Abstract
Background
In adults 1-h plasma glucose concentration cut-point of 155 mg/dL (8.6 mmol/L) during the oral glucose tolerance test (OGTT) is a strong predictor of future diabetes risk.
Objective
We tested the hypothesis that a 1-h glucose concentration ≥ 155 mg/dL is associated with lower β-cell function in overweight/obese youth.
Research design and methods
One hundred and thirteen diabetes free overweight/obese youth aged 10–20 yr, underwent evaluation of β-cell function during a 2 h hyperglycemic clamp ~225 mg/dL (12.5 mmol/L), and insulin sensitivity during a 3 h hyperinsulinemic–euglycemic clamp, and a standard 2 h OGTT. Body composition and abdominal adiposity were determined by DEXA and CT scan. The disposition index (DI) was calculated as the product of first-phase insulin secretion and insulin sensitivity. Subjects were divided into two categories of 1-h plasma glucose concentration: <155 mg/dL (n = 69) and ≥155 mg/dL (n = 44).
Results
Youth with 1-h glucose ≥155 mg/dL had lower DI than those with 1-h glucose <155 mg/dL (295.1 ± 27.4 vs. 498.6 ± 37.7 mg/kg/min, p < 0.001), independent of the glucose tolerance status. In multiple regression models, DI was the strongest contributor to 1-h glucose concentration explaining ~21% of its variance.
Conclusions
Overweight/obese youth with 1-h glucose ≥155 mg/dL during the oral glucose tolerance test have a significantly lower β-cell function relative to insulin sensitivity even within the normal glucose tolerance range. Such youth may be at higher risk of future diabetes.
Keywords: children, disposition index, oral glucose tolerance test, type 2 diabetes mellitus
In 2010, about 285 million people, ages 20–70 yr, are estimated to have diabetes mellitus worldwide (1), representing a 67% increase from the published estimates for the year 2000 (2). Moreover, most disconcerting is that rates of type 2 diabetes mellitus are increasing among youth (3) concomitant with the increase in childhood obesity (4). It is suggested that efforts to lessen the escalating rates of future type 2 diabetes mellitus among adults should target at risk children (5). In order to identify children at risk for type 2 diabetes development, it is paramount to understand the pathogenesis of the disease. Similar to adults, overt type 2 diabetes mellitus in children manifests when there is a significant decline in β-cell function relative to insulin sensitivity (6). The disposition index (DI), a composite measure derived from first-phase insulin secretion, calculated during the hyperglycemic clamp, and insulin sensitivity, calculated during the hyperinsulinemic–euglycemic clamp, is considered the ‘gold standard’ in assessing β-cell function relative to insulin sensitivity (7, 8). DI is highest among children with normal glucose tolerance, lower among those with impaired glucose tolerance (IGT), and lowest in those with type 2 diabetes mellitus despite similar degrees of obesity (9). Thus a progressive decline in DI appears to underlie the progression from normal to impaired glucose tolerance and eventually to overt diabetes (10, 11).
Adults with IGT are at increased risk of future diabetes, with a rate of conversion of 5–10% per year (12). However, studies in adults suggest that the population at risk for developing type 2 diabetes mellitus should not be limited only to subjects with IGT (13). Recent reports from the San Antonio Heart Study and the Botnia Study have shown that the plasma glucose concentration at 1 h during the oral glucose tolerance test (OGTT) is a strong predictor of future type 2 diabetes risk in adults (14, 15). Abdul-Ghani et al. identified 1-h plasma glucose concentration cut-off of 155 mg/dL (8.6 mmol/L) as a useful tool to stratify subjects into high and low-risk groups for developing diabetes, regardless of their glucose tolerance status (14, 15). Moreover the presence of the metabolic syndrome among individuals with 1-h plasma glucose ≥155 mg/dL further increased the risk of diabetes mellitus development (14, 15).
In this study, we investigated the relationship between OGTT 1-h glucose concentration and β-cell function relative to insulin sensitivity expressed as the disposition index, in children. On the basis of adult studies, we hypothesized that a 1-h glucose concentration ≥155 mg/dL is associated with a lower DI in youth, independent of the degree of obesity and glucose tolerance status.
Methods
Study population
One hundred and thirteen overweight/obese, non-diabetic youth, 10–20 years old, who participated in our NIH funded studies to evaluate childhood metabolic markers of adult morbidity, with complete OGTT, hyperglycemic clamp, and hyperinsulinemic–euglycemic clamp data were included in this analysis. Some of the subjects were reported previously (16, 17). Participants in our studies are recruited from the community through local newspaper advertisements, flyers posted at various locations including public bus routes, children’s recreational centers, the medical campus, and the Children’s Hospital of Pitts-burgh. All studies were approved by the Institutional Review Board of the University of Pittsburgh and were in accordance of the Declaration of Helsinki. Informed consent and assent were obtained from each participant and their legal guardians. Fifty-six participants carried a diagnosis of polycystic ovary syndrome (PCOS). None of the participants including the subjects with PCOS were on medications that interfere with glucose metabolism or on oral contraceptives at the time of the study.
