Abstract
Knowledge of mechanisms linking early-life social environment and breast cancer remains limited. We explore direct and indirect effects of early-life socioeconomic status (SES) on breast cancer prevalence in later life. Using 50-year data from the Wisconsin Longitudinal Study (N = 4,275) and structural equation modeling, we found a negative direct effect of early-life SES, indicating that women from higher-SES family background had lower breast cancer prevalence than women from lower-SES families. Additionally, early-life SES has a positive indirect effect on breast cancer via women's adult SES and age at first birth. Were it not for their higher SES in adulthood and delayed childbearing, women from higher-SES families of origin would have had lower breast cancer prevalence than women from lower-SES families. Yet, early-life SES is associated positively with adult SES and age at first birth, and women's higher adult SES and delayed childbearing are related to higher breast cancer prevalence.
Keywords: breast cancer, socioeconomic status, life course, structural equation modeling, aging, demography
From a life course perspective, health of older adults cannot be understood without considering exposures at different stages of the life course (Hayward & Gorman, 2004). Because cancer has a long latency period, an individual's risk of cancer is influenced by genetic predispositions combined with the cumulative exposures in utero, throughout childhood, and during adulthood (Ben-Shlomo & Kuh, 2002; Lynch & Davey Smith, 2005).
DESCRIPTION OF THE PROBLEM
Socioeconomic status (SES) of the family of origin is a fundamental indicator of multiple and diverse environmental conditions that might be implicated in chronic disease incidence and mortality (Power, Hyppönen, & Davey Smith, 2005). Despite the importance of early-life SES in shaping adult health and mortality (Hayward & Gorman, 2004), we have a limited knowledge of the ways in which early-life SES may be related to breast cancer over the life course.
Very few studies have examined the effect of early-life SES on breast cancer. Studies that included early-life SES examined only its overall effect, without decomposing this effect into direct and indirect components (de Kok et al., 2008; Lawlor, Sterne, Tynelius, Davey Smith, & Rasmussen, 2006; Le Marchand, Kolonel, Myers, & Mi, 1988; Næss, Strand, & Davey Smith, 2007; Strand & Kunst, 2007). Thus, existing studies did not explicitly test possible mediating pathways linking early-life SES and breast cancer in later life (Ben-Shlomo & Kuh, 2002). Because cancer etiology is complex, early-life SES is likely to affect breast cancer through multiple pathways, possibly acting in opposite directions. Yet, regression models that estimate only the main effect of early-life SES may not reflect this complexity. To overcome these limitations of previous research, our study integrates the life course theoretical framework with structural equation modeling to explore direct and indirect effects of early-life SES (measured when participants were in late adolescence) on the prevalence of breast cancer in later life.
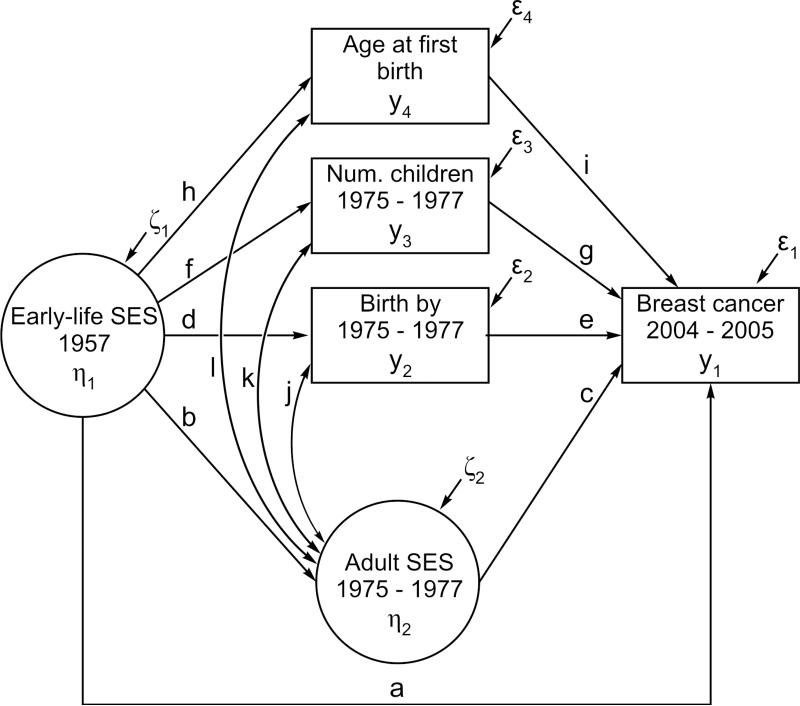
Early-life environmental conditions can influence health in later life both directly and indirectly through their influence on subsequent life course trajectories (Frijters, Hatton, Martin, & Shields, 2010). Based on existing research, we propose specific direct and indirect pathways between early-life socioeconomic conditions and the prevalence of breast cancer. The direct pathway mechanism suggests that early-life SES affects breast cancer directly, and this effect is not mediated by women's characteristics in adulthood. According to the indirect pathway mechanism, the effect of early-life SES is conveyed via women's adult socioeconomic achievement and reproductive behaviors. All hypothesized pathways are shown in Figure 1.
Figure 1.
