Abstract
OBJECTIVES
To assess the ability of an empiric once-daily dosing (ODD) tobramycin regimen to achieve desired serum concentrations in patients with cystic fibrosis (CF); to determine an optimal dosage regimen, using pharmacodynamic parameters; and to evaluate clinical response, adverse effects, and patient/parent satisfaction with ODD.
METHODS
This was a prospective single-center trial in patients with CF who are 5 years of age and older requiring intravenous antibiotics. Tobramycin, 10 mg/kg every 24 hours, was infused over 60 minutes, and two serum concentrations were analyzed using 1-compartment pharmacokinetic modeling. Simulations were performed to identify dosage regimens that maximized desired pharmacodynamic parameters. Other data included demographics, symptoms, spirometry, adverse events, and satisfaction with ODD.
RESULTS
A total of 14 children and 11 adults completed the study. Empiric doses resulted in mean peak tobramycin concentrations of 28.7 ± 5.5 mg/L and undetectable trough concentrations. Only 42% of patients achieved desired peak serum concentrations (20-30 mg/L) with the empiric regimen. A regimen of 12 mg/kg every 24 hours would achieve modified pharmacodynamic goals with an acceptable peak range of 20 to 35 mg/L. Forced expiratory volume in 1 second improved in 15 of 20 (75%) patients with ODD. Two patients experienced reversible vestibular adverse effects attributed to tobramycin. All patients were satisfied or very satisfied with ODD because of convenience and ease of use.
CONCLUSIONS
An empiric tobramycin regimen of 10 mg/kg every 24 hours did not achieve desired serum concentrations for most patients, although all patients demonstrated clinical improvement. Desired tobramycin concentrations with modified pharmacodynamic goals could be achieved by using an empiric dosage of 12 mg/kg every 24 hours. Prospective evaluation of this regimen with individualized patient monitoring is needed to ensure safety and efficacy and to monitor the effects on microbial resistance patterns.
INDEX TERMS: Cystic fibrosis, once-daily, pharmacokinetics, tobramycin
INTRODUCTION
Cystic fibrosis (CF) patients often experience repeated pulmonary exacerbations caused largely by Pseudomonas aeruginosa infection. Aminoglycosides, such as tobramycin, have been the treatment of choice because of their excellent antipseudomonal activity and low potential for causing microbial resistance. Aminoglycosides have historically been administered in multiple daily doses, but their antibacterial activity may be improved by administering larger single daily doses in order to achieve higher peak concentrations that maximize concentration-dependent killing.1,2 Clinical response rates of 90% or greater are achieved when peak aminoglycoside serum concentrations are 8 to 10 times higher than the minimum inhibitory concentration (MIC) of the infecting organism.3 Once-daily dosing (ODD) of aminoglycosides also capitalizes on their concentration-dependent postantibiotic effect (PAE) where microbial growth continues to be inhibited despite drug concentrations that are below the MIC.1,2,4,5 Once-daily administration may also have other advantages including reduced toxicities, reduced drug preparation and administration times, reduced cost, and improved patient satisfaction, particularly for home infusion therapy.1,5,6
Studies of CF patients with pulmonary exacerbations report that tobramycin given once daily has equal efficacy and is no more toxic than when it is given three times daily.7–13 Most of these studies included small numbers of patients (6-60 participants) treated with tobramycin dosages ranging from 9 mg/kg to 15 mg/kg daily.9–11,13 The largest study (the TOPIC study) was a randomized, double-blind, controlled trial in 219 children and adults with CF that reported similar efficacy with once-daily (10 mg/kg) and three times daily tobramycin administration.12 There were statistically significant reductions in serum creatinine and urinary N-acetyl-β-d glucosaminidase concentrations in children treated with once-daily tobramycin compared to those given thrice-daily dosing, but the clinical significance of these changes is questionable. No differences in these parameters were found in adult patients. However, a post hoc analysis found a 30% reduction in tobramycin elimination with ODD compared to three times daily dosage in both children and adults.14 This observation was explained by differences in drug administration times and the effect of circadian rhythm on drug clearance between the two groups.14 Other studies have not found significant differences in rates of nephrotoxicity or ototoxicity between the different dosage regimens.9–11
A few studies have included pediatric patients but related only limited pharmacokinetic analyses, often with suboptimal drug administration and serum sampling techniques that limit the utility of their pharmacokinetic results.9,11,12,15 Several additional pharmacokinetic studies have recommended a variety of ODD tobramycin regimens for CF exacerbations in children and adults.16–22 The optimal tobramycin dosage and monitoring approach for ODD in CF patients remain unclear and may vary with patient age or other factors. Only one study compared ODD tobramycin pharmacokinetics between adults and children with CF.14 While small but statistically significant differences in volumes of distribution were reported, it is unknown whether these differences translate into significant dosing differences required to achieve desired serum concentrations.
The primary objectives of this study were to evaluate the ability of an empiric ODD tobramycin regimen of 10 mg/kg to achieve desired concentrations in both children and adult CF patients with pulmonary exacerbations and to determine an optimal empiric dosage regimen using pharmacokinetic and pharmacodynamic analyses. Secondary objectives were to assess clinical response, adverse effects, and patient and/or parent satisfaction with ODD.
MATERIALS AND METHODS
This was a prospective, open label, single-center study of once-daily tobramycin therapy in a CF population, conducted from April 2005 to February 2007. Patients were identified for enrollment at presentation to the local ambulatory CF care center or at the local hospital emergency department. Criteria for enrollment were a diagnosis of CF pulmonary exacerbation requiring intravenous tobramycin, as determined by the pediatric pulmonologist investigators; age of 5 years or older; and ability of the patient and parent/legal guardian to speak and understand English and provide consent/assent. Patients were not eligible for the study if they were pregnant or lactating, hypersensitive to aminoglycosides, or if they had current renal impairment (defined as a serum creatinine concentration 1.5 times greater than the upper limit of normal for age). Study procedures were followed in accord with the ethical standards and approved by the Institutional Review Board at the local hospital and the Research Committee at the ambulatory clinic. Written informed consent from the adult patient or parents of children and assent from all children were obtained before enrollment. Patients were included only once in the study in order to evaluate achievement of desired serum concentrations with the empiric dosing regimen.