Clamp studies
Participants were admitted twice within 1–4 week period to the Pediatric Clinical and Translational Research Center (PCTRC) the day before the clamp studies, once for a hyperinsulinemic–euglycemic clamp and the other for a hyperglycemic clamp in random order. Each clamp evaluation was performed after a 10–12 h overnight fast. A fasting blood sample was obtained for total cholesterol, HDL, LDL, VLDL, and triglycerides measurements. For each clamp study, two intravenous catheters were inserted after the skin and subcutaneous tissues were anesthetized with EMLA cream (Astra Pharmaceutical Products, West Borough, MA, USA). One catheter was placed in a vein on the forearm for administration of glucose, and insulin; the second catheter was placed in a vein in the dorsum of the contra-lateral heated hand for sampling of arterialized venous blood as reported (6, 9).
Hyperinsulinemic – euglycemic clamp
A 3-h hyperinsulinemic (80 μm/m2/min)–euglycemic clamp was performed with plasma glucose clamped at 100 mg/dL (5.6 mmol/L) with a variable rate infusion of 20% dextrose based on arterialized plasma glucose determinations every 5 min as before (6, 9). Continuous indirect calorimetry by a ventilated hood system (Deltatrac Metabolic Monitor; Sensormedics, Anaheim, CA, USA) was performed to measure CO2 production, O2 consumption, and respiratory quotient for 30 min at baseline and at the end of the clamp (6).
Hyperglycemic clamp
First and second phase insulin secretions were assessed during a 2-h hyperglycemic clamp [225 mg/dL (12.5 mmol/L)]. Glucose and insulin concentrations were measured every 2.5 min (at 2.5, 5, 7.5, 10, and 12.5 min, first phase) and thereafter every 5 min for glucose and every 15 min for insulin, as before (6).
Oral glucose tolerance test
OGTTs were performed during the first admission to the PCTRC, one day prior to the first clamp study. After a 10–12 h overnight fast, the participants underwent a standard 2-h OGTT (1.75 g/kg, maximum 75 g). Blood samples were obtained at −15, 0, 15, 30, 60, 90, and 120 min for determination of glucose and insulin levels.
Body composition
Body composition was determined by dual energy X-ray absorptiometry (DEXA). A single transverse image of the abdomen (L4–L5) was obtained by means of computed tomography in 92 subjects and magnetic resonance imaging in 21 subjects to determine subcutaneous abdominal adipose tissue (SAT) and visceral adipose tissue (VAT).
Blood pressure determination
Resting blood pressure measurements were performed with an automated sphygmomanometer every 10 min for 1 h between 05:00 and 06:00 h, while the participants were asleep in the supine position and before awakening. The mean of seven measurements during each hour was the outcome for statistical analysis.
Biochemical measurements
Plasma glucose was measured with a glucose analyzer (Yellow Springs Instrument Co., Yellow Springs, OH, USA), insulin by radioimmunoassay (RIA) as before (6). HbA1c was measured by high performance liquid chromatography (Tosoh Medics, Inc., San Francisco, CA, USA; 1998) and lipids using the standards of the Centers for Disease Control and Prevention (6).
Calculations
Insulin stimulated glucose disposal rate (Rd) was calculated during the last 30 min of the 3-h hyperinsulinemic–euglycemic clamp to be equal to the rate of exogenous glucose infusion. Peripheral insulin sensitivity was calculated by dividing the Rd by the steady-state clamp insulin level (18). Insulin-stimulated carbohydrate and fat oxidation rates were calculated according to the formulas of Frayn (19) from the indirect calorimetry data. Non-oxidative glucose disposal was estimated by subtracting the rate of glucose oxidation from the total Rd. During the hyperglycemic clamp, the first and second phase insulin concentrations were calculated as described previously (6). Disposition index (DI) was calculated as the product of insulin sensitivity × 1st phase insulin (18).