The Structural Model Representing Causal Relationships Among the Focal Variables and Factors
Parents’ Socioeconomic Status and Early-Life Environment
A direct pathway between early-life SES and breast cancer is represented in life course epidemiology by the critical period model, according to which exposures acting during a specific period have lasting and irreversible effects on organs, tissues, and bodily systems. Moreover, the effects of critical exposures are not modified by experiences later in life (Ben-Shlomo & Kuh, 2002). Consistent with the critical period model, research suggests that infancy, childhood, and adolescence are important stages for the development of breast cancer risk factors (Jeffreys, Warren, Gunnell, McCarron, & Davey Smith, 2004). The breast may be the most vulnerable to potentially carcinogenic effects between menarche and first birth because breast tissue is not fully differentiated until after the first full-term pregnancy (Colditz & Frazier, 1995; Jeffreys et al., 2004). It is possible that early-life socioeconomic characteristics shape the environment that directly affects biological processes in childhood and adolescence, which in turn may reduce or increase breast cancer prevalence later in life.
Parents’ Socioeconomic Status and Women's Roles in Adulthood
Whereas the critical period model posits the direct effect of early-life SES on breast cancer, the accumulation of risks model (Ben-Shlomo & Kuh, 2002) suggests that the effect of early-life environment may be indirect and mediated by the characteristics of women's roles in adulthood. Women's reproductive behavior is one of the “risks” consistently linked to breast cancer. Later age at first birth and low parity or nulliparity are associated with a moderately elevated risk of breast cancer (Britt, Ashworth, & Smalley, 2007; Kelsey & Bernstein, 1996).
Early-life SES may affect women's reproductive behaviors directly by shaping women's childbearing preferences (Barber, 2000; Kahn & Anderson, 1992). In addition, early-life SES may influence women's reproductive behaviors indirectly through women's educational attainment. Parents’ SES is positively associated with children's education (Sewell & Hauser, 1975). In turn, women with higher education have lower fertility, later age at first birth, and a greater prevalence of childlessness (Heck & Pamuk, 1997; Martin, 2000).
PURPOSE
We use data from a 50-year longitudinal cohort study and integrate the life course theoretical framework with structural equation modeling to explore direct and indirect effects of early-life SES on breast cancer prevalence in later life. This study's exploration of life course mechanisms linking early-life socioeconomic environment to breast cancer will provide further insights into breast cancer etiology and identify life stages that may be potentially the most important for prevention efforts (Ben-Shlomo & Kuh, 2002). Consistent with the critical period model, we hypothesize that early-life SES is a marker of diverse exposures in childhood and adolescence that have a long-lasting irreversible effect on the prevalence of breast cancer, and this effect is not explained by women's SES and other characteristics in adulthood. According to the accumulation of risks perspective, we predict that early-life SES affects breast cancer indirectly via women's achieved SES and reproductive behaviors in adulthood.
METHODS
Data
The Wisconsin Longitudinal Study (WLS) is a long-term study of a random sample of 10,317 men and women who graduated from Wisconsin high schools in 1957. Every third graduate from all Wisconsin high schools (class of 1957) was randomly selected to participate in the study. Participants were interviewed at ages 17-18 (in 1957), 36 (in 1975), 53-54 (in 1993), and 64-65 (in 2004). Survey data were also collected from a selected sibling in 1977, 1994, and 2005. The overwhelming majority of the WLS participants are non-Hispanic White because very few members of racial or ethnic minority groups graduated from Wisconsin high schools in the 1950s. The WLS sample retention is exceptionally high. The baseline 1957 sample comprised 5,326 women, over 90% of whom (4,808 women) participated in the 1975 wave. About 90% of the 1975 female participants were re-interviewed in 1993, and 77% of the 1975 women participated in the 2004 interview. In addition, 663 sisters of the main participants were interviewed in all waves between 1977 and 2005. Our analytic subsample contains women who participated in all waves: 275 women (222 main participants and 53 siblings) who have been diagnosed with breast cancer by 2004-2005 and 4,000 women who have never been diagnosed with breast cancer. In addition, we conduct a sensitivity analysis based on 51 main participants and 45 sisters whose death of breast cancer was established via the National Death Index. We have information about mortality status of 94% of all women who did not participate in the 2004-2005 wave. Among 1,209 female participants who are known to be deceased, the cause of death was ascertained for 772 women (64% of all deceased women). Of all women with the known cause of death, 12% died of breast cancer.
We conducted detailed analyses (available upon request) to evaluate how sample attrition can potentially bias our findings. All attrition analyses combined suggest that this study may be more representative of women with a higher income in young adulthood and larger families. Otherwise, there is little evidence that attrition related to cancer or other characteristics can significantly bias our findings.
Measures
The main dependent variable – breast cancer prevalence – is coded 1 for women who were diagnosed with breast cancer by 2004-2005 and 0 for women who have not been diagnosed with breast cancer. This variable excludes 96 women who died of breast cancer. In a sensitivity analysis, we use an additional dependent variable coded 1 for all women diagnosed with breast cancer, both alive in 2004-2005 and deceased by that time.
Life-course biological variables included in all models are age at menarche, age at menopause, the presence and age of hysterectomy/oophorectomy, and family history of breast cancer. In addition, all models adjust for birth year. All main participants were born in 1939, whereas siblings’ birth year ranges from 1929 to 1950, with the median being 1941.
Early-life variables
Sociodemographic family background characteristics were reported in 1957 and 1975 by main participants and in 1977 by siblings. Additional information was obtained from Wisconsin tax records in early 1960s. Sociodemographic characteristics of the family of origin include fathers’ and mothers’ education, family income in 1957 measured in $100's, fathers’ occupation (professional/executive, white collar worker, skilled worker, unskilled worker, and farmer), fathers’ occupational education (the proportion of individuals in a given occupation who completed one or more years of college), and fathers’ occupational income (the proportion of individuals in a given occupation who earned more than $10,000 in 1969). The last two measures are used in sociological research on social stratification and status attainment (Hauser & Warren, 1997; Warren, Sheridan, & Hauser, 1998).
