Abstract
Microparticulate systems for delivery of therapeutics to DCs for immunotherapy have gained attention recently. However, reports addressing the optimization of DC-targeting microparticle delivery systems are limited, particularly for cases where the goal is to deliver payload to DCs in a non-activating fashion. Here, we investigate targeting DCs using poly (d lactide-co-glycolide) microparticles (MPs) in a non-stimulatory manner and assess efficacy in vitro and in vivo. We modified MPs by surface immobilizing DC receptor targeting molecules – antibodies (anti-CD11c, anti-DEC-205) or peptides (P-D2, RGD), where anti-CD11c antibody, P-D2 and RGD peptides target integrins and anti-DEC-205 antibody targets the c-type lectin receptor DEC-205. Our results demonstrate the modified MPs are neither toxic nor activating, and DC uptake of MPs in vitro is improved by the anti-DEC-205 antibody, the anti-CD11c antibody and the P-D2 peptide modifications. The P-D2 peptide MP modification significantly improved DC antigen presentation in vitro both at immediate and delayed time points. Notably, MP functionalization with P-D2 peptide and anti-CD11c antibody increased the rate and extent of MP translocation in vivo by DCs and MΦs, with the P-D2 peptide modified MPs demonstrating the highest translocation. This work informs the design of non-activating polymeric microparticulate applications such as vaccines for autoimmune diseases.
Introduction
Poly (lactic-co-glycolic acid) (PLGA) particulate systems have gained widespread attention as a viable option for delivery of vaccines in the last quarter century.[1] PLGA particles at the micron and sub-micron sizes are contemporary immuno-therapy tools being used to deliver antigen[2,3], adjuvant[4], DNA[5–7] and pharmacological drugs[8,9]. PLGA as a biomaterial has been extensively characterized and has been shown to demonstrate qualities such as biocompatibility, biodegradability[1,10] Additionally, PLGA particulate systems offer control of size and shape of the delivery system, hydrophobicity, loading and release kinetics of a wide range of biomolecules, modulation of immunogenicity, antigen processing and presentation. Furthermore, PLGA particulate matter provide capability for surface functionalization.[11] These qualities combined make PLGA microparticulate systems ideal for vaccine delivery to antigen presenting cells (APCs) including dendritic cells (DCs).
First discovered in 1973 by the Steinman group, it is now well understood that DCs are directly involved in initiation and modulation of T cell and B cell immunity.[12] Dendritic Cells are the most efficient antigen presenting cells due to their exceptional ability to uptake, process and present antigen.[13–15] More recently, it has been recognized that DCs play a critical role in central tolerance and maintenance of peripheral tolerance. The implication is that through DCs, the direction and magnitude of immune response can be manipulated. Therefore, DCs present a therapeutic target for modulation of autoimmune diseases and transplant rejection.[16]
The versatility of DCs to guide immune responses is attributed to its lineage and, maturation state.[12] Immature dendritic cells (iDCs) circulate throughout the body and are able to ‘scavenge’ pathogens, foreign materials, and apoptotic or necrotic cells. They are equipped with a wide array of endocytic and phagocytic surface receptors that recognize a host of molecules including proteins, lipids, sugars, glycoproteins, glycolipids and oligonucleotides.[17,18] Notably, the receptor type engaged during phagocytosis by DCs directs subsequent change in maturation.[18] Researchers have sought to exploit these traits by incorporating targeting molecules such as pathogen-associated molecular patterns, and antibodies against surface receptors in conjunction with proteins, polymeric particles and other drug carriers.[19–21] These approaches are intended to augment drug uptake by DCs as well as bolster adjuvant activity for increased immunogenicity.[20,22–24] However, there are numerous applications in which targeting factors to DCs in a non-stimulating context is perceived to be desirable such as microparticle (MP) -based vaccines correcting T1D.[25] For non-stimulatory applications, DC receptors that do not trigger immuno-stimulatory pathways, or that are tolerance-inducing, are appropriate.
The endocytic receptor, DEC-205 (CD205) represents one such potential candidate for non-activation DC-targeting. DEC-205 is an integral membrane protein highly expressed on the surface of DCs found in lymphoid areas critical for immunity and tolerance.[26] It is a member of the C-type lectin family which binds carbohydrates and mediates endocytosis.[26] Considerable effort has gone towards targeting DCs via DEC-205 antibodies and single-chain fragment variables (scFv).[21,23,27] Bonifaz et al. showed that proteins targeted to this receptor improve antigen presentation by 100-fold.[21] Further, DEC-205 targeting has been linked with DC ability to induce tolerance in vitro as well as in animal models.[28,29] Therefore, iDCs can possibly be primed along a tolerogenic pathway through targeting of the DEC-205 receptor. The implications of this cannot be overstated if the goal is the development of a DC-targeting MP vaccine for autoimmune diseases. Another surface receptor abundantly present on DCs which provides a rational choice for DC targeting is the CD11c surface molecule. The CD11c/CD18 protein is part of the family of β2 integrins expressed exclusively by leukocytes particularly myeloid DCs.[13,30] Targeting of DCs via the CD11c antibodies has been shown to enhance humoral responses in mice.[31] [13,30] In addition to the use of antibodies, DC-specific targeting through the CD11c surface receptor may also be effected through the use of receptor-binding peptides. The P-D2 peptide is derived from the Ig-like domain 2 of intercellular adhesion molecule 4 (ICAM-4).[32] All four members of the β2 integrin family have a strong binding affinity for ICAM-4 which has been shown to be involved in erythrophagocytosis – a process thought to be involved in self recognition and immune homeostasis.[33,34] While we are not aware of any work which directly provides evidence that P-D2 peptide could enhance DC phagocytosis, blocking studies by Ihanus et al. highlight the high affinity that P-D2 peptide has for CD11c, and motivate investigation for use in MP targeting.[32] The use of targeting peptides has the benefit that, unlike whole antibodies, it won’t bind to inflammation-causing Fc receptors and are less expensive to produce than antibodies.[35]
The goal of coupling DC targeting ligands to the surface of MPs is to provide increased payload of drug/biological/antigen delivered, thereby improving response and reducing the number of administrations required. The DC targeting ligands investigated here may function in a non-activating manner, which could prove useful for applications such as immunotherapies to correct autoimmune diseases or promote transplant tolerance.
Methods and Materials
Preparation of Fluorescent Microparticles
A 50:50 polymer composition of poly(d lactide-co-glycolide) (PLGA; average molecular weight ~ 44,0000 g/mol) in methylene chloride (Lactel, AL, USA) was used to generate MPs. Poly-vinyl alcohol (PVA; molecular weight ~ 100,000 g/mol) was purchased from Fisher Scientific (NJ, USA) and was used as an emulsion stabilizer. Distilled water (DiH2O) was used as the aqueous phase to form the emulsions while methylene chloride (Fisher Scientific, NJ, USA) was used as the organic solvent to dissolve PLGA polymer. Microparticles were formed using a standard oil-water solvent evaporation technique.
Briefly, 100 mg of PLGA polymer was dissolved in methylene chloride at 5% w/v ratio. Fluorescent dye (either rhodamine-6g [Sigma-Aldrich, MO,USA], 1,1'-dioctadecyl-3,3,3',3'-tetramethylindodicarbocyanine, 4-chlorobenzenesulfonate salt [DiD; Invitrogen, Karlsruhe, Germany] or Anthracenecarboxylic acid [ANC; Fluka, Buchs, Switzerland]) was directly loaded into 2 ml of the 5% PLGA in methylene chloride solution and emulsified at 35,000 rpm for 180 s using a tissue-miser homogenizer (Fisher Scientific, NJ, USA) to form a primary emulsion. The primary emulsion was added to 2 mL of 5% PVA solution in DiH2O and the homogenizing was continued at 19,500 rpm for 60 s. This was added to 30 mL of 0.5% PVA solution. The particles thus formed were agitated using a magnetic stirrer (Fisher Scientific, NJ, USA) for 24 h to evaporate residual methylene chloride. The remaining solution was centrifuged at 10,000 ×g for 10 min to collect MPs which were subsequently washed three times with DiH2O. The water was aspirated from the centrifuged MPs, which were then flash-frozen in liquid nitrogen and kept under vacuum in dry ice overnight. The MPs were stored at −20 °C until used. Reagents whose vendors were not specified were purchased from Sigma-Aldrich.
