Abstract
Even though Intracytoplasmic Sperm Injection (ICSI) has been widely used for the production of offspring in human infertility clinics and in reproductive research laboratories using mice, many researchers engaged in animal transgenesis still consider it somewhat cumbersome. The greatest advantage of ICSI-mediated transgenesis is that it allows introduction of very large DNA transgenes (e.g., yeast artificial chromosomes), with relatively high efficiency into the genomes of hosts, as compared to pronuclear injection.
Recently, we have developed an active form of ICSI-Tr with fresh sperm utilizing transposons. The transgenic efficiencies rival all transgenic techniques except that of lentiviral methods.
Keywords: piggyBac, transposon, transposase, transgenesis
Introduction
The first attempt of exogenous DNA uptake was performed by incubating fresh spermatozoa with the Simian virus 40 (SV40). No live transgenic offspring were obtained by this method, but the conclusion was reached, whereby a heterologous genome could be incorporated into mammalian spermatozoa and subsequently carried into the ovum during the process of fertilization [1]. The first transgenic mice were obtained by microinjecting SV40 viral DNA into the blastocoel cavity of embryos, and obtaining between 0.5 and 13 SV40 genome equivalents per diploid mouse genome which were indentified in various organs [2]. An improvement to this technique was developed in mice, rabbits, sheep and pigs by injecting exogenous DNA into the male pronucleus of zygotes, resulting in germline transgenic animals [3–5]. Experiments by Hammer et al., also demonstrated that certain promoters can result in transgene expression in the desired tissues [5]. This pronuclear microinjection technique, an example of a passive transgenesis procedure, relies on the injection of a small amount of fluid containing the linearized transgene into the male pronucleus of a zygote and on the repair mechanisms of the zygote nucleus for the insertion of the transgene [6]. It is not a very efficient transgenesis method and usually results in offspring containing multiple concatemerised vector copies [7, 8]. In mice, zygotes transferred to surrogate mothers result in transgenic animals with random integration of the transgene [8] and a transgenic efficiency of at best 3.2% of oocytes injected or 24% of animals born [9]. Many animals produced by this method suffer from mosaicism, thus effecting germline transmission and transgene expression. This mosaicism is due to the integration event(s) occurring after the one cell stage [10], where approximately 70% of the founders produced transmit their transgene to offspring. Furthermore, of the transgenic offspring produced by this method, only about 50% express their transgene at useful levels [8]. The difficulties of the pronuclear microinjection technique are further compounded in such species as bovine and swine, where the oocyte cytoplasm is opaque and requires the centrifugation of the zygote for pronuclei visualization.
To overcome some of the difficulties of pronuclear microinjection our laboratory developed another passive technique for the production of transgenic mice called Intracytoplasmic Sperm Injection-mediated transgenesis (ICSI-Tr) [11], sometimes referred to as metaphase II transgenesis. For this technique mouse spermatozoa are demembranated either by freeze-thawing or by treatment with a detergent such as TritonX-100 [11], then incubated with linear, double stranded DNA that contains the transgene. The rationale for this method was that the exposed perinuclear theca of the sperm head would interact with the DNA and act as a carrier for the transgene. This sperm-DNA complex is then injected into mature metaphase II-oocytes by ICSI, allowing the transgene to be incorporated into the embryonic genome via the DNA repair mechanism [6]. The efficiency of ICSI-Tr is an average of 4.6% of oocytes injected or 45.5% of animals born, with very little mosaicism demonstrated by such founders [11, 12]. One of the main advantages of ICSI-Tr is its higher efficiencies at inserting very large DNA fragments into the host genome, including inbred mice, as compared to pronuclear microinjection [12–14]. Furthermore, non-transgenic ICSI is also able to facilitate the reproductive success of animals which produce spermatozoa that are unable to fertilize oocytes on their own in-vivo or in-vitro. Such spermatozoa may still have chromosomal DNA integrity, therefore, maintaining their ability to generate offspring when microinjected into oocytes [15]. ICSI therefore has become the preferred means of fertilization in most human IVF clinics throughout the world in situations where conventional IVF and other methods have failed [15]. Nevertheless, ICSI has been considered cumbersome for most transgenic laboratories and has not been widely adopted. Therefore, the ICSI-Tr technique needs further improvement before its wide acceptance.
The most successful transgenic method to date, in terms of transgenic offspring produced, is the Lentiviral technique which was first developed in rodents and later extended to farm animals [16, 17]. It makes use of disarmed retroviral vectors to insert a desirable gene(s) into the host organism, usually at the single celled embryo stage [16]. Major drawbacks of this technique are the high embryo lethality (27% survival rate) and the relatively small amount of transgene DNA that can be transported because of the limited physical volume of the viral particle [16]. To improve the size limitations of lentiviral vectors, transgenes have been placed under the control of the long terminal repeats (LTR’s). Although increasing the amount of transgene DNA that can be shuttled by the viral vector, this approach has brought about expression problems that can be alleviated by the use of mutated LTR’s [18]. Additionally, specialized containment facilities for retroviral production are required. These factors preclude many laboratories from using the retroviral-mediated transgenesis approach. There are also lingering concerns about the potential consequences of recombinant events between the viral vector and endogenous retroviruses, leading to the generation of new and more potent pathogenic agents. However, 86.3% of animals born by this procedure are transgenic, representing 23% oocytes injected [16].
How can ICSI transgenesis be improved ?
All transgenic techniques currently utilized need further optimization. For example, pronuclear methods suffer from mechanical damages to the pronucleus, concatamerised insertions, low germline transmissions, reduced transgene expression rates and low transgenic efficiencies [8]. Despite these limitations, the pronuclear microinjection technique is still used by most transgenic laboratories, because of its reliability in producing transgenic offspring and the general ease of implementation over the other methods.
