Abstract
OBJECTIVE
Arginine vasopressin (AVP) is the primary regulator of free water retention through its interactions with the AVP type 2 receptor (V2). As opposed to the natriuresis and diuresis that occur with loop and thiazide diuretics, conivaptan is an AVP V1A/V2 receptor antagonist, which enhances free water excretion while minimizing sodium loss. We report our preliminary experience with conivaptan to promote diuresis in infants with functional or structural cardiac disease.
METHODS
A retrospective cohort study was conducted of infants who had received conivaptan from August 2007 to January 2008. A loading dose of conivaptan (0.3-0.6 mg/kg) was followed by a continuous infusion of 0.01-0.02 mg/kg/hr for 24 hours. Sodium, potassium, chloride, blood urea nitrogen (BUN), creatinine, bicarbonate, and urine output were measured prior to the start of conivaptan and at 24 hours after initiation of the infusion.
RESULTS
Conivaptan was administered intravenously on 6 occasions to 5 patients with hypervolemic hyponatremia. Patients ranged in age from 8 to179 days, and body weight ranged from 3 to 4.12 kg. Mean sodium concentration increased from 130.17 ± 1.94 mEq/L to 133.67 ± 3.88 mEq/L (p=0.048), and median urine output increased from 4.15 to 5.05 mL/kg/hr (p=0.286). No significant changes were noted in serum potassium, bicarbonate, creatinine, or BUN. No adverse effects were noted during conivaptan infusion.
CONCLUSION
Intravenous conivaptan is effective for increasing serum sodium levels and may be a potential adjuvant to enhance diuresis in children with cardiac disease. Given the potential benefits of conivaptan compared to diuretic therapy, with all their potential complications, prospective trials are warranted.
INDEX TERMS: arginine vasopressin receptor antagonist, conivaptan, diuresis, hyponatremia, pediatric
INTRODUCTION
Adequate diuresis is an important component in the management of patients with structural cardiac disease or ventricular dysfunction (functional cardiac disease). Conivaptan (Vaprisol; Astellas Pharma Inc., Deerfield, IL) is a nonpeptide benzazepine derivative that acts as a dual antagonist at the arginine vasopressin (AVP) type V1A and V2 receptors.1 The dual V1A/ V2 receptor blockade provided by conivaptan may offer a therapeutic option in the management of hypervolemia and hyponatremia in pediatric patients with heart failure or low cardiac output states that are unresponsive to conventional diuretic therapy. Currently, these patients are managed by using thiazide and loop diuretics, which have the undesirable adverse effects of hypokalemia and hyponatremia. Studies in adults have demonstrated that although diuretic therapy increases urine output, glomerular filtration rate decreases, and incidence of postoperative renal dysfunction increases.2,3 Additionally, the increase in systemic vascular resistance caused by these agents may compromise cardiac output.3
Fluid retention may be problematic during the postoperative period as pathophysiological changes lead to free water retention, leading to edema and hyponatremia.4 This resultant hyponatremia increases fluid movement into the interstitial space, further complicating the care of patients with decreased cardiac output.5 Conivaptan is currently US Food and Drug Administration (FDA)-approved for use in adults with euvolemic hyponatremia as well as hypervolemic hyponatremia. Although conivaptan has been used to reverse hyponatremia and establish diuresis with reduced risk for hypokalemia in critically ill adults with congestive heart failure of various causes,6 it is not recommended for this specific purpose. This is also highlighted by current product information, which states that conivaptan has not been shown to be effective for the treatment of the signs and symptoms of heart failure and is not approved for this indication.1 The therapeutic role of conivaptan in maintaining fluid and sodium balance in critically ill pediatric patients with potential for hemodynamic instability remains to be determined and needs further study. This retrospective study reviews our experience with conivaptan as adjuvant therapy to promote diuresis in infants with either structural or functional cardiac disease.
