile, while obesity is defined as a BMI at or above the 95th percentile for children of the same age and sex.1 For adults (i.e., >19 years of age), overweight is defined as BMI of 25 to 29.9 kg/m2 and obesity as BMI >30 kg/m2.1 Since 1980, the prevalence of obesity has tripled among school-aged children and adolescents.2 A recent study from 2007 to 2008 reported 16.9% of children and adolescents aged 2 through 19 years were at or above the 95th percentile of the BMI-for-age growth charts, and almost 32% of subjects were at or above the 85th percentile of BMI for age.2
Immediate health consequences of childhood obesity may include social discrimination, low self-esteem, and delayed academic and social functioning.1 Additionally, obese children and adolescents have been found to have risk factors for cardiovascular disease (CVD), including hyperlipidemia, hypertension, and abnormal glucose tolerance. Among 5- to 17-year-old children in a population-based sample, 70% of subjects had at least 1 CVD risk factor, while 39% of subjects had 2 or more CVD risk factors.1 Asthma, hepatic steatosis, sleep apnea, and type 2 diabetes have also been associated with increased weight in children.3 Long-term risks include adult obesity, ischemic stroke, joint disease, cancer, coronary heart disease, and many chronic conditions mentioned above.3,4
Treatment of pediatric obesity is imperative to the overall health and wellness of children and adolescents, in response to which the Endocrine Society, American Academy of Pediatrics (AAP), US Department of Health and Human Services, and Office of the US Surgeon General have published guiding materials.4–6 Support for lifestyle modification (i.e., dietary and behavioral modification and physical activity) for both children and their families is essential for healthy living and a prerequisite for all overweight and obesity treatments. Dietary recommendations include avoidance or reduction of calorie-dense, nutrient-poor foods (i.e., “fast food”) as well as sugar-sweetened beverages, sports drinks, and fruit drinks and juices.7,8 Evidence is growing, especially in regard to sugar-sweetened beverages, that suggests an association with weight gain in children and adolescents. One study observed that for every additional serving of sugar-sweetened drink consumed daily, the odds of becoming obese in childhood increases by 60%.1,9 Sixty minutes of daily moderate to vigorous physical activity is also recommended, and the aim is to find activities that children and their families can do together consistently.4,10 Currently, older children are noted to spend less time engaged in physical activity, specifically during school hours. Daily participation in physical education in schools has dropped from 42% in 1991 to 33% in 2009 among adolescents.11 To augment physical activity, decreasing the time spent in sedentary activities such as watching television and digital versatile disc (DVD)/motion pictures, playing video games, or using computers for recreation should be encouraged.4,12 Reports from the AAP state the average child watches 3 hours of television per day, and, combined with other media, the average time spent is over 6½ hours.12 Additional recommendations for parental modeling of healthy habits are key components to a child's success.4,10 Support for breastfeeding and education of proper nutrition and physical activity for parents is important in addition to the creation of childcare, school, and community programs that implement healthy habits.4,6,13,14
Despite intensive lifestyle modifications and support for healthy practices within children's environment, some children will continue to struggle with extreme excess weight and associated comorbidities.4,15 Because intensive lifestyle modification alone has failed for obese children and adolescents, practice guidelines from the Endocrine Society recommend that a combination of pharmacotherapy and lifestyle modification be considered.4 Overweight children should not be treated with medications unless significant, severe comorbidities persist despite lifestyle modification. The use of pharmacotherapy should also be considered in overweight children with a strong family history of type 2 diabetes or cardiovascular risk factors. Currently, the only FDA-approved prescription drug indicated for the treatment of pediatric obesity is orlistat (Xenical; Genentech USA, Inc., South San Francisco, CA).16 However, metformin and several other drugs have been observed to show promise in their treatment of overweight and obese adolescents. This article reviews the effectiveness and safety of the medications currently being used to treat childhood and adolescent obesity.
METHODS
A comprehensive literature search was performed using Medline (1950-May 2011) and Cochrane Database of Systematic Reviews (2000-May 2011). Search terms included child, adolescent(s), obese, obesity, overweight, treatment, orlistat, sibutramine, metformin, growth hormone, octreotide, and topiramate. To maximize the literature evaluated, all studies published in English were considered for review without regard for study design. Studies were included if they assessed the effects of pharmacological treatment in overweight and/or obese children and adolescents. All data are given as means ± SD, unless otherwise noted.
PHARMACOLOGICAL TREATMENT
Orlistat
Approved for use in children >12 years by the FDA in 2003, orlistat (Genentech USA), 120 mg three times daily, is a reversible gastric and pancreatic lipase inhibitor that limits the gastrointestinal absorption of dietary cholesterols by approximately 30%.16 The resulting decrease in caloric intake is proposed to alter energy balance, thereby, causing a beneficial effect on weight management.16–18 Because this agent causes a reduction in plasma fat-soluble vitamin levels it may also complicate adolescent growth and development; thus, concomitant administration of a daily multivitamin is recommended.16,19,20 Orlistat is currently approved for over-the-counter use (60 mg three times daily; Alli; GlaxoSmithKline, Brentford, Middlesex, UK) and by prescription (120 mg three daily). A number of trials have evaluated the use of orlistat in children and adolescents (Table 1).21–26
Table 1.