In order to make ICSI-Tr more applicable to species where its success was previously limited, we undertook an approach to prevent the freeze-thawing of spermatozoa which was deemed necessary for its success [11]. For regular ICSI procedures where no transgenesis is attempted, the production of normal offspring has proven successful in mice and humans [15, 19]. However, it has been less successful when applied to other species. The reason for this is not clear, but may result from ICSI introducing the sperm plasma membrane and hydrolyzing enzymes of the acrosome which do not enter the oocyte during normal fertilization [15]. For species with small acrosomes such as mice and humans, injection of the acrosome into oocytes does not appear to produce serious problems, but for species like the hamster, with very large acrosomes, injection of intact sperm inevitably results in death of the oocyte [20–22]. In the mouse, the removal of the acrosome is not a prerequisite to produce offspring by ICSI, but it results in earlier onset of oocyte activation and better embryonic development [20]. Since TritonX-100 is not a natural product and its leftover could be toxic to oocytes, we tried lysolecithin as a sperm membrane-removing agent instead of TritonX-100. Lysolecithin is a natural cellular hydrolysis product of membrane phospholipids and is unlikely to be toxic to oocytes at leftover levels. This hypothesis was based upon our knowledge that removal of the plasma membrane and acrosome of the mouse spermatozoa by lysolecithin prior to ICSI results in better embryonic development as compared to TritonX-100 [20]. Two preliminary attempts demonstrated that the application of 0.02% lysolecithin treated spermatozoa to ICSI-mediated transgenesis resulted in high efficiencies of EGFP transgenic mice, 11.4% oocytes injected and 62.5% animals born (Kazuto Morozumi and Stefan Moisyadi, unpublished data: Table 1). These preliminary data represent a higher transgenic efficiency for oocytes injected as compared to reqular freeze-thawed sperm ICSI-Tr which has an efficiency of 4.6% oocytes injected. Once these findings are confirmed, we intend to use it for the insertion of large exogenous DNA (>200kb) into the genomes of mice, as we had done previously with inbred mice [13]. These inbred mouse experiments were done with freeze-thawed sperm, but suffered from embryo developmental problems [23]. Lysolecithin treated sperm could reduce the developmental problems during ICSI-Tr with large transgenes and might also result in a greater percentage of microinjected oocytes developing into live transgenic offspring.
Table 1. Transgenic efficiency of different transgenesis methods.
Trangenesis efficiency comparisons. ICSI-Tr is done with freeze-thawed sperm and linear transgene. TN:ICSI and TN:ROSI is achieved with the Tn5 transposase purchased from manufacturer in the protein form and complexed with a EGFP transgene containing transposon. The transposon-transposase complex along with freshly immobilized sperm is microinjected during ICSI. TP:ICSI is performed by microinjecting the pMMK-2 plasmid construct which contains the donor and helper elements with freshly immobilized sperm. The live sperm were rendered immobile by the removal of their tail during ICSI by brief piezo pulses
| Transgenesis Methods | Mouse strain used for sperm and oocyte | No. of oocytes injected (repetitions) | Total no. of normally fertilized oocytes | No. of embryos transferred (No. recipients) | Births (% transferred) [% oocytes] | Transgenic pups | Publication No. in References | ||
|---|---|---|---|---|---|---|---|---|---|
| Total | Animals born (% births) | Oocytes injected (% oocytes) | |||||||
| Pronuclear | Varius | 4739 (NA) | NA | NA | 626 (NA) [13.2] | 150 | 24.0 | 3.2 | 9 |
| Lentiviral | B6D2F1 hybrid | 270 (NA) | 231 | 197 (NA) | 73 (37.0) [27.0] | 63 | 86.3 | 23.0 | 16 |
| ICSI-Tr | B6D2F1 hybrid | 97 (1) | NA | 53 (3) | 12 (26.6) [12.3] | 2 | 16.6 | 2.0 | 11 |
| ICSI-Tr | B6D2F1 hybrid | NA | 213 | 179 (12) | 14 (7.8) [NA] | 6 | 42.8 | NA | 14 |
| ICSI-Tr | CD1 outbred | 219 (6) | 195 | 163 (8) | 22 (13.5) [10.0] | 10 | 45.5 | 4.6 | 12 |
| Lysolecithin:ICSI | B6D2F1 hybrid | 44 (2) | 36 | 23 (2) | 8 (34.7) [18.2] | 5 | 62.5 | 11.4 | Unpublished |
| TN:ICSI | B6D2F1 hybrid | 204 (7) | 182 | 171 (14) | 107 (62.6) [52.0] | 23 | 21.5 | 11.3 | 24 |
| TN:ICSI | C57BL/6 inbred | 94 (2) | 84 | 77 (6) | 45 (58.4) [47.9] | 4 | 8.8 | 4.3 | 24 |
| TN:ROSI | B6D2F1 hybrid | 120 (5) | 108 | 86 (7) | 40 (46.0) [37.5] | 5 | 16.1 | 4.2 | 24 |
| TP:ICSI | B6D2F1 hybrid | 79 (2) | 72 | 69 (4) | 26 (37.7) [33.0] | 18 | 69.2 | 22.8 | 31 |
Improvement of ICSI transgenesis efficiency with Transposons
A) Proteins
The increased rate of transgenesis with non freeze-thawed spermatozoa during the lysolecithin experiments encouraged us to investigate whether other non freeze-thawed sperm approaches would improve ICSI mediated transgenesis. Non-demembrenated spermatozoa do not facilitate effective transgenesis when mixed with a linear plasmid transgene [11, 24]. However, if such freshly killed spermatozoa by tail removal are incubated with the Tn5 transposome (transposon:transposase), containing the transgene, then ICSI becomes very effective in facilitating the generation of transgenic offspring (TN:ICSI) [24]. In these experiments, the efficiencies of this transposon mediated technique were 11.3% oocytes injected and 21.5% animals born [24]. The oocytes injected transgenic rates are several fold higher than traditional ICSI-Tr performed with freeze-thawed sperm [11, 12]. Furthermore, TN:ICSI performed with fresh sperm is 5 times more efficient as compared to TN:ICSI done with freeze-thawed sperm [24]. During this procedure we obtain 23 born transgenic pups out of 107 live births, resulting in a ratio 21.5% animals born [24]. Although the percentage of transgenic animals born to total animals born by TN:ICSI are lower as compared to other methods, the transgenic animals born to oocyte injected ratio is substantially higher and is a more accurate measure of the transgenic efficiency. These fertilization and development rates are comparable to those obtained by ICSI performed with freshly killed sperm alone [25], indicating that Tn5 complexed DNA is not deleterious to embryo survival at the concentrations used, and results in higher transgenic success rates. In traditional ICSI-Tr experiments done with freeze-thawed sperm, the total animals born to oocytes injected ratio ranges between 10% and 12.3% [11, 12], which pales in comparison to the 52% rate obtained with TN:ICSI [24].