Statistical analyses
Data were examined for normality of distribution. Differences in continuous variables between the two groups were tested using the independent-t test or the Mann–Whitney U test based on non-violation of statistical assumption. Differences in categorical variables were tested using the Chi square test or the Fisher’s exact test. The univariate general linear model (ANCOVA) was used to adjust for potential confounders. Each dependent variable was entered separately to the model, with 1-h glucose category as the fixed factor. Potential confounders adjusted for were entered as covariates in each model, and are specified with the reported results. Linear regression models were used to test the association of 1-h glucose with DI. DI, 1-h glucose, BMI, VAT, fat mass, Triglyceride/HDL ratio, systolic blood pressure, and diastolic blood pressure were log transformed (natural logarithm) before entry into the regression models. Three models were tested with ln (1-h glucose) as the dependent variable and the following independent variables: Model 1 [ln(DI), race, sex, age, ln(SBP), ln(DBP), ln(Triglyceride/HDL), and ln(BMI)]; model 2 was similar to model 1 except that BMI was replaced with ln(Fat mass)], and in model 3 this was replaced with ln(VAT)]. Because blood pressure and triglyceride/HDL ratios were significant contributors to the diabetes mellitus prediction models in adults (14, 15), we included them in the current analysis. Data are presented as mean ± SEM unless otherwise indicated. Statistical significance was set at p ≤0.05. All statistical assumptions were met. All statistical analyses were performed using SPSS software (18th edition SPSS Inc, Chicago, IL, USA).
Results
Study participants
The participants (Table 1) were 10–19.8 years old, overweight/obese individuals with a mean age 14.8 ± 0.2 yr. The group consisted of 38 males (33.6%) and 75 females (66.4%). Forty-one subjects self identified as African-American (36.3%), 65 as Caucasian (57.5%), and 7 as biracial (6.2%). All subjects were pubertal: 12 (10.6%) were in Tanner stages II–III puberty and 101 (89.4%) were in Tanner stages IV–V. Forty-six participants (40.7%) had impaired glucose tolerance as defined by the American Diabetes Association (20). The study participants were divided into two groups based on OGTT 1-h plasma glucose concentration: <155 mg/dL and ≥155 mg/dL. We elected to use this cut-off, because it was shown to be a strong predictor of future diabetes risk in adult studies (14, 15).
Table 1.
Demographic, anthropometric, and fasting metabolic profile of the study participants by 1-h plasma glucose concentration category
| One-hour plasma glucose concentration
|
p | ||
|---|---|---|---|
| <155 mg/dL | ≥155 mg/dL | ||
| n | 69 | 44 | |
| Age (yr) | 15.0 ± 0.2 | 14.5 ± 0.3 | 0.2 |
| Sex (Male/Female), n | 25/44 | 13/31 | 0.5* |
| Race (AA/C/BR), n | 29/33/7 | 12/32/0 | 0.01* |
| Tanner, n | 0.5* | ||
| II–III | 6 | 6 | |
| IV–V | 63 | 38 | |
| BMI (kg/m2) | 35.4 ± 0.7 | 36.6 ± 1.1 | 0.5 |
| BMI % | 98.1 ± 0.2 | 98.3 ± 0.3 | 0.5 |
| Waist circumference (cm) | 105.1 ± 1.9 | 105.6 ± 2.9 | 0.9 |
| Fat mass (kg) | 42.3 ± 1.5 | 41.8 ± 1.9 | 0.8 |
| Free fat mass (kg) | 48.9 ± 1.1 | 49.6 ± 1.6 | 0.8 |
| % Body fat | 45.1 ± 0.7 | 44.3 ± 1.0 | 0.5 |
| VAT (cm2)† | 69.3 ± 4.1 | 80.1 ± 5.4 | 0.1 |
| SAT (cm2)† | 518.5 ± 22.5 | 535.4 ± 28.3 | 0.6 |
| HbA1c (%) | 5.4 ± 0.04 | 5.4 ± 0.07 | 0.4 |
| Cholesterol (mg/dL) | 157.4 ± 3.9 | 165.9 ± 5.6 | 0.2 |
| HDL (mg/dL) | 40.2 ± 1.0 | 39.0 ± 1.4 | 0.7 |
| LDL (mg/dL) | 94.7 ± 3.4 | 97.9 ± 5.5 | 0.6 |
| Triglycerides (mg/dL) | 112.9 ± 6.4 | 145.4 ± 13.9 | 0.04 |
| SBP AM (mmHg) | 111.2 ± 1.5 | 116.0 ± 1.6 | 0.04 |
| DBP AM (mmHg) | 58.8 ± 0.9 | 62.3 ± 1.0 | 0.02 |
| Impaired glucose tolerance, n (%) | 14 (25.5) | 32 (72.7) | <0.001 |
| PCOS, n (%) | 34 (49.3) | 22 (50) | 1.0 |
Data are means ± SEM. AA: African-American, C: Caucasian, BR: Biracial, VAT: visceral adipose tissue, SAT: subcutaneous adipose tissue, HDL: high density lipoprotein, LDL: low density lipoprotein, SBP: systolic blood pressure, DBP: diastolic blood pressure.
p represents Chi-square analysis. To convert to SI units (mmol/L) multiply by 0.05551 for glucose, by 0.02586 for cholesterol, HDL and LDL and by 0.01163 for triglycerides.
