Abstract
Background
The pandemic potential of the influenza A (H5N1) virus is among the greatest public health concerns of the 21st century.
Objective
To determine the effectiveness and cost-effectiveness of alternative pandemic mitigation and response strategies.
Design
Compartmental epidemic model in conjunction with a Markov model of disease progression.
Data Sources
Literature and expert opinion.
Target Population
Residents of a U.S. metropolitan city.
Time Horizon
Lifetime.
Perspective
Societal.
Interventions
One mitigation strategy used non-pharmaceutical interventions, vaccination, and antiviral pharmacotherapy in quantities similar to those available currently in the U.S. stockpile. The second and third strategies used expanded supplies of either antivirals (expanded antiviral prophylaxis strategy) or adjuvanted vaccine (expanded vaccination strategy) in addition to non-pharmaceutical interventions.
Outcome Measures
Infections and deaths averted, costs, quality-adjusted life-years (QALYs), and incremental cost-effectiveness.
Results of Base Case Analysis
The stockpiled strategy averted 44% of infections and deaths, gaining 258,342 QALYs at $8,907 per QALY gained relative to no intervention. Expanded antiviral prophylaxis delayed the pandemic, averting 48% of infections and deaths, and gaining 282,329 QALYs, with a less favorable cost-effectiveness ratio than adjuvanted vaccination. Adjuvanted vaccination was the most effective strategy and was cost-effective, averting 68% of infections and deaths, and gaining 404,030 QALYs at $10,844 per QALY gained relative to stockpiled strategy.
Results of Sensitivity Analysis
Over a wide range of assumptions, the incremental cost-effectiveness ratio of the expanded adjuvanted vaccination strategy was less than $50,000 per QALY gained.
Limitations
Large groups and frequent contacts may spread the virus more rapidly. The model is not designed to target interventions to specific groups.
Conclusions
Expanded adjuvanted vaccination is an effective and cost-effective mitigation strategy for an influenza A (H5N1) pandemic. Expanded antiviral prophylaxis can be beneficial in delaying the pandemic while additional strategies are implemented.
The 2009 (H1N1) Pandemic has highlighted the urgent need for effective mitigation strategies for an influenza pandemic. Despite the appropriate current focus on the (H1N1) Pandemic, the pandemic potential of the influenza A (H5N1) virus remains one of the most important international public health concerns of the 21st century (2). In contrast to Pandemic (H1N1), which has had a low case-fatality to date (1), A (H5N1) is not yet easily transmissible, but is highly lethal. Additionally, A (H5N1) has raised concern by following three patterns historically reminiscent of pandemic viruses: 1) increasing numbers of human infections in Southeast Asia; 2) spread to Europe, Africa, and the Middle East; and 3) accelerated development of distinct genetic groups known as clades and subclades (3). Of the viruses responsible for the three 20th century influenza pandemics, A (H5N1) genetically most closely resembles the A (H1N1) virus which caused the 1918 pandemic (4, 5). This pandemic was one of the most devastating, killing 50-100 million people, with a propensity for pregnant women and young, healthy adults (6).
A virus must meet three conditions to have pandemic potential: high virulence, antigenic uniqueness, and sustained human-to-human transmissibility (8). Existing A (H5N1) meets all of these except one: the ability to spread sustainably among humans (55, 56). It could develop this ability by genetic reassortment via an interspecies link (such as swine, whose trachea contain receptors for both human and avian influenza viruses) or a spontaneous mutation. Owing to its lack of an error-checking mechanism, it is particularly susceptible to such a mutation during replication. The 2009 (H1N1) Pandemic has convincingly demonstrated the extraordinary rapidity of the global spread of a new influenza virus (57), and the World Health Organization (WHO) and World Bank predict an A (H5N1) pandemic could cause hundreds of millions of deaths, with a lasting and crippling impact on global economies (58).
Public health strategies for mitigating an influenza pandemic consist of non-pharmaceutical interventions, such as social distancing, use of masks and respirators, hand hygiene, and cough etiquette, or pharmaceutical interventions such as vaccines and antivirals (59). Previous models have targeted antiviral distribution to close contacts of infected individuals (12, 22, 60, 61), a strategy criticized as having limited usefulness in the 2009 (H1N1) Pandemic (62); researchers have not examined broader distribution strategies for large urban populations with high contact rates between random individuals. Vaccination against A (H5N1) has had limited success in eliciting adequate human antibody response, and designing a vaccine effective against a frequently changing virus has been challenging (63). Few studies have analyzed cost-effectiveness of pandemic mitigation strategies.
Recent studies (36, 64, 65) have overcome limitations of A (H5N1) vaccines by administering them with adjuvants, substances that make them more immunogenic at lower doses. We developed a model of an influenza A (H5N1) pandemic to examine the effectiveness and cost-effectiveness of a pharmaceutical intervention strategy with vaccination and extended-duration antiviral prophylaxis; an expanded antiviral prophylaxis strategy; and an expanded adjuvanted vaccination strategy.
METHODS
Overview
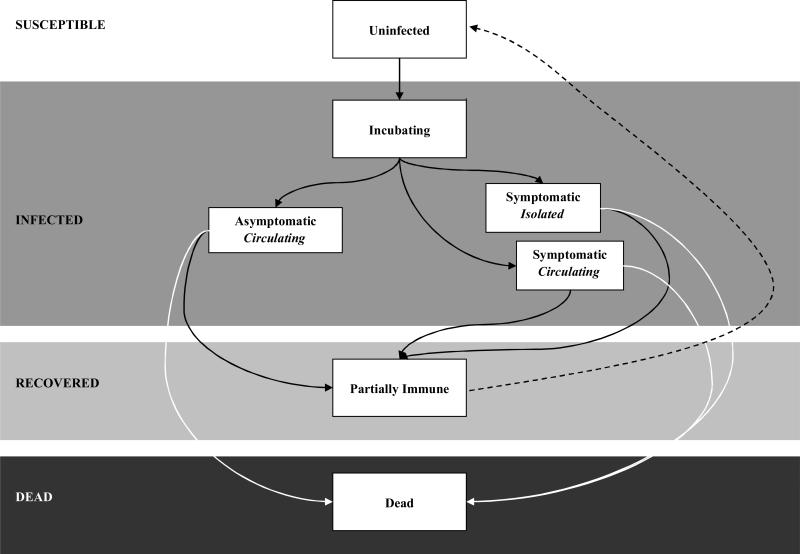
We developed a compartmental epidemic model in conjunction with a Markov model of disease progression to elucidate the spread of A (H5N1) in a susceptible population (Figure 1). We evaluated three mitigation strategies, described further below. Each strategy included non-pharmacologic interventions such as hand washing and social distancing. The first strategy, which we call the stockpiled strategy, was designed to be similar to the U.S. Department of Health and Human Services (HHS) pandemic plan with the use of currently stockpiled vaccine and antivirals. We also evaluated two additional strategies that made use of expanded antiviral drugs (expanded antiviral prophylaxis strategy) or expanded adjuvanted vaccination (expanded adjuvanted vaccination). We modeled the dynamics of disease transmission and progression of the first pandemic wave daily over a period of one year. Following the recommendations of the Panel on Cost-Effectiveness in Health and Medicine (54), we adopted a societal perspective for costs and benefits, discounted at 3% annually. We analyzed outcomes for the remaining lifetime of the individuals. We expressed these outcomes in infections and deaths, costs, quality-adjusted life years (QALYs) and incremental cost-effectiveness ratios. We developed the simulation model and performed analyses with Microsoft Excel (66).
Figure 1. Basic states of the compartmental model.
The infection rate is dynamically related to the number of susceptible, infected, recovered, and dead individuals in the population. All individuals entered the model susceptible to infection. Infected individuals first entered an asymptomatic incubation period and could then progress either to symptomatic or asymptomatic infection. Those with symptomatic infection either isolated or continued to circulate and infect others. Infected individuals either recovered or died. The majority of those who recovered developed complete immunity to the virus; a small minority was susceptible to recurrent infection from a drifted viral strain.
Study Population and Disease Parameters
For the purposes of our analysis (see Figure 1), the population was divided into susceptible, infected, and individuals who had recovered or died from influenza.
Susceptible population
We followed a hypothetical cohort of persons living in a large U.S. city with a sex distribution (53% women), age 0 to 100 years, and average remaining life expectancy similar to the population of New York City (7). We assumed that 1,000 individuals were infected at the start of the pandemic and varied this number from 100 to 10,000 in sensitivity analysis. Lacking prior exposure to A (H5N1) (8), all individuals entered the model susceptible to infection. In sensitivity analysis, we examined scenarios in which 10% of individuals entered immune to the virus.
Infected population
The size of the infected population depends on the ease of influenza transmission. We measure transmissibility by the reproductive number, R0, the average number of secondary infections caused by a single infectious individual in a susceptible population. The reproductive number of a pandemic strain of A (H5N1) virus depends on the unknown transmissibility of a novel human subtype. We assumed an R0 of 1.8, based on the 1918 Spanish Flu Pandemic, corresponding to a Centers for Disease Control and Prevention (CDC) severity category 5 pandemic (60). In sensitivity analysis, we varied R0 from 1.4 (less severe than the 1957 and 1968 pandemics) to 2.2 (more severe than the 1918 pandemic).
Recovered population
Antigenic drift occurs throughout the course of a pandemic; estimations of re-infection with drifted influenza A viruses range from 2-25% (27-30). Most re-infected individuals are either asymptomatic or mildly symptomatic with a shorter duration of illness and less viral shedding, so we assumed that 5% of the recovered population was once again susceptible to infection at an average of 5 months following recovery (Appendix).
Death from influenza
The mortality rate associated with a pandemic strain of A (H5N1) virus is uncertain; a mutated virus capable of efficient human-to-human transmission may develop other mutations affecting its virulence. We modeled a severe (consistent with CDC severity category 5) pandemic, with a 20-40% population illness rate and a 2.5% clinical case-fatality proportion (59). In sensitivity analysis, we modeled a more severe 60% clinical case-fatality proportion (current human case-fatality of A (H5N1) (8)) and less severe (CDC severity category 2, based on the 1957 and 1968 pandemics) 0.5% clinical case-fatality proportion (59). We modeled age-specific mortality, with a “J-shaped” mortality curve, with greater mortality rates in newborns and individuals over 65 years, consistent with the 1957 and 1968 pandemics and seasonal influenza epidemics (67-69). In sensitivity analysis, we examined a “W-shaped” mortality curve, with additional increases in mortality in young adults, as occurred in the 1918 Pandemic (67, 69). Based on population behavior in prior pandemics (31), we assumed that healthy individuals would begin voluntary reactive social distancing as mortality rates in the city increased (Appendix).