Clinical Trials of Orlistat
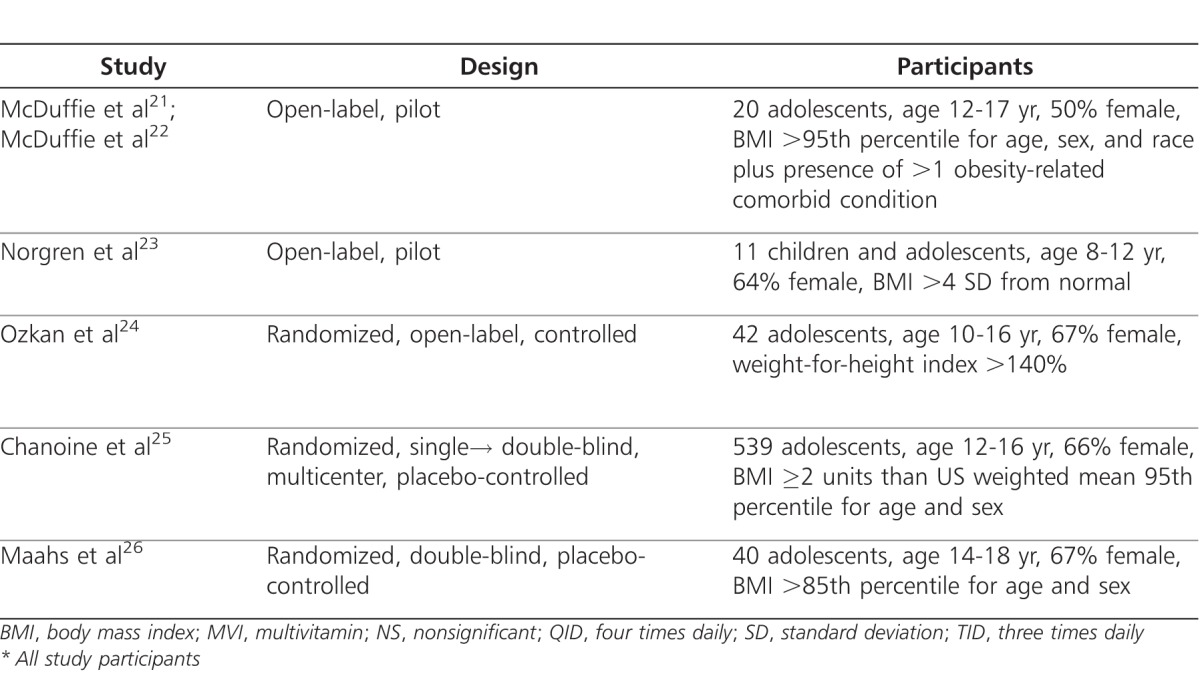
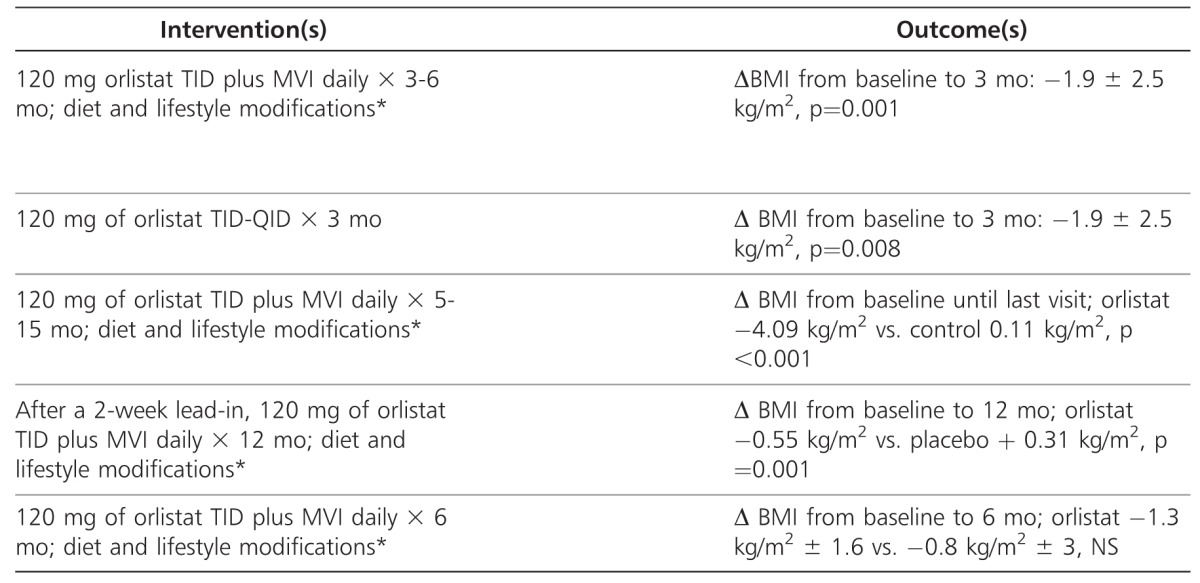
The first US study involving orlistat was a safety and efficacy trial in 20 obese adolescents (mean age, 14.6 ± 2.0 years; mean baseline BMI, 44.1 ± 12.6 kg/m2).21 As an adjunct to a comprehensive behavioral program, patients received orlistat and a daily multivitamin for 3 and 6 months. All patients had one of the following obesity-related comorbidities: hypertension, type 2 diabetes or glucose intolerance, hyperinsulinemia, hyperlipidemia, hepatic steatosis, or documented sleep apnea. Significant decreases in weight (−4.4 ± 4.6 kg; p<0.001) and BMI (−1.9 ± 2.5 kg/m2; p<0.002) were demonstrated after 3 months of orlistat therapy. Significant improvement in plasma lipids, including total cholesterol (p<0.001), low-density lipoprotein (LDL) cholesterol (p<0.0001), and fasting glucose concentration (p<0.003) were also reported. Furthermore, the data suggested that Caucasians may experience a significantly greater weight loss (−7.86 vs. +0.36 kg, p<0.05) and improvement in plasma lipids than African Americans.21,22
A second study evaluated orlistat therapy in 11 severely obese, prepubertal Swedish children (median age, 10.7 years; range, 8.3-12.3 years).23 Although the primary aim of that study was to determine safety, compliance, and psychological well-being of orlistat treatment, a median weight loss of 4 kg was also reported (range, −12.7 kg to +2.5 kg, p=0.016). Although the authors permitted orlistat to be administered up to four times daily with meals, doses above the approved 120 mg three times daily have not been shown to provide additional benefit.21 Overall, compliance was >98%, and favorable effects on psychological well-being were noted. No changes in serum cholesterol or triglycerides values occurred during treatment.23
A prospective, open-label, pilot trial investigated the efficacy and tolerability of orlistat in 42 obese, otherwise healthy, Turkish adolescents.24 In addition to nutritional and lifestyle modification programs, 22 patients were randomized to receive orlistat (median age, 12.9 ± 2.4 years; baseline BMI, 32.5 kg/m2) and a multivitamin, while 20 patients were assigned to the control group (median age, 12.5 ± 2.2 years; baseline BMI, 31.2 kg/m2). Fifteen patients who remained in each group after 1 month of treatment were followed for an average of 1 year (range, 5-15 months). The orlistat group (−6.27 ± 5.4 kg) lost significantly more weight than the control group (+4.16 ± 6.45 kg).
Presently, the largest published trial of orlistat use in adolescents consists of a multicenter, placebo-controlled safety and efficacy study at 32 centers in the United States and Canada.25 In conjunction with diet, behavioral, and lifestyle modifications, patients were randomized to receive orlistat (n=357) or placebo (n=182) three times daily for 1 year. All participants were maintained on a nutritionally balanced, hypocaloric diet designed to produce an initial weight loss of up to 1 kg per week. Both groups experienced a mean decrease in BMI at week 12. However, at the end of the study, the mean BMI was lower by 0.55 kg/m2 in the orlistat group but higher in the placebo group by 0.31 kg/m2 (p=0.001). No significant changes were demonstrated in serum cholesterol levels (i.e., total cholesterol, LDL, high-density lipoprotein [HDL], and LDL-to-HDL ratio) and markers of insulin sensitivity (i.e., insulin, glucose).
Most recently, a randomized, double-blind trial compared the effects of 6 months of orlistat treatment or placebo on BMI in 40 adolescents with a BMI in the >85th percentile for age/sex was conducted.26 In that evaluation, both groups experienced a significant change in BMI from baseline to 6 months (orlistat, −1.3 ± 1.6 kg/m2, p=0.04; placebo, −0.8 ± 3.0 kg/m2, p=0.02) likely due to the dietary and exercise counseling that all patients received. However, there was no statistically significant difference between the two groups. Additionally, no significant differences were noted in serum lipids, glucose, or levels of vitamins A and E related to orlistat administration.