Patients received intravenous tobramycin, 10 mg/kg (using actual body weight) infused over 60 minutes once-daily. This dose was chosen based on studies in CF patients that suggested that larger doses were needed to achieve drug exposure similar to that in patients without CF receiving 7 mg/kg/dose.10,15 Patients were treated as inpatients or as outpatients receiving home infusion therapy, at the discretion of the pulmonologist. Dose administration times were not standardized but rather were based on time of day when hospitalization occurred (for inpatients) or patient convenience (for outpatients). Concurrent antibiotics were prescribed based on the most recent sputum culture and sensitivity results for each patient. Antibiotic selection and duration of therapy were determined by the pulmonologist and were based on clinical response. All chronic medications were continued during the study, and medication changes were made at the discretion of the pulmonologist without regard to inclusion in the study.
Data collection consisted of demographics (age, sex, weight, height), current medications, clinical parameters (symptoms and spirometry results), laboratory values (serum creatinine value, white blood cell count, sputum culture results), and tobramycin dosage and serum concentrations. Clinical improvement was determined based upon improvements in cough, sputum production, shortness of breath, and improvement in oxygenation. Serum samples were drawn via peripheral venipuncture after the first or second dose to assess tobramycin concentrations. Peak serum samples were drawn 60 minutes after the end of the 60-minute infusion, and a random serum sample was drawn 5 to 7 hours postinfusion to allow for flexibility in scheduling, particularly for outpatients. Serum samples were analyzed using enzyme immunoassay (Cobas; Roche Diagnostics, Indianapolis, IN), where the lower limit of detection was 0.33 mg/L. Desired serum tobramycin concentrations were calculated peaks of maximum concentration (Cmax; extrapolated serum concentration at the end of the infusion) of 20 to 30 mg/L and calculated troughs of minimum concentration (Cmin) less than or equal to 0.05 mg/L. The target peak range was selected based on average P. aeruginosa MIC values in CF patients (80% of patients have an MIC of ≤2 mg/L; susceptibility is defined as MIC of ≤4 mg/L) and a goal serum Cmax:MIC ratio of 8 to 10.3,9,20 This peak range was also used in the TOPIC study and is similar to ranges used in several pharmacokinetic studies.12,18,20–22 The target trough concentration was selected based on the expected 24-hour concentrations in patients with normal renal function and trough concentrations reported in pharmacokinetic studies in CF patients.17,18,21,23 Doses were adjusted to maintain desired serum concentrations when necessary, and serum samples were drawn weekly during therapy in a manner similar to that used for initial samples. Patient serum creatinine values were measured at baseline and were also monitored weekly while they received tobramycin.
One-compartment pharmacokinetic modeling (WinNonLin version 4.0 software) was used to characterize patient data. Simulations were performed to identify dosing regimens that would maximize desired pharmacodynamic parameters for the study population (Cmax:MIC ratio of 8-10; time below MIC [T < MIC] of less than 7 hours; a drug-free interval to minimize tobramycin accumulation; and a daily area under the concentration-versus-time curve [AUC] of ≤100 mg·hr/L to minimize nephrotoxicity).24 Actual MICs were not available from the microbiology laboratory, so MIC values of 1 mg/L, 2 mg/L, and 4 mg/L were assumed in the simulations. Drug-free interval was defined as the time that serum concentrations were below the assay quantification limit of 0.3 mg/L.
A pharmacist investigator interviewed patients at least once during hospitalization or at all scheduled clinic visits while they received intravenous tobramycin for the presence of adverse events, including dizziness, lightheadedness, fainting, tinnitus, reduced hearing ability, balance disturbances, rash, or vein irritation. All patients were interviewed after tobramycin therapy was completed to assess for the presence of adverse events that occurred at any time during therapy. Patients and/or caregivers also completed a satisfaction survey after completion of tobramycin therapy. This survey was a 1-item, nonvalidated 5-point Likert scale, where patients and/or their caregivers rated their satisfaction with once-daily tobramycin administration compared to three times daily administration (1 = very dissatisfied to 5 = very satisfied); all patients had been previously treated with traditional dosing.