Cross-linking of Ligands to Microparticles
Ligands were tethered to the surface of PLGA MPs by conjugation of unbound available amine groups in ligands to free carboxyl terminals of the polymer using carbodiimide chemistry. The free carboxyl groups on PLGA MPs were activated with a 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC) (Acros Organics, Belgium) and N-hydroxysulfosuccinimide (NHS) (Pierce Biotechnology, IL, USA) solution for 15 min while agitating at 50 rpm. The ligand (e.g. Anti-mouse CD11c mAb[clone HL3, IgG1, λ2 (BD Pharmingen)], Anti-mouse DEC-205 mAb [clone 205yekta, IgG2a (Ebioscience)]) was introduced to the suspension which was shaken vigorously for 16 h. After incubation, the particles were centrifuged at 10,000 rpm for 10 min and washed twice with phosphate buffered saline (PBS) (Hyclone, UT, USA) and resuspended for use. The peptides, P-D2 [amino acid sequence: GGVTLTYQFAAGPRDK] and RGD [amino acid sequence: GGGRGDSPCGGDK] were produced by the University of Florida ICBR peptide synthesis core facility. For quantification purposes, a fluorescent tag (either 5-carboxytetramethylrhodamine [5-TAMRA] or 7-amino-4-methylcoumarin [MCA]) was added to the carboxyl terminal of these peptides, leaving the amino terminus free to target for crosslinking to MPs. The concentration of the ligands used during the conjugation step was based on 10:1 mole ratio of the ligand to the amount of PLGA polymer present.
As a control group for some experiments, polyethylene glycol moieties were adsorbed onto the surface of MPs by incubating a known mass of PLGA MPs in a 10% Pluronic F127 (BASF) solution for 16 h under gentle agitation. This group was classified as the PEG MP group.
Quantification of Cross-linked Ligands
The method used to quantify conjugated ligand levels was dependent on ligand type. For peptides, each PLGA MP batch (1 mg dry mass) conjugated with fluorescently-tagged polypeptide was suspended in 100 µL of 6 mg/ml bovine pancreatic trypsin (MP Biomedicals LLC, Solon, OH) to cleave the fluorescent tag portion of the polypeptide derivative from the surface of the PLGA MPs. Standards containing TAMRA-RGD and trypsin in solution were incubated simultaneously. The suspensions were centrifuged to pellet MPs and 75 µL of supernatant were transferred to black, flat-bottom, half area, polystyrene 96 well plates (Corning Inc., Corning, NY) and fluorescence quantified using a SpectraMax M5 plate reader (Molecular Devices, LLC).
Antibodies tethered onto the surface of PLGA MPs were quantified using a dot blot procedure. Antibody-conjugated MPs incubated with 6 mg/mL trypsin overnight. Samples were then boiled in 2% SDS 15 min, cooled and were spotted onto a PVDF membrane (BioRad). Standards consisting of serial dilutions of the trypsinized antibody in 2% SDS were also spotted onto the PVDF membrane. The spotted membranes were allowed to dry for 3 h at room temperature and blocked overnight at 4°C using 20 mL of Blotto (5% milk casein, 0.2% Tween-20, 0.02% sodium azide in PBS). Blocking solution was then decanted off and 20 mL of the antibody solution (1:10,000 dilutions of alkaline phosphatase-tagged antibody raised against the tethered antibody) was incubated with the membrane at room temperature for 1 h with gentle shaking. The antibody solution was then removed and three 20 ml washes with TBS-tween (20 mM Tris pH 7.6, 0.8% NaCl, 0.1% Tween-20 in deionized water) were performed to remove unbound antibody. The membrane was then washed with 20 mL of PBS to remove residual TBS-tween solution. The bottom of a light-shielded container was covered with 1 mL of an alkaline phosphatase colorimetric substrate (ECF™ substrate for Western blotting, GE Healthcare) and the membrane was laid face down onto the substrate. The membrane was incubated at room temperature in the substrate for 25 min then rinsed with DI water. A Molecular Dynamics Storm 850 Imager was used to determine image fluorescence (480/520 nm excitation/emission) which was analyzed with Axio Vision software. Densitometric means were taken by recording average pixel brightness in the spot and were background corrected. Antibody levels were compared to protein antibody standards for quantification.
Size Distribution and ζ-Potential of Ligand-modified Microparticles
The MP size distribution was measured by a Microtrac Nanotrac Dynamic Light Scattering Particle Analyzer (Microtrac, Montgomery, PA). The zeta potential of ligand-conjugated MPs was determined using a Brookhaven ZetaPlus zeta potential analyzer (Brookhaven Instruments Corp., NY, USA). For each experimental coating and control, three samples were analyzed at room temperature in distilled water.
Dendritic Cell and Macrophage Cell Culture
Dendritic Cells and MΦs were generated from bone marrow obtained from 8 – 12 week old, female, C57BL6/j and Non-Obese Diabetic (NOD) mice in accordance with guidelines approved by University of Florida using a modified 10 day protocol. For DC culture, mice were euthanized by CO2 asphyxiation followed by cervical dislocation and tibias and femurs were harvested for isolating marrow cells. The marrow cells were obtained by flushing the shaft of the bones with a 25g needle using RPMI medium (MP Biomedicals, OH, USA) containing 1% fetal bovine serum (Lonza, Walkersville, MD) and 1% penicillin-streptomycin (Hyclone) and mixed to make a homogenous suspension. The suspension was then strained using 70 µm cell strainers (Becton Dickinson, NJ, USA) and cells were collected at 1300 rpm for 7 min. The red blood cells (RBCs) were removed by lysing with ACK lysis buffer (Lonza, Walkersville, MD) followed by centrifugation at 1500 rpm for 5 min to recover leukocytes. Leukocytes were then re-suspended in DMEM/F-12 with L-glutamine (Cellgro, Herndon, VA), 10% fetal bovine serum, 1% sodium pyruvate (Lonza, Walkersville, MD), 1% non-essential amino acids (Lonza, Walkersville, MD), 1% penicillin-streptomycin (Hyclone) and 20 ng/ml GM-CSF (R&D systems, MN, USA) (DC media) and plate on tissue culture flasks for 2 d in order to remove adherent cells. At 2 d the floating cells were transferred to low attachment plates and cultured in fresh DC media for expansion of DC precursor cells. At 7 d, cells were transferred to tissue culture plates to allow for DC adhesion and proliferation. At 10 d, they were lifted from tissue culture plates and used. MΦs were obtained similarly using MΦ media supplemented with 20ng/ml G-CSF (Millipore, MA, USA).[36]
Internalization of Microparticles
Phagocytosis of MPs by DCs and MΦs was confirmed by confocal laser scanning microscopy (Olympus DSU-IX81 Spinning Disc, MA, USA). 2 × 105 cells per well were cultured in LabTek (Nunc, Roskilde, Denmark) eight- well chamber glass slides one day prior to being fed surface-modified rhodamine-loaded MPs at a 10:1 ratio (MPs-cells). After incubation at 37 °C for 1 h, un-phagocytosed particles were removed by washing three times with 1X PBS and fixed using 4% paraformaldehyde at room temperature for 10 min. The morphology of the cell was elucidated by staining with Oregon Green® 488 phalloidin (Invitrogen, Karlsruhe, Germany) while the nucleus was highlighted by Hoechst 33342 nucleic acid stain (Invitrogen, Karlsruhe, Germany).
Dendritic Cell Activation and Cytokine Analysis
DC maturation was quantified by measuring cell surface marker levels using flow cytometry. Briefly, DCs were lifted by incubating with 5 mM Na2EDTA solution in 1 M PBS solution at 37 °C for 20 min. Dendritic cells were then washed with 1% fetal bovine serum in PBS and incubated with antibodies raised against CD16/CD32 (Fcγ III/II Receptor) (clone 2.4G2, IgG2b, κ;(BD Pharmingen, CA, USA) for 40 min at 4 °C in order to block Fcγ receptors. Cells were then washed and stained with antibodies against CD80 (clone 16-10A1, IgG2, κ, CD86 (clone GL1, IgG2a,κ), MHC II (clone M5/114.15.2, IgG2b,κ), and CD11c (clone HL3, IgG1, λ2) (BD Pharmingen) for 40 min at 4°C. Species specific isotypes were used as controls. Data acquisition was performed using (FACScalibur, Becton Dickinson, NJ, USA) flow cytometry and the geometric fluorescent intensities determined. More than 10,000 events were acquired for each sample and data analysis was performed using FCS Express version 3 (De Novo Software, Los Angeles, CA).
Cell culture supernatants were collected after 24 h of cell culture with various surface-modified MPs, centrifuged to remove any cell debris and stored at −20 °C until analysis. The IL-12 cytokine subunit, IL-12p40, and IL-10 cytokine production was analyzed using sandwich enzyme-linked immunosorbant assay (ELISA) kits (Becton Dickinson, NJ, USA) according to manufacturer's directions.
Cell Viability
To examine the potential for cytotoxicity of the surface modified PLGA MPs, a lactate dehydrogenase kit (Roche,Mannheim, Germany) was used per manufacturer instructions. In brief, 1 × 106 cells were plated in 6 well tissue culture plates. After being exposed to the various surface modified PLGA MPs for 24 h, the conditioned media were collected and centrifuged to remove debris and the supernatants were stored at −20 °C until further measurement. Dendritic cells were lysed by adding 10% Triton X-100 to media for 1 h, and the lysates were collected as a ‘dead cell’ control. An additional control was used as the ‘live cell’ control consisting of untreated DCs. Lactate Dehydrogenase activity in supernatants and controls were determined by an incubation with the chromogenic substrate at 37 °C for 1 h, and the optical density value was detected at 490 nm by a microplate reader (MRX II, Dynex technologies, VA, USA). The relative cytotoxicity of each treatment was calculated by finding the difference between the sample and the dead cell control, normalized to the difference between the ‘live cell’ and ‘dead cell’ controls.