Interventions
Non-pharmaceutical interventions
Because they rely on state and local jurisdiction, HHS non-pharmaceutical interventions are not standardized (59). The main non-pharmaceutical interventions that could be undertaken include social distancing such as school closure and workplace reduction of contacts (e.g., telecommuting). In addition, a recent randomized trial suggested that hand washing, use of alcohol hand gels, and use of personal protective equipment such as masks could reduce transmission to household contacts by as much as 66% if they are implemented within 36 hours of an index case becoming ill (10). Our model is not designed to evaluate the impact of social distancing strategies directly. Instead, we used the results of a complex network model developed by Davey et al. (70) at Sandia National Laboratories to estimate the reduction in contacts that would occur if non-pharmaceutical interventions were enacted. Based on this work, we assumed that implementation of non-pharmaceutical interventions would reduce contacts by 25%. We evaluated reduction in contacts from 10% to 70% in sensitivity analyses.
Vaccination
Two approaches to A (H5N1) vaccination are under consideration. Vaccine can be given without an adjuvant in two doses (unadjuvanted), or vaccine can be combined with an adjuvant in each dose that heightens the immune response to the vaccine antigen. The potential advantages of the adjuvanted vaccine are increased effectiveness and the ability to reduce the amount of antigen in each dose, which would allow more individuals to be vaccinated (36, 64, 65).
Based on the 1976 influenza vaccination campaign in New York City (71), we assumed that all individuals in the city could be vaccinated in 10 days. Based on studies of two-dose A (H5N1) vaccination (35), we assumed that the second dose would be administered 21 days later and that individuals were protected after that dose. The effectiveness of any vaccine against a novel human influenza subtype is unknown and unlikely to be complete; we assumed that non-adjuvanted vaccine would not be well-matched to the mutated virus and would be 30% effective. Based on studies showing that adjuvanted vaccines help overcome humans’ poor immunogenic response to novel viruses, elicit antibody responses in much higher percentages than non-adjuvanted vaccines, and can protect against different A (H5N1) clades (36, 64, 65), we assumed that the adjuvanted two-dose vaccination sequence would be 50% effective. We assumed an effectiveness of 40% for individuals who received only one of two adjuvanted doses (72).
Based on adjuvanted and unadjuvanted A (H5N1) vaccination data (35, 36), we assumed that 45% of vaccinated individuals would experience mild to moderate adverse reactions such as pain, redness, swelling, induration, ecchymosis, low-grade fevers, arthralgias, fatigues, headaches, myalgias, shivering, or sweating for up to seven days. Based on seasonal influenza, A (H5N1), and 1976 vaccination data (37, 40), we assumed that 0.001% of the population experienced severe adverse reactions such as angioedema, anaphylaxis, or Guillain-Barré Syndrome.
Antiviral Treatment and Prophylaxis
We evaluated the use of antiviral drugs for both treatment and prophylaxis, and made the following additional assumptions: antiviral distribution would begin on target city pandemic day 10 and be completed by day 19; full antiviral effectiveness would occur on the first day of dosing (plasma levels peaking one hour after administration (73)), and based on a meta-analysis of extended-duration neuraminidase inhibitor prophylaxis against influenza A(32), we assumed 74% effectiveness for zanamivir, and 37% effectiveness for oseltamivir in light of developing resistance (74-76).
Based on neuraminidase inhibitor data (33), we assumed that 10% of the population receiving oseltamivir or zanamivir experienced mild to moderate adverse reactions, such as nausea, vomiting, diarrhea, bronchitis, fatigue, dermatitis, worsening diabetes, rash, seizures, hepatitis, and abdominal pain, for the duration of the 40-day treatment. Based on a meta-analysis of extended-duration neuraminidase inhibitor prophylaxis against influenza (32), we assumed 0.001% of the population experienced severe adverse reactions including systemic allergic reactions, arrhythmias, and psychosis.
Mitigation Strategies
Stockpiled strategy
The stockpiled strategy was designed to assess the impact of U.S. non-pharmaceutical intervention approaches along with the use of the antiviral drugs and vaccines currently stockpiled in the U.S. (Table 1). HHS is stockpiling 3.6 billion μg of A (H5N1) vaccine antigen and 2.6 million two-dose courses of vaccine adjuvant (44), a quantity of adjuvant sufficient for 1% of the U.S. population. Given studies showing efficacy of two-dose adjuvanted A (H5N1) vaccines with 3.8μg of antigen (36, 64, 65), and based on the U.S. adjuvant stockpile, we assumed that two-dose 3.8μg adjuvanted vaccine was administered to 1% of individuals in the target city, and that the remaining unadjuvanted antigen was used to vaccinate another 7% of the population with a two-dose 90μg (minimal effective non-adjuvanted dose (35)) vaccine.
Table 1.
Strategies and Population Coverage*
| Strategy | Vaccine Component | Antiviral Component | Non-pharmaceutical Intervention Component† |
|---|---|---|---|
| Stockpiled strategy | 90μg non-adjuvanted vaccine‡ to 7% 3.8μg adjuvanted vaccine to 1% |
5-day treatment§ to 28% 40-day prophylaxis** to 5% |
25% reduction in contacts |
| Expanded antiviral prophylaxis | 90μg non-adjuvanted vaccine to 7% 3.8μg adjuvanted vaccine to 1% |
5-day treatment to 28% 40-day prophylaxis to 40% |
25% reduction in contacts |
| Expanded adjuvanted vaccination | 3.8μg adjuvanted vaccine to 40% | 5-day treatment to 28% 40-day prophylaxis to 5% |
25% reduction in contacts |
Strategies are layered on stockpiled strategy. Components of strategies which differ from stockpiled strategy are denoted in italics.
Includes personal protective equipment, cough etiquette, hand-washing, alcohol hand gels, school and workplace closures
All two-dose sequences of vaccine consist of a primer and booster dose received three weeks apart as described in stockpiled strategy.
Oseltamivir (75mg orally) or zanamivir (10mg inhaled) twice daily as described in stockpiled strategy.
Oseltamivir (75mg orally) or zanamivir (10mg inhaled) once daily as described in stockpiled strategy.
HHS and individual states are stockpiling 2.7 billion doses of neuraminidase inhibitors for treatment and prophylaxis (44). Based on published HHS distribution plans (77), we assumed that 28% of the city's population would receive five-day treatment courses of neuraminidase inhibitors, and that 5% would receive neuraminidase inhibitor prophylaxis daily for 40 days.
Expanded Adjuvanted Vaccination Strategy
In the expanded adjuvanted vaccination strategy, we assumed that sufficient adjuvanted vaccine had been stockpiled to vaccinate 40% of the population with the adjuvanted vaccine. We examined the range of vaccination coverage required to end the pandemic (defined as an effective R0 ≤ 1) in sensitivity analysis. We assumed that non-adjuvanted vaccine was not administered. The non-pharmaceutical intervention component and use of antiviral drugs were the same as in the stockpiled strategy.
Expanded Antiviral Prophylaxis Strategy
In the expanded antiviral prophylaxis strategy, we assumed sufficient antiviral drugs had been stockpiled to provide 40% of individuals 40 days of antiviral prophylaxis. We evaluated a 50/50 stockpile of oseltamivir and zanamivir, as zanamivir has remained effective against influenza subtypes which have developed oseltamivir resistance (75), and might be less likely to develop in-vivo resistance (78). The non-pharmaceutical intervention component and use of vaccination were the same as in the stockpiled strategy.
Costs and Utilities
Our analysis included the costs of interventions, based upon wholesale pricing available to the U.S. government, and average hospitalization costs. Costs and sources are described in the Appendix. All costs were expressed in 2009 U.S. dollars using the GDP deflator. We made adjustments for quality of life (Table 2 and Appendix).
Table 2.