1975-1977 variables
Education was assessed as the total completed years of schooling. Women's occupation is represented with several categories: housewife; professional occupation; sales, administrative, or service occupation; other occupation (laborer, farmer, operative, etc.). Household income reflects a natural log of total earnings of all family members in $100's. Marital status is coded 1 if a woman was married and 0 if she was unmarried. Parental status is represented with three variables: a dummy variable coded 1 if a woman had born at least one child by 1975-1977, age at first birth, and the total number of children. We use the 1975-1977 measures rather than measures obtained later in the life course to improve causal inference because the 1975-1977 variables had been assessed before the first woman in our sample was diagnosed with breast cancer.
Analytic Plan
We start the analysis with a comparison of summary statistics for the focal study variables between women who were diagnosed with breast cancer and women without breast cancer. Further, to examine life course pathways linking early-life SES to women's breast cancer, we use structural equation modeling (SEM). This approach enables us to explore hypothesized causal relationships among the variables and to model direct and indirect pathways between early-life SES and breast cancer. Our structural equation model involves two components: the structural part and the measurement part.
The structural model of early-life SES and breast cancer is presented in Figure 1. The model contains two unobserved latent factors: early-life SES (η1) and women's SES in adulthood in 1975-1977 (η2). There are also four observed variables in the structural model: breast cancer diagnosed by 2004-2005 (Y1), whether a woman had given at least one birth by 1975-1977 (Y2), age at first birth (Y3), and the number of biological children in 1975-1977 (Y4). In addition, the model adjusts for the main respondent or sibling status, birth year, age at menarche, age at menopause, hysterectomy and/or oophorectomy, and family history of breast cancer. Direct paths a-l in combination with indirect paths bc, de, and fg describe the relationships among the focal variables. Each path is described in detail in the Note to Table 2.
Table 2.
Standardized Regression Coefficients and Model Fit Statistics for the Focal Structural Equation Models
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | |
|---|---|---|---|---|---|
| Path a | 0.019 | -0.091* | -0.085* | -0.103* | -0.093* |
| Path b | 0.595*** | 0.596*** | 0.603*** | 0.619*** | |
| Path c | 0.185*** | 0.173*** | 0.198*** | 0.080 | |
| Path d | -0.048 | ||||
| Path e | -0.037 | ||||
| Path f | -0.178*** | ||||
| Path g | -0.011 | ||||
| Path h | 0.181*** | ||||
| Path i | 0.072* | ||||
| Path j | -0.344*** | ||||
| Path k | -0.598*** | ||||
| Path l | 0.454*** | ||||
| Path bc | 0.110*** | 0.103*** | 0.120*** | 0.049 | |
| Path de | 0.002 | ||||
| Path fg | 0.002 | ||||
| Path hi | 0.013* | ||||
| Model fit: | |||||
| χ2 (df) | 229.82 (26) | 500.11 (58) | 724.48 (68) | 749.08 (67) | 470.98 (66) |
| RMSEA | 0.043 | 0.042 | 0.048 | 0.049 | 0.040 |
| CFI | 0.963 | 0.942 | 0.917 | 0.916 | 0.943 |
| N | 4,275 | 4,275 | 4,275 | 4,275 | 3,750 |
Note:
p < .05.
**p < .01.
p < .001.
RMSEA = root mean square error of approximation; CFI = comparative fit index; df = degrees of freedom.
Paths: Path a: SES in 1957 → breast cancer (BC) in 2004-2005. Path b: SES in 1957 → SES in 1975-1977. Path c: SES in 1975-1977 → BC in 2004-2005. Path d: SES in 1957 → birth by 1975-1977. Path e: birth by 1975-1977 → BC in 2004-2005. Path f: SES in 1957 → number of children in 1975-1977. Path g: number of children in 1975-1977 → BC in 2004-2005. Path h: SES in 1957 → age at first birth in 1975-1977. Path i: age at first birth in 1975-1977 → BC in 2004-2005. Path j: SES in 1975-1977 ↔ birth by 1975-1977. Path k: SES in 1975-1977 ↔ number of children in 1975-1977. Path l: SES in 1975-1977 ↔ age at first birth in 1975-1977. Path bc: SES in 1957 → SES in 1975-1977 → BC in 2004-2005. Path →: SES in 1957 → birth by 1975-1977 → BC in 2004-2005. Path fg: SES in 1957 → number of children in 1975-1977 → BC in 2004-2005. Path hi: SES in 1957 → age at first birth in 1975-1977 → BC in 2004-2005. All paths are shown in Figure 1.
Models: Model 1: SES in 1957. Model 2: SES in 1957 and 1975-1977. Model 3: SES in 1957 and 1975-1977, and birth by 1975-1977. Model 4: SES in 1957 and 1975-1977, and the number of children in 1975-1977. Model 5: SES in 1957 and 1975-1977, and age at first birth in 1975-1977 (mothers only). Model 5 is illustrated in Figure 2.
Source: The Wisconsin Longitudinal Study, 1957-2005.