RESULTS
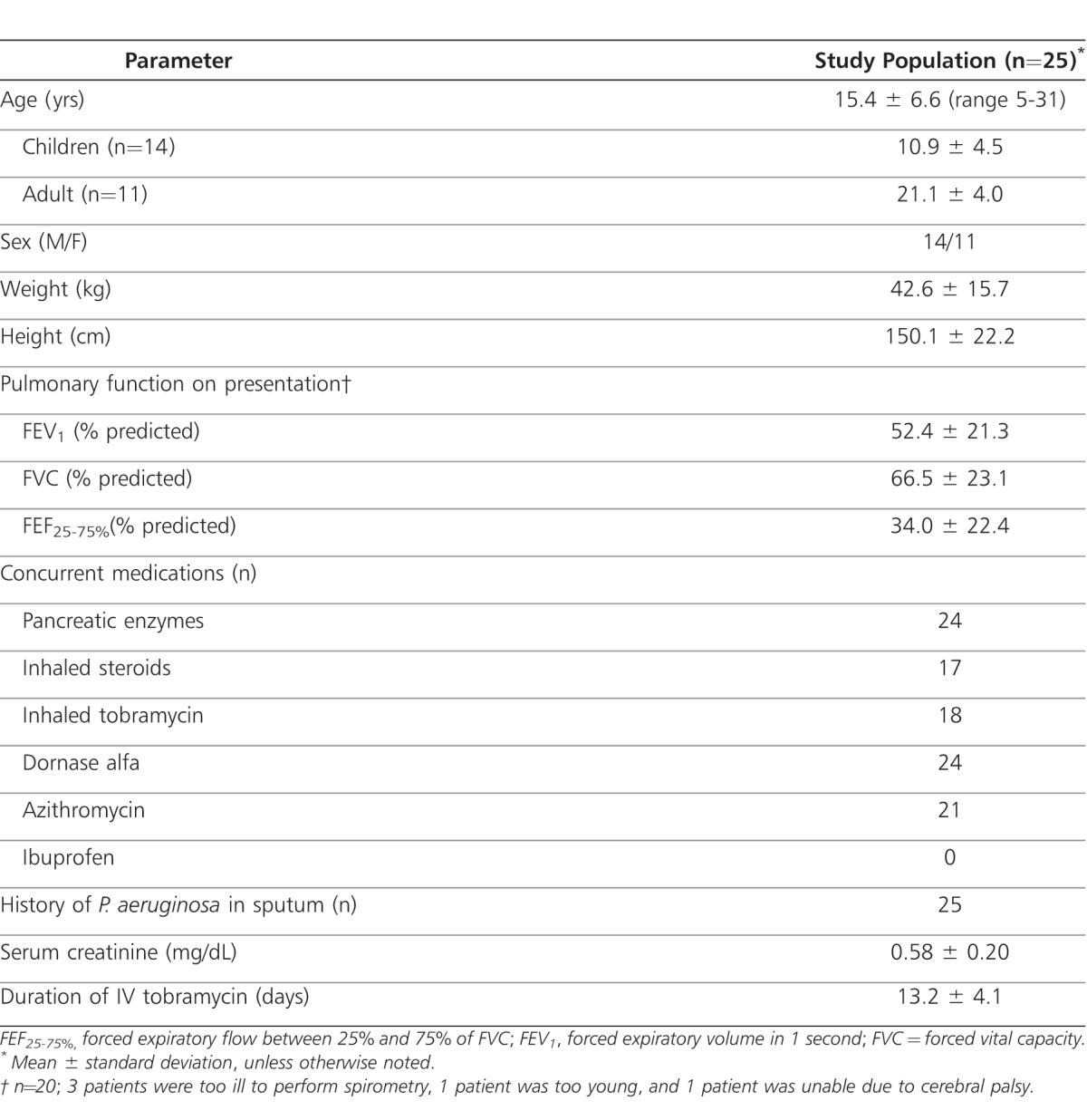
Twenty-five patients were enrolled between April 2005 and February 2007 (Table 1). Most patients had mild to moderate impairment of lung function as measured by spirometry upon study entry (mild impairment was defined as forced expiratory volume in 1 second (FEV1) of 70%-89% predicted, and moderate impairment was defined as FEV1 of 40%-69% predicted). Five patients were unable to perform spirometry. All patients had a previous sputum culture positive for P. aeruginosa, and most were receiving inhaled dornase alfa and oral azithromycin. All patients received concomitant antibiotic therapy that included ceftazidime (n=12), meropenem (n=5), oral fluoroquinolones (n=5), vancomycin (n=3), and/or linezolid (n=1). Eighteen patients (72%) received inhaled tobramycin (TOBI; Novartis, Basel, Switzerland) as chronic therapy in a 28-day-on/28-day-off rotating schedule that was not interrupted during the study.
Table 1.
Patient Demographics
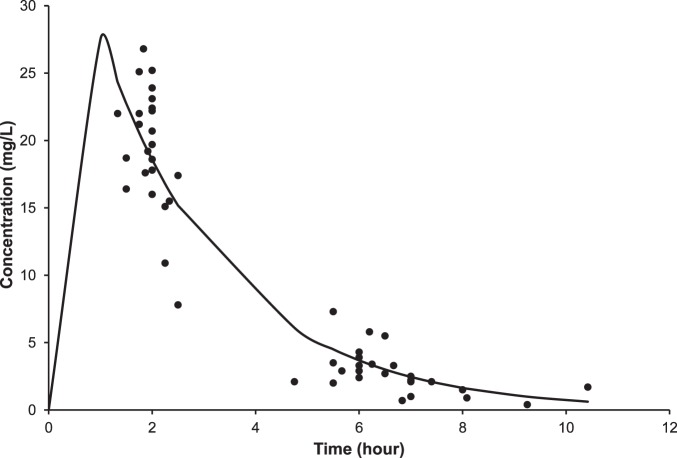
Tobramycin pharmacokinetics were determined after 1 to 2 doses of the empiric regimen designed to deliver 10 mg/kg every 24 hours. Individual patient serum concentrations as a function of time are displayed in the Figure, and calculated pharmacokinetic parameters are shown in Table 2. Data from 1 patient (age 16 years) were excluded because one of the serum samples was drawn from the patient's central line and was deemed falsely elevated (calculated peak of 50 mg/L). Average calculated peak and trough tobramycin serum concentrations in the remaining 24 patients were 28.7 ± 5.5 mg/L and 0 mg/L, respectively, with use of the empiric regimen. However, only 10 patients (42%; 8 children and 2 adults) achieved the desired peak level range with the empiric dose. The average age of the 12 patients whose peak level exceeded 30 mg/L with empiric dosage was 18.5 years. This is in comparison to an average age of 13.5 years for the 2 patients whose peak concentrations were less than 20 mg/L. Six patients (25%) had doses adjusted downward by 12% to 27% to achieve concentrations in the desired peak range compared to 3 patients (13%) who had doses adjusted upward by 13% to 30%. One patient had a dose adjusted upward even though the calculated serum peak concentration was 20.1 mg/L. Six patients had calculated peak concentrations that exceeded 30 mg/L, but dose adjustments were not made. If the acceptable peak range is expanded to 20 to 32 mg/L, where dose adjustments may not be necessary because peak concentrations are slightly outside the desired range of 20 to 30 mg/L, 13 patients (54%; 8 children and 5 adults) reached this range with the empiric dose. No significant differences were noted between the calculated pharmacokinetic parameters of patients ≤12 years of age and those of patients >12 years or between the calculated pharmacokinetic parameters of children <18 years and those of adults.
Figure.