Another advantage of Tn5 transposase mediated transgenesis is improving the transgenic rates of inbred mice and round spermatid injections (ROSI). Transposon enhanced ROSI (TN:ROSI) with round spermatids from B6D2F1 hybrid mice resulted in transgenic animals born to oocytes injected ratio of 4.2%. For the C57BL/6 inbred strain, TN:ICSI with fresh sperm had an oocytes injected ratio of 4.3% [24], equaling the success rates reported with traditional ICSI-Tr done with freeze-thawed sperm [11, 12]. As the efficiency of transgenesis by pronuclear microinjection for the C57BL/6 strain mice was reported to be one eighth of that obtained using a hybrid strain [26], the 38% efficiency of TN:ICSI with the same C57BL/6 inbred strain when compared to the B6D2F1 hybrid stain TN:ICSI rates, represents a considerable improvement with inbred mouse transgenesis over pronuclear microinjection produced rates.
B) DNA
Because the Tn5 based TN:ICSI method suffers from cumbersome enzyme preparation techniques, we have now moved away from this technique and have developed DNA based procedures that allow synthesis of the transposase in-situ.
The piggyBac Transposase
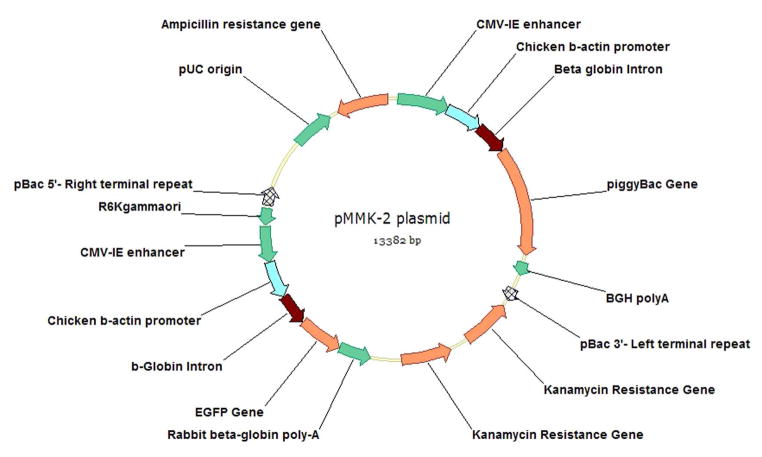
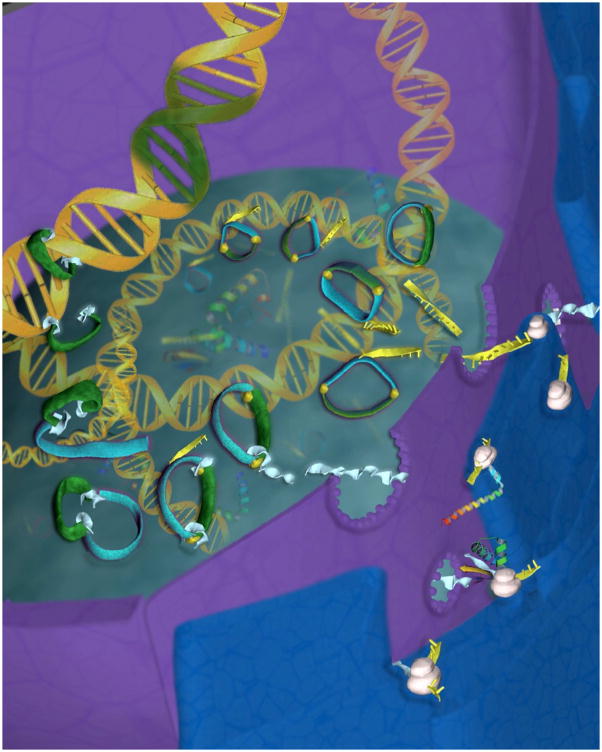
Our previous work showing that Tn5 transposome augments ICSI transgenesis [24], lead us to study several transposases for transgenesis. Of these, the piggyBac transposase isolated from the cabbage looper moth Trichoplusia ni proved the most effective as demonstrated by our laboratories [27], with our results independently confirmed after our publication by Wilson et, al., at Vanderbilt University [28]. piggyBac was shown to transpose efficiently in mice [29] and until recently the piggyBac transposition machinery was described in the literature as a two-component system: a Helper plasmid containing the transposase and a Donor plasmid containing the transposon [30]. We have now simplified this approach by including the Helper and Donor components in a single plasmid named pMMK-2 (Figure 1). This single plasmid approach makes it easier to effect transposition, where if the plasmid has entered the nucleus of a cell, both Helper and Donor components are included in it (Figure 2). The piggyBac gene in the pMMK-2 construct is driven by the CMV-IE+chicken β-actin+β-globin (CAG) promoter. The transposon in this plasmid is flanked by the 5′- and 3′-terminal end (TR) sequences of piggyBac (Figure 1). The in-situ synthesized transposase protein complexes with the TR flanking the transposon and forms a transposome prior to inserting into the host DNA (Figure 2). Since the sperm does not undergo freeze-thawing with TP:ICSI, the development of embryos to live births is more efficient [31], presumably because of reduced chromosomal breakage as observed with traditional ICSI-Tr [23]. The transposon in the pMMK-2 construct is also a rescue plasmid, due to the presence of the bacterial expressed kanamycin genes, and allows identification of the transposon insertion sites [27]. The transgenic rates with piggyBac during TP:ICSI are 22.8% oocytes injected and 69.2% animals born [31], approacing the lentiviral transgenesis efficiency of 23% oocytes injected and 86.3% animals born [16]. The raw data for all the transgenesis techniques described above are tabulated on Table 1. For the piggyBac experiments described, only the results with the highest amount of DNA injected into the oocytes are shown (0.663 pg). When less pMMK-2 was used in these TP:ICSI experiments, the transgenic efficiency decreased. For example, the 0.442 pg pMMK-2 injections gave a transgenic efficiency of 12.1% for oocytes injected and 44.4% for animals born, while 0.221 pg injection resulted in 11.1% oocytes injected and 31% animals born. Thus, the amount of transposon DNA injected into the oocyte directly correlates with higher transgenic rates. At present we have not exceeded 0.663 pg of pMMK-2.