The measurement part is a confirmatory factor analysis of indicators measuring two unobserved latent constructs: early-life SES (η1) and adult SES (η2). The measurement model for early-life SES includes six items loading on one factor: father's education, mother's education, family income, father's occupation, father's occupational education, and father's occupational income. The measurement model for adult SES contains three items loading on one factor: women's education, occupation, and household income.
All structural equation models were estimated using Mplus 5.2 (Munthén & Munthén, 2007). To evaluate the fit of our models, we use three measures of goodness of fit: adjusted χ2, the comparative fit index (CFI), and the root mean square error of approximation (RMSEA).
RESULTS
We compared breast cancer survivors to women who have not been diagnosed with breast cancer by 2004-2005 in terms of the focal study variables. Table 1 indicates that most early-life socioeconomic characteristics did not differ significantly between women with and without breast cancer. These patterns are consistent with existing research showing no overall effect of early-life SES on breast cancer. Family income is the only early-life variable that differs between the two groups of women: women with breast cancer had significantly higher income in adolescence than women without breast cancer (63.7 versus 58.9, p < .01).
Table 1.
Summary Statistics for the Focal Study Variables by Breast Cancer Status
| Variables | Breast cancer (n = 275) | No breast cancer (n = 4,000) |
|---|---|---|
| Early-Life SES: | ||
| Father's education | 9.79 (3.52) | 9.75 (3.37) |
| Mother's education | 10.56 (2.88) | 10.41 (2.84) |
| Father's occupation | 2.49 (1.51) | 2.48 (1.45) |
| Father's occupational education (ln) | 2.67 (.88) | 2.64 (.85) |
| Father's occupational income | 3.05 (.89) | 3.08 (.88) |
| Family income | 63.73** (33.91) | 58.94 (31.29) |
| SES in Adulthood 1975-1977: | ||
| Education | 13.48*** (2.15) | 13.05 (1.82) |
| Professional/executive occupation | .26*** | .16 |
| Household income (ln) | 4.21 (2.42) | 4.33 (2.17) |
| Reproductive Behaviors in 1975-1977: | ||
| At least one birth by 1975-1977 | .84* | .88 |
| Number of children | 2.42** (1.63) | 2.72 (1.65) |
| Age at first birth | 23.88*** (3.84) | 23.03 (3.26) |
Note: Each cell contains proportions or means with standard deviations in parentheses. Asterisks denote a significant difference between women with and without breast cancer:
p < .05.
p < .01.
p < .001.
Source: The Wisconsin Longitudinal Study, 1957-2005.
With respect to women's adult SES in 1975-1977, breast cancer survivors had higher levels of education and were more likely to be in professional or executive occupations than women who were not diagnosed with breast cancer by 2004-2005. Finally, consistent with widely documented reproductive risk factors for breast cancer (Kelsey & Bernstein, 1996), women with breast cancer were significantly less likely to give birth by 1975-1977 (p < .01), had lower parity (p < .01), and later age at first birth (p < .001) than women without breast cancer.
In addition, ANOVA comparisons reveal that age at a breast cancer diagnosis did not differ by women's SES. For example, the mean age at diagnosis of daughters of high school graduates was 53.1 compared to the mean age at diagnosis of daughters of non-graduates at 53.3 years (F = 0.11, p = 0.741). The mean age at diagnosis of women who graduated from college and women who did not graduate from college was 56.3 and 55.8 years, respectively (F = 0.19, p = 0.664).
Regression coefficients and fit statistics for structural equation models are presented in Table 2. All models adjust for life course biological variables, including family history of breast cancer. Model 1 shows that the overall effect of early-life SES on breast cancer is small in magnitude and not statistically significant. Yet, as indicated in Model 2, the effect of early-life SES becomes more complex after the inclusion of the adult SES factor and the decomposition of the early-life effect into a direct component and an indirect component.
In Model 2, path a = -0.091 (p < .05) reflects a direct effect of early-life SES on breast cancer after adjustment for adult SES and biological controls. Path bc = 0.110 (p < .001) represents an indirect effect of early-life SES on breast cancer mediated by adult SES. The negative coefficient for path a means that women from higher-SES families of origin had a lower prevalence of breast cancer in 2004-2005 than women from lower-SES families. The positive coefficient for path bc indicates that women from higher-SES families had an elevated prevalence of breast cancer because early-life SES is related positively to achieved SES in adulthood, and higher adult SES, in turn, increases the prevalence of breast cancer. Thus, higher early-life SES decreases the prevalence of breast cancer directly, while increasing the prevalence of breast cancer indirectly via women's own achieved SES. In other words, adult SES, which is associated positively with the prevalence of breast cancer, suppresses the protective influence of higher early-life SES. This suppression effect suggests that were it not for their higher SES in adulthood, women from higher-SES families of origin would have had a lower prevalence of breast cancer than women from lower-SES background.
Model 3 adds a binary indicator of whether a woman had given at least one birth by 1975-1977. As shown by small and nonsignificant paths d and e, having given a birth versus being nulliparous is neither affected by early-life SES nor affects the prevalence of breast cancer. Therefore, this measure does not mediate the effect of early-life SES on breast cancer.
Similar to the previous model, Model 4 in Table 2 shows that the effect of early-life SES is not mediated by the number of children. After the inclusion of the number of children in Model 4, the direct negative effect of early-life SES (path a) increases slightly in magnitude and becomes -0.103 (p < .05). The coefficient for path f (βf = -0.178, p < .001) indicates that early-life SES is associated negatively with the number of children a woman had born by 1975-1977. Yet, the small and not significant coefficient for path g (βg = -0.011) suggests that parity is unrelated to breast cancer prevalence net of adult SES and life course biological variables. Not surprisingly, the effect of early-life SES on breast cancer is not mediated by the number of children (βfg = 0.002, ns). In contrast, the mediating effect of adult SES remains strong (βbc = 0.120, p < .001).