Tobramycin serum concentration versus time with empiric dosage of 10 mg/kg given every 24 hours.
Table 2.
Tobramycin Pharmacokinetics with Empiric Dosage Regimen (10 mg/kg every 24 hours)
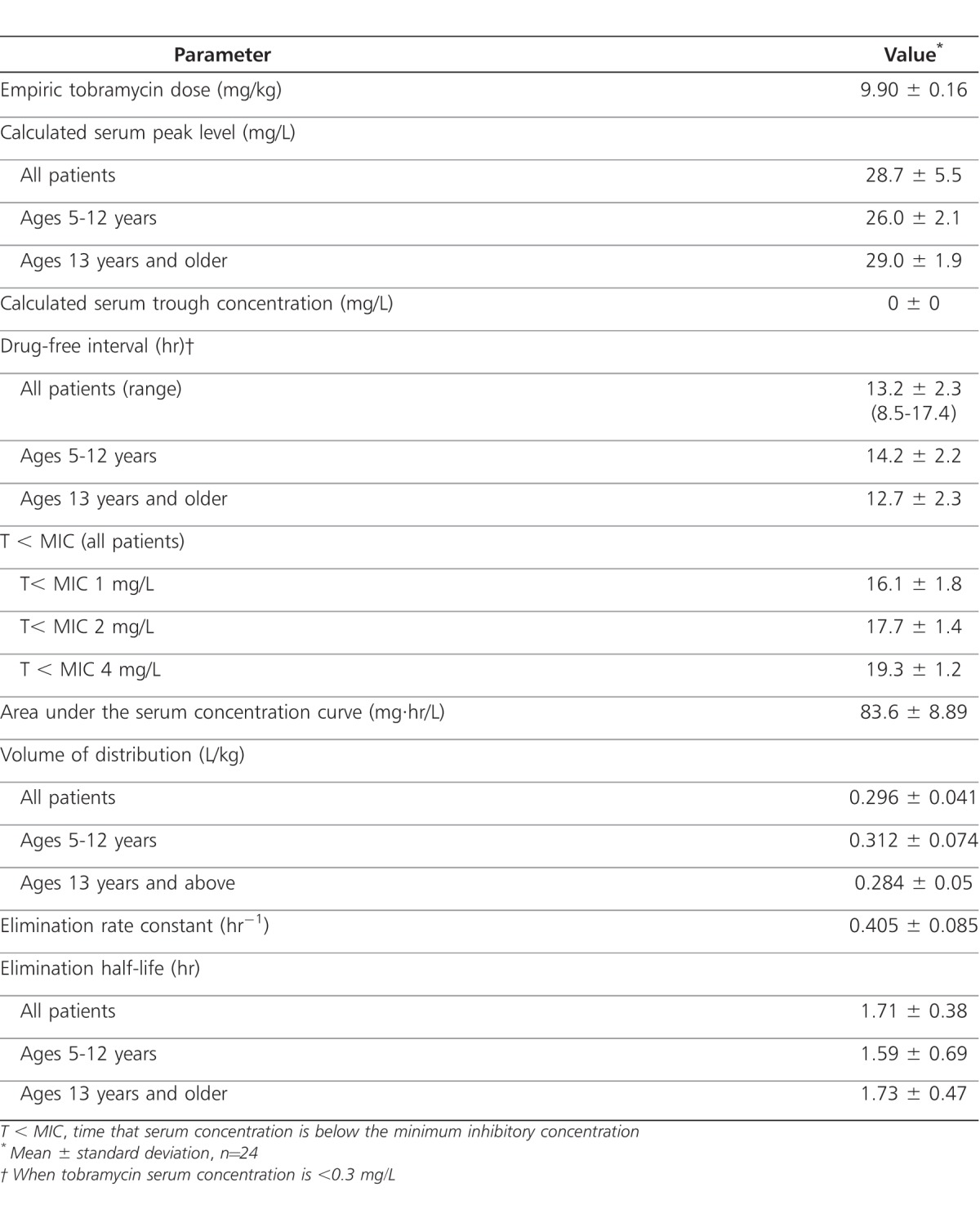
All patients had calculated trough concentrations that were less than 0.05 mg/L. Twenty-two patients (92%) had a drug-free interval of greater than 10 hours within the 24-hour dosing interval. When assumed MIC values of 1 mg/L, 2 mg/L, and 4 mg/L were used, the average T < MICs were 16.1 hours, 17.7 hours, and 19.3 hours, respectively, for the study population.
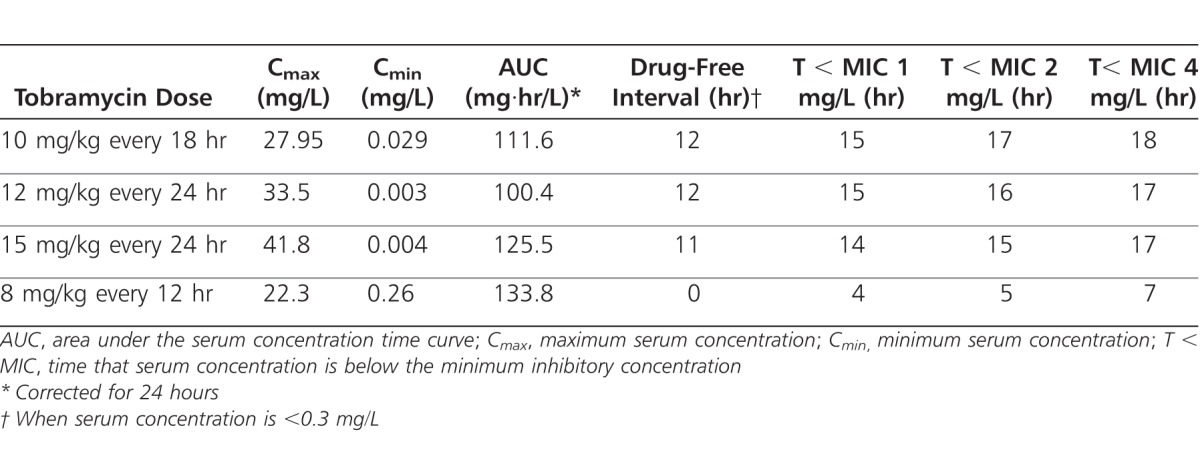
Pharmacokinetic simulations were performed to determine an optimal empiric dosage regimen that would maximize desired pharmacodynamic goals in most patients (Table 3). Tobramycin, 10 mg/kg given every 18 hours, would provide both peak and trough serum concentrations in the study goal ranges, while tobramycin, 12 mg/kg every 24 hours, would provide peak concentrations slightly above the desired 30 mg/L concentration with undetectable trough concentrations. The 12 mg/kg/24 hour regimen provided the best fit with prespecified pharmacodynamic goals. Most of the simulated extended interval dosage regimens would provide prolonged drug-free intervals and time periods where serum concentrations would be lower than average P. aeruginosa MIC values, exceeding the known PAE time duration. Tobramycin, 8 mg/kg given every 12 hours, would provide adequate peak serum concentrations and the shortest T < MIC but higher than desired trough concentrations and a daily AUC of >130 mg·hr/L, the highest of the simulated regimens.