Variables and Sources
| Variable | Base Case (Range) | Source |
|---|---|---|
| Susceptible Population Parameters | ||
| Population | 8,300,000 | New York Vital Statistics (7) |
| Age (range, years) | 0-100 | New York Vital Statistics (7) |
| Percent female | 53 | New York Vital Statistics (7) |
| Pre-existing population immunity | 0% (0-10%) | Assumed, WHO (8) |
| Infected Population Parameters | ||
| R0 | 1.8 (1.4-2.2) | Assumed, CDC Severity Index 5 (9) |
| Non-pharmaceutical interventions reduction in contacts | 25% (10-70%) | Assumed, Cowling et al. (10) |
| Number of infected individuals at start of pandemic | 1,000 (100-10,000) | Assumed |
| Probability of symptomatic infection | 67% (50-90%) | Ferguson et al. (11), Longini et al. (12), Katz et al. (13), Dinh et al (14)., Vong et al. (15), Buxton Bridges et al. (16), Aparnthanarak et al (17)., Liem et al (18)., Wang et al.(19) |
| Reduced infectiousness by incubating | 50% (10-62.5%) | Hayden et a (20)., Wein et al. (21) |
| Reduced infectiousness by asymptomatic | 25% (10-50%) | Hayden et al (20)., Wein et al. (21) |
| Probability of isolating given symptomatic infection | 50% (37.5-62.5%) | Longini et al.(22) |
| Mean incubation time (days) | 2 (1-9) | CDC seasonal influenza data (23), WHO influenza A (H5N1) data (8) |
| Mean duration of infectiousness (days) | 4 (3-10) | Hayden et al. (20), Leekha et al. (24) |
| Mean duration of symptomatic illness (days) | 10 (7.5-12.5) | CDC (23) |
| Proportion of symptomatic patients requiring inpatient care | 10% (7.5-12.5%) | CDC (25), HHS (9) |
| Mean duration of non-ICU inpatient stay (days) | 5 (3.75-6.25) | CDC (25), HHS (9) |
| Proportion of inpatients admitted to Influenza Care Center | 90% (0-95%) | Assumed, NYC Department of Mental Health and Hygiene (26) |
| Proportion of inpatients admitted to hospital | 10% (5-100%) | Assumed, NYC Department of Mental Health and Hygiene (26) |
| Proportion of hospital patients requiring ICU care | 10% (7.5-12.5%) | CDC (25), HHS (9) |
| Mean duration of ICU stay (days) | 10 (7.5-12.5) | CDC (25), HHS (9) |
| Recovered Population Parameters | ||
| Susceptibility to re-infection following recovery | 5% (2-25%) | Smith et al. (27) Monto et al. (28) Sonoguchi et al (29). Davies et al.(30) |
| Timing of waning immunity (months) | 5 (2-8) | Smith et al. (27) Monto et al. (28) Sonoguchi et al (29). Davies et al. (30) |
| Mortality | ||
| Case-fatality proportion | 2.5% (0.5%-60%) | Seasonal flu clinical case-fatality (23), CDC Severity Index 5 Case Fatality (9), Current influenza A (H5N1) Case Fatality (8) |
| Mortality threshold for reactive social distancing | 10 per 10,000 (5-50 per 10,000) | Bootsma and Ferguson (31) |
| Reactive social distancing memory period | 30 days (1-40) | Bootsma and Ferguson (31) |
| Intervention Effectiveness | ||
| Zanamivir prophylaxis | 74% (63%-82%) | Khazeni et al. (32) |
| Oseltamivir prophylaxis | 37% (32-41%) | Khazeni et al. (32) |
| Non-adjuvanted one-dose vaccine | 20% (10-80%) | Assumed |
| Non-adjuvanted two-dose vaccine | 30% (10-80%) | Assumed |
| Adjuvanted one-dose vaccine | 40% (10-80%) | Assumed |
| Adjuvanted two-dose vaccine | 50% (10-80%) | Assumed |
| Intervention side effects | ||
| Neuraminidase Inhibitor | ||
| Mild-moderate side effects | 10% (5-20%) | Tamiflu and Relenza Package Inserts (33, 34) |
| Severe side effects | 0.001% (0-0.01%) | Assumed, Khazeni et al. (32) |
| Risk of death from severe side effects | 5% (1-10%) | Assumed |
| Vaccination | ||
| Mild-moderate side-effects | 45% (5-75%) | Treanor et al. (35), Leroux-Roels et al. (36) |
| Severe side effects for non-adjuvanted vaccine | 0.001% (0-0.01%) | Neustadt and Fineberg (37) |
| Severe side effects for adjuvanted vaccine | 0.001% (0-0.01%) | Neustadt and Fineberg (37) |
| Risk of death from severe side effects | 5% (1-10%) | Chio et al. (38) |
| Risk of long-term care from severe side effects | 5% (1-10%) | Kissel et al. (39) |
| Intervention side effects reduction in quality of life* | ||
| Neuraminidase Inhibitor | ||
| Mild-moderate side effects | 0.05 (0-0.1) | Assumed, Tamiflu and Relenza Package Inserts (33, 34) |
| Severe side effects | 0.5 (0-1) | Assumed, Tamiflu and Relenza Package Inserts (33, 34) |
| Duration of mild-moderate side effects (days) | 40 (10-100) | Assumed, Tamiflu and Relenza Package Inserts (33, 34) |
| Duration of hospitalization for severe side effects (days) | 14 (7-28) | Assumed |
| Vaccination | ||
| Mild-moderate side-effects | 0.05 (0-0.1) | Assumed, Treanor et al. (35), Leroux-Roels et al. (36), CDC(40) |
| Severe side effects | 0.5 (0-1) | Assumed, Neustadt and Fineberg (37) |
| Duration of mild-moderate side effects (days) | 2 (1-7) | Treanor et al. (35), Leroux-Roels et al. (36), CDC(40) |
| Duration of hospitalization for severe side effects (days) | 14 (7-28) | Chio et al. (38) |
| Influenza-related quality of life | ||
| Uninfected/Asymptomatic | 0.96 (0.92-1.00) | New York Census (7), Beaver Dam Health Outcomes (41) |
| Symptomatic Influenza | 0.8 (0.7-0.9) | Turner et al. (42) |
| Post-influenza disabled state for patients requiring ICU care | 0.9 (0.85-0.95) | Assumed |
| Costs | ||
| Vaccine | ||
| Antigen per μg ($) | 0.45 (0.33-0.55) | HHS (43) |
| Adjuvant ($) | 7.00 (5.25-8.75) | BARDA (personal communication – Michael Perdue) |
| μg adjuvant per vaccine | 3.8 (1.9-90) | HHS (44) |
| Stockpiling (annual, $) | 1.00 (0.01-2.00) | Assumed, Sangrujee et al. (45) |
| Administration | ||
| Antigen | 8.73 (6.54-10.91) | Calculated: 10 minutes of nurse wages (46) |
| Adjuvant | 8.73 (6.54-10.91) | Calculated: 10 additional minutes of nurse wages for mixing (46) |
| Patient Time | 10.55 (5.28-21.10) | U.S. Bureau of Labor Statistics (47) |
| Antiviral (per 40-day prophylactic course, $) | ||
| Oseltamivir | 165.78 (48.00-331.56) | Veterans Affairs (personal communication – Mark Hlodniy) |
| Zanamivir | 91.08 (48.00-182.16) | HHS (48) |
| Proportion oseltamivir vs. zanamivir | 50% (0-100%) | Assumed |
| Stockpiling (annual, $) | 0.23 (0.01-1.00) | Sangrujee et al.(45) |
| Dispensing | 10.49 (7.86-13.11) | Bravata et al. (49) |
| Rotation of stockpile (years) | ||
| Antigen | 2 (1-10) | HHS (44) |
| Adjuvant | 3 (1-10) | BARDA (personal communication – Michael Perdue) |
| Antivirals | 5 (1-10) | Tamiflu and Relenza expirations (33, 34) |
| Daily health care costs ($) | ||
| Patient with severe side effects (treated in ICU) | 3,739.05 (2,804.29 – 4,673.82) | Desta et al. (50) |
| General medical hospitalized patient | 1,830.46 (1429.37-1870.54) | Talbird et al. (51) |
| ICU hospitalized patient | 3,739.05 (2,804.29 – 4,673.82) | Desta et al. (50) |
| Long-term treatment facility costs | 313.05 (234.79-391.31) | Metlife Survey (52) |
| Influenza Care Center patient | 100.00 (75.00-1659.00) | Santa Clara County Public Health Department (personal communication – Sara Cody) |
| Normal health care expenditures | 19.56 (14.67-24.45) | Statistical Abstract of the United States(53) |
| Other variables | ||
| Discount Rate (annual %) | 3 (0-5%) | Weinstein et al.(54) |
Quality of life variables represent a person's preference for a given state of health and are scaled form 0 to 1, with 1 equivalent to perfect health.
RESULTS
Base-Case Analysis
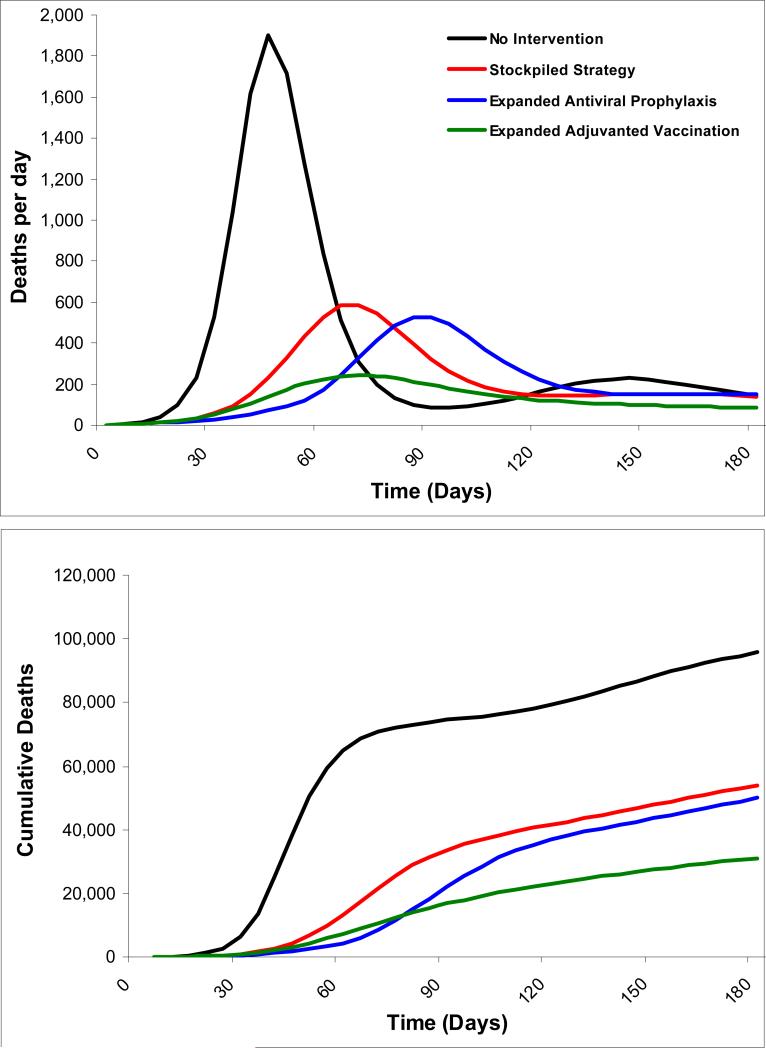
With no intervention, the first pandemic wave (defined as greater than 1% of the population infected) would last 50 days and wane as a result of voluntary social distancing. A second wave, lasting 32 days, would occur as a result of decreases in voluntary social distancing and re-infection as the virus undergoes drift changes. Of the city's 8.3 million individuals, an estimated 2.74 million would become symptomatically infected and 67,822 would die (Figure 2 and Table 3).
Figure 2. Pandemic waves and cumulative mortality for a city of 8.3 million individuals under different strategies.
Expanded adjuvanted vaccination results in the shortest duration pandemic wave with the smallest area under the curve. Expanded antiviral prophylaxis extends time to the first pandemic wave and modestly reduces mortality as compared with stockpiled strategy. Additional waves occur in all strategies as a result of decreases in voluntary social distancing as well as a low re-infection rate as the virus undergoes drift changes
Table 3.
