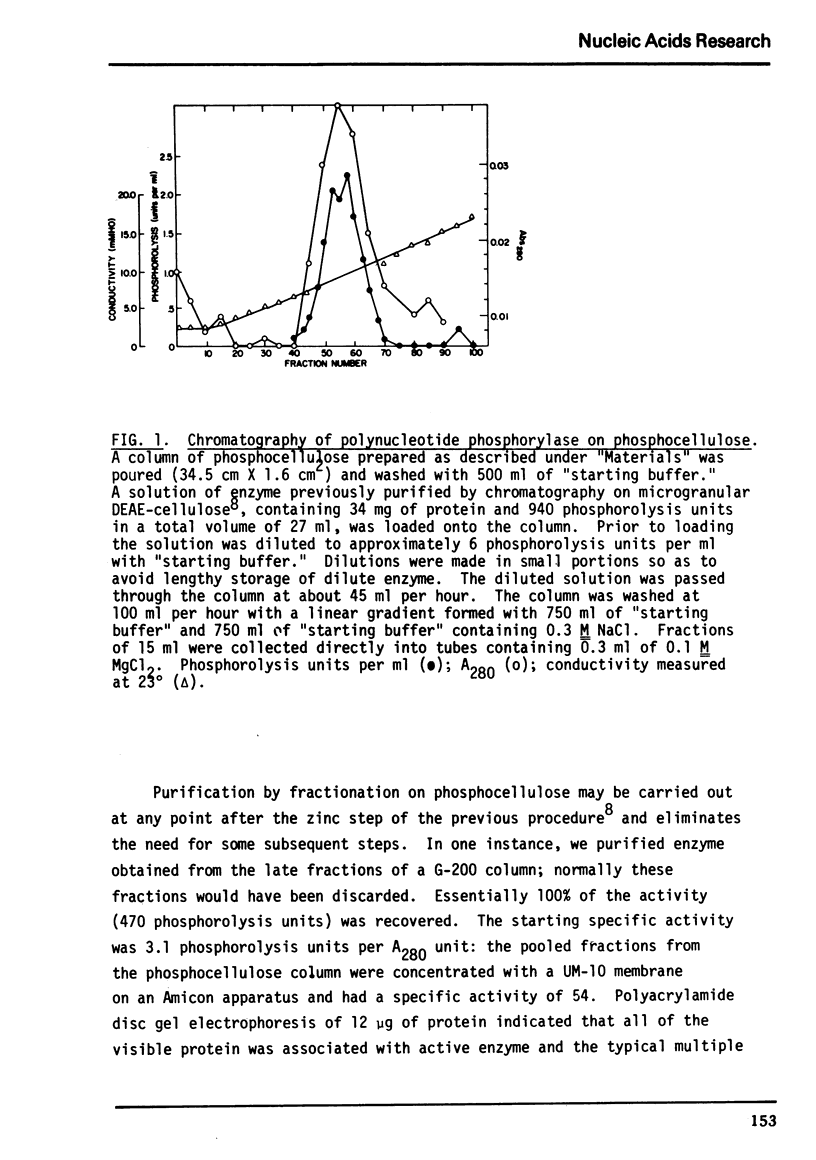
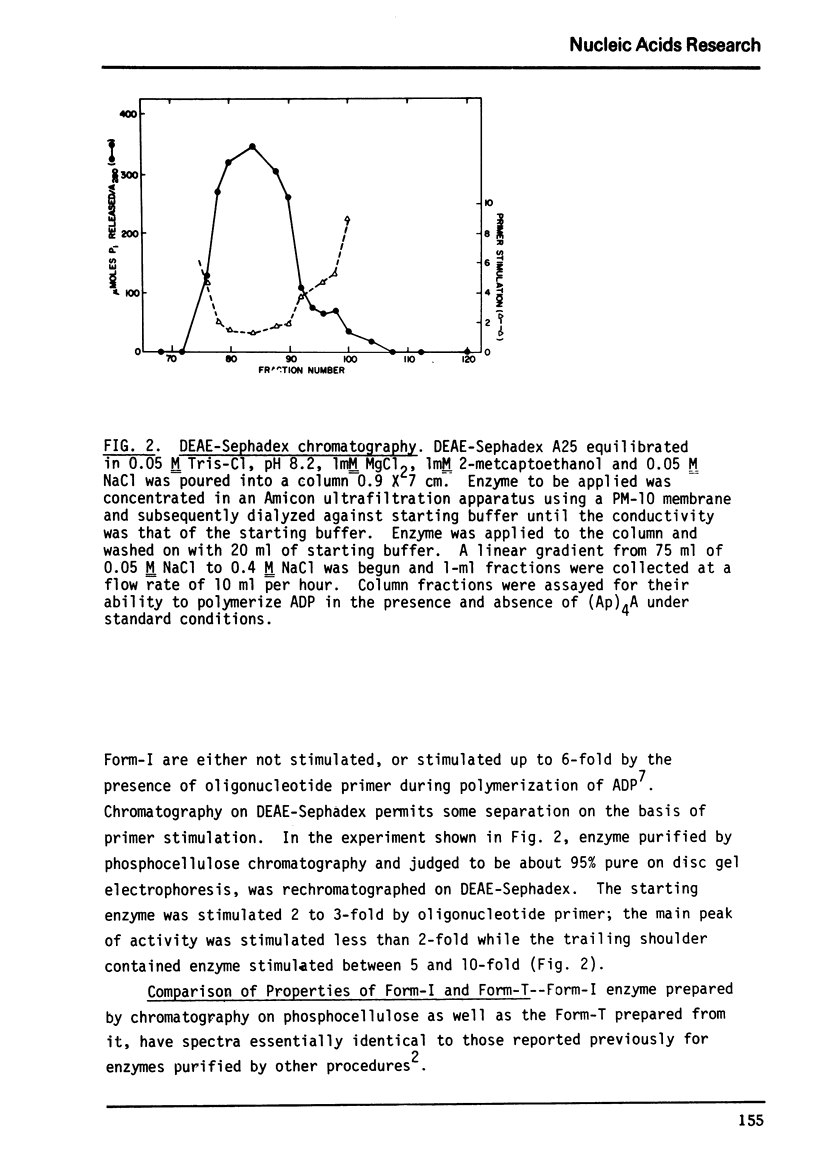
Abstract
The purification of polynucleotide phosphorylase from Micrococcus luteus by chromatography on phosphocellulose columns is described. This procedure offers several advantages over previous procedures.