Twenty-one subjects had abdominal adiposity measurements by magnetic resonance imaging, and 92 subjects by computed tomography. VAT and SAT differences remained non-significant when these subjects were analyzed separately.
Age, sex, and Tanner stage distribution were similar between the two groups (Table 1). Body mass index (BMI), BMI percentiles, waist circumference, visceral adipose tissue, fat mass, and percent body fat were not different between the two groups (Table 1) before and after adjustment for race (p = 0.3 for BMI, p = 0.5 for BMI percentile, p = 0.1 for VAT, p = 0.8 for fat mass and p = 0.5 for % body fat). There were similar numbers of participants with PCOS diagnosis in the two groups.
Blood pressure and fasting metabolic profile
Resting systolic and diastolic blood pressures were significantly higher among the participants with 1-h glucose ≥155 mg/dL (Table 1). The differences remained significant after adjustment for race (ANCOVA p = 0.04 and p = 0.01 for systolic and diastolic blood pressures, respectively). Triglyceride levels were significantly higher among the participants with 1-h plasma glucose ≥155 mg/dL compared to those with concentrations <155 mg/dL (Table 1). There were no statistically significant differences in fasting total cholesterol, low density lipoprotein (LDL) or high density lipoprotein (HDL) between the two groups (Table 1).
In vivo insulin sensitivity and insulin secretion
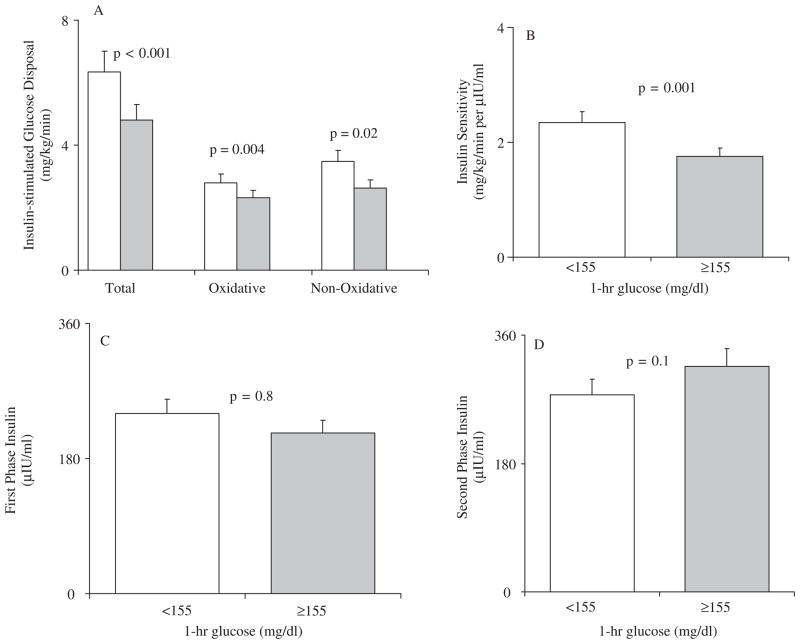
Insulin stimulated total, oxidative and non-oxidative glucose disposal were significantly lower in the participants with 1-h glucose ≥155 mg/dL versus <155 mg/dL (Fig. 1A). Similarly insulin sensitivity during the hyperinsulinemic–euglycemic clamp was significantly lower in the participants with higher 1-h glucose concentrations (Fig. 1B). First-phase and second-phase insulin levels were not significantly different between the two groups (Fig. 1, panels C and D).
Fig. 1.
Insulin stimulated glucose disposal, total, oxidative and non-oxidative, (panel A), and insulin sensitivity (panel B) during the hyperinsulinemic–euglycemic clamp; and first and second phase insulin levels (panels C and D) during the hyperglycemic clamp in youths with 1-h plasma glucose concentration <155 mg/dL (blank bars, n = 69) and ≥155 mg/dL (gray bars, n = 44). Data represent the unadjusted means ± SEM. To convert to SI units multiply by 5.551 for insulin stimulated glucose disposal (μmol/kg/min), by 0.9252 for insulin sensitivity (μmol/kg/min per pmol/L), and by 6.0 for insulin (pmol/L).