METHODS
Patient Selection
The University of Tennessee Health Science Center Institutional Review Board approved the study, considering it minimal risk. All patients with cardiovascular disease being managed by pediatric cardiology, who received conivaptan as an adjunct diuretic over an 18-month period from August 2007 to January 2009 were included. Structural cardiac disease was determined by clinical examination and echocardiography. Functional cardiac disease, including right ventricular dysfunction, was determined by decreased ventricular systolic shortening as noted with echocardiography by an attending cardiologist. Demographic information including age at time of infusion, sex, weight, diagnoses, and other diuretic use during the time of infusion were obtained. Values of heart rate, maximum and minimum mean arterial blood pressures (MAP), urine output (mL/kg/hr); concentrations of serum sodium (mEq/L), potassium (mEq/L), chloride (mEq/L), and bicarbonate (mEq/L); blood urea nitrogen (BUN) and creatinine concentration (mg/dL) were collected for the purpose of this study for the 24 hours before and after infusion of conivaptan was started. Patients were monitored for hypertension, defined as blood pressure >95% for age and height requiring antihypertensive therapy, and hypotension, defined as blood pressure requiring inotropic support, discontinuation of the infusion or fluid bolus, during the use of conivaptan.
In all cases, conivaptan was administered in addition to the diuretic regimen after hyponatremia had been diagnosed (serum sodium, <134 mEq/L). The dose used in our cohort was extrapolated from adult studies, which have used a 20- to 40-mg loading dose followed by a continuous infusion of 40 mg/day. Assuming an average adult weight of 70 kg, we determined a loading dose of 0.3 to 0.6 mg/kg, followed by a continuous infusion of 0.01 to 0.02 mg/kg/hr.1,8–10,19