Table 3.
Simulated Pharmacokinetic Parameters Using Different Tobramycin Regimens
Clinical improvement was noted in all patients treated with once-daily dose (ODD) tobramycin, as demonstrated by reductions in cough, sputum production, and shortness of breath and improvements in oxygenation. Fifteen patients (75%) had improvements in FEV1 during treatment or at the end of antibiotic therapy; the remaining 5 patients remained at their pretreatment FEV1 levels. Two female patients (8%) experienced adverse events while receiving ODD tobramycin. One 25-year-old patient reported diarrhea and transient tinnitus, and a 31-year-old patient reported dizziness, nausea, and weakness, which subsided with subsequent dose reduction. No patients experienced nephrotoxicity (defined as a rise in baseline serum creatinine ≥50%) during the study. Most patients were satisfied (n = 4) or very satisfied (n = 20) with ODD compared to multiple daily doses, because of convenience and ease of use, when asked to compare the two dosage regimens based on historical experiences (n = 1 with missing data).
DISCUSSION
Use of an empiric tobramycin dosage regimen of 10 mg/kg given every 24 hours did not achieve serum concentrations in the desired peak range for most patients in this population with CF pulmonary exacerbations. Despite achieving a mean peak serum level similar to that reported by Smyth et al. (28.4 mg/L),12 the empiric regimen used in this study achieved peak serum concentrations that were within the specified goal range for only 42% of patients. A desired peak serum tobramycin range of 20 to 30 mg/L was selected in order to achieve a high Cmax:MIC ratio for P. aeruginosa infection. This peak range was based on the pharmacodynamic characteristic of aminoglycosides that demonstrates maximal concentration-dependent killing when the Cmax:MIC ratio is 8 to 10.3 Susceptible P. aeruginosa isolates have MICs of ≤4 mg/L, and most isolates from patients with CF have MICs of ≤2 mg/L.9,20 Therefore, a target tobramycin peak range of 16 to 40 mg/L should ensure achievement of serum concentrations that are associated with high clinical response rates, even for infections caused by organisms with MICs near the susceptibility breakpoint. The target peak range in this study was selected because of uncertainty regarding potential toxicity with higher peak concentrations, particularly ototoxicity. Additionally, actual MICs were not available to allow calculation of a target peak range specifically for the study subjects. If a slightly wider peak range of 20 to 32 mg/L was acceptable where dosage adjustments might not be necessary, 54% of patients achieved target peak concentrations.
Achievement of higher peak concentrations (as high as 64.6 mg/L) than in the current study has been reported without evidence of significant ototoxicity or nephrotoxicity.9,11,15,16 These were small studies (range of 8 to 29 patients each) where tobramycin was infused over 5 to 30 minutes and where peak concentrations may have been obtained during the drug distribution phase in one of the studies. The long-term effects of high peak concentrations after repeated drug exposure are unknown. Once-daily aminoglycoside doses are theorized to be less toxic than multiple daily doses because of saturable uptake in inner ear and renal tubular cells and prolonged drug-free intervals that promote aminoglycoside clearance and minimize accumulation. A trend toward reduced hearing loss has been reported with ODD of tobramycin in adult and pediatric patients with CF,25 but the current study did not assess hearing with audiometry because it was not part of routine care. Some patients appear to be more susceptible to vestibular effects from high peak concentrations and short administration times of large doses, but they respond well to dose reduction.15 Extending the duration of infusion to 60 minutes has also been proposed to minimize vestibular effects from high aminoglycoside doses.10 The current study used 60-minute infusions to help prevent these adverse effects. However, two patients still experienced vestibular symptoms that subsided after dose reduction. These patients were the oldest in the study cohort (25 and 31 years of age) and were both female. Their peak serum concentrations from empiric doses were 33.5 mg/L and 34.4 mg/L but were 28 to 30 mg/L after dose reduction. Four other patients in the study ranging in age from 7 to 23 years achieved similar or higher peak concentrations without observed toxicities. Females older than 14 years have previously been found to have smaller volumes of distribution and higher peak serum concentrations than younger or male patients receiving equivalent weight-based doses, which may be explained by a higher proportion of body fat in older females.20 While the average peak serum level achieved in this study population was 28.7 mg/L and pharmacokinetic parameters were similar between different age cohorts, the patients who did not achieve a minimum peak of 20 mg/L were younger than patients whose peak concentrations exceeded 30 mg/L. It is expected that younger patients with larger volumes of distribution will achieve lower peak serum concentrations than older patients with smaller volumes of distribution when using the same weight-based dose. It may, therefore, be appropriate to consider different weight-based doses of ODD of tobramycin for older female CF patients, as suggested by Lam et al20 but this requires further study.