Disposition index
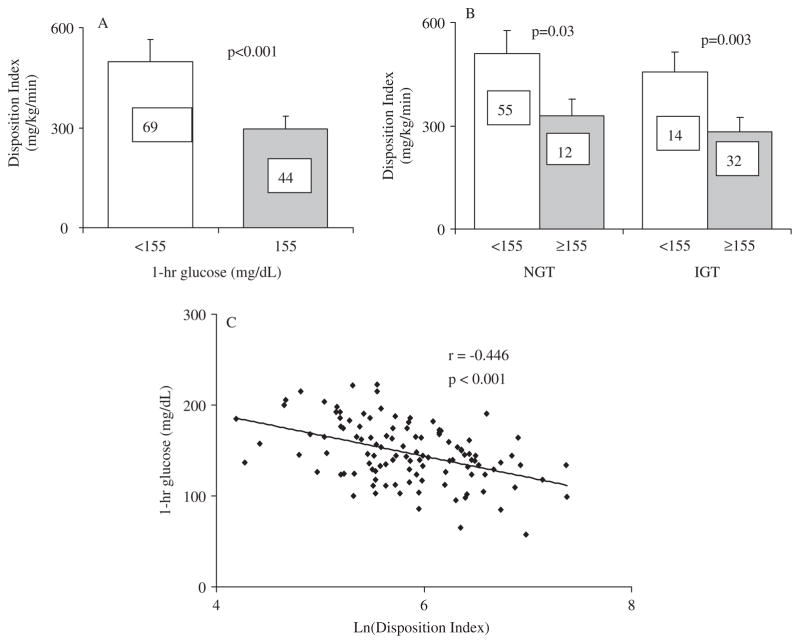
The disposition index was lower among youth with 1-h glucose ≥155 mg/dL compared to those with 1-h glucose <155 mg/dL (295.1 ± 27.4 vs. 498.6 ± 37.7 mg/kg/min, p < 0.001 respectively) (Fig. 2A). The difference remained significant after adjustment for sex, race, BMI, VAT, and % body fat (ANCOVA p = 0.002). Among participants with normal glucose tolerance, DI was still significantly lower in the group with 1-h glucose ≥155 mg/dL (328.6 ± 75.5 vs. 509.6 ± 45.3 mg/kg/min, p = 0.03). Similarly, among participants with impaired glucose tolerance DI was significantly lower in the 1-hr glucose category ≥155 mg/dL (282.5 ± 25.7 vs. 455.4 ± 54.9 mg/kg/min, p = 0.003) (Fig. 2B).
Fig. 2.
(A) Disposition index (mg/kg/min) in youths with 1-h plasma glucose concentration <155 mg/dL (blank bars) and ≥155 mg/dL (gray bars). Numbers in bars represent n in each category. (B) Disposition index (mg/kg/min) in youths with 1-h plasma glucose concentration <155 mg/dL (blank bars) and ≥155 mg/dL (gray bars), separated according to glucose tolerance status. NGT: normal glucose tolerance. IGT: impaired glucose tolerance. Data represent the unadjusted means ± SEM. (C) Pearson correlation between 1-h plasma glucose concentration and ln(Disposition Index). To convert to SI units multiply by 5.551 for disposition index (μmol/kg/min).
Ninety-eight of our subjects had normal fasting glucose defined as plasma glucose <100 mg/dL. Within this group, DI was significantly lower among participants with 1-h glucose ≥155 mg/dL (293.9 ± 25.9 vs. 507.4 ± 40.7 mg/kg/min, p < 0.001). The number of subjects with impaired fasting glucose, defined as fasting glucose 100 to <126 mg/dL, was small (n = 15) precluding meaningful analysis in the sub-groups of 1 h glucose categories.
DI correlated inversely and significantly with 1-h glucose (Fig. 2C). Moreover, in the three linear regression models DI had the strongest partial correlation with 1h glucose, and only DI, age, and sex contributed significantly to the models. SBP, DBP, and triglycerides/HDL ratio did not contribute significantly to the models. Model 1 [ln(DI), race, sex, age, ln(SBP), ln(DBP), ln(Triglyceride/HDL), and ln(BMI)]: R2 = 0.321, p < 0.001 (partial correlations: ln(DI) −0.464, p < 0.001; age −0.284, p = 0.004; sex −0.205, p = 0.04). Model 2 with ln(Fat mass) instead of BMI: R2 = 0.334, p < 0.001, (partial correlations: ln(DI) −0.471, p < 0.001; age −0.292, p = 0.003; sex −0.227, p = 0.02). Model 3 with ln(VAT) instead of BMI: R2 = 0.319, p < 0.001 (partial correlations: ln(DI) −0.442, p < 0.001; age −0.260, p = 0.01; sex −0.203, p = 0.05).