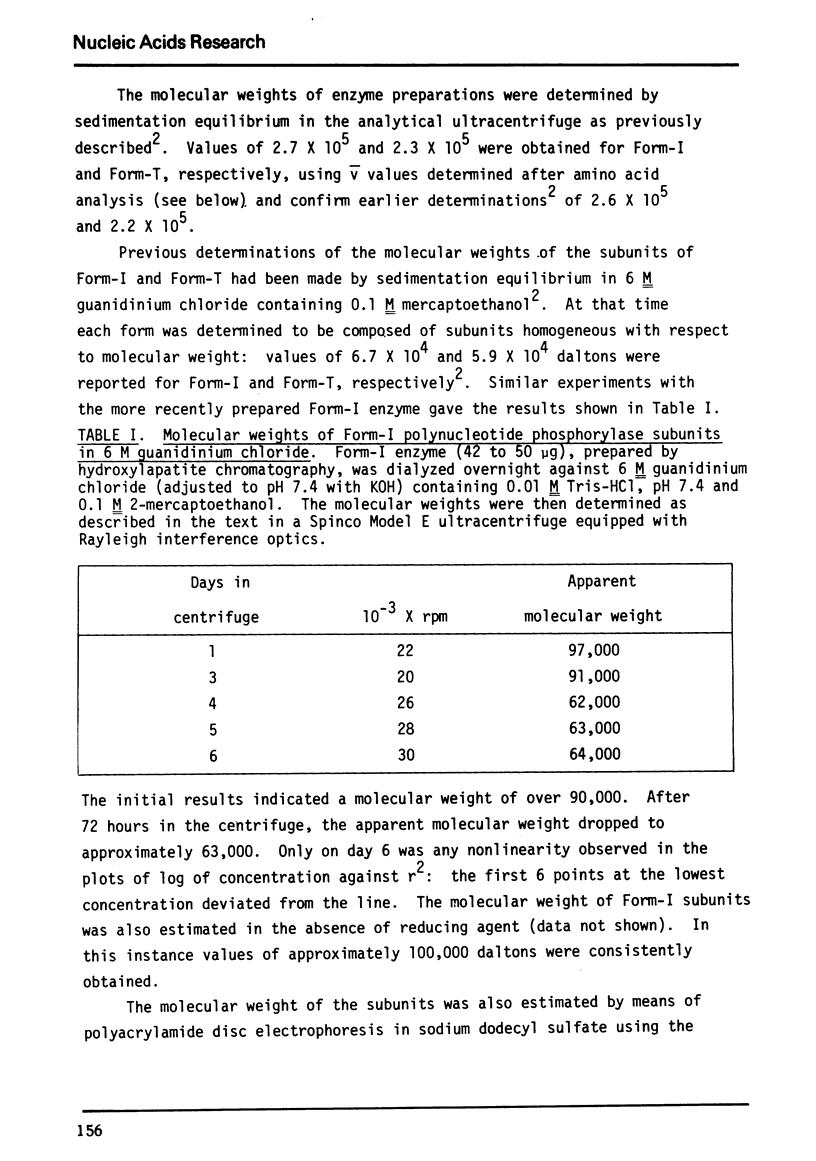
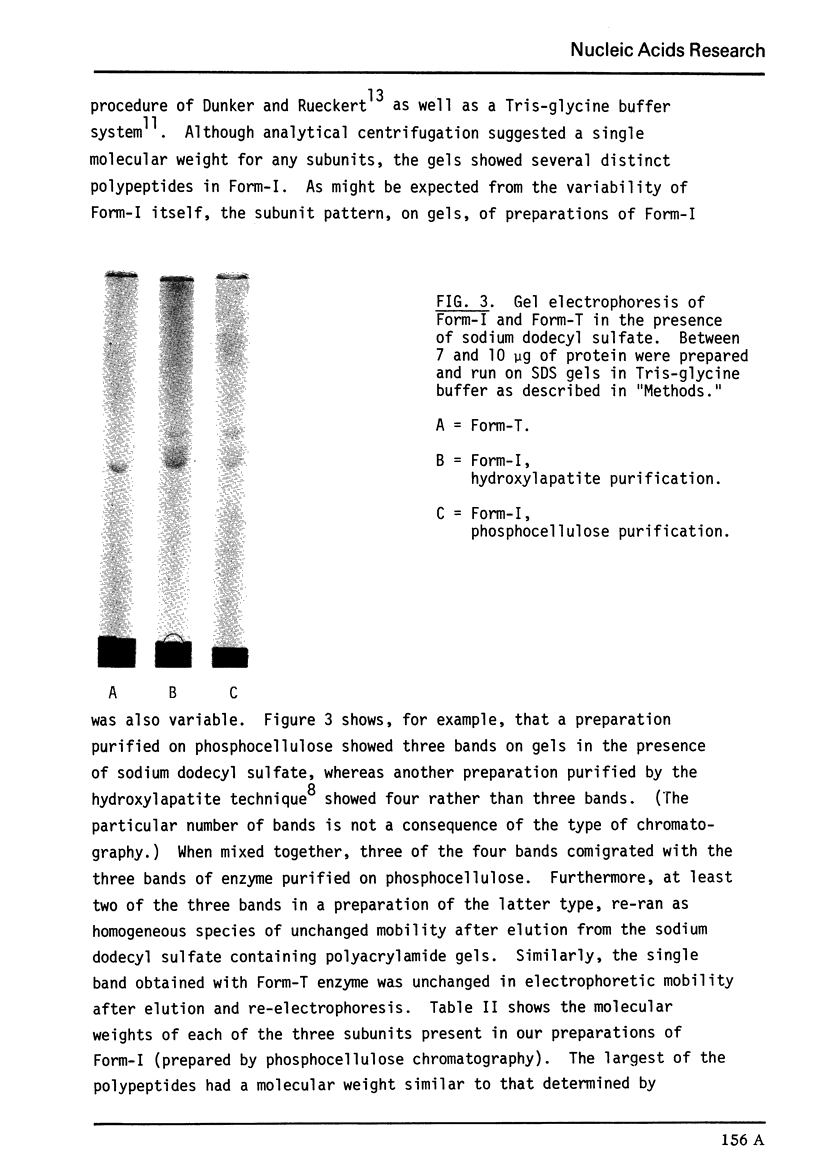
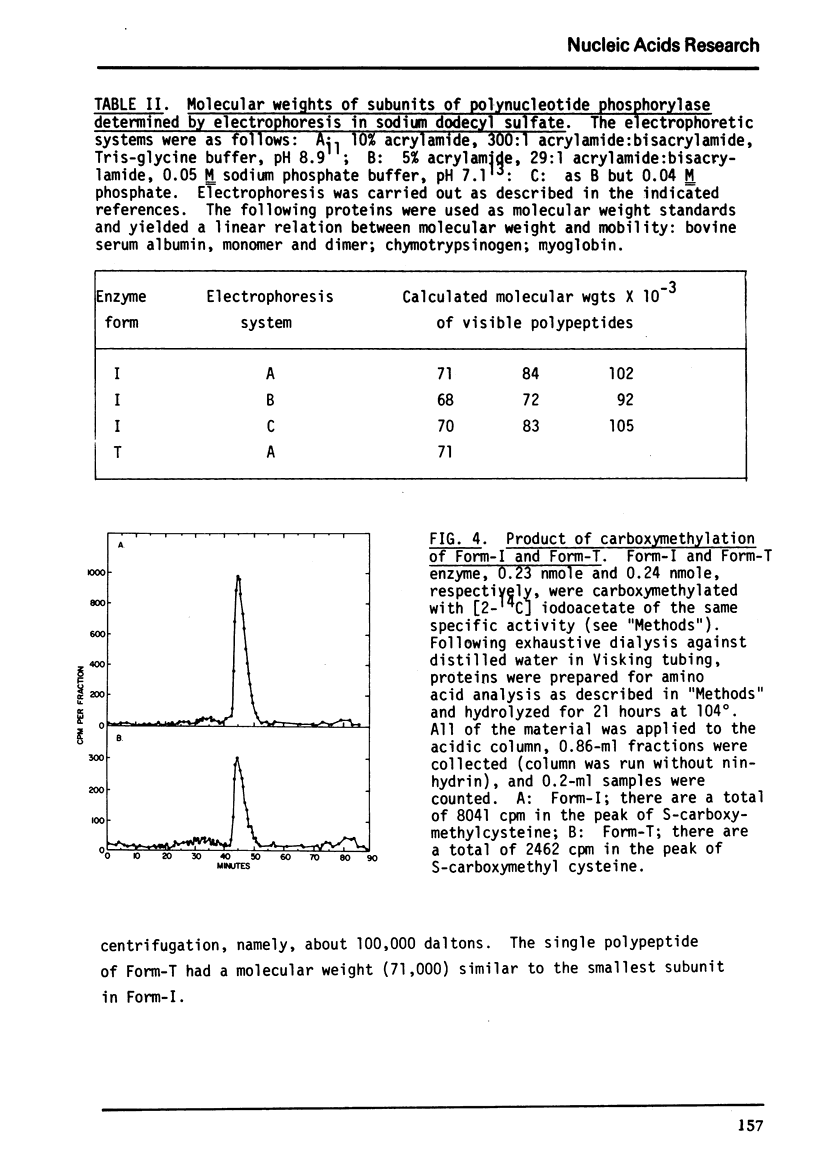
Previously determined molecular weights for Form-I enzyme and Form-T enzyme derived from Form-I by limited tryptic hydrolysis were confirmed as 2.7 and 2.3 × 105, respectively. Form-I appears homogeneous in the ultracentrifuge, but multiple active protein species are separable by polyacrylamide gel electrophoresis. The multiple species are probably the result of proteolysis. On polyacrylamide gel electrophoresis under denaturing conditions, Form-T yielded a single size of subunit of 71,000 daltons, and Form-I yielded several bands of different molecular sizes. These results differ from earlier determinations.