Figure 1.
Map of pMMK-2, which contains both the donor and helper elements of the piggyBac transposon. The donor element (the portion which is integrated) includes the CAG promoter driving the EGFP transgene which is flanked by the 5′-Right terminal and 3′-Left terminal repeats. The piggyBac transposase gene also driven by its own CAG promoter is outside the terminal repeats. The region outside the terminal repeats, including the transposase source is not inserted into the genome of the host and is eventually degraded.
Figure 2.
Diagram depicting insertion of the piggyBac transposon into the host cell genome. After introduction of the plasmid containing both the donor and helper transposon components into the nucleus, the helper part of the construct containing the piggyBac gene (sky blue) participates in the synthesis of mRNA (yellow) which is subsequently translated into protein in the cytoplasmic ribosomes (white). The newly synthesized piggyBac transposase (light blue) then makes its way into the nucleus and couples to specific DNA binding domains at the 5′-Right and 3′-Left terminal repeats of piggyBac (yellow). The transposase then cleaves the DNA outside the terminal repears, forming the transposon-transposase complex (green and light blue) which then inserts the transgene (green) into the genomic DNA of the host cell (Artwork by Krystian Paczkowski).
Since, transposase DNA could integrate into the genome via non-homologous recombination resulting in genomic toxicity, we are now supplying cRNA for the piggyBac transposase into oocytes together with the Donor only plasmid. This method will likely overcome any risks of the transposase gene integrating into the genome; however, there is still a very small possibility that the RNA could undergo reverse transcription resulting in possible recombination into the genome. However, with this approach we should be able to titrate the concentrations of DNA and cRNA in the hope of obtaining the optimal concentrations for effective transagenesis.
Why make transgenic animals?
A transgenic animal is defined as one which has been genetically altered to have specific characteristics that it would otherwise not have. The alteration mentioned in this case is brought about by the insertion of foreign genetic material into the oocyte (i.e., pronuclear injection, ICSI-Tr, lentiviral, TN:ICSI, TP:ICSI). Alternatively, embryonic stem cells in mice, or fibroblast cells in farm animals can be transfected and then injected into blastocyst, or their nuclei can be transferred into enucleated oocytes by nuclear transfer [32–34]. The question of “Why make transgenic animals?” was well answered in a recent review [35]. In it, the authors answer the question by stating “(1) gain new knowledge, (2) decipher the genetic code, (3) study the genetic control of physiological systems, (4) build genetic disease models, (5) improve animal production traits, and (6) produce new animal products.” These answers provide the tenets for those in the animal transgenesis field.
Virtually every medical breakthrough in the 20th century came about as a result of research with animals. The list is almost endless, but includes the discovery of insulin in 1921 by Frederick G. Banting working with dogs [36] and the development of the polio vaccine by Jonas Salk in 1955 working with monkeys [37]. Today, transgenic mice modified to be susceptible to viral infections can frequently replace primate use in the testing of vaccines [38, 39]. For example, transgenic mice expressing the human poliovirus receptor have been used as a model to test oral polio vaccines [40].
The characterization of the mouse genome sequence along with comparative human sequence analysis has brought about much excitement in the functional genomics field. Therefore, many scientists have generated mutant mice defective in the expression of one or more genes through a variety of methods which have most commonly utilized random mutagenesis (e.g., ENU or gene trap mutagenesis) followed by phenotypic and then genotypic analysis. However, these methods lack efficiency when one wishes to analyze rapidly the function of gene(s) of interest. Thus, methods have been developed to directly alter a gene of interest by knocking out or suppressing it followed by phenotypic analysis. Knock out animals produced in part from targeted homologous recombination in ES cells can be expensive, complex, and time consuming [41]. RNA interference may provide a more viable alternative because it is relatively simple, inexpensive, faster, and may in fact provide a more suitable model in those diseases where decreased expression of the gene accounts for certain disease phenotypes [41]. If such gene product studies were restricted to cultured cells, the activity of a specific gene at the cellular level would not yield satisfactory information about the regulation of the gene among the complex physiological interactions of the whole animal. Therefore, it must be assumed that cell cultures cannot possibly simulate tissues and organ systems and predict responses to sophisticated environmental stimuli.