Due to its minimal systemic absorption, orlistat provides a relatively favorable side effect profile compared to many other appetite-suppressant drugs.27,28 Gastrointestinal complaints were most common and documented in up to 100% of patients in the aforementioned pediatric evaluations.21–26 Specifically, increased stool frequency, fatty/oily stools, and oily spotting on clothes were most frequently reported.21–23,25,26 Such side effects were usually mild to moderate and generally transient; nearly half of all gastrointestinal complaints resolved in <1 week, and the majority resolve by 1 month of treatment.16.22,26 Some data also suggest that these gastrointestinal events may be minimized by a reduction in dietary fat intake and/or use of supplemental psyllium mucilloid or dietary fibers.29
Although orlistat has been studied in a relatively small number of pediatric patients, a regimen of 120 mg three times daily in combination with dietary and lifestyle modifications has resulted in a significant decrease in BMI from baseline ranging from 0.5 to 4.09 kg/m2 in trials. Despite the frequent nature of gastrointestinal complaints, study completion rates ranged from 65% to 100% with reported medication adherence rates between 73% and 98%; this variance is likely the result of differences in study design and patient populations between authors. Notably, two reports were randomized, blinded studies including the largest evaluation in over 500 adolescents.25,26 However, true blinding may have been difficult to accomplish given the higher incidence of orlistat-associated gastrointestinal complaints.25,26 At present, data supporting extended orlistat use is lacking, with the evaluations ranging from 3 to 15 months.21–26 More data are needed to clarify differences in weight-loss and improvement in lipids and cholesterol profiles between the aforementioned reports. Combined data from pediatric trials suggest that orlistat may be a safe and effective adjunct to dietary and behavioral modifications in the treatment of obesity for patients >8 years old. Close provider follow-up is warranted until additional longer-term studies confirm the safety of orlistat in this population.21,22
Metformin
Metformin (Glucophage; Bristol-Myers Squibb Company, Princeton, NJ) is a biguanide derivative used for the treatment of type 2 diabetes mellitus in adults and children at least 10 years of age.30 Excess body fat is associated with insulin resistance and dysglycemia and may predict the development of type 2 diabetes and/or metabolic syndrome in children.31–33 Metformin activates adenosine monophosphate-activated protein kinase to reduce hepatic glucose production, decrease intestinal glucose absorption, and increase insulin sensitivity by way of improved peripheral glucose uptake and utilization.30 Additionally, metformin inhibits fat cell lipogenesis and may reduce food intake by increasing a glucogen-like peptide.34 Although metformin is not FDA-approved for treating pediatric obesity, it has been evaluated in several small clinical trials for weight reduction in children and adolescents who are obese, obese with hyperinsulinemia, or who have gained excessive weight secondary to treatment with an atypical antipsychotic agent. Table 2 summarizes the use of metformin for the treatment of obesity in children and adolescents.35–48
Table 2.
Clinical Trials of Metformin
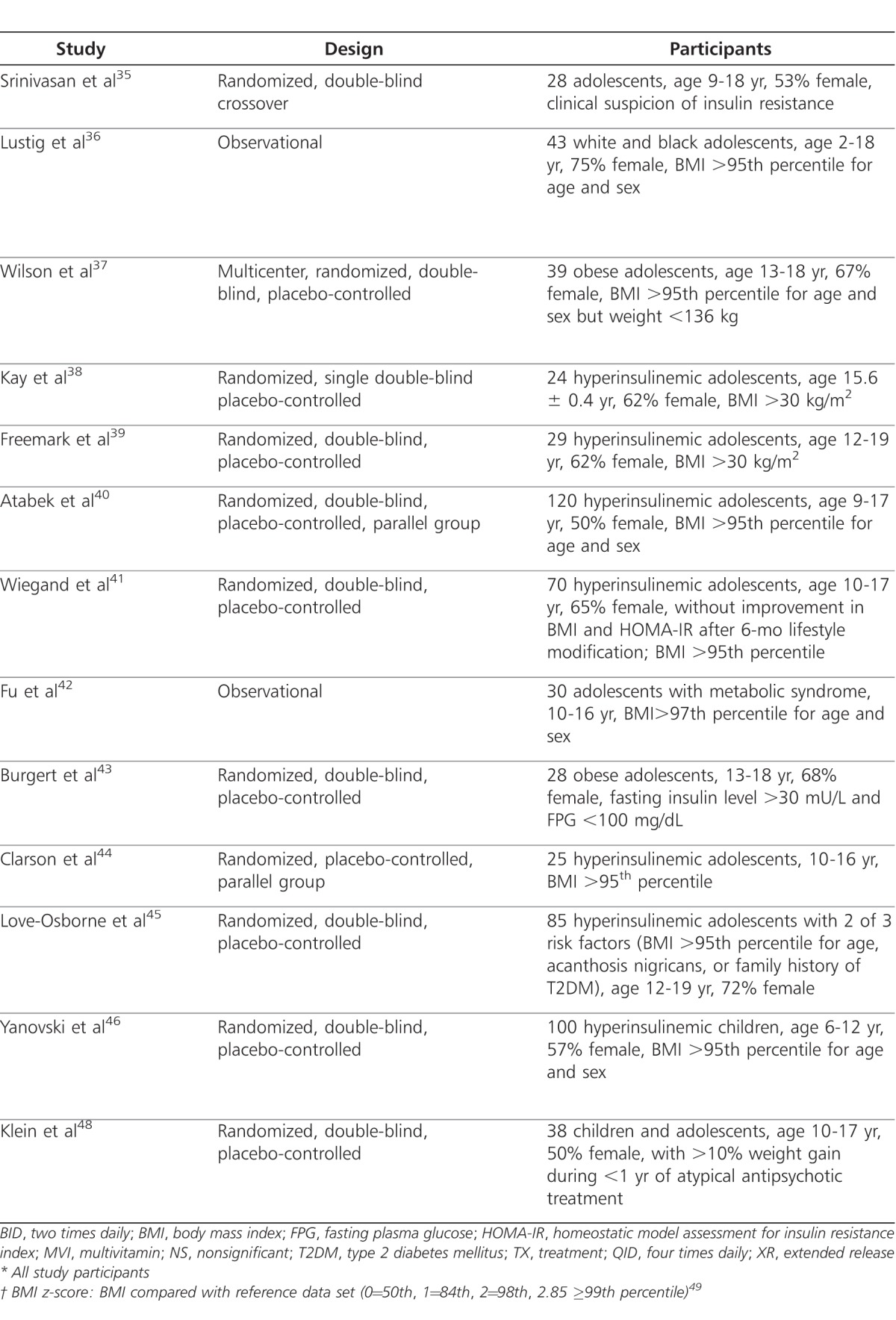

In 2006, a crossover trial was conducted to evaluate the effect on body composition and insulin sensitivity in 28 children and adolescents (mean age, 12.5 ± 2.2 years) who were referred to an endocrine clinic for obesity (baseline BMI, 35.2 ± 5.1 kg/m2) and clinical suspicion of insulin resistance.35 Of the patients included in the trial, 89% had a family history of metabolic syndrome as well as acanthosis nigricans. Patients received metformin or placebo for 6 months, each with a 2-week washout period between medications. No diet or lifestyle modifications were implemented. Metformin had a greater effect than placebo on weight (−4.35 kg, p=0.02), BMI (−1.3 kg/m2, p=0.002), waist circumference (−2.8 cm, p=0.003), and subcutaneous abdominal adipose tissue (−52.5 cm2, p=0.002). However, no significant benefits for insulin sensitivity were noted, although a beneficial treatment effect was observed for fasting insulin (−2.2 mU/L, p=0.011) and fasting glucose (−0.2mmol/L, p=0.048).