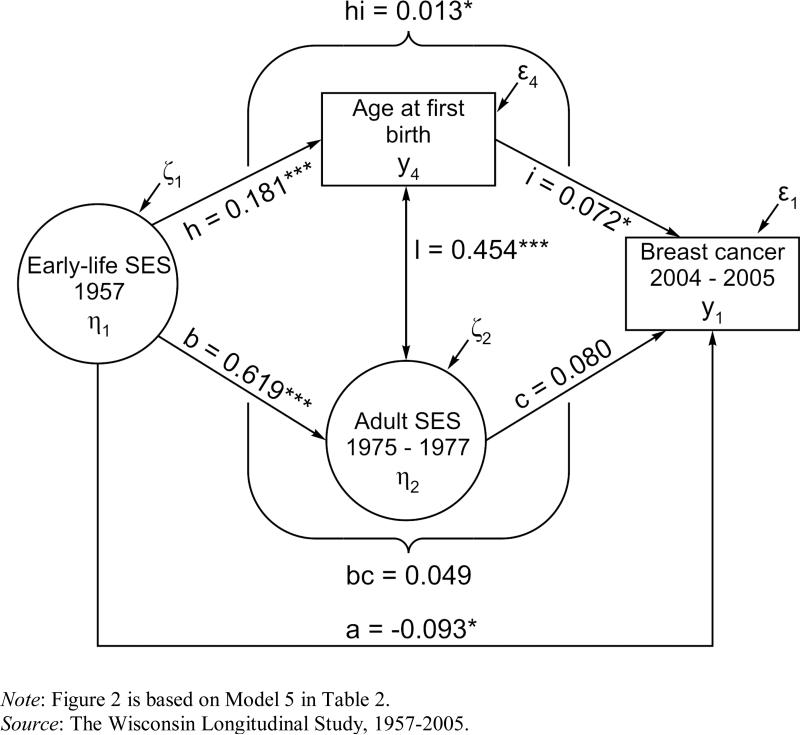
Model 5 adds the effect of age at first birth and, thus, is based on mothers only. This model is graphically represented in Figure 2. Path h (βh = 0.181, p < .001) shows that early-life SES is strongly and positively associated with age at first birth. In turn, path i (βi = 0.072, p < .05) reveals that later age at first birth moderately increases the prevalence of breast cancer. Because the regression coefficients are standardized, this coefficient suggests that as age at first birth increases by one standard deviation (SD = 3.3 years), the prevalence of breast cancer rises by 7%. A comparison of paths a (the direct effect of early-life SES) and hi (the indirect effect of early-life SES via age at first birth) shows that whereas the direct effect is still negative and significant (βa = -0.093, p < .05), the indirect effect is positive and significant (βhi = 0.013, p < .05). Finally, path bc = 0.049 reflects the indirect effect of early-life SES on breast cancer mediated by women's SES in adulthood. Path bc becomes not significant in Model 5, whereas path hi is significant, which suggests that early-life SES operates via adult SES because of age at first birth. Notably, Model 5 is the best fitting model, as indicated by the combination of RMSEA and CFI statistics.
Figure 2.
The Structural Model Representing Direct and Indirect Effects of Early-Life SES via Women's SES in Adulthood and Age at First Birth
Sensitivity Analyses
To address the possibility that higher-SES women are more likely to have regular screening mammograms and, thus, to be diagnosed with breast cancer than lower-SES women (Meissner, Breen, Taubman, Vernon, & Graubard, 2007), we conducted a sensitivity analysis using a measure of self-reported screening mammography utilization that is available only in the most recent wave (2004-2005) of the WLS. Among women who were not diagnosed with breast cancer by 2004-2005, SES at different life stages was not associated with the likelihood of getting a screening mammogram in 2004-2005 because the overwhelming majority of women (over 75%) had a screening mammogram in 2004-2005. Thus, it is unlikely that our findings are driven by the inflation of breast cancer incidence in higher-SES groups compared to lower-SES groups due to screening mammography.
Because the main outcome in our structural equation models is breast cancer prevalence in 2004-2005, we estimated two alternative models to explore whether using measures of breast cancer incidence during specific period might alter our conclusions. First, we limited our analysis to women who were diagnosed with breast cancer between 1993-1994 and 2004-2005 to reflect the incidence of breast cancer between the two most recent waves (N = 191). Second, we estimated the same models for women who were diagnosed with breast cancer between 2000 and 2005 to reflect the five-year incidence of breast cancer (N = 97). The results of these two analyses (available upon request) are very similar to those presented in Table 2, which suggests that our findings are not specific to the measure of breast cancer prevalence in 2004-2005.
Finally, SES differences in breast cancer survival could have potentially affected our findings. To address this possibility, we conducted a sensitivity analysis including all women diagnosed with breast cancer (N = 371)––those who survived until the most recent wave and those who died of breast cancer in the course of the study, as established via the National Death Index. The results from the sensitivity analysis (available upon request) were very similar to the results reported in this study. Thus, there is no evidence that our findings may reflect the SES difference in breast cancer mortality, in particular, poorer survival of lower-SES women.