It is also thought that undetectable serum trough concentrations with ODD minimize the risk of nephrotoxicity through reduced drug accumulation compared to traditional aminoglycoside dosages, which is supported by studies in CF patients.1,7,9–13,15,16 In the current study, all calculated serum tobramycin trough concentrations were less than 0.05 mg/L, supporting the fast renal clearance previously reported in this patient population.15,17 There was a drug-free interval of 13.2 hours in this study population, adding further evidence of rapid drug clearance and lack of aminoglycoside accumulation. No evidence of nephrotoxicity was noted in the current study or in previous reports of CF patients treated with dosages of ≥10 mg/kg/day.9–13,15,16
Another advantage of ODD is the PAE of aminoglycosides, where there is sustained suppression of microbial growth after the serum concentration falls below MIC for the organism. This effect is reported to last up to 3 hours in vitro and up to 7.5 hours in a neutropenic animal model of P. aeruginosa infection.26,27 Therefore, a T < MIC of 3 to 7 hours in a 24-hour administration schedule is a reasonable target to maintain the PAE while minimizing drug accumulation and limiting toxicities. This time interval is likely to be longer in CF patients receiving ODD therapy because they exhibit rapid drug clearance. In this study, actual MICs were not available, so various MICs were used to estimate T < MIC. This timeframe ranged from 16 hours to more than 19 hours, which is similar to or longer than other studies and greatly exceeds the PAE.10,17,20 Bacterial regrowth and antibiotic resistance with long T < MIC intervals have not been well studied. Increased P. aeruginosa resistance was observed after 14 days of ODD in 17 adult CF patients compared to thrice daily tobramycin dosage in 13 patients.13 Unfortunately, patients were not recultured within weeks to months after study completion, so the long-term effects of ODD on resistance could not be characterized. Also, most CF patients being treated for pulmonary exacerbations are given 2 or more antibiotics in combination, so the clinical significance of prolonged aminoglycoside-free intervals and their effects on microbiology and clinical outcomes remains unknown.
It has been proposed that use of the pharmacokinetic parameter daily tobramycin serum AUC of ≤100 mg·hr/L may limit nephrotoxicity.24 A dosage regimen of 12 mg/kg every 24 hours would meet this goal and should not result in a higher risk of nephrotoxicity compared to the studied empiric regimen of 10 mg/kg given every 24 hours. However, the association between AUC and toxicity has not been validated in CF patients, who may be able to tolerate higher daily doses of aminoglycosides than non-CF patients without experiencing renal effects.
There is no clear consensus on the best way to administer and monitor ODD of tobramycin in CF patients. Interpatient variability precludes nomogram-based dosing, and therapeutic drug monitoring with 2 serum concentrations is still recommended for dose individualization.7,14,18,21 Proper serum sampling times for peak concentrations and use of appropriate modeling (1 compartment versus 2 compartment) must be followed in order to avoid incorrect assessment of pharmacokinetic parameters.7,18,21 The current study used a 1-compartment model with peak level measurement at 2 hours after initiation of a 60-minute infusion, which assured serum sampling times were postdistributional. Random concentrations were drawn 5 to 7 hours postinfusion to allow for pharmacokinetic analysis. It is recommended that monitoring trough serum concentrations with ODD in CF patients be avoided because they are likely to be below the lower limit of detection for commercial tobramycin assays and unable to be used for pharmacokinetic analysis.20–22 Use of AUC methods is recommended by some practitioners as a way to standardize drug exposure with different dosage strategies where the target daily AUC is approximately 100 mg·hr/L, but it is not common clinical practice to adjust dosages based on AUC values.7,11,21,28 Most CF centers use 10 mg/kg/day with 60-minute infusions, where doses are adjusted using pharmacokinetic analysis derived by linear regression or Bayesian analysis based on two postinfusion serum concentrations.28,29 Approximately half of CF centers using ODD of tobramycin report a desired peak concentration range of 20 to 29 mg/L, while another 20% target peak ranges that include up to 49 mg/L.28 Use of a peak target range broader or higher than 20 to 30 mg/L may be appropriate based on tobramycin pharmacodynamics, particularly for more resistant strains of P. aeruginosa, and clinical experience with minimal reports of ototoxicity or nephrotoxicity.
Pharmacokinetic simulations revealed two alternative regimens that would achieve appropriate serum tobramycin concentrations in most patients, similar to those included in this study. Tobramycin at 10 mg/kg given every 18 hours would result in improved achievement of peak concentrations in the current target peak range with slightly lower T < MIC values. However, the possibility of confusion and dosage error with this type of administration schedule discourages it from being used more widely and impairs its practicality, particularly in outpatients. In addition, if the desired T < MIC is 3 to 7 hours, use of this dosage schedule would not significantly improve the achievement of this parameter, and daily AUC values would exceed 100 mg·hr/L. Tobramycin at 12 mg/kg given every 24 hours would result in peak serum concentrations that are only slightly outside the target peak range used in this study. A small increase in acceptable peak serum concentrations up to 35 mg/L is not likely to result in more toxicity for most patients and would improve the ability to reach a peak level that maximizes pharmacodynamics, particularly for more resistant P. aeruginosa strains and when MIC data may not be readily available for individual patient dosage considerations. This regimen would also result in slightly lower T < MIC intervals than the current 10 mg/kg/day dosage, but this change is probably not clinically important if the PAE only lasts up to 7 hours. Individualization of doses through therapeutic drug monitoring remains necessary to ensure achievement of target serum concentrations, which may be different in patients with more resistant infections.