Discussion
In the present study, overweight/obese children with 1-h plasma glucose concentration ≥155 mg/dL during the OGTT had ~41% lower glucose disposition indexes than those with 1-h glucose <155 mg/dL regardless of the glucose tolerance status. When analyzed separately, participants with normal glucose tolerance (defined as 2-h plasma glucose concentration <140 mg/dL (7.8 mmol/L)) and 1-h glucose ≥155 mg/dL, still had ~35.5% lower DI than those with 1-h glucose <155 mg/dL. In addition, in the linear regression models, DI negatively correlated with 1-hour glucose concentration and contributed ~21% to the variance in 1-h glucose concentration. The current findings suggest that regardless of the 2-h plasma glucose concentration, overweight/obese children whose 1-h OGTT glucose levels are 155 mg/dL and above have a significantly lower β-cell reserve compared with those below that level.
Adults with impaired glucose tolerance (IGT) are at higher risk of future diabetes (12, 21), however only 35–50% of individuals with IGT progress to diabetes after 10–20 years of follow-up. More importantly, while in most populations studied about 60% of individuals with diabetes had either impaired glucose tolerance or impaired fasting glucose ~5 years prior to diagnosis, the other 40% had normal glucose tolerance by standard definitions (21). These observations led to several studies investigating models for prediction of type 2 diabetes mellitus. Recent reports identified the plasma glucose concentration at 1-h during the oral glucose tolerance test as a strong predictor of future type 2 diabetes risk in adults of different ethnic backgrounds, independent of glucose tolerance status (14, 15). A cut-point of 155 mg/dL for the 1-h plasma glucose concentration had 50% more sensitivity, but 15% less specificity than the 2-h plasma glucose concentration cut-point of 140 mg/dL in predicting the progression to diabetes among participants in the San Antonio Heart Study (22). Our current findings of a significant impairment in DI in youth with 1-h plasma glucose concentration ≥155 mg/dL, regardless of glucose tolerance status, are inline with these reports. Youth with 1-h plasma glucose concentration ≥155 mg/dL in our study had lower insulin-stimulated glucose disposal (total, oxidative, and non-oxidative) compared to those with 1-h plasma glucose concentration <155 mg/dL, however they failed to mount a higher compensatory first-phase and second-phase insulin secretion response. This phenomenon of impaired pancreatic β-cell response relative to the degree of insulin sensitivity is believed to be the major pathophysiologic defect leading to type 2 diabetes development in both children and adults (6, 23).
Our findings of lower DI in the 1-h glucose ≥155 mg/dL category regardless of the glucose tolerance status are consistent with data from adult studies (14, 15). Adults with normal glucose tolerance (NGT) and 1-h glucose ≥155 mg/dL had an 8.5–15.3% risk for future diabetes development, which was significantly higher than the 1.3–2.9% risk among NGT with 1-h glucose <155 mg/dL (14, 15). Similarly the risk of diabetes development was higher, 35.5 versus 17.8% among IGT subjects with 1-h glucose ≥155 mg/dL compared to those with 1-h glucose <155 mg/dL (14). The presence of features of the metabolic syndrome together with a 1-h glucose concentration ≥155 mg/dL further increased the risk of type 2 diabetes in adults by an additional 5–16% (14, 15).
The difference in DI between the two categories of 1-h plasma glucose in our study could not be accounted for by differences in BMI, visceral adipose tissue or fat mass which were not different between the two groups. The youth with 1-h plasma glucose ≥155 mg/dL had features of the metabolic syndrome, manifested in significantly higher systolic and diastolic blood pressures, and higher triglycerides and triglyceride/HDL ratio than those with 1-h glucose <155 mg/dL. Our findings resonate with some longitudinal studies that investigated childhood predictors of young-adult type 2 diabetes mellitus development (24–26). A recent report from the longitudinal prospective National Growth and Health Study (9 years follow-up) and the Princeton Follow-up study (20 years follow-up) identified systolic blood pressure and BMI in the top fifth percentiles along with African-American race to be independently associated with young-adult diabetes mellitus development (25). Glucose levels >100 mg/dL (5.6 mmol/L) and triglyceride concentrations in the top 5th percentile increased the likelihood of diabetes development in young-adults in the Princeton Follow-up Study (25). Data on glucose tolerance status were not available in this study. Franks et al. identified BMI, fasting glucose, 2-h glucose and HDL cholesterol in a cohort of 1,604 Pima Indian children ages 5–19 yr as independent predictors of type 2 diabetes development in young adulthood (24). Nguyen et al. identified consistently higher fasting glucose levels from childhood through adulthood, higher BMI, triglycerides, fasting insulin levels and lower HDL concentrations as risk factors for diabetes development in a retrospective analysis of 1988 participants in the Bogalusa Heart Study (26). Data on glucose tolerance status were not available in this study (26), and neither of the aforementioned studies had data on 1-h plasma glucose concentrations. In our study, despite the higher systolic and diastolic blood pressure and triglycerides/HDL ratio in the 1-h glucose group >155 mg/dL, they did not contribute significantly to 1-h glucose concentration in the linear regression models. Thus, it is possible that metabolic syndrome features are just phenotypic characteristics of obese youth with higher 1-h glucose levels during the OGTT without necessarily contributing or enhancing the risk of abnormal glucose regulation. It is also possible that the duration of exposure to such adverse metabolic profiles may translate over time to enhanced risk as is the case in the longitudinal observations of adult studies. Similarly, BMI did not contribute significantly to the linear regression model in our participants. This is likely because of the fact that all of our participants were overweight or obese with a relatively narrow range of BMIs without youth with normal BMIs. It remains to be determined if our current observations with regards to 1-h glucose levels and β-cell function hold true in normal weight youth.