The patients received a loading dose of either 0.3 (n=4) or 0.6 (n=2) mg/kg, which was administered over 30 minutes. This was followed by an infusion of 0.01 (n=3) to 0.02 (n=3) mg/kg/hr. The diuretic regimen was continued prior to and during the administration of conivaptan. The drug is supplied as a 100-mL, single-use premixed solution containing 20 mg of conivaptan hydrochloride. The pharmacy then prepared the medication into an exact premixed pediatric weight-based dose, separately for the loading dose and the continuous infusion for a 24-hour period.
Statistical Analysis
Mean serum sodium, potassium, BUN, creatinine, bicarbonate, and maximum and minimum values during the study period for systolic blood pressure, diastolic blood pressure, mean blood pressure, and heart rate for 24 hours before starting and during use of conivaptan were analyzed using a paired t-test. Urine output was expressed as a median and analyzed with an unpaired t-test, as it does not exhibit a normal distribution. Data were analyzed using Statistical Package for the Social Sciences version 17 software (SPSS; 2008, IBM).
RESULTS
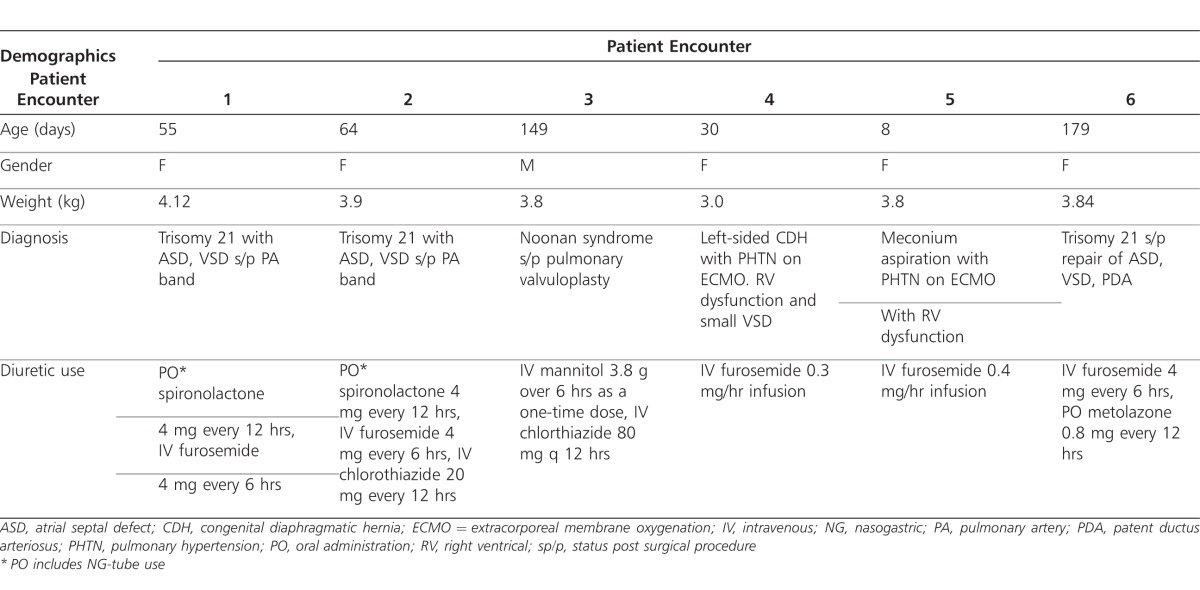
Conivaptan was administered on 6 separate occasions to 5 patients during the study. It should be noted that all patients were not receiving maximum diuretic therapy; however, with the resultant hyponatremia, additional conventional diuretics were deemed ineffective by the attending physician. Patients were either being managed after cardiac surgery for structural disease (n=3) or were receiving venoarterial extracorporeal membrane oxygenation (VA ECMO) with significant right ventricular dysfunction (n=2). Median age was 80.8 days (range, 8-179 days) and median weight was 3.74 kg (range, 3-4.1 kg). The median duration of infusion was 48 hours (range, 16-72 hr). Clinical and demographic data are presented in Table 1.
Table 1.
Clinical data and demographics of conivaptan encounters
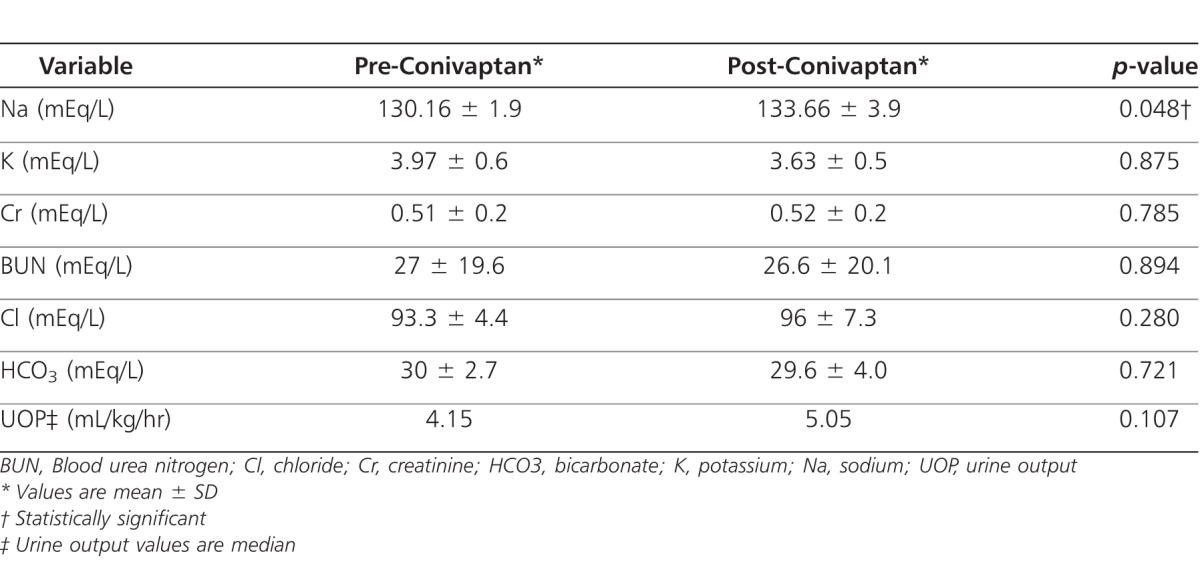
Over the time of conivaptan infusion, mean serum sodium increased by 3.5 ± 3.3 mEq/L, (p=0.048), and median urine output increased by 18% to 5.05 mL/kg/hr (p=0.107) during the administration of conivaptan (Figures 1 and 2). Serum potassium decreased from 3.97 prior to 3.63 after 24 hours of infusion (p=0.875) (Table 2). No statistically significant differences were noted in serum creatinine, bicarbonate, and BUN after 24 hours of infusion (Table 2).