Single Cell and Mixed Culture Preferential Phagocytosis Studies
Microparticle phagocytosis by DCs and MΦs in suspension was investigated. Dendritic cells or MΦs were incubated in a DiD solution (1 µM) for 1 h to track live cells. Following a washing to remove free DiD, cells (1 × 105 cells in 0.2 ml) were co-cultured with various surface-modified rhodamine-loaded MPs for 1 h while being gently agitated at 50 rpm at 37 °C in a 2 mM dextrose solution. Microparticles outnumbered cells by a 10:1 ratio. Flow cytometric analysis was then carried out to determine the mean fluorescent intensity of cells in the rhodamine channel as a measure of phagocytic activity. Controls including cell-only and MP-only suspensions were also assessed by flow cytometry.
A similar method was used to study preferential phagocytosis in a mixed culture of DCs and MΦs. Cells were pre-labeled with either calcein (Invitrogen) - DCs or DiD – MΦs, counted and subsequently mixed in a 1:1 ratio in a 2 mM dextrose solution. Experimental and control ANC-loaded MPs were added to cells in a 10:1 ratio. These suspensions were agitated at 50 rpm for 1 h at 37 °C and flow cytometric analysis to determine the mean fluorescent intensity of cells in the ANC channel after incubation as a measure of MP uptake levels. The appropriate controls including cell-only and MP-only suspensions were also assessed by flow cytometry.
In order to block Fc receptors from binding the Fc portion of surface-immobilized antibodies, Fc Block (anti-CD16/32, clone 2.4G2) was added to the suspensions that contained MPs modified with antibodies. All experiments were repeated using three separate preparations with three replicates each time for each ligand-modified MP group.
Antigen Presentation by Dendritic Cells After Phagocytosis of Surface-modified Microparticles
NOD DCs (2.5 × 104/ well) were co-incubated with surface modified, 1040-55 mimetope-loaded MPs, as well as the relevant control treatments, in a 96 well tissue culture plate for 1 h in 2 mM dextrose at 37 °C. MPs outnumbered NOD DCs by 100:1. After thoroughly washing away all un-phagocytosed and unbound MPs, NOD-BDC2.5 CD4+ T-cells (1.25 × 105/ well) were added to each well and incubated at 37 °C for 3 d. Bromodeoxyuridine (BrdU) (kit from Beckton Dickinson) was added to the culture for the last 4 h. T-cells were then immunofluorescently stained for BrdU according to manufacturer’s specifications. Flow cytometry was then used to quantify T cell proliferation for the different treatments.
In order to determine how prolonged antigen presentation was impacted by MP surface modification, MP-treated DCs after washing were left in DC culture media for 4 d. Subsequent to this period, NOD-BDC2.5 CD4+ T cells were added for a 3 day mixed lymphocyte coupling (MLC) followed by BrdU inoculation and staining for quantification of proliferation.
Uptake and Translocation of Microparticles to Lymph Nodes
This experiment was designed to determine the targeting efficacy of our ligand-modified MPs in an in vivo environment. Female, 6 week old C57Bl/6 mice were divided into 4 experimental groups (based on MP surface ligand type) and three control groups (n ≥ 4 for each group). Each animal from the experimental groups received a subcutaneous injection into the footpad of both hind limbs, containing 0.5 mg of ligand-conjugated DiD-loaded MPs suspended in 50 µl PBS. The animals in the control groups were: unmodified DiD-loaded MPs, 50 µm diameter DiD-loaded MPs (which are too large to be phagocytosed), and unmodified unloaded MPs. The 50 µm diameter DiD-loaded MP was included as a control to locally release DiD staining cells at the injection site without being taken up in order to determine if this effect contributed to the number of DiD-positive cells translocated to the LN.
At 24, 48 and 72 h post-injection, mice were sacrificed by CO2 asphyxiation and cervical dislocation, and the draining popliteal lymph nodes from each hind limb harvested and prepared into single cell suspensions. Cells were then prepped for flow cytometry by incubation at 4 °C for 0.5 h in a cocktail of antibodies specific for DCs (anti-CD11c, clone HL3), MΦs (anti-F4/80, clone A3-1) and T cells (anti-CD3, clone 17A2). All antibodies were from BD Biosciences Pharmingen (CA, USA) except anti-F4/80 which was from AbD Serotec (Oxford, UK). Appropriate isotypes were used for each antibody species as negative controls.
Following staining, multi-color flow cytometry was used to determine the fraction of each cell type stained with the DiD dye as a measure of the extent particle uptake and translocation by DCs and MΦs.
Significance Testing
Statistical analyses were performed using general linear nested model ANOVA. Based on the overall ANOVA p value (p ≤ 0.05), a posthoc assessment using Tukey test was subsequently performed to make pair-wise comparisons. Differences were considered significant when p ≤ 0.05 using Systat (Version 12, Systat Software, Inc., San Jose, CA).
Results
Microparticle Characterization
We characterized surface-modified MPs, determining size ditribution, ligand surface loading and zeta potentials. PLGA MPs were prepared via a double emulsion solvent evaporation technique. Fabricated MPs were determined to have an average diameter of ~ 1 µm via dynamic light scattering testing, calculated by volume with a representative plot shown in Figure 1A. Conjugation of ligands to the MP surface did not alter the size distribution of the particles (data not shown). Ligands were tethered to the surface of PLGA MPs. Carboxyl groups present at the MP surface were activated by EDC/ NHS and coupled to primary amine groups on the decorating molecules (except PEG, which was surface-adsorbed). Surface modification of MPs by PEG, DEC-205 and CD11c anti-bodies, P-D2 and RGD peptides was characterized by zeta potential analysis. Unmodified PLGA MPs demonstrated a negative zeta potential (~ −46 mV). Surface conjugation with CD11c antibody further reduced the zeta potential to approximately −59 mV. Conversely coating with PEG masked the negative surface charge to −20 mV. These observations are consistent with similar published studies.[37] Conjugation of Anti- DEC-205, P-D2 and RGD peptides only slightly increase the surface charge (~ −37 to −42 mV). We verified antibody conjugation by enzymatic cleaving of the proteins. Cleaved antibodies were spotted onto PDVF membranes and quantified by dot blot analysis. Peptide conjugation was also validated by enzymatic cleavage of fluorescently-tagged peptides from the particle surface followed by detection using a plate reader. The surface loading of the CD11c Ab, DEC-205 Ab, P-D2 peptide and RGD peptide were revealed to be substantial at 109 ng/mg, 88 ng/mg, 4359 ng/mg and 19 ng/mg PLGA respectively as tabulated in Figure 1B. It was also determined, that the surface density of all ligands was substantially increased by crosslinking. The surface density of the CD11c Ab, DEC-205 Ab, P-D2 peptide and RGD peptide, in the absence of the cross linker, was determined to be 77 ng/mg, 60 ng/mg, 3373 ng/mg and 11 ng/mg PLGA respectively.
Figure 1.
Poly (d lactide-co-glycolide) microparticle (MP) characterization – (A) Microparticle size was determined to be 1.15µm using DLS. (B) PLGA MPs surface-modified with various ligands and PEG were characterized by zeta potential measurement and fluorescent quantification techniques to confirm surface loading. Zeta potential data shown represent the mean ± standard error for five readings of each sample. The ligand surface loading data shown represent mean ± standard deviation for three replicates of each sample.
Internalization of Microparticles
Confocal laser scanning microscopy was used to confirm the internalization of MPs by DCs and MΦs. The morphology of the cell was elicited by staining filamentous actin while the nucleus was highlighted by a nucleic acid stain and rhodamine-loaded MPs were used to show particle engulfment. Figure 2 is a representative confocal microscopic image of the DC cytoskeleton (green) surrounding the rhodamine-loaded MP (orange) in both the x-y and x-z planes, confirming MP internalization.
Figure 2.