Another trial included 43 obese children and adolescents (mean age, 12.5 ± 3.6 years) who continued to have increasing BMI despite at least 3 months of outpatient exercise and nutritional counseling.36 Patients who received metformin plus a multivitamin for up to 16 months were studied. Efficacy was assessed based on changes in BMI. Patients were stratified based on race and their pretreatment insulin sensitivity, which was determined by the use of oral glucose tolerance testing. The study concluded that metformin was effective in promoting a decrease in BMI and BMI z-scores, provided the patient was insulin resistant and Caucasian (−2.7 kg/m2 at 4-months, p<0.001, and −1.6 kg/m2 at 12-months, p=0.32). African-American patients did not respond to metformin and had increases in BMI at both 4-month and 12-month follow-up visits (+0.5 kg/m2, p=0.57; and +4.6 kg/m2, p=0.53, respectively). This trial had a higher percentage of African-American patients (i.e., 38%) than similar studies,37 which may have affected the outcome of BMI z-scores. Thus, the study chose to report results by ethnicity and not for the entire study population.
A long-term trial evaluated the effects on BMI in 39 obese, euglycemic adolescents with a mean age of 14.8 ± 1.3 years).37 Patients were randomized 1:1 to 48 weeks of treatment with metformin extended-release (XR) (Glucophage XR; Bristol-Myers Squibb Company, Princeton, NJ) or placebo, followed by an additional 48 weeks of monitoring. At the end of treatment, the metformin XR group had significantly decreased BMI compared to increased BMI in the placebo group (p = 0.03). This difference in BMI persisted for 12 to 24 weeks after the treatment period ended. Secondary outcomes included body composition, abdominal fat, and insulin resistance index; all were found to be not statistically significant.
Additional trials have investigated the effects of metformin with daily dosages of 1 to 1.7 g per day for obese children and adolescents with hyperinsulinemia who may be at increased risk for obesity-related comorbidities.1 Morbidly obese, hyperinsulinemic adolescents (mean weight 116 ± 5.1 kg) were evaluated for the weight, lipid and insulin sensitivity effects of metformin.38 Twenty-four patients were placed on a low-calorie diet in addition to metformin or placebo for 8 weeks. Compared to the placebo group, the metformin group had greater weight loss (p <0.01), greater decrease in body fat (p<0.001), and greater attenuation of area under the curve insulin response to an oral glucose tolerance test (p<0.001). In the metformin-treated subjects, this was associated with enhanced insulin sensitivity determined by fasting plasma glucose and insulin ratios and corresponding reductions in cholesterol, triglycerides, and free fatty acid levels (p<0.01, p<0.05, respectively).
Another study assessed the effect of metformin on BMI, glucose tolerance, and serum lipids in 29 obese adolescents (mean age, 14.4 ± 0.6 years).39 Inclusion criteria included fasting hyperinsulinemia and a family history of type 2 diabetes. However, patients were determined to be nondiabetic based on fasting serum glucose and hemoglobin A1c concentrations. Patients were randomized to receive either metformin or placebo for 6 months with no calorie restrictions. Metformin caused a decline in BMI (−1.3% from baseline; SD, 0.12) and fasting glucose levels (84.9 ± 2.2 mg% vs. 75.1 ± 1.6 mg%; both, p<0.02). Fasting insulin concentrations also declined in the metformin-treated group from baseline (p<0.01). In contrast, BMI and fasting glucose levels rose in the placebo group, while fasting insulin levels did not change from baseline. Additionally, serum lipids declined in both placebo and metformin-treated groups, although neither was statistically significant.
The largest metformin trial to date included 120 obese Turkish children and adolescents (mean age 11.8 ± 2.8 years) and sought to determine the effectiveness of low doses of metformin combined with individually tailored diet, exercise and behavioral therapy for 6 months compared to placebo for weight gain and hyperinsulinemia.40 In the metformin group, there was a significant decline in BMI (28.5 ± 3.4 to 26.7 ± 4 kg/m2, p<0.001), fasting, and postprandial insulin and insulin sensitivity indices (p<0.001). However, no significant changes were noted between the groups in secondary outcomes of systolic and diastolic blood pressure or serum lipids.
In a smaller Swiss trial, 70 obese, insulin-resistant children and adolescents (mean age, 13.7 ± 2.1 years) received low doses of metformin versus placebo.41 All patients presented with obesity-related comorbidities, including features of metabolic syndrome. Outcomes for metabolic parameters, including homeostasis model assessment for insulin resistance index (HOMA-IR) and insulin sensitivity index, both are biomarkers for insulin resistance, improved in 73% of the metformin-treated patients compared to 54% of the placebo group (p=0.048); BMI remained unchanged.
A separate trial evaluated metformin combined with lifestyle modification in 30 obese Chinese adolescents (mean age, 12 ± 1.7 years) with metabolic syndrome defined as insulin resistance, hypertension, and dyslipidemia. Twenty of the 30 participants who finished a 3-month follow-up visit experienced reduction in BMI, blood pressure, triglyceride and cholesterol serum levels, and HOMA-IR (all p<0.001).42
Further studies have examined metformin at higher daily doses (i.e., 1.5-2 g daily) in older children and adolescents with insulin resistance. Twenty-eight adolescents (mean age, 15 ± 2 years) were recruited from an obesity clinic to received metformin or placebo for 4 months.43 Patients had hyperlipidemia, cardiovascular risk factors, and increased insulin sensitivity indices. Results found significant change in BMI (p=0.02) and fasting insulin levels (p=0.05); however, a decrease in insulin sensitivity was not significant (p = 0.1) when adjusted for baseline differences. Additionally, heart rate recovery after step exercise improved significantly after metformin compared with placebo (p = 0.03). Because heart rate recovery has been shown to be a powerful predictor of overall mortality and the development of type 2 diabetes a decrease suggests a possible reduction in cardiovascular risk factors.49,50
Two other trials have been conducted with large metformin daily doses in insulin-resistant adolescents. Twenty-five patients (mean age 13.1 ± 3 years) with significant family histories of type 2 diabetes were randomized to receive structured lifestyle intervention in addition to metformin or placebo.44 Outcomes were significant for decreased BMI and serum lipids (p< 0.05) including triglycerides, HDL, and LDL levels in the pharmacologic treatment group. Fasting insulin levels and HOMA-IR both decreased in the lifestyle intervention group from baseline, but levels did not significantly change with lifestyle and metformin compared to control. Similarly, 85 adolescents (mean age, 15.5 ± 1.7 years) with significant family history of type 2 diabetes received metformin or placebo along with monthly goal setting for diet and exercise modification. 45 There were no differences observed between groups in weight loss or measures of glucose metabolism. However, there was a noted difference between the sexes, as females receiving metformin had a significant decrease in BMI (p =0.02). Furthermore, when patients were found to adhere to metformin and decrease meal portion size, BMI reduction was ≥5%.