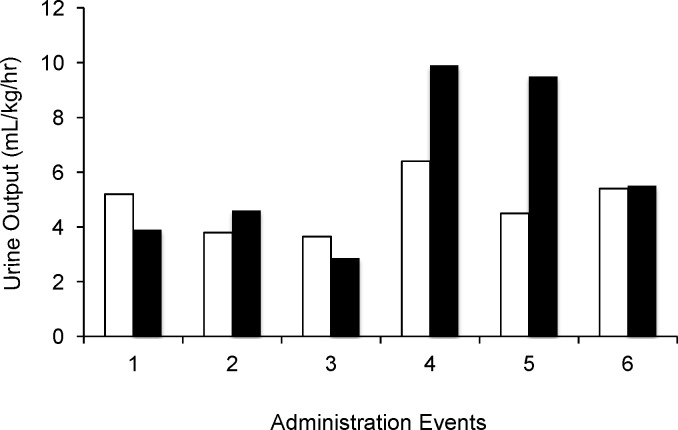
Figure 1.
Urine output before and after the 24-hour infusion of conivaptan
Urine output for 6 encounters in 5 patients raw data (p=0.107)
Before conivaptan (white bars); After conivaptan (black bars)
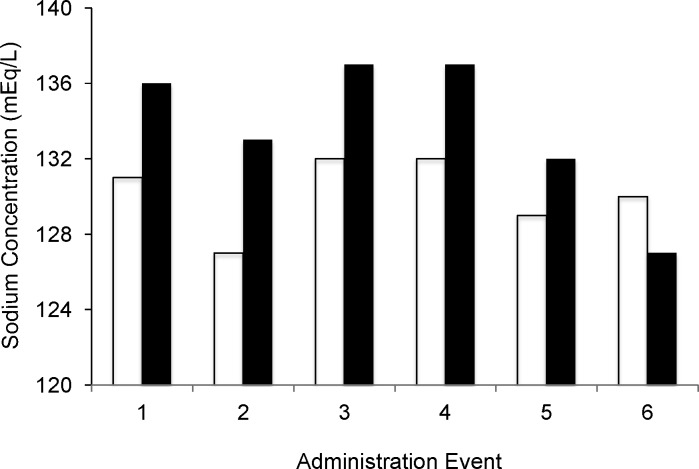
Figure 2.
Serum sodium before and after the 24-hour infusion of conivaptan
Serum sodium for 6 encounters in 5 patients raw data (p=0.048)
Before conivaptan (white bars); After conivaptan (black bars)
Table 2.
Change in various biochemical parameters before and after the 24 hour infusion of conivaptan in 5 patients receiving 6 courses of conivaptan
No significant changes were seen in MAP, systolic blood pressure, or diastolic blood pressure during the administration of conivaptan or for the 24-hour period postinfusion. Before conivaptan was administered, maximum MAP was 67 ± 9 mm Hg and 74 ± 5.1 mm Hg during the infusion (p=0.803). Minimum MAP was 49 ± 8 mm Hg before, and 40 ± 7 mm Hg during the administration of conivaptan (p=0.878). Heart rate increased during the use of conivaptan from a baseline of 157 ± 23 beats/minute to 172 ± 18 beats/minute (p=0.003).