Internalization of surface-modified MPs by dendritic cells (DCs) is demonstrated via confocal microscopy. DCs were incubated rhodamine-loaded surface-modified MPs (orange) for 1 h, fixed and stained for the actin cytoskeleton (green) and the nucleus (blue). This image shows the x-y optical section as well as x-z projection, showing engulfment of the MPs
Dendritic Cell Maturation and Viability
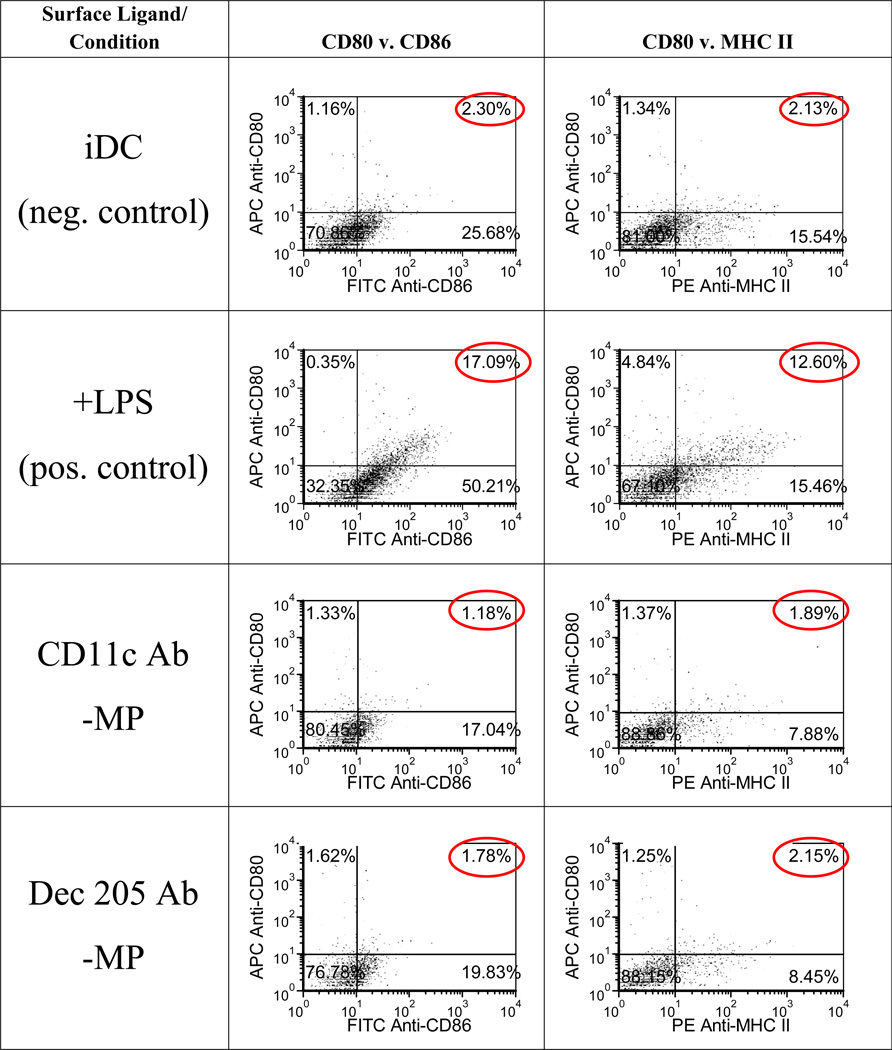
To assess whether our MP surface modifications were relevant for non-activating applications, we examined their effect on DC maturation. Specifically, we investigated whether or not the various ligand-grafted MPs increased expression of the DC maturation markers, CD80, CD86 and MHC-II on bone marrow-derived iDCs.
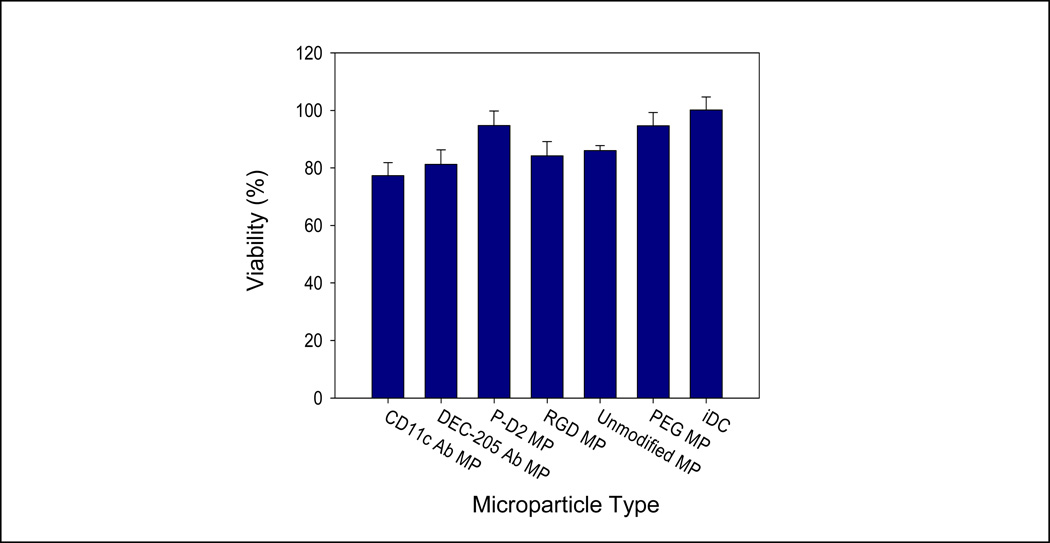
The levels of expression of CD80, CD86 and MHCII on DCs co-cultured with any of the surface-grafted MPs were found to be not statistically (p-value > 0.05) different to that of iDCs and representative dot plots are shown (Figure 3). In contrast, DCs challenged with LPS as a positive control, show significantly higher expression levels of CD80, CD86 and MHCII. These results suggest these ligand-grafted MP modifications do not activate DCs. Furthermore, we demonstrated that there is no DC toxicity associated with MP co-incubation (p-value > 0.05) (Figure 4).
Figure 3.
Immature dendritic cell phenotype is maintained even after exposure to ligand-conjugated MP modifications. Dendritic cells were incubated with various surface-modified MPs for 24h at 37 °C. MPs outnumbered cells by a 10:1 ratio. Cells were then extensively washed to remove unbound MPs and stained with fluorescently-tagged anti- CD80, CD86, MHC II and CD11c antibodies. Flow cytometric assessment revealed the levels of expression for these molecules on DCs. Immature DCs (iDCs) and +LPS groups are included as negative and positive controls, respectively. Representative plots from three separate experiments are shown
Figure 4.
Co-culture of surface-modified MPs with dendritic cells (DCs) has negligible effects on cell viability. Dendritic cells were incubated with various surface-modified MPs for 24 h at 37 °C. Microparticles outnumbered cells by a 10:1 ratio. Cell supernatants were collected after 24 h for a cytotoxicity assay. The percentage viability for each specimen was calculated based on live/dead cell controls. No differences between groups were found by ANOVA (p > 0.1)
Microparticle Phagocytosis by Dendritic Cells and Macrophages
In order to investigate the in vitro uptake of MPs surface modified with ligands by DCs and MΦs, we incubated cells with fluorescently-labeled MPs in suspension, serum-free. Fc Block (anti-CD16/32, clone 2.4G2) was included to the antibody-modified MP groups to block recognition by Fc receptors in order to drive uptake by the targeted receptors. The percent of cells that phagocytosed MPs was not affected by MP treatment, and ranged between 94% – 97% for DCs, and 91% – 96% for MΦs.
Cellular uptake levels were determined based on the mean intensity of MP fluorescence associated with cells. The level of phagocytosis of MPs by DCs was altered based on the surface modification of the MP (Figure 5A). All surface modifications, with the exception of the PEG and RGD modified MPs, demonstrated the capacity to significantly increase MP uptake by DCs compared to the unmodified MP. The antibody groups, anti-CD11c and anti-DEC-205 both significantly improved DC uptake by ~50% compared to unmodified MP. The P-D2 peptide enhanced DC phagocytosis the most, doubling the number of MPs phagocytosed over the unmodified MPs.
Figure 5.
Microparticle (MP) phagocytosis by (A) dendritic cells (DCs) and (B) macrophages MΦs is modulated by MP surface ligand. Cells were cultured with surface-modified rhodamine-loaded MPs for 1 h with agitation at 37 °C. Microparticles outnumbered cells by a 10:1 ratio. Flow cytometric analysis was carried out to determine the mean fluorescent intensity of cells (in the rhodamine channel) after incubation as a measure of MP uptake. Pair-wise significant difference from cell uptake of unmodified PLGA microparticles is denoted by the * symbol (p value < 0.05).
Modulation of MΦ uptake of surface-modified MPs was also investigated (Figure 5B). MPs surface-conjugated with the P-D2 peptide substantially increased phagocytosis by ~ 40% over uptake levels for unmodified MPs. All other surface modifications yielded similar levels of MP uptake as the unmodified.
Because in vivo, MΦs and DCs can compete for MP uptake, we were interested in determining if these ligands demonstrate targeting specificity for either DCs or MΦs. Using a mixed culture of DCs and MΦs (1:1 ratio), we investigated uptake when these cells have equal access to MPs (Figure 6). We found that under these conditions, DCs take up more MPs than MΦs regardless of the surface modification, and the extent of MP uptake is influenced by ligand surface modification. Similar trends, based on the type of surface modification, seen for uptake in both single cell suspension uptake systems were observed for uptake in the mixed culture competitive uptake system. Anti-CD11c and anti-DEC-205 antibodies and P-D2 peptide significantly improved uptake for both DCs and MΦs when compared to uptake of unmodified MPs. Moreover, differences in uptake specificity were observed, suggesting that compared to other treatments, the anti-DEC-205 antibody MP modification provided both high levels of uptake and specificity of DC targeting. In contrast, while MPs surface-modified with anti-CD11c antibody and P-D2 demonstrated high levels of uptake, DC selectivity was low in this in vitro model
Figure 6.