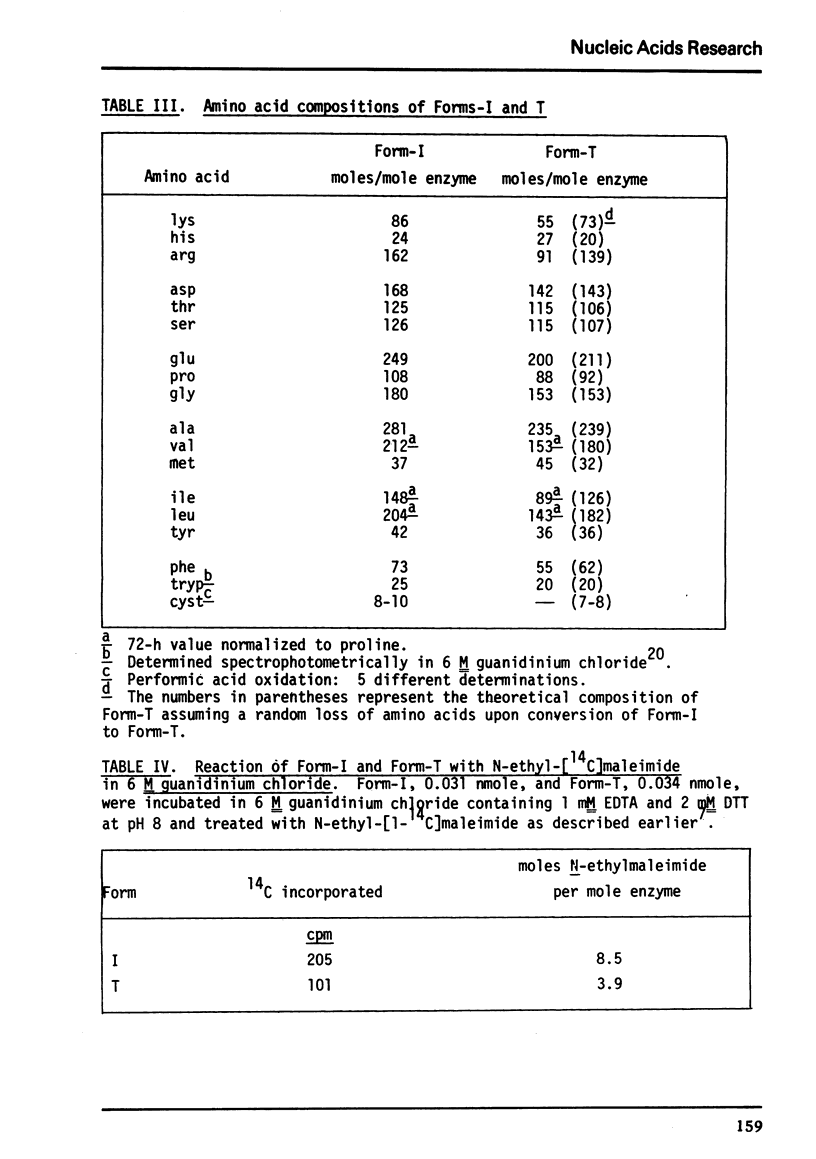
The amino acid compositions of Form-I and Form-T are reported. Form-I contains only between 8 and 10 cysteine residues per molecule and Form-T half that many.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burgess R. R. A new method for the large scale purification of Escherichia coli deoxyribonucleic acid-dependent ribonucleic acid polymerase. J Biol Chem. 1969 Nov 25;244(22):6160–6167. [PubMed] [Google Scholar]
- Burns D. J., Turner N. A. Peptide mapping on cellulose thin layers. J Chromatogr. 1967 Oct;30(2):469–475. doi: 10.1016/s0021-9673(00)84179-5. [DOI] [PubMed] [Google Scholar]
- Chou J. Y., Singer M. F. The effect of chain length on the phosphorolysis of oligonucleotides by polynucleotide phosphorylase. J Biol Chem. 1970 Mar 10;245(5):1005–1011. [PubMed] [Google Scholar]
- Dunker A. K., Rueckert R. R. Observations on molecular weight determinations on polyacrylamide gel. J Biol Chem. 1969 Sep 25;244(18):5074–5080. [PubMed] [Google Scholar]
- Edelhoch H. Spectroscopic determination of tryptophan and tyrosine in proteins. Biochemistry. 1967 Jul;6(7):1948–1954. doi: 10.1021/bi00859a010. [DOI] [PubMed] [Google Scholar]
- Fitt P. S., Wille H. The preferential loss of the polylysine- or polyornithine-stimulated activity of Clostridium perfringens polynucleotide phosphorylase during proteolysis. Biochem J. 1969 May;112(4):489–495. doi: 10.1042/bj1120489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajda A. T., Zaror de Behrens G., Fitt P. S. Preparatin, proteolysis and reversible oxidationof highly purified Azotobacter vinelandii polynucleotide phosphorylase. Biochem J. 1970 Dec;120(4):753–761. doi: 10.1042/bj1200753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klee C. B., Singer M. F. A convenient method for the preparation of primer-dependent polynucleotide phosphorylase from Micrococcus lysodeikticus. Biochem Biophys Res Commun. 