There are some limitations to this study that are worth noting. As previously mentioned, actual MICs for P. aeruginosa from CF sputum cultures were not determined routinely by the microbiology laboratory. T < MIC intervals were estimated using previously reported P. aeruginosa MICs in CF patients, so it is possible that the calculated T < MIC intervals were overestimates. Similarly, achievement of appropriate Cmax:MIC ratios could not be verified without actual MIC determinations. Individual dose adjustments were made to target peak tobramycin serum concentrations of 20 to 30 mg/L, but higher peak concentrations may be appropriate for patients with more resistant organisms. Dose reductions were not always performed when peak concentrations exceeded 30 mg/L in this study, suggesting that higher peak concentrations may be acceptable if tolerated. Also, patients were allowed to continue scheduled inhaled tobramycin therapy cycles to avoid disruption of outpatient therapy. It has been previously reported that inhaled tobramycin has little effect on peak serum concentrations, where 1 hour after a 300-mg dose, the average serum concentration is 0.92 mg/L.30 It was not determined if inhaled therapy had an effect on tobramycin serum concentrations in individual patients in this study. Administration dates and times of inhaled tobramycin were not readily available because, at the time of this study, documentation of inhaled therapies was performed in an administration record that was separate from the patient medical chart. Similarly, documentation of inhaled tobramycin dates and times was not routinely performed in outpatients. Satisfaction with ODD was measured with a nonvalidated scale, but this is the only study of ODD in CF patients that evaluated patient or caregiver satisfaction with this dosing approach. Patients and their families were highly satisfied with ODD in this study and preferred it to more frequent administration, even though most patients were receiving concurrent intravenous antibiotics that required multiple daily doses. Finally, because the primary focus of this study was an evaluation of serum concentrations achieved with an empiric dosage regimen, the long-term effects of ODD on clinical outcomes, toxicities, and microbial resistance could not be determined.
CONCLUSIONS
An empiric tobramycin regimen of 10 mg/kg every 24 hours did not achieve desired peak serum concentrations for most patients, although all patients demonstrated clinical improvements. Achievement of appropriate tobramycin concentrations in cystic fibrosis patients similar to those in this study could be improved by using an empiric dosage of 12 mg/kg every 24 hours, particularly if tobramycin peak concentrations of up to 35 mg/L are acceptable and patients are monitored closely for vestibular toxicity. Once-daily administration is preferable to limit potential drug administration errors and to maximize patient satisfaction with therapy, particularly for outpatients. Prospective evaluation of this regimen (12 mg/kg every 24 hours) with individualized pharmacokinetic monitoring and dose adjustment is needed to ensure safety and efficacy for all patients and to monitor the effects on microbial resistance patterns over time.
ACKNOWLEDGEMENTS
Portions of this research project were presented in abstract form at the 21st Annual North American Cystic Fibrosis Conference, Anaheim, CA, October 4-5, 2007, and at the 14th Annual Pediatric Pharmacy Advocacy Group Meeting, Chicago, IL, October 11, 2005. The authors acknowledge the contributions of the following individuals to this project: Angela Amble, PharmD; John H. Marks, MD; Margaret Schulte, PharmD; and Curtis Smith, PharmD.
ABBREVIATIONS
- CF
cystic fibrosis
- FEV1
Forced expiratory volume in one second
- MIC
minimum inhibitory concentration
- ODD
once-daily dosing
- PAE
post-antibiotic effect
Footnotes
DISCLOSURE The authors declare no conflicts or financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employment, gifts, and honoraria. As primary author, Heather L. VandenBussche had full access to the data in this study and was responsible for the integrity of the data and the accuracy of the data analysis.
REFERENCES
- 1.Kraus D, Manjunath P, Rodvold K, et al. Efficacy and tolerability of extended-interval aminoglycoside administration in pediatric patients. Paediatr Drugs. 2002;4(7):469–484. doi: 10.2165/00128072-200204070-00005. [DOI] [PubMed] [Google Scholar]
- 2.Craig WA. Once-daily versus multiple-daily dosing of aminoglycosides. J Chemother. 1995;7(suppl 2):S47–S52. [PubMed] [Google Scholar]
- 3.Moore RD, Lietman PS, Smith CR. Clinical response to aminoglycoside therapy: importance of the ratio of peak concentration to minimal inhibitory concentration. J Infect Dis. 1987;155(1):93–99. doi: 10.1093/infdis/155.1.93. [DOI] [PubMed] [Google Scholar]
- 4.Craig WA. Post-antibiotic effects in experimental infection models: relationship to in-vitro phenomena and to treatment of infections in man. J Antimicrob Chemother. 1993;31(suppl D):S149–S158. doi: 10.1093/jac/31.suppl_d.149. [DOI] [PubMed] [Google Scholar]
- 5.Spivey JM. The postantibiotic effect. Clin Pharm. 1992;11(10):865–875. [PubMed] [Google Scholar]
- 6.Contopoulos-Ioannidis DG, Giotis ND, Baliatsa DV, Ioannidis JP. Extended-interval aminoglycoside administration for children: a meta-analysis. Pediatrics. 2004;114(1):e111–e118. doi: 10.1542/peds.114.1.e111. [DOI] [PubMed] [Google Scholar]
- 7.Prescott WA, Nagel JL. Extended-interval once-daily dosing of aminoglycosides in adult and pediatric patients with cystic fibrosis. Pharmacotherapy. 2010;30(1):95–108. doi: 10.1592/phco.30.1.95. [DOI] [PubMed] [Google Scholar]