While the number of subjects in our study is significantly large for detection of differences in DI between the two categories of 1-h glucose based on the clamp studies, the sample size may not be large enough to detect additional effects of features of the metabolic syndrome. One limitation of our study is that it included more females than males; however the sex distribution between the two categories of 1-h glucose was not different. On the other hand, there were seven biracial participants in the lower 1-h glucose category and none in the higher category. Exclusion of those seven participants did not alter the results of our analysis. The differences in DI remained significant after adjustment for both sex and race, and could not be attributed to these parameters.
In summary, youth with 1-h plasma glucose ≥155 mg/dL have significantly lower pancreatic β-cell function relative to insulin sensitivity compared to those with 1-h plasma glucose <155 mg/dL, independent of their glucose tolerance status. This may constitute a very early metabolic signal for an enhanced risk of future diabetes development. Longitudinal studies are needed to investigate whether or not this early impairment in DI will translate into higher rates of type 2 diabetes later on. However until such studies are available, overweight/obese children with 1-hr plasma glucose response during the OGTT exceeding 155 mg/dL may require more vigilant follow-up and therapeutic interventions regardless of their glucose tolerance status.
Acknowledgments
These studies would not have been possible without the nurses and the staff of the Pediatric Clinical and Translational Research Center, the devotion of Sabrina Kadri and Nancy Guerra, CRNP, (project coordinators), the laboratory expertise of Resa Stauffer and Katie McDowell, but most importantly, the commitment of the study participants and their parents.
This work was supported by United States Public Health Service grant RO1 HD27503 (SA), K24 HD01357 (SA), Richard L. Day Endowed Chair (SA), MO1 RR00084 (GCRC), UL1 RR024153 (CTSA) and the Thrasher Research Fund (FB).
Footnotes
Conflict of interests
The authors have nothing to disclose.
References
- 1.Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87:4–14. doi: 10.1016/j.diabres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 2.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 3.Dabelea D, Bell RA, D’Agostino RB, Jr, et al. Incidence of diabetes in youth in the United States. Jama. 2007;297:2716–2724. doi: 10.1001/jama.297.24.2716. [DOI] [PubMed] [Google Scholar]
- 4.Ogden CL, Carroll MD, Flegal KM. High body mass index for age among US children and adolescents, 2003–2006. Jama. 2008;299:2401–2405. doi: 10.1001/jama.299.20.2401. [DOI] [PubMed] [Google Scholar]
- 5.Gregg EW. Are children the future of type 2 diabetes prevention? N Engl J Med. 2010;362:548–550. doi: 10.1056/NEJMe0912192. [DOI] [PubMed] [Google Scholar]
- 6.Gungor N, Bacha F, Saad R, Janosky J, Arslanian S. Youth type 2 diabetes: insulin resistance, beta-cell failure, or both? Diabetes Care. 2005;28:638–644. doi: 10.2337/diacare.28.3.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bergman RN, Ader M, Huecking K, Van Citters G. Accurate assessment of beta-cell function: the hyperbolic correction. Diabetes. 2002;51(Suppl 1):S212–S220. doi: 10.2337/diabetes.51.2007.s212. [DOI] [PubMed] [Google Scholar]
- 8.Arslanian SA. Clamp techniques in paediatrics: what have we learned? Horm Res. 2005;64(Suppl 3):16–24. doi: 10.1159/000089313. [DOI] [PubMed] [Google Scholar]
- 9.Bacha F, Gungor N, Lee S, Arslanian SA. In vivo insulin sensitivity and secretion in obese youth: what are the differences between normal glucose tolerance, impaired glucose tolerance, and type 2 diabetes? Diabetes Care. 2009;32:100–105. doi: 10.2337/dc08-1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saad R, Gungor N, Arslanian S. Progression from normal glucose tolerance to type 2 diabetes in a young girl: longitudinal changes in insulin sensitivity and secretion assessed by the clamp technique and surrogate estimates. Pediatr Diabetes. 2005;6:95–99. doi: 10.1111/j.1399-543X.2005.00097.x. [DOI] [PubMed] [Google Scholar]