Health and economic outcomes for a city of 8.3 million individuals
| Strategy | Clinical attack rate | Infections | Deaths | Deaths averted | Pre-pandemic costs (millions of $) | Pandemic intervention costs (millions of $) | Pandemic treatment costs (millions of $) | Normal healthcare expenditures (millions of $) | Total Costs (millions of $) | QALYs* gained | Incremental cost-effectiveness ratio ($ per QALY) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Expanded adjuvanted vaccination | 11% | 889,908 | 21,882 | 45,941 | 306 | 203 | 188 | 1,498,439 | 1,501,284 | 404,030 | 10,844† |
| Expanded antiviral prophylaxis | 17% | 1,429,934 | 35,077 | 32,745 | 455 | 66 | 301 | 1,496,074 | 1,500,339 | 282,329 | Dominated‡ |
| Stockpiled strategy | 19% | 1,547,648 | 38,061 | 29,761 | 75 | 34 | 326 | 1,495,532 | 1,499,704 | 258,342 | 8,907§ |
| No intervention | 33% | 2,739,242 | 67,822 | - | - | 582 | 1,490,164 | 1,497,403 | - | - |
QALY = quality-adjusted life-year
Incremental to stockpiled strategy
Expanded adjuvanted vaccination provides more QALYs at a lower incremental cost effectiveness ratio
Incremental to no intervention
Health Outcomes
The alternative strategies have varying effects on the course of the influenza epidemic (Figure 2 and Table 3). The stockpiled strategy results in a 19% clinical attack rate and prevents 1.19 million infections and 29,761 deaths relative to no intervention.
The expanded antiviral prophylaxis strategy is more effective than the stockpiled strategy, decreasing the clinical attack rate to 17%, and averting 1.31 million infections and preventing 32,745 deaths relative to no intervention. This strategy's main effect is to delay the pandemic during the 40 days it is implemented. Following prophylaxis, individuals who have not had sufficient contact with the virus to develop protective antibodies are once again fully susceptible, with consequent infections and deaths.
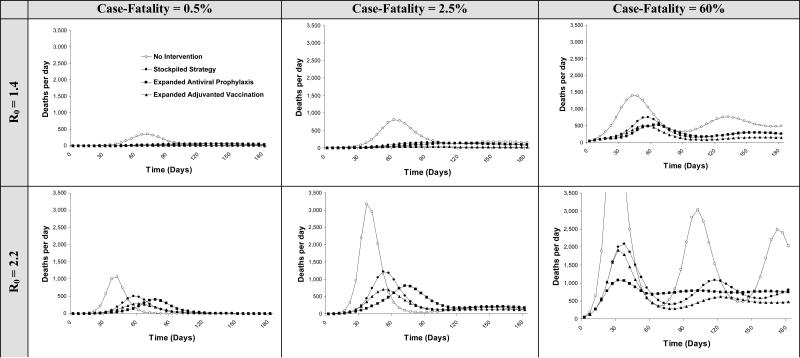
Expanded adjuvanted vaccination is the most effective strategy, with an 11% clinical attack rate. It averts 1.85 million infections and 45,941 deaths relative to no intervention, and remains the most effective strategy across a range of influenza infectivity (R0 1.4 to 2.2) and case-fatality proportions (from 0.5% to 60%) (Figure 3). Administering the first dose of the vaccination sequence prior to the pandemic is more effective than administering both doses at the onset of the pandemic, with a 6.9% decrease in infections (61,708 fewer) and deaths (1,584 fewer) relative to post-pandemic administration. If both vaccine doses could be administered before the onset of the pandemic, infections would fall by 7.6% (68,036 fewer) and 1,747 deaths would be averted relative to post-pandemic administration.
Figure 3. Health outcomes for a city of 8.3 million individuals with varying pandemic severity.
Daily deaths are shown for varying values of R0 and case-fatality proportions. As the case-fatality proportion rises, deaths increase and subsequent waves become more apparent. However, because of reactive social distancing in response to mortality, the peaks in the waves are not proportional to the increase in case fatality. As mortality increases, the population reacts by reducing social interactions, which reduces the spread of infection. Because reactive social distancing occurs in response to mortality rather than infections, the effects of reactive social distancing are more apparent with high case-fatality proportions. Waves in the pandemic occur because social distancing is in response to average mortality over the past 30 days. As reactive social distancing decreases mortality, the population begins to return to higher, more normal levels of social interaction, causing another upswing in mortality (further described in Appendix).
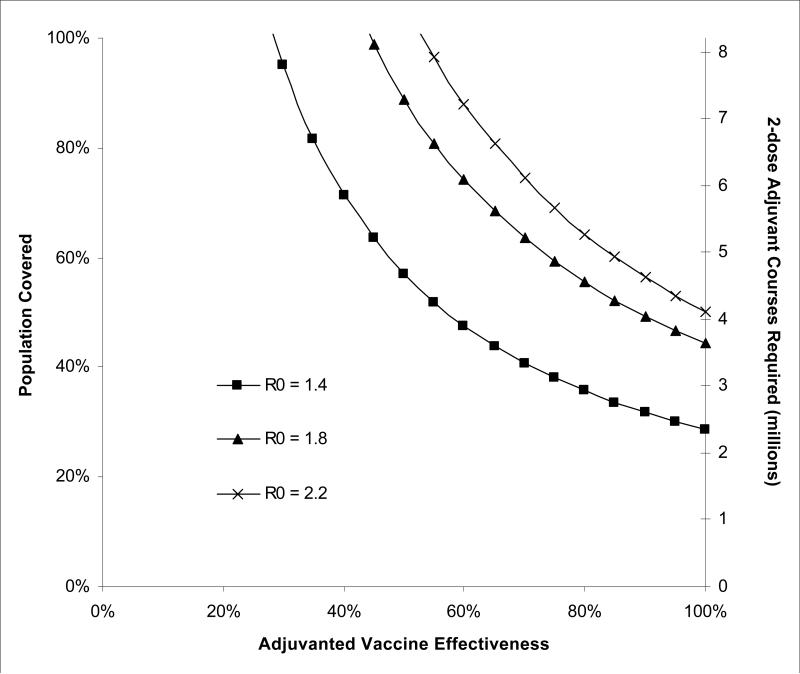
Greater vaccine effectiveness and broader population coverage are the principal reasons why adjuvanted vaccination strategy is more effective that the stockpiled strategy. We identified vaccine effectiveness and adjuvant doses required to avert the pandemic (defined as R0≤1) within the target population (Figure 4). The pandemic would be averted with a vaccine of 70% effectiveness if 70% of the population were vaccinated, which would require about 5.7 million 2-dose courses of vaccine adjuvant. For less than 70% effective vaccines, a greater proportion of the population would require vaccination. Every 10% increase in vaccine effectiveness requires approximately 10% less population coverage to avert the pandemic.
Figure 4. Combinations of vaccine effectiveness and population coverage/adjuvant doses required to avert the pandemic for a city of 8.3 million individuals.
Areas to the right of the curves represent combinations of vaccine effectiveness and population coverage/adjuvant doses under which the pandemic is averted under different R0s.
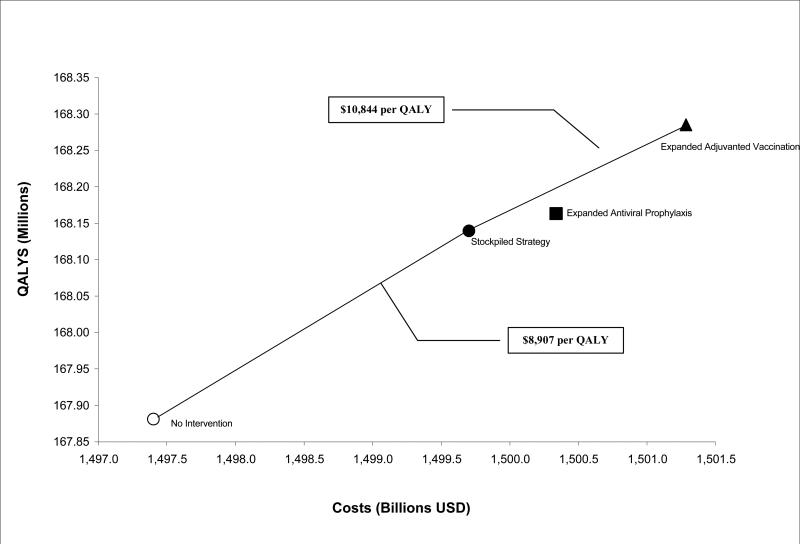
Cost-Effectiveness
The stockpiled strategy increases costs by $2.30 billion and adds 258,342 QALYs, for a cost of $8,907 per QALY gained relative to no intervention (Table 3 and Figure 5). The antiviral prophylaxis strategy is more effective than stockpiled strategy, adding 23,987 QALYs relative to stockpiled strategy, but increases costs by $635 million, and has a less favorable cost per QALY than expanded adjuvanted vaccination.
Figure 5. Cost-effectiveness of strategies for a city of 8.3 million individuals.
Expanded adjuvanted vaccination dominates expanded antiviral prophylaxis strategy through extended dominance and is cost-effective as compared with stockpiled strategy. (QALY = Quality-Adjusted Life-Year)
Expanded adjuvanted vaccination adds 145,688 QALYs at $1.58 billion, for a cost of $10,844 per QALY gained relative to stockpiled strategy. The effectiveness of this strategy is a crucial determinant of cost-effectiveness. At approximately 10% effectiveness, expanded antiviral prophylaxis becomes more cost-effective than expanded adjuvanted vaccination, relative to stockpiled strategy. At 80% effectiveness, the cost-effectiveness ratio of adjuvanted vaccination decreases to $9,704 per QALY gained relative to stockpiled strategy. Appendix Figure A3 depicts changes in cost-effectiveness with variations in efficacy of adjuvanted vaccination as compared with non-adjuvanted vaccine effectiveness.
For short-term budgetary considerations, federal costs for expanded adjuvanted vaccination for a city of 8.3 million individuals would be $231 million to purchase additional adjuvant and ongoing stockpiling costs, city costs would be $102 million to administer the vaccines, city and individual costs would be $56 million in vaccine recipient time and $10 million treating short-term severe side effects, relative to stockpiled strategy. Savings to the city and individuals would be $139 million in pandemic treatment costs relative to stockpiled strategy (Table 3).
Allocating 5-day treatment courses of neuraminidase inhibitors to 28% of the population is adequate to cover symptomatic patients seeking treatment under all scenarios; additional neuraminidase inhibitors allocated to 40-day prophylaxis do not reduce quantities of neuraminidase inhibitors required for treatments.
Although variations in R0 and case-fatality change the number of infections and deaths (Figure 3), they do not change the strategies that would be selected by cost-effectiveness criterion (Appendix Table A1).
Sensitivity to Pre-Existing Immunity
In the base case, we assumed that all individuals were susceptible to the virus. If 10% of the population had pre-existing immunity to A (H5N1), 49% fewer infections and deaths would occur under stockpiled strategy, and 76% fewer infections and deaths would occur under the expanded adjuvanted vaccination strategy, relative to no immunity as compared with no intervention. There is no change in the strategies that would be selected by cost-effectiveness criterion (Appendix Table A1).