Microparticle (MP) uptake in a mixed culture (DCs and MΦs) is influenced by MP surface modification. An equal number of DCs and MΦs were co-cultured with various surface-modified AMC-loaded MPs for 1 h at 37 °C while being gently agitated. Microparticles outnumbered cells by a 10:1 ratio. Flow cytometric analysis was carried out to determine the mean fluorescent intensity (in the AMC channel) of DCs and MΦs after incubation as a measure of MP uptake level. Pair-wise significant difference of MP uptake between DCs and MΦs is denoted by the * symbol (p value < 0.05).
Improved Antigen Presentation by DCs after Phagocytosis of Surface-modified MPs
In order to determine efficacy of DC antigen presentation following phagocytosis of antigen-loaded MPs, NOD-derived DCs cells were allowed to take up ligand-grafted MPs encapsulating the peptide antigen, 1040-55 mimetope, then subsequently incubated with NOD-BDC2.5 T-cells. The NOD-BDC2.5 mouse is engineered with T-cell receptors that are specifically engaged by the 1040-55 mimetope. Upon binding the 1040-55 peptide (e.g. when presented on APCs), T-cells are stimulated to proliferate. In our experimental setup, MPs loaded with 1040-55 peptide were incubated with DCs for 1 h, unbound MPs removed, and then DCs were either immediately co-cultured with BDC 2.5 T-cells, or were cultured for 4 d before the addition of BDC 2.5 T-cells. By comparing the 4 d delayed experiment to the no delay test, the ability for MP-loading to provide prolonged antigen presentation by DCs to T cells was investigated (Figure 7).
Figure 7.
(A) P-D2 peptide surface-modified PLGA MPs loaded with antigenic peptide leads to increased antigen presentation and (B) improved prolonged antigen presentation. Non-obese diabetic mouse DCs were cultured with either MPs loaded with 1040-55 mimitope or soluble peptide (as a control, at an equal mass to that loaded in the MPs) at a 100:1 MP to DC ratio for 1 h, followed by washing to remove unbound MPs. Subsequently, freshly-isolated BDC2.5 CD4+ T cells were added to culture wells (either immediately (early) or after 4 d (prolonged)) and co-cultured a 3-day mixed lymphocyte reaction. T-cell proliferation was then measured using a BrdU proliferation assay as a measure of functional antigen presentation. Data shown represent the mean proliferation indices ± standard error (n = 3). Pair-wise significant difference from the unmodified PLGA MP group (by ANOVA and Tukey Significance Test) is denoted by the * symbol (p value < 0.05).
Regarding the early antigen presentation of the no-delay experiment, the P-D2- modified MPs was the only group that significantly improved T-cell response above the level observed for unmodified, 1040-55 peptide-loaded MPs (Figure 7A). The positive control, a soluble bolus with the equivalent amount of peptide encapsulated in the MPs was included. This control provoked the largest T cell response, over 2-fold higher than the P-D2 modification, while unloaded MPs showed minimal T cell proliferation.
Investigating prolonged antigen presentation by the inclusion of a 4 d delay, MP surface modification was found to impact the response. The incubation delay resulted in a dramatic decline in the response of T-cells to the soluble antigenic peptide control. Most MP modifications maintained a low-magnitude T cell response similar to that seen without the incubation delay. Only the P-D2 peptide-conjugated MP loaded with antigen significantly increased proliferation (Figure 7B). The unloaded, unmodified MP group was included as a control to demonstrate that T cell proliferation was indeed to the encapsulation of 1040-55 peptide in other MP modifications.
In Vivo Uptake and Translocation of Surface-modified Microparticles to Lymph Node
For applications targeting phagocytes in vivo, it is critical to investigate the effect of MP surface modifications using an in vivo model. We therefore used a mouse footpad injection model to determine the rate and extent of surface-modified MP translocation from the injection site to proximal lymph nodes, via uptake from DCs and MΦs. DiD-loaded MPs were surface-modified and injected into the footpad of mice. Subsequently, proximal draining lymph nodes were excised and analyzed for cell-MP co-localization. The percent of MP+ cells harvested from draining lymph nodes was used as a measure of uptake and translocation from the injection site. DCs and MΦs were designated by staining positive for either CD11c or F4/80, respectively. At 24 h after injection, no modification showed distinction in improving trafficking to the draining lymph node (Figure 8A). However, by 48 h, lymph node harvested DCs recovered from mice injected with anti-CD11c- and P-D2- modified MPs displayed a marked increase in the percent of MP+ DCs to 4.8 % MP+ and 6.2% MP+ respectively compared to 2% MP+ for unmodified MPs. A similar trend was observed for MΦs at this 48 h time point, but with significance only for the CD11c antibody modification, which increased the number of cells with MPs to 2% MP+ compared to the 0.5% MP+ seen for unmodified MPs. After 72 h the P-D2 modified particles substantially improved trafficking to draining lymph nodes for DCs (4.4% MP+) and MΦs (2.3% MP+) cells compared to the controls (1.8% MP+ and 0.8% MP+, respectively). Unexpectedly, the isotype antibody for CD11c also improved the number of MP+ trafficked DCs at 72 h implicating the role of DCs Fc receptors at this time point. This illustrates the influence the choice of antibody species and IgG type have when using whole antibodies for in vivo targeting.
Figure 8.
Phagocytosis and trafficking of microparticles (MPs) by dendritic cells (DCs) and macrophages (MΦs) is enhanced through MP surface modification. Female, 6 week old C57Bl/6 mice were given footpad injections containing 0.5 mg of ligand-conjugated DiD-MPs along with control MPs. At different time points (24 h, 48 h, 72 h) the draining lymph nodes were recovered, processed and stained for CD11c (DC) and F4/80 (MΦ) surface markers followed by flow cytometry analysis. The percentage of each cell type MP+ (DiD channel) was determined and analyzed by ANOVA. Microparticle uptake for both DCs and MΦs is summarized for all MP groups (A), as well as detailed separately for DCs (B) and MΦs (C). The * symbol denotes pair-wise comparisons using the Tukey Test showing significant difference in comparison to unmodified MPs (p < 0.05).
In addition to elucidating the extent of MP trafficking, this time series study also contextualizes the impact of MP surface modification on the kinetics of MP trafficking. These data strongly suggest that the rate of uptake and translocation of MPs is greatly superior when MPs are surface-modified, primarily with the P-D2 peptide and to a lesser extent the CD11c antibody, for DCs (Figure 8B) and MΦs (Figure 8C) over a 3 day period. The CD11c antibody-grafted MP shows improvement for both DCs and MΦs which peaks at 48 h but then drops to control levels at 72 h. The P-D2 MP also shows improved uptake and trafficking for DCs which peaks at 48 h but then drops to control levels at 72 h. In contrast, for MΦs, the P-D2 MP maintains increasing levels throughout the experimental time period. In general, these in vivo results correlate well with the in vitro results, except that the modification with the anti-DEC-205 antibody did not translate to uptake and trafficking levels as high as indicated in vitro.