- 11.Weiss R, Cali AM, Dziura J, Burgert TS, Tamborlane WV, Caprio S. Degree of obesity and glucose allostasis are major effectors of glucose tolerance dynamics in obese youth. Diabetes Care. 2007;30:1845–1850. doi: 10.2337/dc07-0325. [DOI] [PubMed] [Google Scholar]
- 12.Gerstein HC, Santaguida P, Raina P, et al. Annual incidence and relative risk of diabetes in people with various categories of dysglycemia: a systematic overview and meta-analysis of prospective studies. Diabetes Res Clin Pract. 2007;78:305–312. doi: 10.1016/j.diabres.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 13.Abdul-Ghani MA, DeFronzo RA. Plasma glucose concentration and prediction of future risk of type 2 diabetes. Diabetes Care. 2009;32(Suppl 2):S194–S198. doi: 10.2337/dc09-S309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abdul-Ghani MA, Abdul-Ghani T, Ali N, Defronzo RA. One-hour plasma glucose concentration and the metabolic syndrome identify subjects at high risk for future type 2 diabetes. Diabetes Care. 2008;31:1650–1655. doi: 10.2337/dc08-0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abdul-Ghani MA, Lyssenko V, Tuomi T, DeFronzo RA, Groop L. Fasting versus postload plasma glucose concentration and the risk for future type 2 diabetes: results from the Botnia Study. Diabetes Care. 2009;32:281–286. doi: 10.2337/dc08-1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burns SF, Lee S, Arslanian SA. In vivo insulin sensitivity and lipoprotein particle size and concentration in black and white children. Diabetes Care. 2009;32:2087–2093. doi: 10.2337/dc09-0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tfayli H, Lee S, Arslanian S. Declining beta-cell function relative to insulin sensitivity with increasing fasting glucose levels in the nondiabetic range in children. Diabetes Care. 2010;33:2024–2030. doi: 10.2337/dc09-2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arslanian SA, Saad R, Lewy V, Danadian K, Janosky J. Hyperinsulinemia in African-American children: decreased insulin clearance and increased insulin secretion and its relationship to insulin sensitivity. Diabetes. 2002;51:3014–3019. doi: 10.2337/diabetes.51.10.3014. [DOI] [PubMed] [Google Scholar]
- 19.Frayn KN. Calculation of substrate oxidation rates in vivo from gaseous exchange. J Appl Physiol. 1983;55:628–634. doi: 10.1152/jappl.1983.55.2.628. [DOI] [PubMed] [Google Scholar]
- 20.Diagnosis and classification of diabetes mellitus. Diabetes Care. 2009;32(Suppl 1):S62–S67. doi: 10.2337/dc09-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Unwin N, Shaw J, Zimmet P, Alberti KG. Impaired glucose tolerance and impaired fasting glycaemia: the current status on definition and intervention. Diabet Med. 2002;19:708–723. doi: 10.1046/j.1464-5491.2002.00835.x. [DOI] [PubMed] [Google Scholar]
- 22.Abdul-Ghani MA, Williams K, DeFronzo RA, Stern M. What is the best predictor of future type 2 diabetes? Diabetes Care. 2007;30:1544–1548. doi: 10.2337/dc06-1331. [DOI] [PubMed] [Google Scholar]
- 23.Kahn SE. The relative contributions of insulin resistance and beta-cell dysfunction to the pathophysiology of Type 2 diabetes. Diabetologia. 2003;46:3–19. doi: 10.1007/s00125-002-1009-0. [DOI] [PubMed] [Google Scholar]
- 24.Franks PW, Hanson RL, Knowler WC, et al. Childhood predictors of young-onset type 2 diabetes. Diabetes. 2007;56:2964–2972. doi: 10.2337/db06-1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morrison JA, Glueck CJ, Horn PS, Wang P. Childhood predictors of adult type 2 diabetes at 9- and 26-year follow-ups. Arch Pediatr Adolesc Med. 164:53–60. doi: 10.1001/archpediatrics.2009.228. [DOI] [PubMed] [Google Scholar]
- 26.Nguyen QM, Srinivasan SR, Xu JH, Chen W, Berenson GS. Changes in risk variables of metabolic syndrome since childhood in pre-diabetic and type 2 diabetic subjects: the Bogalusa Heart Study. Diabetes Care. 2008;31:2044–2049. doi: 10.2337/dc08-0898. [DOI] [PMC free article] [PubMed] [Google Scholar]