The well-characterized physiology, genetics, and short lifespan of mice allow for rapid analysis of the phenotypic changes associated with the transgene over their entire lifespan. These characteristics facilitate the accelerated development of new diagnostic and therapeutic treatments for human diseases. There are many transgenic rodents that model human diseases such as sickle cell anemia [42], AIDS [43], amylotropic lateral sclerosis (ALS), also known as Lou Gehrigs disease [44], hepatitis B infection [44], ankylosing spondylitis [44] and cancer [45]. Most of these are produced by the highly inefficient but preferred pronuclear microinjection technique.
Eventually transgenesis was extended to larger animals to produce transgenic pigs and sheep for the study of genetically modified livestock and the improvements in their growth and body composition [46]. The literature contains transgenic livestock models produced by pronuclear microinjection for sheep, pigs and rabbits [5], cows [47], goats [48] and several excellent reviews cover the field thoroughly [8, 46, 49, 50]. Additionally, retroviruses have been used for gene insertion in chickens [51], cows [52] and pigs [17]. Cattle were produced resistant to mastitis by genetically modifying bovine fibroblast cells and then using their nuclei for nuclear transfer [34]. Therefore, transgenic research in livestock is now progressing rapidly, which may result in improved animal health and the nutritional value of animal products. Transgenic livestock acting as tissue and organ donors may have the potential to improve human health. Furthermore, if very large fragments of DNA need to be inserted into livestock for correct expression of transgenes, the improvements to ICSI-Tr described earlier might prove invaluable in generating such animals where other gene insertion techniques prove inadequate.
Erratic and low success rates of ICSI other than in the mouse and human could be due to persistence of sperm plasma membrane and introduction of acrosomes into oocytes during ICSI [15]. Injection of spermatozoa free of plasma membranes and acrosomes would be less stressful to oocytes as these structures never enter oocytes during normal fertilization. In the mouse, removal of large tails from spermatozoa facilitates ICSI, but for most animals with small spermatozoa (e.g., humans and common farm animals), injection of the whole body of spermatozoa would impose no technical problems.
Although gene modified livestock research is in its infancy compared to mice, the more efficient ICSI transgenesis techniques might allow effective, less costly production of large animal models that simulate certain human diseases more adequately than rodent models. When large transgene size insertion becomes necessary for the correct expression of genes, ICSI-Tr has proven much more efficient than pronuclear microinjection for the insertion of such large DNA fragments at the 500 kb range [12, 13], with a greater number of founder animals being produced in comparison to pronuclear microinjection [53].
Large DNA inserts (e.g., yeast articificial chromosomes) frequently result in correct gene expression, regardless of position, unlike the limited promoter-driven cDNA transgenes. The transgene expression on the large constructs mimics the endogenous expression pattern of the homologous locus, because their large size usually ensures the inclusion of all regulatory elements and enough separation from the endogenous regulatory sequences for proper gene expression [53]. This point becomes very important when genes are transferred across species barriers. If cDNA transgenes end up in environments and tissues different than the one they originated from, they may lack the sufficient regulatory elements critical to their expression due to size limitations. Evolutionary changes in the new species and organs in which the transgene now resides may require different regulatory elements for correct expression, and if absent, may cause aberrant expression [44]. The introduction of the entire genome locus for correct expression of the transgene may overcome this abberant pattern of expression, in certain cases, and allow organ specific regulation of the transgene.
Trangenic farm animals as bioreactors for recombinant proteins
The production of valuable pharmaceutical human enzymes, hormones, antibodies and growth factors currently use large-scale cell cultures to generate products in biological systems. This method requires the production of transfected eukaryotic or bacterial cell lines which contain transgene constructs for the generation of recombinant gene products. By necessity, the model requires large scale bioreactors to aseptically culture the cell lines in nutrient medium which necessitates continual replacement and from which the bioengineered product is then refined. The cost for producing biomolecules is relatively inexpensive in microorganisms (e.g., bacteria and yeast). However, the necessary post-translational processing and proper folding does not occur in these organisms, thus frequently rendering many mammalian proteins nonfunctional. However, the production process for recombinant proteins in vitro in mammalian cell culture is very expensive due to the requirement of pathogen-free conditions with constant monitoring, buffering and temperature regulation of the medium. Therefore, the concept of “pharmaceutical pharming” where large transgenic mammals are used as bioreactors for protein production is very appealing to the pharmaceutical industry, because it represents a cost-effective alternative to the cell culture methods. The impetus of using animals as bioreactors originates from the experiments where transgenic mice for growth hormone (GH) expression demonstrated remarkable growth [54]. Mammary gland transgene expression currently is the preferred option because it allows mass production of large amounts of correctly processed proteins in a temperature-regulated fluid that may be collected daily in a non-invasive fashion [55]. ICSI-mediated transgenesis with improvements described previously, offers an opportunity to insert large transgenes into livestock animals for the correct expression and processing of gene products. Producing large quantities of biological products in animal bioreactors may help alleviate the big demand for biomolecules that are currently synthesized by very expensive procedures.
Conclusion
Many laboratories have not adopted animal ICSI-Tr due to limited success except for the mouse. At least in the mouse, ICSI-Tr with transposons has improved transgenic efficiencies to the level that approach retroviral methods. Furthermore, thus far ICSI-Tr is the only alternative when very large transgenes of ~500 kb need to be inserted for correctly regulated gene expression.