Sensitivity to Antigen Dose
We performed additional analyses based on a study showing decreased effectiveness with a 1.9μg (as compared with 3.8 μg) antigen dose (79). At this dose, with lower vaccine efficacy (Appendix), twice as many individuals could be vaccinated. Vaccination would avert 1.2 million infections and 29,435 deaths with a symptomatic attack rate of 4% in the population at a cost of $11,080 per QALY relative to the stockpiled strategy. However, this intervention would be limited by the need for twice as many doses of adjuvant.
Sensitivity to Severe Vaccine Side Effects
In the base case, we assumed that the probability of severe side effects from an adjuvanted vaccine was the same as that from a non-adjuvanted vaccine. In this case, about 6,000 lives are saved for each death from severe adverse effects. If an adjuvanted vaccine were to increase the incidence of severe side effects 150-fold, 33 lives would be saved for each death from severe adverse effects, and the incremental cost-effectiveness ratio (ICER) of expanded adjuvanted vaccination compared to expanded non-adjuvanted vaccination would be $49,570 per QALY. If the adjuvanted vaccine caused severe side effects 650 times more frequently than a non-adjuvanted vaccine, 8 lives would be saved for each death from severe adverse events. In this case, a non-adjuvanted vaccine would provide higher QALYs and a lower ICER than the adjuvanted vaccine.
Studies of adjuvanted A (H5N1) vaccination in humans examining adverse events (36, 79-91) have reported no severe adverse events from vaccination in 6,095 participants. Based on these data, the highest incidence rate of serious adverse events where zero cases would still fall within the 95% confidence interval would be 49 per 100,000 individuals (49 times more frequently than a non-adjuvanted vaccine).
Sensitivity to Non-pharmaceutical Interventions
In the base case, we assumed a 25% reduction in contacts due to non-pharmaceutical interventions. If contacts were only reduced by 10%, 44% of infections and deaths would be averted compared to no intervention, with little change in the ICER. If contacts fell by 70%, 99% of infections and deaths would be averted with expanded adjuvanted vaccination versus no intervention. However, vaccination would then provide less additional benefit, at a costs of $101,682 per QALY (Appendix Table A1).
Sensitivity to Age-Specific Mortality
In the base case analysis, we had examined increased deaths in newborns and individuals 65 years of age and older (J-shaped mortality). We also performed sensitivity analyses to determine the effect of W-shaped mortality (increased deaths in adults 20-50 years, newborns, and individuals older than 65 years), such as that seen in the 1918 pandemic (Appendix Figure A1) (69). With W-shaped mortality, the increase in deaths among young adults means that significantly more QALYs are lost per death (22.1 vs. 8.2). Averting these deaths and QALY losses makes our strategies more cost-effective, with expanded adjuvanted vaccination costing $8,674 per QALY gained relative to stockpiled strategy.
Monte Carlo Probabilistic Sensitivity Analysis
In 89% of Monte Carlo probabilistic sensitivity analysis simulations (Appendix Figure A2), expanded adjuvanted vaccination has an estimated incremental cost less than $50,000 per QALY saved, and in 95% of simulations, an estimated incremental cost less than $100,000 per QALY saved. Expanded adjuvanted vaccination costs more than $7,000 per QALY gained in 95% of the simulations. In 5% of simulations, another strategy dominates expanded adjuvanted vaccination.
DISCUSSION
We examined the costs and benefits of antiviral and vaccination strategies for an influenza A (H5N1) pandemic in a metropolitan city and defined ranges of vaccine effectiveness and population coverage necessary to avert the pandemic. An expanded adjuvanted vaccination strategy layered onto existing pharmaceutical and non-pharmaceutical HHS pandemic mitigation strategies is the most effective strategy and is cost-effective. A strategy of increasing the number of individuals receiving extended-duration antiviral prophylaxis delays the pandemic.
Higher vaccine effectiveness and greater population coverage are the two most important factors in the adjuvanted vaccination strategy's relative effectiveness and cost-effectiveness. Our assumption about effectiveness is supported by studies suggesting that adjuvanted vaccination increases human A (H5N1) antibody responses and provides cross-protection across multiple clades and subclades (36, 65, 72). We found that vaccinating 60% of the population with an 80% effective vaccine (similar to a well-matched seasonal influenza vaccine in adults) averts the pandemic. The 50% effective vaccine we modeled would require 90% population coverage, a level that could be attained by supplementing the current national HHS vaccine antigen supply with 530 million doses of adjuvant. This relationship allows policymakers to define target population adjuvant and antigen stockpile goals as vaccine technology progresses and more effective vaccines are developed.
Pre-pandemic administration of the primer vaccine is feasible, as studies have shown effective antibody responses in individuals receiving booster vaccination as late as eight years following the primer (72, 92). However, our analysis shows that pre-pandemic primer administration would provide a modest increase in effectiveness. Pre-pandemic vaccination may also not be widely accepted in light of historical pre-pandemic vaccination efforts (93).
An expanded antiviral prophylaxis strategy will delay the pandemic while prophylaxis is implemented, but the health benefits relative to the stockpiled strategy are modest, and it is less cost-effective than the expanded adjuvanted vaccine strategy. This antiviral strategy could be considered as a bridge to development and administration of a well-matched pandemic vaccine, particularly if novel vaccine production strategies (such as cell-based and DNA-based vaccines as described in HHS goals (44)) (86, 94, 95) reduce the time required for vaccine development.
Our analysis has several limitations. Our deterministic modeling approach is a general population model of influenza transmission that assumes homogenous mixing; all individuals have the same frequency of contacts; there may be increased spread associated with large groups or frequent contacts, resulting in a more rapid initial spread of the epidemic, followed by slowing as it spreads to lower contact rates (96). We assume that a fixed fraction of individuals seek inpatient care; this number may vary as healthcare resources become more limited. Recent studies have shown that simple classical compartmental models are likely to be sufficient for these types of policy decisions (97), so these concerns are unlikely to affect our conclusions.
We did not model children or individuals older than 65 separately with regards to spread of infection. Patterns of influenza transmission among children and the elderly may not be the same as for the general population, but have been different in different pandemics: Children may have transmitted virus more efficiently than adults in the 1918 and 1957 pandemics, but had similar attack rates to adults in the 1968 pandemic (98). Additionally, adjuvanted A (H5N1) vaccines have not yet been studied extensively in children and the elderly (99), and zanamivir prophylaxis is not approved in children under five years (34). Our expanded stockpiling strategies only address the stockpiling of additional adjuvant and antivirals at this time. A decision to target interventions to particular age groups will need to be made as data regarding disproportionately affected groups becomes available after the outbreak of a pandemic. In light of the possibility of more efficient transmission by children, or increased mortality in young children and the elderly, we encourage ongoing efforts to establish the safety and efficacy of A (H5N1) vaccination in children and elderly, and the safety of zanamivir prophylaxis in children under five.
An assumption of continued neuraminidase inhibitor effectiveness may not apply to a (mutated) pandemic strain. Neuraminidase inhibitors have demonstrated effectiveness across a wide range of viral mutations including the 1918 A (H1N1) (100) and the current pandemic novel A (H1N1) (101), but some A (H5N1) strains are resistant to oseltamivir (102, 103). Resistance may be less likely to occur with zanamivir; our model's 50/50 oseltamivir and zanamivir stockpile can be adjusted to include higher proportions of zanamivir without changing effectiveness or cost-effectiveness.
We accounted for lost productivity with reduced QALYs in our analysis, but we did not include all costs to uninfected individuals in the setting of a pandemic; these may be greater than costs to sick individuals (104). We also did not include several potential net savings of adjuvanted vaccination, such as limiting displacement of hospitalized patients and disruptions to trading and payments systems from decreased investment. Some analyses suggest that such costs could exceed the direct medical costs that we included in our analysis (104). However, including these costs and savings would only make the pandemic mitigation strategies we examined more cost-effective, or even cost-saving.
Adjuvanted vaccination is a feasible, effective, and cost-effective pandemic mitigation strategy with advantages over non-adjuvanted vaccination, including the potential to protect across different A (H5N1) clades and subclades, a crucial consideration in vaccinating against a mutated pandemic influenza strain. An extended-duration antiviral prophylactic strategy can serve to delay the pandemic as vaccination strategies are implemented. Expanded stockpiles of vaccine adjuvant and neuraminidase inhibitors could be used in pandemics caused by influenza strains other than A (H5N1), as well as in seasonal influenza epidemics. Indeed, current mitigation plans for pandemic novel influenza A (H1N1) include adjuvanted vaccination (105), and the use of neuraminidase inhibitors (101). Our finding that the expanded adjuvanted vaccination strategy's advantage was due to increased effectiveness and population coverage is encouraging, as it demonstrates that ongoing HHS efforts to increase stockpiles of adjuvant can substantially reduce the morbidity and mortality of a severe influenza pandemic. The recently approved U.S. Omnibus Appropriations Bill devotes $700 million in additional funding to pandemic preparedness (106); a significant percentage of these funds should be dedicated to expanding the current HHS adjuvant stockpile.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank Anne Berry, J.D., M.B.A. and Edward Sheen, M.D., M.B.A. of Stanford University for their contributions to the early design of this project; Sara Cody, M.D. of the Santa Clara County Public Health Department for her assistance with the design of our Influenza Care Center; Mark Holodniy, M.D. of the Veterans Affairs Palo Alto Healthcare System for his assistance with cost information; and Michael Gould, M.D., M.S. of Stanford University, Timothy Uyeki, M.D., M.P.H., M.P.P. of the Centers for Disease Control and Prevention, and David Fedson, M.D. for their thoughtful reviews of an earlier version of the manuscript. None of the listed individuals received additional compensation in association with this work.
Funding/Support and Role of Sponsor
This research was supported by the National Institutes of Health (Stanford University T32 HL007948, Dr. Khazeni), the Agency for Healthcare Research and Quality (1 F32 HS018003-01A1, Dr. Khazeni), the Helena Anna Henzl Gabor Travel Award (Dr. Khazeni), the National Institute on Drug Abuse (2 R01 DA15612-016, Dr. Owens), a Stanford Graduate Fellowship (Mr. Hutton), and the Department of Veterans Affairs (Drs. Owens and Garber). The funding organizations had no role in the design and conduct of the study; collection, management, analysis, or interpretation of the data; and preparation, review, or approval of the manuscript.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research and Quality.