The most recent metformin trial is noteworthy as it is the first trial to assess the effects on body weight and composition in obese, insulin-resistant children (mean age 10.1 ± 1.6 years).46 Patients included had significant family history of type 2 diabetes, hyperlipidemia, and 26.4% in the metformin group and 31.9% in the placebo group had a diagnosis of pediatric metabolic syndrome. Results were similar to adolescent studies as the metformin group had significantly greater decreases in BMI, BMI z-score, and body weight (difference −3.38 kg, p<0.001).47 Fasting plasma glucose (p=0.007) and HOMA-IR-IR (p=0.006) also improved more in the metformin groups than in the placebo group.
Last, metformin has also been evaluated for its weight management effects for adolescents (mean age, 13.3 ± 2.4 years) receiving atypical antipsychotics (i.e., olanzapine, risperidone, or quetiapine).48 Over the 16-week treatment period there was little change in the weight of adolescents treated with metformin (mean = −0.03 kg/week), while those receiving placebo continued to gain weight (mean = +0.31kg/week).
In summary, Obese children and adolescents given metformin 1-2 g /day for up to 48 weeks had a reduction in BMI compared to placebo or the baseline group by −0.16 to 3.2 kg/m2.35–40,42,43–46,48 However, one trial did observe a slight increase in BMI (+0.07 kg/m2) over a 6-month treatment period, although the result was found to be nonsignificant.41 Length of treatment seems to have contributed to increased effectiveness, while the size of the metformin dose was not influential. Greater changes in BMI were seen over 3- to 4-month treatment periods with lesser effect noted at 6 to 12 months after metformin initiation.35–46,48 Additionally, one trial observed a significant difference in BMI in the metformin-treatment group (+0.5 kg/m2) compared to the placebo group (−0.8 kg/m2) at the 1-year follow-up giving consideration to lifestyle modification having a larger impact on long-term goals.37
There are several limitations of the studies. The collectively small numbers of participants may have obscured possible differences between the two groups. The trials also enrolled severely obese patients, most of whom continued to gain weight with lifestyle modification prior to initiating treatment. One would expect to see greater change in BMI given the excessive weight of the patients. However, self-motivation and understanding of lifestyle modification counseling were not addressed, giving thought that patients enrolled may be more difficult to treat both, with or without medication.
Although mild gastrointestinal effects including nausea, loose stools, and abdominal discomfort were reported, metformin was well tolerated in most patients.35,37–43,45 Additionally, no serious adverse effects were observed, and medication adherence rates were similar to those of placebo (78%-94%).35,39–41,43,45,46,48 Higher rates of gastrointestinal effects were observed with metformin in the pediatric trial (children ages 6-12 years); however, by one month of treatment, gastrointestinal side effects were no different than placebo, and only 1 child dropped out due to medication intolerance.46 Two additional trials noted only 3 other patients who dropped out due gastrointestinal side effects.41,45 Most participants who dropped out of studies did so due to continued weight gain, noncompliance, or other non-study-related factors.37,44,45,48
Differences in ethnicity and race may also play a role in metformin treatment. In two US trials, metformin was shown to be more effective in Caucasians than African-Americans and/or Hispanics.36,45 Beyond BMI differences, serum lipids, insulin sensitivity, insulin resistance index, fasting insulin, and glucose were measured in the above-described trials, yielding results that varied. With wide variations in outcomes and small sample sizes, there may be differences in specific race or ethnic groups that the current trials have not detected. Therefore, the effects of metformin efficacy should be studied in larger groups of ethnically diverse children and adolescents.
Long-term treatment and follow-up were also not studied as the longest treatment period was 48 weeks, followed by an additional 48 weeks of monitoring. Although metformin produces modest weight loss during short-term treatment, it is not known if it has promise for sustaining weight loss after years of use in children and adolescents. Adverse effects, such as elevated lactate or liver enzymes, or side effects, especially gastrointestinal, are necessary to evaluated during and after long-term use. Additionally, metformin may benefit impaired glucose homeostasis or elevation of serum lipids in children and adolescents at high risk for the development of type 2 diabetes or cardiovascular disease, although again long-term studies are needed.
Additional Pharmacological Treatments
As the incidence of pediatric obesity continues to rise in United States, there is growing interest in expanding the pharmacotherapeutic options available for treatment. Formerly, sibutramine was considered a potential treatment in children via its ability to promote the enhancement of early satiety and/or stimulate energy expenditure through thermogenic effects.51,52 Adolescent data suggested a 1 to 4 kg/m2 reduction in BMI from placebo or baseline with daily doses of 5 to 15 mg.53–58 However, on October 8, 2010, the FDA recommended that Abbott Laboratories voluntary withdraw sibutramine from the market, due to concerns of increased cardiovascular events from the Sibutramine Cardiovascular Outcomes (SCOUT) trial.59,60 Despite a small improvement in mean body weight at 60 months compared to placebo, the use of sibutramine in 9804 overweight or obese patients >55 years with preexisting cardiovascular disease or type 2 diabetes or both was associated with a 16% increase in risk of major adverse cardiovascular events (hazard ratio 1.16; 95% confidence interval 1.03 to 1.31; p=0.02). Therefore, the FDA has concluded that the risk for an adverse cardiovascular event from sibutramine outweighed potential weight-loss benefits and thus, it no longer has a role in treatment of pediatric obesity and will not be reviewed in depth.60
Although not FDA-approved, there are limited data for the use of agents such as growth hormone, octreotide, and topiramate in obese children. Growth hormone (Genotropin, Pharmacia and Upjohn Co., New York, NY; Omnitrope, Sandoz, Inc., Princeton, NJ) administration in children with Prader-Willi syndrome has been associated with decreases body fat percentage and weight loss.61,62 However, adult studies have yet to demonstrate results.63 Beneficial effects of octreotide (Sandostatin; Novartis Pharmaceuticals Corp., East Hanover, NJ) on weight, BMI, and insulin suppression in pediatric patients with hypothalamic obesity have been published.64 Although the anticonvulsant topiramate (Topamax; Janssen Pharmaceuticals Inc., Titusville, NJ) has been proposed to induce insulin sensitivity in adipocytes, use is limited by its high incidence of adverse effects and lacking pediatric data.65,66 Until further large scale trials in children are conducted, these agents should be considered experimental.