In comparing transgenic methods, the reader must not just look at the percentage of transgenic animals born, but more importantly the number of oocytes that are manipulated to obtain that percentage. For example, a method that results in 100% of transgenic animals born but requires injection of 100 oocytes to obtain 1 transgenic animal is inferior in efficiency to one that results in 50% of transgenic animals born but only requires 4 oocytes injected to obtain 1 transgenic animal. In these cases, the percent of oocytes micromanipulated that result in transgenic animals are 1% and 25%, respectively. Based upon both definitions, active ICSI-transgenesis in the mouse is approaching the efficiencies of the lentiviral method (Table 1).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brackett BG. Uptake of Heterologous Genome by Mammalian Spermatozoa and Its Transfer to Ova through Fertilization. PNAS. 1971;68(2):353–357. doi: 10.1073/pnas.68.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jaenisch R, Mintz B. Simian virus 40 DNA sequences in DNA of healthy adult mice derived from preimplantation blastocysts injected with viral DNA. Proc Natl Acad Sci U S A. 1974;71(4):1250–1254. doi: 10.1073/pnas.71.4.1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gordon JW, Ruddle FH. Integration and stable germ line transmission of genes injected into mouse pronuclei. Science. 1981;214(4526):1244–1246. doi: 10.1126/science.6272397. [DOI] [PubMed] [Google Scholar]
- 4.Gordon JW, Scangos GA, Plotkin DJ, Barbarosa JA, Ruddle FH. Genetic Transformation of Mouse Embryos by Microinjection of Purified DNA. PNAS. 1980:777380–7384. doi: 10.1073/pnas.77.12.7380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hammer RE, Pursel VG, Rexroad CE, Jr, Wall RJ, Bolt DJ, Ebert KM, Palmiter RD, Brinster RL. Production of transgenic rabbits, sheep and pigs by microinjection. Nature. 1985;315(6021):680–683. doi: 10.1038/315680a0. [DOI] [PubMed] [Google Scholar]
- 6.Perry AC. Hijacking oocyte DNA repair machinery in transgenesis? Mol Reprod Dev. 2000;56(S2):319–324. doi: 10.1002/(SICI)1098-2795(200006)56:2+<319::AID-MRD24>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 7.Muller U. Ten years of gene targeting: targeted mouse mutants, from vector design to phenotype analysis. Mech Dev. 1999;82(1–2):3–21. doi: 10.1016/s0925-4773(99)00021-0. [DOI] [PubMed] [Google Scholar]
- 8.Wall RJ. Pronuclear microinjection. Cloning Stem Cells. 2001;3(4):209–220. doi: 10.1089/15362300152725936. [DOI] [PubMed] [Google Scholar]
- 9.Nakanishi T, Kuroiwa A, Yamada S, Isotani A, Yamashita A, Tairaka A, Hayashi T, Takagi T, Ikawa M, Matsuda Y, Okabe M. FISH analysis of 142 EGFP transgene integration sites into the mouse genome. Genomics. 2002;80(6):564–574. doi: 10.1006/geno.2002.7008. [DOI] [PubMed] [Google Scholar]
- 10.Lewis-Williams J, Harvey M, Wilburn B, Destrempes M, Echelard Y. Analysis of Transgenic Mosaicism in Microinjected Mouse Embryos Using Fluorescence In Situ Hybridization at Various Developmental Time Points. Theriogenology. 1996;45(1):335. [Google Scholar]
- 11.Perry AC, Wakayama T, Kishikawa H, Kasai T, Okabe M, Toyoda Y, Yanagimachi R. Mammalian transgenesis by intracytoplasmic sperm injection. Science. 1999;284(5417):1180–1183. doi: 10.1126/science.284.5417.1180. [DOI] [PubMed] [Google Scholar]
- 12.Moreira PN, Giraldo P, Cozar P, Pozueta J, Jimenez A, Montoliu L, Gutierrez-Adan A. Efficient generation of transgenic mice with intact yeast artificial chromosomes by intracytoplasmic sperm injection. Biol Reprod. 2004;71(6):1943–1947. doi: 10.1095/biolreprod.104.032904. [DOI] [PubMed] [Google Scholar]
- 13.Osada T, Toyoda A, Moisyadi S, Akutsu H, Hattori M, Sakaki Y, Yanagimachi R. Production of inbred and hybrid transgenic mice carrying large (> 200 kb) foreign DNA fragments by intracytoplasmic sperm injection. Mol Reprod Dev. 2005;72(3):329–335. doi: 10.1002/mrd.20319. [DOI] [PubMed] [Google Scholar]
- 14.Perry AC, Rothman A, de las Heras JI, Feinstein P, Mombaerts P, Cooke HJ, Wakayama T. Efficient metaphase II transgenesis with different transgene archetypes. Nat Biotechnol. 2001;19(11):1071–1073. doi: 10.1038/nbt1101-1071. [DOI] [PubMed] [Google Scholar]
- 15.Yanagimachi R. Intracytoplasmic injection of spermatozoa and spermatogenic cells: its biology and applications in humans and animals. Reprod Biomed Online. 2005;10(2):247–288. doi: 10.1016/s1472-6483(10)60947-9. [DOI] [PubMed] [Google Scholar]
- 16.Lois C, Hong EJ, Pease S, Brown EJ, Baltimore D. Germline transmission and tissue-specific expression of transgenes delivered by lentiviral vectors. Science. 2002;295(5556):868–872. doi: 10.1126/science.1067081. [DOI] [PubMed] [Google Scholar]