Footnotes
Publisher's Disclaimer: This is the prepublication, author-produced version of a manuscript accepted for publication in Annals of Internal Medicine. This version does not include post-acceptance editing and formatting. The American College of Physicians, the publisher of Annals of Internal Medicine, is not responsible for the content or presentation of the author-produced accepted version of the manuscript or any version that a third party derives from it. Readers who wish to access the definitive published version of this manuscript and any ancillary material related to this manuscript (e.g., correspondence, corrections, editorials, linked articles) should go to www.annals.org or to the print issue in which the article appears. Those who cite this manuscript should cite the published version, as it is the official version of record.
Author Contributions
Nayer Khazeni:
I conceptualized the model and wrote the manuscript, including the final version. I made substantial contributions to data collection and data analysis. I had full access to all of the data in the study and I take responsibility for the integrity of the data and the accuracy of the data analysis. This manuscript represents valid work and neither this manuscript nor one with substantially similar content under our authorship has been published or is being considered for publications elsewhere. I have no conflicts of interest.
David W Hutton:
I constructed the model and wrote sections of the appendix pertaining to the model equations. I made substantial contributions to data collection and data analysis. I have seen and approved the final version. I have no conflicts of interest.
Alan M Garber:
I participated in reviewing and conceptualizing the model, in advising on issues related to cost-effectiveness analysis, and making substantial contributions to revisions of the manuscript. I have seen and approved the final version. I have no conflicts of interest.
Douglas K Owens:
I participated in reviewing and conceptualizing the model, in advising on issues related to cost-effectiveness analysis, and making substantial contributions to revisions of the manuscript. I have seen and approved the final version. I have no conflicts of interest.
References
- 1.World Health Organization [June 22, 2009];Epidemic and Pandemic Alert and Response (EPR): Swine Influenza. http://www.who.int/csr/disease/swineflu/en/index.html.
- 2.Binder S, Levitt AM, Sacks JJ, Hughes JM. Emerging Infectious Diseases: Public Health Issues for the 21st century. Science. 1999;284(5418):1311–13. doi: 10.1126/science.284.5418.1311. [DOI] [PubMed] [Google Scholar]
- 3.Webster RG, Govorkova EA. H5N1 influenza--continuing evolution and spread. N Engl J Med. 2006;355(21):2174–7. doi: 10.1056/NEJMp068205. [DOI] [PubMed] [Google Scholar]
- 4.Seo SH, Hoffmann E, Webster RG. Lethal H5N1 influenza viruses escape host antiviral cytokine responses. Nat Med. 2002;8(9):950–4. doi: 10.1038/nm757. [DOI] [PubMed] [Google Scholar]
- 5.Taubenberger JK. The virulence of the 1918 pandemic influenza virus: unraveling the enigma. Arch Virol Suppl. 2005;(19):101–15. doi: 10.1007/3-211-29981-5_9. [DOI] [PubMed] [Google Scholar]
- 6.Kobasa D, Takada A, Shinya K, Hatta M, Halfmann P, Theriault S, et al. Enhanced virulence of influenza A viruses with the haemagglutinin of the 1918 pandemic virus. Nature. 2004;431:703–707. doi: 10.1038/nature02951. [DOI] [PubMed] [Google Scholar]
- 7.Bureau of Vital Statistics - New York City Department of Health and Mental Hygiene [17 July, 2009];SUMMARY OF VITAL STATISTICS, THE CITY OF NEW YORK. 2007 http://www.nyc.gov/html/doh/downloads/pdf/vs/2007sum.pdf.
- 8.World Health Organization [June 25, 2009];Epidemic and Pandemic Alert and Response (EPR): Avian Influenza. http://www.who.int/csr/disease/avian_influenza/en/
- 9.U.S. Department of Health and Human Services [June 17, 2009];Pandemic Influenza Plan. http://www.hhs.gov/pandemicflu/plan/
- 10.Cowling BJ, Chan K-H, Fang VJ, Cheng CKY, Fung ROP, Wai W, et al. Facemasks and Hand Hygiene to Prevent Influenza Transmission in Households: A Randomized Trial. Ann Intern Med. 2009:0000605–200910060-00142. doi: 10.7326/0003-4819-151-7-200910060-00142. [DOI] [PubMed] [Google Scholar]
- 11.Ferguson NM, Cummings DA, Fraser C, Cajka JC, Cooley PC, Burke DS. Strategies for mitigating an influenza pandemic. Nature. 2006;442(7101):448–52. doi: 10.1038/nature04795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Longini IM, Jr., Nizam A, Xu S, Ungchusak K, Hanshaoworakul W, Cummings DA, et al. Containing pandemic influenza at the source. Science. 2005;309(5737):1083–7. doi: 10.1126/science.1115717. [DOI] [PubMed] [Google Scholar]
- 13.Katz JM, Lim W, Bridges CB, Rowe T, Hu-Primmer J, Lu X, et al. Antibody response in individuals infected with avian influenza A (H5N1) viruses and detection of anti-H5 antibody among household and social contacts. J Infect Dis. 1999;180(6):1763–70. doi: 10.1086/315137. [DOI] [PubMed] [Google Scholar]
- 14.Dinh PN, Long HT, Tien NTK, Hien NT, Mai LTQ, Phong LH, et al. Risk factors for human infection with avian influenza A H5N1, Vietnam, 2004. Emerg Infect Dis. 2006;12(12):1841–1847. doi: 10.3201/eid1212.060829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vong S, Coghlan B, Mardy S, Holl D, Seng H, Ly S, et al. Low frequency of poultry-to-human H5NI virus transmission, southern Cambodia, 2005. Emerg Infect Dis. 2006;12(10):1542–7. doi: 10.3201/eid1210.060424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buxton Bridges C, Katz JM, Seto WH, Chan PK, Tsang D, Ho W, et al. Risk of influenza A (H5N1) infection among health care workers exposed to patients with influenza A (H5N1), Hong Kong. J Infect Dis. 2000;181(1):344–8. doi: 10.1086/315213. [DOI] [PubMed] [Google Scholar]
- 17.Apisarnthanarak A, Erb S, Stephenson I, Katz JM, Chittaganpitch M, Sangkitporn S, et al. Seroprevalence of anti-H5 antibody among Thai health care workers after exposure to avian influenza (H5N1) in a tertiary care center. Clin Infect Dis. 2005;40(2):e16–8. doi: 10.1086/427034. [DOI] [PubMed] [Google Scholar]
- 18.Liem NT, Lim W. Lack of H5N1 avian influenza transmission to hospital employees, Hanoi, 2004. Emerg Infect Dis. 2005;11(2):210–5. doi: 10.3201/eid1102.041075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang M, Di B, Zhou DH, Zheng BJ, Jing H, Lin YP, et al. Food markets with live birds as source of avian influenza. Emerg Infect Dis. 2006;12(11):1773–5. doi: 10.3201/eid1211.060675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hayden FG, Fritz R, Lobo MC, Alvord W, Strober W, Straus SE. Local and systemic cytokine responses during experimental human influenza A virus infection. Relation to symptom formation and host defense. J Clin Invest. 1998;101(3):643–649. doi: 10.1172/JCI1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Atkinson MP, Wein LM. Quantifying the routes of transmission for pandemic influenza. Bull Math Biol. 2008;70(3):820–67. doi: 10.1007/s11538-007-9281-2. [DOI] [PubMed] [Google Scholar]
- 22.Longini IM, Jr., Halloran ME, Nizam A, Yang Y. Containing pandemic influenza with antiviral agents. Am J Epidemiol. 2004;159(7):623–33. doi: 10.1093/aje/kwh092. [DOI] [PubMed] [Google Scholar]
- 23.Centers for Disease Control and Prevention [June 17, 2009];Influenza: The Disease. 2007 http://www.cdc.gov/flu/about/disease.htm.
- 24.Leekha S, Zitterkopf N, Espy M, Smith T, Thompson R, Sampathkumar P. Duration of Influenza A Virus Shedding in Hospitalized Patients and Implications for Infection Control. Infection Control and Hospital Epidemiology. 2007;28(9):1071–1076. doi: 10.1086/520101. [DOI] [PubMed] [Google Scholar]
- 25.Coordinating Center for Infectious Diseases . FluSurge, Version 2.0. Atlanta, Georgia: 2006. [Google Scholar]
- 26.New York City Department of Health and Mental Hygeine [May 6, 2009];2009 http://www.nyc.gov/html/doh/html/home/home.shtml.
- 27.Smith CB, Cox NJ, Subbarao K, Taber LH, Glezen WP. Molecular epidemiology of influenza A (H3N2) virus reinfections. J Infect Dis. 2002;185(7):980–5. doi: 10.1086/339416. [DOI] [PubMed] [Google Scholar]
- 28.Monto AS, Davenport FM, Napier JA, Francis T. Modification of an outbreak of influenza in Tecumseh, Michigan by vaccination of schoolchildren. The Journal of Infectious Diseases. 1970;122(1):16–25. doi: 10.1093/infdis/122.1-2.16. [DOI] [PubMed] [Google Scholar]
- 29.Sonoguchi T, Sakoh M, Kunita N, Satsuta K, Noriki H, Fukumi H. Reinfection with influenza A (H2N2, H3N2, and H1N1) viruses in soldiers and students in Japan. The Journal of Infectious Diseases. 1986;153(1):33–40. doi: 10.1093/infdis/153.1.33. [DOI] [PubMed] [Google Scholar]
- 30.Davies JR, Grilli EA, Smith AJ. Influenza A: infection and reinfection. The Journal of hygiene. 1984;92(1):125–7. doi: 10.1017/s002217240006410x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bootsma MCJ, Ferguson NM. From the Cover: The effect of public health measures on the 1918 influenza pandemic in U.S. cities. Proceedings of the National Academy of Sciences. 2007;104(18):7588–7593. doi: 10.1073/pnas.0611071104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khazeni N, Bravata DM, Holty J-EC, Uyeki TM, Stave CD, Gould MK. Safety and Efficacy of Extended-Duration Antiviral Chemoprophylaxis Against Pandemic and Seasonal Influenza. Ann Intern Med. 2009:0000605–200910060-00143. doi: 10.7326/0003-4819-151-7-200910060-00143. [DOI] [PubMed] [Google Scholar]
- 33.Roche Pharmaceuticals [17 June, 2009];Tamiflu Package Insert. 2008 Aug; http://www.rocheusa.com/products/Tamiflu/PI.pdf.