- 8.Smyth AR, Bhatt J. Once-daily versus multiple-daily dosing with intravenous aminoglycosides for cystic fibrosis. Cochrane Database Syst Rev. 2010;1:CD002009. doi: 10.1002/14651858.CD002009.pub3. [DOI] [PubMed] [Google Scholar]
- 9.Vic P, Ategbo S, Turck D, et al. Efficacy, tolerance, and pharmacokinetics of once daily tobramycin for pseudomonas exacerbations in cystic fibrosis patients. Arch Dis Child. 1998;78(6):536–539. doi: 10.1136/adc.78.6.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Whitehead A, Conway S, Etherington C, et al. Once-daily tobramycin in the treatment of adult patients with cystic fibrosis. Eur Respir J. 2002;19(2):303–309. doi: 10.1183/09031936.02.00221602. [DOI] [PubMed] [Google Scholar]
- 11.Master V, Roberts G, Coulthard K, et al. Efficacy of once-daily tobramycin monotherapy for acute pulmonary exacerbations of cystic fibrosis: a preliminary study. Pediatr Pulmonol. 2001;31(5):367–376. doi: 10.1002/ppul.1060. [DOI] [PubMed] [Google Scholar]
- 12.Smyth A, Tan K, Hyman-Taylor P, et al. Once versus three-times daily regimens of tobramycin treatment for pulmonary exacerbations of cystic fibrosis-the TOPIC study: a randomised control trial. Lancet. 2005;365(9459):573–578. doi: 10.1016/S0140-6736(05)17906-9. [DOI] [PubMed] [Google Scholar]
- 13.Burkhardt O, Lehmann C, Madabushi R, et al. Once-daily tobramycin in cystic fibrosis: better for clinical outcome than thrice-daily tobramycin but more resistance development? J Antimicrob Chemother. 2006;58(4):822–829. doi: 10.1093/jac/dkl328. [DOI] [PubMed] [Google Scholar]
- 14.Touw D, Knox A, Smyth A. Population pharmacokinetics of tobramycin administered thrice daily and once daily in children and adults with cystic fibrosis. J Cyst Fibros. 2007;6(5):327–333. doi: 10.1016/j.jcf.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 15.Bragonier R, Brown N. The pharmacokinetics and toxicity of once-daily tobramycin therapy in children with cystic fibrosis. J Antimicrob Chemother. 1998;42(1):103–106. doi: 10.1093/jac/42.1.103. [DOI] [PubMed] [Google Scholar]
- 16.Bates RD, Nahata MC, Jones JW, et al. Pharmacokinetics and safety of tobramycin after once-daily administration in patients with cystic fibrosis. Chest. 1997;112(5):1208–1213. doi: 10.1378/chest.112.5.1208. [DOI] [PubMed] [Google Scholar]
- 17.Beringer PM, Vinks AA, Jelliffe RW, Shapirio BJ. Pharmacokinetics of tobramycin in adults with cystic fibrosis: implications for once-daily administration. Antimicrob Agents Chemother. 2000;44(4):809–813. doi: 10.1128/aac.44.4.809-813.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aminimanizani A, Beringer PM, Kang J, et al. Distribution and elimination of tobramycin administered in single or multiple daily doses in adult patients with cystic fibrosis. J Antimicrob Chemother. 2002;50(4):553–559. doi: 10.1093/jac/dkf168. [DOI] [PubMed] [Google Scholar]
- 19.Massie J, Cranswick N. Pharmacokinetic profile of once daily intravenous tobramycin in children with cystic fibrosis. J Paediatr Child Health. 2006;42(10):601–605. doi: 10.1111/j.1440-1754.2006.00944.x. [DOI] [PubMed] [Google Scholar]
- 20.Lam W, Tjon J, Seto W, et al. Pharmacokinetic modelling of a once-daily dosing regimen for intravenous tobramycin in paediatric cystic fibrosis patients. J Antimicrob Chemother. 2007;59(6):1135–1140. doi: 10.1093/jac/dkm097. [DOI] [PubMed] [Google Scholar]
- 21.Coulthard KP, Peckham DG, Conway SP, et al. Therapeutic drug monitoring of once daily tobramycin in cystic fibrosis-caution with trough concentrations. J Cyst Fibros. 2007;6(2):125–130. doi: 10.1016/j.jcf.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 22.Hennig S, Norris R, Kirkpatrick C. Target concentration intervention is needed for tobramycin dosing in paediatric patients with cystic fibrosis–a population pharmacokinetic study. Br J Clin Pharmcol. 2007;65(4):502–510. doi: 10.1111/j.1365-2125.2007.03045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Begg EJ, Barclay ML, Duffull SB. A suggested approach to once-daily aminoglycoside dosing. Br J Clin Pharmacol. 1995;39(6):605–609. [PMC free article] [PubMed] [Google Scholar]
- 24.Murry KR, McKinnon PS, Mitrzyk B, Rybak MJ. Pharmacodynamic characterization of nephrotoxicity associated with once-daily aminoglycoside. Pharmacotherapy. 1999;19(11):1252–1260. doi: 10.1592/phco.19.16.1252.30876. [DOI] [PubMed] [Google Scholar]
- 25.Mulheran M, Hyman-Taylor P, Tan KH, et al. Absence of cochleotoxicity measured by standard and high-frequency pure tone audiometry in a trial of once- versus three-times daily tobramycin in cystic fibrosis patients. Antimicrob Agents Chemother. 2006;50(7):2293–2299. doi: 10.1128/AAC.00995-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fantin B, Ebert S, Leggett J, et al. Factors affecting duration of in-vivo postantibiotic effect for aminoglycosides against gram-negative bacilli. J Antimicrob Chemother. 1991;27(6):829–836. doi: 10.1093/jac/27.6.829. [DOI] [PubMed] [Google Scholar]
- 27.Vogelman B, Gudmundsson S, Turnidge J, et al. In vivo postantibiotic effectin a thigh infection in neutropenic mice. J Infect Dis. 1988;157(2):287–298. doi: 10.1093/infdis/157.2.287. [DOI] [PubMed] [Google Scholar]
- 28.Van Meter DJ, Corriveau M, Ahern JW, Lahiri T. A survey of once-daily dosage tobramycin therapy in patients with cystic fibrosis. Pediatr Pulmonol. 2009;44(4):325–329. doi: 10.1002/ppul.20985. [DOI] [PubMed] [Google Scholar]
- 29.Phillips JA, Bell SC. Aminoglycosides in cystic fibrosis: a descriptive study of current practice in Australia. Intern Med J. 2001;31(1):23–26. doi: 10.1046/j.1445-5994.2001.00010.x. [DOI] [PubMed] [Google Scholar]
- 30.Geller DE, Pitlick WH, Nardella PA, et al. Pharmacokinetics and bioavailability of aerosolized tobramycin in cystic fibrosis. Chest. 2002;122(1):219–226. doi: 10.1378/chest.122.1.219. [DOI] [PubMed] [Google Scholar]