- 17.Hofmann A, Kessler B, Ewerling S, Weppert M, Vogg B, Ludwig H, Stojkovic M, Boelhauve M, Brem G, Wolf E, Pfeifer A. Efficient transgenesis in farm animals by lentiviral vectors. EMBO Rep. 2003;4(11):1054–1060. doi: 10.1038/sj.embor.7400007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wall RJ. New gene transfer methods. Theriogenology. 2002;57(1):189–201. doi: 10.1016/s0093-691x(01)00666-5. [DOI] [PubMed] [Google Scholar]
- 19.Nagy ZP, Liu J, Joris H, Verheyen G, Tournaye H, Camus M, Derde MC, Devroey P, Van Steirteghem AC. The result of intracytoplasmic sperm injection is not related to any of the three basic sperm parameters. Hum Reprod. 1995;10(5):1123–1129. doi: 10.1093/oxfordjournals.humrep.a136104. [DOI] [PubMed] [Google Scholar]
- 20.Morozumi K, Shikano T, Miyazaki S, Yanagimachi R. Simultaneous removal of sperm plasma membrane and acrosome before intracytoplasmic sperm injection improves oocyte activation/embryonic development. Proc Natl Acad Sci U S A. 2006;103(47):17661–17666. doi: 10.1073/pnas.0608183103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morozumi K, Yanagimachi R. Incorporation of the acrosome into the oocyte during intracytoplasmic sperm injection could be potentially hazardous to embryo development. Proc Natl Acad Sci U S A. 2005;102(40):14209–14214. doi: 10.1073/pnas.0507005102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamauchi Y, Yanagimachi R, Horiuchi T. Full-term development of golden hamster oocytes following intracytoplasmic sperm head injection. Biol Reprod. 2002;67(2):534–539. doi: 10.1095/biolreprod67.2.534. [DOI] [PubMed] [Google Scholar]
- 23.Szczygiel MA, Moisyadi S, Ward WS. Expression of foreign DNA is associated with paternal chromosome degradation in intracytoplasmic sperm injection-mediated transgenesis in the mouse. Biol Reprod. 2003;68(5):1903–1910. doi: 10.1095/biolreprod.102.012377. [DOI] [PubMed] [Google Scholar]
- 24.Suganuma R, Pelczar P, Spetz JF, Hohn B, Yanagimachi R, Moisyadi S. Tn5 transposase-mediated mouse transgenesis. Biol Reprod. 2005;73(6):1157–1163. doi: 10.1095/biolreprod.105.044669. [DOI] [PubMed] [Google Scholar]
- 25.Kimura Y, Yanagimachi R. Intracytoplasmic sperm injection in the mouse. Biol Reprod. 1995;52(4):709–720. doi: 10.1095/biolreprod52.4.709. [DOI] [PubMed] [Google Scholar]
- 26.Brinster RL, Chen HY, Trumbauer ME, Yagle MK, Palmiter RD. Factors affecting the efficiency of introducing foreign DNA into mice by microinjecting eggs. Proc Natl Acad Sci U S A. 1985;82(13):4438–4442. doi: 10.1073/pnas.82.13.4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu SC, Meir YJ, Coates CJ, Handler AM, Pelczar P, Moisyadi S, Kaminski JM. piggyBac is a flexible and highly active transposon as compared to Sleeping Beauty, Tol2, and Mos1 in mammalian cells. Proc Natl Acad Sci U S A. 2006;103(41):15008–15013. doi: 10.1073/pnas.0606979103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilson MH, Coates CJ, George AL., Jr PiggyBac Transposon-mediated Gene Transfer in Human Cells. Mol Ther. 2007;15(1):139–145. doi: 10.1038/sj.mt.6300028. [DOI] [PubMed] [Google Scholar]
- 29.Ding S, Wu X, Li G, Han M, Zhuang Y, Xu T. Efficient transposition of the piggyBac (PB) transposon in mammalian cells and mice. Cell. 2005;122(3):473–483. doi: 10.1016/j.cell.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 30.Wilson M, HCCJ, George AL., Jr piggyBac Transposon-mediated Gene transfer in Human Cells. Molecular Therapy. 2007;15(1):139–145. doi: 10.1038/sj.mt.6300028. [DOI] [PubMed] [Google Scholar]
- 31.Shinohara ET, Kaminski JM, Segal DJ, Pelczar P, Kolhe R, Ryan T, Coates CJ, Fraser MJ, Handler AM, Yanagimachi R, Moisyadi S. Active integration: new strategies for transgenesis. Transgenic Res. 2007;16(3):333–339. doi: 10.1007/s11248-007-9077-z. [DOI] [PubMed] [Google Scholar]
- 32.Denning C, Priddle H. New frontiers in gene targeting and cloning: success, application and challenges in domestic animals and human embryonic stem cells. Reproduction. 2003;126(1):1–11. doi: 10.1530/rep.0.1260001. [DOI] [PubMed] [Google Scholar]
- 33.Robl JM, Wang Z, Kasinathan P, Kuroiwa Y. Transgenic animal production and animal biotechnology. Theriogenology. 2007;67(1):127–133. doi: 10.1016/j.theriogenology.2006.09.034. [DOI] [PubMed] [Google Scholar]
- 34.Wall RJ, Powell AM, Paape MJ, Kerr DE, Bannerman DD, Pursel VG, Wells KD, Talbot N, Hawk HW. Genetically enhanced cows resist intramammary Staphylococcus aureus infection. Nat Biotechnol. 2005;23(4):445–451. doi: 10.1038/nbt1078. [DOI] [PubMed] [Google Scholar]
- 35.Wheeler MB, Walters EM, Clark SG. Transgenic animals in biomedicine and agriculture: outlook for the future. Anim Reprod Sci. 2003;79(3–4):265–289. doi: 10.1016/s0378-4320(03)00168-4. [DOI] [PubMed] [Google Scholar]