CONCLUSIONS
Numerous factors contribute to pediatric obesity causing difficulty for the success of prevention and treatment of the disorder. Lifestyle intervention can reduce rates of weight gain and fat deposition in children and may delay or prevent some long-term risks such as type 2 diabetes.67 However, lifestyle modification needs to be intensive (i.e., calorie restriction, individual and family counseling, and daily exercise) and continued to be effective which is challenging to maintain in children and adolescents.4 Pharmacotherapy may be considered as an adjunct if children or adolescents are not reaching weight loss goals with lifestyle modification or have significant comorbidities. Both orlistat and metformin have demonstrated modest to moderate reduction in BMI (−0.55 to −4.09 kg/m2 and −0.16 to −3.2 kg/m2, respectively) in several, short-term obesity studies supporting their use in conjunction with lifestyle modification. Though adult literature has noted reduced lipid profiles and glucose levels with orlistat, the reviewed pediatric studies did not prove beneficial.68 Results with on lipids and insulin sensitivity in pediatric patients given metformin have been variable.35–43,46–48,50 However, it may be a beneficial treatment option for obese pediatric patients with severe insulin resistance or impaired glucose tolerance although larger trials are needed.35,38–41,43,48 Adverse effects of orlistat and metformin are commonly gastrointestinal related, although for both medications symptoms usually resolve completely within a month following treatment initiation.16,21–23,25,26,35,37–43,47,48 Multivitamin supplementation is recommended when either orlistat or metformin are used due to possible vitamin malaborption.4 When orlistat is used the patients 25OHD3 level should be monitored.
In the future, long-term use of pharmacological agents may be needed to insure lasting benefit of weight loss since discontinuation of medications after short-term use has been associated with weight gain.37 Long-term assessment of health (i.e., incidence of type 2 diabetes and cardiovascular disease) as well as weight outcomes and adverse effects are needed in children and adolescents. Additionally, direct comparison studies of orlistat, metformin and other novel pharmacological agents, including growth hormone, octreotide, and topiramate are needed to fully evaluate treatment options in this population.
ABBREVIATIONS
- AAP
American Academy of Pediatrics
- BMI
body mass index
- CVD
cardiovascular disease
- FDA
Food Drug Administration
- HDL
high-density lipoprotein
- HOMA-IR
homoestatis model assessment for insulin resistance index
- LDL
low-density lipoprotein
- US
United States
Footnotes
DISCLOSURE The authors declare no conflicts or financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employment, gifts, and honoraria.
REFERENCES
- 1.Centers for Disease Control and Prevention US Department of Health and Human Services. Overweight and Obesity. 2011 http://www.cdc.gov/obesity/index.html. Accessed November 19. [Google Scholar]
- 2.Ogden CL, Carroll MD, Curtin LR, et al. Prevalence of high body mass index in US children and adolescents, 2007-2008. JAMA. 2010;303(3):242–249. doi: 10.1001/jama.2009.2012. [DOI] [PubMed] [Google Scholar]
- 3.Gaziano JM. Fifth phase of the epidemiologic transition. JAMA. 2010;303(3):275–276. doi: 10.1001/jama.2009.2025. [DOI] [PubMed] [Google Scholar]
- 4.August GP, Caprio S, Fennoy I, et al. Prevention and treatment of pediatric obesity: an endocrine society clinical practice guideline based on expert opinion. J Clin Endocrinol Metab. 2008;93(12):4576–4599. doi: 10.1210/jc.2007-2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.American Academy of Pediatrics Committee on Nutrition. Prevention of pediatric overweight and obesity. Pediatrics. 2003;112(2):424–430. doi: 10.1542/peds.112.2.424. [DOI] [PubMed] [Google Scholar]
- 6.Office of the Surgeon General US Department of Health and Human Services. The Surgeon General's Vision for a Healthy and Fit Nation 2010. 2011 www.surgeongeneral.gov/library/obesityvision/obesityvision2010.pdf. Accessed May 17. [PubMed] [Google Scholar]
- 7.American Academy of Pediatrics Committee on Nutrition. The use and misuse of fruit juice in pediatrics. Pediatrics. 2001;107(5):1210–1213. doi: 10.1542/peds.107.5.1210. [DOI] [PubMed] [Google Scholar]
- 8.Gidding SS, Dennison BA, Birch LL, et al. for the American Heart Association. Dietary recommendations for children and adolescents: a guide for practitioners. Pediatrics. 2006;117(2):544–559. doi: 10.1542/peds.2005-2374. [DOI] [PubMed] [Google Scholar]
- 9.Ludwig DS, Peterson KE, Gortmaker SL. Relation between consumption of sugar-sweetened drinks and childhood obesity: a prospective, observational analysis. Lancet. 2001;357(9255):505–508. doi: 10.1016/S0140-6736(00)04041-1. [DOI] [PubMed] [Google Scholar]
- 10.Council on Sports Medicine and Fitness; Council on School Health. Active healthy living: prevention of childhood obesity through increased physical activity. Pediatrics. 2006;117(5):1834–1842. doi: 10.1542/peds.2006-0472. [DOI] [PubMed] [Google Scholar]
- 11.Eaton DK, Kann L, Linchen S, et al. Youth risk behavior surveillance: United States, 2009. MMWR Surveill Summ. 2010;59(5):1–142. [PubMed] [Google Scholar]
- 12.Committee on Public Education. American Academy of Pediatrics: children, adolescents, and television. Pediatrics. 2001;107(2):423–426. doi: 10.1542/peds.107.2.423. [DOI] [PubMed] [Google Scholar]
- 13.Gartner LM, Morton J, Lawrence RA, et al. Breastfeeding and the use of human milk. Pediatrics. 2005;115(2):496–506. doi: 10.1542/peds.2004-2491. [DOI] [PubMed] [Google Scholar]
- 14.Khan LK, Sobush K, Keener D, et al. Recommended community strategies and measurements to prevent obesity in the United States. MMWR Recomm Rep. 2009;58(RR-7):1–26. [PubMed] [Google Scholar]
- 15.Yanovski JA, Yanovski SZ. Treatment of pediatric and adolescent obesity. JAMA. 2003;289(14):1851–1853. doi: 10.1001/jama.289.14.1851. [DOI] [PubMed] [Google Scholar]
- 16.US Food and Drug Administration. Orlistat Update Pediatric Advisory Committee Meeting. March 22, 2010. 2011 http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/PediatricAdvisoryCommittee/UCM205380.pdf. Accessed November 25. [Google Scholar]