No adverse events were noted during the use of conivaptan. There were no episodes of hypertension or hypotension during the study period. Also, there were no instances of hypernatremia, defined as serum sodium greater than 148 mEq/L. There was a tendency toward hypokalemia, which was not statistically significant.
DISCUSSION
Our study represents the first reported use of conivaptan in a series of critically ill infants with structural or functional cardiac disease. To date, there is only one case report of the use of conivaptan in pediatrics.7 Conivaptan has been used in adult studies primarily to correct hyponatremia and elevate serum Na in clinically divergent conditions such as heart failure and neurotrauma. In this study, conivaptan seemed to be well tolerated, increasing mean serum sodium by 3.5 ± 3.3 mEq/L (p=0.048) and median urine output by 18% (p=0.107). Serum potassium did decrease from 3.9 to 3.6 mEq/L (p=0.875). Studies of conivaptan use in adults did note hypokalemia was a relatively frequent adverse effect.8,9
AVP acts on the V2 receptors, which are coupled to aquaporin channels in the principal cells of the collecting ducts in the kidney, to promote the reabsorption of free water.11 Antagonism of the V2 receptors by conivaptan produces aquaresis, increased urine output, and decreased urine osmolality with potentially less electrolyte derangements.12 The use of an AVP antagonist may be particularly beneficial in patients with congestive heart failure, as elevated AVP levels, with accompanying fluid retention and hyponatremia, have been demonstrated in this population.8,14–16 Pilot studies of conivaptan in the adult population have shown increased effectiveness with even higher doses of 80 to 120 mg/day.8,9,14–16,18
The 1 patient who did not have an increase in serum sodium was also receiving metolazone and spironolactone as part of the diuretic regimen. Spironolactone competitively inhibits the aldosterone-dependent sodium/potassium exchange sites in the distal tubules, leading to increased secretion of water and sodium and decreased excretion of potassium.20 It is possible that the tubular effects of spironolactone, in combination with the potent natriuretic effect of metolazone, did not allow for an increase in serum sodium in this patient. The increase in serum sodium alone could prove beneficial in that hyponatremia is associated with resistance to conventional diuretics.22 In addition, hyponatremia has been linked to morbidity and even mortality in congestive heart failure.23 Even though the rationale for symptomatic benefit exists in heart failure, the data need validation by randomized prospective trials, as shown by the product information that still considers conivaptan to be unproven therapy in heart failure.1
Conivaptan, as adjunct diuretic therapy, increased urine output by 25%. This value, while not statistically significant, may be clinically relevant in volume-overloaded infants. In caring for hypervolemic infants with risk for hemodynamic instability, there are few diuretics available for fluid removal. Drugs such as conivaptan that seem to be well tolerated could be clinically useful as adjunct therapy but require prospective trials meeting statistical significance to become recommended therapy.
The 2 patients with the greatest increase in urine output were receiving VA ECMO for pulmonary hypertension. Although the primary diagnosis was pulmonary hypertension, both patients had significant right ventricular dysfunction demonstrated by decreased systolic shortening fraction by echocardiography. This contrasts with a previous report that showed that antidiuretic hormone levels decrease in the setting of neonatal VA ECMO,17 which theoretically should make conivaptan less effective. Our understanding of conivaptan's mechanism of action could be enhanced by including antidiuretic hormone levels as well as urine sodium levels in future studies.
The patients in our cohort were admitted after cardiac operations or with right ventricular dysfunction requiring VA ECMO. In this high-risk group prone to hemodynamic instability, it would appear that conivaptan used as adjuvant diuretic therapy was reasonably tolerated with some efficacy. Our study though inadequately powered with a relatively diverse sample, should generate impetus for larger prospective randomized studies that investigate the safety, efficacy, adverse effects and define a role for this novel agent in the pediatric population. Also, the short window of time of conivaptan use limits the identification of the medication's long-term effects on the patient's cohort.