- 34.GlaxoSmithKline Pharmaceuticals [17 June, 2009];Relenza Package Insert. 2008 Feb; http://us.gsk.com/products/assets/us_relenza.pdf.
- 35.Treanor JJ, Campbell JD, Zangwill KM, Rowe T, Wolff M. Safety and immunogenicity of an inactivated subvirion influenza A (H5N1) vaccine. N Engl J Med. 2006;354(13):1343–51. doi: 10.1056/NEJMoa055778. [DOI] [PubMed] [Google Scholar]
- 36.Leroux-Roels I, Borkowski A, Vanwolleghem T, Drame M, Clement F, Hons E, et al. Antigen sparing and cross-reactive immunity with an adjuvanted rH5N1 prototype pandemic influenza vaccine: a randomised controlled trial. The Lancet. 370(9587):580–589. doi: 10.1016/S0140-6736(07)61297-5. [DOI] [PubMed] [Google Scholar]
- 37.Neustadt RE, Fineberg HV. The Swine Flu Affair: Decision Making on a Slippery Disease. 1978 www.nap.edu/catalog/12660.html National Academies Press Online. [PubMed]
- 38.Chio A, Cocito D, Leone M, Giordana MT, Mora G, Mutani R, et al. Guillain-Barre syndrome: A prospective, population-based incidence and outcome survey. Neurology. 2003;60(7):1146–1150. doi: 10.1212/01.wnl.0000055091.96905.d0. [DOI] [PubMed] [Google Scholar]
- 39.Kissel JT, Cornblath DR, JR M. Diagnosis and management of peripheral nerve disorders. Oxford University Press; New York, NY: 2001. Guillain-Barre Syndrome. [Google Scholar]
- 40.Centers for Disease Control and Prevention [June 25, 2009];Key Facts About Flu Vaccination. 2006 http://www.cdc.gov/flu/protect/keyfacts.htm.
- 41.Fryback DG, Dasbach EJ, Klein R, Klein BEK, Dorn N, Peterson K, et al. The Beaver Dam Health Outcomes study: Initial Catalog of Health-state Quality Factors. Medical Decision Making. 1993;13(2):89–102. doi: 10.1177/0272989X9301300202. [DOI] [PubMed] [Google Scholar]
- 42.Turner DA, Wailoo AJ, Cooper NJ, Sutton AJ, Abrams KR, Nicholson KG. The cost-effectiveness of influenza vaccination of healthy adults 50-64 years of age. Vaccine. 2006;24(7):1035–43. doi: 10.1016/j.vaccine.2004.12.033. [DOI] [PubMed] [Google Scholar]
- 43.U.S. Department of Health and Human Services [26 July, 2009];Press Release. HHS Buys Additional Vaccine for Potential Use in an Influenza Pandemic. 2006 Nov 20; www.hhs.gov/news/press/2006pres/20061120.html.
- 44.U.S. Department of Health and Human Services [29 July, 2009];Pandemic Planning Update VI. 2009 www.pandemicflu.gov/plan/pdf/panflureport6.pdf.
- 45.Sangrujee N, Cáceres VM, Cochi SL. Cost analysis of post-polio certification immunization policies. Bulletin of the World Health Organization. 2004;82:9–15. [PMC free article] [PubMed] [Google Scholar]
- 46.County of Santa Clara Santa Clara County Employee Earnings. San Jose Mercury News. 2007 Jun 17; http://www.bayareanewsgroup.com/multimedia/mn/news/scc_salaries_090507.pdf.
- 47.U.S. Department of Labor - Bureau of Labor Statistics [June 26, 2009]; www.bls.gov.
- 48.CIDRAP [21 July, 2009];HHS adds Relenza to state stockpile. 2006 Jul 21; http://www.cidrap.umn.edu/cidrap/content/influenza/panflu/news/jul2106relenza.html.
- 49.Bravata D, Zaric G, Holty J, Brandeau M, Wilhelm E, McDonald K, et al. Reducing Mortality from Anthrax Bioterrorism: Strategies for Stockpiling and Dispensing Medical and Pharmaceutical Supplies. Biosecur Bioterror. 2006;4(3):244–62. doi: 10.1089/bsp.2006.4.244. [DOI] [PubMed] [Google Scholar]
- 50.Desta JF, Mclaughlin TP, Mody SH, Piech CT. Daily cost of an intensive care unit day: The contribution of mechanical ventilation. Crit Care Med. 2005;33(6):1266–71. doi: 10.1097/01.ccm.0000164543.14619.00. [DOI] [PubMed] [Google Scholar]
- 51.Talbird SE, Brogan AJ, Winiarski AP, Sander B. Cost-effectiveness of treating influenzalike illness with oseltamivir in the United States. Am J Health Syst Pharm. 2009;66(5):469–480. doi: 10.2146/ajhp080296. [DOI] [PubMed] [Google Scholar]
- 52.MetLife Survey of Nursing Homes and Home Care Costs and MetLife Market Survey of Assisted Living Costs. [17 June, 2009];What Does Long Term Care Cost in Your State. 2006 2006 Sep;Oct; http://www.aarp.org/families/caregiving/state_ltc_costs.html.
- 53.United States Census Bureau. Statistical Abstract of the United States [17 June, 2009];2009 http://www.census.gov/compendia/statab/2009edition.html.
- 54.Weinstein MC, Siegel JE, Gold MR, Kamlet MS, Russell LB. Recommendations of the Panel on Cost-effectiveness in Health and Medicine. JAMA. 1996;276(15):1253–1258. [PubMed] [Google Scholar]
- 55.Ungchusak K, Auewarakul P, Dowell SF, Kitphati R, Auwanit W, Puthavathana P, et al. Probable person-to-person transmission of avian influenza A (H5N1). N Engl J Med. 2005;352(4):333–40. doi: 10.1056/NEJMoa044021. [DOI] [PubMed] [Google Scholar]
- 56.Kandun IN, Wibisono H, Sedyaningsih ER, Yusharmen, Hadisoedarsuno W, Purba W, et al. Three Indonesian clusters of H5N1 virus infection in 2005. N Engl J Med. 2006;355(21):2186–94. doi: 10.1056/NEJMoa060930. [DOI] [PubMed] [Google Scholar]
- 57.Mangili A, Gendreau MA. Transmission of infectious diseases during commercial air travel. The Lancet. 2005;365(9463):989–996. doi: 10.1016/S0140-6736(05)71089-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brahmbhatt M. Economic Impacts of Avian Influenza Propagation. [17 June, 2009];Speech at the First International Conference on Avian Influenza in Humans. 2006 http://web.worldbank.org/WBSITE/EXTERNAL/NEWS/0,,contentMDK:20978927~menuPK:34472~pagePK:34370~piPK:34424~theSitePK:4607,00.html.
- 59.U.S. Department of Health and Human Services [June 17, 2009];Community Strategy for Pandemic Influenza Mitigation. http://pandemicflu.gov/plan/community/commitigation.html#XVI.
- 60.Ferguson NM, Cummings DA, Cauchemez S, Fraser C, Riley S, Meeyai A, et al. Strategies for containing an emerging influenza pandemic in Southeast Asia. Nature. 2005;437(7056):209–14. doi: 10.1038/nature04017. [DOI] [PubMed] [Google Scholar]
- 61.Germann TC, Kadau K, Longini IM, Jr., Macken CA. Mitigation strategies for pandemic influenza in the United States. Proc Natl Acad Sci U S A. 2006;103(15):5935–40. doi: 10.1073/pnas.0601266103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cheng M. [17 June, 2009];UK's attempts to stop swine flu called flawed. 2009 May 21; http://news.yahoo.com/s/ap/20090521/ap_on_re_eu/med_britain_swine_flu.
- 63.Nichol KL, Treanor JJ. Vaccines for seasonal and pandemic influenza. J Infect Dis. 2006;194(Suppl 2):S111–8. doi: 10.1086/507544. [DOI] [PubMed] [Google Scholar]
- 64.Baras Bt Stittelaar KJ, Simon JH Thoolen RJMM, Mossman SP Pistoor FHM, et al. Cross-Protection against Lethal H5N1 Challenge in Ferrets with an Adjuvanted Pandemic Influenza Vaccine. PLoS ONE. 2008;3(1):e1401. doi: 10.1371/journal.pone.0001401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Leroux-Roels I, Bernhard R, Gerard P, Drame M, Hanon E, Leroux-Roels G. Broad Clade 2 Cross-Reactive Immunity Induced by an Adjuvanted Clade 1 rH5N1 Pandemic Influenza Vaccine. PLoS ONE. 2008;3(2):e1665. doi: 10.1371/journal.pone.0001665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Excel, Version 2003. Redmond; Washington: [Google Scholar]
- 67.Taubenberger JK, Morens DM. 1918 Influenza: the mother of all pandemics. Emerg Infect Dis. 2006;12(1):15–22. doi: 10.3201/eid1201.050979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kung HC, Hoyert DL, Xu J, Murphy SL. Deaths: Final Data for 2005. National Vital Statistics Reports. 2008;56(10) [PubMed] [Google Scholar]
- 69.U.S. Department of Health and Human Services [June 26, 2009];Pandemics and Pandemic Scares in the 20th Century. http://www.hhs.gov/nvpo/pandemics/flu3.htm.
- 70.Davey VJ, Glass RJ, Min HJ, Beyeler WE, Glass LM. Effective, Robust Design of Community Mitigation for Pandemic Influenza: A Systematic Examination of Proposed US Guidance. PLoS ONE. 2008;3(7):e2606. doi: 10.1371/journal.pone.0002606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Thorpe LE, Mostashari F, Karpati AM, Schwartz SP, Manning SE, Marx MA, et al. Mass smallpox vaccination and cardiac deaths, New York City, 1947. Emerg Infect Dis. 2004;10(5):917–920. doi: 10.3201/eid1005.040119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Goji N, Nolan C, Hill H, Wolff M, Noah D, Williams T, et al. Immune Responses of Healthy Subjects to a Single Dose of Intramuscular Inactivated Influenza A/Vietnam/1203/2004 (H5N1) Vaccine after Priming with an Antigenic Variant. The Journal of Infectious Diseases. 2008;198(5):635–641. doi: 10.1086/590916. [DOI] [PubMed] [Google Scholar]
- 73.Massarella JW, He GZ, Dorr A, Nieforth K, Ward P, Brown A. The pharmacokinetics and tolerability of the oral neuraminidase inhibitor oseltamivir (Ro 64-0796/GS4104) in healthy adult and elderly volunteers. J Clin Pharmacol. 2000;40(8):836–43. doi: 10.1177/00912700022009567. [DOI] [PubMed] [Google Scholar]
- 74.World Health Organization [June 17, 2009];Influenza A (H1N1) Virus Resistance to Oseltamivir. 2009 http://www.who.int/csr/disease/influenza/oseltamivir_summary/en/index.html.