- 36.Banting FGBC, Collip JB, Campbell WR, Fletcher AA. Pancreatic extracts in the treatment of diabetes mellitus: Preliminary report. Canadian Medical Association Journal. 1922:12141–146. [PMC free article] [PubMed] [Google Scholar]
- 37.Salk JE. Poliomyelitis vaccination; practicalities for the practicing physician. Pa Med J. 1955;58(12):1321–1324. [PubMed] [Google Scholar]
- 38.Racaniello VR. One hundred years of poliovirus pathogenesis. Virology. 2006;344(1):9–16. doi: 10.1016/j.virol.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 39.Dragunsky EM, Ivanov AP, Abe S, Potapova SG, Enterline JC, Hashizume S, Chumakov KM. Further development of a new transgenic mouse test for the evaluation of the immunogenicity and protective properties of inactivated poliovirus vaccine. J Infect Dis. 2006;194(6):804–807. doi: 10.1086/506949. [DOI] [PubMed] [Google Scholar]
- 40.Nagata N, Iwasaki T, Ami Y, Sato Y, Hatano I, Harashima A, Suzaki Y, Yoshii T, Hashikawa T, Sata T, Horiuchi Y, Koike S, Kurata T, Nomoto A. A poliomyelitis model through mucosal infection in transgenic mice bearing human poliovirus receptor, TgPVR21. Virology. 2004;321(1):87–100. doi: 10.1016/j.virol.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 41.Xia XG, Zhou H, Xu Z. Transgenic RNAi: Accelerating and expanding reverse genetics in mammals. Transgenic Res. 2006;15(3):271–275. doi: 10.1007/s11248-006-0023-2. [DOI] [PubMed] [Google Scholar]
- 42.Ryan TM, Townes TM, Reilly MP, Asakura T, Palmiter RD, Brinster RL, Behringer RR. Human sickle hemoglobin in transgenic mice. Science. 1990;247(4942):566–568. doi: 10.1126/science.2154033. [DOI] [PubMed] [Google Scholar]
- 43.Vogel J, Hinrichs SH, Reynolds RK, Luciw PA, Jay G. The HIV tat gene induces dermal lesions resembling Kaposi’s sarcoma in transgenic mice. Nature. 1988;335(6191):606–611. doi: 10.1038/335606a0. [DOI] [PubMed] [Google Scholar]
- 44.Gordon JW. Transgenic Technology and Laboratory Animal Science. Ilar J. 1997;38(1):32–41. doi: 10.1093/ilar.38.1.32. [DOI] [PubMed] [Google Scholar]
- 45.Sinn E, Muller W, Pattengale P, Tepler I, Wallace R, Leder P. Coexpression of MMTV/v-Ha-ras and MMTV/c-myc genes in transgenic mice: synergistic action of oncogenes in vivo. Cell. 1987;49(4):465–475. doi: 10.1016/0092-8674(87)90449-1. [DOI] [PubMed] [Google Scholar]
- 46.Melo EO, Canavessi AM, Franco MM, Rumpf R. Animal transgenesis: state of the art and applications. J Appl Genet. 2007;48(1):47–61. doi: 10.1007/BF03194657. [DOI] [PubMed] [Google Scholar]
- 47.Roschlau K, Rommel P, Andreewa L, Zackel M, Roschlau D, Zackel B, Schwerin M, Huhn R, Gazarjan KG. Gene transfer experiments in cattle. J Reprod Fertil Suppl. 1989:38153–160. [PubMed] [Google Scholar]
- 48.Denman J, Hayes M, O’Day C, Edmunds T, Bartlett C, Hirani S, Ebert KM, Gordon K, McPherson JM. Transgenic expression of a variant of human tissue-type plasminogen activator in goat milk: purification and characterization of the recombinant enzyme. Biotechnology (N Y) 1991;9(9):839–843. doi: 10.1038/nbt0991-839. [DOI] [PubMed] [Google Scholar]
- 49.Pursel VG, Pinkert CA, Miller KF, Bolt DJ, Campbell RG, Palmiter RD, Brinster RL, Hammer RE. Genetic engineering of livestock. Science. 1989;244(4910):1281–1288. doi: 10.1126/science.2499927. [DOI] [PubMed] [Google Scholar]
- 50.Pursel VG, Rexroad CE, Jr, Bolt DJ, Miller KF, Wall RJ, Hammer RE, Pinkert CA, Palmiter RD, Brinster RL. Progress on gene transfer in farm animals. Vet Immunol Immunopathol. 1987;17(1–4):303–312. doi: 10.1016/0165-2427(87)90149-8. [DOI] [PubMed] [Google Scholar]
- 51.Salter DW, Smith EJ, Hughes SH, Wright SE, Fadly AM, Witter RL, Crittenden LB. Gene insertion into the chicken germ line by retroviruses. Poult Sci. 1986;65(8):1445–1458. doi: 10.3382/ps.0651445. [DOI] [PubMed] [Google Scholar]
- 52.Hofmann A, Zakhartchenko V, Weppert M, Sebald H, Wenigerkind H, Brem G, Wolf E, Pfeifer A. Generation of transgenic cattle by lentiviral gene transfer into oocytes. Biol Reprod. 2004;71(2):405–409. doi: 10.1095/biolreprod.104.028472. [DOI] [PubMed] [Google Scholar]
- 53.Moreira PN, Pozueta J, Perez-Crespo M, Valdivieso F, Gutierrez-Adan A, Montoliu L. Improving the generation of genomic-type transgenic mice by ICSI. Transgenic Res. 2007;16(2):163–168. doi: 10.1007/s11248-007-9075-1. [DOI] [PubMed] [Google Scholar]
- 54.Palmiter RD, Brinster RL, Hammer RE, Trumbauer ME, Rosenfeld MG, Birnberg NC, Evans RM. Dramatic growth of mice that develop from eggs microinjected with metallothionein-growth hormone fusion genes. Nature. 1982;300(5893):611–615. doi: 10.1038/300611a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kolb AF, Coates CJ, Kaminski JM, Summers JB, Miller AD, Segal DJ. Site-directed genome modification: nucleic acid and protein modules for targeted integration and gene correction. Trends Biotechnol. 2005;23(8):399–406. doi: 10.1016/j.tibtech.2005.06.005. [DOI] [PubMed] [Google Scholar]