- 17.Mittendorfer B, Ostlund RE, Jr, Patterson BW, et al. Orlistat inhibits dietary cholesterol absorption. Obes Res. 2001;9(10):599–604. doi: 10.1038/oby.2001.79. [DOI] [PubMed] [Google Scholar]
- 18.Guerciolini R. Mode of action of orlistat. Int J Obes Relat Metab Disord. 1997;21(suppl 3):S12–S23. [PubMed] [Google Scholar]
- 19.Melia AT, Koss-Twardy SG, Zhi J. The effect of orlistat, an inhibitor of dietary fat absorption, on the absorption of vitamins A and E in healthy volunteers. J Clin Pharmacol. 1996;36(7):647–653. doi: 10.1002/j.1552-4604.1996.tb04230.x. [DOI] [PubMed] [Google Scholar]
- 20.McDuffie JR, Calis KA, Booth SL, et al. Effects of orlistat on fat-soluble vitamins in obese adolescents. Pharmacotherapy. 2002;22(7):814–822. doi: 10.1592/phco.22.11.814.33627. [DOI] [PubMed] [Google Scholar]
- 21.McDuffie JR, Calis KA, Uwaifo GI, et al. Three-month tolerability of orlistat in adolescents with obesity-related comorbid conditions. Obes Res. 2002;10(7):642–650. doi: 10.1038/oby.2002.87. [DOI] [PubMed] [Google Scholar]
- 22.McDuffie JR, Calis KA, Uwaifo GI, et al. Efficacy of orlistat as an adjunct to behavioral treatment in overweight African American and Caucasian adolescents with obesity-related co-morbid conditions. J Pediatr Endocrinol Metab. 2004;17(3):307–319. doi: 10.1515/jpem.2004.17.3.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Norgren S, Danielsson P, Jurold R, et al. Orlistat treatment in obese prepubertal children: a pilot study. Acta Paediatr. 2003;92(6):666–670. doi: 10.1080/08035250310002353. [DOI] [PubMed] [Google Scholar]
- 24.Ozkan B, Bereket A, Turan S, et al. Addition of orlistat to conventional treatment in adolescents with severe obesity. Eur J Pediatr. 2004;163(12):738–741. doi: 10.1007/s00431-004-1534-6. [DOI] [PubMed] [Google Scholar]
- 25.Chanoine J, Hampl S, Jensen C, et al. Effect of orlistat on weight and body composition in obese adolescents: a randomized controlled trial. JAMA. 2005;293(23):2873–2883. doi: 10.1001/jama.293.23.2873. [DOI] [PubMed] [Google Scholar]
- 26.Maahs D, Gonzalez de Serna D, Kolotkin RL, et al. Randomized, double-blind, placebo-controlled trial of orlistat for weight loss in adolescents. Endocr Pract. 2006;12(1):18–28. doi: 10.4158/EP.12.1.18. [DOI] [PubMed] [Google Scholar]
- 27.Zhi J, Melia AT, Eggers J, et al. Review of limited systemic absorption of orlistat, a lipase inhibitor, in healthy human volunteers. J Clin Pharmacol. 1995;35(11):1103–1108. doi: 10.1002/j.1552-4604.1995.tb04034.x. [DOI] [PubMed] [Google Scholar]
- 28.Van-Gaal LF, Broom JI, Enzi G, et al. Efficacy and tolerability of orlistat in the treatment of obesity: a 6-month dose-ranging study. Orlistat dose-ranging study group. Eur J Clin Pharmacol. 1998;54(2):125–132. doi: 10.1007/s002280050433. [DOI] [PubMed] [Google Scholar]
- 29.Cavaliere J, Floriano I, Medeiros-Neto G. Gastrointestinal side effects of orlistat may be prevented by concomitant prescription of natural fibers (psyllium mucilloid) Int J Obes Relat Metab Disord. 2001;25(7):1095–1099. doi: 10.1038/sj.ijo.0801645. [DOI] [PubMed] [Google Scholar]
- 30.Metformin. In: DRUGDEX System [intranet database]. Version 5.1. Greenwood Village, CO: Thomson Reuters (Healthcare) Inc; [Google Scholar]
- 31.Weiss R, Dziura J, Burgert TS, et al. Obesity and the metabolic syndrome in children and adolescents. N Engl J Med. 2004;350(23):2362–2374. doi: 10.1056/NEJMoa031049. [DOI] [PubMed] [Google Scholar]
- 32.Weyer C, Tataranni PA, Bogardus C, et al. Insulin resistance and insulin secretory dysfunction are independent predictors of worsening of glucose tolerance during each stage of type 2 diabetes development. Diabetes Care. 2001;24(1):89–94. doi: 10.2337/diacare.24.1.89. [DOI] [PubMed] [Google Scholar]
- 33.Sun SS, Liang R, Huang TT, et al. Childhood obesity predicts adult metabolic syndrome: the Fels Longitudinal Study. J Pediatr. 2008;152(2):191–200. doi: 10.1016/j.jpeds.2007.07.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou G, Myers R, Li Y, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108(8):1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Srinivasan S, Ambler GR, Baur LA, et al. Randomized, controlled trial of metformin for obesity and insulin resistance in children and adolescents: improvement in body composition and fasting insulin. J Clin Endocrinol Metab. 2006;91(6):2074–2080. doi: 10.1210/jc.2006-0241. [DOI] [PubMed] [Google Scholar]
- 36.Lustig RH, Mietus-Snyder ML, Bacchetti P, et al. Insulin dynamics predict body mass index and z-score response to insulin suppression or sensitization pharmacotherapy in obese children. J Pediatr. 2006;148(1):23–29. doi: 10.1016/j.jpeds.2005.08.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wilson DM, Abrams SH, Aye T, et al. Metformin extended release treatment of adolescent obesity. Arch Pediatr Adolesc Med. 2010;164(2):116–123. doi: 10.1001/archpediatrics.2009.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kay JP, Alemzadeh R, Langley G, et al. Beneficial effects of metformin in normoglycemic morbidly obese adolescents. Metabolism. 2001;50(12):1457–1461. doi: 10.1053/meta.2001.28078. [DOI] [PubMed] [Google Scholar]
- 39.Freemark M, Bursey D. The effects of metformin on body mass index and glucose tolerance in obese adolescents with fasting hyperinsulinemia and a family history of type 2 diabetes. Pediatrics. 2001;107(4):e55. doi: 10.1542/peds.107.4.e55. [DOI] [PubMed] [Google Scholar]
- 40.Atabek ME, Pirgon O. Use of metformin in obese adolescents with hyperinsulinemia: a 6-month, randomized, double-blind, placebo-controlled clinical trial. J Pediatr Endocrinol Metab. 2008;21(4):339–348. doi: 10.1515/jpem.2008.21.4.339. [DOI] [PubMed] [Google Scholar]
- 41.Wiegand S. l'Allemand D, Hübel H, et al. Metformin and placebo therapy both improve weight management and fasting insulin in obese insulin-resistant adolescents: a prospective, placebo-controlled, randomized study. Eur J Endocrinol. 2010;163(4):585–592. doi: 10.1530/EJE-10-0570. [DOI] [PubMed] [Google Scholar]
- 42.Fu JF, Liang L, Zou CC, et al. Prevalence of the metabolic syndrome in Zhejiang Chinese obese children and adolescents and the effect of metformin combined with lifestyle modification. Int J Obes. 2007;31:15–22. doi: 10.1038/sj.ijo.0803453. [DOI] [PubMed] [Google Scholar]