1967 Nov 17;29(3):356–361. doi: 10.1016/0006-291x(67)90462-7. [DOI] [PubMed] [Google Scholar]
- Klee C. B. The proteolytic conversion of polynucleotide phosphorylase to a primer-dependent form. J Biol Chem. 1969 May 25;244(10):2558–2566. [PubMed] [Google Scholar]
- Lehrach H., Schäfer K., Scheit K. H. Concerning the subunit structure of polynucleotide phosphorylase from E. coli. FEBS Lett. 1971 May 20;14(5):343–345. doi: 10.1016/0014-5793(71)80296-x. [DOI] [PubMed] [Google Scholar]
- Portier C., van Rapenbusch R., Minh-Nguy-Thang, Grunberg-Manago M. Quaternary structure of polynucleotide phosphorylase from Escherichia coli. Eur J Biochem. 1973 Dec 3;40(1):77–87. doi: 10.1111/j.1432-1033.1973.tb03170.x. [DOI] [PubMed] [Google Scholar]
- Shapiro A. L., Viñuela E., Maizel J. V., Jr Molecular weight estimation of polypeptide chains by electrophoresis in SDS-polyacrylamide gels. Biochem Biophys Res Commun. 1967 Sep 7;28(5):815–820. doi: 10.1016/0006-291x(67)90391-9. [DOI] [PubMed] [Google Scholar]
- Summers M. R., Smythers G. W., Oroszlan S. Thin-layer chromatography of sub-nanomole amounts of phenylthiohydantoin (PTH) amino acids on polyamide sheets. Anal Biochem. 1973 Jun;53(2):624–628. doi: 10.1016/0003-2697(73)90114-0. [DOI] [PubMed] [Google Scholar]
- Thang M. N., Dondon L., Godefroy-Colburn T. Degradation of Escherichia coli polynucleotide phosphorylase by E. coli endogenous proteases and by trypsin. Biochimie. 1971;53(3):291–302. doi: 10.1016/s0300-9084(71)80095-0. [DOI] [PubMed] [Google Scholar]