- 75.Hayden F. Developing New Antiviral Agents for Influenza Treatment: What Does the Future Hold? Clinical Infectious Diseases. 2009;48(s1):S3–S13. doi: 10.1086/591851. [DOI] [PubMed] [Google Scholar]
- 76.Moscona A. Global Transmission of Oseltamivir-Resistant Influenza. N Engl J Med. 2009;360(10):953–956. doi: 10.1056/NEJMp0900648. [DOI] [PubMed] [Google Scholar]
- 77.U.S. Department of Health and Human Services [June 17, 2009];Pandemic Flu Plan. Appendix D. http://www.hhs.gov/pandemicflu/plan/appendixd.html.
- 78.Zurcher T, Yates PJ, Daly J, Sahasrabudhe A, Walters M, Dash L, et al. Mutations conferring zanamivir resistance in human influenza virus N2 neuraminidases compromise virus fitness and are not stably maintained in vitro. J Antimicrob Chemother. 2006;58(4):723–32. doi: 10.1093/jac/dkl321. [DOI] [PubMed] [Google Scholar]
- 79.Levie K, Leroux-Roels I, Hoppenbrouwers K, Kervyn AD, Vandermeulen C, Forgus S, et al. An Adjuvanted, Low-Dose, Pandemic Influenza A (H5N1) Vaccine Candidate Is Safe, Immunogenic, and Induces Cross-Reactive Immune Responses in Healthy Adults. The Journal of Infectious Diseases. 2008;198(5):642–649. doi: 10.1086/590913. [DOI] [PubMed] [Google Scholar]
- 80.Galli G, Hancock K, Hoschler K, DeVos J, Praus M, Bardelli M, et al. Fast rise of broadly cross-reactive antibodies after boosting long-lived human memory B cells primed by an MF59 adjuvanted prepandemic vaccine. Proceedings of the National Academy of Sciences. 2009;106(19):7962–7967. doi: 10.1073/pnas.0903181106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Banzhoff A, Gasparini R, Laghi-Pasini F, Staniscia T, Durando P, Montomoli E, et al. MF59-Adjuvanted H5N1 Vaccine Induces Immunologic Memory and Heterotypic Antibody Responses in Non-Elderly and Elderly Adults. PLoS ONE. 2009;4(2):e4384. doi: 10.1371/journal.pone.0004384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chotpitayasunondh T, Thisyakorn U, Pancharoen C, Pepin S, Nougarede N. Safety, Humoral and Cell Mediated Immune Responses to Two Formulations of an Inactivated, Split-Virion Influenza A/H5N1 Vaccine in Children. PLoS ONE. 2008;3(12):e4028. doi: 10.1371/journal.pone.0004028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lin J, Zhang J, Dong X, Fang H, Chen J, Su N, et al. Safety and immunogenicity of an inactivated adjuvanted whole-virion influenza A (H5N1) vaccine: a phase I randomised controlled trial. The Lancet. 2006;368(9540):991–997. doi: 10.1016/S0140-6736(06)69294-5. [DOI] [PubMed] [Google Scholar]
- 84.Keitel Wendy A, Campbell James D, Treanor John J, Walter Emmanuel B, Patel Shital M, He F, et al. Safety and Immunogenicity of an Inactivated Influenza A/H5N1 Vaccine Given with or without Aluminum Hydroxide to Healthy Adults: Results of a Phase III Randomized Clinical Trial. The Journal of Infectious Diseases. 2008;198(9):1309–1316. doi: 10.1086/592172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nolan T, Richmond PC, Formica NT, Höschler K, Skeljo MV, Stoney T, et al. Safety and immunogenicity of a prototype adjuvanted inactivated split-virus influenza A (H5N1) vaccine in infants and children. Vaccine. 2008;26(50):6383–6391. doi: 10.1016/j.vaccine.2008.08.046. [DOI] [PubMed] [Google Scholar]
- 86.Ehrlich HJ, Muller M, Oh HML, Tambyah PA, Joukhadar C, Montomoli E, et al. A Clinical Trial of a Whole-Virus H5N1 Vaccine Derived from Cell Culture. N Engl J Med. 2008;358(24):2573–2584. doi: 10.1056/NEJMoa073121. [DOI] [PubMed] [Google Scholar]
- 87.Rümke HC, Bayas J-M, de Juanes J-R, Caso C, Richardus JH, Campins M, et al. Safety and reactogenicity profile of an adjuvanted H5N1 pandemic candidate vaccine in adults within a phase III safety trial. Vaccine. 2008;26(19):2378–2388. doi: 10.1016/j.vaccine.2008.02.068. [DOI] [PubMed] [Google Scholar]
- 88.Bernstein David I, Edwards Kathryn M, Dekker Cornelia L, Belshe R, Talbot Helen K B, Graham Irene L, et al. Effects of Adjuvants on the Safety and Immunogenicity of an Avian Influenza H5N1 Vaccine in Adults. The Journal of Infectious Diseases. 2008;197(5):667–675. doi: 10.1086/527489. [DOI] [PubMed] [Google Scholar]
- 89.Zverev VV, KatlinskiÄ- AV, Kostinov MP, Zhirova SN, Erofeeva MK, Stukova MA, et al. [Comparative clinical trial of vaccines against avian influenza]. Zhurnal mikrobiologii, Ä—pidemiologii i immunobiologii. 2007;(3):10–6. [PubMed] [Google Scholar]
- 90.Bresson JL, Perronne C, Launay O, Gerdil C, Saville M, Wood J, et al. Safety and immunogenicity of an inactivated split-virion influenza A/Vietnam/1194/2004 (H5N1) vaccine: phase I randomised trial. Lancet. 2006;367(9523):1657–64. doi: 10.1016/S0140-6736(06)68656-X. [DOI] [PubMed] [Google Scholar]
- 91.Stephenson I, Nicholson KG, Colegate A, Podda A, Wood J, Ypma E, et al. Boosting immunity to influenza H5N1 with MF59-adjuvanted H5N3 A/Duck/Singapore/97 vaccine in a primed human population. Vaccine. 2003;21(15):1687–1693. doi: 10.1016/s0264-410x(02)00632-1. [DOI] [PubMed] [Google Scholar]
- 92.Stephenson I, Nicholson KG, Hoschler K, Zambon MC, Hancock K, DeVos J, et al. Antigenically Distinct MF59-Adjuvanted Vaccine to Boost Immunity to H5N1. N Engl J Med. 2008;359(15):1631–1633. doi: 10.1056/NEJMc0805274. [DOI] [PubMed] [Google Scholar]
- 93.Sencer DJ, Millar JD. Reflections on the 1976 swine flu vaccination program. Emerg Infect Dis. 2006;12(1):29–33. doi: 10.3201/eid1201.051007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wright PF. Vaccine Preparedness -- Are We Ready for the Next Influenza Pandemic? N Engl J Med. 2008;358(24):2540–2543. doi: 10.1056/NEJMp0803650. [DOI] [PubMed] [Google Scholar]
- 95.Matassov D CA, Galarza J. A Novel Intranasal Virus-Like Particle (VLP) Vaccine Designed to Protect against the Pandemic 1918 Influenza A Virus (H1N1). Viral Immunol. 2007;20(3):441–452. doi: 10.1089/vim.2007.0027. [DOI] [PubMed] [Google Scholar]
- 96.Larson RC. Simple Models of Influenza Progression Within a Heterogeneous Population. Operations Research. 2007;55(3):399–412. [Google Scholar]
- 97.Arino J, Brauer F, van den Driessche P, Watmough J, Wu J. Simple models for containment of a pandemic. J R Soc Interface. 2006;3(8):453–7. doi: 10.1098/rsif.2006.0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Glass R, Glass L, Beyeler W, Min H. Targeted social distancing design for pandemic influenza. Emerg Infect Dis. 2006;12(11):1671–81. doi: 10.3201/eid1211.060255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Evans D, Cauchemez S, Hayden FG. “Prepandemic” Immunization for Novel Influenza Viruses, “Swine Flu” Vaccine, Guillain Barre Syndrome, and the Detection of Rare Adverse Events. Journal of Infectious Disease. 2009;(200):321–28. doi: 10.1086/603560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tumpey TM, Garcia-Sastre A, Mikulasova A, Taubenberger JK, Swayne DE, Palese P, et al. Existing antivirals are effective against influenza viruses with genes from the 1918 pandemic virus. Proc Natl Acad Sci U S A. 2002;99(21):13849–54. doi: 10.1073/pnas.212519699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Centers for Disease Control and Prevention Centers for Disease Control and Prevention. Update: Drug Susceptibility of Swine-Origin Influenza A (H1N1) Viruses, April 2009. MMWR Morb Mortal Wkly Rep. 2009;58(16):433–5. [PubMed] [Google Scholar]
- 102.Chutinimitkul S, Suwannakarn K, Chieochansin T, Mai le Q, Damrongwatanapokin S, Chaisingh A, et al. H5N1 Oseltamivir-resistance detection by real-time PCR using two high sensitivity labeled TaqMan probes. J Virol Methods. 2007;139(1):44–9. doi: 10.1016/j.jviromet.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.de Jong MD, Tran TT, Truong HK, Vo MH, Smith GJ, Nguyen VC, et al. Oseltamivir resistance during treatment of influenza A (H5N1) infection. N Engl J Med. 2005;353(25):2667–72. doi: 10.1056/NEJMoa054512. [DOI] [PubMed] [Google Scholar]
- 104.McKibbin WJ, Sidorenko A. Global Macroeconomic Consequences of Pandemic Influenza. 2006 Feb 1; http://www.brookings.edu/papers/2006/02development_mckibbin.aspx.
- 105.World Health Organization [June 17, 2009];Vaccine for the new influenza A (H1N1) 2009 http://www.who.int/csr/disease/swineflu/frequently_asked_questions/vaccine_prepared ness/en/index.html.
- 106.U.S. Congress Omnibus Appropriations Bill. 2009:111–8. http://www.govtrack.us/congress/bill.xpd?bill=h111-1105.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.