- 43.Burgert TS, Duran EJ, Goldberg-Gell R, et al. Short-term metabolic and cardiovascular effects of metformin in markedly obese adolescents with normal glucose tolerance. Pediatr Diabetes. 2008;9(6):567–576. doi: 10.1111/j.1399-5448.2008.00434.x. [DOI] [PubMed] [Google Scholar]
- 44.Clarson CL, Mahmud FH, Baker JE, et al. Metformin in combination with structured lifestyle intervention improved body mass index in obese adolesents, but did not improve insulin resistance. Endocrine. 2009;6(1):141–146. doi: 10.1007/s12020-009-9196-9. [DOI] [PubMed] [Google Scholar]
- 45.Love-Osborne K, Sheeder J, Zeitler P. Addition of metformin to a lifestyle modification program in adolescents with insulin resistance. J Pediatr. 2008;152(6):817–822. doi: 10.1016/j.jpeds.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yanovski JA, Krakoff J, Salaita CG, et al. Effects of metformin on body weight and body composition in obese insulin-resistant children: a randomized clinical trial. Diabetes. 2011;60(2):477–485. doi: 10.2337/db10-1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lobstein T, Baur L, Uauy R. Obesity in children and young people: a crisis in public health. Obes Rev. 2004;5(suppl 1):S4–S85. doi: 10.1111/j.1467-789X.2004.00133.x. [DOI] [PubMed] [Google Scholar]
- 48.Klein DJ, Cottingham EM, Sorter M, et al. A randomized, double-blind, placebo-controlled trial of metformin treatment of weight gain associated with initiation of atypical antipsychotic therapy in children and adolescents. Am J Psychiatry. 2006;163(12):2072–2079. doi: 10.1176/ajp.2006.163.12.2072. [DOI] [PubMed] [Google Scholar]
- 49.Cole CR, Blackstone EH, Pashkow FJ, et al. Heart-rate recovery immediately after exercise as a predictor of mortality. N Engl J Med. 1999;341(18):1351–1357. doi: 10.1056/NEJM199910283411804. [DOI] [PubMed] [Google Scholar]
- 50.Carnethon MR, Gidding SS, Nehgme R, et al. Cardiorespiratory fitness in young adulthood and the development of cardiovascular disease risk factors. JAMA. 2003;290(23):3092–3100. doi: 10.1001/jama.290.23.3092. [DOI] [PubMed] [Google Scholar]
- 51.Nisoli E, Carruba MO. An assessment of the safety and efficacy of sibutramine, an anti-obesity drug with a novel mechanism of action. Obes Rev. 2000;1(2):127–139. doi: 10.1046/j.1467-789x.2000.00020.x. [DOI] [PubMed] [Google Scholar]
- 52.Lean MEJ. How does sibutramine work? Int J Obes Relat Metab Disord. 2001;25(suppl 4):S8–S11. doi: 10.1038/sj.ijo.0801931. [DOI] [PubMed] [Google Scholar]
- 53.Berkowitz RI, Fujioka K, Daniels SR, et al. Effects of sibutramine treatment in obese adolescents: a randomized trial. Ann Intern Med. 2006;145(2):81–90. doi: 10.7326/0003-4819-145-2-200607180-00005. [DOI] [PubMed] [Google Scholar]
- 54.Berkowitz RI, Wadden TA, Tershakovec AM, et al. Behavior therapy and sibutramine for the treatment of adolescent obesity: a randomized controlled trial. JAMA. 2003;289(14):1805–1812. doi: 10.1001/jama.289.14.1805. [DOI] [PubMed] [Google Scholar]
- 55.Godoy-Matos A, Carraro L, Vieira A, et al. Treatment of obese adolescents with sibutramine: a randomized, double-blind, controlled study. J Clin Endocrinol Metab. 2005;90(3):1460–1465. doi: 10.1210/jc.2004-0263. Epub 2004 Dec 21. [DOI] [PubMed] [Google Scholar]
- 56.Violante-Ortìz R, Del-Rio-Navarro BE, Lara-Esqueda A, et al. Use of sibutramine in obese Hispanic adolescents. Adv Ther. 2005;22(6):642–649. doi: 10.1007/BF02849958. [DOI] [PubMed] [Google Scholar]
- 57.García-Morales LM, Berber A, Macias-Lara CC, et al. Use of sibutramine in obese Mexican adolescents: a 6-month, randomized, double-blind, placebo-controlled, parallel-group trial. Clin Ther. 2006;28(5):770–782. doi: 10.1016/j.clinthera.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 58.Reisler G, Tauber T, Afriat R, et al. Sibutramine as an adjuvant therapy in adolescents suffering from morbid obesity. Isr Med Assoc J. 2006;8(1):30–32. [PubMed] [Google Scholar]
- 59.Food and Drug Administration. Drug Safety Communication: FDA Recommends Against the Continued use of Meridia (sibutramine) 2011 www.fda.gov/drugs/drugsafety/ucm228746.htm. Accessed May 17. [Google Scholar]
- 60.James WPT, Caterson I, Coutinho W, et al. Effect of sibutramine on cardiovascular outcomes in overweight and obese subjects. N Engl J Med. 2010;363(10):905–917. doi: 10.1056/NEJMoa1003114. [DOI] [PubMed] [Google Scholar]
- 61.Angulo M, Castro-Magana M, Mazur B, et al. Growth hormone secretion and effects of growth hormone therapy on growth velocity and weight gain in children with Prader-Willi syndrome. J Pediatr Endocrinol Metab. 1996;9(3):393–400. doi: 10.1515/JPEM.1996.9.3.393. [DOI] [PubMed] [Google Scholar]
- 62.Carrel AL, Myers SE, Whitman BY, et al. Benefits of long-term GH therapy in Prader-Willi syndrome. J Pediatr. 2004;145(4):744–749. [Google Scholar]
- 63.Shadid S, Jensen MD. Effects of growth hormone administration in human obesity. Obes Res. 2003;11(2):170–175. doi: 10.1038/oby.2003.27. [DOI] [PubMed] [Google Scholar]
- 64.Lustig RH, Hinds PS, Ringwald-Smith K, et al. Octreotide therapy of pediatric hypothalamic obesity: a double-blind, placebo-controlled trial. J Clin Endocrinol Metab. 2003;88(6):2586–2592. doi: 10.1210/jc.2002-030003. [DOI] [PubMed] [Google Scholar]
- 65.Bray GA, Hollander P, Klein S, et al. A 6-month randomized, placebo-controlled, dose-ranging trial of topiramate for weight loss in obesity. Obes Res. 2003;11(6):722–733. doi: 10.1038/oby.2003.102. [DOI] [PubMed] [Google Scholar]
- 66.Aarsen FK, van den Akker EL, Drop SL, et al. Effect of topiramate on cognition in obese children. Neurology. 2006 Oct 10;67(7):1307–1308. doi: 10.1212/01.wnl.0000238099.36998.6b. [DOI] [PubMed] [Google Scholar]
- 67.Ritchie LD, Crawford PB, Hoelscher DM, et al. Position of the American Diabetes Association: individual-, family-, school- and community-based interventions for pediatric overweight. J Am Diet Assoc. 2006;106(6):925–945. doi: 10.1016/j.jada.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 68.Torgersen JS, Hauptman J, Boldrin MN, et al. XENical in the prevention of diabetes in obese subjects (XENDOS) study : a randomized study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients. Diabetes Care. 2004;27(1):155–161. doi: 10.2337/diacare.27.1.155. [DOI] [PubMed] [Google Scholar]