DISCUSSION
Using data from the Wisconsin Longitudinal Study (WLS) and structural equation modeling, we examine life course mechanisms linking women's early-life SES to the prevalence of breast cancer in later life. Our results contribute to understanding socioeconomic patterns of breast cancer in later life from a life course perspective. To our knowledge, this is the first study to analyze life course pathways incorporating both direct and indirect effects of early-life SES on the prevalence of breast cancer. Moreover, because existing studies of risk factors for breast cancer have overwhelmingly focused on women's characteristics in adulthood (Heck & Pamuk, 1997; Kelsey & Bernstein, 1996), our findings emphasize the importance of early-life environment and mechanisms through which it operates for understanding the etiology of breast cancer.
Our study extends existing research by documenting the complexity of the association between early-life SES and breast cancer. Specifically, we found a negative direct effect, indicating that women from higher-SES families of origin had lower breast cancer prevalence in 2004-2005 than women from lower-SES families. In addition to the direct effect, early-life SES has positive indirect effects on breast cancer via women's adult SES and age at first birth. Women from higher-SES families achieved higher SES in adulthood and had later age at first birth than women from lower-SES family background. In turn, higher SES in adulthood and delayed childbearing are related to a higher prevalence of breast cancer, with women's adult SES operating through age at first birth. It should be noted that the term “effect” is used in a statistical sense and does not imply definitive causal relationships.
The Direct Negative Effect of Early-Life SES on Breast Cancer
The direct effect of early-life SES is consistent with the critical period model, according to which exposures in childhood and adolescence have lasting and irreversible effects on health in later life (Ben-Shlomo & Kuh, 2002). Existing research suggests three potential explanations for why early-life SES may decrease the prevalence of breast cancer directly. One reason may be strenuous physical activity in childhood and adolescence. The incidence and prevalence of breast cancer is lower among women who were athletes in college compared to women who were not, net of exercise patterns in adulthood (Wyshak & Frisch, 2000). Girls from higher-SES families are more likely to exercise vigorously, whereas children of low-SES parents are more likely to experience physical inactivity (Chen, Matthews, & Boyce, 2002). Thus, vigorous exercise of higher-SES girls may protect them against the development of breast cancer later in life.
Another possible explanation may reflect a more healthy diet of girls in higher-SES families. Women who had frequently consumed French fries at preschool age were at an increased risk of breast cancer compared to women with a low consumption of fried foods (Michels, Rosner, Cameron, Colditz, & Willett, 2006). Because daughters of higher-SES parents are less likely to consume fatty and energy dense unhealthy foods (Lioret, Touvier, Lafay, Volatier, & Maire, 2008; Mendoza, Drewnowski, Cheadle, & Christakis, 2006), they may have a lower breast cancer prevalence in adulthood.
Finally, early-life SES may decrease the prevalence of breast cancer by decreasing chronic inflammation. Women from families with less educated parents had higher levels of circulating C-reactive protein (CRP) at midlife than women with higher parental education, independent of their own educational attainment (Phillips et al., 2009). CRP is a measure of chronic low-grade inflammation, and there is evidence that chronic inflammation may contribute to the development and progression of breast cancer (Allin, Bojesen, & Nordestgaard, 2009; Coussens & Werb, 2002). It is possible that higher SES of the family of origin may decrease chronic inflammation over the life course and, thus, reduce the risk of breast cancer in adulthood and later life. We could not test these explanations because our data do not contain relevant measures of early-life environment. Thus, an important direction for future inquiry will be an empirical verification of the three mechanisms we propose based on existing research.
Women's Adult SES, Age at First Birth, and Breast Cancer
Consistent with the accumulation of risks model, we also found that the effect of early-life SES on breast cancer is mediated by women's SES in adulthood and age at first birth, with women's adult SES operating through age at first birth. This finding is in accord with previous research, which suggests that the positive association between women's SES and breast cancer incidence is largely explained by reproductive factors (de Kok et al., 2008; Heck & Pamuk, 1997). Women with higher SES have lower fertility, later age at first birth, and greater prevalence of childlessness (Heck & Pamuk, 1997). College-educated women are likely to remain childless throughout their 20s and delay childbearing into their 30s (Martin, 2000).
Later age at first birth (especially after age 30) and lower parity are associated with a moderately elevated prevalence of breast cancer (Kelsey & Bernstein, 1996). About 70% of breast cancers are hormone-dependent and express estrogen (ER) and/or progesterone (PR). Reproductive factors are more strongly related to ER+/PR+ tumors than ER- and/or PR- tumors (Trivers et al., 2009). Earlier age at first birth decreases women's chances of developing ER+/PR+ breast tumors (Britt et al., 2007). In addition, earlier first birth leads to an earlier differentiation of epithelial breast cells, which makes them less proliferative and less susceptible to malignant transformations (Britt et al., 2007). Although our data do not contain information on the tumor type, based on existing research we speculate that the positive indirect pathway linking early-life SES to breast cancer is particularly relevant for hormone-positive tumors.
Limitations and Future Research
This study is based on women who came of age in the 1950s and early 1960s. Pathways linking early-life SES and breast cancer may be different for women of recent cohorts who differed from our participants in terms of status attainment and childbearing (Casper & Bianchi, 2002). An important direction for future research is to explore cohort differences in the life course pathways analyzed in this study.