This in vivo study also illustrates differences in the kinetics of uptake and translocation between DCs and MΦ. It is evident that for all modifications, the maximum number of MP+ CD11c+ cells occurs at 48 h. For F4/80+ cells, there is no peak for the duration of the study for any of the modifications with the exception of P-D2-grafted MPs in the study time period. These findings corroborate reports of slower lymph node homing of MΦs compared to DCs. [38]
Discussion
PLGA particulate systems have been identified as a valuable immuno-therapeutic tool. Particles at the micron and sub-micron level allow for targeted delivery of a plethora of immuno-modulating agents to critical elements of the immune system.[1,2] Targeted delivery of factors to DCs is of particular interest as DCs play a pivotal role in the activation and maintenance of suppressive networks within the immune system.[12] Efforts at controlling DC polarity for mitigation of autoimmune disorders, such as type 1 diabetes (T1D) have intensified in the past decade.[25] While MΦs are more abundant than DCs and compete for uptake of particulate matter, MΦs are less efficient at antigen presentation and therefore may be considered to be less potent at manipulation of the immune system.[13,39] Notably, researchers have begun to incorporate PLGA MPs as a cell immuno-modulatory system into DC immunotherapy.[9,11] Phillips and associates[40] demonstrated prevention of T1D in NOD mice by polymeric microspheres loaded with anti-sense oligonucleotides that passively targeted DCs by virtue of being micron range particulate, and which were demonstrated to manipulate the immuno-regulatory function of DCs. Others have shown that active targeting of DCs using surface-modified particulate systems improves DC uptake and trafficking, and escalating downstream immuno-stimulatory responses, primarily for cancer vaccine applications. [41]
Active targeting of MPs is accomplished by surface ligation of ligands that specifically bind molecules on the surface of DCs. Prior work has demonstrated efficacious targeting of MPs to DC receptors such as toll-like receptors, CD40, αvβ3 and αvβ5 integrins.[42] However, binding these receptors can result in DC activation. For example, it is well established that toll-like receptors and CD40 receptor are stimulatory in nature.[23] Additionally, we have recently shown that DC adhesion to various adhesive substrates, as well as RGD peptide surface density gradients through the αv integrins are also activating.[43–45] Similarly, others have shown that targeting MPs to αv integrins results in DC activation.[42] Given the stimulatory pathways induced by these receptors, other candidates which are either non-inflammatory or pro-tolerogenic are of interest for non-activating applications. Ligands that target DC surface receptors such as the integrin CD11c/CD18 and the DEC-205 receptor have been identified as potential candidates for this goal. We were therefore interested in the use of antibodies against CD11c and DEC-205 as well as the CD11c-binding peptide, P-D2 peptide as ligands in this class. Both CD11c and DEC-205 antibodies have been previously coupled to antigen-loaded liposomes for targeting of DCs to enhance protection from tumor in mice challenged with malignant melanoma cells. [46,47] Bonifaz et al. [48] and Birkholz et al. [49] conjugated DEC-205 monoclonal antibody and anti-DEC-205 single chain fragment variable directly to antigenic proteins for targeting to DCs to improve antigen-specific immune stimulation. More recently, Fahmy and associates reported grafting of anti-DEC-205 to PLGA nanoparticles and its effect on DC uptake and function. Their study demonstrated that surface grafted DEC-205 nanoparticles induce IL-10 expression but are only modestly effective in improving nanoparticle internalization by DCs.[50] As the popularity of particulate systems as contemporary medical drug delivery devices increases, the need for particle optimization arises (including active targeting, release kinetics, protein repulsion, etc). This study begins to address these parameters, principally for applications requiring targeting to phagocytes while maintaining a non-activated state. Another consideration is that MP modification with targeting peptides instead of antibodies is a particularly attractive candidate as it will not target Fc receptors and has a lower production cost. [51]
To promote phagocytosis, we fabricated our particles with a range of 0.5 – 2.5 µm in size with an average diameter of 1.15 µm and confocal microscopy confirmed that the MPs were readily taken up by DCs. Below this micron size range, particles can be taken up by pinocytosis which is not limited to antigen presenting cells. In this regard, nanoparticles are therefore not as selective as microparticles for phagocytes. [52,53] Well-established EDC/NHS chemistry was used to ligate ligands to the surfaces of PLGA MPs. Verification of conjugation was accomplished by peptide and protein quantitative methods, and corroborated by zeta potential measurement. These results show that a substantial amount of each molecule is immobilized on the surface of the MP, at levels comparable to those previously reported.[42,54] Differences in uptake observed between the different surface modifications of MPs can be attributed to factors affecting the activity of the immobilized ligand including the quantity of immobilized ligand, the specific receptor targeted, the site of ligand binding to the receptor (i.e., does binding activate/de-activate the receptor) and the effective binding affinity of the immobilized ligand.[39,42,55] These factors can explain why modifications in our hands may act differently from prior reports, and their potential influence over our results is discussed further below.
In vitro testing to determine the stimulatory capacity of MP modifications showed there is no significant change in the expression of DC activation markers investigated with any of the surface-modified MPs. Further, neither the modified or unmodified MPs showed any toxic effects. These outcomes are important considerations for designing DC-targeting MPs for applications such as vaccines for amelioration of autoimmune disorders.
We also determined that secretion of cytokines, IL-12 and IL-10, does not vary significantly for DCs exposed to any of the MP modifications (data not shown). With regard to DEC-205 MPs, this result is seemingly contrary to work by the Fahmy group which described an increase in DC secretion of IL-10 upon DEC-205 nanoparticle exposure.[50] This disparity may be explained by the fact that their reported surface density was 50-fold greater than the density measured on our DEC-205 MP, the size of their particle was much smaller at 200 – 250 nm, and the DEC-205 presentation on their particle was different, consisting of palmitate-avidin molecules incorporated into the surface of the particle binding a biotinylated DEC-205 antibody.
The effect of surface functionalization on early uptake of MPs by DCs and MΦs was initially evaluated in vitro. The CD11c and DEC-205 antibody-modified and P-D2 peptide surface functionalized MPs provided significant enhancement of DC phagocytosis while CD11c and P-D2 surface grafting augmented MΦ uptake of MPs. RGD peptide grafting failed to increase either DC or MΦ phagocytosis of MPs. Peptide quantification methods revealed that the surface density of the RGD peptide was 19 ng/ mg which equates to approximately 11,500 fmol/cm2. Petrie et al. [56] reported that an RGD surface density of approximately 2,000 fmol/cm2 on solid substrates promoted murine adhesion. Therefore, it was expected our RGD-modified MPs should encourage MP phagocytosis. Given that our RGD modification did not improve uptake may suggest that peptide presentation in our formulation was sub-optimal. Alternatively, increasing MP phagocytosis may simply require higher threshold levels of RGD surface density than levels required only for adhesion, as Corre and associates [51] reported that microspheres functionalized with 20-fold higher RGD surface density influences MΦ phagocytosis. Whilst ligand quantity and presentation are limiting factors, differences in DC and MΦ levels of expression and activation of the targeted receptors also influence DC and MΦ uptake. [57, 58] Notably, these receptor levels and activation states can certainly differ between in vitro and in vivo scenarios.
Particulate-based antigen delivered to APCs can serve as an intracellular antigen depot providing prolonged antigen presentation due to sustained release. [59] We investigated MP surface modification to increase intracellular stores of antigen thereby increasing prolonged presentation of antigen. We determined that at an initial time point, ligand-directed MP uptake influences antigen presentation significantly for the P–D2 peptide ligand and to a lesser extent and significance level (p <0.09) for the DEC-205 antibody modified MP group. Further, we demonstrated that microparticulate systems allow for prolonged, continuous presentation of antigen, and that ligand surface modification, particularly with P-D2 peptide, increases the level of this protracted presentation. This is important, because taking into account time considerations after subcutaneous injection for APC antigen interception, uptake and translocation, antigen administered in soluble form can be diluted and degraded before effective functional interaction by APCs can occur in T cell rich regions. [2] This result indicates controlled release packaging of antigen in targeted PLGA MPs can overcome this issue.
The in vitro studies provided a well-controlled platform to identify the best candidates for DC uptake. Additionally, these results can guide applications utilizing in vitro/ex vivo manipulation of APCs, which can be enhanced using polymeric microspheres.[17,59] However, applications aimed at in vivo targeting of MPs to DCs require use of in vivo experimental systems to determine efficacy. Based on the low in vitro uptake levels, we eliminated the RGD modified group and compared in vivo uptake and translocation to draining lymph node after subcutaneous injection of our best MP modifications. The P-D2 peptide-conjugated MPs in particular, and to a lesser extent, the anti-CD11c-conjugated MPs were effective in increasing in vivo trafficking of MPs to draining lymph nodes by both DCs and MΦs at 2 d and 3 d, respectively. Notably, inconsistent with our in vitro result, the anti-DEC-205-conjugated MPs failed to alter MP trafficking by DCs in vivo. This may be explained by differences in DC receptors (expression levels and activation states) present in the in vitro and in vivo conditions.[30] For instance, Jefford et al. demonstrated that peripheral blood DCs generated in vivo differed from in vitro monocyte-derived DCs in phenotype, migratory capacity and T-cell stimulation.[60] Furthermore, DC subpopulations in vivo show phenotypic diversity.[61,62] While Steinman and colleagues[21] reported that antigen-coupled DEC-205 antibody injected intravenously successfully targeted lymphoid DCs, it is possible that the DCs present at the subcutaneous injection site have a lower expression of DEC-205.[61] Consequently, subcutaneously injected-MPs modified with the anti-DEC-205 antibody for non-activating DC-targeting may prove ineffective.
Conclusion
In this study, we investigated strategies to achieve active targeting of MPs to DCs in a non-activating mode and assessed the efficacy of each of these approaches in vitro as well as in vivo. Moreover, we have demonstrated that ligation of the P-D2 peptide (and to a lesser extent, the anti-CD11c and anti-DEC-205 antibodies) to the surface of PLGA MPs is a promising, new approach for active DC targeting. In general, conjugation of these ligands to PLGA MPs were non-stimulating to DCs, enhanced DC MP uptake, improved antigen presentation level and duration by DCs and also boosted in vivo trafficking of MPs to the draining lymph node. The knowledge garnered from this study is instructive for the design of non-activating polymeric microparticulate applications such as vaccines for auto-immune disease.