ACKNOWLEDGEMENTS
The authors would like to thank Dr. Gregory Stidham for valuable assistance in getting this project started and Carol Looney for reviewing the manuscript.
ABBREVIATIONS
- AVP
Arginine vasopressin
- AVP V1A
AVP type 1a receptor
- AVP V2
AVP type 2 receptor
- ECMO
Extra Corporeal Membrane Oxygenation
- MAP
mean arterial blood pressure
Footnotes
DISCLOSURE The authors declare no conflicts or financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employment, gifts, and honoraria.
REFERENCES
- 1.Product information: Vaprisol injection, conivaptan HCl injection. Astellas Pharma US, Inc; Deerfield, IL: May, 2010. [Google Scholar]
- 2.Gottlieb SS, Brater DC, Thomas I, et al. BG9719 (CVT-124), an A1 adenosine receptor antagonist, protects against the decline in renal function observed with diuretic therapy. Circulation. 2002;105(11):1348–1353. doi: 10.1161/hc1102.105264. [DOI] [PubMed] [Google Scholar]
- 3.Connelly TP, Francis GS, Williams KJ, et al. Acute vasoconstrictor response to intravenous furosemide in patients with chronic congestive heart failure. Activation of the neurohumoral axis. Am Heart J. 1994;127:392–399. doi: 10.7326/0003-4819-103-1-1. [DOI] [PubMed] [Google Scholar]
- 4.Judd BA, Haycock GB, Dalton N, Chantler C. Hyponatraemia in premature babies and following surgery in older children. Acta Paediatr Scand. 1987;76(3):385–393. doi: 10.1111/j.1651-2227.1987.tb10487.x. [DOI] [PubMed] [Google Scholar]
- 5.Rasool A, Palevsky PM. Treatment of edematous disorders with diuretics. Am J Med Sci. 2000;319(1):25–37. doi: 10.1097/00000441-200001000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Ferguson-Myrthil N. Novel agents for the treatment of hyponatremia: a review of conivaptan and tolvaptan. Cardiol Rev. 2010;18(6):313–321. doi: 10.1097/CRD.0b013e3181f5b3b7. [DOI] [PubMed] [Google Scholar]
- 7.Rianthavorn P, Cain JP, Turman MA. Use of conivaptan to allow aggressive hydration to prevent tumor lysis syndrome in a pediatric patient with large-cell lymphoma and SIADH. Pediatr Nephrol. 2008;23(8):1367–1370. doi: 10.1007/s00467-008-0809-y. [DOI] [PubMed] [Google Scholar]
- 8.Goldsmith SR, Elkayam U, Haught WH, et al. Efficacy and safety of the vasopressin V1A/V2-receptor antagonist conivaptan in acute decompensated heart failure: A dose-ranging pilot study. J Card Fail. 2008;14(8):641–647. doi: 10.1016/j.cardfail.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 9.Arai Y, Fujimori A, Sasamata M, et al. New topics in vasopressin receptors and approach to novel drugs: research and development of conivaptan hydrochloride (YM087), a drug for the treatment of hyponatremia. J Pharmacol Sci. 2009;109(1):53–59. doi: 10.1254/jphs.08r17fm. [DOI] [PubMed] [Google Scholar]
- 10.Burnier M, Fricker AF, Hayoz D, et al. Pharmacokinetic and pharmacodynamic effects of YM087, a combined V1/V2 vasopressin receptor antagonist in normal subjects. Eur J Clin Pharmacol. 1999;55(9):633–637. doi: 10.1007/s002280050685. [DOI] [PubMed] [Google Scholar]