The diagnosis of breast cancer in our study is self-reported; therefore, report bias may potentially present a problem for our analysis. Women may have breast cancer but do not report it simply because they are unaware of it. Yet, research linking self-reports of cancer to state cancer registries suggests that individuals accurately report a past diagnosis of cancer (Bergmann et al., 1998). In addition, we use Monte Carlo (MC) simulations to examine how our findings may change under different scenarios of underreporting and overreporting of a breast cancer diagnosis. Results from MC simulations (available upon request) suggest that our findings are robust to the report bias and, thus, are unlikely to be an artifact of breast cancer misreporting.
Early-life SES may be associated with breast cancer prevalence via women's lifestyle and health behaviors in adulthood. The WLS assessed women's health behaviors in 1993-1994 and 2004-2005 but not at earlier life course stages. An additional analysis (available upon request) showed that health behaviors measured in 1993-1994 were unrelated to subsequent breast cancer incidence and mortality. It is possible that health behaviors at earlier life stages are more important than lifestyle in midlife. Ideally, future studies of breast cancer should include information about diet, body mass index, and physical activity assessed prospectively in childhood, adolescence, and young adulthood.
Further, the WLS contains only White non-Hispanic participants. Given racial and ethnic differences in SES (Hayward & Gorman, 2004) and biological differences in breast cancer etiology and progression (Baquet & Commiskey, 2000; Gerend & Pai, 2008), the proposed mechanisms cannot be directly extrapolated to minority women. Moreover, two-thirds of women lived in Wisconsin in 2004-2005, which makes our findings specific to a particular geographic location. Thus, an important direction for future inquiry will be to compare pathways linking early-life SES and breast cancer in later life among different racial and ethnic groups using nationally representative data sets.
CONCLUSION
Consistent with the life course framework, our analysis identified mechanisms linking social environment early in life to breast cancer decades later. Using data from a 50-year longitudinal cohort study and structural equation modeling, we found a negative direct effect of early-life SES, indicating that women from higher-SES families of origin had lower breast cancer prevalence than women from lower-SES families. In addition, early-life SES has a positive indirect effect on breast cancer via women's adult SES and age at first birth. Were it not for their higher SES in adulthood and delayed childbearing, women from higher-SES families of origin would have had lower breast cancer prevalence than women from lower-SES families. Yet, early-life SES is associated positively with adult SES and age at first birth, and women's higher SES in adulthood and delayed childbearing are related to higher breast cancer prevalence. The present study documents the importance of socioeconomic and reproductive conditions in adolescence and young adulthood for the development of breast cancer later in life.
REFERENCES
- Allin KH, Bojesen S, Nordestgaard B. Baseline C-reactive protein is associated with incident cancer and survival in patients with cancer. Journal of Clinical Oncology. 2009;27:2217–24. doi: 10.1200/JCO.2008.19.8440. [DOI] [PubMed] [Google Scholar]
- Baquet C, Commiskey P. Socioeconomic factors and breast carcinoma in multicultural women. Cancer. 2000;88:1256–64. doi: 10.1002/(sici)1097-0142(20000301)88:5+<1256::aid-cncr13>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Barber JS. Intergenerational influences on the entry into parenthood: Mothers’ preferences for family and nonfamily behavior. Social Forces. 2000;79:319–48. [Google Scholar]
- Ben-Shlomo Y, Kuh D. A life course approach to chronic disease epidemiology: Conceptual models, empirical challenges and interdisciplinary perspectives. International Journal of Epidemiology. 2002;31:285–93. [PubMed] [Google Scholar]
- Bergmann MM, Calle EE, Mervis CA, Miracle-McMahill HL, Thun MJ, Heath CW. Validity of self-reported cancers in a prospective cohort study in comparison with data from state cancer registries. American Journal of Epidemiology. 1998;147:556–62. doi: 10.1093/oxfordjournals.aje.a009487. [DOI] [PubMed] [Google Scholar]
- Britt K, Ashworth A, Smalley M. Pregnancy and the risk of breast cancer. Endocrine-Related Cancer. 2007;14:907–33. doi: 10.1677/ERC-07-0137. [DOI] [PubMed] [Google Scholar]
- Casper LM, Bianchi SM. Continuity and change in the American family. Sage Publications; 2002. [Google Scholar]
- Chen E, Matthews K, Boyce W. Socioeconomic differences in children's health: How and why do these relationships change with age? Psychological Bulletin. 2002;128:295–29. doi: 10.1037/0033-2909.128.2.295. [DOI] [PubMed] [Google Scholar]
- Colditz GA, Frazier L. Models of breast cancer show that risk is set by events of early life: Prevention efforts must shift focus. Cancer Epidemiology, Biomarkers & Prevention. 1995;4:567–71. [PubMed] [Google Scholar]
- Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–67. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kok I, van Lenthe F, Avendano M, Louwman M, Coebergh J-W, Mackenbach J. Childhood social class and cancer incidence: Results of the GLOBE study. Social Science & Medicine. 2008;66:1131–39. doi: 10.1016/j.socscimed.2007.11.035. [DOI] [PubMed] [Google Scholar]