Acknowledgement
This work was supported in part by the National Institutes of Health (R01 DK091658, and R56 DK091658), and by an Innovative Grant from the Juvenile Diabetes Research Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jiang W, Gupta RK, Deshpande MC, Schwendeman SP. Biodegradable poly(lactic-co-glycolic acid) microparticles for injectable delivery of vaccine antigens. Adv Drug Deliv Rev. 2005 Jan 10;57(3):391–410. doi: 10.1016/j.addr.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 2.Ohagan DT, Rahman D, Mcgee JP, Jeffery H, Davies MC, Williams P, et al. Biodegradable microparticles as controlled release antigen delivery dystems. Immunology. 1991;73(2):239–242. [PMC free article] [PubMed] [Google Scholar]
- 3.Jaganathan KS, Vyas SP. Strong systemic and mucosal immune responses to surface-modified PLGA microspheres containing recombinant Hepatitis B antigen administered intranasally. Vaccine. 2006;24(19):4201–4211. doi: 10.1016/j.vaccine.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 4.Rajapaksa TE, Lo DD. Microencapsulation of vaccine antigens and adjuvants for mucosal targeting. Cur Immunol Rev. 2010;6(1):29–37. [Google Scholar]
- 5.Perez C, Sanchez A, Putnam D, Ting D, Langer R, Alonso MJ. Poly(lactic acid)-poly(ethylene glycol) nanoparticles as new carriers for the delivery of plasmid DNA. J Control Release. 2001;75(1–2):211–224. doi: 10.1016/s0168-3659(01)00397-2. [DOI] [PubMed] [Google Scholar]
- 6.Tinsley-Bown AM, Fretwell R, Dowsett AB, Davis SL, Farrar GH. Formulation of poly(D,L-lactic-co-glycolic acid) microparticles for rapid plasmid DNA delivery. J Control Release. 2000;66(2–3):229–241. doi: 10.1016/s0168-3659(99)00275-8. [DOI] [PubMed] [Google Scholar]
- 7.Walter E, Moelling K, Pavlovic J, Merkle HP. Microencapsulation of DNA using poly(DL-lactide-co-glycolide): stability issues and release characteristics. J Control Release. 1999;61(3):361–374. doi: 10.1016/s0168-3659(99)00151-0. [DOI] [PubMed] [Google Scholar]
- 8.Mohammadi G, Valizadeh H, Barzegar-Jalali M, Lotfipour F, Adibkia K, Milani M, et al. Development of azithromycin-PLGA nanoparticles: physicochemical characterization and antibacterial effect against Salmonella typhi. Colloids Surface B. 2010;80(1):34–39. doi: 10.1016/j.colsurfb.2010.05.027. [DOI] [PubMed] [Google Scholar]
- 9.Acharya AP, Clare-Salzler MJ, Keselowsky BG. A high-throughput microparticle microarray platform for dendritic cell-targeting vaccines. Biomaterials. 2009;30(25):4168–4177. doi: 10.1016/j.biomaterials.2009.04.032. [DOI] [PubMed] [Google Scholar]
- 10.Johansen P, Men Y, Merkle HP, Gander B. Revisiting PLA/PLGA microspheres: an analysis of their potential in parenteral vaccination. Eur J Pharm and Biopharm. 2000 Jul 3;50(1):129–146. doi: 10.1016/s0939-6411(00)00079-5. [DOI] [PubMed] [Google Scholar]
- 11.Waeckerle-Men Y, Groettrup M. PLGA microspheres for improved antigen delivery to dendritic cells as cellular vaccines. Adv Drug Deliver Rev. 2005;57(3):475–482. doi: 10.1016/j.addr.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 12.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392(6673):245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 13.Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YT, et al. Immunobiology of dendritic cells. Ann Rev Immunol. 2000;18:767-+. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 14.Matzinger P. Tolerance, danger, and the extended Family. Ann Rev Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 15.Shortman K, Liu YJ. Mouse and human dendritic cell subtypes. Nat Rev Immunol. 2002;2(3):151–161. doi: 10.1038/nri746. [DOI] [PubMed] [Google Scholar]
- 16.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Ann Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 17.Tacken PJ, de Vries I, Torensma R, Figdor CG. Dendritic-cell immunotherapy: from ex vivo loading to in vivo targeting. Nat Rev Immunol. 2007;7(10):790–802. doi: 10.1038/nri2173. [DOI] [PubMed] [Google Scholar]
- 18.Gogolak P, Rethi B, Hajas G, Rajnavolgyi E. Targeting dendritic cells for priming cellular immune responses. J Mol Recognit. 2003;16(5):299–317. doi: 10.1002/jmr.650. [DOI] [PubMed] [Google Scholar]
- 19.Wattendorf U, Coullerez G, Voros J, Textor M, Merkle HP. Mannose-based molecular patterns on stealth microspheres for receptor-specific targeting of human antigen-presenting cells. Langmuir. 2008;24(20):11790–11802. doi: 10.1021/la801085d. [DOI] [PubMed] [Google Scholar]
- 20.Yang L, Yang H, Rideout K, Cho T, Il Joo K, Ziegler L, et al. Engineered lentivector targeting of dendritic cells for in vivo immunization. Nat Biotechnol. 2008;26(3):326–334. doi: 10.1038/nbt1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bonifaz L, Bonnyay D, Mahnke K, Rivera M, Nussenzweig MC, Steinman RM. Efficient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8(+) T cell tolerance. J Exp Med. 2002;196(12):1627–1638. doi: 10.1084/jem.20021598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choe Sw, Acharya AP, Keselowsky BG, Sorg BS. Intravital microscopy imaging of macrophage localization to immunogenic particles and co-localized tissue oxygen saturation. Acta Biomater. 2010;6(9):3491–3498. doi: 10.1016/j.actbio.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 23.Cheong C, Choi JH, Vitale L, He LZ, Trumpfheller C, Bozzacco L, et al. Improved cellular and humoral immune responses in vivo following targeting of HIV Gag to dendritic cells within human anti-human DEC205 monoclonal antibody. Blood. 2010;116(19):3828–3838. doi: 10.1182/blood-2010-06-288068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nchinda G, Kuroiwa J, Oks M, Trumpfheller C, Park CG, Huang Y, et al. The efficacy of DNA vaccination is enhanced in mice by targeting the encoded protein to dendritic cells. J Clin Invest. 2008;118(4):1427–1436. doi: 10.1172/JCI34224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keselowsky BG, Xia CQ, Clare-Salzler M. Multifunctional dendritic cell-targeting polymeric microparticles: Engineering new vaccines for type 1 diabetes. Hum Vaccines. 2011;7(1):37–44. doi: 10.4161/hv.7.1.12916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiang WP, Swiggard WJ, Heufler C, Peng M, Mirza A, Steinman RM, et al. The receptor Dec-205 expressed by dendritic Cells and thymic epithelial-cells is involved in antigen-processing. Nature. 1995;375(6527):151–155. doi: 10.1038/375151a0. [DOI] [PubMed] [Google Scholar]
- 27.Wang B, Kuroiwa JM, He LZ, Charalambous A, Keler T, Steinman RM. The human cancer antigen mesothelin is more efficiently presented to the mouse immune system when targeted to the DEC-205/CD205 receptor on dendritic cells. Ann N Y Acad Sci. 2009;1174:6–17. doi: 10.1111/j.1749-6632.2009.04933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stern JN, Keskin DB, Kato Z, Waldner H, Schallenberg S, Anderson A, et al. Promoting tolerance to proteolipid protein-induced experimental autoimmune encephalomyelitis through targeting dendritic cells. P Natl Acad Sci. 2010;107(40):17280–17285. doi: 10.1073/pnas.1010263107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamazaki S, Dudziak D, Heidkamp GF, Fiorese C, Bonito AJ, Inaba K, et al. CD8(+)CD205(+) splenic dendritic cells are specialized to induce Foxp3(+) regulatory T Cells. J Immunol. 2008;181(10):6923–6933. doi: 10.4049/jimmunol.181.10.6923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ammon C, Meyer SP, Schwarzfischer L, Krause SW, Andreesen R, Kreutz M. Comparative analysis of integrin expression on monocyte-derived macrophages and monocyte-derived dendritic cells. Immunology. 2000;100(3):364–369. doi: 10.1046/j.1365-2567.2000.00056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berry JD, Licea A, Popkov M, Cortez X, Fuller R, Elia M, et al. Rapid monoclonal antibody generation via dendritic cell targeting in vivo. Hybridoma Hybridom. 2003;22(1):23–31. doi: 10.1089/153685903321538053. [DOI] [PubMed] [Google Scholar]
- 32.Ihanus E, Uotila LM, Toivanen A, Varis M, Gahmberg CG. Red-cell ICAM-4 is a ligand for the monocyte/macrophage integrin CD11c/CD18: characterization of the binding sites on ICAM-4. Blood. 2007;109(2):802–810. doi: 10.1182/blood-2006-04-014878. [DOI] [PubMed] [Google Scholar]