- 11.Nielsen S, Frokiaer J, Marples D, et al. Aquaporins in the kidney: from molecules to medicine. Physiol Rev. 2002;82(1):205–244. doi: 10.1152/physrev.00024.2001. [DOI] [PubMed] [Google Scholar]
- 12.Tomura Y, Tahara A, Tsukada J, et al. Pharmacologic profile or orally administered YM087, a vasopressin antagonist, in conscious rats. Clin Exp Pharmacol Physiol. 1999;26(5-6):399–403. doi: 10.1046/j.1440-1681.1999.03045.x. [DOI] [PubMed] [Google Scholar]
- 13.Lee CR, Watkins ML, Patterson JH, et al. Vasopressin: a new target for the treatment of heart failure. Am Heart J. 2003;146(1):9–18. doi: 10.1016/S0002-8703(02)94708-3. [DOI] [PubMed] [Google Scholar]
- 14.Goldsmith SR, Francis GS, Cowley AW, et al. Increased plasma arginine vasopressin levels in patients with congestive heart failure. J Am Coll Cardiol. 1983;1(6):1385–1390. doi: 10.1016/s0735-1097(83)80040-0. [DOI] [PubMed] [Google Scholar]
- 15.Szatalowicz VL, Arnold PE, Chaimovitz C, et al. Radioimmunoassay of plasma arginine vasopressin in hyponatremic patients with congestive heart failure. N Engl J Med. 1981;305(5):263–2666. doi: 10.1056/NEJM198107303050506. [DOI] [PubMed] [Google Scholar]
- 16.Francis GS, Benedict C, Johnstone DE, et al. Comparison of neuroendocrine activation in patients with left ventricular dysfunction with and without congestive heart failure: a substudy of the studies of left ventricular dysfunction (SOLVD) Circulation. 1990;82(5):1724–1729. doi: 10.1161/01.cir.82.5.1724. [DOI] [PubMed] [Google Scholar]
- 17.Semmekrot BA, Pesman GJ, Span PN, et al. Serial plasma concentrations of atrial natriuretic peptide, plasma renin activity, aldosterone, and antidiuretic hormone in neonates on extracorporeal membrane oxygenation. ASAIO J. 2002;48(1):26–33. doi: 10.1097/00002480-200201000-00007. [DOI] [PubMed] [Google Scholar]
- 18.Annane D, Decaux G, Efficacy Smith N. and safety of oral conivaptan, a vasopressin-receptor antagonist, evaluated in a randomized, controlled trial in patients with euvolemic or hypervolemic hyponatremia. Am J Med Sci. 2009;337(1):28–36. doi: 10.1097/MAJ.0b013e31817b8148. [DOI] [PubMed] [Google Scholar]
- 19.Reilly T, Schork MR. Vasopressin antagonists: pharmacotherapy for the treatment of heart failure. Ann Pharmacother. 2010;44(4):680–687. doi: 10.1345/aph.1M660. [DOI] [PubMed] [Google Scholar]
- 20.Horisberger JD, Giebisch G. Potassium-sparing diuretics. Ren Physiol. 1987;10(3-4):198–220. doi: 10.1159/000173130. [DOI] [PubMed] [Google Scholar]
- 21.Paterna S, Di Pasquale P, Parrinello G, Amato P, et al. Effects of high-dose furosemide and small-volume hypertonic saline solution infusion in comparison with a high dose of furosemide as a bolus, in refractory congestive heart failure. Eur J Heart Fail. 2000;2(3):305–313. doi: 10.1016/s1388-9842(00)00094-5. [DOI] [PubMed] [Google Scholar]
- 22.Krämer BK, Schweda F, Riegger GA. Diuretic treatment and diuretic resistance in heart failure. Am J Med. 1999;106(1):90–96. doi: 10.1016/s0002-9343(98)00365-9. [DOI] [PubMed] [Google Scholar]
- 23.Orem RM. Hyponatremia in congestive heart failure. Am J Cardiol. 2005;95(9A):2B–7B. doi: 10.1016/j.amjcard.2005.03.002. [DOI] [PubMed] [Google Scholar]