- Frijters P, Hatton TJ, Martin RM, Shields MA. Childhood economic conditions and length of life: Evidence from the UK Boyd Orr cohort, 1937-2005. Journal of Health Economics. 2010;29:39–47. doi: 10.1016/j.jhealeco.2009.10.004. [DOI] [PubMed] [Google Scholar]
- Gerend MA, Pai M. Social determinants of Black-White disparities in breast cancer mortality: A review. Cancer Epidemiology, Biomarkers & Prevention. 2008;17:2913–23. doi: 10.1158/1055-9965.EPI-07-0633. [DOI] [PubMed] [Google Scholar]
- Hauser RM, Warren JR. Socioeconomic indexes for occupations: A review, update, and critique. Sociological Methodology. 1997;27:177–298. [Google Scholar]
- Hayward MD, Gorman BK. The long arm of childhood: The influence of early-life social conditions on men's mortality. Demography. 2004;41:87–107. doi: 10.1353/dem.2004.0005. [DOI] [PubMed] [Google Scholar]
- Heck KE, Pamuk ER. Explaining the relation between education and postmenopausal breast cancer. American Journal of Epidemiology. 1997;145:366–72. doi: 10.1093/oxfordjournals.aje.a009114. [DOI] [PubMed] [Google Scholar]
- Jeffreys M, Warren R, Gunnell D, McCarron P, Davey Smith G. Life course breast cancer risk factors and adult breast density (United Kingdom). Cancer Causes and Control. 2004;15:947–55. doi: 10.1007/s10522-004-2473-3. [DOI] [PubMed] [Google Scholar]
- Kahn JR, Anderson K. Intergenerational patterns of teenage fertility. Demography. 1992;29:39–57. [PubMed] [Google Scholar]
- Kelsey JL, Bernstein L. Epidemiology and prevention of breast cancer. Annual Review of Public Health. 1996;17:47–67. doi: 10.1146/annurev.pu.17.050196.000403. [DOI] [PubMed] [Google Scholar]
- Lawlor DA, Sterne JA, Tynelius P, Davey Smith G, Rasmussen F. Association of childhood socioeconomic position with cause-specific mortality in a prospective record linkage study of 1,839,384 individuals. American Journal of Epidemiology. 2006;164:907–15. doi: 10.1093/aje/kwj319. [DOI] [PubMed] [Google Scholar]
- Lioret S, Touvier M, Lafay L, Volatier J, Maire B. Dietary and physical activity patterns in French children are related to overweight and socioeconomic status. Journal of Nutrition. 2008;138:101–107. doi: 10.1093/jn/138.1.101. [DOI] [PubMed] [Google Scholar]
- Lynch JW, Davey Smith G. A life course approach to chronic disease epidemiology. Annual Review of Public Health. 2005;26:1–35. doi: 10.1146/annurev.publhealth.26.021304.144505. [DOI] [PubMed] [Google Scholar]
- Martin SP. Diverging fertility of U.S. women who defer childbearing past age 30. Demography. 2000;37:523–33. doi: 10.1353/dem.2000.0007. [DOI] [PubMed] [Google Scholar]
- Meissner HI, Breen N, Taubman ML, Vernon SW, Graubard BI. Which women aren't getting mammograms and why? Cancer Causes and Control. 2007;18:61–70. doi: 10.1007/s10552-006-0078-7. [DOI] [PubMed] [Google Scholar]
- Mendoza J, Drewnowski A, Cheadle A, Christakis DA. Dietary energy density is associated with selected predictors of obesity in U. S. children. Journal of Nutrition. 2006;136:1318–22. doi: 10.1093/jn/136.5.1318. [DOI] [PubMed] [Google Scholar]
- Michels KB, Rosner B, Cameron CW, Colditz GA, Willett WC. Preschool diet and adult risk of breast cancer. International Journal of Cancer. 2006;118:749–54. doi: 10.1002/ijc.21407. [DOI] [PubMed] [Google Scholar]
- Munthén LK, Munthén BO. Mplus user's guide. 5th ed. Munthén & Munthén; Los Angeles, CA: 1998-2007. [Google Scholar]
- Naess O, Strand BH, Davey Smith G. Childhood and adulthood socioeconomic position across 20 causes of death: A prospective cohort study of 800,000 Norwegian men and women. Journal of Epidemiology and Community Health. 2007;61:1004–1009. doi: 10.1136/jech.2006.052811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips JE, Marsland AL, Flory JD, Muldoon MF, Cohen S, Manuck S. Parental education is related to C-reactive protein among female middle-aged community volunteers. Brain, Behavior, and Immunity. 2009;23:677–683. doi: 10.1016/j.bbi.2009.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power C, Hyppönen E, Davey Smith G. Socioeconomic position in childhood and early adult life and risk of mortality: A prospective study of the mothers of the 1958 British birth cohort. American Journal of Public Health. 2005;95:1396–402. doi: 10.2105/AJPH.2004.047340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sewell W, Hauser RM. Education, occupation, and earnings: Achievement in the early career. Academic Press; New York: 1975. [Google Scholar]
- Strand BH, Kunst A. Childhood socioeconomic position and cause-specific mortality in early adulthood. American Journal of Epidemiology. 2007;165:85–93. doi: 10.1093/aje/kwj352. [DOI] [PubMed] [Google Scholar]
- Trivers K, Lund M, Porter P, Liff J, Flagg E, Coates R, Eley W. The epidemiology of triple-negative breast cancer, including race. Cancer Causes and Control. 2009;20:1071–82. doi: 10.1007/s10552-009-9331-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren JR, Sheridan JT, Hauser RM. Choosing a measure of occupational standing: How useful are composite measures in analysis of gender inequality in occupational attainment? Sociological Methods and Research. 1998;27:3–76. [Google Scholar]
- Wyshak G, Frisch RE. Breast cancer among former college athletes compared to non-athletes: A 15-year follow-up. British Journal of Cancer. 2000;82:726–30. doi: 10.1054/bjoc.1999.0987. [DOI] [PMC free article] [PubMed] [Google Scholar]