- 33.Bailly P, Tontti E, Hermand P, Cartron JP, Gahmberg CG. The red cell LW blood group protein is an intercellular adhesion molecule which binds to CD11/CD18 leukocyte integrins. Eur J Immunol. 1995;25(12):3316–3320. doi: 10.1002/eji.1830251217. [DOI] [PubMed] [Google Scholar]
- 34.Ihanus E, Uotila L, Toivanen A, Stefanidakis M, Bailly P, Cartron JP, et al. Characterization of ICAM-4 binding to the I domains of the CD11a/CD18 and CD11b/CD18 leukocyte integrins. Eur J Biochem. 2003;270(8):1710–1723. doi: 10.1046/j.1432-1033.2003.03528.x. [DOI] [PubMed] [Google Scholar]
- 35.Messerschmidt SK, Musyanovych A, Altvater M, Scheurich P, Pfizenmaier K, Landfester K, et al. Targeted lipid-coated nanoparticles: delivery of tumor necrosis factor-functionalized particles to tumor cells. J Control Release. 2009;137(1):69–77. doi: 10.1016/j.jconrel.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 36.Zaveri TD, Dolgova NV, Chu BH, Lee J, Wong J, Lele TP, et al. Contributions of surface topography and cytotoxicity to the macrophage response to zinc oxide nanorods. Biomaterials. 2010;31(11):2999–3007. doi: 10.1016/j.biomaterials.2009.12.055. [DOI] [PubMed] [Google Scholar]
- 37.Li YP, Pei YY, Zhang XY, Gu ZH, Zhou ZH, Yuan WF, et al. PEGylated PLGA nanoparticles as protein carriers: synthesis, preparation and biodistribution in rats. J Control Release. 2001;71(2):203–211. doi: 10.1016/s0168-3659(01)00218-8. [DOI] [PubMed] [Google Scholar]
- 38.Kiama SG, Cochand L, Karlsson L, Nicod LP, Gehr P. Evaluation of phagocytic activity in human monocyte-derived dendritic cells. Journal of aerosol medicine: J Int Soc Aerosols Med. 2001;14(3):289–299. doi: 10.1089/089426801316970240. [DOI] [PubMed] [Google Scholar]
- 39.Thiele L, Merkle HP, Walter E. Phagocytosis and phagosomal fate of surface-modified microparticles in dendritic cells and macrophages. Pharm Res. 2003;20(2):221–228. doi: 10.1023/a:1022271020390. [DOI] [PubMed] [Google Scholar]
- 40.Phillips B, Nylander K, Harnaha J, Machen J, Lakomy R, Styche A, et al. A microsphere-based vaccine prevents and reverses new-onset autoimmune diabetes. Diabetes. 2008;57(6):1544–1555. doi: 10.2337/db07-0507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hamdy S, Haddadi A, Hung RW, Lavasanifar A. Targeting dendritic cells with nano-particulate PLGA cancer vaccine formulations. Adv Drug Deliver Rev. 2011;63(10–11):943–955. doi: 10.1016/j.addr.2011.05.021. [DOI] [PubMed] [Google Scholar]
- 42.Kempf M, Mandal B, Jilek S, Thiele L, Voros J, Textor M, et al. Improved stimulation of human dendritic cells by receptor engagement with surface-modified microparticles. J Drug Target. 2003;11(1):11–18. doi: 10.1080/1061186031000072978. [DOI] [PubMed] [Google Scholar]
- 43.Acharya AP, Dolgova NV, Xia CQ, Clare-Salzler MJ, Keselowsky BG. Adhesive substrates modulate the activation and stimulatory capacity of non-obese diabetic mousederived dendritic cells. Acta Biomater. 2011;7(1):180–192. doi: 10.1016/j.actbio.2010.08.026. [DOI] [PubMed] [Google Scholar]
- 44.Acharya AP, Dolgova NV, Moore NM, Xia CQ, Clare-Salzler MJ, Becker ML, et al. The modulation of dendritic cell integrin binding and activation by RGD-peptide density gradient substrates. Biomaterials. 2010;31(29):7444–7454. doi: 10.1016/j.biomaterials.2010.06.025. [DOI] [PubMed] [Google Scholar]
- 45.Acharya AP, Dolgova NV, Clare-Salzler MJ, Keselowsky BG. Adhesive substrate-modulation of adaptive immune responses. Biomaterials. 2008;29(36):4736–4750. doi: 10.1016/j.biomaterials.2008.08.040. [DOI] [PubMed] [Google Scholar]
- 46.Cheong C, Choi JH, Vitale L, He LZ, Trumpfheller C, Bozzacco L, et al. Improved cellular and humoral immune responses in vivo following targeting of HIV Gag to dendritic cells within human anti-human DEC205 monoclonal antibody. Blood. 2010;116(19):3828–3838. doi: 10.1182/blood-2010-06-288068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Faham A, Altin JG. Ag-bearing liposomes engrafted with peptides that interact with CD11c/CD18 induce potent Ag-specific and antitumor immunity. Int J Cancer. 2011;129(6):1391–1403. doi: 10.1002/ijc.25810. [DOI] [PubMed] [Google Scholar]
- 48.Bonifaz LC, Bonnyay DP, Charalambous A, Darguste DI, Fujii SI, Soares H, et al. In vivo targeting of antigens to maturing dendritic cells via the DEC-205 receptor improves T cell vaccination. J Exp Med. 2004;199(6):815–824. doi: 10.1084/jem.20032220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Birkholz K, Schwenkert M, Kellner C, Gross S, Fey G, Schuler-Thurner B, et al. Targeting of DEC-205 on human dendritic cells results in efficient MHC class II-restricted antigen presentation. Blood. 2010;116(13):2277–2285. doi: 10.1182/blood-2010-02-268425. [DOI] [PubMed] [Google Scholar]
- 50.Bandyopadhyay A, Fine RL, Demento S, Bockenstedt LK, Fahmy TM. The impact of nanoparticle ligand density on dendritic-cell targeted vaccines. Biomaterials. 2011;32(11):3094–3105. doi: 10.1016/j.biomaterials.2010.12.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brandhonneur N, Chevanne F, Vie V, Frisch B, Primault R, Le Potier MF, et al. Specific and non-specific phagocytosis of ligand-grafted PLGA microspheres by macrophages. Eur J Pharm Sci. 2009;36(4–5):474–485. doi: 10.1016/j.ejps.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 52.Singh M, Chakrapani A, O'Hagan D. Nanoparticles and microparticles as vaccine-delivery systems. Expert Rev Vaccines. 2007;6(5):797–808. doi: 10.1586/14760584.6.5.797. [DOI] [PubMed] [Google Scholar]
- 53.Mailender V, Landfester K. Interaction of nanoparticles with cells. Biomacromolecules. 2009;10(9):2379–2400. doi: 10.1021/bm900266r. [DOI] [PubMed] [Google Scholar]
- 54.Tan H, Huang D, Lao L, Gao C. RGD modified PLGA/gelatin microspheres as microcarriers for chondrocyte delivery. J of Biomed Mater ResB. 2009;91B(1):228–238. doi: 10.1002/jbm.b.31394. [DOI] [PubMed] [Google Scholar]
- 55.Lin SX, Mallet WG, Huang AY, Maxfield FR. Endocytosed cation-independent mannose 6-phosphate receptor traffics via the endocytic recycling compartment en route to the trans-golgi network and a subpopulation of late endosomes. Mol Biol Cell. 2004;15(2):721, 733. doi: 10.1091/mbc.E03-07-0497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Petrie TA, Capadona JR, Reyes CD, Garcia AJ. Integrin specificity and enhanced cellular activities associated with surfaces presenting a recombinant fibronectin fragment compared to RGD supports. Biomaterials. 2006;27(31):5459–5470. doi: 10.1016/j.biomaterials.2006.06.027. [DOI] [PubMed] [Google Scholar]
- 57.Kou PM, Babensee JE. Macrophage and dendritic cell phenotypic diversity in the context of biomaterials. J Biomed Mater ResA. 2011;96(1):239–260. doi: 10.1002/jbm.a.32971. [DOI] [PubMed] [Google Scholar]
- 58.Hashimoto D, Miller J, Merad M. Dendritic cell and macrophage heterogeneity in vivo. Immunity. 2011;35(3):323–335. doi: 10.1016/j.immuni.2011.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fredriksen BrN, Grip J. PLGA/PLA micro- and nanoparticle formulations serve as antigen depots and induce elevated humoral responses after immunization of Atlantic salmon (Salmo salar L.) Vaccines. 2012;30(3):656–667. doi: 10.1016/j.vaccine.2011.10.105. [DOI] [PubMed] [Google Scholar]
- 60.Jefford M, Schnurr M, Toy T, Masterman KA, Shin A, Beecroft T, et al. Functional comparison of DCs generated in vivo with Flt3 ligand or in vitro from blood monocytes: differential regulation of function by specific classes of physiologic stimuli. Blood. 2003;102(5):1753–1763. doi: 10.1182/blood-2002-12-3854. [DOI] [PubMed] [Google Scholar]
- 61.Inaba K, Swiggard WJ, Inaba M, Meltzer J, Mirza A, Sasagawa T, et al. Tissue distribution of the Dec-205 protein that is detected by the monoclonal-antibody Nldc-145 .1. Expression on dendritic cells and other subsets of mouse leukocytes. Cell Immunol. 1995;163(1):148–156. doi: 10.1006/cimm.1995.1109. [DOI] [PubMed] [Google Scholar]
- 62.Guo M, Gong S, Maric S, Misulovin Z, Pack M, Mahnke K, et al. A monoclonal antibody to the DEC-205 endocytosis receptor on human dendritic cells. Hum Immunol. 2000;61(8):729–738. doi: 10.1016/s0198-8859(00)00144-0. [DOI] [PubMed] [Google Scholar]